Abstract
Ex vivo lung perfusion (EVLP) is a promising technology that allows the re-evaluation of donor lungs and has the potential to improve marginal lung reconditioning. The present study focused on the effects of milk fat globule epidermal growth factor 8 (MFG‐E8) on the function of donation after circulatory death (DCD) lungs during EVLP and transplant reperfusion. Domestic swine were assigned to 4 groups. In the control group, the donor lungs lacking warm ischemia were preserved in Perfadex for 4 h. The swine in the other three groups underwent hypoxic arrest, followed by 1 h of warm ischemia. The DCD lungs were procured and randomly divided into three groups: cold static preservation (DCD-CSP) group, DCD-EVLP group, and DCD-MFG-E8 group. The left lung of all groups was transplanted and reperfused. During EVLP and reperfusion, lung functions and pathological evaluations were performed. Treatment with MFG-E8 resulted in significantly improved blood oxygenation. The mean pulmonary artery pressure, peak airway pressure, and expression of IL-1β, IL-6, and IL-12 were significantly lower but IL-10 was higher in the DCD -MFG-E8 group. Furthermore, the lung injury severity score, pulmonary edema, and wet-to-dry weight ratio were also reduced in MFG-E8-treated lungs. However, the pulmonary vascular resistance and expression of TNF-α did not differ from the DCD -EVLP group but were significantly lower than in the DCD -CSP group. Adding MFG-E8 into the perfusate during EVLP obtains optimal graft function of lungs from DCD. This finding, if confirmed clinically, can be applied to recondition grafts and expanded use of DCD lungs.
Keywords: MFG-E8, EVLP, Lung transplantation, Marginal donor lungs
1. Introduction
Lung transplantation (LTx) is a lifesaving treatment for patients with end-stage pulmonary disease. However, its benefit is constrained by a significant graft shortage. Additionally, there is only 15–20 % of lungs from eligible multiorgan donors that are considered ideal for transplantation. To solve this problem, donation after cardiac death (DCD) donors are used in many transplant centers [1]. Despite its promising potential, DCD lung brings a higher incidence of primary graft dysfunction (PGD) and decreases early post-transplant outcomes compared to conventional donors [2]. Therefore, improving DCD lung quality before transplantation is a promising strategy.
Ex vivo lung perfusion (EVLP) is a platform of normothermic lung perfusion for graft evaluation, reconditioning, preservation ex vivo, and it has the potential to recruit lung grafts thought unsuitable for transplantation [3]. Recently, the prospect of EVLP has been proved in clinical trials of marginal donor lungs. However, EVLP itself can result in organ damage due to coagulation abnormalities and inflammation. In addition, EVLP alone cannot improve donor lung function pathologically [4]. To overcome this problem, various modalities have been implemented during EVLP.
Milk fat globule-EGF factor 8 (MFG-E8), also called lactadherin, is an anti-inflammatory glycoprotein secreted by the mammary gland and expressed in various types of cells. It has been demonstrated by our lab and others to attenuate inflammation and acute lung injury via its engaging αvβ3 integrin [5]. Moreover, MFG‐E8 expression levels in plasma were manifestly lower in patients with chronic obstructive pulmonary disease [6]. Our recent studies have shown that recombinant human MFG-E8 (rhMFG-E8) can suppress thrombosis in an animal model of deep venous thrombosis by inhibiting inflammatory factors in TLR4/NF-κB signaling pathways [7]. Furthermore, We have also demonstrated that MFG-E8 improves lung graft function and ameliorates pulmonary graft ischemic reperfusion injury by inhibiting pulmonary apoptosis [8]. However, whether MFG-E8 can improve the function of lungs from non–heart-beating donors is currently unclear.
In the current study, we aimed to investigate whether MFG-E8 treatment during EVLP improves the function of lung grafts harvested from a swine DCD model and to address whether this protective effect continues after LTx.
2. Materials and methods
2.1. Animals and experimental design
The present study protocol was approved by the Animal Care and Use Committee (ACUC) of Huazhong University of Science and Technology. All animals received humane care per the “Guidelines for the Care and Use of Laboratory Animals” (National Institutes of Health, Publication No. 86-23, revised in 1996).
Domestic swine of both genders (weight, 21–38 kg) were euthanized and randomly divided into four groups of 8 pigs each. The Heart-Beating Donor (HBD) Group served as the Control Group, the donor lungs lacking warm ischemia were procured, flushed, and preserved conventionally in 4 °C Perfadex(a low potassium dextran solution) for 4 h. In the three DCD Groups, the swine underwent hypoxic arrest, followed by 1 h of warm ischemia. The DCD lungs were procured and randomly divided into three groups: cold static preservation (DCD-CSP) group (preserved in 4 °C Perfadex solution for 4 h), DCD-EVLP group (4 h of normothermic ex vivo perfusion circuit perfused with Steen solution), and DCD-MFG-E8 group (4 h of normothermic ex vivo perfusion circuit perfused with Steen solution supplemented with hMFG-E8 20 μg). The left lungs of all subjects of all groups were subsequently transplanted into a size-matched recipient and reperfused for a continuous 4-h period in vivo.
2.2. Porcine arrest and donor lung procedure
Ketamine (50 mg/kg, YAOPHARMA, China) and xylazine (5 mg/kg, ASIA TALENT CHEMICAL LIMITED) were utilized for induction of anesthesia, and the swine were intubated and ventilated (inspired oxygen fraction (FiO2) 1.0, tidal volume 8–10 mL/kg, respiratory rate 14–18 breaths/min, positive end-expiratory pressure 5.0 cmH2O). Anesthesia was maintained with 3 % isoflurane (ecochem international chemical broker). In the three DCD groups, all donor swine had continuous cardiac monitoring until cardiac arrest occurred. Cardiac arrest was defined as no activity in the electrocardiogram, and the distinction between systolic and diastolic pressure became zero. The endotracheal tube was occluded and disconnected from the ventilator right after an Arterial blood gas measurement was taken, and the swine were euthanized by hypoxic arrest. After the declaration of cardiac death, the pigs were left untouched for 60 min at room temperature, and donor lungs were procured.A median sternotomy was performed and the main pulmonary artery (PA) was cannulated with a Cardioplegia cannula to deliver Prostaglandin-E1 (10 mg/kg) and cold Perfadex (XVIVO Perfusion Inc). The left atrial appendage was incised, the vena cavae were ligated, and the lungs were antegrade flushed with 1.5 L liters of 4 °C Perfadex supplemented with 10,000 IU of heparin. The trachea was clamped mid-inspiration, and the heart-lung block was excised. The heart was discarded, and the lungs were retrograde flushed with an additional 500 mL of cold Perfadex (4 °C) with heparin. For the DCD-CSP group, the lungs were preserved directly in 4 °C Perfadex. For the other groups, the trachea, main pulmonary artery, and left atrium were prepared to be cannulated with EVLP.
