Abstract
Background
Chagas disease (CD) is transmitted by vectors but can also be transmitted orally through contaminated food, drinks, or meat. The One Health perspective aims to understand the complex interaction between human, animal, and environmental health in controlling disease. This study analyzed risk factors and drew lessons from past outbreaks of orally transmitted CD to develop effective preventive strategies.
Methods
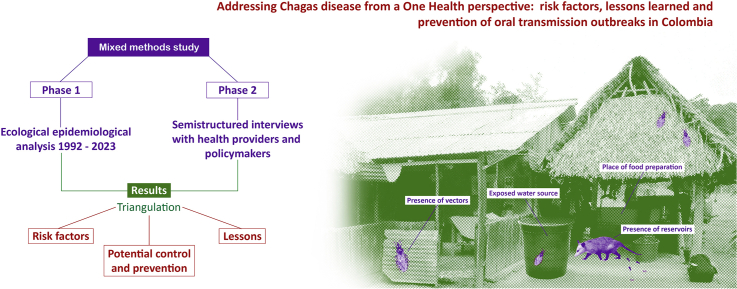
A simultaneous mixed methods study was conducted. The study consisted of two phases: an ecological epidemiological analysis at the municipal level using secondary data spanning from 1992 to 2023, and semistructured interviews with health providers and policymakers at the national level in Colombia. The results from both phases were triangulated to gain a comprehensive understanding of the topic.
Results
A total of 64 outbreaks, infecting 302 individuals, were reported. Most of these outbreaks (89.2%) were classified as family-related, and they occurred most frequently during the months of April to June (46.6%). It is worth noting that a significant number of these outbreaks took place in municipalities that lacked vector control plans. Risk factors for oral transmission included the location of food preparation, poor housing quality, food preparation water source, the presence of vectors/marsupials, forest type, and climatic variables. Interviews conducted emphasized the importance of implementing outbreak plans and providing staff training to effectively address the issue.
Conclusion
A One Health approach strengthening prevention, surveillance, case management and cross-sectoral collaboration is needed to control outbreaks and reduce transmission in Colombia. Preparedness plans and education of health professionals are also important. This study identified modifiable risk factors to guide public health interventions.
Keywords: Chagas disease, Disease outbreaks, Foodborne diseases, Oral transmission
Graphical abstract
1. Introduction
Chagas disease (CD), caused by Trypanosoma cruzi, is a neglected tropical disease primarily transmitted by triatomine bugs in Latin America. However, it can also be transmitted through various routes, including blood transfusions, organ and tissue transplantation, congenital transmission, and laboratory accidents [1]. Additionally, oral transmission is also possible through contaminated food, drinks, or meat from infected wild animals [2]. The One Health perspective aims to understand the complex interaction among human, animal, and environmental health, and its impact on controlling and preventing CD [3,4]. By considering these interconnected factors, comprehensive strategies can be developed to effectively combat the disease [4].
Oral transmission outbreaks of CD present a major public health challenge, impacting individuals and communities throughout the Americas [5]. These outbreaks are most prevalent in nonendemic rural and peri-urban areas of countries such as Argentina, Bolivia, Colombia, Ecuador, and Venezuela [5,6]. Oral transmission of CD exhibits distinct epidemiological and clinical characteristics compared to vector-transmitted infections, including a higher case fatality rate and worse prognosis [2,7]. Detecting oral transmission is challenging due to the absence of specific symptoms in the early stages, which complicates prevention and treatment efforts [2,7]. Additionally, a considerable number of affected individuals are unaware of the oral transmission route of the disease [8].
Colombian health authorities have taken proactive measures to prevent and control oral transmission outbreaks of CD. The National CD Control Program was implemented with the goal of reducing the incidence of disease through comprehensive prevention and control measures. Additionally, awareness and education campaigns have been established in communities affected by CD [9,10].
Colombia is currently facing a critical epidemiological situation, with oral transmission outbreaks playing a major role in the spread of CD. From 2012 to 2019, a total of 248 cases of acute CD were confirmed, and oral transmission outbreaks were responsible for 40.3% of these cases. Since 2008, over 21 outbreaks have been reported in different parts of the country [11]. Understanding the risk factors associated with oral transmission and implementing effective preventive measures are crucial steps in addressing this public health challenge.
To effectively mitigate transmission risks and prevent future outbreaks of CD, the adoption of a One Health approach is crucial. This approach fosters interdisciplinary collaboration between professionals in human, animal, and environmental health, enabling a comprehensive understanding and response to public health challenges [3].
In line with this, this study aimed to analyze risk factors and draw lessons from past outbreaks of orally transmitted CD in Colombia. By examining the risk factors associated with these outbreaks, this study was to provide valuable insights that can contribute to the development of effective preventive strategies. This study also drew from past experiences to identify key lessons that can guide future interventions and mitigate the impact of potential outbreaks.
2. Methods
2.1. Study setting
Colombia, located in northwestern South America, has an area of 1,141,748 km2 and a population of approximately 48.2 million. The country is characterized by the Andes Mountain range, which runs from south to north and contributes to its unique geographical features. These features have resulted in six natural regions defined by their interactions with bioclimatic characteristics: the Caribbean Region along the Atlantic coast, the Pacific Region along the Pacific coast, the Andean Region in the center comprising the Andes Mountains and inter-Andean valleys, the Orinoco Region to the east of the Andes Mountains, the Amazonia Region to the east of the Andes Mountains, and the insular region consisting of islands, cays, and islets. Administratively, Colombia is divided into 32 departments, 9 districts, and 1122 municipalities.
2.2. Study design
A simultaneous mixed methods study was conducted. The first phase involved an ecological epidemiological analysis at the municipal level using secondary information. Municipalities were chosen as the unit of analysis due to their central political–administrative role, allowing for effective data aggregation and analysis. In the second phase, semistructured interviews were conducted with health providers and policymakers at the national level in Colombia. The results from both stages were then presented and triangulated to gain a comprehensive understanding of the risk factors and potential prevention and control measures for oral transmission of CD.
