Abstract
OBJECTIVE:
To assess the association between cerebral saturation (crSO2) using Near-Infrared Spectroscopy (NIRS) and brain injury in extremely preterm infants.
STUDY DESIGN:
This retrospective study includes 62 infants (<28 weeks gestation) who underwent continuous NIRS monitoring in the first 5 days after birth. Median crSO2 were compared in 12 h increments between infants with and without germinal matrix/intraventricular hemorrhage (GM/IVH). crSO2 was also compared by IVH severity, onset, and by grade of injury on term equivalent MRI.
RESULTS:
After 48 h of life (HOL), infants with GM/IVH had significantly lower crSO2 than those without GM/IVH in analysis adjusted for potential confounding e.g., at 49–60 HOL (69.5 (66.2, 72.8) vs. 74.7 (71.8, 77.6), p = 0.023). There were no significant differences in crSO2 by IVH subcategory or injury severity on MRI.
CONCLUSION:
Clinical use of NIRS has the potential to identify crSO2 patterns associated with development of GM/IVH.
INTRODUCTION
The rates of germinal matrix and intraventricular hemorrhage (GM/IVH) in extremely preterm infants (<28 weeks) have not reduced in recent decades, despite improvements in neonatal care, and continue to affect approximately one-third of extremely preterm infants with more severe injury being associated with adverse neurodevelopmental outcomes [1–5]. While the pathogenesis of GM/IVH is multifactorial, a major contributor to development of GM/IVH in extremely preterm infants is cerebral blood flow (CBF) dysregulation. In particular, cerebral pressure passive circulation contributes to decreases, increases, and large fluctuations in CBF in the first few days of life [6–15].
Continuous monitoring of CBF has the potential to guide future preventive and therapeutic strategies for GM/IVH. While near-infrared spectroscopy (NIRS) measures regional cerebral saturation (crSO2), crSO2 may be used as a surrogate for CBF when stable oxygen content and extraction can be reasonably assumed [16]. Although normative values of crSO2 in preterm infants in the first few days of life have been published [17], there are a limited number of studies that have explored the relationship between changes in crSO2 and development of GM/IVH, and these provide conflicting reports on whether relative increases or decreases in crSO2 reflect risk for GM/IVH [14, 18–22]. A few studies have shown increased crSO2 in the first few DOL is associated with GM/IVH [14, 18], while others have found that GM/IVH is associated with decreased crSO2 immediately after birth [19, 20] and within the first few days after birth [21, 23]. In addition to the conflicting prior results, many of these studies have been limited by small sample size and case-control design.
Therefore, the aim of this study was to compare the crSO2 values over the first 5 days of life among extremely preterm infants with and without GM/IVH. In addition, given the known association between brain injury score on term equivalent (TE) MRI and long-term outcomes, we also aimed to explore the relationship between crSO2 patterns and brain injury score on TE MRI.
METHODS
Patients
This is a retrospective cohort study which includes infants born <28 weeks gestational age with NIRS data recorded within the first 108 h of life (HOL). At our institution, beginning January 1st, 2018, all infants born before 28 weeks GA were monitored with NIRS for at least the first 3 days of life as part of the Neonatal Neurocritical Care Program at Brigham and Women’s Hospital (BWH) Neonatal Intensive Care Unit, a single tertiary level unit. We included infants <28 weeks gestational age born between January 2018 and August 2020. Institutional review board approval was obtained with a waiver of consent.
Demographic and basic clinical data
Demographic and basic clinical data were extracted from the medical record and Vermont Oxford Network (VON) database, to which the hospital is a contributor. All clinical definitions are concurrent with the VON database definitions. If chorioamnionitis was recorded in either the mother’s or the infant’s medical record, the infant was identified as having chorioamnionitis. Patent ductus arteriosus (PDA) was defined as left to right or bidirectional ductal shunt on Doppler echo, and/or a systolic or continuous murmur, plus at least two of the following: (1) hyperdynamic precordium, (2) bounding pulses, (3) wide pulse pressure, (4) pulmonary vascular congestion, cardiomegaly, or both. Sepsis was defined as having a bacterial pathogen detected in blood and/or cerebrospinal fluid culture. Intubated refers to intubation throughout the entire first 108 HOL.
NIRS data
CrSO2 was monitored by an INVOS 5100 C Cerebral/Somatic Oximeter (Medtronic, USA) using neonatal sensors applied according to a locally developed Clinical Practice Guideline. This guideline includes continuous NIRS monitoring of all preterm infants <28 weeks GA for at least the first 3 days of life (https://www.brighamandwomens.org/assets/BWH/pediatric-newborn-medicine/pdfs/nirs-cpg.pdf) for clinical purposes. Using the algorithms included in these guidelines, the clinician utilizes crSO2 data to assess cerebral oxygen delivery and/or extraction. For example, a patient with significantly decreased crSO2 relative to baseline or absolute crSO2 < 60% is evaluated for anemia, hypoxia, hypotension, chest hyperinflation, and hypocarbia and is treated accordingly. One sensor was placed on the frontoparietal side of the infant’s head and alternated between right and left sides every 4–6h (https://www.brighamandwomens.org/assets/BWH/pediatric-newborn-medicine/pdfs/nirs-appendix.pdf). These left and right-sided measurements were treated equally in our analysis. Measurements were performed at a sampling rate of 0.03 Hz.
Since the NIRS data were analyzed retrospectively from data collected clinically, we used analytic methodologies to clean the data and remove potential artifacts. Raw crSO2 data were cleaned using MATLAB (ver. R2019b, Mathworks, Inc., Natick, MA) with the following rules in place: (1) to remove sporadic data points and step changes that likely represent detachment of the sensing probe in the last few hours of recording, we rejected a data period at the end of the measurement if it had >50% of points missing within 2 h from the last data point, and in the last 2 h of valid data we rejected the data following an abrupt change (defined as >1.5 times the interquartile range or the signal mean, identified using MATLAB’s isoutlier or ischange functions, respectively); (2) to remove unreliable data points during the measurements, we rejected data if there were more than 90% of missing points in a non-overlapping 1 h sliding window. We also excluded data points >2.5 standard deviations from the mean using a 1 h sliding window, extreme crSO2 values (defined as ≤25% and ≥95%), and all data within 3 min before and after the extreme values. The rejected data points were replaced with NaN (Not a number in MATLAB, which serves as a ‘void’ place holder). The final signal was summarized by calculating the median of the crSO2 values, excluding the rejected data points, in each hourly time bin from birth, using the retime function in MATLAB. Subjects with a total measurement of <1 h within the first 108 HOL were excluded from the analysis. The signal cleaning of NIRS data resulted in an average rejection rate of 5.4 ± 7.9% in all subjects. (Supplemental Explanation of Data Cleaning and Supplemental Table 1). The number of infants with crSO2 data available in first 108 HOL by hour and by 12 h interval is demonstrated in Supplemental Fig. 1A, B.
