Abstract
A recombinant fusion protein (BBG2Na) comprising the central conserved domain of the respiratory syncytial virus subgroup A (RSV-A) (Long) G protein (residues 130 to 230) and an albumin binding domain of streptococcal protein G was shown previously to protect mouse upper (URT) and lower (LRT) respiratory tracts against intranasal RSV challenge (U. F. Power, H. Plotnicky-Gilquin, T. Huss, A. Robert, M. Trudel, S. Stahl, M. Uhlén, T. N. Nguyen, and H. Binz, Virology 230:155–166, 1997). Panels of monoclonal antibodies (MAbs) and synthetic peptides were generated to facilitate dissection of the structural elements of this domain implicated in protective efficacy. All MAbs recognized native RSV-A antigens, and five linear B-cell epitopes were identified; these mapped to residues 152 to 163, 165 to 172, 171 to 187 (two overlapping epitopes), and 196 to 204, thereby covering the highly conserved cysteine noose domain. Antibody passive-transfer and peptide immunization studies revealed that all epitopes were implicated in protection of the LRT, but not likely the URT, against RSV-A challenge. Pepscan analyses of anti-RSV-A and anti-BBG2Na murine polyclonal sera revealed lower-level epitope usage within the central conserved region in the former, suggesting diminished immunogenicity of the implicated epitopes in the context of the whole virus. However, Pepscan analyses of RSV-seropositive human sera revealed that all of the murine B-cell protective epitopes (protectopes) that mapped to the central conserved domain were recognized in man. Should these murine protectopes also be implicated in human LRT protection, their clustering around the highly conserved cysteine noose region will have important implications for the development of RSV vaccines.
Respiratory syncytial virus (RSV) is a member of the genus Pneumovirus and the family Paramyxoviridae. It is the principle etiologic agent of serious respiratory disease in infants and young children (reviewed in references 11 and 25). RSV is characterized by the ability to repeatedly infect the upper respiratory tracts (URTs) of humans throughout their lives, and an important role for this organism in severe respiratory illness of immunocompromised adults and the elderly is becoming increasingly evident (14–17, 19, 34). Furthermore, a primary RSV infection in infants which results in serious lower respiratory tract (LRT) pathology does not necessarily prevent a second serious infection (26). However, the frequency of serious LRT disease in infants progressively diminishes upon subsequent infections, suggesting accumulation of LRT-protective immunity upon repeated virus exposure (for a review, see reference 13). Despite the medical importance of this virus, many aspects of RSV-induced immunity remain unresolved.
RSV encodes two major surface glycoproteins, G and F, which are incorporated into the viral envelope. The G protein is a highly glycosylated type II glycoprotein that functions in viral attachment to an unknown cell receptor (31). The fusion (F) protein is a type I glycoprotein that mediates virus and cell membrane fusion and syncytium formation (52). Both proteins induce neutralizing antibodies and protection against RSV challenge in animal models (reviewed in reference 11). Despite the high degree of conservation of the F protein (29), RSV clinical isolates were classified into two subgroups, A and B, based originally on the antigenic diversity of the G protein (2, 35). Indeed, there is 47% amino acid sequence diversity between prototype RSV subgroup A (RSV-A) and RSV-B G proteins (28). Furthermore, up to 20% amino acid differences in the G protein have been reported within the subgroups (7, 48), with corresponding antigenic divergence also being evident (20). This antigenic and genetic diversity among the RSV G proteins, together with the generally low-level immunogenicity of F and G proteins in infants less than 8 months old, is hypothesized to play an important role in repeat infections (3, 8, 9, 20, 37, 46).
Sequence analyses of RSV clinical isolates (both subgroups) revealed that the G protein contains two hypervariable regions separated by a central conserved domain (7, 20, 48). While monoclonal antibody (MAb) epitopes have been mapped to many regions of the G protein (reviewed in reference 33), several lines of evidence indicate that the C-terminal hypervariable region is immunogenic and may even be immunodominant in the context of the whole G protein: (i) convalescent-phase human infant sera reacted with recombinant fragments of the C terminus in a strain-specific manner (10); (ii) the majority of reactivity escape mutants derived from a panel of G protein-specific murine MAbs were found to have mutations in the variable C terminus (43); (iii) one escape mutant contained a frameshift mutation which resulted in a completely altered C-terminal one-third of the molecule, and this mutant lost reactivity to many MAbs and, importantly, a polyclonal antiserum raised against affinity-purified G protein (22); (iv) the greatest differences between infecting and heterologous RSV groups in terms of antibody titers are for anti-G protein antibodies (27); and (v) the native G protein induces subgroup-specific protection (41, 47). Immunodominance of the hypervariable C terminus would concur with the hypothesis that G protein variability is implicated in repeated RSV infections. A corollary of this is that a single full-length native G protein would be of limited value for vaccine purposes.
We (42) and others (45) have previously demonstrated that recombinant fragments containing G protein residues 130 to 230 and 124 to 204, respectively, which contain the central conserved domain, are sufficient to elicit protective immune responses in rodent models. Indeed, in contrast to the native G protein, the former induced protection against both RSV-A and -B prototype strains when injected in the context of the recombinant fusion protein BBG2Na. Trudel et al. (51) mapped a subgroup-specific protective epitope (protectope) to the cysteine noose region (residues 174 to 187) by MAb passive-transfer experiments and active immunization with a synthetic peptide (coupled to keyhole limpet hemocyanin [KLH]). Interestingly, an equivalent peptide derived from the bovine RSV G protein reduced the incidence of bovine RSV-associated pneumonia in calves (5). An anti-G protein MAb that reacts with both RSV-A and -B G proteins was shown to be cross-protective after passive transfer (53) and mapped to residues 165 to 174 based on sequencing of neutralization escape mutants (54). Other groups have also mapped murine MAb epitopes to this central conserved region, although the role of these epitopes in protection is not yet known (for a review, see reference 33). Finally, human sera were found to react with synthetic peptides derived from this region, indicating immunogenicity in humans (39). Therefore, direction of primary immune responses to the central conserved region, which may be poorly immunogenic in the context of the whole G protein, is conceivable and may be important and sufficient for the induction of LRT protection that is not RSV strain restricted.
The goal of the present study was to dissect the structural elements implicated in inducing protective immune responses against RSV-A challenge. To do so, we generated a panel of MAbs and RSV-A G protein-derived synthetic peptides. MAb epitope mapping was undertaken by Pepscan analyses and/or peptide reactivity. Protectopes were identified by MAb passive-transfer studies and/or active immunization with carrier-protein-conjugated synthetic peptides in mice. Finally, mouse and human polyclonal sera were subjected to Pepscan analyses to determine epitope usage following BBG2Na immunization and RSV infection in mice and humans, respectively. Interestingly, they were found to recognize common epitopes, some of which were protective in mice.
(This work was presented in part at the 16th Annual Meeting of the American Society for Virology, Bozeman, Mont., 19 to 23 July 1997.
MATERIALS AND METHODS
Viruses, cells, and ELISA antigens.
Propagation of RSV-A (Long strain; ATCC VR-26 [American Type Culture Collection, Rockville, Md.]) in HEp-2 cells (ECACC 86030501; European Collection of Animal Cell Cultures, Porton Down, Salisbury, United Kingdom), as well as the production of viral protein and uninfected-cell enzyme-linked immunosorbent assay (ELISA) antigens, was performed as previously described (42). Recombinant protein G2ΔCa was produced by expressing and purifying BBG2ΔCa as previously described (36), cleaving BBG2ΔCa by cyanogen bromide hydrolysis into BB and G2ΔCa components, and purifying the products by reverse-phase high-performance liquid chromatography (RP-HPLC). Proteins BBG2Na and G2Na were produced and purified as previously described (12).
