Abstract
Coxsackievirus B3 (CVB3) infection induces myocardial inflammation and myocyte necrosis in some, but not all, strains of mice. C57BL/6 mice, which inherently lack major histocompatibility complex (MHC) class II IE antigen, develop minimal cardiac lesions despite high levels of virus in the heart. The present experiments evaluate the relative roles of class II IA and IE expression on myocarditis susceptibility in four transgenic C57BL/6 mouse strains differing in MHC class II antigen expression. Animals lacking MHC class II IE antigen (C57BL/6 [IA+ IE−] and ABo [IA− IE−]) developed minimal cardiac lesions subsequent to infection despite high concentrations of virus in the heart. In contrast, strains expressing IE (ABo Eα [IA− IE+] and Bl.Tg.Eα [IA+ IE+]) had substantial cardiac injury. Myocarditis susceptibility correlated to a Th1 (gamma interferon-positive) cell response in the spleen, while disease resistance correlated to a preferential Th2 (interleukin-4-positive) phenotype. Vγ/Vδ analysis indicates that distinct subpopulations of γδ+ T cells are activated after CVB3 infection of C57BL/6 and Bl.Tg.Eα mice. Depletion of γδ+ T cells abrogated myocarditis susceptibility in IE+ animals and resulted in a Th1→Th2 phenotype shift. These studies indicate that the MHC class II antigen haplotype controls myocarditis susceptibility, that this control is most likely mediated through the type of γδ T cells activated during CVB3 infection, and finally that different subpopulations of γδ+ T cells may either promote or inhibit Th1 cell responses.
Myocarditis is characterized as inflammation of the myocardium associated with microbial infections (7, 22, 31). Enteroviruses, including group B coxsackieviruses, are frequently implicated in this disease, yet only a small proportion of enterovirus-infected individuals contract clinical myocarditis. Various factors, including viral tropism, type and severity of cardiac infection (persistent versus nonpersistent), and characteristics of the host response to the virus, contribute to pathogenicity. The final outcome of the disease results from interactions between the virus, the infected cell, and the immune response. Studies using a murine model of coxsackievirus B3 (CVB3) myocarditis show that tissue injury depends predominantly on T-lymphocyte responses (11, 32). T-cell-deficient mice develop minimal cardiac damage even though virus continues to replicate in the heart. Furthermore, the type of T-cell response is crucial to pathogenicity. Mice can respond to infection with either Th1 or Th2 cell profiles (25). During Leishmania major infections, certain mouse strains mount dominant Th1 cell responses which are characterized by the production of interleukin-2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor alpha; this pattern is associated with delayed hypersensitivity reactions (1, 12). Other strains preferentially develop Th2 cell responses, which are characterized by production of IL-4, IL-5, and IL-10, a pattern characteristic of T-cell-dependent humoral immunity and eosinophil-mediated inflammation. In cutaneous leishmaniasis, Th1 cell responses confer disease resistance whereas Th2 cell responses result in death. In experimental myocarditis, the opposite pattern holds true, with Th1 cells promoting susceptibility and Th2 cells conferring resistance. T cells expressing the γδ T-cell receptor (TcR) determine Th1 responsiveness possibly by selectively killing Th2 cells (12). The present study provides new evidence that γδ+ T-cell control of myocarditis susceptibility involves major histocompatibility complex (MHC) class II antigens.
MHC molecules are responsible for graft rejection, limit T-cell recognition and effector function, and serve as signaling receptors capable of triggering cell death (5, 21, 28). MHC class II alleles act as major genetic elements in susceptibility to a variety of autoimmune diseases (20). MHC class I molecules are expressed on all nucleated cells, whereas MHC class II molecules are constitutively expressed on dendritic cells, B cells, and monocytic cells, but expression can be induced on mesenchymal and epithelial cells under conditions of inflammation. The genetic background of the host, especially the allele of MHC class II (IA or IE in the mouse; HLA-DR, -DP, or -DQ in humans), affects the cytokine bias (immune deviation) of the T-cell response to antigenic stimulus in vivo, as demonstrated in several mouse systems of parasitic and autoimmune disease (1, 1a, 7a, 33). How class II molecules influence immune deviation is poorly understood. Epitope presentation by IA and IE antigens differ, and IE-restricted epitopes may be more apt to stimulate Th2 differentiation (33). Alternatively, IE expression could alter γδ+ T-cell repertoire through clonal selection in the thymus. Activating γδ+ T cells in myocarditis-susceptible mouse strains favors Th1 cell differentiation (12). In contrast, myocarditis resistance in other mouse strains may result from activating different γδ+ T cells in these animals. In this report, we show that the γδ+ T-cell repertoires in the hearts and spleens of CVB3-infected C57BL/6 (IA+ IE−) and transgenic C57BL/6 mice expressing IE (Bl.Tg.Eα) differ substantially, that γδ+ T cells directly or indirectly determine myocarditis susceptibility to CVB3 infection, and that disease susceptibility depends upon the dominant CD4+ Th phenotype in the animals.
MATERIALS AND METHODS
Mice.
Genetically modified C57BL mice (males, 5 to 7 weeks of age) were bred and housed at the University of Vermont Animal Care facility. MHC class II transgenic mice have been well characterized as described elsewhere (3, 4, 15, 16, 29). Briefly, C57BL/6 mice inherently lack MHC class II IE because of a naturally occurring defect in the Eα gene making these animals class II IA+ IE−. MHC class II knockout (ABo) mice were made by mutating the Aβ loci by homologous recombination, thus disrupting the gene. Clones of the disrupted Aβ gene were injected into C57BL/6 blastocysts, and chimeric males were bred to C57BL/6 females. Progeny were backcrossed to C57BL/6 mice (3, 4). Animals expressing class II IE (IA+ IE+ or IA− IE+) were made by injecting a cloned Eαk gene from A/J mice into male pronucleus of F2 hybrids from C57BL/6 × SJL animals. After the 18th generation of backcrossing transgenic Eαk mice to C57BL/6 (IA+ IE+) mice, the animals were bred with ABo mice to make the IA− IE+ strain (15). Thus, all animals used in these studies are congenic to the C57BL/6 parental strain except for variations in MHC class II gene.