2.3. EVLP
The EVLP system was prepared and conducted in conformity with the Toronto protocol. The EVLP circuit consisted of a centrifugal pump (Maquet Cardiopulmonary AG, Germany), a membrane oxygenator, a heat exchanger, a venous reservoir (Sorin Group, Arvada, Colo), and polyethylene tubing. The perfusate of EVLP comprised 2L of acellular Steen solution supplemented with heparin (10,000 IU), methylprednisolone (500 mg), and cefazolin (500 mg). hMFG-E8 (20 μg, SantaCruz, Shanghai, China) was added to the perfusate of group DCD-MFG-E8 at the start of the ex vivo perfusion period. A funnel-shaped plastic cannula was sutured to the left atrial cuff, a routine plastic cannula was sewn to the main pulmonary artery, and an 8-0 endotracheal tube was secured into the trachea. Flow was initiated slowly at 150mL/min at 20 °C, and then the perfusate temperature was gradually increased to 37 °C over 30 min as the flow was titrated up to 40 % of the estimated cardiac output (100 mL/kg). As the perfusate temperature reached 32 °C, lungs were ventilated with room air (FiO2 of 0.21) with the ventilator settings (respiratory rate 7 breaths/min, tidal volume 7 mL/kg, positive end-expiratory pressure 5.0 cmH2O). Simultaneously, mixed gas (a mixture of 86 % nitrogen, 6 % oxygen, and 8 % carbon dioxide) was used to deoxygenate the perfusate and maintain a PCO2 between 35 and 45 mmHg. During the full period of EVLP, the pulmonary artery was maintained between 10 and 15 mmHg and the left atrial pressure between 3 and 5 mmHg, by aligning the reservoir level. Samples of the perfusate from the pulmonary artery inflow and left atrium outflow were collected hourly after a 10-min challenge with a 100 % fraction of inspired oxygen for arterial blood gas analysis. Airway pressures were measured hourly and used to calculate dynamic compliance. At the end of EVLP, the pulmonary artery and left atrium cannulas were disconnected from the EVLP circuit, and the lungs were flushed anterograde with 500 mL of 4 °C cold Perfadex (XVIVO Perfusion Inc). After infusion of cold Perfadex, the trachea was clamped to keep the lungs inflated. The left lung was separated off and stored in 4 °C Perfadex before transplantation.
2.4. Lung transplantation procedures
Recipient pigs were anesthetized and ventilated as described above for donors. Orthotopic left single lung transplantation was performed using a simplified continuous two-stitch suture for bronchial anastomosis as per our previous study [9].
2.5. Lung physiologic features during EVLP and after transplantation
Lung functional parameters were measured every hour both during EVLP and reperfusion in vivo. The functional parameters included blood oxygenation (PaO2/FiO2 ratio), mean pulmonary artery pressure (MPAP), pulmonary vascular resistance (PVR), peak airway pressure (PAP), and dynamic lung compliance were evaluated. The functional parameters were measured using a Swan-Ganz catheter and the airway pressure was measured using a pressure transducer attached to the endotracheal tube. At the end of 3h of reperfusion, the endotracheal tube was inserted into the left main bronchus, and the right main pulmonary artery was clamped to evaluate only the left lung graft function. The transplanted lung was excised after 4 h of reperfusion and the swine euthanized.
2.6. Histological assessment of the lungs
At the end of 4h of reperfusion, specimens from the lower lobe were prepared for pathological assessment by instilled with 10 % buffered formalin and stained with hematoxylin and eosin. The lung sections were assessed by a pathologist per the lung injury severity (LIS) score, which is based on the total neutrophil counts per high power field(HPF)(score of 0,<5; 1, 6 to 10; 2,11to20; and score of3, >20), extent of alveolar edema(score of 0,<5 %; 1, 6 %–25 %; 2, 26%–50 %; and 3, >50 %), and degree of interstitial infiltration(score of 0, none; 1, minimal; 2, moderate; and 3, severe). The wet-to-dry weight ratio was calculated as an estimate of pulmonary edema after reperfusion, by dividing the wet weight by the final dry weight before and after storage at 80 °C for 72 h. A composite score was calculated by summation of these 3 components. For each variable, the average of the 3 sample values was taken for group comparisons.
2.7. Cytokine measurement
On completion of EVLP and transplantation, Bronchoalveolar lavage (BAL) of the upper lobe was performed in all groups with 30 mL of normal saline, centrifuged, and the supernatant was stored at-80 °C. The cytokine levels in BAL samples were measured using a commercially available porcine cytokine multiplex immunoassay kit (Quantibody®,antpedia, Shanghai, China) according to the manufacturer's instructions.
2.8. Statistical analysis
All experimental results were expressed as the means standard deviation. Data containing a time component, such as PAP, MPAP, PVR, and repeated measures were analyzed using analysis of variance (ANOVA). For comparison of the wet/dry ratio, a Mann–Whitney test was performed. Fischer's exact test was employed to compare the LIS score. All data analyses were performed with SPSS 26.0 software (SPSS Inc, IBM). Differences were considered to be statistically significant as the p-value is less than 0.05.
