Abstract
Background
Zoonotic diseases originating in animals pose a significant threat to global public health. Recent outbreaks, such as coronavirus disease 2019 (COVID-19), have caused widespread illness, death, and socioeconomic disruptions worldwide. To cope with these diseases effectively, it is crucial to strengthen surveillance capabilities and establish rapid response systems.
Aim
The aim of this review to examine the modern technologies and solutions that have the potential to enhance zoonotic disease surveillance and outbreak responses and provide valuable insights into how cutting-edge innovations could be leveraged to prevent, detect, and control emerging zoonotic disease outbreaks. Herein, we discuss advanced tools including big data analytics, artificial intelligence, the Internet of Things, geographic information systems, remote sensing, molecular diagnostics, point-of-care testing, telemedicine, digital contact tracing, and early warning systems.
Results
These technologies enable real-time monitoring, the prediction of outbreak risks, early anomaly detection, rapid diagnosis, and targeted interventions during outbreaks. When integrated through collaborative partnerships, these strategies can significantly improve the speed and effectiveness of zoonotic disease control. However, several challenges persist, particularly in resource-limited settings, such as infrastructure limitations, costs, data integration and training requirements, and ethical implementation.
Conclusion
With strategic planning and coordinated efforts, modern technologies and solutions offer immense potential to bolster surveillance and outbreak responses, and serve as a critical resource against emerging zoonotic disease threats worldwide.
Keywords: Modern technologies, Solutions, Surveillance, Response systems, Emerging zoonotic diseases
1. Introduction
Emerging zoonotic diseases pose significant threats to human health and wellbeing globally [1]. Zoonotic diseases are infectious diseases transmitted from animals to humans, and approximately 75% of new infectious diseases identified in the past decade have originated in animals [2]. Emerging zoonotic diseases, such as coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and Ebola virus disease, can cause severe illness with high fatality rates [3]. Furthermore, they cause economic losses by disrupting trade, travel, and supply chains [[4], [5], [6]]. With increasing populations and human–animal interactions and climate change, the risk of zoonotic disease outbreaks is on the rise [7,8]. Global environmental changes have led to ecological problems such as climate change, resource changes, and ecosystem disruptions, which all significantly impact human and animal health [9,10]. For example, global warming has increased the prevalence of disease-carrying vectors, such as mosquitoes and rodents, thereby increasing the risk of disease transmission [11,12]. Additionally, deforestation and ecosystem degradation have caused habitat loss for numerous wildlife species, forcing them in contact with humans and livestock, thus facilitating the spread of diseases to both humans and livestock [13,14]. Furthermore, as the risk of zoonotic diseases continues to increase, early detection has become increasingly crucial. Detecting and controlling outbreaks at an early stage can control the spread of the disease and decrease the harm caused [15]. For example, during the COVID-19 pandemic, early detection aided health authorities in identifying infected individuals, thereby slowing the spread of the disease [16,17]. Similarly, for diseases transmitted between animals and humans, early detection and timely implementation of control measures can prevent large-scale transmission within populations [[18], [19], [20]]. Therefore, it is crucial to establish robust surveillance and response systems that enable early detection and rapid response to emerging zoonotic disease outbreaks before they escalate into epidemics or pandemics [5].
Modern technologies and solutions have great potential to enhance our capacity to monitor and create response systems for emerging zoonotic diseases [21]. These technological advancements show potential as feasible approaches for enhancing emerging zoonotic disease control; however, further research and evidence synthesis are required to establish their effectiveness and practical implementation. Advanced analytics, artificial intelligence (AI), the Internet of Things (IoT), remote sensing, and molecular tools can strengthen surveillance capabilities by enabling real-time monitoring, predicting outbreak risks, and detecting anomalies at an early stage [[22], [23], [24]]. Moreover, solutions such as point-of-care diagnostics, telemedicine, and digital contact tracing can significantly improve the speed and effectiveness of the outbreak response [25,26]. Although these technologies have significant potential, implementation challenges related to aspects such as data integration, interoperability, cybersecurity, ethics, and policies must be addressed [27].
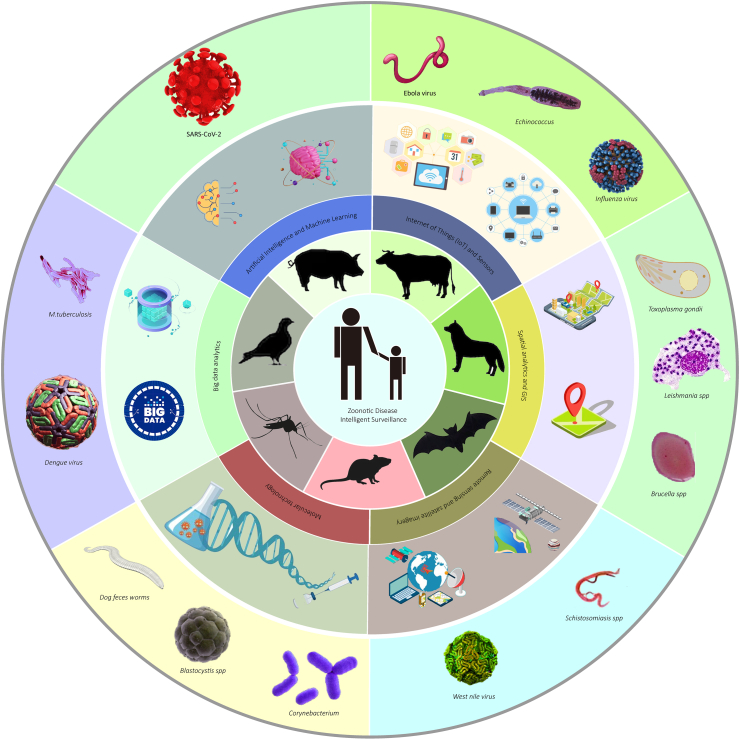
In this review, we examined various modern technologies and solutions that can enhance surveillance and response systems for emerging zoonotic diseases. The applications, benefits, and potential challenges are summarized (Fig. 1). The aim was to provide valuable insights into how cutting-edge innovations could be leveraged to prevent, detect, and control emerging zoonotic disease outbreaks.
Fig. 1.
The overview of modern technologies to enhance zoonotic disease surveillance.
2. Modern technologies to enhance zoonotic disease surveillance
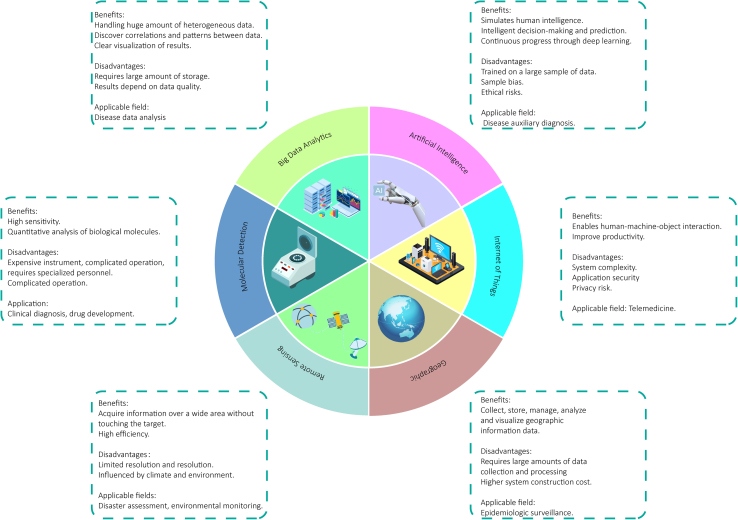
In this era of rapid technological advancement, tools available for disease surveillance are evolving. This section discusses the use of modern technologies that have the potential to enhance the surveillance of emerging zoonotic diseases. We explore the roles of big data analytics, AI and machine learning (ML), IoT, spatial analysis and geographic information systems (GIS), remote sensing, satellite imagery, and molecular technologies (Fig. 2). Each of these technologies is discussed in terms of their functionality, potential benefits, practical applications, and challenges that must be addressed for effective implementation.
Fig. 2.
A summary of the advantages and disadvantages of several technologies.
2.1. Big data analytics
In May 2011, big data was declared the next frontier for productivity and innovation. Big data has various definitions [28]. Strawn described big data as the “fourth paradigm of science”, while Hagstrom suggested that big data played the role of a new paradigm in knowledge assets [29,30]. Big data is a new-era technology that aims to extract value more economically by capturing, discovering, and analyzing large volumes of diverse data [31]. Big data analytics is a comprehensive approach that involves analyzing the quantity, variety, velocity, veracity, and value of different types of data [32]. This approach can be used to act, provide insights, measure performance, and establish competitive advantages. Big data possesses characteristics such as a large quantity, high velocity, and diverse variety, requiring specific technologies and analytical methods to transform its value [33].
A study conducted in 2021 quantified the volume of big data. It included network traffic data, data generated by various institutions, and image data provided by media companies [34]. For example, the Large Hadron Collider of the European Organization for Nuclear Research generated approximately 40,000 exabytes (EB) of raw data [35]. Amazon web services' simple storage service contains more than a quadrillion data points [36]. In the context of zoonotic diseases, a platform for collecting large-scale data typically records patient-related information, such as daily outpatient data from local healthcare institutions, electronic medical records, immunization records, chronic and infectious disease reporting data, and mortality monitoring data related to public health [[37], [38], [39]]. In a study on Dengue fever, data were screened from the Integrated Health Big Data Platform (IHBDP), where 11,900 potential cases were identified among 983,000 children with medical records from healthcare institutions [39]. In zoonotic disease surveillance, big data analytics can help detect outbreaks earlier by identifying patterns in the vast amounts of health data [40,41]. Multiple data streams, including medical records, prescriptions, searches, and social media, can be continuously analyzed to identify anomalies indicative of clusters or outbreaks [42,43]. Sophisticated algorithms can incorporate geographic data into pinpointed locations in real-time [44]. Historical data enables comparisons to identify emerging outliers. Forecasting models can predict the progression of an outbreak based on past patterns [45].
Another study analyzed the effectiveness of an integrated big data platform in China for infectious disease control by comparing its ability to identify cases of Dengue fever and tuberculosis, as well as the number of unvaccinated migrant children using traditional methods [46]. They found that the big data approach detected cases more efficiently. The Yinzhou Center for Disease Prevention and Control in China implementedthe IHBDP. This platform has been successful in detecting additional cases compared to manual diagnosis in specific disease scenarios. For example, among 3,972 suspected cases of Dengue fever, the IHBDP identified six cases, two more than those identified through manual diagnosis. The IHBDP detected 288 suspected cases of tuberculosis among 43,521 university students based on information obtained during their medical examinations. Subsequently, three of these patients were confirmed as tuberculosis carriers through follow-up CT or T-SPOT.TB tests. Furthermore, in immunization screening, the IHBDP identified 240 immunization-deficient mobile children, whereas traditional door-to-door screening methods identified only 20 cases [46]. Furthermore, big data analytics has allowed health authorities in Taiwan, China to proactively warn medical centers about surging Dengue fever cases, demonstrating their potential for outbreak prediction [47]. However, the challenges related to data quality, sharing, security, and privacy must be addressed [48]. Overall, big data analytics shows immense promise in strengthening real-time, predictive disease surveillance if implemented carefully [41].