Cranial ultrasound
Cranial ultrasonography (cUS) was performed by either the GE LOGIQ™ E9 or the GE LOGIQ™ E10, using both the S4–10 baby head ultrasound transducer and the L9 linear probe. The cUS protocol routinely acquires six coronal images (anterior to posterior), coronal clips (frontal through parietal-temporal), eight sagittal images (including midline, right and left parasagittal, occipital horn view from posterior fontanelle), sagittal clips, and axial view of posterior fossa from the mastoid region. cUS was performed based on a local Clinical Practice Guideline (https://www.brighamandwomens.org/assets/BWH/pediatric-newborn-medicine/pdfs/hus-cpg.pdf), which recommends a cUS at one day, 3 days, 1 week and 1 month after birth. An additional cUS at 36 weeks postmenstrual age is obtained if TE magnetic resonance imaging (MRI) is not performed. Clinical cUS reports were used for study analysis. We classified the grade of GM/IVH as the most severe GM/IVH detected on cUS. The cohort was separated into GM/IVH and no GM/IVH. Those with GM/IVH were classified as mild (grade I (germinal matrix hemorrhage) or II) and severe (grade III or IV (periventricular hemorrhagic infarction)) [24]. GM/IVH was also classified into early GM/IVH if the hemorrhage was detected on cUS within the first 36 HOL, and late GM/IVH if there was a cUS performed within 36 HOL that did not show hemorrhage, with a subsequent cUS after the first 36 HOL showing hemorrhage.
Magnetic resonance imaging (MRI)
All TE MRI scans were performed on a 3-T Siemens scanner (Siemens, Erlangen, Germany). The standard clinical imaging protocol includes sagittal motion-corrected magnetization prepared rapid gradient echo T1-weighted images, axial turbo spin echo T1-weighted images, axial turbo spin echo T2-weighted images, and coronal turbo spin echo T2- weighted images. Diffusion-weighted imaging used multidirectional diffusion-weighted measurements. The pattern and severity of brain injury on the TE MRI were assessed via the Kidokoro scoring system, a validated scoring system for evaluating cerebral white matter (WM), cortical gray matter, basal ganglia and thalami, and cerebellum abnormalities. The measurements were corrected for postmenstrual age, and a global brain abnormality score was calculated as the sum of the regional total scores and classified as normal (total score of 0–3), mild (total score of 4–7), moderate (total score of 8–11), or severe (total score of 12 or more) [25].
Statistical analyses
Descriptive statistics of key demographic and clinical characteristics were calculated overall and by IVH status. Differences by IVH status were assessed using chi-square (exact for tests of small sample size, n < 5) or Wilcoxon rank sum, as appropriate. The median crSO2 at each hour was calculated for each infant (see section NIRS Data above). Using these values, we calculated the median crSO2 in each of nine 12 h intervals from the first to 108th HOL. The association between presence of IVH and crSO2 values over time was modeled using linear mixed regression to take into account repeated measures within subjects nested within intrafamilial clusters (twins). The fixed-effects model included terms for IVH status and time (categorical) as well as their interaction. Models were adjusted for potential confounding by GA at birth, birth weight z score, and baseline hematocrit, base deficit, and pCO2. Baseline values of these latter three factors were measured as the mean value from the first 12 HOL. Trajectories of crSO2 over time for each group (GM/IVH vs. no IVH) were generated using the least square means and 95% confidence intervals for each group at each time point estimated from the analytic models. Similar trajectories were created for subgroups of IVH (severity (mild and severe) and time of onset (early (<36 HOL) and late (≥36 HOL))) as well as for total Kidokoro brain injury score severity categories. Due to reduced sample sizes among subgroup analyses, we focused our results on models unadjusted for potential confounding. Because these analyses were exploratory, we did not adjust for multiple comparisons.
For the earliest 12 h block where we identified a significant difference in crSO2 by IVH status, we generated a receiver operating characteristic (ROC) curve to assess the accuracy of GM/IVH prediction using crSO2. We used an unadjusted model and identified the cutpoint that optimized the Youden index. Based on this ROC curve and the optimal cutpoint, we calculated the diagnostic metrics (area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). All analyses were run using SAS v9.4 Software (SAS Institute Inc., Cary, NC, USA).
RESULTS
Demographic and clinical results
During the study period, 65 extremely preterm infants (<28 weeks GA) were eligible for inclusion with NIRS data collected during the first 108 HOL. Of these 65, three infants were excluded due to <1 h of NIRS measurements recorded. Thus, 62 infants were included in our cohort. There were 28 (45.2%) infants who developed GM/IVH and 34 (54.8%) who did not. Demographic and clinical data by GM/IVH status are presented in Table 1. Compared with infants with no GM/IVH, those who developed GM/IVH were of younger GA (p = 0.018), had lower baseline HCT (p = 0.0351), were more likely to be intubated (p = 0.0025), and had higher rates of complications such as sepsis (p = 0.0334) and retinopathy of prematurity (ROP) (p = 0.0139). Among the infants with GM/IVH, 14 (50.0%) had mild GM/IVH (4 grade I and 10 grade II) and 14 (50.0%) had severe GM/IVH (3 grade III and 11 grade IV). The median hours (IQR) of life until first detection of hemorrhage did not differ significantly between the mild (36.8 h (17.7–91.0)) and severe (42.0 h (18.7–65.4)) groups (p = 0.9795).
Table 1.
