Abstract
Background
Although conventional non‐pharmacological and pharmacological treatments for insomnia are effective in many people, alternative therapies such as acupuncture are widely practised. However, it remains unclear whether current evidence is rigorous enough to support acupuncture for the treatment of insomnia.
Objectives
To determine the efficacy and safety of acupuncture for insomnia.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO, Dissertation Abstracts International, CINAHL, AMED, the Traditional Chinese Medical Literature Analysis and Retrieval System (TCMLARS), the World Health Organization (WHO) Trials Portal (ICTRP) and relevant specialised registers of the Cochrane Collaboration in October 2011. We screened reference lists of all eligible reports and contacted trial authors and experts in the field.
Selection criteria
Randomised controlled trials evaluating any form of acupuncture for insomnia. They compared acupuncture with/without additional treatment against placebo or sham or no treatment or same additional treatment. We excluded trials that compared different acupuncture methods or acupuncture against other treatments.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias. We used odds ratio (OR) and mean difference for binary and continuous outcomes respectively. We combined data in meta‐analyses where appropriate.
Main results
Thirty‐three trials were included. They recruited 2293 participants with insomnia, aged 15 to 98 years, some with medical conditions contributing to insomnia (stroke, end‐stage renal disease, perimenopause, pregnancy, psychiatric diseases). They evaluated needle acupuncture, electroacupuncture, acupressure or magnetic acupressure.
Compared with no treatment (two studies, 280 participants) or sham/placebo (two studies, 112 participants), acupressure resulted in more people with improvement in sleep quality (compared to no treatment: OR 13.08, 95% confidence interval (CI) 1.79 to 95.59; compared to sham/placebo: OR 6.62, 95% CI 1.78 to 24.55). However, when assuming that dropouts had a worse outcome in sensitivity analysis the beneficial effect of acupuncture was inconclusive. Compared with other treatment alone, acupuncture as an adjunct to other treatment might marginally increase the proportion of people with improved sleep quality (13 studies, 883 participants, OR 3.08, 95% CI 1.93 to 4.90). On subgroup analysis, only needle acupuncture but not electroacupuncture showed benefits. All trials had high risk of bias and were heterogeneous in the definition of insomnia, participant characteristics, acupoints and treatment regimen. The effect sizes were generally small with wide confidence intervals. Publication bias was likely present. Adverse effects were rarely reported and they were minor.
Authors' conclusions
Due to poor methodological quality, high levels of heterogeneity and publication bias, the current evidence is not sufficiently rigorous to support or refute acupuncture for treating insomnia. Larger high‐quality clinical trials are required.
Keywords: Humans, Acupressure, Acupressure/methods, Acupuncture Therapy, Acupuncture Therapy/methods, Randomized Controlled Trials as Topic, Sleep Initiation and Maintenance Disorders, Sleep Initiation and Maintenance Disorders/therapy
Plain language summary
Acupuncture for insomnia
Although conventional non‐pharmacological and pharmacological treatments for insomnia are effective in many people, alternative therapies such as acupuncture are widely practised. This review was conducted to examine the efficacy and safety of acupuncture in treating insomnia. Thirty‐three randomised controlled trials were eligible for inclusion in the review, involving 2293 participants. We considered all studies to have a high risk of bias. They were diverse in the types of participants, acupuncture treatments and sleep outcome measures used, which limited our ability to draw reliable conclusions. Currently there is a lack of high‐quality clinical evidence to inform us about the efficacy and safety of acupuncture.
Background
Description of the condition
Insomnia may be defined as a complaint of disturbed sleep in the presence of adequate opportunity and circumstance for sleep (NIH 2005). The revised edition of the International Classification of Sleep Disorders lists more than 100 differential diagnoses of insomnia (AASM 2005). The 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) defines primary insomnia as the subjective difficulty in initiating or maintaining sleep or non‐restorative sleep that lasts for at least one month. The sleep disturbance causes clinically significant distress or impairment in social, occupational or other important areas of functioning and is not associated with another mental disorder, substance‐related disorder, general medical disorder or other specific sleep disorder (APA 1994).
A review on the epidemiology of insomnia found that the prevalence of insomnia symptoms that occurred at least three nights per week or often or always occurred was between 16% and 21% (Ohayon 2002). The prevalence of insomnia symptoms with daytime consequences ranged from 9% to 15%, while 8% to 18% of the population had dissatisfaction with sleep quality and quantity. The prevalence of insomnia diagnoses according to the DSM‐IV classification was 6%. Primary insomnia was the most frequent diagnosis, with prevalence ranging between 2% and 4%, followed by insomnia related to another mental disorder, with prevalence ranging from 1% to 3% (Ohayon 2002). Insomnia may result in fatigue, irritability and impaired concentration and is associated with reduced quality of life, increased risk of traffic accidents, impaired job performance and absenteeism (Kleinman 2009; Leger 2001; Walsh 2004). The economic impact of insomnia is high, with an estimated total cost ranging from USD 30 to 35 billion annually in 1994 (Chilcott 1996). A review found that the six‐month predicted direct and indirect costs for adults with insomnia were USD 1253 greater than that for matched control without insomnia (Ozminkowski 2007). For the elderly with insomnia, it was USD 1143 greater than that for matched non‐insomniacs (Ozminkowski 2007). A more recent review found that the annual mean incremental costs to employers were USD 2053 greater for employees with insomnia compared with controls (Kleinman 2009). In addition, there is a strong relationship between insomnia and depression, anxiety disorders, other psychological disorders, alcohol and drug abuse or dependence, suicide and decreased immune functioning (Taylor 2007). Insomnia might also be a risk factor for obesity (Patel 2008), hypertension (Knutson 2009; Lanfranchi 2009; Vgontzas 2009a), diabetes (Vgontzas 2009b), cardiovascular disease (King 2008) and mortality but the data are inconclusive (Taylor 2007). It is important to evaluate and treat insomnia early, since acute untreated insomnia can progress into chronic insomnia that might be more difficult to treat (Ancoli‐Israel 2000).
Description of the intervention
The general approach in the management of insomnia is to evaluate the cause of the symptom before an appropriate treatment is proposed. The underlying medical, psychiatric or behavioural causes of insomnia are usually treated in the first place (Hajak 2000). However, it is not always possible to abolish or alleviate the primary disease process. In such situations, the approach is to focus on interventions that will positively promote sleep. Regardless of the cause of insomnia, physicians treating sleep disorders almost always counsel patients about sleep hygiene. Some key sleep hygiene instructions include regular bedtime and wake up time, avoiding daytime napping, using the bedroom only for sleep, keeping the bedroom dark, quiet, well ventilated and at a comfortable temperature throughout the night, practising a bedtime ritual, getting regular exercise each day, avoiding alcohol and nicotine four to six hours before bedtime, avoiding caffeine entirely or limiting caffeine use to no more than three cups no later than 10 a.m., and avoiding heavy meals and strenuous exercise in the evening (Zarcone 2000).
A recent review found types of psychological and behavioural treatment including cognitive behavioural therapy, stimulus control therapy, sleep restriction therapy, relaxation training and paradoxical intention to have empirical evidence for the treatment of insomnia (Morin 2006). However, such non‐pharmacological therapies have remained largely under‐utilised in primary care, perhaps because they are time consuming and require significant training for effective implementation (Krystal 2004).
Many prescription and over‐the‐counter medications are available for treating insomnia. Benzodiazepine receptor agonists are the only agents currently approved by the US Food and Drug Administration (FDA) for the treatment of insomnia. There are two broad groups of benzodiazepine receptor agonists: benzodiazepines and the non‐benzodiazepine hypnotics (e.g. zaleplon, zolpidem, zopiclone, eszopiclone). Although insomnia is often a chronic condition, the FDA has only approved eszopiclone for use without a specified time limit. The other medications have approved use limited to 35 days or less. The limits on prescription of these medications are due to concerns about its potential for abuse, dependence and adverse effects, such as residual daytime sedation, cognitive impairment and poor motor co‐ordination (NIH 2005). Faced with the limitations of pharmacological and psychological treatments of insomnia, many alternative therapies have been used to treat insomnia and acupuncture is one of the commonly used treatment modalities.
Acupuncture is well accepted by many people and is widely used in treating various illnesses including back pain, arthritis, headache, asthma, digestive disorders, alcohol and substance dependence, and other psychiatric disorders (Johansson 1993; Vickers 1999). Acupuncture is one of the most commonly used complementary therapies in many Western countries (Thomas 2003; Zollman 1999).
Acupuncture is a procedure in which specific body areas, the acupoints (also called meridian points), are pierced with fine needles for therapeutic purposes. Acupoints are points on the body surface that when stimulated are thought to cause therapeutic effects. Acupuncture is one of the major modalities of treatment in Traditional Chinese Medicine. Its theory of diagnosis and treatment is based on the systems of medicine and philosophy of ancient China and its use in China can be traced back more than 2000 years (Wu 1996). Acupuncture involves complex theories of regulation of Yin and Yang forces, Qi (air), blood and body fluids. According to Traditional Chinese Medicine an imbalance in the Yin and Yang forces of the body, or an excess or a deficiency of Qi, blood or body fluids, are the main causes of pain or diseases. Acupuncture treats illness by recreating the balance between the Yin and Yang forces and restoration of normal Qi, blood and body fluids through stimulation of different acupoints which govern different parts of the body and their interaction (Maciocia 1989).
Apart from traditional needle acupuncture, various forms of acupuncture have been developed, including electroacupuncture, laser acupuncture, acupressure, auricular therapy, magnetic acupressure and transcutaneous electrical acupoints stimulation (TEAS). Electroacupuncture is a form of acupuncture in which acupuncture needles are attached to a device that generates continuous electric pulses, generating a small electric current that flows between pairs of needles. Laser acupuncture employs laser beam to stimulate the acupoints instead of stimulation using fine needles. Acupressure is a technique that involves firm manual pressure on the acupoints. Auricular therapy employs acupuncture needles, seeds or magnetic peals to stimulate the acupoints located on the auricles. Magnetic acupressure employs small magnets to provide pressure and magnetic stimulation of acupoints. TEAS combines the technique of both acupuncture and transcutaneous electrical nerve stimulation by using electrode pads placed on the skin to stimulate the acupoints.
How the intervention might work
The exact physiological or biochemical mechanisms by which acupuncture might improve sleep are not completely understood. However, many studies have demonstrated that acupuncture can cause multiple biological responses (Ulett 1998). A review article has summarised how the nervous system, neurotransmitters and endogenous substances could respond to needling stimulation and electroacupuncture, thereby mediating pain relief and other therapeutics (Ma 2004). Acupuncture causes stimulation of the opiodergic neurons in rats resulting in increased concentrations of beta‐endorphin which might have a sleep promoting effect (Cheng 2009). Acupuncture is also found to increase melatonin secretion, which is associated with improvement in sleep (Spence 2004). Stimulation of certain acupoints is found to increase nitric oxide in the brain and the blood, which is associated with sleep improvement clinically (Li 2003). Acupuncture can also cause up‐regulation of an important inhibitory neurotransmitter, gamma‐aminobutyric acid (GABA) that may promote sleep (Fu 2009). Acupuncture also results in modulation of the autonomic nervous system, affecting both sympathetic and parasympathetic activities, which may be associated with its sleep‐promoting effect (Huang 2011).
Why it is important to do this review
Acupuncture is widely used for treatment of insomnia. Anecdotal reports suggest that acupuncture may improve sleep and relieve insomnia. Many clinical trials have also been performed to study the efficacy of acupuncture for insomnia. It remains uncertain whether the existing evidence is rigorous enough to reach a definitive conclusion. For this reason, we undertook a systematic review of randomised controlled trials of acupuncture therapy for insomnia in 2006. However, this question is still unanswered and we performed an update of the review to look for new evidence.
Objectives
To assess the effects of acupuncture therapy for people with insomnia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials were included in the review. We excluded quasi‐randomised trials.
Studies comparing acupuncture or its variants with at least one control group that used no treatment, placebo treatment or sham treatment were included.
Parallel‐group or cross‐over designs were included.
Types of participants
People of any age and gender with insomnia explicitly documented by standardised measures (e.g. the Pittsburgh Sleep Quality Index (Buysse 1989)), objective measures in a sleep laboratory (e.g. polysomnography, actigraphy) or by reports/diaries kept by patients, partners, other informants or nursing staff; or patients with insomnia diagnosed by standard diagnostic criteria such as the Diagnostic and Statistical Manual of Mental Disorders (APA 1994), International Classification of Sleep Disorders (AASM 2005) or International Classification of Diseases (WHO 1992), or with a complaint of sleep difficulties. We also included participants with comorbid psychiatric disorders or physical conditions.
Types of interventions
Trials evaluating all forms of acupuncture therapy including acupressure, laser acupuncture, electroacupuncture, auricular therapy, magnetic acupressure or transcutaneous electrical acupoints stimulation were included in the review, regardless of the number of times of treatment or the length of treatment period. The different forms of acupuncture were grouped together in comparisons.
The control interventions could be no treatment, placebo acupuncture or sham acupuncture. Placebo acupuncture referred to a needle attached to the skin surface (not penetrating the skin but at the same acupoints) (Van Tulder 2004). Sham acupuncture referred to a needle placed in an area close to but not in acupoints (Van Tulder 2004) or subliminal skin electrostimulation via electrodes attached to the skin (SCSSS 1999).
Comparisons investigated were:
acupuncture alone versus no treatment;
acupuncture alone versus placebo or sham treatment;
acupuncture adjunctive to other treatment versus other treatment alone;
acupuncture adjunctive to other treatment versus placebo or sham treatment adjunctive to other treatment.
'Other treatment' mentioned in point 3 and 4 above referred to any treatment, including medication, psychological treatment or alternative complementary treatment, provided that both the intervention and the control groups received the same treatment.
We excluded trials comparing only different forms of acupuncture or acupuncture with other forms of treatment, since these studies could not yield the net effect of acupuncture and could not inform the conclusion as to whether acupuncture per se was efficacious or not.
Types of outcome measures
Primary outcomes
Frequency of improvement in sleep quality (proportion of participants satisfied with insomnia improvement), measured as a dichotomous outcome of improvement. Since improvement in sleep quality is subjective, for the purposes of this review it could be variably defined, with or without the use of a sleep score or other sleep parameters (e.g. sleep onset latency, total sleep duration, total wake time, wake after sleep onset).
Secondary outcomes
-
Sleep parameters, as measured by sleep diary or other objective measurements, such as actigraphy, electroencephalography or polysomnography
Sleep onset latency
Total sleep duration
Total wake‐time
Wake after sleep onset (WASO)
Nocturnal and early morning wakening (defined by the trialist)
Sleep efficiency (ratio of time asleep to time in bed)
Sleep scores, as measured by standardised scales related to sleep, e.g. the Pittsburgh Sleep Quality Index (Buysse 1989)
Daytime functioning, as measured by attentional tasks tests, self report using a standardised measure, e.g. the Stanford Sleepiness Scale (Hoddes 1973), the Epworth Sleepiness Scale (Johns 1991)
Quality of life, as measured by validated scales
Frequency of adverse effects
We divided outcomes, where possible, into immediate post‐treatment, medium‐term (3 to 12 months) and long‐term (more than 12 months).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (to September 2010), MEDLINE (1966 to October 2010), EMBASE (1980 to October 2010), PsycINFO (1887 to October 2010), Dissertation Abstracts International (1861 to October 2010), CINAHL (1982 to October 2010), AMED (the Allied and Complementary Medicine Database, 1985 to October 2010) and TCMLARS (Traditional Chinese Medical Literature Analysis and Retrieval System, 1984 to October 2010) which is a database of Chinese biomedical research literature. We also searched relevant clinical trials and research databases including the WHO International Clinical Trial Registry Platform (ICTRP) (October 2010), the Trials Register of the Cochrane Complementary Medicine Field (September 2010) and the Cochrane Collaboration Depression, Anxiety and Neurosis Group Controlled Trials Register (CCDANCTR) (September 2010).
The search terms for each database are stated in the Appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5). We applied no language restrictions. We translated relevant non‐English articles for this review.
Searching other resources
We searched the reference lists of all relevant reports for further studies (although none were identified by this process). In addition, we contacted colleagues and experts in the field to identify additional unpublished or ongoing studies. We counted multiple publications reporting the same group of participants (or their subsets) as one single study.
Data collection and analysis
Selection of studies
Two review authors independently examined titles and abstracts retrieved from the search and selected all potentially relevant studies. We obtained copies of these articles and the same review authors reviewed them independently against the inclusion criteria used in each study. Review authors were not blinded to the names of the authors, institutions or journal of publication. We resolved all disagreements by consensus.
Data extraction and management
The review authors then extracted data from included trials and assessed trial quality independently. We resolved all disagreements by consensus.
We extracted the following data:
-
Study methods
Design (e.g. parallel or cross‐over design)
Randomisation method (including list generation)
Method of allocation concealment
Blinding method
Stratification factors used if stratified randomisation was employed
-
Participants
Inclusion/exclusion criteria
Number (total/per group)
Age and sex distribution
Specific diagnosis/diagnostic subtypes
Associated physical or neuropsychiatric diseases
Duration of disorder
Previous treatments
-
Intervention and control
Type of acupuncture
Details of treatment regime including duration of treatment
Type of control
Details of control treatment including drug dosage
Details of co‐interventions
Washout period in cross‐over design
-
Follow‐up data
Duration of follow‐up
Dates of treatment withdrawal and reasons for treatment withdrawal
Withdrawal rates
Outcome data as described above in the Types of outcome measures section
-
Analysis data
Methods of analysis (intention‐to‐treat/per‐protocol analysis)
Comparability of groups at baseline (yes/no)
Statistical techniques
Data were entered into the Review Manager 5 software (RevMan 2011) by one review author and then checked by the second review author.
Assessment of risk of bias in included studies
Two review authors independently carried out assessment of risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We resolved all disagreements by consensus.
Sequence generation
We assessed the method used to generate the allocation sequence to determine whether it was truly random and produced comparable groups. We judged whether the allocation sequence was adequately generated.
Ratings: low risk of bias, high risk of bias and unclear risk of bias.
Allocation concealment
We assessed the method used to conceal allocation sequence to determine whether the allocation could have been predicted or known prior to or during recruitment. We judged whether allocation was adequately concealed.
Ratings: low risk of bias, high risk of bias and unclear risk of bias.
Blinding
We assessed any measures used to blind participants, personnel and outcome assessors to determine whether these parties were aware of the treatment allocation prior to or during treatment. We judged whether knowledge of the allocated intervention was adequately prevented during the study.
Ratings: low risk of bias, high risk of bias and unclear risk of bias.
Incomplete outcome data
We assessed data on attrition and exclusions and reasons to determine whether they would introduce bias. We judged whether incomplete data were dealt with adequately.
Ratings: low risk of bias, high risk of bias and unclear risk of bias.
We judged there to be high risk of bias if any of the following occurred.
Reasons for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is enough to induce clinically relevant bias in the intervention effect estimate.
For continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in the observed effect size.
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Selective outcome reporting
We assessed reporting of outcomes to determine whether there was selective reporting by investigators which might introduce bias. We judged whether reports of the study were free of suggestion of selective outcome reporting.
Ratings: low risk of bias, high risk of bias and unclear risk of bias.
We judged there to be high risk of bias if any of the following occurred.
Not all of the study's pre‐specified primary outcomes had been reported.
One or more primary outcomes was reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting was provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Other sources of bias
We also assessed whether the study contained other problems that could put it at a high risk of bias.
Ratings: low risk of bias, high risk of bias and unclear risk of bias.
We judged there to be high risk of bias if any of the following occurred:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problems.
Summary of biases
We summarised the overall risk of bias into one of the three following categories.
A. Low risk of bias: low risk of bias was found in all areas. B. Moderate risk of bias: unclear risk of bias was found in one or more areas but no area was assessed to carry high risk of bias. C. High risk of bias: high risk of bias was found in one or more areas.
Measures of treatment effect
We summarised data using odds ratio (OR) with 95% confidence intervals (CI) for dichotomous outcomes. We used mean difference (MD) with 95% CI for continuous outcomes, analysing with the inverse variance method. All analyses included all participants in the treatment groups to which they were allocated, provided that data were available.
Unit of analysis issues
When cross‐over trials were included in the meta‐analysis, we needed to use the correct unit of analysis to avoid bias. Where appropriate, the results of the cross‐over trials would be combined with results of parallel‐group trials. When the results of cross‐over trials were combined in meta‐analyses with results from parallel trials, we would use the inverse variance methods recommended by Elbourne (Elbourne 2002). We would use the presented data within the first phase only if data available from a cross‐over trial were restricted.
If there were two different control groups in a trial of parallel‐group design, e.g. one sham control and one no treatment control, pair‐wise comparison results would be separately reported under different comparisons, e.g. acupuncture versus sham control and acupuncture versus no treatment, as stated in the Types of interventions section above. If there were two different intervention groups, e.g. one acupuncture group and one electroacupuncture group, pair‐wise comparison results would be reported under different subgroups of intervention in a particular comparison, and their results would not be combined into a single summary measure, but only subgroup summaries would be presented, to preserve the identification of different intervention subgroups, as well as to avoid incorrect unit of analysis.
Dealing with missing data
We contacted authors of included studies to supply missing information on study methods, participants, intervention and control, follow‐up data, outcome data and statistical summary indices such as means and standard deviations. We assessed missing data and dropouts/attrition for each included study, and we assessed the extent to which the results/conclusions of the review could be altered by the missing data in sensitivity analysis. For dichotomous outcomes, we would impute the missing data in the best‐case scenario and the worst‐case scenario to assess whether the missing data could have caused clinically relevant bias in the result. For continuous outcomes, we would impute the missing data as the most extreme values observed in the trial (also as best and worst‐case scenarios) or as two standard deviations away from the means if extreme values were not available, to assess whether the missing data could have caused clinically relevant bias in the result.
Assessment of heterogeneity
We assessed clinical heterogeneity by noting the difference in the distribution of important participant factors between trials (age, gender, specific diagnosis/diagnostic subtypes, duration of disorder, associated diseases), and assessed methodological heterogeneity by noting different trial design factors (randomisation concealment, blinding, losses to follow‐up, treatment type, co‐interventions). We assessed statistical heterogeneity by examining the I2 statistic (Deeks 2009), a quantity that describes approximately the proportion of variation in point estimates due to heterogeneity rather than sampling error.
Thresholds for the interpretation of I2 could be misleading, since the importance of inconsistency depends on several factors. A rough guide to interpretation is as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
In addition, we employed the Chi2 test of homogeneity to determine the strength of evidence that the heterogeneity is genuine. We considered a P value < 0.1 significant.
Assessment of reporting biases
We generated funnel plots (effect size against standard error) if sufficient studies (more than five) were found for the same outcome. Asymmetry could be due to publication bias, but could also be due to a relationship between trial size and effect size. In the event that a relationship was found, clinical diversity of the studies would have been examined (Egger 1997).
If there were multiple publications reporting on the same trial, only one set of data was included and analysed. We contacted authors for information about multiple publications in case of doubts.
Language bias might occur if inclusion of studies in a systematic review was limited by certain language of publication. There was no language restriction in the current review. However, we have not exhaustively searched for studies published in certain languages in local journals not indexed in the databases that we searched and language bias might still have occurred.
Location bias might occur as different trials might have different accessibility and accessibility might be associated with effect size.
Citation bias might occur as some studies were more likely to be cited than others and therefore more likely to be included in the systematic review.
Data synthesis
Where the interventions were the same or similar enough, we synthesised results in a meta‐analysis. We used the random‐effects model in the meta‐analysis since there was substantial heterogeneity in the included studies.
Subgroup analysis and investigation of heterogeneity
Analysis of a number of subgroups could lead to misleading conclusions (Deeks 2009) and were best kept to a minimum. If data permitted, we planned to conduct subgroup analyses for different age groups, diagnostic subtypes or severity of disease to assess whether the treatment effects were different in different subgroups. However, insufficient data reported in the studies meant subgroup analysis was not performed in this review.
Sensitivity analysis
We planned to conduct sensitivity analyses to assess the impact of study quality, provided that sufficient studies were available. These included:
excluding those using inadequate methods of allocation concealment;
excluding those with a lower than 70% follow‐up rate;
excluding those in which insomnia was not diagnosed with standardised criteria.
To assess the effect of dropouts on the outcome, we performed another sensitivity analysis including the following.
1. Best‐case scenario:
Dichotomous outcome: we assumed all the dropouts from the treatment group had a positive outcome and all the dropouts from the control group had a negative outcome
Continuous outcome: we assumed all the dropouts from the treatment group to have the best outcome values or at two standard deviations above the mean and assumed all the dropouts from the control group to have the worst outcome values or at two standard deviations below the mean.
2. Worst‐case scenario:
Dichotomous outcome: we assumed all the dropouts from the treatment group had a negative outcome and all the dropouts from the control group had a positive outcome
Continuous outcome: we assumed all the dropouts from the treatment group to have the worst outcome values or at two standard deviations above the mean and assumed all the dropouts from the control group to have the best outcome values or at two standard deviations below the mean.
Results
Description of studies
Results of the search
Our previous search in 2006 retrieved 1119 results while the current updated search obtained an additional 934 results (a total of 2053 results) on electronic search of the databases, and we identified six additional articles from references of relevant results. After duplicates were removed, there were 1485 articles. We screened the titles and abstracts of these against the inclusion and exclusion criteria for study selection and excluded 1366 references based on titles or abstracts alone. We obtained and assessed the full text of the remaining 119 articles for eligibility. We excluded 78 of these papers describing 76 studies (two studies were published in two papers each) with reasons stated in the table of Characteristics of excluded studies. Five of the remaining studies were ongoing or with results not published yet and were described in the table of Characteristics of ongoing studies. The remaining 33 studies described by 36 papers (three studies were published in two papers each) fulfilled the inclusion criteria and were included for further review. They are described in the table of Characteristics of included studies. The flow of records is summarised in Figure 1.
1.
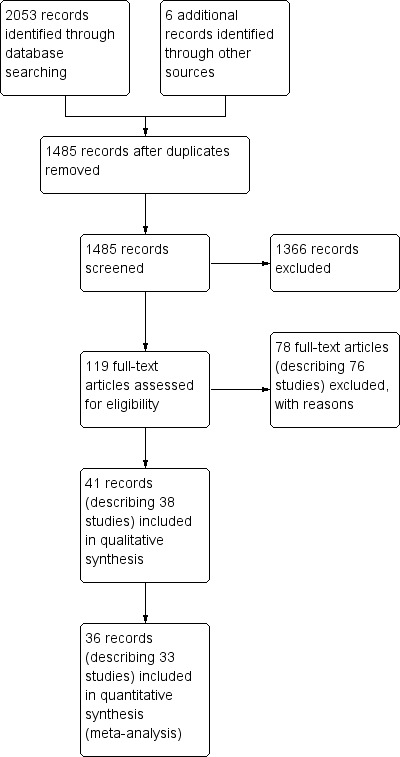
Study flow diagram.
Included studies
We had tried to contact authors of all included studies regarding missing information that was required for data analyses and assessment of risk of bias. Authors of two included studies (Nordio 2008; Sun 2010a) provided additional useful information.
Populations
The included trials were performed in China (n = 19) (Chen 2009; Cui 2003; Du 2007; Guo 2009; Jian 2005; Jin 2003; Lai 2010; Li 2005a; Lin 2007; Liu 2001; Lu 1998; Luo 2006; Lv 2007; Ma 2006a; Tang 2007a; Tian 2006; Ye 2008; Zhao 2010; Zhu 2005), Taiwan (n = 4) (Chen 1999; Sun 2010a; Tsay 2003; Tsay 2004), Korea (n = 4) (Hwang 2007; Kim 2004; Lee 2009; Sok 2005), Hong Kong (n = 2) (Suen 2002; Yeung 2009a), Brazil (n = 1) (Kaiser‐Pagliarini 2009), USA (n = 1) (Hisghman 2006), Italy (n = 1) (Nordio 2008) and Iran (n = 1) (Reza 2010). The trials included a total of 2293 participants. Twenty‐seven trials divided the participants into one intervention and one control group, whilst two trials included two intervention and one control groups (Suen 2002; Tsay 2004) and four trials included one intervention and two control groups (Chen 1999; Kaiser‐Pagliarini 2009; Tsay 2003; Zhao 2010). The target populations in these trials were diverse. The participants' age ranged from 15 to 98 years and the duration of insomnia varied from six months to 19 years. Six trials recruited only elderly patients with different age criteria (Chen 1999; Hisghman 2006; Liu 2001; Reza 2010; Sok 2005; Suen 2002), while one trial recruited only post‐menopausal women (Kaiser‐Pagliarini 2009), and one trial recruited only perimenopausal women (Zhao 2010). The remaining trials did not limit to a particular age group. Four trials recruited only hospitalised inpatients (Jin 2003; Kim 2004; Lee 2009; Tang 2007a), two trials recruited only nursing home residents (Reza 2010; Sok 2005) and one trial recruited only residents of long‐term care facilities (Sun 2010a). Most trials recruited patients without major co‐morbid conditions, while one trial recruited only patients with depression (Li 2005a), one trial recruited only patients with schizophrenia (Ma 2006a), one trial recruited only patients with heroin withdrawal (Zhu 2005), two trials recruited only patients with end‐stage renal disease (Tsay 2003; Tsay 2004) and two trials recruited only patients post‐stroke (Kim 2004; Lee 2009).
The diagnostic criteria for insomnia were variable among the included studies. The diagnosis of insomnia was based on the Chinese Classification of Mental Disorder in five trials (Chen 2009; Guo 2009; Lai 2010; Li 2005a; Lin 2007), Diagnostic and Statistical Manual‐IV (DSM‐IV) in three trials (Kaiser‐Pagliarini 2009; Ye 2008; Yeung 2009a) and International Classification of Disease‐10 (ICD‐10) in one trial (Zhu 2005). The diagnosis of insomnia was based solely on the score on the Pittsburgh Sleep Quality Index (PSQI) in six trials (Chen 1999; Hwang 2007; Reza 2010; Sun 2010a; Tsay 2003; Tsay 2004), Insomnia Severity Index in two trials (Kim 2004; Lee 2009), Spiegel's questionnaires in one trial (Tian 2006), and sleep efficiency estimation in two trials (Cui 2003; Suen 2002). In the remaining 13 trials the diagnosis of insomnia was made on patients' complaints alone without reference to any diagnostic criteria (Du 2007; Hisghman 2006; Jian 2005; Jin 2003; Liu 2001; Lu 1998; Luo 2006; Lv 2007; Ma 2006a; Nordio 2008; Sok 2005; Tang 2007a; Zhao 2010).
Interventions
Interventions tested in the trials included needle acupuncture alone in three trials (Kim 2004; Kaiser‐Pagliarini 2009; Lee 2009), needle acupuncture plus benzodiazepines in four trials (Cui 2003; Luo 2006; Ma 2006a; Ye 2008), needle acupuncture plus zolpidem in one trial (Guo 2009), needle acupuncture plus different Chinese herbs in seven trials (Du 2007; Jian 2005; Liu 2001; Lu 1998; Lv 2007; Tian 2006; Zhao 2010), electroacupuncture alone in three trials (Tsay 2004; Yeung 2009a; Zhu 2005), electroacupuncture plus fluoxetine in one trial (Li 2005a), electroacupuncture plus Chinese herbs in one trial (Lai 2010), acupressure alone in 12 trials (Chen 1999; Hwang 2007; Jin 2003; Lin 2007; Nordio 2008; Reza 2010; Sok 2005; Suen 2002; Sun 2010a; Tang 2007a; Tsay 2003; Tsay 2004), and magnetic acupressure alone in two trials (Hisghman 2006; Suen 2002). The control group received sham or placebo treatments in 12 trials (Chen 1999; Hwang 2007; Kaiser‐Pagliarini 2009; Kim 2004; Lee 2009; Lin 2007; Nordio 2008; Reza 2010; Suen 2002; Sun 2010a; Tsay 2003; Yeung 2009a), and additional treatment alone the same as that in the intervention group or no specific treatment in 24 trials (Chen 1999; Chen 2009; Cui 2003; Du 2007; Guo 2009; Jian 2005; Jin 2003; Kaiser‐Pagliarini 2009; Lai 2010; Li 2005a; Liu 2001; Lu 1998; Luo 2006; Lv 2007; Ma 2006a; Reza 2010; Sok 2005; Tang 2007a; Tian 2006; Tsay 2003; Tsay 2004; Ye 2008; Zhao 2010; Zhu 2005).
Acupoints chosen and acupuncture administrative methods and duration of therapy were highly variable in the 33 included trials and only two trials reported the same acupuncture regimen (Kim 2004; Lee 2009). Nine trials allowed some flexibility in the acupuncture methods or use of additional acupoints on top of the protocol acupoints set for the participants, depending on individual clinical situations (Du 2007; Guo 2009; Jian 2005; Jin 2003; Lin 2007; Liu 2001; Lv 2007; Ma 2006a; Tian 2006). The most frequently used acupoints were: Shenmen on hands (HT7) (Chen 1999; Cui 2003; Guo 2009; Jian 2005; Kim 2004; Lai 2010; Lee 2009; Liu 2001; Lv 2007; Ma 2006a; Nordio 2008; Reza 2010; Sun 2010a; Tian 2006; Tsay 2003; Yeung 2009a; Zhu 2005), Neiguan (PC6) (Cui 2003; Kim 2004; Lee 2009; Lu 1998; Lv 2007; Ma 2006a; Reza 2010; Tian 2006; Zhu 2005), Baihui (GV20) (Chen 1999; Chen 2009; Cui 2003; Jian 2005; Li 2005a; Lv 2007; Ma 2006a; Tian 2006; Yeung 2009a), and Shenmen on ears (Chen 1999; Chen 2009; Hisghman 2006; Jin 2003; Lin 2007; Suen 2002; Tang 2007a; Tsay 2003). The total treatment duration ranged from two days (Kim 2004; Lee 2009) to 10 weeks (Zhu 2005).
Outcomes
The duration of follow‐up for outcome assessment ranged from two days to 12 weeks. Sixteen trials reported the frequency of insomnia improvement as outcome, with variable definitions of different degrees of improvement (Chen 2009; Cui 2003; Du 2007; Guo 2009; Jian 2005; Lai 2010; Li 2005a; Lin 2007; Liu 2001; Lu 1998; Luo 2006; Lv 2007; Ma 2006a; Tang 2007a; Tian 2006; Zhao 2010). Several validated scales measuring insomnia were used as outcomes in some trials, including the Pittsburgh Sleep Quality Index (PSQI) (Chen 1999; Hwang 2007; Kaiser‐Pagliarini 2009; Lai 2010; Lin 2007; Luo 2006; Nordio 2008; Reza 2010; Tsay 2003; Tsay 2004; Ye 2008; Yeung 2009a; Zhao 2010), Insomnia Severity Index (ISI) (Hisghman 2006; Kim 2004; Lee 2009; Yeung 2009a), Athens Insomnia Scale (AIS) (Kim 2004; Lee 2009; Sun 2010a), Sleep Quality Scale (SQS) (Hwang 2007) and Morning Questionnaire (MQ) (Kim 2004). Numerical rating scales of sleep quality which had not been validated previously were also used in three trials (Hisghman 2006; Sok 2005; Yeung 2009a). Some trials reported post‐treatment or improvement in sleep parameters, including total sleep duration (Hisghman 2006; Hwang 2007; Jin 2003; Kim 2004; Li 2005a; Lin 2007; Lv 2007; Suen 2002; Yeung 2009a), sleep onset latency (Hwang 2007; Kim 2004; Lin 2007; Suen 2002; Yeung 2009a), number of awakenings or arousal index (Hisghman 2006; Hwang 2007; Lin 2007; Suen 2002), total wake time (Suen 2002), wake after sleep onset (Suen 2002; Yeung 2009a) and sleep efficiency (Hisghman 2006; Hwang 2007; Lin 2007; Yeung 2009a).
In most sleep trials these parameters were obtained by self report, except two trials which employed electroencephalography (Hwang 2007; Lin 2007) and two trials which employed actigraphy (Suen 2002; Yeung 2009a). Polysomnographic parameters were used as outcomes in one trial but no details were provided (Kaiser‐Pagliarini 2009).
One study reported daytime functioning as measured by Sheehan Disability Index (SDI) (Yeung 2009a). Four studies reported quality of life or general health scores (Hisghman 2006; Kaiser‐Pagliarini 2009; Nordio 2008; Tsay 2003). Adverse effects were reported in three trials (Guo 2009; Hisghman 2006; Kim 2004).
Other outcome measures included Self rated Depression Scale (SDS) (Chen 2009; Lai 2010), Self rated Anxiety Scale (SAS) (Chen 2009; Lai 2010), Beck Depression Inventory (BDI) (Kaiser‐Pagliarini 2009; Tsay 2004), State Trait Anxiety Inventory (STAI) (Kaiser‐Pagliarini 2009; Nordio 2008), Hospital Anxiety and Depression Scale (HADS) (Yeung 2009a), Piper Fatigue Scale (PFS) (Tsay 2004), Credibility of Treatment Rating Scale (CTRS) (Yeung 2009a), Rating Scale for Protracted Withdrawal Symptoms (Zhu 2005), other cognitive tests (Kaiser‐Pagliarini 2009) and the frequency of body comfort (Chen 1999). However, these were not included in our predefined secondary outcomes and were not analysed in the current systematic review.
Excluded studies
We excluded a total of 76 studies after full texts were obtained. The most common reason for exclusion was absence of placebo or sham or no treatment control group (Chen 2007; Ding 2006; Ding 2008; Dong 2008; Fan 2006; Gao 1995; Gong 2009; He 2009; Hong 2005; Hou 2005; Huang 2007; Huang 2009a; Hui 2006; Ju 2009; Kang 2006; Li 2005b; Li 2007a; Li 2007b; Li 2007c; Li 2010; Lian 1990; Liu 2000; Liu 2006; Liu 2007; Lu 2002; Luo 1993; Ma 2006b; Ni 2006; Pan 2005; Qi 2008; Qiu 1999; Sang 2004; Su 2004; Tang 2007b; Wang 1993; Wang 2002; Wang 2003a; Wang 2003b; Wang 2004; Wang 2008; Wei 2006; Wei 2010; Weng 2007; Xiong 2003; Xuan 2007; Yan 2010; Zhang 2000; Zhang 2003b; Zhang 2005; Zhang 2008; Zhang 2010; Zhou 2010; Zhu 2002; Zou 2008). Other reasons included not being a comparative clinical trial (Cummings 2003; Gao 1997; Phillips 2001; Shang 2000; Shen 2004; Shi 2003; Suen 2003; Wang 2000; Xu 1997; Yao 1999; Yu 1997; Zhang 2002; Zhang 2003a), use of quasi‐randomisation (Chen 2003; Da Silva 2005; Sjoling 2008; Wang 2006; Zhong 2008), non‐random allocation of participants (Becker‐Carus 1985; Lu 2008; Ruan 2001) and primary complaint not being insomnia (Cohen 2003).