2.3. Quantitative data collection
Data on orally transmitted Chagas outbreaks were obtained from two sources: the National Public Health Surveillance System (SIVIGILA) for the period 2007–2023 and the Parasitology Group of the National Institute of Health for the period 1992–2006. SIVIGILA is a nationwide platform that systematically collects relevant information for public health in Colombia, including mandatory reporting of certain communicable diseases such as CD (https://www.ins.gov.co/buscador-eventos/Paginas/Info-Evento.aspx). In Colombia, all cases of acute CD must be reported and confirmed using direct parasitological methods, polymerase chain reaction, or evidence of IgG antibody seroconversion. Information on housing conditions, including wall and floor materials, cooking locations, access to electricity, and water sources for food preparation, was derived from the 2018 National Population and Housing Census conducted by the National Administrative Department of Statistics (DANE) (https://www.dane.gov.co/index.php/en/). Information on Chagas reservoirs and vectors was obtained from the Global Invasive Species Database (GIBIF) (https://www.gbif.org/), while CD-related mortality cases were collected from SIVIGILA. Data on temperature, precipitation, vegetation, and altitude were obtained from the Institute of Hydrology, Meteorology, and Environmental Studies (IDEAM) (http://www.ideam.gov.co).
2.4. Definition of orally transmitted CD outbreaks
A CD outbreak is defined as an unexpected increase in the disease's incidence, with one or more confirmed acute cases. These cases are epidemiologically related, located in a specific area, and exhibit severe clinical symptoms. The absence of triatomines in the intra- or peridomicile within the affected area is also a characteristic of such outbreaks. Oral transmission of CD can occur through various means, including direct ingestion of the parasite through accidental consumption of infected triatomines or their feces, contact with blood from wild reservoirs during animal slaughter, consumption of spoiled cooked meat from infected wild animals, consumption of contaminated water, food, drinks, or preparations, contamination of kitchen utensils with triatomine feces or odoriferous secretions from reservoirs, inadequate handling of infected mammal corpses, and cultural practices associated with the direct consumption of blood from wild reservoirs.
2.5. Qualitative data collection
Semistructured interviews were conducted with 12 key informants, including health policymakers, vector-borne disease coordinators, and experienced physicians managing orally transmitted CD cases. These interviews were based on the WHO's framework and toolkit for infection prevention and control at healthcare facilities. The interview guide consisted of seven sections: basic information about the interviewee, guidelines for designing preparedness and response plans for orally transmitted CD outbreaks, actions for staff training and equipping, improving prevention measures and patient participation, managing patients during an outbreak, strategies to reduce oral transmission in communities, and enhancing communication and collaboration during an outbreak. The interviews, primarily conducted in Spanish, lasted under an hour, and provided a structure for in-depth discussions.
To establish contact with initial interviewees, in-country key informants were utilized. Subsequent interviews were obtained using a snowball sampling method, where participants facilitated contact with other relevant actors. The sampling strategy aimed for saturation rather than a specific number of interviews at each level, ensuring that interviews continued until responses became repetitive or all relevant actors had been engaged.
2.6. Data analysis
All data were saved in Excel (Microsoft, Redmond, USA) and analyzed using R v.2.15 software (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria). GIS software v 10.5 (ESRI, Redlands, CA, USA) was used to generate maps. Summary statistics were calculated using absolute and relative frequency measures. Bivariate and multivariate analyses were conducted using Poisson regression models to assess the association between the number of CD cases in outbreaks caused by oral transmission and several independent variables. These variables included the location of food preparation, the source of water used for food preparation, and housing indicators such as wall and floor materials, as well as the presence of electricity service. Additionally, due to their direct association with oral CD outbreaks in Colombia, the presence of Didelphis marsupialis and the vector Panstrongylus geniculatus were considered [12,13]. Altitude, precipitation, temperature, and forest type were also included as variables, following the classification from a previous study [14].
Altitude was categorized into specific ranges of meters above sea level (m.a.s.l.) as follows:
-
•
High areas (>600 m.a.s.l.): Corresponding to mountainous regions.
-
•
Medium areas (>400–600 m.a.s.l.): Representing hilly landscapes.
-
•
Transition areas (200–400 m.a.s.l.): Referring to regions between medium and low zones, characterized by foothills.
-
•
Low areas (<200 m.a.s.l.): Comprising flat and low plains.
Precipitation was classified based on the monthly average into the following categories:
-
•
High (>1600 mm)
-
•
Medium–high (>1400–1600 mm)
-
•
Medium–low (1200–1400 mm)
-
•
Low (<1200 mm)
Temperature was categorized into specific ranges based on the monthly average as follows:
-
•
Low (<22 °C)
-
•
Medium (22–25 °C)
-
•
High (>25–28 °C)
-
•
Very high (>28 °C)
For the vegetation variable, the following forest types were considered:
-
•
Dense forest associated with palm trees
-
•
Sparse forest associated with palm trees
-
•
Gallery forest
Variables with a P value ≤ 0.10 in the bivariate analysis were selected as candidates for the final regression model. Both crude and adjusted incidence rate ratios (IRRs) were calculated along with 95% confidence intervals for each covariate. A P value < 0.05 indicated a significant association. Regarding qualitative analysis, content and thematic analyses were performed using ATLAS.ti Scientific Software Development GmbH. Qualitative findings were then presented alongside quantitative results through data triangulation to assess the convergence of themes. The codes “Agreement”, “Partial agreement”, and “Disagreement” were used to display and interpret the findings.
