Abstract
Herpes simplex virus type 1 (HSV-1) infection alters the phosphorylation of the large subunit of RNA polymerase II (RNAP II), resulting in the depletion of the hypophosphorylated and hyperphosphorylated forms of this polypeptide (known as IIa and IIo, respectively) and induction of a novel, alternatively phosphorylated form (designated IIi). We previously showed that the HSV-1 immediate-early protein ICP22 is involved in this phenomenon, since induction of IIi and depletion of IIa are deficient in cells infected with 22/n199, an HSV-1 ICP22 nonsense mutant (S. A. Rice, M. C. Long, V. Lam, P. A. Schaffer, and C. A. Spencer, J. Virol. 69:5550–5559, 1995). However, depletion of IIo still occurs in 22/n199-infected cells. This suggests either that another viral gene product affects the RNAP II large subunit or that the truncated ICP22 polypeptide encoded by 22/n199 retains residual activity which leads to IIo depletion. To distinguish between these possibilities, we engineered an HSV-1 ICP22 null mutant, d22-lacZ, and compared it to 22/n199. The two mutants are indistinguishable in their effects on the RNAP II large subunit, suggesting that an additional viral gene product is involved in altering RNAP II. Two candidates are UL13, a protein kinase which has been implicated in ICP22 phosphorylation, and the virion host shutoff (Vhs) factor, the expression of which is positively regulated by ICP22 and UL13. To test whether UL13 is involved, a UL13-deficient viral mutant, d13-lacZ, was engineered. This mutant was defective in IIi induction and IIa depletion, displaying a phenotype very similar to that of d22-lacZ. In contrast, a Vhs mutant had effects that were indistinguishable from wild-type HSV-1. Therefore, UL13 but not the Vhs function plays a role in modifying the RNAP II large subunit. To study the potential role of UL13 in viral transcription, we carried out nuclear run-on transcription analyses in infected human embryonic lung cells. Infections with either UL13 or ICP22 mutants led to significantly reduced amounts of viral genome transcription at late times after infection. Together, our results suggest that ICP22 and UL13 are involved in a common pathway that alters RNAP II phosphorylation and that in some cell lines this change promotes viral late transcription.
Herpes simplex virus type 1 (HSV-1), a human herpesvirus which grows robustly in cell culture, provides a valuable system for studying how nucleus-replicating DNA viruses regulate their genetic information during infection of host cells. The genome of HSV-1 consists of a linear, 152-kb double-stranded DNA molecule encoding approximately 80 proteins. Soon after the virus enters susceptible cells, the genome is transported to the cell nucleus, where its five major immediate-early (IE) genes are recognized and transcribed by the host RNA polymerase II (RNAP II). During the remainder of the infection, HSV-1 effectively commandeers the cell’s RNAP II transcription machinery to express its remaining delayed-early (DE) and late (L) genes at high levels and in a temporally orchestrated cascade (26) (reviewed in references 59, 67, and 74). At the same time, HSV-1 inhibits the action of RNAP II on most host cell genes (53, 70). Understanding the mechanisms by which HSV-1 subverts the RNAP II transcription machinery will shed light on virus replication strategies and may also provide valuable insights into mechanisms that regulate cellular gene transcription.
The mechanisms that regulate transcription of the IE genes immediately following HSV-1 lytic infection are understood in some detail (reviewed in reference 20). Transcription of the IE genes is stimulated by a virion tegument protein, VP16, which interacts with the cellular factors Oct-1 and HCF. The resulting complex binds to cis-acting DNA sequences in IE promoters, allowing transcriptional activation via recruitment and/or stabilization of RNAP II transcription preinitiation complexes at IE promoters. In contrast, the transcriptional induction of the HSV-1 DE and L genes during productive infection is not well understood. One fact which has emerged, however, is that the IE protein ICP4 is absolutely required for efficient DE and/or L transcription (18, 24, 52). ICP4 is a nucleus-localized, sequence-specific DNA-binding protein which is able to form in vitro complexes with the cellular transcription factors TBP (TATA-binding protein), TFIIB, and TAF250 (8, 68). Although ICP4’s DNA-binding activity is necessary for its ability to activate DE and/or L genes (2, 64), high-affinity ICP4 DNA-binding sites do not appear to be required for the ICP4-responsiveness of certain DE and/or L gene promoters (66). Thus, the mechanism(s) by which ICP4 is directed to viral promoters may involve determinants other than DNA sequence. In addition to ICP4, the IE proteins ICP0 (28), ICP22 (57), and ICP27 (36, 48) may also help activate transcription of DE and/or L genes during productive infection.
We have been investigating whether HSV-1 promotes the transcription of its genes by altering the enzyme which mediates mRNA transcription, RNAP II. A great deal is known about the biochemistry of this enzyme (reviewed in reference 77). RNAP II consists of at least 10 subunits ranging in size from 10 to 240 kDa. The largest subunit, which possesses catalytic activity, contains a unique carboxy-terminal structure called the C-terminal domain, or CTD (for reviews, see references 16 and 19). The CTD consists of up to 52 repeats of the consensus sequence YSPTSPS, is highly conserved in eukaryotes, and is essential for cell viability. Moreover, the CTD is the site of extensive phosphorylation, which occurs predominantly on serine residues and to a lesser extent on threonine and tyrosine residues. As a result of this modification, the large subunit is normally found in either of two states, designated IIa and IIo. IIa is hypophosphorylated, whereas IIo is heavily phosphorylated on the CTD. Thus, RNAP II also exists in two forms, designated RNAP IIA (containing IIa) and RNAP IIO (containing IIo). Interestingly, RNAP IIA and RNAP IIO are associated with distinct aspects of transcription. RNAP IIA is involved in transcription initiation, being the form which is preferentially recruited into preinitiation complexes (33, 49). In contrast, RNAP IIO is believed to be involved in transcription elongation (6, 33, 49). The transition between RNAP IIA and RNAP IIO occurs early in the transcription cycle, i.e., at or shortly after the initiation step (43, 49). After completion of a round of transcription, RNAP IIO must be converted to RNAP IIA before reinitiation of transcription can occur (12). The CTD and its phosphorylation are required in vivo for efficient transcription elongation and for response to transcription activators (1, 23, 45, 76). Various in vitro CTD kinases and phosphatases have been identified, and several kinases have been verified as in vivo kinases that influence transcription initiation and elongation (3, 21, 25, 34, 35). In addition to its direct role in transcription regulation, recent evidence indicates that the CTD may also play a role in pre-mRNA processing by recruiting pre-mRNA processing factors to nascent transcripts (reviewed in references 5, 14, 40, 72).
Previously, we found that HSV-1 infection alters the phosphorylation state of the large subunit of RNAP II (58). Specifically, infection results in the general depletion of the IIo and IIa forms and the appearance of abundant new species which have intermediate electrophoretic mobilities. For convenience, we have referred to these as a single form, designated IIi (for intermediately migrating), and have designated the form of RNAP II bearing IIi as RNAP III. In vitro, IIi can be converted to IIa by treatment with calf intestinal phosphatase, indicating that it is an alternatively phosphorylated form of the large subunit, with phosphorylation likely occurring on the CTD. Given the suspected role of the CTD in transcription regulation, we hypothesized that HSV-1-induced changes to CTD phosphorylation alter the function of RNAP II in a manner which promotes viral gene transcription.
We previously asked whether specific IE proteins are required for HSV-1’s effects on RNAP II (57). We found that HSV-1-induced changes to the large subunit occur apparently normally in cells infected with HSV-1 mutants defective in ICP4, ICP0, or ICP27. In contrast, IIi formation and IIa depletion do not occur efficiently in cells infected with 22/n199, an HSV-1 mutant with a nonsense codon insertion in the ICP22 gene. Therefore, ICP22 plays an important role in the modification of RNAP II. However, we noted that significant changes to the RNAP II large subunit still occur in 22/n199-infected cells in that the IIo form is depleted and low but detectable levels of IIi are induced. Since 22/n199 encodes the N-terminal half of ICP22 (199 of 420 residues), it is possible that the N-terminal fragment retains some function and is able to mediate the observed alterations. Alternatively, HSV-1 may encode or induce additional factors which mediate changes to RNAP II. To distinguish between these two possibilities, we have constructed an HSV-1 ICP22 null mutant and analyzed its effects on RNAP II. Our results demonstrate conclusively that other viral factors affect RNAP II modification. We have identified one such factor as UL13, a virion-localized protein kinase which has been previously implicated in the posttranslational modification of ICP22.
MATERIALS AND METHODS
Cells, viruses, and infections.
African green monkey kidney (Vero) and human embryonic lung 299 (HEL) cells were used for infections. Both lines were obtained from the American Type Culture Collection. Cells were grown as monolayer cultures and were propagated in Dulbecco modified Eagle’s medium containing 5% heat-inactivated fetal calf serum, 50 U of penicillin/ml, and 50 μg of streptomycin/ml. All tissue culture reagents were purchased from Gibco-BRL.
KOS1.1 (27) was the wild-type (WT) strain of HSV-1 used in this study. The HSV-1 ICP22 mutant 22/n199, which has been described previously, contains a nonsense codon insertion following codon 199 of the ICP22 gene (4, 57). 22/n199R is a marker-rescued derivative of 22/n199 (4, 57). Both 22/n199 and 22/n199R were grown and titered on Vero cells. The construction of d22-lacZ, R22-lacZ, d13-lacZ, and R13-lacZ is described in detail below. The HSV-1 Vhs mutant, vhsA (65), a derivative of strain KOS, was obtained from Jim Smiley (University of Alberta).
