Abstract
Background
d-alanine administration prevented kidney damage in a murine acute kidney injury model. Further data are needed on the influence of d-alanine on kidney function in humans.
Objective
This study investigated the effects of d-alanine intake on amino acid metabolism and kidney function in healthy volunteers.
Methods
This multicenter pilot study randomly assigned individuals from the general Japanese population to receive 3 g or 6 g of d-alanine intake per day for 7 d in a 1:1 ratio. The primary endpoint was the mean change in plasma and urine d-alanine levels from baseline to 7 d after intake. The secondary endpoints were mean changes in kidney function and other clinical factors. Safety was assessed by evaluating adverse events and clinical parameters.
Results
We randomly assigned 24 participants to the 3-g (n = 12) and 6-g d-alanine (n = 12) groups. The mean baseline estimated glomerular filtration rate (eGFR) was 73 mL/min/1.73 m2. The mean plasma d-alanine concentration increased from baseline by 77.5 ± 34.3 and 192.1 ± 80.9 nmol/mL in the 3-g and 6-g d-alanine groups (both p < 0.0001), respectively, in a dose-dependent manner (between-group difference: 114.6 nmol/mL; 95% CI: 62.1–167.2; P = 0.0002). A similar increase was observed for the urine d-alanine to creatinine ratio. The mean eGFR was elevated by 5.7 ± 8.8 mL/min/1.73 m2 in the 6-g d-alanine group (P = 0.045) but did not significantly change in the 3-g d-alanine group. Nonserious adverse events were reported in 11 participants.
Conclusions
d-alanine intake increased plasma and urine d-alanine levels and was well tolerated in participants with normal kidney function. These results will be useful in future trials investigating the effects of d-alanine intake on kidney disease progression in patients with chronic kidney disease.
This trial was registered at the UMIN Clinical Trials Registry as UMIN000051466.
Keywords: amino acid, d-alanine, prospective study, chronic kidney disease, healthy volunteers
Introduction
Chronic kidney disease (CKD) has been reported to affect over 10% of the general population and can cause adverse outcomes such as end-stage kidney disease and cardiovascular disease [1]. Studies have demonstrated that several therapeutic agents, such as renin-angiotensin system inhibitors and sodium-glucose cotransporter-2 inhibitors, can prevent CKD progression [2,3]. However, further therapeutic approaches are still needed to reduce the persistent risk of CKD development and progression in the general population.
With recent improvements in techniques for detecting and measuring specific d-amino acid levels, which are enantiomers of l-amino acids, the potential roles of d-amino acids as biomarkers and therapeutic targets in kidney diseases have been demonstrated [[4], [5], [6], [7]]. Of the 20 amino acids found in proteins in human body, previous clinical studies demonstrated that some plasma d-alanine and d-serine levels were particularly higher in patients with CKD than in healthy adults [4,[8], [9], [10]]. Furthermore, in a murine acute kidney injury model after the induction of ischemia-reperfusion, the oral administration of d-alanine and d-serine was shown to attenuate kidney damage [4,9,11]. These findings indicate that d-alanine and d-serine are potential therapeutic agents in patients with CKD. Alanine is commonly used as a food additive, and its safety has been confirmed in humans [12,13]. However, further data are needed regarding the influence of d-alanine intake on kidney function in humans.
Therefore, this multicenter, randomized pilot study aimed to investigate the effects of d-alanine administration on amino acid metabolism and kidney function in healthy adults. The results obtained in this study will be useful for dose setting in future clinical trials of d-alanine in patients with CKD to assess the potential of d-alanine administration to prevent the progression of kidney disease.
Methods
Study design and participants
This multicenter, open-label, randomized, pilot study evaluated the efficacy of d-alanine intake for 7 d in healthy volunteers. The study protocol was approved by the medical ethics committee of Kanazawa University and was registered at UMIN-CTR (UMIN ID UMIN000051466). The study was conducted in accordance with the Declaration of Helsinki.
Eligible participants included those who were aged ≥20 y and who had provided informed consent before participation at Kanazawa University Hospital and Kouryo Clinic, Japan. We excluded individuals who were pregnant, lactating, using antimicrobials or lactobacillus preparations, or considered unsuitable as participants by the study investigators (who would be unlikely to comply with d-alanine intake and follow-up).
As this was a pilot trial, no formal sample size calculation was performed. A sample size of 24 participants (12 per group) was chosen based on the recommendations for pilot studies [14,15].
Interventions
Eligible participants were randomly assigned in a 1:1 ratio to receive either 3 g or 6 g of d-alanine for 7 d within 30 d of the study enrollment (Supplemental Figure 1). Randomization was performed centrally in sequence using the UMIN INDICE cloud web system.