3. Results
3.1. Characteristics of orally transmitted CD outbreaks in Colombia
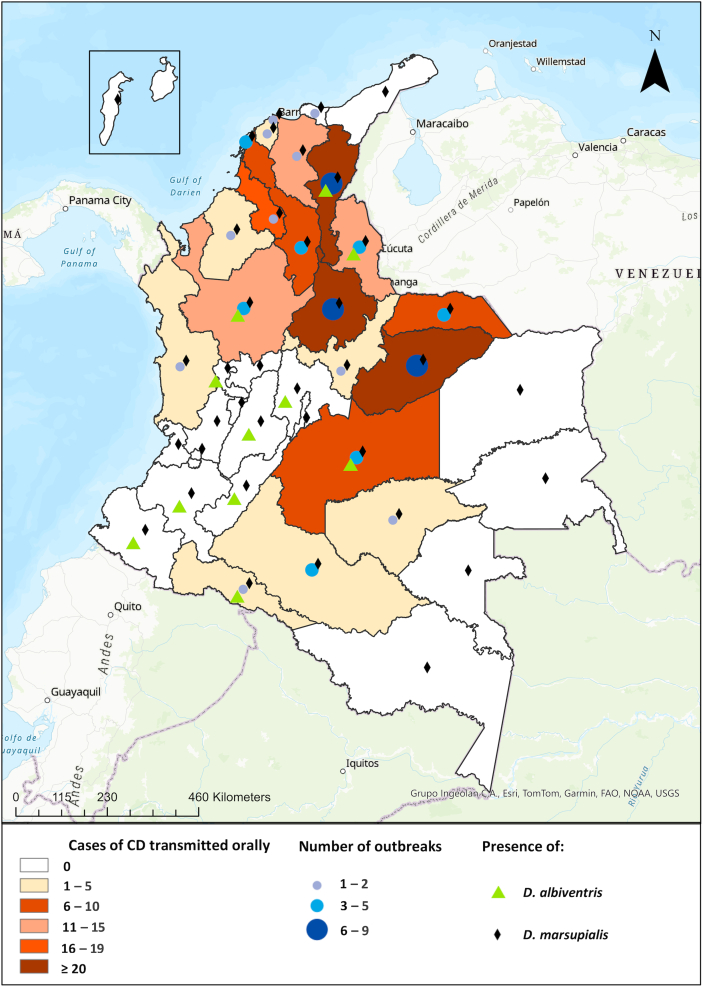
From 1992 to 2023, a total of 64 outbreaks of orally transmitted CD were recorded in Colombia, affecting 302 individuals across 50 municipalities. The smallest outbreaks, occurring between 2020 and 2023, only affected one person each in various municipalities such as San Juan de Uraba in Antioquia, Tame in Arauca, Arenal in Bolivar, and several others. The municipalities of Paz de Ariporo, Pore, and Lebrija have experienced the highest number of outbreaks, with Paz de Ariporo having the most significant impact in 2014, affecting 41 people, followed by Mani in 2019, impacting 22 individuals. In terms of regions, the Andean Region accounted for 35.9% of the outbreaks, followed by the Orinoco region with 31.3%. The most affected departments were Santander (23.4%), Casanare (21.9%), and Cesar (12.5%), as shown in Fig. 1. Notably, 58.7% of the affected individuals came from 10 specific municipalities, including Paz de Ariporo, Maní, Trinidad, Aguachica, Becerril, Valledupar, El Roble, Guamal, Lebrija, and Turbo.
Fig. 1.
Spatial distribution of oral transmission Chagas disease outbreaks in Colombia (1992–2023).
Most infections occurred in males, with 217 cases (71.9%), and the median age of those affected was 29.1 years, ranging from newborns to 84-year-olds. The case fatality rate of the disease was 10.9%, varying from 0 to 50%. Most outbreaks were classified as familial (89.2%) and were more common between April and June (46.6%). It is noteworthy that many of these outbreaks occurred in municipalities without vector control plans in place.
3.2. Risk factors
The bivariate analysis showed important associations between the place of food preparation, housing conditions (inappropriate walls and flooring), water source, the presence of D. marsupialis, and environmental factors (altitude, precipitation, temperature, forest type) and the number of cases of orally transmitted CD (Table 1).
Table 1.
Risk factors for orally transmitted CD outbreaks: bivariate and multivariate analysis using Poisson distribution.
| Covariate | Bivariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | P value | IRR | 95% CI | P value | |
| Food preparation area | ||||||
| Dedicated kitchen | Ref | Ref | ||||
| Other locations | 1.8 | 1.3–2.9 | 0.001 | 2.7 | 1.7–6.1 | 0.019 |
| Flooring material | ||||||
| Synthetic | Ref | Ref | ||||
| Earthen floor | 0.8 | 0.6–1.3 | 0.040 | 1.2 | 1.1–2.8 | 0.032 |
| Electricity availability | ||||||
| No | Ref | Ref | ||||
| Yes | 0.7 | 0.5–1.6 | 0.114 | 0.8 | 0.5–1.9 | 0.896 |
| Source of water for cooking | ||||||
| Public/Private | Ref | Ref | ||||
| Others | 1.4 | 1.1–2.2 | 0.023 | 2.3 | 1.3–3.9 | 0.011 |
| Exterior wall material | ||||||
| Masonry | Ref | Ref | ||||
| Wattle-and-daub | 1.2 | 1.1–1.9 | 0.081 | 1.6 | 1.1–2.6 | 0.035 |
| Presence of P. geniculatus | ||||||
| No | Ref | Ref | ||||
| Yes | 1.3 | 1.1–2.6 | 0.015 | 2.1 | 1.2–3.4 | 0.027 |
| Presence of D. marsupialis | ||||||
| No | Ref | Ref | ||||
| Yes | 1.5 | 1.1–1.9 | 0.011 | 2.4 | 1.5–4.3 | 0.008 |
| Altitude (m.a.s.l.) | ||||||
| <200 | Ref | Ref | ||||
| 200–400 | 0.9 | 0.4–2.3 | 0.829 | 1.7 | 0.7–4.1 | 0.221 |
| >400–600 | 0.7 | 0.6–1.8 | 0.642 | 2.4 | 0.9–7.2 | 0.089 |
| >600 | 1.5 | 1.1–2.1 | 0.045 | 3.5 | 1.4–9.2 | 0.010 |
| Precipitation (mm) | ||||||
| <1200 | Ref | Ref | ||||
| 1200–1400 | 0.8 | 0.5–1.1 | 0.206 | 0.9 | 0.3–2.4 | 0.986 |
| >1400–1600 | 0.6 | 0.4–1.6 | 0.110 | 0.8 | 0.4–2.7 | 0.883 |
| >1600 | 2.5 | 1.4–3.7 | <0.001 | 3.4 | 1.2–9.3 | 0.018 |
| Temperature (°C) | ||||||
| <22 | Ref | Ref | ||||
| 22–25 | 0.6 | 0.4–1.1 | 0.251 | 0.6 | 0.3–1.6 | 0.140 |
| 25–28 | 1.1 | 0.6–1.4 | 0.734 | 1.5 | 0.8–2.9 | 0.133 |
| >28 | 2.9 | 2.1–4.2 | <0.001 | 3.4 | 1.2–9.3 | <0.001 |
| Forest type | ||||||
| Gallery forest | Ref | Ref | ||||
| Sparse forest, palm trees | 2.2 | 1.4–3.6 | 0.001 | 3.3 | 1.5–7.4 | 0.003 |
| Dense forest, palm trees | 3.3 | 2.3–4.9 | <0.001 | 3.7 | 1.2–6.8 | <0.001 |
Note: IRR: incidence rate ratio; CI: confidence interval; Ref: reference; m.a.s.l.: meters above sea level. Synthetic: Hardwood laminate, ceramic, porcelain, vinyl; Earthen floor: is a type of flooring constructed using natural materials such as dirt, raw earth, or unworked ground materials. Masonry: brick, block, prefabricated materials, and stone. Wattle-and-daub: combination of wet soil, clay, sand, animal dung and straw. adobe, rammed earth.
