Abstract
A novel hantavirus, first detected in Siberian lemmings (Lemmus sibiricus) collected near the Topografov River in the Taymyr Peninsula, Siberia (A. Plyusnin et al., Lancet 347:1835–1836, 1996), was isolated in Vero E6 cells and in laboratory-bred Norwegian lemmings (Lemmus lemmus). The virus, named Topografov virus (TOP), was most closely related to Khabarovsk virus (KBR) and Puumala viruses (PUU). In a cross focus reduction neutralization test, anti-TOP Lemmus antisera showed titers at least fourfold higher with TOP than with other hantaviruses; however, a rabbit anti-KBR antiserum neutralized TOP and KBR at the same titer. The TOP M segment showed 77% nucleotide and 88% amino acid identity with KBR and 76% nucleotide and 82% amino acid identity with PUU. However, the homology between TOP and the KBR S segment was disproportionately higher: 88% at the nucleotide level and 96% at the amino acid level. The 3′ noncoding regions of KBR and the TOP S and M segments were alignable except for 113- and 58-nucleotide deletions in KBR. The phylogenetic relationships of TOP, KBR, and PUU and their respective rodent carriers suggest that an exceptional host switch took place during the evolution of these viruses; while TOP and KBR are monophyletic, the respective rodent host species are only distantly related.
The members of the genus Hantavirus, family Bunyaviridae, are each primarily carried by a different, specific rodent host species. The phylogeny of the hantaviruses has been shown to mirror the relatedness of their respective carrier species, suggesting coevolution of the viruses with their hosts (35). The permanent transmission of hantavirus to another rodent species has been documented only once (27); permanent transmission between different rodent genera has never been documented. However, temporary spillover to secondary hosts, such as other rodent species or humans, where hantaviruses can cause hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome, may occur (for a review, see references 16, 23, 35, and 40). In northern Europe, the predominant pathogenic hantavirus is Puumala virus (PUU), carried by the bank vole Clethrionomys glareolus. Other known hantavirus carrier rodents on the Eurasian continent include species of Rattus, Apodemus, and Microtus.
Lemmings are arvicoline rodents inhabiting the Arctic tundra. Lemmus lemmus of Fennoscandia (the area comprising Norway, Sweden, Finland, and adjacent areas of Russia) is closely related to the western type Lemmus sibiricus, whose distribution extends to the Taymyr Peninsula in Russia to the east (Fig. 1). Together, these two are genetically more distantly related to the central type of L. sibiricus (found from the Lena to the Kolyma Rivers) and to the eastern type (found east of the Kolyma River and in Alaska), also known as Lemmus trimucronatus) (8). L. lemmus is well known for its drastic population fluctuations and mass migrations (12). The spring migration of lemmings during one peak population year, 1942, coincided with an outbreak of more than 1,000 cases of a mild hemorrhagic fever-like illness among Finnish and German troops stationed in Salla, Finland (14, 44).
FIG. 1.
Geographic origin of the infected wild rodents (localities indicated by arrows) and the geographical distribution of (i) L. lemmus and L. sibiricus western type (found west of the Lena River) (ii) L. sibiricus central type (found between the Lena and Kolyma Rivers), and (iii) L. sibiricus eastern type (or L. trimucronatus, found east of the Kolyma River) (8, 10). Localities from which lemming samples were analyzed are shown by dots. In addition, Finse, Norway, (origin of the lemming colony), and Salla, Finland (the site of a putative lemming-borne outbreak in 1942), are indicated.
The presence of hantaviral antigen in lemmings in Arctic Siberia has been previously reported (28). To screen for a hantavirus in lemmings, we obtained lemming liver samples that were collected in several locations along the Siberian coast during the Swedish-Russian Tundra Ecology Expedition of 1994 (Fig. 1).
Our aim was to isolate the hantavirus present in this evolutionarily distinct arvicoline rodent in cell culture and to characterize it genetically and antigenically. When this was achieved, the isolated virus was found to have striking similarities to another hantavirus, Khabarovsk virus (KBR), isolated from an evolutionarily distant arvicoline rodent species, Microtus fortis, in the Russian far east (7, 13). This led us to further compare the phylogenetic relationships between the hantavirus genomes from the carrier rodents and their mitochondrial DNA sequences.
MATERIALS AND METHODS
Viruses.
All hantavirus strains were propagated in a biosafety level 3 laboratory in Vero E6 cells cultivated in Eagle’s minimal essential medium supplemented with 2% fetal calf serum, 2 mM l-glutamine, penicillin, and streptomycin. The following hantavirus strains were used: Hantaan/76-118 (HTN) (17), Puumala/Sotkamo (PUU) (3, 41), Prospect Hill/PH-1 (PH) (18), KBR/MF-43 (KBR) (7, 13), Tula/M02V (TUL) (48), and Sin Nombre/CC107 (SN) (39).
Collection and screening of rodent samples.
The lemming liver samples from Siberia were collected during the Swedish-Russian Tundra Ecology Expedition of 1994 (10). Altogether, 231 samples from 12 localities (Fig. 1) were screened by immunoblotting for hantavirus N antigen with a rabbit antiserum raised against recombinant PUU N antigen (anti-GST-PUU-N2/3) (47).
Virus isolation and production of antisera.
Isolation of hantavirus in Vero E6 cells was performed by inoculating diluted, homogenized lemming liver tissue (stored for 2 years at −70°C) into Vero E6 cells and passaging the cells by trypsinization and the addition of fresh cells (at a 1 to 3 ratio to the rest of the cells) every 3 weeks as described previously (48). The cells were checked by immunofluorescence assay (IFA) with monoclonal antibody (MAb) 1C12 (21) for hantavirus antigen. The isolated virus was purified by a sucrose gradient as described before (48).
