Abstract
Background:
Neisseria gonorrhoeae cross-protection was suggested in a New Zealand meningitis B vaccine. We modeled the potential impact of similar vaccines on gonorrhea prevalence in heterosexuals in the United States.
Methods:
Our mathematical model incorporated infection, behavior, and vaccination dynamics. Approximate Bayesian Computation calibrated our model to US prevalence. Primary analyses assumed New Zealand vaccine characteristics: 30% efficacy and 2-year duration of protection. We estimated impact under two vaccine coverages (20%, 50%).
Results:
Reduction in gonorrhea prevalence ranged from 4.8 to 39.4%, depending on vaccine coverage. Vaccine impact was correlated with both size of the highly sexually active subpopulation and sexual mixing between high and low activity subpopulations.
Conclusions:
A meningitis vaccine providing low efficacy cross-protection against gonorrhea acquisition and short duration of protection could result in a large reduction in gonorrhea prevalence in the United States. Potential dual protective effects can be considered when making vaccine recommendations.
1. Introduction
Neisseria gonorrhoeae (NG) reported cases have risen 92 % between 2009 and 2019 [1]. About half of NG infections in the United States are resistant to at least one antibiotic [2]. As a result, NG was identified as a priority pathogen for vaccine development in 2019 [3]. NG vaccine development is challenging since there are no known correlates of protection and no natural non-human NG hosts [4,5]. Despite some progress [4], there are currently no NG-specific vaccines. However, an outer membrane vesicle (OMV) meningococcal B vaccine for a specific epidemic strain in New Zealand (MeNZB, Chiron) suggested cross-protection against NG with an estimated 30 % efficacy and 2-year duration of protection [6]. This is also supported by decreases in NG incidence after vaccination campaigns against meningitis B using other OMV-based vaccines in Cuba and Norway [7,8].
The 2018 uptake of meningitis B vaccination among 17-year-olds in the United States was 17.2 % [9] across two vaccine types: a 4-component meningococcal serogroup B OMV vaccine (4CMenB, Bexsero, GSK) [10] and a recombinant protein-based vaccine (MenB-FHbp, Trumemba, Pfizer) [11]. 4CMenB contains OMVs and three recombinant proteins; cross-protection against NG is induced through homologous OMV recombinant proteins and Neisseria heparin binding antigen [12]. MenB-FHbp is unlikely to exhibit the same cross-protection as 4CMenB because the factor H binding protein (fHbp) of N. meningitidis is not expressed on the surface of NG [12].
Mathematical modeling studies have shown a vaccine with characteristics similar to MeNZB could reduce NG prevalence and incidence in a variety of populations. In Australia, modeled vaccination during adolescence with a 20 % efficacious vaccine that provided permanent protection resulted in a 40 % reduction in modeled NG prevalence after 20 years. In a similar study with a vaccine that provided 10 years of protection a decrease of 83,167 NG infections in a single birth cohort was estimated. Other modeling studies have evaluated the impact of NG-vaccines on drug resistant NG and vaccine optimization. None of these models assessed whether uncertainty in their underlying NG transmission parameters affected predicted vaccine impact [13–17].
With the growing burden of NG, the Health and Human Services Office of Disease Prevention and Health Promotion set a Healthy People 2030 ten-year objective to reduce gonorrhea rates 10 % in adolescent and young males 15 to 24 years old by 2030 [18]. We assessed whether a vaccine similar to 4CMenB could help achieve a similar objective of a 10 % reduction in males and females ages 15–24 years in the United State by constructing and analyzing a mathematical model involving heterosexual NG transmission and vaccination. We also assessed the benefits of a vaccine with either improved efficacy or duration of protection. Finally, we assessed how NG input parameter uncertainty affected our estimates.