Expression and purification of the carrier proteins P40 and BB.
Gene assembly, vector construction, and P40 and BB protein expression in Escherichia coli were undertaken as previously described (38, 40). P40, an outer membrane protein A from Klebsiella pneumonia with carrier-protein properties, was purified to homogeneity by two ion-exchange chromatography steps (24). BB, the albumin binding domain of streptococcal protein G, was purified by affinity chromatography on albumin-Sepharose followed by cation-exchange chromatography and RP-HPLC. Protein purity was >95% as assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% homogeneous gels under reducing conditions.
Synthetic peptides.
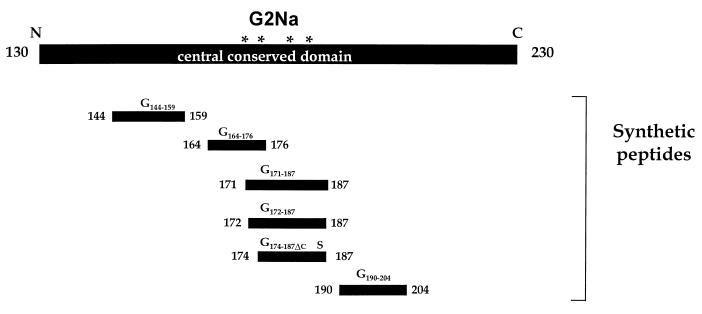
Synthesis of individual peptides was performed by a solid-phase method on an Applied Biosystems 433A synthesizer, using fluorenylmethoxycarbonyl/t-butyl (FMOC/tBu) chemistry. The synthesized peptides, cleaved from resin by trifluoroacetic acid in the presence of scavengers, were lyophilized and purified by preparative RP-HPLC. The purity of the peptides was greater than 90% according to both RP-HPLC and free-zone capillary electrophoresis analyses. Peptides G174–187ΔC, G172–187, G171–187, G144–159Cys and CysG144–159, G190–204Cys and CysG190–204, and G164–176 and G164–176ΔC corresponded to residues 174 to 187, 172 to 187, 171 to 187, 144 to 159, 190 to 204, and 164 to 176, respectively, of the RSV-A (Long) G protein (Fig. 1). Peptides G144–159Cys, CysG144–159, G190–204Cys, and CysG190–204 each contain an extra cysteine residue added either N terminally or C terminally to facilitate orientation of peptide coupling to carrier proteins. Peptides G172–187 and G171–187, each of which contains 4 cysteines, were obtained after oxidation of the crude mercaptoethanol-reduced derivatives with dimethyl sulfoxide (49). All three possible oxidized isomers of the latter peptides were synthesized under various oxidation conditions, resulting in different ratios of isomers. Each isolated isomer was characterized by RP-HPLC, free-zone capillary electrophoresis, and electrospray mass spectrometry after purification by preparative RP-HPLC. The different cysteine pairings were unambiguously established by enzymatic digestion, liquid chromatography-mass spectrometry analysis, and peptide microsequencing (data not shown). Peptides G174–187ΔC and G164–176ΔC contained Cys-Ser substitutions at residues 186 and 173, respectively. G174–187ΔC corresponds precisely to the protective peptide identified by Trudel et al. (51), with the substitution inserted to facilitate formation of the native Cys176-Cys182 disulfide bond (32). The substitution in G164–176ΔC (a Ser at Cys173 [Cys173Ser]) was inserted to prevent nonnative Cys173-Cys186 disulfide bridge formation and to facilitate orientation of peptide coupling to the carrier protein.
FIG. 1.
Schematic representation of synthetic peptides used to characterize murine MAbs and polyclonal sera and to identify protectopes in the central conserved region of the RSV-A G protein. The asterisks denote the locations of the conserved Cys residues. The S in peptide G174–187ΔC signifies a Cys-to-Ser substitution at position 186.
Peptide-carrier protein coupling.
Peptides G174–187ΔC, G172–187, G171–187, and G164–176 were coupled to P40 by treatment with glutaraldehyde as previously described (24). The amino acid sequences of these peptides indicated that glutaraldehyde coupling would result in a population of both N- and C-terminus-coupled G174–187ΔC and G171–187 while G172–187 and G164–176 would be coupled uniquely by their C and N termini, respectively. Peptides CysG144–159, G144–159Cys, CysG190–204, and G164–176ΔC were conjugated by using N-hydroxysuccinimidyl bromoacetate as a coupling reagent (6, 24), thereby specifically controlling their coupling orientation. For hybridoma screening and immunization purposes, all peptides were also coupled to BB, KLH (both from Calbiochem, Meudon, France), and/or bovine serum albumin (Sigma, Saint Quentin Fallavier, France) by the same methods. P40 and BB conjugates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% homogeneous gels under reducing conditions.
Animals.
Female BALB/c mice, aged 6 to 9 weeks, were purchased from Iffa Credo (L’Arbresle, France) and kept under specific-pathogen-free conditions. They were confirmed as seronegative vis-à-vis RSV before inclusion in the studies. All animals were fed rat and mouse maintenance diet A04 (UAR, Villemoissin-sur-Orge, France) and water ad libitum and were housed and manipulated according to French and European guidelines.
MAbs and polyclonal sera.
MAbs 11F7, 5B7, 6H66, and 6H69 were produced by immunizing BALB/c mice twice, subcutaneously (s.c.), with 30 μg of BBG2Na in phosphate-buffered saline (PBS) containing 20% (vol/vol) Alhydrogel [Al(OH)3]; Superfos BioSector a/s, Vedbaek, Denmark). Four days after a further, intravenous inoculation of BBG2Na, the mice were killed and spleen cells were fused with SP2/0-Ag14 myeloma cells (American Type Culture Collection) by using polyethylene glycol (Serva, Heidelberg, Germany). Resultant hybridomas were initially screened for secretion of G2Na- and RSV-A-specific antibodies by ELISA. Positive reactors were subsequently screened for reactivity against KLH-conjugated G172–187, G144–159, G190–204, and G164–176ΔC. A panel of hybridomas was selected on the basis of their peptide reactivity patterns and cloned three times by serial limiting dilution. Four independent clones were expanded and injected into pristane (Sigma) primed mice to induce the production of ascitic fluid. Ascites fluids were purified, isotyped, and used in subsequent studies.
MAbs 18D1 and 5C2 were produced by immunizing BALB/c mice intraperitoneally (i.p.) with 50 μg of KLH-G174–187ΔC or G2ΔCa in complete Freund’s adjuvant. A second immunization was performed with incomplete Freund’s adjuvant. Four days after a further, intravenous inoculation of 10 μg of each peptide, the mice were killed and spleen cells were fused with SP2/0-Ag14 myeloma cells as described above. The resulting hybridomas were screened for antibody secretion by ELISA with bovine serum albumin-G174–187ΔC or G2ΔCa, respectively, as the coating antigen. Positive reactors were cloned, expanded, and used for ascites fluid production, as described above.
Murine polyclonal serum against G190–204 was produced by immunizing 25 BALB/c mice i.p. three times at 2-week intervals with 20 μg of BB-CysG190–204. Three weeks after the last immunization, the mice were sacrificed and exsanguinated by cardiac puncture. Blood samples were collected in serum separation tubes (Beckton Dickinson, Meylan, France) and centrifuged at 1,850 × g for 10 min, and pooled sera were stored at −80°C until used for titration in ELISAs and neutralization assays or in passive-transfer studies. Production of murine anti-BBG2Na and BB polyclonal sera was previously described (42), while anti-RSV-A polyclonal sera were prepared by immunizing mice three times i.p. (in Alhydrogel) or five times intranasally (i.n.) (in 50-μl volumes) with 105 50% tissue culture infectious doses (TCID50) at 2-week intervals. The human RSV-positive sera (kindly provided by Michel Segondy, Centre Hospitalier Universitaire, Montpellier, France) were derived from two individuals demonstrating high anti-RSV-A ELISA titers (log10 3.8 and 4, respectively).
