Abstract
Background
Limited data exist for management strategies targeting immunotherapy-related enteritis (irEnteritis). Systemic corticosteroids are commonly used but often are limited by adverse events. Enteric corticosteroids such as budesonide offer an attractive alternative; however, the ileocolonic release of enteric-coated budesonide has limited utility for diffuse enteritis. Open-capsule budesonide (OCB) is a novel therapeutic approach that offers drug delivery throughout the small bowel. We report outcomes in patients treated with OCB for confirmed or suspected irEnteritis.
Methods
This retrospective cohort included all individuals treated with OCB for irEnteritis at Memorial Sloan Kettering from July 2018 to August 2023. Primary outcomes included clinical response, clinical remission, and corticosteroid-free remission following OCB. Secondary outcomes were OCB-related adverse events and efficacy by gastrointestinal toxicity location.
Results
19 patients (53% female) with irEnteritis were treated with OCB. All patients presented with diarrhea; 15 (79%) reported anorexia with median 6 kg weight loss. 17 patients (89%) underwent esophagogastroduodenoscopy with biopsies revealing enteritis in all; 8 (42%) had concomitant colitis. 15 (79%) patients were treated previously with systemic corticosteroids: 8 (53%) were corticosteroid-dependent while 7 (47%) demonstrated non-response. 18 patients (95%) achieved clinical response, 15 (79%) attained clinical remission, and 11 (58%) had corticosteroid-free remission. Response to OCB was rapid with improvement noted after a median 4 days. 14 (74%) patients restored their pre-irEnteritis weight by OCB cessation. One mild, self-resolving adverse event was reported.
Conclusions
OCB is a safe and effective therapy for irEnteritis. OCB avoids systemic immunosuppression and successfully achieves clinical response and remission even in patients previously nonresponsive to systemic corticosteroids. Future studies are needed to optimize indications and duration.
Keywords: Immunotherapy, STEROIDS, Immune related adverse event - irAE, Immune Checkpoint Inhibitor, Immunosuppression
WHAT IS ALREADY KNOWN ON THIS TOPIC
Immune-related enteritis (irEnteritis) is an understudied cause of immune checkpoint inhibitor (ICI)-induced diarrhea that can present with the additional symptoms of nausea, vomiting, anorexia, and early satiety. Current management guidelines for irEnteritis simply extrapolate recommendations for immune-related colitis, typically consisting of systemic corticosteroids and biological agents that are not only linked to numerous adverse effects but also potentially blunted antitumor ICI efficacy. Open-capsule budesonide (OCB), a novel mechanism previously described as a promising treatment for refractory celiac disease and autoimmune enteropathy, may provide improved drug delivery and therapeutic localization for irEnteritis.
WHAT THIS STUDY ADDS
In patients with irEnteritis, including those with prior inadequate response to systemic corticosteroids, OCB was found to be a safe and effective treatment regimen, achieving clinical response and remission in 95% and 79% of patients, respectively, with a median time to improvement of 4 days. While early satiety, anorexia, and significant weight loss were common in patients with irEnteritis, 74% of patients on OCB were able to restore their pre-irEnteritis weight by OCB cessation, providing novel data on the total impact of irEnteritis on weight loss and the efficacy of OCB on weight gain.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
We suggest that OCB may be optimally indicated for patients presenting with a high clinical suspicion for irEnteritis, such as predominant upper GI symptoms including anorexia, early satiety, nausea, vomiting and diarrhea, ideally with endoscopic and/or histological confirmation of enteritis. OCB’s advantages of greater control over drug delivery localization and minimal risk for systemic adverse effects make it an attractive alternative to more intensive immunosuppressive regimens.
Introduction
Immune checkpoint inhibitors (ICIs) targeting key checkpoint receptors responsible for immune system downregulation are efficacious agents offering remarkable clinical and survival benefit across a variety of cancer types.1 However, through their blockade of inhibitory immune signaling pathways such as programmed death (PD)-1, its ligand PD-L1, and cytotoxic T lymphocyte-associated protein (CTLA)-4, ICI therapy can also lead to side effects, collectively referred to as immune-related adverse events (irAEs).2,4 Among these, the gastrointestinal (GI) tract is among the most common sites of inflammatory toxicity and represents the most common type of severe irAEs requiring cessation of ICI therapy.1 4 5 Immune-related diarrhea can impact up to 40% of patients on ICI therapy6 with its toxicities ranging from mild diarrhea to a life-threatening condition, which results in substantial morbidity, treatment interruption, and discontinuation.7 While immune-related colitis is the best-studied cause of immune-related diarrhea, upper GI toxicity, including gastritis, gastroenteritis, and enteritis, has also been reported and can exist in isolation or in combination with lower GI toxicity.
Current practice guidelines recommend the prompt initiation of empiric immunosuppression, typically with moderate to high doses of systemic corticosteroids, for most patients with diarrhea suspected secondary to immunotherapy.8,10 Significant concerns have been raised, however, regarding the detrimental impact of systemic corticosteroids in the treatment of various irAEs. High-dose corticosteroid use has been linked to impaired antitumor response11,13 and an increased risk of severe infection in patients on ICIs.14 15 Furthermore, prolonged steroid usage even at low to moderate doses has been linked to numerous adverse effects.16 In addition, data on the management and response of symptoms associated with upper GI toxicity, such as nausea, vomiting, anorexia and early satiety, remain extremely sparse. Identifying new treatment strategies for immunotherapy-related GI toxicity that minimize prolonged systemic corticosteroid exposure, effectively target upper GI toxicities and enable patients to continue or resume ICI therapy is therefore of significant clinical importance.
Budesonide is a second-generation corticosteroid that offers important advantages over conventional systemic corticosteroids due to potent anti-inflammatory effect in the GI tract with minimal systemic absorption due to extensive first-pass metabolism in the liver.17 Currently, available oral formulations are designed for distal small bowel and proximal colonic release (enteric-coated (EC) budesonide formulation)) or pan-colonic release (budesonide-MMX), which limits their utility for small bowel enteropathies.18 Open-capsule budesonide (OCB) is a simple, yet novel delivery mechanism whereby some of the daily capsules are opened and/or the contents chewed to facilitate more proximal GI release. OCB has been previously described as a promising treatment option for other small bowel diseases such as refractory celiac disease and autoimmune enteropathy19 20 and may, therefore, provide improved drug delivery and therapeutic localization for immunotherapy-related enteritis (irEnteritis). In this study, we report our experience with the use of OCB for patients with suspected or confirmed irEnteritis based on symptoms and endoscopic evaluation.