2. Materials and methods
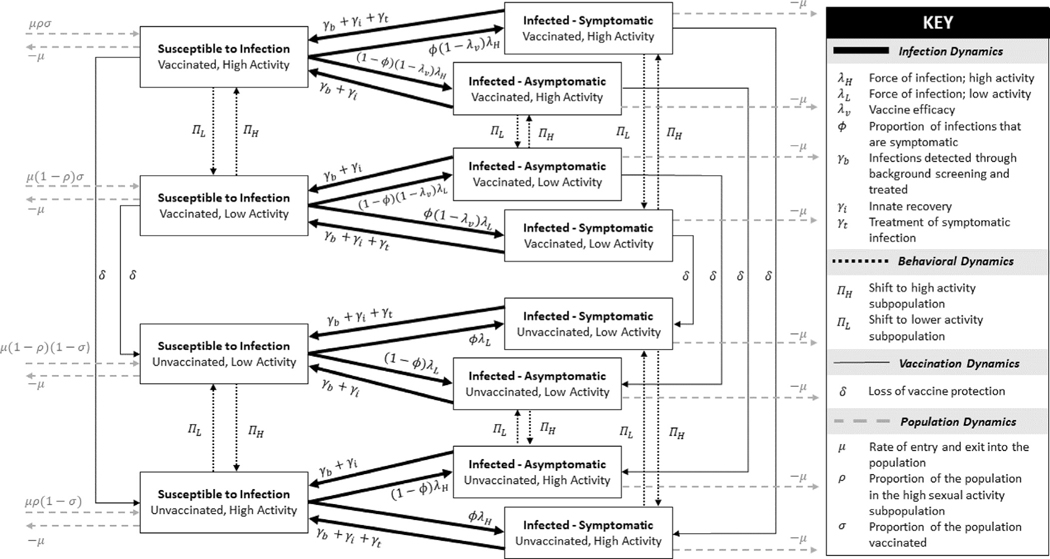
We constructed a heterosexual NG transmission model using ordinary differential equations. In the model people are categorized by sex, infection status, sexual activity level, and vaccination status (Fig. 1). We assumed equivalent and constant male and female population sizes with model entry balanced by exit. For infection status, we classified people as either susceptible, symptomatically infected, or asymptomatically infected, modeling only urogenital NG infection, ignoring extragenital infection. Infection recovery occurred through either seeking treatment (symptomatic infections only), detection of infection through routine screening, or natural clearance; reinfections could occur. Activity levels were defined by the average sexual contact rate per year, and people in the population were categorized into either a high or low activity sub-population. A mixing parameter was used to determine the proportion of sexual contacts between people in the same activity level. Higher levels of this mixing parameter caused higher assortativity, where more sexual contacts occurred between people in the same activity sub-population (more high-high and low-low contacts); lower levels of the mixing parameter caused lower assortativity, where more sexual contacts were with people of the opposite activity sub-population. A full list of parameter values can be found in Supplemental Table S1.
Fig. 1. Model dynamics.
Four types of dynamics are modeled: infection, behavioral, vaccination, and population. Infection dynamics (thick black line) account for infection and recovery; behavioral dynamics (dotted black line) account for movement between the high and low sub-populations; vaccine dynamics (thin black line) account for the loss of vaccine protection; population dynamics (dashed grey line) account for entry to and exit from the population. Vaccine administration is assumed to occur just prior to sexual debut. Most parameters take sex-specific values (not shown; Supplemental materials summarize these details). Unvaccinated states include people who have never received the vaccine and also people previously vaccinated who have lost vaccine protection due to waning.
Uncertainty exists around key parameter values that describe gonorrhea transmission such as rates of natural clearance, rates of routine background screening, the proportion of infections that are symptomatic, and the number of contacts per year (Table 1). Because of this uncertainty, we used Approximate Bayesian Computation to identify 10,000 uncorrelated parameter sets with the same baseline equilibrium prevalence: 1.125 % in females and 0.75 % in males. Gonorrhea prevalence has previously been estimated between 2008 and 2018 among 15–24 year-olds to range from 0.1 to 7.6 % for females and 0.0–4.8 % for males [19,20].
Table 1.
