Abstract

Peel and seeds are the main byproducts from tomato (Lycopersicon esculentum P. Mill) processing with high concentrations of polyphenols that have been underexploited. Herein, polyphenolic profiles in tomato peel and seeds were elucidated by untargeted liquid chromatography coupled to high-resolution mass spectrometry (LC-HRMS) with an LTQ Orbitrap analyzer. Samples from two Spanish regions—“Murcia” and “Almería”—were analyzed to obtain complementary results. 57 compounds were found, mainly phenolic acids and flavonoids, of which eight were identified for the first time in tomato. Polyphenols were more abundant in byproducts from “Murcia” samples than in those from“Almería” samples, where the abundance of compounds like coutaric, caffeic, neochlorogenic, dicaffeoylquinic and ferulic acids, vanillic acid hexoside, catechin, naringenin, prunin, apigenin-O-hexoside, rutin, and rutin-O-pentoside was even much higher in byproducts than that in whole fruits. These results reveal the wide range of polyphenols found in tomato byproducts, with potential applications in pharmaceutical research, food preservation, and cosmetic development, among others.
Keywords: phenolic compounds, Lycopersicon esculentum P. Mill, tomato byproducts, LC-HRMS, chemometrics
1. Introduction
The tomato processing industry produces different residues/wastes with dangerous economic and environmental implications, such as release of greenhouse gases and billions of liters of water waste, loss of gross domestic product, etc.1,2 According to official data from the Food and Agriculture Organization of the United Nations (FAO, UN), tomato is the second most distributed horticultural crop worldwide, with a production rate of more than 186 million tons per year.3 However, it also generates around 8.5 million tons of byproducts, where peel and seeds represent 61% and 38%, respectively, of the total amount. Therefore, research on their exploitation is preconized to enforce the UN sustainable development agenda in the framework of circular economy.4
Despite that tomato byproducts have been mainly employed for animal feed or compost production, disposal of such wastes is currently a costly issue. However, applying tomato peel and seeds in the pharmaceutical, food, and cosmetic fields is a reality now. For example, tomato seed extract was recently commercialized as a nutritional supplement, claiming the improvement of sport performance in users;5 refined flour substituted with around 40% of tomato seeds exhibited higher amounts of dietary fiber and vitamin C, as well as longer shelf life, than those in traditional bakery products;6 addition of dried peel in raw and cooked beef burgers (at 4%) improved their sensorial and physicochemical characteristics like color and texture.7
Utilizing tomato byproducts in all mentioned areas is possible since peel and seeds are rich sources of bioactive compounds. Like tomato fruits, byproducts show exceptional concentrations of phytochemicals (carotenoids and polyphenols), vitamins (ascorbic acid, tocopherols, and provitamin A), glycoalkaloids (tomatine), pectin, fatty acids, and minerals.8,9 Coelho and coworkers have encouraged the integral valorization of tomato byproducts through the recovery of bioactive compounds, indicating that the mainly exploited compounds from tomato byproducts are carotenoids which account for around 70% of the total chemical composition of tomato matrices, but a promising minor fraction with exploitation potential includes phenolic compounds.10
The main body of studies on polyphenols from tomato byproducts has been focused on the extraction processing of such molecules and, a priori, their identification and quantification using liquid chromatography (LC) with UV detection or low-resolution mass spectrometry (LRMS). Ferreres and coworkers identified 14 flavonols in tomato seeds, including quercetin, kaempferol, and isorhamnetin derivatives, employing an LC-LRMS with an ion trap mass analyzer.11 On the other hand, Tamasi and coworkers reported six polyphenols in tomato peel using an LC-LRMS system with a triple-quadrupole mass analyzer, finding that caffeic acid, chlorogenic acid, and rutin were the most abundant polyphenols.12 By this way, Kalogeropoulos and coworkers carried out the most exhaustive identification panel of polyphenols, determining 18 polyphenols within phenolic acid and flavonoid families, where a selective ion monitoring (SIM) gas chromatography–mass spectrometry (GC-MS) method was used.13
The extensive knowledge of the extraction processing of polyphenols contrasts with the need for more information about the phenolic composition in tomato byproducts, which should be as broad as possible. López-Yerena reviewed and summarized the literature on identifying polyphenols in tomato byproducts and outlined their extraordinary exploitation potential in different industrial areas;14 however, the exhaustive polyphenolic profile has not yet been evaluated.
Thus, the main objective of this study was to characterize the polyphenolic profile in tomato peel and seeds by the analysis of hydroethanolic extracts using an untargeted analytical strategy based on LC coupled to high-resolution MS (LC-HRMS) with an LTQ Orbitrap analyzer. The combination of full-scan and data-dependent acquisition modes was employed to increase the detection coverage of polyphenols in the extracts. To understand the importance of their valorization, the polyphenolic profile obtained from these two byproducts was compared to the profile of the whole fruit, namely, a solid matrix with all components – like peel, pulp, seeds, and other tissues – which is regularly consumed fresh. For this purpose, an unsupervised multivariate analysis was carried out to find patterns that can be used to sum up differences and a univariate statistical analysis was then employed to profoundly investigate the changes on the leading phenolic families and representative polyphenols. This is the first report regarding the comprehensive elucidation of the phenolic composition of tomato peel and seeds, as well as the investigation of significant changes on the abundances of representative polyphenols in such byproducts with respect to the whole fruit. It was also intended to identify a higher number of phenolic compounds in these matrices in order to increase the knowledge of tomato composition. All these results had the goal to contribute with relevant information in the polyphenols present in these kinds of waste which could help to propose new routes for their valorization in the framework of circular economy.
2. Materials and Methods
2.1. Chemicals and Reagents
Standards of the following polyphenols were used for confirmation purposes: gallic acid, caffeic acid, ferulic acid, vanillic acid, syringic acid, 4-hydroxybenzoic acid, p-coumaric acid, protocatechuic acid, homovanillic acid, sinapic acid, apigenin, quercetin, and kaempferol were supplied by Sigma-Aldrich (St. Louis, MO, USA); catechin, rutin, and myricetin were provided by TCI (Tokyo, Japan); quercetin and chlorogenic acid were from Merck (Darmstadt, Germany); epigallocatechin and naringenin were from Biosynth Carbosynth (Berkshire, United Kingdom); diosmin was from Alfa Aesar (Kandel, Germany); galangin was from Cymit (Barcelona, Spain). A stock solution of each polyphenol was prepared in DMSO (Panreac, Barcelona, Spain) at a concentration of 5000 mg L–1. Working solutions for LC-HRMS analysis were prepared at 5 and 10 mg L–1 with acetonitrile/water (50/50, v/v) from the stock solutions.
For chromatographic separation, the following solvents were used: water purified with an Elix 3 coupled to a Milli-Q system (Bedford, USA), formic acid (≥95%, Sigma-Aldrich, St Louis, USA), and acetonitrile (99.9%, UHPLC Supergradient, Panreac, Barcelona, Spain).
2.2. Instrumentation and LC-HRMS Analysis
A Dionex UHPLC system coupled to an LTQ Orbitrap Velos mass spectrometer with an ESI-II electrospray ionization source (Thermo Scientific, Ca, USA) was used for the analysis of extracts.
The chromatographic separation was carried out with a Kinetex C18 column (150 mm length × 4.6 mm I·D, 2.6 μm partially porous particle size) from Phenomenex (Torrance, CA, USA) equipped with a SecurityGuard ULTRA cartridge C18 (Phenomenex). The mobile phase consisted of 0.1% (v/v) formic acid (solvent A) and acetonitrile (solvent B). A constant flow rate of 0.7 mL min–1 was used. The gradient elution program employed was as follows: initially, 3% B was maintained for 3 min, then, from 3% to 30% B was applied for the next 18 min, and the percentage of B was increased linearly to 65% from 18 to 23 min. Then, the percentage of B was increased up to 90% in 2 min and kept constant for additional 2.5 min. Finally, the percentage of B was decreased to initial conditions (3%) in 0.5 min, and the column was conditioned for 7 min before the next injection. The injection volume was 10 μL.
