Abstract
Out of all emerging infectious diseases, approximately 75% are of zoonotic origin, with their source often traced back to animals. The emergence of zoonoses is driven by a complex interplay between anthropogenic, genetic, ecological, socioeconomic, and climatic factors. This intricate web of influences poses significant challenges for the prediction and prevention of zoonotic outbreaks. Effective coordination and collaboration among the animal, human, and environmental health sectors are essential for proactively addressing major zoonotic diseases. Despite advancements in surveillance and diagnostic practices, the emergence of zoonoses continues to be a pressing global concern. Therefore, prioritizing zoonotic disease surveillance is of paramount importance as part of a comprehensive disease prevention and containment strategy. Furthermore, evaluating existing surveillance systems provides insights into the challenges faced, which can be mitigated through implementation of One Health principles involving relevant stakeholders. To initiate multisectoral partnerships, it is crucial to identify the priorities and core themes of surveillance systems with equitable inputs from various sectors. Strengthening surveillance, promoting data sharing, enhancing laboratory testing capabilities, and fostering joint outbreak responses in both the human and animal health sectors will establish the necessary infrastructure to effectively prevent, predict, detect, and respond to emerging health threats, thereby reinforcing global health security. This review assesses existing surveillance approaches by offering an overview of global agencies engaged in monitoring zoonoses and outlines the essential components required at the human–animal–environment interface for designing comprehensive surveillance networks. Additionally, it discusses the key steps necessary for executing effective zoonotic disease surveillance through a One Health approach, while highlighting the key challenges encountered in establishing such a robust surveillance system.
Keywords: Emerging infectious diseases, Global health, One Health, Surveillance, Zoonoses
Highlights
-
•
Prioritizing zoonoses within ‘One Health’ approach is essential.
-
•
Shifting from reactive to proactive zoonotic risk mitigation.
-
•
Early warning system for timely detection and response to zoonotic threats.
-
•
Global health security requires transparent data sharing and global commitment.
-
•
Multisectoral collaboration is crucial for effective zoonotic surveillance.
1. Introduction
A major worldwide threat that is well acknowledged is the spread of infectious disease(s). With 2.5 billion infection incidents and 2.7 million fatalities each year, zoonoses (those diseases which can be naturally transmitted from animals to humans) account for 60% of known infectious diseases and up to 75% of emerging infectious diseases (EIDs) [1]. The list of zoonotic diseases is exhaustive and ever-evolving [2], with coronavirus disease 2019 (COVID-19) being the latest in the list [3]. By the mid-2023, the COVID-19 pandemic has resulted in the loss of 6.9 million lives [4]. The COVID-19 pandemic has clearly underscored the critical importance of implementing evidence-based multisectoral measures to prevent, detect, and mitigate the impact of EIDs. This urgency arises not only from immediate threats, but also from historical precedents. Historically, zoonotic spillover of pathogens has led to substantial outbreaks. Examples of zoonotic outbreaks in the 21st century include severe acute respiratory syndrome (SARS), H5N1 variant of highly pathogenic avian influenza (HPAI), swine flu (H1N1), Middle East respiratory syndrome (MERS), Zika, Ebola, Nipah, and COVID-19, all of which pose severe risks to human health and, in some cases, have also affected animal health, given their zoonotic nature [[5], [6], [7], [8]].
Understanding the underlying causes and origins of zoonotic outbreaks is necessary to mitigate the risk of disease transmission, particularly considering the observed upward trend in the number of zoonotic outbreaks over time. Numerous factors, such as international trade and travel, intensive animal husbandry practices, increasing animal food demand and related ecological disturbances, exploitation of wildlife, antimicrobial resistance (AMR), deforestation, climate change, global warming, and ecotourism have collectively precipitated the emergence of several critical global public health challenges [[9], [10], [11]].
The expansion of agriculture has resulted in encroachment into wildlife habitats, altering ecosystems and bringing humans and livestock into closer contact with wildlife and disease vectors, thereby potentially increasing the risk of zoonotic pathogen transmission through sylvatic cycles [5]. The risk of the spread of zoonoses at the environment–wildlife interface is further enhanced by anthropogenic activities such as hunting and consuming game meat, selling wild animals, and exploiting animal parts for trade [7]. Furthermore, improper use of antibiotics in animal husbandry exacerbates the global issue of AMR. By 2050, up to 10 million lives could be lost globally owing to AMR-related illnesses, and the economic burden associated with AMR is estimated at USD 100 trillion from 2015 to 2050 [12].
In our globalized world influenced by ever-evolving anthropogenic activities, the emergence of zoonoses underscores the need for robust surveillance systems. Identifying risk factors is crucial for determining potential hotspots and addressing these concerns necessitates evidence-based strategies and data-driven decision making. A rapid cross-border response that transcends geographical and political divides is vital, with effective communication as a linchpin to seamlessly connect stakeholders, healthcare providers, and the public.
Considering these challenges and the context, this review comprehensively assesses the existing surveillance strategies for zoonotic diseases. It provides an overview of global agencies engaged in monitoring zoonotic outbreaks and outlines the essential components required at the human–animal–environment interface for designing comprehensive surveillance networks. The aim of this review is to enhance the comprehension of zoonotic dynamics and support the development of effective surveillance strategies to safeguard global public health.
2. Socio-economic burden of emerging zoonoses
Since 1980, more than 87 new vectors and zoonotic infectious diseases have been documented [1]. These EIDs account for 26% of annual global deaths, encompassing various pathogen categories. Among these, viruses (including prions) account for 37% of emerging and re-emerging pathogens, while protozoa account for 25% [13]. Notably, viruses predominate as the primary causative agents of most emerging and re-emerging diseases. The impact of infectious diseases extends beyond mortality, leading to a substantial loss of disability-adjusted life years (DALYs) exceeding 30% of the 1.49 billion DALYs lost annually [14,15].
Zoonotic diseases have a significant impact on the global economy. Previous outbreaks from 1997 to 2009, including Nipah (Malaysia), West Nile fever (USA), SARS (Asia, Canada), HPAI (Asia, Europe), bovine spongiform encephalopathy (United Kingdom), and Rift Valley fever (Tanzania, Kenya, Somalia), resulted in an estimated financial toll of approximately USD 80 billion [16]. Moreover, other estimates indicate that EIDs have resulted in direct expenses exceeding $100 billion in the past two decades, with potential pandemic scenarios posing losses that reach trillions of dollars [17]. The International Livestock Research Institute (ILRI) acknowledges zoonotic diseases as a major factor affecting the livelihoods of one billion livestock keepers and resulting in 2.7 million human fatalities and approximately 2.5 billion cases of human illness annually [18]. Furthermore, in resource-limited regions, more than one in seven animals are estimated to be affected by these zoonoses [19]. Given the substantial socioeconomic costs associated with zoonotic outbreaks, policymakers should consider prioritizing investments for an effective holistic approach aimed at disease prevention rather than relying solely on response measures [17,20,21]. The persistent emergence of new EIDs poses an enduring threat to the integrity of the healthcare system and has the potential to hinder economic growth unless comprehensive mitigation strategies are formulated [6,9,22]. Addressing these challenges requires substantial investment in infrastructure development, encompassing early disease detection capabilities, integrated laboratory testing facilities, robust surveillance systems, vaccine production, and the integration of artificial intelligence to proactively manage zoonotic risks [[23], [24], [25], [26]].
To this end, the World Health Organization (WHO) has articulated a series of recommendations for national-level preparedness. These include enhancing the public health infrastructure, fortifying risk communication mechanisms, bolstering epidemic preparedness and response mechanisms, advancing research and its practical application, and advocating for political commitment and collaborative partnerships [27]. Collectively, these measures form a proactive framework for mitigating the impact of emerging zoonotic diseases on both healthcare and socioeconomic systems.
3. Prioritizing surveillance of zoonotic diseases
The spectrum of zoonotic diseases encompassing both age-old afflictions such as rabies and recent outbreaks exemplified by COVID-19 continues to expand. The emergence of zoonotic diseases typically follows a phased progression pattern, commencing with initial instances of spillover from animal reservoirs to humans, followed by sporadic small-scale outbreaks within human populations, and culminating in pathogen adaptation for sustained human-to-human transmission [28]. Each of these sequential phases may be driven by distinct underlying factors necessitating tailored, context-specific control measures.
In cases where emerging zoonoses shift to an endemic state, they persistently afflict both human and animal populations and concurrently pose the ongoing risk of future epidemic resurgence when the conditions conducive to rapid transmission are reinstated. A concerning observation gathered from the EIDs trend is that the frequency of zoonotic outbreaks is not anticipated to decrease in the near future. This is especially pronounced in light of expanding human and animal populations coupled with dynamic changes in the environmental landscape such as the loss of biodiversity [29].