The pure alanine powder formulation (Direct Alanine; KAGAMI INC.) used in this study was a mixture of 1-g d-alanine and 1-g l-alanine in a single packet in which l-alanine was used to stabilize the structure of d-alanine. The 3-g and 6-g d-alanine groups took one and 2 packets (1-g and 2-g d-alanine), respectively, 3 times a day after each meal for 7 d. Participants were restricted from using antimicrobials, lactobacillus preparations, and probiotic-containing foods (e.g., fermented foods) that can affect the gut microbiota from 7 d before to 10 d after the first day of d-alanine intake.
Laboratory measurements
Participants underwent blood tests (fasting or ≥6 h after meals) and early morning urine tests at baseline and on days 2, 7, and 9. Four dl-amino acid levels, namely, alanine, serine, asparagine, and proline, were determined and quantified in the plasma and urine at baseline and on days 2, 7, and 9, using a two-dimensional high-performance liquid chromatography system (DASH 27B3X00322000001; KAGAMI INC.), as previously described with modifications [9,11,13].
We assessed other blood parameters, including leukocytes, erythrocytes, hemoglobin, hematocrit, platelets, total protein, albumin, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, creatinine, uric acid, sodium, potassium, calcium, inorganic phosphorus, fasting blood glucose, hemoglobin A1c, total cholesterol, high-density lipoprotein cholesterol, and triglycerides. The estimated glomerular filtration rate (eGFR) was calculated using the following equation proposed by the Japanese Society of Nephrology: eGFR (mL/min/1.73 m2) = 194 × Serum creatinine (mg/dL)−1.094 × Age (y)−0.287 × 0.739 (if female) [16]. Moreover, we assessed urine parameters, including urine protein to creatinine ratio (UPCR), β2 microglobulin, and N-acetyl-beta-glucosaminidase (NAG). The evaluation of the blood and urine parameters was performed by an independent laboratory in BML, Inc., using in-house developed automated clinical testing technology. All urine parameter values, including dl-amino acid, were normalized with regard to urine creatinine to correct for differences in concentrations related uniquely to the hydration status or urinary volume of the participant [17].
End points
The primary end point was the mean change in plasma and urine amino acid levels from baseline to 7 d after d-alanine intake. Intention-to-treat analyses were performed according to the randomly assigned groups. The secondary end points were the mean changes in the following parameters of kidney function and the associated factors with kidney disease progression from baseline to 7 d after d-alanine intake: blood pressure, BMI, eGFR, blood glucose, hemoglobin A1c, UPCR, urine β2 microglobulin, and urine NAG.
Statistical analysis
For baseline participant characteristics, continuous variables are presented as means with standard deviations, and categorical variables are presented as numbers with percentages.
For the primary and secondary end points, clinical and laboratory parameters were compared between baseline and 7 d after d-alanine intake using a paired t-test in each of the 3-g and 6-g d-alanine groups. Next, to assess dose-dependent responses, mean changes in parameters from baseline to 7 d after d-alanine intake were compared between the 3-g and 6-g d-alanine groups using Student’s t-test.
In an additional analysis, all parameters evaluated during follow-up (baseline, days 2, 7, and 9) were compared using repeated-measures analysis of variance (ANOVA) in the 3-g and 6-g d-alanine groups, respectively. If the p value of the ANOVA test was <0.05, a paired t-test was performed to compare parameters between baseline and either day 2, 7, or 9 after d-alanine intake.
Safety was monitored by assessing adverse events, a medical interview, and a review of laboratory values ≤2 d after the end of d-alanine intake.
All analyses were performed using Stata version 18 (Stata Corp; College Station). A two-sided P value of <0.05 was considered statistically significant.
Results
Baseline characteristics of the participants
From June to August 2023, a total of 24 participants were randomly assigned (12 participants in each of the 3-g and 6-g d-alanine groups) and completed the protocol treatment (Supplemental Figure 2). In the 3-g and 6-g d-alanine groups, the mean participant age was 43 and 45 y, there were 4 (33%) and 3 (25%) male participants, and the mean eGFR was 72.3 and 73.1 mL/min/1.73 m2, respectively (Table 1). The baseline characteristics of the participants were similar in the 2 groups, except for total cholesterol and UPCR. Total cholesterol and UPCR values were likely to be higher in the 3-g d-alanine group than in the 6-g d-alanine group.
TABLE 1.