Risk of bias in included studies
We assessed all 33 included trials to be of poor methodological quality with high risk of bias. The sample size varied from 22 to 200 participants (10 to 100 participants in treatment groups and 11 to 100 participants in control groups). None of the studies reported sample size calculations, essential for ensuring adequate statistical power. The distribution of bias is shown in Figure 2.
2.
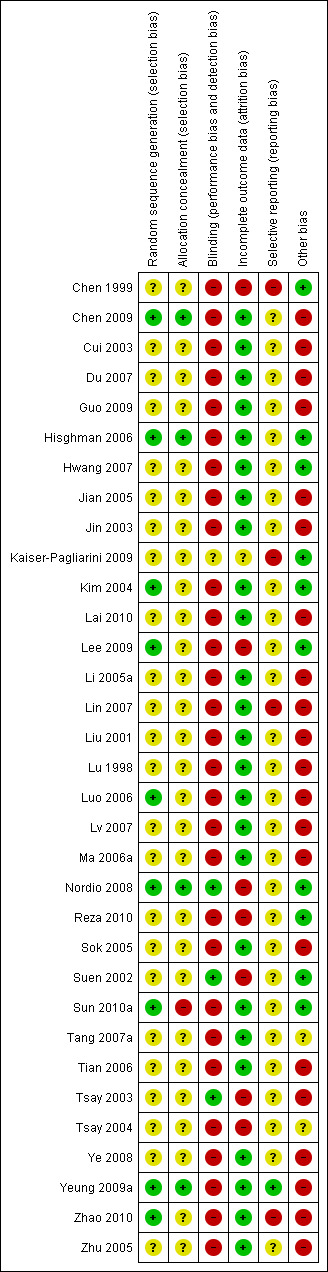
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only nine of the 33 studies described the randomisation procedure and sequence generation (Chen 2009; Hisghman 2006; Kim 2004; Lee 2009; Luo 2006; Nordio 2008; Sun 2010a; Yeung 2009a; Zhao 2010). Sequence generation and allocation concealment was therefore unlikely to be adequate in the remaining 24 studies. We considered only four studies (Chen 2009; Hisghman 2006; Nordio 2008; Yeung 2009a) to have adequate allocation concealment.
Blinding
Only three studies (Nordio 2008; Suen 2002; Tsay 2003) blinded the physicians, the participants and outcome assessors. One study (Kaiser‐Pagliarini 2009) was described as double‐blind (both physicians and patients were blinded) but details of blinding were not provided and therefore it was unclear whether blinding was adequate. Five studies were single‐blind (only patients were blinded) (Chen 1999; Hisghman 2006; Hwang 2007; Sun 2010a; Yeung 2009a). However, the placebo or sham treatment in some of these studies might not be able to ensure adequate blinding. Two studies only blinded the outcome assessors (Kim 2004; Lee 2009). The remaining studies either explicitly denied blinding or did not describe blinding and were therefore considered likely to be unblinded since the intervention involved an invasive procedure. Significant bias was likely to occur in the subjective report of insomnia outcomes where participants were not blinded to treatments. Performance bias might occur if care takers were not blinded. Assessment bias was also possible if the outcome assessors were not blinded to the treatment groups.
Incomplete outcome data
In 23 studies there were no dropouts (Chen 2009; Cui 2003; Du 2007; Guo 2009; Hisghman 2006; Hwang 2007; Jian 2005; Jin 2003; Kaiser‐Pagliarini 2009; Lai 2010; Li 2005a; Lin 2007; Liu 2001; Lu 1998; Luo 2006; Lv 2007; Ma 2006a; Sok 2005; Tang 2007a; Tian 2006; Ye 2008; Zhao 2010; Zhu 2005). In these studies, either the authors stated no dropouts explicitly or the results presented clearly showed the complete number of participants. In three studies (Kim 2004; Sun 2010a; Yeung 2009a), dropouts constituted a small proportion of participants with reasons provided and sensitivity analyses on best and worst‐case scenarios showed that dropouts were unlikely to alter the conclusion of these trials. In three studies (Chen 1999; Suen 2002; Tsay 2003), dropouts were excluded from analyses but reasons were not provided or clearly described and might cause bias. In two studies (Nordio 2008; Reza 2010), dropouts were excluded but the reasons might be related to treatment and caused bias. In two studies (Lee 2009; Tsay 2004), sensitivity analyses on best and worst‐case scenarios suggested that dropouts might have affected the conclusion.
Selective reporting
In one study (Kaiser‐Pagliarini 2009), only PSQI scores among all different outcomes were provided. Such selective reporting might have caused bias. In two studies (Lin 2007; Zhao 2010), PSQI was one of the secondary outcomes but only some sub‐scores which were statistically significantly different between the treatment groups were reported; some sub‐scores were not reported. Such selective reporting might have caused bias. In one study (Chen 1999), data on the frequency of improvement in sleep quality were not available for the no treatment control group and might have caused bias. In most other studies (Chen 2009; Cui 2003; Du 2007; Guo 2009; Hisghman 2006; Hwang 2007; Jian 2005; Jin 2003; Kim 2004; Lai 2010; Lee 2009; Li 2005a; Liu 2001; Lu 1998; Luo 2006; Lv 2007; Ma 2006a; Nordio 2008; Reza 2010; Sok 2005; Suen 2002; Sun 2010a; Tang 2007a; Tian 2006; Tsay 2003; Tsay 2004; Ye 2008; Zhu 2005) the trial protocols were not available to judge whether there might have been selective reporting of outcomes. In the remaining trial done by one of the current review authors (Yeung 2009a), all pre‐specified outcomes were reported.
Other potential sources of bias
In four studies (Chen 2009; Tsay 2003; Ye 2008; Yeung 2009a), the baseline characteristics of the intervention and the control groups might not be comparable and might introduce bias. In two studies (Tang 2007a; Tsay 2004), information was not available to judge whether the baseline characteristics of the intervention and the control groups were comparable. In nine studies (Du 2007; Guo 2009; Jian 2005; Jin 2003; Lin 2007; Liu 2001; Lv 2007; Ma 2006a; Tian 2006), acupoints chosen or acupuncture regimen were variable among the intervention group or the control group and might introduce bias. In 18 studies (Chen 2009; Cui 2003; Du 2007; Guo 2009; Jian 2005; Jin 2003; Lai 2010; Li 2005a; Liu 2001; Lu 1998; Luo 2006; Lv 2007; Ma 2006a; Sok 2005; Tian 2006; Ye 2008; Zhao 2010; Zhu 2005) no sham or placebo control was used and the placebo effect might cause bias with over‐estimation of the effect size. If acupuncture is compared to no treatment and the result shows that acupuncture is more effective than no treatment, we are actually not sure whether this is just an attention placebo effect or a genuine effect of acupuncture (Koog 2011). Thus the conclusion that acupuncture is effective is potentially biased. In addition, the effect size of acupuncture compared to no treatment is likely to be greater than the effect size of acupuncture compared to placebo acupuncture, because the potential placebo effect of acupuncture has not been controlled for (Koog 2011).
Effects of interventions
Comparison 1: Acupuncture versus no treatment
There were 10 trials comparing acupuncture versus no treatment. Eight trials evaluated acupressure (Chen 1999; Chen 2009; Jin 2003; Reza 2010; Sok 2005; Tang 2007a; Tsay 2003; Tsay 2004), one trial evaluated needle acupuncture (Kaiser‐Pagliarini 2009) and one trial evaluated electroacupuncture (Zhu 2005). One of the 10 trials also had a third group testing electroacupuncture (Tsay 2004). Two trials (Kaiser‐Pagliarini 2009; Zhu 2005) did not provide useful information on our pre‐specified outcomes.
Primary outcome
1.1 Frequency of improvement in sleep quality
The pooled results of two trials on acupressure (Chen 2009; Tang 2007a) showed that more participants in the intervention group receiving acupressure had improvement compared to the control group (odds ratio (OR) 13.08, 95% confidence interval (CI) 1.79 to 95.59, P = 0.03) (Analysis 1.1; Figure 3). Assuming the probability of improvement in the control group ranged from 0.35 to 0.6 as occurred in the included trials, the risk ratios for improvement would range from 1.58 to 2.5, indicating moderate likelihood of some benefit from acupressure. However, there was substantial heterogeneity between the trials (I2 = 86%, Chi2 test P = 0.008), which might be explained by the difference in participants, treatment regimens and definition of improvement in sleep quality.
1.1. Analysis.
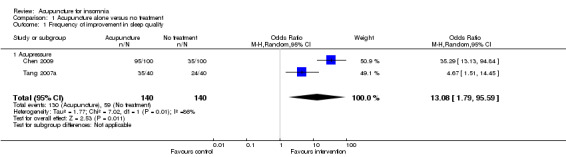
Comparison 1 Acupuncture alone versus no treatment, Outcome 1 Frequency of improvement in sleep quality.
3.
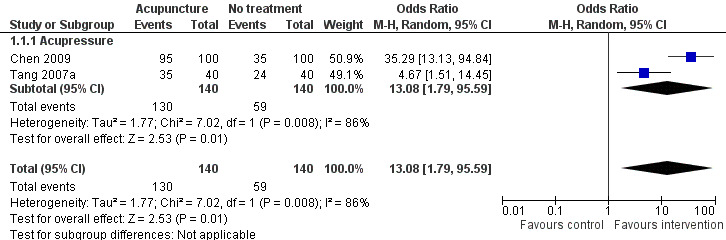
Forest plot of comparison: 1 Acupuncture alone versus no treatment, outcome: 1.1 Frequency of improvement in sleep quality.
Secondary outcomes
1.2 Sleep parameters
Total sleep duration (hours)
One trial (Jin 2003) reported that the intervention group treated with acupressure had longer total sleep duration after treatment compared to the control group (mean difference (MD) 0.80, 95% CI 0.14 to 1.46, P = 0.02) (Analysis 1.2). However, the difference was less than one hour and might not be clinically relevant.
1.2. Analysis.
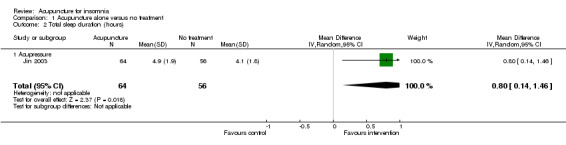
Comparison 1 Acupuncture alone versus no treatment, Outcome 2 Total sleep duration (hours).
1.3 Sleep score
1.3.1 Total score on the Pittsburgh Sleep Quality Index (PSQI)
The pooled results of five trials on acupressure (Chen 1999; Chen 2009; Reza 2010; Tsay 2003; Tsay 2004) showed that the total score on the PSQI was better in the intervention group compared to the control group (MD ‐3.87, 95% CI ‐5.14 to ‐2.60, P < 0.00001) (Analysis 1.3). The mean reduction of PSQI score was approximately one standard deviation which indicated a moderate improvement that might be clinically relevant. However, the results were substantially heterogeneous among the trials (I2 = 72%, Chi2 test P = 0.006), which might be explained by the difference in participants, treatment regimen and the ways outcome was measured.
1.3. Analysis.
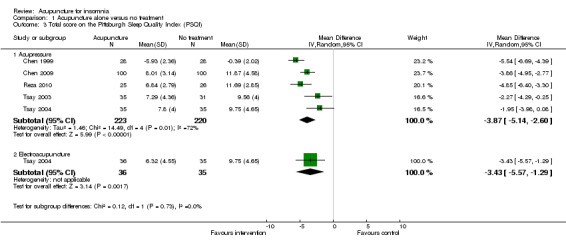
Comparison 1 Acupuncture alone versus no treatment, Outcome 3 Total score on the Pittsburgh Sleep Quality Index (PSQI).
One of the trials also evaluated electroacupuncture (Tsay 2004) and reported a better post‐treatment total score on the PSQI in the intervention group compared to the control group (MD ‐3.43, 95% CI ‐5.57 to ‐1.29, P = 0.002). The difference indicated a moderate improvement that might be clinically relevant.
1.3.2 Sleep score
One study on acupressure (Sok 2005) reported that the intervention group had a better sleep score after treatment compared to the control group (MD 30.65, 95% CI 30.52 to 30.78, P < 0.00001) (Analysis 1.4). The difference appeared to represent tremendous benefit as the sleep score in the intervention group almost doubled the pre‐treatment score while it remained similar before and after treatment in the control group. However, the standard deviation of the sleep score appeared disproportionately small in each group, and we were uncertain whether the sleep score in this study was a validated measure.
1.4. Analysis.
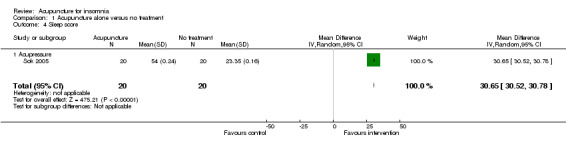
Comparison 1 Acupuncture alone versus no treatment, Outcome 4 Sleep score.
1.4 Daytime functioning
No study in this comparison reported this outcome.
1.5 Quality of life
1.5.1 Scores on the SF‐36
One trial on acupressure (Tsay 2003) reported that the intervention group had some improvement in the physical component score on the SF‐36 (MD 3.57, 95% CI ‐0.51 to 7.65, P = 0.09) (Analysis 1.5). However, the confidence interval was wide and the benefit was inconclusive. The difference was just about half of one standard deviation and might not be clinically relevant. There was similar improvement in the mental component score on the SF‐36 which was inconclusive and unlikely to be clinically relevant (MD 3.69, 95% CI ‐1.13 to 8.51, P = 0.13) (Analysis 1.6).
1.5. Analysis.
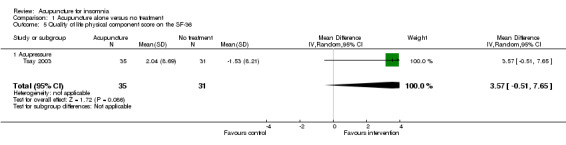
Comparison 1 Acupuncture alone versus no treatment, Outcome 5 Quality of life physical component score on the SF‐36.
1.6. Analysis.
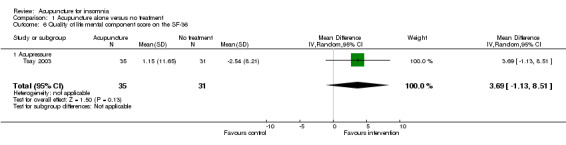
Comparison 1 Acupuncture alone versus no treatment, Outcome 6 Quality of life mental component score on the SF‐36.
1.6 Frequency of adverse effects
None of the included studies reported adverse effects.
Comparison 2: Acupuncture versus placebo or sham acupuncture
There were 13 trials comparing acupuncture versus placebo or sham acupuncture, including eight trials on acupressure (Chen 1999; Hwang 2007; Lin 2007; Nordio 2008; Reza 2010; Suen 2002; Sun 2010a; Tsay 2003), three trials on needle acupuncture (Kaiser‐Pagliarini 2009; Kim 2004; Lee 2009), one trial on electroacupuncture (Yeung 2009a) and one trial on magnetic acupressure (Hisghman 2006). One of these trials had a third group testing magnetic acupressure (Suen 2002). One trial (Kaiser‐Pagliarini 2009) did not provide useful information on our pre‐specified outcomes.
Primary outcome
2.1 Frequency of improvement in sleep quality
The pooled results of two trials on acupressure (Chen 1999; Lin 2007) showed that the intervention group was more likely to have improvement in sleep quality compared to the control group (OR 6.62, 95% CI 1.78 to 24.55, P = 0.005) (Analysis 2.1; Figure 4). Assuming the probability of improvement in the control group ranged from 0.038 to 0.67 as occurred in the included trials, the risk ratios for improvement would range from 1.39 to 5.46, indicating mild to moderate likelihood of some benefit from acupressure. There was no significant heterogeneity between the trials (I2 = 0%, Chi2 test P = 0.91).
2.1. Analysis.
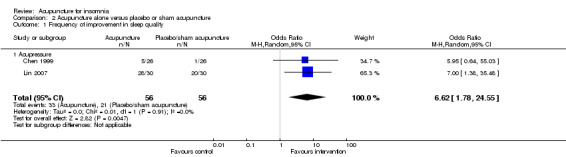
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 1 Frequency of improvement in sleep quality.
4.
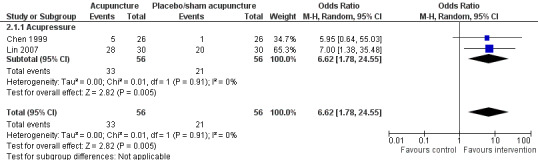
Forest plot of comparison: 2 Acupuncture alone versus placebo or sham acupuncture, outcome: 2.1 Frequency of improvement in sleep quality.
Secondary outcomes
2.2 Sleep parameters
Sleep onset latency (minutes)
One study on needle acupuncture (Kim 2004) reported this outcome and found that the intervention group had shorter sleep onset latency compared to the control group (MD ‐59.30, 95% CI ‐156.02 to 37.42, P = 0.23) (Analysis 2.2). Although the difference of about one hour in sleep onset latency might be clinically relevant, the benefit was inconclusive as the confidence interval included both positive and negative effects.
2.2. Analysis.
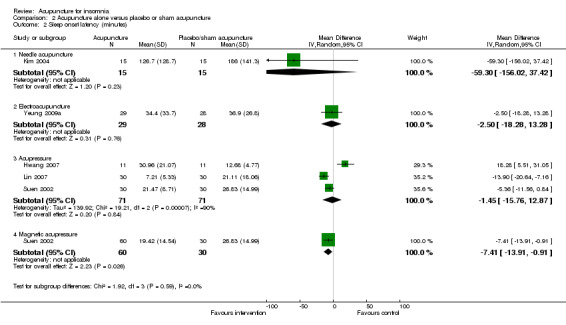
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 2 Sleep onset latency (minutes).
The trial on electroacupuncture (Yeung 2009a) reported no clinically relevant difference between the intervention and the control groups in sleep onset latency after treatment (MD ‐2.50, 95% CI ‐18.28 to 13.28, P = 0.76) (Analysis 2.2).
The pooled results of three trials on acupressure (Hwang 2007; Lin 2007; Suen 2002) also showed no clinically relevant difference between the intervention and the control groups in sleep onset latency after treatment (MD ‐1.45, 95% CI ‐15.76 to 12.87, P = 0.84) (Analysis 2.2). The results were considerably heterogeneous (I2 = 90%, Chi2 test P < 0.0001), which might be explained by the difference in the participants, acupressure regimen and measurement of sleep onset latency.
One trial also evaluated magnetic acupressure (Suen 2002) and reported that sleep onset latency was shorter after treatment in the intervention group compared to the control group (MD ‐7.41, 95% CI ‐13.91 to ‐0.91, P = 0.03) (Analysis 2.2). However, the difference of seven minutes was not clinically relevant.
Total sleep duration (hours)
The trial on needle acupuncture (Kim 2004) showed that total sleep duration was longer after treatment in the intervention group compared to the control group (MD 1.43, 95% CI 0.43 to 2.43, P = 0.005) (Analysis 2.3). The difference of more than one hour in total sleep duration might be clinically relevant.
2.3. Analysis.
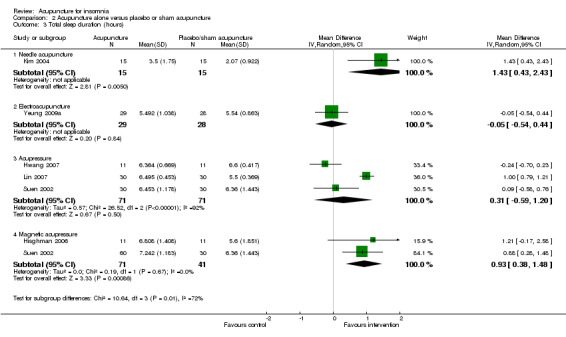
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 3 Total sleep duration (hours).
However, the trial on electroacupuncture (Yeung 2009a) reported no clinically relevant difference between the intervention and the control groups in total sleep duration after treatment (MD ‐0.05, 95% CI ‐0.54 to 0.44, P = 0.84) (Analysis 2.3).
The pooled results of three trials on acupressure (Hwang 2007; Lin 2007; Suen 2002) showed no clinically relevant difference in total sleep duration between the intervention and the control groups (MD 0.31, 95% CI ‐0.59 to 1.20, P = 0.50) (Analysis 2.3). The results were considerably heterogeneous (I2 = 92%, Chi2 test P < 0.00001), which might be explained by the difference in the participants, acupressure regimen and measurement of total sleep duration.
The pooled results of two trials on magnetic acupressure (Hisghman 2006; Suen 2002) showed that total sleep duration was longer after treatment in the intervention group compared to the control group (MD 0.93, 95% CI 0.38 to 1.48, P = 0.0009) (Analysis 2.3). The difference of less than one hour in total sleep duration might not be clinically relevant. There was no significant heterogeneity between the trial results (I2 = 0%, Chi2 test P = 0.67).
Total wake time (minutes)
One trial (Suen 2002) reported shorter total wake time after treatment in patients who received acupressure compared to the control group (MD ‐25.80, 95% CI ‐69.84 to 18.24, P = 0.25) (Analysis 2.4). The difference was below half an hour and probably not clinically relevant. The benefit was also inconclusive as the confidence interval included both positive and negative effects.
2.4. Analysis.
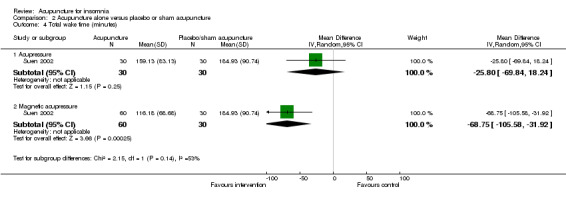
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 4 Total wake time (minutes).
The same trial (Suen 2002) also compared magnetic acupressure with placebo acupressure and found that the intervention group had shorter total wake time (MD ‐68.75, 95% CI ‐105.58 to ‐31.92, P = 0.0003) (Analysis 2.4). The difference was more than one hour which was probably clinically relevant.
Wake after sleep onset (minutes)
One trial on electroacupuncture (Yeung 2009a) reported shorter wake after sleep onset in the intervention group compared to the control groups (MD ‐33.60, 95% CI ‐74.16 to 6.96, P = 0.10) (Analysis 2.5). The clinical relevance of the modest difference was uncertain. The benefit was also inconclusive as the confidence interval included both positive and negative effects.
2.5. Analysis.
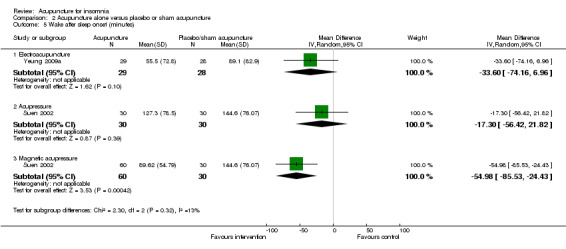
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 5 Wake after sleep onset (minutes).
Another trial on acupressure (Suen 2002) found no clinically relevant difference in wake after sleep onset between the intervention and the control groups (MD ‐17.30, 95% CI ‐56.42 to 21.82, P = 0.39) (Analysis 2.5).
The same trial (Suen 2002) also compared magnetic acupressure with placebo acupressure and found that the intervention group had shorter wake after sleep onset (MD ‐54.98, 95% CI ‐85.53 to ‐24.43, P = 0.0004) (Analysis 2.5). The difference of nearly one hour might be clinically relevant.
Number of awakenings
The pooled results of two trials on acupressure (Lin 2007; Suen 2002) showed a lower number of awakenings after treatment in the intervention group compared to the control group (MD ‐4.1, 95% CI ‐6.7 to ‐1.5, P = 0.002) (Analysis 2.6). The difference was modest and of uncertain clinical relevance. There was some heterogeneity in the results (I2 = 27%, Chi2 test P = 0.24), which might not be important.
2.6. Analysis.
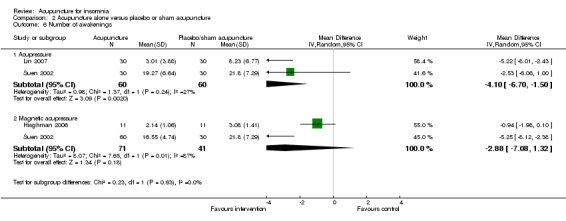
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 6 Number of awakenings.
The pooled results of two trials on magnetic acupressure (Hisghman 2006; Suen 2002) showed a lower number of awakenings after treatment in the intervention group compared to the control group (MD ‐2.88, 95% CI ‐7.08 to ‐1.32, P = 0.18) (Analysis 2.6). The difference was small and probably not clinically relevant. There was substantial heterogeneity in the results (I2 = 87%, Chi2 test P = 0.006), which might be due to differences in participants, treatment regimen and measurement methods.
Arousal index (number of arousals per hour)
One trial on acupressure (Hwang 2007) reported no clinically relevant differences in arousal index between in the intervention group compared to the control group (MD 0.08, 95% CI ‐7.37 to 7.53, P = 0.98) (Analysis 2.7).
2.7. Analysis.
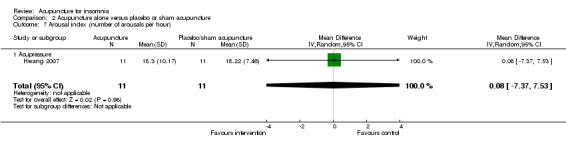
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 7 Arousal index (number of arousals per hour).
Sleep efficiency (%)
One trial on electroacupuncture (Yeung 2009a) reported that the post‐treatment sleep efficiency was higher in the intervention group compared to the control group (MD 7.50, 95% CI 1.39 to 13.61, P = 0.02) (Analysis 2.8). The difference was just modest and might not be clinically relevant.
2.8. Analysis.
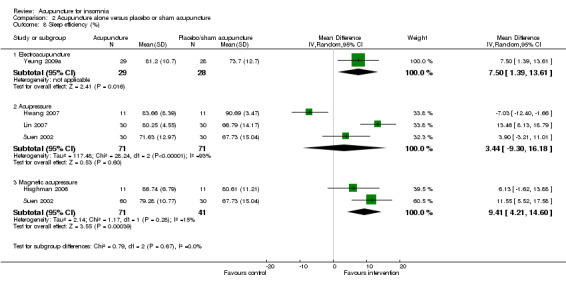
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 8 Sleep efficiency (%).
The pooled results of three trials on acupressure (Hwang 2007; Lin 2007; Suen 2002) showed no clinically relevant difference in the post‐treatment sleep efficiency between the intervention and the control groups (MD 3.44, 95% CI ‐9.30 to 16.18, P = 0.60) (Analysis 2.8). The results were considerably heterogeneous (I2 = 93%, Chi2 test P < 0.00001). The heterogeneity might be explained by the difference in the participants, acupressure regimen and measurement of sleep efficiency.
The pooled results of two trials on magnetic acupressure (Hisghman 2006; Suen 2002) showed that the post‐treatment sleep efficiency was higher in the intervention group compared to the control group (MD 9.41, 95% CI 4.21 to 14.60, P = 0.0004) (Analysis 2.8). The difference was just modest with doubtful clinical relevance. There was no significant heterogeneity in the results (I2 = 15%, Chi2 test P = 0.28).
2.3 Sleep score
Total score on the Pittsburgh Sleep Quality Index (PSQI)
The pooled results of one trial on electroacupuncture (Yeung 2009a) and six trials on acupressure (Chen 1999; Hwang 2007; Lin 2007; Nordio 2008; Reza 2010; Tsay 2003) showed that the intervention group had a better PSQI total score compared to the control group (MD ‐2.11, 95% CI ‐3.39 to ‐0.83, P = 0.001) (Analysis 2.9). However, the difference was small and unlikely to be clinically relevant. There was substantial heterogeneity between the trials (I2 = 73%, Chi2 test P = 0.001), which might be explained by the difference in the participants, acupuncture regimen and the ways outcome was measured.
2.9. Analysis.
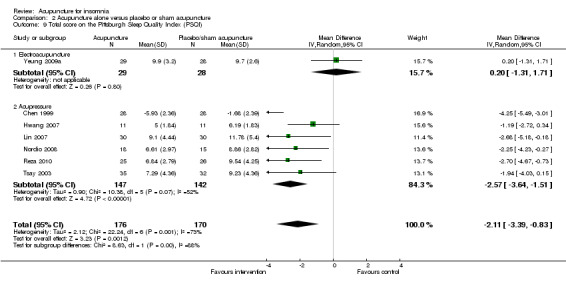
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 9 Total score on the Pittsburgh Sleep Quality Index (PSQI).
Total score on the Insomnia Severity Index (ISI)
The pooled result of two trials on needle acupuncture (Kim 2004; Lee 2009) and one trial on electroacupuncture (Yeung 2009a) showed that the intervention group had a better ISI score after treatment compared to the control group (MD ‐3.38, 95% CI ‐6.24 to ‐0.52, P = 0.02) (Analysis 2.10). The difference was just modest with uncertain clinical relevance. There was substantial heterogeneity between the results (I2 = 72%, Chi2 test P = 0.03), which might be explained by the difference in the participants and acupuncture regimen.
2.10. Analysis.
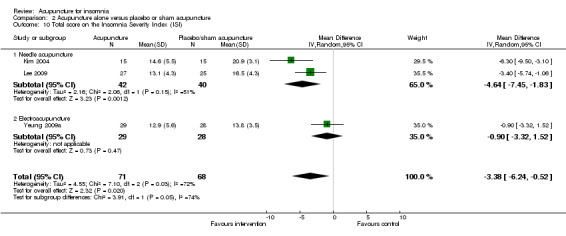
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 10 Total score on the Insomnia Severity Index (ISI).
Total score on the Athens Insomnia Scale (AIS)
The pooled result of two trials on needle acupuncture (Kim 2004; Lee 2009) and one trial on acupressure (Sun 2010a) showed that the intervention group had a better AIS score after treatment compared to the control group (MD ‐2.39, 95% CI ‐4.69 to ‐0.09, P = 0.04) (Analysis 2.11). The difference was small and probably not clinically relevant. There was substantial heterogeneity between the trial results (I2 = 69%, Chi2 test P = 0.04), which might be explained by the difference in the participants and acupuncture regimen.
2.11. Analysis.
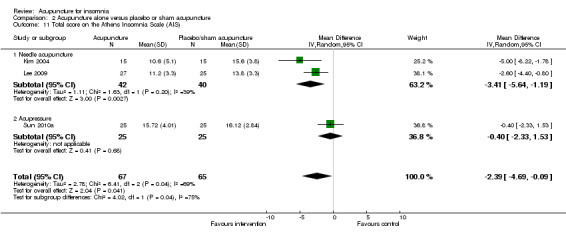
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 11 Total score on the Athens Insomnia Scale (AIS).
Total score on the Sleep Quality Scale (SQS)
One trial on acupressure (Hwang 2007) reported no clinically relevant difference between the intervention and the control group in total score on the SQS (MD ‐7.91, 95% CI ‐17.16 to 1.34, P = 0.09) (Analysis 2.12).
2.12. Analysis.
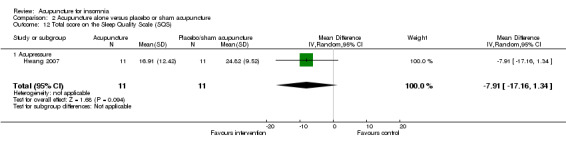
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 12 Total score on the Sleep Quality Scale (SQS).
Quality of sleep score on the Morning Questionnaire (MQ)
One trial on needle acupuncture (Kim 2004) reported that the intervention group had a better quality of sleep score on the MQ compared to the control group (MD 34.60, 95% CI 19.06 to 50.12, P < 0.0001) (Analysis 2.13). The difference was moderate and probably of clinical relevance.
2.13. Analysis.
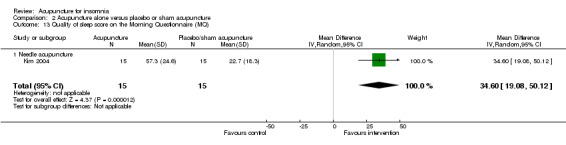
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 13 Quality of sleep score on the Morning Questionnaire (MQ).
Sleep quality score from sleep diary
One trial on electroacupuncture (Yeung 2009a) reported no clinically relevant difference in post‐treatment sleep quality score from sleep diary between the intervention and the control groups (MD 0.03, 95% CI ‐0.20 to 0.26, P = 0.80) (Analysis 2.14).
2.14. Analysis.
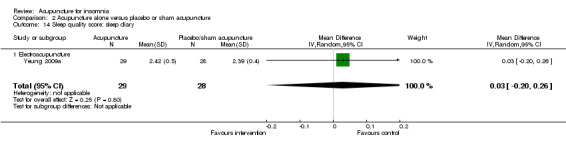
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 14 Sleep quality score: sleep diary.
2.4 Daytime functioning
Scores on the Sheehan Disability Index (SDI)
The same trial (Yeung 2009a) reported post‐treatment scores on the SDI and found no clinically relevant difference between the intervention and the control groups in the work score (MD 0.90, 95% CI ‐0.16 to 1.96, P = 0.09) (Analysis 2.15), social score (MD 0.30, 95% CI ‐0.69 to 1.29, P = 0.54) (Analysis 2.16) or family score (MD 0.50, 95% CI ‐0.46 to 1.46, P = 0.30) (Analysis 2.17).
2.15. Analysis.
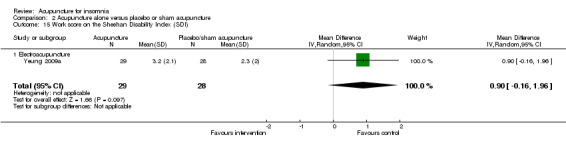
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 15 Work score on the Sheehan Disability Index (SDI).
2.16. Analysis.
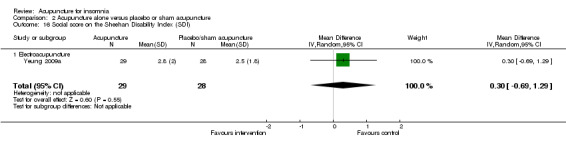
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 16 Social score on the Sheehan Disability Index (SDI).
2.17. Analysis.
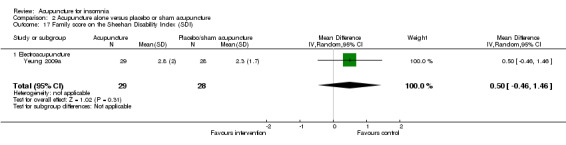
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 17 Family score on the Sheehan Disability Index (SDI).
2.5 Quality of life
Total score on the General Health Questionnaires (GHQ28)
One trial on acupressure (Nordio 2008) reported no clinically relevant difference in quality of life score on the GHQ28 between the intervention and the control groups (MD ‐1.41, 95% CI ‐3.07 to 0.25, P = 0.10) (Analysis 2.18).
2.18. Analysis.
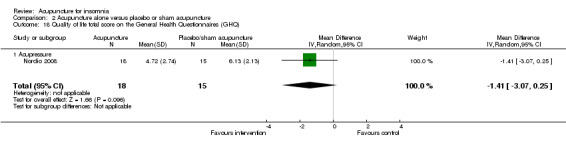
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 18 Quality of life total score on the General Health Questionnaires (GHQ).
Scores on the SF‐36
One trial on acupressure (Tsay 2003) reported a lower improvement in the physical component score in the intervention group compared to the control group (MD ‐6.45, 95% CI ‐10.45 to ‐2.45, P = 0.002) (Analysis 2.19). However, the difference was modest and probably not clinically relevant. There was also no clinically relevant difference in the mental component score between the intervention and the control groups (MD ‐0.70, 95% CI ‐5.44 to 4.04, P = 0.77) (Analysis 2.20).
2.19. Analysis.
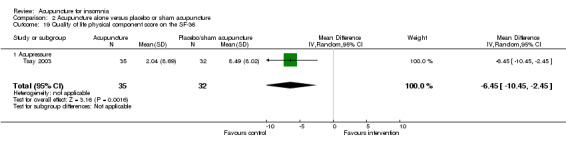
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 19 Quality of life physical component score on the SF‐36.
2.20. Analysis.
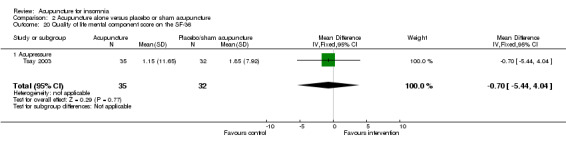
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 20 Quality of life mental component score on the SF‐36.
2.6 Frequency of adverse effects
One study on needle acupuncture (Kim 2004) reported adverse effect in one participant in the intervention group who experienced intolerable pain induced by the needle insertion and withdrew from further treatment. There was no conclusive difference in the frequency of adverse effects between the intervention and the control groups (OR 3.19, 95% CI 0.12 to 84.43, P = 0.49) (Analysis 2.21).
2.21. Analysis.
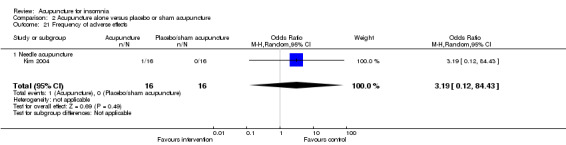
Comparison 2 Acupuncture alone versus placebo or sham acupuncture, Outcome 21 Frequency of adverse effects.
Another trial on acupressure (Hisghman 2006) mentioned adverse effects and found no participant experienced any.