Methods
Study population
This single-center, retrospective analysis included all individuals treated with OCB for irEnteritis at Memorial Sloan Kettering Cancer Center from July 2018 to August 2023. Patients with histopathological confirmation of irEnteritis obtained via esophagogastroduodenoscopy (EGD) (n=17) and those clinically suspected to have irEnteritis (diarrhea plus one additional upper GI symptom, including anorexia, nausea, vomiting, epigastric pain, and/or early satiety, and/or imaging findings suggesting enteritis; n=2) were included.
Open-capsule budesonide
Initial OCB dosing is three 3 mg oral budesonide capsules taken once daily. EC budesonide is available as a 3 mg hard gelatin capsule containing the drug in 1 mm diameter round pellets with the active drug contained in an insoluble ethylcellulose polymer, which provides time-dependent release.21 Opening the capsule with or without grinding the drug contents between the teeth before swallowing is theorized to free the budesonide contents from the ethylcellulose matrix and facilitate earlier drug delivery to the more proximal GI tract. Directions for the modified OCB regimen have been previously described19 and include (1) first capsule: open the capsule, empty and stir the contents into applesauce, grind the contents between the teeth, then rinse and swallow with water (targeting gastric and proximal small bowel); (2) second capsule: open the capsule, empty and stir the contents into applesauce and swallow with water (targeting mid-small bowel) and (3) third capsule: swallow the whole capsule (targeting distal small bowel and proximal colon). Initiation, cessation, and duration of OCB therapy were determined by the patient’s treating gastroenterologist. Taper strategy was guided by the location of inflammation when known and/or by dominant symptoms (eg, first discontinuing the whole capsule when primarily upper GI disease and/or anorexia are present).
Data collection
Data regarding demographics, irEnteritis, immunosuppressive therapy, endoscopic evaluations, and clinical outcomes were collected. Weight loss, diarrhea, and colitis were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), V.5.0. Endoscopic and histological features of GI toxicity were extracted from medical reports. Colitis scoring was adapted from the Mayo endoscopic scoring system.22 In the absence of a validated scoring system, enteritis severity was graded on a qualitative scale similar to the Mayo system of “normal,” “erythema,” “marked erythema and edema,” and “erosions/ulcerations.”
Outcomes
Primary outcomes included clinical response, clinical remission, and corticosteroid-free clinical remission following treatment with OCB. Clinical response to OCB was defined as at least one CTCAE grade decrease in symptoms. Clinical remission was defined as return to baseline stool frequency of diarrhea and the resolution of any additional presenting GI symptoms, such as abdominal pain, nausea, vomiting, or anorexia. Corticosteroid-free remission was defined as clinical remission 4 weeks after stopping OCB therapy. Secondary outcomes included time to each of the primary outcomes, OCB-related adverse events, and differences in clinical outcomes by GI toxicity location.
Statistical analysis
Continuous variables were summarized as mean and SD if normally distributed and as median and IQR or range if not normally distributed. Categorical variables were summarized as counts and percentages. Student’s t-tests and Wilcoxon’s rank sum tests were used to compare continuous variables while χ2 tests were used for categorical variables. Baseline parameters were defined at the time of OCB initiation. An alpha of 0.05 was considered significant. Statistical calculations were performed using Stata Statistical Software: Release V.17 (StataCorp).
Results
Baseline characteristics and ICI treatment history
19 patients were treated with OCB and included in the study. Baseline characteristics are summarized in table 1. 10 female patients (53%, n=10/19) and 9 (47%, n=9/19) male patients were analyzed. Melanoma (32%, n=6/19) was the most common malignancy, and anti PD-(L)1 immunotherapy (63%, n=12/19) was the most common regimen used prior to irEnteritis. The median number of days of ICI therapy prior to irEnteritis onset was 116 (IQR 57–151). Eight of 19 (63%) patients discontinued ICIs due to irEnteritis while the remainder were able to resume immunotherapy after temporary discontinuation during treatment. Three patients ultimately discontinued ICIs due to progression of disease, two due to non-GI irAEs (pulmonary and endocrine), and two are still currently receiving immunotherapy.
Table 1. Baseline patient and ICI regimen characteristics (n=19).
| Characteristics | No. of patients |
| Age at irEnteritis, median (range) | 62 (29–86) |
| Female sex | 10 (53%) |
| Race | |
| White | 16 (84%) |
| Black | 1 (5%) |
| Other/unknown | 2 (11%) |
| Cancer type | |
| Melanoma | 6 (32%) |
| Lung | 5 (26%) |
| Genitourinary | 3 (16%) |
| Gynecologic | 2 (11%) |
| Hematologic | 1 (5%) |
| Breast | 1 (5%) |
| Angiosarcoma | 1 (5%) |
| Cancer stage | |
| I–III | 6 (32%) |
| IV | 13 (68%) |
| Cancer status | |
| Stable disease | 10 (52%) |
| Progressive disease | 3 (16%) |
| Partial response | 3 (16%) |
| Complete response | 3 (16%) |
| Concurrent chemotherapy | 2 (11%) |
| ICI regimen | |
| PD-(L)1 | 12 (63%) |
| CTLA-4 + PD-(L)1 | 7 (37%) |
| Total days on ICI therapy prior to irEnteritis, median (IQR) | 116 (57–151) |
| No. of ICI cycles prior to irEnteritis onset | |
| 1–2 | 6 (32%) |
| 3–5 | 4 (21%) |
| 6–10 | 7 (36%) |
| >10 | 2 (11%) |
| ICI regimen outcome | |
| Cessation due to irEnteritis | 12 (63%) |
| Cessation due to progression of malignancy | 1 (5%) |
| Cessation due to other irAEs | 4 (21%) |
| Still receiving ICI after temporary interruption | 2 (11%) |
ICI, immune checkpoint inhibitor; irAE, immune-related adverse eventsirEnteritis, immune-related enteritis
Immune-related enterocolitis clinical characterization
Median time from ICI initiation to irEnteritis onset was 138 days (IQR 50–186) (table 2). All 19 (100%) patients developed diarrhea while 14 (74%) developed nausea and/or vomiting, 15 (79%) developed anorexia and/or early satiety, and only 9 (47%) developed abdominal pain. Median weight loss noted from irEnteritis onset to nadir was 6 kg (IQR 4–8) with 7 patients (37%) experiencing CTCAE grades 2–3 weight loss related to irEnteritis totaling ≥10% of their total body weight. Most patients also had CTCAE grades 1–2 diarrhea (79%, n=15/19) and grades 1–2 colitis (94%, n=18/19). The median total duration of irEnteritis symptoms in this cohort was 123 days (IQR 35–182). For 10 patients who had fecal calprotectin checked at the time of irEnteritis onset, the median was 134 µg/mg (IQR 49–241). Six patients had fecal calprotectin values available at the time of OCB cessation of whom 5 (83%, n=5/6) had normalization to <50 µg/g. Finally, five patients underwent evaluation for celiac disease serologies of whom one patient had an elevated tissue transglutaminase IgA (71.7 units/mL) and was treated additionally with a gluten-free diet.
