Abstract
Rockery constitutes one of the significant elements of Chinese royal garden design, and it becomes an emerging interdisciplinary topic covering both science and art. In this work, we report an attempt to restore the color of the ancient rockeries by finding the appropriate inorganic pigments. Chromium is doped as activators into strontium melilites with varying Si/Ge ratio, and the synthesized powders give a series of color changing from ice blue to dark-sea green. With the structure and spectroscopic characterizations, we discuss the substitutions of Cr in Mg/Si/Ge sites of the melilite host. The Tanabe-Sugano diagram of 3d2 configuration is employed to explain the change of diffuse reflectance spectra caused by Si/Ge substitution. With the work, we hope to inspire the interdisciplinary research and promote the rockery heritage restoration and protection.
Keywords: Rockery, Mililite, Cr4+, Color restoration
Highlights
-
•
Cr doped strontium melilite for rockery color restoration is studied.
-
•
Site occupancy and valence state of Cr are explored.
-
•
Color change is analyzed by Tanabe-Sugano diagram.
Graphical abstract
1. Introduction
Rockery is one of the most unique elements in ancient Chinese garden design, and also an important means of creating naturalistic landscapes [1,2]. Among them, the artificial rock formations in the royal gardens of the Ming and Qing dynasties of China, such as the Three Seas, Three Mountains and Five Gardens, are particularly magnificent, with distinctive shapes, novel colors, and rich variations, making them the top examples of ancient Chinese rockeries [3]. In fact, the selection and construction of rockwork in royal gardens pay attention to the choice of stone colors and mountain colors, reflecting the elegance and luxury of the imperial gardens. However, due to the destruction by war and natural weathering, the existing rockery material has deteriorated, causing the colors to become darker and rustier, often appearing in dark or gray tones, losing the original color information, as depicted in Fig. 1. Therefore, the color restoration and reproduction of rockery is one of the important tasks in the preservation of historical gardens and cultural heritage [4]. Finding restoration pigments that are the same or similar to the original colors has become an urgent research task.
Fig. 1.
The verdant stone rockery with well-preserved colors in (a) Kanhua Corridor of Beihai Park and (b) the upright peak of the green stone rockery in Xiangshan Temple of Jinyi Garden. The green stone rockwork with color deterioration in (c) Zibishanfang of Yuanmingyuan and (d) the upright peak of the green stone rockery in Qiyun Building of Jinyi Garden.
To find the appropriate material for the restoration pigments, we focus on the rare-earth or transition metal elements doped inorganic materials [[5], [6], [7]]. Strontium magnesium melilites have been thoroughly investigated for rare-earth doped luminescence as wLED (white light emitting diode) or persistence phosphors [8,9]. Both structure and luminescence properties of this host material with Eu2+ dopant have been widely investigated [[10], [11], [12]]. Some general rules have been outlined which state that the substitution of Ca/Sr could create a continuous solid solution, in which the crystal filed strength changes successively, and the emission peak shifts to longer wavelengths from blue of Ca series to yellow of Sr series. This phenomenon could be successfully explained by the effects of the crystal field splitting of the d-manifold of Eu2+ and electron-phonon coupling effect (Stokes shift) originating from relaxation in the 4f65d1 excited state [[13], [14], [15]]. This is applicable to the end member components for a continuous solid solution. With Eu2+ and Dy3+ co-doping, (Sr,Ca)MgSi2O7 can present blue–green afterglow for more than 20 h under the ultra-violet-light excitation, and Eu3+/Dy2+ serve as the stable trapping centers [16,17]. Nevertheless, when doped with Eu2+ or Ce3+, the strong absorption and emission associated with parity-allowed f-d transition presents too vivid blueish or greenish color of the powders, making them inappropriate for rockery color-restoration pigments. On the contrary, for transition-metal ions, the low oscillator strength of the parity-forbidden d-d transitions could be explored for the potential color-restoration. For example, chromium doped inorganic compounds display relatively low visible light absorption [18,19]. It is therefore possible to explore the restoration pigments by doping Cr into strontium magnesium melilites.
