Abstract
This protocol describes how to obtain high-quality retinal cryosections in larger animals, such as rabbits. After enucleation, the eye is briefly immersed in the fixative. Then, the cornea and iris are removed and the eye is left overnight for additional fixation at 4 °C. Following fixation, the lens is removed. The eye is then placed in a cryomold and filled with an embedding medium. By removing the lens, the embedding medium has better access to the vitreous and leads to better retinal stability. Importantly, the eye should be incubated in embedding medium overnight to allow complete infiltration throughout the vitreous. Following overnight incubation, the eye is frozen on dry ice and sectioned. Whole retinal sections may be obtained for use in immunohistochemistry. Standard staining protocols may be utilized to study the localization of antigens within the retinal tissue. Adherence to this protocol results in high-quality retinal cryosections that may be used in any experiment utilizing immunohistochemistry.
Introduction
The retina is composed of several layers of specialized cells within the eye that together work to convert light into neural signals. Because the retina plays a critical role in vision, understanding its structure and function can provide valuable insights into some of the most common causes of vision loss such as macular degeneration and diabetic retinopathy, among others.
Rabbits serve as a convenient animal model in retinal research as they offer several advantages compared to other models. Rabbit eyes are relatively similar in anatomy to human eyes1,2. For example, rabbits have an area of increased photoreceptor density, known as the horizontal visual streak, that is analogous to the fovea in humans. Other commonly used animal models, such as rodents, do not have an anatomic equivalent. In addition, compared to rodents, the retinal vasculature in rabbits is fairly similar to that in humans. Rabbit eyes are relatively large as well. This makes them particularly suitable for studies that involve drug administration or surgical intervention within the vitreous or retina that may otherwise be difficult or impossible in a smaller eye3.
Immunohistochemistry (IHC) is a widely used technique to study the localization of antigens within a tissue and has broad applications in retinal research4,5,6. Because the retina is a delicate structure, obtaining useful results via IHC requires careful tissue processing. Retinal detachment and other tissue artifacts such as retinal breaks or folds commonly occur during processing and may interfere with the interpretation of results. Successful processing depends on a variety of factors, including tissue manipulation, type and duration of fixation, type of embedding media, and sectioning techniques7,8,9,10. Despite the advantages of using rabbits as an animal model in retinal research, very few protocols describing successful tissue processing of the rabbit retina exist. This paper describes a reliable method for obtaining high-quality retinal sections from whole rabbit eyes for use in IHC.
Protocol