Correlation of fitted parameters with predicted vaccine impact for a vaccine with 30% efficacy and a 2-year duration of protection by vaccine coverage assuming a baseline NG prevalence of 1.125% in women and 0.75% in men.
| Parameter Description | Parameter | Low Coverage (20 %) | High Coverage (50 %) |
|---|---|---|---|
|
| |||
| Probability of transmission per contact, female to male transmission compared to male to female transmission | −0.005 | −0.010 | |
| Proportion of infections that are symptomatic in males | −0.001 | −0.013 | |
| Proportion of infections that are symptomatic in females compared to males | 0.045 | 0.041 | |
| Symptomatic treatment rate | 0.062 | 0.071 | |
| Proportion of the population in the high activity subgroup | 0.303 | 0.306 | |
| Number of contacts per year, low activity subgroup | −0.073 | −0.076 | |
| Number of contacts per year, high activity subgroup compared to the low activity subgroup | −0.101 | −0.103 | |
| Proportion of contacts reserved for assortative mixing | −0.908 | −0.906 | |
| Routine recommended screening rate for females | −0.009 | −0.010 | |
| Routine recommended screening rate for males compared to females | −0.016 | −0.016 | |
| Natural clearance rate for males (days) | 0.072 | 0.076 | |
| Natural clearance rate for females compared to males | −0.065 | −0.058 | |
We defined hypothetical vaccine candidates by their efficacy and duration of protection. Efficacy was modeled as a reduction in susceptibility to infection and was assumed to be constant throughout the entire duration of protection. To reflect the vaccine performance described in New Zealand, a modeled OMV vaccine candidate had 30 % efficacy and 2-year duration of protection. A hypothetical NG-specific vaccine could have improved performance, so we also evaluated hypothetical candidates with improved efficacy (50 %, 70 %) and durations of protection (5 years, 8 years).
All vaccine candidates were modeled at a low (20 %) and high (50 %) population coverage, representing the percent of the population vaccinated prior to sexual debut (Fig. 1). These values were selected to approximate current coverage of meningitis B vaccination in 17-year-olds (17.2 %) and human papillomavirus (HPV) vaccination in 13-year-olds in the United States in 2018 (50.8 %) [9].