Infection.
Animals were infected by intraperitoneal (i.p.) (11) injection in 0.5 ml of phosphate-buffered saline (PBS) containing 5 × 104 PFU CVB3 (H3 variant) derived from Cos cells transfected with the infectious cDNA of this virus (13).
Organ viral titer.
Hearts were homogenized in 0.9 ml of RPMI 1640 containing penicillin (100 U/ml), streptomycin (100 μg/ml), and 5% fetal bovine serum (FBS). Cellular debris was removed by centrifugation at 1,045 × g for 10 min. The supernatant was titered by the plaque-forming assay on HeLa cell monolayers as described previously (11).
Histology.
Hearts were removed, fixed in 10% buffered formalin, paraffin embedded, sectioned, and stained with hematoxylin and eosin. Stained sections were used for image analysis in transmitted light mode with an Olympus BX50 compound light microscope (4× objective lens; numerical aperture, 0.13). True color digital images (640 by 480 pixels) were captured with a Sony DXC-960MD/LLP video camera connected via an RS170 cable to a video frame grabber on a Sun SPARCstation 5. Image processing and analysis were accomplished with IMIX software (Princeton Gamma Tech, Inc., Princeton, N.J.). Final percent cardiac injury was calculated by dividing the area of injury by the total area of the heart.
Preparation of lymphocytes.
Mice were euthanized by injecting sodium pentobarbital (120 mg/kg of body weight in PBS) i.p. The spleens were removed, disrupted to produce single-cell suspensions, and washed in RPMI 1640 medium containing 5% FBS and antibiotics. After removal of tissue debris by sedimentation, the cells were centrifuged at 225 × g for 10 min at 5°C. The cell pellet was treated with RBC (erythrocyte) lysing solution (Sigma), washed with medium, resuspended in medium, and counted by trypan blue exclusion. For preparation of lymphoid cells infiltrating the heart, hearts were removed, minced finely with scissors, and digested sequentially three times with 10 ml of 0.4% collagenase. Cells in the supernatant were washed twice and centrifuged on Histopaque (Sigma). Lymphoid cells at the interface were retrieved, washed, and counted by trypan blue exclusion.
Antibodies.
Isotype control and antigen-specific antibodies were obtained from Pharmingen (San Diego, Calif.) unless otherwise stated. These included fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated rat immunoglobulin G1 (IgG1) (clone R3-34); PE-rat anti-mouse IFN-γ (clone XMG 1.2); PE-rat anti-mouse IL-4 (clone BVD4-1D11); FITC-rat anti-mouse CD4 (clone GK 1.5); Red613-rat anti-mouse CD8 (clone 53-6.7; Gibco BRL); PE-mouse anti-IAb (clone AF6-120.1); PE-mouse anti-IEk (clone 14-4-4S); FITC-hamster IgG; and FITC-hamster anti-γδ TcR (clone GL3) antibodies. Additional FITC- and biotin-conjugated antibodies to Vγ1 (clone 2.11), Vγ4 (clone UC3), Vδ4 (clone GL2), and Vδ6.3 (clone 17C) were obtained from Rebecca O’Brien. Ascites antibody to γδ TcR was made by injecting approximately 107 GL3-3A hybridoma cells (clone originally obtained from Ralph Budd, Department of Medicine, University of Vermont) i.p. into BALB/c mice given 0.5 ml of pristane (2,6,10,14-tetramethyl-pentadecane) 10 to 20 days earlier and 550 R on the day of inoculation. Immunoglobulin was purified by 50% ammonium sulfate precipitation and Sepharose S-200 chromatography. Protein determination was done by spectrophotometry at 280 nm.
Cell surface marker staining.
Lymphocytes (105) were washed in PBS containing 1% bovine serum albumin (BSA) and 0.1% sodium azide (PBS-BSA) and resuspended in 0.1 ml PBS-BSA containing a 1:100 dilution of fluorochrome-labeled antibody and a 1:100 dilution of Fc-Block (Pharmingen). After incubation for 30 min on ice, the cells were washed twice in PBS-BSA and fixed in 2% formaldehyde for flow analysis.
Intracellular cytokine staining.
A modification of the method of Picker et al. (24) was used to evaluate intracellular cytokines in splenocytes. Briefly, 106 spleen cells were cultured in medium containing brefeldin A (10 μg/ml), phorbol myristate acetate (50 ng/ml), and ionomycin (500 ng/ml) (Sigma Chemical Co., St. Louis, Mo.) for 4 h at 37°C in 5% CO2. The cells were subsequently resuspended in medium containing rat polyclonal IgG (50 μg/ml; Zymed, San Francisco, Calif.) and brefeldin A, incubated for 10 min at 5°C, washed, and resuspended in medium containing Fc-Block (Pharmingen) and either fluorochrome-labeled surface marker antibodies or appropriate immunoglobulin isotype controls. After incubation on ice for 30 min, the cells were washed in PBS-BSA-brefeldin A and fixed for 10 min in 2% paraformaldehyde. The cells were then washed once in PBS-BSA, incubated for 10 min in PBS-BSA containing 0.5% saponin, and stained for intracellular cytokines, using either PE-anti-IFN-γ or PE-anti-IL-4. Isotype control for intracellular staining was PE-rat IgG. All staining was performed in buffer containing Fc-Block and polyclonal rat IgG (50 μg/ml) to block nonspecific antibody binding. The cell membranes were permeabilized in saponin permeabilization solution. After incubation for 30 min, the cells were washed twice in PBS-BSA-saponin and once in saponin-free PBS to close the membrane and then resuspended in PBS-azide containing 1% paraformaldehyde.