Specifically, the multivariate analysis showed a significant association between the place of food preparation, food preparation water source, and walls and flooring of the home and the number of orally transmitted CD cases. The number of cases were also associated with the presence of the vector P. geniculatus and marsupial species D. marsupialis. Furthermore, altitude, precipitation, temperature, and forest type were associated with the disease. However, no association was found with the presence of electric light (Table 1).
3.3. Lessons learned and outbreak prevention
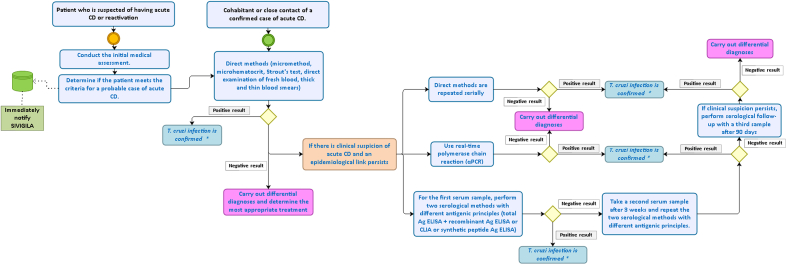
In interviews with managers and health professionals, insights were gained regarding the diagnostic tools available for acute CD in the Colombian health system (Fig. 2). Key informants unanimously reported that their organizations provided diagnoses for the disease, but access to health care and integration were impacted by community services and external factors beyond their control.
Fig. 2.
Diagnostic flow chart for acute-phase Chagas disease diagnosis in Colombia.
∗ Adjust the information regarding probable or confirmed cases of acute Chagas disease in SIVIGILA.
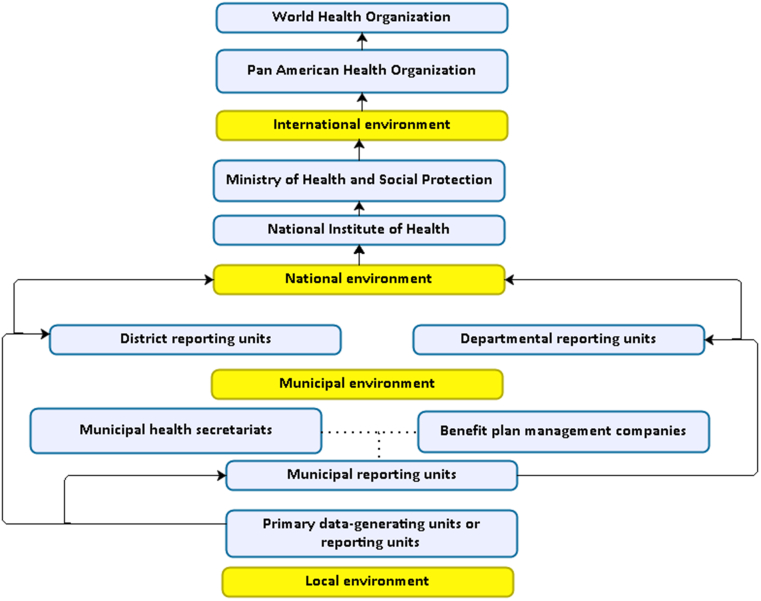
Additionally, a pathway for reporting acute CD cases to the public health surveillance system was established (Fig. 3).
Fig. 3.
Flow chart for the notification of a case of Chagas disease to the public health surveillance system in Colombia.
Below are the responses to the top questions regarding infection prevention and control tools, aimed at preparing for and responding to outbreaks at the health care facility level (Table 2).
Table 2.
Lessons learned and prevention strategies for oral transmission Chagas disease outbreaks in Colombia.
