Abstract
Background
The ketone body 3‐hydroxybutyrate (3‐OHB) increases cardiac output (CO) by 35% to 40% in healthy people and people with heart failure. The mechanisms underlying the effects of 3‐OHB on myocardial contractility and loading conditions as well as the cardiovascular effects of its enantiomeric forms, D‐3‐OHB and L‐3‐OHB, remain undetermined.
Methods and Results
Three groups of 8 pigs each underwent a randomized, crossover study. The groups received 3‐hour infusions of either D/L‐3‐OHB (racemic mixture), 100% L‐3‐OHB, 100% D‐3‐OHB, versus an isovolumic control. The animals were monitored with pulmonary artery catheter, left ventricle pressure‐volume catheter, and arterial and coronary sinus blood samples. Myocardial biopsies were evaluated with high‐resolution respirometry, coronary arteries with isometric myography, and myocardial kinetics with D‐[11C]3‐OHB and L‐[11C]3‐OHB positron emission tomography. All three 3‐OHB infusions increased 3‐OHB levels (P<0.001). D/L‐3‐OHB and L‐3‐OHB increased CO by 2.7 L/min (P<0.003). D‐3‐OHB increased CO nonsignificantly (P=0.2). Circulating 3‐OHB levels correlated with CO for both enantiomers (P<0.001). The CO increase was mediated through arterial elastance (afterload) reduction, whereas contractility and preload were unchanged. Ex vivo, D‐ and L‐3‐OHB dilated coronary arteries equally. The mitochondrial respiratory capacity remained unaffected. The myocardial 3‐OHB extraction increased only during the D‐ and D/L‐3‐OHB infusions. D‐[11C]3‐OHB showed rapid cardiac uptake and metabolism, whereas L‐[11C]3‐OHB demonstrated much slower pharmacokinetics.
Conclusions
3‐OHB increased CO by reducing afterload. L‐3‐OHB exerted a stronger hemodynamic response than D‐3‐OHB due to higher circulating 3‐OHB levels. There was a dissocitation between the myocardial metabolism and hemodynamic effects of the enantiomers, highlighting L‐3‐OHB as a potent cardiovascular agent with strong hemodynamic effects.
Keywords: 3‐hydroxybutyrate, heart failure, hemodynamics, ketone, metabolism, pressure‐volume loop, pharmacokinetics
Subject Categories: Metabolism
Nonstandard Abbreviations and Acronyms
- 3‐OHB
3‐hydroxybutyrate
- CO
cardiac output
- D‐[11C]3‐OHB
D‐[11C]3‐hydroxybutyrate
- D‐3‐OHB
D‐3‐hydroxybutyrate
- E a
arterial elastance
- E es
end‐systolic elastance
- L‐[11C]3‐OHB
L‐[11C]3‐hydroxybutyrate
- L‐3‐OHB
L‐3‐hydroxybutyrate
- LVESP
left ventricle end‐systolic pressure
- LVPmax
left ventricle maximal pressure
- PV
pressure volume
- PVA
pressure‐volume area
- PVR
pulmonary vascular resistance
- V t
volume of distribution
Clinical Perspective.
What Is New?
Increased cardiac output was primarily mediated by afterload reduction rather than by changes in left ventricular contractility or preload.
Enantiomer‐selective cardiovascular effects were observed with 3‐hydroxybutyrate (3‐OHB) infusion. D/L‐3‐OHB and L‐3‐OHB increased cardiac output by 2.7 L/min, whereas D‐3‐OHB had a minor, nonsignificant effect.
This study emphasizes the important and unexpected role of L‐3‐OHB in securing a beneficial hemodynamic response to 3‐OHB infusion; a dissociation was observed between the myocardial metabolism of D‐ and L‐3‐OHB and their corresponding hemodynamic responses.
What Are the Clinical Implications?
The enantiomer‐specific pharmacokinetic effects of 3‐OHB suggest that the L‐enantiomer has greater potential for enhancing cardiac output than the D‐enantiomer has.
The hemodynamic response during 3‐OHB infusion is driven primarily by afterload reduction, suggesting potential benefits in conditions where it is desirable to reduce afterload, for example, in chronic and acute heart failure.
The ketone body 3‐hydroxybutyrate (3‐OHB) increases cardiac output (CO) by 33% 1 and myocardial blood flow by 75% in healthy participants. 2 In patients with chronic heart failure, 3‐OHB increases CO by 40% and left ventricular ejection fraction (LVEF) by 8 percentage points. 1 We recently reported beneficial hemodynamic effects of 3‐OHB treatment in patients with cardiogenic shock 3 and pulmonary hypertension. 4 Our findings suggested that 3‐OHB may represent a promising therapeutic target for treatment of heart failure, although further studies are needed to fully understand its mechanisms of action. 5 , 6 , 7
3‐OHB exists as 2 enantiomers, D‐3‐OHB and L‐3‐OHB. The D‐3‐OHB enantiomer is the main physiologically active enantiomer in humans and can be metabolized by most cells and organs in the body. 8 In the basal state, the concentration of circulating D‐3‐OHB is around 100 μmol/L, whereas L‐3‐OHB constitutes only 4% (4 μmol/L) of the total amount of 3‐OHB in human plasma 9 and 2% (3 μg/mg) in the liver. Intriguingly, heart tissue has the highest fraction of L‐3‐OHB of any body organ, 34% (38 μg/mg), 10 , 11 but the metabolism of L‐3‐OHB remains unclarified.
3‐OHB improves mitochondrial function, especially in neuronal cells. 12 , 13 Few studies have investigated myocardial mitochondrial function during ketonemia. One study showed no change in isolated rat ventricular myocyte excitation‐contraction during ketonemia, 14 whereas other studies showed increased ATP production during ketonemia. 15 , 16 Furthermore, 3‐OHB's potential stereoselective effect on mitochondrial function has yet to be investigated.
In previous studies, 3‐OHB infusion 1 , 2 consisted of a racemic mixture of 50% L‐3‐OHB and 50% D‐3‐OHB. It remains unknown whether the cardiovascular effects of 3‐OHB are stereoselective. Therefore, we investigated the unique cardiovascular effects of D/L‐3‐OHB, L‐3‐OHB, and D‐3‐OHB infusions in a porcine model. The main purposes of this study were (1) to study whether the hemodynamic changes of 3‐OHB were mediated through changes in myocardial contractility, preload, or afterload; (2) to separately investigate the specific hemodynamic responses of each 3‐OHB enantiomer; (3) to address the potential effects on myocardial mitochondrial respiratory function; (4) to study the direct effects of each 3‐OHB enantiomer on isolated coronary vessels; and (5) to examine the myocardial kinetics of each 3‐OHB enantiomer by positron emission tomography (PET) using dedicated D‐[11C]3‐OHB and L‐[11C]3‐OHB radiotracers.
METHODS
Animals
We used 60‐kg female Danish Landrace×Yorkshire pigs. Premedication with Zoletil Vet 50 (Virbac, Carros, France) was administered intramuscularly (Data S1). The pigs were intubated and mechanically ventilated. Tidal volume was maintained at 8 mL/kg, fraction of inspired oxygen at 40%, positive end‐expiratory pressure at 5 cm H2O, and a respiratory rate of 12 to 16 breaths/min. Intravenous propofol (3.5 mg/kg per h) and fentanyl (15 μg/kg per h) were administered for anesthesia and analgesia. Throughout the entire study, all animals received a continuous isotonic saline infusion of 7.6 mL/kg per h to sustain euvolemia. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Design
The study was a randomized, controlled, assessor‐blinded crossover study (Figure 1). A total of 24 pigs were randomly and equally divided into 3 groups. No inclusion or exclusion criteria were applied. One group received infusion of 0.36 mg/kg per hour D/L‐3‐OHB (racemic mixture of 50% D‐3‐OHB and 50% L‐3‐OHB, sodium 3‐OHB, GoldBio, US) and an isovolumic control (Ringer‐acetate, Fresenius Kabi, Norway) for 3 h in a balanced random order. Another group received infusion of 100% L‐3‐OHB (0.36 mg/kg per hour, sodium‐3‐OHB, Glpbio, USA) and isovolumic control (Ringer‐acetate, Fresenius Kabi, Norway) for 3 hours a in balanced random order, whereas a third group received infusion of 100% D‐3‐OHB (0.36 mg/kg per hour, sodium (R)‐3‐OHB, Sigma‐Aldrich, USA) and isovolumic control (Ringer‐acetate, Fresenius Kabi, Norway) for 3 hours in random order. Dobutamine (3.0 μg/kg per min, STADA Nordic, Denmark) was administered as positive inotropic control after the 180‐minute measurements at the end of each infusion period for 20 minutes in all animals.
Figure 1. Study design.