Flow cytometry.
We used a Coulter Epics Elite instrument with a single excitation wavelength (488 nm) and band filters for PE (575 nm), FITC (525 nm), and Red613 (613 nm). Each sample population was classified for cell size (forward scatter) and complexity (side scatter) and gated on a population of interest; data for 10,000 cells were evaluated. Criteria for positive staining were established based on the intensity of the isotype controls. The results were expressed as the percentage of cells within the size/complexity gate of interest that stained positively for each marker, or as the percentage of positive cells with gating on a second marker, after subtraction of the percent positive cells in the isotype control. The specificity of intracellular cytokine staining was demonstrated by negative results or by decreased percentages of stained cells when brefeldin A was omitted, the saponin permeabilization step was omitted, or the cells were stained with unlabeled antibody before staining with fluorochrome-linked antibody (data not shown).
Each study was repeated at least two times, and the data from a representative experiment are presented.
Statistics.
Statistical evaluation was performed by the Wilcoxon ranked score method.
RESULTS
Mortality and myocarditis in transgenic mice.
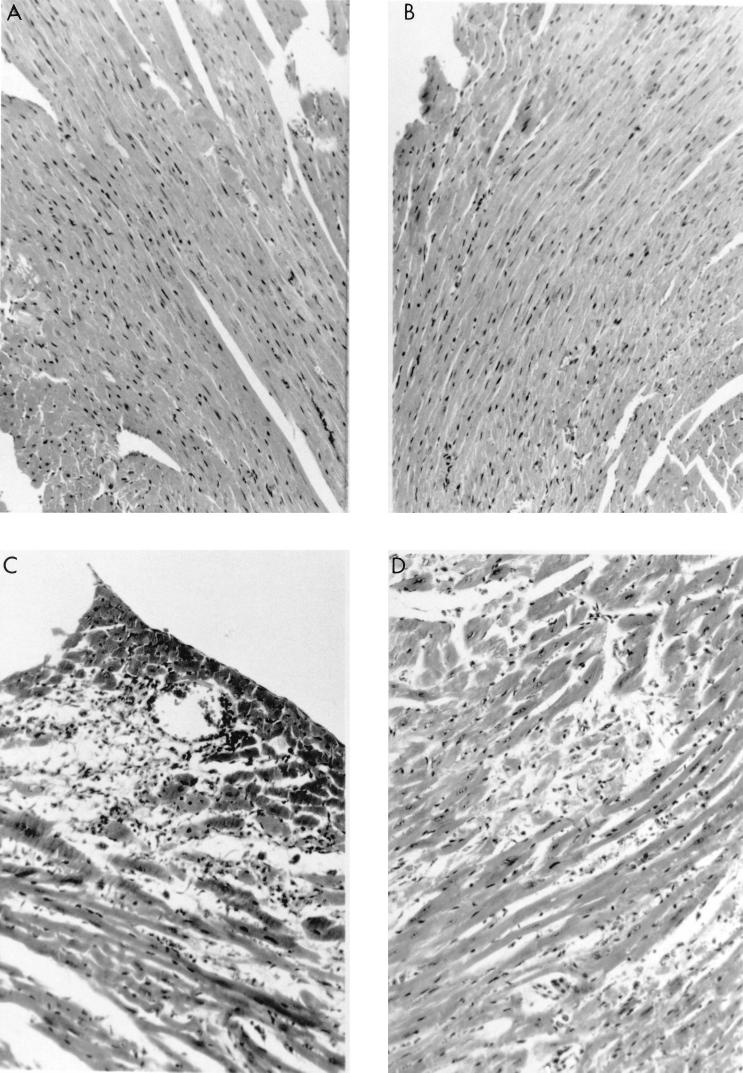
Transgenic mice differing in class II MHC antigen expression were infected i.p. with CVB3. Surviving mice were euthanized 7 days later for evaluation of myocarditis. Figure 1 shows representative histological sections of infected mice. Table 1 summarizes the mortality and extent of myocardial inflammation for each strain and treatment. ABo (IA− IE−) and C57BL/6 (IA+ IE−) mice had little or no cardiac inflammation and no mortality. In contrast, IE-bearing (Bl.Tg.Eα [IA+ IE+] and ABoEα [IA− IE+]) mice showed increased mortality accompanied by substantial myocardial necrosis or inflammation. ABoEα mice began dying earlier (day 3 postinfection) and had more extensive coagulative myocardial necrosis with limited cardiac inflammation compared to Bl.Tg.Eα mice. Cardiac lesions in Bl.Tg.Eα mice were consisted of extensive regions of mononuclear cell infiltration and myocyte dropout. Viral titers also differed between mouse strains, with the highest titers occurring in ABo and ABoEα mice. This finding suggests that IA expression is important in virus clearance. Also, the elevated viral titers in ABoEα mice must not be directly responsible for the necrotic heart lesions in this strain since ABo mice also had elevated virus concentrations but no histological evidence of cardiac injury.
FIG. 1.