All procedures were carried out in compliance with and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Southern California. Fourteen (n = 14) Dutch-belted rabbits between 4 and 6 months of age were used in the development of this protocol. Both male and female animals were used. All animals weighed between 2.0 and 2.5 kg. All animals were singly housed. A list of recommended materials and equipment can be found in the Table of Materials.
Table of Materials.
The list of materials used.
| Name of Material/Equipment | Company | Catalog Number | Comments/Description |
|---|---|---|---|
| 100 mm culture dish | Corning | 353025 | Used for dissection (steps 1.3, 3 and 5) |
| 50 mL tube | Genesee Scientific | 28–106 | For fixation and cryoprotection (step 1) |
| Cryostat | Leica | CM1850 | For cryosectioning (step 7) |
| Curved scissors | Fine Science Tools | 91500-09 | Used for dissection (steps 1.3, 3 and 5) |
| DAPI | Fisher Scientific | D3571 | Diluted 1:1,000 in blocking buffer |
| Dissection microscope | Zeiss | Stemi 2000-C | Used for dissection (steps 1.3, 3 and 5) |
| Donkey anti-Goat 488 | Fisher Scientific | A-11055 | Diluted 1:1,000 in blocking buffer |
| Donkey anti-Mouse 555 | Fisher Scientific | A-31570 | Diluted 1:1,000 in blocking buffer |
| Forceps | Fine Science Tools | 91150-20 | Used for dissection (steps 1.3, 3 and 5) |
| Glass Slide Cover | VWR | 48404-453 | For cryosectioning (step 7) |
| Goat anti-SOX2 | R&D Systems | AF2018 | Diluted 1:100 in blocking buffer |
| High-profile disposable cryostat blades | Leica Microsystems Inc. | 14035838926 | For cryosectioning (step 7) |
| Kimwipe | Fisher Scientific | 06-666-A | Used to wipe away excess PBS or OCT (steps 3 and 6) |
| Mouse anti-RPE65 | Novus Bio | NB100-355SS | Diluted 1:100 in blocking buffer |
| OmniPur Sucrose | Millipore | 167117 | Used for cryoprotectant (step 1.2) |
| Paraformaldehyde 20% solution | Electron Microscopy Sciences | 15713 | Used as tissue fixative (diluted to 4% in step 1.1) |
| Peel-A-Away Disposable Embedding Mold (22x22x20 mm Deep) | Polysciences, Inc. | 18646A | Used as embedding mold (step 6) |
| Phosphate buffered saline, 1x | Corning | 21-030-CV | Used in preparation of fixative (step 1.1) and cryoprotectant (step 1.2) |
| Scalpel blade no. 15 | Feather | 08-916-5D | Used for dissection (steps 1.3, 3 and 5) |
| Superfrost Plus Microscope Slides | Fisher Scientific | 12-550-15 | For cryosectioning (step 7) |
| Tissue-Tek O.C.T. Compound | Sakura | 4583 | Used as embedding media (step 6) |
1. Preparation
- Fixative preparation.
- Prepare 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). At a minimum, 50 mL of fixative is required per eye.
- Cryoprotective agent preparation.
- Prepare 30% weight by volume (w/v) sucrose in PBS. Place on a shaker for at least 15 min to ensure that the sucrose is completely dissolved. At a minimum, 50 mL of cryoprotective agent is required per eye.
- Dissection instrument preparation.
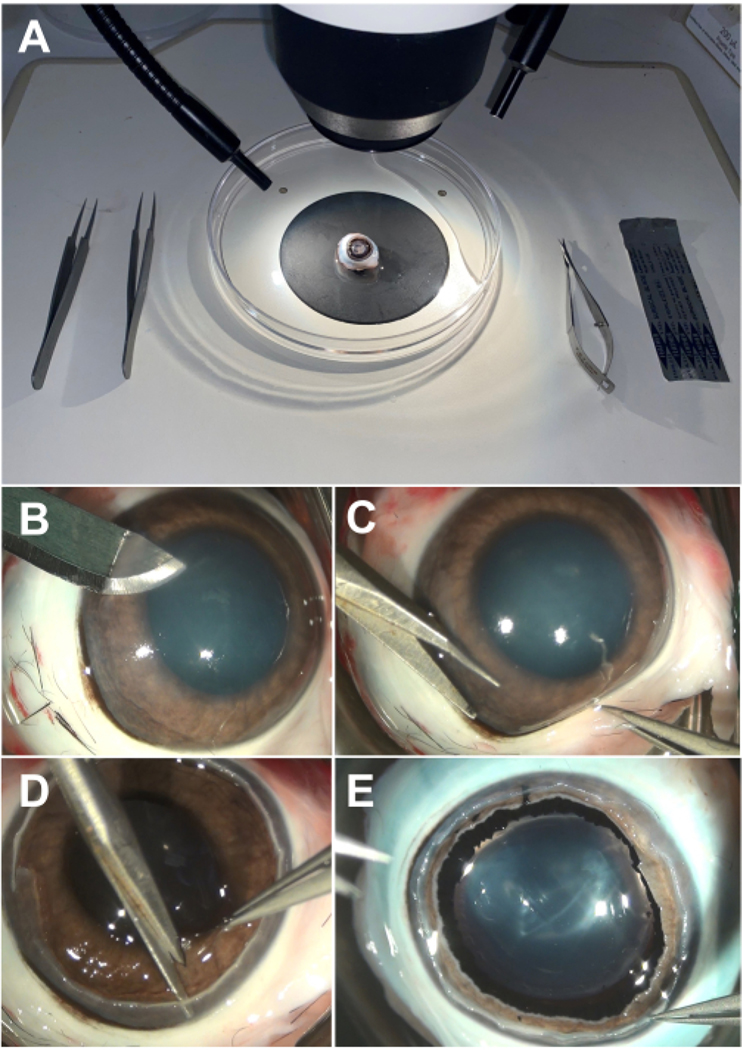
- Gather two pairs of forceps, one pair of curved scissors, one scalpel blade, and a 100 mm dissection dish and place them near a dissection microscope. An example setup is shown in Figure 1A.
