Abstract
Background
Human and mouse natural killer (NK) cells have been shown to develop memory-like function after short-term exposure to the cocktail of IL-12/15/18 or to overnight co-culture with some tumor cell lines. The resulting cells retain enhanced lytic ability for up to 7 days as well as after cryopreservation, and memory-like NK cells (mlNK) have been shown to induce complete remissions in patients with hematological malignancies. No single phenotype has been described for mlNK and the physiological changes induced by the short-term cytokine or tumor-priming which are responsible for these enhanced functions have not been fully characterized. Here, we have generated mlNK by cytokine and tumor-priming to find commonalities to better define the nature of NK cell “memory” in vitro and, for the first time, in vivo.
Methods
We initiated mlNK in vitro from healthy donors with cytokines (initiated cytokine-induced memory-like (iCIML)-NK) and by tumor priming (TpNK) overnight and compared them by high-dimensional flow cytometry, proteomic and metabolomic profiling. As a potential mechanism of enhanced cytolytic function, we analyzed the avidity of binding of the mlNK to NK-resistant tumors (z-Movi). We generated TpNK from healthy donors and from cancer patients to determine whether mlNK generated by interaction with a single tumor type could enhance lytic activity. Finally, we used a replication-incompetent tumor cell line (INKmune) to treat patients with myeloid leukaemias to potentiate NK cell function in vivo.
Results
Tumor-primed mlNK from healthy donors and patients with cancer showed increased cytotoxicity against multiple tumor cell lines in vitro, analogous to iCIML-NK cells. Multidimensional cytometry identified distinct memory-like profiles of subsets of cells with memory-like characteristics; upregulation of CD57, CD69, CD25 and ICAM1. Proteomic profiling identified 41 proteins restricted to mlNK cells and we identified candidate molecules for the basis of NK memory which can explain how mlNK overcome inhibition by resistant tumors. Finally, of five patients with myelodysplastic syndrome or refractory acute myeloid leukemia treated with INKmune, three responded to treatment with measurable increases in NK lytic function and systemic cytokines.
Conclusions
NK cell “memory” is a physiological state associated with resistance to MHC-mediated inhibition, increased metabolic function, mitochondrial fitness and avidity to NK-resistant target cells.
Keywords: Leukemia, Memory, Myelodysplastic Syndrome, Natural killer - NK, Adoptive cell therapy - ACT
WHAT IS ALREADY KNOWN ON THIS TOPIC
Natural killer (NK) cells with memory properties have been described in the context of viral infections; cytokine-induced memory-like NK cells; and tumor-memory NK cells. All of these share a common characteristic, that they show enhanced cytolytic and/or cytokine secretion responses on restimulation, many days after the initial cytokine or cellular stimulation has been removed. How these different NK cell types relate to each other is unknown as is the molecular signature of NK “memory”.
WHAT THIS STUDY ADDS
We described the commonalities on the initiation of memory-like NK (mlNK) cells generated by multicytokine stimulation and by tumor-mediated priming. These mlNK cells share common phenotypical clusters, proteomic changes, and metabolic function. This study also shows that tumor-mlNK cells can be generated in vivo in a clinical trial in patients with hematological malignancies.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Contributes to the understanding of NK cell immunological memory by expanding in the characteristics of tumor-mlNK cells and their use as a treatment for cancer.
Introduction
Natural killer (NK) cells mediate defenses against pathogens and cancer cells.1 During the past two decades, we have seen a paradigm shift with the observations of memory-like properties in NK cell subsets. First described as “tumor-activated” NK cells2 which retained ability to lyse NK-resistant tumor cells after cryopreservation and in absence of cytokines, these were followed by reposts of “adaptive” NK cells in the context of viral infections, where both murine and human NK cells show long-lasting, enhanced secondary responses against cytomegalovirus (CMV)-infected target cells compared with resting NK (rNK) cells.3,6 In addition, NK cells activated with the cytokine cocktail interleukin (IL)-12/15/18 showed enhanced proliferative capacity, cytokine secretion and cytotoxicity against various targets after a period of rest and consecutive ex vivo restimulation, and termed cytokine-induced memory-like (CIML) NK cells.7,9 These cytokine-induced NK cells appeared analogous to the cells described by our group2 10 in which the activated state was retained after cryopreservation; the cells being able to lyse NK-resistant tumor cell lines and primary tumor cells, overcoming inhibition by MHC molecules on the tumor cells. These tumor-primed, memory-like NK cells (TpNK), are characterized by a distinct genomic profile compared with resting and IL-2 activated NK cells and to NK cells exposed to other tumor cells such as K562.11 We isolated a unique subclone of cells from the CTV-1 parent cell line and named it INB16. These cells have been used throughout to generate TpNK in vitro and, after manufacture into a replication-incompetent drug product (INKmune), generated the same TpNK cells in vivo in our first human trial subject in patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) (NCT05933070).
The concept of mlNK is well established, but a phenotype has yet to be determined and the mechanisms underpinning the “memory” remain undefined. Both cytokine and tumor-induced mlNK are initiated by overnight stimulation by a cytokine cocktail or by relevant tumor cells. Here, we hypothesized that commonalities between mlNK immediately postinitiation by cytokine-priming (iCIML) and tumor-priming (TpNK) could be used to identify common characteristics of NK cell memory. We report that, despite the different mode of generation, these mlNK cells show shared phenotypic clusters characterized by populations of mature CD57high KIRhigh NK cells. Furthermore, proteomic analysis also identified shared protein changes within the two memory conditions, including proteins involved in NK cell cytotoxicity and DNA repair in response to hypoxia. Metabolomic analyses showed enhanced glycolysis by mlNK cells. These are characteristics of “fitter” NK cells that may overcome the suppressive tumor microenvironment (TME). We also identified unique differences between cytokine-primed and tumor-primed mlNK cells, specifically, the greater upregulation of mitochondrial survival proteins and nutrient receptors in TpNK cells and an associated increase in metabolic function. This culminates with our in vivo study, where we show that INKmune generates tumor-mlNK cells in vivo which gain NK lytic function and show a phenotype that resembles the one we identified in vitro. Here, we show that, despite different origins, memory-like NK cells that arise from tumor-priming show many shared characteristics with iCIML-NK cells and these may define NK “memory” and explain the physiological nature of the phenomenon.
Materials and methods
Additional methods can be found in online supplemental materials.
Patient samples
Peripheral blood samples from patients were taken into lithium heparin Vacutainer (BD Biosciences) and processed within 6–24 hours. Mononuclear cells were analyzed immediately or cryopreserved in 10% DMSO containing cryoprotectant media. Cryopreserved cells were recovered by rapid thawing at 37°C and resuspended in complete growth medium (CM) (RPMI 1640 GlutaMax supplemented with 10% v/v fetal bovine serum and 1% antibiotic-antimycotic (all from Life Technologies).
NK cell purification and culture
Freshly or frozen isolated NK cells were cultured in CM. We produced four types of NK cells from each donor; directly isolated NK cells were considered to be at rest (rNK). NK+IL15 cells were generated by addition of low dose IL-15 (1 ng/mL) (R&D Systems). To generate TpNK cells, rNK cells were co-incubated with INKmune at a 1:2 ratio. iCIML-NK cells were generated according to the protocol for clinical trial9 by priming with the cytokine cocktail IL-12 (10 ng/mL), IL-15 (1 ng/mL) and IL-18 (50 ng/mL) (R&D Systems). All NK cell stimulations were performed for 16 hours at 37 °C in CM. For some functional assays, overnight stimulation was removed on the second day by depleting dead cells with Dead Cell Removal Kit (Miltenyi Biotec) and positive NK cell isolation using CD56 MicroBeads (Miltenyi Biotec).