Baseline participant characteristics in the 3-g and 6-g d-alanine groups
| d-alanine3 g (n = 12) | d-alanine6 g (n = 12) | |
|---|---|---|
| Age (y) | 43 (9) | 45 (9) |
| Male | 4 (33) | 3 (25) |
| Current smoking | 0 (0) | 2 (17) |
| Drinking habit | 5 (42) | 6 (50) |
| Body weight (kg) | 53.9 (12.4) | 59.7 (9.9) |
| BMI (kg/m2) | 20.7 (3.3) | 23.1 (2.2) |
| Systolic blood pressure (mmHg) | 123 (14) | 120 (9) |
| Diastolic blood pressure (mmHg) | 77 (14) | 78 (13) |
| AST (U/L) | 22 (6) | 24 (12.2) |
| ALT (U/L) | 18 (6) | 22 (17) |
| eGFR (mL/min/1.73 m2) | 72 (17) | 73 (11) |
| Uric acid (mg/dL) | 5.2 (1.6) | 5.4 (1.6) |
| Fasting blood glucose (mg/dL) | 93 (12) | 91 (4) |
| Hemoglobin A1c (%) | 5.4 (0.3) | 5.3 (0.2) |
| Total cholesterol (mg/dL) | 211 (33) | 177 (26) |
| HDL-cholesterol (mg/dL) | 68 (17) | 61 (16) |
| Triglycerides (mg/dL) | 115 (74) | 73 (44) |
| Urine protein/Cr (mg/g Cr) | 55.3 (30.2) | 32.1 (20.9) |
| Urine β2 microglobulin/Cr (ug/g Cr) | 141.5 (82.2) | 159.6 (130.7) |
| Urine NAG/Cr (IU/g Cr) | 5.6 (2.6) | 5.1 (3.3) |
| Plasma amino acid | ||
| d-alanine (nmol/mL) | 2.1 (2.5) | 0.8 (0.4) |
| l-alanine (nmol/mL) | 355.8 (80.2) | 325.8 (58.1) |
| d/l-alanine (%) | 0.6 (0.7) | 0.3 (0.1) |
| d-serine (nmol/mL) | 2.1 (0.4) | 1.8 (0.4) |
| l-serine (nmol/mL) | 119.6 (31.7) | 116.8 (17.3) |
| d/l-serine (%) | 1.8 (0.4) | 1.5 (0.4) |
| d-asparagine (nmol/mL) | 0.2 (0.1) | 0.2 (0.1) |
| l-asparagine (nmol/mL) | 45.4 (5.4) | 52.0 (16.2) |
| d/l-asparagine (%) | 0.5 (0.1) | 0.4 (0.1) |
| d-proline (nmol/mL) | 0.6 (0.2) | 0.5 (0.4) |
| l-proline (nmol/mL) | 150.1 (43.9) | 135.9 (28.6) |
| d/l-proline (%) | 0.4 (0.1) | 0.4 (0.3) |
| Urine amino acid | ||
| d-alanine/Cr (mmol/g Cr) | 60.8 (72.3) | 26.8 (14.4) |
| l-alanine/Cr (mmol/g Cr) | 118.7 (58.7) | 141.0 (56.1) |
| d-serine/Cr (mmol/g Cr) | 139.8 (38.2) | 143.2 (35.4) |
| l-serine/Cr (mmol/g Cr) | 172.5 (20.0) | 235.5 (169.4) |
| d-asparagine/Cr (mmol/g Cr) | 15.6 (4.0) | 16.1 (3.3) |
| l-asparagine/Cr (mmol/g Cr) | 54.2 (36.3) | 65.6 (27.8) |
| d-proline/Cr (mmol/g Cr) | 0.10 (0.17) | 0.06 (0.02) |
| l-proline/Cr (mmol/g Cr) | 5.1 (1.5) | 4.6 (1.3) |
Data are described as mean (standard deviation) or n (%).
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; Cr, creatinine; NAG, N-acetyl-β-glucosaminidase.