Vaccine impact was measured as the percent reduction of NG prevalence after 10 years. Impact predictions were made for each scenario (of the 10,000 parameter sets) for all combinations of vaccine candidates and coverage. The effect of parameter uncertainty on predicted impact was assessed individually using correlation coefficients. All models and analyses were conducted in R using version 3.6.1. A full description of the model equations and parameters can be found in Supplemental Materials.
2.1. Sensitivity analysis
To assess how baseline prevalence affected vaccine impact, we performed the same analyses assuming a higher baseline prevalence: 2.25 % in females and 1.5 % in males.
3. Results
3.1. Predicted impact
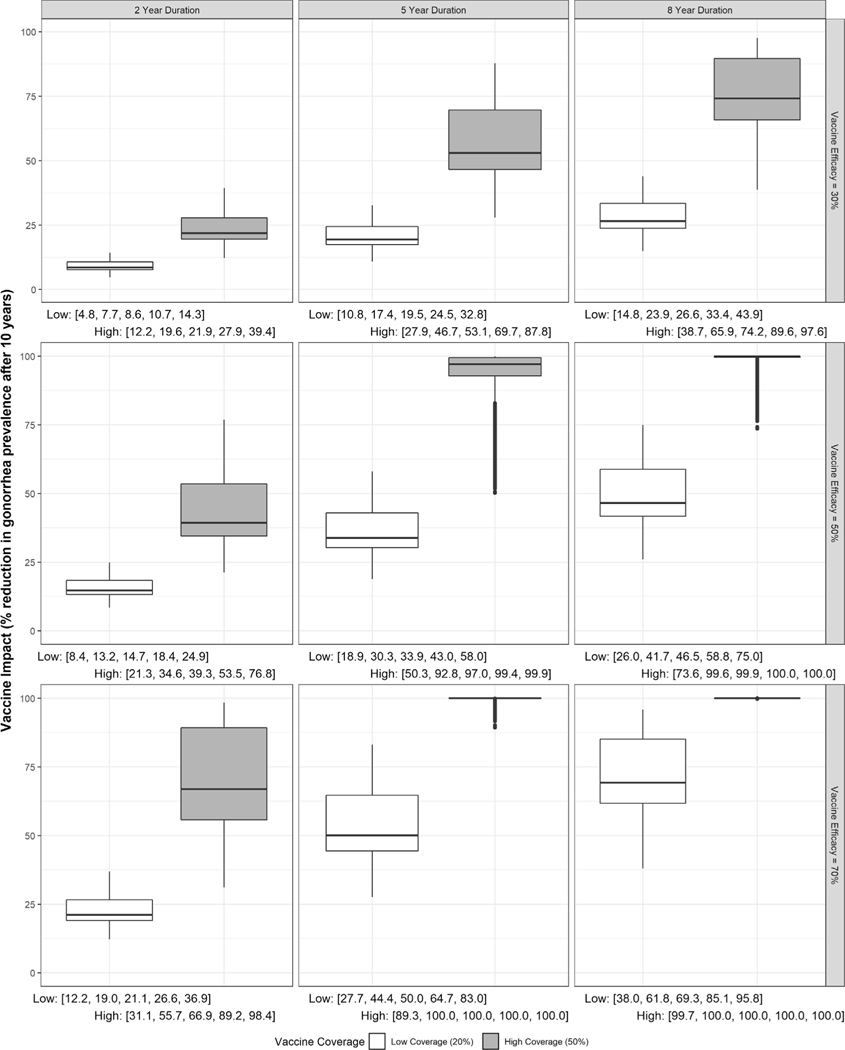
Our model estimated a vaccine with 30 % efficacy and 2-year duration of protection would reduce NG prevalence by 4.8–39.4 % depending on coverage and scenario (Fig. 2). Under higher vaccine coverage, >10 % prevalence reduction was predicted in all scenarios with the impact ranging from 12.2 to 39.4 %. However, with low vaccine coverage, >10 % prevalence reduction was achieved in about 1/3 of scenarios with impact ranging from 4.8 to 14.3 %.
Fig. 2. Distribution of predicted impact for vaccines with 30%, 50%, or 70% efficacy and 2, 5, or 8-year duration under low (20%) or high (50%) and vaccine coverage assuming a baseline prevalence of 1.125% in women and 0.75% in men.
Below each panel are the minimum, 25th percentile, median, 75th percentile, and maximum predicted impact at each coverage level.
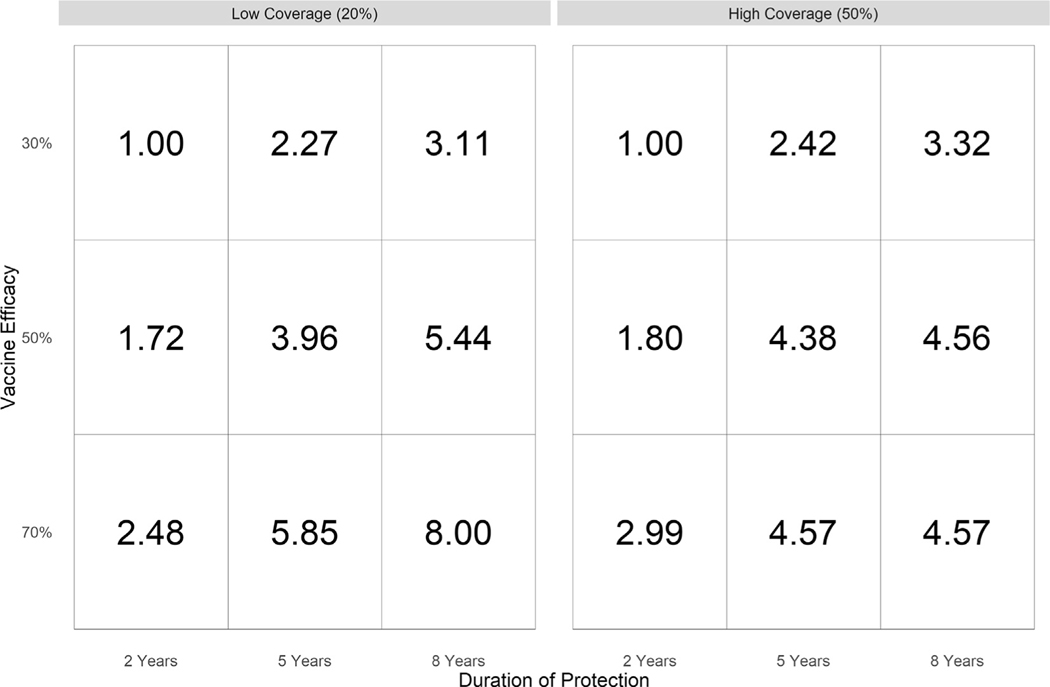
A better vaccine candidate with up to 8-year duration and 70 % efficacy could increase impact up to 8-fold (Fig. 3). Increasing vaccine efficacy from 30 % to either 50 % or 70 % for vaccine candidates with a 2-year duration of protection increased vaccine impact to 8.4–36.9 % under low coverage and 21.3–98.4 % under high coverage. An increase in the duration of protection from 2 years to either 5 years or 8 years for vaccine candidates with 30 % efficacy increased vaccine impact to 10.8–43.9 % for low coverage and 27.9–97.6 % for high coverage (Fig. 2).