Figure 1: Dissection of rabbit eye.
(A) An example setup of necessary equipment, including forceps, curved scissors, a scalpel blade, a 100 mm dissection dish, and a dissection microscope. (B) Initial corneal incision may be made with a scalpel blade and should be parallel to the plane of the iris. (C) The cornea may be removed by making a circumferential cut with curved scissors. (D) The iris may be removed by making a circumferential cut in a similar fashion to the cornea. (E) The lens may be mobilized and removed by cutting the zonules with curved scissors.
2. Initial fixation
Following trans-conjunctival enucleation11, immediately place the eye in at least 50 mL of 4% PFA in PBS on ice. Ensure that the eye is completely submerged. If air bubbles collect on the outer surface of the eye, remove them by gently shaking.
3. Cornea and iris removal
After 15 min, remove the eye from 4% PFA in PBS and place it in a 100 mm dissection dish under a dissecting microscope. Fill the dish with PBS to prevent the eye from drying out.
- Remove the cornea.
- Begin by creating an incision with a surgical blade 1 mm anterior to the limbus, parallel to the iris plane to avoid accidentally puncturing underlying structures (Figure 1B).
- Insert curved scissors into the initial incision and continue cutting circumferentially while remaining 1 mm from the limbus. Use forceps to stabilize the eye while cutting (Figure 1C).
- Once the circumferential cut is complete, remove the cornea with forceps and gently wipe away excess PBS with a laboratory wipe. Place the cornea in 30% sucrose solution at room temperature (RT) for cryoprotection or discard. Cryoprotection is complete when the cornea sinks, which typically takes less than 1 h.
- Remove the iris.
- Begin by making an initial cut with curved scissors through the iris. Completely separate the iris from the rest of the tissue by cutting circumferentially similar to cornea removal (Figure 1D).
- Once the circumferential cut is complete, remove the iris with forceps and gently wipe away excess PBS with a laboratory wipe. Place the iris in 30% sucrose solution at RT for cryoprotection or discard as above. Cryoprotection is complete when the iris sinks, which typically takes less than 30 min.
4. Completion of fixation
With the cornea and iris removed, place the eye in at least 50 mL of freshly prepared 4% PFA in PBS and put it on a shaker for gentle agitation. Ensure the eye remains submerged during agitation.
-
Keep the eye in 4% PFA in PBS at 4 °C overnight.
NOTE: We have found that 4% PFA results in excellent IHC staining if used between 16 h and 24 h at 4 °C, with longer fixation durations resulting in increased nonspecific background staining and epitope masking.
5. Lens removal
The following morning, remove the eye from 4% PFA in PBS and place it in a 100 mm dissection dish under a dissecting microscope. Fill the dish with PBS to prevent the eye from drying out.
- Remove the lens.
-
Begin by carefully grasping the anterior lens capsule with forceps.NOTE: Be extremely careful not to forcefully push or pull on the lens with the forceps as this can create a retinal detachment.
-
Carefully insert the scissors into the posterior chamber with the blades curved posteriorly along the lens. Make an initial cut through the lens zonules with curved scissors.NOTE: Be extremely careful not to forcefully push or pull on the zonules with the scissors as this can create a retinal detachment (Figure 1E).
- Continue to make small cuts through the lens zonules in a circumferential pattern. Make three to five cuts per clock hour.
- To check if the lens is separated from the rest of the tissue, gently grasp the anterior lens capsule with forceps and attempt to lift it gently. If the lens does not immediately lift, do not attempt to pull more as this can create a retinal detachment. Instead, make more cuts through the remaining lens zonules and reattempt.
- Once separated from the rest of the tissue, place the lens in 30% sucrose solution at RT for cryoprotection or discard as above. Cryoprotection is complete when the lens sinks, which typically takes a few hours.