2.2. Artificial intelligence and machine learning
AI and ML are computational techniques that analyze data, identify patterns, and make predictions. By processing surveillance data, AI/ML algorithms can enhance human analysis by automating the detection of zoonotic disease outbreaks [49,50]. The term “machine learning” was coined by Samuel in 1959 [51]. ML is the practice of using algorithms to analyze data, learn from them, and make predictions or judgments regarding the future state of any new dataset. Therefore, instead of manually coding software routines with a specific set of instructions predetermined by programmers to accomplish a particular task, machines are taught how to perform the task using large amounts of data and algorithms. Common ML algorithms include supervised (using labeled data for training) and unsupervised learning [51]. ML enables systems to make better decisions and predictions using new data, and continuously improves and enhances system performance by identifying complex patterns in the data. With the development of AI, ML techniques have continuously improved and are now applied in various fields such as discovering and predicting new materials, molecular properties, quantum chemistry research, and drug design [[52], [53], [54], [55]]. Specific applications include natural language processing to extract insights from unstructured text-based reports, and deep neural networks to integrate and analyze genetic, epidemiological, and climatic data [56,57]. Supervised ML algorithms can be trained on labeled outbreak datasets to identify similar signals [58,59]. Furthermore, AI models can forecast outbreak trajectories based on transmission dynamics [60].
The key benefits of AI/ML are their ability to rapidly process massive datasets that cannot be analyzed manually, and the capacity to detect subtle signals that may indicate early-stage outbreaks [50,61]. Currently, multiple studies have reported the use of AI to monitor and predict the spread of COVID-19 [[62], [63], [64], [65]]. For example, BlueDot's AI algorithm provided an early warning of the COVID-19 outbreak by scanning news reports [66]. EPIWATCH is another AI-based system that generated automatic early warnings for COVID-19 using open-source data [66]. The public dashboard offers free analytics, including searchable and categorizable outbreak report tables, analytical capabilities, and GIS mapping. EPIWATCH provides AI-based event filtering, priority sorting, management, and reporting through manual review. These functionalities ensure that users are not overwhelmed by unrealistic volumes of data and provide more reliable and trustworthy predictions of disease outbreaks [66]. The system uses AI technology combined with contemporary natural language processing and named entity recognition algorithms to automatically scan articles for data points, evaluating whether the articles contain relevant outbreak information with 88.2% accuracy. Additionally, studies have utilized artificial neural networks to predict the incidence of Salmonella infection [67]. Pillai et al. employed a set-based approach, combining random forest, XGBoost, and three other deep learning techniques to analyze the physicochemical indicators of chicken farm soil, feces, and feed [68]. These indicators include the sodium and potassium contents in the soil and, the carbon/nitrogen ratio in the feed. By predicting the prevalence of Salmonella, the team provided management recommendations to reduce Salmonella outbreaks, such as increasing the sodium content in the soil to 10 mg/L, maintaining manganese levels between 40–100 mg/L, and ensuring a feed carbon/nitrogen ratio of ≤15 [68].
Similarly, various AI models have been developed to monitor and predict the occurrence of Dengue fever, a zoonotic disease that has long plagued humans [[69], [70], [71]]. For example, a model created by Raja et al. used Bayesian network ML techniques and trained the model with predictive variables such as temperature, rainfall, onset date, notification date, and vector indices like Aedes albopictus, Aedes aegypti , and their larva [69]. The model achieved a prediction accuracy of 79%–84% in estimating the mosquito population and the results could be used to infer the likelihood of Dengue fever outbreaks in selected areas surrounding the Klang Valley in Malaysia. However, challenges related to the model interpretability, bias, and data privacy remain [72].
2.3. The internet of things and sensors
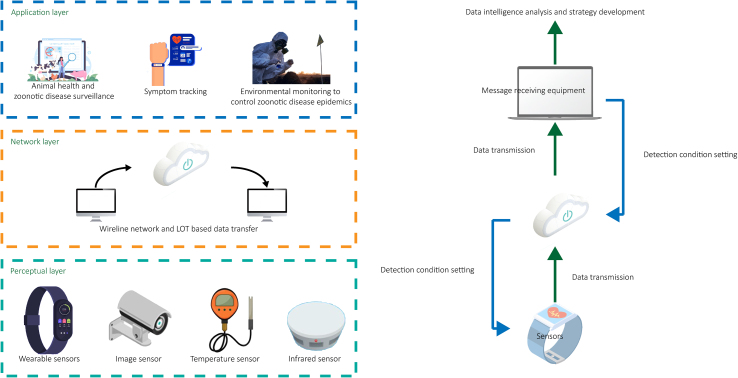
The concept of IoT was established in the 1990s, but it did not fully develop until after 2000 [73]. The IoT refers to a large-scale infrastructure where various physical objects ('things') are connected by sensors, software, and networks, enabling physical objects to generate, exchange and consume data, thus achieving intelligent recognition, positioning, tracking, monitoring, and management [74]. The IoT connects things in the physical world to the internet, enabling intelligent perception, interaction, and communication between 'things, people, and people with things'. This provides a deep integration of the physical and digital worlds. As shown in Fig. 3, IoT systems for disease surveillance consist of various components including biosensors, environmental sensors, agricultural sensors, wearable devices, and backend analytical platforms. These components enable the continuous real-time monitoring of parameters related to wildlife, livestock, and human health. In disease surveillance, deploying IoT-based sensors in key locations allows continuous real-time monitoring of environmental parameters, animal health, and human activity [[75], [76], [77]]. Specific applications include the use of wildlife biosensors to detect ecological disruptions that lead to zoonotic spillovers, agricultural IoT devices to monitor livestock health, and wearable sensors to track human vitals and symptoms [[78], [79], [80]]. Early anomaly detection enables targeted investigation and preventive measures [81].
Fig. 3.
Schematic diagram of the basic working principle of the Internet of Things (IoT).
The key benefits of the IoT are real-time data for rapid detection, ease of integrating multiple data streams, scalability across regions, and reduced costs compared to manual data collection [82,83]. IoT technology has also been applied to deal with the Ebola virus. Research has been conducted to combine IoT technology with other smart devices to sense and identify suspected cases of the Ebola virus [84]. Sareen et al. proposed a healthcare service based on IoT and cloud computing [85]. They utilized wearable sensor technology to obtain real-time data from Ebola patients, thereby enabling the detection and monitoring of Ebola infections. Furthermore, by employing radio frequency identification devices and temporal network analysis, the automatically sensed and analyzed proximity interactions between users, enabling them to monitor the epidemic situation. The team evaluated the model's performance and accuracy using synthetic data from two million users on the Amazon EC2 cloud, achieving a classification accuracy of 94% and a resource utilization rate of 92% [85]. Real-time data generated from suspected cases can be reported anytime and anywhere, facilitating rapid response and intervention [84]. Furthermore, a method to control and remotely monitor echinococcosis, based on IoT, is being designed and implemented [86]. Khan et al. proposed an influenza pandemic prediction modeling system based on a multi-stage deep interpretable inference feedforward neural network [87]. During the validation phase, the system model will be updated via the cloud as long as inputs are submitted through IoT devices, providing predictions for influenza pandemic alerts [87].
Additionally, wearable sensors and IoT can be used for accurate COVID-19 prediction and symptom analysis [88,89]. In the context of the COVID-19 pandemic, Aqeel-ur-Rehman et al. proposed an intelligent solution for statistical analysis and prediction of virus transmission [90]. The approach involved collecting the temperature data of individuals using devices such as infrared sensors at the entrances of public places. IoT systems are responsible for transmitting data, such as temperature, ID, age, gender, location, and phone numbers, to specific locations and organizations [90]. Finally, AI-based analytical software was used to perform statistical analysis and predict the extent and manner in which the virus would spread. A wireless, patch-like sensor has been developed to continuously monitor the vital signs of patients, including heart rate, blood oxygen levels, body temperature, and respiratory rate, and depth. The sensor transmits data wirelessly and in real-time using Bluetooth technology. ML algorithms are used to detect subtle changes in an individual's physiological features. Compared to traditional intermittent monitoring (typically conducted every 8 h), continuous remote monitoring of various vital signs in patients with Ebola, along with personalized data analysis, can provide early warnings of significant changes in a patient's condition, without compromising the safety of healthcare workers [90]. However, challenges regarding data security, privacy, transmission, and quality remain.
2.4. Geographic information systems
GIS is an emerging interdisciplinary field that emerged in the late 1960s with the development of computer technology [91]. It integrates various disciplines such as geography, statistics, computer science, and surveying and mapping, and primarily focuses on theoretical methods and technological means for the collection, storage, management, analysis, and display of geographic spatial information [92,93]. It uses information technology to digitally describe and quantitatively analyze various geographic elements and phenomena, revealing the spatiotemporal relationships between geographic elements and supporting decision-making and scientific research. In the context of disease monitoring applications, the predecessor of GIS is the visualization of maps showing the relationship between location and health, with the earliest example dating back to 1694, which was a map depicting the control of the plague in Italy [94]. Over the next 225 years, maps have been a significant communication tool for understanding and tracking infectious diseases such as yellow fever, cholera, and the 1918 influenza pandemic [95]. After GIS emerged, a significant portion of studies focused on disease mapping [96,97]. A review of GIS-related literature published in 2014 found that 28.7% of studies were related to infectious disease mapping [98].
GIS has been widely used to elucidate zoonotic transmission. Researchers have used GIS to map the prevalence zones and special areas of Toxoplasma gondii infection in the Khyber Pakhtunkhwa Province and to determine the spatial distribution of cutaneous leishmaniasis in an endemic area of Central Iran [99,100]. Leishmaniasis is a vector-borne disease influenced by various environmental factors. Maracy et al. used GIS analysis to examine the spatial distribution characteristics of cutaneous leishmaniasis in Isfahan, Iran, from 2014 to 2018 [100]. They utilized data from the provincial health center's database and archives, which recorded information on leishmaniasis patients from 44 counties in Isfahan. Hotspot analysis was conducted using the Getis-Ord-Gi software and the highest incidence rates were observed in the age group of 18–64 years, with males accounting for 61.6% of the cases. The disease exhibited seasonal distribution, with the highest occurrence in autumn (58.6%). The north-central and southeastern regions of Isfahan Province were identified as high-risk hotspots [100]. Using this information in epidemiological research can assist public health decision-makers in enhancing disease control and prevention strategies.
In addition, a GIS-based adaptive neuro-fuzzy inference system was employed to explore the spatial distribution patterns of human brucellosis (HB) in Mazandaran Province, Iran [101]. In 2012 and 2013, most of the hotspots were in the western region of the province, whereas in 2018, they were predominantly concentrated in the eastern region. Various factors, including demographic data, disease incidence rates, ecology, climate, topography, and vegetation, influence the incidence of HB. A linear regression model revealed that these parameters influencing HB incidence were independent of each other and could only account for 25.3% of the total variation in the HB incidence logarithm. Pearson's correlation analysis showed the strongest positive correlation between vegetation and the logarithm of population size and the number of HB cases. These findings may be attributed to the presence of suitable grazing areas in vegetated regions, which attract large populations of grazing animals, thereby increasing the number of HB cases. The results of the study could be used by health authorities to predict the transmission of HB and implement prevention programs. Furthermore, they could investigate factors influencing disease prevalence, identify high-risk areas, and allocate resources accordingly [101].