Fig. 3. Median increased vaccine impact of improved vaccines compared to a vaccine with 30% efficacy and 2-year duration, assuming either low (20%) or high (50%) coverage and assuming a baseline NG prevalence of 1.125% in women and 0.75% in men.
“Increased vaccine impact” is calculated as the ratio of the reduction in prevalence in the improved vaccine compared to the reduction in prevalence of the vaccine with 30% efficacy and 2-year duration of protection.
Predicted vaccine impact was affected by the underlying scenario (i.e., uncertainty in input parameter values). With a vaccine candidate similar to 4CMenB, some input parameters were strongly correlated with vaccine impact, namely the mixing parameter ( = −0.91) and the size of the high activity sub-population ( = 0.30) (Table 1). Thus, higher vaccine impact resulted from scenarios with lower assortativity and larger size of the high activity sub-population. Similar correlations were estimated for all vaccine efficacy, duration of protection, and coverage combinations except for vaccine candidates that had a better vaccine (70 % efficacy and 8-year duration of protection) and higher coverage, which had a weaker correlation between the mixing parameter and impact ( = −0.47) (Supplemental Table S2).
3.2. Sensitivity analysis
When our model assumed a higher baseline prevalence, the relative median impact was always smaller (Supplemental Fig. S1). A better vaccine candidate, in terms of either improved efficacy or increased duration of protection showed similar improvements under the higher baseline prevalence sensitivity analysis (Supplemental Fig. S2). Vaccine impact remained correlated with the mixing parameter for all vaccine scenarios ( = −0.94 to −0.96) but was no longer strongly correlated with the high activity population size for any vaccine scenario ( = 0.01 to 0.12) (Supplemental Table S3). Full results can be found in the High Prevalence: Results section of the supplemental materials.
4. Discussion
A low efficacy vaccine with a short duration of protection could reduce gonorrhea prevalence by > 10 % given high enough coverage if its vaccine efficacy is confirmed to be 30 % or greater. This could be achieved with the currently available 4CMenB in the United States with > 50 % vaccine coverage. At lower vaccine coverage, a 10 % reduction could be met if the population has a larger high activity subpopulation and lower assortativity. Our sensitivity analysis indicates that if the baseline prevalence is two-times higher than we considered, >10 % reduction could only be achieved with additional types of intervention such as broader or more frequent screening.
A vaccine with improved performance would make it easier to achieve > 10 % reduction in prevalence. For a vaccine with 30 % efficacy, increasing the duration of protection to 5 years doubles the vaccine impact (10.8–43.9 %); increasing to 8 years triples impact (27.9–97.6 %). For a vaccine with a 2-year duration of protection, increasing efficacy to 50 % nearly doubles vaccine impact (8.3–76.8 %); increasing efficacy to 80 % nearly triples impact (26.0–98.4 %). Previous heterosexual models found meaningful reductions in gonorrhea prevalence for vaccine candidates with low efficacy and high duration of protection but only negligible impact with high efficacy and short duration of protection [13]. Extended protection could be achieved through vaccine development targeted to NG or by incorporating boosters into the current vaccine schedule.