Demographic and clinical data of the study cohort (n = 62).
| No GM/ IVH (n = 34) | GM/ IVH (n = 28) | p value | |
|---|---|---|---|
| GA (weeks) | 26.1 (25.3–27.1) | 25.1 (24.1–26.1) | 0.0180a |
| Birth weight (g) | 805 (690–930) | 697.5 (582.5–858.5) | 0.1431 |
| Multiple Births | 11 (32.4) | 6 (21.4) | 0.3373 |
| Sex (male) | 14 (41.2) | 18 (64.3) | 0.0700 |
| Chorioamnionitis | 3 (8.8) | 6 (21.4) | 0.2773 |
| Cesarean section | 30 (88.2) | 20 (71.4) | 0.1165 |
| Apgar 1 min | 4.0 (3.0–6.0) | 3.0 (1.5–5.0) | 0.0641 |
| Apgar 5 min | 7.0 (6.0–8.0) | 6.0 (4.0–7.5) | 0.1414 |
| Chest compressions in DR | 3 (8.8) | 3 (10.7) | 1.0000 |
| Baseline Base deficit (mEq/L) | 2.5 (1.4–4.4) | 3.5 (1.8–5.6) | 0.2211 |
| Baseline pCO2 (mmHg) | 48.8 (43.2–53.7) | 50.2 (43.1–55.3) | 0.5812 |
| Baseline Hematocrit (%) | 44.4 (39.0–47.8) | 40.0 (35.8–45.3) | 0.0351a |
| Intubated | 10 (29.4) | 19 (67.9) | 0.0025a |
| PDA | 24 (70.6) | 20 (71.4) | 0.9422 |
| Sepsis | 2 (5.9) | 8 (28.6) | 0.0334a |
| ROPb | 19/32 (59.4) | 20/22 (90.9) | 0.0139a |
| Death | 1 (2.9) | 4 (14.3) | 0.1658 |
| HOL until first detection of hemorrhage | N/A | 41.2 (18.7–66.2) | 0.9795 |
| HOL NIRS measurements began | 9.5 (5–19) | 7 (4.5–18.5) | 0.6099 |
| HOL NIRS measurements ended | 80.5 (73–100) | 78 (67.5–126.5) | 0.9211 |
| Median duration (hours) of measurement | 68 (54–89) | 70 (48–109) | 0.5714 |
Categorical and binary data presented as n (%), continuous data as medians (Interquartile range (IQR)).
Differences among categories were assessed using chi-square (exact for tests of small sample size, n < 5), Wilcoxon rank sum.
GA gestational age, DR delivery room, PDA Patent Ductus Arteriosus, ROP Retinopathy of Prematurity.
p value < 0.05.
Not all subjects had data on ROP status. In the Table we have specified the denominator for this variable to provide the total number of subjects on which our percentages are based.
Among the 28 infants who developed GM/IVH, both early and late cUS was available for 24 (85.7%) infants. Of these, 11 (45.8%) had early GM/IVH (I (n = 2), II (n = 4), III (n = 0), IV (n = 5), and 13 (54.2%) had late GM/IVH (I (n = 2), II (n = 4), III (n = 3), IV (n = 4)). The median age in hours (IQR) at detection of GM/IVH in the early group was 18.1 (14.4–23.6), compared with 94.7 (60.6–156) in the late group (p < 0.0001). Of note, five infants received a diagnosis of GM/IVH after the 108 h evaluated in this study.