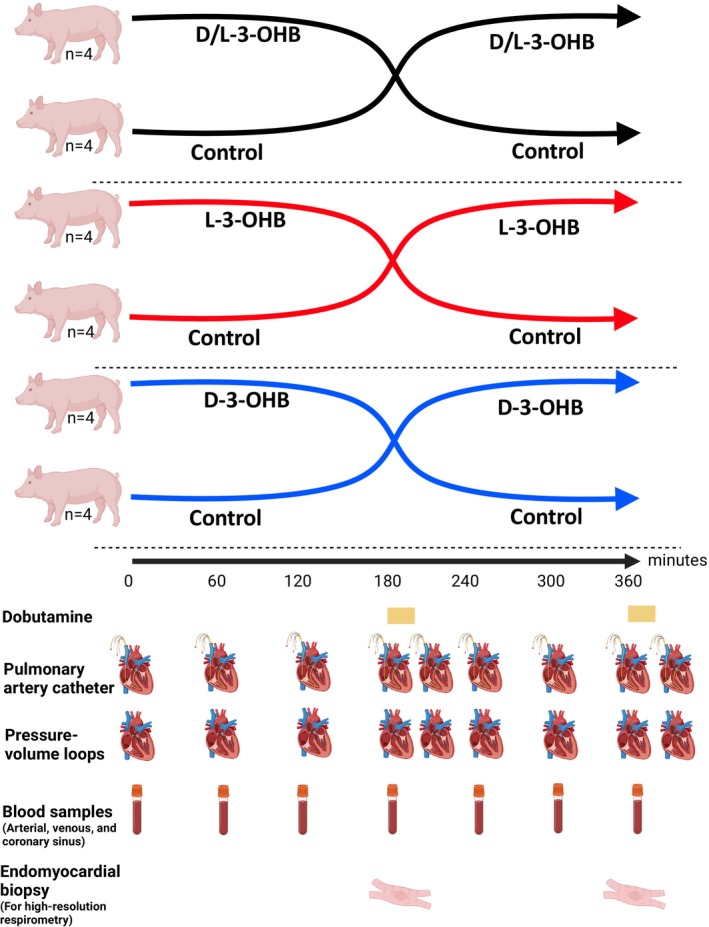
Study overview. The pigs were divided into 3 groups of 8 pigs each. The groups received D/L‐3‐OHB, L‐3‐OHB, or D‐3‐OHB for 180 minutes and the control infusion for 180 minutes in random order. Invasive hemodynamic measurements with a pulmonary artery catheter and pressure‐volume loops were performed hourly and after each dobutamine infusion. Blood samples were collected hourly. Endomyocardial biopsies for high‐resolution respirometry were performed after each infusion period. D‐3‐OHB indicates D‐3‐hydroxybutyrate; and L‐3‐OHB, L‐3‐hydroxybutyrate.
End Points and Measurements
All pigs were monitored using invasive blood pressure measurements: a pulmonary artery catheter, a pressure‐volume (PV) catheter in the left ventricle (LV), and arterial, venous, and coronary sinus blood sampling. Measurements were performed at baseline and every hour during the 6‐hour study period. Additionally, after a 20‐minute dobutamine infusion at the end of each infusion period, hemodynamic measurements were obtained (Figure 1). The primary end point was CO changes during the 3‐OHB infusion compared with the control period. Secondary outcomes were stroke volume, end‐systolic elastance (E es), arterial elastance (E a), LVEF, LV end‐systolic pressure (LVESP), LV end‐diastolic volume (LVEDV), LV maximal pressure (LVPmax), mixed venous saturation, pulmonary artery wedge pressure, mean pulmonary artery pressure, pulmonary vascular resistance (PVR), and systemic vascular resistance (SVR).
Right Heart Catheterization
A pulmonary artery catheter was placed, pressure guided, through the external jugular vein to the pulmonary artery. Right atrial pressure, mean pulmonary artery pressure, pulmonary artery wedge pressure, mixed venous saturation, and CO were measured at baseline and hourly after infusion initiation. CO was measured by the bolus thermodilution technique as the mean of 3 consecutive measurements. The SVR was calculated as mean arterial pressure‐right atrial pressure/CO. The PVR was calculated as mean pulmonary artery pressure‐pulmonary artery wedge pressure/CO.
Pressure‐Volume Catheter
A PV admittance catheter (7 Fr, Transonic Systems, Ithaca, NY) was inserted into the LV through the left common carotid artery. 17 , 18 Cardiac function measurements were collected at baseline and hourly after infusion initiation. An occlusion balloon was placed through the femoral vein into the inferior vena cava at the level of the diaphragm. Balloon inflation caused preload decline and allowed measurement of the slope (E es) of the end‐systolic PV relationship as a load‐independent contractility parameter. PV loops were also used to quantify the afterload estimates, E a, and LVESP. LVEF, LVPmax, LVEDV, PV area (PVA), and cardiac efficiency were also assessed using the PV loops.
Blood Samples
Blood samples were drawn from the central venous and arterial catheters of the femoral vein and artery, respectively. A Judkins right coronary artery catheter was placed in the coronary sinus by fluoroscopy‐guidance through the external jugular vein. The catheter was used for coronary sinus blood sampling. The arteriovenous gradients of circulating metabolites across the heart were calculated by subtracting the concentration in the arterial blood from that in the coronary sinus. The extraction ratio was determined as the ratio of the arteriovenous gradient to the arterial concentration. All samples were collected at baseline and hourly after infusion was initiated. The pH, glucose, and lactate levels were analyzed immediately after sampling using a YSI STAT 2100 (YSI Inc., Netherlands). All other samples were kept at −80 °C and analyzed at study closure. Total 3‐OHB, that is, the sum of D‐ and L‐3‐OHB, was measured by hydrophilic interaction liquid chromatography–tandem mass spectrometry, as previously described. 19 D‐3‐OHB levels were measured using a Beta‐hydroxybutyrate Assay Kit (Sigma‐Aldrich, USA). The L‐3‐OHB level was calculated as the difference between total 3‐OHB and D‐3‐OHB.
High‐Resolution Respirometry of Endomyocardial Biopsies
Endomyocardial biopsies (0.5–2.0 mg) were obtained from the LV through the common carotid artery using a bioptome catheter. Biopsies were taken at the end of each intervention period (180 minutes) as shown in Figure 1. High‐resolution respirometry (Oxygraph‐2k; Oroboros, Innsbruck, Austria) was used to evaluate the mitochondrial respiratory capacity, as previously described. 20 , 21 , 22 , 23 The chambers were hyperoxygenated to avoid any O2‐limitation and all measurements were conducted in duplicate. Respiratory rates are expressed as O2 flux and normalized to the cardiac muscle mass of the individual fibers (detailed description in Data S1). The oxidative phosphorylation capacity was calculated as the absolute difference between complex I+II coupled respiration and basal, nonphosphorylating respiration. All measurements were corrected for residual oxygen consumption.
PET Substudy: Pharmacokinetics of D‐[ 11C]3‐OHB and L‐[ 11C]3‐OHB
Four additional 40‐kg female Danish Landrace×Yorkshire crossbred pigs were premedicated with ketamine and midazolam. The pigs were studied under propofol anesthesia in a nonrecovery setup with D‐[11C]3‐OHB and L‐[11C]3‐OHB 24 radiotracers under various conditions (detailed description in Data S1). The pigs were PET scanned to determine (1) the biodistribution of D‐[11C]3‐OHB; (2) the myocardial kinetics of D‐[11C]3‐OHB; (3) the myocardial kinetics of L‐[11C]3‐OHB; and (4) the myocardial kinetics of D‐[11C]3‐OHB during conditions of low ketone levels (<50 μmol/L) and high ketone levels (~3 mmol/L) achieved by D/L‐3‐OHB infusion (75 g/L). The pigs were euthanized with an overdose of pentobarbitone (100 mg/kg, IV).
Pharmacokinetics were estimated for D‐[11C]3‐OHB and L‐[11C]3‐OHB. All input functions were based on a time‐activity curve from the volume of interest placed in the LV. One‐tissue and 2‐tissue compartmental models were applied to the dynamic data. In addition, graphical analyses with linearization models for irreversible uptake (Patlak plots) and reversible uptake (Logan plots) were applied to obtain simple estimates of flux and volume of distribution (V t), respectively. Flux is a measure of the net uptake rate of the tracer, and V t is a measure of the ratio of the total amount of tracer distributed in the tissue to the total amount of tracer in the plasma.
Myograph: Coronary Artery Tension
Porcine heart tissue was collected from a local abattoir and transported to the laboratory in ice‐cold physiological saline solution. 25 Small side‐branches of the left anterior descending artery were isolated under a stereo microscope and mounted on 40 μm wires in physiological saline solution‐filled myograph chambers (DMT 610M and 620M, Denmark) for isometric evaluation (detailed description in Data S1). The coronary arteries were precontracted with the thromboxane analog U46619 and then exposed to 3 mmol/L Na‐D‐3‐OHB or Na‐L‐3‐OHB. Relative active tension changes of the isolated coronary arteries were reported.
Statistical Analysis
The coefficient of variation of the primary end point CO is 4%. 26 By enrolling 8 pigs in each group, we expected to detect a relative difference of minimum 10% with a power of 80% and a 2‐sided significance level of 5%. The data were tested for normality by qq plots. Data are presented as the mean±SD. A priori, all animals completing both intervention periods were included in the analyses. A linear mixed model with repeated measurements was performed. To compare the effects of each intervention with their control, treatment, time, treatment‐by‐time interaction, period, and treatment sequence were defined as fixed effects, whereas animals and period nested within animals were selected as random effects. The residuals were tested for normality and homoscedasticity. Data were log transformed if needed. To compare the effects between each ketone infusion (L‐3‐OHB versus D‐3‐OHB), the ketone infusion type was added to the fixed effects. Pearson's coefficient was applied to assess the correlation between change in 3‐OHB levels and hemodynamic changes. During control infusion, the mean±SE is presented. The effect sizes of each ketone infusion are presented as the mean with 95% CI. Vascular responses and mitochondrial functions were compared using repeated measures 2‐way ANOVA. Statistical significance was set at P<0.05. LabChart (ADInstruments, New Zealand) was used to record and analyze all PV measurements. Data analyses and figures were made with R (Rstudio, PBC) and GraphPad Prism (GraphPad Software, USA). Randomization sequence generation was accomplished with www.randomizer.org. The investigators were blinded to the randomization order during the experiments and outcome assessments.