Higher vaccine impact was strongly correlated with lower assortativity and larger high-activity sub-populations. This high-lights the importance of the comprehensive uncertainty analyses we conducted. Our boundary values for the mixing parameter allowed for fully assortative or disassortative contact and our model analyzed each vaccine candidate across 10,000 parameter sets fit to the same baseline prevalence. Our analysis showed substantial variation in predicted vaccine impact caused by this underlying uncertainty, unrelated to vaccine efficacy, duration of protection, and coverage. This suggests that future modeling work predicting NG vaccine impact could either utilize nationally representative data for these parameters, which may not be available, or consider broad ranges. Failure to do so could lead to substantial over- or underestimation of potential vaccine impact. Incorporating more detailed contact network data, such as the frequency, duration, and sex of sex partners may add credibility to predictions, but only if input data are representative of the population in question or if sufficiently broad ranges or heterogeneity is incorporated.
Our model does not address potential uses of booster opportunities to extend the duration of protection or catch-up opportunities to vaccinate people who may not receive the vaccine prior to sexual debut. With a short duration of protection, vaccine administration prior to sexual debut may not be a feasible roll-out strategy. As such, our work should be interpreted cautiously, as an exploration of effective coverage, though achievement of this coverage was not modeled in a feasible manner. Future modeling work that explicitly accounts for age and gradual waning of vaccine effects will be necessary to make stronger recommendations regarding broader vaccination administration strategies.
Besides vaccine characteristics and delivery, our model is also simplified in terms of NG natural history and sexual behavior: we ignored extragenital infection as well as transmission between men. Currently available data are unable to inform transmission strength between all possible routes of transmission when extragenital infection is being modeled, which is of particular concern for men who have sex with men [21]. Because of this, we limited our scope to only urogenital heterosexual transmission. Future modeling work will be critical for exploring how extragenital infection affects vaccine impact.
Currently meningitis B vaccine recommendations in the United States do not state a preference for one meningitis B vaccine over another as both are similarly protective against meningitis B infection [22]. If one vaccine were definitively shown to provide cross-protection against NG, the relative costs and benefits of the two meningitis vaccines might differ. Cost-effectiveness work accounting for the dual protection will be critical to inform this issue. Additional work is also needed to provide evidence for use of this vaccine in high NG burden populations such as STD clinic attendees.
5. Conclusion
A meningitis B OMV vaccine, such as 4CMenB, with a low efficacy against gonorrhea acquisition and a short duration of protection could meet a targeted 10 % reduction in gonorrhea prevalence in the United States with 50 % or greater vaccine coverage. OMV vaccines with greater efficacy or longer duration of protection against gonorrhea could have an even greater impact. Potential dual protective effects of OMV vaccines can help to inform vaccine recommendations.
Supplementary Material
Funding
This research as supported in part by an appointment to the National Institute of Allergy and Infectious Diseases (NIAID) Emerging Leaders in Data Science Research Participation Program. This program is administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the National Institutes of Health.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.10.031.
Data availability
Data will be made available on request.
References
- [1].Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2019. Atlanta; 2021. [Online]. Available from: https//www.cdc.gov/std/statistics/2019/default.htm. [Google Scholar]
- [2].Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States 2019. Atlanta; 2019. [Online]. Available from: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. [Google Scholar]
- [3].U.S. Department of Health and Human Services. Sexually transmitted infections national strategic plan for the United States: 2021–2025. Washington D.C.; 2020. [Online]. Available from: https://www.hhs.gov/sites/default/files/STI-National-Strategic-Plan-2021-2025.pdf. [Google Scholar]
- [4].Gottlieb SL, Jerse AE, Delany-Moretlwe S, Deal C, Giersing BK. Advancing vaccine development for gonorrhoea and the Global STI Vaccine Roadmap CSIRO. Sexual Health 2019;16(5):426–32. 10.1071/SH19060. [DOI] [PubMed] [Google Scholar]
- [5].Russell MW, Jerse AE, Gray-Owen SD. Progress toward a gonococcal vaccine: the way forward. Front Immunol 10;2019. Frontiers Media S.A. doi: 10.3389/fimmu.2019.02417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Petousis-Harris H et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 2017;390(10102):1603–10. 10.1016/S0140-6736(17)31449-6. [DOI] [PubMed] [Google Scholar]
- [7].Pérez O, Cuello M, González E. Mucosal approaches in Neisseria Vaccinology. Accessed: May 07, 2021. [Online]. Available from: https://www.researchgate.net/publication/26636153.