NIRS measurements
NIRS monitoring began at a median (IQR) eight (5–19) h old and ended at 79.5 (72–107) h old, with a median duration of 69 h (52–93). Time to start and duration of NIRS monitoring did not differ based on IVH status (Table 1).
crSO2 comparison between no IVH and IVH
Based on estimates from the unadjusted linear mixed model, the crSO2 trajectory for the GM/IVH group exhibited a steady decrease after 36 h while that for the no GM/IVH group was relatively stable over time. Infants with GM/IVH had significantly lower crSO2 values than those with no GM/IVH after 36 HOL (Fig. 1). When the model was adjusted for potential confounding, there was attenuation of the difference between groups as compared with the adjusted model, but the crSO2 trajectories still exhibited significantly lower levels among the GM/IVH infants compared with the no GM/IVH infants after 48 h (Fig. 2).
Fig. 1. Trajectories of %crSO2 (least square means and 95% CIs) over time by IVH status.
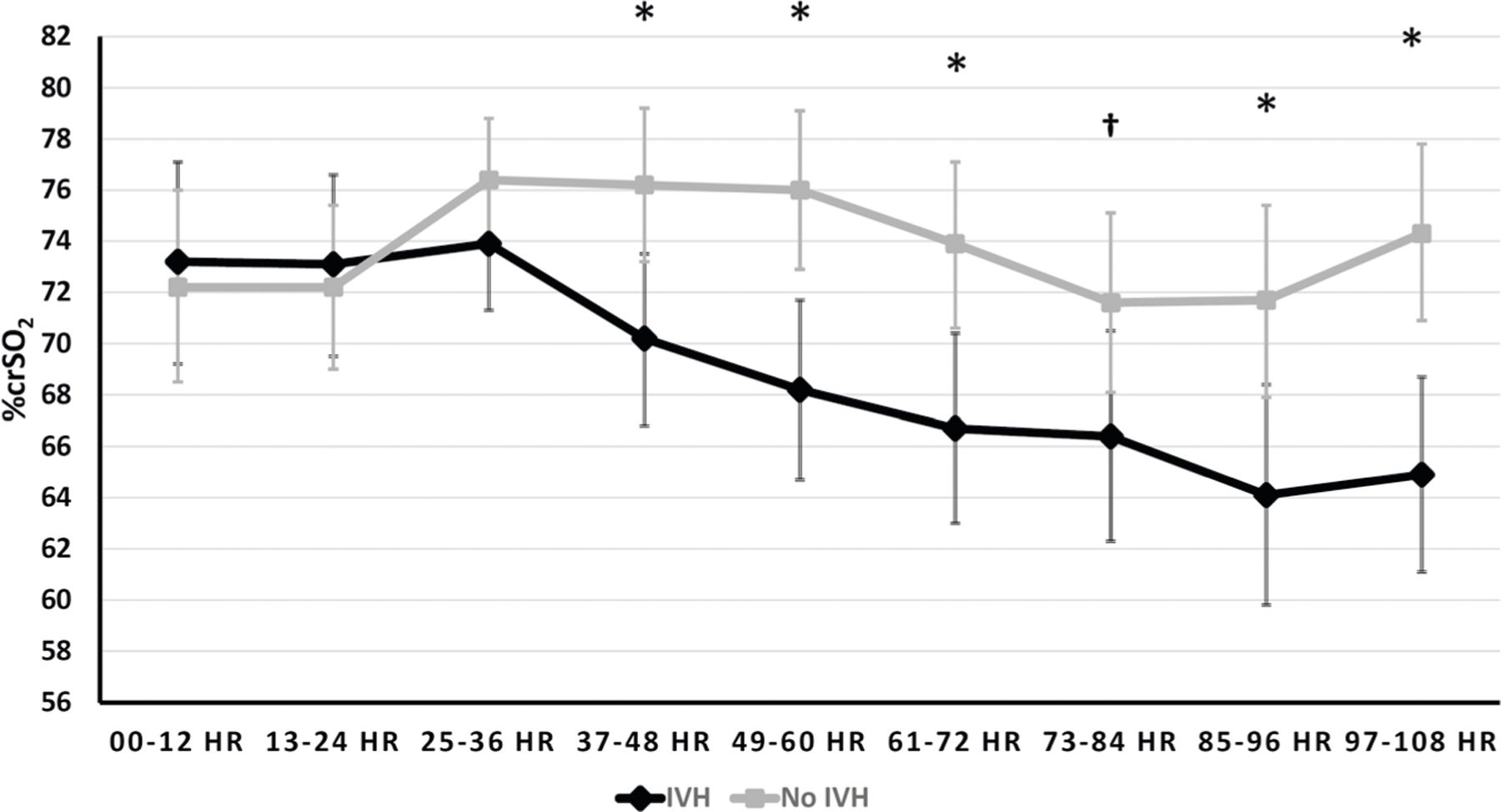
Repeated measures analysis with intrafamilial clustering among subjects using linear mixed models unadjusted for potential confounding. *p < 0.05; Least square means (95% CI): 37–48 h (IVH 70.2 (66.8,73.5), no IVH 76.2 (73.2, 79.2), p = 0.0094); 49–60 h (IVH 68.2 (64.7, 71.7); no IVH 76 (72.9, 79.1), p = 0.0015); 61–72 h (IVH 66.7 (63,70.4); no IVH 73.9 (70.6,77.1), p = 0.0053); 85–96 h (IVH 64.1 (59.8, 68.4), no IVH 71.7 (67.9,75.4), p = 0.0097); 97–108 h (IVH 64.9 (61.1, 68.7), no IVH 74.3 (70.9, 77.8), p = 0.0005). †p < 0.1; Least square means (95% CI): 73–84 h (IVH 66.4 (62.3,70.5), no IVH 71.6 (68.1,75.1), p = 0.0603).
Fig. 2. Trajectories of %crSO2 (least square means and 95% CIs) over time by IVH status.
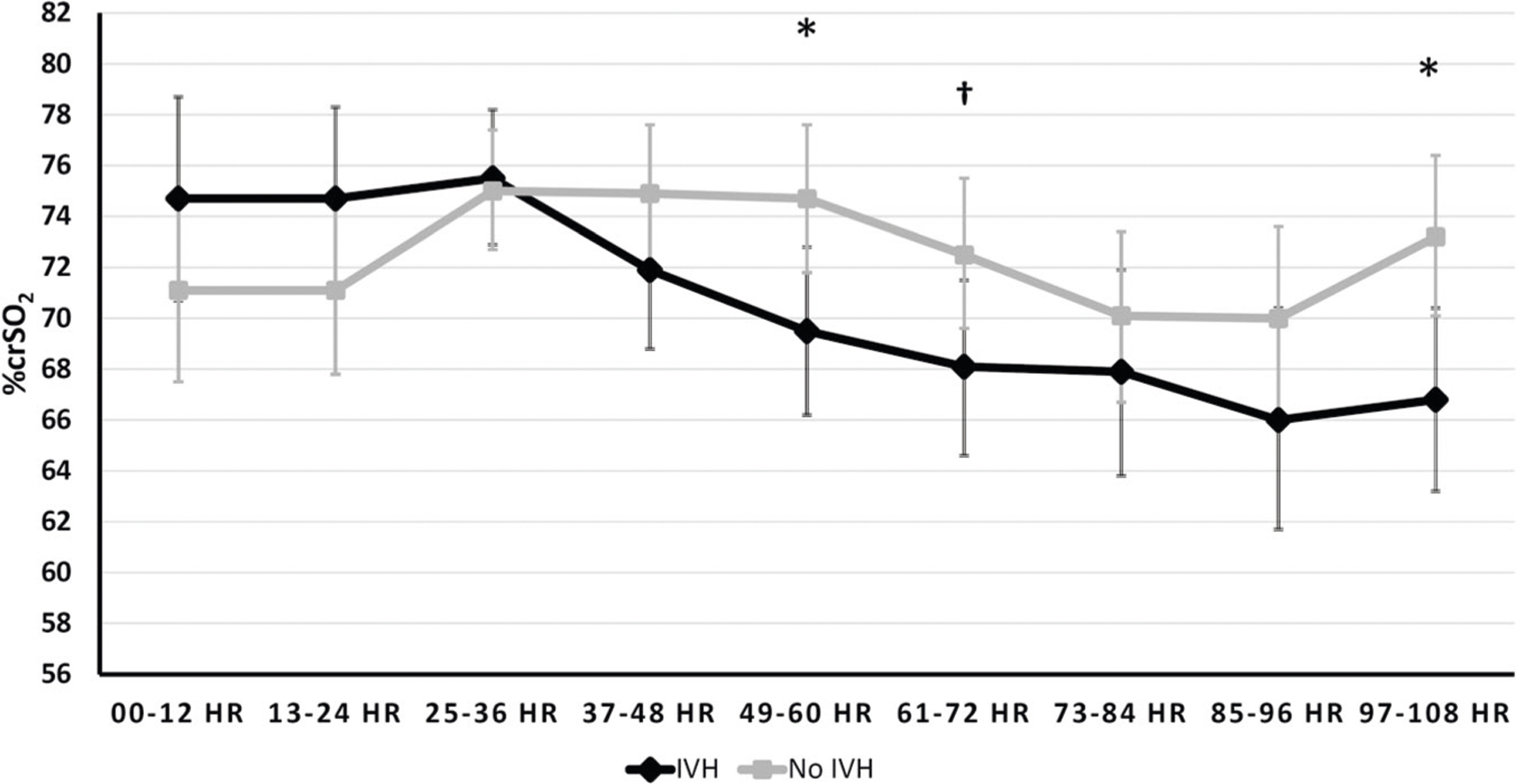
Repeated measures analysis with intrafamilial clustering among subjects using linear mixed models adjusted for potential confounding by gestational age at birth, birth weight z score & baseline hematocrit, base deficit and pCO2. *p < 0.05; Least square means (95% CI): 49–60 h (IVH 69.5 (66.2, 72.8); no IVH 74.7 (71.8, 77.6), p = 0.0232); 97–108 h (IVH 66.8 (63.2, 70.4), no IVH 73.2 (70.1, 76.4), p = 0.0104). †p < 0.1; Least square means (95% CI): 61–72 h (IVH 68.1 (64.6,71.5), no IVH 72.5 (69.6,75.5), p = 0.0584).