Comparison 3: Acupuncture as an adjunct to other treatment versus other treatment alone
There were 14 trials comparing acupuncture as an adjunct to other treatment versus other treatment alone, including 12 trials on needle acupuncture (Cui 2003; Du 2007; Guo 2009; Jian 2005; Liu 2001; Lu 1998; Luo 2006; Lv 2007; Ma 2006a; Tian 2006; Ye 2008; Zhao 2010) and two trials on electroacupuncture (Lai 2010; Li 2005a).
Primary outcome
3.1 Frequency of improvement in sleep quality
The pooled results of 11 studies on needle acupuncture (Cui 2003; Du 2007; Guo 2009; Jian 2005; Liu 2001; Lu 1998; Luo 2006; Lv 2007; Ma 2006a; Tian 2006; Zhao 2010) and two studies on electroacupuncture (Lai 2010; Li 2005a) showed that the intervention group was more likely to have improvement in sleep quality compared to the control groups (OR 3.08, 95% CI 1.93 to 4.90, P < 0.00001) (Analysis 3.1; Figure 5). Assuming the probability of improvement in the control group ranged from 0.48 to 0.91 as occurred in the included trials, the risk ratios for improvement would range from 1.06 to 1.54, indicating mild likelihood of some benefit from acupuncture. There was mild heterogeneity between the trials (I2 = 28%, Chi2 test P = 0.16), which might be explained by differences in participants, treatment regimen and definition of improvement in sleep quality. Publication bias might be present as suggested by asymmetry of the funnel plot (Figure 6).
3.1. Analysis.
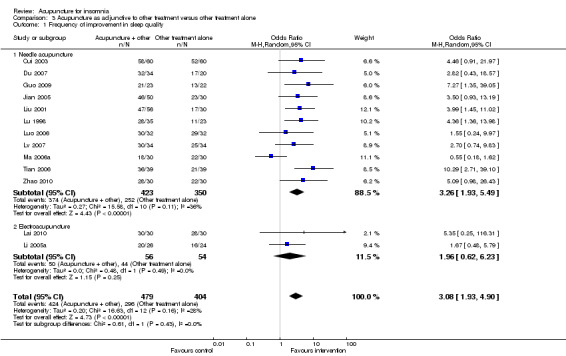
Comparison 3 Acupuncture as adjunctive to other treatment versus other treatment alone, Outcome 1 Frequency of improvement in sleep quality.
5.
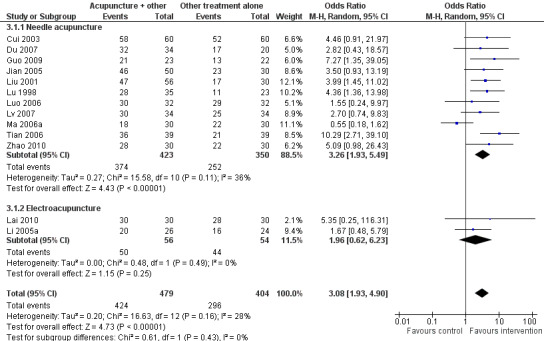
Forest plot of comparison: 3 Acupuncture as adjunctive to other treatment versus other treatment alone, outcome: 3.1 Frequency of improvement in sleep quality.
6.
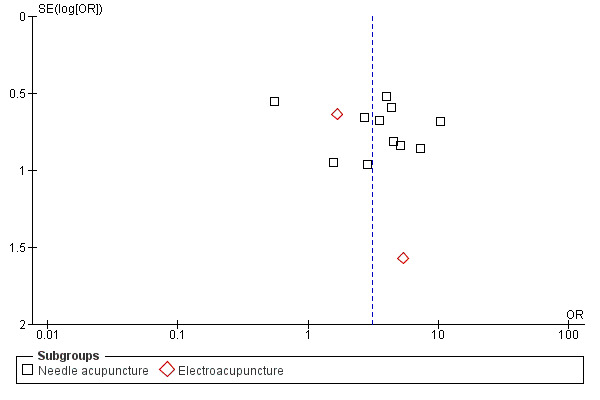
Funnel plot of comparison: 3 Acupuncture as adjunctive to other treatment versus other treatment alone, outcome: 3.1 Frequency of improvement in sleep quality.
Secondary outcomes
3.2 Sleep parameters
Sleep onset latency (minutes)
One study on needle acupuncture (Zhao 2010) reported that the intervention group had shorter sleep onset latency after treatment compared to the control group (MD ‐21.32, 95% CI ‐24.85 to ‐17.79, P < 0.00001) (Analysis 3.2). The difference of about 20 minutes was modest and of doubtful clinical relevance.
3.2. Analysis.
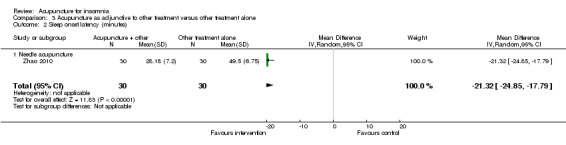
Comparison 3 Acupuncture as adjunctive to other treatment versus other treatment alone, Outcome 2 Sleep onset latency (minutes).
Total sleep duration (hours)
The pooled results of two studies on needle acupuncture (Lv 2007; Zhao 2010) and one study on electroacupuncture (Li 2005a) showed that the total sleep duration was longer after treatment in the intervention group compared to the control group (MD 1.28, 95% CI 0.90 to 1.66, P < 0.00001) (Analysis 3.3). The improvement of more than one hour was probably clinically relevant. However, there was moderate heterogeneity between the trials (I2 = 45%, Chi2 test P = 0.16), which might be explained by differences in participants and treatment regimen.
3.3. Analysis.
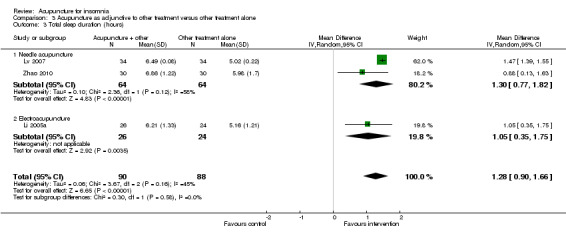
Comparison 3 Acupuncture as adjunctive to other treatment versus other treatment alone, Outcome 3 Total sleep duration (hours).
Sleep efficiency (%)
The pooled results of one study on needle acupuncture (Zhao 2010) and one study on electroacupuncture (Lai 2010) reported that the post‐treatment sleep efficiency was better in the intervention group compared to the control group (MD 5.06, 95% CI 2.14 to 7.99, P = 0.0007) (Analysis 3.4). However, the small difference was not clinically relevant. There was no significant heterogeneity in the results (I2 = 0%, Chi2 test P = 0.50).
3.4. Analysis.
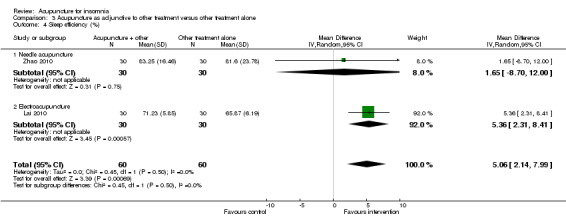
Comparison 3 Acupuncture as adjunctive to other treatment versus other treatment alone, Outcome 4 Sleep efficiency (%).
3.3 Sleep score
Total score on the Pittsburgh Sleep Quality Index (PSQI)
The pooled results of two studies on needle acupuncture (Luo 2006; Ye 2008) and one study on electroacupuncture (Lai 2010) showed better post‐treatment total score on the PSQI compared to the control group (MD ‐2.57, 95% CI ‐3.41 to ‐1.74, P < 0.00001) (Analysis 3.5). The difference was modest and might not be clinically relevant. There was no significant heterogeneity in the results (I2 = 0%, Chi2 test P = 0.63).
3.5. Analysis.
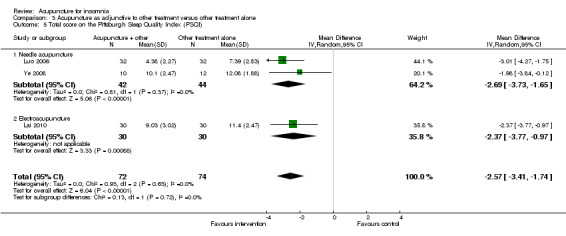
Comparison 3 Acupuncture as adjunctive to other treatment versus other treatment alone, Outcome 5 Total score on the Pittsburgh Sleep Quality Index (PSQI).
3.4 Daytime functioning
None of the included studies in this comparison reported this outcome.
3.5 Quality of life
None of the included studies in this comparison reported this outcome.
3.6 Frequency of adverse effects
One trial on needle acupuncture (Guo 2009) reported adverse effects. Six patients in the control group experienced adverse effects, including three patients with headache, two patients with dizziness and one patient with gastrointestinal upset. These might be caused by zolpidem used in the control group. The increase in adverse effects in the control group was not conclusive as the confidence interval included both increase and decrease in likelihood (OR 0.05, 95% CI 0.00 to 1.03, P = 0.05) (Analysis 3.6).
3.6. Analysis.
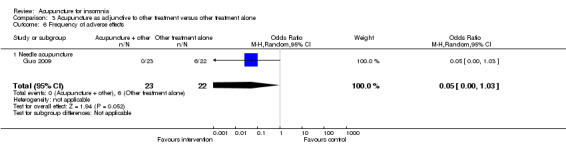
Comparison 3 Acupuncture as adjunctive to other treatment versus other treatment alone, Outcome 6 Frequency of adverse effects.
Sensitivity analysis
Only four included studies had adequate allocation concealment and all studies had more than 70% follow‐up. Therefore sensitivity analyses in these respects were not performed.
Sensitivity analysis excluding trials in which insomnia was not diagnosed by standardised criteria
To investigate the influence of studies in which insomnia was not stringently diagnosed by standardised criteria, we performed these sensitivity analyses. The outcomes for which one or more studies were removed are reported below.
1. Acupuncture versus no treatment
Frequency of improvement in sleep quality
One (Tang 2007a) of the two included studies did not have standardised criteria for diagnosis of insomnia. The result of the remaining trial on acupressure (Chen 2009) still showed more participants in the intervention group having improvement compared to the control group (OR 35.29, 95% CI 13.13 to 94.84, P < 0.00001) (Analysis 4.1). Assuming the probability of improvement in the control group ranged from 0.35 to 0.6, the risk ratios for improvement would range from 1.64 to 2.71, indicating moderate likelihood of some benefit from acupressure. This was not substantially different from the original analysis.
4.1. Analysis.
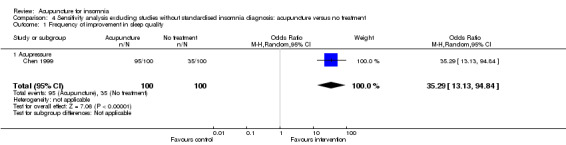
Comparison 4 Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture versus no treatment, Outcome 1 Frequency of improvement in sleep quality.
2. Acupuncture versus sham or placebo acupuncture
Total sleep duration (hours)
One trial of magnetic acupressure (Hisghman 2006) did not have standardised criteria for diagnosis of insomnia and was removed. The remaining trial (Suen 2002) showed slightly longer total sleep duration in the intervention group compared to the control group (MD 0.88, 95% CI 0.28 to 1.48, P = 0.004) (Analysis 5.1), which was probably not clinically relevant. This was not substantially different from the original analysis.
5.1. Analysis.
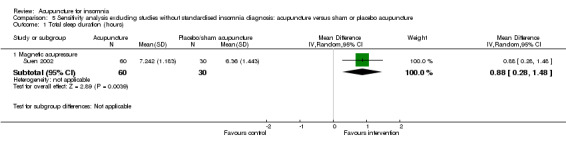
Comparison 5 Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture versus sham or placebo acupuncture, Outcome 1 Total sleep duration (hours).
Number of awakenings
One trial of magnetic acupressure (Hisghman 2006) did not have standardised criteria for diagnosis of insomnia and was removed. The result of the remaining trial on magnetic acupressure (Suen 2002) showed a lower number of awakenings in the intervention group compared to the control group (MD ‐5.25, 95% CI ‐8.12 to ‐2.38, P = 0.0003) (Analysis 5.2). The difference was moderate and bigger than the original analysis and might be clinically relevant.
5.2. Analysis.
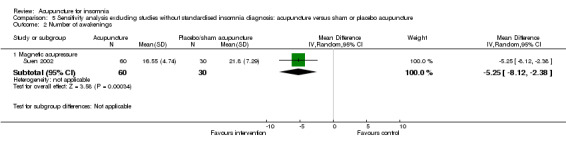
Comparison 5 Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture versus sham or placebo acupuncture, Outcome 2 Number of awakenings.
Sleep efficiency (%)
One trial of magnetic acupressure (Hisghman 2006) did not have standardised criteria for diagnosis of insomnia and was removed. The remaining trial (Suen 2002) showed that the post‐treatment sleep efficiency was higher in the intervention group compared to the control group (MD 11.55, 95% CI 5.52 to 17.58, P = 0.0004) (Analysis 5.3). The difference was just modest with doubtful clinical relevance. There was no substantial change from the original analysis.
5.3. Analysis.
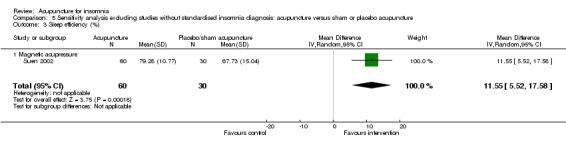
Comparison 5 Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture versus sham or placebo acupuncture, Outcome 3 Sleep efficiency (%).
Total score on the Pittsburgh Sleep Quality Index (PSQI)
One (Nordio 2008) of the seven studies did not have standardised criteria for diagnosis of insomnia. The pooled results of the remaining six trials on electroacupuncture (Yeung 2009a) and acupressure (Chen 1999; Hwang 2007; Lin 2007; Reza 2010; Tsay 2004) still showed a better total score on the PSQI in the intervention group compared to the control group (MD ‐2.08, 95% CI ‐3.58 to ‐0.59, P = 0.006) (Analysis 5.4). However, the difference was small and probably not clinically relevant. There was no substantial change from the original analysis.
5.4. Analysis.
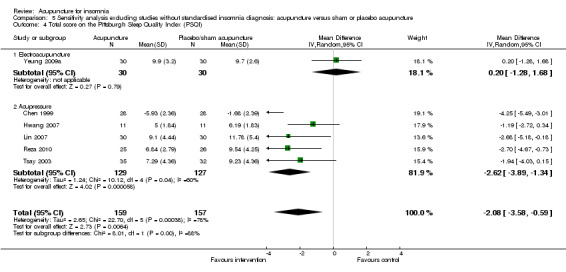
Comparison 5 Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture versus sham or placebo acupuncture, Outcome 4 Total score on the Pittsburgh Sleep Quality Index (PSQI).
3. Acupuncture as an adjunct to other treatment versus other treatment alone
Frequency of improvement in sleep quality
Eight trials (Du 2007; Jian 2005; Liu 2001; Lu 1998; Lv 2007; Luo 2006; Ma 2006a; Zhao 2010) did not have well‐defined criteria for diagnosing insomnia and were removed. The pooled result of the remaining three studies on needle acupuncture (Cui 2003; Guo 2009; Tian 2006) and two trials on electroacupuncture (Lai 2010; Li 2005a) showed more patients in the intervention group having improvement in sleep quality compared to the control group (OR 4.55, 95% CI 2.20 to 9.39, P < 0.0001) (Analysis 6.1). Assuming the probability of improvement in the control group ranged from 0.48 to 0.91, the risk ratios for improvement would range from 1.08 to 1.68, indicating only mild likelihood of some benefit from acupuncture. There was no substantial change from the original analysis.
6.1. Analysis.
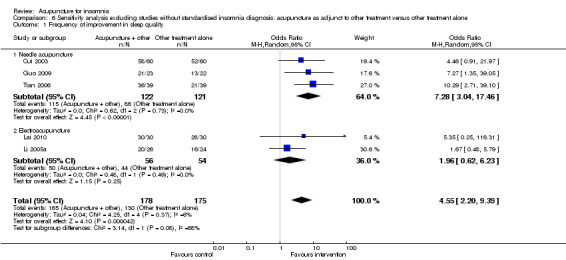
Comparison 6 Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture as adjunct to other treatment versus other treatment alone, Outcome 1 Frequency of improvement in sleep quality.
Total sleep duration (hours)
Two studies on needle acupuncture (Lv 2007; Zhao 2010) did not have well‐defined criteria for diagnosing insomnia and were removed. The remaining study on electroacupuncture (Li 2005a) showed that the intervention group had longer total sleep duration compared to the control group (MD 1.05, 95% CI 0.35 to 1.75, P = 0.003) (Analysis 6.2). The improvement of more than one hour might still be clinically relevant although not as much as in the original analysis.
6.2. Analysis.
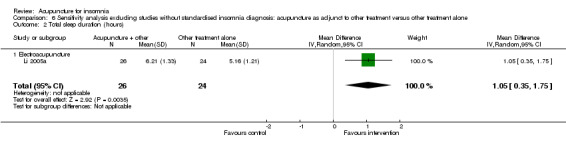
Comparison 6 Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture as adjunct to other treatment versus other treatment alone, Outcome 2 Total sleep duration (hours).
Sleep efficiency (%)
The study on needle acupuncture (Zhao 2010) did not have well‐defined criteria for diagnosing insomnia and was removed. The remaining study on electroacupuncture (Lai 2010) reported that the post‐treatment sleep efficiency was better in the intervention group compared to the control group (MD 5.36, 95% CI 2.31 to 8.41, P = 0.0006) (Analysis 6.3). The result was similar to the original analysis and the small difference was not clinically relevant.
6.3. Analysis.
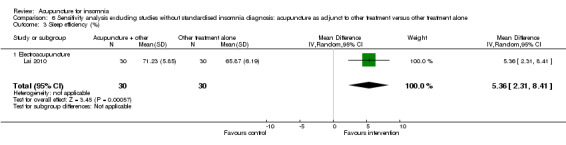
Comparison 6 Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture as adjunct to other treatment versus other treatment alone, Outcome 3 Sleep efficiency (%).
Total score on the Pittsburgh Sleep Quality Index (PSQI)
One (Luo 2006) of the three studies reporting this outcome did not have well‐defined criteria for diagnosing insomnia. When the results of the remaining two studies on needle acupuncture (Ye 2008) and electroacupuncture (Lai 2010) were pooled, the intervention group still showed a better total score on the PSQI compared to the control group (MD ‐2.23, 95% CI ‐3.35 to ‐1.11, P < 0.0001) (Analysis 6.4). The difference was smaller than the original analysis and might not be clinically relevant.
6.4. Analysis.
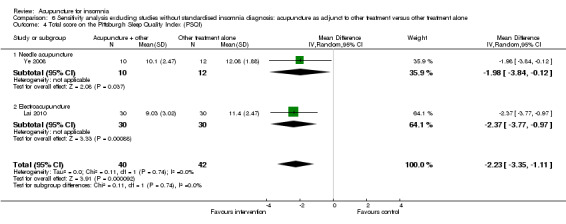
Comparison 6 Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture as adjunct to other treatment versus other treatment alone, Outcome 4 Total score on the Pittsburgh Sleep Quality Index (PSQI).
Sensitivity analysis for dropouts
To investigate the influence of dropouts, we performed this sensitivity analysis for best and worst‐case scenarios.
1. Acupuncture versus no treatment
Four (Chen 1999; Reza 2010; Tsay 2003; Tsay 2004) of 10 trials in this comparison had dropouts. Sensitivity analyses on best and worst‐case scenarios for outcomes involving these studies are described below.
Total score on the Pittsburgh Sleep Quality Index (PSQI)
In the best scenario, the pooled results of five trials on acupressure (Chen 1999; Chen 2009; Reza 2010; Tsay 2003; Tsay 2004) showed that total score on the PSQI was better in the intervention group compared to the control group (MD ‐4.71, 95% CI ‐6.50 to ‐2.91, P < 0.00001) (Analysis 7.1). The mean reduction of PSQI score was approximately one standard deviation which indicated a moderate improvement that might be clinically relevant. One of the trials also evaluated electroacupuncture (Tsay 2004) and reported a better score on the PSQI in the intervention group compared to the control group (MD ‐3.69, 95% CI ‐5.86 to ‐1.52, P = 0.0009) (Analysis 7.1). The difference indicated a moderate improvement that might be clinically relevant.
7.1. Analysis.
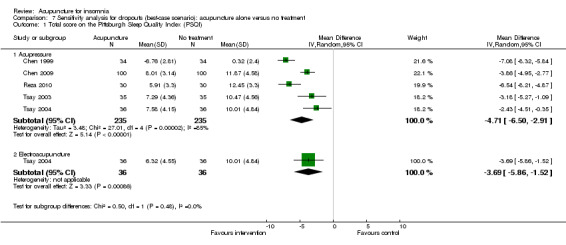
Comparison 7 Sensitivity analysis for dropouts (best‐case scenario): acupuncture alone versus no treatment, Outcome 1 Total score on the Pittsburgh Sleep Quality Index (PSQI).
However, in the worst scenario, the pooled results on acupressure (Chen 1999; Chen 2009; Reza 2010; Tsay 2003; Tsay 2004) showed a much lower difference in total score on the PSQI between the intervention and the control group that was not clinically relevant (MD ‐2.77, 95% CI ‐3.70 to ‐1.84, P < 0.00001) (Analysis 8.1). The slightly better total score on the PSQI for electroacupuncture (Tsay 2004) compared with no treatment was also probably not clinically relevant (MD ‐3.17, 95% CI ‐5.34 to ‐1.00, P = 0.004) (Analysis 8.1).
8.1. Analysis.
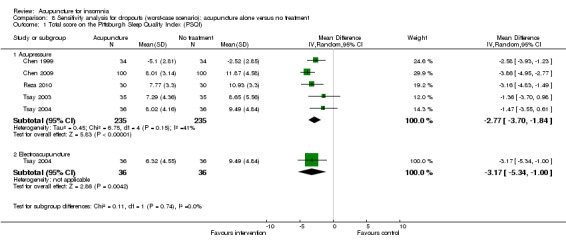
Comparison 8 Sensitivity analysis for dropouts (worst‐case scenario): acupuncture alone versus no treatment, Outcome 1 Total score on the Pittsburgh Sleep Quality Index (PSQI).
Quality of life scores on the SF‐36
In the best scenario, the trial on acupressure (Tsay 2003) showed some improvement in the physical component score on the SF‐36 in the intervention group compared to the control group (MD 5.09, 95% CI 1.27 to 8.91, P = 0.009) (Analysis 7.2). However, the benefit was small and probably not clinically relevant. There was similar improvement in the mental component score on the SF‐36 which was again unlikely to be clinically relevant (MD 5.57, 95% CI 0.62 to 10.62, P = 0.03) (Analysis 7.3).
7.2. Analysis.
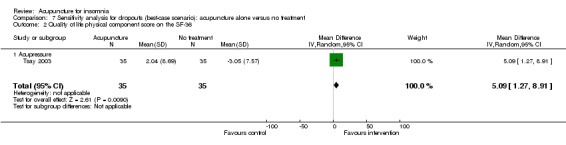
Comparison 7 Sensitivity analysis for dropouts (best‐case scenario): acupuncture alone versus no treatment, Outcome 2 Quality of life physical component score on the SF‐36.
7.3. Analysis.
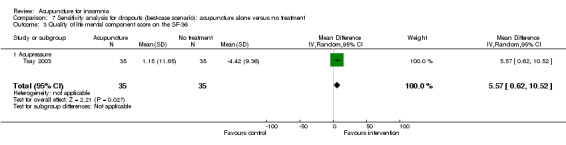
Comparison 7 Sensitivity analysis for dropouts (best‐case scenario): acupuncture alone versus no treatment, Outcome 3 Quality of life mental component score on the SF‐36.
In the worst scenario, the differences in the physical and mental component score on the SF‐36 between the intervention group and the control group were even smaller and without clinical relevance (MD 2.05, 95% CI ‐1.77 to 5.87, P = 0.29 for physical component score (Analysis 8.2); and MD 1.81, 95% CI ‐3.14 to 6.76, P = 0.47 for mental component score (Analysis 8.3)).
8.2. Analysis.
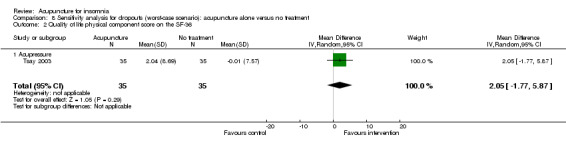
Comparison 8 Sensitivity analysis for dropouts (worst‐case scenario): acupuncture alone versus no treatment, Outcome 2 Quality of life physical component score on the SF‐36.
8.3. Analysis.
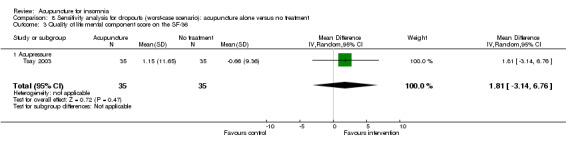
Comparison 8 Sensitivity analysis for dropouts (worst‐case scenario): acupuncture alone versus no treatment, Outcome 3 Quality of life mental component score on the SF‐36.
2. Acupuncture versus sham or placebo acupuncture
Nine (Chen 1999; Kim 2004; Lee 2009; Nordio 2008; Reza 2010; Suen 2002; Sun 2010a; Tsay 2003; Yeung 2009a) of the 13 trials in this comparison had dropouts. Sensitivity analyses on best and worst‐case scenarios for outcomes involving these studies are described below.
Frequency of improvement in sleep quality
In the best scenario, the pooled results of two trials on acupressure (Chen 1999; Lin 2007) showed that the intervention group was more likely to have improvement in sleep quality compared to the control group (OR 10.43, 95% CI 2.88 to 37.73, P = 0.0004) (Analysis 9.1). Assuming the probability of improvement in the control group ranged from 0.029 to 0.67 as occurred in the included trials, the risk ratios for improvement would range from 1.43 to 8.19, indicating mild to moderate likelihood of some benefit from acupressure.
9.1. Analysis.
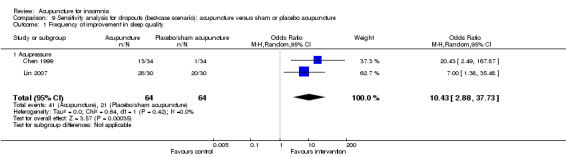
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 1 Frequency of improvement in sleep quality.
However, in the worst scenario, the pooled results showed a much lower and inconclusive odds ratio in favour of intervention (OR 1.73, 95% CI 0.12 to 24.31, P = 0.68) (Analysis 10.1). Assuming the probability of improvement in the control group ranged from 0.26 to 0.67 as occurred in the included trials, the risk ratios for improvement would range from 1.16 to 1.45, indicating only mild likelihood of some inconclusive benefit from acupressure.
10.1. Analysis.
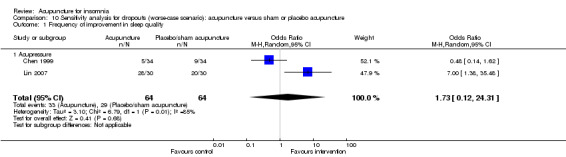
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 1 Frequency of improvement in sleep quality.
Sleep onset latency (minutes)
In the best scenario, the study on needle acupuncture (Kim 2004) showed that the intervention group had shorter sleep onset latency compared to the control group (MD ‐84.9, 95% CI ‐183.0 to 13.2, P = 0.09) (Analysis 9.2). Although the difference of more than one hour in sleep onset latency was clinically relevant, the benefit was inconclusive as the confidence interval included both positive and negative effects. The trial on electroacupuncture (Yeung 2009a) showed no clinically relevant difference between the intervention and the control groups (MD ‐7.20, 95% CI ‐23.16 to 8.76, P = 0.38) (Analysis 9.2). The pooled results of three trials on acupressure (Hwang 2007; Lin 2007; Suen 2002) also showed no clinically relevant difference between the intervention and the control groups in sleep onset latency (MD ‐4.17, 95% CI ‐20.33 to 12.00, P = 0.61) (Analysis 9.2). The trial on magnetic acupressure (Suen 2002) also did not show a clinically relevant difference in sleep onset latency between the intervention and the control groups (MD ‐13.53, 95% CI ‐20.26 to ‐6.80, P < 0.0001) (Analysis 9.2).
9.2. Analysis.
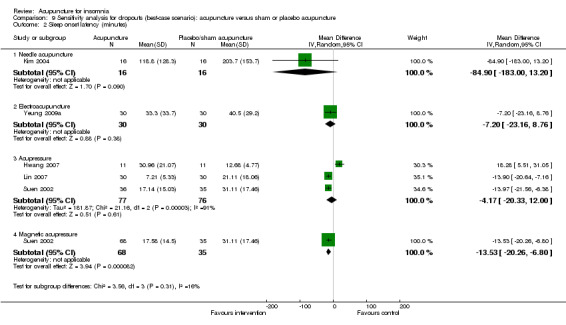
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 2 Sleep onset latency (minutes).
In the worst scenario, the study on needle acupuncture (Kim 2004) showed that the intervention group had shorter sleep onset latency compared to the control group (MD ‐31.60, 95% CI ‐130.08 to 66.88, P = 0.53) (Analysis 10.2). The difference of half an hour in sleep onset latency might not be clinically important, and the benefit was inconclusive. The trial on electroacupuncture (Yeung 2009a) showed no clinically relevant difference between the intervention and the control groups (MD 2.20, 95% CI ‐13.81 to 18.21, P = 0.79) (Analysis 10.2). The pooled results of three trials on acupressure (Hwang 2007; Lin 2007; Suen 2002) also showed no clinically relevant difference between the intervention and the control groups in sleep onset latency (MD 1.10, 95% CI ‐14.62 to 16.82, P = 0.89) (Analysis 10.2). The trial on magnetic acupressure (Suen 2002) also did not show a clinically relevant difference in sleep onset latency between the intervention and the control groups (MD ‐0.16, 95% CI ‐6.98 to 6.66, P = 0.96) (Analysis 10.2).
10.2. Analysis.
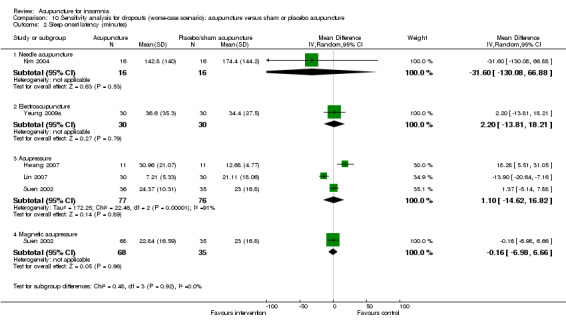
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 2 Sleep onset latency (minutes).
Total sleep duration (hours)
In the best scenario, the trial on needle acupuncture (Kim 2004) showed that total sleep duration was longer after treatment in the intervention group compared to the control group (MD 1.77, 95% CI 0.71 to 2.82, P = 0.001) (Analysis 9.3). The difference of more than one hour in total sleep duration was clinically relevant. In contrast, the trial on electroacupuncture (Yeung 2009a) reported no clinically relevant difference between the intervention and the control groups in total sleep duration (MD 0.13, 95% CI ‐0.38 to 0.65, P = 0.61) (Analysis 9.3). The pooled results of three trials on acupressure (Hwang 2007; Lin 2007; Suen 2002) showed no clinically relevant difference in total sleep duration between the intervention and the control groups (MD 0.55, 95% CI ‐0.30 to 1.40, P = 0.20) (Analysis 9.3). The pooled results of two trials on magnetic acupressure (Hisghman 2006; Suen 2002) showed that the total sleep duration was longer after treatment in the intervention group compared to the control group (MD 1.50, 95% CI 0.92 to 2.09, P < 0.00001) (Analysis 9.3). The difference was clinically relevant.
9.3. Analysis.
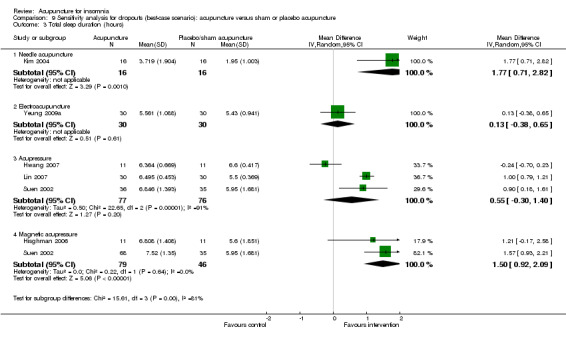
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 3 Total sleep duration (hours).
However, in the worst scenario, the trial on needle acupuncture (Kim 2004) showed a much smaller difference in total sleep duration between the intervention and the control groups (MD 1.10, 95% CI 0.04 to 2.15, P = 0.04) (Analysis 10.3). However, the difference of more than one hour in total sleep duration might still be clinically relevant. In contrast, the trial on electroacupuncture (Yeung 2009a) reported no clinically relevant difference between the intervention and the control groups in total sleep duration (MD ‐0.24, 95% CI ‐0.75 to 0.28, P = 0.37) (Analysis 10.3). The pooled results of three trials on acupressure (Hwang 2007; Lin 2007; Suen 2002) also showed no clinically relevant difference in total sleep duration between the intervention and the control groups (MD 0.05, 95% CI ‐1.05 to 1.15, P = 0.93) (Analysis 10.3). The pooled results of two trials on magnetic acupressure (Hisghman 2006; Suen 2002) still showed that total sleep duration was longer in the intervention group compared to the control group (MD 0.52, 95% CI ‐0.39 to 1.42, P = 0.26) (Analysis 10.3). However, the difference was inconclusive and it was much smaller and probably not clinically relevant.
10.3. Analysis.
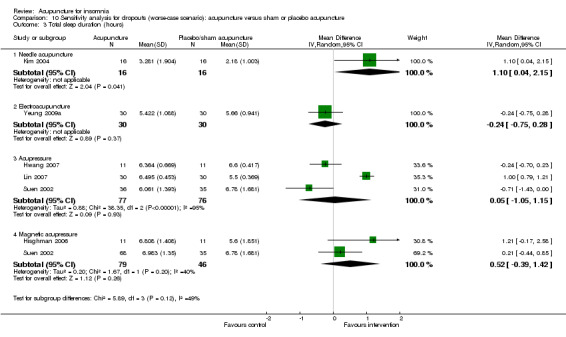
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 3 Total sleep duration (hours).
Total wake time (minutes)
In the best scenario, one trial (Suen 2002) reported shorter total wake time after treatment in participants who received acupressure compared to the control group (MD ‐77.06, 95% CI ‐123.85 to ‐30.27, P = 0.001) (Analysis 9.4). The difference of more than one hour in total wake time was probably clinically relevant. The same trial on magnetic acupressure (Suen 2002) also found that the intervention group had shorter total wake time (MD ‐108.35, 95% CI ‐147.61 to ‐69.09, P < 0.00001) (Analysis 9.4). The difference was clinically relevant.
9.4. Analysis.
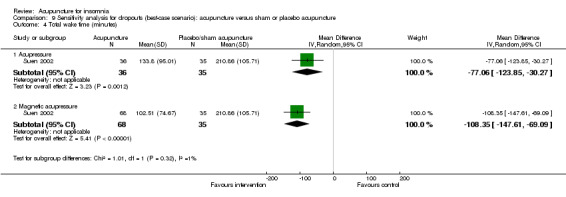
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 4 Total wake time (minutes).
However, in the worst scenario, the direction of effect reversed in that total wake time was better in participants who received no treatment compared to those who received acupressure (MD 27.84, 95% CI ‐19.69 to 75.37, P = 0.25) (Analysis 10.4), although the difference might not be clinically relevant. The difference in total wake time between the magnetic acupressure and the control groups was much smaller and might not be clinically relevant (MD ‐26.66, 95% CI ‐66.33 to 13.01, P = 0.19) (Analysis 10.4). The difference was also inconclusive.
10.4. Analysis.
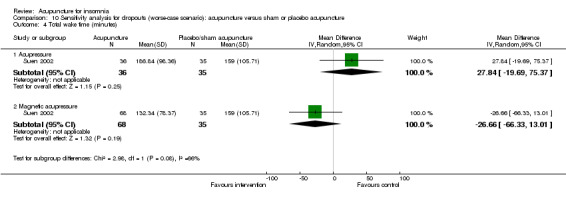
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 4 Total wake time (minutes).
Wake after sleep onset (minutes)
In the best scenario, the trial on electroacupuncture (Yeung 2009a) showed shorter wake after sleep onset in the intervention group compared to the control group (MD ‐46.5, 95% CI ‐87.9 to ‐5.1, P = 0.03) (Analysis 9.5). The clinical relevance of the modest difference was uncertain. The trial on acupressure (Suen 2002) found shorter wake after sleep onset in the intervention group compared to the control groups (MD ‐60.25, 95% CI ‐100.92 to ‐19.58, P = 0.004) (Analysis 9.5). The difference of more than one hour might be clinically relevant. The same trial (Suen 2002) also reported that participants who received magnetic acupressure had shorter wake after sleep onset compared to the control group (MD ‐87.25, 95% CI ‐119.79 to ‐54.71, P < 0.00001) (Analysis 9.5). The difference of more than one hour was clinically relevant.
9.5. Analysis.
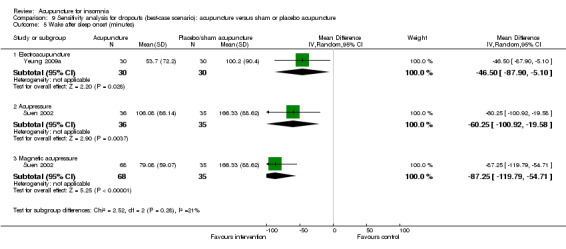
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 5 Wake after sleep onset (minutes).
In the worst scenario, the trial on electroacupuncture (Yeung 2009a) showed no clinically relevant difference in wake after sleep onset between the intervention and the control groups (MD ‐22.80, 95% CI ‐63.17 to 17.57, P = 0.27) (Analysis 10.5). Participants who received acupressure (Suen 2002) had shorter wake after sleep onset in the control group compared to the intervention group (MD 29.53, 95% CI ‐12.32 to 71.38, P = 0.17) (Analysis 10.5), but the difference was not clinically relevant. Participants who received magnetic acupressure had shorter wake after sleep onset compared to the control group (MD ‐21.43, 95% CI ‐53.86 to 11.00, P = 0.20) (Analysis 10.5), but the difference was not clinically relevant.