Cell culture
The following cell lines were used: K562, Raji, U266, SKOV-3, OVCAR (all from ATCC); 786-O, ACHN (kindly provided by Maxine G. B. Tran); MDS-L (kindly provided by Kaoru Tohyama); INB16 (kindly provided by INmuneBio Inc).
Flow cytometry
NK cell phenotype was evaluated using standard protocols for flow cytometry analysis by Novocyte/NovoExpress (Agilent) or Aurora (Cytek)/FlowJo (Tree Star). Isotype and fluorescence minus one controls were included in each experiment. Stochastic neighbor embedding (SNE) analysis and flowSOM analysis were performed in Cytobank (Beckman Coulter). Flow cytometry antibodies are available in online supplemental sTable 1.
Flow cytometry-based killing assay
Flow cytometric killing assays were performed by co-incubating rNK or TpNK cells as indicated with target cells labeled with PKH67 dye (Sigma Aldrich) at an effector and target (E:T) ratio of 5:1 for 4 hours at 37°C. The ovarian cancer patients’ NK cells were tested in hypoxia (1% O2).
Avidity measurements using z-Movie Cell Avidity Analyzer (Lumicks)
Target cells were adhered to a temperature-controlled microfluidic and co-incubated with CellTrace Far Red (ThermoFisher) labeled NK cells in the different conditions. At different time points, an acoustic force ramp was applied and displaced NK cells identified by automated fluorescence microscopy at a fixed focal point above the target cell monolayer. Data were analyzed by Oceon software (Lumicks) by automated counting of the % of cells detached.
Assessment of proteomics profile
Different types of stimulated NK cells were prepared as indicated. Cell pellets were analyzed by an independent third party (Biognosys AG, Switzerland) by mass spectrometry for proteome-wide protein profiling.
Extracellular flux assays
Different types of stimulated NK cells were prepared as indicated. Oxygen consumption rates (OCR) and extracellular acidification rate (ECAR) were measured at basal level and following the addition of inhibitors using an XFp Analyzer as indicated by the manufacturer (Seahorse XF Technology, Agilent). OCR and ECAR were normalized to cell count and cell viability per well using normalization function in Wave software.
Statistical analysis
Data analyses were performed using GraphPad Prism V.10.1 software. Graphs represent individual values±SD. Paired Student’s t-tests were used for paired comparisons and analysis of variance with post hoc analysis was used to compare more than two groups. Differences were considered significant at p<0.05, and p values are denoted with asterisks as follows: *p<0.05, **p<0.01, and ***p<0.001.
Results
Tumor-mlNK and cytokine-mlNK cells show a unique phenotypical metacluster containing mature NK cells
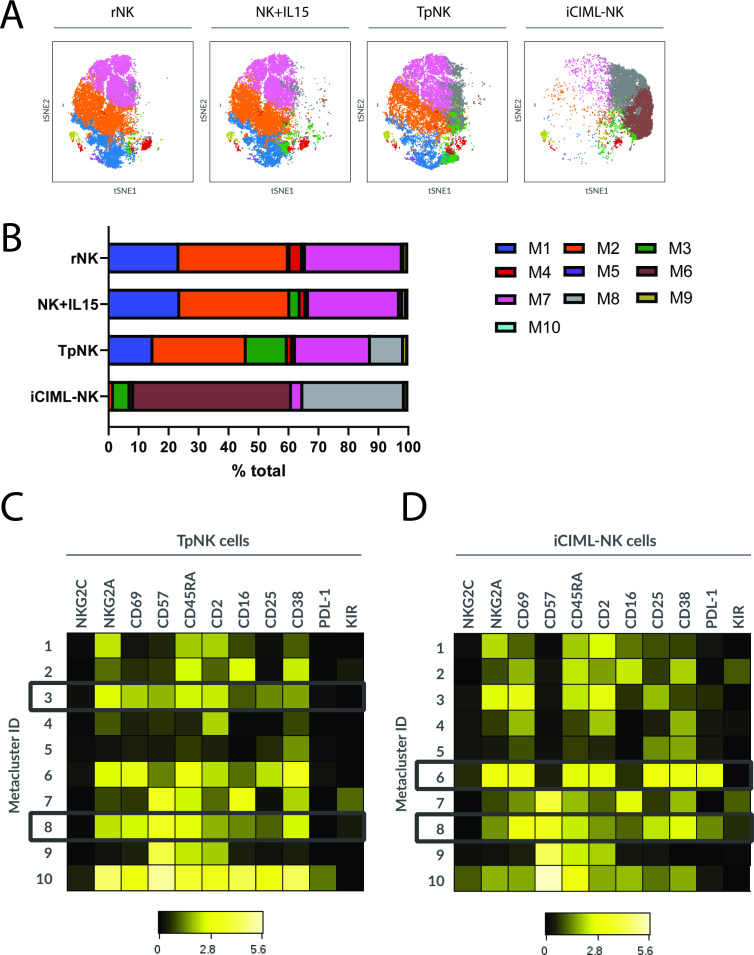
To investigate the phenotypic changes in NK cells following tumor-priming, we performed multiparameter spectral flow cytometry and compared it to the previously reported iCIML-NK9 by conventional flow cytometry analysis and using the viSNE algorithm (figure 1).
Figure 1. Tumor-mlNK and cytokine-mlNK cell display their own unique phenotype but show characteristics of activation. Freshly isolated peripheral blood NK cells from HD were incubated as indicated overnight at 37 °C. The next day, cells were washed and the expression of 30 different NK cell markers was analyzed using spectral flow cytometry. viSNE analysis of multiparametric flow data from three concatenated files was performed on the indicated markers. Each point on the viSNE map represents a single cell and color depicts intensity of protein expression. Graphs show the analysis of the % expression and MeFI of the positive population on the indicated markers. Bars represent the means±SD of three different donors. Comparisons were made between rNK and the different stimulatory conditions using a one-way ANOVA with a Dunnett’s multiple comparison test. Statistical significance is indicated as *p<0.05, **p<0.01, ***p<0.001. The absence of asterisk indicates non-significance. ANOVA, analysis of variance; HD, healthy donor; iCIML, initiated cytokine-induced memory-like; mlNK, memory-like natural killer cell; viSNE, visualization stochastic neighbor embedding.
As reported previously, tumor-priming of NK cells led to a significant upregulation of CD25, and CD69,11 and a small increase in the expression of CD39 (29% increase and 49% on TpNK and iCIML-NK cells, respectively). We also observed downregulation of CD16 in both TpNK and iCIML-NK cells. The adhesion molecule ICAM1 was upregulated in both mlNK cells, as well as the downregulation of CD62-L (figure 1). Interestingly, the percentage of NK cells expressing CD57 increased but there was a reduction in intensity. This suggests that tumor-priming may initiate maturation of CD57-ve NK cells,12 13 leading to a gradual increase in CD57 expression to levels associated with fully mature NK cells (figure 1 and online supplemental figure S1). As previously reported,9 iCIML-NK cells showed a significant upregulation of TRAIL; as well as an increase in PDL-1 expression (figure 1).
Unsupervised clustering analysis using the FlowSOM algorithm allowed us to identify metaclusters based on coexpression patterns, to identify phenotypical markers that are shared or acquired within the conditions.