Cells were infected at a multiplicity of infection (MOI) of 10 PFU per cell in phosphate-buffered saline containing 0.1% glucose and 1% heat-inactivated newborn calf serum. The virus inoculum was allowed to adsorb to the cells for 1 h at 37°C. The inoculum was then replaced with 199 medium containing 2% heat-inactivated newborn calf serum, 50 U of penicillin/ml, and 50 μg of streptomycin/ml, and the infected cells were reincubated at 37°C. For viral plaque assays, the medium was the same as for virus infections except that heat-inactivated normal pooled human serum (ICN Pharmaceuticals) was added to 1%. Plaque assay cultures were incubated at 37°C for 3 to 4 days to allow plaques to develop.
Plasmids.
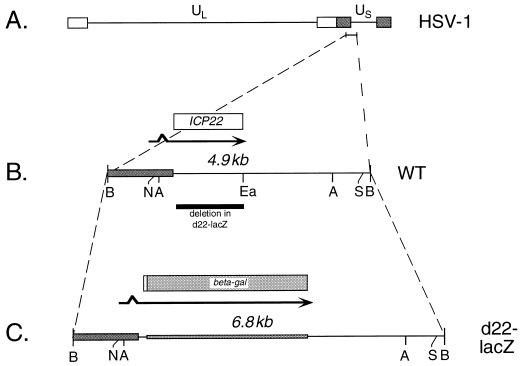
To engineer a plasmid bearing a null allele of the HSV-1 ICP22 gene, the following manipulations were carried out. First, the 4.9-kb BamHI N fragment of the WT HSV-1 strain (KOS1.1) genome, which contains the ICP22 gene, was cloned into pUC19. This generated plasmid pBamN, the insert of which is shown in Fig. 1B. pBamN was then digested with NruI and SspI, liberating a 3.9-kb fragment containing the ICP22 gene. This was cloned into the SmaI site of pSELECT-1 (Promega), generating pNS22. Oligonucleotide mutagenesis (Altered States kit; Promega) was used to alter codons 5 to 7 of the ICP22 gene in pNS22 from CCA-GGC-GCT to CCA-GAT-CTT (nucleotide changes are underlined), creating a BglII site (AGATCT). This plasmid was designated pNS-Bgl. The modified gene was subcloned into pUC19 by using the unique EcoRI and BamHI sites present in the polylinkers of both plasmids. This generated plasmid pUCNS. Next, a plasmid lacking nearly all of the ICP22 open reading frame (ORF) was constructed. This was accomplished by digesting pUCNS with EagI, which cuts in the last two codons of the ICP22 ORF. The DNA ends were made blunt by using the Klenow enzyme, BglII linkers were ligated on, and the DNA was digested with BglII. After religation and transformation into Escherichia coli, the plasmid pUCNSΔ was obtained. This plasmid has a deletion of all but the first six codons of the ICP22 ORF. Finally, a 3.1-kb BamHI fragment containing the E. coli lacZ gene, obtained from plasmid pMC1871 (63), was cloned into the unique BglII site of pUCNSΔ. This generated plasmid pUCNS-lacZ, which has the first six codons of the ICP22 ORF fused in frame to the β-galactosidase coding region.
FIG. 1.
Schematic representation of WT and mutant ICP22 alleles. (A) Representation of the prototypical form of the HSV-1 genome. Unique DNA sequences are represented by horizontal lines, and inverted repeated DNA sequences are shown as open and gray bars. The position of the BamHI N fragment, containing the ICP22 gene, is indicated and enlarged below in panel B. (B) Map of the WT BamHI N fragment. Unique and repeated HSV-1 DNA sequences are denoted by the horizontal line and gray bar, respectively. Above the DNA representation, the spliced ICP22 transcript is denoted by an arrow and the ICP22 ORF is indicated by an open bar. Below the representation, the sequences which are deleted in d22-lacZ are shown as a black bar. (C) Map of the altered BamHI fragment present in d22-lacZ. DNA sequences are shown at the bottom. Unique HSV-1 sequences are represented by a horizontal line, repeated HSV-1 sequences are denoted by a gray bar, and E. coli lacZ sequences are represented by a crosshatched bar. Above, the transcript and ORF of the ICP22–β-galactosidase fusion protein (beta-gal) are indicated by an arrow and bar, respectively. Restriction sites relevant to the engineering of d22-lacZ are indicated in panels B and C: A, AgeI; B, BamHI; Ea, EagI; N, NruI; S, SspI).
To engineer a plasmid bearing a mutated UL13 gene, the following steps were performed. First, a derivative of pUC19 in which the HindIII site was destroyed was constructed. This was accomplished by cleaving pUC19 with HindIII, generating blunt ends by using the Klenow enzyme, and religating the resulting DNA. This plasmid was designated pUC19-Hind(−). Next, the 5.3-kb BglII O fragment from the HSV-1 (KOS1.1) genome, which contains the UL13 gene, was cloned into the BamHI site of pUC19-Hind(−), creating pBglO, the insert of which is shown in Fig. 5B. To delete a large portion of the UL13 ORF, pBglO was cut to completion with HindIII, then cut partially with BstEII. After repair of the DNA ends with the Klenow enzyme, 8-bp BglII linkers (NEB) were ligated on, and the DNA was cut with BglII. The resulting mixture of DNA fragments was resolved on an agarose gel, and the 7.2-kb fragment was isolated and religated. The resulting plasmid was designated pBglOΔUL13. It contains a 780-bp deletion of a 3′ portion of the UL13 gene, with a unique BglII site at the site of the deletion. To create a lacZ gene-containing derivative, the 3.1-kb BamHI fragment from pMC1871 was cloned into the BglII site, generating pBglOZ, the insert of which is shown in Fig. 5C. This plasmid encodes a hybrid protein in which the first 155 residues of UL13 are fused to β-galactosidase.
FIG. 5.
Schematic representation of WT and mutant UL13 alleles. (A) Representation of the prototypical form of the HSV-1 genome. Unique DNA sequences are represented by horizontal lines, and inverted-repeat DNA sequences are shown as open and gray bars. The position of the BglII O fragment, containing the UL13 gene, is indicated, and this fragment is enlarged in panel B. (B) Map of the WT BglII O fragment. HSV-1 DNA sequences are denoted by the horizontal line. Above, the UL13 and UL14 transcripts are denoted by arrows, and the UL13 and UL14 ORFs are indicated by open bars. Below, the DNA sequences which are deleted in d13-lacZ are shown as a black bar. (C) Map of the altered BglII O fragment present in d13-lacZ. DNA sequences are shown at the bottom. HSV-1 sequences are represented by a horizontal line, and E. coli lacZ sequences are denoted by a crosshatched bar. Above, transcripts and ORFs of UL14 and the UL13–β-galactosidase fusion protein (beta-gal) are indicated as in panel B. Restriction sites relevant to the engineering and analysis of d13-lacZ are indicated in panels A and B: A, AgeI; Bg, BglII; Bs, BstEII; E, EcoRI; Ea, EagI; H, HindIII; Nh, NheI).
Isolation of mutant and marker-rescued viruses.
The isolation of d22-lacZ, R22-lacZ, d13-lacZ, and R13-lacZ was carried out by a marker transfer procedure (56). To construct d22-lacZ, pUCNS-lacZ was cleaved with AgeI, liberating the modified ICP22 gene along with flanking viral sequences. The digested DNA was then cotransfected with the infectious KOS1.1 virus DNA into Vero cells. The progeny of the cotransfection were plated on Vero cells in the presence of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). A blue plaque was picked, plaque purified three additional times, and designated d22-lacZ. To construct R22-lacZ, infectious-virus DNA was prepared from d22-lacZ. This was cotransfected into Vero cells with AgeI-cleaved pBamN. The progeny were plated on Vero cells in the presence of X-Gal, and nonblue plaques were picked from the background of parental blue plaques. One plaque was further plaque purified and designated R22-lacZ.
To construct d13-lacZ, the pBglOZ plasmid was cleaved with AgeI and NheI, which liberates the modified UL13 gene along with flanking viral sequences. This DNA was cotransfected with infectious KOS1.1 strain DNA into Vero cells. A blue plaque was isolated by using the protocol described above for the isolation of d22-lacZ. After plaque purification, this isolate was designated d13-lacZ. To engineer a marker-rescued derivative of d13-lacZ, infectious-mutant d13-lacZ DNA was cotransfected into Vero cells with AgeI- plus NheI-digested pBglO DNA. A nonblue plaque was isolated, plaque purified, and designated R13-lacZ.
Analysis of mutant viruses.
To confirm the genomic structures of the engineered viruses, Southern blot analysis was performed. Total DNA was isolated from infected cells by the procedure described by Gao and Knipe (22). Southern blotting and hybridizations were carried out by standard procedures (61). The DNA probes used were linearized pBamN and pBglO DNAs, uniformly labeled with 32P by using the random-primer labelling method (61).
For analysis of viral growth, Vero or HEL cells were infected at an MOI of 10. At 2 h postinfection (p.i.), the cells were washed with glycine–saline buffer (pH 3.0) to inactivate extracellular virus (7). The infections were terminated at 24 h p.i. by freezing the cultures at −70°C. Virus was released by three cycles of freeze-thawing, and the titers in the lysates were determined by plaque assay on Vero cells.