HRMS with the LTQ Orbitrap was carried out in negative full scan mode (from m/z 100 to 1500) using a resolution of 60 000 full-width at half-maximum (FWHM) at m/z 200. In addition, a data-dependent product ion scan was activated when the full scan signal was higher than 1.0 × 105 (peak intensity threshold). Stepped normalized collision energies (NCEs) of 17.5, 35.0, and 52.5 were applied, and HRMS/MS spectra were recorded from an m/z of 50 Da. A mass resolution of 17 500 FWHM at m/z 200 was used for data-dependent analysis. Nitrogen (purity higher than 99.98%) was used as ESI sheath gas, ion-sweep gas, and auxiliary gas, at flow rates of 60, 0, and 10 arbitrary units, respectively. Capillary and S-lens RF voltages were set at −2.5 kV and 50 V, respectively. The source temperature was maintained at 25 °C, and the capillary temperature at 320 °C. The HRMS analyzer was tuned and calibrated every 3 days by using the calibration solution supplied by Thermo Fisher Scientific. Initially, the tentative identification (based on accurate mass errors below 5 ppm) of polyphenols was performed with fragmentation patterns from HRMS and HRMS/MS spectra; when available, they were compared with those of pure standards for a definitive confirmation.
LC-HRMS data were acquired and processed with Xcalibur 2.2 (Thermo Scientific, Ca, USA). The peak areas of the compounds from extracted ion chromatograms were integrated using OriginPro 8 software (OriginLab Corporation, USA). For the different statistical analyses, SOLO (eigenvector Research, USA), Statgraphics Centurion 19 (STATPOINT Inc., USA), and Microsoft Excel 2019 (Microsoft Corporation, USA) software were used.
2.3. Samples and Sample Treatment
Three sets of 2-kg oblong tomato fruit samples were acquired at local markets from Barcelona, Spain (September 2023); they were produced at two different Spanish regions (Murcia and Almería). The samples were treated within 24 h from purchase and cleaned with distilled water. After that, one set was pooled to represent an analytical sample of fruit, one for a peel sample, and one for a sample of seeds. This step was independently carried out for each region. All samples were frozen at −20 °C and lyophilized for 48 h using a freeze-dryer HT 40 from Telstar LyoQuest (Barcelona, Spain). Finally, samples were ground and stored at −20 °C in darkness until analysis.
Extraction of polyphenolic compounds from solid matrices was carried out using a solid–liquid procedure previously developed.15 Briefly, 0.5 g of sample were extracted with 30 mL of ethanol/water (75/25, v/v) at 40 °C under continuous stirring. Subsequently, extracts were centrifuged for 15 min at 3500 rpm and filtered in a 0.22-μm membrane, and then placed in 2 mL LC vials.
3. Results and Discussion
3.1. Examination of LC-HRMS Data
An outlook of total ion chromatograms from extracts is shown in Figure S1, with complex chemical fingerprints of all samples. The chromatographic elution profiles show two predominant elution windows; the first was from 4 to 15 min, attributed to polar features, and the second was in the range from 20 to 27 min, attributed to semipolar ones. Moreover, an elution window was detected between these ranges with few peaks. Thus, a total window, which involves the three mentioned, from 4 to 27 min, was chosen to extract the molecular features, whereby the most significant number of components could be evaluated, excluding death time.
The identification scheme of the molecular features was carried out as follows: possible phenolic constituents were first summarized based on published literature and available databases in terms of their chemical family, molecular formula, molecular mass, and fragment ion information. After that, total ion chromatograms were examined to detect peaks by matching with those of possible constituents, and extracted ion chromatograms were then used to obtain retention time, accurate mass, error, and HRMS/MS fragments of each feature.
As a result, the tentative identification of polyphenols was achieved by comparing the experimental HRMS data and the previously summarized information, and possible fragmentation pathways were elucidated employing characteristic fragments and neutral losses. Available polyphenol standards were analyzed to confirm the presence of some compounds, and the obtained HRMS/MS spectra were compared with those of the extracts.
Under this scheme, the total number of identified polyphenols was 57, and the assigned compounds and their LC-HRMS information are described in Table 1. To the best of our knowledge, from the whole list of polyphenolic compounds, it was the first time that epigallocatechin, arbutin, diosmin, diosmetin-O-hexoside, galangin 3-[galactosyl-(1→4)-rhamnoside], homovanillic acid, two coumaroyltartaric acid isomers, and dihydroferulic acid glucuronide were found in tomato or related matrices, whereby this paper contributes to increase the knowledge on the metabolic profile of those matrices.
Table 1. Polyphenolic Compounds Found in Hydroethanolic Extracts from Tomato Fruit, Peel, and Seeds and the Main LC-HRMS/MS Parameters that Support their Identification*.
| compound | chemical formula | retention time (min) | precursor ion m/z calculated value | precursor ion m/z observed value | adduct | error mass (ppm) | main MS/MS fragments |
|---|---|---|---|---|---|---|---|
| gallic acida | C7H6O5 | 6.32 | 169.01314 | 169.01466 | [M-H]− | 2.446 | 125.02372 |
| cinnamic acid | C9H8O2 | 6.78 | 193.04953 | 193.05109 | [M-H+HCOOH]− | 2.372 | 147.02982 |
| 129.01892 | |||||||
| 113.02410 | |||||||
| 103.03966 | |||||||
| hydroxybenzoic acid-O-hexoside | C13H16O8 | 8.01 | 299.07614 | 299.07790 | [M-H]− | 2.205 | 137.02432 |
| 93.08644 | |||||||
| vanillic acida | C8H8O4 | 8.30 | 167.03388 | 167.03532 | [M-H]− | 2.023 | 152.01074 |
| 123.04369 | |||||||
| coumaroyltartaric acid isomer | C13H12O8 | 9.68 | 295.04592 | 295.04562 | [M-H]− | –1.086 | 163.04472 |
| 132.03430 | |||||||
| 119.03445 | |||||||
| 101.02397 | |||||||
| vanillic acid hexoside | C14H18O9 | 9.74 | 329.08670 | 329.08650 | [M-H]− | –3.966 | 285.09784 |
| 167.04552 | |||||||
| 123.04502 | |||||||
| dihydroxybenzoic acid isomer I | C7H6O4 | 9.95 | 153.01823 | 153.01923 | [M-H]− | –0.666 | 109.02962 |
| arbutin | C12H16O7 | 10.56 | 317.08670 | 317.08847 | [M-H+HCOOH]− | 2.096 | 227.08145 |
| 109.02852 | |||||||
| monocaffeoylquinic acid isomer I (neochlorogenic acid) | C16H18O9 | 10.82 | 353.08670 | 353.08820 | [M-H]− | 1.118 | 191.05640 |
| 179.03545 | |||||||
| 173.04575 | |||||||
| 135.04498 | |||||||