RESULTS
Twenty‐four animals were included and randomized 1:1:1 to the 3 interventions (D/L 3‐OHB, L‐3‐OHB, and D‐3‐OHB). Each animal was further randomized to start with either the control or one of the ketone infusions in a balanced order (Figure 1).
Effects of the Racemic Mixture of 50% D‐3‐Hydroxybutyrate and 50% L‐3‐Hydroxybutyrate
Total circulating 3‐OHB increased from 0.00 mmol/L to 5.71 mmol/L (95% CI, 4.50–6.93 mmol/L) compared with controls (Table 1; Figure S1). D‐3‐OHB increased by 1.11 mmol/L (95% CI, 0.90–1.31 mmol/L); and L‐3‐OHB, by 4.61 mmol/L (95% CI, 3.52–5.69 mmol/L) (Table 1). CO increased by 2.8 L/min (95% CI, 1.5–4.1 L/min) during D/L‐3‐OHB infusion from 4.7±0.2 L/min during control infusion (Table 1; Figure 2). LVEF increased by 13 percentage points (95% CI, 4–22 percentage points), whereas mean arterial pressure decreased from 87±5 mm Hg during control infusion by 14 mm Hg (95% CI, −26 to −2 mm Hg) during D/L‐3‐OHB infusion. Heart rate was 87±6 beats per minute (bpm) during control infusion and exhibited a nonsignificant 18 bpm increase (95% CI, −1 to 36 bpm) during D/L‐3‐OHB infusion. E a, SVR, LVESP, LVPmax, and PVR decreased significantly during D/L‐3‐OHB infusion (Table 1; Figure S2). No significant changes were observed in E es, whereas dP/dtmax increased during D/L‐3‐OHB infusion compared with control infusion (Table 1). PVA and cardiac efficiency remained unchanged during D/L‐3‐OHB infusion.
Table 1.
Hemodynamic Measurements
| D/L‐3‐OHB vs control (n=8) | L‐3‐OHB vs control (n=8) | D‐3‐OHB vs control (n=8) | L vs D | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control infusion | D/L‐3‐OHB effect size (95% CI) | P value | Control infusion | L‐3‐OHB effect size (95% CI) | P value | Control infusion | D‐3‐OHB effect size (95% CI) | P value | ||
| Pulmonary artery catheter measurements | ||||||||||
| Cardiac output, L/min | 4.7±0.2 | 2.8 (1.4 to 4.2) | <0.001 | 5.0±0.4 | 2.7 (1.2 to 4.2) | 0.003 | 5.3±0.4 | 0.6 (−0.4 to 1.6) | 0.2 | > |
| Stroke volume, mL | 57.2±6.8 | 6.7 (−15.6 to 29.1) | 0.6 | 69.5±5.0 | 12.1 (−0.3 to 24.5) | 0.072 | 62.4±5.1 | −12.1 (−26.2 to 2.0) | 0.11 | |
| Heart rate, bpm | 87±6 | 18 (−1 to 36) | 0.079 | 70±5 | 12 (1 to 22) | 0.038 | 87±8 | 31 (9 to 53) | 0.015 | |
| Mean arterial pressure, mm Hg | 87±5 | −14 (−26 to −2) | 0.037 | 82±4 | −6 (−17 to 6) | 0.4 | 94±6 | 2 (−10 to 14) | 0.7 | |
| Systemic vascular resistance, WU | 18±1 | −12 (−17 to −8) | <0.001 | 17±1 | −6 (−11 to 0) | 0.069 | 18±2 | −1 (−6 to 4) | 0.6 | |
| Right atrial pressure, mm Hg | 4±1 | 1 (−2 to 4) | 0.5 | 5±1 | −1 (−3 to 0) | 0.11 | 4±1 | −1 (−2 to 0) | 0.2 | |
| Mean pulmonary arterial pressure, mm Hg | 29±2 | −5 (−8 to −1) | 0.013 | 19±1 | 0 (−3 to 3) | >0.9 | 26±2 | −6 (−9 to −2) | 0.006 | > |
| Pulmonary artery wedge pressure, mm Hg | 6±1 | 3 (0 to 6) | 0.061 | 7±1 | 1 (0 to 3) | 0.2 | 7±1 | −1 (−3 to 1) | 0.3 | |
| Pulmonary vascular resistance, WU | 5±0 | −4 (−6 to −2) | <0.001 | 3±0 | −2 (−4 to 0) | 0.060 | 4±1 | −2 (−3 to 0) | 0.049 | |
| Pressure: volume loop measurements | ||||||||||
| End‐systolic elastance, mm Hg/mL | 0.91±0.11 | −0.26 (−0.76 to 0.24) | 0.3 | 0.56±0.08 | −0.19 (−0.33 to −0.05) | 0.021 | 0.72±0.12 | 0.18 (−0.25 to 0.60) | 0.4 | |
| dP/dt (max), mm Hg/s | 1363.3±86.3 | 525.2 (107.2 to 943.3) | 0.023 | 1501.8±81.0 | 340.1 (92.9 to 587.2) | 0.014 | 1702.8±101.1 | 461.2 (129.0 to 793.5) | 0.017 | |
| Arterial elastance, mm Hg/mL | 2.74±0.52 | −1.38 (−2.10 to −0.66) | 0.002 | 1.72±0.18 | −0.91 (−1.66 to −0.17) | 0.025 | 1.91±0.33 | −0.06 (−0.68 to 0.55) | 0.8 | |
| LV end‐systolic pressure, mm Hg | 103.8±4.6 | −16.1 (−29.9 to −2.3) | 0.033 | 100.2±4.5 | −10.9 (−23.9 to 2.0) | 0.12 | 115.0±6.1 | −1.4 (−15.2 to 12.4) | 0.8 | |
| LV max pressure, mm Hg | 107.0±4.5 | −15.6 (−30.0 to −1.3) | 0.046 | 104.7±4.3 | −8.1 (−18.8 to 2.6) | 0.2 | 119.6±6.1 | 0.1 (−12.0 to 12.3) | >0.9 | |
| LV end‐diastolic volume, mL | 104.3±12.3 | −27.6 (−52.4 to −2.8) | 0.041 | 137.0±8.5 | −13.5 (−35.0 to 8.0) | 0.2 | 116.1±12.6 | 3.8 (−27.6 to 35.1) | 0.8 | |
| LV ejection fraction % | 32±4 | 13 (4 to 22) | 0.012 | 32±3 | 7 (1 to 14) | 0.033 | 38±4 | 4 (−5 to 12) | 0.4 | |
| Tau, ms | 32.4±1.7 | −1.4 (−5.2 to 2.4) | 0.5 | 33.0±1.7 | −4.7 (−8.4 to −1.1) | 0.020 | 33.5±1.4 | −1.5 (−6.3 to 3.4) | 0.6 | |
| End‐diastolic pressure‐volume relationship, mm Hg×mL | 0.049±0.015 | 0.023 (−0.018 to 0.065) | 0.3 | 0.032±0.013 | 0.018 (0.001 to 0.035) | 0.046 | 0.031±0.010 | −0.008 (−0.030 to 0.014) | 0.5 | |
| Stroke work, mm Hg×mL | 4940.2±746.2 | 2125.9 (−442.9 to 4694.6) | 0.12 | 6065.0±867.8 | 376.3 (−1253.2 to 2005.7) | 0.7 | 7717.5±959.6 | 652.5 (−1625.5 to 2930.5) | 0.6 | |
| Pressure‐volume area, mm Hg×mL | 9392.9±1181.2 | −79.6 (−4353.0 to 4193.8) | >0.9 | 9833.2±1122.9 | 2621.8 (−1564.5 to 6808.2) | 0.2 | 14206.5±1880.4 | −3729.5 (−8737.8 to 1278.8) | 0.2 | |
| Potential energy, mm Hg×mL | 5477.6±927.6 | −938.3 (−4396.5 to 2519.9) | 0.6 | 5536.1±1158.3 | 3132.5 (−78.1 to 6343.2) | 0.070 | 7988.8±1185.5 | −3286.0 (−7021.1 to 449.2) | 0.11 | > |
| Cardiac efficiency % | 44.8±4.0 | 3.8 (−9.5 to 17.1) | 0.6 | 48.5±4.5 | −4.9 (−13.4 to 3.6) | 0.3 | 45.1±2.4 | 7.0 (−3.4 to 17.3) | 0.2 | |
The control infusion is shown as the mean±SE during 180‐minute control infusion. The effect size is shown as mean (95% CI). The effect size is the mean change observed during the intervention period compared with the control during the180‐minute infusion period. The symbols “<” and “>” indicate whether there was a statistically significant difference between L‐3‐OHB and D‐3‐OHB. 3‐OHB indicates 3‐hydroxybutyrate; bpm, beats per minute; D‐3‐OHB, D‐3‐hydroxybutyrate; L‐3‐OHB, L‐3‐hydroxybutyrate; LV, left ventricular; and WU, Wood units.
Figure 2. Hemodynamic changes during D/L‐3‐OHB, L‐3‐OHB, and D‐3‐OHB infusions compared with control.