Another study proposed a novel Web GIS architecture for disease mapping that focused on the diagnosis and prevention of echinococcosis [102]. However, the effect of climate change on the risk of zoonotic diseases is not yet fully understood. Lima et al. deployed a newly developed model within a GIS to accurately identify future high-risk areas of Japanese encephalitis in Victoria [103]. This model considers several parameters, including rainfall, temperature, elevation, and distance to water bodies and pig farms, to assess the suitability of the vector mosquito, Culex annulirostris, for Japanese encephalitis transmission. Future climate predictions incorporated into the model were generated using the global climate model ACCESS-CM2 and were driven by worst-case (SSP126) and best-case (SSP585) scenarios. Risk maps for each month were created for the current and projected climates for 2040 and 2060.
2.5. Remote sensing and satellite imagery
Remote sensing technology originated in the mid- to late-19th century when ground photography was used for surveying. In the 1960s, the first Earth resources satellite allowed space-based remote sensing [104]. Remote sensing technology refers to the technique of collecting target information using various sensors without direct contact with the target. It primarily detects electromagnetic radiation in different regions of the electromagnetic spectrum using sensors placed on aircraft and satellite platforms to acquire information about the object being measured [105]. With the development of various remote sensing technologies such as optical, infrared, and microwave technologies, the spatial, spectral, temporal, and radiometric resolutions of remote sensing have continuously improved [105]. Remote sensing has been widely used in fields such as resource investigation, environmental monitoring, weather forecasting, ocean monitoring, and land management [106,107]. It is also being developed as a continuous observation system for the Earth, providing support for Earth science research and global change monitoring. Remote sensing and satellite imagery are crucial tools for monitoring and controlling zoonotic diseases. These technologies provide a comprehensive, real-time view of the environment, enabling researchers and public health officials to track the movement and distribution of disease vectors [108,109].
Remote sensing involves the use of devices or sensors to detect and measure objects or areas from a distance and offers valuable insights into the habitats and breeding grounds of disease-carrying organisms. For example, leveraging remote sensing data to identify environmental risk factors for mosquito-borne diseases in different geographical settings helps understand how land cover and the geographic environment affect the spread of vector-borne diseases [110]. Cunha et al. developed a model based on remote sensing and deep learning to help control Egyptian mosquitoes and Dengue fever in Campinas [111]. They used unmanned aerial vehicles and deep learning algorithms to capture images for the purpose of detecting water containers installed on rooftops and swimming pools, respectively, as potential breeding sites for Aedes aegypti. Additionally, a chi-square distribution model was employed to estimate the ratio of water containers/pools per square kilometer in each study area. This information is significant for vector control planning, as it helps identify areas with a high prevalence of Ae. aegypti, which is crucial for determining regions susceptible to vector-borne diseases [111]. Moreover, it contributes to the development of more appropriate response strategies for outbreaks of diseases such as Zika, Dengue fever, and Chikungunya.
Satellite imagery provides a bird's eye view of the Earth's surface, which can be used to map the spread of diseases and identify hotspots of disease outbreaks. For example, deep neural networks (DNN) have been used to predict the spread of the West Nile virus (WNV) by analyzing satellite imagery [112]. The West Nile fever is one of the most common mosquito-borne zoonotic diseases worldwide. Bonicelli et al. employed satellite imagery and DNN to predict the circulation of WNV. Their assessment focused on the Italian peninsula, which is characterized by high climatic variability and diversity. Specifically, they used graph neural networks to aggregate features from neighboring locations and extended these modules to consider multiple relationships, such as temperature and soil humidity differences between two locations, geographic distance and seasonality [112]. This study demonstrates the impact of multi-band satellite imagery on the circulation of the WNV, providing further insights into the relevance of circulation and seasonality models.
2.6. Molecular technologies
Molecular technologies have revolutionized the field of zoonotic disease surveillance and response. These advanced techniques allow for the rapid and accurate detection, identification, and characterization of pathogens, enabling timely and targeted interventions.
The polymerase chain reaction (PCR) is a key molecular technology used in zoonotic disease surveillance. It amplifies specific DNA or RNA sequences, allowing the detection of pathogens in various samples, such as blood, saliva, or environmental samples [113]. Modise et al. developed and validated a novel multiplex real-time quantitative PCR method based on high-resolution melting curve analysis [114]. This method allowed the simultaneous detection and differentiation of four abortive zoonotic agents in cattle, sheep, and goats [114]. They examined 216 DNA samples, and detected 45, 57, 12, and 19 cases of Brucella, Burkholderia, Leptospira, and Listeria monocytogenes, respectively, as well as 41 cases of mixed infection with Brucella and Burkholderia.. There were 42 negative results. All findings were confirmed using established testing methods, and the confirmation was 100% consistent [114]. It is a rapid, cost-effective, and reliable diagnostic tool for detecting abortive pathogens that cause zoonotic diseases in ruminants. Two Taq-Man-based multiplex PCR detection methodswere developed and validated to detect and differentiate all four canine hookworm species in naturally infected dog feces [115]. This technique not only enables the early detection of zoonotic diseases but also provides valuable information about the genetic characteristics of pathogens, aiding in their surveillance and monitoring of potential drug resistance.
Next-generation sequencing (NGS) is a molecular technology that has transformed zoonotic disease surveillance. NGS allows for the rapid sequencing of entire genomes, providing a comprehensive understanding of the genetic makeup of pathogens [116]. Blastocystis spp. are common intestinal protozoan parasites found in the feces of humans and animals worldwide. Limited reports are available on the subtypes and prevalence of avian Blastocystis infections. Maloney et al. revealed the extensive genetic diversity of Blastocystis subtypes in chickens using NGS, implying that chickens may serve as a source of human infection [117]. Analysis of molecular characteristics of the small subunit rRNA gene confirmed the existence of a new ST29 subtype in chickens. Additionally, all positive samples contained one or both potential zoonotic subtypes, ST6 and ST7, suggesting that chickens could be a source of human infection and environmental contamination.
Compared with traditional epidemiological studies that use multilocus sequence typing, NGS offers a higher resolution. Meinel et al. revealed a novel pathway for the zoonotic transmission of Corynebacterium ulcerans using NGS analysis [118]. Using NGS and comparative genomics analysis of nine isolates obtained from human patients and their livestock, they identified an isolate from the corresponding livestock that had with a prophage that was absent from the human isolates. This prophage harbors a putative novel virulence factor that is highly homologous to the Salmonella RhuM virulence factor, which has not been previously identified in Corynebacterium species. Additionally, a pathogenic island carrying the diphtheria toxin gene was identified in C. ulcerans. These findings suggest that the virulence of C. ulcerans can change rapidly through the acquisition of new virulence genes. This technique helps identify new strains or variants of zoonotic pathogens, track their transmission routes, and assess their potential to cause outbreaks or drug resistance.
In addition to PCR and NGS, other molecular technologies, such as loop-mediated isothermal amplification (LAMP) and enzyme-linked immunosorbent assay (ELISA) have also contributed to improving zoonotic disease surveillance. LAMP is a molecular biology method used for rapid and sensitive amplification of nucleic acid sequences [119]. Compared to traditional PCR methods, LAMP operates under isothermal conditions without the need for complex temperature cycling steps, resulting in faster reaction speed and higher specificity. LAMP has the potential to rapidly diagnose Leptospirosis disease [120]. Hamer et al. conducted LAMP testing on serum, clot, kidney, spleen, liver, and lung samples from golden Syrian hamsters [120]. All infected samples showed positive results, whereas amplification was observed only in the kidney, spleen, liver, and lung samples using PCR lipL32. Furthermore, they optimized the LAMP technique using different colorimetric indicators such as BFo and calcium green dye. Compared with LAMP combined with hydroxy naphthol blue or endpoint PCR lipL32 (10 ng DNA per reaction), the detection limit of LAMP combined with calcein green (1 ng DNA per reaction) increased 10-fold. Thisindicates that LAMP is an effective method for detecting Leptospira DNA in clinical samples.
The application of ELISA for zoonotic diseases involves the detection and quantification of specific antibodies or antigens in humans and animals for diagnostic, surveillance, and epidemiological purposes [121]. An indirect ELISA (iELISAINTA) developed by Novoa et al. can detect antibodies against Brucella in milk [122]. Brucellosis is an abortion-causing zoonotic disease prevalent in low- and middle-income countries, highlighting the importance of developing and validating sensitive, specific, and cost-effective diagnostic methods for Brucella. In cattle, it is primarily caused by Brucella abortus. They purified the lipopolysaccharide antigen from B. abortus in cows and generated monoclonal antibodies against bovine IgG to establish the iELISAINTA. Furthermore, they compared the iELISAINTA results with those of Canadian iELISACFIA, serum buffered plate antigen (BPA), and complement fixation test (CFT) using 4385 bulk milk samples and 968 individual milk samples to evaluate the performance of the method in the field. The kappa value for bulk milk samples between iELISAINTA and iELISACFIA was 0.86, whereas for individual milk samples, the kappa values for iELISAINTA with iELISACFIA, BPA, and CFT were 0.79, 0.85, and 0.82, respectively [122]. These results indicate that iELISAINTA performs well, and reduces stress on cows and sampling personnel.
Molecular technologies, such as PCR, NGS, LAMP, and ELISA, play vital roles in enhancing zoonotic disease surveillance. These technologies enable early detection, accurate identification, and characterization of pathogens, providing valuable insights for targeted interventions and control measures. However, implementation of these technologies require specialized laboratory infrastructure, trained personnel, and quality control measures. Overcoming these challenges and ensuring the accessibility and affordability of molecular technologies in resource-limited settings are crucial for their widespread adoption and effective use in zoonotic disease surveillance and response.
3. Solutions to improve response to zoonotic disease outbreaks
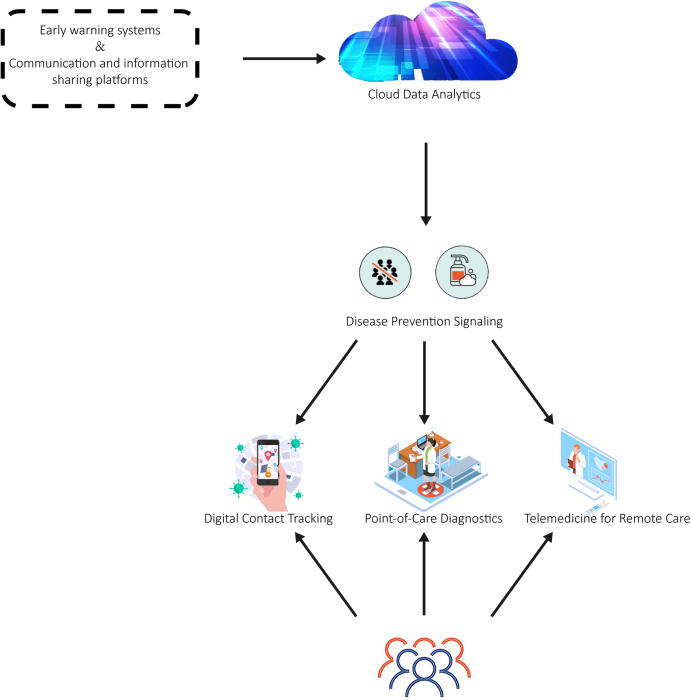
Robust response systems are crucial during zoonotic disease outbreaks. This section discusses various solutions that have been proposed or implemented to improve outbreak response. These include early warning systems, communication, information sharing platforms, point-of-care diagnostics (POC), telemedicine for remote care, and digital contact tracing (Fig. 4).