To analyze the RNAP II large-subunit forms, immunoblotting was performed as previously described (57, 58). Briefly, mock- or virus-infected cells were scraped in phosphate-buffered saline containing protease inhibitors (50 μg of N-α-p-tosyl-l-lysine chloromethyl ketone per ml and 25 μg of phenylmethylsulfonyl fluoride per ml), pelleted by low-speed centrifugation, and lysed in sodium dodecyl sulfate (SDS)-polyacrylamide gel sample buffer. In some experiments, the following phosphatase inhibitors were included in the scraping buffer: 1 mM sodium orthovanadate, 25 mM sodium fluoride, 50 mM tetrasodium phosphate, and 50 mM sodium pyrophosphate. Proteins were separated by SDS-6% polyacrylamide gel electrophoresis (PAGE), transferred to Hybond ECL membranes, and probed with anti-RNAP II large subunit antibodies 8WG16 (73) or ARNA3 (30). The secondary antibody was horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G, which was detected by the enhanced chemiluminescence (ECL) detection system (Amersham). 8WG16 was obtained from Nancy Thompson (University of Wisconsin), ARNA3 was purchased from Cymbus Bioscience Ltd. (Southampton, United Kingdom), and horseradish peroxidase-conjugated secondary antibody was purchased from Jackson ImmunoResearch Labs (West Grove, Pa.).
Nuclear run-on transcription assays were performed as previously described (70, 71). In brief, transcription in isolated nuclei was allowed to proceed in the presence of [32P]UTP, and RNA products were purified and hybridized to DNA probes on nitrocellulose filters. The probes were single-stranded bacteriophage M13 clones containing HSV-1 genomic inserts and were designed to detect either sense or antisense transcription in the gene region of interest. All probes have been described previously (24, 57).
RESULTS
Construction and growth characteristics of an HSV-1 ICP22 null mutant.
Previously, we analyzed RNAP II modifications in cells infected with the HSV-1 mutant 22/n199 (57). This mutant possesses a nonsense codon insertion in the middle of the ICP22 gene, resulting in the expression of a truncated ICP22 polypeptide corresponding to the N-terminal 199 (of 420) residues. In contrast to WT HSV-1 infection, 22/n199 infection does not result in the depletion of the normal hypophosphorylated IIa form of the RNAP II large subunit, nor does it efficiently induce the IIi form. However, 22/n199 infection does result in depletion of the hyperphosphorylated IIo form and the induction of low but detectable levels of IIi-like forms. One possible explanation is that another HSV-1 gene product in addition to ICP22 affects RNAP II phosphorylation. An alternate explanation is that the N-terminal ICP22 fragment encoded by 22/n199 retains residual activity. To distinguish between these possibilities, we engineered and analyzed an HSV-1 mutant that is unable to express any significant portion of ICP22.
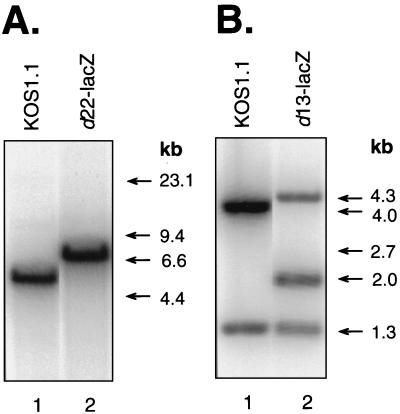
To construct such a mutant, we first mutated the ICP22 gene on a plasmid (Fig. 1). All but the first six codons of the ICP22 ORF were deleted, and the E. coli lacZ ORF was inserted in frame. The resulting allele, illustrated in Fig. 1C, encodes a hybrid protein in which the first six residues of ICP22 are fused to β-galactosidase. The mutant allele was then transferred to the viral genome by a marker transfer protocol (see Materials and Methods). Southern blot analysis of BamHI-restricted viral DNAs indicated that the d22-lacZ genome possesses the expected mutant BamHI N fragment (Fig. 2A, lane 2), which is 6.8 kb in size, instead of the WT, 4.9-kb fragment (lane 1). In addition, immunoblot analysis performed by using a β-galactosidase-specific monoclonal antibody (MAb) indicated that d22-lacZ-infected Vero cells express a β-galactosidase protein of the expected size, ∼115 kDa (data not shown). These results demonstrate that the d22-lacZ genome contains the engineered ICP22 gene alteration. As a control for the phenotypic analysis of d22-lacZ, we also constructed a marker-rescued derivative of d22-lacZ, designated R22-lacZ. Southern blot analysis confirmed that the R22-lacZ genome possesses the WT-sized, 4.9-kb BamHI N fragment (not shown).
FIG. 2.
Southern blotting analyses of HSV-1 ICP22 and UL13 mutant viral genomes. (A) Viral DNA preparations from WT strain (KOS1.1)-infected (lane 1) or d22-lacZ-infected (lane 2) Vero cells were digested with BamHI and subjected to Southern blotting. The filter was probed with 32P-labeled pBamN DNA, which has an insert of the WT HSV-1 BamHI N fragment. (B) Viral DNA preparations from WT-infected (lane 1) or d13-lacZ-infected (lane 2) Vero cells were doubly digested with BglII and EcoRI. The filter was probed with 32P-labeled pBglO DNA, which has an insert of the WT HSV-1 BglII O fragment. For both panels A and B, the positions of DNA size standards are shown on the right.
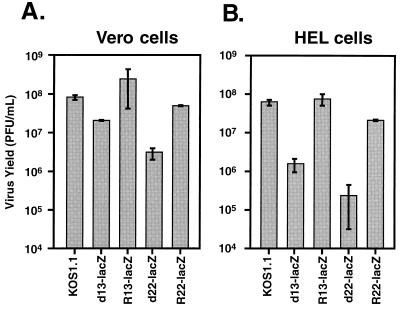
Previous studies have demonstrated that HSV-1 ICP22 mutants exhibit a cell type-dependent growth defect, in that they replicate normally or near-normally in some cultured cell lines, including Vero cells, but inefficiently in others, including HEL cells (51, 62). To determine whether d22-lacZ exhibits this host-range phenotype, single-cycle growth experiments were performed. Vero or HEL cells were infected in duplicate with the WT strain KOS1.1, 22/n199, 22/n199R (a marker-rescued derivative of 22/n199 [4, 57]), d22-lacZ, or R22-lacZ at an MOI of 10. At 24 h p.i., the cultures were harvested, and virus yield was determined by plaque assay of the infected-cell lysates on Vero cells. When the infections were carried out in Vero cells (Fig. 3A), both 22/n199 and d22-lacZ showed a modest (3.5- to 10-fold) replication defect compared to WT HSV-1, whereas the marker-rescued derivatives replicated as well as or slightly better than the WT. A similar, modest replication defect has previously been observed for an ICP22 null mutant in Vero cells (51). In contrast, when the infections were carried out in HEL cells (Fig. 3B), both 22/n199 and d22-lacZ replicated >100-fold less efficiently than WT HSV-1 or the marker-rescued derivatives. These results indicate that both ICP22 mutants exhibit the expected cell type-dependent replication defect. In addition, since the marker-rescued viruses replicate similarly to the WT, we conclude that the replication defects of the mutants are due to their engineered ICP22 gene mutations.
FIG. 3.
Growth of ICP22 mutants in Vero and HEL cells. Confluent monolayers of Vero (A) or HEL (B) cells in 25-cm2 flasks were infected in duplicate with WT HSV-1 strain (KOS1.1) or various HSV-1 mutants at an MOI of 10 and incubated at 37°C for 24 h. Virus yield in the infected-cell lysates was determined by plaque assay on Vero cells. Each bar denotes the mean virus yield for the cells infected with the indicated virus, with the error bars indicating the values of the two duplicate infections.
Alterations to the RNAP II large subunit following d22-lacZ infection.
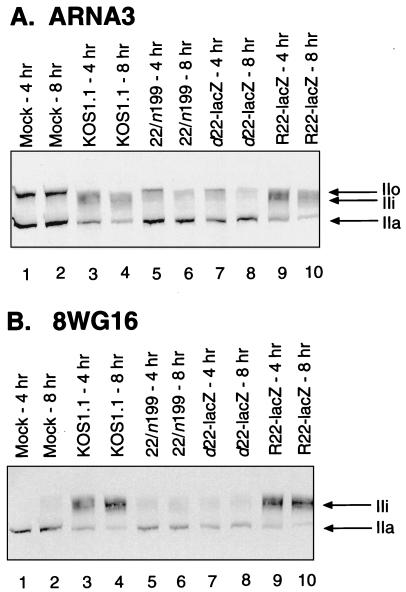
We next examined d22-lacZ to see if its effects on RNAP II large-subunit phosphorylation were similar to those of 22/n199. Vero cells were mock infected or were infected with the WT strain KOS1.1, 22/n199, d22-lacZ, or R22-lacZ at an MOI of 10. At 4 and 8 h p.i., total protein extracts were prepared and subjected to immunoblot analysis by using MAbs directed against the large subunit of RNAP II. The first MAb used was ARNA3 (30), which recognizes an epitope on the body of the large subunit and reacts with all phosphorylation variants of the protein. Mock-infected Vero cells contain approximately equal amounts of IIa and IIo, as detected by ARNA3 (Fig. 4A, lanes 1 and 2). As observed previously, infection with WT HSV-1 resulted in substantial changes to these phosphorylated forms (lanes 3 and 4), with loss of IIo and, to a lesser extent, IIa. In addition, large amounts of the novel IIi form were induced by WT HSV-1 infection. In contrast, both 22/n199 and d22-lacZ (lanes 5 and 6 and lanes 7 and 8, respectively) exhibited phenotypes distinct from that of the WT but very similar to each others. Neither mutant efficiently induced IIi, although low levels could be observed, particularly at 4 h p.i. The mutant infections also differed from the WT infection in that they resulted in significantly less depletion of the hypophosphorylated IIa form. Both mutants appeared to be as efficient as the WT in depleting the hyperphosphorylated IIo form, which was undetectable in all infected cells by 8 h p.i. As expected, the marker-rescued virus, R22-lacZ, was similar to the WT virus in its effects on the RNAP II large subunit (lanes 9 and 10).