The same liver samples were inoculated subcutaneously and intranasally into laboratory-bred hantavirus antibody-negative L. lemmus lemmings from Finse, Norway. The inoculated lemmings were then sacrificed 30 days later and checked for anti-PUU antibodies by IFA and for N antigen by immunoblotting with a rabbit antiserum as described above. The generation of polyclonal antisera for a focus reduction neutralization test (FRNT) was achieved by the intranasal inoculation of New Zealand White rabbits as described previously (30). In addition, the sera of experimentally infected L. lemmus lemmings were used.
Reverse transcription-PCR, cloning, and sequencing of viral sequences.
RNA extraction (5) was done similarly for infected Vero E6 cell cultures and rodent samples. The entire S segment was amplified by using one primer and was cloned as described before (32, 34). The M segment was amplified and cloned in three parts; for the L segment (nucleotides [nt] 181 to 514), a pair of nested primers was used (sequences are available upon request). For this study, the KBR (13) total M segment sequence was also completed, and a partial L segment sequence of KBR and PH was determined. The PCR amplicons were separated in agarose gels and purified with the QIAquick kit (Qiagen GmbH). Direct sequencing was performed with an ABI Prism dye terminator sequencing kit (Perkin-Elmer Applied Biosystems Division [PE/ABI], Foster City, Calif.) according to the manufacturer’s instructions, and reactions were run on an ABI 373 A sequencer (PE/ABI). Cloning of the PCR products was done with a PGEM-T cloning kit (Promega). Plasmids were purified with either the Wizard Mini-preps kit (Promega) or the QIAprep kit (Qiagen GmbH) and sequenced either with Sequenase, version 2.0 (United States Biochemicals), or automatically. In the latter case, sequencing was performed with either an ABI Prism dye terminator or ABI Prism M13F and M13R dye primer sequencing kit (PE/ABI).
Rodent sequences.
Mitochondrial cytochrome b gene (cyt b) DNA sequences were either downloaded from GenBank or generated in the laboratory. The rodent data are part of a larger study on the evolution of arvicoline genera and species of Microtus. They will be published in complete form elsewhere (6). Samples were derived from different rodent species from various collections as follows (sample number in parentheses). (i) Apodemus flavicollis (AF 3326), Clethrionomys glareolus (AF 3133), Clethrionomys rufocanus (AF 3783), L. sibiricus (AF 15478), L. trimucronatus (AF 4077), Microtus californicus (AF 15891), Microtus pennsylvanicus (AF 2511), and Peromyscus maniculatus (AF 17750) samples were from the Alaska Frozen Tissue Collection, University of Alaska Museum, Fairbanks; (ii) a Reithrodontomys megalotis sample (812 bases, UAM 38139 [skin]) was from the University of Alaska Museum; and (iii) an M. fortis sample (MVZ 1524) was from the Museum of Vertebrate Zoology, University of California, Berkeley. DNA was extracted from samples of heart or skin via a modified salt method (25). Symmetric PCR (37) was used to amplify portions of the cyt b gene (Mus mitochondrial DNA, base pairs [bp] 14139 to 15282) (1) with standard cyt b primers (6, 42) and PCR protocols. Sequences for both strands were determined on an ABI 373a stretch DNA sequencer with a Prism dye terminator kit (Fst-RR, 402119; Perkin-Elmer).
Phylogenetic analyses.
The PHYLIP program package (9) was used to make 200 bootstrap replicates of the sequence data (Seqboot). Distance matrices were calculated by using Kimura’s two-parameter model (Dnadist) and analyzed by the Fitch-Margoliash tree-fitting algorithm (Fitch). The bootstrap support percentages of particular branching points were calculated from these trees (Consense). For comparison, existing sequence data were obtained from GenBank.
The S segment sequences include KBR/MF-43 (GenBank accession no. U35255), PUU/Sotkamo (X61035), PUU/Vindeln/83-L20 (Z48586), PUU/Bashkiria/CG1820 (M32750), PUU/France/90-13 (U22423), PUU/Tobetsu (B010731), PUU/Udmurtia/894Cg/91 (Z21497), TUL/Moravia/5286Ma/94 (Z48573), TUL/76Ma/87 (Z30941), PH/PH-1 (Z49098), Isla Vista (ILV)/MC-SB-1 (U31534), SN/HlO (L25784), New York (NY)/RI-1 (U09488), El Moro Canyon (ELMC)/RM-97 (U11427), Laguna Negra (LN)/510B (AF005727), Rio Segundo (RIOS)/RMx-Costa-I (U18100), Rio Mamore (RIOM) (U52136), Bayou (BAY)/Louisiana (L36929), Black Creek Canal (BCC) (L39949), Seoul (SEO)/SR-11 (M34882), HTN/76-118 (M146271), and Dobrava (DOB)/Slovenia (L41916).
The M segment sequences included PUU/Sotkamo (X61034), PUU/Vindeln/83-L20 (Z49214), PUU/Bashkiria/CG1820 (M29979), PUU/Kazan (Z84205), PUU/France90-13 (U22418), TUL/Moravia/5286Ma/94 (Z66538), PH/PH-1 (Z55129), SN/Hl0 (L25783), NY/RI-1 (U36801), ELMC/RM-97 (U26828), LN/510B (AF005728), BAY/Louisiana (L36930), BCC (L39950), SEO/SR-11 (M34881), HTN/76118 (M14627), Dobrava (DOB) (L33685), and Thailand (THAI)/749 (L08756). The L segment sequences included PUU/Sotkamo (Z66548), PUU/Bashkiria/CG1820 (M63194), SN/H10 (L37901), BCC (L39951), SEO/80-39 (X56492), and HTN/76-118 (X55901).