Receiver operating characteristic (ROC) curve
We generated an ROC curve to assess the accuracy of GM/IVH prediction between 37 and 48 h using crSO2 (Supplemental Fig. 2). The area under the ROC curve (AUC) was 0.7141 (95% CI: 0.5710, 0.8571, p = 0.0037) indicating that the model has a 71.41% chance to distinguish infants by GM/IVH status and that the estimate is significantly better than by chance (50%) alone. A crSO2 cutoff at 73% optimized the Youden index and the distance to the (0,1) point. This cutoff further gave the highest correct classification rate, 70% (along with the cutoff at 71.5%). The diagnostic metrics at the 73% threshold were as follows: sensitivity: 75%, specificity: 66%, PPV: 64.5%, and NPV: 76%.
crSO2 comparison between subgroups of IVH
In linear mixed models both unadjusted (Supplemental Fig. 3) and adjusted (not shown) for potential confounding, there were no significant differences between median crSO2 levels among infants with mild GM/IVH compared to those with severe GM/IVH. The trajectories of the two IVH groups exhibited a decrease in crSO2 values over time after 36 ho compared to the no IVH group. However, there was overlap between the mild and severe IVH trajectories and a consistent difference in crSO2 between them was not evident.
In models unadjusted (Fig. 3) and adjusted (not shown) for potential confounding, comparing early GM/IVH and late GM/IVH, crSO2 values among early GM/IVH infants were higher than those among late GM/IVH infants in the initial 24 h of life (e.g., between 13 and 24 HOL, 76.4% (early) vs. 69.5% (late), p = 0.066). This trend was also observed when the model was adjusted for potential confounding (p = 0.094).
Fig. 3. Trajectories of crSO2 (least square means and 95% CIs) over time by Early/Late/No IVH status.
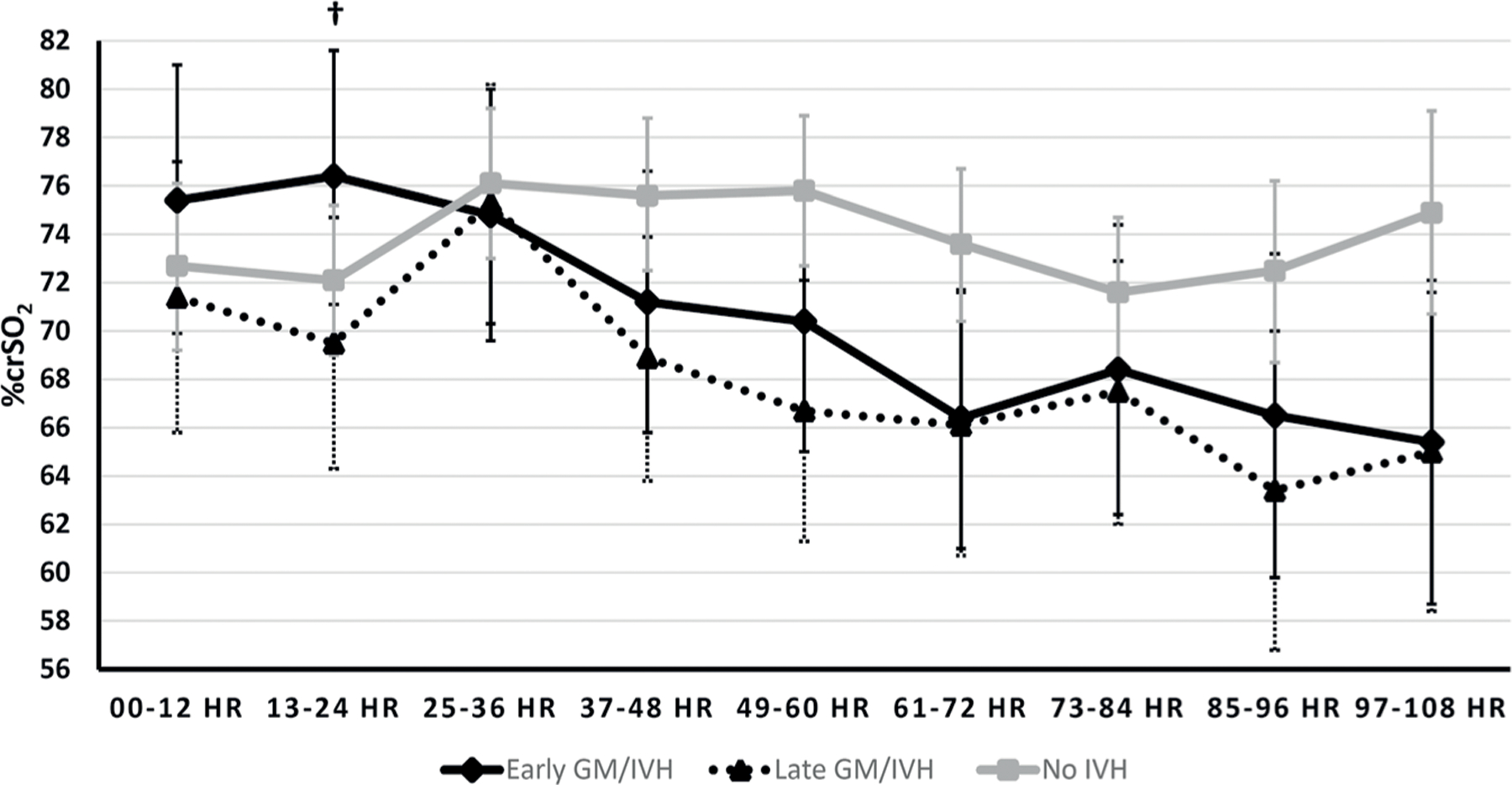
Repeated measures analysis with intrafamilial clustering among subjects using linear mixed models unadjusted for potential confounding. †p < 0.1; Least square means (95% CI): 13–24 h (Early GM/IVH 76.4 (71.1,81.6), Late GM/ IVH 69.5 (64.3,74.7), p = 0.0660).