Effects on amino acid levels
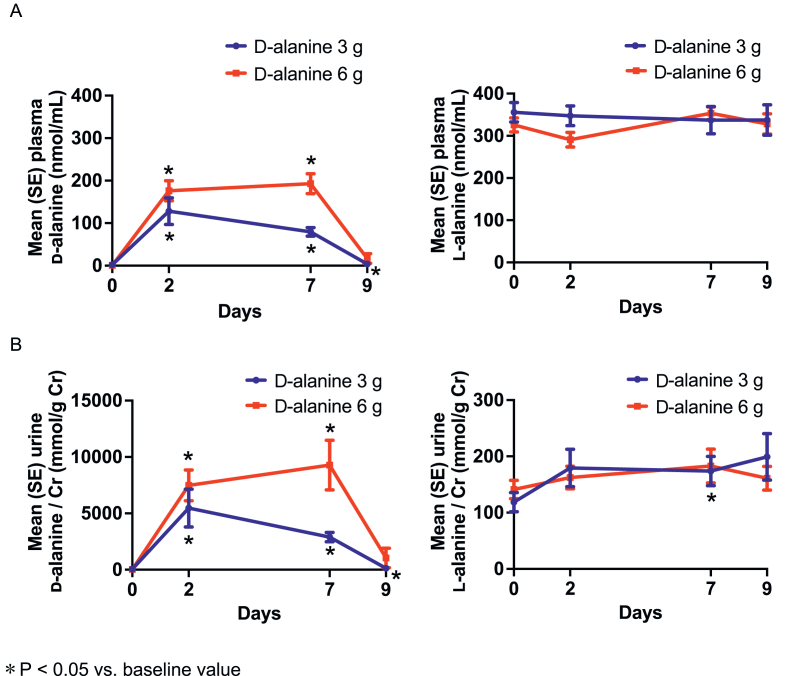
At baseline, in the 3-g and 6-g d-alanine groups, the mean(SD) plasma d-alanine levels were 2.1(2.5) nmol/mL and 0.8(0.4) nmol/mL, respectively. After 7 d of d-alanine intake, the mean plasma d-alanine concentration increased from baseline by 77.5 ± 34.3 nmol/mL in the 3-g d-alanine group (P < 0.0001) and by 192.1 ± 80.9 nmol/mL in the 6-g d-alanine group (P < 0.0001) (Figure 1A). These levels were higher in the 6-g d-alanine group than in the 3-g d-alanine group (between-group difference: 114.6 nmol/mL; 95% CI: 62.1–167.2 nmol/mL; P = 0.0002).
FIGURE 1.
Mean plasma (A) and urine (B) dl-alanine levels during follow-up in the 3-g and 6-g d-alanine groups.
The mean urine d-alanine to creatinine ratio dose-dependently increased by 2835.6 ± 1409.0 mmol/g Cr in the 3-g d-alanine group (P < 0.0001) and by 9249.3 ± 7623.5 mmol/g Cr in the 6-g d-alanine group (P = 0.002) after 7 d of d-alanine intake (between-group difference: 6413.6 mmol/g Cr; 95% CI: 1772.3–11055.0 mmol/g Cr; P = 0.009) (Figure 1B). The mean d-alanine levels in the plasma and urine returned to baseline or near baseline levels 2 d after the end of intake (day 9).
On the other hand, the mean plasma l-alanine concentration did not significantly change during d-alanine intake in both groups, whereas the mean urine l-alanine to creatinine ratio slightly increased by 55.1 ± 83.8 mmol/g Cr in the 3-g d-alanine group (P = 0.04) after 7 d of d-alanine intake (Figure 1). Moreover, in the 3 g d-alanine group, the mean plasma d-serine concentration moderately reduced by 0.20 ± 0.25 nmol/mL (P = 0.02) after 7 d of d-alanine intake whereas the mean urine d-serine to creatinine ratio increased by 35.5 ± 55.9 mmol/g Cr (P = 0.049) after 2 d of d-alanine intake. No significant changes were observed for other amino acids, such as l-serine, dl-asparagine, and dl-proline, during d-alanine intake in either of the d-alanine groups (Supplemental Figure 3 and Supplemental Table 1).
Effects on clinical and laboratory parameters
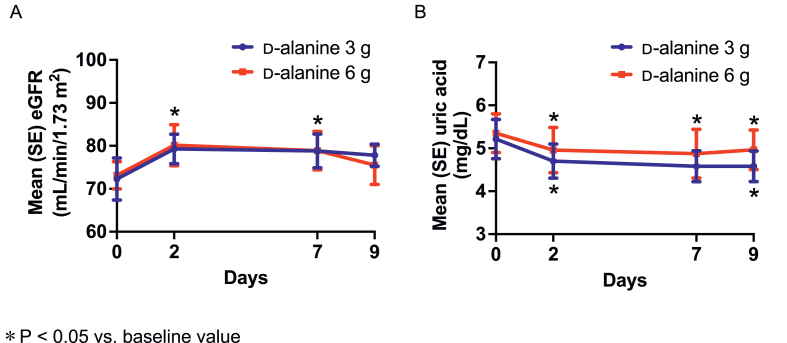
After 7 d of d-alanine intake, the mean eGFR increased in the 6-g d-alanine group [mean(SD) change from baseline: 5.7(8.8) mL/min/1.73 m2; P = 0.045] but did not significantly change in the 3-g d-alanine group (6.5 ± 12.9 mL/min/1.73 m2; P = 0.11) (Figure 2A and Table 1). A dose-dependent increase was not observed in the mean change in eGFR (between-group difference: 0.8 mL/min/1.73 m2; 95% CI: −8.5 to 10.2; P = 0.86). There were no significant differences in mean changes in blood pressure, BMI, blood glucose, hemoglobin A1c, UPCR, urine β2 macroglobulin, and NAG between baseline and 7 d of d-alanine intake in the 2 intake groups (Table 2).