3. Results
3.1. Lung function during EVLP
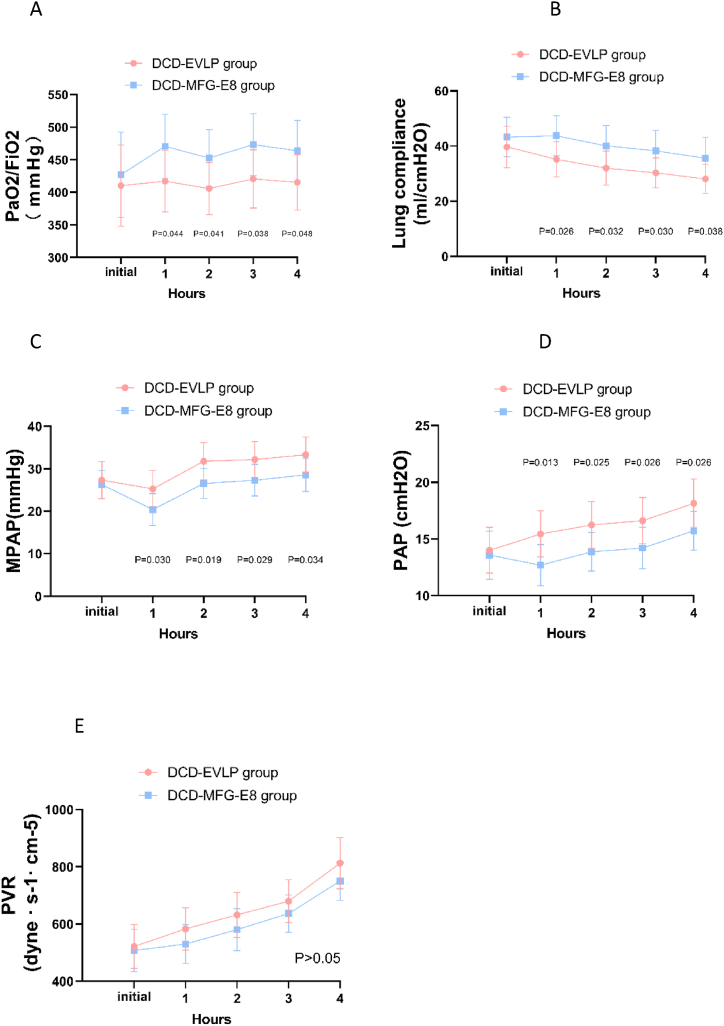
Ventilated and perfused lungs underwent 4 h of normothermic EVLP (Fig. 1). Lung physiologic features were monitored during the 4 h EVLP period. The oxygenation (PaO2/FiO2) (P = 0.048), mean pulmonary artery (PA) pressure (MPAP) (P = 0.034), dynamic lung compliance(P = 0.038), and peak airway pressure (PAP) (P = 0.026) differed significantly between the DCD-EVLP and DCD-MFG-E8 group at the end of EVLP. However, the pulmonary vascular resistance (PVR) in the MFG-E8 group did not differ from the EVLP group(P = 0.136). (Fig. 2A–E).
Fig. 1.
Lungs being ventilated and perfused.
Fig. 2.
Lung physiologic features during ex vivo lung perfusion.
A. oxygenation (PaO2/FiO2), B. lung compliance, C. mean pulmonary artery (PA) pressure (MPAP), D. peak airway pressure (PAP), E. pulmonary vascular resistance (PVR). The oxygenation (PaO2/FiO2), mean pulmonary artery pressure, dynamic lung compliance, and peak airway pressure were significantly better in the DCD-MFG-E8 group over time. The pulmonary vascular resistance (PVR) was lower in the DCD-MFG-E8 group but the differences did not reach statistical significance.
3.2. Lung function after LTx
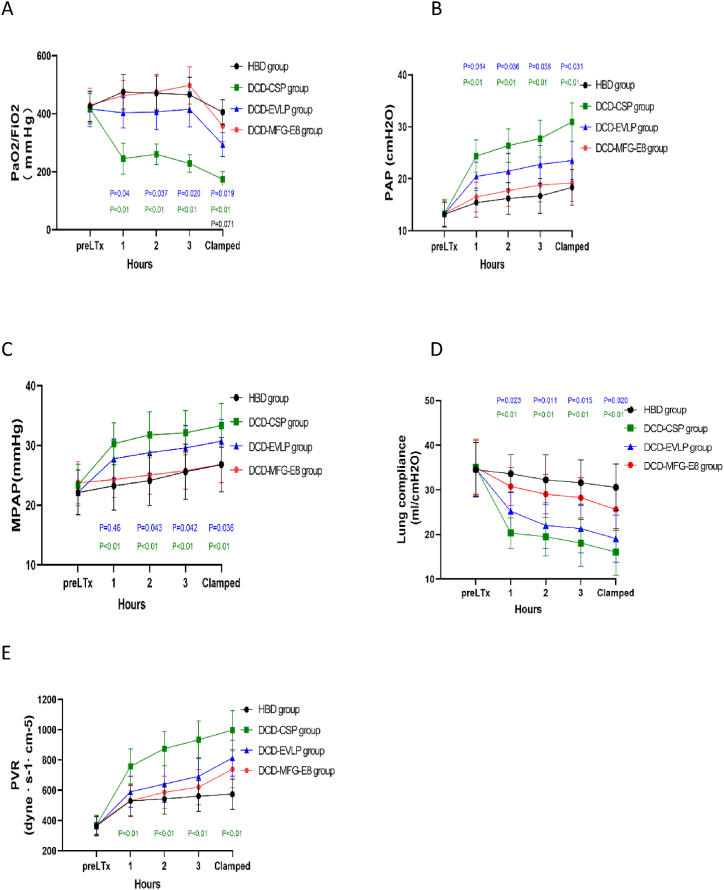
Orthotopic left single lung transplantation was performed successfully(Fig. 3). After transplantation and 4 h of reperfusion, the oxygenation (PaO2/FiO2) was better in the DCD-MFG-E8 group than in the DCD-EVLP (P = 0.019) group. Lung dynamic compliance was also significantly better in group DCD-MFG-E8 compared with the DCD-EVLP group (P = 0.020) as well as MPAP (P = 0.036) and PAP (p = 0.031). Moreover, no significant differences were observed between the HBD and DCD-MFG-E8 groups. The PVR in the DCD-MFG-E8 group did not differ from the DCD-EVLP group but was significantly lower than in the DCD-CSP group (P = 0.001). (Fig. 4A–E).