-
Place the eye in at least 50 mL of 30% sucrose in PBS at 4 °C for cryoprotection. Cryoprotection is complete when the eye sinks, which typically takes 24–48 h. To expedite cryoprotection, exchange the initial sucrose solution with a fresh one after 8–12 h and/or keep the eye in sucrose solution at RT.
6. Embedding
-
Once cryoprotection is complete, remove the eye from 30% sucrose solution and place it in a 100 mm dissection dish under a dissecting microscope. Carefully flip the eye so it faces downward to remove excess sucrose solution from the inner part of the eye.
NOTE: Do not attempt to remove excess solution from the inner part of the eye with a laboratory wipe as the wipe can stick to the vitreous body and result in a retinal detachment.
With the eye facing downward, gently wipe away excess sucrose solution from the outer part of the eye using a wipe.
-
Fill a disposable embedding mold with optimal cutting temperature (OCT) compound to a depth of 0.5–1.0 cm.
NOTE: This base ensures the eye will not touch the bottom of the mold, which interferes with cryosectioning later.
Place the eye in the disposable embedding mold facing upward. Slowly fill the inner part of the eye with OCT taking special care to avoid bubble formation within the eye. If bubbles do form, carefully remove them or push them to the edge of the mold using forceps.
-
Finish filling the disposable embedding mold with OCT. Ensure the eye is completely submerged in OCT and is not touching the inner surfaces of the mold or exposed to the air. Wrap the disposable embedding mold containing the eye in OCT in parafilm; place at 4 °C overnight.
NOTE: In our experience, shorter incubation periods do not allow OCT to completely infiltrate the vitreous. As a result, a layer of sucrose often remains in between the retina and OCT. When this occurs, the retina often detaches or completely tears away from the rest of the tissue during cryosectioning as frozen sucrose likely provides less structural support than frozen OCT, making high-quality sections difficult or even impossible to obtain.
-
The following morning, place the embedding mold containing the eye in OCT in a 100 mm dissection dish under a dissecting microscope. Confirm that the posterior hyaloid membrane appears lifted upward from the inner retina.
NOTE: This upward lifting may obscure the view of the retina, making orientation difficult to determine.
-
To better determine the orientation of the eye, carefully grasp the posterior hyaloid membrane anteriorly and attempt to peel it away with forceps to expose the underlying retina while in OCT.
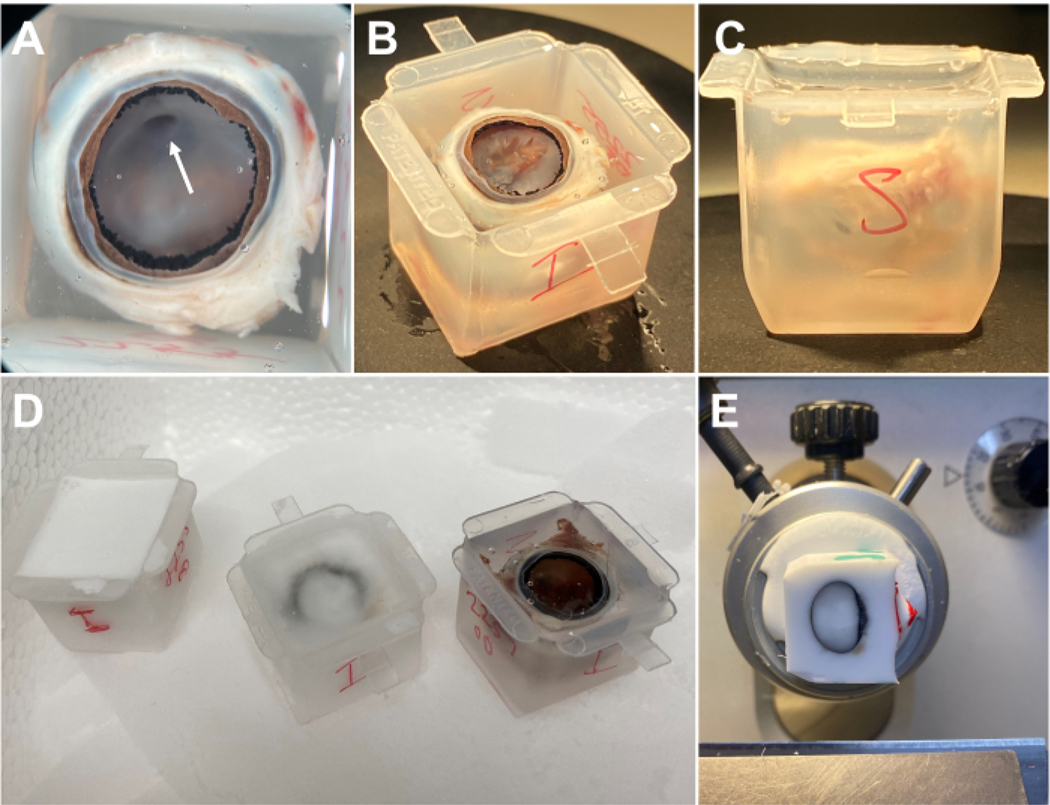