Globally, the significance of robust surveillance in the battle against zoonotic diseases is crucial. Surveillance systematically generates critical data that underpin strategic efforts to confine, manage, and alleviate the impact on susceptible human and animal populations [30]. Early detection assumes significant importance in the containment of infections within their geographical origin and is a necessity accentuated by the contemporary global mobility of populations. Furthermore, early detection is imperative for averting life-threatening illnesses, such as Zika, Nipah, Ebola, and yellow fever [31]. Timely disease detection through surveillance coupled with swift deployment of responsive interventions can effectively curtail the magnitude, severity, and financial burden associated with emergency outbreak responses [32].
Confronting zoonotic diseases poses a distinctive challenge, necessitating targeted attention to the factors involved in their emergence. The intricate interplay between ecosystems and human activities amplifies the potential for disease transmission from animals to humans. Therefore, surveillance mechanisms should elucidate the pivotal role of certain animal species in bridging the gap between wildlife reservoirs and human populations, thereby enabling precise interventions. Many zoonoses are characterized by their insidious nature, often manifesting as asymptomatic or subclinical in both animal hosts and human carriers, thereby facilitating subtle transmission and covert dissemination. The COVID-19 pandemic has exemplified how asymptomatic individuals act as carriers, contributing to the rapid dissemination of the virus. Consequently, surveillance systems capable of identifying carriers with mild or absent symptoms are of fundamental significance for timely containment.
A robust scientific framework is required to enhance our ability to predict and implement proactive measures against zoonotic disease outbreaks. Such a framework should entail systematic acquisition and comprehensive understanding of data concerning the intricacies of disease emergence and transmission. This provides multifaceted insights into the genetic, demographic, sociological, and ecological determinants that facilitate the transmission of zoonotic pathogens to human hosts. Such scientific approaches are pivotal for developing a holistic strategy for early outbreak detection and containment, thereby enhancing preparedness to anticipate, prevent, and respond to future zoonotic disease threats [33].
4. Assessment of existing surveillance system/ongoing surveillance approach
Effective public health strategies for managing and controlling emerging pathogens rely heavily on surveillance systems [10]. This involves establishing event- and indicator-based surveillance, enhancing laboratory capabilities in both the human and animal sectors, ensuring a well-trained epidemiological workforce, and providing proper communication mechanisms [34]. Surveillance systems are typically overseen by a multitude of departments or ministries at the national level, including human infectious disease surveillance under health departments, food and animal disease surveillance under agriculture or livestock departments, and wildlife disease surveillance under natural resources, wildlife, and aquaculture departments. Moreover, some food production companies involved in raising livestock for international trade have also undertaken surveillance [35]. While companion animals can play a role in zoonotic disease transmission, surveillance of these animals is often conducted as a special study and lacks designated departmental responsibility in most countries [36].
Globally, numerous disease surveillance systems and networks have been established with a primary emphasis on detecting emerging zoonotic diseases in human and animal populations. Notable human disease surveillance systems include the WHO Global Outbreak and Response Network (GOARN), the U.S. Department of Defense's surveillance initiatives facilitated by the Global Emerging Infection Surveillance and Response System. These efforts, particularly those concentrated in high-risk regions across Africa, the Middle East, and Asia (particularly in the Southeast regions) are instrumental in the prompt identification of zoonotic diseases [37]. Other initiatives that collaborate closely with communities to reinforce zoonotic disease surveillance endeavors include the PREDICT project, funded by United States Agency for International Development (USAID) and the Global Early Warning and Response System for major animal diseases including zoonoses (GLEWS+), the joint initiative by the Food and Agriculture Organization (FAO), World Organization for Animal Health (WOAH), and WHO. These initiatives also provide comprehensive training for field and laboratory personnel, spanning both the human and animal health domains [38]. The overarching objective of the PREDICT project is the rapid detection of emerging novel viral threats to human and animal health. These methodologies focus on detecting hitherto unidentified infections and fostering the capacity to assess risks and manage epidemics that could potentially become pandemic [39]. The aim of GLEWS+ is to facilitate prevention and control measures by the rapid detection and assessment of health threats and potential concerns at the human–animal–ecosystem interface. Specifically, for zoonotic events, alerts of animal outbreaks can provide direct early warnings, enabling the enhancement of human surveillance and the implementation of preventive actions [16]. Table 1 provides a comprehensive overview of the selected global agencies and coordinated initiatives that serve as early warning systems, preparedness platforms, and data repositories for monitoring zoonotic outbreaks and the associated public health threats worldwide.
Table 1.
List of global agencies and initiatives for monitoring zoonotic outbreaks and other public health threats.
| Program | Key area(s) | Aim/objective(s) | Reference |
|---|---|---|---|
| Africa Centres for Disease Control and Prevention (CDC) Institute of Pathogen Genomics | ‘Hub-and-spoke’ model advancing data collection and surveillance capacity at the regional level. | To improve disease monitoring and public health collaborations by establishing integrated, cross-continent laboratory networks that are equipped with the tools, human resource capacity, and data infrastructure needed to fully harness essential genome sequencing technologies. | [40] |
| ArboNET | National arboviral surveillance system managed by the CDC. | Maintains data on arboviral infections among human viraemic blood donors, non-human mammals, sentinel animals, dead birds, and mosquitoes. | [41] |
| Coalition for Epidemic Preparedness Innovations (CEPI) | An innovative global partnership with a blend of financial commitments. | To finance and coordinate the development of new vaccines to prevent and control infectious disease outbreaks. | [42] |
| Connecting Organisations for Regional Disease Surveillance (CORDS) | CORDS is a program of Ending Pandemics, comprised of six regional member networks, working in 28 countries in Africa, Asia, the Middle East and Europe. | To detect and control the spread of infectious diseases by catalyzing exchange and collaboration among regional surveillance networks globally. | [43] |
| Global Disease Detection Program (GDD) | Program for developing and strengthening global public health capacity to rapidly identify and contain disease threats globally. | To detect, monitor, and support responses to global public health events of international importance by conducting event-based surveillance (EBS). | [44] |
| Global Food Infections Network (GFN) | Network to better detect and control foodborne and other enteric infections through integrated laboratory-based surveillance, and collaboration among human, veterinary, and food sectors. | Aims in building capacity to monitor, detect, analyze, characterize and respond to outbreaks of foodborne disease. It also provides training courses focus on regional or national needs ranging from basic to advanced laboratory practices, surveillance, outbreak detection and response, and attribution. | [45] |
| Global Health Security Agenda (GHSA) | An international effort to prevent, detect, and respond to infectious disease threats. | Aims to catalyze a collaborative, multisectoral initiative uniting countries, regions, international organizations, and the non-governmental sectors to enhance and expedite global health security efforts. | [46] |
| Global Influenza Surveillance and Response System (GISRS) | Global system focused on surveillance, preparedness and response for seasonal, pandemic and zoonotic influenza. | Aims for effective collaboration and sharing of viruses, data and benefits based on member states' commitment to a global public health model. | [47] |
| Global Initiative on Sharing All Influenza Data (GISAID) | An initiative to provide access to genomic data of influenza viruses. | Promotes the rapid sharing of data from all influenza viruses and the coronavirus causing COVID-19. | [48] |
| Global Outbreak Alert and Response Network (GOARN) | A WHO network that responds to acute public health events with the deployment of staff and resources to affected countries. | To examine and study diseases, evaluate the risks, and improve international capability to deal with diseases. The aim is to deliver rapid and effective support to prevent and control infectious diseases outbreaks and public health emergencies when requested. | [49] |
| Global Public Health Intelligence Network (GPHIN) | Electronic public health early warning system developed by Canada's Public Health Agency, and is part of the WHO GOARN. | Monitors internet media, such as news wires and websites, in nine languages in order to help detect and report potential disease or other health threats around the world. | [50] |
| Global Virome Project (GVP) | An innovative network partnership among public, private, philanthropic, and civil organizations to detect the planet's unknown viral threats to human health and food security to prepare for and stop future epidemics. | GVP is a strategic response to better predict, prevent, and respond to future viral pandemic threat. Through a collaborative, multidisciplinary approach, this global consortium fosters coordination, allowing partners and participants to leverage shared global and regional strategies while retaining the flexibility to address local needs. | [51] |
| International Food Safety Authorities Network (INFOSAN) | A global voluntary network, led jointly by FAO and WHO, comprises national Food Safety authorities from almost all member States of both organizations. | Aims to strengthen prevention, preparedness and response to food safety incidents and emergencies through fostering a global community of practice among food safety professionals. | [52] |