| Lesson | Strategy |
|---|---|
| Guidelines for health care facilities to design preparedness and response plans for orally transmitted CD outbreaks | |
| 1. Early detection and diagnosis | Establish clear policies and procedures for the early detection and diagnosis of suspected and confirmed cases of CD. |
| 2. Outbreak response plan | Develop a plan that considers the severity and oral transmission of the disease, including an outbreak response committee with representatives from various areas. |
| 3. Vulnerable populations | Identify high–risk geographic areas and populations to promote prevention and early detection. |
| 4. Regular assessment and updates | Regularly assess outbreak response capacity and update the plan accordingly. |
| 5. Training and education | Provide training to medical and laboratory personnel on the early detection, treatment, and prevention of CD. |
| Actions that health care facilities can take to ensure staff are adequately trained and equipped to detect and treat orally transmitted CD cases | |
| 1. Regular training | Provide ongoing training to health care personnel on the proper diagnosis and treatment of CD. |
| 2. Clear protocols | Establish protocols for the identification and management of suspected and confirmed cases. |
| 3. Access to treatment | Ensure health care facilities have the necessary tools and supplies for diagnosis and treatment, with treatment access available to the public. |
| 4. Mandatory case notification | Emphasize the importance of mandatory notification of cases to the epidemiological surveillance system. |
| Improving orally transmitted CD prevention measures and encouraging active participation of patients and visitors in infection prevention efforts | |
| 1. Education and awareness | Provide clear educational materials about the disease and prevention measures. |
| 2. Communication with patients and visitors | Engage medical staff and social workers in conversations about the disease and prevention measures. |
| 3. Promote self-education | Encourage individuals to educate themselves on avoiding exposure to insect vectors and safe food preparation. |
| 4. Community involvement | Encourage patients and visitors participation in disease prevention efforts through awareness campaigns and community programs. |
| 5. Establish advisory committees | Create patient safety and infection prevention advisory committees to encourage participation and strengthen relationships. |
| Measures to effectively manage patients with CD during an outbreak of oral transmission in a health center | |
| 1. Identification and notification | Identify and notify all patients seeking care with CD. |
| 2. Monitoring and recording | Establish a system to monitor and record cases in the health facility. |
| 3. Communication with epidemiological team | Maintain close communication with the local epidemiological team. |
| 4. Treatment provision and monitoring | Provide treatment to identified patients, monitor effectiveness, and adjust if necessary. |
| 5. Hospitalization decisions | Make decisions regarding hospitalization based on clinical assessment. |
| 6. Home-based treatment | Treat most patients at home under medical supervision. |
| 7. Education and training | Provide adequate education and training to health center staff on CD. |
| 8. Collaboration and coordination | Work collaboratively with local health authorities and organizations to coordinate outbreak management. |
| Strategies and interventions to reduce the risk of oral transmission of CD in local communities | |
| 1. Education and awareness | Promote education about the disease and preventive measures. |
| 2. Improving housing and hygiene conditions | Take measures to improve housing and hygiene, including vector control. |
| 3. Source control | Implement measures to control the source of infection, such as eliminating animal reservoirs and fumigating infested homes. |
| 4. Epidemiological surveillance | Strengthen community surveillance to detect symptoms and report suspected cases. |
| 5. Research and development | Promote research to advance understanding and prevention. |
| 6. Regulatory policies | Develop specific policies to ensure food and beverage safety. |
| Improving communication and collaboration during a CD outbreak | |
| 1. Up-to-date information dissemination | Provide timely and accurate information about the outbreak through various communication channels. |
| 2. Collaboration networks | Strengthen collaboration between stakeholders involved in the outbreak response. |
| 3. Community participation | Encourage active participation of the community in prevention and control efforts. |
| 4. Engagement of health care providers | Ensure health care providers are well–informed and equipped to respond effectively. |
| 5. Coordination with public health authorities | Maintain close coordination with public health authorities to share information and align messaging |
Note: CD: Chagas disease.
3.4. Data integration
The results obtained from the triangulation of risk factors across different perspectives reveal interesting patterns. Table 3 demonstrates agreement, partial agreement, and disagreement among the health professionals, administrators, and quantitative study. Agreement was observed for various risk factors, including the food preparation area, source of water for cooking, presence of P. geniculatus, presence of D. marsupialis, altitude, forest type, and exterior wall material. These factors consistently emerged as potential risk factors across the different health perspectives. Partial agreement was found for risk factors such as floor material, presence of D. marsupialis, altitude, precipitation, and temperature. While there were areas of overlap and similarity between perspectives, there were also differences in the level of agreement or disagreement. Disagreement was evident for precipitation, with contrasting views between the health professionals and quantitative study perspectives (Table 3).
Table 3.
Triangulation results: Risk factors for oral transmission outbreaks of Chagas disease from a One Health perspective in Colombia.
| Risk factor | Human health perspective | Animal health perspective | Environmental health perspective |
|---|---|---|---|
| Food preparation area | HP: agreement Admin: agreement Study: agreement |
- | - |
| Floor material | HP: partial agreement Admin: agreement Study: agreement |
- | - |
| Source of water for cooking | HP: agreement Admin: agreement Study: agreement |
- | - |
| Presence of P. geniculatus | - | HP: agreement Admin: agreement Study: agreement |
- |
| Presence of D. marsupialis | - | HP: partial agreement Admin: partial agreement Study: agreement |
- |
| Altitude | - | - | HP: agreement Admin: partial agreement Study: agreement |
| Precipitation | - | - | HP: disagreement Admin: partial agreement Study: agreement |
| Temperature | - | - | HP: partial agreement Admin: agreement Study: agreement |
| Forest type | - | - | HP: agreement Admin: agreement Study: agreement |
| Exterior wall material | - | - | HP: agreement Admin: agreement Study: agreement |
HP: Health professionals.
Admin: Administrators.
Study: Quantitative study.
- : Not applicable
4. Discussion
This study investigated the risk factors and lessons learned from epidemic outbreaks of orally transmitted CD in Colombia over the past 31 years. The findings suggest that there are modifiable factors that can be addressed to prevent future outbreaks. The research identified deficiencies in housing, both qualitative and quantitative, as significant factors related to the spread of the disease. This emphasizes the importance of improving housing conditions to prevent the transmission of CD.
This study also found that environmental factors, such as the presence of triatomines and wild mammals, as well as altitude, precipitation, temperature, and vegetation, contribute to the risk of transmission. The location of the kitchen and the source of water used for food preparation are both crucial factors contributing to the risk of oral transmission of CD. When the kitchen is located outside the home, there is a higher chance of food and drinks coming into contact with vectors and their feces, increasing the risk of disease transmission [15,16]. It is essential to emphasize the importance of covering food left outside the house to prevent contamination and reduce the risk of disease transmission. Ideally, promoting safe kitchens inside homes would be the best approach. Additionally, the presence of walls with inadequate material and dirt floors in many homes encourages the proliferation and entry of triatomines, further increasing the risk of transmission [17].
The lack of adequate and safe housing is a concerning reality in Latin America, leading countries in the region to implement various actions to improve housing conditions [18]. These measures aim to meet people's basic needs for well–being, protection, security, and health, as well as address poverty, which affects approximately 33.7% of the population in the region [18]. Governments and organizations in the region are implementing policies and programs to improve housing situations [[18], [19], [20]]. These initiatives include constructing affordable housing, improving existing infrastructure, implementing building standards, and promoting community participation in decision-making. Efforts are also being made to provide access to financing for home acquisition or improvement and to provide training in construction and maintenance. These initiatives aim to ensure that people have real opportunities to access decent and affordable housing, which will contribute to improving their quality of life and promoting sustainable development in the region [21].
This study found that the presence of synanthropic wild animals, specifically the common opossum, is crucial in the transmission of CD. Opossums act as both reservoirs and vectors of the T. cruzi parasite, maintaining disease transmission cycles. Consuming opossum meat in rural areas near tropical forests can also lead to infection. It is crucial to raise awareness about this risk and promote safe food handling and cooking practices to prevent CD transmission.