Pepscan analysis.
Ninety-four overlapping 8-mer peptides or 90 overlapping 12-mer peptides spanning residues 130 to 230 (G2Na) of the human RSV-A G protein were synthesized on noncleavable derivatized rods (Chiron Technologies, Emeryville, Calif.) according to established procedures. The peptides were tested for their reactivities with MAbs or sera by ELISA. Briefly, nonspecific binding was blocked by incubation for 1 h at 37°C with PBS containing 0.1% Tween (Sigma) and 1% gelatin. The rods were subsequently incubated at 4°C overnight with the sera or MAbs, washed three times, and incubated 1 h at room temperature with a horseradish peroxidase-conjugated goat anti-mouse antibody (1/5,000; Southern Biotechnology Associates, Birmingham, Ala.). After being washed four times, the rods were transferred to a microtiter plate (Nunc, Roskilde, Denmark) containing 100 μl of tetramethyl benzidine (Dynatech, Chantilly, Va.). The reaction was terminated with 100 μl of 1 M H2SO4 per well. Optical densities were measured at 450 nm.
ELISA titrations and neutralization assays.
MAb characterization and RSV-A-specific serum immunoglobulin G (IgG) determinations were accomplished by ELISA as previously described (42), except that for MAb titrations, blocking and washing solutions consisted of PBS–0.1% (wt/vol) gelatin and PBS–0.05% Tween 20 (Sigma)–0.01% (wt/vol) gelatin, respectively. MAb isotyping was undertaken with an ImmunoPure MAb isotyping kit (Pierce, Rockford, Ill.). ELISA titers were expressed as the reciprocal of the last dilution with an optical density of ≥0.15 and at least twofold higher than that of the negative control. Because the virus stocks contained cellular antigen, RSV-A-specific antibody titers were calculated by subtracting anti-HEp-2 titers from those of anti-RSV-A. Neutralization assays were undertaken in triplicate as previously described (42). Neutralization titers were expressed as the reciprocal of the highest dilution that reduced positive-control syncytium numbers by at least 60%.
Active and passive immunization and challenge procedures.
Mice were immunized i.p. with 200-μl volumes of peptide-carrier protein conjugate solutions containing 20% (vol/vol) Alhydrogel in PBS. Second and subsequent immunizations were given at 2-week intervals. Animals were bled 2 weeks after the last immunization to determine RSV-A-specific serum antibody titers and neutralizing activity. They were challenged 3 weeks postimmunization with 105 TCID50 of RSV-A i.n. after anesthetization with 2.5 ml of a 4/1 mixture of ketamine (Imalgène 500; Rhône Mérieux, Lyon, France) and xylazine (Rompun at 2%; Bayer, Puteaux, France) per kilogram of body weight. Immunoprophylaxis studies were undertaken by passively transferring various dilutions of MAbs or polyclonal sera in 200-μl volumes by the i.p. route to mice 1 day before challenging with RSV-A as described above. Mice were sacrificed 5 days after challenge.
Animal sample preparation and virus titration.
Animals were anesthetized as described above and exsanguinated by cardiac puncture. Lung removal, lung homogenate preparation, nasal tract lavage (NTL), and virus titrations were undertaken as previously described, except that NTL fluids were snap frozen without being subjected to centrifugation (42). The limit of detection for lung tissues was ≤1.45 log10 TCID50/g of lung tissue, except when insufficient lung homogenate was available. The limit of detection for NTL samples was invariably log10 ≤0.45/ml. When no virus was detected, actual detection limits were used for statistical analyses. Thus, standard deviations of >0 were occasionally recorded for lung titers of some virus-free animal groups. Animal organs were considered protected when virus titers were reduced by at least 2 log10 relative to PBS-immunized control mouse levels.
Statistics.
Statistical analyses were done with the t test or Kolmogorov-Smirnov nonparametric test (for small sample sizes) of the Statigraphic software program (Manugistics, Rockville, Md.). Probability values of greater than 0.05 were considered insignificant.
RESULTS
Isolation and characterization of RSV-A G protein-specific MAbs.
To localize B-cell determinants on the central conserved domain of the RSV (Long) G protein, MAbs were generated by immunizing mice with BBG2Na (MAb 5B7, 6H66, 6H69, and 11F7), G2ΔCa (MAb 5C2), KLH-G174–187ΔC (MAb 18D1), or KLH-G190–204 (MAb 8A3). Because of difficulties in establishing hybridomas that secrete MAbs specific for the G190–204 region (residues 190 to 204), a polyclonal antiserum was also produced in parallel by immunizing mice with BB-G190–204. All isolated MAbs and the polyclonal antiserum recognized both BBG2Na and RSV-A, while the MAbs were exclusively of the IgG1 isotype (Table 1). With the exception of MAb 8A3, all had anti-RSV-A ELISA titers of >4 log10. Pepscan analyses identified three independent linear epitopes (detailed in Table 1) recognized by MAbs 5B7, 5C2, 11F7, and 18D1, with the last two MAbs showing reactivities to the same region (Table 1). These results were confirmed by ELISA titrations against relevant synthetic peptides. MAb 8A3 and anti-BB-G190–204 recognized a fourth, independent linear epitope as determined by peptide ELISA reactivities. Surprisingly, anti-BB-G190–204 also reacted with P40-G144–159Cys, although the ELISA titer was considerably lower than those against P40-G190–204Cys, BBG2Na, and RSV-A. The nature of this cross-reactivity is under investigation. Interestingly, MAb 6H66 and 6H69 did not show any reactivity in Pepscan analysis, but both demonstrated high peptide G171–187-specific ELISA titers. This peptide is conformational in that it contains two disulfide bridges. Since none of the Pepscan peptides contain all 4 Cys residues, and therefore the disulfide bridges, these results indicate that MAbs 6H66 and 6H69 recognize contiguous residues in the residue 171 to 187 region in a conformation-dependent manner. Furthermore, MAbs 6H66 and 6H69 reacted very weakly with peptide G172–187, indicating that residue 171V is a critical component and hence identifying a fifth epitope. Competition ELISAs demonstrated that these MAbs recognized the same epitope while MAbs 11F7 and 18D1 also recognized a single epitope that overlapped with the 6H66/6H69 epitope (data not shown). Thus, five independent B-cell epitopes were mapped on the G2Na fragment of the RSV-A G protein. None of the MAbs was capable of neutralizing RSV-A in vitro, as demonstrated in microneutralization assays (Table 1).
TABLE 1.