The rodent cyt b sequences were aligned by eye. The following rodent cyt-b sequences were downloaded from GenBank (full cyt b sequence if not otherwise indicated): Calomys lepidus (801 bases, U03544), Microtus arvalis (U54488), Oligoryzomys microtis (801 bases, U58381), Oryzomys palustris (401 bases, L37388), Peromyscus leucopus (321 bases, X89790), and Rattus rattus (X14848). By using the PHYLIP program package (9), Kimura two-parameter distances (Dnadist) were analyzed by the Fitch-Margoliash tree-fitting algorithm (Fitch). Bootstrap percentages for 250 iterations were calculated (Seqboot), and TreeMap software (31) was used to construct a “tanglegram” of murid rodents and associated hantaviruses.
MAbs and IFA.
A panel of MAbs (21, 24) and recombinant Fab fragments (38) was used in a standard IFA on acetone-fixed, hantavirus-infected Vero E6 cells (21).
FRNT.
Endpoint titers of neutralizing antibodies were determined by FRNT (30). Dilutions of sera were mixed with an equal volume containing 30 to 70 focus-forming units of virus/100 μl. The mixture was incubated for 1 h and subsequently inoculated onto confluent Vero E6 cell monolayers in six-well plates. After adsorption for 1 h, the wells were overlaid with a mixture of agarose and basal Eagle’s medium. Plates were incubated for 9 (HTN), 10 (KBR), or 12 (PUU, PH, Topografov virus [TOP], TUL, and DOB) days. Virus-infected cells were detected with hantavirus-specific polyclonal antisera, followed by peroxidase-labeled goat antibodies and a substrate. An 80% reduction in the number of foci was the criterion for virus neutralization titers.
HI test.
A goose erythrocyte hemagglutination inhibition (HI) test was performed essentially as described previously (4). PUU-, TOP-, and KBR-infected Vero E6 cell culture supernatants (for TUL, a sucrose gradient peak fraction) treated with Tween 20-ether (1:1 ratio) were used as hemagglutination antigens with 3 hemagglutination units of antigen per well.
Nucleotide sequence accession numbers.
The TOP S, M, and partial L sequences are deposited in GenBank with accession no. AJ011646, AJ011647, and AJ011649, respectively; the KBR total M genome and partial L segment sequences are deposited with accession no. AJ011648 and AJ011650, respectively; and the PH partial L sequence is deposited with accession no. AJ011651.
RESULTS AND DISCUSSION
Isolation of TOP.
Altogether, 231 liver samples from Siberian lemmings (L. sibiricus), collected in 12 localities during the Swedish-Russian Tundra Ecology Expedition of summer 1994, were screened for hantavirus antigen by immunoblotting; 6 specimens were positive. These animals were among 61 collected from two localities (no. 8 and 10) on the Taymyr Peninsula (Fig. 1) (33).
Each positive sample was injected into Norwegian lemmings (L. lemmus) originating from Finse, Norway. The virus was successfully passaged in (four of six) laboratory lemmings, with the presence of antigen in the lungs demonstrated by immunoblotting (four of six) and/or with the development of anti-PUU antibodies demonstrated by IFA (three of six). The lung tissues of antigen-positive lemmings were used for RNA isolation, followed by reverse transcription and nested PCR. Initially, amplicons (354 bp in length) from the S genomic segment from two specimens were prepared. Subsequent sequencing revealed a previously unknown hantavirus genotype, named TOP according to its geographic origin, the area around the Topografov River (33) (locality 10) (Fig. 1). Identical sequences were also derived from the original lemming samples.
We also applied the same liver samples from wild lemmings onto Vero E6 cells without a prior passage in laboratory lemmings. After 9 weeks and two cell passages, four of six isolations were clearly positive for hantavirus N antigen, including isolates from individual samples from the two different locations. One of the isolates, designated TOP/Ls136V5/94 and originating from locality 10, was selected as the reference strain. The virus supernatant could be passaged several times in Vero E6 cells, and the virus was further purified by sucrose gradient ultracentrifugation; the viral peak was recovered at 42% (wt/vol) sucrose, where hantavirus-like particles could be distinguished by electron microscopy. TOP G1 migrated more slowly in a sodium dodecyl sulfate-polyacrylamide gel than did PUU G1, whereas TOP G2 and N showed apparent molecular weights similar to those of PUU G2 and N (data not shown).
Antigenic properties.
When studied with a panel of nucleocapsid protein-specific MAbs by IFA, TOP was found to be antigenically most closely related to KBR (Table 1). Two MAbs could be used to distinguish PUU from TOP and KBR. The nucleocapsid proteins of TOP and KBR differed only in reactivity to one anti-PUU MAb, 3H9, the epitope of which has been mapped to the most variable part of N, amino acids (aa) 251 to 260 (20, 22). The reactivity of 3H9 with TOP was very weak: aa 252 to 257 for TOP N were KPGAPA instead of KPGTPA in PUU and KBR, which most probably explains the different reactivity. We noted also that native SN was detected by two MAbs (1C8 and 3C11) that were not reactive with recombinant SN nucleocapsid protein (24) (Table 1).
TABLE 1.
Selected panel of nucleocapsid protein-specific MAbs and Fab fragments showing reactivities in an IFA
| Hantavirus strain | Reactivity with MAb or Fab fragment:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1C12 | 3F10 | 6A6 | 7A4 | 1C8 | 3C11 | 3H9 | Fab 41 | |
| TOP | + | − | − | + | + | + | (+/−)a | − |
| KBR | + | − | − | + | + | + | + | − |
| PUU | + | − | − | − | + | + | + | + |
| TUL | + | + | + | + | + | + | − | − |
| PH | + | − | + | + | + | + | − | − |
| SN | + | − | − | + | + | + | − | − |
(+/−), very weak reactivity.
In a cross-FRNT, TOP was distinguishable from all other hantaviruses with at least a two-way fourfold titer difference (Table 2) except for a one-way titer difference for KBR, as the anti-KBR rabbit antiserum neutralized KBR and TOP with the same titer (1:1,280). A similar pattern was obtained in an HI test.
TABLE 2.