Fig. 3.
Lungs being transplanted.
Fig. 4.
Lung physiologic features after lung transplantation.
A. oxygenation (PaO2/FiO2), B. peak airway pressure (PAP), C. mean pulmonary artery (PA) pressure (MPAP), D. lung compliance, E. pulmonary vascular resistance (PVR). During 4 h of reperfusion, the oxygenation (PaO2/FiO2) and lung compliance were significantly better in the DCD-MFG-E8 group compared with DCD-EVLP group, as well as MPAP and PAP. The PVR in the DCD-MFG-E8 group did not differ from the DCD -EVLP group but was significantly lower than in the DCD-CSP group.
3.3. Cytokine expression
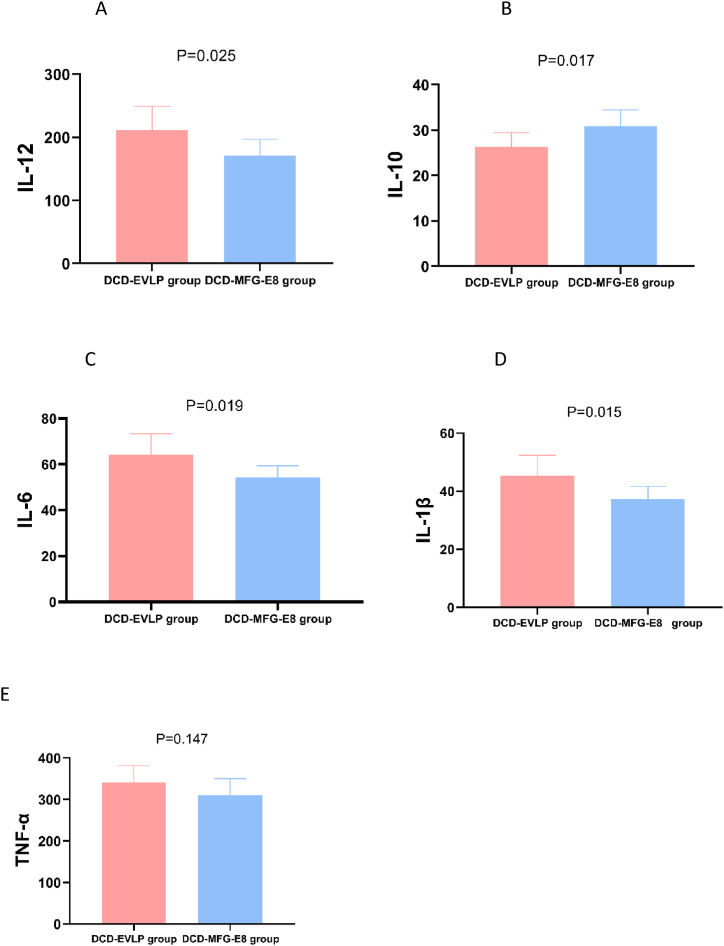
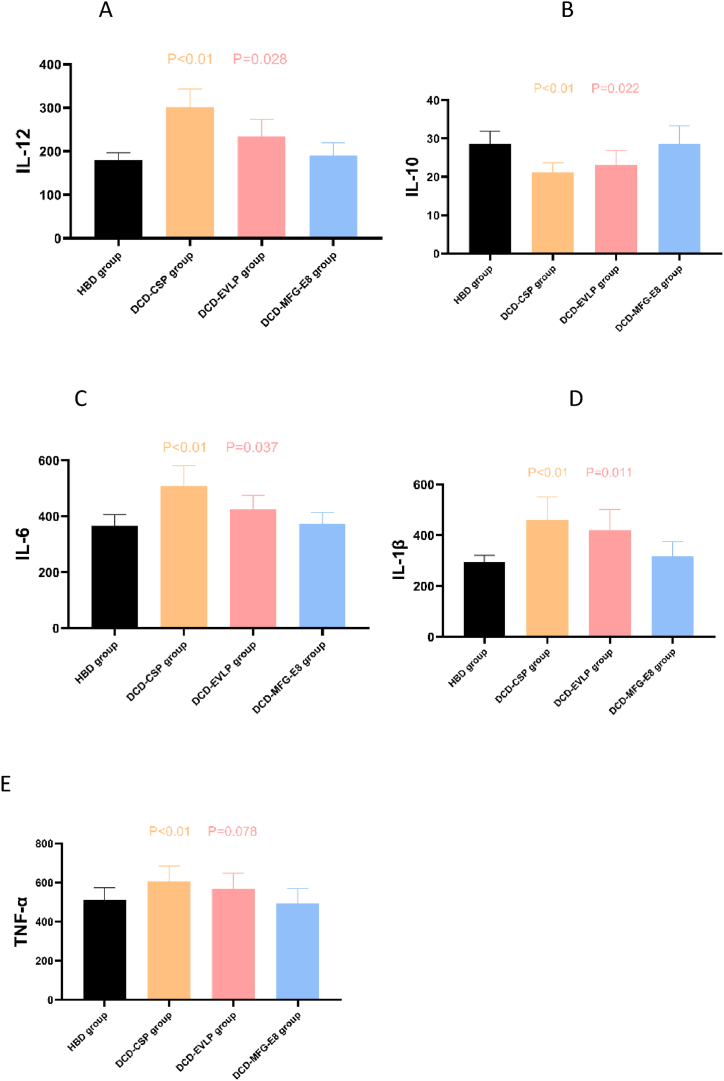
The effect of the various strategies on the expression of cytokines in BAL samples was evaluated at the end of EVLP and transplant reperfusion. Compared with DCD-EVLP, a significant decrease was confirmed in levels of IL-1β(p = 0.015), IL-6(p = 0.019) and IL-12(p = 0.025) in the DCD-MFG-E8 Group at the end of EVLP, as well as in levels of IL-1β(p = 0.011), IL-6(p = 0.037) and IL-12(p = 0.028) after reperfusion in vivo, but significant increase in IL-10 (at the end of EVLP: p = 0.017; after reperfusion in vivo: p = 0.022) was found in the DCD-MFG-E8 Group. However, there was no statistically significant difference in TNF-α neither at the end of EVLP nor after reperfusion in vivo when we compared both groups. (at the end of EVLP: p = 0.147; after reperfusion in vivo: p = 0.078). There was no statistically significant difference between HBD and DCD-MFG-E8 in the expression of above-mentioned cytokines in BAL. (Fig. 5A–E, Fig. 6A–E).