| dihydroxybenzoic acid isomer II | C7H6O4 | 10.89 | 153.01823 | 153.01961 | [M-H]− | 1.817 | 109.02959 |
| dihydroxybenzoic acid-O-pentoside | C12H14O8 | 11.10 | 285.06049 | 285.06107 | [M-H]− | –1.826 | 153.01904 |
| 109.02907 | |||||||
| coumaric acid isomer I | C9H8O3 | 11.31 | 163.03897 | 163.04053 | [M-H]− | 2.837 | 119.04977 |
| dihydroferulic acid glucuronide | C16H19O10 | 11.31 | 371.09727 | 371.0997 | [M-H]− | 3.584 | 325.09152 |
| 163.03935 | |||||||
| homovanillic acid-O-hexoside | C15H20O9 | 11.65 | 343.10235 | 343.10452 | [M-H]− | 3.103 | 181.05069 |
| 137.06046 | |||||||
| eriodictyol | C15H12O6 | 11.73 | 287.05501 | 287.05530 | [M-H]− | –2.826 | 151.00370 |
| 135.04545 | |||||||
| 125.02450 | |||||||
| 4-hydroxybenzoic acida | C7H6O3 | 11.78 | 137.02332 | 137.02403 | [M-H]− | –2.827 | 93.03410 |
| caffeic acid-O-hexoside | C15H17O9 | 11.81 | 341.0867 | 341.08746 | [M-H]− | –1.012 | 179.03436 |
| 135.04460 | |||||||
| catechina | C15H14O6 | 12.15 | 289.07066 | 289.07839 | [M-H]− | 1.036 | 245.07366 |
| 203.05232 | |||||||
| 123.04862 | |||||||
| apigenin-O-hexoside isomer I | C21H20O10 | 12.25 | 431.09727 | 431.09726 | [M-H]− | –2.575 | 269.04119 |
| 225.05496 | |||||||
| 175.01565 | |||||||
| coumaric acid-O-hexoside | C15H17O8 | 12.45 | 325.09179 | 325.09430 | [M-H]− | 4.335 | 163.03976 |
| 119.04962 | |||||||
| protocatechuic acida | C7H6O4 | 12.52 | 153.01823 | 153.01878 | [M-H]− | –0.552 | 109.02956 |
| homovanillic acida | C9H10O4 | 12.82 | 227.05501 | 227.05516 | [M-H]− | –4.190 | 137.05551 |
| monocaffeoylquinic acid isomer II (cryptochlorogenic acid) | C16H18O9 | 13.11 | 353.08670 | 353.08734 | [M-H]− | –1.318 | 191.05614 |
| 179.03503 | |||||||
| 173.04565 | |||||||
| 135.04512 | |||||||
| apigenin-O-hexoside isomer II | C21H20O10 | 13.56 | 431.09727 | 431.09726 | [M-H]− | –2.575 | 269.04095 |
| 225.05487 | |||||||
| 175.01572 | |||||||
| ferulic acid-O-hexoside | C16H20O9 | 13.68 | 355.10235 | 355.10269 | [M-H]− | –2.155 | 193.05016 |
| 178.04008 | |||||||
| 149.06064 | |||||||
| caffeic acida | C9H8O4 | 13.69 | 179.03388 | 179.03522 | [M-H]− | 1.329 | 135.04501 |
| epigallocatechina | C15H14O7 | 13.76 | 305.06557 | 305.06645 | [M-H]− | –1.593 | 305.06123 |
| 125.02442 | |||||||
| 109.06123 | |||||||
| monocaffeoylquinic acid isomer III (chlorogenic acid a) | C16H18O9 | 14.00 | 353.08670 | 353.08759 | [M-H]− | –0.610 | 191.05566 |
| 179.03439 | |||||||
| 4-hydroxybenzoic acid isomer | C7H6O3 | 14.13 | 137.02332 | 137.02414 | [M-H]− | –2.024 | 93.03695 |
| syringic acida | C9H10O5 | 14.13 | 197.04444 | 197.04588 | [M-H]− | 1.692 | 182.05101 |
| 153.05028 | |||||||
| coumaroyltartaric acid isomer II | C13H12O8 | 15.11 | 295.04592 | 295.04562 | [M-H]− | –1.086 | 163.04489 |
| 132.03421 | |||||||
| 119.03456 | |||||||
| 101.02384 | |||||||
| myricetina | C15H10O8 | 15.36 | 317.02919 | 317.02917 | [M-H]− | –3.534 | 301.04257 |
| 273.03082 | |||||||
| 151.00361 | |||||||
| coumaroylquinic acid | C16H18O8 | 15.75 | 337.09179 | 337.09225 | [M-H]− | –1.901 | 191.05507 |
| 163.04123 | |||||||
| rutin-O-pentoside | C32H38O20 | 15.87 | 741.18726 | 741.18781 | [M-H]− | NA | 609.14453 |
| 301.03421 | |||||||
| 300.02655 | |||||||
| 178.99792 | |||||||
| kaempferol-O-hexoside | C21H20O11 | 16.49 | 447.09218 | 447.09283 | [M-H]− | –1.017 | 285.03851 |
| 175.03975 | |||||||
| galangin 3-[galactosyl-(1→4)-rhamnoside] | C27H30O14 | 16.52 | 577.15518 | 577.15698 | [M-H]− | 1.215 | 341.10760 |
| 269.04443 | |||||||
| 179.05547 | |||||||
| 161.04498 | |||||||
| p-coumaric acida | C9H8O3 | 16.76 | 163.03897 | 163.04019 | [M-H]− | 0.752 | 119.05045 |
| rutina | C27H29O16 | 16.86 | 609.14501 | 609.14514 | [M-H]− | NA | 301.03452 |
| 300.02689 | |||||||
| 178.99789 | |||||||
| phloretin-C-diglucoside | C27H34O15 | 16.93 | 597.18139 | 597.18219 | [M-H]− | –0.508 | 597.18159 |
| 477.18023 | |||||||
| 273.07483 | |||||||
| sinapic acida | C11H12O5 | 17.53 | 223.06009 | 223.06059 | [M-H]− | –2.720 | 208.03522 |
| 179.05569 | |||||||
| quercetin-O-hexoside isomer I | C21H19O12 | 17.53 | 463.08710 | 463.08917 | [M-H]− | 2.096 | 301.03343 |
| 300.99658 | |||||||
| 178.99796 | |||||||
| 151.00313 | |||||||
| kaempferol-O-rutinoside | C27H30O15 | 17.99 | 593.15009 | 593.15002 | [M-H]− | –1.978 | 431.11533 |
| 285.03867 | |||||||
| 175.03958 | |||||||
| naringenin-O-hexoside (Prunin) | C21H22O10 | 18.82 | 433.11292 | 433.11304 | [M-H]− | –2.263 | 271.08127 |
| 177.08153 | |||||||
| 161.04480 | |||||||
| diosmina | C28H32O15 | 18.92 | 607.16574 | 607.16608 | [M-H]− | –1.257 | 299.05726 |
| 151.00312 | |||||||
| diosmetin-O-hexoside | C22H22O11 | 19.28 | 461.10783 | 461.10809 | [M-H]− | –1.832 | 299.05618 |
| 151.00365 | |||||||
| 149.02641 | |||||||
| dicaffeoylquinic acid isomer I | C25H24O12 | 19.36 | 515.11840 | 515.12158 | [M-H]− | 4.039 | 353.08768 |
| 191.05626 | |||||||
| 173.04650 | |||||||
| kaempferol isomer | C15H10O6 | 21.45 | 285.03936 | 285.03995 | [M-H]− | –1.794 | 285.03969 |
| 175.03948 | |||||||
| 151.00324 | |||||||
| quercetin-O-hexoside isomer II | C21H20O12 | 21.78 | 463.08710 | 463.08737 | [M-H]− | –1.791 | 301.03345 |
| 300.99658 | |||||||
| 178.99796 | |||||||
| 151.00325 | |||||||
| dicaffeoylquinic acid isomer II | C25H24O12 | 21.85 | 515.11840 | 515.12158 | [M-H]− | 4.039 | 353.08779 |
| 191.05615 | |||||||
| 173.04642 | |||||||
| quercetina | C15H10O7 | 22.12 | 301.03427 | 301.03598 | [M-H]− | 2.007 | 300.99658 |
| 178.99796 | |||||||
| 151.00313 | |||||||
| 149.02365 | |||||||
| kaempferola | C15H10O6 | 22.31 | 285.03936 | 285.03995 | [M-H]− | –1.794 | 285.03955 |
| 175.03938 | |||||||
| 151.00310 | |||||||
| naringenina | C15H12O5 | 23.12 | 271.06009 | 271.06122 | [M-H]− | 0.086 | 271.06095 |
| 177.08135 | |||||||
| 161.04358 | |||||||
| 151.03003 | |||||||
| 119.00356 | |||||||
| apigenina | C15H10O5 | 23.21 | 269.04444 | 269.04522 | [M-H]− | –1.214 | 269.04801 |
| 225.05506 | |||||||
| 175.01542 | |||||||
| 151.00226 | |||||||
| 149.02408 | |||||||
| quercetin isomer | C15H10O7 | 23.25 | 301.03427 | 301.03482 | [M-H]− | –1.847 | 300.99672 |
| 178.99782 | |||||||
| 151.00329 | |||||||
| 149.02381 | |||||||
| apigenin isomer | C15H10O5 | 23.80 | 269.04444 | 269.04514 | [M-H]− | –1.512 | 269.04801 |
| 225.05506 | |||||||
| 151.00226 | |||||||
| 149.02408 | |||||||
| ferulic acida | C10H10O4 | 24.23 | 193.04953 | 193.05039 | [M-H]− | –1.254 | 178.04015 |
| 149.04507 | |||||||
| isorhamnetin | C16H12O7 | 24.57 | 315.04992 | 315.04953 | [M-H]− | 2.679 | 315.04912 |
| 151.00325 | |||||||
| 119.00263 |
Polyphenolic compounds confirmed by LC-HRMS analysis of reference standards.