Characterization of the anti-RSV-A monoclonal antibodies and anti-BB-G190–204 polyclonal serum
| MAb or polyclonal seruma | Ig isotype | Pepscan reactivities (amino acid residues)b | ELISA titer (log10) vs:
|
In vitro neutralizing activity/25 μl | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RSV-A | BBG2Na | P40-G172–187 | P40-G171–187 | P40-G144–159Cys | P40-G190–204Cys | P40-G164–176 | ||||
| 5B7 (a) | IgG1 | 163–174 | 4.58 | 6.25 | <1.95 | <1.95 | <1.95 | <1.95 | 5.53 | <8 |
| 5C2 (b) | IgG1 | 150–157 | 5.77 | 5.77 | 1.95 | <1.95 | 5.77 | <1.95 | <1.95 | <8 |
| 6H66 (a) | IgG1 | NRc | 4.82 | 6.25 | 2.90 | 5.53 | <1.95 | <1.95 | <1.95 | <8 |
| 6H69 (a) | IgG1 | NR | 4.10 | 5.77 | 2.90 | 4.58 | <1.95 | <1.95 | <1.95 | <8 |
| 11F7 (a) | IgG1 | 176–187 | 5.29 | 6.73 | 6.25 | 5.29 | 2.43 | 2.19 | <1.95 | <8 |
| 18D1 (c) | IgG1 | 178–185 | 4.58 | 6.73 | 6.73 | 6.01 | 2.90 | 2.43 | 2.90 | <8 |
| 8A3 (d) | IgG1 | NDd | 3.38 | 4.82 | <1.95 | <1.95 | <1.95 | 4.34 | <1.95 | <8 |
| Anti-BB-G190–204 | ND | ND | 5.22 | 6.25 | 1.95 | <1.95 | 3.38 | 5.59 | 2.90 | <8 |
MAbs produced by immunizing BALB/c mice with BBG2Na (a), G2ΔC (b), KLH-G1ΔCa (c), or P40-G190–204 (d).
Reactivities against overlapping 8-mer peptides spanning the region containing residues 130 to 230 of the RSV-A(Long) G protein.
NR, no reactivity.
ND, not determined.
Protective efficacy of RSV-A G protein specific polyclonal antibodies and MAbs.
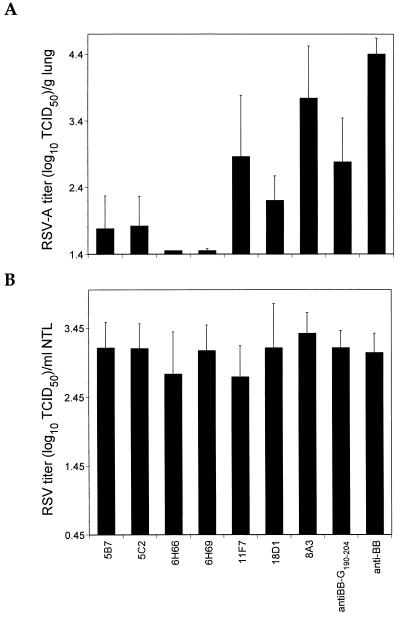
Passive-transfer experiments with the MAbs and anti-BB-G190–204Cys polyclonal serum were undertaken with groups of six to seven mice to determine if the identified B-cell epitopes were protective (protectopes). As indicated in Fig. 2A, MAbs 5B7, 5C2, 6H66, 6H69, and 18D1 protected the lungs from RSV-A challenge, although most of the mice contained infectious virus. MAb 11F7 was less protective under these conditions, since only three of six animals were considered protected while a fourth animal demonstrated a virus titer reduction of log10 1.7 relative to control animals. Nonetheless, MAb 11F7 has the capacity to protect mouse lungs from RSV-A challenge. Like MAb 11F7, anti-BB-G190–204 passive transfer resulted in significant virus titer reductions in the lungs of recipient mice (P < 0.001). However, only one of six mice was considered protected. MAb 8A3, which maps to the G190–204 peptide, had little impact on the prevention of lung infection in most animals under these experimental conditions, although one of six mice was protected. Interestingly, both anti-BB-G190–204 and MAb 8A3 were found to be highly labile, demonstrating diminished reactivities against both BBG2Na and RSV-A with time in ELISAs (data not shown). These data therefore demonstrate that all five B-cell epitopes identified above are LRT protectopes, albeit with considerably different efficacies under these experimental conditions. Unlike lung protection, and consistent with previous observations (42), none of the MAbs or polyclonal sera protected URTs from RSV-A challenge (Fig. 2B).
FIG. 2.
LRT (A) and URT (B) prophylactic efficacies of murine anti-RSV-A MAbs and polyclonal sera. Groups of six or seven mice received a single dose each of 104 anti-RSV-A ELISA titer equivalents of antibody 24 h before challenge with 105 TCID50 of RSV-A by i.n. instillation. Control mice received murine anti-BB polyclonal serum (at an anti-BB ELISA titer equivalent of 104). Mice were sacrificed 5 days later, at which point the lungs were removed and 10% lung homogenates were prepared. Nasal tracts were rinsed by forcibly injecting 1.5 ml of virus transport medium through the nasopharyngeal cavity and recovering exudate from the nares. Virus titers were determined as described in Materials and Methods.
Immunogenicity and protective efficacy of RSV-A G protein-derived synthetic peptides.
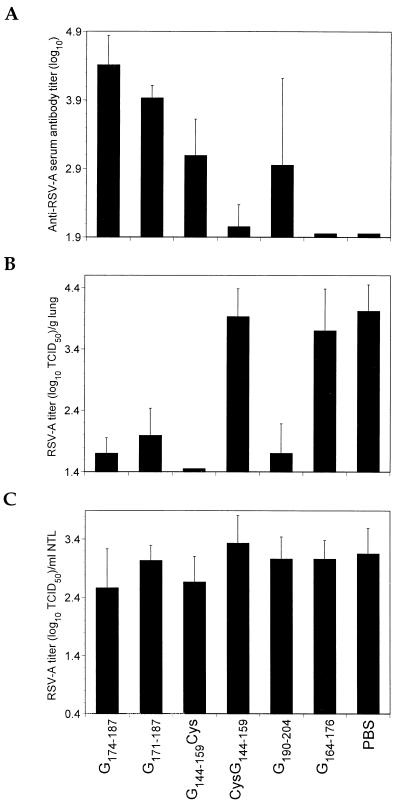
To confirm the results of the antibody passive-transfer studies, a series of peptides was synthesized and coupled to either P40 or BB by their N termini (CysG144–159, CysG190–204, and G164–176), C termini (G144–159Cys and G190–204Cys), or both (G174–187ΔC and G171–187) and injected into BALB/c mice. Mice primed with 20 μg of P40-G174–187ΔC and subsequently given two boosters of 100 μg each developed elevated RSV-A serum antibody titers, and their lungs were protected from challenge (Fig. 3A and B). In our hands, P40-G171–187 was less immunogenic than P40-G174–187ΔC under similar immunization conditions (P < 0.02). The differential immunogenicity was reflected in the respective protective efficacies of the peptides; the P40-G171–187 was slightly less efficacious in reducing virus titers in the lungs following RSV-A challenge. However, differences in protective efficacy were not statistically significant.
FIG. 3.
Humoral immune responses (A) and LRT- (B) and URT- (C) protective efficacies in mice following immunization with carrier-protein-coupled synthetic peptides. Groups of 7 to 12 mice were immunized i.p. at 2-week intervals two or three times with peptide coupled to P40 carrier protein in 200-μl volumes containing 20% (vol/vol) Alhydrogel. Mice immunized with P40-G174–187ΔC, P40-G172–187, or P40-G164–176 were primed with 20 μg of protein followed by two boosters of 100 μg each. Those immunized with P40-CysG190–204 received three doses of 20 μg of protein. Finally, mice immunized with P40-CysG144–159 or P40-G144–159Cys were immunized twice with 20 μg of protein. Controls included animals immunized with PBS. All mice were bled 2 weeks after the last immunization, challenged 1 week later with 105 TCID50 of RSV-A, and sacrificed 5 days postchallenge. Sera were tested in ELISAs against RSV-A. Ten percent lung homogenates were prepared. Nasal tracts were rinsed by forcibly injecting 1.5 ml of virus transport medium through the nasopharyngeal cavity and recovering exudate from the nares. LRT and URT virus titers were determined as described in Materials and Methods.