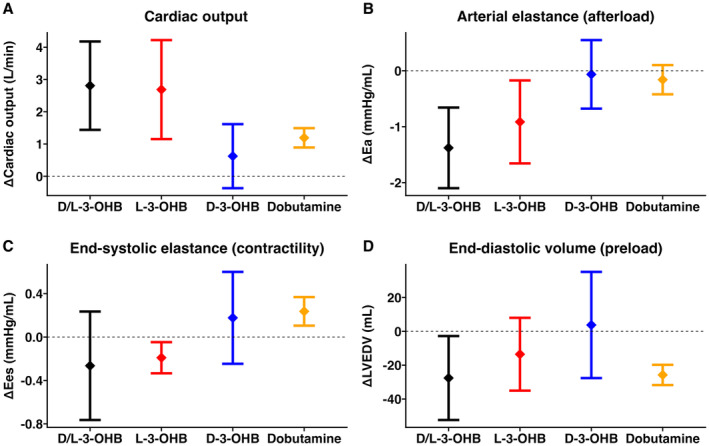
Error bars indicate the mean change and 95% CI for cardiac output (A), arterial elastance (B), end‐systolic elastance (C), and end‐diastolic volume (D) for D/L‐3‐OHB, L‐3‐OHB and D‐3‐OHB compared with their respective control. D‐3‐OHB indicates D‐3‐hydroxybutyrate; E a, arterial elastance; E es, end‐systolic elastance; L‐3‐OHB, L‐3‐hydroxybutyrate; and LVEDV, left ventricular end‐diastolic volume.
No significant changes in glucose, lactate, or free fatty acid (FFA) plasma levels were observed. pH increased by 0.12 (95% CI, 0.07–0.17), and acetoacetate increased by 3.08 mmol/L (95% CI, 2.24–3.92 mmol/L) during the D/L‐3‐OHB infusion compared with controls (Table 2). The arteriovenous gradients of the circulating metabolites across the heart are presented in Table 3. Arteriovenous gradients of 3‐OHB and glucose were significantly increased during D/L‐3‐OHB compared with controls. No change was observed in the arteriovenous gradients of lactate, oxygen, or FFA during D/L‐3‐OHB infusion compared with controls. The extraction ratio of 3‐OHB and glucose also rose by 15 percentage points (95% CI, 11–18 percentage points) and 28 percentage points (95% CI, 3–52 percentage points), respectively, during D/L‐3‐OHB infusion compared with controls. No changes were recorded in the extraction ratios of the other metabolites (Table 3).
Table 2.
Blood Samples
| D/L‐3‐OHB vs placebo (n=8) | L‐3‐OHB vs placebo (n=8) | D‐3‐OHB vs placebo (n=8) | L vs D | ||||
|---|---|---|---|---|---|---|---|
| Effect size (95% CI) | P value | Effect size (95% CI) | P value | Effect size (95% CI) | P value | ||
| 3‐OHB, mmol/L | 5.71 (4.50 to 6.93) | <0.001 | 6.59 (5.16 to 8.02) | <0.001 | 4.83 (3.81 to 5.85) | <0.001 | |
| D‐3‐OHB, mmol/L | 1.11 (0.90 to 1.31) | <0.001 | 0.49 (0.34 to 0.64) | <0.001 | NA | NA | |
| L‐3‐OHB, mmol/L | 4.61 (3.52 to 5.69) | <0.001 | 6.10 (4.80 to 7.41) | <0.001 | NA | NA | |
| Acetoacetate, mmol/L | 3.08 (2.24 to 3.92) | <0.001 | 1.11 (0.81 to 1.42) | <0.001 | 3.51 (2.58 to 4.44) | <0.001 | < |
| pH | 0.12 (0.07 to 0.17) | <0.001 | 0.06 (0.03 to 0.09) | <0.001 | 0.12 (0.09 to 0.16) | <0.001 | < |
| Glucose, mmol/L | 1.7 (0.0 to 3.5) | 0.070 | −1.3 (−2.3 to −0.4) | 0.017 | −1.6 (−2.3 to −0.8) | <0.001 | |
| Lactate, mmol/L | 0.4 (−0.7 to 1.4) | 0.5 | 0.4 (0.0 to 0.8) | 0.051 | 0.4 (−0.2 to 1.0) | 0.3 | |
| Free fatty acid, mmol/L | 0.11 (−0.31 to 0.53) | 0.6 | −0.33 (−0.65 to −0.02) | 0.052 | −0.48 (−0.79 to −0.18) | 0.005 | |
| Insulin, mU/L | 1.79 (−2.55 to 6.14) | 0.4 | 5.28 (2.77 to 7.79) | <0.001 | 3.40 (0.75 to 6.05) | 0.021 | |
The effect size is shown as mean (95% CI). The effect size indicates the mean change observed for each outcome during the intervention period compared with the control during the 180‐minute infusion period. The symbols “<” and “>” indicate whether there was a statistically significant difference between L‐3‐OHB and D‐3‐OHB. 3‐OHB indicates 3‐hydroxybutyrate; D‐3‐OHB, D‐3‐hydroxybutyrate; L‐3‐OHB, L‐3‐hydroxybutyrate; and NA, not available.
Table 3.
Arteriovenous Gradients of Metabolites Across the Heart and Cardiac Extraction Ratio
| D/L‐3‐OHB vs placebo (n=8) | L‐3‐OHB vs placebo (n=8) | D‐3‐OHB vs placebo (n=8) | L vs D | ||||
|---|---|---|---|---|---|---|---|
| Effect size (95% CI) | P value | Effect size (95% CI) | P value | Effect size (95% CI) | P value | ||
| 3‐OHB, mmol/L | 0.67 (0.50 to 0.83) | <0.001 | 0.47 (0.22 to 0.71) | 0.003 | 0.79 (0.62 to 0.95) | <0.001 | < |
| Lactate, mmol/L | −0.2 (−0.6 to 0.2) | 0.3 | 0.1 (−0.3 to 0.5) | 0.6 | −0.5 (−0.7 to −0.2) | 0.008 | > |
| Glucose mmol/L | 2.0 (0.3 to 3.7) | 0.035 | −0.6 (−1.4 to 0.1) | 0.11 | 0.1 (−0.7 to 1.0) | 0.7 | |
| Oxygen, mg/dL | −2.1 (−4.0 to −0.1) | 0.052 | −1.3 (−4.1 to 1.6) | 0.4 | 0.2 (−0.7 to 1.1) | 0.7 | |
| FFA, mmol/L | −0.1 (−0.3 to 0.1) | 0.3 | 0.0 (−0.3 to 0.3) | >0.9 | 0.1 (−0.1 to 0.2) | 0.4 | |
| Extraction ratio | |||||||
| 3‐OHB, % | 15 (11 to 18) | <0.001 | 7 (−2 to 16) | 0.15 | 20 (14 to 26) | <0.001 | < |
| Lactate, % | −14 (−33 to 6) | 0.2 | 0 (−27 to 26) | >0.9 | −29 (−47 to −12) | 0.006 | |
| Glucose, % | 28 (3 to 52) | 0.038 | −11 (−24 to 2) | 0.13 | 4 (−26 to 33) | 0.8 | |
| Oxygen, % | 6 (−9 to 22) | 0.5 | 15 (−2 to 33) | 0.11 | −4 (−9 to 1) | 0.13 | |
| FFA, % | −17 (−90 to 55) | 0.6 | −41 (−157 to 76) | 0.5 | 14 (−47 to 74) | 0.7 | |
The effect size is shown as mean (95% CI). The effect size indicates the variation in change observed for each outcome during the intervention compared with the control infusion. The symbols “<” and “>” indicate whether there was a statistically significant difference between L‐3‐OHB and D‐3‐OHB. 3‐OHB indicates 3‐hydroxybutyrate; D‐3‐OHB, D‐3‐hydroxybutyrate; FFA, free fatty acid; and L‐3‐OHB, L‐3‐hydroxybutyrate.
Effects of 100% L‐3‐Hydroxybutyrate
L‐3‐OHB infusion increased total 3‐OHB from 0.00 mmol/L to 6.59 mmol/L (95% CI, 5.16 –8.02 mmol/L), D‐3‐OHB by 0.49 mmol/L (95% CI, 0.34–0.64 mmol/L), and L‐3‐OHB by 6.10 mmol/L (95% CI, 4.80–7.41 mmol/L). CO increased from 5.0±0.4 L/min during control infusion by 2.7 L/min (95% CI, 1.2–4.1 L/min) during L‐3‐OHB infusion. LVEF increased by 7 percentage points (95% CI, 1–14 percentage points) (Table 1; Figure 2). Mean arterial pressure did not change significantly. Heart rate increased from 70±5 bpm by 12 bpm (95% CI, 1–22 bpm). E a decreased significantly during L‐3‐OHB infusion compared with controls, whereas SVR, LVESP, LVPmax, and PVR did not reach statistical significance (Table 1; Figure 2). E es was 0.56±0.08 mm Hg/mL during control infusion and decreased by 0.19 mm Hg/mL (95% CI, −0.33 to −0.05 mm Hg/mL) during L‐3‐OHB infusion, whereas dp/dtmax increased significantly during L‐3‐OHB infusion (Table 1). PVA and cardiac efficiency were unchanged during L‐3OHB infusion.