FIG. 4.
Immunoblot analysis of RNAP II large-subunit forms in Vero cells infected with ICP22 mutants. Vero cells were mock infected (lanes 1 and 2) or were infected with the various HSV-1 strains indicated (lanes 3 to 10) for 4 or 8 h (odd-numbered and even-numbered lanes, respectively) at an MOI of 10. Cells were scraped, washed, and lysed directly into SDS-PAGE sample buffer. Extracts were run on SDS–6% PAGE, transferred to filters, and probed with either MAb ARNA3 (A), which reacts with the body of the RNAP II large subunit, or MAb 8WG16 (B), which reacts with the large-subunit CTD. All lanes in panel A or B contain extracts from the same numbers of cells. Subunit IIa migrates at approximately 200 kDa; IIo migrates at approximately 240 kDa.
Immunoblot analysis of the same samples was also performed by using 8WG16 (73), a MAb which recognizes an epitope on the CTD and reacts with both the IIa and IIi forms of the RNAP II large subunit but not with the hyperphosphorylated IIo form (58). The results were consistent with the results of ARNA3 blotting. In both KOS1.1- and R22-lacZ-infected cells (Fig. 4B, lanes 3 and 4 and lanes 9 and 10, respectively), large amounts of IIi were induced. As expected, IIi was not efficiently induced in either the 22/n199 or d22-lacZ infections (lanes 5 and 6 and lanes 7 and 8, respectively).
Similar immunoblotting experiments were carried out in HEL cells, in which the growth of ICP22 mutants is more restricted. The results (not shown) were qualitatively similar to those obtained in Vero cells in that (i) neither ICP22 mutant efficiently caused IIi induction or IIa depletion, (ii) both mutants efficiently depleted IIo, and (iii) the two mutants could not be distinguished from each other in their effects. Together, these experiments confirm that ICP22 plays an important role in the alteration of RNAP II phosphorylation. We also conclude that the truncated ICP22 polypeptide expressed by 22/n199 does not appreciably affect RNAP II, since the null mutant d22-lacZ is indistinguishable from 22/n199. Since infection by the null mutant is still able to effect the depletion of the hyperphosphorylated IIo form and induce low but detectable amounts of IIi, at least one additional viral factor besides ICP22 must be involved in HSV-1’s effects on the RNAP II large subunit.
Construction and growth characteristics of an HSV-1 UL13 mutant.
One candidate HSV-1 gene product which might affect phosphorylation of RNAP II is the UL13 protein kinase, which has been implicated in the phosphorylation of ICP22 (54, 55). To see whether UL13 plays a role, we engineered a UL13 mutation into the strain KOS1.1 (Fig. 5). It was not feasible to delete the entire UL13 gene, since the N-terminal portion of the UL13 ORF overlaps with the C-terminal segment of the UL14 ORF (Fig. 5B). Therefore, we altered a cloned UL13 gene so that its 3′ portion was replaced by lacZ-coding sequences (Fig. 5C). This mutant gene encodes a hybrid protein in which the N-terminal 155 residues of UL13 are fused to β-galactosidase. Importantly, the region of the UL13 gene encoding the conserved protein kinase motifs (11, 69) is absent, so the hybrid protein is not expected to retain any protein kinase activity. Marker transfer was used to introduce the mutant allele into the viral genome (see Materials and Methods), generating d13-lacZ. To confirm the genomic structure of d13-lacZ, Southern blotting analysis of BglII- plus EcoRI-digested viral DNAs was carried out, using the cloned UL13 gene as a probe. The mutant DNA yielded the expected 4.3-, 2.0-, and 1.3-kb hybridizing fragments (Fig. 2B). In addition, immunoblot analysis of d13-lacZ-infected Vero cells indicated that the virus expresses a β-galactosidase-related polypeptide of the expected size, ∼135 kDa (data not shown). As a control for further experiments, a marker-rescued derivative of d13-lacZ, designated R13-lacZ, was also generated. Southern blot analysis indicated that the R13-lacZ genome possesses a UL13 gene with a WT genomic structure (data not shown).
Previous studies by Purves et al. have indicated that HSV-1 UL13 mutants possess a cell type-dependent replication defect similar to that of ICP22 mutants (54). To test whether d13-lacZ exhibits this host-range phenotype, we studied its replication in Vero and HEL cells. Cells were infected in duplicate at an MOI of 10 and incubated for 24 h. Virus yields were determined by plaque assay of the infected-cell lysates on Vero cells. In Vero cells (Fig. 6A), d13-lacZ replicated only fourfold less efficiently than WT HSV-1, indicating at most only a modest growth defect. d22-lacZ was somewhat more deficient than d13-lacZ for growth in Vero cells in this experiment, replicating 25-fold less efficiently than the WT. In HEL cells, d13-lacZ exhibited a much more severe growth defect, replicating 40-fold less well than the WT (Fig. 6B). d22-lacZ grew even more poorly, showing an approximately 250-fold growth defect. As expected, R13-lacZ replicated similarly to the WT HSV-1 in both Vero and HEL cells (Fig. 6A and B), indicating that the observed deficiencies in d13-lacZ growth are due to the engineered UL13 mutation. These results indicate that d13-lacZ possesses the expected cell type-dependent replication defect. However, it appears that the d13-lacZ mutant is less compromised in its growth in HEL cells than is d22-lacZ.
FIG. 6.
Growth of d13-lacZ in Vero and HEL cells. Confluent monolayers of Vero (A) or HEL (B) cells in 25-ml flasks were infected in duplicate with WT HSV-1 strain (KOS1.1), d13-lacZ, or R13-lacZ at an MOI of 10 and incubated at 37°C for 24 h. Virus yield in the infected-cell lysates was determined by plaque assay on Vero cells. The bars denote the mean virus yields for cells infected with the indicated viruses, with the error bars indicating the values of the two duplicate infections.
UL13 is involved in altering the RNAP II large subunit.
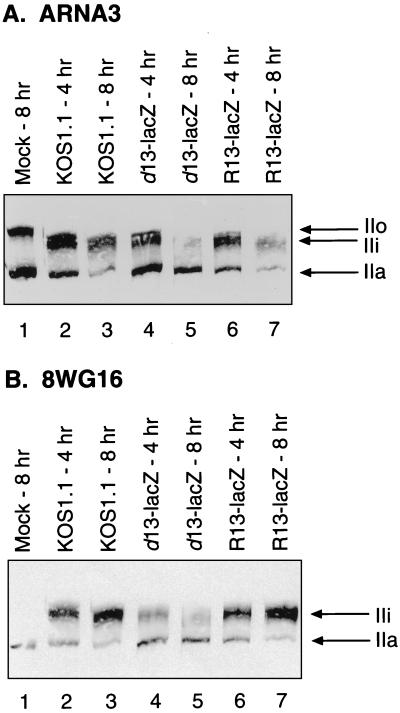
To determine whether UL13 plays a role in HSV-1-induced alterations to RNAP II, we analyzed large-subunit modifications in cells infected with d13-lacZ. Vero cells were mock infected or were infected with KOS1.1, d13-lacZ, or R13-lacZ at an MOI of 10. Protein extracts were prepared at 4 and 8 h p.i. and subjected to immunoblotting with ARNA3 or 8WG16. The results of the ARNA3 blotting are shown in Fig. 7A. As expected, both KOS1.1 (lanes 2 and 3) and R13-lacZ (lanes 6 and 7) infections resulted in IIa and IIo depletion and induction of IIi, although the IIi induction by R13-lacZ did not appear quite as robust. In contrast, d13-lacZ infection (lanes 4 and 5) did not result in efficient IIi induction or IIa depletion but did result in efficient IIo depletion. The results of the 8WG16 blotting (Fig. 7B) were consistent with the ARNA3 analysis, but the difference between d13-lacZ and the other two viruses was more dramatic. Large amounts of IIi were induced by both the KOS1.1 and R13-lacZ by 8 h p.i. (lanes 3 and 7, respectively), but d13-lacZ-infected cells were unable to induce large amounts of IIi (lanes 4 and 5). Similar experiments were carried out in HEL cells. The results (not shown) were qualitatively similar to those obtained in Vero cells. Based on these analyses, we conclude that UL13 is required for the efficient induction of IIi and for the depletion of IIa but not for the depletion of the hyperphosphorylated (IIo) form of the RNAP II large subunit.
FIG. 7.
Immunoblot analysis of RNAP II large-subunit forms in Vero cells infected with d13-lacZ. Vero cells were mock infected (lanes 1) or were infected with the virus strains indicated for 4 or 8 h. Cells were scraped, washed, and lysed directly into SDS-PAGE sample buffer. Protein extracts were run on SDS–6% PAGE, transferred to filters, and probed with ARNA3 (A) or 8WG16 (B). All lanes in panel A or B contain extracts from the same numbers of cells. The positions at which various RNAP II large-subunit forms migrate are indicated.
The Vhs function is not required for altered phosphorylation of RNAP II.