In this work, we dope Cr into strontium melilites with varying Si/Ge ratio, and obtain light blue-green powders which have potential applications in the restoration pigments. We discuss in detail the site occupation of Cr and its valence states. The color change with compositional variation from Si to Ge is explained by the diffuse reflection spectra, and a crystal field analysis is conducted by the Tanabe-Sugano diagram of 3d2 configuration in the tetrahedron environment. With this structure-property relationship, we hope to provide a useful guidance to tune the color of Cr doped strontium magnesium melilites.
2. Experimental
2.1. Materials and Synthesis Procedures
A series of Sr2Mg1-xSi2O7:xCr4+ (x = 0, 0.01, 0.03, and 0.05) and Sr2Mg1-xGe2O7:xCr4+ (x = 0, 0.01, 0.03, and 0.05) phosphors were synthesized using the traditional high-temperature solid-state reaction method. The raw materials of SrCO3, MgO, SiO2, GeO2, and Cr2O3 were of high purity (99.99 %, Aladdin) and weighed in accordance with the stoichiometric ratio. Additionally, H3BO3 (99 %, Aladdin) was added with a mass fraction of 5 % to enhance the crystallinity. The raw materials were thoroughly mixed by grinding for 30 min in an agate mortar. Subsequently, the mixtures were placed in alumina crucibles and sintered at 1350 °C (Sr2MgSi2O7:Cr4+) and 1300 °C (Sr2MgGe2O7:Cr4+) for 4 h under air atmosphere. Finally, the resulting products were finely ground into powders after cooling down to room temperature for further characterization.
2.2. Characterization
The powder X-ray diffraction (XRD) patterns of all the samples were obtained using a diffractometer (TTR-III, Rigaku, Japan) with Cu Kα radiation (λ = 1.5406 Å) operating at 40 kV and 200 mA. The Rietveld whole pattern refinements were conducted using Fullprof Suite [20]. The diffuse reflection (DR) spectra were measured using a UV–Vis–NIR spectrophotometer (Hitachi High-Tech Science Corporation, UH4150), with BaSO4 as the standard. The powders are loaded in a built-in integrated sphere. The scanning electron microscopy (SEM) and element mapping images were obtained using a JEOL JSM-6510A equipped with an energy dispersive X-ray analyzer. The sample photos were taken using a Huawei P30 mobile phone.
3. Results and discussion
3.1. Crystal structure
Both Sr2MgSi2O7 (SMS) and Sr2MgGe2O7 (SMG) exhibit tetragonal system crystallization with a Space group of P 21 m (No. 113). The structure is layered, as depicted in Fig. 2a, with the Sr layer and Mg–Si/Ge layer interwoven along the a and b directions. The larger Sr cations occupy the Wyckoff site 4e, coordinated by eight oxygen ligands. The Si/Ge at 4e site and Mg at 2a site have a tetrahedral ligand environment. When viewed from the c direction, the Sr cations are situated in the columns of tetrahedron rings. The unique structure poses the question of which site the chromium activator may substitute for. As the raw material for chromium is Cr2O3 and the samples are sintered in air, the stabilized valence could be +3 or +4. However, given the larger ionic radius of Cr3+ and the valence difference between Cr3+ and Si4+/Ge4+, chromium has a higher likelihood of being stabilized in the +4 valence state.
Fig. 2.
Crystal structure of SMS/SMG viewed from (a) [010] direction, and (b) [001] direction.