NA: not applicable.
Furthermore, three peaks with high abundance were found but could not be assigned to any known compound. The peak at 6.92 min showed a precursor ion at m/z 164.07195 and fragments at m/z 147.04472 and 120.05328 in its HRMS/MS spectra; given masses correspond to Δm/z 17 and 44, attributed to dihydroxylation and decarboxylation, respectively, which are typical for phenolic acids. The peak at 18.19 min showed a precursor ion at m/z 741.19135 and HRMS/MS fragments at m/z, 807.31563, 779.61233, 747.36616, and 682.33643. Finally, the peak at 26.10 showed a precursor ion at m/z 353.20111 with HRMS/MS fragments at m/z 352.20041, 332.20013, 302.23311, and 122.20031. This information is for further studies that could be carried out to elucidate the molecular structure of the found compounds using a battery of spectroscopic techniques.
In the following sections, the characteristic fragmentation patterns supporting the identification of the detected phenolic compounds are discussed with various meaningful cases (see the Supporting Information).
3.2. Identification of Phenolic Acids and Derivatives
12 hydroxybenzoic acids and 18 hydroxycinnamic acids were detected, most of them first assigned to their [M-H]− ions in HMRS full scan and by the monitoring of the decarboxylation process in the HRMS/MS spectra as primary fragmentation.16,17 For instance, the peak at 6.32 min showed a m/z value of 169.01466, which matched the gallic acid [M-H]− ion with an error of 2.446 ppm. In the analysis of HRMS/MS spectra (Figure S2A), a single ion of m/z 125.02372 was detected, which corresponds to the loss of the carboxylic moiety as −COO (Δm/z = 44). The retention time and HRMS/MS spectra (Figure S2B) agree with those obtained in the pure standard, confirming the identification of this compound as gallic acid. In the same way, two hydroxybenzoic acid isomers, three dihydroxybenzoic acid isomers, two coumaric acid isomers, syringic acid, ferulic acid, and caffeic acid were assigned.
In another illustrative case, the HRMS full-scan spectra of the chromatographic peak at 12.99 min showed an ion of m/z 223.06059 with an error of −2.720 ppm, tentatively matching with the sinapic acid [M-H]− chemical formula. As shown in Figure S3A, an ion of m/z 179.05569 was found in its HRMS/MS spectrum, corresponding to the decarboxylation process, and an ion of m/z 208.03522 was also detected which corresponds to the demethylation −CH3 (Δm/z = 15) of the structure.18 When the pure standard was analyzed, retention time and MS/MS fragments were the same as those detected in the extracts (Figure S3B); thereby, the assignment of sinapic acid was confirmed. Analogously, vanillic acid was confirmed in the extracts.
Although [M-H]− is the main ion of phenolic compounds in negative ESI, the [M-H+HCOOH]− adduct could be potentially detected.19 For example, the peak at 6.78 min with the HRMS spectra (Figure 1A) showed a precursor ion of m/z 193.05109 and with an error of 2.372 ppm, which was not primarily assigned to any compound; however, in the HRMS/MS spectra (Figure 1B), an ion at m/z 147.02982 was detected and matched with a molecular formula of the cinnamic acid moiety. Besides, two ions at m/z 129.01892 and m/z 103.03966 were observed, which are neutral losses of −H2O (Δm/z = 18) and −COO, respectively, so this species was tentatively identified as cinnamic acid. For arbutin, this fragmentation pattern was also observed; the peak at 10.56 min showed HRMS spectra (Figure S4A) with a precursor ion at m/z 317.08847 which matches with [M-H+HCOOH]− of arbutin (error: 2.096 ppm). During the exploration of its HRMS/MS spectra (Figure S4B), it was noted that a fragment ion at m/z 227.08145 corresponded to formula C12H15O7 for arbutin, and an ion at m/z 109.02852 corresponded to the catechol moiety after the neutral loss of hexoside. This fragmentation pattern was like that proposed by Song and coworkers.20
Figure 1.
HRMS spectra (A) and HRMS/MS spectra (B) of cinnamic acid from tomato and byproducts extracts. Dotted line indicates a fragment of ion at m/z 147.
Another fragmentation that could be found is that in the de-esterification; this is the cleavage of O-linkage between a phenolic acid moiety and a glycoside moiety, or two phenolic acid moieties, with different similar structures.21−23 In this sense, eight glycoside derivatives, three monocaffeoylquinic acid isomers, two dicaffeoylquinic acid isomers, two coumaroyltartaric acid isomers, and a monocoumaroylquinic acid were identified in the extracts.
For the assignation of glycoside derivatives, neutral losses of hexose (Δm/z = 162), pentose (Δm/z = 132), rhamnose (Δm/z = 146), and glucuronide (Δm/z = 176) moieties were investigated as the main fragmentations.17 An example of this is the peak at 11.10 min; this showed an ion at m/z 285.06107 in HRMS spectra, resulting in a chemical formula of C12H13O8 with an error of −1.826 ppm. Exploration of its HRMS/MS spectra (Figure S5A) led to relayed in ions at m/z 153.01904 and m/z 109.02907, meaning neutral losses of −C5H8O4 (Δm/z = 132) and −COO, respectively, and matching with the fragmentation pattern of dihydroxybenzoic acid-O-pentoside. Similarly, homovanillic acid-O-hexoside was elucidated. The HRMS spectra of the peak at 11.65 min denoted an ion at m/z at 343.10452 with C15H19O9 as the proposed formula and an error of 3.103 ppm, and the HRMS/MS spectra of this ion (Figure S5B) showed fragments of m/z 181.05069 and m/z 137.06046, representing −C6H10O5 (Δm/z = 162) and −COO, respectively, and matching with the fragmentation pattern of that polyphenol.
Coumaroyltartaric acid (coutaric acid) isomers were identified as follows: peaks at 9.68 and 15.11 min, which showed [M-H]− of m/z 295.04562 with an error of −1.086 ppm (Figure S6A), corresponded to the loss of the tartaric acid moiety (Δm/z = 132) and the base peak of the coumaric acid moiety (m/z 163.04472) in their HRMS/MS spectra (Figure S6B).24 Besides, the ion at m/z 119.03445 was observed in that spectra because of the neutral loss of −COO in the coumaric acid moiety, and then, the ion at m/z 101.02397 can be attributed to structural rearrangement of such an ion by −H2O loss.
In the case of monocaffeoylquinic acid isomers, peaks at 10.82, 13.11, and 14.00 min had [M-H]− of m/z 353.08820, 353.08734, and 353.08759, with errors of 1.118, −1.318, and −0.610 ppm, respectively, proposing C16H17O9 as the formula. It could be observed ions at m/z 191, 179, 173, and 135, which correspond to the quinic acid moiety, caffeic acid moiety, −H2O loss of the quinic acid moiety, and −COO loss of the caffeic acid moiety, respectively. According to the literature,25,26 three main isomers, chlorogenic, cryptochlorogenic, and neochlorogenic acids, can be distinguished by comparing the relative intensity of those ions. For the compound at 10.82 min (Figure S7A), the ratio of m/z 191 and 179 was approximately 100/20, while the ratio of m/z 191 and 135 was 100/30, conjecturing the presence of neochlorogenic acid. For the compound at 13.11 min (Figure S7B), the ion at m/z 173 showed 100-% intensity, being very specific for cryptochlorogenic acid. Finally, in the HRMS/MS spectra of compound at 14.00 min (Figure S7C), it was noticed that a ratio of m/z 191 and 179 was 100/<10, and this information matched with retention time and HRMS/MS spectra ions when the chlorogenic acid standard was analyzed (Figure S7D).