As indicated in Fig. 3, peptide G144–159 was immunogenic and protected the LRT from RSV-A challenge. However, these properties depended entirely on the orientation of peptide coupling to the carrier protein. Mice immunized twice with 20 μg of P40-G144–159Cys (C-terminal coupling) developed moderate RSV-A serum antibody titers, and their lungs were protected from RSV-A challenge; most lacked detectable virus. In contrast, 2 doses of 20 μg of P40-CysG144–159 (N-terminal coupling) were poorly immunogenic, and no lung protection was observed. Similar results were obtained when BB was used (data not shown), indicating that the results were independent of the carrier protein. In addition to the G174–187ΔC/G171–187 and G144–159 regions, G190–204 was also found to be immunogenic and protective. Mice immunized three times with 20 μg of conjugate demonstrated potent lung protection (Fig. 3B) that was independent of peptide orientation (data not shown). These results are consistent with and confirm the antibody passive-transfer study results described above. In contrast to the MAb passive-transfer results, peptide G164–176 was poorly immunogenic in BALB/c mice that were primed with 20 μg and boosted twice with 100 μg of conjugate. Their poor immunogenicity was reflected in the lack of significant lung virus titer reductions in mice immunized with either P40-G164–176 (Fig. 3B) or P40-G164–176ΔC (data not shown).
In contrast to the lung-protective efficacy observed for most of the peptides, none induced URT protection (Fig. 3C). This is also in agreement with the antibody passive-transfer study data. The combined results suggest that serum antibodies and linear RSV-A-specific B-cell epitopes in the G2Na fragment are insufficient for URT protection.
Reactivities of murine polyclonal sera with G protein-derived synthetic peptides.
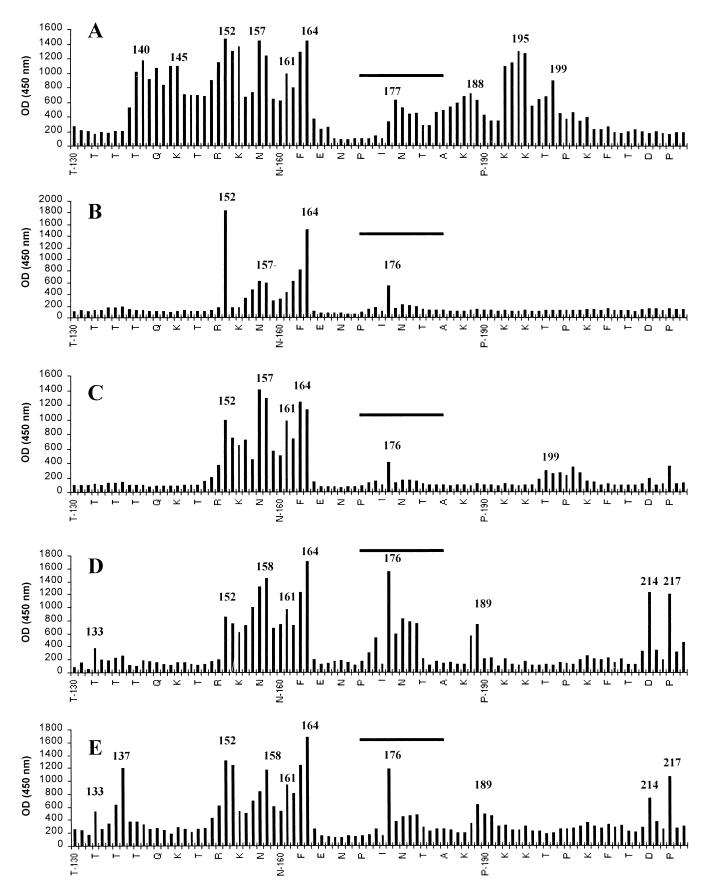
To determine and compare the epitope usage in mice following BBG2Na or RSV-A immunizations or infections, relevant sera were screened in a Pepscan assay using a series of overlapping dodecapeptides that spanned the central conserved domain (residues 130 to 230). As evidenced in Fig. 4A, sera derived from mice immunized i.p. with BBG2Na demonstrated six independent major peak reactivities, including peptides 140 to 151, 145 to 156, 152 to 163, 157 to 168, 164 to 175, and 195 to 206. Four independent minor reactivity peaks were also evident, including peptides 161 to 172, 177 to 188, 188 to 199, and 199 to 210. There was a noticeable absence of reactivity with peptides spanning the conserved cysteine-rich region. However, as indicated above to explain the lack of Pepscan reactivity of MAbs 6H66 and 6H69, this may be an artifact of the Pepscan assay. Therefore, with the exception of this region, and as expected, the protectopes identified above are clearly immunogenic after immunization with BBG2Na.
FIG. 4.
Reactivities of polyclonal sera from BALB/c mice immunized two times i.p. with 20 μg of BBG2Na (A) or three times i.p. (B) or i.n. (C) with RSV-A (105 TCID50) against overlapping 12-mer peptides spanning the region containing residues 130 to 230 of the RSV-A (Long) G protein. Intraperitoneal immunizations were undertaken in 200-μl volumes containing 20% (vol/vol) Alhydrogel. Mice were bled 10 days after the last immunization, and sera were tested in Pepscan analyses. The sera were diluted 1:5,000, 1:5,000, and 1:2,500 for anti-BBG2Na, anti-RSV-A i.p., and anti-RSV-A i.n., respectively. (D and E) Reactivities of two RSV-convalescent-phase human sera diluted 1:5,000. N-terminal residues of every third peptide are indicated on the x axis. Reactivity peaks are numbered to facilitate direct comparison of the various sera; the numbers correspond to the first amino acid residues of the implicated peptides. The conserved cysteine domains are indicated by horizontal bars. OD, optical density.
Sera from mice immunized i.p. (Fig. 4B) or infected i.n. (Fig. 4C) with RSV-A demonstrated far less epitope usage within the G2Na fragment than the anti-BBG2Na serum. Nonetheless, common reactivities with anti-BBG2Na serum were identified and mapped to the regions 152 to 163, 157 to 168, 164 to 175, and 176 to 187. Two other common reactivities with anti-BBG2Na serum were evident only in sera from mice infected i.n. with RSV-A; these mapped to residues 161 to 172 and 199 to 210 and coincided with three of the protectopes described above.
Mapping of B-cell epitopes in RSV-convalescent-phase human sera.
Pepscan analyses were undertaken with sera from humans with RSV infections to determine whether the human immune responses following infection result in antibodies reactive with the protectopes defined by mouse MAbs and anti-BBG2Na sera. Two sera obtained from patients recovering from RSV infections and selected because of their relatively high anti-RSV-A titers, 3.8 log10 and 4 log10, were tested. These sera had several independent peak reactivities in common, most notably peptides 133 to 144, 152 to 163, 158 to 169, 161 to 172, 164 to 175, 176 to 187, 189 to 200, 214 to 225, and 217 to 228 (Fig. 4D and E). Six of these reactivities coincided with those found in sera from BBG2Na-immunized mice. More importantly, the reactivities coincided with all five murine protectopes described above, even, surprisingly, those mapping to the cysteine-rich region.