Term equivalent brain abnormalities
MRI data for Kidokoro scorings were available for 44 (77.2%) of the 57 surviving infants among the original 62. Median (IQR) of the global brain abnormality score in our cohort was in the normal (scores 0–3)-to-mild (scores 4–7) range (3 (1–6)). The majority of MRIs (28 (63.6%)) were categorized as normal, while the rest had abnormalities categorized as mild in eight (18.2%) infants, moderate in four (9.1%), and severe in four (9.1%). There were no significant differences in crSO2 levels by Kidokoro severity category (normal, mild, moderate, severe) using linear mixed models unadjusted (Supplemental Fig. 4) or adjusted (not shown) for potential confounding overall or within any single time interval.
DISCUSSION
In a contemporary cohort of extremely preterm newborns utilizing NIRS for clinical brain monitoring, exploratory analysis showed that infants with no GM/IVH had relatively stable crSO2 in the first 108 h after birth, while those who developed GM/IVH showed a decline in their crSO2 after the second day of life.
These results are consistent with other studies that have demonstrated that GM/IVH is associated with lower crSO2 immediately after birth [19, 20] and in the first 2–15 DOL [21, 23]. Other authors who extended NIRS monitoring beyond this period have demonstrated a lower crSO2 in babies with GM/IVH up to 68 postnatal days, suggesting that these early deviations in crSO2 may persist for prolonged periods, well beyond the time when the hemorrhage actually occurred [22].
Identifying a cut-off value of crSO2 that represents increased risk for GM/IVH would be of great clinical value. One prior prospective study using crSO2 attempted to identify a risk cutoff for the period immediately after birth among preterm infants <32 weeks. Using an outcome of death or severe IVH in the first 72 h of life, the authors calculated a ROC curve and demonstrated a crSO2 threshold of 66 during the period from seven to 10 min of life, with a sensitivity of 89% and specificity of 81% in predicting poor outcome [19], although the number of infants with the outcome was very small (n = 4). In our study, we found a threshold of 73% at 37–48 h to predict GM/IVH, with a sensitivity of 75% and specificity of 66%. This difference in threshold could be explained by the fact that in our study we evaluated any degree of IVH and not severe IVH/death as in the aforementioned study. In addition, caution is necessary when using NIRS absolute values, especially when different devices and sensors are used for measurements. In the prior study, crSO2 was measured via a FORE-SIGHT Elite Absolute Tissue Oximeter by CASMED combined with EEG, so the absolute values may not be directly comparable to ours.
In our study, the severity of GM/IVH did not affect the magnitude of decline in crSO2. This finding is consistent with those from some previous studies with similar sample size to ours [21]. For example, in a case-control study of 34 preterm infants (mean GA 29 weeks) using NIRS for 2 h a day over the first 2 weeks of life, crSO2 was lower and fractional tissue oxygen extraction was higher in infants with GM/IVH throughout the first 15 days of life, regardless of the IVH grade [21]. However, other reports from larger cohorts found a negative correlation between the severity of GM/IVH and crSO2 [22, 26]. It is possible that the sample size of our cohort may have limited our ability to detect small differences in crSO2 between infants with varying severity of GM/IVH.
The mechanism of the association of GM/IVH with low crSO2 is not well defined, and it is unknown whether the association between low crSO2 and GM/IVH is causal. The low saturation very early in life prior to the development of hemorrhage may relate to low cardiac output with a resulting cerebral hypoperfusion leading to GM/IVH. Later in the first few days of life, with declining pulmonary pressure, a hemodynamically significant PDA could be associated with a significant left to right shunt and a cerebral “steal phenomenon,” leading to decreased cerebral perfusion [27–29] or fluctuations increasing risk for GM/IVH [30]. Disturbance of cerebrovascular autoregulation is thought to be a key factor in GMH/IVH as impaired autoregulation limits the buffering of fluctuations in systemic blood pressure resulting in cerebral ischemic-reperfusion and hemorrhagic injury [31, 32]. Fluctuating pressure passivity occurs in the majority of very low birth weight infants [10], and increased passivity in this population has been associated with GM/IVH [11, 14, 23]. One prior study using NIRS in the immediate postpartum period among infants born less than 32 weeks GA showed that crSO2 was lower in GM/IVH group [20] despite no differences in systemic oxygen saturation between groups, supporting the premise that clinically significant differences in crSO2 likely represent differences in CBF. In the presence of established GM/IVH, low crSO2 could also be related to impaired autoregulation with an altered brain tissue metabolism in injured tissue leading to increased oxygen extraction [22]. Alternatively, low crSO2 may be due to altered regional blood flow affecting cortical and subcortical regions in the presence of GM/IVH [33]. Finally, low crSO2 could also reflect an associated developing anemia, although we controlled for baseline hematocrit in our study.
Although we did not capture a clear sequence of an initial hypoperfusion followed by reperfusion, crSO2 values for early GM/IVH tended to be higher than for late (and no) GM/IVH in the first 24 HOL, particularly between 13 and 24 h (p = 0.066). This finding among those who developed early GM/IVH is similar to results reported from previous studies which demonstrated an increase in crSO2 prior to development of hemorrhage [14, 15, 34]. In one prospective study, newborns who developed severe GM/IVH after 12 HOL had higher crSO2 preceding the development of GM/IVH when compared to matched controls without IVH (cUS was done on admission and then daily) [14]. This finding was also reported in a small, well-designed prospective observational study of infants with no IVH who had cardiac function, CBF and IVH status monitored every 12 h for 72 h beginning within 4–6 HOL. Although this study only had five infants with GM/IVH, it observed a hypoperfusion-reperfusion pattern before development of GM/IVH. While infants without IVH had stable myocardial function and cerebral perfusion, those who developed GM/IVH had low initial stroke volume and lower crSO2 followed by improved systemic and cerebral perfusion before development of GM/IVH [15]. Similarly, a recent study using daily cUS and echocardiogram showed that although infants with GM/IVH had overall lower crSO2, a temporary rise in crSO2 was noted between the 12 and 36 h preceding GM/IVH detection. That study also showed that those with IVH had increased pressure passive circulation using the heart rate reactivity index, but there was no change in left ventricular output [34]. In our study, a trend of increased crSO2 was only observed in the early group, but not the late group. An explanation for this difference could be that the late group developed GM/IVH over a longer time period, making it difficult to detect a clear trend in crSO2. This late IVH group includes five patients who were diagnosed with GM/IVH after 108 HOL, which goes beyond the time of NIRS monitoring. In addition, this difference in trends could point to a difference in pathogenesis of GM/IVH that happens early (with more pronounced impact of hemodynamic changes) vs. late (with more impact from other comorbid factors e.g., sepsis, etc.).