Cross-FRNT and cross-HI comparison of TOP with other hantaviruses
| Test and hantavirusa | Antibody titer with antiserum to:
|
|||||
|---|---|---|---|---|---|---|
| TOP/ LS136 | KBR/ MF43 | PUU/ Vindeln/ 83-L20 | TUL/ M02V | PH/ PH-1 | HTN/ 76-118 | |
| FRNT | ||||||
| TOP | 2,560 | 1,280 | 80 | 160 | 40 | 40 |
| KBR/MF-43 | 640 | 1,280 | 160 | 80 | 80 | <20 |
| PUU/Vindeln/83-L20 | 160 | <20 | 1,280 | 20 | 80 | <20 |
| TUL/M02V | 80 | 20 | 20 | 5,120 | 160 | 80 |
| PH/PH-1 | 80 | <20 | <20 | 160 | 1,280 | <20 |
| HTN/76-118 | NDb | <20 | <20 | <20 | <20 | 320 |
| HI | ||||||
| TOP/76-118 | 160 | 320 | 40 | 80 | 20 | 20 |
| KBR/MF-43 | 80 | 320 | 40 | 40 | 20 | 10 |
| PUU/PH-1 | 40 | 160 | 320 | 20 | 20 | 20 |
| TUL/M02V | 160 | 40 | 80 | 320 | 80 | 80 |
Strains described in Materials and Methods.
ND, not determined.
Genetic properties.
Sequencing of TOP S showed that the S segment consists of 1,953 nt and codes for a nucleocapsid protein of 433 aa and a putative nonstructural protein, NSs, of 90 aa. The sequence was most closely related to that of KBR (82% nucleotide and 96% amino acid identity for the S segment) and PUU (77% nucleotide and 87% amino acid identity) (Table 3). The 3′ noncoding region (according to the messenger sense) of TOP S was alignable with that of KBR, showing 77% nucleotide homology. However, the KBR S noncoding region had a deletion of 113 nt compared to the TOP S sequence.
TABLE 3.
S, M, and L segment nucleotide and amino acid identities of TOP with other hantaviruses
| Segment (corresponding protein) | % nt (aa) TOP identity with:
|
|||||
|---|---|---|---|---|---|---|
| KBR | PUU | TUL | SN | HTN | SEO | |
| S (N) | 82 (96) | 77 (87) | 76 (82) | 67 (72) | 62 (64) | 62 (63) |
| M (G1 and G2) | 77 (88) | 76 (82) | 73 (83) | 66 (68) | 60 (55) | 60 (55) |
| La (L) | 81 (90) | 78 (90) | 74 (84) | 69 (76) | 59 (58) | 64 (61) |
Partial sequence (nt 181 to 514).
The M segment consisted of 3,735 nt and coded for a G1-G2 precursor of 1,142 aa. The M segment showed considerably more variation than the S segment, but the pattern of relatedness to different hantaviruses was the same, showing 77% nucleotide and 88% amino acid identity between TOP and KBR and 76% nucleotide and 83% amino acid identity between TOP and PUU (Table 3). Similarly to the S segment, a deletion of 55 nt was found in the 3′ noncoding region of KBR compared to TOP. The putative Asn-linked glycosylation sites in G1 and G2 were identical among TOP, PUU/Sotkamo, and KBR. The L segment sequence was analyzed from nt 181 to 514. This region showed 81% nucleotide and 90% amino acid homology to the KBR L segment. Although the M segment tends to be the most variable of the hantavirus genome segments, the difference between the M and S segment amino acid homologies for TOP and KBR (88 and 96%, respectively) is exceptionally high. While this could be explained by reassortment, no sequence data currently available on related viruses support this hypothesis. The discordant homologies for the two genome segments could mean that either (i) a recombinant virus would have had better capabilities for a permanent host switch or (ii) after the host switch, an evolutionary pressure in a new host has generated more amino acid changes in the glycoproteins than in the nucleocapsid protein.
TOP strain variation.
TOP strains derived from the two localities (no. 8 and 10 [Fig. 1]) were analyzed by reverse transcription-PCR of a 354-nt fragment (nt 4 to 357) of the S segment and subsequent sequencing. Interestingly, in locality 8, two lineages showing 7.5% nucleotide divergence cocirculated. The other lineage (represented by strain 29) was surprisingly closely related (1% nucleotide divergence) to the TOP strains from locality 10 (no. 136 and 137). As localities 8 and 10 are about 600 km apart, the lack of clear geographic clustering of genetic variants might reflect the dispersal capacity of lemmings (8).
Phylogenetic relationships of hantaviruses and respective rodent carriers.