Ng et al. have demonstrated that both ICP22 and UL13 positively regulate expression of the UL41 gene (41). The UL41 gene encodes the virion host shutoff (Vhs) factor, a virion-localized protein that destabilizes mRNAs following infection (59). As a result of reduced UL41 gene expression, ICP22 and UL13 mutants contain less Vhs protein in their virions and hence do not efficiently induce the inhibition of cellular protein synthesis. It is thus conceivable that at least some of the effects of ICP22 and UL13 on the RNAP II large subunit might occur indirectly via their abilities to promote the Vhs effect. To test whether Vhs is involved in the RNAP II alterations, we analyzed RNAP II large-subunit forms in cells infected with the HSV-1 Vhs mutant vhsA (65). Vero cells were mock infected or were infected with KOS1.1, vhsA, d22-lacZ, or d13-lacZ, and protein extracts were prepared at 4 and 8 h p.i. Immunoblotting was performed with either ARNA3 MAb or 8WG16 MAb. The results of both the ARNA3 (Fig. 8A) and 8WG16 (Fig. 8B) blottings demonstrated that the vhsA mutant (lanes 5 and 6) is very similar to WT HSV-1 (lanes 3 and 4) in its effects on RNAP II, with both viral infections resulting in efficient IIa and IIo depletion and robust IIi induction. Therefore, the Vhs function is not required for HSV-1’s ability to modify the RNAP II large subunit. This experiment also allowed us to directly compare the d22-lacZ and d13-lacZ mutants for their effects on the RNAP II large subunit. Compared to WT virus (Fig. 8, lanes 3 and 4), both d22-lacZ (lanes 7 and 8) and d13-lacZ (lanes 9 and 10) were similarly deficient at IIi induction and IIa depletion (Fig. 8). However, like the WT, both mutants were able to cause IIo depletion by 8 h p.i. We conclude that inactivation of either the ICP22 gene or the UL13 gene leads to a very similar defect in the ability of HSV-1 to alter the phosphorylation of the RNAP II large subunit.
FIG. 8.
Vhs activity is not required for alteration of the RNAP II large subunit. Vero cells were mock infected (lanes 1) or were infected with the virus strains indicated for 4 or 8 h. Cells were scraped, washed, and lysed directly into SDS-PAGE sample buffer. Protein extracts were run on SDS–6% PAGE, transferred to filters, and probed with ARNA3 (A) or 8WG16 (B). All lanes in panel A or B contain extracts from the same numbers of cells. The positions at which various RNAP II large-subunit forms migrate are indicated.
UL13 is required for normal late viral genome transcription patterns in HEL cells.
Nuclear run-on analysis of transcription in HSV-1-infected cells has suggested that the HSV-1 genome undergoes extensive genome-wide transcription by RNAP II after the commencement of viral DNA replication (24, 75). This is indicated by high transcription signals from nearly all regions of the HSV-1 genome, including both the transcribed and nontranscribed strands (detected by using sense and antisense single-stranded DNA probes, respectively) of known IE, DE, and L genes. The significance of this extensive late transcription, including apparent IE gene transcription and antisense transcription, is not known. We previously observed that both sense and antisense nuclear run-on transcription signals are reduced at late times after infection in 22/n199-infected HEL cells (57), indicating that ICP22 plays a role in promoting RNAP II transcription in this cell line. The reduction in transcription was not evident in 22/n199-infected Vero cells (57), suggesting that ICP22’s effect on transcription may correlate with its cell type-dependent replication effect.
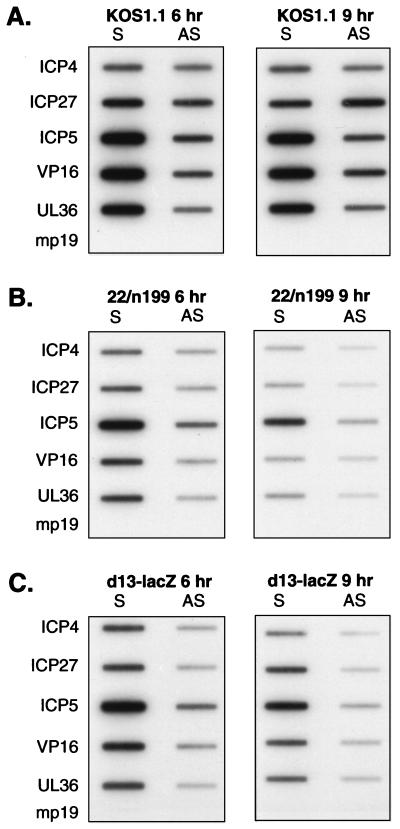
Given the involvement of UL13 in altering RNAP II large-subunit phosphorylation, it was of interest to examine viral transcription in d13-lacZ-infected HEL cells. HEL cells were infected with KOS1.1, 22/n199, or d13-lacZ, and nuclei were isolated at 6 and 9 h p.i. Nuclear run-on transcription was performed on equal numbers of nuclei per sample by allowing RNA transcripts initiated in vivo to be elongated in vitro in the presence of [32P]UTP. The radiolabeled run-on transcripts were purified and hybridized to single-stranded bacteriophage M13 DNA probes detecting sense and antisense RNAs. The probes used detected two IE transcripts (ICP4 and ICP27) and three L transcripts (ICP5, VP16, and UL36). The transcription pattern for the WT virus (Fig. 9A) was consistent with the results of our previous analysis in HEL cells (57). At both 6 and 9 h p.i., high levels of labeled transcripts hybridizing to all sense probes were observed, although the L gene probes gave somewhat higher signals than the IE probes. In addition, readily detectable signals were observed for the antisense probes. The transcription pattern seen in 22/n199-infected cells (Fig. 9B) was also consistent with our previous analysis. The pattern differed significantly from the WT pattern in that both sense and antisense transcription levels were significantly reduced. Interestingly, the transcription pattern seen in d13-lacZ-infected HEL cells (Fig. 9C) was much more similar to the ICP22 mutant pattern than to the WT pattern. Both sense and antisense transcription signals were reduced, although the sense signals appeared somewhat higher than those observed for the 22/n199 infection.
FIG. 9.
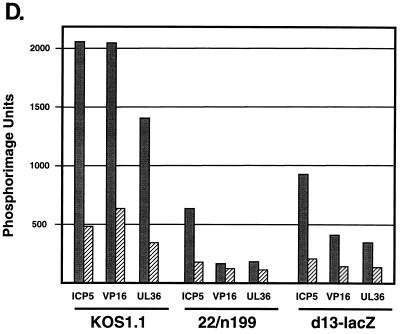
Nuclear run-on transcription analysis of viral gene transcription in d13-lacZ-infected HEL cells. HEL cells were infected with the WT virus strain KOS1.1 (A), the ICP22 mutant 22/n199 (B), or the UL13 mutant d13-lacZ (C) for the times indicated. Nuclei were isolated, and transcription was allowed to proceed in the presence of [32P]UTP as described in Materials and Methods. RNA products from equal numbers of nuclei per sample were hybridized to immobilized single-stranded DNA probes which detect sense (S) or antisense (AS) transcripts arising from the IE genes ICP4 and ICP27 and the L genes ICP5, VP16 (ICP25), and UL36 (ICP1-2). Single-stranded DNA of M13mp19 was included as a background hybridization control. Nuclear run-on transcription assays of mock-infected cells yielded no hybridization to these probes (data not shown). (D) Quantitation of the relative 32P-labeled hybridization signals to the ICP5, VP16, and UL36 gene probes by phosphorimager analysis at 9 h p.i. The values shown are in arbitrary units. Sense transcription levels are denoted by solid bars; antisense transcription levels are indicated by crosshatched bars.
To better analyze these data with respect to late transcription, the radioactive signals for the L genes at the 9-h time point were quantitated by phosphorimager analysis. The results are shown graphically in Fig. 9D. Sense transcription of the three L genes was significantly lower in 22/n199-infected cells than in WT-infected cells, with ICP5, VP16, and UL36 sense transcription being reduced 3-, 12-, and 5-fold, respectively, compared to the WT levels. Antisense transcription (Fig. 9D) was also reduced, although to a lesser extent than the sense transcription. It is notable that the levels of sense transcription of the VP16 and UL36 genes in 22/n199-infected cells were not significantly higher than their levels of antisense transcription, suggesting that there is only a very low level of promoter-specific transcription of these two genes in the 22/n199 infected cells at 9 h p.i. For the d13-lacZ infection, the levels of sense transcription of the three L genes were also significantly reduced compared to those for the WT infection, with ICP5, VP16, and UL36 sense signals being reduced approximately two-, five-, and fourfold compared to the WT levels. However, sense transcription of these genes in d13-lacZ-infected cells was approximately twofold higher than in 22/n199-infected cells, suggesting that the d13-lacZ transcriptional defect was somewhat less severe than that of 22/n199. In conclusion, nuclear transcription run-on analysis indicates that both the 22/n199 and d13-lacZ mutants exhibit diminished L gene sense and antisense transcription in HEL cells compared to WT HSV-1. Thus, both UL13 and ICP22 are required in HEL cells for the normal pattern of late viral genome transcription.
DISCUSSION
Modifications to RNAP II following HSV-1 infection.
The infection of susceptible cells with HSV-1 results in dramatic subversion of the cell’s transcription machinery, with virus transcription overtaking that of the host cell in a few hours. The mechanisms for this switch are not fully understood, but they could involve virus-induced modifications to cellular components. Consistent with this, we previously found that HSV-1 infection rapidly alters the phosphorylation state of the large subunit of RNAP II, generating a novel form that we have designated IIi (58). We have been unable to detect IIi in uninfected cells, leading us to suspect that it is a form unique to HSV-infected cells. However, we cannot eliminate the possibility that IIi is a normal, rare variant which is strongly induced by viral infection.