DISCUSSION
We previously demonstrated that a recombinant protein (BBG2Na) incorporating the central conserved region of a prototype RSV-A G protein protected mouse and cotton rat lungs from virus challenge (42). In the present study, using a panel of murine MAbs, synthetic peptides derived from this G protein fragment, and Pepscan analyses, we determined the locations of five independent murine B-cell epitopes implicated in this protection. Two overlap with previously described protectopes (51, 53)—one in the completely conserved region and one in the cysteine noose domain—and map to residues 163 to 174 and 178 to 185, respectively. A third protectope (residues 171 to 187) overlaps with protectope 176 to 187, but its critical dependence on residue Val171 for reactivity with MAbs 6H66 and 6H69 established it as an independent epitope. Finally, two other novel protectopes, mapping to residues 150 to 157 and 190 to 204, were identified, although the immunogenicity of peptides (residues 144 to 159 and 187 to 200) overlapping these protectopes was previously reported (21, 51). Interestingly, the dependence of the immunogenicity and protective efficacy of peptide G144–159 (residues 144 to 159) on the orientation of coupling to the carrier protein may explain the lack of protection reported by Trudel et al. (51), in which N-terminal peptide coupling was likely. The importance of the G144–159 peptide’s orientation to its immunogenicity and protective efficacy is consistent with previous studies using tandem T- and B-cell epitopes (18).
Three protectopes map to a region of the prototype RSV-A (Long) G protein that is completely conserved among all known subgroup A isolates (residues 158 to 190). Indeed, it corresponds to the region known to contain subgroup-specific and conserved B-cell epitopes (32, 33). It seems reasonable to suggest, therefore, that these protectopes would be functional against all RSV-A isolates. Indeed, protectope 163 to 174, which maps to the completely conserved region of the G protein, may even explain the previously reported capacity of BBG2Na to protect against RSV-B challenge (42). Studies to evaluate this hypothesis are underway. Furthermore, protectopes 150 to 157 and 190 to 204 correspond to regions in which the prototype sequence is very highly conserved in recent clinical isolates (8, 20), and amino acid changes are usually conservative. Thus, these protectopes are also likely to elicit LRT protection against a broad spectrum of RSV-A isolates.
In contrast to the LRT protection, no prophylactic URT protection was evident after antibody passive transfer or peptide vaccination. There are several possible explanations for the URT protection failure. First and most simply, the epitopes concerned are incapable alone of inducing URT-protective immune responses. Second, at least as far as the MAb passive-transfer experiments are concerned, the doses employed were insufficient to achieve URT protection. Indeed, it was previously shown that URT protection requires significantly higher antibody doses than LRT protection (44). However, that peptide vaccination in some cases induced high anti-RSV-A antibody titers, similar to those previously described for BBG2Na (which demonstrates URT-protective efficacy) (42), without evidence of URT protection is more in accordance with our first hypothesis than the second. Finally, mechanisms other than antibody mediation, such as T-cell-mediated URT protection, may be implicated in BBG2Na-induced URT protection (42). This implies, however, that the hypothetical T-cell URT protectope(s) is not a component of the peptides used but is located elsewhere on the G2Na fragment.
Although most of the MAbs employed in this study prophylactically protected LRTs against virus challenge, none neutralized virus in vitro. This is consistent with previous reports (50, 51) and confirms the discordance between in vitro virus neutralization and in vivo protection. The results suggest that either the in vitro neutralization assay is a poor correlate for in vivo neutralization or mechanisms other than direct virus neutralization by the MAbs are employed in vivo. Such mechanisms may include antibody-dependent complement lysis of infected cells and/or antibody-dependent cellular cytotoxicity. These hypotheses are presently being evaluated. The low-level protective efficacies of MAb 8A3 and anti-P40-G190–204Cys polyclonal serum were surprising in view of the potent LRT protection induced by the corresponding G190–204 peptide. However, since both of these reagents are highly labile, it is likely that the protection attributed to these antibodies is underestimated. In contrast, the discordance between the levels of protection afforded by MAb 5B7 and peptide G164–176 is more difficult to explain. Since MAb 5B7 was derived from BBG2Na-immunized mice, the relevant epitope is clearly employed in the context of this RSV G fragment. However, peptide G164–176 (residues 164 to 176) corresponds to the hydrophobic domain of the central conserved region and is indeed very hydrophobic. It is possible that the hydrophobicity of this domain is accentuated in the peptide context, rendering it poorly immunogenic.
Since protectopes 163 to 174, 171 to 187, and 178 to 185 were identified by MAbs derived from BBG2Na-immunized mice, they are evidently employed upon BBG2Na immunization. Pepscan analysis of anti-BBG2Na polyclonal serum confirmed this for protectopes 163 to 174 and 178 to 185. However, this was not possible for protectope 171 to 187 due to its conformational dependence. Furthermore, Pepscan analysis of the serum indicated that epitopes overlapping with or incorporating protectopes 150 to 157 and 190 to 204 were also employed upon BBG2Na immunization. Interestingly, a lower level of epitope usage was evident in the G2Na region by mice following immunizations or infections with RSV-A than by those receiving BBG2Na immunizations (only three of five protectopes were employed). This is not surprising, considering the much higher number of RSV-specific epitopes on both the G and other viral proteins available to RSV-primed mice, which undoubtedly influences the hierarchy of epitope usage. It is also consistent with the hypothesis that the central conserved region of the G protein is poorly immunogenic in the context of the whole virion and G protein. The lack of reactivity with the other protectopes suggests that they represent subdominant epitopes in the context of the whole virus. These results are also consistent with the fact that in two independent panels of RSV-A G protein-specific murine MAbs, most MAbs are directed against the C-terminal hypervariable one-third of the protein (32; reviewed in reference 33).
Finally, Pepscan analyses of human RSV-convalescent-phase sera demonstrated considerable conservation of B-cell epitope usage in two individuals. Importantly, the epitope usage included all of the murine B-cell protectopes identified in this communication. Interestingly, the reactivities of both sera seemed to be intensified in the 152 to 175 region, which includes the completely conserved domain. Although more pronounced than that observed with murine anti-BBG2Na and RSV-A polyclonal sera, the Pepscan data suggest poor reactivity of the human sera within the cysteine noose region. This contrasts with previous reports (1, 39) in which peptide reactivities suggested that residues 174 to 188 constituted an immunodominant domain of the native G protein. However, care must be taken in interpreting our Pepscan data because the assay likely interferes with the noose conformation and thereby the associated epitopes. It is also noteworthy that more reactivities were observed in the Pepscan assay than previously reported when using peptides (1, 39). The discrepancies may be due to our use of 12-mer peptides that overlapped by 11 residues, thereby facilitating a more complete dissection of the epitopes in the conserved region than that achieved by Norrby et al. (39), who employed 15-mer peptides that overlapped by 5 residues.
In conclusion, we identified five independent B-cell protectopes capable of preventing RSV infection of mouse LRTs. Immunizing mice with BBG2Na increases the protectope usage in this central conserved region compared with RSV-A-infected or immunized mice. The use of these epitopes was evident in convalescent-phase human sera following RSV infection. Since anti-RSV polyclonal sera are known to be sufficient to reduce the incidence of RSV disease in the LRTs of high-risk children (4, 23), it is conceivable that the murine protectopes are also implicated in this prophylactic protection in view of the conserved epitope usage in humans. Should this hypothesis be proven, and in view of their linear nature, the murine protectopes may constitute important correlates of protective immunity in the development of RSV vaccines for humans. Conversely, a correlation with LRT protection in humans suggests that the central conserved region of the RSV G protein may constitute an important component of such vaccines.
ACKNOWLEDGMENTS
H.P.-G. and L.G. contributed equally to this work.
Excellent technical assistance was provided by Marie-Claire Bussat, Dominique Cyblat, Francis Derouet, and Nathalie Herbault. We thank A. Van Dorsselaer and N. Zorn for performing mass spectrometry. We are most grateful to Michel Segondy, Centre Hospitalier Universitaire, Montpellier, France, who provided the human RSV-positive sera.