We also analyzed the TE MRIs of study patients. Although low crSO2 was associated with WM injury in a previous study [22], in our study, we did not identify a relationship between crSO2 and total brain abnormality score. Although the Kidokoro score reflects WM injury as well as other causes of altered brain growth which are common in this population, these causes may not be related to NIRS measurements in the first few days of life.
A key feature of crSO2 is that it is not only a marker of perfusion, but also of oxygen delivery and extraction. Low crSO2 is associated with short and long-term adverse outcomes [35, 36]. Whether low crSO2 is a marker for GM/IVH or if it is a modifiable risk factor is not fully understood. Other authors have shown that although established practice algorithms could reduce the burden of cerebral hypoxia immediately after birth [37] and in the first few DOL [38], no improvement was noted in imaging, EEG, or biomarkers of brain injury [39, 40]. We await the results of ongoing trials to determine if correcting for cerebral hypoxia immediately after birth [41] or in the first few DOL [42] can prevent or reduce the occurrence of GM/IVH and improve neurologic outcomes.
The limitations of this study include the retrospective nature of the data collected. The objective nature of the exposure variable (crSO2), however, limits potential bias related to the retrospective design. To further mitigate these effects, we adjusted for possible confounding due to key demographic and clinical differences between groups. Although a hemodynamically significant PDA could affect crSO2 values, we did not add PDA as a confounder due to absence of consistent echocardiography findings in first few days of life. It is a strength of our study that these data were collected clinically, which demonstrates that clinical NIRS data were of adequate quality to detect differences between infants with or without IVH, highlighting the potential clinical utility of the NIRS device. Another limitation is that there was no cUS performed on admission. In most cases, there were only two cUS available in the first four DOL and nearly 50% of these with GM/IVH developed it prior to the first cUS. This infrequent imaging does not allow for an accurate estimation as to the timing of GM/IVH development. In the absence of accurate timing of IVH, a cause-effect relationship between the onset of IVH and the crSO2 changes described in the study cannot be presumed. In addition, since simultaneous systemic oxygen saturation data were unavailable, we were unable to calculate oxygen extraction. Furthermore, there was no long-term developmental follow-up of the study population. However, it is known that the degree of GMH/IVH impacts neurodevelopmental outcomes.
In summary, infants with GM/IVH exhibit divergent crSO2 trajectories in the first few DOL when compared to infants without hemorrhage. In those with GM/IVH, crSO2 levels decline after the second DOL, whereas infants without hemorrhage have stable crSO2 levels. Thus, non-invasive monitoring of crSO2 using NIRS may provide a useful bedside tool to monitor interventions aimed at prevention of GM/ IVH.
Supplementary Material
Footnotes
COMPETING INTERESTS
MEl-D discloses he is the PI of an investigator-initiated research funded by Medtronic and served once on an advisory board for Radiometer but these are not related to this work. Other authors declare that they have no competing interests.
ADDITIONAL INFORMATION
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41372-022-01447-w.
DATA AVAILABILITY
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Bell EF, Hintz SR, Hansen NI, Bann CM, Wyckoff MH, DeMauro SB, et al. Mortality, In-Hospital Morbidity, Care Practices, and 2-Year Outcomes for Extremely Preterm Infants in the US, 2013–2018. JAMA 2022;327:248–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal Outcomes of Extremely Preterm Infants From the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotardo JW, Volkmer NFV, Stangler GP, Dornelles AD, Bohrer BBA, Carvalho CG. Impact of peri-intraventricular haemorrhage and periventricular leukomalacia in the neurodevelopment of preterms: a systematic review and meta-analysis. PLoS ONE. 2019;14:e0223427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin PY, Hagan K, Fenoglio A, Grant PE, Franceschini MA. Reduced cerebral blood flow and oxygen metabolism in extremely preterm neonates with low-grade germinal matrix- intraventricular hemorrhage. Sci Rep 2016;6:25903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inder TE, Perlman JM, Volpe JJ. Chapter 24 - Preterm Intraventricular Hemorrhage/Posthemorrhagic Hydrocephalus. In: Volpe JJ, Inder TE, Darras BT, de Vries LS, du Plessis AJ, Neil JJ, et al. (eds). Volpe’s Neurology of the Newborn. 6th ed. Elsevier; 2018. pp 637–98.e621. [Google Scholar]
- 7.Pryds O Control of cerebral circulation in the high-risk neonate. Ann Neurol 1991;30:321–9. [DOI] [PubMed] [Google Scholar]
- 8.Blankenberg FG, Loh NN, Norbash AM, Craychee JA, Spielman DM, Person BL, et al. Impaired cerebrovascular autoregulation after hypoxic-ischemic injury in extremely low-birth-weight neonates: detection with power and pulsed wave Doppler US. Radiology. 1997;205:563–8. [DOI] [PubMed] [Google Scholar]
- 9.Lightburn MH, Gauss CH, Williams DK, Kaiser JR. Cerebral blood flow velocities in extremely low birth weight infants with hypotension and infants with normal blood pressure. J Pediatr 2009;154:824–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res 2007;61:467–73. [DOI] [PubMed] [Google Scholar]
- 11.O’Leary H, Gregas MC, Limperopoulos C, Zaretskaya I, Bassan H, Soul JS, et al. Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics. 2009;124:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meek JH, Tyszczuk L, Elwell CE, Wyatt JS. Low cerebral blood flow is a risk factor for severe intraventricular haemorrhage. Arch Dis Child: Fetal Neonatal Ed 1999;81:F15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child: Fetal Neonatal Ed 2000;82:F188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alderliesten T, Lemmers PMA, Smarius JJM, Van De Vosse RE, Baerts W, Van Bel F. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr 2013;162:698–704.e692 [DOI] [PubMed] [Google Scholar]
- 15.Noori S, McCoy M, Anderson MP, Ramji F, Seri I. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. J Pediatr 2014;164:264–70.e261–3 [DOI] [PubMed] [Google Scholar]
- 16.El-Dib M, Soul JS. Monitoring and management of brain hemodynamics and oxygenation. Handb Clin Neurol 2019;162:295–314. [DOI] [PubMed] [Google Scholar]