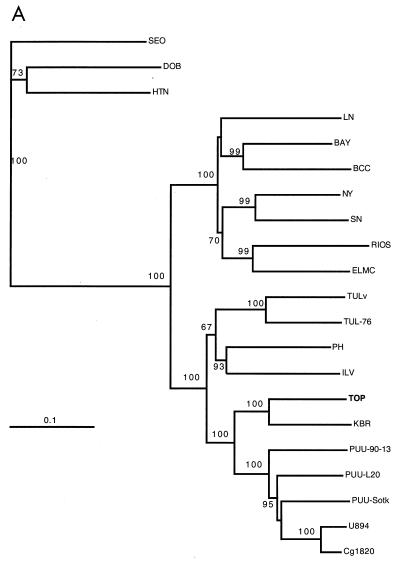
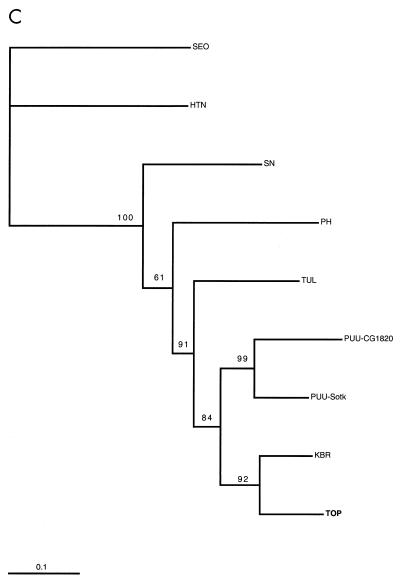
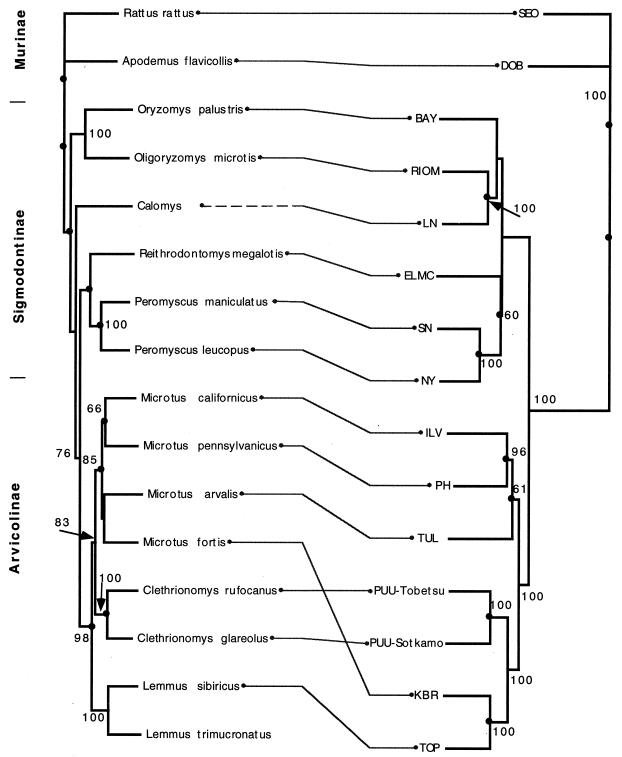
In the phylogenetic trees created for all three genome segments (Fig. 2), TOP was monophyletic with KBR. Furthermore, the TOP-KBR clade had a common origin with the PUU viruses with a high branching probability. A comparison of the phylogenetic trees of hantaviruses and their known carriers shows (Fig. 3) that coevolution is a general rule (resulting in similar branching order for the rodents and their respective hantaviruses). Also, a heuristic search for the best match between trees (31) suggested nine cospeciations. However, an evident host switch has occurred for the ancestor of KBR from a PUU lineage, as KBR does not group with other Microtus-borne viruses but instead has a common node of origin, firstly with TOP and secondly with PUU, which are carried by Lemmus and Clethrionomys, respectively. Another clear discrepancy in the arvicoline rodent phylogeny is that the branching of TOP from the PUU lineage is distal to the main Microtus-derived virus cluster (PH, TUL, and ILV), although the Clethrionomys and Microtus species are more closely related to each other and have a common evolutionary origin (83% bootstrap support) compared to the more ancestral Lemmus (Fig. 3) (26). In fact, the branching position of the common ancestor of KBR and TOP is not in line with the phylogeny with either of the host rodents.
FIG. 2.
Phylogenetic trees (PHYLIP) of hantavirus S (A), M (B), and partial L segments (C) (see Materials and Methods). Kimura two-parameter distances (Dnadist) were used to construct the Fitch-Margoliash tree (Fitch). Branching probabilities of >60% for different hantaviruses are indicated. PUU-Sotk, PUU-Sotkamo; TUL-02, Tula/M0ZV. Hantavirus strains are described in Materials and Methods.
FIG. 3.
Tanglegram constructed with TreeMap software (31), showing murid rodents and associated hantaviruses. The host tree on the left was generated from cyt b sequences, and the hantavirus S segment tree on the right was generated from S segment nucleotide sequences. Kimura two-parameter distances (Dnadist) were used to construct the Fitch-Margoliash tree (Fitch). Values indicate bootstrap percentages of >50% for 250 iterations (Seqboot). Trees were rerooted with Rattus rattus and SEO as outgroups. Hantavirus strains are described in Materials and Methods.
A hypothetical scenario for the host switch events might be that the ancestor of Clethrionomys, clearly after the divergence of the rapidly radiating Microtus species approximately 1.5 million years ago (26), was the donor of an ancestral virus which became the common ancestor of both TOP and KBR. This ancestral virus branch is separate from all PUU viruses from Japan to Belgium, which have a distinct, common node of origin. It is not certain if the ancestral virus was first transmitted to Lemmus; the long deletions in the S and M segment 3′ noncoding regions of KBR compared to TOP suggest that TOP could represent a more ancestral virus than KBR. However, the knowledge of the genetic diversity and geographical distribution of TOP is limited. We screened more than 400 L. lemmus samples from Fennoscandia and a total of 177 lemmings from other locations in the Russian Arctic (Fig. 1) without finding evidence of hantavirus antigen.
The geographic distributions of M. fortis and L. sibiricus do not overlap today (43), meaning that both species should permanently carry their respective viruses; i.e., TOP and KBR do not represent spillover viruses. During the more than 3 million years that the Clethrionomys-Microtus branch evolved from the common ancestor to Lemmus (36), the geographic ranges of the host rodent species have probably varied considerably, and the host switch(es) may have taken place, for example, in the southern ranges frequented by the central L. sibiricus group.