3.3. Identification of Flavonoids and Derivatives
Additionally, flavonoids were identified utilizing their characteristic fragments, attributed to the rupture of the B ring bond and the retro Diels–Alder fragmentation (m/z 151).17,27 Neutral losses like de-esterification can also be found by rupture of glycoside bonds and ruptures of −CO (Δm/z = 28) and −CH3 (Δm/z = 15).28,29
Peaks at 17.53 min showed a precursor ion at m/z 463.08917 with the formula of C21H18O12 (error: 2.096 ppm), whereas the precursor ion of the peak at 22.12 min was at m/z 301.03598 with the formula of C21H18O12 (error: 2.096 ppm). As shown in Figure S8, HRMS/MS spectra of ions at m/z 463 and m/z 301 show fragments at m/z 300.99658, 178.99796, and 151.00313. For the compound at 17.53 min, a difference of m/z 162 (−C6H10O5) was found, determining a rupture of the hexoside bond. In both compounds, spectra showed fragments at m/z 300.99658, 178.99796, 151.00313, and 149.02365; all these results matched with retention time and fragments found in the HRMS/MS spectra of quercetin standard solution (Figure S8C), so this compound was assigned as quercetin, showing a quercetin isomer and two quercetin-O-hexosides.
In addition, apigenin was found in peak at 23.21 min, which showed the precursor ion at m/z 269.04522 with the formula of C15H9O5 (errors: −1.214 and 1.512 ppm). HRMS/MS spectra are shown in Figure S9A. Fragments at m/z 269.04801, 225.05506, 175.01542, 151.002265, and 149.02408 were correlated with the fragmentation patterns of apigenin observed by Chiriac29 and Kečkeš.30 The fragment at m/z 175 was related explicitly to the rupture of bond of phenol-type B ring in apigenin structure. Furthermore, retention times and fragments matched with HRMS/MS spectra from apigenin standard (Figure S9B).
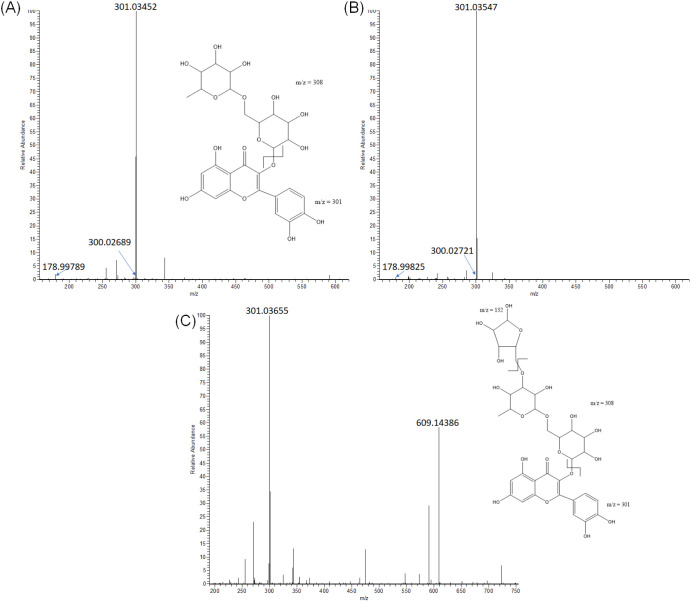
On the other hand, a particular case was the identification of rutin and rutin-O-pentoside. HRMS spectra of the peak at 16.86 min showed a precursor ion at m/z 609.14514 which did not match with any chemical formula; however, during the visualization of its HRMS/MS spectrum (Figure 2A), we observed ions at 301.03452 and 300.02689 which mean [M-H]− and [M-2H]− of quercetin with Δm/z = 308 due to the rupture of rutinoside bond, as well as fragment at m/z 178. The retention time and HRMS/MS fragmentation pattern (Figure 2B) were in concordance with those of the rutin standard. Likewise, peak at 15.87 min with a precursor ion at m/z 741.18781 did not match with any chemical formula, but HRMS/MS shown in Figure 2C enabled the detection of ions at m/z 609.14453, with Δm/z = 132 attributed to the rupture of the pentoside bond, and at m/z 301.03421 and 300.02655, which are parallel to data obtained in the HRMS/MS spectra of rutin, and enabled the assignation of this compound such as rutin-O-pentoside.
Figure 2.
HRMS/MS spectra of rutin from tomato and byproducts extracts (A), rutin standard solution (B), and rutin-O-pentoside (C) from tomato and byproducts extracts.
3.4. Unsupervised Exploratory Analysis
To evaluate differences between the polyphenolic profiles in the six classes of samples under study, an unsupervised principal component analysis (PCA) was conducted using a data set constructed from peak abundances (peak areas in extracted ion chromatograms) of the 57 found polyphenolic compounds. Data were preprocessed by autoscaling, and the number of principal components (PC) was set at 3.
The obtained model explains a total accumulative variance with a Q-residuals value of 10.99% and a Hotelling T2 value of 89.01%. PC1 and PC2 support 55.60% and 22.33% of variance, respectively. When data were analyzed along the six different classes of samples (Figure 3A), the complete discrimination of’Murcia’ fruit and peel was done in PC1, whereas’Murcia’ peel,’Almería’ peel, and’Almería’ fruit were differentiated, along PC2. However, the PCA did not discriminate all the samples between the two analyzed regions (Figure 3B). On the other hand, a study of the differences between fruit, peel, and seeds, without the dependency of regions, showed a clear tendency of agglomeration for seeds (Figure 3C). The loadings revealed 14 polyphenolic compounds responsible for the discrimination among samples, including 4-hydroxybenzoic acid, a dihydroxybenzoic acid isomer, homovanillic acid-O-hexoside, caffeic acid, 4-O-caffeoylquinic acid, p-coumaric acid, dihydroferulic acid-O-glucuronide, eriodictyol, prunin, diosmetin-O-hexoside, quercetin, rutin, kaempferol, and phloretin-C-dihexoside.
Figure 3.
Unsupervised exploration of polyphenolic profiles in samples by use of principal component analysis and hierarchical clustering analysis. Score plots from the principal component analysis of samples divided in 6 different classes (A), regions (B), and groups (C). Dendrogram plot from hierarchical clustering analysis for the 6 classes of samples (D). Data set was formed with peak abundances of the 57 identified polyphenolic compounds in LC-HRMS/MS.
The hierarchical clustering analysis (HCA) with k-nearest neighbors using results from the PCA set at the Mahalanobis distance enables an understanding of the found differences. The resulted dendrogram is shown in Figure 3D. The analysis separated the samples into three main groups. The “Murcia” fruit was the most different sample, where the abundances of the detected phenolic compounds were extensively different to the rest of the samples. Also, the total number of elucidated polyphenolic compounds was found in this sample. Besides, it was noticed that the abundances on all’Almería’ samples were different to “Murcia” samples, enabling a differentiation of both classes. The exploratory results indicate significant changes on their polyphenolic profiles, mainly attributed to the class of sample (fruit, peel, or seed).
3.5. Changes in the Abundances of the Main Phenolic Families between Tomato Fruit, Peel, and Seeds from “Murcia” and “Almería” Regions
Apart from the information obtained in exploratory analysis, the variations in the abundances of phenolic families were studied to deeply evaluate the differences in the polyphenolic profiles in the whole set of samples. The 57 polyphenols were classified into eight phenolic families (Table S1), and the total abundance of a phenolic family was estimated by the sum of the peak areas of all compounds belonging to such family. With the given scope in mind, data were subjected to one-way ANOVA at 95-% confidence, and then the Tukey HSD test was performed.31
Figure 4 shows the results of the distribution of the families among the samples. As the graphs imply, both phenolic acids were 4-fold higher in “Murcia” fruit than those in its respective peels and 2-fold higher than those in seeds. Contrarily, “Almería” samples showed different tendencies; peel showed a 50% increase in the content of hydroxycinnamic acids compared to seeds and fruit, but hydroxybenzoic acids were 1.6 times more concentrated in seeds than those in the other samples. All flavonoid classes show better distribution for both “Murcia” and “Almería” peels (up to 5 times concentrated) than those in respective fruit and seeds; only slight discrepancies, as in the case of flavones, were observed where this family was 50% more concentrated in “Murcia” fruit than that in the respective peel. In a similar way, other phenols were up to 3 times more distributed in peel than those in fruit and seeds for both regions.