Fig. 4.
Solutions to improve response to zoonotic disease outbreaks.
3.1. Early warning systems
Early warning systems play a crucial role in the prevention and control of zoonotic diseases. These systems are designed to detect potential outbreaks or changes in disease patterns at an early stage, and provide valuable time for public health officials to implement effective response measures [123].
The early detection and warning of poultry diseases significantly improve animal welfare and reduce losses. Current early warning technologies offer the capability to continuously and automatically monitor the health status of chickens in a non-invasive manner [124]. Banakar et al. utilized data mining methods and the Dempster-Shafer evidence theory to develop an intelligent poultry disease warning device [125]. The device uses a fast fourier transform and discrete wavelet transform to process the sound signals of chickens in both the frequency and time-frequency domains. It can differentiate between Newcastle disease, infectious bronchitis virus, and avian influenza, and monitor chickens infected with the viruses within two days, achieving an accuracy rate of 91.15% and serve as an early warning system [125].
With increasing globalization, the probability of the spread of emerging infectious diseases (EIDs) has greatly increased, posing new challenges to existing surveillance and early warning systems in countries worldwide. Hui-min et al. developed a comprehensive monitoring and early warning system that demonstrated practicality and promising application prospects [126]. This system provides valuable guidance for the prevention and control of COVID-19 and future EIDs [126]. Their EID surveillance and early warning system, based on the concept of comprehensive health, was built on the scientific foundation of detection technology and early warning models to establish a monitoring and early warning network. It relies on external support and guarantees from the education, policy, and research sectors to form a sustainable and dynamic monitoring and early warning system. Simultaneously, a comprehensive surveillance and early warning system for EID requires substantial investments in manpower, resources, and finances; however, there is a lack of communication between laboratories in different fields and countries. Interdisciplinary and cross-sectoral exchanges and cooperation are increasing, allowing improved application and implementation of comprehensive health concepts [126]. However, translating warnings into effective action requires further optimization of early warning systems and tighter integration with outbreak response networks.
3.2. Communication and information sharing platforms
Effective communication and information sharing platforms play a crucial role in the surveillance and response to zoonotic diseases. These platforms enable various stakeholders, including public health officials, healthcare providers, researchers, and the public, to exchange timely and accurate information about disease outbreaks [25].
The COVID-19 pandemic has highlighted the effectiveness of information exchange and sharing. During this crisis, the U.S. Centers for Disease Control and Prevention (CDC) communicated directly with the public on COVID-19 related issues through Facebook, and effectively conveyed scientific information by addressing community concerns, promoting self-protection, encouraging action, and addressing emerging frames of understanding and fear [127]. This study provided a valuable reference for how public entities can engage with the public during an epidemic.
Similarly, network platforms established in Southeast Asia have prioritized diseases such as brucellosis, coxiellosis, and foot-and-mouth disease (FMD), thereby strengthening the country's ability to monitor zoonotic diseases [128]. Monitoring animal diseases in resource-limited countries poses challenges, and cost-effectiveness and sustainability remain significant limiting factors. Siengsanan-Lamont and Blacksell examined the development and implementation of a slaughterhouse-based national surveillance network in Laos and central Cambodia, consisting of an information exchange platform and a sample collection and submission system [128]. These networks provide information on the seroprevalence of specific “One Health” and high-consequence veterinary pathogens, including Q fever, brucellosis, and FMD. However, in animals sampled in both countries, over 40% exhibited FMD antibodies from natural infections rather than from vaccine administration, indicating a significant underreporting of outbreak events. Despite the support from multiple international organizations and partnerships over the years for animal disease surveillance activities in the Lao People's Democratic Republic and Cambodia, such networks require further improvement [128]. However, to fully realize the benefits of shared surveillance, deeper community engagement is essential to build trust and capacity.
3.3. Point-of-care diagnostics
Point-of-care (POC) diagnostics play a critical role in the rapid detection and management of zoonotic diseases [129]. These medical tests are performed at or near the point of patient care, such as in a doctor's office, bedside, or field, rather than in a centralized laboratory [130].
POC testing offers several key advantages, including immediate results, ease of operation, and portability. For example, the POC circulating cathodic antigen (POC-CCA) test can be utilized for diagnosing Japanese schistosomiasis [131]. The potential contribution of livestock schistosomiasis to the transmission of human diseases has affected the design of effective disease management and elimination programmes. As emphasized in the recently released “WHO Roadmap on Neglected Tropical Diseases 2021–2030” and revised schistosomiasis guidelines, there is a need for sensitive and specific tools for the diagnosis of animal schistosomiasis [131]. Calvo-Urbano et al. used POC-CCA to detect intestinal schistosomiasis in humans from Senegal [132]. The sensitivity of POC-CCA varies depending on the ruminant population and the parasite species and location. Overall, cattle showed higher sensitivity than sheep and goats, whereas the specificity of POC-CCA did not differ among the different populations of small ruminants.
POC diagnostics have also found widespread applications for COVID-19 testing, with nasopharyngeal and oropharyngeal swabs being the most commonly used collection methods [133]. The use of nasopharyngeal and oropharyngeal swabs is recommended for diagnostic testing of COVID-19; however, it increases the risk of transmission to healthcare workers [133]. Additionally, specimen collection can cause discomfort and bleeding in patients with low platelet counts [134]. In such cases, the availability of saliva samples has gained increasing attention. Sorelle developed a POC isothermal amplification platform called Abbott ID NOW (Abbott Diagnostics) for the detection of COVID-19 in saliva samples, providing results for SARS-CoV-2 in approximately 5 min. This platform is used in pharmacies, hospitals, and outpatient facilities across all 50 states in USA. They also conducted studies on the preservation of samples at room temperature for up to five days, and the results remained positive [133]. Notably, compared to the viral transport media nasopharyngeal swabs used in RT-PCR testing, the positive percent agreement for POC saliva testing was 82% (32/39), and the negative percent agreement was 100% (44/44). This suggests that testing should be conducted within two weeks of symptom onset to reduce the probability of false negatives.
However, challenges concerning test accuracy, quality control, and result connectivity remain [135]. Despite these issues, POC diagnostics remain essential tools for zoonotic disease surveillance and response. They enable prompt on-site pathogen detection, facilitate timely treatment and inform disease control strategies. Ongoing advancements in POC technology, along with efforts to improve its accessibility and usage, hold promise for enhancing the management of zoonotic diseases.
3.4. Telemedicine for remote care
Telemedicine has emerged as an instrumental tool for managing zoonotic diseases, particularly in regions with limited access to healthcare services. This study provides significant evidence for surveillance and response strategies in the context of zoonotic diseases. For instance, telemedicine can be utilized to remotely monitor and diagnose patients in areas experiencing outbreaks, eliminating the need for individuals to travel to healthcare facilities [136,137]. This has been particularly beneficial during the COVID-19 pandemic and was highly beneficial for patients, especially those with cancer [138]. Evidence from China suggests that COVID-19 poses a significant risk to patients with cancer, particularly those undergoing chemotherapy, who have a higher risk of severe illness or death from COVID-19. Digital platforms such as Oncopadi already exist and provide remote medical services, consultations, symptom control, and advice for patients with cancer and their caregivers [139]. By facilitating remote healthcare delivery, telemedicine not only helps contain the spread of diseases but also enhances patient outcomes.
Telemedicine plays a pivotal role in developing preventive measures. It is used in the management of close monkey pox contacts, enabling effective prevention and monitoring [140]. For example, the successful treatment of live subretinal cysticercosis using smartphone-assisted telemedicine highlights its potential [141].
Telemedicine offers a flexible and efficient approach for strengthening zoonotic disease surveillance and response. It enables the direct delivery of essential health services to individuals, facilitates the early detection and management of disease outbreaks, and fosters the integration of human and animal health services. Despite these challenges, the potential impact of telemedicine on disease management and public health underscores its value for combating zoonotic diseases.
3.5. Digital contact tracing
Digital contact tracing has become an essential tool in the battle against zoonotic diseases because of its ability to track disease transmission quickly and accurately. This technique uses digital technologies, such as mobile applications and data analytics, to identify interactions between individuals and the potential transmission chains of infectious diseases [142,143].
Traditional contact tracing methods involve manual techniques to identify individuals who have had close contact with infected individuals. However, manual contact tracing has two significant limitations. First, a substantial number of trained personnel were required to conduct interviews. Second, it fails to identify individuals who may have had close contact with an infected person but are unknown to them; for example, while using public transportation or dining at restaurants [144]. To overcome these limitations, Luo et al. conducted a study using epidemiological data from 278 officially reported COVID-19 cases in Chengdu and Tianjin between January 21 and February 22, 2020. They constructed a visual transmission network based on the relationships among cases. Among the cases examined, 45 had missing information, and 24 had vague exposure details. Due to the absence of contact history data, approximately one-third of cases could not be traced, highlighting the need to enhance case tracing methods [145]. In the context of zoonotic diseases, digital contact tracing can expedite surveillance and response efforts. It allows health authorities to promptly identify and notify individuals who have had close contact with an infected person, thereby preventing further spread of the disease [146]. In 2020, the Singapore government introduced a nationwide Bluetooth contact tracing application called TraceTogether. It used the OpenTrace code to implement the BlueTrace protocol, aiming to achieve privacy-preserving community-driven contact tracing across borders [147]. BlueTrace devices exchange information via Bluetooth to record encounters. A unique personal identifier is securely stored by the health authorities, and the encounter history of each user is stored only on their own device. Health authorities have access to this information only when a user is confirmed to have encountered an infected individual.
In addition, Rodríguez evaluated the epidemiological impact of the Spanish digital contact-tracing application Radar Covid [148]. They conducted a four-week population-controlled trial in La Gomera, Canary Islands, Spain, and estimated that at least 33% of the population used this technology. The application detected approximately 6.3 close contacts per initial simulated infection, which was nearly twice the median number of contacts detected manually by professional contact tracers, with a significant proportion of these contacts being with strangers. Notably, the infection in this controlled experiment was simulated, which has certain limitations. Overall, these results provide empirical evidence for the potential usefulness of digital contact tracing during outbreaks in real populations [148]. However, concerns regarding user adoption, transparency, equity, and data privacy remain.