Recent data on SN-like viruses of P. leucopus and P. maniculatus have shown that horizontal transmission between closely related species can occur (27). Another likely hantavirus host transfer (or possibly duplication) is suggested by the existence of Dobrava virus in both A. flavicollis and Apodemus agrarius in Europe (28a), while the latter carries the distinct Hantaan virus in far eastern Asia. Data on South American hantaviruses and their host species, both of which have radiated rapidly (15, 19), are not in full agreement with the coevolution in rodent host species (see, e.g., the Laguna Negra virus, [Fig. 3]) (29). In the cases of TOP and KBR, while the sequence of events remains unclear, these are the first clear indications that a hantavirus host switch event can indeed occur among more distantly related rodent species and even between different rodent genera. Yet a host switch seems to be a rare event, and cospeciation remains the rule. An analogy to the phylogeny of rodent carriers can be drawn, for example, with Arenaviridae (2) and even with some ectoparasites such as lice (11), which show cospeciation with rodents and occasional host switches, resulting in new adaptive peaks.
The taxonomic relationships of TOP and KBR are of special interest. If our hypothesis about a horizontal transmission, or host switch, during evolution of these viruses is correct, it seems logical to assume that TOP and KBR are still radiating from each other. Ongoing radiation by the two viruses could be reflected by different degrees of diversity for the M and S segments of TOP and KBR. Nevertheless, based on an analysis of the combination of properties of TOP and KBR, we believe that TOP and KBR fulfill the criteria for demarcation (45) and should thus be considered distinct virus species. The strongest argument for such a conclusion is that TOP and KBR have clearly distinct host species in which they are constantly maintained. The identification of additional sequences from related hantaviruses might shed light on the origin and relatedness of these viruses. For example, another hantavirus identified from M. fortis in the Russian far east, Vladivostok, seems at least as divergent from KBR as TOP is (15a).
Is TOP a pathogen?
We screened serum samples of World War II veterans who had been stationed in Salla, Finland, during the putative lemming-borne hantavirus outbreak of 1942 for hantavirus antibodies. While hantavirus antibodies were found in one-third of the samples, no difference in reactivity or antibody titers could be demonstrated between TOP, PUU, and KBR antigens by FRNT or with truncated recombinant antigens in an enzyme immunoassay (data not shown). However, similar results were also found in samples from people with decades-old PUU immunity who had no association with lemmings. It remains to be determined whether a hantavirus actually circulates in lemmings in Fennoscandia and if TOP can be transmitted to humans. However, it is possible that the outbreak of 1942 was caused by a hantavirus carried by lemmings, since (i) the clinical symptoms mimicked those later described for nephropathia epidemica, including high fever, acute renal failure, and, occasionally, myopia (14, 44); (ii) the year 1942 coincided with a mass migration of lemmings in the region; and (iii) the incidence of the disease peaked in May and June 1942, which coincides with the lemming spring migration and contrasts with the epidemiology of the Clethrionomys-borne disease, which normally peaks in November or December in Fennoscandia (46). As the population density of lemmings fluctuates strongly, it may be that only during occasional peak years do rodent densities reach the threshold necessary for the virus to spread efficiently.
ACKNOWLEDGMENTS
We thank Carl-Henrik von Bonsdorff and Anssi Mörttinen for electron micrographs and Tytti Manni, Leena Kostamovaara, and Mari Gilljam for expert technical assistance. We thank Brian Hjelle for sharing primer sequences that were used for the initial detection of the TOP S segment in lemming samples. Hiroaki Kariwa is acknowledged for sharing his data on Vladivostok virus and Stuart Nichol for helpful discussions and for sharing the complete sequence of the KBR S segment and a manuscript before publication.
This work was supported by grants from the Wilhelm Stockmann Foundation, the Sigrid Jusélius Foundation, the European Commission (BMH4-CT97-2499), and the Swedish Medical Research Council (12177 and 12642).
REFERENCES
- 1.Bibb M J, Van Etten R A, Wright C T, Walberg M W, Clayton D. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981;26:167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- 2.Bowen M, Peters C J, Mills J M, Nichols S T. Oliveros virus: a novel arenavirus from Argentina. Virology. 1996;217:362–366. doi: 10.1006/viro.1996.0124. [DOI] [PubMed] [Google Scholar]
- 3.Brummer-Korvenkontio M, Henttonen H, Vaheri A. Hemorrhagic fever with renal syndrome in Finland: ecology and virology of nephropathia epidemica. Scand J Infect Dis Suppl. 1982;36:S88–S91. [PubMed] [Google Scholar]
- 4.Brummer-Korvenkontio M, Manni T, Ukkonen S, Vaheri A. Detection of hemagglutination-inhibiting antibodies in patients with nephropathia epidemica and Korean hemorrhagic fever by using Puumala virus cell culture antigen. J Infect Dis. 1986;153:997–998. doi: 10.1093/infdis/153.5.997-a. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Conroy, C. J., and J. A. Cook. MtDNA evidence for repeated pulses of speciation within arvicoline and murid rodents. J. Mamm. Evol., in press.