Table 2. Immune-related enteritis (irEnteritis) clinical, endoscopic, and histological findings (n=19).
| Characteristics | No. of patients |
| Days from ICI therapy initiation to irEnteritis onset, median (IQR) | 138 (50–186) |
| Presenting associated symptoms | |
| Diarrhea | 19 (100%) |
| Abdominal pain | 9 (47%) |
| Nausea/vomiting | 14 (74%) |
| Anorexia/early satiety | 15 (79%) |
| Weight loss (kg) from irEnteritis onset to nadir, median (IQR) | 6 (4–8) |
| Highest CTCAE grade of weight loss | |
| I (<10% total body weight) | 12 (63%) |
| II (10%–20% total body weight) | 5 (26%) |
| III (>20% total body weight) | 2 (11%) |
| Highest CTCAE grade of anorexia* | |
| I | 2 (11%) |
| II | 13 (68%) |
| III | 4 (21%) |
| Highest CTCAE grade of nausea† | |
| I | 2 (11%) |
| II | 14 (74%) |
| III | 3 (15%) |
| Highest CTCAE grade of abdominal pain‡ | |
| None | 4 (21%) |
| I | 3 (16%) |
| II | 12 (63%) |
| Highest CTCAE grade of diarrhea§ | |
| I/II | 15 (79%) |
| III/IV | 4 (21%) |
| Highest CTCAE grade of colitis¶ | |
| I/II | 18 (94%) |
| III/IV | 1 (6%) |
| Pre-OCB fecal calprotectin, median (IQR) (n=10) | 134 (49–241) |
| Post-OCB fecal calprotectin normalization** (n=6) | 5 (86%) |
| Duration (days) of irEnteritis, median (IQR) | 123 (35–182) |
| Hospitalization for irEnteritis | 6 (32%) |
| Adverse events on systemic corticosteroids prior to OCB (n=15) | 6 (40%) |
| Days of continuous steroids prior to OCB, median (IQR) | 46 (15–99) |
| Days from last biological dose to OCB, median (IQR) | 64 (14–158) |
| Days from irEnteritis onset to first endoscopy, median (IQR) | 34 (19–95) |
| Endoscopic evaluation | |
| Sigmoidoscopy only | 2 (11%) |
| EGD only | 4 (21%) |
| Colonoscopy and EGD | 8 (42%) |
| Sigmoidoscopy and EGD | 5 (26%) |
| EGD endoscopic findings (n=17) | |
| Normal | 2 (12%) |
| Gastritis | 3 (18%) |
| Duodenitis | 6 (35%) |
| Gastritis and duodenitis | 5 (29%) |
| Esophagitis, gastritis, and duodenitis | 1 (6%) |
| EGD endoscopic severity (n=17) | |
| Normal mucosa | 5 (29%) |
| Erythema | 4 (24%) |
| Marked erythema and edema | 5 (29%) |
| Erosions/ulcerations | 3 (18%) |
| EGD histological findings (n=17) | |
| Duodenitis | 6 (35%) |
| Gastritis and duodenitis | 9 (53%) |
| Esophagitis, gastritis, and duodenitis | 2 (12%) |
| Lower endoscopy Mayo score (n=15) | |
| 0 | 8 (53%) |
| 1 | 4 (27%) |
| 2 | 3 (20%) |
| Lower endoscopy histological findings (n=15) | |
| Active colitis | 2 (13%) |
| Chronic active colitis | 2 (13%) |
| Microscopic colitis (lymphocytic/collagenous) | 3 (20%) |
| GVHD-like/apoptosis-predominant colitis | 2 (13%) |
| Normal mucosa | 6 (40%) |
| GI toxicity location | |
| Upper GI toxicity alone | 11 (58%) |
| Combined upper and lower GI toxicity | 8 (42%) |
| Small bowel inflammation | |
| Noted on EGD | 17 (89%) |
| Suspected but no EGD | 2 (11%) |
| Treatments for irEnteritis prior to OCB | |
| None/symptomatic | 2 (11%) |
| Systemic corticosteroids | 15 (79%) |
| Standard budesonide | 3 (16%) |
| Biological agents | 4 (21%) |
Definitions of CTCAE anorexia grading: I – —loss of appetite without alteration in eating habits; II – —oral intake altered without significant weight loss or malnutrition; III – —associated with significant weight loss or malnutrition.
Definitions of CTCAE nausea grading: I – —loss of appetite without alteration in eating habits; II – —oral intake decreased without significant weight loss, dehydration, or malnutrition; III – —inadequate oral caloric or fluid intake.
Definitions of CTCAE abdominal pain grading: I – —mild pain; II – —moderate pain, limiting instrumental ADL.