10.5. Analysis.
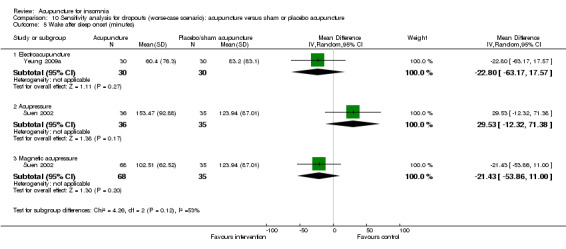
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 5 Wake after sleep onset (minutes).
Number of awakenings
In the best scenario, the pooled results of two trials on acupressure (Lin 2007; Suen 2002) showed a lower number of awakenings after treatment in the intervention group compared to the control group (MD ‐5.78, 95% CI ‐8.03 to ‐3.53, P < 0.00001) (Analysis 9.6). The difference was modest and of uncertain clinical relevance. The pooled results of two trials on magnetic acupressure (Hisghman 2006; Suen 2002) showed a lower number of awakenings in the intervention group compared to the control group (MD ‐4.55, 95% CI ‐11.90 to 2.81, P = 0.23) (Analysis 9.6). The difference was again of uncertain clinical relevance. There was considerable heterogeneity in this outcome.
9.6. Analysis.
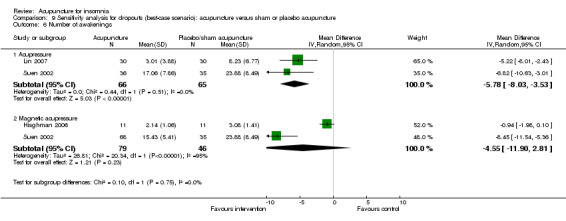
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 6 Number of awakenings.
However, in the worst scenario, the pooled results of two trials on acupressure (Lin 2007; Suen 2002) showed no clinically relevant difference in number of awakenings between the intervention and the control groups (MD ‐1.86, 95% CI ‐8.69 to 4.98, P = 0.59) (Analysis 10.6). The difference was small and not clinically relevant. The pooled results of two trials on magnetic acupressure (Hisghman 2006; Suen 2002) also showed no clinically relevant difference in number of awakenings between the intervention and the control groups (MD ‐1.05, 95% CI ‐2.04 to ‐0.07, P = 0.04) (Analysis 10.6).
10.6. Analysis.
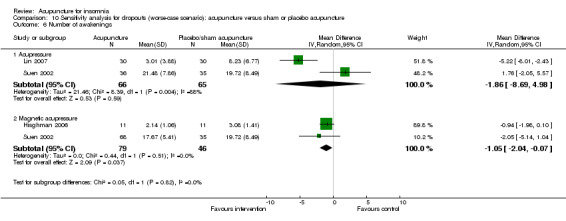
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 6 Number of awakenings.
Sleep efficiency (%)
In the best scenario, the trial on electroacupuncture (Yeung 2009a) showed that sleep efficiency was higher in the intervention group compared to the control group (MD 9.80, 95% CI 3.43 to 16.17, P = 0.003) (Analysis 9.7). The difference was just modest and might not be clinically relevant. The pooled results of three trials on acupressure (Hwang 2007; Lin 2007; Suen 2002) showed no clinically relevant difference in sleep efficiency between the intervention and the control groups (MD 6.22, 95% CI ‐7.82 to 20.25, P = 0.39) (Analysis 9.7). The pooled results of two trials on magnetic acupressure (Hisghman 2006; Suen 2002) showed that sleep efficiency was higher in the intervention group compared to the control group (MD 12.40, 95% CI 0.49 to 24.31, P = 0.04) (Analysis 9.7). The difference of more than 10% might be clinically relevant.
9.7. Analysis.
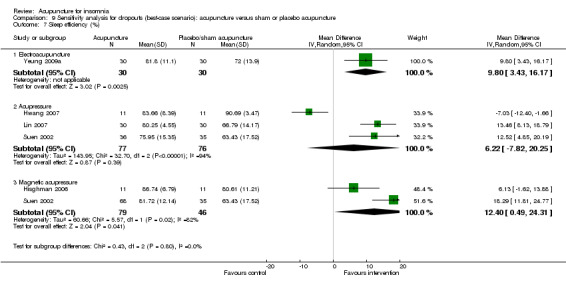
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 7 Sleep efficiency (%).
In the worst scenario, the trial on electroacupuncture (Yeung 2009a) showed no clinically relevant difference in sleep efficiency between the intervention and the control groups (MD 5.10, 95% CI ‐1.26 to 11.46, P = 0.12) (Analysis 10.7). The pooled results of three trials on acupressure (Hwang 2007; Lin 2007; Suen 2002) showed no clinically relevant difference in sleep efficiency between the intervention and the control groups (MD 0.66, 95% CI ‐13.14 to 14.47, P = 0.92) (Analysis 10.7). The pooled results of two trials on magnetic acupressure (Hisghman 2006; Suen 2002) also showed no clinically relevant difference in sleep efficiency between the intervention and the control groups (MD 5.30, 95% CI 0.32 to 10.28, P = 0.04) (Analysis 10.7).
10.7. Analysis.
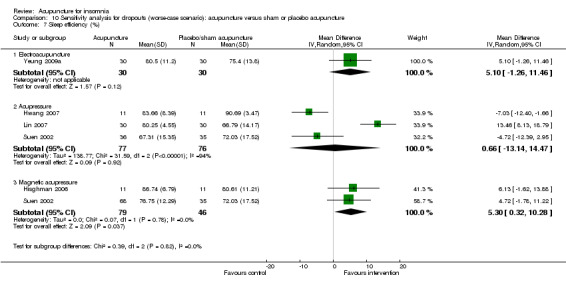
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 7 Sleep efficiency (%).
Total score on the Pittsburgh Sleep Quality Index (PSQI)
In the best scenario, the pooled results of one trial on electroacupuncture (Yeung 2009a) and six trials on acupressure (Chen 1999; Hwang 2007; Lin 2007; Nordio 2008; Reza 2010; Tsay 2003) showed that the intervention group had a better PSQI total score compared to the control group (MD ‐3.10, 95% CI ‐4.86 to ‐1.35, P < 0.001) (Analysis 9.8). However, the difference was just modest and of doubtful clinical relevance.
9.8. Analysis.
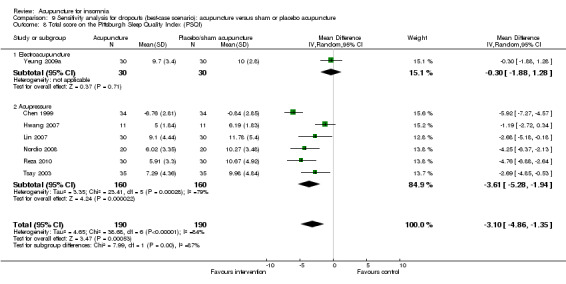
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 8 Total score on the Pittsburgh Sleep Quality Index (PSQI).
However, in the worst scenario, the pooled results showed no clinically relevant difference in the total PSQI score between the intervention and the control groups (MD ‐1.10, 95% CI ‐2.08 to ‐0.12, P = 0.001) (Analysis 10.8).
10.8. Analysis.
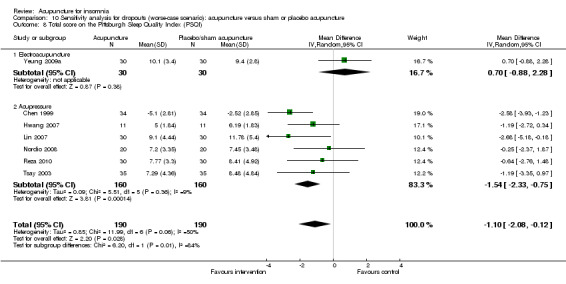
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 8 Total score on the Pittsburgh Sleep Quality Index (PSQI).
Total score on the Insomnia Severity Index (ISI)
In the best scenario, the pooled result of two trials on needle acupuncture (Kim 2004; Lee 2009) and one trial on electroacupuncture (Yeung 2009a) showed that the intervention group had a better ISI score compared to the control group (MD ‐4.74, 95% CI ‐7.86 to ‐1.63, P = 0.003) (Analysis 9.9). The difference was approximately one standard deviation and might be clinically relevant.
9.9. Analysis.
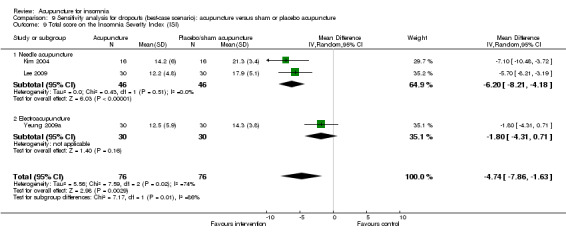
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 9 Total score on the Insomnia Severity Index (ISI).
However, in the worst scenario, the pooled results showed no clinically relevant difference in the ISI score between the intervention and the control groups (MD ‐1.78, 95% CI ‐4.40 to ‐0.84, P = 0.18) (Analysis 10.9).
10.9. Analysis.
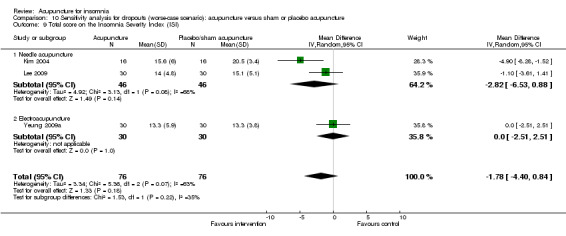
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 9 Total score on the Insomnia Severity Index (ISI).
Total score on the Athens Insomnia Scale (AIS)
In the best scenario, the pooled result of two trials on needle acupuncture (Kim 2004; Lee 2009) and one trial on acupressure (Sun 2010a) showed that the intervention group had a better AIS score after treatment compared to the control group (MD ‐3.46, 95% CI ‐6.76 to ‐0.16, P = 0.04) (Analysis 9.10). The difference was small and probably not clinically relevant.
9.10. Analysis.
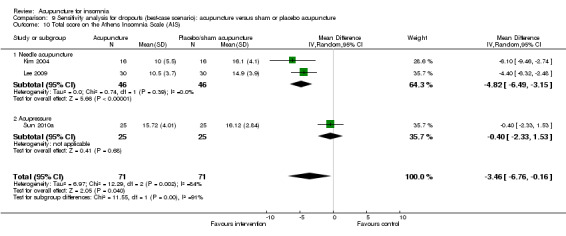
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 10 Total score on the Athens Insomnia Scale (AIS).
In the worst scenario, the difference in AIS score between the intervention and the control groups was even smaller and not clinically relevant (MD ‐1.24, 95% CI ‐2.92 to 0.44, P = 0.15) (Analysis 10.10).
10.10. Analysis.
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 10 Total score on the Athens Insomnia Scale (AIS).
Quality of sleep score on the Morning Questionnaire (MQ)
In the best scenario, the trial on needle acupuncture (Kim 2004) showed that the intervention group had better quality of sleep score on the MQ after treatment compared to the control group (MD 39.10, 95% CI 23.12 to 55.08, P < 0.00001) (Analysis 9.11). The difference was moderate and probably clinically relevant.
9.11. Analysis.
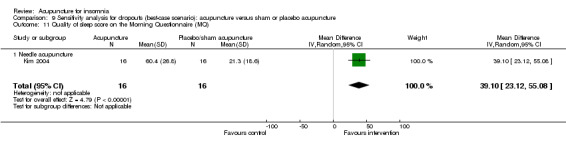
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 11 Quality of sleep score on the Morning Questionnaire (MQ).
In the worst scenario, the difference in quality of sleep score on the MQ between the intervention and the control group was smaller (MD 29.20, 95% CI 12.84 to 45.56, P = 0.0005) (Analysis 10.11). This difference was more than one standard deviation and might be clinically relevant.
10.11. Analysis.
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 11 Quality of sleep score on the Morning Questionnaire (MQ).
Sleep quality score from sleep diary
In the best scenario, the trial on electroacupuncture (Yeung 2009a) showed no clinically relevant difference in sleep quality score from sleep diary between the intervention and the control groups (MD ‐0.05, 95% CI ‐0.29 to 0.19, P = 0.69) (Analysis 9.12).
9.12. Analysis.
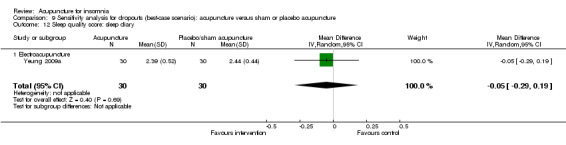
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 12 Sleep quality score: sleep diary.
In the worst scenario, the control group had a better score compared to the intervention group (MD 0.11, 95% CI ‐0.13 to 0.35, P = 0.38) (Analysis 10.12), but the difference was small and not clinically relevant.
10.12. Analysis.
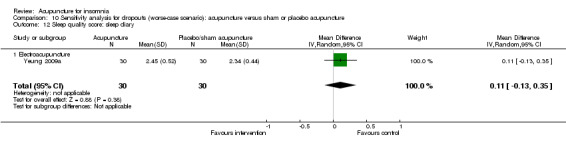
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 12 Sleep quality score: sleep diary.
Scores on the Sheehan Disability Index (SDI)
In the best scenario, the trial on electroacupuncture (Yeung 2009a) showed no clinically relevant differences between the intervention and the control groups in work score (MD 0.50, 95% CI ‐0.59 to 1.59, P = 0.37) (Analysis 9.13), social score (MD 0.00, 95% CI ‐1.01 to 1.01, P = 1.00) (Analysis 9.14) or family score (MD 0.20 95% CI ‐0.79 to 1.19, P = 0.69) (Analysis 9.15).
9.13. Analysis.
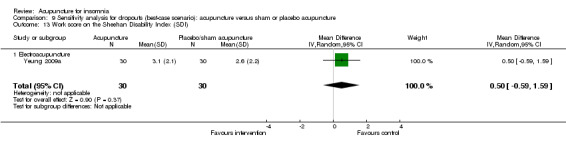
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 13 Work score on the Sheehan Disability Index (SDI).
9.14. Analysis.
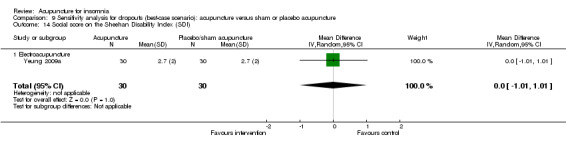
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 14 Social score on the Sheehan Disability Index (SDI).
9.15. Analysis.
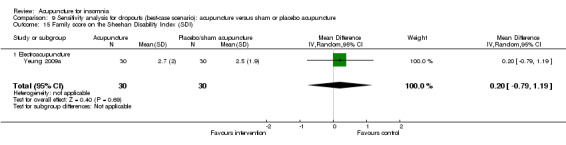
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 15 Family score on the Sheehan Disability Index (SDI).
In the worst scenario, all results favoured the control group but the differences were again not clinically relevant (MD 1.20, 95% CI 0.14 to 2.26, P = 0.03 for work score (Analysis 10.13); MD 0.60, 95% CI ‐0.39 to 1.59, P = 0.23 for social score (Analysis 10.14); MD 0.80 95% CI ‐0.17 to 1.77, P = 0.10 for family score (Analysis 10.15)).
10.13. Analysis.
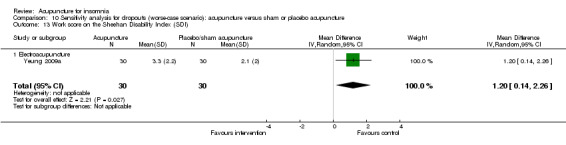
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 13 Work score on the Sheehan Disability Index (SDI).
10.14. Analysis.
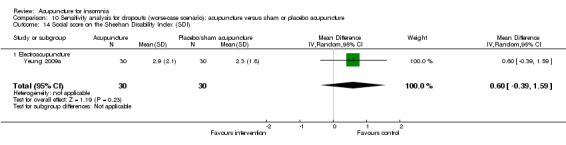
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 14 Social score on the Sheehan Disability Index (SDI).
10.15. Analysis.
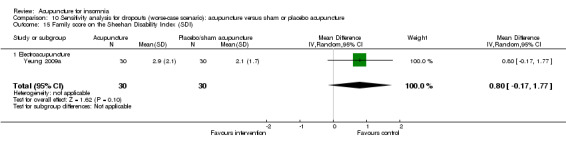
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 15 Family score on the Sheehan Disability Index (SDI).
Quality of life score on the General Health Questionnaires (GHQ28)
In the best scenario, the trial on acupressure (Nordio 2008) showed the intervention group had a better quality of life score on the GHQ28 compared to the control group (MD ‐2.95, 95% CI ‐4.69 to ‐1.21, P = 0.0009) (Analysis 9.16). The difference was approximately one standard deviation and might be clinically relevant.
9.16. Analysis.
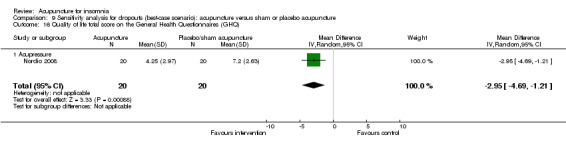
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 16 Quality of life total score on the General Health Questionnaires (GHQ).
However, in the worst scenario, there was no clinically relevant difference in quality of life score on the GHQ28 between the intervention and the control groups (MD 0.20, 95% CI ‐1.58 to 1.98, P = 0.83) (Analysis 10.16).
10.16. Analysis.
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 16 Quality of life total score on the General Health Questionnaires (GHQ).
Quality of life scores on the SF‐36
In the best scenario, the trial on acupressure (Tsay 2003) showed a worse physical component score on the SF‐36 in the intervention group compared to the control group (MD ‐5.07, 95% CI ‐9.19 to ‐0.95, P = 0.02) (Analysis 9.17). However, the difference was small and probably not clinically relevant. There was also no clinically relevant difference in mental component score between the intervention and the control groups (MD ‐0.66, 95% CI ‐4.18 to 5.50, P = 0.79) (Analysis 9.18).
9.17. Analysis.
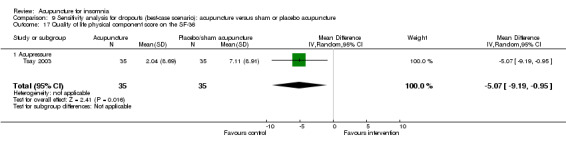
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 17 Quality of life physical component score on the SF‐36.
9.18. Analysis.
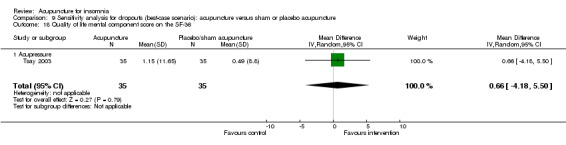
Comparison 9 Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 18 Quality of life mental component score on the SF‐36.
In the worst scenario, there were also no clinically relevant differences between the intervention and the control groups in physical component score (MD ‐7.82, 95% CI ‐11.94 to ‐3.70, P = 0.0002) (Analysis 10.17) and mental component score (MD ‐2.06, 95% CI ‐6.90 to 2.78, P = 0.40) (Analysis 10.18).
10.17. Analysis.
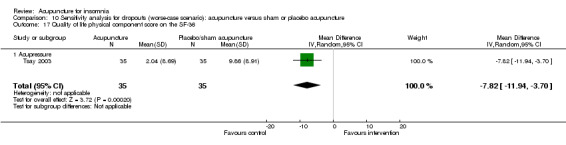
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 17 Quality of life physical component score on the SF‐36.
10.18. Analysis.
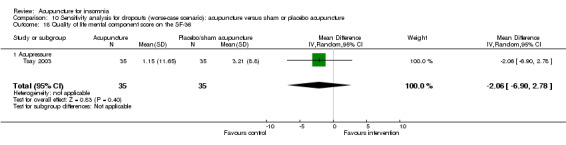
Comparison 10 Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture, Outcome 18 Quality of life mental component score on the SF‐36.
3. Acupuncture as adjunct to other treatment versus other treatment alone
There were no dropouts in the studies in this comparison and hence we did not perform sensitivity analysis for dropouts for this comparison.
Discussion
Summary of main results
There were a total of 33 randomised controlled trials (RCTs) included in the current review. They evaluated different forms of acupuncture (needle acupuncture, electroacupuncture, acupressure and magnetic acupressure) with or without additional treatment compared to sham or placebo control or no specific treatment. For the primary outcome, frequency of improvement in sleep quality, we found that when compared with no specific treatment, acupressure was associated with mild to moderate improvement in sleep quality (two studies). When compared with sham or placebo, acupressure also appeared to be associated with mild to moderate improvement in sleep quality (two studies). However, sensitivity analysis suggested that the beneficial effect was inconclusive if dropouts were taken into consideration. When evaluating the intervention as an adjunct to other treatment compared with other treatment alone, acupuncture (including needle acupuncture (11 studies) and electroacupuncture (two studies)) as an adjunct to other treatment mildly increased the proportion of people with improved sleep quality. However, in the subgroup analyses, only needle acupuncture, not electroacupuncture, showed conclusive benefit.
The results for secondary outcomes were mixed but most did not show clinically relevant or consistent benefits of acupuncture. Compared with no specific treatment, acupressure showed no clinically relevant benefit in total sleep duration (one study). Although primary analyses suggested participants who received acupuncture (including electroacupuncture (one study) and acupressure (five studies)) might have a better Pittsburgh Sleep Quality Index (PSQI) score, sensitivity analyses demonstrated no consistent benefit if dropouts were assumed to have a poor outcome. Acupressure might be associated with clinically relevant benefit on another sleep score. However, this was reported by one study only and we were uncertain whether the sleep score in this study was a validated measure. Acupressure showed no clinically relevant benefit on quality of life.
Compared to sham or placebo, acupuncture showed no clinically relevant benefit in most sleep parameters, including sleep onset latency (needle acupuncture (one study), electroacupuncture (one study), acupressure (three studies) and magnetic acupressure (one study)), number of awakenings (acupressure (two studies) and magnetic acupressure (two studies)), arousal index (acupressure (one study)) and sleep efficiency (electroacupuncture (one study), acupressure (three studies) and magnetic acupressure (two studies)). There was some evidence that needle acupuncture might result in clinically relevant improvement in total sleep duration, but this was based on one study only. Magnetic acupressure might reduce total wake time (one study), but sensitivity analysis on worst‐case scenario for dropouts showed no consistent, clinically relevant benefit. Acupuncture also did not show clinically relevant benefit in most sleep scores, including PSQI (electroacupuncture (one study) and acupressure (six studies)), ISI (needle acupuncture (two studies) and electroacupuncture (one study)), AIS (needle acupuncture (two studies) and acupressure (one study)), SQS (acupressure (one study)), and sleep quality score (electroacupuncture (one study)). Only needle acupuncture showed consistent, clinically relevant benefit in the sleep quality score on the Morning Questionnaire. However, this was reported by just one study. Acupuncture did not result in a clinically relevant benefit in daytime functioning (electroacupuncture (one study)) or quality of life (acupressure (two studies)).
Compared with other treatment alone, acupuncture as an adjunct to other treatment showed a clinically relevant benefit in total sleep duration (needle acupuncture and electroacupuncture). However, the benefit on sleep onset latency (needle acupuncture) was only modest with uncertain clinical relevance. There was no clinically relevant benefit on sleep efficiency or sleep score (needle acupuncture and electroacupuncture).
Overall completeness and applicability of evidence
Although 33 studies were included, the evidence regarding the effectiveness of acupuncture for insomnia was incomplete. Only 17 studies provided data on our pre‐specified primary outcome, i.e. frequency of patients with improved sleep quality. What constituted improvement of sleep quality was variably defined in these studies or not clearly defined. This made the results difficult to interpret and apply. Many studies did not report outcomes on sleep parameters. For those that reported on these parameters, most relied on participants' subjective report instead of objective measurements and hence the validity and reliability of the reported sleep parameters were doubtful. Other clinically important outcomes such as daytime functioning and quality of life were reported in only a few studies. Due to the limited availability of valid and reliable outcomes in the included trials, the power of the current meta‐analyses to draw conclusions on the effectiveness of acupuncture with respect to clinically important outcomes was limited.
In the majority of included studies the participants' characteristics were not described in sufficient detail. We did not know the causes of insomnia in the participants and whether the participants had comorbid physical or psychological symptoms that might have affected their response to treatment. Many studies did not describe the duration of insomnia or the treatments that participants had previously received. Therefore we cannot draw any conclusions related to the effects of acupuncture on different population groups.
The duration of follow‐up was short in all included studies. This might partially explain why a large proportion of studies had no dropouts. Whether acupuncture is efficacious in the long run is uncertain and needs further investigations.
Only a few studies reported adverse effects of acupuncture. It is uncertain whether adverse effects were entirely absent in the other trials or if there has been a failure to report them. Although adverse effects were not among the primary objectives of the individual trials focusing on the efficacy of acupuncture, it is considered unethical not to report adverse effects that occur during the trial. Furthermore, all trials included only small numbers of participants, so rare adverse effects might not have been experienced. We therefore do not have any evidence to inform us as to whether the potential benefits of acupuncture outweigh the potential risks.
There were no randomised controlled trials on acupuncture as an adjunct to other treatment compared with placebo/sham acupuncture as an adjunct to other treatment. Therefore we could not answer the question of whether acupuncture is an effective adjunctive treatment for insomnia. There were also no randomised controlled trials on certain forms of acupuncture such as laser acupuncture or auricular therapy and so the effectiveness of these types of acupuncture could not be evaluated.
Adverse events were only reported in a few trials and there were only a small number of minor adverse events that occurred. Although the existing literature on acupuncture in patients with insomnia and patients with a variety of other conditions has found that acupuncture is a relatively safe treatment modality which obviates the side effects of sleeping pills, acupuncture is not without risks. Numerous very serious adverse effects of acupuncture have been reported. Apart from pain, there are occasional instances of infections and inappropriate needle placement causing inadvertent damage. Acupressure or electrical or magnetic stimulation of acupoints might cause less pain and might be safer as no breach of skin occurs. However, excessive pressure applied to skin for a long period might result in tissue ischaemia or necrosis and electrical or magnetic stimulation might have other untoward effects. Therefore, all forms of acupuncture should only be performed by a well‐trained therapist who is experienced, understands the theories underpinning the practice of acupuncture, and takes the necessary precautions.
Quality of the evidence
Overall the quality of the evidence was poor, as all included studies were considered to have high risk of bias. Important potential biases in the studies included problems in sequence generation which might have led to some trials not being truly randomised. Allocation concealment and blinding were absent in most trials. These would likely result in an overestimation of treatment effects. As improvement in insomnia is largely subjective, proper randomisation concealment, blinding and standardised assessment of outcomes are extremely important. Some trials also had attrition and reporting biases. Baseline characteristics might not be comparable in several trials, making comparisons of outcomes prone to bias. Acupuncture methods used were not standardised or stratified within individual trials, making it difficult to draw conclusions on the effectiveness of a particular acupuncture protocol. We used funnel plots to assess publication bias, which appeared to be highly likely judging from the asymmetry. It was also noted that all the studies from mainland China had positive findings.
Many trials did not include a sham or placebo control group and therefore a placebo effect could not be excluded and the results of these trials might therefore be biased in favour of acupuncture. On the other hand, pragmatic trials without the use of sham or placebo acupuncture control can address different research questions. Although the non‐acupoints selected in sham control may not produce the specific effects that the real acupoints do, the sham acupoints may still be physiologically active and may produce non‐specific effects in addition to the placebo effects (Birch 2003). In other words, sham acupuncture might not be an entirely physiologically inert treatment and hence researchers need a larger sample size to detect a significant difference when a sham acupuncture control group is used. Similarly, placebo acupuncture, with needles attached to but not penetrating the skin surface on acupoints, can also have non‐specific effects. Therefore, interpreting randomised controlled trials of complex physical therapies such as acupuncture has inherent difficulties, as placebo and Hawthorn effects can mask the results (Mason 2002). Incidental factors are not clear in this type of study in contrast with pharmacological treatments (Paterson 2005). In these non‐pharmaceutical therapies the characteristic and incidental factors are intertwined and elements categorised as incidental in the context of drug trials are integral to complex non‐pharmaceutical interventions such as acupuncture. Therefore, the choice of a randomised design with pragmatic characteristics is important (Vickers 2004).
Some results had wide confidence intervals encompassing both positive and negative effects and were therefore inconclusive. The included studies were heterogeneous with respect to participant characteristics (age and gender of patients, underlying cause for insomnia and co‐morbidities), type of acupuncture, acupoints used, treatment regimen and duration, co‐interventions, control treatment and outcome measures. Therefore, only a small number of studies could be grouped together for analysis. Even then the studies within each analysis were heterogeneous with respect to patient population, acupoints used, treatment regimen and duration, and outcome definition. Therefore, it is impossible to draw any robust conclusions on the effectiveness of a particular acupuncture regimen for a particular patient population.
The definition of insomnia varied among the studies. There was no precise clinical diagnosis of insomnia. Some studies recruited participants who had a complaint of insomnia, whilst others used validated questionnaires or diagnostic criteria. The variation in the diagnosis of insomnia further increased the baseline differences among the studies and made the results difficult to interpret.
In addition, none of the trials provided a calculation of sample size before initiation of recruitment, and most of the studies were small with fewer than 100 participants, limiting the precision of effect size estimates and generalisability of the results. In addition, multiple comparisons with vast numbers of secondary outcome measures had inflated the alpha errors and hence the probability of false positive results.
Potential biases in the review process
We searched extensively in the international and Chinese literature. The current review is therefore more comprehensive than existing reviews, and it is likely that the majority of relevant studies were identified. However, studies that were not published in English or Chinese might have been missed. Information contained in the published reports of included trials was often inadequate and only a few authors responded to our enquiries about missing information. The missing information might potentially cause bias in this review. On the other hand, we tried to reduce the risk of bias in the current review by excluding quasi‐randomised controlled trials which are at higher risk of bias compared to truly randomised controlled trials. The study selection, data collection and analyses fully adhered to the pre‐defined protocol, which should have further reduced bias. However, we identified significant risk of bias in most of the included studies and therefore the results derived from meta‐analyses of these trials were susceptible to bias.
Agreements and disagreements with other studies or reviews
There are other systematic reviews of acupuncture or acupressure in the literature (Cao 2009; Huang 2009b; Kalavapalli 2007; Lee 2008; Sun 2010b; Yeung 2009b). These reviews had different inclusion and exclusion criteria and therefore included different sets of primary studies for analyses. However, most reviews had a similar conclusion, namely that there is no high‐quality evidence supporting the effectiveness of various forms of acupuncture for treatment of insomnia.
Only one review (Cao 2009) concluded that acupuncture appears to be effective in the treatment of insomnia. In the comparison of acupuncture versus no treatment in that review, meta‐analysis of three trials (Chen 1999; Tsay 2003; Tsay 2004) showed a better PSQI score (mean difference (MD) ‐3.28, 95% confidence interval (CI) ‐6.10 to ‐0.46, P = 0.02). All three of these trials were also included in the current review (which included two additional trials published after the review by Cao (Chen 2009; Reza 2010)) and the results were similar. In the comparison of acupuncture versus sham acupuncture in the review by Cao, meta‐analysis of two trials (Chen 1999; Tsay 2003) again showed better PSQI scores (MD ‐2.94, 95% CI ‐5.77 to ‐0.11, P = 0.04). These two trials were also included in the current review (which included four additional trials published later (Hwang 2007; Lin 2007; Nordio 2008; Reza 2010)) and the results were similar. In the comparison of acupuncture as an adjunct to other treatment versus other treatment alone, the review by Cao separated the comparison into two, namely acupuncture as an adjunct to Western medicine versus Western medicine alone, and acupuncture as an adjunct to Chinese herbs versus Chinese herbs alone. In the comparison of acupuncture as an adjunct to Western medicine versus Western medicine alone, meta‐analysis of three trials (Luo 2006; Ye 2008; Zhong 2008) showed a better PSQI score in the intervention group compared to the control group (MD ‐2.02, 95% CI ‐2.81 to ‐1.24, P < 0.00001). Meta‐analysis of two trials (Chen 2003; Li 2005a) showed better total sleep duration in the intervention group compared to the control group (MD 1.09, 95% CI 0.56 to 1.61, P < 0.0001). Meta‐analysis of four trials (Chen 2003; Li 2005a; Luo 2006; Ma 2006a) showed a higher likelihood of sleeping for more than three hours in the intervention group compared to the control group (risk ratio (RR) 1.33, 95% CI 1.03 to 1.71, P = 0.03). In the comparison of acupuncture as an adjunct to Chinese herbs versus Chinese herbs alone, meta‐analysis of two trials (Du 2007; Lu 1998) showed a higher likelihood of sleeping efficiency above 60% in the intervention group compared to the control group (RR 1.67, 95% CI 1.12 to 2.50, P = 0.01). Two of the trials (Chen 2003; Zhong 2008) in these comparisons of acupuncture as an adjunct to other treatment versus other treatment alone were excluded in the current review because of pseudo‐randomisation. We also did not include the outcomes of the frequency of people achieving sleep duration above three hours or sleep efficiency above 60%. In the current review, we showed similar improvement in PSQI score and total sleep duration in the group that received acupuncture as an adjunct to other treatment compared to other treatment alone.
Despite similar results in meta‐analyses in all comparisons between the review by Cao and the current review, the interpretation or results and conclusion were different. In the review by Cao, methodological quality of the included trials was assessed in a different way. Seven criteria were used: 1. clear description of population, setting, interventions and comparison groups; 2. appropriate measurement of outcomes; 3. appropriate statistical and analytical methods; 4. no reporting errors; 5. less than 20% dropouts; 6. clear reporting of dropouts; and 7. appropriate consideration and adjustment for potential confounders. According to these seven criteria, primary studies were categorised as good, fair or poor. However, how these seven criteria were integratively judged to the three categories of overall assessment was not clearly described and which study was assigned to which category was not reported in the review. Hence it was not clear whether the apparently better results in the acupuncture groups were based on good primary studies or not. In addition, the effect size and its clinical relevance was not considered in the Cao review, and no sensitivity analyses were performed to examine the robustness of the results with respect to the dropouts. The conclusion in the Cao review was also based on positive results only, disregarding the negative results in multiple other outcomes that showed no significant difference between the intervention and the control groups. All existing systematic reviews, including the review by Cao, suggested that further rigorously designed RCTs were warranted.
The effect size of acupuncture compared to other treatment modalities was similar in some outcomes. In a systematic review on sedative hypnotics for older people with insomnia, benzodiazepines were associated with an increase in total sleep duration by 34 minutes on average compared with placebo (Glass 2005). When all sedatives were combined, total sleep duration was 25 minutes longer in the intervention group compared to the placebo group. This appeared similar to the results of the current review. However, the number of awakenings was lowered by a mean of only 0.6 in those who received sedatives or benzodiazepines compared with placebo. This was significantly lower than the improvement in the number of awakenings (2.9 to 4.1) found in the current review for acupuncture compared with placebo. In another systematic review on cognitive behavioural therapy for people above 60 years old with insomnia (Montgomery 2009), sleep onset latency was reduced by an average of three minutes in the intervention group compared to the control group, which was similar to the effect size of acupuncture in the current review. Total sleep duration and sleep efficiency were increased by an average of 7.5% and 32 minutes respectively in people who received cognitive behavioural therapy compared to the control group, which were also similar to the effect size of acupuncture. Total wake time and wake after sleep onset were reduced by 62 minutes and 22 minutes respectively in people who received cognitive behavioural therapy, which were not markedly different from people who received acupuncture. PSQI score was reduced by 2.8 points in people who received cognitive behavioural therapy, which was slightly better than people who received acupuncture.
Authors' conclusions
Implications for practice.
This review suggests that there is insufficient high‐quality evidence to support or refute the use of acupuncture to improve people's self rated sleep quality. Although some forms of acupuncture might improve sleep parameters in the short term in some people, the current evidence is not rigorous enough to allow recommendation to be made about the wide application of any form of acupuncture with or without additional therapies for the treatment of insomnia of any aetiology in people of any age group. The long‐term effect of acupuncture is not known and its potential adverse effects are not entirely clear. People who seek to receive acupuncture for insomnia should be informed of the uncertainty of its effectiveness and potential risks.
Implications for research.
Existing randomised controlled trials of acupuncture for treatment of insomnia are of small size and low methodological quality. Further high‐quality studies of larger sample size are needed to assess the effectiveness of acupuncture for treating insomnia. Randomisation methods need to be more rigorous and concealed. Although blinding of the therapist who applied acupuncture might be very difficult, blinding of the patients, the other care providers and outcome assessors should be attempted as far as possible to minimise performance and assessment biases. In the current review, only three included studies achieved blinding of patients, care takers and outcome assessors (Nordio 2008; Suen 2002; Tsay 2003). They employed sham acupuncture methods in the control group that were essentially indistinguishable from real acupuncture by lay people without good knowledge about genuine acupoints. We advise future studies to employ a similar design, such that only the acupuncturist knows who receives genuine acupuncture and who receives sham acupuncture; and the acupuncturist should not be involved in other aspects of participants' care or assessment of outcomes.
Analysis of outcomes based on intention‐to‐treat principle is important. All outcomes specified in the trial protocol should be reported. A standardised set of outcome measures is important for comparison among different trials. These should include measurements of sleep parameters objectively using actigraphy or polysomnography so that even if the person administering and receiving acupuncture is not blind, the outcome assessors could be blinded to treatment. Other well‐known standardised sleep scores such as the Pittsburgh Sleep Quality Index could also be used. Daytime functioning and quality of life are also important outcomes to be included in future trials, and these should be measured using validated instruments. Adverse effects should be closely monitored prospectively and reported.
Since insomnia is a highly heterogeneous disease with different aetiology and severity, acupuncture is likely to have different effects on different subgroups of patients. Therefore, future clinical trials should be focused on a particular subgroup or include a very large sample size to delineate the effect of acupuncture on different types of patients. In addition, well‐defined diagnostic criteria, such as DSM‐IV or ICSD, should be employed to make a precise clinical diagnosis of insomnia, and hence increase the comparability between studies. Since insomnia may wax and wane with or without treatment, a longer follow‐up period with serial measurements of outcomes is important to determine the genuine effectiveness of acupuncture and its long‐term effect. It might also be worthwhile to further examine the effectiveness of combination therapy for insomnia using acupuncture with other non‐pharmacological or pharmacological treatments.