Glucose levels decreased by 1.3 mmol/L (95% CI, −2.3 to −0.4) during L‐3‐OHB infusion compared with control infusion. Lactate and acetoacetate levels increased with L‐3‐OHB infusion compared with controls (Table 2). Arteriovenous gradients of 3‐OHB across the heart increased by 0.47 mmol/L (95% CI, 0.22–0.71 mmol/L) during L‐3‐OHB infusion compared with controls (Table 3). Arteriovenous gradients of other metabolites remained unchanged. Additionally, the extraction ratio of 3‐OHB and the other metabolites across the heart were unchanged.
Effects of 100% D‐3‐Hydroxybutyrate
D‐3‐OHB infusion increased total 3‐OHB from 0.00 mmol/L to 4.83 mmol/L (95% CI, 3.81–5.85 mmol/L). CO and LVEF did not increase significantly during D‐3‐OHB infusion compared with controls (0.6 L/min, 95% CI, −0.4 to 1.6 L/min and 3.6 percentage points, 95% CI, −4.6 to 11.9 percentage points, respectively). Heart rate was 87±8 bpm during control infusion and increased by 31 bpm (95% CI, 9–53 bpm) during D‐3‐OHB infusion. E a, SVR, LVESP, mean arterial pressure, and LVPmax remained unchanged during D‐3‐OHB infusion compared with control. PVR decreased 2 WU (95% CI, −3 to 0 WU) during the D‐3‐OHB infusion from 4±1 WU during control infusion. E es was unchanged during D‐3‐OHB infusion, whereas dp/dtmax increased significantly (Table 1). The PVA and cardiac efficiency changes did not meet statistical significance (Table 1).
Glucose and FFA levels decreased during the D‐3‐OHB infusion compared with controls. Acetoacetate levels were elevated during the D‐3‐OHB infusion compared with controls (Table 2). Arteriovenous gradients of 3‐OHB across the heart were increased by 0.79 mmol/L (95% CI, 0.62–0.95 mmol/L) and lactate decreased by 0.45 mmol/L (95% CI, −0.74 to −0.16 mmol/L) during D‐3‐OHB compared with controls (Table 3). The arteriovenous gradients of the other metabolites were similar during the D‐3‐OHB infusion compared with controls. The extraction ratios of 3‐OHB and lactate increased by 20.0 percentage points (95% CI, 14–26 percentage points) and decreased by 29 percentage points (95% CI, −47 to −12 percentage points), respectively, during D‐3‐OHB infusion compared with control infusion.
Differential Cardiovascular Effects of the 2 Enantiomers of 3‐Hydroxybutyrate
L‐3‐OHB infusion produced the highest concentrations of circulating 3‐OHB compared with D‐3‐OHB infusion. However, the difference was not significant (Table 1). L‐3‐OHB infusion increased CO significantly more than D‐3‐OHB infusion. The increase in circulating total 3‐OHB levels exhibited a positive correlation with CO changes during both L‐3‐OHB and D‐3‐OHB infusions (P<0.001; Figure 3A). Similarly, the increase in circulating total 3‐OHB levels exhibited a significant negative correlation with SVR changes (Figure 3B). The slope of the regression lines for CO and SVR did not differ significantly between L‐3‐OHB and D‐3‐OHB infusions (P=0.134 and P=0.876, respectively). No major changes were observed in the PV measurements between interventions (Table 1). Acetoacetate and pH levels were higher during D‐3‐OHB infusion. The arteriovenous gradients across the heart showed an increased extraction ratio of 3‐OHB during D‐3‐OHB infusion compared with L‐3‐OHB (Table 3). No significant differences were observed in the arteriovenous gradients of other metabolite extraction ratios between L‐3‐OHB and D‐3‐OHB (Table 3).
Figure 3. Correlation of circulating 3‐OHB levels and hemodynamic measurements.
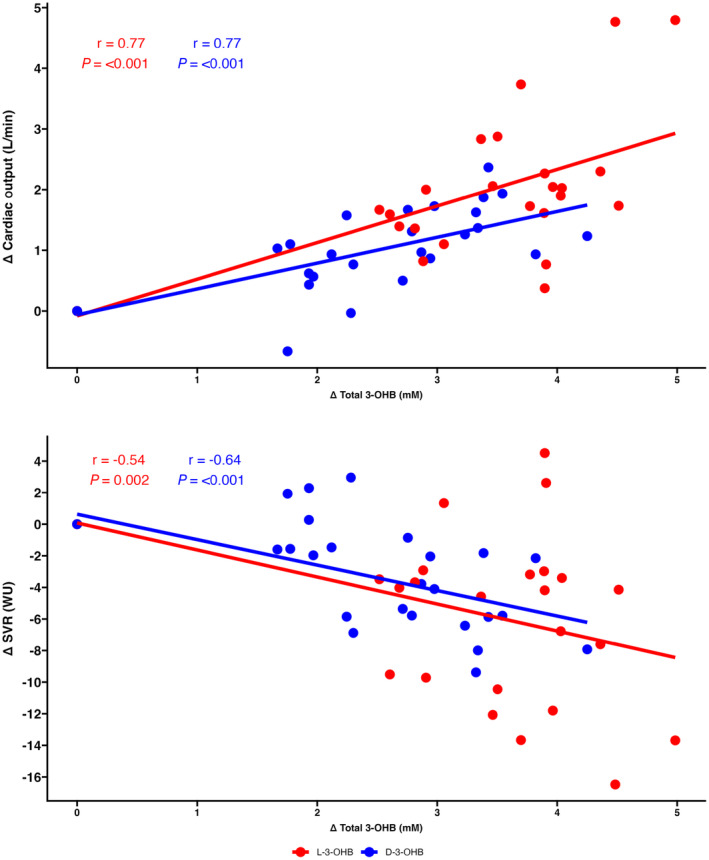
Correlation between changes in total 3‐OHB concentration and changes in cardiac output (A) and changes in SVR (B). There were no significant differences in the slopes of the regression lines (P=0.134 and P=0.876, respectively). 3‐OHB indicates 3‐hydroxybutyrate; D‐3‐OHB, D‐3‐hydroxybutyrate; L‐3‐OHB, L‐3‐hydroxybutyrate; SVR, systemic vascular resistance; and WU, Wood units.
Dobutamine Challenge
Dobutamine was chosen as a positive inotropic control. Dobutamine increased CO by 1.2 L/min (95% CI, 0.9–1.5 L/min) from 5.8±1.4 L/min during the ketone and control infusions alone (Figure 2). LV contractility measurement, E es, increased significantly (0.24 mm Hg/mL, 95% CI, 0.10–0.38 mm Hg/mL). Heart rate and LVEF increased significantly, whereas SVR, mean pulmonary artery pressure, and PVR decreased (Table S1).
Myocardial Mitochondrial Function
High‐resolution respirometry was performed to estimate myocardial mitochondrial function. Compared with controls, neither D/L‐3‐OHB, L‐3‐OHB or D‐3‐OHB infusions had sustained effects on glucose‐linked oxidative phosphorylation capacity that could be detected in tissue samples evaluated without 3‐OHB present in the oxygraphy (Figure 4A). The presence of 3‐OHB in the oxygraphy chamber elicited no differences in oxidative phosphorylation capacity (Figure 4B). Oxidative phosphorylation capacity measured with and without 3‐OHB present did not differ significantly between tissues sampled from pigs treated with D/L‐3‐OHB, D‐3‐OHB, or L‐3‐OHB, and control infusions (P>0.05).
Figure 4. Myocardial mitochondrial function measured by high‐resolution respirometry.
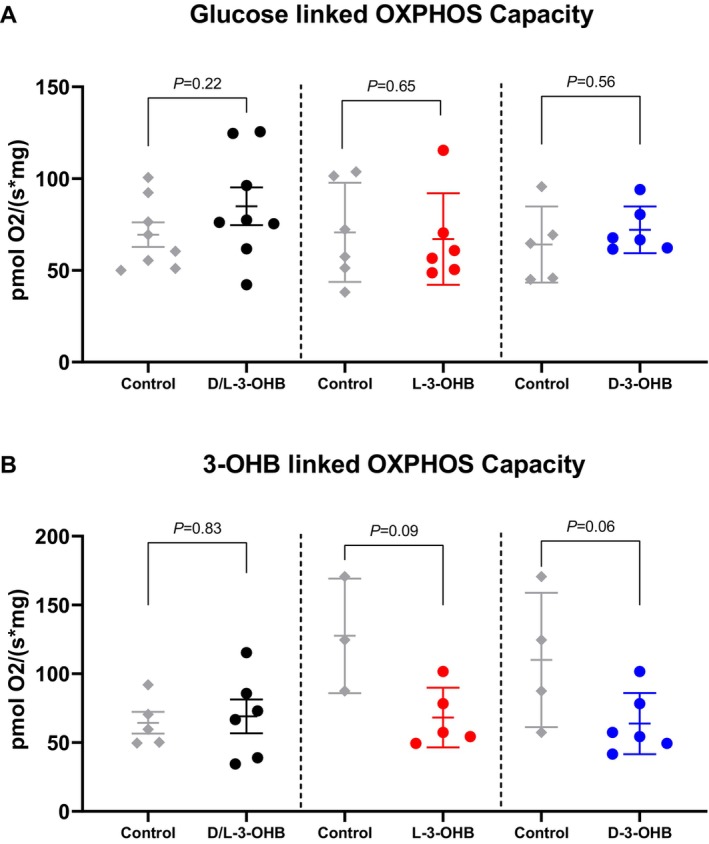
High‐resolution respirometry of myocardial biopsies after ketone infusion and control infusion. The oxidative phosphorylation (OXPHOS) capacity after 180 minutes of 3‐OHB infusion was compared with that after 180 minutes of control infusion. Glutamate, malate, and ADP were added to 1 high‐resolution respirometry chamber (A), whereas glutamate, 3‐OHB, ADP, and succinate were added to another high‐resolution respirometry chamber (B). 3‐OHB indicates 3‐hydroxybutyrate; D‐3‐OHB, D‐3‐hydroxybutyrate; L‐3‐OHB, L‐3‐hydroxybutyrate.