Myocarditis in MHC class II antigen transgenic mice. Male ABo (IA− IE−) (A), C57BL/6 (IA+ IE−) (B), Bl.Tg.Eα (IA+ IE+) (C), and ABoEα (IA− IE+) (D) mice were infected i.p. with 104 PFU of CVB3 and killed 7 days later. Heart sections were stained with hematoxylin and eosin. Magnification, ×80.
TABLE 1.
Effect of depleting γδ+ T cells on CVB3-induced myocarditisa
| Strain | Antibody treatment | Cumulative mortality (day 7) | Mean virus titer (log 10 PFU) ± SEM | % Myocardium inflamed (mean ± SEM) |
|---|---|---|---|---|
| C57BL/6 (IA+ IE−) | None | 0 | 5.1 ± 0.7 | 0.5 ± 0.3 |
| Anti-γδ TcR | 0 | 5.5 ± 0.9 | 0 ± 0 | |
| ABo (IA− IE−) | None | 0 | 6.5 ± 1.4 | 0 ± 0 |
| Anti-γδ TcR | 0 | 7.1 ± 0.8 | 1.3 ± 0.8 | |
| ABoEαk (IA− IE+) | None | 100 | 6.2 ± 0.9 | 5.1 ± 2.0b |
| Anti-γδ TcR | 25 | 6.5 ± 0.7 | 1.8 ± 1.1c | |
| BL.Tg.Eαk (IA+ IE+) | None | 50 | 4.3 ± 0.5 | 8.3 ± 1.6b |
| Anti-γδ TcR | 0 | 5.3 ± 0.4c | 1.7 ± 0.5bc |
Male mice, 4 to 5 weeks of age, were injected i.p. with 100 μg of GL3-3A (anti-γδ) monoclonal antibody in 0.5 ml of PBS or isotype hamster IgG on days −1 and −2 relative to virus infection. Animals received 5 × 7 104 PFU of CVB3 on day 0, and surviving animals were euthanized on day 7. Hearts were removed from animals dying between days 5 and 7 for analysis. Hearts were divided, and the apex was formalin fixed, sectioned, and evaluated by image analysis for percentage of myocardium affected. The remaining tissue was titered by plaque-forming assay for virus. Groups consisted of four mice each.
Significantly different from C57BL/6 at P ≤ 0.05.
Significantly different from non-antibody-treated mice at P ≤ 0.05 by Wilcoxon ranked score.
Table 2 summarizes the characteristics of the splenocyte populations in the various CVB3-infected transgenic mouse groups. As expected, no IE+ splenocytes were observed in ABo mice; however, approximately 5% of these lymphoid cells expressed IAb. This low-level class II antigen expression most likely explains the presence of small numbers of CD4+ T cells in the spleen (1.5 × 106 cells, or approximately 14% of the number of CD4+ T cells in parental C57BL/6 mice). Total numbers of splenocytes in ABo mice were only slightly lower than in parental animals, due to increased proportions of other cell types such as CD8+ T cells in these animals (data not shown). IE+ strains (Bl.Tg.Eα and ABoEα) generally had substantially fewer splenocytes than IE− strains (C57BL/6 and ABo). Interestingly, depletion of γδ+ T cells in Bl.Tg.Eα mice restored splenocyte numbers, implying that these effectors directly or indirectly might regulate lymphocyte numbers in peripheral lymphoid organs. Numbers of γδ+ T cells were unexpectedly lower in ABo (IA− IE−) mice than in other strains but tended to be higher in Bl.Tg.Eα animals. The reason for these differences is not known.
TABLE 2.
Characterization of splenocyte populations in transgenic micea
| Strain | Mean ± SEM (n = 3–6 mice/group)
|
||||
|---|---|---|---|---|---|
| Total splenocytes (106) | Positive cells/spleen (106)
|
||||
| CD4+ | γδ+ | IA+ | IE+ | ||
| C57BL/6 | 98.3 ± 5.5 | 10.88 ± 2.7 (11b) | 4.0 ± 2.5 (4) | 53.8 ± 10.4 (55) | 0 ± 0 |
| ABo | 77.6 ± 16.2 | 1.5 ± 0.48c (2) | 1.7 ± 0.8 (2) | 5.5 ± 1.2c (7) | 0 ± 0 |
| ABoEα | 48.1 ± 9.2c | 6.0 ± 0.70c (12) | 2.6 ± 1.3 (5) | 4.3 ± 1.2c (9) | 23.6 ± 3.9c (49) |
| Bl.Tg.Eα | |||||
| Untreated | 59.9 ± 18.4c | 8.2 ± 2.1 (14) | 8.8 ± 3.9 (15) | 43.2 ± 8.6 (72) | 38.9 ± 4.6c (65) |
| Treated with anti-γδ TcR | 71.1 ± 10.3 | 10.6 ± 1.1 (15) | 0.5 ± 0.1cd (0.1) | ND | ND |
Spleen cells from individual animals infected 7 days earlier with 5 × 104 PFU of CVB3 were treated with RBC lysing solution (Sigma) and counted by trypan blue exclusion, and aliquots of the cells were stained for the cell surface markers as described in Materials and Methods. ND, not determined.
Percentage of total splenocytes positive for the marker.
Total cells per spleen differ from C57BL/6 group at P ≤ 0.05 by Wilcoxon ranked score.
Anti-γδ treatment significantly reduced total γδ+ T cells in spleens of Bl.Tg.Eα mice compared to isotype antibody-treated control animals of the same strain (P ≤ 0.05).
When Bl.Tg.Eα (IA+ IE+) mice were treated with 200 μg of monoclonal antibody to anti-γδ TcR antibody, cardiac injury was substantially reduced, demonstrating the importance of these cell populations in viral pathogenesis (Table 1). Efficacy of cell depletion was determined to be greater than 90% in three experiments (Table 2). These results demonstrate that γδ+ cells must affect myocarditis susceptibility in IE-bearing animals.