Figure 4.
Relative abundances of the main phenolic families in tomato fruit, peel, and seeds from “Murcia” and “Almería” regions. (A) Hydroxybenzoic acids, (B) hydroxycinnamic acids, (C) flavanols, (D) flavanones, (E) flavones, (F) flavonols, (G) chalcones, and (H) other phenols.
The high abundance of these phenolic families in peel is well justified due to its role in attracting pollinator insects and protecting against biotic and abiotic stresses.32 Other phenols did not contribute significantly to the phenolic composition of any sample, and this family was more abundant in “Murcia” peel. The results show that tomato byproducts are excellent sources of phenolic acids and flavonols.
3.6. Changes in the Abundances of the Representative Polyphenols between Tomato Fruit, Peel, and Seeds from “Murcia” and “Almería” Regions
After evaluating the behavior of the phenolic families, the abundances of some representative polyphenols were also tested through comparing their peak abundance area obtained in extracted ion chromatograms by statistical analysis as indicated in the previous case. While’Murcia’ fruit has the highest concentration of most of the identified polyphenols, the principal goal of this work is to demonstrate the capability of tomato byproducts like enriched sources of bioactive polyphenols; thus, the studied polyphenols in this section were selected because they showed the highest content in any byproduct sample, and the results are depicted in Figure 5.
Figure 5.
Relative abundances of polyphenolic compounds in tomato fruit, peel, and seeds from “Murcia” and “Almería” regions. (A) Vanillic acid hexoside, (B) coutaric acid isomer, (C) caffeic acid, (D) neochlorogenic acid, (E) dicaffeoylquinic acid, (F) ferulic acid, (G) catechin, (H) naringenin, (I) prunin, (J) apigenin-O-hexoside, (K) rutin, and (L) rutin-O-pentoside.
Vanillic acid hexoside (Figure 5A) was the hydroxybenzoic acid with more content in “Murcia” seeds, where the difference between the abundances of those samples was 97.27%; this compound was also more concentrated in “Almería” seeds with an increase of 98.48% and 42.22% in comparison to that in fruit and peel samples, respectively. The presence of this compound in tomato byproducts is reported for the first time herein. However, vanillic acid was previously detected in tomato wastes from industries after processing, where it can be hypothesized that employed processes could promote the rupture of the hexoside bonds, producing the release of aglycone form.13,33,34
Regarding hydroxycinnamic acids, five compounds were more abundant in tomato byproducts than those in the respective whole fruit: coutaric acid isomer (Figure 5B), caffeic acid (Figure 5C), neochlorogenic acid (Figure 5D), dicaffeoylquinic acid (Figure 5E), and ferulic acid (Figure 5F). Coutaric acid was 61.17% more concentrated in’Almería’ seeds than that in’Murcia’ and’Almería’ fruits and’Murcia’ peel. Caffeic acid showed a tendency where the abundance increased in the order of fruit < peel < seeds for both regions, with’Murcia’ byproducts overcoming up to 81.43% of the abundance of’Almería’ byproducts. In addition, this compound is the most detected one in a wide range of tomato byproduct samples from different environmental origins.12,13,34−38 By this way, the abundance of neochlorogenic acid in’Murcia’ peel was 1.57 times higher than that in the respective fruit. These phenolic acid and other monocaffeoylquinic acid isomers have been identified in tomato byproducts according to the literature.12,13,33,35−37,39−41 “Murcia” peel was notably the sample with the highest content of dicaffeoylquinic acid, with 83.18% more abundance than that in other samples. It is relevant to mention that coutaric acid and dicaffeoylquinic acid were found in tomato byproducts for the first time. In the case of ferulic acid, the concentration of this polyphenol in peel and seeds from the two regions was highly superior in comparison to the respective fruits (∼98.57%), and, specifically, peel was around 30% more concentrated than seeds. Ferulic acid is a polyphenol found in tomato byproducts from markets, cultivars, and factories.13,34,36,37,42
For flavonoids, catechin (Figure 5G) was slightly (12.76%) more abundant in “Almería” peel than other samples, and it has been described that its abundance is low in comparison with other polyphenols.13,34,36,38 On the other hand, a high abundance of naringenin and quercetin, as well as their derivatives like prunin and rutin, in tomato seeds and peel was reported before.11,43,44 Naringenin (Figure 5H) and prunin (Figure 5I) showed a higher abundance in peel from both regions than those in the whole fruit, with increases of 65.08% and 83.61%, respectively. For the first time, apigenin-O-hexoside (Figure 5J) was found in tomato byproducts, exceptionally concentrated in’Murcia’ seeds (∼90%), compared to that in other samples. Concerning rutin (Figure 5K) and rutin-O-pentoside (Figure 5L), their maximum abundance was observed in “Murcia” peel, even though it was up to 45% higher than that in “Almería” peel. Rutin has also been extensively found in tomato byproducts,2,12,33,34,36−38,40,45,46 and a rutin derivative was reported before, but its identity is not clear.37
To sum up, tomato peel and seeds – from two Spanish cultivars – contained a wide variety of polyphenolic compounds, where a significant number of them was found in these kinds of samples for the first time. Of the 57 detected compounds, most of them belong to phenolic acids and flavonoids, mainly flavonols. Among the most abundant compounds found in the byproducts, seven aglycones (coutaric acid, caffeic acid, neochlorogenic acid, dicaffeoylquinic acid, ferulic acid, catechin, and naringenin) and five glycoside derivatives (vanillic acid hexoside, prunin, apigenin-O-hexoside, rutin and rutin-O-pentoside) stand out. In this context, this paper offers relevant information to a wide range of people, like tomato producers, environmentalists, and other scientists, interested in the comprehensive valorization of tomato wastes through obtaining high-value natural products with elevated bioactive properties from these matrices.
Acknowledgments
Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT) is acknowledged for the PhD scholarship (CVU: 957048), and Coordinación General de Estudios de Posgrado – UNAM is acknowledged for the Apoyo de Movilidad de Larga Duración given to J.M.L.-T.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.4c02126.
Total ion chromatograms of all extracts with LC-HRMS, HRMS, and HRMS/MS spectra for representative polyphenolic compounds; classification of identified polyphenolic compounds (PDF)
This research was supported by the project PID2020–114401RB-C22 financed by the Agencia Estatal de Investigación (AEI/10.13039/501100011033), the Agency for Administration of University and Research Grants (Generalitat de Catalunya, Spain) under the project 2021SGR–00365, and María de Maeztu Unit of Excellence (Research Institute of Nutrition and Food Safety, INSA-UB, University of Barcelona), grant CEX2021–001234-M, funded by MCIN/AEI/10.13039/501100011033
The authors declare no competing financial interest.
Supplementary Material
References
- Silva P. A.; Borba B. C.; Pereira V. A.; Reis M. G.; Caliari M.; Brooks M. S.-L.; Ferreira T. A. P. C. Characterization of Tomato Processing By-Product for Use as a Potential Functional Food Ingredient: Nutritional Composition, Antioxidant Activity and Bioactive Compounds. Int. J. Food Sci. Nutr. 2019, 70 (2), 150–160. 10.1080/09637486.2018.1489530. [DOI] [PubMed] [Google Scholar]
- Szabo K.; Catoi A.-F.; Vodnar D. C. Bioactive Compounds Extracted from Tomato Processing By-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. 10.1007/s11130-018-0691-0. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of United Nations (FAO). FAOSTAT. https://www.fao.org/faostat/es/#data/QCL (accessed 2023 November 23).