Fig. 5.
The effects of MFG-E8 on inflammation of lung grafts at the end of ex vivo lung perfusion.
A. The expression level of interleukin (IL)-12, B. IL-10, C. IL-6, D. IL-1β, E. tumor necrosis factor-alpha (TNF-α). The expression level of proinflammatory cytokine(IL-12, IL-6, IL-1β) was significantly lower in the DCD-MFG-E8 Group, but the expression level of anti-inflammatory cytokine(IL-10) was significantly higher in the DCD-MFG-E8 Group. However, there was no statistically significant difference in TNF-α between the DCD-EVLP and DCD-MFG-E8 group.
Fig. 6.
The effects of MFG-E8 on inflammation of lung grafts after lung transplantation.
A. The expression level of interleukin (IL)-12, B. IL-10, C. IL-6, D. IL-1β, E. TNF-α. After transplant reperfusion, the DCD-MFG-E8 Group demonstrated significantly decreased proinflammatory cytokine (IL-12, IL-6, IL-1β) and increased anti-inflammatory cytokine(IL-10) compared with the DCD-EVLP group. The levels of TNF-α in the DCD-MFG-E8 group did not differ from the DCD-EVLP group but was significantly lower than in the DCD-CSP group. There was no statistically significant difference between HBD and DCD-MFG-E8 in the expression of above-mentioned cytokines in BAL.
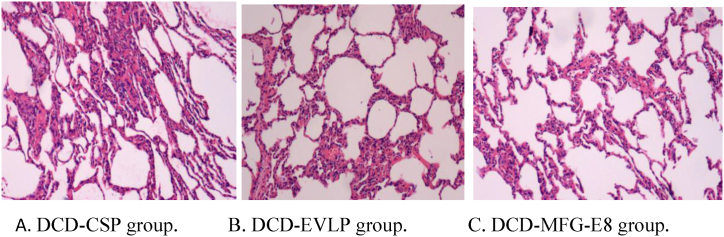
3.4. Histologic evidence of lung injury
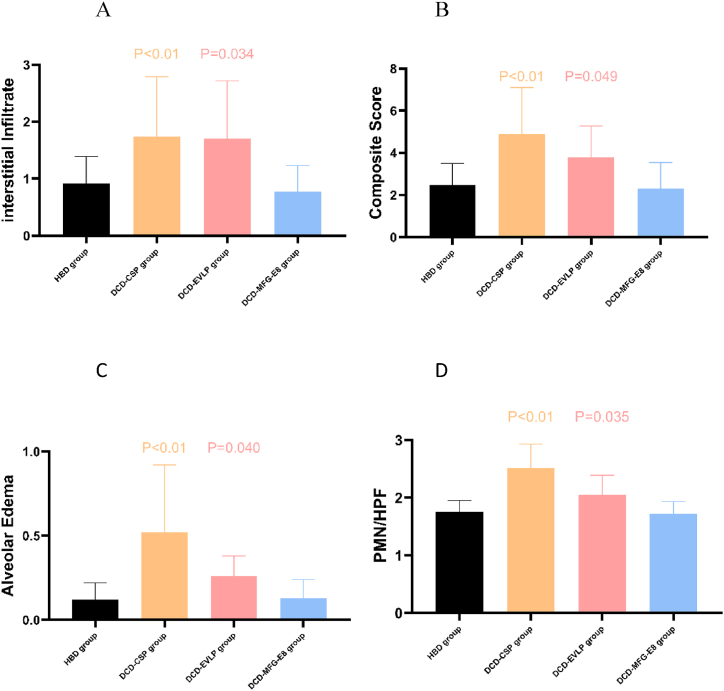
The light microscopic histological findings for each group are revealed in Fig. 7. The DCD-CSP group exhibited a more severe lung injury with extensive alveolar hemorrhage and neutrophil emigration than the other groups (Fig. 7A). As far as the histology of the DCD-MFG-E8 group, only a mild neutrophil infiltration within alveolar capillaries and no obvious alveolar exudates and hemorrhage in the perivascular space (Fig. 7B) have been noted. By contrast, neutrophil infiltration and alveolar edema were markedly reduced in the DCD-MFG-E8 group compared to the DCD-EVLP group (Fig. 7C), indicating that MFG-E8 treatment during EVLP attenuated these pulmonary histological changes. In addition, the DCD-MFG-E8 group had decreased lung injury scores (2.30 ± 1.24), compared with the DCD-EVLP(3.78 ± 1.50, P = 0.049)and DCD-CSP (4.90 ± 2.20, P < 0.01)groups. Significantly fewer neutrophils PMN (polymorphonuclear leukocytes)/HPF(high power field) were revealed in the DCD-MFG-E8 group(1.72 ± 0.21) compared with DCD-CSP (2.51 ± 0.42, P = 0.001) and DCD-EVLP (2.05 ± 0.34, P = 0.035). The DCD-MFG-E8 group also had significantly decreased alveolar edema (DCD-MFG-E8, 0.13 ± 0.11; DCD-EVLP, 0.26 ± 0.12; P = 0.040) and less interstitial infiltrate than the DCD-EVLP group. (DCD-MFG-E8, 0.77 ± 0.46; DCD-EVLP, 1.70 ± 1.02; P = 0.034) These results showed that the degree of pulmonary injury was statistically lower in DCD-MFG-E8 group.(Fig. 8A–D).
Fig. 7.