- 7.Dzagurova T, Tkachenko E, Slonova R, Ivanov L, Ivanidze E, Markeshin S, Dekonenko A, Niklasson B, Lundkvist A. Antigenic relationships of hantavirus strains analysed by monoclonal antibodies. Arch Virol. 1995;140:1763–1773. doi: 10.1007/BF01384340. [DOI] [PubMed] [Google Scholar]
- 8.Fedorov V, Goropashnaya A, Jarrell G H, Fredga K. Phylogeographic structure and mitochondrial DNA variation in true lemmings (Lemmus) from the Eurasian Arctic. Biol J Linn Soc. 1999;66:357–371. [Google Scholar]
- 9.Felsenstein J. PHYLIP (Phylogeny Inference Package), version 3.5c. Seattle: University of Washington; 1993. [Google Scholar]
- 10.Fredga K, Fedorov V, Gelter H, Jarrell G, Thulin C-G. Genetic studies in lemmings. In: Grönlund E, Melander O, editors. Swedish-Russian tundra ecology expedition ’94. Stockholm, Sweden: Swedish Polar Research Secretariat; 1994. pp. 235–242. [Google Scholar]
- 11.Hafner M S, Sudman P D, Villablanca F X, Spradling T A, Demastes J W, Nadler S A. Disparate rates of molecular evolution in cospeciating hosts and parasites. Science. 1994;265:1087–1090. doi: 10.1126/science.8066445. [DOI] [PubMed] [Google Scholar]
- 12.Henttonen H, Kaikusalo A. Lemming movements. In: Stenseth N C, Inns R A, editors. The biology of lemmings. New York, N.Y: Academic Press, Inc.; 1993. pp. 157–186. [Google Scholar]
- 13.Hörling J, Chizhikov V, Lundkvist Å, Jonsson M, Ivanov L, Dekonenko A, Niklasson B, Dzagurova T, Peters C J, Tkachenko E, Nichol S T. Khabarovsk virus: a phylogenetically and serologically distinct hantavirus isolated from Microtus fortis trapped in far-east Russia. J Gen Virol. 1996;77:687–694. doi: 10.1099/0022-1317-77-4-687. [DOI] [PubMed] [Google Scholar]
- 14.Hortling H. En epidemi av fältfäber (?) i finska Lappland. Nord Med. 1946;30:1001–1004. [Google Scholar]
- 15.Johnson A M, Bowen M D, Ksiazek T G, Williams R J, Bryan R, Mills J, Peters C J, Nichol S T. Laguna Negra virus associated with HPS in western Paraguay and Bolivia. Virology. 1997;238:115–127. doi: 10.1006/viro.1997.8840. [DOI] [PubMed] [Google Scholar]
- 15a.Kariwa, H. Personal communication.
- 16.Khan A S, Ksiazek T G, Peters C J. Hantavirus pulmonary syndrome. Lancet. 1996;347:739–741. doi: 10.1016/s0140-6736(96)90082-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee H W, Lee P W, Johnson K M. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis. 1978;137:289–308. doi: 10.1093/infdis/137.3.298. [DOI] [PubMed] [Google Scholar]
- 18.Lee P W, Amyx H L, Gajdusek D C, Yanagihara R T, Goldgaber D, Gibbs C J., Jr New hemorrhagic fever with renal syndrome-related virus in rodents in the United States. Lancet. 1982;ii:1405. . (Letter.) [PubMed] [Google Scholar]
- 19.Levis S, Morzunov S, Rowe J, Enria D, Pini N, Calderon G, Sabattini M, St. Jeor S C. Genetic diversity and epidemiology of hantaviruses in Argentina. J Infect Dis. 1998;177:529–538. doi: 10.1086/514221. [DOI] [PubMed] [Google Scholar]
- 20.Lundkvist Å, Björsten S, Niklasson B, Ahlborg N. Mapping of B-cell determinants in the nucleocapsid protein of Puumala virus: definition of epitopes specific for acute immunoglobulin G recognition in humans. Clin Diagn Lab Immunol. 1995;2:82–86. doi: 10.1128/cdli.2.1.82-86.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundkvist Å, Fatouros A, Niklasson B. Antigenic variation of European haemorrhagic fever with renal syndrome virus strains characterized using bank vole monoclonal antibodies. J Gen Virol. 1991;72:2097–2103. doi: 10.1099/0022-1317-72-9-2097. [DOI] [PubMed] [Google Scholar]
- 22.Lundkvist Å, Kallio-Kokko H, Brus Sjölander K, Lankinen H, Niklasson B, Vaheri A, Vapalahti O. Characterization of Puumala virus nucleocapsid protein: identification of B-cell epitopes and domains involved in protective immunity. Virology. 1996;216:397–406. doi: 10.1006/viro.1996.0075. [DOI] [PubMed] [Google Scholar]
- 23.Lundkvist Å, Niklasson B. Haemorrhagic fever with renal syndrome and other hantavirus infections. Rev Med Virol. 1994;4:177–184. [Google Scholar]
- 24.Lundkvist Å, Vapalahti O, Plyusnin A, Brus Sjölander K, Niklasson B, Vaheri A. Characterization of Tula virus antigenic determinants defined by monoclonal antibodies raised against baculovirus-expressed nucleocapsid protein. Virus Res. 1996;45:29–44. doi: 10.1016/0168-1702(96)01360-3. [DOI] [PubMed] [Google Scholar]
- 25.Medrano J, Aasen E, Sharrow L. DNA extraction from nucleated red blood cells. BioTechniques. 1990;8:43. [PubMed] [Google Scholar]
- 26.Modi W S. Phylogenetic history of LINE-1 among arvicolid rodents. Mol Biol Evol. 1996;13:633–641. doi: 10.1093/oxfordjournals.molbev.a025623. [DOI] [PubMed] [Google Scholar]
- 27.Morzunov S P, Rowe J E, Ksiazek T G, Peters C J, St. Jeor S C, Nichol S T. Genetic analysis of the diversity and origin of hantaviruses in Peromyscus leucopus mice in North America. J Virol. 1998;72:57–64. doi: 10.1128/jvi.72.1.57-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myasnikov Y A, Apekina N S, Zuevskii A P, Khitrin A V, Bernshtein A D. The disposition of natural foci of hemorrhagic fever with renal syndrome in different landscape areas of Tyumen Province. Vopr Virusol. 1992;37:161–165. . (In Russian.) [PubMed] [Google Scholar]
- 28a.Nemirov, K., O. Vapalahti, Å. Lundkvist, V. Vasilenko, I. Golovljova, A. Plyusnina, J. Niemimaa, J. Laakkonen, H. henttonen, A. Vaheri, A. Plyusnin. 1999. Isolation and characterisation of Dobrava hantavirus carried by the striped field mouse (Apodemus agrarius) in Estonia. J. Gen. Virol. 80:371–379. [DOI] [PubMed]
- 29.Nichol, S. T. Genetic analysis of hantaviruses and their host relationships. In J. F. Saluzz and B. Dodet (ed.), Factors in the emergence and control of rodent-borne viral diseases (hantaviruses and arenaviruses), in press. Elsevier, Paris, France.