Digital contact tracing offers a robust and efficient approach to enhance the surveillance and response to zoonotic diseases. Despite the challenges, finding a right balance between public health interests and individual privacy rights can significantly contribute to the management of zoonotic diseases and mitigate their impact on communities.
4. Implementation challenges and recommendations
Implementation of modern technologies and solutions to enhance surveillance and response systems for emerging zoonotic diseases presents several challenges. These challenges range from logistical and technical difficulties to financial constraints and regulatory hurdles. For example, deploying technologies such as big data analytics, AI, and IoT in remote or underserved areas can be challenging owing to limited infrastructure and connectivity [[22], [23], [24]]. Similarly, the utilization of advanced technologies like automation may require significant financial investment and specialized training for healthcare staff, posing challenges in resource-limited settings [149,150].
Moreover, the integration of human and animal health services, which is crucial for managing zoonotic diseases, can lead to challenges related to coordination, data sharing, and policy alignment across different sectors. Additionally, issues concerning data security, privacy, and ethical considerations can pose significant challenges to the use of digital technologies for disease surveillance and response [151].
Several recommendations are proposed to address this issue. First, enhancing the infrastructure and connectivity in remote and underserved areas will facilitate the deployment of digital technologies [152]. Second, providing training and capacity building for healthcare staff will ensure the effective use of advanced technologies [153]. Third, fostering cross-sector collaboration and coordination enhances integration of human and animal health services. Fourth, establishing robust data governance frameworks can address security and privacy concerns. Finally, leveraging public-private partnerships can help mobilize resources and expertise for implementing implementation these technologies and solutions [154].
Overall, although the implementation of modern technologies and solutions for zoonotic disease surveillance and response faces challenges, strategic planning, resource allocation, and cross-sector collaboration can pave the way for effective and sustainable implementation. With appropriate strategies and resources, these technologies and solutions can significantly enhance our ability to detect, respond to, and control emerging zoonotic diseases.
5. Conclusions
The integration of modern technologies and solutions into surveillance and response systems for emerging zoonotic diseases has significant potential for improving disease control and prevention. By harnessing the power of data, enhancing communication and collaboration, and adopting innovative approaches, we can enhance our ability to detect, respond to, and mitigate the impact of zoonotic disease outbreaks. Continued research, investment, and collaboration are crucial for realizing the full potential of these technologies and solutions in safeguard public health.
Author contributions
L.Z.: Conceptualization, Writing—original draft preparation, Methodology; W.G. and C.L.: Suggestions for revision. All authors have read and agreed to publish this version of the manuscript.
Conflict of interest
The authors declare that there are no conflict of interest.
Acknowledgements
We would like to thank all those who advised and helped us, especially those who studied with us in Hainan. We appreciate the imaging materials provided by Bing. If any copyright concerns exist, please contact us for modifications. This study did not receive any funding.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.soh.2023.100061.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Liu Q., Cao L., Zhu X.Q. Major emerging and re-emerging zoonoses in China: a matter of global health and socioeconomic development for 1.3 billion. Int. J. Infect. Dis. 2014;25:65–72. doi: 10.1016/j.ijid.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomori O., Oluwayelu D.O. Domestic animals as potential reservoirs of zoonotic viral diseases. Annu. Rev. Anim. Biosci. 2023;11:33–55. doi: 10.1146/annurev-animal-062922-060125. [DOI] [PubMed] [Google Scholar]
- 3.Di Bari C., Venkateswaran N., Fastl C., Gabriel S., Grace D., Havelaar A.H., Huntington B., Patterson G.T., Rushton J., Speybroeck N., Torgerson P., Pigott D.M., Devleesschauwer B. The global burden of neglected zoonotic diseases: current state of evidence. One Health. 2023;17 doi: 10.1016/j.onehlt.2023.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohr J.R., Barrett C.B., Civitello D.J., Craft M.E., Delius B., DeLeo G.A., Hudson P.J., Jouanard N., Nguyen K.H., Ostfeld R.S., Remais J.V., Riveau G., Sokolow S.H., Tilman D. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019;2(6):445–456. doi: 10.1038/s41893-019-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaheen M.N.F. The concept of one health applied to the problem of zoonotic diseases. Rev. Med. Virol. 2022;32(4):e2326. doi: 10.1002/rmv.2326. [DOI] [PubMed] [Google Scholar]
- 6.Allen T., Murray K.A., Zambrana-Torrelio C., Morse S.S., Rondinini C., Di Marco M., Breit N., Olival K.J., Daszak P. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 2017;8(1):1124. doi: 10.1038/s41467-017-00923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naguib M.M., Li R., Ling J., Grace D., Nguyen-Viet H., Lindahl J.F. Live and wet markets: food access versus the risk of disease emergence. Trends Microbiol. 2021;29(7):573–581. doi: 10.1016/j.tim.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plowright R.K., Parrish C.R., McCallum H., Hudson P.J., Ko A.I., Graham A.L., Lloyd-Smith J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017;15(8):502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappaioanou M., Kane T.R. Addressing the urgent health challenges of climate change and ecosystem degradation from a One Health perspective: what can veterinarians contribute? J. Am. Vet. Med. Assoc. 2023;261(1):49–55. doi: 10.2460/javma.22.07.0315. [DOI] [PubMed] [Google Scholar]
- 10.Abbass K., Qasim M.Z., Song H., Murshed M., Mahmood H., Younis I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Control Ser. 2022;29(28):42539–42559. doi: 10.1007/s11356-022-19718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinz R., Frickmann H., Krüger A. In: International Climate Protection. Palocz-Andresen M., Szalay D., Gosztom A., Sípos L., Taligás T., editors. Springer International Publishing; Cham: 2019. Climate change and infectious diseases; pp. 269–276. [Google Scholar]
- 12.Bartlow A.W., Manore C., Xu C., Kaufeld K.A., Del Valle S., Ziemann A., Fairchild G., Fair J.M. Forecasting zoonotic infectious disease response to climate change: mosquito vectors and a changing environment. Vet. Sci. 2019;6(2):40. doi: 10.3390/vetsci6020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortiz D.I., Piche-Ovares M., Romero-Vega L.M., Wagman J., Troyo A. The impact of deforestation, urbanization, and changing land use patterns on the ecology of mosquito and tick-borne diseases in Central America. Insects. 2022;13(1):20. doi: 10.3390/insects13010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellwanger J.H., Kulmann-Leal B., Kaminski V.L., Valverde-Villegas J., Veiga A.B.G., Spilki F.R., Fearnside P.M., Caesar L., Giatti L.L., Wallau G.L. Beyond diversity loss and climate change: impacts of Amazon deforestation on infectious diseases and public health. An Acad. Bras Ciências. 2020;92(1) doi: 10.1590/0001-3765202020191375. [DOI] [PubMed] [Google Scholar]
- 15.Sim S., Cho M. Convergence model of AI and IoT for virus disease control system. Personal Ubiquitous Comput. 2023;27(3):1209–1219. doi: 10.1007/s00779-021-01577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albahri A.S., Hamid R.A., Alwan J.k., Al-qays Z.T., Zaidan A.A., Zaidan B.B., Albahri A.O.S., AlAmoodi A.H., Khlaf J.M., Almahdi E.M., Thabet E., Hadi S.M., Mohammed K.I., Alsalem M.A., Al-Obaidi J.R., Madhloom H.T. Role of biological data mining and machine learning techniques in detecting and diagnosing the novel coronavirus (COVID-19): a systematic review. J. Med. Syst. 2020;44(7):122. doi: 10.1007/s10916-020-01582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraemer M.U.G., Yang C.-H., Gutierrez B., Wu C.-H., Klein B., Pigott D.M., Open C.-D.W.G., du Plessis L., Faria N.R., Li R., Hanage W.P., Brownstein J.S., Layan M., Vespignani A., Tian H., Dye C., Pybus O.G., Scarpino S.V. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science. 2020;368(6490):493–497. doi: 10.1126/science.abb4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acharya K.P., Acharya N., Phuyal S., Subramanya S.H. Human infection with Avian influenza A virus in Nepal: requisite for timely management and preparedness. VirusDisease. 2020;31(3):244–248. doi: 10.1007/s13337-020-00593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung Kjær L., Ward M.P., Boklund A.E., Larsen L.E., Hjulsager C.K., Kirkeby C.T. Using surveillance data for early warning modelling of highly pathogenic avian influenza in Europe reveals a seasonal shift in transmission, 2016–2022. Sci. Rep. 2023;13(1) doi: 10.1038/s41598-023-42660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guinat C., Tago D., Corre T., Selinger C., Djidjou-Demasse R., Paul M., Raboisson D., Nguyen Thi Thanh T., Inui K., Pham Thanh L., Padungtod P., Vergne T. Optimizing the early detection of low pathogenic avian influenza H7N9 virus in live bird markets. J. R. Soc. Interface. 2021;18(178) doi: 10.1098/rsif.2021.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh K.B., Parekh F.K., Tabynov K., Tabynov K., Hewson R., Fair J.M., Essbauer S., Hay J. Operationalizing cooperative research for infectious disease surveillance: lessons learned and ways forward. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.659695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farahani B., Firouzi F., Luecking M. The convergence of IoT and distributed ledger technologies (DLT): opportunities, challenges, and solutions. J. Netw. Comput. Appl. 2021;177 [Google Scholar]
- 23.Firouzi F., Rahmani A.M., Mankodiya K., Badaroglu M., Merrett G.V., Wong P., Farahani B. Internet-of-Things and big data for smarter healthcare: from device to architecture, applications and analytics. Future Generat. Comput. Syst. 2018;78:583–586. [Google Scholar]
- 24.Huang S.-C., Chaudhari A.S., Langlotz C.P., Shah N., Yeung S., Lungren M.P. Developing medical imaging AI for emerging infectious diseases. Nat. Commun. 2022;13(1):7060. doi: 10.1038/s41467-022-34234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asadzadeh A., Kalankesh L.R. A scope of mobile health solutions in COVID-19 pandemics. Inform. Med. Unlocked. 2021;23 doi: 10.1016/j.imu.2021.100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Niakan Kalhori S., Bahaadinbeigy K., Deldar K., Gholamzadeh M., Hajesmaeel-Gohari S., Ayyoubzadeh S.M. Digital health solutions to control the COVID-19 pandemic in countries with high disease prevalence: literature review. J. Med. Internet Res. 2021;23(3) doi: 10.2196/19473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dabla P.K., Gruson D., Gouget B., Bernardini S., Homsak E. Lessons learned from the COVID-19 pandemic: emphasizing the emerging role and perspectives from artificial intelligence, mobile health, and digital laboratory medicine. Ejifcc. 2021;32(2):224–243. [PMC free article] [PubMed] [Google Scholar]