| International Pathogen Surveillance Network (IPSN) | A global network of pathogen genomic surveillance actors, brought together by the WHO Hub for Pandemic and Epidemic Intelligence. | Aims to accelerates progress in pathogen genomics and enhance public health decision-making by collecting, sequencing, and analyzing pathogen genomes to gain insights into their genetics, evolution, and transmission. | [53] |
| Pan American Center for Foot-and-Mouth Disease and Veterinary Public Health (PANAFTOSA/VPH) | A specialized center of the Pan American Health Organization/WHO linked to the communicable diseases' prevention, control, and elimination department. | Coordinates the Veterinary Public Health program, offering technical cooperation for zoonosis prevention, surveillance, control, and elimination, as well as promoting initiatives to enhance food safety systems and eradicate foot-and-mouth disease. | [54] |
| PREDICT | PREDICT empowered global surveillance for pathogens with zoonotic potential by enhancing capabilities for detecting and identifying potentially pandemic viruses. This initiative is a key component of USAID's Emerging Pandemic Threats program. | Strengthening worldwide monitoring and laboratory diagnostic capabilities for both known and newly discovered viruses within several important virus groups, such as filoviruses (including ebolaviruses), influenza viruses, paramyxoviruses, and coronaviruses. | [55] |
| Surveillance system for attack on health care (SSA) | Global and standardized monitoring system coordinated by WHO for the collection of primary data about attacks on health care. | The purpose is to systematically collect and make available data on attacks on health care, and their immediate impact on health care in countries facing emergencies. | [56] |
| The Global Early Warning System (GLEWS) | Joint mission of WHO, FAO, and WOAH to integrate alert mechanisms for emerging zoonotic diseases in the Global Early Warning System for major animal disease. | Aims to notify the global community and stakeholders about the emerging zoonoses, facilitate prediction, prevention, and control efforts, and deploy collaborative field missions for outbreak assessment and containment. | [57] |
| The Program for Monitoring Emerging Diseases (ProMED) | ProMED is the largest publicly available system conducting global reporting of infectious disease outbreaks. | Rapid global dissemination of information on outbreaks of infectious diseases and acute exposures to toxins that affect human health (including those in animals and in plants grown for food or animal feed). | [58] |
| The UK's Infection Innovation Consortium (iiCON) | Public-private-philanthropic partnership for pandemic preparedness by accelerating the discovery and development of new treatments, diagnostics, vaccines, and preventative products for infectious diseases. | Accelerate the research and development pipeline for drugs, vaccines, diagnostics, and public health interventions to address high-priority pandemics such as AMR and coronavirus. | [59] |
| Unitaid | A global health initiative collaborates to innovate disease prevention, diagnosis, and treatment in low- and middle-income countries (LMICs), with a primary focus on tuberculosis, malaria, HIV/AIDS, and associated deadly co-infections. | Finances late-stage research and development of novel drugs, diagnostics, and disease prevention tools. Also, facilitates data generation to support usage guidelines and promotes the availability of cost-effective generic medicines in LMICs. | [60] |
| WHO Mediterranean Zoonosis Control Programme (MZCP) | To strengthen zoonoses prevention and control, it collaborates closely with the WHO Regional Office for the Eastern Mediterranean, specialized WHO collaborating centers, national institutions, and maintains strong ties with the WOAH and FAO. | Aims to promote national and regional programs, strengthen collaboration between animal and public health services, offer training courses, support public health education, and foster international cooperation among MZCP member nations | [61] |
| WOAH and FAO global network of expertise on animal influenza: OFFLU | To promptly identify and analyze emerging strains of influenza viruses in animal populations. | Aims to reduce the negative impacts of animal influenza viruses by promoting effective collaboration between animal health experts and human health sector. OFFLU is to collaborate with the WHO on issues related to the animal–human interface, including pandemic preparedness for the early preparation of human vaccines. |
[62] |
| World Animal Health Information System (WAHIS) | WOAH offers computer tools for free global animal health data access via WAHIS portal, enhancing transparency in the world animal health situation. | Aims to provide an early warning system for listed and emerging diseases, a monitoring system for regular updates on these diseases, and collects additional data from national authorities on animal diseases impacting both human and animal populations, as well as veterinary service capacities. | [63] |
| Zoonotic Disease Integrated Action (ZODIAC) | An initiative by International Atomic Energy Agency (IAEA) to help countries prevent pandemics caused by zoonotic agents. | Aims to strengthen the preparedness and capabilities of Member States to rapidly detect and timely respond to the outbreaks. | [64] |
Human surveillance systems have effectively identified various zoonotic disease outbreaks in human populations. However, the global capacity to promptly detect infections within animal populations (including wildlife) to pre-empt transmission to humans remains limited [65]. Disease surveillance initiatives in livestock, poultry, and wildlife often lack unified oversight and have historically received inadequate funding compared to human surveillance efforts [66]. Consequently, it is frequently the identification of disease outbreaks in humans that catalyzes the subsequent discovery of outbreaks within animal populations, rather than a more proactive approach in which animal surveillance serves as an early warning system for potential human infections.
To mitigate the adverse effects of biological threats including the inadvertent or deliberate release of pathogenic or toxic agents, the international community ratified a legally binding framework, the ‘International Health Regulations 2005’ (IHR2005). Effective execution of the WHO's IHR requires active engagement from each nation's animal health sector [67]. For the animal sector, the WOAH offers the Performance of Veterinary Services (PVS) pathway, a comprehensive mechanism that enables countries to conduct in-depth assessments of their veterinary services and identify areas that warrant enhancement. Table 2 provides a comparative delineation of the core capacity prerequisites for disease surveillance stipulated in the IHR2005 and the quality evaluation criteria explained in the WOAH PVS tool. Notably, WOAH and WHO have thoroughly scrutinized their respective monitoring tools, revealing a spectrum of similarities, complementarities, and synergies.
Table 2.
Comparison of WHO's International Health Regulations and WOAH's Performance of Veterinary Services.
| Aspect of Comparison | International Health Regulations (IHR) | Performance of Veterinary Services (PVS) |
|---|---|---|
| Scope | Human health | Animal health |
| Member States | 196 member states | 182 member countries |
| Primary focus | Human diseases and public health | Animal diseases and animal health |
| Legal basis | Legally binding treaty (IHR2005) | Non-binding international standards |
| Purpose | Prevention and response to international public health emergencies | Evaluation and improvement of national veterinary services |
| Reporting requirements | Mandatory reporting of specified diseases and events with potential international impact | Voluntary reporting of animal health data |
| Disease categories | Focus on human diseases such as pandemics, epidemics, and specific diseases of international concern | Focus on animal diseases, including notifiable terrestrial and aquatic animal diseases |
| Monitoring and evaluation | WHO conducts assessments and provides guidance on capacity-building | WOAH conducts evaluations and offers support for improving veterinary services |
| Capacity building | WHO supports member states in building capacity for disease surveillance, detection, and response | WOAH assists member countries in enhancing their veterinary infrastructure and capabilities |
| Notification time frames | Immediate notification of certain diseases or events (within 24 h) | Reporting of animal diseases and events may not have strict timeframes |
| Sanitary measures | Recommendations for travel and trade restrictions to prevent the international spread of diseases | Recommendations for sanitary measures in animal populations to control disease spread |
| Zoonotic diseases | Includes zoonotic diseases that can impact human health | Focuses on diseases that affect animals, but zoonotic diseases are indirectly addressed due to their impact on animal health and trade |
| Communication | Emphasis on international coordination and communication between member states and WHO | Emphasis on national veterinary authorities' communication with WOAH and other relevant organizations |
| Transparency | IHR provisions require transparency in disease reporting and response measures | WOAH promotes transparency in the evaluation of veterinary services and encourages member countries to share relevant information |
Leveraging the insights and outcomes gathered from these assessment and gap analysis tools, which are specifically employed within the IHR monitoring framework and the WOAH PVS pathway, WHO and the WOAH have jointly devised methodologies to foster cross-sectoral communication between animal health and human health [68,69]. This elevated level of dialogue enhances operational coordination and furnishes policymakers with more comprehensive insights to guide strategic investments in surveillance and monitoring programs, ultimately strengthening preparedness to contain the spillover and spread of zoonotic diseases.
5. Understanding disease pathways and surveillance interconnections
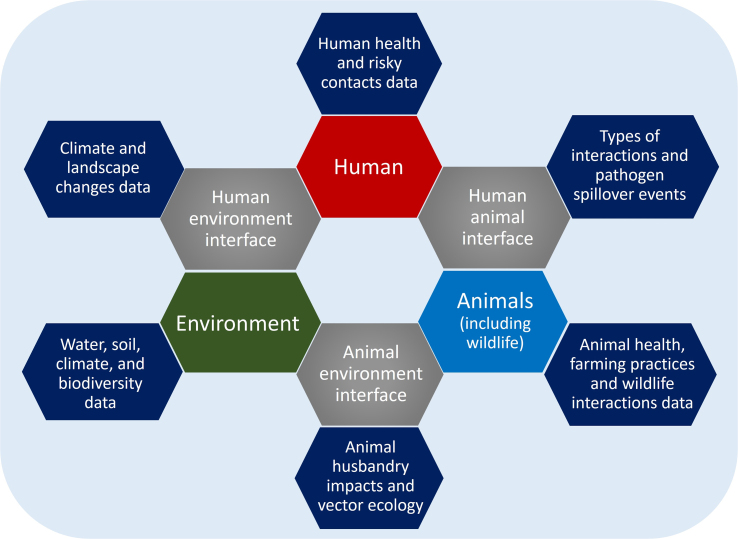
To enhance our capacity to predict and proactively manage disease outbreaks, it is essential to explore the intricate biological pathways of diseases and conceptual interdependencies that link the human, animal, and environmental surveillance sectors. These interconnections are pivotal in preventing the emergence of diseases. Typically, there are six distinct stages (Fig. 1) that shape the dynamics of disease in both human and animal populations. Disease dynamics are intrinsically linked to various phases of zoonotic transmission.