We identified 10 metaclusters (figure 2A) with the changes in % cell numbers of each metacluster shown in figure 2B. iCIML-NK cells clustered in a very unique manner compared with the rest of the conditions with metaclusters 1, 2 and 7 considerably reduced frequency to 0.3%, 1.4% and 3.8%, respectively, and the expansion of a unique metacluster 6, which was not observed in rNK but represented 52.4% of the cells. Despite having a distinct clustering signature, iCIML-NK cells expanded metacluster 8 (from 0.63% in rNK to 33.8%), which was also expanded in TpNK cells (from 0.63% in rNK to 11.5% in TpNK) but not in the rest of the conditions. TpNK cells uniquely expanded metacluster 3, increasing from 0.53% in rNK to 13.74% (figure 2B).
Figure 2. Tumor-mlNK and cytokine-mlNK cells share a unique phenotypical metacluster containing mature NK cells. Freshly isolated peripheral blood NK cells from HD were incubated overnight at 37 °C as indicated. The next day, cells were washed and the expression of 30 different NK cell markers was analyzed using spectral flow cytometry. After that, viSNE and FlowSOM analysis clustered on 30 NK cell phenotypic markers was performed (A) viSNE plots of the different metaclusters across all conditions. (B) Stacked bars show changes in % cell numbers of each metacluster for every condition. (C, D) Heatmaps show fluorescence intensity of the total of cells on the indicated markers of metaclusters 1–10, with metaclusters of interest highlighted in (C) TpNK and (D) iCIML-NK. Plots show the data from three concatenated donors. HD, healthy donor; iCIML, initiated cytokine-induced memory-like; NK, natural killer; viSNE, visualization stochastic neighbor embedding.
Because of the relative expansion of metaclusters 3 and 8 in TpNK and 6 and 8 in iCIML-NK, we examined their phenotype further by analyzing the fluorescence intensity of each marker (figure 2C,D and online supplemental figure S2). Metacluster 3, expanded in TpNK, was characterized by high expression of CD45RA, CD2, CD57, CD69, NKG2A and medium CD38 (figure 2C and online supplemental figure S2). The expansion of a group of NK cells that highly express CD2 confirms our previous findings, where we defined the importance of the interaction of CD2-CD15 for the generation of TpNK.10 In metacluster 6, which was only expanded in iCIML-NK cells, cells were characterized by high expression of CD45RA, CD2, CD25, CD69, NKG2A, CD38 and PDL-1 (figure 2D and online supplemental figure S2); indicative of a highly cytotoxic phenotype. Metacluster 8 was restricted to TpNK and iCIML-NK (figure 2C,D and online supplemental figure S2) and defined by high expression of CD45RA, CD69, and CD38; and expression of KIRs. One of the most characteristic features of this metacluster was the high expression of CD57, which together with the acquisition of KIRs suggests that these cells are highly differentiated and mature.12 13
Metacluster 8 was present at very low, but detectable, frequencies in rNK and NK+IL15 and was expanded in the memory-like populations; suggesting that mlNK may be a physiologically normal subset in vivo. mlNK cells exhibit a unique proteomic profile characterized by the upregulation of mitochondrial survival proteins.
To further characterized the distinct types of mlNK cells, we next explored the proteomic profiles, comparing iCIML-NK, TpNK and NK+IL15 versus directly isolated rNK. In total, 9253 proteins represented by 204,407 peptide ion variants were quantified across all samples. Hierarchical clustering analysis using the Manhattan distance measure using all protein z-score values across all samples was carried out and the clustered data are displayed in a heat map (figure 3A) showing strong separation according to treatment group. We observed the differential abundance of 1420 proteins between samples from TpNK cells and untreated controls, and the volcano plot shows the 9253 proteins quantified with the red dots showing protein candidates significantly changed after exposure to INB16 (online supplemental figure S3).
Figure 3. Memory-like NK cells exhibit a unique proteomic profile characterized by the upregulation of mitochondrial survival proteins. Freshly isolated NK cells from three healthy donors were incubated overnight at 37°C as indicated. The next day, cells were prepared for mass spectrometry for proteome-wide protein profiling by removing dead cells and positively selecting NK cells to remove stimulatory agents. (A) Hierarchical clustering analysis using the Manhattan distance measure using all protein z-score values across all samples was carried out. (B) Venn diagram generated by the intersection list of the proteins significantly (p<0.001) upregulated by more than four log compared with NK cells incubated in medium alone. (C, D) Graph showing the average Log ratio from rNK in TpNK, iCIML-NK and IL-15 treated NK cells corresponding to (C) mitochondrial survival proteins and (D) lytic function related proteins. iCIML, initiated cytokine-induced memory-like; NK, natural killer; viSNE, visualization stochastic neighbor embedding.
We identified statistically significantly upregulated proteins (p<0.001) and with an absolute average log ratio of >4 log and compared the three conditions (figure 3B). Of the 249 proteins upregulated in TpNK, 41 were shared with iCIML-NK (online supplemental sTable 2) but not with NK+IL15 cells and may be characteristics of NK cell “memory”. Notable proteins were ADAM28, APOC2, FERM2, INF-G, LOXL3, RM14, RM21 and SL9A9; each of which may contribute to memory-like function.
Gene Ontology enrichment analysis identified 411 biological processes, 130 cellular components and 260 molecular functions significantly enriched in TpNK cells relative to untreated controls. The top 20 enriched biological processes in TpNK cells were those associated with mitochondrial function (online supplemental figure S4), and we observed how TpNK and iCIML-NK upregulated 60 and 36 proteins respectively (figure 3C). All stimulating conditions upregulated proteins related to lytic function equally (figure 3D). Previous studies on single cytokine-activated NK cells reported higher mTOR activity, the excessive activation of which can lead to mitochondrial fragmentation and damaged mitochondrial function.14 15 Thus, the ability of TpNK and, to a lesser extent, iCIML-NK cells to activate metabolic pathways that promote and restore mitochondrial health and survival may provide an opportunity for NK cell activation without mitochondrial damage, allowing them to survive the removal of cytokine support.