What's new
| Date | Event | Description |
|---|---|---|
| 23 May 2012 | New search has been performed | New studies incorporated |
| 23 May 2012 | New citation required but conclusions have not changed | Methods updated |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 30 March 2011 | New search has been performed | Search and review were updated |
| 31 October 2008 | Amended | Converted to new review format |
| 4 May 2007 | New search has been performed | Minor update |
| 24 March 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Sarah Dawson, Trials Search Co‐ordinator, for her help in searching various databases. We are also grateful to the staff of the Medical Library of the University of Hong Kong for their help in finding the articles relevant to this review. We thank Dr Maurizio Nordio and Dr Chia‐Chin Lin for providing further information about their trials.
Appendices
Appendix 1. Search strategy for CENTRAL
#1 MeSH descriptor Acupuncture, this term only #2 MeSH descriptor Acupuncture Therapy, explode all trees [Acupuncture Therapy / or Acupuncture Analgesia/ or Acupuncture, Ear/ or Electroacupuncture/ or Meridians / or Acupuncture Points/ or Moxibustion/] #3 (acupunct* or acupress* or acupoints* or electroacupunct* or electro‐acupunct*):ti,ab,kw #4 (auriculotherap* or auriculoacupunct* or moxibust*):ti,ab,kw #5 (#1 or #2 or #3 or #4) #6 insomnia*:ti,ab,kw #7 sleep:ti,ab,kw #8 MeSH descriptor Sleep Initiation and Maintenance Disorders, this term only #9 MeSH descriptor Insomnia, Fatal Familial, this term only #10 MeSH descriptor Sleep, this term only #11 MeSH descriptor Sleep Deprivation, this term only #12 MeSH descriptor Sleep Stages, this term only #13 MeSH descriptor Wakefulness, this term only #14 (#6 or #7 or #8 or #9 or #10 or #11 or #12 or #13) #15 (#5 or #14)
Appendix 2. Search strategy for MEDLINE, EMBASE, PsycINFO, Dissertation Abstracts International, CINAHL and AMED
1. exp acupuncture/ 2. acupunc$.mp 3. acupress$.mp 4. electroacupunc$.mp 5. meridian$.mp 6. acupoints$.mp 7. or/1‐6 8. exp sleep/ 9. sleep$.mp 10. insomnia$.mp 11. wakeful$.mp 12. sleepless$.mp 13. somnambul$.mp 14. or/8‐13 15. 7 and 14
Appendix 3. Search strategy for TCMLARS
1. "ZhenJiu" (acupuncture) or "ZhenCi" (acupuncture) or "DianZhen" (electroacupuncture) or "ZhenYa" (acupressure) or "ErZhen" (auricular acupuncture) or "XueWei" (acupoints) 2. "Shimian" (insomnia) or "Shuimian" (sleep) 3. 1 and 2
Appendix 4. Search strategy for ICTRP
(acupuncture and insomnia)
Appendix 5. Search strategy for Trials Register of the Cochrane Complementary Medicine Field and CCDANCTR
(acupunct* or acupress* or acupoints* or electroacupunct* or electro‐acupunct* or auriculotherap* or auriculoacupunct* or moxibust*) and (insomnia* or "sleep initiation and maintenance disorder*" or sleep or wakefulness)
Data and analyses
Comparison 1. Acupuncture alone versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of improvement in sleep quality | 2 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 13.08 [1.79, 95.59] |
| 1.1 Acupressure | 2 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 13.08 [1.79, 95.59] |
| 2 Total sleep duration (hours) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.14, 1.46] |
| 2.1 Acupressure | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.14, 1.46] |
| 3 Total score on the Pittsburgh Sleep Quality Index (PSQI) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Acupressure | 5 | 443 | Mean Difference (IV, Random, 95% CI) | ‐3.87 [‐5.14, ‐2.60] |
| 3.2 Electroacupuncture | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐3.43 [‐5.57, ‐1.29] |
| 4 Sleep score | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 30.65 [30.52, 30.78] |
| 4.1 Acupressure | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 30.65 [30.52, 30.78] |
| 5 Quality of life physical component score on the SF‐36 | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 3.57 [‐0.51, 7.65] |
| 5.1 Acupressure | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 3.57 [‐0.51, 7.65] |
| 6 Quality of life mental component score on the SF‐36 | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 3.69 [‐1.13, 8.51] |
| 6.1 Acupressure | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 3.69 [‐1.13, 8.51] |
Comparison 2. Acupuncture alone versus placebo or sham acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of improvement in sleep quality | 2 | 112 | Odds Ratio (M‐H, Random, 95% CI) | 6.62 [1.78, 24.55] |
| 1.1 Acupressure | 2 | 112 | Odds Ratio (M‐H, Random, 95% CI) | 6.62 [1.78, 24.55] |
| 2 Sleep onset latency (minutes) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Needle acupuncture | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐59.30 [‐156.02, 37.42] |
| 2.2 Electroacupuncture | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐2.5 [‐18.28, 13.28] |
| 2.3 Acupressure | 3 | 142 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐15.76, 12.87] |
| 2.4 Magnetic acupressure | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐7.41 [‐13.91, ‐0.91] |
| 3 Total sleep duration (hours) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Needle acupuncture | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 1.43 [0.43, 2.43] |
| 3.2 Electroacupuncture | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.54, 0.44] |
| 3.3 Acupressure | 3 | 142 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.59, 1.20] |
| 3.4 Magnetic acupressure | 2 | 112 | Mean Difference (IV, Random, 95% CI) | 0.93 [0.38, 1.48] |
| 4 Total wake time (minutes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Acupressure | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐25.80 [‐69.84, 18.24] |
| 4.2 Magnetic acupressure | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐68.75 [‐105.58, ‐31.92] |
| 5 Wake after sleep onset (minutes) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Electroacupuncture | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐33.60 [‐74.16, 6.96] |
| 5.2 Acupressure | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐17.30 [‐56.42, 21.82] |
| 5.3 Magnetic acupressure | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐54.98 [‐85.53, ‐24.43] |
| 6 Number of awakenings | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Acupressure | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐6.70, ‐1.50] |
| 6.2 Magnetic acupressure | 2 | 112 | Mean Difference (IV, Random, 95% CI) | ‐2.88 [‐7.08, 1.32] |
| 7 Arousal index (number of arousals per hour) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐7.37, 7.53] |
| 7.1 Acupressure | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐7.37, 7.53] |
| 8 Sleep efficiency (%) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Electroacupuncture | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 7.5 [1.39, 13.61] |
| 8.2 Acupressure | 3 | 142 | Mean Difference (IV, Random, 95% CI) | 3.44 [‐9.30, 16.18] |
| 8.3 Magnetic acupressure | 2 | 112 | Mean Difference (IV, Random, 95% CI) | 9.41 [4.21, 14.60] |
| 9 Total score on the Pittsburgh Sleep Quality Index (PSQI) | 7 | 346 | Mean Difference (IV, Random, 95% CI) | ‐2.11 [‐3.39, ‐0.83] |
| 9.1 Electroacupuncture | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.31, 1.71] |
| 9.2 Acupressure | 6 | 289 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐3.64, ‐1.51] |
| 10 Total score on the Insomnia Severity Index (ISI) | 3 | 139 | Mean Difference (IV, Random, 95% CI) | ‐3.38 [‐6.24, ‐0.52] |
| 10.1 Needle acupuncture | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐4.64 [‐7.45, ‐1.83] |
| 10.2 Electroacupuncture | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐3.32, 1.52] |
| 11 Total score on the Athens Insomnia Scale (AIS) | 3 | 132 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐4.69, ‐0.09] |
| 11.1 Needle acupuncture | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐3.41 [‐5.64, ‐1.19] |
| 11.2 Acupressure | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐2.33, 1.53] |
| 12 Total score on the Sleep Quality Scale (SQS) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐7.91 [‐17.16, 1.34] |
| 12.1 Acupressure | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐7.91 [‐17.16, 1.34] |
| 13 Quality of sleep score on the Morning Questionnaire (MQ) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 34.60 [19.08, 50.12] |
| 13.1 Needle acupuncture | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 34.60 [19.08, 50.12] |
| 14 Sleep quality score: sleep diary | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.20, 0.26] |
| 14.1 Electroacupuncture | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.20, 0.26] |
| 15 Work score on the Sheehan Disability Index (SDI) | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.16, 1.96] |
| 15.1 Electroacupuncture | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.16, 1.96] |
| 16 Social score on the Sheehan Disability Index (SDI) | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.69, 1.29] |
| 16.1 Electroacupuncture | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.69, 1.29] |
| 17 Family score on the Sheehan Disability Index (SDI) | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.46, 1.46] |
| 17.1 Electroacupuncture | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.46, 1.46] |
| 18 Quality of life total score on the General Health Questionnaires (GHQ) | 1 | 33 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐3.07, 0.25] |
| 18.1 Acupressure | 1 | 33 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐3.07, 0.25] |
| 19 Quality of life physical component score on the SF‐36 | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐6.45 [‐10.45, ‐2.45] |
| 19.1 Acupressure | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐6.45 [‐10.45, ‐2.45] |
| 20 Quality of life mental component score on the SF‐36 | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐5.44, 4.04] |
| 20.1 Acupressure | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐5.44, 4.04] |
| 21 Frequency of adverse effects | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 3.19 [0.12, 84.43] |
| 21.1 Needle acupuncture | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 3.19 [0.12, 84.43] |
Comparison 3. Acupuncture as adjunctive to other treatment versus other treatment alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of improvement in sleep quality | 13 | 883 | Odds Ratio (M‐H, Random, 95% CI) | 3.08 [1.93, 4.90] |
| 1.1 Needle acupuncture | 11 | 773 | Odds Ratio (M‐H, Random, 95% CI) | 3.26 [1.93, 5.49] |
| 1.2 Electroacupuncture | 2 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 1.96 [0.62, 6.23] |
| 2 Sleep onset latency (minutes) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐21.32 [‐24.85, ‐17.79] |
| 2.1 Needle acupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐21.32 [‐24.85, ‐17.79] |
| 3 Total sleep duration (hours) | 3 | 178 | Mean Difference (IV, Random, 95% CI) | 1.28 [0.90, 1.66] |
| 3.1 Needle acupuncture | 2 | 128 | Mean Difference (IV, Random, 95% CI) | 1.30 [0.77, 1.82] |
| 3.2 Electroacupuncture | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 1.05 [0.35, 1.75] |
| 4 Sleep efficiency (%) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | 5.06 [2.14, 7.99] |
| 4.1 Needle acupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.65 [‐8.70, 12.00] |
| 4.2 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 5.36 [2.31, 8.41] |
| 5 Total score on the Pittsburgh Sleep Quality Index (PSQI) | 3 | 146 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐3.41, ‐1.74] |
| 5.1 Needle acupuncture | 2 | 86 | Mean Difference (IV, Random, 95% CI) | ‐2.69 [‐3.73, ‐1.65] |
| 5.2 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.37 [‐3.77, ‐0.97] |
| 6 Frequency of adverse effects | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 1.03] |
| 6.1 Needle acupuncture | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 1.03] |
Comparison 4. Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of improvement in sleep quality | 1 | 200 | Odds Ratio (M‐H, Random, 95% CI) | 35.29 [13.13, 94.84] |
| 1.1 Acupressure | 1 | 200 | Odds Ratio (M‐H, Random, 95% CI) | 35.29 [13.13, 94.84] |
Comparison 5. Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture versus sham or placebo acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total sleep duration (hours) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Magnetic acupressure | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.88 [0.28, 1.48] |
| 2 Number of awakenings | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Magnetic acupressure | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐5.25 [‐8.12, ‐2.38] |
| 3 Sleep efficiency (%) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 11.55 [5.52, 17.58] |
| 3.1 Magnetic acupressure | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 11.55 [5.52, 17.58] |
| 4 Total score on the Pittsburgh Sleep Quality Index (PSQI) | 6 | 316 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐3.58, ‐0.59] |
| 4.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.28, 1.68] |
| 4.2 Acupressure | 5 | 256 | Mean Difference (IV, Random, 95% CI) | ‐2.62 [‐3.89, ‐1.34] |
Comparison 6. Sensitivity analysis excluding studies without standardised insomnia diagnosis: acupuncture as adjunct to other treatment versus other treatment alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of improvement in sleep quality | 5 | 353 | Odds Ratio (M‐H, Random, 95% CI) | 4.55 [2.20, 9.39] |
| 1.1 Needle acupuncture | 3 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 7.28 [3.04, 17.46] |
| 1.2 Electroacupuncture | 2 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 1.96 [0.62, 6.23] |
| 2 Total sleep duration (hours) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 1.05 [0.35, 1.75] |
| 2.1 Electroacupuncture | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 1.05 [0.35, 1.75] |
| 3 Sleep efficiency (%) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 5.36 [2.31, 8.41] |
| 3.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 5.36 [2.31, 8.41] |
| 4 Total score on the Pittsburgh Sleep Quality Index (PSQI) | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐2.23 [‐3.35, ‐1.11] |
| 4.1 Needle acupuncture | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐3.84, ‐0.12] |
| 4.2 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.37 [‐3.77, ‐0.97] |
Comparison 7. Sensitivity analysis for dropouts (best‐case scenario): acupuncture alone versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total score on the Pittsburgh Sleep Quality Index (PSQI) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Acupressure | 5 | 470 | Mean Difference (IV, Random, 95% CI) | ‐4.71 [‐6.50, ‐2.91] |
| 1.2 Electroacupuncture | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐3.69 [‐5.86, ‐1.52] |
| 2 Quality of life physical component score on the SF‐36 | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 5.09 [1.27, 8.91] |
| 2.1 Acupressure | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 5.09 [1.27, 8.91] |
| 3 Quality of life mental component score on the SF‐36 | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 5.57 [0.62, 10.52] |
| 3.1 Acupressure | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 5.57 [0.62, 10.52] |
Comparison 8. Sensitivity analysis for dropouts (worst‐case scenario): acupuncture alone versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total score on the Pittsburgh Sleep Quality Index (PSQI) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Acupressure | 5 | 470 | Mean Difference (IV, Random, 95% CI) | ‐2.77 [‐3.70, ‐1.84] |
| 1.2 Electroacupuncture | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐3.17 [‐5.34, ‐1.00] |
| 2 Quality of life physical component score on the SF‐36 | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 2.05 [‐1.77, 5.87] |
| 2.1 Acupressure | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 2.05 [‐1.77, 5.87] |
| 3 Quality of life mental component score on the SF‐36 | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐3.14, 6.76] |
| 3.1 Acupressure | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐3.14, 6.76] |
Comparison 9. Sensitivity analysis for dropouts (best‐case scenario): acupuncture versus sham or placebo acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of improvement in sleep quality | 2 | 128 | Odds Ratio (M‐H, Random, 95% CI) | 10.43 [2.88, 37.73] |
| 1.1 Acupressure | 2 | 128 | Odds Ratio (M‐H, Random, 95% CI) | 10.43 [2.88, 37.73] |
| 2 Sleep onset latency (minutes) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Needle acupuncture | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐84.90 [‐183.00, 13.20] |
| 2.2 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐7.20 [‐23.16, 8.76] |
| 2.3 Acupressure | 3 | 153 | Mean Difference (IV, Random, 95% CI) | ‐4.17 [‐20.33, 12.00] |
| 2.4 Magnetic acupressure | 1 | 103 | Mean Difference (IV, Random, 95% CI) | ‐13.53 [‐20.26, ‐6.80] |
| 3 Total sleep duration (hours) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Needle acupuncture | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 1.77 [0.71, 2.82] |
| 3.2 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.38, 0.65] |
| 3.3 Acupressure | 3 | 153 | Mean Difference (IV, Random, 95% CI) | 0.55 [‐0.30, 1.40] |
| 3.4 Magnetic acupressure | 2 | 125 | Mean Difference (IV, Random, 95% CI) | 1.50 [0.92, 2.09] |
| 4 Total wake time (minutes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Acupressure | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐77.06 [‐123.85, ‐30.27] |
| 4.2 Magnetic acupressure | 1 | 103 | Mean Difference (IV, Random, 95% CI) | ‐108.35 [‐147.61, ‐69.09] |
| 5 Wake after sleep onset (minutes) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐46.5 [‐87.90, ‐5.10] |
| 5.2 Acupressure | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐60.25 [‐100.92, ‐19.58] |
| 5.3 Magnetic acupressure | 1 | 103 | Mean Difference (IV, Random, 95% CI) | ‐87.25 [‐119.79, ‐54.71] |
| 6 Number of awakenings | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Acupressure | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐5.78 [‐8.03, ‐3.53] |
| 6.2 Magnetic acupressure | 2 | 125 | Mean Difference (IV, Random, 95% CI) | ‐4.55 [‐11.90, 2.81] |
| 7 Sleep efficiency (%) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 9.80 [3.43, 16.17] |
| 7.2 Acupressure | 3 | 153 | Mean Difference (IV, Random, 95% CI) | 6.22 [‐7.82, 20.25] |
| 7.3 Magnetic acupressure | 2 | 125 | Mean Difference (IV, Random, 95% CI) | 12.40 [0.49, 24.31] |
| 8 Total score on the Pittsburgh Sleep Quality Index (PSQI) | 7 | 380 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐4.86, ‐1.35] |
| 8.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.88, 1.28] |
| 8.2 Acupressure | 6 | 320 | Mean Difference (IV, Random, 95% CI) | ‐3.61 [‐5.28, ‐1.94] |
| 9 Total score on the Insomnia Severity Index (ISI) | 3 | 152 | Mean Difference (IV, Random, 95% CI) | ‐4.74 [‐7.86, ‐1.63] |
| 9.1 Needle acupuncture | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐6.20 [‐8.21, ‐4.18] |
| 9.2 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐4.31, 0.71] |
| 10 Total score on the Athens Insomnia Scale (AIS) | 3 | 142 | Mean Difference (IV, Random, 95% CI) | ‐3.46 [‐6.76, ‐0.16] |
| 10.1 Needle acupuncture | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐4.82 [‐6.49, ‐3.15] |
| 10.2 Acupressure | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐2.33, 1.53] |
| 11 Quality of sleep score on the Morning Questionnaire (MQ) | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 39.1 [23.12, 55.08] |
| 11.1 Needle acupuncture | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 39.1 [23.12, 55.08] |
| 12 Sleep quality score: sleep diary | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.29, 0.19] |
| 12.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.29, 0.19] |
| 13 Work score on the Sheehan Disability Index (SDI) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.59, 1.59] |
| 13.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.59, 1.59] |
| 14 Social score on the Sheehan Disability Index (SDI) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.01, 1.01] |
| 14.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.01, 1.01] |
| 15 Family score on the Sheehan Disability Index (SDI) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.79, 1.19] |
| 15.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.79, 1.19] |
| 16 Quality of life total score on the General Health Questionnaires (GHQ) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐2.95 [‐4.69, ‐1.21] |
| 16.1 Acupressure | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐2.95 [‐4.69, ‐1.21] |
| 17 Quality of life physical component score on the SF‐36 | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐5.07 [‐9.19, ‐0.95] |
| 17.1 Acupressure | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐5.07 [‐9.19, ‐0.95] |
| 18 Quality of life mental component score on the SF‐36 | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐4.18, 5.50] |
| 18.1 Acupressure | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐4.18, 5.50] |
Comparison 10. Sensitivity analysis for dropouts (worse‐case scenario): acupuncture versus sham or placebo acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of improvement in sleep quality | 2 | 128 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [0.12, 24.31] |
| 1.1 Acupressure | 2 | 128 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [0.12, 24.31] |
| 2 Sleep onset latency (minutes) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Needle acupuncture | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐31.60 [‐130.08, 66.88] |
| 2.2 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐13.81, 18.21] |
| 2.3 Acupressure | 3 | 153 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐14.62, 16.82] |
| 2.4 Magnetic acupressure | 1 | 103 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐6.98, 6.66] |
| 3 Total sleep duration (hours) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Needle acupuncture | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 1.10 [0.04, 2.15] |
| 3.2 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.75, 0.28] |
| 3.3 Acupressure | 3 | 153 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐1.05, 1.15] |
| 3.4 Magnetic acupressure | 2 | 125 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.39, 1.42] |
| 4 Total wake time (minutes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Acupressure | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 27.84 [‐19.69, 75.37] |
| 4.2 Magnetic acupressure | 1 | 103 | Mean Difference (IV, Random, 95% CI) | ‐26.66 [‐66.33, 13.01] |
| 5 Wake after sleep onset (minutes) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐22.80 [‐63.17, 17.57] |
| 5.2 Acupressure | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 29.53 [‐12.32, 71.38] |
| 5.3 Magnetic acupressure | 1 | 103 | Mean Difference (IV, Random, 95% CI) | ‐21.43 [‐53.86, 11.00] |
| 6 Number of awakenings | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Acupressure | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐8.69, 4.98] |
| 6.2 Magnetic acupressure | 2 | 125 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐2.04, ‐0.07] |
| 7 Sleep efficiency (%) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 5.10 [‐1.26, 11.46] |
| 7.2 Acupressure | 3 | 153 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐13.14, 14.47] |
| 7.3 Magnetic acupressure | 2 | 125 | Mean Difference (IV, Random, 95% CI) | 5.30 [0.32, 10.28] |
| 8 Total score on the Pittsburgh Sleep Quality Index (PSQI) | 7 | 380 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.08, ‐0.12] |
| 8.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.88, 2.28] |
| 8.2 Acupressure | 6 | 320 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐2.33, ‐0.75] |
| 9 Total score on the Insomnia Severity Index (ISI) | 3 | 152 | Mean Difference (IV, Random, 95% CI) | ‐1.78 [‐4.40, 0.84] |
| 9.1 Needle acupuncture | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐2.82 [‐6.53, 0.88] |
| 9.2 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐2.51, 2.51] |
| 10 Total score on the Athens Insomnia Scale (AIS) | 3 | 142 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.92, 0.44] |
| 10.1 Needle acupuncture | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐2.03 [‐5.00, 0.94] |
| 10.2 Acupressure | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐2.33, 1.53] |
| 11 Quality of sleep score on the Morning Questionnaire (MQ) | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 29.20 [12.84, 45.56] |
| 11.1 Needle acupuncture | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 29.20 [12.84, 45.56] |
| 12 Sleep quality score: sleep diary | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.13, 0.35] |
| 12.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.13, 0.35] |
| 13 Work score on the Sheehan Disability Index (SDI) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.20 [0.14, 2.26] |
| 13.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.20 [0.14, 2.26] |
| 14 Social score on the Sheehan Disability Index (SDI) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.39, 1.59] |
| 14.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.39, 1.59] |
| 15 Family score on the Sheehan Disability Index (SDI) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.17, 1.77] |
| 15.1 Electroacupuncture | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.17, 1.77] |
| 16 Quality of life total score on the General Health Questionnaires (GHQ) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.58, 1.98] |
| 16.1 Acupressure | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.58, 1.98] |
| 17 Quality of life physical component score on the SF‐36 | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐7.82 [‐11.94, ‐3.70] |
| 17.1 Acupressure | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐7.82 [‐11.94, ‐3.70] |
| 18 Quality of life mental component score on the SF‐36 | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐6.90, 2.78] |
| 18.1 Acupressure | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐6.90, 2.78] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 1999.
| Methods | Design: parallel‐group
Randomisation method: block randomisation Method of allocation concealment: information not available Blinding: single‐blind (patients) Stratification: according to combinations of hypertension, exercise, use of hypnotics and naps |
|
| Participants | Inclusion: PSQI score > 5, with clear mental status, without dementia, able to communicate in Mandarin or Taiwanese, not out of town during weekdays, age > 60 years, able to sit > 15 minutes, absence of any amputations of the upper extremities and no infection, injury, bleeding, thrombophlebitis or tumours nearby the chosen acupressure points in the head, neck and hands
Exclusion: nil Number of participants: intervention: 34; control 1 (sham acupressure): 34; control 2 (no treatment): 34 Number of males: 52 of 84 who remained in study Age (years): range 61 to 98, mean 79.04 (SD 7.77) Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder: information not available Previous treatments: information not available |
|
| Interventions | Intervention group (acupressure): massage to the following acupoints: Baihui, Fengchi, Anmian and Shenmen, and hands for 15 minutes, which consisted of 5 minutes of finger massage and 10 minutes of acupoints massage (2 minutes per each acupoints). The correctness of acupressure was confirmed if the participants felt sore, numb, heavy, distended and/or warm. Administration time of interventions was between 1 pm and prior to sleep (before 10 pm) Control group 1 (sham acupressure): sham acupressure, at non‐acupressure points Control group 2 (no specific treatment): conversation only Duration of treatment: 15 minutes per session, 5 sessions per week for 3 weeks | |
| Outcomes | 1. Frequency of improvement in sleep quality 2. Pittsburgh Sleep Quality Index (PSQI) |
|
| Notes | Duration of follow‐up: 5 weeks
Dropouts: intervention group: 6; control 1: 6; control 2: 6. Reasons not described Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, living conditions, current use of drugs, numbers of chronic diseases, admission time to the living‐assisted facility, habits of naps, exercise, time in bed, consumption of milk, tea and coffee, smoking or baseline PSQI scores Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only patients were blinded. Treating physicians were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were excluded but reasons not provided. There were no data for 18% of participants initially randomised. |
| Selective reporting (reporting bias) | High risk | Data on the frequency of improvement of sleep quality were not reported for the no treatment control group |
| Other bias | Low risk | No other bias was apparent |
Chen 2009.
| Methods | Design: parallel‐group
Randomisation method: block randomisation (4 participants in 1 block), used random number to generate allocation sequence Method of allocation concealment: allocation numbers were put in sealed, numbered, opaque envelopes Blinding: single‐blind (patients) Stratification: not used |
|
| Participants | Inclusion: diagnosis of insomnia according to Chinese Classification of Mental Disorder‐2 Revised (CCDM‐2R), age 18 to 65 years, no ulcers on head or ears
Exclusion: patients who are receiving other therapies that might affect evaluation of study therapy were excluded. Patients with severe diseases in cardiovascular, cerebrovascular, hepatic, renal or blood diseases or psychiatric diseases were excluded. Pregnant or lactating women were excluded. Patients whose major symptom was not insomnia were excluded. Number of participants: intervention: 100; control: 100 Number of males: intervention: 42; control: 44 Age: intervention: 16 to 30 years (22), 31 to 45 years (30), 46 to 60 years (48), range 19 to 58 years; control: 16 to 30 years (20), 31 to 45 years (31), 46 to 60 years (49), range 18 to 56 years Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder (years): intervention: mean 2.5, range 0.6 to 10; control: mean 2.3, range 0.5 to 9 Previous treatments: intervention: 11 participants frequently used hypnotics, 31 participants occasionally used hypnotics; Control: 11 participants frequently used hypnotics, 36 participants occasionally used hypnotics |
|
| Interventions | Intervention group (acupressure): massage to head including the following acupoints: Yintang, Shenting, Baihui, Taiyang, applied alternate day. Acupressure was applied at Shenmen and auricular acupoints of heart, lung, spleen, kidney, liver, endocrine, sympathetic and cortical areas. Seeds were attached to a small bandage to apply pressure 3 times daily.
Control group (no specific therapy): no specific therapy was given Duration of therapy: 1 month |
|
| Outcomes | 1. Frequency of improvement in sleep quality: cure was defined as normalised total sleep duration or total sleep duration at least 6 hours, with deep and refreshing sleep. Moderate improvement was defined as obvious improvement in sleep, with increase in total sleep duration by at least 3 hours and increase in depth of sleep. Some improvement was defined as symptom improvement with increase in total sleep duration by less than 3 hours. No improvement was defined as no change in symptoms or worsening symptoms. 2. Pittsburgh Sleep Quality Index (PSQI) 3. Self rated Depression Scale (SDS) 4. Self rated Anxiety Scale (SAS) |
|
| Notes | Duration of follow‐up: 1 month
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, occupation, education levels and previous treatments. However, patients in the control group appeared to have lower (better) scores in sleep onset latency of PSQI at baseline. Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number was used to generate randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Allocation number was put in numbered, sealed and opaque envelope |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only patients were blinded. Treating physicians were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | No placebo or sham control was used and hence there might be a placebo effect. The 2 groups might not have comparable sleep onset latency at baseline. |
Cui 2003.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: diagnosis of insomnia with sleep efficiency < 60%, with Traditional Chinese Medicine type of interior stirring by phlegm‐heat
Exclusion: patients with severe diseases of heart, liver, kidney or brain or patients who could not follow through whole treatment were excluded Number of participants: intervention: 60; control: 60 Number of males: intervention: 34; control: 28 Age (years): intervention: mean 43.2; control: mean 38.45 Specific diagnoses/diagnostic subtypes: all patients had insomnia with Traditional Chinese Medicine type of interior stirring by phlegm‐heat Associated disease: information not available Duration of disorder (years): information not available Previous treatments: information not available |
|
| Interventions | Intervention group (needle acupuncture + estazolam): acupuncture to the following acupoints: Baihui, Shenting, Sishencong, Shenmen, Neiguan, Zhongwan, Fenglong and Gongsun. A filiform needle 1 to 1.5 inches in length was inserted to the acupoints with uniform reinforcing‐reducing manoeuvre. Treatment was applied daily for 30 days. Estazolam was given 1 to 2 mg orally every night for 30 days. Control group (estazolam alone): estazolam was given as in intervention group | |
| Outcomes | Frequency of improvement in sleep quality. Cure was defined as sleep efficiency > 75% without hypnotics use. Marked improvement was defined as increase in sleep efficiency by 10% to 20% without hypnotics use. Some improvement was defined as increase in sleep efficiency by less than 10% with hypnotics reduced by 75% in dosage. No improvement was defined as no change in sleep efficiency. Deterioration was defined as worsening of symptoms, decrease in sleep efficiency or increase in estazolam use. | |
| Notes | Duration of follow‐up: 30 days
Dropouts: none Comparability of groups at baseline: no information was available on baseline severity of insomnia of the treatment groups Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | No placebo or sham control was used and hence there might be a placebo effect. Baseline severity of insomnia was not described and the comparability of the treatment groups was questionable. |
Du 2007.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: participants with insomnia
Exclusion: nil Number of participants: intervention: 34; control: 20 Number of males: intervention: 12; control: 7 Age (years): intervention: mean 55.3, range 20 to 82; control: mean 54, range 20 to 79 Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder: 1 week to 40 years Previous treatments: information not available |
|
| Interventions | Intervention group (needle acupuncture + Chinese herb): acupuncture to the following acupoints: Yintang, Sanyinjiao and 1 inch above medial malleolus. Patients with deficiencies in heart and spleen were additionally treated at Xinshu and Pishu. Patients with deficiency in liver with heat were additionally treated at Ganshu, Shenshu and Taixi. Patients with deficiencies in heart and kidney were additionally treated at Xinshu, Shenshu and Zhishi. Patients with heat in liver and gallbladder were additionally treated at Ganshu, Xingjian and Xuqiaoyin. The needles were left in place for 30 to 60 minutes. Treatment was applied 3 times per week. Chinese herbs contain amber, cortex of Albiziae, Paeoniae alba. Patients with deficiencies in heart and spleen were additionally given Guipitang. Patients with deficiency in liver with heat were additionally given Jujube seed decoction. Patients with deficiencies in heart and kidney were additionally given Shengdi, Baiziren. Patients with heat in liver and gallbladder were additionally given Wendantang. Herbs were boiled and taken twice daily.
Control group (Chinese herb alone): Chinese herb alone as in intervention group Duration of treatment: 3 weeks |
|
| Outcomes | Frequency of improvement in sleep quality. Cure was defined as sleep efficiency > 75% without hypnotics use. Moderate improvement was defined as sleep efficiency > 65% without hypnotics use. Some improvement was defined as symptom improvement with sleep efficiency > 55% with hypnotics reduced by > 75% in dosage. No improvement was defined as no change in symptoms with sleep efficiency <40% or requires hypnotics. | |
| Notes | Duration of follow‐up: 3 weeks
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, and severity of insomnia at baseline. Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | Treatment regimen varied among patients and might introduce bias. No placebo or sham control was used and hence there might be a placebo effect. |
Guo 2009.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: age 18 to 65 years, diagnosis of insomnia by Chinese Classification of Mental Disorder‐III (CCM‐III), duration of insomnia > 1 year, score on the Pittsburgh Sleep Quality Index (PSQI) > 7
Exclusion: nil Number of participants: intervention: 23; control: 22 Number of males: information not available Age (years): overall mean 39 (SD 15.27) Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder (years): overall mean 6.8 (SD 4.29), range 1 to 15 Previous treatments: information not available |
|
| Interventions | Intervention group (needle acupuncture + zolpidem): acupuncture to the following acupoints: Shenmen, Sanyinjiao, Guanyuan and Sishenchong. Patients with deficiencies in heart and spleen were additionally treated at Xinshu and Pishu. Patients with deficiencies in heart and kidney were additionally treated at Xinshu, Shenshu and Taixi. Patients with dysharmony of spleen and stomach were additionally treated at Weishu and Zusanli. Patients with elevation of liver heat were additionally treated at Ganshu and Taichong. Patients with deficiencies in heart and liver were additionally treated at Xinshu and Ganshu. The needles were left in place for 30 minutes. Treatment was applied daily for 6 days and then rest for 1 day (1 course). Four courses of treatment were given in total. Zolpidem was given 10 mg every night for 4 weeks. Control group (Zolpidem alone): zolpidem as in intervention group | |
| Outcomes | 1. Frequency of improvement in sleep quality. Cure was defined as normalised total sleep duration or total sleep duration at least 6 hours, with deep and refreshing sleep, and improvement of PSQI by > 80%. Moderate improvement was defined as obvious improvement in sleep, with increase in total sleep duration by at least 3 hours and increase in depth of sleep, and improvement of PSQI by 60% to 80%. Some improvement was defined as symptom improvement with increase in total sleep duration by less than 3 hours, and improvement of PSQI by 30% to 60%. No improvement was defined as no change in symptoms or worsening symptoms, and improvement of PSQI by < 30%. 2. Adverse effects |
|
| Notes | Duration of follow‐up: 4 weeks
Dropouts: none Comparability of groups at baseline: no significant differences between the groups but data not shown Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | Treatment regimen varied among patients and might introduce bias. No placebo or sham control was used and hence there might be a placebo effect. |
Hisghman 2006.
| Methods | Design: parallel‐group
Randomisation method: randomisation schedule generated by a computer program Method of allocation concealment: group assignment was contained in sealed, numbered, opaque envelope Blinding: single‐blind (patients) Stratification: not used |
|
| Participants | Inclusion: age 50 to 70 years, complaints of sleep quality at least 3 nights per week, difficulty initiating or maintaining sleep for at least 1 month, not received acupuncture for past 30 days, willing not to initiate any new sleeping therapy during the course of study Exclusion: patients using a medical device such as pacemaker, defibrillator or insulin pump were excluded. Patients who were participating in another trial or who were anticipated to receive an investigational drug, vaccine or medical device within 30 days prior to the first acupuncture treatment or during the study were excluded. Patients having a life‐threatening or serious underlying disease particularly renal or hepatic impairment were excluded. Patients with history of psychotic episodes, or currently being treated for epilepsy were excluded. Pregnant women, patients who were unable to speak English, or unable to understand the questions or provide informed consent were excluded. Number of participants: intervention: 11; control: 11 Number of males: intervention: 1; control 1 Age (years): intervention: mean 61.55 (SD 5.52, range 52 to 70; control: mean 56 (SD 3.256), range 51 to 61 Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder (years): information not available Previous treatments: intervention: prescribed sleep aid (2), alternative product (3); control: prescribed sleep aid (2), alternative product (1), alcohol (1) |
|
| Interventions | Intervention group (magnetic acupressure): magnetic acupressure to the following auricular acupoints: Shenmen, auricular areas of heart, kidney, liver, spleen, occiput and subcortex. Helio gold‐plated 800 gauss magnetic pellets, 1.7 mm in diameter, with random magnetic pole orientation were used. The negative pole was placed directly on the auricular acupoints unilaterally with hypoallergic, tan‐coloured surgical tape with the starting ear chosen randomly. The magnets were retained in place for 3 days until next visit. At consecutive appointments every 3 days, the pellets were placed on identical acupoints on opposite ear. If it was not possible to alternate ears, the same ear was used but the pellets replaced.