Histological findings in lung grafts(H&E, × 100). A. DCD-CSP group. Severe lung injury with extensive alveolar hemorrhage and many neutrophil emigration were found. B. DCD-EVLP group. Moderate pulmonary edema and inflammatory cells infiltration were found. C. DCD-MFG-E8 group. A mild neutrophil infiltration within alveolar capillaries and no obvious alveolar exudates and hemorrhage in the perivascular space.
Fig. 8.
Mean lung injury severity scores of lung grafts stratified by histological feature. A. interstitial infiltrate. B. composite lung injury severity scores. C. alveolar edema. D. PMN (polymorphonuclear leukocytes)/HPF(high power field).The DCD-MFG-E8 group had decreased alveolar edema, lung injury scores, and less interstitial infiltrate compared with the DCD-EVLP and DCD-CSP groups(p < 0.05).
3.5. Wet-to-dry weight ratio
Compared with DCD-EVLP, the wet-to-dry (W/D) weight ratio was significantly lower in the DCD-MFG-E8 Group (2.23 ± 0.26 vs 2.89 ± 0.30, p = 0.035), reflecting that the degree of pulmonary edema was the lowest. There were no statistically significant differences between HBD Control and DCD-MFG-E8 (1.99 ± 0.23 vs 2.23 ± 0.26, p = 0.071).
4. Discussion
Since the introduction of standard criteria donation (SCD), existing surgical techniques, postoperative care, and immunosuppressive therapy have seen major improvement and have led to significant improvements in both short and long-term outcomes after LTx. Although SCD remains the starting point for donor evaluation, extended criteria donor (ECD) is used nowadays as the first step to maximizing the donor pool. ECD further supports the use of EVLP and DCD lungs in patients with ABO blood group incompatibility [10]. Recent studies have been using cohorts of unexploited donors, for instance, ECD for potential contribution to overcoming the chronic organ shortage in LTx to allow safer use of lungs from high-risk donors and graft modification to match graft recipients that may also improve post-transplant outcomes.
DCD is seeking to have a considerable impact on the expansion of the donor pool and compensate for the limitation of SCD. A recent cohort study estimated that all potential donors with controlled DCD under Maastricht classification category III could upregulate the lung supply up to 22.7 % for optimal donor lungs and 50 % for sub-optimal donor lungs [11].In contrast to donation after brain death(DBD), controlled DCD lungs are an effective and safe method with good survival outcomes, and its broad implementation over the last decade has been very significant. However, for donation after uncontrolled DCD, data are still insufficient, and further investigations are needed.
Using a preclinical porcine model of DCD left lung transplantation, the present study attempts to assess the effect of MFG-E8 treatment during EVLP on ex vivo pulmonary ischemia-reperfusion injury and graft function. The results demonstrate that MFG-E8 treatment during EVLP can rehabilitate lungs from uncontrolled DCD donors (Maastricht category I) with 1h of warm ischemia to an admissible functional condition for successful transplantation.MFG-E8 treatment during EVLP resulted in superior pulmonary function (oxygenation and dynamic compliance) and post reperfusion analyses (infiltration of neutrophils and inflammatory cytokine expression) compared with cold static preservation and EVLP treatment of DCD lungs respectively. These findings encourage us and may form a foundation for new therapeutic strategies to improve donor lung utilization and expand the pool of available donor lungs.
Today, EVLP has emerged as a platform that allows for accurate lung evaluation and improvement of lung function by providing oxygen and adequate nutrients [12]. Additionally, various modified techniques have been applied to improve marginal lung function. However, the results of the present study were contrary to our initial hypothesis regarding the benefit of EVLP itself. Compared with DCD-CSP, the lung physiology, and pro-inflammatory cytokine expression were not significantly better in the DCD-EVLP Group, which was in agreement with the report of Stone [13]. The results reflect that neither 4 h of CSP nor EVLP alone can effectively improve the function of the donor lung with 1 h of warm ischemia. It has been verified that cold injury impairs the function of the endothelium, thus leading to graft tissue interstitial edema and cell swelling following reperfusion, and early graft dysfunction [14]. In addition, whether EVLP itself can improve lung physiology is still controverted, as it can result in lung injury associated with the pump or mechanical ventilator. Therefore, in most recent reports, various medications and additional procedures such as surfactant injections, fibrinolytic treatment (urokinase), nitric oxide (NO) or hydrogen gas inhalation, and gene therapy (IL-10) have been applied in combination with the EVLP system to improve marginal lung function [15].
One aspect of the present study is the use of MFG-E8 in the perfusion circuit during EVLP to reduce donor lung inflammation and IR injury by a preclinical animal transplant model for the first time. Our laboratory has rich experience using MFG-E8 in animal models of lung ischemia-reperfusion injury and deep venous thrombosis [7,8].
During the assessment of pulmonary function during EVLP, the PaO2/FiO2 and MPAP were significantly better in the DCD-MFG-E8 group, as well as the lung compliance and peak airway pressure (PAP). Additionally, such a better function of the transplanted lung during EVLP could be sustained after LTx. All PaO2/FiO2, MPAP, lung compliance, and PAP reflected superior lung function except for PVR. These results demonstrate that MFG-E8 treatment during EVLP exhibits significantly more protective functions than cold static preservation or EVLP alone.
Lung I/R resulted from oxidative stress and a systemic inflammatory reaction because of the release of proinflammatory cytokines. Moreover, a pivotal aspect of lung I/R injury is an increase in apoptotic cell death of type II alveolar epithelial cells and bronchial epithelial cells [16]. Furthermore, lacking clearance of apoptotic cells after ischemic injury probably causes increased inflammation and impaired tissue repair. MFG-E8, a secretory molecule, that is mainly produced by macrophages and dendritic cells, is crucial for apoptotic cell clearance [17,18]. Hanayama and colleagues found that MFG-E8 plays a crucial part in the clearance of apoptotic B cells in the spleen and prevents proinflammatory immune responses and the development of autoimmune diseases [19]. In addition, MFG-E8 KO mice revealed remarkably potentiated lung inflammatory responses and apoptosis, providing evidence for the crucial role of MFG-E8 in lung I/R [20].