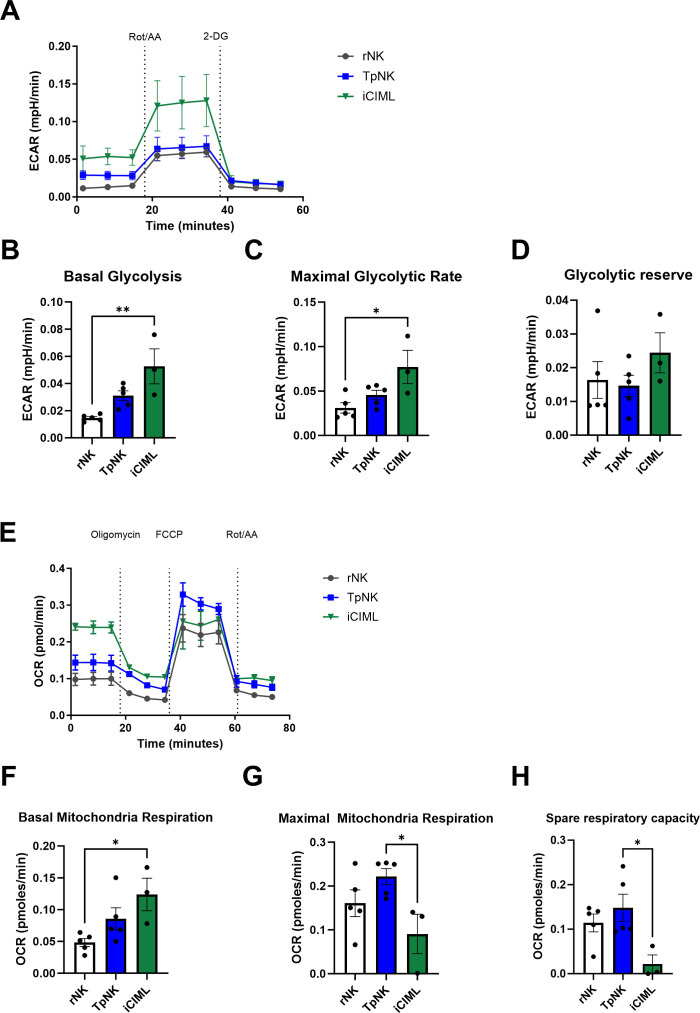
Tumor-priming increases spare respiratory capacity of NK cells
To investigate whether changes in mitochondrial survival proteins translate into improved metabolic fitness, we evaluated NK cell metabolism in real time by measuring glycolysis and mitochondrial respiration of NK cells in a Seahorse assay. The two main energy-producing pathways that cells use are glycolysis and oxidative phosphorylation (Oxphos); rNK cells mostly use Oxphos to meet their energy needs but when activated, they strongly upregulate both glycolysis and Oxphos pathways.15,17 This allows the NK cells to increase their energy production in parallel with the synthesis of molecules needed for effector functions.15 17 18 First, we estimated the ECAR, a measure of lactate production and glycolysis at the basal state and after the addition of rotenone and antimycin A, which interfere with complex I and complex III of the electron transport chain, respectively, and 2-deoxy-glucose (2-DG), a competitive inhibitor of glycolysis in resting compared with TpNK and iCIML-NK cells (figure 4A). As expected, iCIML-NK cells had higher basal glycolysis and maximal glycolytic rate compared with resting and TpNK cells, yet maintaining their glycolytic reserve (figure 4B–D). Next, we assessed mitochondrial respiration by measuring OCR, a measure of Oxphos, at basal levels and following the addition of a stressor mix (figure 4E). iCIML-NK cells exhibited higher basal OCR compared with both resting and TpNK cells (figure 4F). However, maximal respiration was not significantly increased, and spare respiratory capacity (SRC) was repressed in iCIML-NK cells (figure 4G,H). By contrast, TpNK cells showed higher maximal respiration and higher SRC suggestive of better metabolic fitness compared with iCIML-NK cells (figure 4H).
Figure 4. Tumor-priming increases spare respiratory capacity of NK cells Freshly isolated NK cells were incubated overnight at 37°C as indicated. The next day, cells were prepared by removing dead cells and positively selecting NK cells to remove stimulatory agents. After that, (A–D) glycolytic function and (E–H) mitochondrial respiration were measured according to manufacturer’s protocol in Seahorse XF platform (Agilent). Data were normalized to cell count and cell viability per well using normalization function. Bars represent the means±SD of five different donors. Comparisons were made between all conditions using a one-way ANOVA with a Tukey multiple comparison test. Statistical significance is indicated as *p<0.05, **p<0.01. The absence of asterisk indicates non-significance. ANOVA, analysis of variance; NK, natural killer.
Tumor-primed NK cells from healthy donors and patients with cancer lyse NK-resistant hematological and solid tumor cells
One of the hallmarks of NK cell memory is the enhanced cytotoxicity of these cells on a second encounter with a target. It has been described that adaptive NK cells display enhanced antibody-dependent cell cytotoxicity and IFN-γ secretion after second-time exposure, this has been observed in NK cells from CMV+ individuals but also in HIV and hepatitis C infected individuals.519,21 CIML-NK cells are also characterized by their enhanced IFN-γ secretion and antitumor cytotoxicity.7,9 We and others have described that tumor-priming of NK cells results in NK cells with memory-like properties characterized by enhanced cytotoxicity on exposure to a second target.2 11 22
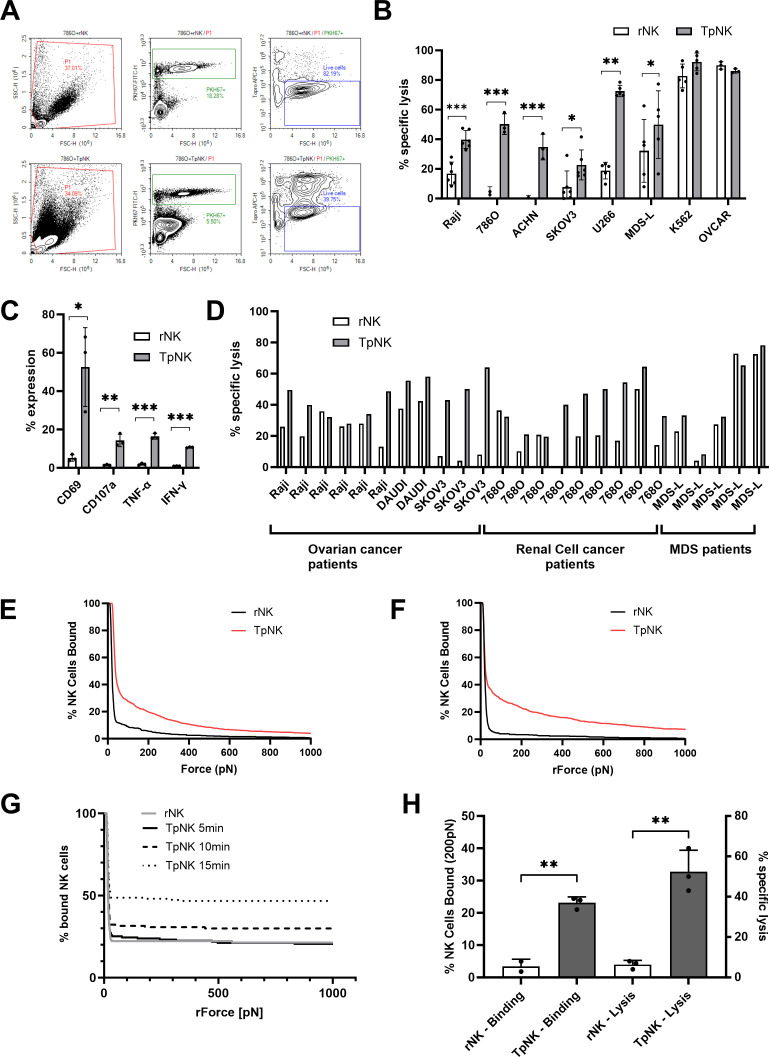
We next wanted to further characterize the cytotoxic capacity of TpNK cells. In line with our previous published data,2 10 11 23 NK cells from HD primed with INB16 showed enhanced lysis in vitro of tumor cell lines of hematologic cancers (Raji, U266, MDS-L), renal cell carcinoma (786O and ACHN), and ovarian cancer (SKOV3) (figure 5A,B). TpNK did not show enhanced killing of NK-sensitive cell lines (K562 and OVCAR) (figure 5B). Tumor-priming was associated with rapid upregulation of CD69 and evidence of low-level degranulation (CD107a expression) and induction of IFN-γ and tumor necrosis factor (TNF)-α secretion (figure 5C). Furthermore, TpNK cells also showed the ability to proliferate on a 5-day assay (online supplemental figure S5), similar to what has been reported on CIML-NK cells8 and adaptive NK cells.3
Figure 5. Tumor-primed NK cells from healthy donors and cancer patients lyse hematological and solid tumor cells. (A) Representative example of flow cytometry plots for cytotoxicity assay against 786O. Top plot shows the gating strategy for rNK cells and bottom plot for TpNK cells. NK cells and target cells are gated in P1; in the second plot, target cells are gated as PKH67+; in the third plot, live cells are gated as Topro−. Cell counts are obtained from the ‘Live cells gate’. (B, C) Freshly isolated peripheral blood NK cells from HD were incubated as indicated overnight at 37 °C. (B) The next day, cells were harvested and set-up for a 4h-cytotoxicity assay against hematological cancer cell lines (Raji, U266, MDS-L and K562), renal cell carcinoma (786-O and ACHN) and ovarian cancer (SKOV3 and OVCAR) at E:T ratio 5:1. Bars represent the means±SD of 3–6 different donors represented by the individual dots. (C) Surface and intracellular expression of different markers was analyzed in NK cells the next day by flow cytometer. Bars represent the means±SD of 2–3 different donors. (D) Freshly isolated peripheral blood NK cells from patients were incubated overnight at 37°C as indicated. The ovarian cancer patient NK cells were isolated from the ascites and were tested in hypoxia (1% O2). The next day, cells were harvested and set-up for a 4h-cytotoxicity assay as described against hematological cancer cell lines (Raji, K562 and MDS-L), renal cell carcinoma (786-O) and ovarian cancer (SKOV3) at E:T ratio 5:1. To measure avidity (E–H), NK cells from HD were stimulated overnight as indicated. The next day, chips were coated and target cells were seeded. NK cells were prepared by removing overnight stimulatory agents and labeled using CellTrace Far Red dye, before placing into the chips. (E, F) Avidity measure of (E) Raji and (F) SKOV3 at 10 min. (G) Avidity measure of Raji at various timepoints. (H) Avidity of Raji conjugation was compared with lytic function. For measuring cytotoxicity, rNK and TpNK cells from the same donor were set-up for a 4h-cytotoxicity assay against Raji at E:T ratio 5:1. Bars represent the means±SD of three different donors. (B, C, H) Data were tested for normality and comparisons were made between rNK and TpNK cells using the paired t-test. Statistical significance is indicated as *p<0.05, **p<0.01, ***p<0.001. The absence of asterisk indicates non-significance. E:T, effector and target; HD, healthy donor; NK, natural killer.