The consumption of contaminated foods or beverages with triatomine feces is a major source of infection for oral CD. As human settlements expand into rural areas with diverse triatomine communities, the risk of accidental consumption of contaminated foods increases [6]. Deforestation and the reduction of wildlife populations also contribute to the distribution of triatomines. The species richness of triatomines raises the likelihood of contact with humans, facilitating both oral and vector transmission pathways [22]. Particularly, the results from this study support that the departments in Colombia with the highest triatomine species richness, specifically Casanare and Santander, also reported the most cases of acute CD [23]. These departments experienced outbreaks associated mainly with contaminated food and beverages like mandarin orange and soursop juice, highlighting the presence of P. geniculatus, Rhodnius prolixus, Rhodnius pallescens and Eratyrus cuspidatus [4]. Quindío is the only department where no triatomines or acute CD cases have been reported. However, departments with fragmented distribution of triatomine species across municipalities, such as Amazonas, Vaupes, Risaralda, and Nariño, tend to have a lower number of acute CD cases. In these regions, triatomine species may be present but have sylvatic life cycles, rarely invading the domicile and therefore being less likely to come in contact with humans.
Triatomine species found near human dwellings have the potential to contaminate food and become the source of oral CD infections. However, species like R. pallescens have primarily been associated with oral CD in scenarios where palm trees are present [24]. This study emphasized the importance of the P. geniculatus species in the oral transmission of CD. While commonly associated with opossums, armadillos, and bats, recent studies have shown that it also feeds on rodents, canines, ruminants, and primates. P. geniculatus has been reported to invade human homes, with evidence of intradomicile colonization in various regions [[25], [26], [27]]. Its ability for passive dispersal through human activity and animal transport contributes to its long-distance migration. P. geniculatus can fly long distances and is attracted to artificial lights in houses, facilitating the introduction of T. cruzi from sylvan foci [28]. In Colombia and Venezuela, P. geniculatus has been associated with multiple outbreaks of oral CD, making it essential to consider its presence and behavior in vector control strategies.
The natural environment plays an important role in CD transmission [29,30]. Higher altitudes and warmer temperatures are associated with a greater presence of triatomines, increasing the risk of transmission. The optimal temperatures for the development of triatomines range between 22 and 28 °C, while temperatures above 30 °C adversely affect their development, and at 15 °C, there is no development [31]. Vegetation availability and precipitation also influence vector proliferation [30]. Conducting epidemiological studies that consider environmental characteristics is essential for designing effective control strategies [32].
Despite advancements in CD management and control, the high fatality rate of the disease remains a concern for public health [10,33]. Addressing this challenge requires a comprehensive approach involving human, veterinary, and environmental health professionals, as well as affected communities [34]. Strengthening epidemiological surveillance, improving access to diagnosis and treatment, promoting education and awareness, and implementing prevention and control strategies based on interdisciplinary collaboration are necessary [35]. Integration of efforts is crucial to reducing the burden of CD.
Continued training of health professionals responsible for diagnosis and treatment is crucial for the timely detection and appropriate management of the disease [8]. Creating a core group of highly trained professionals to provide expert advice and support to colleagues facing complex cases is suggested. This initiative should be led by the national CD program of the Ministry of Health and Social Protection to strengthen the disease response and improve patient care.
Community participation is vital to the prevention and control of CD [36]. Promoting community awareness of risk factors and preventive measures, encouraging participation in vector surveillance and control programs, and establishing education and training strategies to identify signs and symptoms of the disease and seek early medical attention are essential [37,38].
International collaborations are necessary to comprehensively address CD [39]. Coordination between endemic countries and cooperation with international health organizations facilitate the exchange of experiences, resources, and technical knowledge [40]. This allows for the implementation of more efficient control strategies, the sharing of best practices, and the promotion of collaborative research. Global cooperation is crucial to effectively and sustainably addressing this global challenge [41].
This study has certain limitations and biases concerning the quality and reliability of the available information during the study period. These limitations mainly pertain to the uniformity and completeness of crucial variables related to morbidity and mortality within the databases of the information systems used. However, it is important to note that the selected secondary sources were reputable and authoritative, ensuring the capture of necessary information. It is crucial to recognize that these results cannot be generalized to other countries in the region, as transmission patterns in Colombia are influenced by familial and housing risk factors and reservoirs, unlike the Amazon region of Brazil where transmission occurs in a wilder context. Nevertheless, we believe that the findings of this study constitute the most reliable technical evidence available, which should be considered by decision-makers and those responsible for the national CD prevention and control program.
5. Conclusion
The prevention of epidemic outbreaks of oral transmission of CD in Colombia requires coordinated efforts at the national, regional, and local levels. It is essential to implement prevention and control measures in the proper management of the preparation and storage of food and beverages for consumption, improving hygiene and sanitary conditions in places where food is handled and stored, in addition to raising awareness among the population about the ways of disease transmission. These findings emphasize the need to implement measures to improve housing and vector control to prevent future outbreaks.
It is essential that governments, organizations, and society work in a collaborative and coordinated manner to achieve significant results in this crucial area. In addition, epidemiological surveillance, and the capacity to diagnose and treat CD cases in the health system must be strengthened. Interdisciplinary collaboration plays a key role in effectively addressing the challenges associated with the oral transmission of CD in Colombia and other Latin American countries [42].
This study sought to contribute to scientific knowledge and public health policies with the aim of preventing future health crises related to CD. To achieve this, it is essential to continue investing in research and education to improve the understanding of this disease and its mode of transmission, as well as develop effective prevention and control strategies.
Ethics approval and consent to participate
Approval was granted by the Research Ethics and Methodology Committee of the National Institute of Health in Bogotá, Colombia (Protocol CMIN-332017). Participation was voluntary and informed consent was obtained from all interviewees.
Availability of data and materials
The results presented are sufficient to support the conclusion of this study. Nonetheless, the lead author is available to provide additional data upon request.
Consent for publication
Not applicable.
Funding
This work was supported by the Instituto Nacional de Salud de Colombia.
Author's contributions
MJO conceptualized and designed the research. All authors contributed to data collection and analysis. The initial manuscript draft was carried out by MJO, CYRC, SMC, while AJO and MJVS provided critical review and editing. All authors participated in the final approval of the manuscript and made substantial contributions to its publication.