REFERENCES
- 1.Åkerlind-Stopner B, Utter G, Mufson M A, Örvell C, Lerner R A, Norrby E. A subgroup-specific antigenic site in the G protein of respiratory syncytial virus forms a disulfide-bonded loop. J Virol. 1990;64:5143–5148. doi: 10.1128/jvi.64.10.5143-5148.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson L J, Hierholzer J C, Tsou C, Hendry R M, Fernie B F, Stone Y, McIntosh K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985;151:626–633. doi: 10.1093/infdis/151.4.626. [DOI] [PubMed] [Google Scholar]
- 3.Anderson L J, Hendry R M, Pierik L T, Tsou C, McIntosh K. Multicenter study of strains of respiratory syncytial virus. J Infect Dis. 1991;163:687–692. doi: 10.1093/infdis/163.4.687. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics. 1997;99:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 5.Bastien N, Taylor G, Thomas L H, Wyld S G, Simard C, Trudel M. Immunization with a peptide derived from the G glycoprotein of bovine respiratory syncytial virus (BRSV) reduces the incidence of BRSV-associated pneumonia in the natural host. Vaccine. 1997;15:1385–1390. doi: 10.1016/s0264-410x(97)00033-9. [DOI] [PubMed] [Google Scholar]
- 6.Bernatowicz M S, Matsueda G R. Preparation of peptide-protein immunogens using N-succinimidyl bromoacetate as a heterobifunctional crosslinking reagent. Anal Biochem. 1986;155:95–102. doi: 10.1016/0003-2697(86)90231-9. [DOI] [PubMed] [Google Scholar]
- 7.Cane P A, Matthews D A, Pringle C R. Identification of variable domains of the attachment (G) protein of subgroup A respiratory syncytial viruses. J Gen Virol. 1991;72:2091–2096. doi: 10.1099/0022-1317-72-9-2091. [DOI] [PubMed] [Google Scholar]
- 8.Cane P A, Matthews D A, Pringle C R. Analysis of relatedness of subgroup A respiratory syncytial viruses isolated worldwide. Virus Res. 1992;25:15–22. doi: 10.1016/0168-1702(92)90096-r. [DOI] [PubMed] [Google Scholar]
- 9.Cane P A, Pringle C R. Evolution of subgroup A respiratory syncytial virus: evidence for progressive accumulation of amino acid changes in the attachment protein. J Virol. 1995;69:2918–2925. doi: 10.1128/jvi.69.5.2918-2925.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cane P A, Thomas H M, Simpson A F, Evans J E, Hart C A, Pringle C R. Analysis of the human serological immune response to a variable region of the attachment (G) protein of respiratory syncytial virus during primary infection. J Med Virol. 1996;48:253–261. doi: 10.1002/(SICI)1096-9071(199603)48:3<253::AID-JMV7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1313–1351. [Google Scholar]
- 12.Corvaïa N, Tournier P, Nguyen T N, Haeuw J F, Power U F, Binz H, Andreoni C. Challenge of BALB/c mice with respiratory syncytial virus does not enhance the Th2 pathway induced after immunization with a recombinant G fusion protein, BBG2Na, in aluminum hydroxide. J Infect Dis. 1997;176:560–569. doi: 10.1086/514075. [DOI] [PubMed] [Google Scholar]
- 13.Crowe J E J. Immune responses of infants to infection with respiratory viruses and live attenuated respiratory virus candidate vaccines. Vaccine. 1998;16:1423–1432. doi: 10.1016/s0264-410x(98)00103-0. [DOI] [PubMed] [Google Scholar]
- 14.Dowell S F, Anderson L J, Gary H E J, Erdman D D, Plouffe J F, File T M J, Marston B J, Breiman R F. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174:456–462. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- 15.Falsey A R, Treanor J J, Betts R F, Walsh E E. Viral respiratory infections in the institutionalized elderly: clinical and epidemiologic findings. J Am Geriatr Soc. 1992;40:115–119. doi: 10.1111/j.1532-5415.1992.tb01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falsey A R, Cunningham C K, Barker W H, Kouides R W, Yuen J B, Menegus M, Weiner L B, Bonville C A, Betts R F. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis. 1995;172:389–394. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- 17.Falsey A R, Walsh E E. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis. 1998;177:463–466. doi: 10.1086/517376. [DOI] [PubMed] [Google Scholar]
- 18.Fernández I M, Snijders A, Benaissa-Trouw B J, Harmsen M, Snippe H, Kraaijeveld C A. Influence of epitope polarity and adjuvants on the immunogenicity and efficacy of a synthetic peptide vaccine against Semliki Forest virus. J Virol. 1993;67:5843–5848. doi: 10.1128/jvi.67.10.5843-5848.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouillard L, Mouthon L, Laporte J P, Isnard F, Stachowiak J, Aoudjhane M, Lucet J C, Wolf M, Bricourt F, Douay L. Severe respiratory syncytial virus pneumonia after autologous bone marrow transplantation: a report of three cases and review. Bone Marrow Transplant. 1992;9:97–100. [PubMed] [Google Scholar]
- 20.García O, Martín M, Dopazo J, Arbiza J, Frabasile S, Russi J, Hortal M, Perez-Breńa P, Martínez I, García-Barreno B, Melero J A. Evolutionary pattern of human respiratory syncytial virus (subgroup A): cocirculating lineages and correlation of genetic and antigenic changes in the G glycoprotein. J Virol. 1994;68:5448–5459. doi: 10.1128/jvi.68.9.5448-5459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-Barreno B, Delgado T, Åkerlind-Stopner B, Norrby E, Melero J A. Location of the epitope recognized by monoclonal antibody 63G on the primary structure of human respiratory syncytial virus G glycoprotein and the ability of synthetic peptides containing this epitope to induce neutralizing antibodies. J Gen Virol. 1992;73:2625–2630. doi: 10.1099/0022-1317-73-10-2625. [DOI] [PubMed] [Google Scholar]
- 22.García-Barreno B, Portela A, Delgado T, Lopez J A, Melero J A. Frame shift mutations as a novel mechanism for the generation of neutralization resistant mutants of human respiratory syncytial virus. EMBO J. 1990;9:4181–4187. doi: 10.1002/j.1460-2075.1990.tb07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groothuis J R, Simoes E A, Levin M J, Hall C B, Long C E, Rodriguez W J, Arrobio J, Meissner H C, Fulton D R, Welliver R C. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 24.Haeuw J-F, Rauly I, Zanna L, Libon C, Andreoni C, Nguyen T N, Baussant T, Bonnefoy J-Y, Beck A. The recombinant Klebsiella pneumoniae outer membrane protein OmpA has carrier properties for conjugated antigenic peptides. Eur J Biochem. 1998;255:446–454. doi: 10.1046/j.1432-1327.1998.2550446.x. [DOI] [PubMed] [Google Scholar]
- 25.Hall C B. Prospects for a respiratory syncytial virus vaccine. Science. 1994;265:1393–1394. doi: 10.1126/science.7915433. [DOI] [PubMed] [Google Scholar]
- 26.Henderson F W, Collier A M, Clyde W A J, Denny F W. Respiratory syncytial virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 27.Hendry R M, Burns J C, Walsh E E, Graham B S, Wright P F, Hemming V G, Rodriguez W J, Kim H W, Prince G A, McIntosh K. Strain-specific serum antibody responses in infants undergoing primary infection with respiratory syncytial virus. J Infect Dis. 1988;157:640–647. doi: 10.1093/infdis/157.4.640. [DOI] [PubMed] [Google Scholar]