- Schroeder P.; Anggraeni K.; Weber U. The Relevance of Circular Economy Practices to the Sustainable Development Goals. J. Ind. Ecol. 2019, 23 (1), 77–95. 10.1111/jiec.12732. [DOI] [Google Scholar]
- Ciriminna R.; Fidalgo A.; Meneguzzo F.; Ilharco L. M.; Pagliaro M. Lycopene: Emerging Production Methods and Applications of a Valued Carotenoid. ACS Sustainable Chem. Eng. 2016, 4 (3), 643–650. 10.1021/acssuschemeng.5b01516. [DOI] [Google Scholar]
- Mehta D.; Prasad P.; Sangwan R. S.; Yadav S. K. Tomato Processing Byproduct Valorization in Bread and Muffin: Improvement in Physicochemical Properties and Shelf Life Stability. J. Food Sci. Technol. 2018, 55 (7), 2560–2568. 10.1007/s13197-018-3176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisa García M.; Calvo M. M. Beef Hamburgers Enriched in Lycopene Using Dry Tomato Peel as an Ingredient. Meat Sci. 2009, 83 (1), 45–49. 10.1016/j.meatsci.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Ali M. Y.; Sina A. A. I.; Khandker S. S.; Neesa L.; Tanvir E. M.; Kabir A.; Khalil M. I.; Gan S. H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2021, 10, 1. 10.3390/foods10010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats S.; Bansal R.; Rana N.; Kumawat S.; Bhatt V.; Jadhav P.; Kale V.; Sathe A.; Sonah H.; Jugdaohsingh R.; et al. Unexplored Nutritive Potential of Tomato to Combat Global Malnutrition. Crit. Rev. Food Sci. Nutr. 2022, 62 (4), 1003–1034. 10.1080/10408398.2020.1832954. [DOI] [PubMed] [Google Scholar]
- Coelho M. C.; Rodrigues A. S.; Teixeira J. A.; Pintado M. E. Integral Valorisation of Tomato By-Products towards Bioactive Compounds Recovery: Human Health Benefits. Food Chem. 2023, 410, 135319. 10.1016/j.foodchem.2022.135319. [DOI] [PubMed] [Google Scholar]
- Ferreres F.; Taveira M.; Pereira D. M.; Valentão P.; Andrade B. Tomato (Lycopersicon Esculentum) Seeds: New Flavonols and Cytotoxic Effect. J. Agric. Food Chem. 2010, 58 (5), 2854–2861. 10.1021/jf904015f. [DOI] [PubMed] [Google Scholar]
- Tamasi G.; Pardini A.; Bonechi C.; Donati A.; Pessina F.; Marcolongo P.; Gamberucci A.; Leone G.; Consumi M.; Magnani A.; et al. Characterization of Nutraceutical Components in Tomato Pulp, Skin and Locular Gel. Eur. Food Res. Technol. 2019, 245 (4), 907–918. 10.1007/s00217-019-03235-x. [DOI] [Google Scholar]
- Kalogeropoulos N.; Chiou A.; Pyriochou V.; Peristeraki A.; Karathanos V. T. Bioactive Phytochemicals in Industrial Tomatoes and Their Processing Byproducts. LWT - Food Sci. Technol. 2012, 49 (2), 213–216. 10.1016/j.lwt.2011.12.036. [DOI] [Google Scholar]
- López-Yerena A.; Domínguez-López I.; Abuhabib M. M.; Lamuela-Raventós R. M.; Vallverdú-Queralt A.; Pérez M. Tomato Wastes and By-Products: Upcoming Sources of Polyphenols and Carotenoids for Food, Nutraceutical, and Pharma Applications. Crit. Rev. Food Sci. Nutr. 2023, 1–18. 10.1080/10408398.2023.2226211. [DOI] [PubMed] [Google Scholar]
- Singh J. M.; Sá M.; Sharma S.; Nadimi M.; Paliwal J.; House J. D.; Koksel F. Effects of Feed Moisture Content on the Physical and Nutritional Quality Attributes of Sunflower Meal-based High-Moisture Meat Analogues. Food Bioproc Tech. 2024, 17 (7), 1897–1913. 10.1007/s11947-023-03225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Chan B. L. S.; Mitchell A. E. Identification/Quantification of Free and Bound Phenolic Acids in Peel and Pulp of Apples (Malus Domestica) Using High Resolution Mass Spectrometry (HRMS). Food Chem. 2017, 215, 301–310. 10.1016/j.foodchem.2016.07.166. [DOI] [PubMed] [Google Scholar]
- Dou Y.; Shu L.; Jia X.; Yao Y.; Chen S.; Xu Y.; Li Y. Rapid Classification and Identification of Chemical Constituents in Leonurus Japonicus Houtt Based on UPLC-Q-Orbitrap-MS Combined with Data Post-Processing Techniques. J. Mass Spectrom. 2023, 58 (11), e4978 10.1002/jms.4978. [DOI] [PubMed] [Google Scholar]
- Lorigooini Z.; Jamshidi-Kia F.; Hosseini Z.. Chapter 4 - Analysis of Aromatic Acids (Phenolic Acids and Hydroxycinnamic Acids). In Recent Advances in Natural Products Analysis; Sanches Silva A.; Nabavi S. F.; Saeedi M.; Nabavi S. M.. Chapter 4 - Analysis of Aromatic Acids (Phenolic Acids and Hydroxycinnamic Acids). In Recent Advances in Natural Products Analysis; Elsevier, 2020; pp. 199–219.. 10.1016/B978-0-12-816455-6.00004-4. [DOI] [Google Scholar]
- Hofmann T.; Nebehaj E.; Albert L. The High-Performance Liquid Chromatography/Multistage Electrospray Mass Spectrometric Investigation and Extraction Optimization of Beech (Fagus Sylvatica L.) Bark Polyphenols. J. Chromatogr. A 2015, 1393, 96–105. 10.1016/j.chroma.2015.03.030. [DOI] [PubMed] [Google Scholar]
- Song X.-C.; Canellas E.; Dreolin N.; Nerin C.; Goshawk J. Discovery and Characterization of Phenolic Compounds in Bearberry (Arctostaphylos Uva-Ursi) Leaves Using Liquid Chromatography–Ion Mobility–High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2021, 69 (37), 10856–10868. 10.1021/acs.jafc.1c02845. [DOI] [PubMed] [Google Scholar]
- Schütz K.; Kammerer D. R.; Carle R.; Schieber A. Characterization of Phenolic Acids and Flavonoids in Dandelion (Taraxacum Officinale WEB. Ex WIGG.) Root and Herb by High-Performance Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2005, 19 (2), 179–186. 10.1002/rcm.1767. [DOI] [PubMed] [Google Scholar]
- Mallmann L. P.; Rios A. O.; Rodrigues E. MS-FINDER and SIRIUS for Phenolic Compound Identification from High-Resolution Mass Spectrometry Data. Food Res. Int. 2023, 163, 112315. 10.1016/j.foodres.2022.112315. [DOI] [PubMed] [Google Scholar]
- Álvarez-Fernández M. A.; Hornedo-Ortega R.; Cerezo A. B.; Troncoso A. M.; García-Parrilla M. C. Determination of Nonanthocyanin Phenolic Compounds Using High-Resolution Mass Spectrometry (UHPLC-Orbitrap-MS/MS) and Impact of Storage Conditions in a Beverage Made from Strawberry by Fermentation. J. Agric. Food Chem. 2016, 64 (6), 1367–1376. 10.1021/acs.jafc.5b05617. [DOI] [PubMed] [Google Scholar]
- Mir-Cerdà A.; Carretero I.; Coves J. R.; Pedrouso A.; Castro-Barros C. M.; Alvarino T.; Cortina J. L.; Saurina J.; Granados M.; Sentellas S. Recovery of Phenolic Compounds from Wine Lees Using Green Processing: Identifying Target Molecules and Assessing Membrane Ultrafiltration Performance. Sci. Total Environ. 2023, 857, 159623. 10.1016/j.scitotenv.2022.159623. [DOI] [PubMed] [Google Scholar]