Definitions of CTCAE diarrhea grading: I – —increase of <4 stools/day over baseline, II – —increase of 4–6 stools/day over baseline, III – —increase of ≥7 stools/day over baseline, IV – —life-threatening consequences.
Definitions of CTCAE colitis grading: I – —asymptomatic, clinical or diagnostic observations only; II – —abdominal pain, mucus or blood in stool; III – —severe abdominal pain, peritoneal signs; IV – —life-threatening consequences.
Normal fecal calprotectin levels are defined by our lab as <50 mcgµg/gm.
CTCAE, Common Terminology Criteria for Adverse EventsEGD, esophagogastroduodenoscopy; GVHD, graft-versus-host disease; ICI, immune checkpoint inhibiitor; IQR, interquartile range; irEnteritis, immune-related enteritis; OCB, open-capsule budesonide
Endoscopic and histological findings
All 19 patients underwent endoscopic evaluation via lower endoscopy (colonoscopy or flexible sigmoidoscopy), EGD, or a combination of the two for evaluation of suspected immunotherapy-related GI toxicity. Endoscopic and histological characteristics are summarized in table 2. 17 of 19 (90%) patients underwent EGD with the most common finding being isolated duodenitis (35%, n=6/17). On histological assessment of patients with upper GI biopsies, all 17 patients had evidence of enteritis, thereby prompting the indication for treatment with OCB as opposed to standard budesonide EC or MMX formulations used for ileocolitis. Concomitant gastritis was noted in 11 patients (65%, n=11/17) and concomitant gastritis and esophagitis in 2 patients (12%, n=2/17). Enteritis severity was varied with nearly half of patients found to have marked erythema and edema (29%, n=5/17) or erosions and/or ulcerations (18%, n=3/17) on endoscopic evaluation.
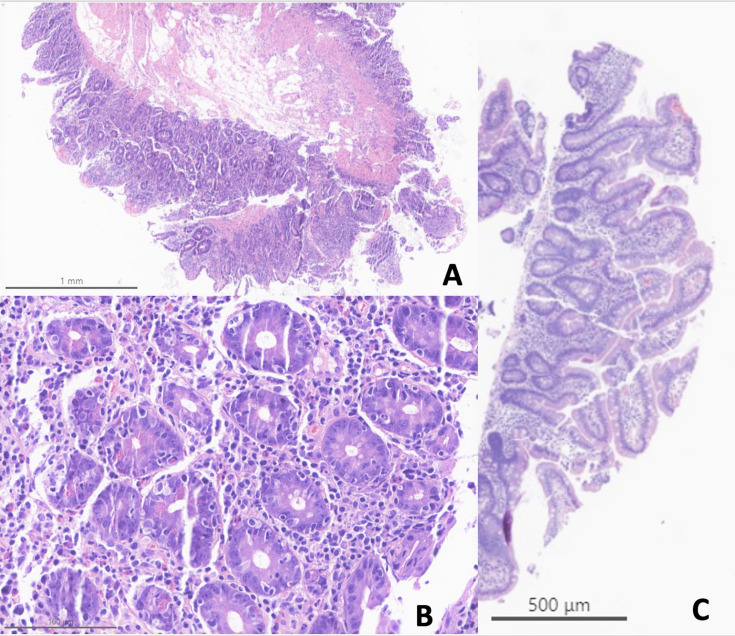
Of the 15 patients who underwent lower endoscopy, histological evidence of colitis was noted in 9 (60%) patients. Of note, the two patients in this cohort without an EGD underwent endoscopic evaluation via flexible sigmoidoscopy, which is often performed in our clinical practice as part of the initial consultation for suspected immune-related colitis, which demonstrated normal endoscopic and histological colonic findings. The first patient presented with diarrhea, abdominal pain, anorexia, and 15-lb weight loss over 3 weeks after ICI initiation. The second patient presented with grade 3 diarrhea, frequent nausea and vomiting, anorexia, and a 6-lb weight loss over 2 months after ICI initiation. Due to their constellation of upper GI symptoms (anorexia, weight loss) and lack of endoscopic or pathological evidence of colitis, both patients were treated empirically with OCB for suspected irEnteritis. Both patients ultimately achieved clinical response, remission, and corticosteroid-free remission on OCB. Thus, upper GI toxicity alone was proven or suspected in 11 patients (58%, n=11/19) while combined upper and lower GI toxicity was shown in the remaining 8 (42%). Figure 1 shows representative histological images from one patient (patient #7) before and after OCB treatment.
Figure 1. Representative histological images of immune-related enteritis pretreatment and post-treatment with open-capsule budesonide. (A) (Patient #7, pretreatment): Jejunal biopsies with near complete villous blunting and marked expansion of the lamina propria by a mixed inflammatory cell infiltrate. (B) (Patient #7, pretreatment): Higher power examination shows active neutrophilic infiltration, apoptotic bodies and intraepithelial lymphocytes, the classic triad typically seen in immunotherapy enteritis. (C) (Patient #7, post-treatment): Post-treatment jejunal biopsy with normalization of villous architecture and complete resolution of the abnormal inflammatory lamina propria infiltrate.
Systemic immunosuppression
Prior to initiating OCB, 15 of 19 (79%) patients had received systemic corticosteroids for irEnteritis including eight (42%) intravenously (table 2). Eight of 15 (53%) patients were systemic corticosteroid-dependent (defined as symptomatic relapse within 3 months of tapering or stopping systemic corticosteroid therapy) while the remaining 7 (47%) demonstrated non-response to systemic corticosteroids. Of these 15 patients, 6 (40%) also reported adverse events associated with their systemic steroid regimen, including impaired glycemic control (n=2), skin striae and thinning (n=2), worsening hand tremor (n=1), and myopathy (n=1).