- 28.Johnson P R, Spriggs M K, Olmsted R A, Collins P L. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci USA. 1987;84:5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson P R, Collins P L. The fusion glycoproteins of human respiratory syncytial virus of subgroups A and B: sequence conservation provides a structural basis for antigenic relatedness. J Gen Virol. 1988;69:2623–2628. doi: 10.1099/0022-1317-69-10-2623. [DOI] [PubMed] [Google Scholar]
- 30.Langedijk J P M, Meloen R H, Taylor G, Furze J M, van Oirschot J T. Antigenic structure of the central conserved region of protein G of bovine respiratory syncytial virus. J Virol. 1997;71:4055–4061. doi: 10.1128/jvi.71.5.4055-4061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine S, Klaiber-Franco R, Paradiso P R. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987;68:2521–2524. doi: 10.1099/0022-1317-68-9-2521. [DOI] [PubMed] [Google Scholar]
- 32.Martínez I, Dopazo J, Melero J A. Antigenic structure of the human respiratory syncytial virus G glycoprotein and relevance of hypermutation events for the generation of antigenic variants. J Gen Virol. 1997;78:2419–2429. doi: 10.1099/0022-1317-78-10-2419. [DOI] [PubMed] [Google Scholar]
- 33.Melero J A, García-Barreno B, Martínez I, Pringle C R, Cane P A. Antigenic structure, evolution and immunobiology of human respiratory syncytial virus attachment (G) protein. J Gen Virol. 1997;78:2411–2418. doi: 10.1099/0022-1317-78-10-2411. [DOI] [PubMed] [Google Scholar]
- 34.Mlinaric-Galinovic G, Falsey A R, Walsh E E. Respiratory syncytial virus infection in the elderly. Eur J Clin Microbiol Infect Dis. 1996;15:777–781. doi: 10.1007/BF01701518. [DOI] [PubMed] [Google Scholar]
- 35.Mufson M A, Orvell C, Rafnar B, Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985;66:2111–2124. doi: 10.1099/0022-1317-66-10-2111. [DOI] [PubMed] [Google Scholar]
- 36.Murby M, Samuelsson E, Nguyen T N, Mignard L, Power U, Binz H, Uhlén M, Ståhl S. Hydrophobicity engineering to increase solubility and stability of a recombinant protein from respiratory syncytial virus. Eur J Biochem. 1995;230:38–44. doi: 10.1111/j.1432-1033.1995.tb20531.x. [DOI] [PubMed] [Google Scholar]
- 37.Murphy B R, Graham B S, Prince G A, Walsh E E, Chanock R M, Karzon D T, Wright P F. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J Clin Microbiol. 1986;23:1009–1014. doi: 10.1128/jcm.23.6.1009-1014.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen T N, Samuelson P, Sterky F, Merle-Poitte C, Robert A, Baussant T, Haeuw J-F, Uhlén M, Binz H, Ståhl S. Chromosomal sequencing using a PCR-based biotin-capture method allowed isolation of the complete gene for the outer membrane protein A of Klebsiella pneumoniae. Gene. 1998;210:93–101. doi: 10.1016/s0378-1119(98)00060-2. [DOI] [PubMed] [Google Scholar]
- 39.Norrby E, Mufson M A, Alexander H, Houghten R A, Lerner R A. Site-directed serology with synthetic peptides representing the large glycoprotein G of respiratory syncytial virus. Proc Natl Acad Sci USA. 1987;84:6572–6576. doi: 10.1073/pnas.84.18.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nygren P A, Eliasson M, Abrahmsen L, Uhlén M, Palmcrantz E. Analysis and use of the serum albumin binding domains of streptococcal protein G. J Mol Recognit. 1988;1:69–74. doi: 10.1002/jmr.300010204. [DOI] [PubMed] [Google Scholar]
- 41.Olmsted R A, Elango N, Prince G A, Murphy B R, Johnson P R, Moss B, Chanock R M, Collins P L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci USA. 1986;83:7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Power U F, Plotnicky-Gilquin H, Huss T, Robert A, Trudel M, Ståhl S, Uhlén M, Nguyen T N, Binz H. Induction of protective immunity in rodents by vaccination with a prokaryotically expressed recombinant fusion protein containing a respiratory syncytial virus G protein fragment. Virology. 1997;230:155–166. doi: 10.1006/viro.1997.8465. [DOI] [PubMed] [Google Scholar]
- 43.Rueda P, Delgado T, Portela A, Melero J A, García-Barreno B. Premature stop codons in the G glycoprotein of human respiratory syncytial viruses resistant to neutralization by monoclonal antibodies. J Virol. 1991;65:3374–3378. doi: 10.1128/jvi.65.6.3374-3378.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siber G R, Leombruno D, Leszczynski J, McIver J, Bodkin D, Gonin R, Thompson C M, Walsh E E, Piedro P A, Hemming V G, Prince G A. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J Infect Dis. 1994;169:1368–1373. doi: 10.1093/infdis/169.6.1368. [DOI] [PubMed] [Google Scholar]
- 45.Simard C, Nadon F, Seguin C, Trudel M. Evidence that the amino acid region 124–203 of glycoprotein G from the respiratory syncytial virus (RSV) constitutes a major part of the polypeptide domain that is involved in the protection against RSV infection. Antivir Res. 1995;28:303–315. doi: 10.1016/0166-3542(95)00053-4. [DOI] [PubMed] [Google Scholar]
- 46.Storch G A, Anderson L J, Park C S, Tsou C, Dohner D E. Antigenic and genomic diversity within group A respiratory syncytial virus. J Infect Dis. 1991;163:858–861. doi: 10.1093/infdis/163.4.858. [DOI] [PubMed] [Google Scholar]
- 47.Stott E J, Taylor G, Ball L A, Anderson K, Young K K-Y, King A M Q, Wertz G W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987;61:3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullender W M, Mufson M A, Anderson L J, Wertz G W. Genetic diversity of the attachment protein of subgroup B respiratory syncytial viruses. J Virol. 1991;65:5425–5434. doi: 10.1128/jvi.65.10.5425-5434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tam J P, Wu C-R, Liu W, Zhang J-W. Disulfide bond formation in peptides by dimethyl sulfoxide: scope and applications. J Am Chem Soc. 1991;113:6659–6662. [Google Scholar]
- 50.Taylor G, Stott E J, Bew M, Fernie B F, Cote P J, Collins A P, Hughes M, Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984;52:137–142. [PMC free article] [PubMed] [Google Scholar]
- 51.Trudel M, Nadon F, Seguin C, Binz H. Protection of BALB/c mice from respiratory syncytial virus infection by immunization with a synthetic peptide derived from the G glycoprotein. Virology. 1991;185:749–757. doi: 10.1016/0042-6822(91)90546-n. [DOI] [PubMed] [Google Scholar]
- 52.Walsh E E, Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol. 1983;47:171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walsh E E, Hall C B, Schlesinger J J, Brandriss M W, Hildreth S, Paradiso P. Comparison of antigenic sites of subtype-specific respiratory syncytial virus attachment proteins. J Gen Virol. 1989;70:2953–2961. doi: 10.1099/0022-1317-70-11-2953. [DOI] [PubMed] [Google Scholar]
- 54.Walsh E E, Falsey A R, Sullender W M. Monoclonal antibody neutralization escape mutants of respiratory syncytial virus with unique alterations in the attachment (G) protein. J Gen Virol. 1998;79:479–487. doi: 10.1099/0022-1317-79-3-479. [DOI] [PubMed] [Google Scholar]