Next, we evaluated responses in NK cells isolated from patients with ovarian cancer (n=6), clear cell renal cell cancer (n=9) and MDS (n=5), at rest and after priming with INB16 (figure 5D). NK cells were isolated from peripheral blood except those from patients with ovarian cancer, where NK cells were isolated from ascites and tested in hypoxia (1% O2) to recapitulate the peritoneal microenvironment. Raji cell lysis was enhanced in three out of six patients with ovarian cancer and these NK cells also acquired the ability to lyse the NK-resistant SKOV3 ovarian cancer cell line (p<0.0001) following INB16 priming (figure 5D). Peripheral blood NK cells from patients with clear cell renal cell carcinoma were tested against the 786-O cell line, which was completely resistant to lysis by rNK cells from HD (figure 5B). Notably, patients’ rNK cells showed higher lytic activity against 786-O than HD NK cells but, in 7/9 cases, this lysis was significantly enhanced by INB16 priming. In line with our previously published data,24 the MDS patients fell into two groups with respect to NK cell function. In 4/6 cases, the patients showed low rNK cell function which could be enhanced by INB16 priming. In contrast, 2/6 patients had rNK cells which showed high level killing of the MDS-L cell line and this was not enhanced by tumor-mediated priming (figure 5D).
To further investigate the mechanisms behind the increased lytic function induced by mlNK cells, we analyzed the formation of the synapse between TpNK cells and a range of tumor cell lines. The Z-Movi analyses the avidity of the binding between E:T cells in real time where the avidity of the immune synapse is proportional to the amount of force required to disrupt the effector cells. We used Raji and SKOV3 cells as known “NK-resistant” target cells. For Raji cells, it was apparent that rNK cells from healthy donors formed very weak synapses with almost 100% disruption with as little as 50pN of acoustic force. In contrast, over 40% of TpNK cells remained attached to the Raji cells at 50pN and over 10% remained attached to target cells even at the maximal force of 1000pN (figure 5E). Similar results were obtained when using SKOV3 (figure 5F), where the formation of a stable immune synapse by TpNK was rapid and sustained. Next, we determined the temporal dynamics of TpNK conjugation to Raji cells at 5, 10 and 15 min compared with rNK from the same donors at 15 min (figure 5G). Stable synapse formation was already apparent at 10 min and increased substantially over the next 5 min to form stable conjugates which resisted even maximal acoustic disruption. The formation of a stable and mature immunological synapse is a critical step in the successful delivery of lytic granules into the target cell.25 We, therefore, studied how avidity and lysis correlate within the same donor NK cells against Raji cells. We observed low levels of lysis and avidity by rNK cells which was overcome after tumor-priming, where both avidity and lytic capacity dramatically increased (figure 5H). The ability to lyse, otherwise resistant, tumor cells is a hallmark of mlNK and this increased avidity may be a result of the upregulation of FERM2 seen in the proteomics analysis, which is a regulator of integrin activation.
INKmune generates NK cells with features of immunological memory in vivo in patients with MDS and AML
Our group has used tumor-priming of allogeneic NK cells previously in two phase I/II, open-label trials of adoptive immunotherapy in patients with AML in partial remission (PR) or in complete remission (CR) but with a high risk of relapse.26 27 Allogeneic NK cells from haploidentical donors were incubated with an INB16 lysate that was removed. After that, NK cells were cryopreserved, shipped and infused into the patients 1 day after completion of lymphoreductive chemotherapy. These were the first trials of an adoptive NK cell therapy that did not require cytokine support. A total of 19 patients were treated in the two trials with one patient with chemoresistant AML achieving CR which was sustained for over 11 months, three patients achieving CR and two patients remaining relapse-free for 32.6 to 47.6+months.
Having shown previously that NK cell function in patients with AML28 and MDS24 is predictive of duration of remission, we postulated that activating endogenous NK cells in patients with AML/MDS, in whom NK cell function is defective, could boost their activity and improve overall survival. Tumor-priming of endogenous NK cells in vivo is being trialed currently in patients with advanced MDS or multiply relapsed AML (NCT05933070). Patients receive three, weekly, infusions of a replication-incompetent preparation of the INB16 cell line (INKmune). These patients receive no lymphoreductive conditioning chemotherapy and no cytokine support; resulting in a treatment which is well tolerated and compatible with an elderly patient cohort.
Here, we present the data from one patient with advanced, trilineage MDS and two patients with treatment-refractory AML who have completed treatment and follow-up for 4 months (table 1). All patients received three doses of 1×108 INKmune on days 1, 8 and 15 and we performed a longitudinal assessment of NK cell activation and lytic function throughout treatment (figure 6A). The frequencies of activated NK cells increased in all patients until day 29 (figure 6B) when monitoring of the compassionate cases ended. The MDS trial patient was followed according to protocol and showed increased percentage of activated NK until the end of monitoring on day+119, over 100 days after the last INKmune treatment.