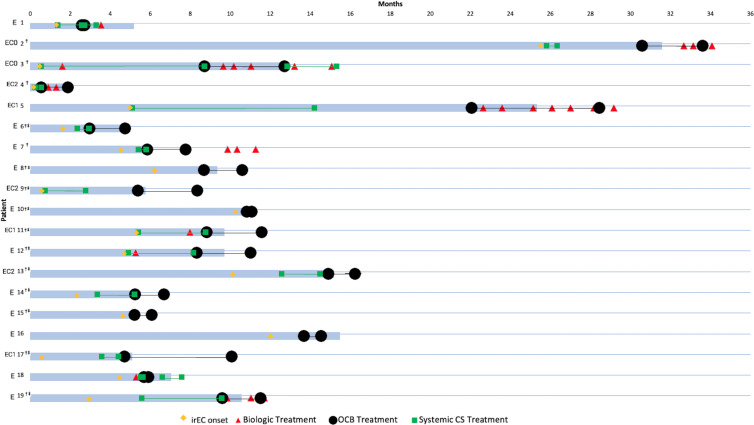
Each patient’s treatment history with systemic corticosteroids and/or biological agents in relation to their OCB treatment duration and overall irEnteritis clinical course is shown in figure 2. The median number of days of continuous systemic corticosteroids prior to OCB was 46 (IQR 15–99) and almost half (47%, n=7/15) had been on systemic steroids for over a month before starting OCB. In all these cases except one, systemic corticosteroids were discontinued the same day that OCB was started. In this patient (patient #1), he continued his pre-existing systemic corticosteroids for 18 overlapping days. Although this patient initially attained clinical response, he relapsed on this dual steroid regimen and subsequently required one dose of infliximab to achieve symptom resolution. One other patient (patient #18) was started on a second course of systemic corticosteroids due to OCB treatment failure and attained symptom resolution. Furthermore, four patients (21%, n=4/19) received at least one dose of biological therapy with inadequate response prior to OCB with the median time from last biological dose to OCB treatment being 64 days (IQR 14–158).
Figure 2. Clinical presentation and immunosuppressive treatment regimens of patients treated with open-capsule budesonide (OCB) for immune-related enteritis (irEnteritis). Blue bar: Time from immune checkpoint inhibitor initiation to immune-related enteritis clinical remission. Yellow diamond: Time of irEnteritis onset. Black circles: OCB start and end dates. Green squares: systemic corticosteroid (CS) start and end dates. Red triangle: biological (infliximab or vedolizumab) treatment dates. E: Patient diagnosed with enteritis alone. EC#: Patient diagnosed with concomitant enteritis and colitis (Mayo score). †Patient achieved irEnteritis clinical remission on OCB. ‡Patient achieved irEnteritis corticosteroid-free clinical remission on OCB.
Six of 19 (32%) patients received vedolizumab at some point during and/or after OCB therapy. Three of these patients (patients #3, #5, and #19) were started on vedolizumab concurrent with OCB due to high irEnteritis symptom severity and received a range of 3–7 doses. The three other patients (patients #2, #4, and #7) attained initial clinical remission on OCB but later had relapse of irEnteritis subsequently treated with vedolizumab. Five (83%) of these six patients ultimately attained or reachieved clinical remission on a dual OCB-vedolizumab regimen. Notably, five of six (83%) patients achieved clinical response on OCB before receiving their first dose of biologics; the one remaining attained clinical response on OCB after four concurrent doses of vedolizumab. In these 5 patients, OCB therefore served as an effective bridge therapy while awaiting biological initiation.
Treatment and clinical course
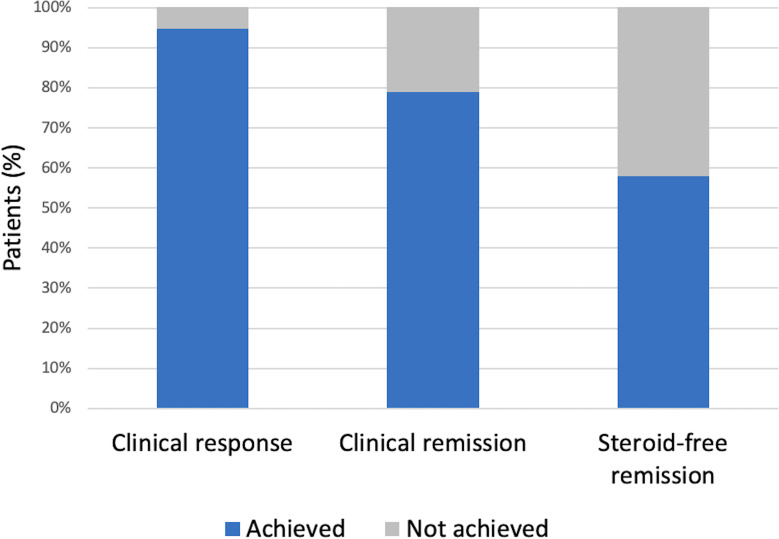
Treatment with OCB was initiated at a median of 62 days (IQR 27–134) after irEnteritis onset and 16 days (IQR 10–44) from last endoscopic evaluation (table 3). Clinical response was noted in 18 of 19 (95%) patients (figure 3). All 11 patients (100%) with isolated upper GI toxicity attributed to immunotherapy demonstrated clinical response while seven of eight patients (88%) with combined upper and lower GI toxicity attained response (p=0.26). Median time to clinical response was 4 days after OCB start (IQR 1–7 days) with 15 patients (79%, n=15/19) demonstrating response in ≤7 days. 15 of 19 (79%) patients demonstrated clinical remission with a median time to remission of 27 days (IQR 8–36 days). Patients with isolated upper GI toxicity and combined upper and lower GI toxicity demonstrated similar remission rates of 80% and 88%, respectively (p=0.67). Of the 15 patients attaining clinical remission, 5 (33%) underwent follow-up endoscopic evaluation after OCB initiation with pathological assessment confirming resolution of irEnteritis in all cases.