- [8].Whelan J, Kløvstad H, Haugen IL, Robert-Du Ry van Beest Holle M, Storsaeter J. Ecologic study of meningococcal B vaccine and Neisseria gonorrhoeae infection. Norway. Emerg Infect Dis 22(6);2016:1137–9. Centers for Disease Control and Prevention (CDC). doi: 10.3201/eid2206.151093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Fredua B, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2018. MMWR Morb Mortal Wkly Rep 2019;68(33):718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Patton ME, Stephens D, Moore K, MacNeil JR. Updated recommendations for use of MenB-FHbp Serogroup B meningococcal vaccine — advisory committee on immunization practices, 2016. MMWR Morb Mortal Wkly Rep 2017;66 (19):509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wilkins AL, Snape MD. Emerging clinical experience with vaccines against group B meningococcal disease. Vaccine 2018;36(36):5470–6. 10.1016/j.vaccine.2017.07.056. [DOI] [PubMed] [Google Scholar]
- [12].Semchenko EA, Tan A, Borrow R, Seib KL. The serogroup B meningococcal vaccine bexsero elicits antibodies to Neisseria gonorrhoeae. Clin Infect Dis 2019;69(7):1101–11. 10.1093/cid/ciy1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Craig AP et al. The potential impact of vaccination on the prevalence of gonorrhea. Vaccine 2015;33(36):4520–5. 10.1016/j.vaccine.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Régnier SA, Huels J. Potential impact of vaccination against Neisseria meningitidis on Neisseria gonorrhoeae in the United States: Results from a decision-analysis model. Hum Vaccines Immunother 2014;10(12):3737–45. 10.4161/hv.36221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Seedat S, Abu-Raddad L. P653 Modeling the impact of partially efficacious gonorrhea vaccines. In: Sexually Transmitted Infections, Jul. 2019, vol. 95, no. Suppl 1. p. A288.1–A288. doi: 10.1136/sextrans-2019-sti.721. [DOI] [Google Scholar]
- [16].Heijne J, Xiridou M, Turner K, Van Benthem B, Low N. P505 The impact of gonorrhoea vaccination in men who have sex with men on prevalence and resistance: mathematical modelling study. In: Sexually Transmitted Infections, Jul. 2019, vol. 95, no. Suppl 1. p. A232.2–A232. doi: 10.1136/sextrans-2019-sti.585. [DOI] [Google Scholar]
- [17].Whittles LK, White PJ, Didelot X. Assessment of the potential of vaccination to combat antibiotic resistance in Gonorrhea: a modeling analysis to determine preferred product characteristics. Clin Infect Dis 2020;71(8):1912–9. 10.1093/cid/ciz1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].U.S. Department of Health and Human Services. Reduce gonorrhea rates in male adolescents and young men — STI 02. Health People 2030, 2020. Available from: https://health.gov/healthypeople/objectives-and-data/browse-objectives/sexually-transmitted-infections/reduce-gonorrhea-rates-male-adolescents-and-young-men-sti-02.
- [19].Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MCB, et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sex Transm Dis 2013;40(3):187–93. [DOI] [PubMed] [Google Scholar]
- [20].Kreisel KM, Weston EJ, St Cyr SB, Spicknall IH. Estimates of the prevalence and incidence of Chlamydia and Gonorrhea among US Men and Women, 2018. Sex Transm Dis 2021;48(4):222–31. 10.1097/OLQ.0000000000001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Spicknall IH, Mayer KH, Aral SO, Romero-Severson EO. Assessing uncertainty in an anatomical site-specific gonorrhea transmission model of men who have sex with men. Sex Transm Dis 2019;46(5):321–8. 10.1097/OLQ.0000000000000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, et al. Meningococcal vaccination: recommendations of the advisory committee on immunization practices, United States, 2020. MMWR Recomm Rep 2020;69 (9):1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.