FIGURE 2.
Mean estimated glomerular filtration rate (A) and uric acid (B) levels during follow-up in the 3-g and 6-g d-alanine groups.
TABLE 2.
Mean changes in clinical and laboratory parameters in the 3-g and 6-g d-alanine groups
| Day | Mean (SD) change from baseline |
p | |||
|---|---|---|---|---|---|
| d-alanine 3 g (n = 12) | p | d-alanine 6 g (n = 12) | |||
| Systolic blood pressure (mmHg) | 2 | –4.5 (11.4) | 0.20 | 0.08 (11.1) | 0.98 |
| 7 | –4.9 (10.9) | 0.15 | 1.6 (8.2) | 0.52 | |
| 9 | –5.2 (10.6) | 0.12 | 3.1 (8.1) | 0.22 | |
| Diastolic blood pressure (mmHg) | 2 | –4.9 (8.0) | 0.06 | –2.4 (8.2) | 0.33 |
| 7 | –3.9 (7.9) | 0.11 | –4.1 (8.3) | 0.12 | |
| 9 | –3.6 (7.8) | 0.14 | 1.3 (7.9) | 0.60 | |
| BMI (kg/m2) | 2 | –0.06 (0.2) | 0.42 | –0.04 (0.2) | 0.54 |
| 7 | 0.09 (0.2) | 0.18 | –0.04 (0.2) | 0.35 | |
| 9 | –0.04 (0.3) | 0.63 | 0.004 (0.2) | 0.93 | |
| eGFR (mL/min/1.73 m2) | 2 | 7.0 (12.6) | 0.08 | 7.0 (8.2) | 0.01 |
| 7 | 6.5 (12.9) | 0.11 | 5.7 (8.8) | 0.04 | |
| 9 | 5.6 (15.8) | 0.25 | 2.4 (7.8) | 0.31 | |
| Fasting blood glucose (mg/dL) | 2 | –1.8 (7.7) | 0.45 | 1.3 (6.2) | 0.47 |
| 7 | –1.5 (11.0) | 0.65 | 0.2 (3.8) | 0.88 | |
| 9 | –1.8 (9.0) | 0.49 | –0.8 (4.0) | 0.53 | |
| HbA1c (%) | 2 | –0.03 (0.08) | 0.17 | –0.008 (0.07) | 0.67 |
| 7 | –0.04 (0.10) | 0.18 | –0.04 (0.07) | 0.05 | |
| 9 | 0.01 (0.08) | 0.50 | –0.03 (0.08) | 0.17 | |
| Urine protein/Cr (mg/g Cr) | 2 | –20.0 (33.6) | 0.06 | 12.3 (62.4) | 0.51 |
| 7 | –11.3 (33.9) | 0.27 | 8.4 (35.4) | 0.43 | |
| 9 | –7.3 (39.9) | 0.54 | –3.8 (32.7) | 0.70 | |
| Urine β2 microglobulin/Cr | 2 | 8.3 (93.0) | 0.76 | 146.0 (587.8) | 0.41 |
| (ug/g Cr) | 7 | 2.51 (93.3) | 0.93 | 22.6 (146.2) | 0.60 |
| 9 | 28.5 (93.1) | 0.31 | 20.4 (201.7) | 0.73 | |
| Urine NAG/Cr (IU/g Cr) | 2 | –0.8 (2.1) | 0.22 | 0.05 (2.3) | 0.94 |
| 7 | 0.2 (2.0) | 0.72 | 0.8 (2.3) | 0.24 | |
| 9 | –0.2 (1.9) | 0.69 | 0.7 (3.4) | 0.46 | |
Abbreviations: BMI, body mass index; Cr, creatinine; eGFR, estimated glomerular filtration rate; NAG, N-acetyl-β-glucosaminidase.
Exploratory analysis was performed to assess the effects of d-alanine intake on additional clinical and laboratory parameters (Supplemental Table 2). The mean serum uric acid concentration decreased after d-alanine intake in the 3-g and 6-g d-alanine groups [mean(SD) change from baseline: −0.5(0.5) and −0.4(0.6) mg/dL, P = 0.007 and P = 0.049, respectively] (Figure 2B). There was no dose-dependent reduction in the mean change in serum uric acid levels (between-group difference: −0.1 mg/dL; 95% CI: −0.6 to 0.4; P = 0.60). No significant changes after 7 d of d-alanine intake were observed for other parameters, such as body weight, aspartate aminotransferase, alanine aminotransferase, total and high-density lipoprotein cholesterol, and triglycerides.