Myocardial Kinetics of L‐[ 11C]3‐OHB and D‐[ 11C]3‐OHB
The biodistribution of D‐[11C]3‐OHB showed avid cardiac ketone uptake (Figure 5A). Myocardial kinetics of the physiologically occurring D‐3‐OHB revealed that a simple 1‐tissue model fitted the data best under low ketone conditions (Table S2). The high K1 value of 0.63 corresponded well with the anticipated myocardial blood flow and high myocardial extraction fraction of D‐3‐OHB. During elevated circulating 3‐OHB levels of 3 mmol/L (Figure 5B), a 2‐tissue model fitted the data better (Table S2) with the second tissue compartment, possibly indicating 11C‐isotope trapping in the byproducts of the tricarboxylic acid cycle. The irreversible model for kinetic analysis (Patlak) returned negative k‐values and was deemed unsuitable for the data analysis (Table S2).
Figure 5. Tracer kinetics.
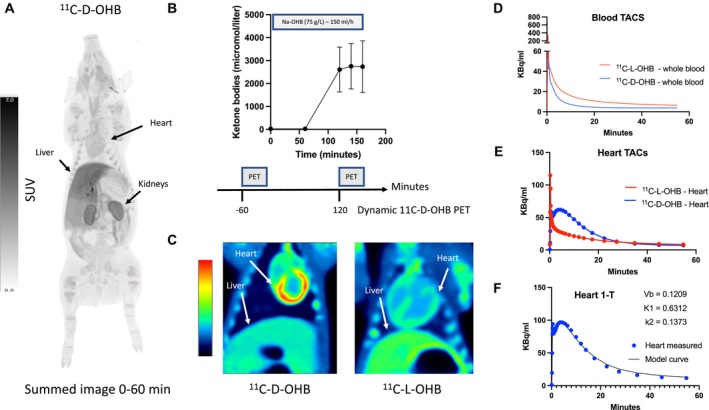
A, Biodistribution of 11C‐D‐OHB after injection of 183 MBq radiotracer (summed image 0–60 minutes). There is avid tracer uptake in the liver, kidneys, and the heart. B, Experimental setup to determine the myocardial kinetics of 11C‐D‐OHB during conditions of low and elevated ketones. C, Summed (6–15 minutes) dynamic images of 11C‐D‐OHB and 11C‐L‐OHB in the heart and liver in the field of view. D, Image‐derived whole blood activity after the injection of 11C‐D‐OHB and 11C‐L‐OHB. The D‐enantiomer was cleared more rapidly than the L‐enantiomer. E, Myocardial TACs of 11C‐D‐OHB and 11C‐L‐OHB showing uptake and clearance of the D‐enantiomer (blue), whereas the L‐enantiomer (red) to a large extent was cleared immediately, leaving only a small fraction that was metabolized or irreversibly bound. V t for the L‐3‐OHB was 146±0.19 and for the D‐3‐OHB was 3.77±0.46. F, One‐tissue compartmental modeling of myocardial 11C‐D‐OHB kinetics yielding a good fit to the measured data. D‐3‐OHB indicates D‐3‐hydroxybutyrate; K1, rate constant for the transfer of a radiotracer from plasma to tissue; k2, rate constant for the transfer of a radiotracer from tissue back to plasma; L‐3‐OHB, L‐3‐hydroxybutyrate; PET, positron emission tomography; SUV, standardized uptake value; TAC, tissue activity curve; Vb, blood volume; and V t, volume of distribution.
L‐[11C]3‐OHB and D‐[11C]3‐OHB had different kinetics as shown in Figure 5D and E. Heart tissue radioactivity from L‐[11C]3‐OHB peaked shortly after injection but decreased rapidly to a plateau. Thus, L‐3‐OHB retention in cardiac tissue was lower than that of the D‐enantiomer, probably reflecting less oxidation via the tricarboxylic acid cycle. When comparing V t, the ratio of the total amount of tracer distributed in the heart tissue (Figure 5E) to the total amount of tracer in the blood (Figure 5D), the V t of the L‐[11C]3‐OHB was about half that of the D‐[11C]3‐OHB (1.46±0.19 versus 3.77±0.46; Table 4). Additionally, circulating radiotracers were higher for L‐[11C]3‐OHB (Figure 5D) throughout the studies, reflecting an attenuated tissue uptake of L‐3‐OHB.
Table 4.
D‐[11C]3‐OHB and L‐[11C]3‐OHB Kinetics
| Flux (Patlak) | V t (Logan) | |
|---|---|---|
| D‐[11C]3‐OHB (n=2) | NA | 3.77±0.46 |
| L‐[11C]3‐OHB (n=3) | 0.39±0.09 | 1.46±0.19 |
A Patlak plot was used to estimate the flux (Ki) for irreversible tracer kinetics, and a Logan plot was used to estimate V t for reversible tracer kinetics. Data are presented as mean±SD. 3‐OHB indicates 3‐hydroxybutyrate; and V t, volume of distribution, the ratio of the total amount of tracer distributed in the tissue to the total amount of tracer in the plasma.
Effects of L‐3‐OHB and D‐3‐OHB on Isolated Coronary Arteries
Isolated porcine coronary arteries were precontracted with the thromboxane analogue U46619 and then exposed to D‐3‐OHB or L‐3‐OHB. Overall, 3‐OHB relaxed arteries compared with equimolar NaCl (P=0.04), but we observed no differences in the vasorelaxant responses of the 2 3‐OHB enantiomers (Figure 6).
Figure 6. Isolated coronary arteries.
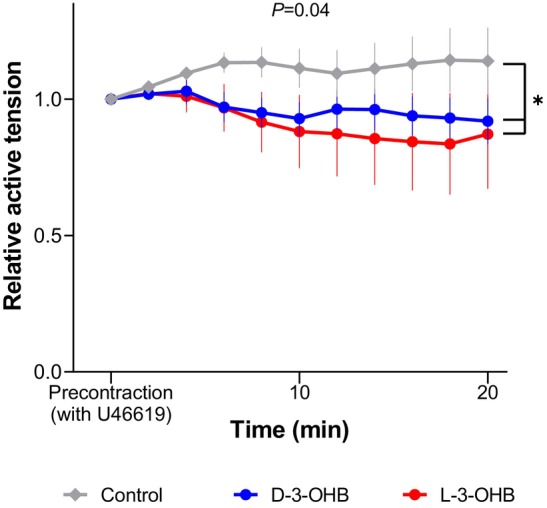
Myograph measures of isolated coronary arteries exposed for 3 mmol/L D‐3‐OHB or L‐3‐OHB and control. The relative active tension for 20 minutes is presented. D‐3‐OHB indicates D‐3‐hydroxybutyrate; and L‐3‐OHB, L‐3‐hydroxybutyrate.
DISCUSSION
In the present study, we investigated and compared the acute cardiovascular effects of D/L‐3‐OHB, L‐3‐OHB, and D‐3‐OHB in 60‐kg pigs. The main findings were as follows. First, the increase in CO during D/L‐3‐OHB infusions was mediated through a reduction in E a (afterload) and not by an increase in E es (left ventricular contractility) or LVEDV (preload). Second, L‐3‐OHB infusion resulted in a greater increase in CO of 2.7 L/min, whereas D‐3‐OHB infusion caused only a minor rise in CO of 0.6 L/min. This difference was explained by higher circulating 3‐OHB levels during L‐3‐OHB due to its slower metabolization at whole body level. Thus, CO and SVR changes during L‐3‐OHB and D‐3‐OHB infusions displayed a similar correlation with the change in circulating 3‐OHB levels. Third, myocardial mitochondrial respiratory capacity did not change significantly after each 3‐OHB infusion compared with their control infusion. Fourth, in experiments on isolated coronary arteries, D‐ and L‐3‐OHB produced similar levels of vasorelaxation. Fifth, D‐[11C]3‐OHB was rapidly taken up by the cardiac tissue and metabolized, whereas L‐[11C]3‐OHB was taken up and metabolized more slowly. This was further supported by the increased arteriovenous extraction ratio of 3‐OHB across the heart during D‐3‐OHB infusion but not during L‐3‐OHB infusion (Figure 7).
Figure 7. Summary of relevant findings of the study.