Table 1. Summary of clinical characteristics of the three patients treated with INKmune.
| Subject | Code | Age | Diagnosis | Stage at treatment | Outcome post-treatment |
| MDS#01 | L003 | Patient in their 70s | MDS-EB2Tri-lineage dysplasia | Tri-lineage dysplastic. Failed 16 cycles of Azacytidine. 19% BM blasts, RAD21 VAF 40%.Weekly red cell and platelet transfusionsECOG 2. | Improvement from ECOG2 to ECOG 0.Discharged from hospital with reduced platelet and RBC transfusion dependence.Alive >2 years. |
| AML#01 | C01 | Patient in their 20s | AML | Congenital GATA2 mutation. Relapsed post 3x allogeneic-HSCT. 8 months as in-patient with cytopaenias, blood product dependent and infections. | Stabilized counts at 2 months, reduced bone pain, neutrophil recovery and discharged home 3 weeks post completion of treatment.Disease recurrence at 6 months and died. |
| AML#02 | C02 | Patient in their 20s | AML-M6 | Relapsed post 2x haploidentical m/m HSCT—5% blasts. | Counts stabilized for 4 months allowing discharge to home. Bridged to third allogeneic HSCT. |
AML, acute myeloid leukemia; BM, bone marrow; EB, excess blasts; ECOG, Eastern Cooperative Oncology Group; HSCT, hematopoietic stem cell transplant; MDS, myelodysplastic syndrome; RBC, red blood cells; VAF, variant allele frequencies
Figure 6. INKmune generates memory-like NK cells in vivo in patients with myelodysplastic syndrome and acute myeloid leukemia. (A) Infographic representing treatment course. Patients receive three infusions of INKmune on days 1, 8 and 15. Blood samples are taken on days 1, 8, 15, 29, 43, 73 and 119. The sample from patient AML#01 on day 140 is from the bone marrow. (B) Percentage of CD69+NK cells in each patient on the indicated dates. (C, D) Percentage of specific lysis of (C) Raji and (D) K562 mediated by the patients NK cells on the indicated days. (E, F) Histograms show the % expression of CD57, CD2 and granzyme B on the indicated days. Heatmap represents median fluorescence intensity of the positive population normalized to the lowest value for each column for patients (E) MDS#01 and (F) AML#01. MDS, myelodysplastic syndrome; NK, natural killer.
Increases in NK cell activation were confirmed by coincident enhancement of lytic activity against the Burkitt’s lymphoma cell line Raji in all three patients after treatment, which was maintained on days 119 in patient MDS#01 and, in bone marrow at day 140 on patient AML#01 (figure 6C). Enhanced lysis was also observed against the K562 cell line in patient MDS#01 up to day 119, and AML#01 on day 140 (figure 6D).
Phenotypical analysis of patient MDS#01 (figure 6E and online supplemental figure S6) and AML#01 (figure 6F and online supplemental figure S6) showed increased intensity of expression of CD57 and CD2. This mirrors the effect that we had previously observed in vitro in the generation of tumor-memory NK cells, where TpNK cells expanded metacluster 8, which was CD57high, and metacluster 3, which was CD2high (figure 2). Furthermore, we observed how both patients had NK cells with granules that were not armed with granzyme B, which increased throughout the course of the treatment, in fluorescence intensity in MDS#01 and in % expression in AML#01. It is known that patients with MDS have NK cells that are deficient in their killing machinery,29 here we observe that this dysfunction can be reversed by INKmune treatment.
We also examined the cytokine and chemokine profile (Cytokine 30-Plex Human Panel, ThermoFisher) in longitudinal serum samples from patient MDS#01. Levels of IL-15, TNF, MIP-1 and MIP-1 increased until day 29 to then droped on day 43. Similarly, IL-8 secretion increased with each INKmune infusion and fell to pretreatment level by day 43 (online supplemental figure S7), suggesting a systemic in vivo effect by tumor-priming.
This result suggests that treatment with INKmune is safe and well tolerated. Furthermore, INKmune can not only reach the NK cells of the patient in vivo but also generates TpNK cells, akin to our observations in vitro. To our knowledge, this is the first-time NK cells with features of tumor-memory have been generated in vivo and sustained without the need for cytokine support. These cells generated in vivo attained the ability to lyse NK-resistant tumor cells in vitro, and express high levels of CD57 and CD2, which are shared with mlNK.
Discussion
The use of cytokines including IL-2, IL-12, IL-15 to activate NK cells in vivo and, more often, ex vivo for clinical use has a long history. Cytokine infusions with IL-2 and IL-12 have been associated with unacceptable toxicity or limited potency. Most recently, IL-15 and functional analogs such as IL-15RA have shown a more favorable side effect profile/better tolerability and some clinical benefit. However, no single cytokine treatment has been shown to generate memory-like NK cells, which appear to be the most potent mediators of antitumor cytotoxicity. In contrast, exposure of NK cells to the triple cytokine combination of IL-12/15/18 or to certain tumor cell lines appears to initiate the acquisition of memory-like phenotype. Here, we show the initiation of tumor-mlNK cells both in vitro and in vivo by exposure to the pharmaceutical-grade tumor cell line, INB16, and compare these with those generated with the cytokine cocktail of IL-12/15/18 to identify commonalities which may define NK memory. We have previously reported the generation of TpNK and their ability to enhance lysis of NK-resistant cancer cell lines2; this recall response has been described by others in the context of cancer, where NK cells exposed to the combination of cytokines IL12/15/18 during a short period of time have an increased recall response when exposed to tumor cells7 8; and also in the context of tumor-priming, where NK cells primed with a tumor cell line were capable of lysis after a second re-exposure.22 The TpNK cells described here resemble iCIML-NK cells, with enhanced capacity to kill a broad spectrum of tumor cell lines not limited to the priming tumor.
Phenotyping data show that both mlNK cell types exhibit traits of cytotoxic NK cells, marked by upregulations of CD25, CD69, ICAM1, and the loss of CD16; nonetheless, iCIML-NK appear to have a more activated phenotype, characterized by an increase in TRAIL. CD39 is known to be expressed on tumor infiltrating T cells and, to a lesser extent on NK cells. Recently this marker has been found to be expressed on iCIML-NK30 and we have confirmed this finding on iCIML-NK and, now TpNK. CD39, with its partner ectonucleotidase CD73, is involved in a co-relationship with heme in the upregulation of ICAM-1 and other adhesion molecules31 32 which we have found to be upregulated on both ml-NK cell types. The appearance of a new metacluster “8” in both the mlNK cell types characterized by CD57high and KIRhigh, despite the different origin of generation, is interesting and suggests that these cells are mature and terminally differentiated.12 13 As a distinguishing element, TpNK cells show expansion of metacluster “3” which is characterized by high expression of CD2, further confirming the importance of the CD2-CD15 interaction in tumor-priming.10
We used proteomic analysis to determine whether there is a proteomic “fingerprint” of NK cell “memory” by comparing TpNK and iCIML-NK versus rNK and NK+IL15. Analysis of upregulated proteins by more than 4 log in the two memory populations compared with NK+IL15 identified 41 proteins shared by iCIML-NK and TpNK.
APOC2 was upregulated in both TpNK and iCIML-NK but not by single cytokine stimulation. Secretion of APOC2 by mlNK cells will increase the availability of fatty acids as nutrients, which are used as substrates for oxidation and energy production, membrane synthesis, energy storage and production of signaling molecules and, therefore, are essential for cell survival and proliferation both under normoxia and hypoxia.33 Fatty acid metabolism has to be modified under hypoxia and alternative sources of fatty acid precursors have to be exploited. In tumor cells, which usually have to grow in a hypoxic microenvironment, these hypoxia-mediated changes in lipid metabolism are especially important in order to maintain the high proliferation rate that characterizes cancer cells.
LOXL3 expression in mlNK may also be part of the memory fingerprint since the disruption of STAT3 dimerization by LOXL3 is likely to enhance NK cell lysis and DNAM-1 expression. In line with this, STAT3-KO mice show increased DNAM-1 expression and increased NK-mediated tumor-cell lysis.34
A number of upregulated proteins shared by both mlNK cell types are associated with DNA repair arising from oxidative stress (NEIL3, RL27, RL38, TYSY) which may be involved in resistance to intratumoral hypoxia. Others, such as RM14 and RM21, are involved in mitochondrial metabolism.