We have previously shown that the form of RNAP II bearing IIi, RNAP III, is the major transcriptionally active form of the enzyme in HSV-1-infected cells (70). We have hypothesized that RNAP III possesses altered functional properties that allow it to preferentially transcribe viral genes (57, 58). For example, the HSV-1-induced changes could modify the transcription initiation or elongation requirements of RNAP II, which might differ between the viral and cellular genomes due to their inherently different chromatin structures (31, 39). Another possibility, not mutually exclusive with the first, is that the modifications to RNAP II alter the ability of the CTD to recruit pre-mRNA processing factors to nascent transcripts. This could promote viral gene expression if pre-mRNA processing pathways differ between viral and host pre-mRNAs. In this regard, it is notable that nearly all HSV-1 DE and L genes are devoid of introns, whereas most cellular genes contain multiple introns and their transcripts need to undergo multiple splicing events.
Involvement of ICP22 and UL13 in RNAP II modifications.
The mechanism by which HSV-1 alters the phosphorylation of the RNAP II large subunit is not yet known. To delineate this mechanism, it will be important to first identify the viral gene products which are involved. We previously found that the HSV-1 protein ICP22 is required for the efficient induction of IIi, as well as the depletion of IIa, the hypophosphorylated form of the RNAP II large subunit (57). In the present work, we have confirmed this finding using d22-lacZ, a newly engineered ICP22 null mutant. This finding shows that loss of IIo during infection with the 22/n199 virus is not due to any residual activity of the truncated ICP22 protein.
To date, not a great deal is known about the function of ICP22, an ∼68 kDa IE protein which is predominantly localized to the nuclei of infected cells (32). Genetic studies demonstrate that ICP22 is essential for productive virus growth in some but not all cultured cells (51, 62). In those cell lines in which it is required, it stimulates the expression of viral genes, including that of the IE ICP0 gene (54) and those of several L genes (41, 50, 62). ICP22 may activate L genes transcriptionally (57), whereas its stimulation of the ICP0 gene may involve a posttranscriptional mechanism (10). It is noteworthy that a second gene, designated US1.5, also maps to the ICP22 locus (9). The US1.5 mRNA is a low-abundance IE transcript which is 3′ coterminal with the ICP22 mRNA but is transcribed from a promoter downstream of the ICP22 gene promoter. Its encoded protein arises from the same reading frame as ICP22 and corresponds to the 273 carboxyl-terminal residues of ICP22. Since the engineered mutations in both 22/n199 and d22-lacZ abrogate expression of the US1.5-encoded protein, we cannot exclude the possibility that the US1.5 protein plays a role in the alteration of RNAP II phosphorylation.
Our analysis of the phenotype of d13-lacZ, a newly engineered HSV-1 UL13 mutant, demonstrates that the UL13 protein kinase is also involved in modifying RNAP II. Three lines of evidence have previously linked the function of UL13 to ICP22. First, ICP22 is hypophosphorylated in UL13 mutant-infected cells (55). Second, HSV-1 UL13 mutants and ICP22 mutants exhibit similar cell type-dependent growth and gene expression (54). Third, UL13 is required for the normal localization of ICP22 to nuclear dense bodies late in infection (32). Given these links, it is perhaps not surprising that mutation of the UL13 gene leads to an RNAP phosphorylation phenotype similar to that of an ICP22 mutant. However, not all of the regulatory effects of ICP22 depend on UL13, as recent work has shown that ICP22 but not UL13 is required for HSV-1’s ability to induce expression of the cellular α-globin gene (13).
The UL13 protein is an ∼56 kDa polypeptide with signature protein kinase motifs (11, 69) and demonstrated protein kinase activity (17). It is expressed with L gene kinetics (46), but it is packaged into the tegument of virus particles (15, 47) and is thus present from the onset of infection. Although the direct in vivo substrates of the UL13 protein are unknown, several proteins are hypophosphorylated in UL13 mutant-infected cells, including the viral proteins ICP22 (55), VP22 (15), ICP0 (44), and glycoproteins E and I (gE and gI) (42) and the cellular translation factor EF-1δ (29). Like ICP22, the role of UL13 in HSV-1 infection is not well understood, but there is evidence that it has multiple functions. First, the protein kinase activity of UL13 may help promote tegument disassembly at the beginning of infection (38). Second, as mentioned above, UL13 stimulates viral gene expression in some cell lines. Third, UL13 appears to have a role in the Vhs-dependent shutoff of host translation (46). This last effect may be due to the ability of UL13 to promote the expression of the UL41 gene (41), which encodes the Vhs function.
In considering the effects of ICP22 and UL13 on RNAP II phosphorylation, it is helpful to consider that there are three definable changes to the RNAP II large subunit that occur soon after HSV-1 infection: (i) depletion of the hyperphosphorylated IIo form, (ii) depletion of the hypophosphorylated IIa form, and (iii) induction of the alternatively phosphorylated IIi species. Together, our past (57, 58) and present results suggest that these changes are due to two distinct effects. The first effect, which does not depend on ICP22 or UL13, is the efficient depletion of IIo. The second effect, which depends on both ICP22 and UL13, is the induction of IIi and depletion of IIa. We discuss each of these two effects separately below.
Depletion of IIo by an ICP22- and UL13-independent mechanism.
The biological significance of the HSV-1-induced depletion of the normal hyperphosphorylated IIo form of the RNAP II large subunit is unknown. It has been established that the CTD and its phosphorylation are necessary for efficient RNAP II transcription elongation, response to transcription activators in vivo, and association with RNA processing factors (1, 5, 14, 23, 40, 45, 72, 76). Therefore, it is conceivable that loss of hyperphosphorylation is crucial for the switch from viral to host genome transcription. Alternately, loss of hyperphosphorylation may be a side effect of virus-induced host transcription repression, brought about by mechanisms unrelated to RNAP II phosphorylation. To date, we have been unable to test these hypotheses, since we have been unsuccessful in identifying conditions or virus mutations which specifically prevent IIo depletion or host transcription repression or that separate these phenomena. We have shown that IIo depletion and host gene transcription repression also occur efficiently in cells infected with an ICP4 null mutant (57), indicating that ICP4, DE and/or L gene expression, and viral DNA replication are not required. IIo depletion and transcription repression occur efficiently in cells infected with HSV-1 strains deficient in the IE functions mediated by ICP0, ICP22, or ICP27, as well as ICP6 (57). However, IIo depletion and host gene transcription repression do not occur in cells infected with UV-inactivated virus or in cells infected in the presence of cycloheximide (58). Together, these results indicate that neither virion components nor viral IE gene transcription are sufficient for IIo depletion and host transcription repression, strongly suggesting that viral IE proteins are required.
Given these results, we suggest two possible explanations for the identity of the viral factor(s) needed to deplete IIo. First, IIo depletion may depend on an IE gene product other than ICP4, ICP0, ICP22, or ICP27, although the only other known major IE protein, ICP47, is not known to have a role in viral gene regulation. Second, IE proteins could be redundant in their abilities to induce IIo depletion. Thus, mutations in more than one IE gene may be required to prevent IIo depletion. Since HSV-1 mutants bearing multiple IE mutations have been isolated (reference 60 and references therein), it should be possible to test this model.
ICP22- and UL13-dependent depletion of IIa and induction of IIi.
Based on our analyses of RNAP II large-subunit phosphorylation in cells infected d22-lacZ and d13-lacZ, we suggest that ICP22 and UL13 function in a common pathway that leads to IIi induction and IIa depletion. This pathway is separable and possibly independent from that which leads to IIo loss. To date, we have been unable to uncouple the induction of IIi from the depletion of IIa. This suggests that the two events are mechanistically linked. Although there are many possibilities, a simple and appealing model is that ICP22 and UL13 together are responsible for inducing a novel CTD kinase activity which converts IIa to IIi. Given that UL13 is a protein kinase, one version of this model posits that UL13 is an ICP22-dependent CTD kinase. An alternative version proposes that ICP22 and UL13 together activate or alter an existing cellular CTD kinase or phosphatase.
ICP22 is hypophosphorylated in UL13 mutant-infected cells, indicating that the phosphorylation of ICP22 is dependent on UL13 (54, 55). Thus, one important question regarding the role of UL13 in RNAP II modifications is whether its effect is a result of its ability to mediate, directly or indirectly, the phosphorylation of ICP22. Based on the following argument, we believe that this is unlikely. First, we have previously shown that HSV-1-induced modifications to RNAP II occur efficiently in cells infected with an ICP4 mutant, demonstrating that DE and L gene expression are not required. Thus, the UL13 protein carried into cells with the virus particle must be sufficient for RNAP II modification, assuming there is no ICP4-independent expression of the UL13 gene. Second, Purves and colleagues have presented strong evidence that UL13’s phosphorylation of ICP22 requires newly expressed UL13 (54). Together, these data strongly indicate that RNAP II modifications can be effected by an ICP22 molecule which has not yet acquired its UL13-dependent phosphorylation. Thus, we suggest that the role of UL13 in altering RNAP II phosphorylation must be other than simply to mediate ICP22 phosphorylation.
Role of ICP22- and UL13-dependent RNAP II modifications in transcription.