NOTE: At times, the hyaloid canal may be visible, which attaches to the optic nerve head and can be used for orientation purposes since the optic nerve head is located in the superior portion of the retina (Figure 2A). It is not necessary to completely remove the posterior hyaloid membrane as OCT has already infiltrated the vitreous body to line the inner retina and any remaining membrane will not affect cryosectioning (Figure 2B). The posterior hyaloid membrane should only be removed for orientation, if necessary. Other options for determining orientation include placing a suture or other type of mark prior to enucleation.
Transfer the eye to a new embedding mold with new OCT filled to a depth of 0.5–1.0 cm and then fill with new OCT as in steps 6.3–6.5 to ensure that the final embedding medium is free of debris and/or other imperfections that may have accumulated overnight. If possible, gently remove OCT with a wipe from the inside of the eye as well, although this is not necessary.
Label the mold for orientation purposes (Figure 2C). Orienting the eye with the eye facing upward in the cryo mold ensures that OCT will line the inner retina. The hyaloid canal, which attaches to the optic nerve head, is an important landmark for determining the superior part of the eye (Figure 2A).
Place the labeled embedding mold containing the eye in OCT on top of dry ice. Ensure that the eye is not touching any of the inner surfaces of the embedding mold or exposed to air. Ensure the mold is directly touching the dry ice to speed up the freezing process (Figure 2D).
Transfer the embedded eye to a −80 °C freezer or directly to the cryostat for sectioning (Figure 2E).
Figure 2: Embedding of rabbit eye.
(A) After overnight incubation in OCT, the hyaloid membrane should appear lifted. The hyaloid canal (arrowhead) may be visible and can be used for orientation purposes. (B) For better orientation, the hyaloid membrane may be dissected to reveal the optic nerve head underneath. (C) The eye should not touch any part of the cryomold and should be sufficiently submerged without significant bubble formation. (D) Cryomolds should be placed on dry ice. The appearance at various stages of freezing is shown. (E) After freezing, the block may be sectioned in the cryostat. This block is oriented with the pupil facing rightward. OCT is visible within the eye and in close apposition to the inner and outer surface of the eye. Abbreviation: OCT = optimal cutting temperature.
7. Cryosectioning
Mount the block with the superior part of the eye facing superiorly and the inferior part of the eye facing inferiorly, with the remaining cornea and iris facing to the right (as in Figure 2E) or left (not shown). Alternatively, mount the block with a different orientation, as desired. If necessary, break the mold to mobilize the block by carefully breaking off each side of the mold one at a time and marking the block directly to not lose the orientation of the eye.
Allow the block to equilibrate to the temperature within the cryostat for 30–60 min; −20 °C is a good starting point.
Once mounted, place a fresh blade at the desired angle, typically parallel to the vertical axis of the eye.