- Moco S.; Bino R. J.; Vorst O.; Verhoeven H. A.; de Groot J.; van Beek T. A.; Vervoort J.; de Vos C. H. R. A Liquid Chromatography-Mass Spectrometry-Based Metabolome Database for Tomato. Plant Physiol. 2006, 141 (4), 1205–1218. 10.1104/pp.106.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallverdú-Queralt A.; Jáuregui O.; Medina-Remón A.; Andrés-Lacueva C.; Lamuela-Raventós R. M. Improved Characterization of Tomato Polyphenols Using Liquid Chromatography/Electrospray Ionization Linear Ion Trap Quadrupole Orbitrap Mass Spectrometry and Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2010, 24 (20), 2986–2992. 10.1002/rcm.4731. [DOI] [PubMed] [Google Scholar]
- Ledesma-Escobar C. A.; Priego-Capote F.; Luque de Castro M. D. Characterization of Lemon (Citrus Limon) Polar Extract by Liquid Chromatography–Tandem Mass Spectrometry in High Resolution Mode. J. Mass Spectrom 2015, 50 (11), 1196–1205. 10.1002/jms.3637. [DOI] [PubMed] [Google Scholar]
- Regueiro J.; Sánchez-González C.; Vallverdú-Queralt A.; Simal-Gándara J.; Lamuela-Raventós R.; Izquierdo-Pulido M. Comprehensive Identification of Walnut Polyphenols by Liquid Chromatography Coupled to Linear Ion Trap–Orbitrap Mass Spectrometry. Food Chem. 2014, 152, 340–348. 10.1016/j.foodchem.2013.11.158. [DOI] [PubMed] [Google Scholar]
- Chiriac E. R.; Chiţescu C. L.; Borda D.; Lupoae M.; Gird C. E.; Geană E.-I.; Blaga G.-V.; Boscencu R. Comparison of the Polyphenolic Profile of Medicago Sativa L. and Trifolium Pratense L. Sprouts in Different Germination Stages Using the UHPLC-Q Exactive Hybrid Quadrupole Orbitrap High-Resolution Mass Spectrometry. Molecules 2020, 25, 2321. 10.3390/molecules25102321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kečkeš S.; Gašić U.; Veličković T. Ć.; Milojković-Opsenica D.; Natić M.; Tešić Ž. The Determination of Phenolic Profiles of Serbian Unifloral Honeys Using Ultra-High-Performance Liquid Chromatography/High Resolution Accurate Mass Spectrometry. Food Chem. 2013, 138 (1), 32–40. 10.1016/j.foodchem.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Miller J. N.; Miller J. C.. Statistics and Chemometrics for Analytical Chemistry, 4th ed. ed.; Prentice Hall, 2000. [Google Scholar]
- Dias M. C.; Pinto D. C. G. A.; Silva A. M. S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26 (17), 17. 10.3390/molecules26175377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour V.; Panaite T. D.; Ropota M.; Turcu R.; Trandafir I.; Corbu A. R. Nutritional and Bioactive Compounds in Dried Tomato Processing Waste. CyTA - J. Food 2018, 16 (1), 222–229. 10.1080/19476337.2017.1383514. [DOI] [Google Scholar]
- Paulino S.-L. J.; Adrián Á.-T. G.; Gabriela E.-A. L.; Maribel V.-M.; Sergio M.-G. Nutraceutical Potential of Flours from Tomato By-Product and Tomato Field Waste. J. Food Sci. Technol. 2020, 57 (9), 3525–3531. 10.1007/s13197-020-04585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninčević Grassino A.; Ostojić J.; Miletić V.; Djaković S.; Bosiljkov T.; Zorić Z.; Ježek D.; Rimac Brnčić S.; Brnčić M. Application of High Hydrostatic Pressure and Ultrasound-Assisted Extractions as a Novel Approach for Pectin and Polyphenols Recovery from Tomato Peel Waste. Innovative Food Sci. Emerging Technol. 2020, 64, 102424. 10.1016/j.ifset.2020.102424. [DOI] [Google Scholar]
- Perea-Domínguez X. P.; Hernández-Gastelum L. Z.; Olivas-Olguin H. R.; Espinosa-Alonso L. G.; Valdez-Morales M.; Medina-Godoy S. Phenolic Composition of Tomato Varieties and an Industrial Tomato By-Product: Free, Conjugated and Bound Phenolics and Antioxidant Activity. J. Food Sci. Technol. 2018, 55 (9), 3453–3461. 10.1007/s13197-018-3269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ćetković G.; Savatović S.; Čanadanović-Brunet J.; Djilas S.; Vulić J.; Mandić A.; Četojević-Simin D. Valorisation of Phenolic Composition, Antioxidant and Cell Growth Activities of Tomato Waste. Food Chem. 2012, 133 (3), 938–945. 10.1016/j.foodchem.2012.02.007. [DOI] [Google Scholar]
- Fernández M. D. L. Á.; Espino M.; Gomez F. J. V.; Silva M. F. Novel Approaches Mediated by Tailor-Made Green Solvents for the Extraction of Phenolic Compounds from Agro-Food Industrial By-Products. Food Chem. 2018, 239, 671–678. 10.1016/j.foodchem.2017.06.150. [DOI] [PubMed] [Google Scholar]
- Szabo K.; Dulf F. V.; Diaconeasa Z.; Vodnar D. C. Antimicrobial and Antioxidant Properties of Tomato Processing Byproducts and Their Correlation with the Biochemical Composition. LWT - Food Sci. Technol. 2019, 116, 108558. 10.1016/j.lwt.2019.108558. [DOI] [Google Scholar]
- Tranfić Bakić M.; Pedisić S.; Zorić Z.; Dragović-Uzelac V. Effect of Microwave-Assisted Extraction on Polyphenols Recovery from Tomato Peel Waste. Acta Chim. Slov 2019, 66 (2), 367–377. 10.17344/acsi.2018.4866. [DOI] [PubMed] [Google Scholar]
- Navarro-González I.; García-Valverde V.; García-Alonso J.; Periago M. J. Chemical Profile, Functional and Antioxidant Properties of Tomato Peel Fiber. Food Res. Int. 2011, 44 (5), 1528–1535. 10.1016/j.foodres.2011.04.005. [DOI] [Google Scholar]
- Vorobyova V.; Skiba M.; Vasyliev G. Extraction of Phenolic Compounds from Tomato Pomace Using Choline Chloride–Based Deep Eutectic Solvents. J. Food Meas. Charact. 2022, 16 (2), 1087–1104. 10.1007/s11694-021-01238-5. [DOI] [Google Scholar]
- Concha-Meyer A.; Palomo I.; Plaza A.; Gadioli Tarone A.; Junior M. R. M.; Sáyago-Ayerdi S. G.; Fuentes E. Platelet Anti-Aggregant Activity and Bioactive Compounds of Ultrasound-Assisted Extracts from Whole and Seedless Tomato Pomace. Foods 2020, 9 (11), 1564. 10.3390/foods9111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azabou S.; Sebii H.; Taheur F. B.; Abid Y.; Jridi M.; Nasri M. Phytochemical Profile and Antioxidant Properties of Tomato By-Products as Affected by Extraction Solvents and Potential Application in Refined Olive Oils. Food Biosci. 2020, 36, 100664. 10.1016/j.fbio.2020.100664. [DOI] [Google Scholar]
- Di Donato P.; Taurisano V.; Tommonaro G.; Pasquale V.; Jiménez J. M. S.; de Pascual-Teresa S.; Poli A.; Nicolaus B. Biological Properties of Polyphenols Extracts from Agro Industry’s Wastes. Waste Biomass Valorization 2018, 9 (9), 1567–1578. 10.1007/s12649-017-9939-4. [DOI] [Google Scholar]
- Coelho M.; Pereira R.; Rodrigues A. S.; Teixeira J. A.; Pintado M. E. Extraction of Tomato By-Products’ Bioactive Compounds Using Ohmic Technology. Food Bioprod. Process. 2019, 117, 329–339. 10.1016/j.fbp.2019.08.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.