ICP22 and UL13 are not essential for virus replication and efficient DE and/or L gene transcription in Vero cells and some other cell lines. Therefore, efficient IIi induction and IIa depletion are clearly not required for productive viral infection in all situations. For ICP22 mutant-infected cells of both the Vero and HEL lines, which show productive and nonproductive infections, respectively, hyperphosphorylated forms of the RNAP II large subunit are not readily evident in Western blots of infected-cell proteins (57, 70). Despite this, in both types of infection, it is the hyperphosphorylated forms which are found to be associated with nascent RNA by UV cross-linking (70). This suggests that, during HSV-1 infection, RNAP II molecules actively transcribing viral genes need to have undergone some CTD hyperphosphorylation. In Vero cells infected with an ICP22 mutant, these nonabundant hyperphosphorylated large-subunit forms do not appear to be limiting for transcription. However, they may be limiting in HEL cells, resulting in less viral transcription and ultimately a nonproductive infection. This possibility is consistent with the results of our nuclear run-on transcription analyses, which indicated that late viral gene transcription is significantly reduced in HEL cells infected with ICP22 or UL13 mutants. One possibility, then, is that HSV-1 has evolved a mechanism, involving ICP22 and UL13, which acts to induce a novel hyperphosphorylated form of the RNAP II large subunit, IIi, to make up for the loss of IIo. IIi may be dispensable in some cell lines, such as Vero cells, where residual hyperphosphorylated forms may still be sufficient for transcription. In other cells, such as HEL cells, these forms may be limiting, making IIi essential. In this regard, it is interesting that ICP22 is required for acute infection and virulence in animal models (37, 50). This suggests that HSV-1’s capacity to replicate in at least some cells of its natural human host may depend on its ability to induce the IIi form of the RNAP II large subunit.
ACKNOWLEDGMENTS
We thank Jim Smiley for providing the vhsA mutant and Nancy Thompson for the gift of the 8WG16 MAb. We are also grateful to Leslie Schiff and Jim Smiley for helpful discussions and to Leslie Schiff for a critical review of the manuscript.
This research was supported by operating grants from the National Cancer Institute of Canada (to S.A.R.) and from the Medical Research Council (to C.A.S.). S.A.R. and C.A.S. are Senior Scholars of the Alberta Heritage Foundation for Medical Research.
REFERENCES
- 1.Akhtar A, Faye G, Bentley D L. Distinct activated and non-activated RNA polymerase II complexes in yeast. EMBO J. 1996;15:4654–4664. [PMC free article] [PubMed] [Google Scholar]
- 2.Allen K E, Everett R D. Mutations which alter the DNA binding properties of the herpes simplex virus type 1 transactivating protein Vmw175 also affect its ability to support virus replication. J Gen Virol. 1997;78:2913–2922. doi: 10.1099/0022-1317-78-11-2913. [DOI] [PubMed] [Google Scholar]
- 3.Archambault J, Pan G, Dahmus G K, Cartier M, Marshall N, Zhang S, Dahmus M E, Greenblatt J. FCP1, the RAP74-interacting subunit of a human protein phosphatase that dephosphorylates the carboxyl-terminal domain of RNA polymerase IIO. J Biol Chem. 1990;273:27593–27601. doi: 10.1074/jbc.273.42.27593. [DOI] [PubMed] [Google Scholar]
- 4.Astor, T. L., S. A. Rundle, C. L. Bogard, W. Cai, and P. A. Schaffer. Unpublished data.
- 5.Bentley D. A tale of two tails. Nature. 1998;395:21–22. doi: 10.1038/25616. [DOI] [PubMed] [Google Scholar]
- 6.Cadena D L, Dahmus M E. Messenger RNA synthesis in mammalian cells is catalyzed by the phosphorylated form of RNA polymerase II. J Biol Chem. 1987;262:12468–12474. [PubMed] [Google Scholar]
- 7.Cai W, Person S, DebRoy C, Gu B. Functional regions and structural features of the gB glycoprotein of herpes simplex virus type 1: an analysis of linker insertion mutants. J Mol Biol. 1998;201:575–588. doi: 10.1016/0022-2836(88)90639-0. [DOI] [PubMed] [Google Scholar]
- 8.Carroza M J, DeLuca N A. Interaction of the viral activator protein ICP4 with TFIID through TAF250. Mol Cell Biol. 1996;16:3085–3093. doi: 10.1128/mcb.16.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter K L, Roizman B. The promoter and transcriptional unit of a novel herpes simplex virus type 1 α gene are contained in, and encode a protein in frame with, the open reading frame of the α22 gene. J Virol. 1996;70:172–178. doi: 10.1128/jvi.70.1.172-178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter K L, Roizman B. Alternatively spliced mRNAs predicted to yield frame-shift proteins and stable intron 1 RNAs of the herpes simplex virus 1 regulatory gene αo accumulate in the cytoplasm of infected cells. Proc Natl Acad Sci USA. 1996;93:12525–12540. doi: 10.1073/pnas.93.22.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chee M S, Lawrence G L, Barrell B G. Alpha-, beta-, and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 1989;70:1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- 12.Chesnut J D, Stephens J H, Dahmus M E. The interaction of RNA polymerase II with the adenovirus-2 major late promoter is precluded by phosphorylation of the C-terminal domain of subunit IIa. J Biol Chem. 1992;267:10500–10506. [PubMed] [Google Scholar]
- 13.Cheung, P., C. Spencer, and J. R. Smiley. Unpublished data.
- 14.Corden J L, Patturajan M. A CTD function linking transcription to splicing. Trends Biochem Sci. 1997;11:413–416. doi: 10.1016/s0968-0004(97)01125-0. [DOI] [PubMed] [Google Scholar]
- 15.Coulter L J, Moss H W M, McGeoch D J. A mutant of herpes simplex virus in which the UL13 protein kinase gene is disrupted. J Gen Virol. 1993;74:387–395. doi: 10.1099/0022-1317-74-3-387. [DOI] [PubMed] [Google Scholar]
- 16.Dahmus M E. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J Biol Chem. 1996;271:19009–19012. doi: 10.1074/jbc.271.32.19009. [DOI] [PubMed] [Google Scholar]
- 17.Daikoku T, Shibata S, Goshima F, Oshima S, Tsurumi T, Yamada H, Yamashita Y, Nishiyama Y. Purification and characterization of the protein kinase encoded by the UL13 gene of herpes simplex virus type 2. Virology. 1997;235:82–93. doi: 10.1006/viro.1997.8653. [DOI] [PubMed] [Google Scholar]
- 18.DeLuca N A, McCarthy A M, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emili A, Ingles C J. The RNA polymerase II carboxyl-terminal domain: links to a bigger and better “holoenzyme”. Curr Opin Genet Dev. 1995;5:204–209. doi: 10.1016/0959-437x(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 20.Flint J, Shenk T. Viral transactivating proteins. Annu Rev Genet. 1997;31:177–212. doi: 10.1146/annurev.genet.31.1.177. [DOI] [PubMed] [Google Scholar]
- 21.Fujinaga K, Cujec T P, Peng J, Garriga J, Price D H, Graña X, Peterlin B M. The ability of positive transcription factor b to transactivate human immunodeficiency virus transcription depends on a functional kinase domain, cyclin T1, and Tat. J Virol. 1998;72:7154–7159. doi: 10.1128/jvi.72.9.7154-7159.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao M, Knipe D M. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J Virol. 1989;63:5258–5267. doi: 10.1128/jvi.63.12.5258-5267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber H P, Hagmann M, Seipel K, Georgiev O, West M A, Litingtung Y, Schaffner W, Corden J L. RNA polymerase II C-terminal domain required for enhancer-driven transcription. Nature. 1995;374:660–662. doi: 10.1038/374660a0. [DOI] [PubMed] [Google Scholar]
- 24.Godowski P J, Knipe D M. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc Natl Acad Sci USA. 1986;83:256–260. doi: 10.1073/pnas.83.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hengartner C J, Myers V, Liao S-M, Wilson C J, Koh S, Young R A. Temporal regulation of RNAP II by SRB10 and Kin28 cycling dependent kinases. Mol Cell. 1998;2:43–53. doi: 10.1016/s1097-2765(00)80112-4. [DOI] [PubMed] [Google Scholar]
- 26.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;41:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes R G, Munyon W H. Temperature-sensitive mutants of herpes simplex virus type 1 defective in lysis but not in transformation. J Virol. 1975;16:275–283. doi: 10.1128/jvi.16.2.275-283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan R, Schaffer P A. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J Virol. 1997;71:6850–6862. doi: 10.1128/jvi.71.9.6850-6862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawaguchi Y, Van Sant C, Roizman B. Eukaryotic elongation factor 1δ is hyperphosphorylated by the protein kinase encoded by the UL13 gene of herpes simplex virus 1. J Virol. 1998;72:1731–1736. doi: 10.1128/jvi.72.3.1731-1736.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer A, Haars R, Kabisch R, Will H, Bautz F A, Bautz E K F. Monoclonal antibody directed against RNA polymerase II of D. melanogaster. Mol Gen Genet. 1980;180:193–199. doi: 10.1007/BF00267369. [DOI] [PubMed] [Google Scholar]
- 31.Leinbach S S, Summers W C. The structure of the herpes simplex virus type 1 DNA as probed by micrococcal nuclease digestion. J Gen Virol. 1980;51:45–59. doi: 10.1099/0022-1317-51-1-45. [DOI] [PubMed] [Google Scholar]