Control group (sham acupressure): Accu‐Patch stainless steel pellets were used instead of magnetic pellets Duration of treatment: 12 days |
|
| Outcomes | 1. Insomnia Severity Index 2. Total sleep duration 3. Sleep efficiency 4. Number of awakenings 5. Quality of life scores on the SF12 6. Quality of sleep ratings 7. Adverse effects |
|
| Notes | Duration of follow‐up: 12 days
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, education level, work status, marital status, household income, prior sleep aid used or baseline sleep parameters Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule was generated by a computer program |
| Allocation concealment (selection bias) | Low risk | Assignment was put in sealed, numbered, opaque envelope |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only patients were blinded. Treating physicians were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | Low risk | No other bias was apparent |
Hwang 2007.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: single‐blind (patients) Stratification: not used |
|
| Participants | Inclusion: age 30 to 59 years, insomnia with score on the Pittsburgh Sleep Quality Index (PSQI) < 5 Exclusion: patients with history of hypertension, diabetes, stroke or depression were excluded. Patients with magnetic device in the body or work shift duties were excluded. Number of participants: intervention: 11; control: 11 Number of males: intervention: 5; control 4 Age (years): intervention: mean 38.18 (SD 8.21); control: mean 37.55 (SD 6.68) Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder (years): information not available Previous treatments: information not available |
|
| Interventions | Intervention group (acupressure): acupressure to multiple acupoints on hand using New Seoam Press Pellets
Control group (sham acupressure): adhesive tapes of the same shape, size and quality without pellets were applied to the same acupoints as in intervention group Duration of treatment: 4 weeks |
|
| Outcomes | 1. Pittsburgh Sleep Quality Index (PSQI) 2. Sleep Quality Scale (SQS) 3. Total sleep duration by electroencephalography 4. Sleep onset latency by electroencephalography 5. Sleep efficiency by electroencephalography 6. Arousal index (number of arousals per hour) by electroencephalography |
|
| Notes | Duration of follow‐up: 4 weeks
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, education level, job, marital status, living status, monthly income, exercise, alcohol, smoking, coffee drinking, body mass index and general health status. Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only patients were blinded. Treating physicians were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | Low risk | No other bias was apparent |
Jian 2005.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: patients with insomnia dependant on hypnotics, characterised by 2 of the followings and lasted for at least 1 year: difficulty initiating sleep, difficulty maintaining sleep, early wakening, frequent awakenings at night or non‐refreshing sleep
Exclusion: patients with organic disease were excluded Number of participants: intervention: 50; control: 30 Number of males: overall 28 Age (years): overall mean 47, range 29 to 76 Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder: overall range 0.5 to 38 years Previous treatments: hypnotics |
|
| Interventions | Intervention group (needle acupuncture + Chinese herbs): acupuncture to the following acupoints: Xinshu, Anmian, Baihui, Shenting, Zhaohai, Shenmen and Taiyang. Patients with deficiency or excess were punctured with different manipulations. The needles were left in place for 40 minutes. Acanthopanax senticosus 60 ml was added to 500 ml 5% dextrose solution to be infused daily. Treatment was applied daily for 10 days and then rest for 3 to 5 days (1 course). Two courses of treatment were given in total. Control group (Chinese herb alone): Acanthopanax senticosus infusion as in intervention group | |
| Outcomes | Frequency of improvement in sleep quality. Cure was defined as sleep efficiency > 75% without hypnotics use. Some improvement was defined as symptom improvement with sleep efficiency > 55% with hypnotics reduced by > 75% in dosage. No improvement was defined as no change in symptoms with sleep efficiency < 40% or requires hypnotics. | |
| Notes | Duration of follow‐up: 30 days
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, duration of insomnia or severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | Treatment regimen varied among patients and might introduce bias. No placebo or sham control was used and hence there might be a placebo effect. |
Jin 2003.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: inpatients in medical ward with insomnia
Exclusion: nil Number of participants: intervention: 64; control: 56 Number of males: overall 51 Age (years): overall mean 42 (SD 8.7) Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder (years): information not available Previous treatments: information not available |
|
| Interventions | Intervention group (acupressure): acupressure to the following auricular acupoints: Shenmen, occiput and subcortical area. Patients with liver problems were additionally treated at liver and gallbladder areas. Patients with dysharmony in qi of stomach were additionally treated at spleen and stomach areas. Patients with deficiency in Yin were additionally treated at heart and kidney areas. Patients with deficiencies in heart and spleen were additionally treated at heart, liver and spleen areas. Seeds were attached to a small bandage to apply to acupoints with pressure for a few seconds. Participants were advised to apply pressure to the seeds 2 to 3 times per day. Seeds were left in place for 3 to 5 days, then rest for 1 to 2 days (1 course).
Control group (no specific treatment): no specific treatment Duration of treatment: 10 days |
|
| Outcomes | Total sleep duration | |
| Notes | Duration of follow‐up: 10 days
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in total sleep duration at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | Treatment regimen varied among patients and might introduce bias. No placebo or sham control was used and hence there might be a placebo effect. |
Kaiser‐Pagliarini 2009.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: double‐blind (but no description of who were blinded) Stratification: not used |
|
| Participants | Inclusion: post‐menopausal women aged 50 to 67 years with insomnia diagnosed according to Diagnostic and Statistical Manual‐IV (DSM‐IV) criteria, BMI <= 30, follicle‐stimulating hormone level >= 30 mIU/ml, at least 1 year of amenorrhoea
Exclusion: patients using antidepressants, hypnotics or hormonal replacement therapy were excluded Number of participants: intervention: 9; control 1: 9; control 2: 9 Number of males: intervention: 0; control 1: 0; control 2: 0 Age (years): information not available Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder (years): information not available Previous treatments: information not available |
|
| Interventions | Intervention group (needle acupuncture): needle acupuncture was applied in 10 sessions over 5 weeks
Control group 1 (sham acupuncture): details not described Control group 2 (no specific treatment): no specific treatment |
|
| Outcomes | 1. Polysomnography parameters 2. Cognitive tests (Stroop test, story recall, trail test, cancel test) 3. Pittsburgh Sleep Quality Index (PSQI) 4. Beck Depression Inventory (BDI) 5. State Trait Anxiety Inventory (STAI) 6. Quality of life by WHOQOL‐Bref |
|
| Notes | Duration of follow‐up: 10 days
Dropouts: information not available Comparability of groups at baseline: no significant differences between the groups in anthropometric, polysomnographic, cognitive and questionnaire data at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind" but there was no description of who were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts were not described |
| Selective reporting (reporting bias) | High risk | Only PSQI scores were reported |
| Other bias | Low risk | No other bias was apparent |
Kim 2004.
| Methods | Design: parallel‐group
Randomisation method: by random digits Method of allocation concealment: information not available Blinding: single‐blind (assessor) Stratification: not used |
|
| Participants | Inclusion: persistent insomnia for more than 3 days in a row after stroke, Insomnia Severity Index (ISI) > 15, hospitalised at the Department of Cardiovascular and Neurologic Diseases (Stroke Center), Hospital of Oriental Medicine, Kyung Hee Medical Center, Seoul, Korea from 1 Nov 2002 to 31 Jul 2003
Exclusion: patients treated with sedative, antidepressant, tranquilliser, narcotic analgesics, antihistamine or amphetamine‐containing drugs were excluded. Patients who had disorientation, dysphasia or nocturnal voiding frequency were also excluded. Number of participants: intervention: 16; control: 16 Number of males: intervention: 8; control: 9 Age (years): intervention: mean 65.1 (SD 9.0); control: mean 68.3 (SD 10.4) Specific diagnoses/diagnostic subtypes: information not available Associated disease: stroke in all participants; intervention group: hypertension (8), diabetes mellitus (2), ischaemic heart disease (1); control group: hypertension (8), diabetes (5), ischaemic heart disease (3) Duration of disorder (years): information not available Previous treatments: no |
|
| Interventions | Intervention group (needle acupuncture): acupuncture to the following acupoints: Shenmen and Neikuan in both arms, by 4 Dong bang sterile disposable intradermal acupuncture needles (0.18 x 6 mm). A piece of skin tape (1 x 1 cm) was put on each needle to fix it persistently for 2 days. Control group (sham acupuncture): the needles were laid down on the same acupoints as in intervention group, not letting the needles penetrate the skin | |
| Outcomes | 1. Morning Questionnaire (MQ) 2. Insomnia Severity Index (ISI) 3. Athens Insomnia Scale (AIS) 4. Sleep onset latency 5. Total sleep duration 6. Adverse effect |
|
| Notes | Duration of follow‐up: 2 days
Dropouts: intervention: 1 (could not stand the pain induced by needle insertion); control: 1 (was administered psychoactive drug during the study) Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, ischaemic stroke, medical history of hypertension, diabetes or ischaemic stroke, or severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group allocation by random digits |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Outcome assessor (an independent neurologist) was blinded but patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was 1 dropout in each treatment group with reasons provided and missing data constituted only 6% of the data |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | Low risk | No other bias was apparent |
Lai 2010.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: age 18 to 60 years, insomnia diagnosed according to Chinese Classification of Mental Disorder‐III (CCM‐III), Traditional Chinese Medicine diagnosis of deficiencies in heart and spleen, duration of disease more than 1 year, sleep efficiency <= 60%, score on the Pittsburgh Sleep Quality Index (PSQI) > 7, not used hypnotics for 7 days, consented to participate to receive treatment for 1 month
Exclusion: patients with insomnia secondary to physical or psychiatric diseases were excluded. Patients who were pregnant, lactating or having drug allergy or systemic diseases such as pain, fever, cough, head injury, cardiovascular, lung, liver, kidney, blood diseases, severe physical diseases, organ dysfunction, neurological or psychiatric diseases, alcohol or drug dependence, or after operation were excluded. Patients who could not tolerate acupuncture, or did not comply to treatment or had missing data were excluded. Number of participants: intervention: 30; control: 30 Number of males: intervention: 16; control: 13 Age: intervention: 21 to 30 years (3), 31 to 40 years (6), 41 to 50 years (10), 51 to 60 years (11); control: 21 to 30 years (2), 31 to 40 years (8), 41 to 50 years (12), 51 to 60 years (8) Specific diagnoses/diagnostic subtypes: information not available Associated disease: no Duration of disorder: intervention: < 3 years (7), 6 years (10), 9 years (8), 12 years (5); control: <3 years (6), 6 years (14), 9 years (5), 12 years (5) Previous treatments: hypnotics: intervention: 24; control: 22 |
|
| Interventions | Intervention group (electroacupuncture + Chinese herb): acupuncture to the following acupoints: Zhaohai, Shenmai, Shenmen, Yintang, Sishencong, Anmian, Xinshu, Pishu and Zusanli. When getting Qi after needles inserted, G6805 electric acupuncture apparatus was connected and continuous wave of 50 to 100 Hz was applied for 10 minutes, followed by sparse‐dense waves 2 to 4 Hz/50 to 100 Hz for 20 minutes. Treatment was given daily for 5 days a week for 8 weeks. Gan Mai Da Cao Tang 0.05 g/kg was given 3 times per day for 8 weeks. Control group (Chinese herb alone): Gan Mai Da Cao Tang was given as in intervention group | |
| Outcomes | 1. Frequency of improvement in sleep quality: cure was defined as normalised total sleep duration or total sleep duration at least 6 hours, with deep and refreshing sleep. Moderate improvement was defined as obvious improvement in sleep, with increase in total sleep duration by at least 3 hours and increase in depth of sleep. Some improvement was defined as symptom improvement with increase in total sleep duration by less than 3 hours. No improvement was defined as no change in symptoms or worsening symptoms. 2. Pittsburgh Sleep Quality Index (PSQI) 3. Self rated Depression Scale (SDS) 4. Self rated Anxiety Scale (SAS) |
|
| Notes | Duration of follow‐up: 8 weeks
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, occupation, history of hypnotics use, duration or severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | No placebo or sham control was used and hence there might be a placebo effect |
Lee 2009.
| Methods | Design: parallel‐group
Randomisation method: by random digits Method of allocation concealment: information not available Blinding: single‐blind (assessor) Stratification: not used |
|
| Participants | Inclusion: persistent insomnia for more than 3 days in a row after stroke, Insomnia Severity Index (ISI) > 15, hospitalised at the Department of Cardiovascular and Neurologic Diseases (Stroke Center), Hospital of Oriental Medicine, Kyung Hee Medical Center, Seoul, Korea from 1 November 2007 to 31 August 2008
Exclusion: patients treated with sedative, antidepressant, tranquilliser, narcotic analgesics, antihistamine or amphetamine‐containing drugs were excluded. Patients who had disorientation, dysphasia or nocturnal voiding frequency were also excluded Number of participants: intervention: 30; control: 30 Number of males: intervention: 12; control: 12 Age (years): intervention: mean 66.7 (SD 11); control: mean 66 (SD 9.6) Specific diagnoses/diagnostic subtypes: information not available Associated disease: stroke in all participants; intervention group: hypertension (20), diabetes mellitus (7), hyperlipidaemia (4), ischaemic heart disease (4); control group: hypertension (17), diabetes (10), hyperlipidaemia (4), ischaemic heart disease (2) Duration of disorder (years): information not available Previous treatments: no |
|
| Interventions | Intervention group (needle acupuncture): acupuncture to the following acupoints: Shenmen and Neikuan in both arms, by 4 Dong bang sterile disposable intradermal acupuncture needles (0.18 x 6 mm). A piece of skin tape (1 x 1 cm) was put on each needle to fix it persistently for 2 days. Control group (sham acupuncture): the needles were laid down on the same acupoints as in intervention group, not letting the needles penetrate the skin | |
| Outcomes | 1. Insomnia Severity Index (ISI) 2. Athens Insomnia Scale (AIS) |
|
| Notes | Duration of follow‐up: 2 days
Dropouts: intervention: 3 (due to discharge from hospital); control: 5 (due to discharge from hospital) Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, medical history of hypertension, diabetes, hyperlipidaemia or ischaemic stroke, or severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group allocation by random digits |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Outcome assessor (an independent neurologist) was blinded but patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 8 dropouts in total which constituted 13% of the data and had affected the results in sensitivity analyses of best and worse‐case scenarios |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | Low risk | No other bias was apparent |
Li 2005a.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: patients with diagnosis of depressive disorder according to Chinese Classification of Mental Disorder (CCMD) with insomnia, score on the Self rated Depression Scale (SDS) > 40
Exclusion: nil Number of participants: intervention: 26; control: 24 Number of males: intervention: 10; control: 9 Age (years): intervention: range 33 to 80; control: range 31 to 78 Specific diagnoses/diagnostic subtypes: information not available Associated disease: depressive disorder Duration of disorder: intervention: range 3 months to 22 years; control: range 2 months to 20 years Previous treatments: information not available |
|
| Interventions | Intervention group (electroacupuncture + fluoxetine): acupuncture to the following acupoints: Anmian, Baihui and Yintang, by 0.5 inch disposable acupuncture needle. Needles were inserted to 0.3 inch deep and then G6805 electric acupuncture apparatus was connected and continuous wave of 80 to 100 Hz was applied for 45 minutes. Treatment was applied alternate days. Fluoxetine 20 mg daily was given orally 30 minutes after breakfast
Control group (fluoxetine alone): fluoxetine as in intervention group Duration of treatment: 4 weeks |
|
| Outcomes | 1. Frequency of improvement in sleep quality: cure was defined as normalised total sleep duration or total sleep duration at least 6 hours, with deep and refreshing sleep. Moderate improvement was defined as obvious improvement in sleep, with increase in total sleep duration by at least 3 hours and increase in depth of sleep. Some improvement was defined as symptom improvement with increase in total sleep duration by less than 3 hours. No improvement was defined as no change in symptoms or worsening symptoms. 2. Total sleep duration |
|
| Notes | Duration of follow‐up: 4 weeks
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, duration of insomnia, or total sleep duration at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | No placebo or sham control was used and hence there might be a placebo effect |
Lin 2007.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: age 18 to 60 years, diagnosis of insomnia by Chinese Classification of Mental Disorder‐III Revised (CCMD‐IIIR)
Exclusion: pregnant or lactating women, or patients with cerebrovascular, liver, kidney or blood diseases were excluded. Patients participating in another study, or taking hypnotics or other therapies for insomnia were excluded. Patients who could not complete therapy or had missing data were excluded. Number of participants: intervention: 30; control: 30 Number of males: intervention: 16; control: 17 Age (years): intervention: mean 40.3 (SD 12.12), range 19 to 58; control: mean 39.47 (SD 11.99), range 18 to 55 Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder (years): information not available Previous treatments: information not available |
|
| Interventions | Intervention group (acupressure): acupressure to the following auricular acupoints: Shenmen, endocrine and heart areas. Patients with deficiencies in heart and spleen were additionally treated at heart and small intestine areas. Patients with non‐communication of heart and kidney were additionally treated at liver and kidney areas. Patients with deficiencies of heart and gallbladder were additionally treated at liver and gallbladder areas. Patients with heart problems with phlegm and fire were additionally treated at spleen and large intestine areas. Patients with deficiencies in liver and stagnant Qi were additionally treated at liver area and Sanjiao. Small nut seeds were attached to a 0.5 cm x 0.5 cm rubberised fabric to apply to acupoints on right or left ear with pressure for 5 times, each time lasting for 5 minutes. Seeds were left in place for 3 days and then applied to the opposite ear. Treatment was given for 15 days and then rest for 3 days (1 course). Total 3 courses of treatment were given.
Control group (sham acupressure): rubberised fabric alone without nut seeds was applied to the same acupoints as intervention group Duration of treatment: 54 days |
|
| Outcomes | 1. Frequency of improvement in sleep quality: cure was defined as normalised total sleep duration or total sleep duration at least 6 hours, with deep and refreshing sleep. Moderate improvement was defined as obvious improvement in sleep, with increase in total sleep duration by at least 3 hours and increase in depth of sleep. Some improvement was defined as symptom improvement with increase in total sleep duration by less than 3 hours. No improvement was defined as no change in symptoms or worsening symptoms. 2. Pittsburgh Sleep Quality Index (PSQI) 3. Total sleep duration by electroencephalography 4. Sleep onset latency by electroencephalography 5. Number of awakenings by electroencephalography 6. Sleep efficiency by electroencephalography |
|
| Notes | Duration of follow‐up: 54 days
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, or severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | High risk | Some sub‐scores on the PSQI were not reported |
| Other bias | High risk | Treatment regimen varied among patients and might introduce bias |
Liu 2001.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: age > 50 years, insomnia characterised by frequent insufficient sleep, difficulty initiating sleep, early awakening, difficulty reinitiating sleep after awakening, no sleep throughout the night, difficulty maintaining sleep, or dependent on hypnotics for sleep, and total sleep duration less than 4 hours, tired at daytime with impaired memory
Exclusion: nil Number of participants: intervention: 56; control: 30 Number of males: overall 31 Age (years): overall mean 67, range 56 to 82 Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder: overall range 0.5 to 39 years Previous treatments: information not available |
|
| Interventions | Intervention group (needle acupuncture + Chinese herb): acupuncture to the following acupoints: Yintang, Zhaohai, Shenmen and Taiyang. Patients with deficiency in kidney were additionally treated at Taixi. Patients with deficiency in spleen were additionally treated at Zusanli. Patients with excessive liver heat were additionally treated at Taichong. Patients with stomach problems were additionally treated at Zhongwan and Liangmen. Needles were left in place for 40 minutes. Acanthopanax senticosus 60 ml was added to 500 ml 5% dextrose solution to be infused daily. Treatment was applied daily for 10 days and then rest for 3 to 5 days (1 course). Two courses of treatment were given in total. Control group (Chinese herb alone): Acanthopanax senticosus infusion as in intervention group | |
| Outcomes | Frequency of improvement in sleep quality: cure was defined as sleep onset latency no more than 1 hour, with at most 1 awakening at night, able to fall asleep again after awakening, no excessive dreams, independent of hypnotics and normal daytime activity. Some improvement was defined as increase in total sleep duration by more than 2 hours, with reduction of hypnotics by at least 50%, and improved daytime functioning. No improvement was defined as no change in symptoms or use of hypnotics. | |
| Notes | Duration of follow‐up: 30 days
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, or severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | Treatment regimen varied among patients and might introduce bias. No placebo or sham control was used and hence there might be a placebo effect. |
Lu 1998.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: insomnia characterised by difficulty initiating sleep, shallow sleep, lots of dreams, early awakening, difficulty reinitiating sleep after awakening, sleep efficiency below 40%, tired and sleepy at daytime with anxiety, impaired memory or attention affecting work efficiency, duration of insomnia more than 3 months
Exclusion: nil Number of participants: intervention: 35; control: 23 Number of males: intervention: 14; control: 10 Age (years): overall range 16 to 60 Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder: intervention: range 3 months to 5 years; control: range 3 months to 5 years Previous treatments: 80% participants had used hypnotics |
|
| Interventions | Intervention group (needle acupuncture + Chinese herb): acupuncture to Neiguan bilaterally. Needles were left in place for 30 minutes. Treatment was applied daily for 30 days. Qi Ye Shen An Pian 100 mg was given 3 times daily for 30 days. Control group (Chinese herb alone): Qi Ye Shen An Pian as in intervention group | |
| Outcomes | Frequency of improvement in sleep quality: Cure was defined as sleep efficiency more than 70%. Moderate improvement was defined as sleep efficiency 60% to 70%. Some improvement was defined as sleep efficiency 50% to 60%. No improvement was defined as sleep efficiency below 40%. | |
| Notes | Duration of follow‐up: 30 days
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in gender distribution or duration of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | No placebo or sham control was used and hence there might be a placebo effect |
Luo 2006.
| Methods | Design: parallel‐group
Randomisation method: randomisation number generated by computer program Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: individuals with insomnia
Exclusion: nil Number of participants: intervention: 32; control: 32 Number of males: intervention: 13; control: 15 Age (years): intervention: mean 39.53 (SD 13.62); control: 42 (SD 13.02) Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder (months): intervention: mean 34.78 (SD 13.25); control: mean 36.23 (SD 10.54) Previous treatments: information not available |
|
| Interventions | Intervention group (needle acupuncture + clonazepam): acupuncture to all acupoints along Zu Tai Yang Pang Guang Jing and governing vessel. Rolling needles were applied for 15 to 20 minutes. Treatment was applied daily 5 days per week for 2 weeks and then 3 days per week for 2 weeks. Clonazepam was given 4 mg orally 30 minutes before bed every night for 4 weeks. Control group (clonazepam alone): clonazepam as in intervention group | |
| Outcomes | 1. Frequency of improvement in sleep quality: cure was defined as normalised, refreshing sleep with resolution of symptoms. Some improvement was defined as increased total sleep duration by less than 3 hours. No improvement was defined no improvement in symptoms. 2. Pittsburgh Sleep Quality Index (PSQI) |
|
| Notes | Duration of follow‐up: 4 weeks
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, or duration or severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation number was generated by a computer program |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | No placebo or sham control was used and hence there might be a placebo effect |
Lv 2007.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: non‐organic insomnia with difficulty initiating or maintaining sleep, at a frequency of more than 3 times per week for more than 1 month and affecting daily activities
Exclusion: patients with insomnia due to alcohol, drugs, pain, fever, cough, operation or other environmental factors were excluded. Patients who had diseases of the heart, liver, kidney, lungs or other severe systemic diseases were excluded. Number of participants: intervention: 34; control: 34 Number of males: intervention: 13; control: 11 Age (years): intervention: mean 47.9 (SD 4.1), range 20 to 70; control: 46.3 (SD 8.2), range 18 to 67 Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder: intervention: range 28 days to 7.6 years; control: range 25 days to 7 years Previous treatments: information not available |
|
| Interventions | Intervention group (needle acupuncture + Chinese herb): acupuncture to the following acupoints: Shenting, Baihui, Sishenchong, Neiguan, Shenmen, Zusanli, Sanyinjiao, Anmian, Shenmai and Zhaohai. Patients with non‐communication of heart and kidney were additionally treated at Taixi and Daling. Patients with deficiencies in heart and spleen were additionally treated at Xinshu and Shenshu. Patients with liver problems with heat were additionally treated at Hegu, Taichong and Xiaxi. Needles were left in place for 20 minutes. Treatment was applied daily for 8 days and then rest for 2 days (1 course). Three courses of treatment were given in total. Chinese herb consists of Suan Zao Ren 18 g, Poria Cocos 10 g, Zhi Mu 10 g, Ligustici Wallichii 10 g and Glycyrrhizae 5 g to be boiled with 100 ml of water for 45 minutes and taken 150 ml orally twice daily. Control group (Chinese herb alone): Chinese herb as in intervention group | |
| Outcomes | 1. Frequency of improvement in sleep quality: cure was defined as normalised total sleep duration or total sleep duration at least 6 hours, with deep and refreshing sleep. Moderate improvement was defined as obvious improvement in sleep, with increase in total sleep duration by at least 3 hours and increase in depth of sleep. Some improvement was defined as symptom improvement with increase in total sleep duration by less than 3 hours. No improvement was defined as no change in symptoms or worsening symptoms. 2. Total sleep duration |
|
| Notes | Duration of follow‐up: 30 days
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, or duration or severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | Treatment regimen varied among patients and might introduce bias. No placebo or sham control was used and hence there might be a placebo effect. |
Ma 2006a.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: insomnia with schizophrenia
Exclusion: nil Number of participants: intervention: 30; control: 30 Number of males: intervention: 4; control: 5 Age (years): intervention: mean 42.3 (SD 13.6); control: 41.2 (SD 12.8) Specific diagnoses/diagnostic subtypes: information not available Associated disease: schizophrenia Duration of disorder (years): intervention: mean 3.2 (SD 1.1); control: 3.2 (SD 1.1) Previous treatments: information not available |
|
| Interventions | Intervention group (needle acupuncture + alprazolam): acupuncture to the following acupoints: Baihui, Sishenchong, Neiguan, Shenmen, Zusanli and Sanyinjiao. Patients with phlegm and heat were additionally treated at Taichong and Taiyang. Patients with wet phlegm were additionally treated at Fenglong. Patients with deficiency of Yin and excessive heat were additionally treated at Taixi. Patients with stagnant Qi and blood were additionally treated at Xuehai and Xingjian. Patients with deficiency of Yang were additionally treated at Xinshu and Pishu. Needles were left in place for 30 minutes. Treatment was applied daily for 10 days and then rest for 1 day (1 course). Two to three courses of treatment were given in total. Alprazolam 0.4 to 0.8 mg was given every night orally. Control group (alprazolam alone): alprazolam as in intervention group | |
| Outcomes | Frequency of improvement in sleep quality: cure was defined as normalised total sleep duration or total sleep duration at least 6 hours, with deep and refreshing sleep. Moderate improvement was defined as obvious improvement in sleep, with increase in total sleep duration by at least 3 hours and increase in depth of sleep. Some improvement was defined as symptom improvement with increase in total sleep duration by less than 3 hours. No improvement was defined as no change in symptoms or worsening symptoms. | |
| Notes | Duration of follow‐up: 33 days
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, or duration of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | Treatment regimen varied among patients and might introduce bias. No placebo or sham control was used and hence there might be a placebo effect. |
Nordio 2008.
| Methods | Design: parallel‐group
Randomisation method: computer‐generated randomisation sequence Method of allocation concealment: allocation maintained in numbered, sealed, opaque envelopes Blinding: double‐blind (patients and study personnel) Stratification: not used |
|
| Participants | Inclusion: age 18 to 80 years, non‐occasional insomnia for at least 3 months, absence of important pathologies, particularly renal and/or liver failure, normal creatinine level, understand the study and available and affordable, informed consent signed Exclusion: shift workers and those who had intercontinental flights within 30 days were excluded. Patients with habitual used of exogenous melatonin, NSAIDs, beta‐blockers, antidepressants were excluded. Patients with psychiatric illnesses, seasonal affective disorder, drug, alcohol or smoke addiction were excluded. Pregnant and lactating women were excluded. Number of participants: intervention: 20; control: 20 Number of males: intervention: 9; control: 7 Age (years): overall mean 64.05, range 49 to 77 Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder (years): information not available Previous treatments: information not available |
|
| Interventions | Intervention group (acupressure): acupressure to bilateral H7 acupoints on the wrists by disposable acupressure devices. The device was applied to the wrist at 10 pm by patient every night. Written instruction was given on how to assemble and apply the device correctly. Control group (placebo acupressure): participants were instructed to apply the acupressure device to non‐H7 point on a different position on both wrists | |
| Outcomes | 1. Pittsburgh Sleep Quality Index (PSQI) 2. State Trait Anxiety Inventory (STAI) 3. General Health Questionnaires (GHQ28) |
|
| Notes | Duration of follow‐up: 20 days
Dropouts: intervention: 2; control: 5. Reasons for dropout included patients considered treatment annoying or ineffective. Comparability of groups at baseline: no significant differences between the groups in gender distribution or severity of insomnia at baseline Risk of bias: low |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed, opaque envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both study personnel and patients were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts occurred more frequently in control group (25%) compared to intervention group. Dropouts were excluded but reasons were related to treatment effectiveness. |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | Low risk | No other bias was apparent |
Reza 2010.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: nursing home residents in Tehran, Iran, age >= 60 years, score on the Pittsburgh Sleep Quality Index (PSQI) >= 5, normal mental status with no dementia, ability to communicate in Farsi language Exclusion: patients with severe congestive heart failure, severe pulmonary disease, amputation of upper and lower extremity, injury, bleeding, thrombophlebitis, tumours near acupuncture points in head, neck and hands, hearing defects, chronic or acute pain interfering on nocturnal sleep were excluded. Patients who were transferred to acute care unit or received prescription of hypnotics‐sedatives during intervention period were excluded. Number of participants: intervention: 30; control 1 (sham): 30; control 2 (no specific treatment): 30 Number of males: intervention: 13; control 1 (sham): 14; control 2 (no specific treatment): 14 Age (years): intervention: mean 75.44 (SD 8.71); control 1 (sham): mean 73.58 (SD 8.17); control 2 (no specific treatment): mean 76.62 (SD 9.74) Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder (years): information not available Previous treatments: information not available |
|
| Interventions | Intervention group (acupressure): acupressure to the following acupoints: Neiguan, Shenmen, Yungchuan, Sanyinjiao and Anmian by finger massage. The main investigator was trained by an expert a month prior to the study. A scale was used to measure the force of finger pressure between 3 to 4 kg. The force of finger pressure was measured 30 times and the mean forces were from 3.21 to 3.39 kg. Treatment was given 3 times per week for 4 weeks.
Control group 1 (sham acupressure): acupressure to non‐acupoints at 0.5 inch from the true acupoints Control group 2 (no specific treatment): routine care alone |
|
| Outcomes | Pittsburgh Sleep Quality Index (PSQI) | |
| Notes | Duration of follow‐up: 4 weeks
Dropouts: intervention: 5; control 1: 4; control 2: 4. Reasons include transfer to acute care unit, taking sleep medication, pain due to fall and disagreement to continue the study. Comparability of groups at baseline: no significant differences between the groups in gender distribution, or severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐outs were excluded and reasons may be related to treatment outcomes |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | Low risk | No other bias was apparent |
Sok 2005.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: age >= 65 years, nursing home resident with insomnia Exclusion: nil Number of participants: intervention: 20; control: 20 Number of males: intervention: 7; control: 7 Age: intervention: 65 to 74 years (4), 75 to 79 years (8), >= 80 years (8); control: 65 to 74 years (6), 75 to 79 years (6), >= 80 years (8) Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder (years): information not available Previous treatments: information not available |
|
| Interventions | Intervention group (acupressure): acupressure to the following auricular acupoints: Zhenjing and Bailing. Treatment was given for 15 days. Control group (no specific treatment): no specific treatment was given | |
| Outcomes | 1. Sleep score 2. Self satisfaction score |
|
| Notes | Duration of follow‐up: 15 days
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, marital status, presence of spouse, religion, education level, employment and socioeconomic status at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no dropout |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | No placebo or sham control was used and hence there might be a placebo effect |
Suen 2002.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: double‐blind (patients and assessors) Stratification: not used |
|
| Participants | Inclusion: age >= 60, suffering from sleep disturbances, sleeping poorly on at least 3 nights per week, insomnia lasted for a minimum 6 months, actigraph monitoring of sleep efficiency of < 85%
Exclusion: patients with serious physical and psychological illness, such as stroke, mental illness, dementia, major depression and impairment such as being bedridden, sleep apnoea, regular use of a hypnotic medication or other psychotropic medication with an inability or unwillingness to discontinue medication, wearing pacemaker, or implanted electrical device, having a sleep partner, infection or abscess of external ear were excluded Number of participants: intervention 1 (magnetic acupressure): 68; intervention 2 (acupressure): 36; control (placebo acupressure): 35 Number of males: overall 10 Age: overall mean 81.66 (SD 5.8) Specific diagnoses/diagnostic subtypes: "Excess" syndrome and "Deficiency" syndrome based on Traditional Chinese Medicine theory Associated disease: 96% reported having good or acceptable health condition. Diabetes mellitus and hypertension, which were under control by regular treatment, were most commonly reported among the participants Duration of disorder: range 6 months to > 20 years Previous treatments: information not available |
|
| Interventions | Intervention group 1 (magnetic acupressure): auricular acupressure using magnetic pearl with a magnetic flux densities ranging from 0.01 mT to 2 T were stick to seven sterilised auricular points: Shenmen, heart, kidney, liver, spleen, occiput and subcortex areas. The pearls were applied to the most sensitive area of each selected auricular point. The sensitive point was detected by means of an electrical detector (Potentiometer). The pearls were replaced every 3 days. Intervention group 2 (acupressure): auricular acupressure using Semen vaccariae. The procedure and acupoints used were the same as the intervention group 1. Control (placebo acupressure): placebo acupressure using Junci Medulla (placebo), the procedure and acupoints used were the same as the intervention group 1. Junci Medulla is a soft material that does not induce any pressure on the acupoints. Duration of treatment: 3 weeks | |
| Outcomes | 1. Total sleep duration by actigraphy 2. Sleep onset latency by actigraphy 3. Total wake time by actigraphy 4. Wake after sleep onset by actigraphy 5. Number of awakenings by actigraphy 6. Sleep efficiency by actigraphy |
|
| Notes | Duration of follow‐up: 3 weeks
Dropouts: 8 in intervention group 1, 11 in intervention group 2 and control group. Reasons were home leave (n = 8), refusal to wear an actigraph as a monitoring device (n = 3), admission to hospital (n = 2), being treated for influenza (n = 3), unreported reasons (n = 3). Dropouts were replaced. Comparability of groups at baseline: no significant differences between the groups in exercise level, anxiety level, depression level, or sleep parameters at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both the patients and assessors were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Distribution of reasons for dropouts among the treatment groups were not clearly described |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | Low risk | No other bias was apparent |
Sun 2010a.
| Methods | Design: parallel‐group
Randomisation method: allocation to treatment groups by computer generated random function of SPSS Method of allocation concealment: not concealed Blinding: single‐blind (patients) Stratification: not used |
|
| Participants | Inclusion: resided in long‐term care facilities for at least 3 months, scores on the Pittsburgh Sleep Quality Index (PSQI) > 5 in past 3 weeks, scores on the Athens Insomnia Scale (AIS) > 6, communicate well with researchers verbally Exclusion: patients who had heart diseases, acute diseases (e.g. inflammation or upper respiratory disease), trauma, wounds on both wrists, or use of hypnotics for more than 4 times per week were excluded Number of participants: intervention: 25; control: 25 Number of males: intervention: 17; control: 15 Age (years): intervention: mean 73.2 (SD 14.15); control: mean 67.76 (SD 18.7) Specific diagnoses/diagnostic subtypes: information not available Associated disease: intervention: cerebrovascular accidents (18), dementia (3), chronic psychiatric condition or psychosis (2), chronic obstructive pulmonary disease (1), central nervous system injury (1); control: cerebrovascular accidents (14), dementia (3), chronic psychiatric condition or psychosis (2), chronic obstructive pulmonary disease (2), central nervous system injury (4) Duration of disorder: information not available Previous treatments: information not available |
|
| Interventions | Intervention group (acupressure): acupressure to HT7 acupoints on both wrists, with an interval of 5‐second pressure followed by 1‐second rest for 5 minutes. Four trained assistants administered the therapy to participants before bedtime every night. The pressure should be 3 to 5 kg, using a standard scale.