Fig. 1.
Components at the human–animal–environment interface to be considered while designing surveillance networks.
As the disease progresses within both human and animal populations, the goals of disease mitigation and surveillance evolve accordingly. An effective surveillance mechanism can play a crucial role in identifying the presence of a disease in animals before its initial spillover into human populations, during localized human infections resulting from spillovers, or at the stage of sustained human-to-human transmission of zoonotic threats [70]. Each of these phases necessitates distinct public health and animal disease mitigation objectives coupled with tailored interventions and surveillance measures designed specifically for the affected and susceptible populations. This adaptability is founded on the formulation of a risk profile for public health in each scenario, factoring in considerations such as the nature of the pathogen and potential exposure pathways. The significance of the information gathered through surveillance at various stages varies according to the established risk profile. A comprehensive exploration of the human–animal–environment interface and the required surveillance inputs is provided in Table 3.
Table 3.
Human–animal–environment interface and essential surveillance inputs.
| Component | Data needed for surveillance |
|---|---|
| Human |
|
| Animal |
|
| Environment |
|
| Human–Animal Interface |
|
| Human–Environment Interface |
|
| Animal–Environment Interface |
|
6. Need for multisectoral collaboration for zoonotic disease surveillance
To establish an effective zoonotic disease surveillance system, fostering evidence-based multisectoral collaboration with clearly defined roles and responsibilities is essential. Such partnerships have the potential to significantly reduce the impact of diseases on both humans and animals and mitigate social and economic consequences at the household, regional, and national levels [71]. International cooperation in this regard is exemplified by a tripartite partnership involving the WHO, WOAH, and FAO. This collaboration expanded further with the signing of a quadripartite memorandum of understanding on March 17, 2022, which included the UN Environment Programme (UNEP) [72]. This new framework aims to ensure the holistic well-being of humans, animals, plants, and the environment, thereby strengthening national and regional health systems.
The recent COVID-19 pandemic has underscored the need for effective multisectoral partnerships. Human, livestock, wildlife, and environmental health can no longer be discussed in isolation; they fall under one umbrella, which is One Health [73]. Multisectoral collaboration(s) should be tailored to specific needs and available resources, as health is a collective effort that relies on professional trust and expertise sharing. Both the UNEP and ILRI emphasize the significance of using a multisectoral collaborative approach to manage and prevent zoonotic disease outbreaks and pandemics at the human, animal, and environmental interfaces [74]. Under international health regulations, national officials must cooperate with the relevant ministries to promptly identify critical public health events. To execute effective zoonotic disease surveillance through the One Health approach, several key steps as those mentioned below should be considered.
6.1. Identifying, gathering, analyzing, and disseminating information
Timely detection of emerging zoonotic diseases is essential for minimizing transmission and mitigating the associated health and socioeconomic impacts. Effective disease surveillance relies on the ability to distinguish between abnormal and normal disease patterns. Different data collection methods such as active, passive, targeted, risk-based, and syndromic surveillance can be employed depending on requirement and resource availability [75]. Additionally, traditional participatory methods such as interviews, surveys, and discussions, along with the utilization of electronic databases from established surveillance systems, can enhance the overall quality of existing surveillance efforts [34,76].
For decision makers to understand and utilize the collected data, efficient data analysis and presentation in accessible formats are essential [77]. In this regard, collaborations with the information technology sector can help leverage modern technology, such as computers, cell phones, remote sensing, and Internet searches. Notable initiatives in this domain include GOARN, which utilizes web-based data for routine disease surveillance [78]. The revised IHR2005 empowers the WHO to act on informal information, enabling the timely issuance of disease prevention recommendations [79]. Early warning systems such as ProMED and GPHIN also exemplify integrated efforts to harness data from various sectors to enhance overall effectiveness.
6.2. Development of laboratory capacities and networks
Laboratory capacities and networks determine the effective collection of specimens, analysis, and definitive identification of the causative agents [9]. To achieve these objectives, laboratory systems must adhere to critical procedures, some of which are listed below.
-
a)
Sample transportation: The laboratory system should have robust mechanisms for safe and efficient transportation of specimens to designated facilities.
-
b)
Biosafety and biosecurity: Prioritizing biosafety measures is imperative to safeguard the health of investigators and prevent accidental exposure. Robust biosecurity protocols further ensure the safe handling and containment of dangerous pathogens, thereby reducing the risk of intentional harm or misuse.
-
c)
Standardized and adaptable protocols: Standardized laboratory protocols ensure consistency and reliability in diagnostic procedures and foster seamless collaboration among personnel. Designed to be adaptable, these protocols should respond to evolving research needs by ensuring rigorous and responsive diagnostic processes [30].
Incorporating both surveillance and technical-reference capabilities into national, regional, and global networks is a fundamental tenet of effective disease surveillance systems. Since the 1970s, international programs have been initiated to enhance the capacity of field epidemiology, laboratory work, and program management [80]. Notably, organizations such as the FAO, WOAH, and the United States Department of Agriculture, with support from agencies such as the World Bank and USAID, have organized capacity-building initiatives for disease surveillance and response in human and animal populations. The WHO has extended its contribution by founding GOARN, a comprehensive network of laboratories that provides guidance and support to local and national laboratories for disease detection. These collaborative endeavors have trained animal health experts globally, equipping them with diagnostic techniques and expertise in the prevention and control of significant diseases, including zoonotic threats associated with animal trade [81].
6.3. Zoonotic disease prioritization and collaboration for proactive intervention
Efficient coordination and collaborative efforts should encompass multiple sectors and should be initiated for select zoonotic diseases prioritized based on equitable inputs from all stakeholders [82]. Prioritization plays a pivotal role in resource allocation and ensures effective utilization [83]. By integrating disease prioritization strategies with seroprevalence studies, it is possible to identify and target priority zoonotic diseases in specific regions, thereby enabling more efficient prevention and control measures. Effective prioritization also supports the development of laboratory capacities and establishment of a robust surveillance network. Nevertheless, it is noteworthy that the socioeconomic impact of zoonotic infections can vary temporally and spatially and is often underestimated in resource-limited areas owing to restricted surveillance and awareness [28].
To identify high-priority zoonoses requiring multidisciplinary engagement, the CDC's One Health office has devised the One Health Zoonotic Disease Prioritization tool, which employs a semiquantitative approach to ensure equitable participation from all relevant sectors, even when reliable surveillance data are scarce [83,84]. This tool assembles a diverse panel of experts from the human, animal, environmental, and other pertinent domains. Collaboratively, they establish criteria that can be tailored to specific regions or countries and categorize zoonotic diseases of high national or regional significance [85,86]. This tool has been successfully deployed in numerous countries to highlight zoonotic diseases for future engagement programs as part of the GHSA action package for zoonotic diseases [87].
6.4. Human capacity development for intersectoral collaborations
Execution of an integrated zoonotic disease surveillance system requires cooperation at the regional, national, and global levels among professionals from diverse fields and with varied training, such as clinical diagnosis, field epidemiology, laboratory sciences, social sciences, ecology, and information technology [80]. Additionally, a team with good management skills, an understanding of the significance of a nationally and globally integrated system, and the ability to collaborate effectively with experts from different disciplines is essential. Collaboration between public and private sector organizations in human health, agricultural, and natural resource sectors is vital. In the human health sector, this involves physicians, public health professionals, village and community health workers, laboratories, hospitals, and nongovernmental organizations (NGOs) specializing in health education, communication, and training. The agricultural, livestock, and poultry sectors encompass veterinarians, village and community animal-health workers and technicians, animal producers, animal hospitals, and NGOs offering development and capacity-building programs for small-scale livestock and poultry farmers. Initiatives related to wildlife conservation, management, and disease surveillance play crucial roles [70,88].
Over the years, professionals, such as physicians, nurses, veterinarians, animal technicians, and laboratory scientists, have played critical roles in the early detection of emerging zoonotic diseases, as exemplified by the early detection of the monkeypox virus in 2022 [89]. Providing comprehensive in-person hands-on training to manage zoonotic infections in both human and animal populations is essential, particularly for rare, sporadic, and newly emerging diseases. While the use of information technology for such training is promising, further development and evaluation of these tools are necessary to ensure their effectiveness.