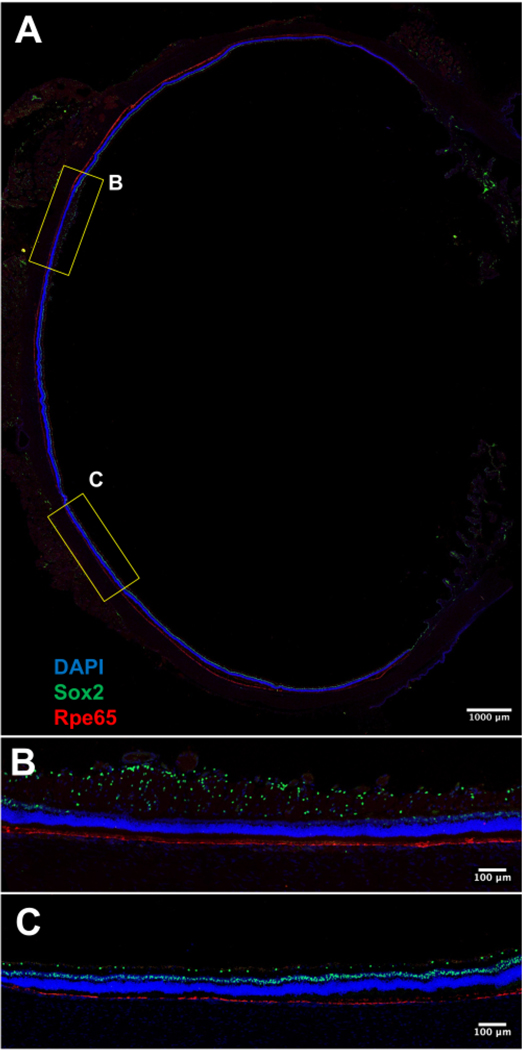
- Set the section thickness as desired: 10–20 μm thick sections are a good starting point. An example of a 14 μm thick section is demonstrated in Figure 3A–C.
- In a slow and controlled manner, begin sectioning through the block. Ensure the blade is cutting the block parallel to the vertical axis of the eye. If not, adjust the angle the blade contacts the block or stage orientation accordingly.
- While sectioning the block, prevent wrinkling or curling of the section with a brush. Quickly flip the section over and apply gentle pressure downward with a long-haired brush to flatten the section and minimize wrinkling or curling. Fashion a separate brush with a pointed tip to help manipulation of the section.
-
Attach the section to a charged glass slide by slowly moving the slide facing downward toward the section. Prevent the section from folding on itself or otherwise distorting itself during attachment by gently rolling the glass slide over the section.
NOTE: Do not hover the slide directly over the section or press it against the section as this can damage or distort the section.
Allow the slides to dry overnight before transferring to a −80 °C freezer or proceeding directly to a standard immunofluorescence protocol.
Figure 3: Representative fluorescence image of a whole retinal cryosection (20x magnification).
(A) The RPE layer is stained with Rpe65 (red). It is continuous and in close apposition to the photoreceptor layer. The nuclei are stained with DAPI (blue). Sox2 (green) shows the location of Müller glia nuclei within the inner nuclear layer and a subset of amacrine cells in the ganglion cell layer. (B) An image of the rabbit medullary ray, a horizontal streak of myelinated retinal nerve fiber layer. (C) An image of mid-peripheral rabbit retina. Scale bars = 1,000 μm (A), 100 μm (B,C). Abbreviations: RPE = retinal pigment epithelium; DAPI = 4’6,-diamidino-2-phenylindole.
Representative Results
After tissue processing, a standard immunofluorescence protocol may be utilized to investigate any number of biological processes within the retina. Figure 3A–C illustrate representative fluorescence images of a retinal section obtained via confocal microscopy. The retinal section was immunostained according to a previously described protocol12.
The representative retinal sections shown in Figure 3A–C are near the optic nerve head and oriented orthogonally to the axis of the retinal vessels (that is, the section contains peripheral retina, retinal vessels, myelinated nerve fibers, and the horizontal visual streak). Sox2+ nuclei may be seen within the nerve fiber layer and inner nuclear layer. RPE65 staining demonstrates a continuous layer of retinal pigment epithelium (RPE) cells adjoining the photoreceptor layer. The fluorescence images demonstrate a uniform pigmented RPE layer without significant detachment from the overlying photoreceptor layer. A brightfield image of the retinal section further demonstrates intact retinal morphology of all layers including the photoreceptor and RPE layers (Supplemental Figure S1). Small focal detachments may be seen in the peripheral retina, likely induced during the removal of the lens or manipulation during sectioning. Fluorescence images demonstrate uniform inner and outer nuclear layers without any significant laminar disruption or distortion, suggesting minimal tissue damage during described processing.