The upregulation of SLC9A9 is particularly intriguing and, we hypothesize, has a major role in generation of mlNK characteristics. This protein not only regulates endosome maturation but is an important promotor of transferrin receptor recycling. Resting NK cells do not express transferrin receptors (TfR) but they are known to be upregulated by activation with IL-2 and the increase in lytic function by addition of IL-2 was further enhanced by the addition of transferrin. Blockade of TfR with monoclonal antibody abrogated this effect.35 The mechanism for this may be via the inhibitory C-type lectin, KLRG1. High expression of TfRs appears to sequester KLRG1 and impair binding to cadherins on tumor cells, thus removing the inhibitory signals through KLRG1 ITIM.36 Cytokine-mediated NK cell activation induces TfR expression, but the recycling of TfRs by SLC9A9 appears unique to mlNK cells since it was not induced by IL-15 in this study, nor by IL-2 in our previous work.11 The continuous expression of TfRs on mlNK would remove a major inhibitory signal and lower the threshold for activation by natural cytotoxicity receptors. The resistance to KIR-mediated, MHC-induced suppression is a shared characteristic between TpNK and iCIML-NK.
A notable differentiation of TpNK and iCIML-NK cells from NK+IL15 cells was the substantial upregulation of mitochondrial survival proteins which may indicate enhanced mitochondrial fitness. Recently, it has been shown that iCIML-NK cells have an accumulation of unfit mitochondria, characterized by an increase of mitochondrial superoxide levels.37
Within the two mlNK cultures, TpNK cells were found to have greater SRC compared with iCIML-NK cells. Mitochondria with a higher SRC are more capable at responding to ATP demands that occur during cellular stress. Furthermore, SRC is increased in adaptive NK cells,38 and it is also important in T cell memory formation.39 Our results show that iCIML-NK cells increase their glycolytic rate on stimulation but have reduced maximal mitochondria respiration and SRC. Work by Terren and colleagues found that iCIML-NK cells have enhanced SRC and Oxphos after being stimulated by IL12/15/18, but these returned to basal levels after a period of rest.40 Interestingly, continuous IL-15 stimulation of NK cells has been shown to decrease NK cell survival, diminishing mitochondrial function and SRC, a profile consistent with exhaustion.41 Overall, these findings suggest that TpNK cells may have a survival advantage especially within the hostile TME.42 43 Future work is required to fully elucidate the metabolic requirements of TpNK cell populations.
There have been many NK cell-clinical trials in both solid and hematological cancers.44 In the context of AML/MDS, several clinical trials have used allogeneic NK cells which have shown a good safety profile and where CRs have been achieved in some patients.45,47 The infusion of cytokines in order to sustain NK cells in vivo continues to be common practice but adds clinical complexity and needs careful monitoring for adverse effects.
We have reported previously, in two clinical trials in AML, that infusions of INB16 primed haploidentical NK cells can lead to sustained in vivo function without the need for cytokine support. This offers an alternative to the use of IL-2, IL-15 or IL-15RA.26 27 Here, we presented the results of three patients treated with intravenous infusions of INKmune with no conditioning and no cytokine support. Doses were well tolerated and clinical improvement was seen in all three. The patients’ NK cell phenotype mirrors the one observed in vitro, characterized by upregulations of CD57 and CD2 and together with the enhancement in NK cell function we report the generation of NK cells with characteristics of immunological memory in vivo. The concept of “NK cell memory” is well established but it is apparent that NK cells with memory function can be derived through more than one mechanism. This may be due to the memory characteristics arising in different NK cell subsets or through different molecular pathways depending on the way in which memory is induced. Memory-like NK cells have been studied extensively in the context of CMV and CIML-NK cells but the idea that mlNK cells can arise from tumor-priming remains far less studied. Here, we show that, despite different origin, TpNK and iCIML-NK cells share similar characteristics both phenotypically and at the proteomics level, and speculate that memory-like properties arise from those groups of cells. Irrespective of the mechanism of generation of mlNK, the subsequent enhanced ability to kill tumor cells is a common factor. Our data identify some important differences but also shared features between mlNK types, in relation to resting cells, which could provide a useful definition of the proteomic signature of NK cell memory.
supplementary material
Acknowledgements
We would like to thank K. Da Costa for her support with high-parameter flow cytometry and Dr Elizabeth Payne, her nursing team and her MDS patients for provision of peripheral blood samples. Biognosys AG was responsible for the proteomics primary data analysis including identifying the protein inventory and calculations of the protein ratios, candidates and intensities.
Footnotes
Funding: This work was supported by grants from the Royal Free Hospital Charity—PhD Studentship Award number 183398 (HA-B), the Myrovlytis Trust—Award number MTT23_2 (MWL), INmuneBio—contracted research through UCL Consultants (MWL) and NIH award R01AI55182 (DP).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Ethics approval: This study involves human participants and patient samples were acquired after informed consent and following ethical approval by the RFH/UCL Research Tissue Bank (UK National Research Ethics IRAS 63407—project codes NC2020.15, NC2021.03). Obtaining blood from healthy donors was approved by the University College London—Royal Free Hospital Biobank Ethical Review Committee (NC.2015.019). The clinical trial monitoring samples (MDS#01) were taken as part of an ethically approved UK national trial of INKmune in MDS/AML (NCT05933070) while the samples from patients with multiply relapsed AML were from patients treated compassionately after local institutional ethical approval. Participants gave informed consent to participate in the study before taking part.
Contributor Information
Helena Arellano-Ballestero, Email: h.arellano@ucl.ac.uk.
Agnieszka Zubiak, Email: a.zubiak@ucl.ac.uk.
Chris Dally, Email: christopher.dally@nhs.net.
Kim Orchard, Email: orchak55@icloud.com.
Aljawharah Alrubayyi, Email: aljawharah.alrubayyi@linacre.ox.ac.uk.
Xenia Charalambous, Email: x.charalambous@ucl.ac.uk.
Melina Michael, Email: melina.michael@ucl.ac.uk.
Robert Torrance, Email: robert.torrance.21@ucl.ac.uk.
Trinity Eales, Email: trinity.eales.19@alumni.ucl.ac.uk.
Kushal Das, Email: k.das@lumicks.com.
Maxine G. B. Tran, Email: m.tran@ucl.ac.uk.
May Sabry, Email: may.sabry.10@alumni.ucl.ac.uk.
Dimitra Peppa, Email: d.peppa@ucl.ac.uk.