6.5. Harnessing artificial intelligence (AI) for pandemic preparedness and response
Shortly after the declaration of the COVID-19 pandemic, the WHO and other health organizations recognized the potential of AI as a valuable tool for addressing the pandemic and preparing for future health crises [90]. AI-powered early warning systems play a pivotal role by analyzing real-time data (from various sources including digital content and information channels) to detect patterns that indicate potential epidemics. These systems provide timely alerts that can aid surveillance, healthcare network management, and data analysis. Notable examples include the WHO Early Warning System and Bluedot [90,91]. Many countries today utilize geolocation data, surveillance camera footage, credit card information, and contact tracing systems to identify potential infection pathways [92]. In certain situations, AI can extract epidemiological data more rapidly than conventional methods of reporting health data [93]. Ongoing technological advancements hold great promise for both human and animal well-being and underscore the notion that the integrating AI into established research frameworks can play a pivotal role in preventing future pandemics [26].
6.6. Assessing the effectiveness of disease surveillance systems
Various attributes must be examined to assess the effectiveness of disease surveillance systems. These include timeliness (in detection, confirmation, and dissemination), simplicity (user-friendliness), flexibility (adaptation and responsiveness to changing circumstances, emerging threats, and evolving data needs), data quality, reliability, acceptability, sensitivity (the ability to identify all disease cases or outbreaks), positive predictive value (indicating the likelihood that the reported outbreaks are genuine), representativeness of the population at risk, stability (the system's resilience under adverse conditions), and overall usefulness [32,88]. In the context of animal disease monitoring and surveillance systems, the key criteria for effectiveness include clarity about the objectives, sampling methods, coordination and awareness, environmental factors, screening and diagnostic protocols, data collection and transmission procedures, data processing and analysis capabilities, and mechanisms for information dissemination [65]. Increasing farmers' awareness to encourage identification and self-reporting can be vital for the success of passive data collection, particularly in resource-limited settings.
The guidelines for the assessment of disease surveillance system effectiveness have been developed by organizations such as the CDC [94,95], WHO [96], and WOAH [97,98]. These guidelines are structured around the components and attributes that are essential for robust surveillance.
7. Key challenges in establishing effective surveillance systems: a way forward
The establishment of a robust surveillance system is inherently complex, necessitating careful consideration of diverse options, effective resource allocation, and management of constraints. Structural and operational intricacies along with regulatory impediments pose significant challenges and can hinder the seamless functioning of these indispensable networks. In this context, we explored some of these challenges, especially in resource-limited settings. We present a potential points for a path forward below.
-
a)
Enhancing data integration: In resource-limited countries, integrating data related to disease outbreaks, surveillance, and mortality and morbidity reports remains challenging. Vital registration systems often operate in isolation from healthcare systems, creating substantial gaps in the understanding of disease patterns and their real-world impacts [28]. These gaps persist because of administrative and social barriers, resulting in a significant underreporting of deaths, even within healthcare facilities. Consequently, the understanding of the link between mortality and morbidity, particularly in infectious diseases, remains limited.
Furthermore, data related to animal trade are frequently inadequate and unreliable, such as inadequacies in the critical information about the species involved, trade volumes, trade routes, and long-term trends in both legal and illegal, national and international trade of domestic animals and wildlife. This deficiency in the comprehensive monitoring and surveillance of wildlife trade elevates the risk of zoonotic disease emergence. A clear illustration of this risk materialized when the monkeypox virus was transmitted from Ghana to Texas through the pet trade involving pouched rats [99]. Therefore, to mitigate the risk of zoonotic disease transmission and to foster a more holistic understanding of disease dynamics, it is essential to strengthen the integration of data systems. This endeavor demands substantial improvements in reporting mechanisms and a commitment to monitoring and surveillance efforts, encompassing both domestic and wildlife trade. The success of these initiatives depends on cooperative and coordinated actions at both national and international levels.
-
b)
Implementation challenges: One of the significant challenges in disease surveillance systems is the separation of data streams. This delineation separates data related to citizens' utilization of healthcare services for disease treatment from the mechanisms employed for reporting disease outbreaks. Consequently, a lack of uniformity has emerged in the investigation and reporting of disease outbreaks. This fragmented approach results in inconsistencies in the collected data, hampering the comprehensiveness of surveillance efforts [69].
To address these issues, pilot projects such as WHO-supported influenza surveillance and the CDC's initiative for acute encephalitis syndrome and acute febrile illnesses have been launched [100]. However, these efforts have mainly remained research-driven pilots owing to resource constraints and varying government commitment levels. This underscores the need for sustained investment and the political will to scale up and strengthen disease surveillance for better detection and response to emerging health threats.
-
c)
Surveillance functions in vertical siloes of programs and institutions: Surveillance programs such as GLEWS and GOARN have demonstrated significant success in addressing disease transmission, enhancing public awareness of zoonoses in affected regions (e.g., COVID-19, rabies, and monkeypox), expanding access to treatment for those infected, and reducing mortality rates. These achievements underscore the importance of targeted surveillance efforts to combat zoonotic threats. Despite these advances, challenges remain in the current disease surveillance landscape. One prominent issue is the fragmentation of surveillance data among vertical programs. Although these programs have made substantial progress individually, their surveillance data often remain segregated and are not fully integrated into a unified surveillance platform. This fragmentation impedes efficient sharing and utilization of critical data. Even when data overlap occurs between programs, there is often no established mechanism for sharing or coordinating the use of these data. This lack of coordination results in inefficiencies and missed opportunities for collaboration. Furthermore, the use of existing data systems to address program- and policy-related inquiries is limited. A disconnect often exists between program implementation structures and research organizations, which limits their capacity to harness data for broader insights and improvements [101]. This gap in coordination and information sharing undermines the potential for a more holistic approach to zoonotic disease surveillance.
-
d)
Limited involvement of private sector: The private healthcare sector encompasses diverse entities from unregistered practitioners to large hospitals that operate as for-profit or not-for-profit institutions [102]. However, their participation in disease surveillance has been limited. Integrating the private sector into public health surveillance requires addressing essential questions such as identifying diseases relevant to private healthcare providers, determining appropriate levels of engagement from primary care clinics to specialized institutions, ensuring data quality and consistency through established standards, and exploring the utility of private sector insurance data for surveillance purposes. A citizen-centric approach that combines public- and private-sector data can enhance real-time surveillance and comprehensive population coverage. Successful integration with the private sector demands meticulous planning, collaboration, and transparent guidelines to ensure the accuracy, consistency, and value of the data collected for public health purposes [1].
-
e)
Human resource challenges: In several countries, the recruitment of state- and district-level surveillance units is decentralized, resulting in inconsistent responses from individual states in addressing human resource shortages for public health surveillance [103]. This decentralization means that health surveillance remains a national priority, whereas health management is a state responsibility. However, persistent vacancies and capacity constraints are significant issues in many regions. A study by Bernstein et al. highlighted a striking disparity in veterinarians' availability across regions, ranging from two veterinarians per 100,000 people in Africa to two per 1000 people in countries such as Spain, Uruguay, and the Falkland Islands. The shortage of well-trained veterinarians, especially in spillover hotspots, is concerning given their critical role in preventing disease transmission from wildlife or livestock to humans [28]. Therefore, exploring and addressing the reasons for the limited emphasis on public health surveillance by state governments is essential. Possible factors include competing priorities, resource constraints, and differing perceptions regarding the importance of surveillance. To enhance public health surveillance and response, proactive engagement with state governments, resource allocation, and national and international collaboration are crucial to bridge human resource gaps and strengthen disease surveillance expertise.
-
f)
Enhancing institutional capacity for training in public health core-capacity: To effectively monitor emerging zoonotic disease risks, there is a global need to reinforce institutional capacity through field-oriented, multidisciplinary training programs. While various training initiatives exist for public health experts with a significant emphasis on surveillance, some noteworthy programs deserve mention. The United States Epidemic Intelligence Service (EIS), conducted by the CDC, offers a comprehensive two-year master's degree in public health programs focused on the EIS, aiming to cultivate highly skilled epidemiologists who specialize in surveillance. The Public Health Agency of Canada's Field Epidemiology Training Program plays a vital role in enhancing the public health readiness to respond to crises. However, it is essential to acknowledge that many countries face a shortage of public health professionals, emphasizing the need for continued investment and collaborative efforts to address this critical gap in public health capacity building [104].