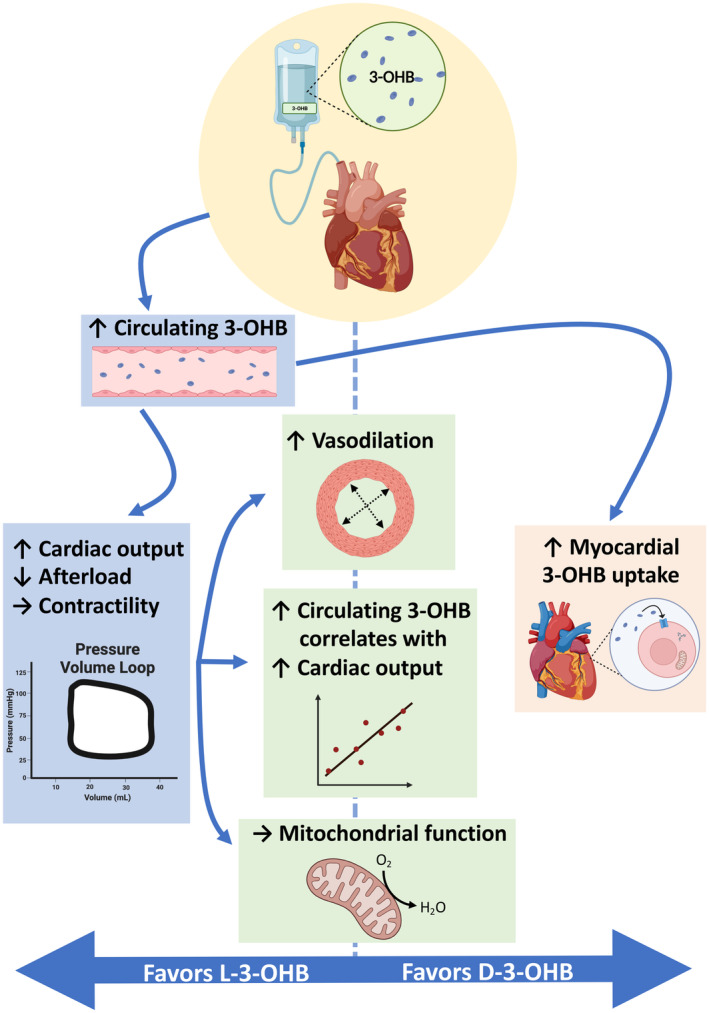
L‐3‐OHB elicits greater increases in circulating 3‐hydroxybutyrate levels and cardiac output than D‐3‐OHB infusion. The isolated coronary artery responses and myocardial mitochondrial function were similar during exposure to L‐3‐OHB and D‐3‐OHB. The correlations between circulating 3‐OHB levels and cardiac output were similar for both L‐3‐OHB and D‐3‐OHB levels. Myocardial 3‐OHB uptake was more pronounced during D‐3‐OHB infusion than L‐3‐OHB infusion. 3‐OHB indicates 3‐hydroxybutyrate; D‐3‐OHB, D‐3‐hydroxybutyrate; and L‐3‐OHB, L‐3‐hydroxybutyrate.
Cardiovascular Effects of D/L‐3‐OHB
Clinical studies have shown that D/L‐3‐OHB infusion increases CO by 33% in healthy participants 1 and by 40% patients with heart failure. 1 , 27 However, the exact mechanisms underlying these hemodynamic changes have not been investigated. The present study aimed to investigate the LV hemodynamic changes contributing to the increase in CO during 3‐OHB infusion. Our findings indicated that afterload reduction, as estimated by reduced E a, LVESP, LVPmax, and SVR, was the primary underlying mechanism involved in the CO increase during D/L‐3‐OHB infusion (Table 1). Meanwhile, the load‐independent LV contractility measurement, E es, exhibited no significant changes during D/L‐3‐OHB infusion. Although the observed 18 bpm increase in heart rate contributed to the increase in CO during 3‐OHB, it does not fully account for the considerably larger increase in CO of 2.8 L/min. During D/L‐3‐OHB infusion, the preload, as measured by LVEDV, decreased compared with the control infusion. Therefore, the preload changes did not explain the CO increase. Furthermore, D/L‐3‐OHB infusion did not alter myocardial mitochondrial oxidative capacity. These findings highlight that the cardiovascular effect is mediated by a vascular effect rather than by a direct myocardial mechanism. Indeed, our study further demonstrates that 3‐OHB relaxes isolated coronary arteries in the relevant concentration range. The potential of 3‐OHB to unload the heart holds promise in that previous studies have demonstrated the beneficial effects of unloading the compromised heart in the context of acute myocardial infarction or chronic heart failure. 28 Notably, unloading the failing heart reversed the cellular and anatomical changes commonly observed in the pathological state of heart failure. 28 Moreover, the arterial elastance reduction by 1.38 mm Hg/mL during 3‐OHB infusion was more pronounced than the reported effects of nitroglycerin infusion in patients with and without heart failure. 29 , 30 This substantiates the notion that 3‐OHB enjoys therapeutic potential for reducing cardiac overload while concurrently increasing CO and LVEF. 1 , 4
Differential Cardiovascular Effects of D‐ and L‐3‐OHB
D‐3‐OHB is the predominant endogenous ketone body in humans, primates, and mammals. 31 However, the stereoisomer L‐3‐OHB has received little attention. Previous animal studies have reported that the highest concentration of L‐3‐OHB is found in cardiac tissue, 10 , 11 , 32 suggesting either active cardiac uptake or production of L‐3‐OHB. Under physiological conditions, the exact mechanism responsible for the formation of L‐3‐OHB is unclear. Some studies suggest hepatic production of L‐3‐OHB by L‐3‐hydroxybutyryl‐CoA dehydrogenase, 33 whereas others have shown that L‐3‐OHB is not metabolized by hepatic dehydrogenase but via mitochondrial activation. 34 , 35 L‐3‐OHB can be converted from D‐3‐OHB by a racemase responsible for the interconversion of L‐and D‐3‐hydroxybutyryl‐CoA dehydrogenase. 36 However, the physiological role of L‐3‐OHB is not fully understood. L‐3‐OHB can be transported into cardiomyocytes through monocarboxylate transporters 37 but is not believed to be metabolized. Some studies have further suggested that L‐3‐OHB is an intracellular intermediate metabolite. 10 The specific impact of L‐3‐OHB on the cardiovascular system has not previously been explored in depth. In the present study, L‐3‐OHB infusion increased CO, whereas D‐3‐OHB infusion produced only a minor increase in CO. This difference in hemodynamic response was due to different pharmacokinetic properties of the 3‐OHB enantiomers. L‐3‐OHB was metabolized more slowly, resulting in higher circulating 3‐OHB levels. The increase in circulating 3‐OHB levels correlated with CO for both enantiomers (Figure 3A). Therefore, the significant increase in CO during L‐3‐OHB infusion may be mediated through higher circulating 3‐OHB levels as compared with D‐3‐OHB infusion. Additionally, we observed similar vasorelaxant effects of the 2 enantiomers on isolated coronary arteries under closely controlled D‐ and L‐3‐OHB concentrations. Furthermore, our findings challenge the concept that D‐3‐OHB is the only enantiomer with cardiovascular effects. The importance of L‐3‐OHB is supported by a previous study showing that glucose use in cardiomyocytes is reduced by D‐3‐OHB and recovered in a dose‐dependent manner by L‐3‐OHB addition. 10 Additionally, although D/L‐3‐OHB has been shown to protect the heart from ischemia–reperfusion‐induced myocardial damage, 16 D‐3‐OHB alone did not protect cardiac tissue during low‐flow ischemia. 38 Finally, the 2 3‐OHB enantiomers have different effects on cardiac myocyte electrophysiology. 39 The stereoselective effect of 3‐OHB has also been observed in other organ tissues. 3‐OHB prevents muscle weakness during sepsis or critical illness. 40 , 41 An animal study showed that L‐3‐OHB reached its maximal muscle‐protective effect at a lower dose than did D‐3‐OHB. 42 These effects are consistent with the pharmacokinetic differences between D‐ and L‐3‐OHB, which results in higher plasma concentrations of 3‐OHB during L‐3‐OHB treatment and leads to stronger cardiovascular effects. Additionally, these data showed that the acute beneficial hemodynamic response to 3‐OHB is mediated through vasodilation and afterload reduction rather than through the direct myocardial metabolism of 3‐OHB.
Mitochondrial Function
Only a few studies have investigated the effect of 3‐OHB on myocardial mitochondrial function. 3‐OHB does not alter excitation‐contraction coupling in isolated rat ventricular myocyte. 14 Similarly, pressure‐overloaded, hypertrophied failing mouse hearts increase cardiac ATP production, whereas cardiac work and cardiac efficiency remain unaltered during ketonemia. 15 Furthermore, in patients with chronic heart failure, 3‐OHB infusion do not change myocardial external energy efficiency. 1 The present study directly examined myocardial mitochondrial oxygen consumption after ketonemia and found no changes when compared with the control infusion as measured with high‐resolution respirometry (Figure 4). These findings support the safety of myocardial tissue when exposed to 3‐OHB as we observed no evidence of harm or damage to mitochondrial function. Furthermore, the findings supported our hemodynamic results, which showed that the effects of 3‐OHB were not driven by enhanced myocardial contractility, that is, a direct cardiac effect. Additionally, PVA (Table 1), which correlates with myocardial oxygen consumption, 43 remained unchanged during 3‐OHB infusion. In conjunction, our results suggest that the hemodynamic effects induced by 3‐OHB are attributable to extracardiac factors, involving vasodilation, which reduces the left ventricular afterload.