- 28.Yaqoob I., Hashem I.A.T., Gani A., Mokhtar S., Ahmed E., Anuar N.B., Vasilakos A.V. Big data: from beginning to future. Int. J. Inf. Manag. 2016;36(6):1231–1247. Part B) [Google Scholar]
- 29.Hagstrom M. High-performance analytics fuels innovation and inclusive growth: use big data, hyperconnectivity and speed to intelligence to get true value in the digital economy. Journal of Advanced Analytics. 2012;2(3):31–44. [Google Scholar]
- 30.Strawn G.O. Scientific research: how many paradigms? Educ. Rev. 2012;47(3):26. [Google Scholar]
- 31.Jin X., Wah B.W., Cheng X., Wang Y. Significance and challenges of big data research. Big Data Research. 2015;2(2):59–64. [Google Scholar]
- 32.Fosso Wamba S., Akter S., Edwards A., Chopin G., Gnanzou D. How ‘big data’ can make big impact: findings from a systematic review and a longitudinal case study. Int. J. Prod. Econ. 2015;165:234–246. [Google Scholar]
- 33.De Mauro A., Greco M., Grimaldi M. A formal definition of Big Data based on its essential features. Libr. Rev. 2016;65(3):122–135. [Google Scholar]
- 34.Sheng J., Amankwah-Amoah J., Khan Z., Wang X. COVID-19 pandemic in the new era of big data analytics: methodological innovations and future research directions. Br. J. Manag. 2021;32(4):1164–1183. [Google Scholar]
- 35.Pace A. Technologies for large data management in scientific computing. Int. J. Mod. Phys. C. 2013;25(2) [Google Scholar]
- 36.Chiu D., Agrawal G. In evaluating caching and storage options on the Amazon Web services cloud, 2010 11th. IEEE/ACM International Conference on Grid Computing. 2010;2010:17–24. 25-28 Oct. 2010. [Google Scholar]
- 37.Kasson P.M. Infectious disease research in the era of big data. Annu. Rev. Biomed. Data Sci. 2020;3(1):43–59. [Google Scholar]
- 38.Asokan G.V., Mohammed M.Y. In: Big Data in Psychiatry #x0026; Neurology. Moustafa A.A., editor. Academic Press; 2021. Chapter 16 - harnessing big data to strengthen evidence-informed precise public health response; pp. 325–337. [Google Scholar]
- 39.Zhou X., Lee E.W.J., Wang X., Lin L., Xuan Z., Wu D., Lin H., Shen P. Infectious diseases prevention and control using an integrated health big data system in China. BMC Infect. Dis. 2022;22(1):344. doi: 10.1186/s12879-022-07316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schantz P.M. Parasitic zoonoses in perspective. Int. J. Parasitol. 1991;21(2):161–170. doi: 10.1016/0020-7519(91)90006-s. [DOI] [PubMed] [Google Scholar]
- 41.Asokan G., Asokan V. Leveraging “big data” to enhance the effectiveness of “one health” in an era of health informatics. J. Epidemiol. Glob. Health. 2015;5(4):311–314. doi: 10.1016/j.jegh.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greene S.K., Huang J., Abrams A.M., Gilliss D., Reed M., Platt R., Huang S.S., Kulldorff M. Gastrointestinal disease outbreak detection using multiple data streams from electronic medical records. Foodborne Pathog. Dis. 2012;9(5):431–441. doi: 10.1089/fpd.2011.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Althouse B.M., Scarpino S.V., Meyers L.A., Ayers J.W., Bargsten M., Baumbach J., Brownstein J.S., Castro L., Clapham H., Cummings D.A. Enhancing disease surveillance with novel data streams: challenges and opportunities. EPJ Data Sci. 2015;4(1):1–8. doi: 10.1140/epjds/s13688-015-0054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng H., Ruan Z., Long F., Simpson J.H., Myers E.W. V3D enables real-time 3D visualization and quantitative analysis of large-scale biological image data sets. Nat. Biotechnol. 2010;28(4):348–353. doi: 10.1038/nbt.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rekatsinas T., Ghosh S., Mekaru S.R., Nsoesie E.O., Brownstein J.S., Getoor L., Ramakrishnan N. vol. 2015. SIAM; 2015. pp. 379–387. (In Sourceseer: Forecasting Rare Disease Outbreaks Using Multiple Data Sources, Proceedings of the 2015 SIAM International Conference on Data Mining). [Google Scholar]
- 46.Zhou X., Lee E.W.J., Wang X., Lin L., Xuan Z., Wu D., Lin H., Shen P. Infectious diseases prevention and control using an integrated health big data system in China. BMC Infect. Dis. 2022;22(1):1–9. doi: 10.1186/s12879-022-07316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S.F., Wang W.H., Chang K., Chen Y.H., Tseng S.P., Yen C.H., Wu D.C., Chen Y.M. Severe dengue fever outbreak in taiwan. Am. J. Trop. Med. Hyg. 2016;94(1):193–197. doi: 10.4269/ajtmh.15-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bertino E. 2016 IEEE 40th Annual Computer Software and Applications Conference (cOMPSAc) IEEE; 2016. In Data security and privacy: concepts, approaches, and research directions; pp. 400–407. 2016. [Google Scholar]
- 49.Bezbaruah R., Ghosh M., Kumari S., Nongrang L., Ali S.R., Lahiri M., Waris H., Kakoti B.B. Role of AI and ML in epidemics and pandemics. Bioinformatics Tools for Pharmaceutical Drug Product Development. 2023:345–369. [Google Scholar]
- 50.Dogan O., Tiwari S., Jabbar M., Guggari S. A systematic review on AI/ML approaches against COVID-19 outbreak. Complex Intell. Syst. 2021;7:2655–2678. doi: 10.1007/s40747-021-00424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Categorical A. Glossary of terms. Mach. Learn. 1998;30(2):271–274. [Google Scholar]
- 52.Butler K.T., Davies D.W., Cartwright H., Isayev O., Walsh A. Machine learning for molecular and materials science. Nature. 2018;559(7715):547–555. doi: 10.1038/s41586-018-0337-2. [DOI] [PubMed] [Google Scholar]
- 53.Nantasenamat C., Isarankura-Na-Ayudhya C., Prachayasittikul V. Advances in computational methods to predict the biological activity of compounds. Expet Opin. Drug Discov. 2010;5(7):633–654. doi: 10.1517/17460441.2010.492827. [DOI] [PubMed] [Google Scholar]
- 54.Nash W., Drummond T., Birbilis N. A review of deep learning in the study of materials degradation. npj Mater. Degrad. 2018;2(1):37. [Google Scholar]
- 55.Wang M., Wang T., Cai P., Chen X. Nanomaterials discovery and design through machine learning. Small Methods. 2019;3(5) [Google Scholar]
- 56.Bellinger C., Mohomed Jabbar M.S., Zaïane O., Osornio-Vargas A. A systematic review of data mining and machine learning for air pollution epidemiology. BMC Publ. Health. 2017;17:1–19. doi: 10.1186/s12889-017-4914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ozturk T., Talo M., Yildirim E.A., Baloglu U.B., Yildirim O., Acharya U.R. Automated detection of COVID-19 cases using deep neural networks with X-ray images. Comput. Biol. Med. 2020;121 doi: 10.1016/j.compbiomed.2020.103792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh R., Singh R. Applications of sentiment analysis and machine learning techniques in disease outbreak prediction–A review. Mater. Today: Proc. 2023;81:1006–1011. [Google Scholar]
- 59.Muhammad L., Algehyne E.A., Usman S.S., Ahmad A., Chakraborty C., Mohammed I.A. Supervised machine learning models for prediction of COVID-19 infection using epidemiology dataset. SN Comput. Sci. 2021;2:1–13. doi: 10.1007/s42979-020-00394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu Z., Ge Q., Li S., Jin L., Xiong M. 2020. Artificial Intelligence Forecasting of Covid-19 in china. arXiv preprint arXiv:2002.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rashidi H.H., Tran N., Albahra S., Dang L.T. Machine learning in health care and laboratory medicine: general overview of supervised learning and Auto-ML. Int. J. Lit. Humanit. 2021;43:15–22. doi: 10.1111/ijlh.13537. [DOI] [PubMed] [Google Scholar]
- 62.Allam Z., Dey G., Jones D.S. Artificial intelligence (AI) provided early detection of the coronavirus (COVID-19) in China and will influence future Urban health policy internationally. Ai. 2020;1(2):156–165. [Google Scholar]
- 63.Dananjayan S., Raj G.M. Artificial Intelligence during a pandemic: the COVID-19 example. Int. J. Health Plann. Manag. 2020;35(5):1260. doi: 10.1002/hpm.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calandra D., Favareto M. Artificial Intelligence to fight COVID-19 outbreak impact: an overview. European J. Soc. Impact Circular Econ. 2020;1(3):84–104. [Google Scholar]
- 65.Khemasuwan D., Colt H.G. Applications and challenges of AI-based algorithms in the COVID-19 pandemic. BMJ Innov. 2021;7(2):387–398. [Google Scholar]
- 66.MacIntyre C.R., Chen X., Kunasekaran M., Quigley A., Lim S., Stone H., Paik H.-y., Yao L., Heslop D., Wei W. Artificial intelligence in public health: the potential of epidemic early warning systems. J. Int. Med. Res. 2023;51(3) doi: 10.1177/03000605231159335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Permanasari A.E., Rambli D.R.A., Dominic P.D.D. IEEE; 2010. In Forecasting of Salmonellosis Incidence in Human Using Artificial Neural Network (ANN), 2010 the 2nd International Conference on Computer and Automation Engineering (ICCAE) pp. 136–139. 2010. [Google Scholar]
- 68.Pillai N., Ayoola M.B., Nanduri B., Rothrock M.J., Jr., Ramkumar M. An ensemble learning approach to identify pastured poultry farm practice variables and soil constituents that promote Salmonella prevalence. Heliyon. 2022;8(11) doi: 10.1016/j.heliyon.2022.e11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raja D.B., Mallol R., Ting C.Y., Kamaludin F., Ahmad R., Ismail S., Jayaraj V.J., Sundram B.M. Artificial intelligence model as predictor for dengue outbreaks. Malaysian J. Public Health Med. 2019;19(2):103–108. [Google Scholar]
- 70.Guo P., Liu T., Zhang Q., Wang L., Xiao J., Zhang Q., Luo G., Li Z., He J., Zhang Y. Developing a dengue forecast model using machine learning: a case study in China. PLoS Neglected Trop. Dis. 2017;11(10) doi: 10.1371/journal.pntd.0005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoyos W., Aguilar J., Toro M. Dengue models based on machine learning techniques: a systematic literature review. Artif. Intell. Med. 2021;119 doi: 10.1016/j.artmed.2021.102157. [DOI] [PubMed] [Google Scholar]
- 72.Saraswat D., Bhattacharya P., Verma A., Prasad V.K., Tanwar S., Sharma G., Bokoro P.N., Sharma R. IEEE Access; 2022. Explainable AI for Healthcare 5.0: Opportunities and Challenges. [Google Scholar]
- 73.Wanasinghe T.R., Gosine R.G., James L.A., Mann G.K.I., Silva O.d., Warrian P.J. The internet of things in the oil and gas industry: a systematic review. IEEE Internet Things J. 2020;7(9):8654–8673. [Google Scholar]
- 74.Aloi G., Caliciuri G., Fortino G., Gravina R., Pace P., Russo W., Savaglio C. Enabling IoT interoperability through opportunistic smartphone-based mobile gateways. J. Netw. Comput. Appl. 2017;81:74–84. [Google Scholar]
- 75.Yu X., Zhang S., Guo W., Li B., Yang Y., Xie B., Li K., Zhang L. Recent advances on functional nucleic-acid biosensors. Sensors. 2021;21(21):7109. doi: 10.3390/s21217109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Javaid M., Khan I.H. Internet of Things (IoT) enabled healthcare helps to take the challenges of COVID-19 Pandemic. J. Oral Biol. Craniofac. Res. 2021;11(2):209–214. doi: 10.1016/j.jobcr.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chude-Okonkwo U.K. One Health-inspired early implementation of airborne disease spread mitigation protocols aided by IoT-based biosensor network. Int. J. Sens. Netw. 2022;39(4):215–226. [Google Scholar]