-
g)
Enhancing disease surveillance financing for effective global health security: Investments in disease surveillance, both in developed and developing countries, often originate as development aid, addressing not only financial and material challenges but also the intricacies of manpower-related difficulties that may arise. According to recent assessments, LMICs typically allocate a modest annual median budget of approximately USD 0.04 per capita for vaccine-preventable disease surveillance [105]. Recent research in the European Union (EU) has shed light on the average willingness to allocate resources for surveillance, revealing EUR 264 per year per individual, equivalent to approximately 5% of the overall healthcare budget [106]. These data underscore the potential disparity in funding allocation for disease surveillance within the EU, and raise questions about the adequacy of resources. Challenges in financing disease surveillance programs can manifest within organizations tasked with implementing national prophylactic plans. These hurdles often emerge when organizations harbor reservations about the effectiveness of these plans or perceive limited direct benefits from their execution. Furthermore, obstacles may arise from a lack of cooperation among diverse professional groups, including breeders, livestock brokers, and veterinarians. In some instances, professionals in these sectors may be reluctant to fully embrace certain disease control programmes, citing the perception that health authorities have inadequate incentives to engage in prophylactic measures. Consequently, addressing these human-related challenges, along with the financial and material aspects, is essential for ensuring the success and sustainability of disease surveillance efforts on a global scale [107].
8. Conclusion
The continuous emergence of new zoonotic diseases presents an ongoing challenge, necessitating the continuous improvement of disease surveillance systems to prevent and manage outbreaks proactively. This vision calls for an unwavering commitment at both national and international levels to establish an efficient surveillance mechanism. Successful leadership and collaborative synergy among diverse stakeholders, ranging from health and agriculture to natural resources, education, and beyond are indispensable aspects of this concerted effort. These efforts must transcend geographical boundaries and unite countries and regions. Furthermore, financial support and commitment are essential not only for the creation of the system but also for its continued operation. The ongoing assessment and evaluation of surveillance mechanisms across human, animal, and interconnected systems are crucial for adapting to evolving threats. This comprehensive evaluation should encompass key considerations, such as the comprehensiveness and quality of surveillance, effectiveness of multisectoral collaboration, and other critical dimensions. While global organizations such as the WHO, FAO, and WOAH have undertaken efforts to strengthen and assess ongoing global surveillance mechanisms, effective national and regional data integration is indispensable for the comprehensive success of zoonotic disease surveillance systems.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Generative AI and AI-assisted technologies in the writing process
While editing the manuscript, the authors employed ChatGPT to improve the language clarity in certain paragraphs. After using this tool, the authors reviewed and edited the content as needed. The authors take full responsibility for the content of the publication.
Declaration of competing interest
None.
Acknowledgements
The authors thank the Guru Angad Dev Veterinary and Animal Sciences University (GADVASU) and Kerala Veterinary and Animal Sciences University (KVASU) for providing the necessary facilities for this research.
References
- 1.Gebreyes W.A., Dupouy-Camet J., Newport M.J., D.-C. J, M.J. Newport The global One Health paradigm: challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Neglected Trop. Dis. 2014;8:e3257. doi: 10.1371/journal.pntd.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedi J.S., Vijay D., Dhaka P. first ed. John Wiley & Sons; 2022. Textbook of Zoonoses. [Google Scholar]
- 3.Tiwari R., Dhama K., Sharun K., Iqbal Yatoo Mohd., Malik Y.S., Singh R., Michalak I., Sah R., Bonilla-Aldana D.K., Rodriguez-Morales A.J. COVID-19: animals, veterinary and zoonotic links. Vet. Q. 2020;40:169–182. doi: 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO, WHO Coronavirus . 2023. COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard with Vaccination Data.https://covid19.who.int/ [Google Scholar]
- 5.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhama K., Chakraborty S., Kapoor S., Tiwari R., Kumar A., Deb R., Rajagunalan S., Singh R., Vora K., Natesan S. One World, One Health - Veterinary Perspectives. Adv. Anim. Vet. Sci. 2013;1:5–13. [Google Scholar]
- 7.Cunningham A.A., Daszak P., Wood J.L.N. vol. 372. Philosophical Transactions of the Royal Society B: Biological Sciences; 2017. (One Health, Emerging Infectious Diseases and Wildlife: Two Decades of Progress?). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong G., Bi Y.-H., Wang Q.-H., Chen X.-W., Zhang Z.-G., Yao Y.-G. Zoonotic origins of human coronavirus 2019 (HCoV-19/SARS-CoV-2): why is this work important? Zool. Res. 2020;41:213–219. doi: 10.24272/j.issn.2095-8137.2020.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergis J. One Health approach: veterinary perspectives in global and Indian context. Adv. Anim. Vet. Sci. 2014;2:11–16. doi: 10.14737/journal.aavs/2014/2.4s.11.16. [DOI] [Google Scholar]
- 10.Belay E.D., Kile J.C., Hall A.J., Barton-Behravesh C., Parsons M.B., Salyer S., Walke H. Zoonotic disease programs for enhancing global health security. Emerg. Infect. Dis. 2017;23:S65. doi: 10.3201/eid2313.170544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UNEP . UN Report; 2020. Unite Human, Animal and Environmental Health to Prevent the Next Pandemic.https://www.unep.org/news-and-stories/press-release/unite-human-animal-and-environmental-health-prevent-next-pandemic-un [Google Scholar]
- 12.O'Neill J. 2016. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. [Google Scholar]
- 13.Woolhouse M.E.J., Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor L.H., Latham S.M., woolhouse M.E.J. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . World Health Organization; 2005. Combating Emerging Infectious Diseases in the South-East Asia Region. [Google Scholar]
- 16.McCloskey B., Dar O., Zumla A., Heymann D.L. Emerging infectious diseases and pandemic potential: status quo and reducing risk of global spread. Lancet Infect. Dis. 2014;14:1001–1010. doi: 10.1016/S1473-3099(14)70846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Bank, World Bank People, pathogens and our planet, The economics of One Health, Volume 2 (2012), pp 65, Washington DC. https://documents1.worldbank.org/curated/en/612341468147856529/pdf/691450ESW0whit0D0ESW120PPPvol120web.pdf. (Accessed 15 September 2023).
- 18.Grace D., Mutua F., Ochungo P., Kruska R.L., Jones K., Brierley L. 2012. Lapar Lucila, Mapping of Poverty and Likely Zoonoses Hotspots. [Google Scholar]
- 19.Das Shagun S. Livestock farming is a crucible for zoonotic disease; here is how. Down Earth. 2022 https://www.downtoearth.org.in/news/health/livestock-farming-is-a-crucible-for-zoonotic-disease-here-is-how-85883 [Google Scholar]
- 20.Sleeman J.M., De Liberto T., Nguyen N. Optimization of human, animal, and environmental health by using the One Health approach. J. Vet. Sci. 2017;18:263–268. doi: 10.4142/jvs.2017.18.S1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghai R.R., Wallace R.M., Kile J.C., Shoemaker T.R., Vieira A.R., Negron M.E., Shadomy S.V., Sinclair J.R., Goryoka G.W., Salyer S.J., Barton Behravesh C. A generalizable One Health framework for the control of zoonotic diseases. Sci. Rep. 2022;12:8588. doi: 10.1038/s41598-022-12619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasobant S., Bruchhausen W., Saxena D., Falkenberg T. One Health collaboration for a resilient health system in India: learnings from global initiatives. One Health. 2019;8:100096. doi: 10.1016/j.onehlt.2019.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blancou J., Chomel B.B., Belotto A., Meslin F.X. Emerging or re-emerging bacterial zoonoses: factors of emergence, surveillance and control. Vet. Res. 2005;36:507–522. doi: 10.1051/vetres:2005008. [DOI] [PubMed] [Google Scholar]
- 24.Vink W.D., McKenzie J.S., Cogger N., Borman B., Muellner P. Building a foundation for “One Health”: an education strategy for enhancing and sustaining national and regional capacity in endemic and emerging zoonotic disease management. Curr. Top. Microbiol. Immunol. 2013;366:185–205. doi: 10.1007/82_2012_241. [DOI] [PubMed] [Google Scholar]