Table 3. Treatment outcomes with open-capsule budesonide (n=19).
| Characteristics | No. of patients |
| Clinical response | 18 (95%) |
| Days from OCB initiation to clinical response, median (IQR) | 4 (1–7) |
| Clinical remission | 15 (79%) |
| Days from OCB initiation to clinical remission, median (IQR) | 27 (8–36) |
| Corticosteroid-free remission | 11 (58%) |
| Restoration of pre-irEnteritis weight on OCB cessation | 14 (74%) |
| Weight gain (kg) from OCB initiation to cessation, median (range) | 4 (0–19) |
| Weight gain (kg) from irEnteritis nadir to remission, median (range) | 5 (1–19) |
| Days from irEnteritis onset to OCB initiation, median (IQR) | 62 (27–134) |
| Days from most recent endoscopic evaluation to OCB initiation, median (IQR) | 16 (10–44) |
| Adverse events from OCB | 1 (1%) |
| Duration (days) of OCB treatment, median (IQR) | 57 (26–89) |
| irEnteritis relapse after OCB treatment | 7 (37%) |
| Days from OCB cessation to irEnteritis relapse, median (IQR) | 26 (4–57) |
| Treatments for irEnteritis relapse on OCB (n=7) | |
| Systemic corticosteroids alone | 1 (14%) |
| Infliximab | 3 (43%) |
| Vedolizumab | 5 (71%) |
| Response after restarting OCB for irEnteritis relapse (n=2) | |
| Clinical response | 2 (100%) |
| Clinical remission | 1 (50%) |
irEnteritis, immune-related enteritis; OCB, open-capsule budesonide
Figure 3. Clinical outcomes following treatment with open-capsule budesonide for immune-related enteritis.
The median time from irEnteritis onset to weight nadir was 30 days (IQR 21–56). All 19 patients treated with OCB were noted to have gained weight from OCB initiation to cessation (median 4 kg) (table 3). Notably, 14 of 19 (74%) patients restored their pre-irEnteritis weight by the time of OCB cessation. Median time from OCB initiation to pre-irEnteritis weight restoration was 20 days (IQR 10–27). Adverse events from OCB were observed in one patient (1%, n=1/19) and included mild scalp and skin peeling, which self-resolved.
Median overall duration of OCB therapy was 57 days (IQR 26–89) (table 3). Corticosteroid-free remission was achieved in 11 patients (58%, n=11/19). There were no clinical or treatment characteristics that differentiated patients who attained corticosteroid-free remission from those who relapsed (online supplemental table 1). 70% of those presenting with upper GI ICI toxicity alone demonstrated remission compared with 50% with combined upper and lower GI toxicity (p=0.39). Among seven patients (37%, n=7/19) with irEnteritis relapse, median time from OCB cessation to relapse was 26 days (IQR 4–57). Therapeutic regimens targeting irEnteritis relapse were vedolizumab alone (57%, n=4/7), vedolizumab and infliximab (14%, n=1/7), infliximab alone (14%, n=1/7), and infliximab and systemic corticosteroids (14%, n=1/7). In two relapsing patients, a second regimen of OCB was also initiated. One patient recaptured clinical response and remission after 11 days and ultimately completed a 61-day course of OCB. The other recaptured clinical response after 9 days but experienced further irEnteritis relapse and OCB ultimately was discontinued after 43 days. She subsequently received three doses of vedolizumab with minimal response, then three doses of infliximab before achieving GI symptom resolution.
Discussion
In our study, we observed that OCB is an effective and safe treatment regimen for achieving both clinical response and remission in patients with irEnteritis with or without concurrent esophagitis, gastritis or colitis. All patients but one (95%) had a decrease in diarrhea frequency with 79% of patients ultimately restoring their baseline stool frequency with resolution of concomitant upper GI symptoms. Upper GI symptoms of ICI toxicity have received little attention, yet here we observed impressive resolution of symptoms including weight gain and frequent weight restoration after OCB treatment. OCB was well tolerated with only one mild, self-limited adverse event that did not lead to discontinuation of therapy. The beneficial impact of OCB is further emphasized by its success in this cohort of patients in which 75% had a prior history of systemic corticosteroid dependence and/or non-response as well as substantial irEnteritis-related weight loss. Importantly, over one-third of the patients in our cohort were able to remain on their ICI regimen after initiating treatment with OCB. Despite most of these patients ultimately discontinuing immunotherapy due to non-GI irAE reasons, it is encouraging OCB may allow some patients to complete their full treatment courses.
Clinical descriptions of upper GI toxicity attributed to immunotherapy have generally been limited to small retrospective analyses and case studies23,25 with even fewer data available on endoscopic and histological findings.26 27 However, upper GI inflammation was shown to occur in about 40% of ICI-treated patients in one study18 and may, therefore, represent an important cause of diarrhea in ICI-treated patients with or without concurrent colitis on lower endoscopy. Diarrhea was the most common clinical symptom reported in our cohort followed by nausea and vomiting, which is consistent with prior studies examining upper GI inflammation related to ICIs.26 27 Decreased appetite has also been posited as a potential clinical presentation of upper GI inflammation8; however, the total impact of irEnteritis on weight loss and the efficacy of various treatment regimens on weight gain has yet to be shown. In our study, we offer novel observations that demonstrate the high frequency (75%) of early satiety and anorexia as presenting symptoms in patients found to have irEnteritis as well as the significant weight loss experienced, with 40% of patients observed to have ≥10% total body weight loss from time of symptom onset to nadir. Additionally, we demonstrate how OCB impacts this understudied manifestation: 14 of 19 patients (74%) restored their pre-irEnteritis weight on cessation of OCB.
Immune-related enteritis has remained a critically understudied disease process, which may be potentially explained by the clinical challenge posed by its diagnosis. Diarrhea may predispose clinicians to workup the patient for immune-related colitis, a more common manifestation, while upper GI symptoms of nausea, anorexia, and weight loss may be incorrectly attributed to active cancer and/or concurrent chemotherapy, which may also prevent clinicians from accurately identifying irEnteritis. These difficulties are reflected in the available literature on the upper GI symptoms of ICI-related toxicity, which consist of relatively small case series and focus on patients who had EGD as part of their evaluation in which prevalence of GI inflammation was >70%.26 27 As such, the true prevalence of upper GI involvement among all patients with ICI-related diarrhea is unknown. Current gastroenterology and oncology society guidelines also provide extremely limited data regarding management of these rarer upper GI irAEs, often recommending use of similar therapies (eg, systemic corticosteroids) as are used for lower GI toxicity.8,1028 This approach reflects the lack of evidence for tailored treatment of irEnteritis but is also problematic due to the numerous and significant safety limitations of systemic corticosteroids described previously. Furthermore, while standard budesonide formulations, such as EC budesonide and budesonide-MMX, have previously been demonstrated to be an effective treatment for subsets of ICI-induced colitis such as microscopic colitis,18 29 the drug’s construction targets delivery to the distal the GI tract and so would likely be ineffective in treating more proximal enteritis.