- 30.Niklasson B, Jonsson M, Lundkvist Å, Horling J, Tkachenko E. Comparison of European isolates of viruses causing hemorrhagic fever with renal syndrome by a neutralization test. Am J Trop Med Hyg. 1991;45:660–665. doi: 10.4269/ajtmh.1991.45.660. [DOI] [PubMed] [Google Scholar]
- 31.Page R D M. Tree map, version 1.0c. Glasgow, Scotland: University of Glasgow; 1997. [Google Scholar]
- 32.Plyusnin A, Vapalahti O, Lankinen H, Lehväslaiho H, Apekina N, Myasnikov Y, Kallio-Kokko H, Henttonen H, Lundkvist Å, Brummer-Korvenkontio M, Gavrilovskaya I, Vaheri A. Tula virus: a newly detected hantavirus carried by European common voles. J Virol. 1994;68:7833–7839. doi: 10.1128/jvi.68.12.7833-7839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plyusnin A, Vapalahti O, Lundkvist Å, Henttonen H, Vaheri A. Newly recognized hantavirus in Siberian lemmings. Lancet. 1996;347:1835–1836. doi: 10.1016/s0140-6736(96)91655-4. [DOI] [PubMed] [Google Scholar]
- 34.Plyusnin A, Vapalahti O, Ulfves K, Lehvaslaiho H, Apekina N, Gavrilovskaya I, Blinov V, Vaheri A. Sequences of wild Puumala virus genes show a correlation of genetic variation with geographic origin of the strains. J Gen Virol. 1994;75:405–409. doi: 10.1099/0022-1317-75-2-405. [DOI] [PubMed] [Google Scholar]
- 35.Plyusnin A, Vapalahti O, Vaheri A. Hantaviruses: genome structure, expression and evolution. J Gen Virol. 1996;77:2677–2687. doi: 10.1099/0022-1317-77-11-2677. [DOI] [PubMed] [Google Scholar]
- 36.Repenning C, Fejfar O, Heinrich W-D. International Symposium on Evolution, Phylogeny, and Biostratigraphy of Arvicolids (Rodentia, Mammalia). 1990. Arvicolid rodent biochronology of the northern hemisphere; pp. 385–418. Pfeil-Verlag, Prague, Czechoslovakia. [Google Scholar]
- 37.Saiki R, Gelfand D, Stoffel S, Scharf S, Higuchi R, Horn G, Mullis K, Erlich H. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1981;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 38.Salonen E-M, Parren P, Graus Y, Lundkvist Å, Fisicaro P, Vapalahti O, Kallio-Kokko H, Vaheri A, Burton D. Human recombinant Puumala virus antibodies: cross reaction with other hantaviruses and use in diagnostics. J Gen Virol. 1998;79:667–671. doi: 10.1099/0022-1317-79-4-659. [DOI] [PubMed] [Google Scholar]
- 39.Schmaljohn A L, Li D, Negley D L, Bressler D S, Turell M J, Korch G W, Ascher M S, Schmaljohn C S. Isolation and initial characterization of a newfound hantavirus from California. Virology. 1995;206:963–972. doi: 10.1006/viro.1995.1019. [DOI] [PubMed] [Google Scholar]
- 40.Schmaljohn C, Hjelle B. Hantaviruses: a global disease problem. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmaljohn C S, Hasty S E, Dalrymple J M, LeDuc J W, Lee H W, von Bonsdorff C-H, Brummer-Korvenkontio M, Vaheri A, Tsai T F, Regnery H L, Goldgaber D, Lee P W. Antigenic and genetic properties of viruses linked to hemorrhagic fever with renal syndrome. Science. 1985;227:1041–1044. doi: 10.1126/science.2858126. [DOI] [PubMed] [Google Scholar]
- 42.Smith M F, Patton J L. The diversification of South American murid rodents: evidence from mitochondrial DNA sequence data for the akodontine tribe. Biol J Linn Soc. 1993;50:149–177. [Google Scholar]
- 43.Stenseth N C, Ims R A, editors. The biology of lemmings. New York, N.Y: Academic Press, Inc.; 1993. [Google Scholar]
- 44.Stuhlfault K. Bericht über ein neues schlammfieberähnliches Krankenheitsbild bei Deutschen Truppen in Lappland. Dtsch Med Wochenschr. 1943;69:439–443. , 474–477. [Google Scholar]
- 45.Van Regenmortel M H V, Bishop D H L, Fauquet C M, Mayo M A, Maniloff J, Calisher C H. Guidelines to the demarcation of virus species. Arch Virol. 1997;142:1505–1528. [PubMed] [Google Scholar]
- 46.Vapalahti, K., M. Paunio, A. Vaheri, and O. Vapalahti. Epidemiology of Puumala virus infections in Finland: increased risk for farmers. Am. J. Epidemiol., in press. [DOI] [PubMed]
- 47.Vapalahti O, Kallio-Kokko H, Närvänen A, Julkunen I, Lundkvist Å, Plyusnin A, Lehväslaiho H, Brummer-Korvenkontio M, Vaheri A, Lankinen H. Human B-cell epitopes of Puumala virus nucleocapsid protein, the major antigen in early serological response. J Med Virol. 1995;46:293–303. doi: 10.1002/jmv.1890460402. [DOI] [PubMed] [Google Scholar]
- 48.Vapalahti O, Lundkvist Å, Kukkonen S K J, Cheng Y, Gilljam M, Kanerva M, Manni T, Pejcoch M, Niemimaa J, Kaikusalo A, Henttonen A, Vaheri A, Plyusnin A. Isolation and characterization of Tula virus, a distinct serotype in the genus Hantavirus, family Bunyaviridae. J Gen Virol. 1996;77:3063–3067. doi: 10.1099/0022-1317-77-12-3063. [DOI] [PubMed] [Google Scholar]