3.2. Tetrahedron occupation by XRD analysis
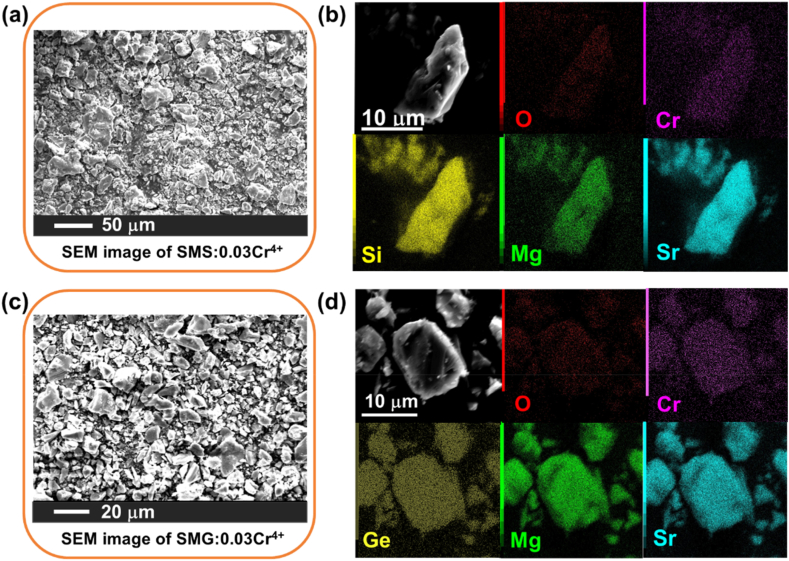
The diffraction peaks of the synthesized Sr2MgSi2O7/Sr2MgGe2O7 correspond well with the standard patterns, as depicted in Fig. 3. In both series, the incorporation of Cr4+ results in a significant shift of diffraction peaks towards large angles, indicating cell contraction according to the Bragg equation. However, for Sr2MgSi2O7:Cr4+, the peak shift displays a consistent trend towards larger angles, while for Sr2MgGe2O7:Cr4+, the shift is postponed at low Cr content, as depicted in the inserts of Fig. 3 a and b. The difference in trend arises from the tetrahedral occupation of Cr4+ in Mg2+/Si4+/Ge4+. Since Mg2+ has larger ionic radius of 0.57 Å than the 0.26/0.39 Å of Si4+/Ge4+ in a tetrahedral environment, the cell contraction suggests that Cr4+ with an ionic radius of 0.41 Å substitutes the Mg2+ sites in the host [21,22]. In Sr2MgSi2O7:Cr, the MgO4 tetrahedron serves as the only substitution site for Cr4+ due to the extreme small size of the Si site. On the other hand, Ge shares a similar ionic radius with Cr4+, and at low doping contents of Sr2MgGe2O7, the substitution of Cr4+ for Ge4+ compensates for the cell shrinkage. Therefore, the co-substitution of MgO4-GeO4 tetrahedra in Sr2MgGe2O7 explains the delayed diffraction peak shift. Further, we extract the cell parameters and volumes by whole pattern Rietveld refinements, as displayed in Fig. 4. It is obvious that the doping of Cr in Sr2MgSi2O7 causes continuous shrinkage, but the shrinkage in Sr2MgGe2O7 is postponed. Since the ionic radius of Cr4+ lies between Mg2+ and Si4+, it is then confirmed that Cr4+ mainly substitutes for Mg2+ not Si4+. It is likely that the valence imbalance caused by the aliovalent substitution between Cr4+ and Mg2+ could be compensated for by the creation of Mg2+ or Si4+/Ge4+ vacancies. The powder morphologies are measured by the scanning electron microscopy (SEM), and the images are displayed in Fig. 5. They have typical shapes of inorganic powders. Meanwhile, the energy dispersive X-ray spectroscopy (EDS) elemental mappings in Fig. 5 show uniform composition distribution of the selected particles.
Fig. 3.
XRD patterns and selected diffraction peaks near 30° of (a) Sr2Mg1-xSi2O7:xCr4+ (x = 0, 0.01, 0.03 and 0.05), and (b) Sr2Mg1-xGe2O7:xCr4+ (x = 0, 0.01, 0.03 and 0.05).
Fig. 4.
(a) Represented Rietveld refinement of Sr2Mg1-xGe2O7 sample; (b) Variations of cell parameters a, c and volumes as a function of x in Sr2Mg1-xSi2O7:xCr4+ and Sr2Mg1-xGe2O7:xCr4+.
Fig. 5.
The SEM and mapping images: (a) and (b) for SMS, (c) and (d) for SMG.