Mark W. Lowdell, Email: m.lowdell@ucl.ac.uk.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.North J, Bakhsh I, Marden C, et al. Tumor-primed human natural killer cells Lyse NK-resistant tumor targets: evidence of a two-stage process in resting NK cell activation. J Immunol. 2007;178:85–94. doi: 10.4049/jimmunol.178.1.85. [DOI] [PubMed] [Google Scholar]
- 3.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nat New Biol. 2009;457:557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Vergès S, Milush JM, Schwartz BS, et al. Expansion of a unique CD57 +NKG2C Hi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108:14725–32. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–74. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Béziat V, Liu LL, Malmberg J-A, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121:2678–88. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper MA, Elliott JM, Keyel PA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–9. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romee R, Schneider SE, Leong JW, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–60. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8:357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabry M, Tsirogianni M, Bakhsh IA, et al. Leukemic priming of resting NK cells is killer IG-like receptor independent but requires CD15-mediated CD2 ligation and natural cytotoxicity receptors. J Immunol. 2011;187:6227–34. doi: 10.4049/jimmunol.1101640. [DOI] [PubMed] [Google Scholar]
- 11.Sabry M, Zubiak A, Hood SP, et al. Tumor- and cytokine-primed human natural killer cells exhibit distinct Phenotypic and transcriptional signatures. PLoS One. 2019;14:e0218674. doi: 10.1371/journal.pone.0218674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Björkström NK, Riese P, Heuts F, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56Dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116:3853–64. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Vergès S, Milush JM, Pandey S, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56DimCD16+ NK-cell subset. Blood. 2010;116:3865–74. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng X, Qian Y, Fu B, et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat Immunol. 2019;20:1656–67. doi: 10.1038/s41590-019-0511-1. [DOI] [PubMed] [Google Scholar]
- 15.Keating SE, Zaiatz-Bittencourt V, Loftus RM, et al. Metabolic reprogramming supports IFN-Γ production by CD56Bright NK cells. J Immunol. 2016;196:2552–60. doi: 10.4049/jimmunol.1501783. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Guan D, Wang S, et al. Glycolysis and oxidative Phosphorylation play critical roles in natural killer cell receptor-mediated natural killer cell functions. Front Immunol. 2020;11:202. doi: 10.3389/fimmu.2020.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnelly RP, Loftus RM, Keating SE, et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193:4477–84. doi: 10.4049/jimmunol.1401558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keppel MP, Saucier N, Mah AY, et al. Activation-specific metabolic requirements for NK cell IFN-Γ production. J Immunol . 2015;194:1954–62. doi: 10.4049/jimmunol.1402099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Amran FS, Kramski M, et al. An NK cell population lacking FcRγ is expanded in chronically infected HIV patients. J Immunol. 2015;194:4688–97. doi: 10.4049/jimmunol.1402448. [DOI] [PubMed] [Google Scholar]
- 20.Oh JS, Ali AK, Kim S, et al. NK cells lacking FcεRIγ are associated with reduced liver damage in chronic hepatitis C virus infection. Eur J Immunol. 2016;46:1020–9. doi: 10.1002/eji.201546009. [DOI] [PubMed] [Google Scholar]
- 21.Peppa D, Pedroza-Pacheco I, Pellegrino P, et al. Adaptive reconfiguration of natural killer cells in HIV-1 infection. Front Immunol. 2018;9:474. doi: 10.3389/fimmu.2018.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal M, Schwab L, Yermakova A, et al. Tumor-priming converts NK cells to memory-like NK cells. Oncoimmunology. 2017;6:e1317411. doi: 10.1080/2162402X.2017.1317411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katodritou E, Terpos E, North J, et al. Tumor-primed natural killer cells from patients with multiple myeloma Lyse Autologous, NK-resistant, bone marrow-derived malignant plasma cells. Am J Hematol. 2011;86:967–73. doi: 10.1002/ajh.22163. [DOI] [PubMed] [Google Scholar]
- 24.Tsirogianni M, Grigoriou E, Kapsimalli V, et al. Natural killer cell cytotoxicity is a predictor of outcome for patients with high risk myelodysplastic syndrome and Oligoblastic acute myeloid leukemia treated with Azacytidine. Leuk Lymphoma. 2019;60:2457–63. doi: 10.1080/10428194.2019.1581935. [DOI] [PubMed] [Google Scholar]
- 25.Orange JS. Formation and function of the Lytic NK-cell immunological Synapse. Nat Rev Immunol. 2008;8:713–25. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kottaridis PD, North J, Tsirogianni M, et al. Two-stage priming of allogeneic natural killer cells for the treatment of patients with acute myeloid leukemia: a phase I trial. PLoS ONE. 2015;10:e0123416. doi: 10.1371/journal.pone.0123416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fehniger TA, Miller JS, Stuart RK, et al. A phase 1 trial of CNDO-109–activated natural killer cells in patients with high-risk acute myeloid leukemia. Biol Blood Marrow Transplant. 2018;24:1581–9. doi: 10.1016/j.bbmt.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowdell MW, Craston R, Samuel D, et al. Evidence that continued remission in patients treated for acute leukaemia is dependent upon Autologous natural killer cells. Br J Haematol. 2002;117:821–7. doi: 10.1046/j.1365-2141.2002.03495.x. [DOI] [PubMed] [Google Scholar]
- 29.Hejazi M, Manser AR, Fröbel J, et al. Impaired cytotoxicity associated with defective natural killer cell differentiation in myelodysplastic syndromes. Haematologica. 2015;100:643–52. doi: 10.3324/haematol.2014.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran J, Foltz JA, Wong P, et al. Mechanisms of cytokine-induced NK cell therapy: IL-12/15/18 induce a unique transcriptional, epigenetic, and functional human NK cell memory program. Blood. 2023;142:2067. doi: 10.1182/blood-2023-182435. [DOI] [Google Scholar]
- 31.Wagener FA, Feldman E, de Witte T, et al. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E Selectin in vascular endothelial cells. Proc Soc Exp Biol Med. 1997;216:456–63. doi: 10.3181/00379727-216-44197. [DOI] [PubMed] [Google Scholar]
- 32.Lee GR, Shaefi S, Otterbein LE. HO-1 and CD39: it takes two to protect the realm. Front Immunol. 2019;10:1765. doi: 10.3389/fimmu.2019.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mylonis I, Simos G, Paraskeva E. Hypoxia-inducible factors and the regulation of lipid metabolism. Cells. 2019;8:1–16. doi: 10.3390/cells8030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotthardt D, Putz EM, Straka E, et al. Loss of STAT3 in murine NK cells enhances NK cell–dependent tumor surveillance. Blood. 2014;124:2370–9. doi: 10.1182/blood-2014-03-564450. [DOI] [PubMed] [Google Scholar]
- 35.Shau HY, Shen D, Golub SH. The role of transferrin in natural killer cell and IL-2-induced cytotoxic cell function. Cell Immunol. 1986;97:121–30. doi: 10.1016/0008-8749(86)90381-3. [DOI] [PubMed] [Google Scholar]
- 36.Schweier O, Hofmann M, Pircher H. KLRG1 activity is regulated by association with the transferrin receptor. Eur J Immunol. 2014;44:1851–6. doi: 10.1002/eji.201344234. [DOI] [PubMed] [Google Scholar]
- 37.Terrén I, Sandá V, Amarilla-Irusta A, et al. IL-12/15/18-induced cell death and mitochondrial dynamics of human NK cells. Front Immunol. 2023;14:1211839. doi: 10.3389/fimmu.2023.1211839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cichocki F, Wu C-Y, Zhang B, et al. ARID5B regulates metabolic programming in human adaptive NK cells. J Exp Med. 2018;215:2379–95. doi: 10.1084/jem.20172168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearce EL, Poffenberger MC, Chang CH, et al. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terrén I, Orrantia A, Mosteiro A, et al. Metabolic changes of Interleukin-12/15/18-stimulated human NK cells. Sci Rep . 2021;11:1–15. doi: 10.1038/s41598-021-85960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felices M, Lenvik AJ, McElmurry R, et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight. 2018;3:1–14. doi: 10.1172/jci.insight.96219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kouidhi S, Noman MZ, Kieda C, et al. Intrinsic and tumor microenvironment-induced metabolism adaptations of T cells and impact on their differentiation and function. Front Immunol. 2016;7 doi: 10.3389/fimmu.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang C-H, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabry M, Lowdell MW. Killers at the crossroads: the use of innate immune cells in adoptive cellular therapy of cancer. Stem Cells Transl Med. 2020;9:974–84. doi: 10.1002/sctm.19-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curti A, Ruggeri L, D’Addio A, et al. Successful transfer of Alloreactive Haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–9. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 46.Vela M, Corral D, Carrasco P, et al. Haploidentical IL-15/41BBL activated and expanded natural killer cell infusion therapy after salvage chemotherapy in children with relapsed and refractory leukemia. Cancer Lett. 2018;422:107–17. doi: 10.1016/j.canlet.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 47.Bachanova V, Cooley S, Defor TE, et al. Clearance of acute myeloid leukemia by Haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood . 2014;123:3855–63. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.