To our knowledge, other than a prior case report featuring a single patient,30 this is the first analysis of OCB use for the treatment of irEnteritis. Prior studies have reported on the high clinical success rates of OCB used for the treatment of other small intestinal inflammatory pathologies such as refractory celiac disease19 and autoimmune enteropathy.20 These conditions demonstrate numerous similarities to irEnteritis due to their involvement of the mucosal layer of the small intestine and often indistinguishable histological appearances.27 We posited OCB’s therapeutic targeting of the small bowel would maximize its potent and directed anti-inflammatory effect while minimizing risks for systemic adverse effects and infection in patients with irEnteritis featuring small bowel inflammation. In accordance with budesonide’s minimal systemic absorption, cumulative toxicities well described with systemic corticosteroids, such as infections, hyperglycemia, osteoporosis and glaucoma among others are notably absent even after prolonged budesonide use for a year or more.31 32 An additional advantage with OCB is that the various steps described in the regimen allow for greater control over drug delivery localization and may allow for more targeted distribution of therapeutic effect depending on the segments of bowel involved.
We suggest that OCB’s optimal place in irEnteritis treatment algorithms is for patients presenting with high clinical suspicion for irEnteritis such as predominant upper GI symptoms including anorexia, early satiety, nausea, vomiting and diarrhea, ideally with endoscopic and/or histological confirmation of enteritis. These recommendations are in line with our data showing that in the seven patients with combined enteritis and colitis who ultimately achieved clinical remission, all received concomitant systemic corticosteroid and/or biological therapy prior to OCB that may have accounted for improvements in their colitis prior to OCB-related enteritis resolution. As such, while the presence of mild or microscopic concomitant colitis would still be appropriate for a trial of OCB, severe concomitant colitis should prompt consideration of more potent therapies such as biological agents rather than upfront treatment with OCB. In addition to histologically confirmed cases of irEnteritis, OCB may be ideally indicated as an empiric treatment for suspected irEnteritis in patients with negative lower endoscopic findings but with a constellation of the upper GI symptoms described above.
Furthermore, given that most patients in our study achieved clinical response within 7 days and clinical remission within 1 month, OCB is likely suitable for acute treatment of patients presenting with irEnteritis. While most patients in this study had received systemic corticosteroids prior to OCB initiation, future studies investigating OCB’s potential as a first-line therapy are warranted. Given its limited systemic absorption and evidence supporting its safety even for long term use, it is an attractive option for patients requiring longer-term irEnteritis therapy, such as those resuming ICI therapy after pausing due to enteritis who may benefit from concomitant use of gut-selective immunosuppressive strategies.33 Finally, these advantages and the importance of future investigation may be further amplified by the cost-effectiveness of OCB in potentially reducing the use of the more expensive biological agents.
There are several limitations to this study. As data were collected from retrospective review of patient health records, the exact timing of endpoints was not available in all cases. Endoscopic and histological assessments following treatment with OCB were also unavailable for most patients, limiting our ability to examine patient responses via these objective endpoints. However, weight restoration was a notable objective metric of treatment success that we observed in our study. Additionally, since OCB was added to ongoing treatment regimens featuring systemic corticosteroids and biological agents in some patients, we cannot exclude the possibility that the clinical improvements observed in these individuals may be attributable to these other treatments rather than OCB. However, the response parameters in our study were specifically measured from initiation of OCB, and as noted above, we observed clinical response to OCB alone in the majority of patients, even if they had previously used other agents or went on to use additional agents subsequently. Celiac disease was also only assessed in a minority of these patients. Since irEC has overlapping features with celiac disease, including immunotherapy-associated celiac disease,34 it is possible OCB was used to treat some patients with new-onset celiac disease. The small sample size also limited the performance of more extensive subgroup analyses to better understand the relationship between OCB efficacy and GI toxicity location. The instructions for the three-step administration process for OCB may also be unfamiliar to some patients, and so we emphasize the importance of clear and comprehensive patient education before initiating this therapy. Finally, the retrospective design and lack of a control group limits the definitive assessment of OCB treatment efficacy and real-world data such as our study are fundamentally more subject to confounding than the controlled environment of a randomized trial. Nonetheless, we believe in the novelty and potential practice-changing impact of even this small cohort in a rapidly emerging disease state, where no similar data or therapeutic options exist, and where controlled studies are challenging and unlikely to be performed in the near future.
In conclusion, OCB is effective and safe in inducing and maintaining clinical response and remission in patients with irEnteritis, including in those with prior inadequate response to systemic corticosteroids. Our results suggest that the use of enteric steroids targeting the sites most affected by inflammation can improve efficacy and minimize systemic toxicity, which makes it an attractive alternative to more intensive and higher-risk immunosuppressive regimens. Future prospective studies are needed to validate these findings and further elucidate OCB’s optimal indications, duration, and place in the irEnteritis treatment algorithm.
supplementary material
Footnotes
Funding: Grant support was received from the NIH/NCI Cancer Center–P30 CA008748.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: Deidentified data will be made available by the corresponding author on reasonable request.
Ethics approval: This study was approved by the Institutional Review Board at MSKCC (19-205A(6)) and patient consent was deemed not required due to the retrospective nature of the study.
Contributor Information
Patrick Tiongco Magahis, Email: patrickmagahis@gmail.com.
Tara Corso, Email: corsot@mskcc.org.
Pamela Livingstone, Email: livingsp@mskcc.org.
Erika Tom, Email: tome@mskcc.org.
Amitabh Srivastava, Email: srivasa2@mskcc.org.
Michael Postow, Email: postowm@mskcc.org.
David Faleck, Email: faleckd@mskcc.org.
Data availability statement
Data are available on reasonable request.