3.3. Color rendering modulation of Cr4+ due to Si/Ge substitution
As depicted in the inserts of Fig. 6 a and b, the visual colors of Sr2MgSi2O7:Cr4+ and Sr2MgGe2O7:Cr4+ stem from the incorporation of Cr4+ into the host, as the undoped samples have a white color. The color of Sr2MgSi2O7:Cr4+ appears as ice blue, while that of Sr2MgGe2O7:Cr4+ presents as dark-sea green. To comprehend the origin of color, DR spectra are measured, as shown in Fig. 6 a and b. The undoped hosts exhibit nearly uniform reflection throughout the visible-light region, resulting in the aforementioned white color. The incorporation of Cr4+ results in a series of absorptions that arise from the intra-configurational transitions of 3d2 electrons. The energy levels of 3d-block transition-metals could be analyzed using Tanabe-Sugano (T-S) diagrams, which, however, are derived under the octahedral environment. In this study, the energy levels of 3 d2 electrons in a tetrahedral environment are required to analyze the color change caused by Si/Ge compositional modification. Although the T-S diagrams of 3d8 in octahedron could be employed as an approximation for 3d2 in tetrahedron, it fails to quantify the crystal-field parameters of Dq/B, as evidenced in numerous Cr4+ doped near-infrared luminescent phosphors.[[23], [24]] Recently, the energy level diagram of 3d2 electrons in the tetrahedron environment has been constructed using the irreducible tensor operator method [25], and it can be employed to conduct a crystal field analysis of Cr4+ in Sr2Mg(Si/Ge)2O7. As shown in Fig. 6 c, the absorption peaks are ascribed to transitions from the ground state 3A2(3F) to excited states 3T1(3P) and 3T1(3F). The ∼430 nm peak due to 3A2(3F)→3T1(3P) transition is overlapped with the host absorption band. The three peaks in the red region belong to 3T1(3F). All those peak wavelengths denoted in Fig. 6 c are used to calculate the Dq/B value according to the following equations [25].
Fig. 6.
DR spectra and the visual colors of (a) SMS:xCr4+ (x = 0, 0.01, 0.03 and 0.05), and (b) SMG:xCr4+ (x = 0, 0.01, 0.03 and 0.05). (c) DR spectra of SMS:0.01Cr4+ and SMG:0.01Cr4+. (d) T-S energy level diagram for Cr4+ ion in tetrahedral coordination environment in SMS:Cr4+ and SMG:Cr4+ phosphors.
The Dq/B values for Sr2MgSi2O7:Cr and Sr2MgGe2O7:Cr are 1.40 and 1.36, respectively. The two values are located in the weak field region as shown in Fig. 6 d. The variation in crystal field splitting could be interpreted by the Si/Ge substitution in the two hosts. The relationship between crystal field splitting Dq and center-vertex distance R could be characterized by the following equation [15].
In this model, the ligand has charge Ze, and r stands for the radial part of the electron wavefunction. As a result, the smaller ionic size of Si over Ge leads to shorter center-vertex distance R, and consequently larger crystal field splitting, as reflected by Fig. 6 d. Therefore, the color change and the subtle difference in DR spectra arise from the crystal field strength modulated by Si/Ge variation in the host composition. It needs to be noted that in Sr2MgGe2O7, Cr4+ in the GeO4 tetrahedron could have a smaller vertex-distance R than that in MgO4 of Sr2MgSi2O7. Considering the overall shrinking trend in Fig. 4, however, it still supports a smaller average Cr4+-O bond length in Sr2MgSi2O7, and thus larger Dq/B value. Generally, the modulation of Dq/B values by compositional modulation has been widely studied in Cr3+ doped phosphors. For example, substituting Cr3+ for larger cations could reduce the Dq/B value, and change the luminescence profile from sharp peaks to the wide-band shape [26].