Lymphoid cells were isolated from spleens and hearts of individual mice 7 days after CVB3 infection and stained with Vγ- and Vδ-specific antibodies. Although there is substantial interanimal variability, the only statistically significant differences between C57BL/6 and Bl.Tg.Eα mice are increased proportions of Vγ1+ cells in the resistant and Vγ4+ cells the susceptible strains of mice (Table 3).
TABLE 3.
Vγ/Vδ usage in transgenic micea
| Strain | No. of mice/group | Tissue | Mean ± SEM
|
|||||
|---|---|---|---|---|---|---|---|---|
| Total lymphoid cells (105) | Total γδ+ (GL3-3A+) cells (105) | Positive cells/animal (105)
|
||||||
| Vγ1+ | Vγ4+ | Vδ4+ | Vδ6.3+ | |||||
| C57BL/6 | 3 | Heart | 8.7 ± 0.04 | 2.8 ± 0.9 (32b) | 1.6 ± 0.03 (18) | 0.2 ± 0.1 (2) | 0.5 ± 0.3 (6) | 0.6 ± 0.2 (7) |
| 4 | Spleen | 715.9 ± 63.5 | 45.2 ± 21.0 (6) | 9.4 ± 3.5c (1) | 2.1 ± 0.09 (0.3) | 4.0 ± 1.6 (0.1) | 4.8 ± 2.0 (0.1) | |
| Bl.Tg.Eα | 4 | Heart | 47.4 ± 1.8 | 18.2 ± 3.9 (39) | 2.3 ± 0.4 (5) | 5.2 ± 1.8c (11) | 3.3 ± 0.8 (7) | 1.4 ± 0.05 (3) |
| 6 | Spleen | 405.06 ± 23.5 | 61.5 ± 16.6 (15) | 4.7 ± 2.0 (1) | 10.3 ± 2.7c (3) | 3.2 ± 1.6 (1) | 5.7 ± 2.3 (1) | |
Lymphoid cells were isolated from spleens and hearts of individual mice 7 days after infection with CVB3 and depleted of RBC as indicated in Materials and Methods. Aliquots of cells were stained for a total γδ+ cells or Vγ/Vδ subpopulations.
Percentage of total lymphoid cells positive for the marker.
Total positive cells recovered per tissue differs from same tissue of other strain at P ≤ 0.05 by Wilcoxon ranked score.
Correlation of myocarditis susceptibility with preferential CD4+ Th1 cell responses.
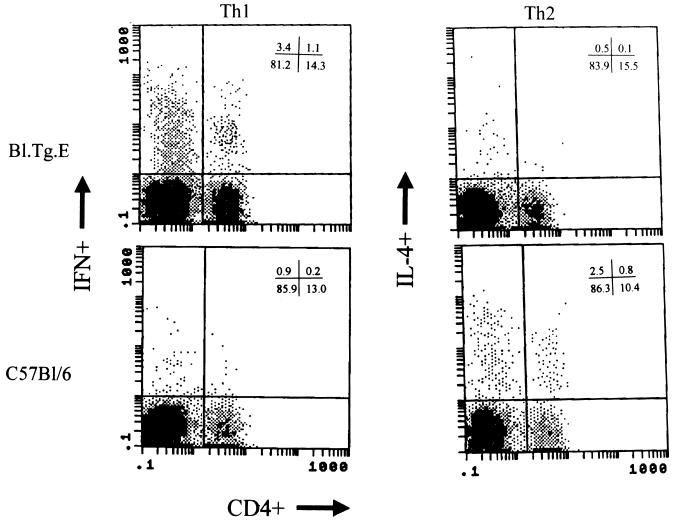
Earlier studies correlated myocarditis susceptibility in BALB/c mice infected with CVB3 with a preferential Th1 CD4+ cell phenotype (12). To evaluate the cytokine responses in C57BL/6 transgenic mice, splenocytes were isolated 7 days after infection and stained for CD4 and for intracellular IL-4 and IFN-γ (Table 4; Fig. 2). C57BL/6 mice had few IFN-γ-producing cells but more IL-4-producing cells than did IE-positive (Bl.Tg.Eα) animals. Depleting γδ+ T cells from Bl.Tg.Eα animals increased numbers of IL-4-producing cells, while numbers of IFN-γ-producing cells were only slightly decreased. Interestingly, most cytokine-producing cells in the spleen are CD4−. Evaluation of various additional lymphoid subsets indicates that approximately two-thirds of non-CD4 cytokine-positive cells are NK1.1+ and one-third are γδ+. Few CD8+ cytokine-producing cells were observed (data not shown). Thus, myocarditis susceptibility in MHC class II transgenic mice correlated with decreased Th2-like or increased Th1-like cell responses.
TABLE 4.
Th1/Th2 phenotype of CD4+ T cells in transgenic micea
| Strain | No. of mice/group | Mean ± SEM
|
|||||
|---|---|---|---|---|---|---|---|
| Total splenocytes (106) | Positive cells/spleen (106)
|
||||||
| CD4+ | IFN-γ+ | IL-4+ | CD4+ IFN-γ+ | CD4+ IL-4+ | |||
| C57BL/6 | 6 | 86.5 ± 13.7 | 11.9 ± 2.5 | 0.7 ± 0.4 (0.39b) | 1.8 ± 0.4 | 0.1 ± 0.1 (0.13) | 0.8 ± 0.3 |
| Bl.Tg.Eα | |||||||
| Untreated | 4 | 50.1 ± 12.4 | 6.0 ± 1.3 | 2.3 ± 0.9c (3.29) | 0.7 ± 0.3c | 0.5 ± 0.2c (2.5) | 0.2 ± 0.1c |
| Treated with anti-γδ TcR | 4 | 68.1 ± 10.7 | 8.9 ± 2.2 | 1.6 ± 0.4 (0.84) | 1.9 ± 0.5 | 0.3 ± 0.2 (0.60) | 0.5 ± 0.2 |
Bl.Tg.Eα mice were treated with a total of 200 μg of anti-γδ TcR antibody or hamster IgG as an isotype control. Spleen cells from individual mice obtained 7 days after infection with CVB3 were stained for CD4 and cytokines as described in Materials and Methods.