Safety
All adverse events are listed in Table 3. Eleven participants (46%) reported a total of 14 nonserious adverse events (6 and 5 participants in the 3-g and 6-g d-alanine groups, respectively). Two participants each experienced hyperphosphatemia, hypertriglyceridemia, creatinine increase, hyperglycemia, and hypocalcemia. One participant in the 6-g d-alanine group reported hypertension above 140/90 mmHg. The mean changes in systolic and diastolic blood pressure were −4.9 and −3.9 mmHg in the 3-g d-alanine group, and 1.6 and −4.1 mmHg in the 6-g d-alanine group, respectively (Table 2). No serious adverse events were recorded.
TABLE 3.
Safety of d-alanine intake in the 3-g and 6-g d-alanine groups
|
N (%) |
||
|---|---|---|
| d-alanine 3 g (n = 12) | d-alanine 6 g (n = 12) | |
| Hyperphosphatemia | 2 (17) | 0 (0) |
| Hypertriglyceridemia | 1 (8) | 1 (8) |
| Creatinine increase | 1 (8) | 1 (8) |
| Hyperglycemia | 1 (8) | 1 (8) |
| Hypocalcemia | 1 (8) | 1 (8) |
| Liver injury | 1 (8) | 0 (0) |
| Hypertension | 0 (0) | 1 (8) |
| Abdominal discomfort | 0 (0) | 1 (8) |
| Sleep disturbed | 0 (0) | 1 (8) |
Discussion
In this multicenter, open-label, randomized, pilot study, d-alanine administration for 7 d selectively increased plasma and urine d-alanine levels and was well tolerated in healthy adults with normal kidney function. There were no serious adverse events after d-alanine intake. Short-term d-alanine intake sustained eGFR levels and did not affect other markers of kidney function.
This study showed that d-alanine administration for 7 days induced an effective d-alanine response in the plasma and urine in participants from the general population. Multiple factors may influence d-alanine levels in humans. d-alanine is usually obtained through food intake and synthesis by gut microbiota [18]. d-amino acids, including d-alanine, have been widely detected in a variety of raw ingredients and processed foods, such as vegetables, fruits, milk, and liquor [[19], [20], [21]]. In particular, fermented foods are known to contain high amounts of d-amino acids, which are produced during microbial fermentation [22]. Furthermore, some lactic acid bacteria synthesize d-alanine and encode alanine racemase, which catalyzes the conversion of l-alanine to d-alanine [23,24]. Although we did not evaluate the d-alanine levels in stool, the protocol of the current study restricted the intake of fermented foods and the use of antimicrobial agents and lactic acid preparations during the follow-up period to minimize their influences. In addition, the specific effects of d-alanine observed in this study are not typically induced by food intake or gut microbiota and therefore could be attributed to d-alanine intake.
To our knowledge, this is the only study that has evaluated the efficacy and safety of d-alanine intake regarding its association with amino acid metabolism and kidney function in humans. A dose-dependent increase in d-alanine levels was observed immediately after d-alanine intake, which was sustained during d-alanine intake. These results are important for the dose and protocol setting in subsequent clinical trials of d-alanine in patients with CKD. Previous studies demonstrated that plasma d-alanine levels were negatively correlated with eGFR [25], and were moderately higher in patients with CKD and acute kidney injury than in healthy controls, which are thought to contribute to kidney protection [9,11]. Additionally, urine d-alanine levels were similar and saliva d-alanine levels were higher in patients with CKD than in healthy controls [8]. These results suggest that the increased plasma d-alanine levels were possibly due to the production by oral microbiota rather than the regulation of urine excretion [25]. A further study is required to investigate how d-alanine administration affects the production of d-alanine by oral microbiota in patients with CKD.
A small increase in eGFR was observed only among participants in the 6-g d-alanine group. It is unknown whether this result reflects the true effect on kidney function or not because we used eGFR instead of the directly measured GFR, which may have led to some misclassification of the true changes in kidney function. In addition, no remarkable changes were observed for other biomarkers of kidney function, such as urinary protein and kidney tubular markers. A recent study has revealed that d-alanine protected tubular epithelial cells after hypoxia and induced their proliferation [9], which is suggested as a key mechanism leading to kidney protection. However, the acute effect of d-alanine administration on glomerular filtration remains unknown, and a future trial should elucidate the short-term response to GFR after the initiation of d-alanine administration.