- 78.Akhigbe B.I., Munir K., Akinade O., Akanbi L., Oyedele L.O. IoT technologies for livestock management: a review of present status, opportunities, and future trends. Big Data Cogn. Comput. 2021;5(1):10. [Google Scholar]
- 79.Sanyal A., Agarwal S., Ramakrishnan U., Garg K.M., Chattopadhyay B. Using environmental sampling to enable zoonotic pandemic preparedness. J. Indian Inst. Sci. 2022;102(2):711–730. doi: 10.1007/s41745-022-00322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lv Z., Li Y. Wearable sensors for vital signs measurement: a survey. J. Sens. Actuator Netw. 2022;11(1):19. [Google Scholar]
- 81.Wendt A., Kreienbrock L., Campe A. Zoonotic disease surveillance–inventory of systems integrating human and animal disease information. Zoonoses Public Health. 2015;62(1):61–74. doi: 10.1111/zph.12120. [DOI] [PubMed] [Google Scholar]
- 82.Cui Y., Liu F., Jing X., Mu J. Integrating sensing and communications for ubiquitous IoT: applications, trends, and challenges. IEEE Network. 2021;35(5):158–167. [Google Scholar]
- 83.Abdul-Qawy A.S., Pramod P., Magesh E., Srinivasulu T. The internet of things (iot): an overview. Int. J. Eng. Res. Afr. 2015;5(12):71–82. [Google Scholar]
- 84.Rahman M.S., Peeri N.C., Shrestha N., Zaki R., Haque U., Ab Hamid S.H. Defending against the novel coronavirus (COVID-19) outbreak: how can the internet of things (IoT) help to save the world? Health Policy Technol. 2020;9(2):136. doi: 10.1016/j.hlpt.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sareen S., Sood S.K., Gupta S.K. IoT-based cloud framework to control Ebola virus outbreak. J. Ambient Intell. Hum. Comput. 2018;9:459–476. doi: 10.1007/s12652-016-0427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang S.-J., Xiao N., Li J.-Z., Feng Y., Ma J.-Y., Quzhen G.-S., Yu Q., Zhang T., Yi S.-C., Zhou X.-N. A remote management system for control and surveillance of echinococcosis: design and implementation based on internet of things. Infect. Dis. Poverty. 2021;10:1–12. doi: 10.1186/s40249-021-00833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan M.A., Abidi W.U.H., Al Ghamdi M.A., Almotiri S.H., Saqib S., Alyas T., Khan K.M., Mahmood N. Forecast the influenza pandemic using machine learning. Comput. Mater. Continua (CMC) 2020;66(1):331–340. [Google Scholar]
- 88.Singh R.P., Javaid M., Haleem A., Suman R. Internet of things (IoT) applications to fight against COVID-19 pandemic. Diabetes Metabol. Syndr.: Clin. Res. Rev. 2020;14(4):521–524. doi: 10.1016/j.dsx.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Otoom M., Otoum N., Alzubaidi M.A., Etoom Y., Banihani R. An IoT-based framework for early identification and monitoring of COVID-19 cases. Biomed. Signal Process Control. 2020;62 doi: 10.1016/j.bspc.2020.102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aqeel-ur-Rehman S.U.R., Khan I.U., Moiz M., Hasan S. Security and privacy issues in IoT. Int. J. Commun. Network. Inf. Secur. 2016;8(3):147–157. [Google Scholar]
- 91.Goodchild M.F. Geographical information science. Int. J. Geogr. Inf. Syst. 1992;6(1):31–45. [Google Scholar]
- 92.Goodchild M.F. Geographic information systems and science: today and tomorrow. Spatial Sci. 2009;15(1):3–9. [Google Scholar]
- 93.Goodchild M. Reimagining the history of GIS. Spatial Sci. 2018;24:1–8. [Google Scholar]
- 94.Waller L.A. The atlas of disease: mapping deadly epidemics and contagion from the plague to the Zika virus. Cartogr. J. 2020;57(1):86. -86. [Google Scholar]
- 95.Engelmann L., Henderson J., Lynteris C. Routledge; 2018. Plague and the City. [Google Scholar]
- 96.Khashoggi B.F., Murad A. vol. 9. 2020. Issues of healthcare planning and GIS: a review. (ISPRS International Journal of Geo-Information). [Google Scholar]
- 97.Shanono N., Frans, Kodong F.R., Aljaberi M. Monitoring infectious diseases diffusion through GIS. J. Sci. Technol. 2020;2:23–33. [Google Scholar]
- 98.Fradelos E.C., Papathanasiou I.V., Mitsi D., Tsaras K., Kleisiaris C.F., Kourkouta L. Health based geographic information systems (GIS) and their applications. Acta Inf. Med. 2014;22(6):402–405. doi: 10.5455/aim.2014.22.402-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan M.J., Mubaraki M.A., Jahan S., Khattak B., Khan M., Fozia, Khokhar M.A.H., Ahmad I. Assessment of geographical distribution of emerging zoonotic Toxoplasma gondii infection in women patients using geographical information system (GIS) in various regions of Khyber Pakhtunkhwa (KP) Province, Pakistan. Trop. Med. Infect. Dis. 2022;7(12):430. doi: 10.3390/tropicalmed7120430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maracy M.R., Jaffary F., Ebrahimi A., Sokhanvari F., Heidari A., Sharifian-Koupaiee H., Fadaei R., Ramazanpour J., Moazeni M. GIS-based risk mapping of cutaneous leishmaniasis: a survey in an endemic area of Central Iran. Environ. Sci. Pollut. Control Ser. 2021;28(41):57470–57485. doi: 10.1007/s11356-021-14455-8. [DOI] [PubMed] [Google Scholar]
- 101.Babaie E., Alesheikh A.A., Tabasi M. Spatial prediction of human brucellosis (HB) using a GIS-based adaptive neuro-fuzzy inference system (ANFIS) Acta Trop. 2021;220 doi: 10.1016/j.actatropica.2021.105951. [DOI] [PubMed] [Google Scholar]
- 102.Kulawiak M., Kulawiak N., Sulima M., Sikorska K. A novel architecture of Web-GIS for mapping and analysis of echinococcosis in Poland. Applied Geomatics. 2022;14(2):181–198. [Google Scholar]
- 103.Lima M.F., Marais M., Cotton J., Sposito V., Faggian R. Modelling the future risk of Japanese encephalitis in Victoria using geographic information system. Population Medicine. 2023;5(Supplement) [Google Scholar]
- 104.Gupta R.P. Springer; 2017. Remote Sensing Geology. [Google Scholar]
- 105.Sabins F.F., Jr., Ellis J.M. Waveland Press; 2020. Remote Sensing: Principles, Interpretation, and Applications. [Google Scholar]
- 106.Gorji T., Sertel E., Tanik A. Monitoring soil salinity via remote sensing technology under data scarce conditions: a case study from Turkey. Ecol. Indicat. 2017;74:384–391. [Google Scholar]
- 107.West H., Quinn N., Horswell M. Remote sensing for drought monitoring & impact assessment: progress, past challenges and future opportunities. Rem. Sens. Environ. 2019;232 [Google Scholar]
- 108.Ford T.E., Colwell R.R., Rose J.B., Morse S.S., Rogers D.J., Yates T.L. Using satellite images of environmental changes to predict infectious disease outbreaks. Emerg. Infect. Dis. 2009;15(9):1341. doi: 10.3201/eid/1509.081334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garni R., Tran A., Guis H., Baldet T., Benallal K., Boubidi S., Harrat Z. Remote sensing, land cover changes, and vector-borne diseases: use of high spatial resolution satellite imagery to map the risk of occurrence of cutaneous leishmaniasis in Ghardaia, Algeria. Infect. Genet. Evol. 2014;28:725–734. doi: 10.1016/j.meegid.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 110.McMahon A. 2021. Earth Observation and Mosquito-Borne Diseases: Assessing Environmental Risk Factors for Disease Transmission via Remote Sensing Data. [Google Scholar]
- 111.Cunha H.S., Sclauser B.S., Wildemberg P.F., Fernandes E.A.M., Dos Santos J.A., Lage M.d.O., Lorenz C., Barbosa G.L., Quintanilha J.A., Chiaravalloti-Neto F. Water tank and swimming pool detection based on remote sensing and deep learning: relationship with socioeconomic level and applications in dengue control. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0258681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bonicelli L., Porrello A., Vincenzi S., Ippoliti C., Iapaolo F., Conte A., Calderara S. Spotting virus from satellites: modeling the circulation of West Nile virus through Graph neural networks. IEEE Trans. Geosci. Rem. Sens. 2023;99:1–11. [Google Scholar]
- 113.Liu X., Wang X., Zhang H., Yan Z., Gaňová M., Lednický T., Řezníček T., Xu Y., Zeng W., Korabečná M., Neužil P. Smartphone integrated handheld (SPEED) digital polymerase chain reaction device. Biosens. Bioelectron. 2023;232 doi: 10.1016/j.bios.2023.115319. [DOI] [PubMed] [Google Scholar]
- 114.Modise B.M., Mpoloka S.W., Settypalli T.B., Hyera J., Natale A., Ceglie L., Gcebe N., Marobela-Raborokgwe C., Viljoen G.J., Cattoli G. A novel multiplex qPCR-HRM assay for the simultaneous detection of four abortive zoonotic agents in cattle, sheep, and goats. Sci. Rep. 2023;13(1) doi: 10.1038/s41598-023-39447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Massetti L., Colella V., Zendejas P.A., Ng-Nguyen D., Harriott L., Marwedel L., Wiethoelter A., Traub R.J. High-throughput multiplex qPCRs for the surveillance of zoonotic species of canine hookworms. PLoS Neglected Trop. Dis. 2020;14(6) doi: 10.1371/journal.pntd.0008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chiara M., D'Erchia A.M., Gissi C., Manzari C., Parisi A., Resta N., Zambelli F., Picardi E., Pavesi G., Horner D.S. Next generation sequencing of SARS-CoV-2 genomes: challenges, applications and opportunities. Briefings Bioinf. 2021;22(2):616–630. doi: 10.1093/bib/bbaa297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maloney J.G., da Cunha M.J., Molokin A., Cury M.C., Santin M. Next-generation sequencing reveals wide genetic diversity of Blastocystis subtypes in chickens including potentially zoonotic subtypes. Parasitol. Res. 2021;120(6):2219–2231. doi: 10.1007/s00436-021-07170-3. [DOI] [PubMed] [Google Scholar]
- 118.Meinel D.M., Margos G., Konrad R., Krebs S., Blum H., Sing A. Next generation sequencing analysis of nine Corynebacterium ulcerans isolates reveals zoonotic transmission and a novel putative diphtheria toxin-encoding pathogenicity island. Genome Med. 2014;6(11):113. doi: 10.1186/s13073-014-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fan Z., Mei Y., Xing J., Chen T., Hu D., Liu H., Li Y., Liu D., Liu Z., Liang Y. Loop-mediated isothermal amplification (LAMP)/Cas12a assay for detection of Ralstonia solanacearum in tomato. Front. Bioeng. Biotechnol. 2023:11. doi: 10.3389/fbioe.2023.1188176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hamer M., Watanabe O., Saraullo V., Ortega F., Sánchez C., Martínez M., Brihuega B., Grune Loffler S. Optimization and comparative analysis of LAMP and PCR techniques for the detection of leptospiral DNA in Golden Syrian hamsters. Vet. Res. Commun. 2023:1–9. doi: 10.1007/s11259-023-10183-1. [DOI] [PubMed] [Google Scholar]
- 121.Yoon B.K., Sut T.N., Yoo K.Y., Lee S.H., Hwang Y., Jackman J.A., Cho N.-J. Lipid bilayer coatings for rapid enzyme-linked immunosorbent assay. Appl. Mater. Today. 2021;24 doi: 10.1016/j.apmt.2021.101128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Novoa M.B., Aguirre N.P., Valentini B., Torioni-de-Echaide S., Signorini M.L., Primo M.E., Elena S., Vanzini V.R. Development, validation and field evaluation of an indirect ELISA for the detection of antibodies against Brucella abortus in bulk and individual milk samples in dairy cattle. Prev. Vet. Med. 2022;208 doi: 10.1016/j.prevetmed.2022.105740. [DOI] [PubMed] [Google Scholar]
- 123.Shi, X.; Shi, Z.; Zhu, Y.; Tian, H.; Zhu, Z.; Zheng, H., Digital Diagnostics and Early Warnings of Infectious Diseases. Available at SSRN 4505899.