We have previously demonstrated that MFG-E8 has anti-inflammatory effects and can reduce the number of apoptotic cells in numerous preclinical models [7,8]. Our current study also found that the ischemia-reperfusion injury of DCD lungs after transplantation was attenuated after treatment with rhMFG-E8, as evidenced by suppressed local inflammation and decreased tissue injury. Therefore, one may speculate that the beneficial effect of MFG-E8 is likely a consequence of enhanced clearance of apoptotic cells. Thus, the reduction of apoptosis with rhMFG-E8 offers an alternative strategy to treat ischemia-reperfusion injury after DCD lung transplantation.
To prove the anti-inflammatory effect of MFG-E8, we evaluated the expression levels of pro- and anti-inflammatory cytokines (including TNF-α, IL-1β, IL-6, IL-12, and IL-10) in the BAL sample of lung grafts. The results revealed that the expression levels of pro-inflammatory cytokines IL-6 and IL-12 were significantly higher in the DCD-CSP and DCD-EVLP groups. On the contrary, IL-10, an anti-inflammatory cytokine, was expressed significantly higher in the DCD-MFG-E8 group. Hence, we could confirm that MFG-E8 treatment might exhibit an anti-inflammatory effect by up-regulating anti-inflammatory cytokines and down-regulating pro-inflammatory cytokines by our previous result.
Previous work from our laboratory showed that administration of rhMFG-E8 dramatically decreased the release of IL-1β and other proinflammatory cytokines induced by pulmonary I/R injury and impeded neutrophil activation and infiltration [7,8]. The current study turned these findings into a preclinical porcine transplant model for the first time. IL-12 is a proinflammatory cytokine excreted by tissue-resident dendritic cells and macrophages, which results in increased interferon-γ by T-helper cells. In addition, IL-12, concomitant with IL-18, a crucial constituent of inflammasome activation, has been involved in T-cell infiltration and the up-regulation of matrix-degrading enzymes in pulmonary injury [21]. The present study shows evidence of a decrease in IL-12 production with rhMFG-E8 treatment during porcine EVLP. Therefore, selective implementation to downregulate IL-12 expression may be a promising therapy during EVLP to prevent inflammation and attenuate IR injury after lung transplantation.
The present study manifested a significant decrease in neutrophil infiltration with MFG-E8 treatment during EVLP. In addition, the lung injury scores were statistically lower in the DCD-MFG-E8 group. Lower wet/dry ratios also indicated that MFG-E8 may also play a part in reducing pulmonary edema. Neutrophil activation and infiltration rely on various signaling pathways with a close relationship to both innate and adaptive immunity. We have previously indicated that MFG-E8 can down-regulate TLR4/NF-κB signaling pathways and attenuate neutrophil infiltration in a rat model of lung IR injury, same as in a porcine transplantation model. The present study shows the first evidence of an attenuation in histological changes of DCD lungs exposed to 1 h of warm ischemia with MFG-E8 treatment during EVLP.
Our study does have limitations. The first concern was the lack of cellular and molecular mechanisms underlying the observed results. However, we would like to reiterate that rhMFG-E8's protective effects on deep venous thrombosis and pulmonary graft ischemic reperfusion injury and mechanisms have already been well documented by our previous works. Secondly, there is natural heterogeneity present in large animal studies. Finally, the current study is limited by the fact that only one dose of rhMFG-E8 (20 μg/kg) was assessed, and that treatment was initiated at the beginning of EVLP. Further investigation, including post-transplantation with increasing doses of rhMFG-E8, is needed to more firmly determine its therapeutic effects.
In conclusion, using a preclinical porcine transplant model, this study demonstrates that rhMFG-E8 treatment during EVLP can effectively reduce the inflammatory response and IR injury of lungs from non–heart-beating donors and rehabilitate DCD lungs to an acceptable functional condition for successful transplantation. The results of the present study suggest that it may be possible to deliver rehabilitated uncontrolled DCD donor lungs to the most appropriate recipient, decrease the wait list times, and save lives. Our findings provide prospects for the employment of uncontrolled DCD donors in future human lung transplantation.
Availability of data and materials
This study's data can be obtained from the corresponding author upon reasonable request.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No. 81973992, 81570090).
CRediT authorship contribution statement
Ping Li: Project administration. Kai Peng: Writing – original draft, Software, Project administration, Data curation. Li Gang Liu: Investigation. Qing Yun Liu: Investigation. Zhen Hua Huang: Investigation. Durgahee Mouniir Sha Ahmad: Writing – review & editing. Xiang Wei: Supervision, Investigation. Si Hai Gao: Writing – review & editing, Writing – original draft, Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors acknowledge Wei Huang, Tianpen Cui and Shuang Zhang for their expert technical assistance and Xuefeng Yang for her statistical advice to this project.