Discussion
Prior to implementing the above protocol, we consistently faced difficulties with the tissue processing of rabbit eyes for IHC. We had adapted several protocols from the eyes of smaller animals such as mice but found these to lead to inadequate fixation and difficulty with tissue sectioning. There are several important considerations that allow for consistent, high-quality sections of the rabbit retina.
One consideration is the large size of the rabbit globe in comparison to other commonly used animal models such as rodents. Because the rabbit eye is similar in size to the human eye, translational studies that utilize ocular procedures commonly done in humans may be readily performed. For example, procedures involving the retina and/or the vitreous such as intravitreal injection, laser photocoagulation, vitrectomy, and surgery for retinal detachment can all be safely and easily performed in rabbits13,14,15. However, the large size of the rabbit globe requires special considerations with regard to tissue fixation and embedding procedures that make the use of protocols developed in smaller animals less effective.
Appropriate fixation depends on several factors related to the type of tissue and what the tissue will ultimately be used for. In a large eye, it is important to ensure sufficient fixative penetration to the retina. Early removal of the cornea and iris allows for a more even fixative penetration to the retina by increasing direct access to the vitreous body. Although removal of the lens may also increase fixative penetration, we have found that early removal of the lens often leads to large retinal detachments that may involve the horizontal visual streak. By removing the lens after fixation, retinal detachments are less likely to occur because of increased crosslinking within the retina, which lends additional rigidity and better toleration of the mechanical forces exerted on the retina during dissection. The type and duration of fixative are also important factors to consider, with protocols recommending anything from 4% PFA, as in this protocol, to alternatives such as Davidson’s fixative or those containing various concentrations of glutaraldehyde. Although other fixatives have been reported to result in less tissue shrinking and may better preserve certain epitopes, we have found that 4% PFA results in excellent IHC staining if used between 16 h and 24 h at 4 °C. It is important to note that minor tissue shrinkage can result in a wave-like appearance of the outer nuclear layer in some areas, although this appearance will likely not affect major results.
Another important point to consider when preparing a larger eye such as a rabbit eye for IHC is the embedding procedure. OCT is a popular embedding material for use in IHC as it preserves tissue morphology and the antigenicity of a wide range of epitopes within the tissue, making it compatible with most IHC protocols and reagents. To preserve retinal morphology, it is important that OCT approximates the outside of the globe (the sclera side) and completely infiltrates and approximates the inside of the globe (the retina side). Incubating the eye in OCT overnight is one easy method to ensure this occurs. This allows sufficient time for the OCT to infiltrate the vitreous and results in detachment of the posterior hyaloid membrane away from the retina, allowing OCT to directly line the innermost surface of the retina. It is likely that OCT liquefies or otherwise causes contraction of the vitreous just as in posterior vitreous detachment.
The protocol described here provides a detailed and readily adaptable method for obtaining consistently high-quality retinal sections from whole rabbit eyes for use in IHC. Although many protocols exist for smaller animals such as rodents, very few protocols exist for processing rabbit retinas. Even in smaller animals, it is often very difficult to reliably obtain whole retinal sections without significant artifact formation. This results in the loss of valuable time and resources, as well as negatively affects the interpretation of results. This method minimizes tissue damage and cryo section loss. Furthermore, this method may easily be adapted for use in other larger mammals such as pigs, cows, or monkeys. Because this protocol requires careful dissection involving the lens and vitreous body, it is less applicable for use in smaller animals such as rodents. Future studies should investigate alternative methods for use in smaller eyes. In addition, future studies should investigate utilizing different fixation or embedding techniques that may further optimize results. Other modifications may be made to further minimize artifacts, such as the use of glue to stabilize the eye cup.
Supplementary Material
Supplemental Figure S1: Representative Brightfield image of a whole retinal cryosection (20x magnification). (A) Retinal layers are intact throughout the majority of the section. Small areas of focal detachment may be seen in the far periphery of the retina. (B) An image of the rabbit medullary ray demonstrating intact morphology of all retinal layers including the photoreceptor and RPE layers. (B) An image of the mid-peripheral rabbit retina demonstrating intact morphology of all retinal layers. Scale bars = 1,000 μm (A), 100 μm (B,C). Abbreviation: RPE = retinal pigment epithelium.