- 17.Alderliesten T, Dix L, Baerts W, Caicedo A, van Huffel S, Naulaers G, et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res 2016;79:55–64. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Chan GS, Tracy MB, Lee QY, Hinder M, Savkin AV, et al. Cerebral near-infrared spectroscopy analysis in preterm infants with intraventricular hemorrhage. Conf Proc: Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf 2011;2011:1937–40. [DOI] [PubMed] [Google Scholar]
- 19.Katheria AC, Harbert MJ, Nagaraj SB, Arnell K, Poeltler DM, Brown MK, et al. The Neu-Prem Trial: Neuromonitoring of Brains of Infants Born Preterm During Resuscitation-A Prospective Observational Cohort Study. J Pediatr 2018;198:209. e203 [DOI] [PubMed] [Google Scholar]
- 20.Baik N, Urlesberger B, Schwaberger B, Schmolzer GM, Avian A, Pichler G. Cerebral haemorrhage in preterm neonates: does cerebral regional oxygen saturation during the immediate transition matter? Arch Dis Child-Fetal Neonatal Ed 2015;100:F422–7. [DOI] [PubMed] [Google Scholar]
- 21.Verhagen EA, Ter Horst HJ, Keating P, Martijn A, Van Braeckel KNJA, Bos AF. Cerebral oxygenation in preterm infants with germinal matrix-intraventricular hemorrhages. Stroke. 2010;41:2901–7. [DOI] [PubMed] [Google Scholar]
- 22.Vesoulis ZA, Whitehead HV, Liao SM, Mathur AM. The hidden consequence of intraventricular hemorrhage: persistent cerebral desaturation after IVH in preterm infants. Pediatr Res 2021;89:869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sortica da Costa C, Cardim D, Molnar Z, Kelsall W, Ng I, Czosnyka M, et al. Changes in hemodynamics, cerebral oxygenation and cerebrovascular reactivity during the early transitional circulation in preterm infants. Pediatr Res 2019;86:247–53. [DOI] [PubMed] [Google Scholar]
- 24.Volpe JJ. Volpe’s neurology of the newborn, Sixth edition. edn. Philadelphia, PA: Elsevier; 2018. [Google Scholar]
- 25.Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am J Neuroradiol 2013;34:2208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen LC, Maroun LL, Borch K, Lou HC, Greisen G. Neonatal cerebral oxygenation is not linked to foetal vasculitis and predicts intraventricular haemorrhage in preterm infants. Acta Paediatr 2008;97:1529–34. [DOI] [PubMed] [Google Scholar]
- 27.Navikiene J, Virsilas E, Vankeviciene R, Liubsys A, Jankauskiene A. Brain and renal oxygenation measured by NIRS related to patent ductus arteriosus in preterm infants: a prospective observational study. BMC Pediatrics. 2021;21:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemmers PMA, Toet MC, Van Bel F. Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics. 2008;121:142–7. [DOI] [PubMed] [Google Scholar]
- 29.Poon WB, Tagamolila V. Cerebral perfusion and assessing hemodynamic significance for patent ductus arteriosus using near infrared red spectroscopy in very low birth weight infants. J Matern-fetal neonatal Med: Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet 2021;34:1645–50. [DOI] [PubMed] [Google Scholar]
- 30.Evans N, Kluckow M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed 1996;75:F183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee CJ, da Costa CS, Austin T, Brady KM, Czosnyka M, Lee JK. Neonatal cerebrovascular autoregulation. Pediatr Res 2018;84:602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goddard Finegold J, Michael LH. Cerebral blood flow and experimental intraventricular hemorrhage. Pediatr Res 1984;18:7–11. [PubMed] [Google Scholar]
- 33.Tortora D, Lo Russo FM, Severino M, Parodi A, Massirio P, Ramenghi LA, et al. Regional impairment of cortical and deep gray matter perfusion in preterm neonates with low-grade germinal matrix-intraventricular hemorrhage: an ASL study. Neuroradiology. 2020;62:1689–99. [DOI] [PubMed] [Google Scholar]
- 34.Cimatti AG, Martini S, Galletti S, Vitali F, Aceti A, Frabboni G, et al. Cerebral Oxygenation and Autoregulation in Very Preterm Infants Developing IVH During the Transitional Period: a pilot study. Front Pediatrics. 2020;8:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alderliesten T, van Bel F, van der Aa NE, Steendijk P, van Haastert IC, de Vries LS, et al. Low Cerebral Oxygenation in Preterm Infants Is Associated with Adverse Neurodevelopmental Outcome. J Pediatr 2019;207:109–16.e102 [DOI] [PubMed] [Google Scholar]
- 36.Pansy J, Baik N, Schwaberger B, Scheuchenegger A, Pichler-Stachl E, Avian A, et al. Cerebral hypoxia during immediate transition after birth and short term neurological outcome. Early Hum Dev 2017;110:13–5. [DOI] [PubMed] [Google Scholar]
- 37.Pichler G, Urlesberger B, Baik N, Schwaberger B, Binder-Heschl C, Avian A, et al. Cerebral Oxygen Saturation to Guide Oxygen Delivery in Preterm Neonates for the Immediate Transition after Birth: A 2-Center Randomized Controlled Pilot Feasibility Trial. J Pediatr 2016;170:73–8.e71–74 [DOI] [PubMed] [Google Scholar]
- 38.Hyttel-Sorensen S, Pellicer A, Alderliesten T, Austin T, Van Bel F, Benders M, et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: Phase II randomised clinical trial. BMJ 2015;350:g7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plomgaard AM, Hagmann C, Alderliesten T, Austin T, van Bel F, Claris O, et al. Brain injury in the international multicenter randomized SafeBoosC phase II feasibility trial: cranial ultrasound and magnetic resonance imaging assessments. Pediatr Res 2016;79:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plomgaard AM, van Oeveren W, Petersen TH, Alderliesten T, Austin T, van Bel F, et al. The SafeBoosC II randomized trial: treatment guided by near-infrared spectroscopy reduces cerebral hypoxia without changing early biomarkers of brain injury. Pediatr Res 2016;79:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pichler G, Baumgartner S, Biermayr M, Dempsey E, Fuchs H, Goos TG, et al. Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): an investigator-initiated, randomized, multi-center, multi-national, clinical trial on additional cerebral tissue oxygen saturation monitoring combined with defined treatment guidelines versus standard monitoring and treatment as usual in premature infants during immediate transition: study protocol for a randomized controlled trial. Trials. 2019;20:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen ML, Pellicer A, Gluud C, Dempsey E, Mintzer J, Hyttel-Sørensen S, et al. Cerebral near-infrared spectroscopy monitoring versus treatment as usual for extremely preterm infants: a protocol for the SafeBoosC randomised clinical phase III trial. Trials. 2019;20:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