4. Conclusion
Cr doped Sr2MgSi2O7 and Sr2MgGe2O7 strontium melilites were synthesized by high-temperature solid-state reaction method. All the samples are single phased and crystallized in the melilite structure. By analyzing the diffraction peak shift, it is concluded that Cr4+ substitute for Mg site in the Sr2MgSi2O7 host, and both Mg/Ge sites in the Sr2MgGe2O7 site. Diffuse reflectance spectra explain the color difference caused by Si/Ge variation. The Tanabe-Sugano diagram is constructed to perform the crystal field analysis. This work will be helpful in applying spectroscopic technologies to the fields of cultural heritage protection.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Fang Wen: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Chenghang Li: Software, Methodology, Investigation. Bo Zhang: Resources, Funding acquisition, Conceptualization. Ke Qin: Investigation, Data curation. Xin Li: Methodology, Investigation. Mingyue Chen: Writing – original draft, Visualization, Funding acquisition. Zhen Song: Validation, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work is supported by the Fundamental Research Funds for the Central Universities (FRF-TP-22-002A1), and Research on post-disaster planning and construction in the Beijing-Tianjin-Hebei region in 2023 - Safety assessment and rescue measures for heritage buildings under flood risk - Taking Mentougou District Beijing as an example (110051360023XN278-21)
Contributor Information
Fang Wen, Email: fangwen@ncut.edu.cn.
Bo Zhang, Email: abaodoc@ncut.edu.cn.
References
- 1.Jun T. China Architecture & Building Press; Beijing: 1984. Annals of Jiangnan Gardens. [Google Scholar]
- 2.Wenfu D. SDX Joint Publishing Company; Beijing: 2002. Classical Chinese Rocks. [Google Scholar]
- 3.Xiangyun J. Shanghai Scientific & Technical Publishers; Shanghai: 2010. The Phylogeny of Chinese Shangshi Culture. [Google Scholar]
- 4.Zhaozhen M. China Forestry Publishing House; Beijing: 2012. Landscape Architecture Engineering. [Google Scholar]
- 5.Shao Y., Cai H., Zhao F., Song Z., Liu Q. Efficient blue–violet phosphor with small Stokes-shift for Full-Spectrum lighting. Laser Photonics Rev. 2023;17 doi: 10.1002/lpor.202300342. [DOI] [Google Scholar]
- 6.Wang S.X., Song Z., Liu Q.L. Recent progress in Ce3+/Eu2+-activated LEDs and persistent phosphors: focusing on the local structure and the electronic structure. J. Mater. Chem. C. 2023;11(1):48–96. doi: 10.1039/D2TC02639B. [DOI] [Google Scholar]
- 7.Liu S.Q., Du J.X., Song Z., Ma C.-G., Liu Q.L. Intervalence charge transfer of Cr3+-Cr3+ aggregation for NIR-II luminescence. Light Sci. Appl. 2023;12(1):181. doi: 10.1038/s41377-023-01219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Tang B., Cao X. The roles of dopant concentration and defect states in the optical properties of Sr2MgSi2O7:Eu2+, Dy3+ J. Alloys Compd. 2023;949 doi: 10.1016/j.jallcom.2023.169841. [DOI] [Google Scholar]
- 9.Srinivasa P.R., Krushna B.R.R., Malleshappa J., Sharma S.C., Manjunatha K., Wu S.Y., et al. Blue light emitting Sr2MgSi2O7:Eu2+ nanophosphor for latent fingerprint, anti-counterfeiting and near UV-LED applications. Colloids Surf. A Physicochem. Eng. Asp. 2023;674 doi: 10.1016/j.colsurfa.2023.131857. [DOI] [Google Scholar]