Acknowledgments
Thanks to Rosanna Calderon, Dominic Shayler, and Rosa Sierra for technical advice. This study was supported in part by an unrestricted grant to the Department of Ophthalmology at the USC Keck School of Medicine from Research to Prevent Blindness (AN), NIH K08EY030924 (AN), the Las Madrinas Endowment in Experimental Therapeutics for Ophthalmology (AN), a Research to Prevent Blindness Career Development Award (AN), Knights Templar Eye Foundation Endowment (AN), and the Edward N. and Della L. Thome Memorial Foundation (AN, KG).
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/66115.
Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Peiffer RL Jr, Pohm-Thorsen L, Corcoran K. Chapter 19-Models in ophthalmology and vision research. In American College of Laboratory Animal Medicine, The Biology of the Laboratory Rabbit. Manning PJ, Ringler DH, Newcomer CE (Eds), Academic Press, 409–433 (1994). [Google Scholar]
- 2.Davis FA The anatomy and histology of the eye and orbit of the rabbit. Trans Am Ophthalmol Soc. 27, 400.2–441 (1929). [PMC free article] [PubMed] [Google Scholar]
- 3.Zernii EY et al. Rabbit models of ocular diseases: New relevance for classical approaches. CNS & Neurological Disorders Drug Targets. 15 (3), 267–291 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Coons AH Labelled antigens and antibodies. Annu Rev Microbiol. 8, 333–352 (1954). [DOI] [PubMed] [Google Scholar]
- 5.Coons AH Fluorescent antibodies as histochemical tools. Fed Proc. 10 (2), 558–559 (1951). [PubMed] [Google Scholar]
- 6.Coons AH, Kaplan MH Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 91 (1), 1–13 (1950). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang J. et al. Step-by-step preparation of mouse eye sections for routine histology, immunofluorescence, and RNA in situ hybridization multiplexing. STAR Protoc. 2 (4), 100879 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorden SD et al. Spontaneous background and procedure-related microscopic findings and common artifacts in ocular tissues of laboratory animals in ocular studies. Toxicol Pathol. 49 (3), 569–580 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Margo CE, Lee A. Fixation of whole eyes: the role of fixative osmolarity in the production of tissue artifact. Graefes Arch Clin Exp Ophthalmol. 233 (6), 366–370 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S. Artefacts in histopathology. J Oral Maxillofac Pathol. 18 (Suppl 1), S111–S116 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell N. Enucleation in companion animals. Ir Vet J. 61 (2), 108–114 (2008). [Google Scholar]
- 12.Kastan NR et al. Development of an improved inhibitor of Lats kinases to promote regeneration of mammalian organs. Proc Natl Acad Sci U S A. 119 (28), e2206113119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baiza-Durán L. et al. Safety and tolerability evaluation after repeated intravitreal injections of a humanized anti-VEGF-A monoclonal antibody (PRO-169) versus ranibizumab in New Zealand white rabbits. Int J Retina Vitreous. 6, 32 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu DY, Cringle SJ, Su E, Yu PK, Humayun MS, Dorin G. Laser-induced changes in intraretinal oxygen distribution in pigmented rabbits. Invest Ophthalmol Vis Sci. 46 (3), 988–999 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Faude F. et al. Facilitation of artificial retinal detachment for macular translocation surgery tested in rabbit. Invest Ophthalmol Vis Sci. 42 (6), 1328–1337 (2001). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1: Representative Brightfield image of a whole retinal cryosection (20x magnification). (A) Retinal layers are intact throughout the majority of the section. Small areas of focal detachment may be seen in the far periphery of the retina. (B) An image of the rabbit medullary ray demonstrating intact morphology of all retinal layers including the photoreceptor and RPE layers. (B) An image of the mid-peripheral rabbit retina demonstrating intact morphology of all retinal layers. Scale bars = 1,000 μm (A), 100 μm (B,C). Abbreviation: RPE = retinal pigment epithelium.