- 124.He P., Chen Z., Yu H., Hayat K., He Y., Pan J., Lin H. Research progress in the early warning of chicken diseases by monitoring clinical symptoms. Appl. Sci. 2022;12(11):5601. [Google Scholar]
- 125.Banakar A., Sadeghi M., Shushtari A. An intelligent device for diagnosing avian diseases: Newcastle, infectious bronchitis, avian influenza. Comput. Electron. Agric. 2016;127:744–753. doi: 10.1016/j.compag.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li H.M., Liu J.S., Wang X.H., Zhao H.Q., Xie Y., Yin J.X., Lv C., Zhou N., Jiang T.G., Gu S.Y., Yin K., Zhou X.N., Guo X.K., Hu Q.Q. Integrated surveillance and early warning system for emerging infectious diseases based on One Health concept: structures and innovations. Chin. J. Parasitol. Parasit. Dis. 2022;40(5):572–578. [Google Scholar]
- 127.Kandzer M., Castano V., Baker L.M., McLeod-Morin A. Framing friction: a content analysis investigating how the CDC framed social media communication with the public during the COVID-19 pandemic. J. Appl. Commun. 2022;106(1):4. [Google Scholar]
- 128.Siengsanan-Lamont J., Blacksell S.D. Surveillance for one health and high consequence veterinary pathogens (brucellosis, Coxiellosis and foot and mouth disease) in Southeast Asia: Lao PDR and Cambodia in focus and the importance of international partnerships. Microbiol. Aust. 2021;42(4):156–160. [Google Scholar]
- 129.Hobbs E.C., Colling A., Gurung R.B., Allen J. The potential of diagnostic point-of-care tests (POCTs) for infectious and zoonotic animal diseases in developing countries: technical, regulatory and sociocultural considerations. Transbound. Emerg. Dis. 2021;68(4):1835–1849. doi: 10.1111/tbed.13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Manessis G., Gelasakis A.I., Bossis I. Point-of-Care diagnostics for farm animal diseases: from biosensors to integrated lab-on-chip devices. Biosensors. 2022;12(7):455. doi: 10.3390/bios12070455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cai P., Mu Y., Weerakoon K.G., Olveda R.M., Ross A.G., McManus D.P. Performance of the point-of-care circulating cathodic antigen test in the diagnosis of schistosomiasis japonica in a human cohort from Northern Samar, the Philippines. Infect. Dis. Poverty. 2021;10(5):40–51. doi: 10.1186/s40249-021-00905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Calvo-Urbano B., Léger E., Gabain I., De Dood C.J., Diouf N.D., Borlase A., Rudge J.W., Corstjens P.L., Sène M., Van Dam G.J. Sensitivity and specificity of human point-of-care circulating cathodic antigen (POC-CCA) test in African livestock for rapid diagnosis of schistosomiasis: a Bayesian latent class analysis. PLoS Neglected Trop. Dis. 2023;17(5) doi: 10.1371/journal.pntd.0010739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sharma S., Kumar V., Chawla A., Logani A. Rapid detection of SARS-CoV-2 in saliva: can an endodontist take the lead in point-of-care COVID-19 testing? Int. Endod. J. 2020;53(7):1017. doi: 10.1111/iej.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S., Tsoi H.-W., Lo S.K.-F., Chan K.-H., Poon V.K.-M., Chan W.-M., Ip J.D., Cai J.-P., Cheng V.C.-C., Chen H., Hui C.K.-M., Yuen K.-Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang S.-M., Lv S., Zhang W., Cui Y. Microfluidic point-of-care (POC) devices in early diagnosis: a review of opportunities and challenges. Sensors. 2022;22(4):1620. doi: 10.3390/s22041620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burhani T.S., Naqvi W.M. Telehealth-A boon in the time of COVID 19 outbreak. J. Evol. Med. Dent. Sci. 2020;9(29):2081–2084. [Google Scholar]
- 137.Sommer A.C., Blumenthal E.Z. Telemedicine in ophthalmology in view of the emerging COVID-19 outbreak. Graefes Arch. Clin. Exp. Ophthalmol. 2020;258:2341–2352. doi: 10.1007/s00417-020-04879-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rahman M., Prasanna N. COVID-19 and cancer: optimizing health communication through telemedicine. Curr. Trends Biotechnol. Pharm. 2022;16(1):108–113. [Google Scholar]
- 139.Salako O., Okunade K., Allsop M., Habeebu M., Toye M., Oluyede G., Fagbenro G., Salako B. Upheaval in cancer care during the COVID-19 outbreak. Ecancermedicalscience. 2020:14. doi: 10.3332/ecancer.2020.ed97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Seah B.Z.Q., Koh E.T.C., Lim E.W.T., Vasoo S., Chong S.J. Applicability and benefits of telemedicine in the monitoring of monkeypox close contacts. J. Telemed. Telecare. 2022;0(0) doi: 10.1177/1357633X221130290. 1357633X221130290. [DOI] [PubMed] [Google Scholar]
- 141.Shashni A., Gaur N., Takkar B., Dhasmana R. Smartphone-assisted telemedicine for a case of live subretinal cysticercosis. BMJ Case Rep. 2020;13(11) doi: 10.1136/bcr-2020-239719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wesolowski A., Buckee C.O., Engø-Monsen K., Metcalf C.J.E. Connecting mobility to infectious diseases: the promise and limits of mobile phone data. J. Infect. Dis. 2016;214(suppl_4):S414–S420. doi: 10.1093/infdis/jiw273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Keddy K.H., Saha S., Kariuki S., Kalule J.B., Qamar F.N., Haq Z., Okeke I.N. Using big data and mobile health to manage diarrhoeal disease in children in low-income and middle-income countries: societal barriers and ethical implications. Lancet Infect. Dis. 2022;22(5):e130–e142. doi: 10.1016/S1473-3099(21)00585-5. [DOI] [PubMed] [Google Scholar]
- 144.Eames K.T.D., Keeling M.J. Contact tracing and disease control. Proc. Roy. Soc. Lond. B Biol. Sci. 2003;270(1533):2565–2571. doi: 10.1098/rspb.2003.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luo C., Ma Y., Jiang P., Zhang T., Yin F. The construction and visualization of the transmission networks for COVID-19: a potential solution for contact tracing and assessments of epidemics. Sci. Rep. 2021;11(1):8605. doi: 10.1038/s41598-021-87802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen M., Xu S., Husain L., Galea G. Digital health interventions for COVID-19 in China: a retrospective analysis. Intell. Med. 2021;1(1):29–36. doi: 10.1016/j.imed.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Michael K., Abbas R. Behind COVID-19 contact trace apps: the google–apple partnership. IEEE Consumer Electronics Magazine. 2020;9(5):71–76. [Google Scholar]
- 148.Rodríguez P., Graña S., Alvarez-León E.E., Battaglini M., Darias F.J., Hernán M.A., López R., Llaneza P., Martín M.C., Ramirez-Rubio O., Romaní A., Suárez-Rodríguez B., Sánchez-Monedero J., Arenas A., Lacasa L. A population-based controlled experiment assessing the epidemiological impact of digital contact tracing. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-020-20817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hayase S., Katayama Y.A., Hatta T., Iwamoto R., Kuroita T., Ando Y., Okuda T., Kitajima M., Natsume T., Masago Y. Near full-automation of COPMAN using a LabDroid enables high-throughput and sensitive detection of SARS-CoV-2 RNA in wastewater as a leading indicator. Sci. Total Environ. 2023;881 doi: 10.1016/j.scitotenv.2023.163454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang Y., Yazid N.B.M., Ho P.-Y., Hu X., Chen S., Vasoo S., Kanitthamniyom P. DropCarba–An automated magnetic digital microfluidic platform for rapid phenotypic testing of carbapenemase-producing Gram-negative bacilli. Biosens. Bioelectron. 2023;225 doi: 10.1016/j.bios.2023.115099. [DOI] [PubMed] [Google Scholar]
- 151.Park Y.J., Cho S.Y., Lee J., Lee I., Park O. Development and utilization of a rapid and accurate epidemic investigation support system for COVID-19. Osong Public Health Res. Perspect. 2020;11(3):118–127. doi: 10.24171/j.phrp.2020.11.3.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shah I., Doshi C., Patel M., Tanwar S., Hong W.-C., Sharma R. A comprehensive review of the technological solutions to analyse the effects of pandemic outbreak on human lives. Medicina. 2022;58 doi: 10.3390/medicina58020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leal Neto O., Paolotti D., Dalton C., Carlson S., Susumpow P., Parker M., Phetra P., Lau E.H.Y., Colizza V., Jan van Hoek A., Kjelsø C., Brownstein J.S., Smolinski M.S. Enabling multicentric participatory disease surveillance for global health enhancement: viewpoint on global flu view. JMIR Public Health Surveill. 2023;9 doi: 10.2196/46644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mnf S. The concept of one health applied to the problem of zoonotic diseases. Rev. Med. Virol. 2022;32(4):e2326. doi: 10.1002/rmv.2326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.