Ratio of IFN-γ+ cells to IL-4+ cells.
Number of positive cells significantly different from C57BL/6 at P ≤ 0.05 by Wilcoxon ranked score.
FIG. 2.
Cytometric analysis of IFN-γ and IL-4 production by splenocytes from CVB3-infected C57BL/6 and Bl.Tg.Eα transgenic mice. Splenocytes were obtained from mice 7 days after infection with 5 × 104 PFU of CVB3 and stained with antibody to CD4 and either IFN-γ or IL-4 intracellular cytokine production. Results are from one mouse for each strain out of a total of four or more mice per strain examined. Percentages of cells in each quadrant are given in the upper right corners of the graphs.
DISCUSSION
This report demonstrates three points. First, MHC class II IE expression promotes myocarditis susceptibility in C57BL/6 mice. Second, myocarditis correlates with induction of a Th1 cytokine phenotype whereas resistance correlates to a Th2 cell phenotype in these animals. Third, both myocarditis resistance and susceptibility apparently depend on γδ+ T cells which may use MHC class II antigens to affect Th subset differentiation.
MHC class II molecules affect disease susceptibility through several distinct mechanisms. The best-known mechanism is through antigenic epitope selection and presentation to T lymphocytes, resulting in biasing of the T-cell repertoire. Thus, individuals of a particular MHC haplotype are more likely to develop pathogenic autoimmune responses due to the ability of their MHC molecules to bind specific self peptides. In this case, myocardial injury ought to be mediated by IE-restricted T cells, while mouse strains lacking the relevant IE molecules are incapable of generating pathogenic T-cell responses. However, while IE− C57BL/6 mice are clearly resistant to CVB3-induced myocarditis, Henke et al. (8) demonstrated that CVB3 infection of C57BL/6 CD4 knockout mice resulted in severe myocarditis mediated by pathogenic CD8+ T-cell responses. This observation indicates that resistance in C57BL/6 mice results from preferential activation of immunoregulatory CD4+ T-cell responses which suppress pathogenic immunity. In this study we demonstrate that CVB3 infection of C57BL/6 mice stimulates primarily Th2-like (IL-4+) cell responses. Since CD4+ Th2 cells will suppress delayed hypersensitivity reactions, responses considered to be important in causing myocardial injury during myocarditis, this most likely explains the “immunosuppressive” CD4+ T cell described in the earlier publication. CVB3-infected Bl.Tg.Eα mice generate a predominant Th1-like response, but eliminating γδ+ T cells in these animals shifts the cytokine response to a Th2 cell phenotype and correlates to acquired resistance of these animals to myocarditis. Thus, the role of MHC class II IE in CVB3-induced myocarditis seems most likely to be in promoting immune deviation toward a Th1 phenotype rather than selecting specific IE-restricted CD4+ T-cell clones.
Studies using different antigenic stimuli variously report that γδ+ T cells promote immune deviation to either the Th1 or Th2 phenotype (2, 6, 9, 18, 34). We believe that the difference in γδ+ T-cell effect in C57BL/6 and Bl.Tg.Eα mice primarily reflects variations in γδ+ T-cell subtypes dominating in these two strains. Unlike T cells expressing the αβ TcR, γδ+ T cells usually react to antigen directly without requiring antigen processing. Frequently, γδ+ T-cell recognition either is MHC antigen independent or occurs in the absence of peptides bound to MHC molecules (27, 30). However, MHC class II IE molecules may determine the γδ+ T-cell repertoire in vivo presumably by affecting thymic differentiation or selection (14, 26). Thus, the subtypes of γδ+ T cells may differ between IE− and IE+ strains of mice. Should different subtypes of γδ+ T cells vary in the ability to influence Th cell differentiation, then the difference in effects of γδ+ T-cell depletion in C57BL/6 and Bl.Tg.Eα mice would reflect the γδ+ T-cell repertoire in these two strains. T cells expressing Vγ1 dominate in C57BL/6 mice. In contrast, cells expressing Vγ4 are more prevalent in Bl.Tg.Eα than parental mice. Differences in Vγ1+ Vγ4+ cell populations were especially evident in the heart as a proportion of the total γδ+ T-cell population. In absolute cell numbers, all γδ+ T-cell subpopulations were increased in hearts of IE+ mice because of the greater cardiac inflammation in this strain.
One potential problem with the γδ+ T-cell depletion studies is that the cells were eliminated with monoclonal anti-γδ TcR. Animals required a total of 200 μg of the monoclonal antibody for effective γδ+ T-cell elimination over the 9 days of the experiment. While this treatment resulted in over 90% depletion of γδ+ T cells in the spleen at the end of the experiment, it is probable that initial antibody-T cell interactions could have activated the γδ+ T-cell population prior to its depletion. Thus, whether the apparent protection observed in Bl.Tg.Eα mice reflects the elimination or the initial activation of this cell population by the antibody is problematic. Because of this problem, animals were treated with the monoclonal antibody for several days prior to infection. This should allow elimination of the γδ+ T cells and any transient effects caused by the antibody treatment before virus stimulation.