Declaration of competing interest
The authors declare no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Acknowledgement
The authors would like to express their gratitude to the knowledge management, research, and innovation network in Chagas for their support during this study.
References
- 1.Rassi A., Jr., Rassi A., Marcondes de Rezende J. American trypanosomiasis (Chagas disease) Infect. Dis. Clin. North Am. 2012;26:275–291. doi: 10.1016/j.idc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Rincón-Acevedo C.Y., Parada-García A.S., Olivera M.J., Torres-Torres F., Zuleta-Dueñas L.P., Hernández C., et al. Clinical and epidemiological characterization of acute chagas disease in Casanare, eastern Colombia, 2012-2020. Front. Med. 2021;8 doi: 10.3389/fmed.2021.681635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adisasmito W.B., Almuhairi S., Behravesh C.B., Bilivogui P., Bukachi S.A., Casas N., et al. One Health: a new definition for a sustainable and healthy future. PLoS Pathog. 2022;18 doi: 10.1371/journal.ppat.1010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-García A., Gilabert J.A. Oral transmission of Chagas disease from a One Health approach: a systematic review. Trop. Med. Int. Health. 2023;28:689–698. doi: 10.1111/tmi.13915. [DOI] [PubMed] [Google Scholar]
- 5.Rueda K., Trujillo J.E., Carranza J.C., Vallejo G.A. [Oral transmission of Trypanosoma cruzi : a new epidemiological scenario for Chagas' disease in Colombia and other South American countries] Biomedica. 2014;34:631–641. doi: 10.1590/S0120-41572014000400017. [DOI] [PubMed] [Google Scholar]
- 6.Franco-Paredes C., Villamil-Gómez W.E., Schultz J., Henao-Martínez A.F., Parra-Henao G., Rassi A.J., et al. A deadly feast: elucidating the burden of orally acquired acute Chagas disease in Latin America - public health and travel medicine importance. Trav. Med. Infect. Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101565. [DOI] [PubMed] [Google Scholar]
- 7.Bruneto E.G., Fernandes-Silva M.M., Toledo-Cornell C., Martins S., Ferreira J.M.B., Corrêa V.R., et al. Case-fatality from orally-transmitted acute chagas disease: a systematic review and meta-analysis. Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am. 2021;72:1084–1092. doi: 10.1093/cid/ciaa1148. [DOI] [PubMed] [Google Scholar]
- 8.Olivera M.J., Villamil J.F.P., Gahona C.C.T., Hernández J.M.R. Barriers to diagnosis access for chagas disease in Colombia. J. Parasitol. Res. 2018;2018 doi: 10.1155/2018/4940796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministerio de Salud y Protección Social . 2013. Plan Decenal de Salud Pública PDSP, 2012-2021.https://www.minsalud.gov.co/PlanDecenal/Paginas/home2013.aspx Bogotá. [Google Scholar]
- 10.Olivera M.J., Buitrago G. Economic costs of Chagas disease in Colombia in 2017: a social perspective. Int. J. Infect. Dis. 2020;91:196–201. doi: 10.1016/j.ijid.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Instiuto Nacional de Salud, Informe de evento Chagas agudo, 2019, Bogotá. 2019. https://www.ins.gov.co/buscador-eventos/Paginas/Info-Evento.aspx
- 12.Gómez Vargas W. GBIF; 2021. Colección de Entomología Médica del Instituto Colombiano de Medicina Tropical. [DOI] [Google Scholar]
- 13.Beatty N.L., Arango-Ferreira C., Gual-Gonzalez L., Zuluaga S., Nolan M.S., Cantillo-Barraza O. Oral chagas disease in Colombia-confirmed and suspected routes of transmission. Trav. Med. Infect. Dis. 2024;9:14. doi: 10.3390/tropicalmed9010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas K. Mal de Chagas y factores geográficos: Propuesta de zonificación del riesgo epidemiológico, municipio Araure, Estado Portuguesa. Venezuela. Terra. 2015;31:109–129. [Google Scholar]
- 15.Díaz M.L., Leal S., Mantilla J.C., Molina-Berríos A., López-Muñoz R., Solari A., et al. Acute chagas outbreaks: molecular and biological features of Trypanosoma cruzi isolates, and clinical aspects of acute cases in Santander, Colombia. Parasites Vectors. 2015;8:608. doi: 10.1186/s13071-015-1218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez C., Vera M.J., Cucunuba Z., Florez C., Cantillo O., Buitrago L.S., et al. High-resolution molecular typing of two large outbreaks of acute Chagas disease in Colombia. J. Infect. Dis. 2016;14:1252–1255. doi: 10.1093/infdis/jiw360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackey T.K., Liang B.A., Cuomo R., Hafen R., Brouwer K.C., Lee D.E. Emerging and reemerging neglected tropical diseases: a review of key characteristics, risk factors, and the policy and innovation environment. Clin. Microbiol. Rev. 2014;27:949–979. doi: 10.1128/CMR.00045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inter-American Development Bank . 2022. Three Strategies to Reduce the Housing Deficit in Latin America and the Caribbean.https://www.iadb.org/en/story/three-strategies-reduce-housing-deficit-latin-america-and-caribbean [Google Scholar]
- 19.OECD Policies for housing and habitat in Colombian cities. 2021. https://www.oecd-ilibrary.org/sites/f09c6cb7-en/index.html?itemId=/content/component/f09c6cb7-en
- 20.Ferreira J.S.W., Rojas E., Carvalho H.R.D.S., Frignani C.R., Lupo L.S. Housing policies and the roles of local governments in Latin America: recent experiences. Environ. Urbanization. 2020;32:333–350. doi: 10.1177/0956247820935699. [DOI] [Google Scholar]