References
- 1.Wigfield C. Donation after cardiac death for lung transplantation: a review of current clinical practice. Curr. Opin. Organ Transplant. 2014 Oct;19(5):455–459. doi: 10.1097/MOT.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 2.Ehrsam J.P., Benden C., Immer F.F., Inci I. Current status and further potential of lung donation after circulatory death. Clin. Transplant. 2021 Jul;35(7) doi: 10.1111/ctr.14335. [DOI] [PubMed] [Google Scholar]
- 3.Iske J., Hinze C.A., Salman J., Haverich A., Tullius S.G., Ius F. The potential of ex vivo lung perfusion on improving organ quality and ameliorating ischemia reperfusion injury. Am. J. Transplant. 2021 Dec;21(12):3831–3839. doi: 10.1111/ajt.16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallinder Andreas, Ricksten Sven-Erik, Silverborn Martin, Hansson Christoffer, Riise Gerdt C., Liden Hans, Jeppsson Anders, Dellgren Göran. Early results in transplantation of initially rejected donor lungs after ex vivo lung perfusion: a case–control study. Eur. J. Cardio. Thorac. Surg. 2014 Jan;45(1):40–44. doi: 10.1093/ejcts/ezt250. ; discussion 44-5. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda A., Jacob A., Wu R., Zhou M., Nicastro J.M., Coppa G.F., Wang P. Milk fat globule-EGF factor VIII in sepsis and ischemia-reperfusion injury. Mol Med. 2011 Jan-Feb;17(1–2):126–133. doi: 10.2119/molmed.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S., Xie J.G., Su B.T., Li J.L., Hu N., Chen J., Luo G.W., Cui T.P. MFG-E8, a clearance glycoprotein of apoptotic cells, as a new marker of disease severity in chronic obstructive pulmonary disease. Braz. J. Med. Biol. Res. 2015 Nov;48(11):1032–1038. doi: 10.1590/1414-431X20154730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Q.Y., Huang Z.H., Liu Y.K., et al. Inhibitory effects of Milk fat globule epidermal growth factor 8 on deep venous thrombosis by modulating Toll-like receptor 4/nuclear factor κB signaling pathway. Chin J Exp Surg. 2022;39(2):250–253. doi: 10.3760/cma.j.cn421213-20210710-01204. [DOI] [Google Scholar]
- 8.Wang R., Liu Q.Y., Li P., et al. Protective effects of milk fat globule epidermal growth factor 8 on ischemic reperfusion injury of pulmonary grafts by modulating hypoxia inducible factor-1α/vascular endothelial growth factor/Notch signaling pathway. Chin J Exp Surg. 2023;40(1):78–82. doi: 10.3760/cma.j.cn421213-20220621-01200. [DOI] [Google Scholar]
- 9.Li P., Zhu L., Tang F.F., Xiong J., Ma M.J., Dsa M., Gao S.H. A simplified continuous two-stitch suture for bronchial anastomosis of left single lung transplant in dogs. Curr Med Sci. 2020 Jun;40(3):548–555. doi: 10.1007/s11596-020-2212-2. [DOI] [PubMed] [Google Scholar]
- 10.Noda Kentaro, Furukawa Masashi, Chan Ernest G., Sanchez Pablo G. Expanding donor options for lung transplant: extended criteria, donation after circulatory death, ABO incompatibility, and evolution of ex vivo lung perfusion. Transplantation. 2023 Jul 1;107(7):1440–1451. doi: 10.1097/TP.0000000000004480. Epub 2023 Jun 20. [DOI] [PubMed] [Google Scholar]
- 11.Scott D Halpern, Hasz Richard D., Abt Peter L. Incidence and distribution of transplantable organs from donors after circulatory determination of death in U.S. intensive care units. Ann Am Thorac Soc. 2013 Apr;10(2):73–80. doi: 10.1513/AnnalsATS.201211-109OC. [DOI] [PubMed] [Google Scholar]
- 12.Cypel M., Rubacha M., Yeung J., Hirayama S., Torbicki K., Madonik M., Fischer S., Hwang D., Pierre A., Waddell T.K., de Perrot M., Liu M., Keshavjee S. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am. J. Transplant. 2009 Oct;9(10):2262–2269. doi: 10.1111/j.1600-6143.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- 13.Mulloy Daniel P., Stone Matthew L., Crosby Ivan K., Lapar Damien J., Sharma Ashish K., Webb David V., Lau Christine L., Laubach Victor E., Kron Irving L. Ex vivo rehabilitation of non–heart-beating donor lungs in preclinical porcine model: delayed perfusion results in superior lung function. J. Thorac. Cardiovasc. Surg. 2012 Nov;144(5):1208–1215. doi: 10.1016/j.jtcvs.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner Cynthia E., Pope Nicolas H., Charles Eric J., Huerter Mary E., Sharma Ashish K., Salmon Morgan D., Carter Benjamin T., Stoler Mark H., Lau Christine L., Laubach Victor E., Kron Irving L. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J. Thorac. Cardiovasc. Surg. 2016 Feb;151(2):538–545. doi: 10.1016/j.jtcvs.2015.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haam S., Lee J.G., Paik H.C., Park M.S., Lim B.J. Hydrogen gas inhalation during ex vivo lung perfusion of donor lungs recovered after cardiac death. J. Heart Lung Transplant. 2018 Oct;37(10):1271–1278. doi: 10.1016/j.healun.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y., Yin H.Q. Dimethyl itaconate inhibits LPS-induced inflammatory release and apoptosis in alveolar type II epithelial and bronchial epithelial cells by activating pulmonary surfactant proteins A and D. Allergol. Immunopathol. 2022 Nov 1;50(6):176–186. doi: 10.15586/aei.v50i6.586. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda A., Jacob A., Wu R., Zhou M., Nicastro J.M., Coppa G.F., Wang P. Milk fat globule-EGF factor VIII in sepsis and ischemia-reperfusion injury. Mol Med. 2011 Jan-Feb;17(1–2):126–133. doi: 10.2119/molmed.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui T., Miksa M., Wu R., Komura H., Zhou M., Dong W., Wang Z., Higuchi S., Chaung W., Blau S.A., Marini C.P., Ravikumar T.S., Wang P. Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am. J. Respir. Crit. Care Med. 2010 Feb 1;181(3):238–246. doi: 10.1164/rccm.200804-625OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanayama R., Tanaka M., Miyasaka K., Aozasa K., Koike M., Uchiyama Y., Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004 May 21;304(5674):1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Luo G., Chen J., Jiang R., Zhu J., Hu N., Huang W., Cheng G., Jia M., Su B., Zhang N., Cui T. Cigarette smoke attenuates phagocytic ability of macrophages through down-regulating Milk fat globule-EGF factor 8 (MFG-E8) expressions. Sci. Rep. 2017 Feb 14;7 doi: 10.1038/srep42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamoto M., Kato S., Oizumi K., Kinoshita M., Inoue Y., Hoshino K., Akira S., McKenzie A.N., Young H.A., Hoshino T. Interleukin 18 (IL-18) in synergy with IL-2 induces lethal lung injury in mice: a potential role for cytokines, chemokines, and natural killer cells in the pathogenesis of interstitial pneumonia. Blood. 2002 Feb 15;99(4):1289–1298. doi: 10.1182/blood.v99.4.1289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study's data can be obtained from the corresponding author upon reasonable request.