- 32.Leopardi R, Ward P L, Ogle W O, Roizman B. Association of herpes simplex virus regulatory protein ICP22 with transcriptional complexes containing EAP, ICP4, RNA polymerase II, and viral DNA requires posttranslational modification by the UL13 protein kinase. J Virol. 1997;71:1133–1139. doi: 10.1128/jvi.71.2.1133-1139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H, Flores O, Weinmann R, Reinberg D. The non-phosphorylated form of RNA polymerase II preferentially associates with the preinitiation complex. Proc Natl Acad Sci USA. 1991;88:10004–10008. doi: 10.1073/pnas.88.22.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancebo H S, Lee G, Flygare J, Tomassini J, Luu P, Zhu Y, Peng J, Blau C, Hazuda D, Price D, Flores O. P-TEFb kinase is required for HIV tat transcriptional activation in vivo and in vitro. Genes Dev. 1997;11:2633–2634. doi: 10.1101/gad.11.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall N F, Dahmus G K, Dahmus M E. Regulation of the carboxy-terminal domain phosphatase by HIV-1 Tat protein. J Biol Chem. 1998;273:31726–31730. doi: 10.1074/jbc.273.48.31726. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy A M, McMahan L, Schaffer P A. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989;63:18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meignier B, Longnecker R, Mavromara-Nazos P, Sears A E, Roizman B. Virulence and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology. 1988;162:251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- 38.Morrison E E, Wang Y-F, Meredith D M. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J Virol. 1998;72:7108–7114. doi: 10.1128/jvi.72.9.7108-7114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muggeridge M I, Fraser N W. Chromosomal organization of the herpes simplex genome during acute infection of the mouse central nervous system. J Virol. 1986;59:764–767. doi: 10.1128/jvi.59.3.764-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neugebauer K M, Roth M B. Transcription units as RNA processing units. Genes Dev. 1997;11:3279–3285. doi: 10.1101/gad.11.24.3279. [DOI] [PubMed] [Google Scholar]
- 41.Ng T I, Chang Y E, Roizman B. Infected cell protein 22 of herpes simplex virus 1 regulates the expression of virion host shutoff gene UL41. Virology. 1997;234:226–234. doi: 10.1006/viro.1997.8659. [DOI] [PubMed] [Google Scholar]
- 42.Ng T I, Ogle W O, Roizman B. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology. 1998;241:39–48. doi: 10.1006/viro.1997.8963. [DOI] [PubMed] [Google Scholar]
- 43.O’Brien T, Hardin S, Greenleaf A, Lis J. Phosphorylation of RNA polymerase II C-terminal domain and transcriptional elongation. Nature. 1994;370:75–77. doi: 10.1038/370075a0. [DOI] [PubMed] [Google Scholar]
- 44.Ogle W O, Ng T I, Carter K L, Roizman B. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology. 1997;235:406–413. doi: 10.1006/viro.1997.8710. [DOI] [PubMed] [Google Scholar]
- 45.Okamoto H, Sheline C T, Corden J, Jones K, Peterlin B M. Transactivation by HIV Tat protein requires the CTD of RNA polymerase II. Proc Natl Acad Sci USA. 1996;93:11575–11579. doi: 10.1073/pnas.93.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Overton H A, McMillan D J, Hope L, Wong-Kai-In P. Production of host shutoff-defective mutants of herpes simplex virus type 1 by inactivation of the UL13 gene. Virology. 1994;202:97–106. doi: 10.1006/viro.1994.1326. [DOI] [PubMed] [Google Scholar]
- 47.Overton H A, McMillan D J, Klavinskis L S, Hope L, Ritchie A J, Wong-Kai-In P. Herpes simplex virus type 1 gene UL13 encodes a phosphoprotein that is a component of the virion. Virology. 1992;190:184–192. doi: 10.1016/0042-6822(92)91204-8. [DOI] [PubMed] [Google Scholar]
- 48.Panagiotidis C A, Lium E K, Silverstein S J. Physical and functional interactions between herpes simplex virus immediate-early proteins ICP4 and ICP27. J Virol. 1997;71:1547–1557. doi: 10.1128/jvi.71.2.1547-1557.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Payne J M, Laybourn P J, Dahmus M E. The transition of RNA polymerase II from initiation to elongation is associated with phosphorylation of the carboxyl-terminal domain of subunit IIa. J Biol Chem. 1989;264:19621–19629. [PubMed] [Google Scholar]
- 50.Poffenberger K L, Idowu A D, Fraser-Smith E B, Raichlen P E, Herman R C. A herpes simplex virus type 1 ICP22 deletion mutant is altered for virulence and latency in vivo. Arch Virol. 1994;139:111–119. doi: 10.1007/BF01309458. [DOI] [PubMed] [Google Scholar]
- 51.Poffenberger K L, Raichlen P E, Herman R C. In vitro characterization of a herpes simplex virus type 1 ICP22 mutant. Virus Genes. 1993;7:171–186. doi: 10.1007/BF01702397. [DOI] [PubMed] [Google Scholar]
- 52.Preston C M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979;29:275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preston C M, Newton A A. The effects of herpes simplex virus type 1 on cellular DNA-dependent RNA polymerase activities. J Gen Virol. 1976;33:471–482. doi: 10.1099/0022-1317-33-3-471. [DOI] [PubMed] [Google Scholar]
- 54.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Purves F C, Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for the posttranslational processing associated with phosphorylation of the regulatory protein α22. Proc Natl Acad Sci USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rice S A, Knipe D M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus α protein ICP27. J Virol. 1990;64:1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rice S A, Long M C, Lam V, Schaffer P A, Spencer C A. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and the establishment of the normal viral transcription cycle. J Virol. 1995;69:5550–5559. doi: 10.1128/jvi.69.9.5550-5559.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rice S A, Long M C, Lam V, Spencer C A. RNA polymerase II is aberrantly phosphorylated and localized to viral replication compartments following herpes simplex virus infection. J Virol. 1994;68:988–1001. doi: 10.1128/jvi.68.2.988-1001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Howley P M, Knipe D M, editors. Virology. 2nd ed. New York, N.Y: Raven Press; 1996. pp. 1795–1841. [Google Scholar]
- 60.Samaniego L A, Neiderhiser L, DeLuca N A. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 62.Sears A E, Halliburton I W, Meignier B, Silver S, Roizman B. Herpes simplex virus 1 mutant deleted in the α22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J Virol. 1985;55:338–346. doi: 10.1128/jvi.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shapira S K, Chou J, Richard F V, Casadaban M J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of β-galactosidase. Gene. 1983;25:71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- 64.Shepard A A, Imbalzano A N, DeLuca N A. Separation of primary structural components conferring autoregulation, transactivation, and DNA-binding properties to the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1989;63:3714–3728. doi: 10.1128/jvi.63.9.3714-3728.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smibert C A, Smiley J R. Differential regulation of endogenous and transduced β-globin genes during infection of erythroid cells with a herpes simplex virus type 1 recombinant. J Virol. 1990;64:3882–3894. doi: 10.1128/jvi.64.8.3882-3894.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smiley J R, Johnson D C, Pizer L I, Everett R D. The ICP4 binding sites in the herpes simplex virus type 1 glycoprotein D (gD) promoter are not essential for efficient gD transcription during virus infection. J Virol. 1992;66:623–631. doi: 10.1128/jvi.66.2.623-631.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smiley J R, Panning B, Smibert C A. Regulation of cellular genes by HSV products. In: Wagner E K, editor. Herpesvirus transcription and its regulation. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 151–179. [Google Scholar]
- 68.Smith C A, Bates P, Rivera-Gonzalez R, Gu B, DeLuca N A. ICP4, the major transcriptional regulatory protein of herpes simplex virus type 1, forms a tripartite complex with TATA-binding protein and TFIIB. J Virol. 1993;67:4676–4687. doi: 10.1128/jvi.67.8.4676-4687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith R F, Smith T F. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J Virol. 1989;63:450–455. doi: 10.1128/jvi.63.1.450-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spencer C A, Dahmus M E, Rice S A. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J Virol. 1997;71:2031–2040. doi: 10.1128/jvi.71.3.2031-2040.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spencer C A, LeStrange R C, Novak U, Hayward W S, Groudine M. The block to transcriptional elongation is promoter dependent in normal and Burkitt’s lymphoma c-myc alleles. Genes Dev. 1990;4:75–88. doi: 10.1101/gad.4.1.75. [DOI] [PubMed] [Google Scholar]
- 72.Steinmetz E J. Pre-mRNA processing and the CTD of RNA polymerase II: the tail that wags the dog. Cell. 1997;89:491–494. doi: 10.1016/s0092-8674(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 73.Thompson N E, Aronson D, Burgess R R. Purification of eukaryotic RNA polymerase II by immunoaffinity chromatography. J Biol Chem. 1990;265:7069–7077. [PubMed] [Google Scholar]
- 74.Wagner E K, Guzowski J F, Singh J. Transcription of the herpes simplex virus genome during productive and latent infection. Prog Nucleic Acid Res Mol Biol. 1995;51:123–165. doi: 10.1016/s0079-6603(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 75.Weinheimer S P, McKnight S L. Transcriptional and post-transcriptional controls establish the cascade of herpes simplex virus protein synthesis. J Mol Biol. 1987;195:819–833. doi: 10.1016/0022-2836(87)90487-6. [DOI] [PubMed] [Google Scholar]
- 76.Yang X, Herrmann C H, Rice A P. The human immunodeficiency virus Tat proteins specifically associate with TAK in vivo and require the carboxyl-terminal domain of RNA polymerase II for function. J Virol. 1996;70:4576–4584. doi: 10.1128/jvi.70.7.4576-4584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young R A. RNA polymerase II. Annu Rev Biochem. 1991;60:689–715. doi: 10.1146/annurev.bi.60.070191.003353. [DOI] [PubMed] [Google Scholar]