Control group (placebo acupressure): light touch was applied to the same acupoints as in intervention group Duration of treatment: 5 weeks |
|
| Outcomes | Athens Insomnia Scale | |
| Notes | Duration of follow‐up: 2 weeks
Dropouts: intervention: 2 (took hypnotics more than 4 times per week); control: 4 (took hypnotics more than 4 times per week) Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, education level, associated diseases, length of stay in long‐term care facilities, or severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to treatment groups was done by a computer‐generated random function |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only patients were blinded. Treating physicians were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All dropouts were explained and their results included in intention‐to‐treat analyses |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | Low risk | No other bias was apparent |
Tang 2007a.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: hospitalised patients with insomnia characterised by one or more of the following: sleep onset latency more than 1 hour, number of mid‐sleep awakenings more than 2, early awakening by more than 2 hours, difficulty falling asleep again after awakening, or total sleep duration less than 5 hours
Exclusion: nil Number of participants: intervention: 40; control: 40 Number of males: intervention: 16; control: 18 Age (years): intervention: range 49 to 84; control: range 47 to 83 Specific diagnoses/diagnostic subtypes: information not available Associated disease: intervention: hypertension (15), coronary heart disease (18), hyperthyroidism (4), diabetes mellitus (3); Control: hypertension (13), coronary heart disease (21), hyperthyroidism (2), diabetes mellitus (4) Duration of disorder: information not available Previous treatments: information not available |
|
| Interventions | Intervention group (acupressure): acupressure to the following auricular acupoints: Shenmen, adrenal, heart, liver, kidney, sympathetic and endocrine areas. A seed was attached to a small bandage 0.5 x 0.7cm to be applied to the acupoints. Participants were instructed to apply pressure to the acupoints 3 to 4 times daily. The seed and bandage were changed every 5 to 7 days. Massage was applied to head every night before sleep. Treatment was given for 3 weeks. Control group (no specific treatment): no specific treatment except sleep hygiene alone | |
| Outcomes | Frequency of improvement in sleep quality: excellent improvement was defined as total sleep duration at least 6 hours, sleep onset latency less than 0.5 hour, no mid‐sleep awakening, early awakening by less than 0.5 hour, and very good refreshment after sleep. Moderate improvement was defined as total sleep duration increased by at least 3 hours, with sleep onset latency 0.5 to 0.75 hour, no more than 1 mid‐sleep awakening, early awakening by less than 1 hour, and better refreshment after sleep. Some improvement was defined as total sleep duration increased by less than 3 hours, sleep onset latency 0.75 to 1 hour, no more than 2 mid‐sleep awakenings, early awakening at by 1 to 2 hours, and some refreshment after sleep. No improvement was defined as no improvement in total sleep duration, sleep onset latency more than 1 hour, more than 2 mid‐sleep awakenings, early awakening at by more than 2 hours, and no refreshment after sleep. | |
| Notes | Duration of follow‐up: 3 weeks
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, duration of hospitalisation, or associated disease at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | Unclear risk | The 2 treatment groups may not be comparable in severity of insomnia at baseline as no information was available |
Tian 2006.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: age 18 to 70 years, insomnia diagnosed according to Spiegel's questionnaires indicating sleep onset latency more than 30 minutes and total sleep duration less than 4 hours, insomnia lasting more than 1 month
Exclusion: patients who had systemic diseases such as pain, fever, cough, operations or insomnia caused by environmental factors were excluded. Pregnant or lactating women were excluded. Patients with severe heart, liver, kidney, blood or psychiatric diseases were excluded. Number of participants: intervention: 39; control: 39 Number of males: overall 36 Age (years): overall 35 to 70 Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder: overall 1 month to 10 years Previous treatments: information not available |
|
| Interventions | Intervention group (needle acupuncture + Chinese herb): acupuncture to the following acupoints: Baihui, Sishenchong, Shenmen, Sanyinjiao and Anmian. Patients with liver problem with heat were additionally treated at Taichong, Fengchi, Yanglingquan and Qimen. Patients with phlegm heat and internal disturbance were additionally treated at Fenglong, Houxi, Shenmai, Daling and Lidui. Patients with deficiency of Yin with excessive heat were additionally treated at Xinshu, Shenshu, Zhaohai, Daling, Taixi and Taichong. Patients with deficiencies of heart and spleen were additionally treated at Xinshu, Pishu, Zusanli and Neiguan. Patients with deficiencies of heart and gallbladder were additionally treated at Xinshu, Danshu, Daling and Fengshi. Needles were left in place for 30 minutes. Treatment was given daily for 10 days then rest for 5 days (1 course). Two courses of treatment was given in total. Different Chinese herbs were given according to diagnostic subtypes in Traditional Chinese Medicine. Chinese herbs were given 3 times daily for 10 days, then rest for 5 days (1 course). Two courses of treatment was given in total. Control group (Chinese herb alone): Chinese herbs alone were given as in intervention group | |
| Outcomes | Frequency of improvement in sleep quality: cure was defined as resolution of symptoms with total sleep duration at least 8 hours and sleep onset latency less than 30 minutes. Some improvement was defined as improvement of symptoms with total sleep duration at least 4 hours and sleep onset latency less than 30 minutes. No improvement was defined as no improvement in symptoms with total sleep duration less than 4 hours and sleep onset latency more than 30 minutes. | |
| Notes | Duration of follow‐up: 30 days
Dropouts: none Comparability of groups at baseline: information not available Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | Acupoints varied among patients and might introduce bias. No placebo or sham control was used and hence there might be a placebo effect. The 2 treatment groups may not be comparable at baseline as no information was available. |
Tsay 2003.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: double‐blind (patients and care providers and assessor) Stratification: not used |
|
| Participants | Inclusion: age 18 to 65 years, scores on the Pittsburgh Sleep Quality Index (PSQI) >= 5, good mental health status without dementia, able to communicate in Chinese, end‐stage renal disease (ESRD) patients routinely receiving afternoon haemodialysis 3 times a week, agreement to participate in the study
Exclusion: patients with psychiatric diagnoses, major chronic illness such as insulin dependent diabetes, cancer or lupus erythematosus were excluded Number of participants: intervention: 35; control 1 (sham acupuncture): 35; control 2 (no specific treatment): 35 Number of males: intervention: 17; control 1 (sham acupuncture): 10; control 2 (no specific treatment): 15 Age (years): overall mean 55.52 (SD 12.98) Specific diagnoses/diagnostic subtypes: information not available Associated disease: end‐stage renal disease Duration of disorder: information not available Previous treatments: information not available |
|
| Interventions | Intervention group (acupressure): acupressure to the following acupoints: Shenmen in ears and hand, and Yungchuan, by finger pressure of 3 to 4 kg. Participants were requested to refrain from massaging any acupoints during the study. Precision of acupressure was confirmed if patients felt sore, numb, heavy, distended and/or warm. The time of interventions consisted of 5 minutes of massage to relax the person and 9 minutes of acupoints massage (3 minutes per acupoints). Treatment was given 3 times per week for 4 weeks Control group 1 (sham acupressure): sham acupressure to locations with no acupoints Control group 2 (no specific treatment): usual care alone | |
| Outcomes | 1. Pittsburgh Sleep Quality Index (PSQI) 2. Quality of life by SF‐36 |
|
| Notes | Duration of follow‐up: 4 weeks
Dropouts: intervention: 0; control 1: 3; control 2: 4. One dropout was due to hospitalisation and others being transferred to other dialysis centre Comparability of groups at baseline: no significant differences between the groups in gender distribution, marital status or severity of insomnia at baseline. However, the education level and working status appeared different between the intervention group and the control group 1. Education level appeared different between the intervention group and the control group 2. Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The patients and care takers and assessors were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were excluded but the distribution of dropouts among the treatment groups was not clearly described |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | The 2 treatment groups might not be comparable at baseline as there were differences in education level and working status |
Tsay 2004.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: ages >= 18 years, diagnosis with end‐stage renal disease (ESRD) and treatment with haemodialysis for at least 3 months, complaints of fatigue symptoms, score on the Pittsburgh Sleep Quality Index (PSQI) scores >= 5, score on the Beck Depression Inventory (BDI) >= 10
Exclusion: patients with lower‐extremity amputations, co‐morbid diagnoses of psychiatric disorders, congestive heart failure, chronic obstructive pulmonary disease, insulin‐dependent diabetes, neuromuscular disease, systemic lupus erythematosus, rheumatoid arthritis, cancer, regular steroid therapy or use of anti‐hypertension medications were excluded Number of participants: intervention 1 (acupressure): 36; intervention 2 (electroacupuncture‐TEAS): 36; control 2 (no specific treatment): 36 Number of males: overall 36 Age (years): overall mean 58.16 (SD 12.19) Specific diagnoses/diagnostic subtypes: information not available Associated disease: end‐stage renal disease Duration of disorder: information not available Previous treatments: information not available |
|
| Interventions | Intervention group 1 (acupressure): acupressure to the following acupoints: Yungchuan in both feet, Zusanli, Yanglingchuan and Sanyingjao in both legs, by finger force between 3 to 4 kg provided by trained investigators and research assistants. The treatment was limited to 15 minutes, consisting of 3 minutes of massage to relax the person and 12 minutes of acupressure (3 minutes per acupoints). Treatment were given 3 times per week for 4 weeks. Intervention group 2 (electroacupuncture‐TEAS): Transcutaneous Electrical Acupoint Stimulation (TEAS) was given to the same acupoints as in intervention group 1. The TEAS was standardised before each treatment and was set at 2/100 (2 Hz alternating with 100 Hz, each lasting for 3 seconds). Paired skin electrodes were placed on the acupoints. Patients were instructed not to massage any acupoints during the study period. Each treatment takes 15 minutes, and 12 minutes of TEAS (3 minutes per acupoints). Treatments were given 3 times per week for 4 weeks Control group (no specific treatment): routine unit care only | |
| Outcomes | 1. Pittsburgh Sleep Quality Index (PSQI) 2. Piper Fatigue Scale (PFS) 3. Beck Depression Inventory (BDI) | |
| Notes | Duration of follow‐up: 12 weeks
Dropouts: intervention 1: 1 (due to medical reasons); intervention 2: 0; control: 1 (due to relocation) Comparability of groups at baseline: information not available Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts might have affected the results substantially as shown in sensitivity analyses of best and worse‐case scenarios |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | Unclear risk | The 2 treatment groups might not be comparable at baseline as no information was available |
Ye 2008.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: insomnia diagnosed according to Diagnostic and Statistical Manual‐IV (DSM‐IV), frequency of symptoms >= 3 per week, lasting more than 1 month
Exclusion: patients with psychiatric diseases, insomnia caused by organic diseases or drugs were excluded Number of participants: intervention: 10; control: 12 Number of males: overall 9 Age (years): intervention: mean 55; control: 44 Specific diagnoses/diagnostic subtypes: information not available Associated disease: information not available Duration of disorder: information not available Previous treatments: information not available |
|
| Interventions | Intervention group (needle acupuncture + estazolam): acupuncture to the following acupoints: Zhongwan, Xiawan, Qihai, Guanyuan, Shangqu, Huaroumen, Xiafengshi point and Qipang, by 0.25 mm x 40 mm acupuncture needles. Needles were left in place for 30 minutes. Treatment was applied daily 5 days per week for 2 weeks. Estazolam was given 2 mg orally every night for 2 weeks. Dose can be decreased if sleep improved. Control group (estazolam alone): estazolam were given as in intervention group | |
| Outcomes | Pittsburgh Sleep Quality Index (PSQI) | |
| Notes | Duration of follow‐up: 2 weeks
Dropouts: none Comparability of groups at baseline: no significant differences between the groups severity of insomnia at baseline. The mean age in intervention group appeared older. Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | No placebo or sham control was used and hence there might be a placebo effect. The 2 treatment groups might not be comparable at baseline as the mean age of the intervention group appeared older. |
Yeung 2009a.
| Methods | Design: parallel‐group
Randomisation method: using a computer‐generated list of numbers Method of allocation concealment: computer‐generated randomisation sequence was kept by an independent researcher and was concealed to participants, treating physicians or acupuncturist Blinding: single‐blind (patients); research assistants who analysed the questionnaires, sleep diaries and actigraphy results were also blinded Stratification: not used |
|
| Participants | Inclusion: ethnic Chinese, age 18 to 65 years, insomnia complaints >= 3 nights per week, diagnosis of primary insomnia by Diagnostic and Statistical Manual‐IV (DSM‐IV) for at least 3 months, total score on the Insomnia Severity Index (ISI) >= 15 Exclusion: patients taking herbal remedies, over‐the‐counter medicines or psychotropic drugs within the past 2 weeks were excluded. Patients who were diagnosed to have concurrent major depressive disorder, generalised anxiety disorder, panic disorder, manic or hypomanic episode, substance use disorder besides caffeine or nicotine use, organic mental disorder, schizophrenia, or any other psychotic disorder were also excluded. Patients who had received any acupuncture treatment in the past 12 months, had apnoea‐hypopnoea index >‐10 or periodic limb movement index with arousal >= 15, had an infection or abscess close to the site of the selected acupoints, were pregnant, lactating or a woman of childbearing potential not using adequate contraception, had valvular heart defects or bleeding disorders or were taking anticoagulant drugs, had a significant risk of suicide, had any serious physical illness, or had participated in any clinical trial within 3 months were also excluded. Number of participants: intervention: 30; control: 30 Number of males: intervention: 8; control: 6 Age (years): intervention: mean 48.3 (SD 9.5); control: mean 47.8 (SD 8.6) Specific diagnoses/diagnostic subtypes: information not available Associated disease: intervention: chronic medical illness (6); control: chronic medical illness (2) Duration of disorder (years): intervention: mean 7.7 (SD 8.1); control: mean 10.8 (SD 16.7) Previous treatments: intervention: Western medication (20), psychological treatment (2), over‐the‐counter drug (14), Chinese herbal medicine (18), other (9); control: Western medication (16), psychological treatment (1), over‐the‐counter drug (17), Chinese herbal medicine (18), other (6) |
|
| Interventions | Intervention group (electroacupuncture): acupuncture to the following acupoints: Yintang, Baihui, bilateral auricular Shenmen, Sishencong and Anmian, using disposable acupuncture needles. The acupoints on the head and ears were treated using 0.25 x 25 mm needles an 0.2 x 25 mm needles (Tai Chi, China), respectively. An irritating feeling considered to be indicative of effective needling was achieved if possible. Surgical tape or hairpins were used to secure the needles. An electric stimulator (CE‐FAR Acus II, Lurd, Sweden) was connected to the needles and delivered a constant current, 0.45 ms, square wave, brief pulse stimulus of 4 Hz frequency to the participants. The needles were left for 30 minutes. The acupuncture was performed by a licensed acupuncturist who had 2 years of experience in providing needle acupuncture. Treatment was given 3 times per week. Control group (placebo acupuncture): acupoints same as intervention group were treated using placebo needles. The blunt needle was not fixed inside the copper handle. When its tip touched the skin, a pricking sensation was felt by the participant, thereby simulating the puncture of the skin. The needle moved inside the handle and appeared to be shortened. The needles were connected to the electric stimulator as in the intervention group. Duration of treatment: 3 weeks | |
| Outcomes | 1. Pittsburgh Sleep Quality Index (PSQI) 2. Insomnia severity Index (ISI) 3. Sleep diary: total sleep duration, sleep onset latency, wake after sleep onset, sleep efficiency, sleep quality 4. Actigraphy: total sleep duration, sleep onset latency, wake after sleep onset, sleep efficiency 5. Hospital Anxiety and Depression Scale (HADS) 6. Sheehan Disability Index (SDI) 7. Credibility of Treatment Rating Scale (CTRS) |
|
| Notes | Duration of follow‐up: 4 weeks
Dropouts: intervention: 1 (due to pregnancy); control: 2 (due to incompatible working schedule (1) and relocation away from treatment centre (1)) Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, education attainment, marital status, occupation, previous treatment for insomnia, alcohol use and polysomnographic parameters at baseline. The intervention group appeared to have shorter duration of insomnia, higher frequency of coffee use and more severe insomnia (higher ISI score) at baseline. Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a computer‐generated list of numbers |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation sequence was kept by an independent researcher and was concealed to participants, treating physicians or acupuncturist |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only patients were blinded. Treating physicians were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts occurred in 3% of intervention group and 7% of control group only, with reasons provided |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in protocol were reported |
| Other bias | High risk | The 2 groups might not be comparable for duration and severity of insomnia at baseline and might introduce bias |
Zhao 2010.
| Methods | Design: parallel‐group
Randomisation method: according to random number table Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: age 40 to 55, diagnosed to have perimenopausal symptoms, deficiencies in kidney and Yin according to Traditional Chinese Medicine Exclusion: patients who had bilateral oophorectomy, breast or ovarian cancer, idiopathic menstrual disturbance, allergy, psychiatric disease, severe cardiovascular, lung, liver, kidney or blood disease were excluded. Patients who had used sex hormones within 3 months, participated in another clinical trial or not signed informed consent were excluded. Number of participants: intervention (acupuncture + Chinese herb): 30; control 1 (Chinese herb alone): 30; control 2 (acupuncture alone): 30 Number of males: intervention: 0; control 1: 0; control 2: 0 Age (years): intervention: mean 44.13 (SD 10.87); control 1: mean 47.02 (SD 7.98); control 2: mean 44.73 (SD 10.26) Specific diagnoses/diagnostic subtypes: deficiencies in kidney and Yin according to Traditional Chinese Medicine Associated disease: perimenopause Duration of disorder: information not available Previous treatments: information not available |
|
| Interventions | Intervention group (needle acupuncture + Chinese herbs): acupuncture to the following acupoints: Zhaohai and Shenmai. Needles were left in place for 45 minutes. Gengniananshensan (a combination of different herbs) was given 3 times daily orally.
Control group 1 (Chinese herbs alone): Chinese herbs alone as in intervention group Control group 2 (needle acupuncture alone): acupuncture alone as in intervention group Duration of treatment: 2 months |
|
| Outcomes | 1. Frequency of improvement in sleep quality. Cure was defined as normalised total sleep duration or total sleep duration at least 6 hours, with deep and refreshing sleep. Moderate improvement was defined as obvious improvement in sleep, with increase in total sleep duration by at least 3 hours and increase in depth of sleep, but total sleep duration less than 6 hours. Some improvement was defined as symptom improvement with increase in total sleep duration by less than 3 hours. No improvement was defined as no change in symptoms or worsening symptoms. 2. Pittsburgh Sleep Quality Index (PSQI) |
|
| Notes | Duration of follow‐up: 2 months
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, or severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | High risk | The total score and some sub‐scores on the PSQI were not reported |
| Other bias | High risk | No placebo or sham control was used and hence there might be a placebo effect |
Zhu 2005.
| Methods | Design: parallel‐group
Randomisation method: information not available Method of allocation concealment: information not available Blinding: information not available Stratification: not used |
|
| Participants | Inclusion: age 18 to 60 years, diagnosis of heroin dependence according to International Classification of Disease‐10 (ICD‐10), history of heroin use for more than 0.5 year, with average daily heroin intake more than 0.5 g, had received detoxification for 1 week but had protracted withdrawal symptoms, negative urine test for morphine, had stopped all therapies that may interfere with evaluation of the current study
Exclusion: patients with hepatitis, AIDS, severe heart, liver, kidney, blood, respiratory, alimentary, psychiatric diseases, malnutrition or injuries were excluded Number of participants: intervention: 25; control: 25 Number of males: intervention: 25; control: 25 Age (years): overall mean 27.32 (SD 8.71), range 18 to 47 Specific diagnoses/diagnostic subtypes: heroin dependence Associated disease: information not available Duration of disorder: information not available Previous treatments: information not available |
|
| Interventions | Intervention group (electroacupuncture): acupuncture to the following acupoints: Shenshu, Neiguan, Shenmen, Zusanli and Sanyinjiao, by 0.3 mm x 50 mm stainless steel needles to be inserted to 1.5 to 2.5 inches deep. The needles were connected to G6805‐2 electric stimulator and delivered sparse wave 10 Hz at 5 mA for 20 minutes. Treatment was given daily 3 times per week for 10 weeks. Control group (no specific treatment): no specific treatment was given | |
| Outcomes | Rating Scale for Protracted Withdrawal Symptoms | |
| Notes | Duration of follow‐up: 10 weeks
Dropouts: none Comparability of groups at baseline: no significant differences between the groups in age and gender distribution, route and duration and amount of heroin, and severity of insomnia at baseline Risk of bias: high |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not described. Since the intervention involves acupuncture, it is highly likely that the treating physicians and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available to judge whether there was selective reporting |
| Other bias | High risk | No placebo or sham control was used and hence there might be a placebo effect |
BMI: body mass index; NSAIDs: non‐steroidal anti‐inflammatory drugs; PSQI: Pittsburgh Sleep Quality Index; SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Becker‐Carus 1985 | Allocation of participant was not randomised |
| Chen 2003 | Quasi‐randomised controlled trial |
| Chen 2007 | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment control group |
| Cohen 2003 | The primary complaint is not insomnia |
| Cummings 2003 | Commentary, not RCT |
| Da Silva 2005 | Quasi‐randomised controlled trial |
| Ding 2006 | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment control group |
| Ding 2008 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment control group |
| Dong 2008 | RCT comparing different acupuncture methods; no placebo or sham or no treatment control group |
| Fan 2006 | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment control group |
| Gao 1995 | RCT comparing acupressure with estazolam; no placebo or sham or no treatment control group |
| Gao 1997 | Case series, not RCT |
| Gong 2009 | RCT comparing acupuncture with alprazolam; no placebo or sham or no treatment control group |
| He 2009 | RCT comparing different acupuncture methods; no placebo or sham or no treatment control group |
| Hong 2005 | RCT comparing acupuncture with trazodone; no placebo or sham or no treatment group |
| Hou 2005 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment control group |
| Huang 2007 | RCT comparing acupuncture with clonazepam; no placebo or sham or no treatment group |
| Huang 2009a | RCT comparing acupuncture with clonopin; no placebo or sham or no treatment group |
| Hui 2006 | RCT comparing moxibustion with diazepam; no placebo or sham or no treatment control group |
| Ju 2009 | RCT comparing moxibustion with estazolam; no placebo or sham or no treatment control group |
| Kang 2006 | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment group |
| Li 2005b | RCT comparing acupressure with estazolam; no placebo or sham or no treatment control group |
| Li 2007a | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment group |
| Li 2007b | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment group |
| Li 2007c | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment group |
| Li 2010 | RCT comparing different acupuncture methods; no placebo or sham or no treatment control group |
| Lian 1990 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment group |
| Liu 2000 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment group |
| Liu 2006 | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment group |
| Liu 2007 | RCT comparing acupuncture with clonazepam; no placebo or sham or no treatment group |
| Lu 2002 | No mention of using randomisation |
| Lu 2008 | RCT comparing different acupuncture methods; no placebo or sham or no treatment control group |
| Luo 1993 | RCT comparing acupressure with barbiturates; no placebo or sham or no treatment group |
| Ma 2006b | RCT comparing acupuncture with clonazepam; no placebo or sham or no treatment group |
| Ni 2006 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment group |
| Pan 2005 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment group |
| Phillips 2001 | Single‐arm trial, not RCT |
| Qi 2008 | RCT comparing acupuncture with alprazolam; no placebo or sham or no treatment group |
| Qiu 1999 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment group |
| Ruan 2001 | No mention of using randomisation |
| Sang 2004 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment control group |
| Shang 2000 | Case series, not RCT |
| Shen 2004 | Case series, not RCT |
| Shi 2003 | Case series, not RCT |
| Sjoling 2008 | Quasi‐randomised controlled trial |
| Su 2004 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment control group |
| Suen 2003 | A study using repeated measures without control group |
| Tang 2007b | RCT comparing acupuncture with herbal medicine; no placebo or sham or no treatment control group |
| Wang 1993 | RCT comparing acupressure with diazepam; no placebo or sham or no treatment group |
| Wang 2000 | Commentary on methodology |
| Wang 2002 | RCT comparing acupressure with diazepam; no placebo or sham or no treatment group |
| Wang 2003a | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment control group |
| Wang 2003b | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment control group |
| Wang 2004 | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment control group |
| Wang 2006 | Quasi‐randomised controlled trial |
| Wang 2008 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment control group |
| Wei 2006 | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment control group |
| Wei 2010 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment control group |
| Weng 2007 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment control group |
| Xiong 2003 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment control group |
| Xu 1997 | Case series, not RCT |
| Xuan 2007 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment control group |
| Yan 2010 | RCT comparing different acupuncture methods; no placebo or sham or no treatment control group |
| Yao 1999 | Case series, not RCT |
| Yu 1997 | Case series, not RCT |
| Zhang 2000 | RCT comparing acupressure with diazepam; no placebo or sham or no treatment control group |
| Zhang 2002 | Case series, not RCT |
| Zhang 2003a | Case series, not RCT |
| Zhang 2003b | RCT comparing acupuncture with clonazepam; no placebo or sham or no treatment control group |
| Zhang 2005 | RCT comparing acupuncture with estazolam; no placebo or sham or no treatment control group |
| Zhang 2008 | RCT comparing acupressure with alprazolam; no placebo or sham or no treatment control group |
| Zhang 2010 | RCT comparing different acupuncture methods; no placebo or sham or no treatment group |
| Zhong 2008 | Quasi‐randomised controlled trial |
| Zhou 2010 | RCT comparing different acupuncture methods; no placebo or sham or no treatment control group |
| Zhu 2002 | RCT comparing acupuncture with diazepam; no placebo or sham or no treatment control group |
| Zou 2008 | RCT comparing acupuncture with alprazolam; no placebo or sham or no treatment control group |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ISRCTN12585433.
| Trial name or title | The effect of acupuncture compared with sham acupuncture and estazolam in primary insomnia |
| Methods | This is a 6‐week, single‐blind, randomised, sham‐controlled study. A total of 150 untreated patients with primary insomnia will be recruited. Under single‐blind conditions, patients will be randomly assigned to one of the 3 groups. |
| Participants | Inclusion criteria: 1. Either gender aged 25 to 75 years 2. Have primary insomnia diagnosed from International Classification of Diseases, 10th Edition (ICD‐10) 3. Patients with insomnia persistent for 4 weeks or longer before the start of observation period 4. Have not yet received any psychoactive medications 5. Patients who submit written informed consent for study entry 6. Able to take part in the entire treatment and data collection procedure Exclusion criteria: 1. Diagnosis of depression, anxiety or schizophrenia 2. Diagnosis of serious disease of heart, brain, kidney or liver 3. History of sleep apnoea (temporary cessation of breathing during sleep) 4. Suffering from insomnia less than 4 weeks 5. Treatment with investigational drugs in past 6 months 6. Ever received acupuncture against insomnia, or during the last year received acupuncture for any indication |
| Interventions | Group A: active acupuncture with estazolam placebo tablet for 6 weeks. Active acupuncture is conducted by stimulating 5 acupoints: Shen‐Ting (DU‐24), Si‐Shen‐Cong (EX‐HN1), San‐Yin‐Jiao (SP‐6), Shen‐Men (HT‐7) and Bai‐Hui (DU‐20) for 30 minutes every other day. Stainless steel needles of 0.35 mm diameter are inserted at a depth of 10 mm obliquely into Bai‐Hui (Du‐20) Shen‐Ting (DU‐24) and Si‐Shen‐Cong (EX‐HN1), 10 mm straightly into San‐Yin‐Jiao (SP‐6) and 5 mm straightly into Shen‐Men (HT‐7). In the day without acupuncture intervention one estazolam placebo tablet should be taken before sleep. Group B: estazolam combined with sham acupuncture for 6 weeks. Sham acupuncture is conducted by stimulating 4 acupoints every other day: Bi‐Nao (LI‐14), Shou‐San‐li (LI‐10), Yu‐Ji (LU‐10) and Feng‐Shi (GB‐31). Stainless steel needles of 0.35 mm diameter are inserted straightly at a depth of 10 mm into the 4 points. According to traditional Chinese medicine theory, these 4 acupoints have no effect for insomnia. Estazolam dose is given 1 mg in the day without acupuncture intervention before sleep. Group C: sham acupuncture with estazolam placebo tablet for 6 weeks. Sham acupuncture is conducted as the group B. In the day without acupuncture one estazolam placebo tablet should be taken before sleep. |
| Outcomes | Primary outcomes: 1. Sleepiness measured using the Epworth Sleepiness Scale. Assessments will be conducted at baseline and at day 7, 14, 28, 42 and 2 months follow‐up. 2. Sleep diary assessments will be conducted every day until 2 months follow‐up Secondary outcomes: 1. Changes in the Pittsburgh Sleep Quality Index (PSQI). Assessments will be conducted at baseline and at day 28, 42 and 2 months follow‐up 2. Changes in the 36‐item Short Form Health Survey (SF‐36) scores. Assessments will be conducted at baseline and at day 28, 42 and 2 months follow‐up. |
| Starting date | 1 August 2009 |
| Contact information | Dr Jing Guo, Acupuncture Department of Beijing TCM Hospital, Meishuguanhoujie Road 23, Beijing, 100011, China. Tel: +86‐0‐1052176910. Email: guojing_2002@163.com. Sponsor website: http://www.bjzhongyi.com/ |
| Notes | Trial completed but results not reported yet |
NCT00838994.
| Trial name or title | A randomized controlled trial of acupuncture for residual insomnia associated with major depressive disorder |
| Methods | This is a randomised, single‐blinded controlled trial. Patients will be randomly assigned to one of the 3 groups. One is the traditional acupuncture treatment group, one is the non‐traditional acupuncture treatment group and the other is an acupuncture‐like placebo treatment. Patients will be put into groups and then compared. The chance of getting into each group is 1:1:1, i.e. equal chance. |
| Participants | Inclusion criteria: 1. Willing to give informed consent 2. Hong Kong resident 3. Age 18 to 65 years 4. Previous DSM‐IV Major Depressive Disorder as confirmed with the Structured Clinical Interview for DSM‐IV 5. Hamilton Depression Rating Scale scores of 18 or below for at screening and baseline visit 6. A chief complaint of insomnia 7. Able to comply with the trial protocol Exclusion criteria: 1. Any symptoms suggestive of specific sleep disorders, including loud snoring, periodic leg movement, parasomnia 2. A diagnosis of sleep apnoea or periodic limb movement disorder (PLMD) as assessed by overnight polysomnography (PSG) 3. Presence of suicidal risk 4. Previous history of schizophrenia, other psychotic disorders and bipolar disorder 5. Pregnant, breast‐feeding or woman of childbearing potential not using adequate contraception 6. Infection or abscess close to the site of selected acupoints and in the investigator's opinion inclusion is unsafe |
| Interventions | Traditional acupuncture: experimental patients will be treated at bilateral Ear Shenmen, Sishencong EX‐HN1, Anmian and unilateral Yintang EX‐HN3 and Baihui GV20. Acupuncture treatment will be performed by a registered Chinese medicine practitioner. "De qi" (an irradiating feeling considered to be indicative of effective needling) is achieved if possible. An electric‐stimulator (CEFAR Acus II, Lund, Sweden) is connected to these needles to give an electric‐stimulation in continuous wave, frequency of 4 Hz, 0.45 ms square wave pulses and constant current. Surgical tape or hair pin will be adhered to the needles. The needles will be left for 30 minutes and then removed. Acupuncture treatment will consist of 3 sessions per week for 3 consecutive weeks. Minimal acupuncture: active comparator patients will be treated superficially at points away from classic acupoints. The points include bilateral "Deltoideus" (in the middle of the line insertion of Binao LI 14 and acromion), "Forearm" (1 inch laterally of the middle point between Shaohai HE3 and Shenmen HE7), "Upper arm" (1 inch laterally of Tianfu LU 3) and "Lower leg" (0.5 inch dorsally of Xuanzhong GB39). "De qi" is avoided during needling. The treatment procedure, electric‐stimulation, frequency, duration and number of treatment sessions will be the same for the traditional acupuncture group. Placebo acupuncture: placebo comparator needles designed by Streitberger (1998) will be used. The placebo needles are blunt needle that will not penetrate the skin during needle insertion. The handles of these placebo needles will slide over the needle when it is compressed, giving it the appearance of penetrating the skin. The placebo needles are inserted to the site 1 inch beside the acupoints in order to avoid the acupressure effect. The needles are held by a surgical tape or hair pin in hairy region to imitate the retention of needles. The needles are connected to an electric‐stimulator with zero frequency and amplitude. The number, duration and frequency of the treatment sessions, and the intervention procedure will be the same for electro‐acupuncture and placebo acupuncture. |
| Outcomes | Primary outcomes: 1. Self rated sleep quality score measured by Insomnia Severity Index questionnaire (time frame: baseline, weekly during the treatment course, 1 week and 4 weeks post‐treatment) 2. Sleep parameters (sleep onset latency, sleep efficiency, total sleep time, times of wakening during sleep) by objective measure ‐ wrist actigraphy (time frame: baseline, 1 week and 4 weeks post‐treatment) 3. Sleep parameters (sleep onset latency, sleep efficiency, total sleep time, times of wakening during sleep) by subjective measures using sleep log (time frame: baseline, weekly during the treatment course, 1 week and 4 weeks post‐treatment) Secondary outcomes: 1. Self rated sleep quality score measured by the Pittsburgh Sleep Quality Index (PSQI) questionnaire (time frame: baseline, weekly during the treatment course, 1 week and 4 weeks post‐treatment) 2. Depression state measured by Hamilton Depression Rating Scale (HAMD) (time frame: baseline, 1 week and 4 weeks post‐treatment) 3. Patients' functioning regarding work/study, social life and family measured by Sheehan Disability Scale (time frame: baseline, weekly during the treatment course, 1 week and 4‐ weeks post‐treatment) 4.Patients' credibility of the treatment measured by credibility of treatment rating scale (time frame: second and the last time of the treatment) |
| Starting date | Oct 2007 |
| Contact information | Dr Ka‐Fai Chung, Western Psychiatry Center, Hong Kong |
| Notes | Study completed in April 2009 but results not reported yet |
NCT00855140.
| Trial name or title | Acupuncture for the treatment of insomnia ‐ a pilot study |
| Methods | This is a randomised, double‐blind, placebo‐controlled trial for treatment of adults with insomnia |
| Participants | Inclusion criteria: 1. Ages 18 to 60 2. Ability to speak, read and write English 3. Insomnia disorder, as defined by RDC, of 3 months or greater duration Exclusion criteria: 1. Presence of serious psychiatric Axis I DSM‐IV disorders such as bipolar or psychotic disorders, as individuals with these conditions may respond differently than those with insomnia disorder to the acupuncture intervention, potentially confounding the results 2. Active suicidal ideation or active psychosis, as this may present a concern regarding safety for a patient's participation in this study 3. Presence of depressive or anxiety disorders of moderate or greater severity based on either HAM‐D scores or HAM‐A scores of 14 or greater 4. Presence of unstable medical conditions commonly associated with significant sleep disturbance, e.g. uncompensated congestive heart failure, as this would not be expected to respond to the acupuncture intervention 5. Presence of other sleep disorder, such as periodic limb movement disorder or sleep apnoea, as these conditions would require other medical treatment; this will be based on known history of sleep disorder or findings on screening PSG of apnoea‐hypopnoea index of > 10 or periodic limb movement index of > 10 6. Alcohol use > 14 beverages/week, as this may impact on response to the intervention and assessment measures 7. Ongoing use of any recreational drugs 8. Ongoing use of benzodiazepines, prescription hypnotic medication, over‐the‐counter hypnotic medication, or nutritional supplements with purported hypnotic effects 9. Ongoing use of other psychotropic medication, such as psychostimulants, antipsychotics or antidepressants 10. Caffeine use > the equivalent of 5 cups of coffee/day 11. Pregnancy, as the safe use of acupuncture in pregnancy has not been established 12. Active malignancy, autoimmune condition or treatment with immunosuppressive drugs 13. Presence of coagulopathy or use of anticoagulant medication 14. Active involvement in any psychotherapy or other treatment specifically directed towards insomnia 15. Prior experience with acupuncture treatment for any condition as such patients may be more likely to determine if they are receiving a sham versus verum acupuncture treatment |
| Interventions | Verum acupuncture (experimental): acupuncture derived from the TCM literature specific for insomnia Sham acupuncture (control): placement of placebo needles |
| Outcomes | Primary outcomes: 1. Sleep efficiency on polysomnography (time frame: baseline and post‐intervention) 2. Pittsburgh Sleep Quality Index (PSQI) (time frame: baseline, end of intervention and 3 months post‐treatment) 3. Insomnia Severity Index (ISI) (time frame: baseline, end of intervention and 3 months post‐treatment) Secondary outcomes: 1. Epworth Sleepiness Scale (ESS) (time frame: baseline, end of intervention and 3 months post‐treatment) 2. Multidimensional Fatigue Inventory (MFI) (time frame: baseline, end of intervention and 3 months post‐treatment) 3. Inventory for Depressive Symptomatology ‐ Self Rated (IDS‐SR) (time frame: baseline, end of intervention and 3 months post‐treatment) 4. State version of the Spielberger State‐Trait Anxiety Inventory (STAI‐s) (time frame: baseline, end of intervention and 3 months post‐treatment) 5. The Pre‐Sleep Arousal Scale (PSAS) (time frame: baseline, end of intervention and 3 months post‐treatment) 6. Pittsburgh Sleep Diary (PghSD) and actigraphy recording (time frame: baseline, end of intervention and 3 months post‐treatment) 7. Adverse event form (AEF) (time frame: weekly during intervention and end of treatment) 8. Autonomic arousal as measured by HRV and Q‐EEG during sleep recording (time frame: baseline and end of intervention) |
| Starting date | March 2009 |
| Contact information | Dr Ronald M Glick, Center for Integrative Medicine at UPMC Shadyside, Pittsburgh, Pennsylvania, United States, 15232 |
| Notes | Still recruiting participants |
NCT00868517.
| Trial name or title | The effect of acupuncture on PTSD‐related insomnia |
| Methods | This is a randomised single‐blind trial comparing 3 treatment groups: acupuncture, sham acupuncture and no treatment, for patients with insomnia related to post‐traumatic stress disorder |
| Participants | Inclusion criteria: 1. Combat veteran of Operation Iraqi Freedom or Operation Enduring Freedom conflicts 2. Diagnosed with PTSD per DSM‐IV criteria 3. Have insomnia as indicated by a score equal to or greater than 8 on the Insomnia Severity Index (ISI) 4. Diagnosis of insomnia made after PTSD diagnosis 5. If on psychotropic medications, must be on stable psychotropic medication regimen for one month prior to enrolment in study Exclusion criteria: 1. Does not speak English 2. Not competent to sign informed consent 3. History of moderate or severe traumatic brain injury 4. Start use of CPAP or BiPAP during the study 5. Experiencing severe psychiatric illness defined as suicidal ideation, homicidal ideation or psychosis 6. History of substance abuse dependence (as defined per DSM‐IV criteria) during the one year preceding enrolment in the study OR history of illicit substance use for 3 months prior to study enrolment OR positive Audit C score at study enrolment or during course of study enrolment (defined as score of 5 and above) 7. Received acupuncture during past 3 months 8. On Coumadin, heparin or Lovenox 9. Pregnancy |
| Interventions | Experimental: will receive true group ear acupuncture Sham comparator: will receive sham group ear acupuncture No intervention strict control group: will receive conventional care only |
| Outcomes | Primary outcome: Sleep quality rating as measured by Insomnia Severity Index and Morin Sleep diary (time frame: t = 0, 1, 2 months) Secondary outcomes: 1. Fragmented sleep patterns as measured by Morin Sleep Diary and Wrist Actigraphs (time frame: t = 0, 1, 2 months) 2. Hypnotic medication use (time frame:t = 0,1,2 months) 3. Attrition rates (time frame: t = 0, 1, 2 months) 4. Veteran Satisfaction Scores (time frame: t = 0, 1, 2 months) |
| Starting date | October 2009 |
| Contact information | Michael C Jecmen, MA, USA. Tel: 202‐745‐8000 ext 6236. Email: michael.jecmen@va.gov |
| Notes | Still recruiting participants |
NCT01162018.
| Trial name or title | Acupuncture for sleep disruption in cancer survivors |
| Methods | This is a randomised, open‐label trial of acupuncture for sleep disruption in female breast cancer survivors. The eligible women would be randomised and stratified by sleep problems to 2 arms: acupuncture versus sham acupuncture. |
| Participants | Inclusion criteria: 1. Female 18 years and older 2. Breast cancer 3. Post‐treatment 4. With insomnia |
| Interventions | Experimental: acupuncture Placebo control: validated sham acupuncture There are 10 sessions each lasting approximately 20 minutes |
| Outcomes | Primary outcomes: 1. Fatigue reduction (time frame: 1 month and 3 months post final acupuncture treatment) 2. Quality of life (time frame: 1 month and 3 months post final acupuncture treatment) 3. Insomnia reduction (time frame: 1 month and 3 months post final acupuncture treatment) |
| Starting date | July 2010 |
| Contact information | Dr David Spiegel, Stanford University School of Medicine, Stanford, California, United States, 94305. Tel: 650‐7236421. Email: dspiegel@stanford.edu |
| Notes | Still recruiting participants |
CPAP: continuous positive airway pressure; PTSD: post‐traumatic stress disorder; TCM: traditional Chinese medicine
Contributions of authors
Cheuk DKL: protocol development, searching for trials, quality assessment of trials, data extraction, data input, data analyses, development of final review, corresponding author.
Yeung J: protocol development, searching for trials, quality assessment of trials, data extraction, data input, data analyses, development of final review.
Chung CF: development of final review.
Wong V: protocol development, development of final review.
Sources of support
Internal sources
The University of Hong Kong, Hong Kong.