- 21.Rolfe S., Garnham L., Godwin J., Anderson I., Seaman P., Donaldson C. Housing as a social determinant of health and wellbeing: developing an empirically-informed realist theoretical framework. BMC Publ. Health. 2020;20:1138. doi: 10.1186/s12889-020-09224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Silva G.G., Lopez V.M., Vilarinho A.C., Datto-Liberato F.H., Oliveira C.J.F., Poulin R., et al. Vector species richness predicts local mortality rates from Chagas disease. Int. J. Parasitol. 2023;54:139–145. doi: 10.1016/j.ijpara.2023.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Méndez-Cardona S., Ortiz M.I., Carrasquilla M.C., Fuya P., Guhl F., González C. Altitudinal distribution and species richness of triatomines (Hemiptera:Reduviidae) in Colombia. Parasites Vectors. 2022;15:450. doi: 10.1186/s13071-022-05574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soto H., Tibaduiza T., Montilla M., Triana-Chávez O., Suárez D.C., Torres M., et al. Lugo, Investigación de vectores y reservorios en brote de Chagas agudo por posible transmisión oral en Aguachica, Cesar, Colombia. Cad. Saúde Pública. 2014;30:746–756. doi: 10.1590/0102-311X00024013. [DOI] [PubMed] [Google Scholar]
- 25.Wolff M., Castillo D. Evidencias de domesticación y aspectos biológicos de Panstrongylus geniculatus (Latreille, 1811) (Hemiptera: Reduviidae) Acta Entomol. Chil. 2000;24:77–83. [Google Scholar]
- 26.Montenegro D., Vera M., Zuleta L., Llanos I., Junqueira A. Estrategia para determinar la línea base en áreas de interrupción vectorial de la enfermedad de Chagas. Rev. Panam. Salud Públic. 2016;39:341–351. [PubMed] [Google Scholar]
- 27.Maestre-Serrano R., Eyes-Escalante M. Vol. 52. Bol Malariol Salud Amb; 2012. pp. 125–128. (Actualización de la presencia y distribución de triatominos en el departamento del Atlántico-Colombia: 2003-2010). [Google Scholar]
- 28.Vivas R.J., García J.E., Guhl F., Hernández C., Velásquez N., Ramírez J.D., et al. Systematic review on the biology, ecology, genetic diversity and parasite transmission potential of Panstrongylus geniculatus (Latreille 1811) in Latin America. Mem. Inst. Oswaldo Cruz. 2021;116 doi: 10.1590/0074-02760200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parra-Henao G., Garzón-Jiménez S.P., Bernal-Rosas Y., Olivera M.J., Salgado M., Torres-García O.A. Risk factors for triatominae infestation in a municipality of Colombia. Ther. Adv. Infect. Dis. 2021;8 doi: 10.1177/20499361211030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quiros-Gomez O., Segura-Cardona Á., Flórez P.A., Pinto N., Medina M., Henao-Martínez A.F., et al. Risk factors and spatial analysis for domiciliary infestation with the Chagas disease vector Triatoma venosa in Colombia. Ther. Adv. Infect. Dis. 2022;9 doi: 10.1177/20499361221084164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eberhard F.E., Cunze S., Kochmann J., Klimpel S. Modelling the climatic suitability of Chagas disease vectors on a global scale. Elife. 2020;9 doi: 10.7554/eLife.52072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivera M.J., Muñoz L. Exploring the latency period in Chagas disease: duration and determinants in a cohort from Colombia. Trans. R. Soc. Trop. Med. Hyg. 2024 doi: 10.1093/trstmh/trae004. [DOI] [PubMed] [Google Scholar]
- 33.Olivera M.J., Porras-Villamil J.F., Villar J.C., Herrera E.V., Buitrago G. Chagas disease-related mortality in Colombia from 1979 to 2018: temporal and spatial trends. Rev. Soc. Bras. Med. Trop. 2021;54 doi: 10.1590/0037-8682-0768-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pettan-Brewer C., Martins A.F., de Abreu D.P.B., Brandão A.P.D., Barbosa D.S., Figueroa D.P., et al. From the approach to the concept: one health in Latin America-experiences and perspectives in Brazil, Chile, and Colombia. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivera M.J., Palencia-Sánchez F., Riaño-Casallas M. The cost of lost productivity due to premature chagas disease-related mortality: lessons from Colombia (2010-2017) Trav. Med. Infect. Dis. 2021;6:17. doi: 10.3390/tropicalmed6010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivera E.P., Arrivillaga M.R., Juárez J.G., De Urioste-Stone S.M., Berganza E., Pennington P.M. Adoption of community-based strategies for sustainable vector control and prevention. BMC Publ. Health. 2023;23:1834. doi: 10.1186/s12889-023-16516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanmartino M., Forsyth C.J., Avaria A., Velarde-Rodriguez M., Gómez I Prat J., Albajar-Viñas P. The multidimensional comprehension of Chagas disease. Contributions, approaches, challenges and opportunities from and beyond the Information, Education and Communication field. Mem. Inst. Oswaldo Cruz. 2022;117 doi: 10.1590/0074-02760200460. [DOI] [PubMed] [Google Scholar]
- 38.Gómez I Prat J., Gregori M.S., Guiu I.C., Choque E., Flores-Chavez M.D., Molina I., et al. Community-based actions in consulates: a new paradigm for opportunities for systematic integration in Chagas disease detection. BMC Infect. Dis. 2023;23:847. doi: 10.1186/s12879-023-08844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monroy M.C., Penados D., Pineda J., Ruiz E.L., Agreda E.O., Alcantara B., et al. A multidisciplinary, collaborative, inter-agency and comprehensive approach for the control of Chagas Disease as a public health problem in Guatemala. Acta Trop. 2022;225 doi: 10.1016/j.actatropica.2021.106157. [DOI] [PubMed] [Google Scholar]
- 40.Abril M.C. Intersectoral partnerships, a necessary path to overcome the challenges presented by Chagas disease. Front. Parasitol. 2023;2:1150041. doi: 10.3389/fpara.2023.1150041. [DOI] [Google Scholar]
- 41.Gascon J., Vilasanjuan R., Lucas A. The need for global collaboration to tackle hidden public health crisis of Chagas disease, Expert Rev. Anti. Infect. Ther. 2014;12:393–395. doi: 10.1586/14787210.2014.896194. [DOI] [PubMed] [Google Scholar]
- 42.Manageiro V., Caria A., Furtado C., Botelho A., Oleastro M., Gonçalves S.C. Intersectoral collaboration in a One Health approach: lessons learned from a country-level simulation exercise. One Heal. 2023;17 doi: 10.1016/j.onehlt.2023.100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The results presented are sufficient to support the conclusion of this study. Nonetheless, the lead author is available to provide additional data upon request.