- 25.Kheirallah K.A., Al-Mistarehi A.-H., Alsawalha L., Hijazeen Z., Mahrous H., Sheikali S., Al-Ramini S., Maayeh M., Dodeen R., Farajeh M., Masadeh N., Alemam A., Alsulaiman J., Samhouri D. Prioritizing zoonotic diseases utilizing the One Health approach: Jordan's experience. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahiwade D., Patle G., Meshram E. 2019 3rd International Conference on Computing Methodologies and Communication (ICCMC) IEEE; 2019. Designing disease prediction model using machine learning approach; pp. 1211–1215. [DOI] [Google Scholar]
- 27.Dikid T., Jain S.K., Sharma A., Kumar A., Narain J.P. Emerging & re-emerging infections in India: an overview, centenary review article. Indian J. Med. Res. 2013;138:19–31. [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein A.S., Ando A.W., Loch-Temzelides T., Vale M.M., V Li B., Li H., Busch J., Chapman C.A., Kinnaird M., Nowak K., Castro M.C., Zambrana-Torrelio C., Ahumada J.A., Xiao L., Roehrdanz P., Kaufman L., Hannah L., Daszak P., Pimm S.L., Dobson A.P. 2022. E P I D E M I O L O G Y the Costs and Benefits of Primary Prevention of Zoonotic Pandemics.https://www.science.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morens D.M., Fauci A.S. Emerging infectious diseases: threats to human health and global stability. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedi J.S., Vijay D., Dhaka P., Singh Gill J., Barbuddhe S. Emergency preparedness for public health threats, surveillance, modelling & forecasting. Indian J. Med. Res. 2021;153:287. doi: 10.4103/ijmr.IJMR_653_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clow K.M., Leighton P.A., Pearl D.L., Jardine C.M. A framework for adaptive surveillance of emerging tick-borne zoonoses. One Health. 2019;7 doi: 10.1016/j.onehlt.2019.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vrbova L., Stephen C., Kasman N., Boehnke R., Doyle-Waters M., Chablitt-Clark A., Gibson B., FitzGerald M., Patrick D.M. Systematic review of surveillance systems for emerging zoonoses. Transbound Emerg. Dis. 2010;57:154–161. doi: 10.1111/j.1865-1682.2010.01100.x. [DOI] [PubMed] [Google Scholar]
- 33.Sokolow S.H., Nova N., Jones I.J., Wood C.L., Lafferty K.D., Garchitorena A., Hopkins S.R., Lund A.J., MacDonald A.J., LeBoa C., Peel A.J., Mordecai E.A., Howard M.E., Buck J.C., Lopez-Carr D., Barry M., Bonds M.H., De Leo G.A. Ecological and socioeconomic factors associated with the human burden of environmentally mediated pathogens: a global analysis. Lancet Planet. Health. 2022;6 doi: 10.1016/S2542-5196(22)00248-0. e870–e879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NRC Sustaining. Google Books; 2010. Global Surveillance and Response to Emerging Zoonotic Diseases - National Research Council, Division on Earth and Life Studies, Board on Agriculture and Natural Resources, Institute of Medicine, Board on Global Health, Committee on Achieving Sustainable Global Capacity for Surveillance and Response to Emerging Diseases of Zoonotic Origin. [Google Scholar]
- 35.Qiu Y., Guitian J., Webster J.P., Musallam I., Haider N., Drewe J.A., Song J. vol. 378. Philosophical Transactions of the Royal Society B: Biological Sciences; 2023. (Global Prioritization of Endemic Zoonotic Diseases for Conducting Surveillance in Domestic Animals to Protect Public Health). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glickman L.T., Moore G.E., Glickman N.W., Caldanaro R.J., Aucoin D., Lewis H.B. Vol. 6. 2006. pp. 14–23. (Purdue university–Banfield national companion animal surveillance program for emerging and zoonotic diseases, vector-borne and zoonotic diseases). [DOI] [PubMed] [Google Scholar]
- 37.IOM N.R.C. National Academies Press; Washington, D.C.: 2008. Achieving Sustainable Global Capacity for Surveillance and Response to Emerging Diseases of Zoonotic Origin. [DOI] [PubMed] [Google Scholar]
- 38.Fearnley L. Viral sovereignty or sequence etiquette? Asian science, open data, and knowledge control in global virus surveillance. East Asian Sci. Technol. Soc.: Int. J. 2020;14:479–505. doi: 10.1215/18752160-8698019. [DOI] [Google Scholar]
- 39.Krofah E., Steven Galson K., Robert Califf M., Gregory Simon. 2021. Clinical Trials in Crisis: Building on COVID-19's Lessons toward A Better Future. [DOI] [Google Scholar]
- 40.Institute of Pathogen Genomics (IPG) 2023. https://africacdc.org/institutes/ipg/
- 41.ArboNET 2023. https://www.cdc.gov/
- 42.CEPI 2023. https://cepi.net/
- 43.Connecting Organisations for Regional Disease Surveillance. 2023. https://www.cordsnetwork.org/ [Google Scholar]
- 44.Global Disease Detection. 2023. https://www.cdc.gov/globalhealth/healthprotection/gddopscenter/index.html [Google Scholar]
- 45.Global Food Infections Network 2023. https://www.emro.who.int/food-chemical-safety/food-infocus/food-infections-network.html
- 46.Global Health Security Agenda (GHSA) 2023. https://www.cdc.gov/globalhealth/security/what-is-ghsa.htm
- 47.Global Influenza Surveillance and Response System. GISRS); 2023. https://www.who.int/initiatives/global-influenza-surveillance-and-response-system [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.GISAID 2023. https://gisaid.org/
- 49.Global Outbreak Alert and Response Network. 2023. https://www.who.int/activities/rapidly-detecting-and-responding-to-health-emergencies/who-global-outbreak-alert-and-response-network-%28goarn%29 [Google Scholar]
- 50.GPHIN 2023. https://gphin.canada.ca/cepr/aboutgphin-rmispenbref.jsp?language=en_CA
- 51.Global Virome Project (GVP) 2023. https://www.globalviromeproject.org/
- 52.International Food Safety Authorities Network (INFOSAN) 2023. https://www.fao.org/food-safety/emergencies/infosan/en/
- 53.International Pathogen Surveillance Network (IPSN) 2023. https://www.who.int/initiatives/international-pathogen-surveillance-network#:∼:text=The%20International%20Pathogen%20Surveillance%20Network%20%28IPSN%29%20is%20a,in%20pathogen%20genomics%2C%20and%20improve%20public%20health%20decision-making
- 54.Pan American Center for Foot-And-Mouth Disease and Veterinary Public Health. PANAFTOSA/VPH); 2023. https://www.paho.org/en/panaftosa [Google Scholar]
- 55.PREDICT 2023. https://p2.predict.global/
- 56.Surveillance System for Attacks on Health Care. SSA); 2023. https://www.who.int/publications/i/item/surveillance-system-for-attacks-on-health-care-(-ssa [Google Scholar]
- 57.GLEWS 2023. http://www.glews.net
- 58.Program for Monitoring Emerging Diseases. ProMED); 2023. https://promedmail.org/about-promed/ [Google Scholar]
- 59.Infection Innovation Consortium. 2023. https://www.infectioninnovation.com/ [Google Scholar]
- 60.Unitaid 2023. https://unitaid.org/#en
- 61.The Activities of the Mediterranean Zoonoses Control Programme. 2023. https://iris.who.int/handle/10665/122022 [Google Scholar]
- 62.OFFLU's Annual Report: Tackling Animal Influenza through Data Sharing. 2023. https://www.woah.org/en/offlus-annual-report-tackling-animal-influenza-through-data-sharing/ [Google Scholar]
- 63.World Animal Health Information System 2023. https://www.woah.org/en/what-we-do/animal-health-and-welfare/disease-data-collection/world-animal-health-information-system/
- 64.Zoonotic Disease Integrated Action (ZODIAC) 2023. https://www.iaea.org/services/zodiac [Google Scholar]
- 65.Morse S.S., Mazet J.A., Woolhouse M., Parrish C.R., Carroll D., Karesh W.B., Zambrana-Torrelio C., Lipkin W.I., Daszak P. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kock R., Caceres-Escobar H. 2022. Situation Analysis on the Roles and Risks of Wildlife in the Emergence of Human Infectious Diseases. Switzerland. [Google Scholar]
- 67.Sturtevant J.L., Anema A., Brownstein J.S. The new international health regulations: considerations for global public health surveillance. Disaster Med. Public Health Prep. 2007;1:117–121. doi: 10.1097/DMP.0b013e318159cbae. [DOI] [PubMed] [Google Scholar]
- 68.Kimball A.M., Moore M., French H.M., Arima Y., Ungchusak K., Wibulpolprasert S., Taylor T., Touch S., Leventhal A. vol. 92. Medical Clinics of North America; 2008. pp. 1459–1471. (Regional Infectious Disease Surveillance Networks and Their Potential to Facilitate the Implementation of the International Health Regulations). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suthar A.B., Allen L.G., Cifuentes S., Dye C., Nagata J.M. Lessons learnt from implementation of the International Health Regulations: a systematic review. Bull. World Health Organ. 2018;96:110–121E. doi: 10.2471/BLT.16.189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morse S.S. Public health surveillance and infectious disease detection. Biosecur. Bioterror. 2012;10:6–16. doi: 10.1089/bsp.2011.0088. [DOI] [PubMed] [Google Scholar]