References
- 1.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dougan M, Luoma AM, Dougan SK, et al. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell. 2021;184:1575–88. doi: 10.1016/j.cell.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 4.Dougan M. Checkpoint blockade toxicity and immune homeostasis in the gastrointestinal tract. Front Immunol. 2017;8:1547. doi: 10.3389/fimmu.2017.01547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–42. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 6.Collins M, Soularue E, Marthey L, et al. Management of patients with immune checkpoint inhibitor-induced enterocolitis: a systematic review. Clin Gastroenterol Hepatol. 2020;18:1393–403. doi: 10.1016/j.cgh.2020.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Pauken KE, Dougan M, Rose NR, et al. Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol. 2019;40:511–23. doi: 10.1016/j.it.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougan M, Wang Y, Rubio-Tapia A, et al. AGA clinical practice update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis. Gastroenterology. 2021;160:1384–93. doi: 10.1053/j.gastro.2020.08.063. [DOI] [PubMed] [Google Scholar]
- 9.Thompson JA, Schneider BJ, Brahmer J, et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw. 2020;18:230–41. doi: 10.6004/jnccn.2020.0012. [DOI] [PubMed] [Google Scholar]
- 10.Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. JCO. 2021;39:4073–126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 11.Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of Ipilimumab-induced Hypophysitis is associated with reduced survival in patients with Melanoma. Cancer. 2018;124:3706–14. doi: 10.1002/cncr.31629. [DOI] [PubMed] [Google Scholar]
- 12.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell Death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–8. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 13.Thompson LL, Katznelson E, Leet DE, et al. Impact of systemic corticosteroids on survival outcomes in immune checkpoint inhibitor-induced gastroenterocolitis. Eur J Cancer. 2021;142:143–6. doi: 10.1016/j.ejca.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Abu-Sbeih H, Mao E, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6:37. doi: 10.1186/s40425-018-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Castillo M, Romero FA, Argüello E, et al. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of Melanoma. Clin Infect Dis. 2016;63:1490–3. doi: 10.1093/cid/ciw539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saag KG, Koehnke R, Caldwell JR, et al. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994;96:115–23. doi: 10.1016/0002-9343(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 17.Miehlke S, Acosta MB, Bouma G, et al. Oral Budesonide in gastrointestinal and liver disease: a practical guide for the clinician. J Gastroenterol Hepatol. 2018 doi: 10.1111/jgh.14151. [DOI] [PubMed] [Google Scholar]
- 18.Hughes MS, Molina GE, Chen ST, et al. Budesonide treatment for microscopic colitis from immune checkpoint inhibitors. J Immunother Cancer. 2019;7:292. doi: 10.1186/s40425-019-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukewar SS, Sharma A, Rubio-Tapia A, et al. Open-capsule Budesonide for refractory celiac disease. Am J Gastroenterol. 2017;112:959–67. doi: 10.1038/ajg.2017.71. [DOI] [PubMed] [Google Scholar]
- 20.Sharma A, Choung RS, Wang XJ, et al. Features of adult autoimmune enteropathy compared with refractory celiac disease. Clin Gastroenterol Hepatol. 2018;16:877–83. doi: 10.1016/j.cgh.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 21.Edsbäcker S, Bengtsson B, Larsson P, et al. A pharmacoscintigraphic evaluation of oral Budesonide given as controlled-release (Entocort) capsules. Aliment Pharmacol Ther. 2003;17:525–36. doi: 10.1046/j.1365-2036.2003.01426.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheung VTF, Gupta T, Olsson-Brown A, et al. Immune checkpoint inhibitor-related colitis assessment and prognosis: can IBD scoring point the way. Br J Cancer. 2020;123:207–15. doi: 10.1038/s41416-020-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Lin P, Ho EY, et al. Nivolumab-associated nausea and vomiting as an immune adverse event. Eur J Cancer. 2017;84:367–9. doi: 10.1016/j.ejca.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi M, Yamaguchi O, Nagata K, et al. Acute hemorrhagic Gastritis after Nivolumab treatment. Gastrointest Endosc. 2017;86:915–6. doi: 10.1016/j.gie.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Collins M, Michot JM, Danlos FX, et al. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol. 2017;28:2860–5. doi: 10.1093/annonc/mdx403. [DOI] [PubMed] [Google Scholar]
- 26.Tang T, Abu-Sbeih H, Luo W, et al. Upper gastrointestinal symptoms and associated endoscopic and histological features in patients receiving immune checkpoint inhibitors. Scand J Gastroenterol. 2019;54:538–45. doi: 10.1080/00365521.2019.1594356. [DOI] [PubMed] [Google Scholar]
- 27.Zhang ML, Neyaz A, Patil D, et al. Immune-related adverse events in the gastrointestinal tract: diagnostic utility of upper gastrointestinal biopsies. Histopathology. 2020;76:233–43. doi: 10.1111/his.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. JCO. 2018;36:1714–68. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic Melanoma. Melanoma Res. 2015;25:321–7. doi: 10.1097/CMR.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 30.Hussain N, Robert M, Al-Bawardy B. Open-capsule Budesonide for the treatment of isolated immune checkpoint inhibitor-induced Enteritis. ACG Case Rep J. 2022;9:e00882. doi: 10.14309/crj.0000000000000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tome J, Sehgal K, Kamboj AK, et al. Budesonide maintenance in microscopic colitis: clinical outcomes and safety profile from a population-based study. Am J Gastroenterol. 2022;117:1311–5. doi: 10.14309/ajg.0000000000001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Münch A, Bohr J, Miehlke S, et al. Low-dose Budesonide for maintenance of clinical remission in Collagenous colitis: a randomised, placebo-controlled, 12-month trial. Gut. 2016;65:47–56. doi: 10.1136/gutjnl-2014-308363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haanen J, Ernstoff M, Wang Y, et al. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: review of the literature and suggested prophylactic strategy. J Immunother Cancer. 2020;8:e000604. doi: 10.1136/jitc-2020-000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badran YR, Shih A, Leet D, et al. Immune checkpoint inhibitor-associated celiac disease. J Immunother Cancer. 2020;8:e000958. doi: 10.1136/jitc-2020-000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.