The effect of d-alanine intake on uric acid levels is particularly noteworthy given that d-alanine supplementation may be effective in patients with hyperuricemia. A previous chiral resolution of amino acids showed no correlation between plasma d-alanine and uric acid levels in healthy populations [26]. However, a previous trial demonstrated that milk intake rapidly decreased serum uric acid levels and increased urine excretion of uric acid in healthy individuals [27]. In another cohort study in an elderly population with metabolic syndrome, high consumption of dairy products, such as milk, yogurt, and cheese, was associated with a lower risk of hyperuricemia [28]. Considering the high content of d-alanine in these dairy products [20], d-alanine may be able to reduce serum uric acid levels, although these studies did not assess d-amino acids, and dietary information was not assessed in the current study.
Our study has several limitations. First, this study used an open-label design, which could introduce performance bias owing to the unblinding of participants. Second, the safety data may be limited owing to the inclusion of a healthy population, a short-term follow-up, and being a pilot study, which might be associated with a lower risk of adverse events. A more conclusive safety assessment is needed in future long-term follow-up clinical trials in patients with CKD. Lastly, considering that this study was performed in the Japanese population, the results may not be generalizable to other ethnic populations.
In conclusion, in the Japanese general population, d-alanine administration at doses of 3 g and 6 g for 7 d was effective at increasing plasma d-alanine levels. Additionally, eGFR increased slightly only at 6 g and other markers of kidney function were stable during d-alanine intake. The demonstrated potential efficacy and safety profile of d-alanine will be useful in future clinical trials of d-alanine in patients with CKD. The findings of these trials are expected to support further development of d-alanine as a novel nutrition management for CKD.
Author contributions
MO, TTad, TW, YI: contributed to the study design; MO, TTad: collected data for the study, contributed to the analysis and interpretation of the data, and wrote the first draft of the manuscript; and all authors: read and approved the final manuscript.
Conflict of interest
This study was supported by KAGAMI INC. MM is the founder and CEO of KAGAMI INC., a startup company working on chiral amino acid analysis and research for medical applications. Other authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
This study was supported by KAGAMI INC. MM is the founder and CEO of KAGAMI INC., a startup company working on chiral amino acid analysis and research for medical applications. Other authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data described in the manuscript will be considered for availability upon request to the corresponding author.
Acknowledgments
We also thank Hiroshi Imoto, Eiichi Negishi, Maiko Nakane, and Shoto Ishigo in KAGAMI INC. who have contributed to the development of alanine powder formulation and chiral amino acid analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cdnut.2024.103787.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.United States Renal Data System . National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2023. 2023 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. [Google Scholar]
- 2.Nuffield Department of Population Health Renal Studies Group SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium, Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–1801. doi: 10.1016/S0140-6736(22)02074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie X., Liu Y., Perkovic V., Li X., Ninomiya T., Hou W., et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am. J. Kidney. Dis. 2016;67(5):728–741. doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Hesaka A., Tsukamoto Y., Nada S., Kawamura M., Ichimaru N., Sakai S., et al. d-Serine mediates cellular proliferation for kidney remodeling. Kidney360. 2021;2(10):1611–1624. doi: 10.34067/KID.0000832021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karakawa S., Shimbo K., Yamada N., Mizukoshi T., Miyano H., Mita M., et al. Simultaneous analysis of D-alanine, D-aspartic acid, and D-serine using chiral high-performance liquid chromatography-tandem mass spectrometry and its application to the rat plasma and tissues. J. Pharm. Biomed. Anal. 2015;115:123–129. doi: 10.1016/j.jpba.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Kimura T., Hamase K., Miyoshi Y., Yamamoto R., Yasuda K., Mita M., et al. Chiral amino acid metabolomics for novel biomarker screening in the prognosis of chronic kidney disease. Sci. Rep. 2016;6 doi: 10.1038/srep26137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furusho A., Koga R., Akita T., Mita M., Kimura T., Hamase K. Three-dimensional high-performance liquid chromatographic determination of Asn, Ser, Ala, and Pro enantiomers in the plasma of patients with chronic kidney disease. Anal. Chem. 2019;91(18):11569–11575. doi: 10.1021/acs.analchem.9b01615. [DOI] [PubMed] [Google Scholar]