- 10.Zhang M., Wang J., Ding W., Zhang Q., Su Q. Luminescence properties of M2MgSi2O7:Eu2+ (M=Ca, Sr) phosphors and their effects on yellow and blue LEDs for solid-state lighting. Opt. Mater. 2007;30(4):571–578. doi: 10.1016/j.optmat.2007.01.008. [DOI] [Google Scholar]
- 11.Wu H., Wang Y., Hu Y., Deng L., Xie W. Controllable optical properties by ratio of Sr/Ca in Sr1.97−xCaxMgSi2O7:Eu0.012+, Dy0.023+ phosphors. J. Phys. Appl. Phys. 2009;42(12) doi: 10.1088/0022-3727/42/12/125406. [DOI] [Google Scholar]
- 12.Kim T., Kim Y., Kang S. Luminescence properties of Eu2+ in M2MgSi2O7 (M=Ca, Sr, and Ba) phosphors. Appl. Phys. B. 2012;106(4):1009–1013. doi: 10.1007/s00340-012-4914-z. [DOI] [Google Scholar]
- 13.Volker B., Cees R., Oliver O., Wolfgang S., Andries M. Color Point tuning for (Sr,Ca,Ba)Si2O2N2:Eu2+for white light LEDs. Chem. Mater. 2009;21(2):316–325. [Google Scholar]
- 14.Song Z., Liu Q.L. Basic crystal field theory—a simple and useful tool to understand the structure–property relationship in luminescent materials. Opt. Mater. X. 2022;16 doi: 10.1016/j.omx.2022.100189. [DOI] [Google Scholar]
- 15.Song Z., Liu Q.L. Crystal-field splitting in Regular Polyhedron. Chin. J. Lumin. 2022;43(9):1428–1435. doi: 10.37188/CJL.20220190. [DOI] [Google Scholar]
- 16.Liu B., Shi C., Yin M., Dong L., Xiao Z. The trap states in the Sr2MgSi2O7 and (Sr,Ca)MgSi2O7 long afterglow phosphor activated by Eu2+ and Dy3+ J. Alloys Compd. 2005;387(1):65–69. doi: 10.1016/j.jallcom.2004.06.061. [DOI] [Google Scholar]
- 17.Dorenbos P. Mechanism of persistent luminescence in Sr2MgSi2O7:Eu2+; Dy3+ Phys. Status Solidi B. 2005;242(1):R7–R9. doi: 10.1002/pssb.200409080. [DOI] [Google Scholar]
- 18.Wang X., Zhao Y., Yin M., Zhou T., Xie R.-J. Cr3+-Doped La2MgSnO6 Double Perovskite phosphors for near-infrared pc-LEDs. J. Phys. Chem. C. 2023;127(46):22799–22807. doi: 10.1021/acs.jpcc.3c05834. [DOI] [Google Scholar]
- 19.Sun Z., Zhou T., Liu R., Tang X., Xie R. Ultrawide near‐infrared SrHfO3:Cr3+ phosphor with dual emission bands. J. Am. Ceram. Soc. 2023;106(6):3446–3454. doi: 10.1111/jace.18995. [DOI] [Google Scholar]
- 20.Rodríguez-Carvajal J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter. 1993;192(1):55–69. doi: 10.1016/0921-4526(93)90108-I. [DOI] [Google Scholar]
- 21.Shannon R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A. 1976;32(5):751–767. doi: 10.1107/s0567739476001551. [DOI] [Google Scholar]
- 22.Shannon R.D., Prewitt C.T. Effective ionic radii in oxides and fluorides. Acta Crystallogr. Sect. B. 1969;25(5):925–946. doi: 10.1107/S0567740869003220. [DOI] [Google Scholar]
- 23.Kück S., Hartung S. Comparative study of the spectroscopic properties of Cr4+-doped LiAlO2 and LiGaO2. Chem. Phys. 1999;240(3):387–401. [Google Scholar]
- 24.Sharonov M.Y., Bykov A., Owen S., Petricevic V., Alfano R. Crystal growth and optical properties of Cr4+: Li2TiGeO5. J. Appl. Phys. 2003;93(2):1044–1047. [Google Scholar]
- 25.Song Z., Liu Q.L. Energy level diagram of 3d2 configuration in tetrahedral crystal field and its applications to Cr4+/Mn5+ -Doped luminescent materials. Adv. Theory Simul. 2022;5(11) doi: 10.1002/adts.202200466. [DOI] [Google Scholar]
- 26.Zhao F.Y., Song Z., Liu Q.L. Advances in chromium‐activated phosphors for near‐infrared light sources. Laser Photonics Rev. 2022;16(11) doi: 10.1002/lpor.202200380. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.