External sources
No sources of support supplied
Declarations of interest
One included trial was published by co‐authors of this review. They were not involved in data extraction or risk of bias assessment of their own study.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Chen 1999 {published data only}
- Chen ML, Lin LC, Wu SC, Lin JG. The effectiveness of acupressure in improving the quality of sleep of institutionalized residents. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 1999;54(8):389‐4. [DOI] [PubMed] [Google Scholar]
Chen 2009 {published data only}
- Chen X. The clinical study of head massage with ear application therapy in treating patients of insomnia with depressive and anxiety disorder. Thesis (M. Phil) Guanzhou University of Chinese Medicine.
Cui 2003 {published data only}
- Cui R, Zhou D. Treatment of phlegm‐ and heat‐induced insomnia by acupuncture in 120 cases. Journal of Traditional Chinese Medicine 2003;23(1):57‐8. [PubMed] [Google Scholar]
- Cui R, Zhou DA. Treatment of phlegm and heat induced insomnia by acupuncture in 120 cases. Beijing Journal of Traditional Chinese Medicine 2001;20:38‐9. [PubMed] [Google Scholar]
Du 2007 {published data only}
- Du MX. Effect of acupuncture and medicine on treatment of insomnia: 34 cases clinical observation. Journal of Practical Traditional Chinese Medicine 2007;23:18‐9. [Google Scholar]
Guo 2009 {published data only}
- Guo A, Li A. Observation on treatment of primary insomnia by acupuncture combined with zolpidem. Medical Journal of Communications 2009;23(4):434‐8. [Google Scholar]
Hisghman 2006 {published data only}
- Hisghman VI. Effects of a standardized auricular point prescription in adults with insomnia. Thesis (PhD) University of Virginia 2006.
Hwang 2007 {published data only}
- Hwang EH. Effects of hand acupuncture therapy on sleep quality in sleep disrupted adults. Journal of Korean Academy of Nursing 2007;37(7):1108‐18. [DOI] [PubMed] [Google Scholar]
Jian 2005 {published data only}
- Jian J, Liu J. 50 cases observation on effect of acupuncture combined medicine on treatment of refractory insomnia. Henan Traditional Chinese Medicine 2005;25:62‐3. [Google Scholar]
Jin 2003 {published data only}
- Jin RF. Stimulating auricular acupoint by Semen Vaccariae to treat internal diseases complicated with insomnia in 64 cases. Zhejiang Journal of Traditional Chinese Medicine 2003;36:213. [Google Scholar]
Kaiser‐Pagliarini 2009 {published data only}
- Kaiser‐Pagliarini TG, Hachul HC, Maciel AL, Yagihara FT, Garbuio SA, Freire AO, et al. Acupuncture in insomnia and its consequences in postmenopausal women. Sleep Medicine 2009;10(Suppl 2):S18. [Google Scholar]
Kim 2004 {published data only}
- Kim YS, Lee SH, Jung WS, Park SU, Moon SK, Ko CN, et al. Intradermal acupuncture on shen‐men and nei‐kuan acupoints in patients with insomnia after stroke. American Journal of Chinese Medicine 2004;32(5):771‐8. [DOI] [PubMed] [Google Scholar]
Lai 2010 {published data only}
- Lai MY. Clinical research on the treatment of insomnia with electroacupuncture and Gan Mai Da Cao Tang (Kanbakutaisoto). Thesis (PhD) Guangzhou University of Chinese Medicine 2010.
Lee 2009 {published data only}
- Lee SY, Baek YH, Park SU, Moon SK, Park JM, Kim YS, et al. Intradermal acupuncture on Shen‐Men and Nei‐Kuan acupoints improves insomnia in stroke patients by reducing the sympathetic nervous system activity. American Journal of Chinese Medicine 2009;37(6):1013‐21. [DOI] [PubMed] [Google Scholar]
Li 2005a {published data only}
- Li QY, Dong YF. 26 cases observation on electroacupuncture and Western medicine combined on treatment of dyssomnia of depression. Chinese Journal of Information on Traditional Chinese Medicine 2005;12:71‐2. [Google Scholar]
Lin 2007 {published data only}
- Lin B. Clinical research on treatment of insomnia by auricular acupressure. Thesis (M. Phil) Beijing University of Chinese Medicine 2007.
Liu 2001 {published data only}
- Liu Y, Jiang ZQ. Acupuncture combined Ciwujia injection in treatment of senile insomnia. Practical Journal of Rural Doctor 2001;8:26. [Google Scholar]
Lu 1998 {published data only}
- Lu WW. Effect of acupuncture on Neiguan and Chinese medicine named Qiyeanshen tablet used on treatment of insomnia: 35 cases clinical observation. Journal of Nanjing University Traditional Chinese Medicine 1998;14:164. [Google Scholar]
Luo 2006 {published data only}
- Luo L, Hu YP, Yu SG, Li N. Observation on therapeutic effect of rolling needle therapy on insomnia. Chinese Acupuncture Moxibustion 2006;26(3):183‐5. [PubMed] [Google Scholar]
Lv 2007 {published data only}
- Lv YE. Effect observation of acupuncture combined with TCM therapy on 68 cases of insomnia. World Journal of Integrated Traditional and Western Medicine 2007;2:355‐6. [Google Scholar]
Ma 2006a {published data only}
- Ma X. Effect of acupuncture combined with Western medicine on treatment of dyssomnia of patients of paracme of schizophrenia. Henan Traditional Chinese Medicine 2006;26(3):57‐8. [Google Scholar]
Nordio 2008 {published data only}
- Nordio M, Romanelli F. Efficacy of wrists overnight compression (HT7 point) on insomniacs: possible role of melatonin?. Minerva Medicine 2008;99(6):539‐47. [PubMed] [Google Scholar]
Reza 2010 {published data only}
- Reza H, Kian N, Pouresmail Z, Masood K, Bagher MSS, Cheraghi MA. The effect of acupressure on quality of sleep in Iranian elderly nursing home residents. Complementary Therapies in Clinical Practice 2010;16:81‐5. [DOI] [PubMed] [Google Scholar]
Sok 2005 {published data only}
- Sok SR, Kim KB. Effects of auricular acupuncture on insomnia in Korean elderly. Journal of Korean Academy of Nursing 2005;35(6):1014‐24. [DOI] [PubMed] [Google Scholar]
Suen 2002 {published data only}
- Suen LK, Wong TK, Leung AW. Effectiveness of auricular therapy on sleep promotion in the elderly. American Journal of Chinese Medicine 2002;30(4):429‐49. [DOI] [PubMed] [Google Scholar]
Sun 2010a {published data only}
- Sun JL, Sung MS, Huang MY, Cheng GC, Lin CC. Effectiveness of acupressure for residents of long‐term care facilities with insomnia: a randomized controlled trial. International Journal of Nursing Studies 2010;47:798‐805. [DOI] [PubMed] [Google Scholar]
Tang 2007a {published data only}
- Tang WH. Clinical observation of auricular therapy and head's message on treatment of patients with insomnia. Hunan Journal of Traditional Chinese Medicine 2007;23:75‐6. [Google Scholar]
Tian 2006 {published data only}
- Tian HZ, Li JQ. Clinical observation of acupuncture combined with medicine on treatment of insomnia. Chinese Archives of Traditional Chinese Medicine 2006;24(6):1146‐7. [Google Scholar]
Tsay 2003 {published data only}
- Tsay SL, Chen ML. Acupressure and quality of sleep in patients with end‐stage renal disease‐‐a randomized controlled trial. International Journal of Nursing Studies 2003;40(1):1‐7. [DOI] [PubMed] [Google Scholar]
- Tsay SL, Rong JR, Lin PF. Acupoints massage in improving the quality of sleep and quality of life in patients with end‐stage renal disease. Journal of Advanced Nursing 2003;42(2):134‐42. [DOI] [PubMed] [Google Scholar]
Tsay 2004 {published data only}
- Tsay SL, Cho YC, Chen ML. Acupressure and Transcutaneous Electrical Acupoint Stimulation in improving fatigue, sleep quality and depression in hemodialysis patients. American Journal of Chinese Medicine 2004;32(3):407‐16. [DOI] [PubMed] [Google Scholar]
Ye 2008 {published data only}
- Ye TS, Wang QJ, Xiew WX, Chen Y, He JC. A randomized control study of abdominal acupuncture treatment for primary insomnia. Shanhai Journal of Acupuncture Moxibustion 2008;27(2):3‐5. [Google Scholar]
Yeung 2009a {published data only}
- Yeung WF, Chung KF, Zhang SP, Yap TG, Law ACK. Electroacupuncture for primary insomnia: a randomized controlled trial. Sleep 2009;32(8):1039‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2010 {published data only}
- Zhao Y. Controlled clinical trial of acupuncture combined with Gengniananshensan for treatment of perimenopausal insomnia. Thesis (PhD) Guangzhou University of Chinese Medicine 2010.
Zhu 2005 {published data only}
- Zhu ZC, Mu JP, Liang Y, Zong L, Hu J, Xu P. Clinical observation on treatment of dyssomnia of heroin addicts after detoxification by electro‐acupuncture. Shanghai Journal of Acupuncture and Moxibustion 2005;24:6‐8. [Google Scholar]
References to studies excluded from this review
Becker‐Carus 1985 {published data only}
- Becker‐Carus C, Heyden T, Kelle A. Effectiveness of acupuncture and attitude‐relaxation training in the treatment of primary sleep disorders. Zeitschrift fur Klinische Psychologie und Psychotherapie 1985;33(2):161‐72. [PubMed] [Google Scholar]
Chen 2003 {published data only}
- Chen XH. Clinical observation of therapeutic effect of acupuncture according to syndrome differentiation combined with medicine on treatment of asthenic insomnia. Guangxi Journal of Traditional Chinese Medicine 2003;26(3):36‐7. [Google Scholar]
- Chen XH. Observation on the curative effect of combined acupuncture and medicine on insomnia. Shanghai Journal of Acupuncture Moxibustion 2003;22(11):30‐1. [Google Scholar]
Chen 2007 {published data only}
- Chen FK, Xu XH, Chen HL, Wang QQ. Clinical observation of effect of electroacupuncture combined frequency spectrum therapy on treatment of 40 patients of insomnia. Journal of Clinical Acupuncture Moxibustion 2007;23:28. [Google Scholar]
Cohen 2003 {published data only}
- Cohen SM, Rousseau ME, Carey BL. Can acupuncture ease the symptoms of menopause?. Holistic Nursing Practice 2003;17(6):295‐9. [DOI] [PubMed] [Google Scholar]
Cummings 2003 {published data only}
- Cummings M. Acupressure improves sleep quality in patients with end‐stage renal disease compared with no treatment controls (but not compared with sham acupressure). Focus on Alternative and Complementary Therapies 2003;8(2):210‐1. [Google Scholar]
Da Silva 2005 {published data only}
- Silva JB, Nakamura MU, Cordeiro JA, Kulay LJ. Acupuncture for insomnia in pregnancy‐a prospective, quasi‐randomised, controlled study. Acupuncture in Medicine 2005;23(2):47‐51. [DOI] [PubMed] [Google Scholar]
Ding 2006 {published data only}
- Ding WC. Treatment of 84 cases of insomnia using acupuncture on Shenmen and Sanyinjiao as main acupoints. Jilin Journal of Traditional Chinese Medicine 2006;26:46. [Google Scholar]
Ding 2008 {published data only}
- Ding DG, Luo HP, Jiao Y. Clinical research of long‐playing acupuncture on insomnia. Journal of Clinical Acupuncture Medicine 2008;24:10‐1. [Google Scholar]
Dong 2008 {published data only}
- Dong JP, Wang S, Sun WY, Liu F. Randomized controlled observation on head point‐through‐point therapy for treatment of insomnia. Chinese Acupuncture and Moxibustion 2008;28(3):159‐62. [PubMed] [Google Scholar]
Fan 2006 {published data only}
- Fan D, Sun BY, Zhao X, Wang FC. Clinical observation of acupuncture with tranquillizing and allaying excitement method on treatment of 40 cases of insomnia. Journal of Changchun University Traditional Chinese Medicine 2006;22:28. [Google Scholar]
Gao 1995 {published data only}
- Gao Y. Insomnia treated by auricular acupressure in 128 cases. Shanghai Journal of Acupuncture and Moxibustion 1995;14(4):161‐2. [Google Scholar]
Gao 1997 {published data only}
- Gao QW. Acupuncture treatment of insomnia: clinical observation of 288 cases. International Journal of Clinical Acupuncture 1997;8(2):183‐5. [Google Scholar]
Gong 2009 {published data only}
- Gong YL, Zhang YB, Han C, Jiang YY, Li Y, Chen SC, et al. Clinical observation on therapeutic effect of the pressing plantar reflex area with wooden needle for treatment of patients with insomnia. Chinese Acupuncture and Moxibustion 2009;29(11):935‐7. [PubMed] [Google Scholar]
He 2009 {published data only}
- He Y, Li X, Wang D, Zhang G. Acupuncture with cupping for treatment of 48 cases of insomnia. Shanghai Journal of Acupuncture and Moxibustion 2009;28(11):659. [Google Scholar]
Hong 2005 {published data only}
- Hong YB, Zhu W, Yao WH. Clinical therapeutic controlled study of electroacupuncture combined medicine on treatment of insomnia. Bulletin of Medical Research 2005;34:72‐4. [Google Scholar]
Hou 2005 {published data only}
- Hou C, Liu Q. 150 cases of insomnia treated by acupuncture. New Journal of Traditional Chinese Medicine 2005;37(3):61. [Google Scholar]
Huang 2007 {published data only}
- Huang LS, Wang DL, Wang CW, Hu YP, Zhou JW, Li N. Clinical study on rolling needle therapy for treatment of cases of nonorganic chronic insomnia. Journal of Traditional Clinical Medicine 2007;48:331‐4. [Google Scholar]
Huang 2009a {published data only}
- Huang LS, Wang DL, Wang CW, Hu YP, Zhou JW, Li N. The needle‐rolling therapy for treatment of non‐organic chronic insomnia in 90 cases. Journal of Traditional Chinese Medicine 2009;29(1):19‐23. [DOI] [PubMed] [Google Scholar]
Hui 2006 {published data only}
- Hui JP, Zhao YD, Hui JR. Treatment of 30 cases of obstinate insomnia with penetrating Bei Shu point to Jiaji (EX‐B2). Journal of Gansu College of Traditional Chinese Medicine 2006;23(2):47‐8. [Google Scholar]
Ju 2009 {published data only}
- Ju YL, Chi X, Liu JX. Forty cases of insomnia treated by suspended moxibustion at Baihui (GV 20). Journal of Traditional Chinese Medicine 2009;29(2):95‐6. [DOI] [PubMed] [Google Scholar]
Kang 2006 {published data only}
- Kang FH. 78 cases clinical observation of effect of auricular therapy combined acupuncture on treatment of insomnia. Journal of Tianjin University Traditional Chinese Medicine 2005;25:62‐3. [Google Scholar]
Li 2005b {published data only}
- Li HT, Liu JH, Zhu QX. Clinical observation on treatment of senile insomnia with application therapy on shenque acupoint with Ginkgo leaf preparation: a report of 25 cases. Journal of Chinese Integrative Medicine 2005;3(5):398‐9. [DOI] [PubMed] [Google Scholar]
Li 2007a {published data only}
- Li XJ, Bi LP. Differentiation of syndromes and use acupuncture combined auricular therapy on treatment of insomnia: 133 cases clinical observation. Lishizhen Medicine Materia Medica Research 2007;18(3):678. [Google Scholar]
Li 2007b {published data only}
- Li TB. Effect of acupuncture on post‐stroke insomnia. Clinical Journal of Rehabilitation Theory and Practice 2007;13:656‐7. [Google Scholar]
Li 2007c {published data only}
- Li JH, Li JZ. Clinical observation of electroacupuncture treatment for insomnia. Journal of Changzhi Medical College 2007;21:465‐6. [Google Scholar]
Li 2010 {published data only}
- Li LF, Lu JH. Clinical observation on acupuncture treatment of intractable insomnia. Journal of Traditional Chinese Medicine 2010;30(1):21‐2. [DOI] [PubMed] [Google Scholar]
Lian 1990 {published data only}
- Lian N, Yan Q. Insomnia treated by auricular pressing therapy. Journal of Traditional Chinese Medicine 1990;10(3):174‐5. [PubMed] [Google Scholar]
Liu 2000 {published data only}
- Liu Q. Acupuncture and auricular therapy for 36 cases of insomnia. Jilin Journal of Traditional Chinese Medicine 2000;20:63. [Google Scholar]
Liu 2006 {published data only}
- Liu JH, Huang XH, Chen XH. 32 cases clinical observation of effect of acupuncture on treatment of post‐stroke insomnia. International Medicine and Health Guidance News 2006;12:107‐8. [Google Scholar]
Liu 2007 {published data only}
- Liu XB, Niu WM. Hairline acupuncture treatment on 160 cases of insomnia. Shanxi Journal of Chinese Medicine 2007;28:716‐7. [Google Scholar]
Lu 2002 {published data only}
- Lu Z. Scalp and body acupuncture for treatment of senile insomnia ‐ a report of 83 cases. Journal of Traditional Chinese Medicine 2002;22(3):193‐4. [PubMed] [Google Scholar]
Lu 2008 {published data only}
- Lu M, Liu X. Insomnia due to deficiency of both the heart and spleen treated by acupuncture‐moxibustion and Chinese Tuina. Journal of Traditional Chinese Medicine 2008;28(1):10‐2. [DOI] [PubMed] [Google Scholar]
Luo 1993 {published data only}
- Luo ZP, Li XD, He YN, Han XY. Clinical observation of 367 cases of treating insomnia by oto‐pressure. Heilongjiang Journal of Traditional Chinese Medicine 1993;1:45‐8. [Google Scholar]
Ma 2006b {published data only}
- Ma ZX, Li GH, Duan HM. 31 cases of insomnia treated by acupuncture. Guangming Journal of Chinese Medicine 2006;21(4):25‐6. [Google Scholar]
Ni 2006 {published data only}
- Ni JX, Zhu WZ. Clinical study of 76 cases of insomnia with head acupoints penetration. Journal of Clinical Acupuncture‐Moxibustion 2006;22(12):35‐6. [Google Scholar]
Pan 2005 {published data only}
- Pan QL, Zhang LM, Yang JY, He YL. Results in treatment of obstinate insomnia by acupuncture. China Tropical Medicine 2005;5(8):1705‐6. [Google Scholar]
Phillips 2001 {published data only}
- Philips KD, Skelton WD. Effects of individualized acupuncture on sleep quality in HIV disease. Journal of the Association of Nurses in AIDS Care 2001;12(1):27‐39. [DOI] [PubMed] [Google Scholar]
Qi 2008 {published data only}
- Qi LZ, Ma XP, Yang L. Observation on the therapeutic effect of neck clustered needling on insomnia. Chinese Acupuncture Moxibustion 2008;28(12):861‐4. [PubMed] [Google Scholar]
Qiu 1999 {published data only}
- Qiu RJ, Zheng CM, Lun ZX, Zeng WH, Ding MZ. Observation of effect of a kind of massage on treatment of 53 patients of insomnia. New Journal of Traditional Chinese Medicine 1999;31:23. [Google Scholar]
Ruan 2001 {published data only}
- Ruan J. Clinical observation on the treatment of 102 cases of insomnia with point‐application. International Journal of Clinical Acupuncture 2001;12(2):109‐13. [Google Scholar]
Sang 2004 {published data only}
- Sang P, Wang S. 40 cases of insomnia treated by head acupuncture. Heilongjiang Journal of Traditional Chinese Medicine 2004;33(3):43. [Google Scholar]
Shang 2000 {published data only}
- Shang YT. Ear pressing for insomnia: observation of 82 cases. International Journal of Clinical Acupuncture 2000;11(1):65‐7. [Google Scholar]
Shen 2004 {published data only}
- Shen P. Two hundred cases of insomnia treated by otopoint pressure plus acupuncture. Journal of Traditional Chinese Medicine 2004;24(3):168‐9. [PubMed] [Google Scholar]
Shi 2003 {published data only}
- Shi D. Acupuncture treatment of insomnia. Journal of Traditional Chinese Medicine 2003;23(2):136‐7. [PubMed] [Google Scholar]
Sjoling 2008 {published data only}
- Sjoling M, Rolleri M, Englund E. Auricular acupuncture versus sham acupuncture in the treatment of women who have insomnia. Journal of Alternative and Complementary Medicine 2008;14(1):39‐46. [DOI] [PubMed] [Google Scholar]
Su 2004 {published data only}
- Su X, Wu Z. 166 cases of secondary insomnia caused by cervical spondylosis using acupuncture at neck Jiaji. Chinese Journal of Current Traditional and Western Medicine 2005;3:426. [Google Scholar]
Suen 2003 {published data only}
- Suen LK, Wong TK, Leung AW, Ip WC. The long‐term effects of auricular therapy using magnetic pearls on elderly with insomnia. Complementary Therapies in Medicine 2003;11(2):85‐92. [DOI] [PubMed] [Google Scholar]
Tang 2007b {published data only}
- Tang SC, Liu JM, Liu GL. Clinical observation on effect of electric acupuncture at Sishencong in treating insomnia. Chinese Journal of Integrated Traditional and Western Medicine 2007;27(11):1030‐2. [PubMed] [Google Scholar]
Wang 1993 {published data only}
- Wang XM. The comparative analysis between using auriculo‐pressing therapy and oral diazepam for treating insomnia. Journal of Clinical Psychiatry 1993;3:189. [Google Scholar]
Wang 2000 {published data only}
- Wang F. Treatment of insomnia by pressing Yongquan and pinching toes. International Journal of Clinical Acupuncture 2000;11(1):69‐70. [Google Scholar]
Wang 2002 {published data only}
- Wang ST. An observation on efficacy of auricular acupressure on insomnia using magnetic pearls. Anhui Clinical Journal of Traditional Chinese Medicine 2002;14:165. [Google Scholar]
Wang 2003a {published data only}
- Wang MZ, Wei Y. 115 cases of insomnia treated by acupuncture in Jiannao using Anshen granule. Journal of Clinical Acupuncture and Moxibustion 2003;19:32‐3. [Google Scholar]
Wang 2003b {published data only}
- Wang QQ, Chen HL. Observations on the curative effect of combined acupoint injection and electroacupuncture on 40 insomnia cases. Shanghai Journal of Acupuncture and Moxibustion 2003;22:27‐8. [Google Scholar]
Wang 2004 {published data only}
- Wang Y, Zhao ZF, Wu Y. Clinical therapeutic effect of acupuncture on post‐stroke depression with insomnia. Chinese Acupuncture and Moxibustion 2004;24:603‐6. [Google Scholar]
Wang 2006 {published data only}
- Wang J, Jiang JF, Wang LL. Clinical observation on Governor vessel Daoqi method for treatment of dyssomnia in the treatment of depression. Chinese Acupuncture and Moxibustion 2006;26(5):328‐30. [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang XY, Yuan SH, Yang HY, Sun YM, Cheng FP, Zhang CL, et al. Abdominal acupuncture for insomnia in women: a randomized controlled clinical trial. Acupuncture and Electrotherapeutics Research 2008;33(1‐2):33‐41. [DOI] [PubMed] [Google Scholar]
Wei 2006 {published data only}
- Wei J, Zhao Y, Wei J. 30 cases of obstinate insomnia treated by acupuncture through Beixue and jiaji acupoints. Journal of Gansu College of Traditional Chinese Medicine 2006;23(2):47. [Google Scholar]
Wei 2010 {published data only}
- Wei Y. Clinical observation on acupoint catgut embedding at head‐acupoint combined with massage of sole for treatment of refractory insomnia. Chinese Acupuncture and Moxibustion 2010;30(2):117‐20. [PubMed] [Google Scholar]
Weng 2007 {published data only}
- Weng M, Liao HQ. Efficacy of electroacupuncture treatment for insomnia in the elderly using scale analysis. Journal of Clinical Acupuncture and Moxibustion 2007;23:33‐4. [Google Scholar]
Xiong 2003 {published data only}
- Xiong X, Chen Y, Liang D, Xu X, Fang Y. 45 cases of insomnia treated by shallow acupuncture. Journal of Fujian College of Traditional Chinese Medicine 2003;13:44‐5. [Google Scholar]
Xu 1997 {published data only}
- Xu G. 45 cases of insomnia treated by acupuncture. Shanghai Journal of Acupuncture and Moxibustion 1997;16(6):10. [Google Scholar]
Xuan 2007 {published data only}
- Xuan YB, Guo J, Wang LP, Wu X. Randomized and controlled study on effect of acupuncture on sleep quality in the patient of primary insomnia. Chinese Acupuncture and Moxibustion 2007;27(12):886‐8. [PubMed] [Google Scholar]
Yan 2010 {published data only}
- Yan XK, Zhang Y, Yu L, Yang B, Chen C, Wang FC. Effect of "tranquillization needling" on the sleep quality in patients with insomnia of heart‐spleen deficiency type. Acupuncture Research 2010;35(3):222‐5. [PubMed] [Google Scholar]
- Yan XK, Zhang Y, Yu L, Yue GL, Li T, Chen C, et al. Effect on tranquillizing and allaying excitement needling method on brain blood flow in the patients of insomnia of heart and spleen deficiency. Chinese Acupuncture and Moxibustion 2010;30(2):113‐6. [PubMed] [Google Scholar]
Yao 1999 {published data only}
- Yao S. 46 cases of insomnia treated by semiconductor laser irradiation on auricular points. Journal of Traditional Chinese Medicine 1999;19(4):298‐9. [PubMed] [Google Scholar]
Yu 1997 {published data only}
- Yu GX. Through puncture to Huatuojiaji points plus wrist ‐ ankle needling in treatment of insomnia. International Journal of Clinical Acupuncture 1997;8(1):69‐71. [Google Scholar]
Zhang 2000 {published data only}
- Zhang J, Li J, Li YS, Pan JL, Zhang M. A controlled study of treating insomnia by auricular acupressure and diazepam. Yunan Journal of Traditional Chinese Medicine 2000;21:37‐8. [Google Scholar]
Zhang 2002 {published data only}
- Zhang Q. Clinical observation on intractable insomnia treated by point pressure in 42 cases. Journal of Traditional Chinese Medicine 2002;22(4):276‐7. [PubMed] [Google Scholar]
Zhang 2003a {published data only}
- Zhang Q. Clinical observation on acupuncture treatment of insomnia in 35 cases. Journal of Traditional Chinese Medicine 2003;23(2):125‐6. [PubMed] [Google Scholar]
Zhang 2003b {published data only}
- Zhang H, Deng H, Xiong K. Acupuncture regulating Yinqiao meridians for treatment of 89 cases of insomnia. Chinese Acupuncture and Moxibustion 2003;23:394‐6. [Google Scholar]
Zhang 2005 {published data only}
- Zhang XY. Effect of acupuncture on 45 cases of insomnia. Journal of Clinical Acupuncture and Moxibustion 2005;21:24. [Google Scholar]
Zhang 2008 {published data only}
- Zhang GX. Analysis on the treatment of insomnia by auricular point imbedding therapy in college students. Chinese Journal of School Health 2008;29(6):568. [Google Scholar]
Zhang 2010 {published data only}
- Zhang YF, Ren GF, Zhang XC. Acupuncture plus cupping for treating insomnia in college students. Journal of Traditional Chinese Medicine 2010;30(3):185‐9. [DOI] [PubMed] [Google Scholar]
Zhong 2008 {published data only}
- Zhong ZG, Cai H, Li XL, Lv D. Effect of acupuncture combined with massage of the sole on sleeping quality of the patient with insomnia. Chinese Acupuncture and Moxibustion 2008;28(6):4113. [PubMed] [Google Scholar]
Zhou 2010 {published data only}
- Zhou ZL, Shi X, Li SD, Guan L. Effect of scalp point penetration needling on sleep quality and sleep structure of insomnia patients. Chinese Acupuncture and Moxibustion 2010;30(9):721‐4. [PubMed] [Google Scholar]
- Zhou ZL, Shi X, Li SD, Guan L. Scalp penetration acupuncture for insomnia: a randomized controlled trial. Journal of Chinese Integrative Medicine 2010;8(2):126‐30. [DOI] [PubMed] [Google Scholar]
Zhu 2002 {published data only}
- Zhu H. Acupuncture treating refractory insomnia by balancing Yin Yang Qiao Meridian. Journal of Traditional Chinese Medicine 2002;22:55‐6. [Google Scholar]
Zou 2008 {published data only}
- Zou Y. Clinical observation on the treatment of insomnia by tranquillizing electroacupuncture. Heilongjiang Journal of Traditional Chinese Medicine 2008;37:40‐1. [Google Scholar]
References to ongoing studies
ISRCTN12585433 {published data only}
- Guo J. The effect of acupuncture compared with sham acupuncture and estazolam in primary insomnia. World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) 2010.
NCT00838994 {published data only}
- Chung KF. Acupuncture for residual insomnia associated with major depressive disorder. World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) 2009.
NCT00855140 {published data only}
- Glick RM. Acupuncture for the treatment of insomnia. World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) 2010.
NCT00868517 {published data only}
- Prisco MK. Examining the effect of acupuncture on sleep difficulties related to post traumatic stress disorder. World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) 2010.
NCT01162018 {published data only}
- Spiegel D. Acupuncture for sleep disruption in cancer survivors. World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) 2010.
Additional references
AASM 2005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd Edition. Westchester, IL: American Academy of Sleep Medicine, 2005. [Google Scholar]
Ancoli‐Israel 2000
- Ancoli‐Israel S. Insomnia in the elderly: a review for the primary care practitioner. Sleep 2000;23(Suppl 1):S23‐28. [PubMed] [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington: American Psychiatric Association, 1994. [Google Scholar]
Birch 2003
- Birch S. Controlling for non‐specific effects of acupuncture in clinical trials. Clinical Acupuncture and Oriental Medicine 2003;4(2):59‐70. [Google Scholar]
Buysse 1989
- Buysse DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28:193‐213. [DOI] [PubMed] [Google Scholar]
Cao 2009
- Cao H, Pan X, Li H, Liu J. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. Journal of Alternative and Complementary Medicine 2009;15(11):1171‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2009
- Cheng CH, Yi PL, Lin JG, Chang FC. Endogenous opiates in the nucleus tractus solitarius mediate electroacupuncture‐induced sleep activities in rats. Evidence Based Complementary Alternative Medicine 2009;Sep 3:[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chilcott 1996
- Chilcott LA, Shapiro CM. The socioeconomic impact of insomnia. An overview. Pharmacoeconomics 1996;10 Suppl 1:1‐14. [DOI] [PubMed] [Google Scholar]
Deeks 2009
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphic test. BMJ 1997;315(7):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fu 2009
- Fu LW, Longhurst JC. Electroacupuncture modulates vlPAG release of GABA through presynaptic cannabinoid CB1 receptors. Journal of Applied Physiology 2009;106(6):1800‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glass 2005
- Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits. BMJ 2005;19(331):1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajak 2000
- Hajak G. Insomnia in primary care. Sleep 2000;23 Suppl 3:S54‐63. [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. [Google Scholar]
Hoddes 1973
- Hoddes E, Zarconi V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychopathology 1973;10:431‐6. [DOI] [PubMed] [Google Scholar]
Huang 2009b
- Huang W, Kutner N, Bliwise DL. A systematic review of the effects of acupuncture in treating insomnia. Sleep Medicine Reviews 2009;13:73‐104. [DOI] [PubMed] [Google Scholar]
Huang 2011
- Huang W, Kutner N, Bliwise DL. Autonomic activation in insomnia: the case for acupuncture. Journal of Clinical Sleep Medicine 2011;7(1):95‐102. [PMC free article] [PubMed] [Google Scholar]
Johansson 1993
- Johansson K, Lindgren I, Widner H, Wiklund I, Johansson BB. Can sensory stimulation improve the functional outcome in stroke patients?. Neurology 1993;43:2189‐92. [DOI] [PubMed] [Google Scholar]
Johns 1991
- Johns MW. A new method of measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14(6):540‐5. [DOI] [PubMed] [Google Scholar]
Kalavapalli 2007
- Kalavapalli R, Singareddy R. Role of acupuncture in the treatment of insomnia: a comprehensive review. Complementary Therapies in Clinical Practice 2007;13:184‐93. [DOI] [PubMed] [Google Scholar]
King 2008
- King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA 2008;300:2859‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleinman 2009
- Kleinman NL, Brook RA, Doan JF, Melkonian AK, Baran RW. Health benefit costs and absenteeism due to insomnia from the employer's perspective: a retrospective, case‐control, database study. Journal of Clinical Psychiatry 2009;70(8):1098‐104. [DOI] [PubMed] [Google Scholar]
Knutson 2009
- Knutson KL, Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Archives of Internal Medicine 2009;169:1055‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koog 2011
- Koog YH, We SR, Min BI. Three‐armed trials including placebo and no‐treatment groups may be subject to publication bias: systematic review. PLoS One 2011;6(5):e20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krystal 2004
- Krystal AD. The changing perspective on chronic insomnia management. Journal of Clinical Psychiatry 2004;65 Suppl 8:20‐5. [PubMed] [Google Scholar]
Lanfranchi 2009
- Lanfranchi PA, Pennestri MH, Fradette L, Dumont M, Morin CM, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep 2009;32:760‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2008
- Lee MS, Chin BC, Suen LKP, Park TY, Ernst E. Auricular acupuncture for insomnia: a systematic review. International Journal of Clinical Practice 2008;62(11):1744‐52. [DOI] [PubMed] [Google Scholar]
Leger 2001
- Leger D, Scheuermaier K, Philip P, Paillard M, Guilleminault C. SF‐36: evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosomatic Medicine 2001;63(1):49‐55. [DOI] [PubMed] [Google Scholar]
Li 2003
- Li S, Chen K, Wu Y, Jiao J, Tao L. Effects of warm needling at zusanli (ST 36) on NO and IL‐2 levels in the middle‐aged and old people. Journal of Traditional Chinese Medicine 2003;23(2):127‐8. [PubMed] [Google Scholar]
Ma 2004
- Ma SX. Neurobiology of acupuncture: toward CAM. Evidence Based Complementary and Alternative Medicine 2004;1(1):41‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maciocia 1989
- Maciocia G. Foundations of Chinese Medicine: A comprehensive Text for Acupuncturists and Herbalists. Edinburgh: Churchill Livingstone, 1989. [Google Scholar]
Mason 2002
- Mason S, Tovey P, Long AF. Evaluation complementary medicine: methodological challenges of randomized controlled trials. BMJ 2002;325:832‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 2009
- Montgomery P, Dennis JA. Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003161] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morin 2006
- Morin AK. Strategies for treating chronic insomnia. American Journal of Managed Care 2006;12(8 Suppl):S230‐45. [PubMed] [Google Scholar]
NIH 2005
- National Institute of Health. NIH State‐of‐the‐Science Conference Statement on manifestations and management of chronic insomnia in adults. NIH State‐of‐the‐Science Statement 2005;22(2):1‐30. [PubMed] [Google Scholar]
Ohayon 2002
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Medicine Reviews 2002;6(2):97‐111. [DOI] [PubMed] [Google Scholar]
Ozminkowski 2007
- Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep 2007;30(3):263‐73. [DOI] [PubMed] [Google Scholar]
Patel 2008
- Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16(3):643‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paterson 2005
- Paterson C, Dieppe P. Characteristical and incidental (placebo) effects in complex interventions such as acupuncture. BMJ 2005;330:1202‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
SCSSS 1999
- Swedish Collaboration on Sensory Stimulation in Stroke. Sensory stimulation after stroke: a randomized controlled trial. Cerebrovascular Disease 1999;9 Suppl 11:28. [Google Scholar]
Spence 2004
- Spence DW, Kayumov L, Chen A, Lowe A, Jain U, Katzman MA, et al. Acupuncture increases nocturnal melatonin secretion and reduces insomnia and anxiety: a preliminary report. Journal of Neuropsychiatry and Clinical Neuroscience 2004;16(1):19‐28. [DOI] [PubMed] [Google Scholar]
Sun 2010b
- Sun JQ, Guo J. Evaluation on acupuncture treatment of primary insomnia. Acupuncture Research 2010;35(2):151‐5. [PubMed] [Google Scholar]
Taylor 2007
- Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep 2007;30(2):213‐8. [DOI] [PubMed] [Google Scholar]
Thomas 2003
- Thomas KJ, Coleman P, Nicholl JP. Trends in access to complementary or alternative medicines via primary care in England: 1995‐2001 results from a follow‐up national survey. Family Practice 2003;20:575‐7. [DOI] [PubMed] [Google Scholar]
Ulett 1998
- Ulett GA, Han S, Han JS. Electroacupuncture: mechanisms and clinical application. Biological Psychiatry 1998;44(2):129‐38. [DOI] [PubMed] [Google Scholar]
Van Tulder 2004
- Tulder MW, Cherkin DC, Berman B, Lao L, Koes BW. Acupuncture for low back pain. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD001351] [DOI] [PubMed] [Google Scholar]
Vgontzas 2009a
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela‐Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. 2009 2009;32:491‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vgontzas 2009b
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population‐based study. Diabetes Care 2009;32:1980‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vickers 1999
- Vickers A, Zollman C. ABC of complementary medicine. Acupuncture.. BMJ 1999;319(7215):973‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vickers 2004
- Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al. Acupuncture for chronic headache in primary care: large, pragmatic, randomised trial. BMJ 2004;328:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2004
- Walsh JK. Clinical and socioeconomic correlates of insomnia. Journal of Clinical Psychiatry 2004;65 Suppl 8:13‐9. [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. Tenth Revision. Geneva: World Health Organization, 1992. [PubMed] [Google Scholar]
Wu 1996
- Wu JN. A short history of acupuncture. Journal of Alternative and Complementary Medicine 1996;2:19‐21. [DOI] [PubMed] [Google Scholar]
Yeung 2009b
- Yeung WF, Chung KF, Leung YK, Zhang SP, Law ACK. Traditional needle acupuncture treatment for insomnia: a systematic review of randomized controlled trials. Sleep Medicine 2009;10:694‐704. [DOI] [PubMed] [Google Scholar]
Zarcone 2000
- Zarcone VP. Sleep hygiene. In: Kryger MH, Roth T, Dement WC editor(s). Principles and Practice of Sleep Medicine. 3rd Edition. WB Saunders, 2000:657‐61. [Google Scholar]
Zollman 1999
- Zollman C, Vickers A. ABC of complementary medicine. Users and practitioners of complementary medicine. BMJ 1999;319(7213):836‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]