- 8.Nakade Y., Iwata Y., Sakai N., Mita M., Nakane M., Hamase K., et al. Increased levels of oral Streptococcus-derived D-alanine in patients with chronic kidney disease and diabetes mellitus. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-26175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwata Y., Nakade Y., Kitajima S., Yoneda-Nakagawa S., Oshima M., Sakai N., et al. Protective effect of d-alanine against acute kidney injury. Am. J. Physiol. Renal. Physiol. 2022;322(6):F667–F679. doi: 10.1152/ajprenal.00198.2021. [DOI] [PubMed] [Google Scholar]
- 10.Okushima H., Iwata Y., Hesaka A., Sugimori E., Ikeda T., Nakane M., et al. Intra-body dynamics of D-serine reflects the origin of kidney diseases. Clin. Exp. Nephrol. 2021;25(8):893–901. doi: 10.1007/s10157-021-02052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakade Y., Iwata Y., Furuichi K., Mita M., Hamase K., Konno R., et al. Gut microbiota-derived D-serine protects against acute kidney injury. JCI. Insight. 2018;3(20) doi: 10.1172/jci.insight.97957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joint FAO/WHO Expert Committee on Food Additives (JECFA) NaFSN, Standards & Scientific Advice on food nutrition (SSA) World Health Organization; Geneva: 2006. Safety evaluation of certain food additives / prepared by the sixty-third meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA) [PubMed] [Google Scholar]
- 13.Ishii C., Akita T., Mita M., Ide T., Hamase K. Development of an online two-dimensional high-performance liquid chromatographic system in combination with tandem mass spectrometric detection for enantiomeric analysis of free amino acids in human physiological fluid. J. Chromatogr. A. 2018;1570:91–98. doi: 10.1016/j.chroma.2018.07.076. [DOI] [PubMed] [Google Scholar]
- 14.Julious S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005;4(4):287–291. doi: 10.1002/pst.185. [DOI] [Google Scholar]
- 15.Moore C.G., Carter R.E., Nietert P.J., Stewart P.W. Recommendations for planning pilot studies in clinical and translational research. Clin. Transl. Sci. 2011;4(5):332–337. doi: 10.1111/j.1752-8062.2011.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K., et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney. Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Bienaimé F., Muorah M., Metzger M., Broeuilh M., Houiller P., Flamant M., et al. Combining robust urine biomarkers to assess chronic kidney disease progression. EBioMedicine. 2023;93 doi: 10.1016/j.ebiom.2023.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi J. d-Amino acids and lactic acid bacteria. Microorganisms. 2019;7(12) doi: 10.3390/microorganisms7120690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brückner H., Westhauser T. Chromatographic determination of L- and D-amino acids in plants. Amino Acids. 2003;24(1–2):43–55. doi: 10.1007/s00726-002-0322-8. [DOI] [PubMed] [Google Scholar]
- 20.Tian H., Zheng N., Li S., Zhang Y., Zhao S., Wen F., Wang J. Characterization of chiral amino acids from different milk origins using ultra-performance liquid chromatography coupled to ion-mobility mass spectrometry. Sci. Rep. 2017;7 doi: 10.1038/srep46289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato S., Ishihara T., Hemmi H., Kobayashi H., Yoshimura T. Alterations in D-amino acid concentrations and microbial community structures during the fermentation of red and white wines. J. Biosci. Bioeng. 2011;111(1):104–108. doi: 10.1016/j.jbiosc.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Mutaguchi Y., Ohmori T., Akano H., Doi K., Ohshima T. Distribution of D-amino acids in vinegars and involvement of lactic acid bacteria in the production of D-amino acids. Springerplus. 2013;2:691. doi: 10.1186/2193-1801-2-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi J., Yukimoto J., Shimizu Y., Ohmori T., Suzuki H., Doi K., Ohshima T. Characterization of Lactobacillus salivarius alanine racemase: short-chain carboxylate-activation and the role of A131. Springerplus. 2015;4:639. doi: 10.1186/s40064-015-1335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu-Ibuka A., Sato A., Ichimura H., Hiraga H., Nakayama S., Nishiwaki T. Regulation of alanine racemase activity by carboxylates and the d-type substrate d-alanine. F.E.B.S. J. 2023;290(11):2954–2967. doi: 10.1111/febs.16745. [DOI] [PubMed] [Google Scholar]
- 25.Nagata Y., Higashi M., Ishii Y., Sano H., Tanigawa M., Nagata K., et al. The presence of high concentrations of free D-amino acids in human saliva. Life. Sci. 2006;78(15):1677–1681. doi: 10.1016/j.lfs.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M., Shimizu-Hirota R., Mita M., Hamase K., Sasabe J. Chiral resolution of plasma amino acids reveals enantiomer-selective associations with organ functions, Amino. Acids. 2022;54(3):421–432. doi: 10.1007/s00726-022-03140-w. [DOI] [PubMed] [Google Scholar]
- 27.Dalbeth N., Wong S., Gamble G.D., Horne A., Mason B., Pool B., et al. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann. Rheum. Dis. 2010;69(9):1677–1682. doi: 10.1136/ard.2009.124230. [DOI] [PubMed] [Google Scholar]
- 28.Mena-Sánchez G., Babio N., Becerra-Tomás N., Martínez-González M.Á., Díaz-López A., Corella D., et al. Association between dairy product consumption and hyperuricemia in an elderly population with metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2020;30(2):214–222. doi: 10.1016/j.numecd.2019.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be considered for availability upon request to the corresponding author.