A separate question is how γδ+ T cells modulate Th cell responses. Most studies suggest that these effectors release cytokines favoring one or the other type of Th cell response (6). Our studies indicate γδ+ T-cell regulation in CVB3-induced myocarditis may depend on direct interactions between these effectors and the CD4+ T-cell population (10). Although many investigators have not demonstrated MHC class II antigen expression on activated CD4+ T cells, studies by Osborne and Rudikoff (23) show the presence of IA on this population. Our own experience indicates that MHC class II antigen expression on activated CD4+ T cells is restricted to IE molecules, while IA is often not induced. Thus, individuals staining only for IA could miss MHC class II upregulation in CD4+ T cells. Since a population of γδ+ T cells are known to recognize IE (14, 26), γδ+ T cells might influence modulation of immune deviation through the direct interaction of γδ+ T cells and activated CD4+ T cells, using IE expressed by the latter cells.
While there clearly is a role for IE molecules in myocarditis, IA molecules must also have some impact. Both Bl.Tg.Eα (IA+ IE+) and ABoEα (IA− IE+) mice develop significant cardiac lesions, but the lesions differ in nature. In the presence of IA, a highly cellular inflammatory lesion is observed, but in the absence of IA, the lesions are extremely necrotic yet have few infiltrating lymphoid cells. Furthermore, animal mortality is accelerated in IA− IE+ mice. Thus, IA-dependent responses modulate the character of the myocardial disease, although these molecules appear inherently less important in conferring overall disease susceptibility. One interesting note is that ABo mice, which should lack MHC class II molecules and be CD4+ T-cell deficient, are not myocarditis susceptible even though previous studies demonstrated that CD4 knockout mice developed myocarditis (8). One would think that MHC class II knockout and CD4 knockout mice should behave similarly. However, the ABo strain appears slightly leaky for MHC class II antigen expression, and significant numbers of CD4+ T cells remain in the spleen. Because C57BL/6 mice have a natural defect in the Eα gene, and the ABo strain was produced by disruption of the Aβ gene, chimeric molecules are possible between the Aα and Eβ chains (19). These chimeric molecules could allow some CD4+ T-cell selection, which might make the MHC class II knockout mice functionally different from CD4 knockout animals.
In conclusion, these studies are important because they demonstrate the complexity of genetic control of viral myocarditis. Clinically, HLA-DR4/1 and histidine at position 36 of the HLA-DQ β1 gene have been associated with increased susceptibility to myocarditis (17). Such associations can be controversial, however, and may not be found in all studies. One factor which could complicate MHC associations is that specific MHC haplotypes could contribute to either susceptibility or resistance in distinct ways. Thus, while some MHC haplotypes may promote myocarditis through presentation of heart-specific antigens and stimulation of pathogenic autoimmune responses, other MHC haplotypes may modulate susceptibility through effects on other types of cells, such as the γδ+ T cell, or on immune deviation.
ACKNOWLEDGMENTS
This work was supported by the following grants and institutional support: RO1 HL58583 (S.A.H.); RO1 HL47069 (G.S.D.); CA 24473 (C.D.); RO1 AI 33470 (M.K.N.); KO4 AI01291-01; and a grant from the Rocky Mountain Chapter of the Arthritis Foundation (R.L.O.).
We gratefully acknowledge the expert secretarial assistance of Roberta Christie and Debbie Perrotte. We are grateful for the expert flow cytometric analyses performed by Colette Charland.
REFERENCES
- 1.Bretscher P A. An hypothesis to explain why cell-mediated immunity alone can contain infections by certain intracellular parasites and how immune class regulation of the response against such parasites can be subverted. Immunol Cell Biol. 1992;70:343–351. doi: 10.1038/icb.1992.44. [DOI] [PubMed] [Google Scholar]
- 1a.Chakkalath H, Titus R. Leishmania major-parasitized macrophages augment Th2-type T cell activation. J Immunol. 1994;153:4378–4386. [PubMed] [Google Scholar]
- 2.Chomarat P, Kjeldsen-Kragh J, Quayle A, Natvig J, Miossec P. Different cytokine production profiles of gamma delta T cell clones: relation to inflammatory arthritis. Eur J Immunol. 1994;24:2087–2091. doi: 10.1002/eji.1830240923. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove D, Bodmer H, Bogue M, Benoist C, Mathis D. Evaluation of the functional equivalence of major histocompatibility complex class A and E complexes. J Exp Med. 1992;176:629–634. doi: 10.1084/jem.176.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 5.Doherty P C, Zinkernagel R M. A biological role for the major histocompatibility antigens. Lancet. 1975;i:1406–1409. doi: 10.1016/s0140-6736(75)92610-0. [DOI] [PubMed] [Google Scholar]
- 6.Ferrick D, Schrenzel M, Mulvania T, Hsieh B, Ferlin W, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 7.Friman G, Wesslen L, Fohlman J, Karjalainen J, Rolf C. The epidemiology of infectious myocarditis, lymphocytic and dilated cardiomyopathy. Eur Heart J. 1995;16:36–41. doi: 10.1093/eurheartj/16.suppl_o.36. [DOI] [PubMed] [Google Scholar]
- 7a.Fritz R B, Skeen M J, Chou C H, Garcia M, Egorov I K. Major histocompatibility complex-linked control of the murine immune response to myelin basic protein. J Immunol. 1985;134:2328–2332. [PubMed] [Google Scholar]