- 71.Coker R., Rushton J., Mounier-Jack S., Karimuribo E., Lutumba P., Kambarage D., Pfeiffer D.U., Stärk K., Rweyemamu M. Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect. Dis. 2011;11:326–331. doi: 10.1016/S1473-3099(10)70312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.WHO . 2022. Strengthening the Global Architecture for Health Emergency Preparedness, Response and Resilience.https://www.who.int/publications/m/item/strengthening-the-global-architecture-for-health-emergency-preparedness-response-and-resilience [Google Scholar]
- 73.Nik Hasan N.N., Hamzah A. 2021. Global Partnership under a New Normal: Challenges and Response to COVID-19; pp. 511–522. [DOI] [Google Scholar]
- 74.MacMillan S. International Livestock Research Institute; 2020. A One-Health Approach Is Needed to Protect Both People and the Planet—ILRI and UNEP Leaders. [Google Scholar]
- 75.Dhaka P., Bedi J., Vijay D. In: ICT Tools for Management and Control of Emerging Zoonoses and Animal Health Threats. first ed. Narayanan M., Shahaji P., Prejit J. Vergis, A. K, V. P, editors. Directorate of Entrepreneurship, KVASU, COHEART, KVASU & MANAGE; Hyderabad: 2021. Epidemiological surveillance and disease modelling; pp. 57–61. [Google Scholar]
- 76.Swaminathan B., Barrett T.J., Hunter S.B., V Tauxe R., CDC PulseNet Task Force PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg. Infect. Dis. 2001;7:382–389. doi: 10.3201/eid0703.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mandl K.D. Implementing syndromic surveillance: a practical guide informed by the early experience. J. Am. Med. Inf. Assoc. 2003;11:141–150. doi: 10.1197/jamia.M1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keating P., Murray J., Schenkel K., Merson L., Seale A. Electronic data collection, management and analysis tools used for outbreak response in low- and middle-income countries: a systematic review and stakeholder survey. BMC Publ. Health. 2021;21:1741. doi: 10.1186/s12889-021-11790-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.WHO . 2008. Second Meeting of Pacific National Focal Points for the International Health Regulations. Rarotonga, Cook Islands. [Google Scholar]
- 80.Keusch G.T., Pappaioanou M., Gonzalez M.C., Scott K.A., Tsai P. National Academies Press; 2010. Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases. [DOI] [PubMed] [Google Scholar]
- 81.Christensen R., Fisher D., Salmon S., Drury P., Effler P. Training for outbreak response through the global outbreak alert and response network. BMC Med. 2021;19:123. doi: 10.1186/s12916-021-01996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salyer S.J., Silver R., Simone K., Barton Behravesh C. Prioritizing zoonoses for global health capacity building—themes from One Health zoonotic disease workshops in 7 countries, 2014–2016. Emerg. Infect. Dis. 2017;23(13):S55–S64. doi: 10.3201/eid2313.170418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rist C.L., Arriola C.S., Rubin C. Prioritizing zoonoses: a proposed One Health tool for collaborative decision-making. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.CDC . 2020. One Health Zoonotic Disease Prioritization Workshop. [Google Scholar]
- 85.Rabaa M.A., Tue N.T., Phuc T.M., Carrique-Mas J., Saylors K., Cotten M., Bryant J.E., Nghia H.D.T., Van Cuong N., Pham H.A., Berto A., Phat V.V., Dung T.T.N., Bao L.H., Hoa N.T., Wertheim H., Nadjm B., Monagin C., van Doorn H.R., Rahman M., Tra M.P.V., Campbell J.I., Boni M.F., Tam P.T.T., van der Hoek L., Simmonds P., Rambaut A., Toan T.K., Van Vinh Chau N., Hien T.T., Wolfe N., Farrar J.J., Thwaites G., Kellam P., Woolhouse M.E.J., Baker S. The Vietnam initiative on zoonotic infections (VIZIONS): a strategic approach to studying emerging zoonotic infectious diseases. EcoHealth. 2015;12:726–735. doi: 10.1007/s10393-015-1061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X., Rainey J.J., Goryoka G.W., Liang Z., Wu S., Wen L., Duan R., Qin S., Huang H., Kharod G., Rao C.Y., Salyer S.J., Behravesh C.B., Jing H. Using a One Health approach to prioritize zoonotic diseases in China. PLoS One. 2019;16(2021) doi: 10.1371/journal.pone.0259706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Talisuna A., Yahaya A.A., Rajatonirina S.C., Stephen M., Oke A., Mpairwe A., Diallo A.B., Musa E.O., Yota D., Banza F.M., Wango R.K., Roberts N.A., Sreedharan R., Kandel N., Rashford A.M., Boulanger L.L., Huda Q., Chungong S., Yoti Z., Fall I.S. Joint external evaluation of the International Health Regulation (2005) capacities: current status and lessons learnt in the WHO African region. BMJ Glob. Health. 2019;4 doi: 10.1136/bmjgh-2018-001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Halliday J., Daborn C., Auty H., Mtema Z., Lembo T., Bronsvoort B.M. deC., Handel I., Knobel D., Hampson K., Cleaveland S. Bringing together emerging and endemic zoonoses surveillance: shared challenges and a common solution. Phil. Trans. Biol. Sci. 2012;367:2872–2880. doi: 10.1098/rstb.2011.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.WHO . 2022. Multi-country Monkeypox Outbreak in Non-endemic Countries.https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 [Google Scholar]
- 90.Khan M., Mehran M.T., Haq Z.U., Ullah Z., Naqvi S.R., Ihsan M., Abbass H. Applications of artificial intelligence in COVID-19 pandemic: a comprehensive review. Expert Syst. Appl. 2021;185 doi: 10.1016/j.eswa.2021.115695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mbunge E., Akinnuwesi B., Fashoto S.G., Metfula A.S., Mashwama P. A critical review of emerging technologies for tackling COVID -19 pandemic. Hum. Behav. Emerg. Technol. 2021;3:25–39. doi: 10.1002/hbe2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lalmuanawma S., Hussain J., Chhakchhuak L. Applications of machine learning and artificial intelligence for Covid-19 (SARS-CoV-2) pandemic: a review. Chaos, Solit. Fractals. 2020;139 doi: 10.1016/j.chaos.2020.110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tayarani N M.-H. Applications of artificial intelligence in battling against covid-19: a literature review. Chaos, Solit. Fractals. 2021;142 doi: 10.1016/j.chaos.2020.110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.CDC, Surveillance Resource Center 2023. https://www.cdc.gov/surveillancepractice/index.html
- 95.Updated Guidelines for Evaluating Public Health Surveillance Systems. 2001. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5013a1.htm [PubMed] [Google Scholar]
- 96.WHO Recommended Surveillance Standards. 1999. https://www.who.int/publications/i/item/who-recommended-surveillance-standards [Google Scholar]
- 97.Guidelines for Wildlife Disease Surveillance: An Overview. 2015. https://www.woah.org/fileadmin/Home/eng/Internationa_Standard_Setting/docs/pdf/WGWildlife/OIE_Guidance_Wildlife_Surveillance_Feb2015.pdf [Google Scholar]
- 98.Animal Health Surveillance. 2022. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_surveillance_general.pdf [Google Scholar]
- 99.CDC . 2018. 2003 United States Outbreak of Monkeypox.https://stacks.cdc.gov/view/cdc/108095 [Google Scholar]
- 100.WHO . GISRS); 2023. Global Influenza Surveillance and Response System.https://www.who.int/initiatives/global-influenza-surveillance-and-response-system [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Asokan G.V., Mohammed M.Y. Big Data in Psychiatry #x0026. Elsevier; Neurology: 2021. Harnessing big data to strengthen evidence-informed precise public health response; pp. 325–337. [DOI] [Google Scholar]
- 102.Bennett A., Avanceña A.L.V., Wegbreit J., Cotter C., Roberts K., Gosling R. Engaging the private sector in malaria surveillance: a review of strategies and recommendations for elimination settings. Malar. J. 2017;16:252. doi: 10.1186/s12936-017-1901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jayatilleke K. Challenges in implementing surveillance tools of high-income countries (HICs) in low middle income countries (LMICs) Curr. Treat. Options Infect. Dis. 2020;12:191–201. doi: 10.1007/s40506-020-00229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Doble A., Sheridan Z., Razavi A., Wilson A., Okereke E. The role of international support programmes in global health security capacity building: a scoping review. PLOS Global Public Health. 2023;3 doi: 10.1371/journal.pgph.0001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hossain A., Politi C., Mandalia N., Cohen A.L. Expenditures on vaccine-preventable disease surveillance: analysis and evaluation of comprehensive multi-year plans (cMYPs) for immunization. Vaccine. 2018;36:6850–6857. doi: 10.1016/j.vaccine.2018.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Himmler S., Van Exel J., Perry-Duxbury M. vol. 21. 2020. pp. 763–773. (Werner Brouwer, Willingness to Pay for an Early Warning System for Infectious Diseases). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Vries L., Koopmans M., Morton A., Van Baal P. The economics of improving global infectious disease surveillance. BMJ Glob. Health. 2021;6(9) doi: 10.1136/bmjgh-2021-006597. [DOI] [PMC free article] [PubMed] [Google Scholar]