- 8.Henke A, Huber S, Stelzner A, Whitton J. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J Virol. 1995;69:6720–6728. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh J, Schrenzel M, Mulvania T, Lepper H, DiMolfetto-Landon L, Ferrick D. In vitro cytokine production in murine listerosis. Evidence for immunoregulation by gamma delta+ T cells. J Immunol. 1996;156:232–237. [PubMed] [Google Scholar]
- 10.Huber, S., R. Budd, K. Rossner, and M. Newell. Apoptosis in coxsackievirus B3-induced myocarditis and dilated cardiomyopathy. Submitted for publication. [DOI] [PubMed]
- 11.Huber S, Lodge P. Coxsackievirus B3 myocarditis in Balb/c mice: evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984;116:21–29. [PMC free article] [PubMed] [Google Scholar]
- 12.Huber S, Mortensen A, Moulton G. Modulation of cytokine expression by CD4+ T cells during coxsackievirus B3 infections of BALB/c mice initiated by cells expressing the γδ+ T-cell receptor. J Virol. 1996;70:3039–3045. doi: 10.1128/jvi.70.5.3039-3044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowlton K, Jeon E, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996;70:7811–7818. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefrancois L, LeCorre R, Mayo J, Bluestone J, Goodman T. Extrathymic selection of TCR gamma delta+ T cells by class II major histocompatibility complex molecules. Cell. 1990;63:333–340. doi: 10.1016/0092-8674(90)90166-c. [DOI] [PubMed] [Google Scholar]
- 15.LeMeur M, Gerlinger P, Benoit C, Mathis D. Correcting an immune-response deficiency by creating Ea gene transgenic mice. Nature. 1985;316:38–42. doi: 10.1038/316038a0. [DOI] [PubMed] [Google Scholar]
- 16.LeMeur M, Waltzinger C, Gerlinger P, Benoist C, Mathis D. Restricted assembly of MHC class II molecules in transgenic mice. J Immunol. 1989;142:323–327. [PubMed] [Google Scholar]
- 17.Limas C. Autoimmunity in dilated cardiomyopathy and the major histocompatibility complex. Int J Cardiol. 1996;54:113–116. doi: 10.1016/0167-5273(96)02587-9. [DOI] [PubMed] [Google Scholar]
- 18.McMenamin C, Pimm C, McKersey M, Holt P. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma-delta+ T cells. Science. 1994;265:1869–1873. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 19.McNicholas J M, Murphy D B, Matis L A, Schwartz R H, Lerner E A, Janeway C A, Jr, Jones P P. Immune response gene function correlates with the expression of an Ia antigen. I. Preferential association of certain Ae and Ealpha chains results in a quantitative deficiency in expression of an Ae:Ealpha complex. J Exp Med. 1982;155:490–507. doi: 10.1084/jem.155.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nepom G, Erlich H. MHC class II molecules and autoimmunity. Annu Rev Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- 21.Newell M, Justement L, Lehmann K, Caldwell K, Cooper D, Freed J, Cambier J. Do class II major histocompatibility molecules function as signal transducers during B lymphocyte activation? In: David C S, editor. Class II major histocompatibility complex genes: roles in immune function. New York, N.Y: Plenum Press; 1987. pp. 531–540. [Google Scholar]
- 22.Olsen E G J. Prognosis of dilated cardiomyopathy: the value of endomyocardial biopsies. In: Sekiguchi S, Richardson P J, editors. Prognosis and treatment of cardiomyopathies and myocarditis. Tokyo, Japan: University of Tokyo Press; 1994. pp. 121–127. [Google Scholar]
- 23.Osborne B, Rudikoff S. Murine thymocyte and splenocyte Ia antigens are indistinguishable by two-dimensional gel electrophoresis. J Immunol. 1983;131:1386–1390. [PubMed] [Google Scholar]
- 24.Picker L J, Singh M K, Zdraveski Z, Treer J R, Maino V C. Demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–1419. [PubMed] [Google Scholar]
- 25.Romagnani S. Induction of Th1 and Th2 responses: a key role for the “natural” immune response. Immunol Today. 1992;13:379–385. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- 26.Schild H, Mavaddat N, Litzenberger C, Ehrlich E, Davis M, Bluestone J, Matis L, Draper R, Chien Y-H. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1995;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 27.Schild H, Mavaddat N, Litzenberger C, Ehrlich E W, Davis M M, Bluestone J A, Matis L, Draper R K, Chien Y-H. The nature of major histocompatibility complex recognition by gamma-delta T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 28.Snell G. Studies in histocompatibility. Science. 1981;213:172–177. doi: 10.1126/science.7017931. [DOI] [PubMed] [Google Scholar]
- 29.Taneja V, Hansen J, Smart M, Griffiths M, Luthra H, David C. Expression of H-2E molecule mediates protection to collagen induced arthritis in HLA-DQ8 transgenic mice: role of cytokines. Int Immunol. 1997;9:1213–1219. doi: 10.1093/intimm/9.8.1213. [DOI] [PubMed] [Google Scholar]
- 30.Weintraub B C, Jackson M R, Hedrick S M. Gamma delta T cells can recognize nonclassical MHC in the absence of conventional antigenic peptides. J Immunol. 1994;153:3051–3058. [PubMed] [Google Scholar]
- 31.Woodruff J. Viral myocarditis. Am J Pathol. 1980;101:425–483. [PMC free article] [PubMed] [Google Scholar]
- 32.Woodruff J, Woodruff J. Involvement of T lymphocytes in the pathogenesis of coxsackievirus B3 heart disease. J Immunol. 1974;113:1726–1734. [PubMed] [Google Scholar]
- 33.Zamvil S, Mitchell D, Moore A, Kitamura K, Steinman L, Rothbard J. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–261. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- 34.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig B, Pereira P, Pretolani M. Requirement for gammadelta T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]