Arteriovenous Gradients of Metabolites Across the Heart and Extraction Ratio
The arteriovenous gradients across the heart of 3‐OHB were substantial for D/L‐3‐OHB, D‐3‐OHB, and L‐3‐OHB (Table 3). The arteriovenous gradient of 3‐OHB was significantly higher during D‐3‐OHB infusion than during L‐3‐OHB infusion. The extraction ratios of 3‐OHB significantly increased during D/L‐3‐OHB and D‐3‐OHB infusion but not during L‐3‐OHB infusion (Table 3). These findings are consistent with observations from our radiotracer PET study, demonstrating a slower cardiac uptake of L‐[11C]3‐OHB than of D‐[11C]3‐OHB (Figure 5C and 5E). Notably, the arteriovenous gradients of other metabolites across the heart remained largely unchanged during each intervention when compared with the control infusion (Table 3), although the glucose arteriovenous gradients during D/L‐3‐OHB were significantly increased. These findings run counter to those of other clinical studies. 2 , 44 In healthy volunteers, infusion of D/L‐3‐OHB halved myocardial glucose uptake, whereas palmitate uptake remained unaltered. 2 Another study, examining oral ketone ester treatment in healthy volunteers and patients with heart failure using arterial and coronary sinus blood sampling, demonstrated that 3‐OHB, FFA, and lactate are taken up by the myocardial tissue. 44 However, in a mouse model with pressure‐overloaded heart failure and in healthy mice, ketonemia did not affect cardiac glucose oxidation or palmitate oxidation. 15 , 45 Accordingly, there are inconsistencies in the studies examining cardiac metabolism during ketonemia. The present study highlighted the distinctive metabolic effects of 3‐OHB enantiomers and the dissociation between their metabolic and hemodynamic responses. D‐3‐OHB administration significantly increased the extraction ratio of 3‐OHB across the heart while merely exhibiting a modest hemodynamic response. In contrast, L‐3‐OHB demonstrated no significant change in the extraction ratio of 3‐OHB across the heart while demonstrating pronounced hemodynamic effects.
Cardiac Uptake and Metabolism of D‐[ 11C]3‐OHB—and L‐[ 11C]3‐OHB
The present proof‐of‐concept PET study with dedicated D‐[11C]3‐OHB tracers provided evidence that the cardiac metabolism of D‐3‐OHB and L‐3‐OHB were markedly different. The metabolization of D‐[11C]3‐OHB in heart tissue closely resembles that of [11C]‐acetate, suggesting that the k2‐value reflected the oxidation of D‐3‐OHB to [11C]CO2 and not the resecretion of unmetabolized D‐[11C]3‐OHB back into circulation. This notion was supported by rapid appearance of metabolites in the form of [11C]CO2 in plasma (Figure S3). The myocardial metabolism of D‐[11C]3‐OHB is similar in healthy participants. 46 Whereas D‐3‐OHB was taken up and metabolized by the myocardial tissue, L‐3‐OHB was metabolized at a much slower rate or bound irreversibly to the tissue, suggesting a receptor‐binding effect. A slow metabolization of L‐3‐OHB was supported by a low rate of appearance of [11C]CO2 in plasma (Figure S3). Both enantiomers are transported across the plasma membrane through monocarboxylate transporters, which have no stereoselective abilities. 37 Thus, the high L‐3‐OHB levels in cardiac tissue may be due to a dissociation between cardiac uptake and metabolization. In a rat study, high L‐3‐OHB levels in heart tissue were observed after a single oral D/L‐3‐OHB dose. 32 Overall, our PET findings documented a dissociation between the rather slow cardiac metabolism of the L‐3‐OHB enantiomer and its profound hemodynamic effects. This further supports that acute cardiovascular effects are mediated by noncardiac mechanisms (ie, vasodilation). Intriguingly, the slow metabolization of L‐3‐OHB at the whole‐body level 47 also yielded a more favorable pharmaco‐kinetic and ‐dynamic profile. It would be worth exploring a novel L‐3‐OHB ester in future studies owing to the potential L‐3‐OHB harbors as a novel cardiovascular treatment option.
Metabolic Changes During D‐3‐OHB and L‐3‐OHB Infusion
The influence of 3‐OHB on FFA release is extensively documented and involves a negative feedback loop acting through the hydroxycarboxylic acid receptor 2 on the surface of adipocytes. 48 This receptor exhibits affinity for the 3‐OHB enantiomers. 49 In the present study, both L‐3‐OHB and D‐3‐OHB infusions suppressed circulating FFA levels consistent with the observations in clinical studies using D/L‐3‐OHB infusions. 1 , 4 , 27
Additionally, our study revealed that both L‐3‐OHB and D‐3‐OHB infusions decreased glucose levels and increased insulin levels. These changes have not been investigated in the clinical studies during D/L‐3‐OHB infusion as an euglycemic clamp was used. 1 , 4 , 27 , 50 However, during oral 3‐OHB treatment, glucose levels were reduced in healthy participants and patients with type 2 diabetes. 51 , 52 The perceived beneficial effects of 3‐OHB on glucose metabolism warrant further investigation, particularly regarding the distinct metabolic effects of 3‐OHB enantiomer infusion in clinical studies.
Limitations
The results of this study were obtained from a healthy animal model. Thus, the hemodynamic effects of 3‐OHB infusion may be different in a cardiac‐diseased animal model and in humans. However, clinical studies have shown that the cardiovascular effects of D/L‐3‐OHB are similar in patients with heart failure and healthy participants. This demonstrates that the ketonemic response is induced irrespective of preexisting cardiac disease. 1 , 2 Presently, 100% pure D‐ and L‐3‐OHB are not available for human use. Therefore, we chose to study enantiomer‐specific effects in human‐sized pigs. Furthermore, the current investigation focused on examining the acute impacts of 3‐OHB infusion; however, we recognize that the outcomes of long‐term 3‐OHB treatment might diverge and that the enantiomer‐specific effects of 3‐OHB may exhibit different actions.
The present study was structured as a crossover trial, which carries a well‐established risk of carryover bias. To mitigate this risk, a mixed‐effects model was used to evaluate the interaction between period and treatment order. No significant carryover bias was observed in the hemodynamic measurements.
The power calculation was based on the primary end point CO and therefore interpretations of the secondary outcomes should be done with caution and investigated further in separate studies. The radiotracer PET study was designed as a proof‐of‐concept study with only 4 animals. Despite the small sample size, the results clearly demonstrated differential cardiac uptake of D‐3‐OHB and L‐3‐OHB.
The afterload reducing effects of 3‐OHB were not compared with other afterload reducing agents, such as sodium nitrite. The choice of dobutamine as a positive control was deliberate, aligning with its common use in heart failure and allowing us to emphasize the unique impact of 3‐OHB on the primary end point CO. Future research endeavors may explore direct comparisons with afterload reducing agents to better elucidate the distinctive properties of 3‐OHB in this context.
The animals were anesthetized using propofol and fentanyl, both of which may cause vasodilation. Despite administering minimal doses to mitigate these effects, it is possible that the observed hemodynamic effects during the interventions may be more pronounced without anesthesia.
In the porcine model, termination of the azygos vein in the coronary sinus raises the concern that blood samples collected from this location may not accurately reflect the heart's use of metabolites. Nevertheless, efforts were made to accurately place the catheter in the coronary sinus by using fluoroscopy, positioning it beyond the entrance of the azygos vein.
CONCLUSIONS
3‐OHB increased CO by reducing afterload rather than enhancing contractility or preload. The myocardial uptake and metabolism of D‐3‐OHB exceeded those of L‐3‐OHB, but the hemodynamic response was stronger during L‐3‐OHB infusion. The correlation between changes in CO and SVR was similar for both enantiomers. Neither of the enantiomers of 3‐OHB affected myocardial mitochondrial respiratory capacity, and both enantiomers caused equal dilation of the coronary arteries. The enhanced hemodynamic response during L‐3‐OHB infusion was caused by higher circulating 3‐OHB levels due to slower whole‐body metabolization. These findings suggest that L‐3‐OHB may be a potent cardiovascular agent with strong hemodynamic effects.
Sources of Funding
The study was supported by Aarhus University, the Independent Research Fund Denmark (grant no. 8020‐00120A), the Novo Nordisk Foundation (grant no. NNF17OC0028230), the Lundbeck Foundation (grant no. R231‐2016‐2716), and director Kurt Boennelycke and wife Grethe Boennelycke's Foundation.
Disclosures
Henrik Wiggers has been the principal or a subinvestigator in studies involving the following pharmaceutical companies: MSD, Bayer, Daiichi‐Sankyo, Novartis, Novo Nordisk, Sanofi‐Aventis, and Pfizer. Roni Nielsen has been the principal or a subinvestigator in studies involving the following pharmaceutical companies: Imbria, Medtrace, and Janssen. The remaining authors have no disclosures to report.
Ethics Approval
This study was approved by the Danish Animal Experiments Inspectorate (2021‐15‐0201‐00853, 2019‐15‐0201‐01609). The Animal Research: Reporting of In Vivo Experiments guidelines were followed in this study. The first author has full access to all study data and takes responsibility for their integrity and data analysis.
Supporting information
Data S1
Tables S1–S2
Figures S1–S3
References 53–56
Acknowledgments
The authors would like to extend their sincere appreciation to Oskar Kjærgaard Hørsdal, Lasse Juul Christensen, Rasmus Gebauer, and Maya Jensen for their invaluable assistance in the animal laboratory facilities. Furthermore, we extend our sincere gratitude to Lene Trudsø, Eva Mølgaard Jensen, and Casper Elkjær for their invaluable assistance with blood and tissue sample collection and analysis.
This article was sent to Julie K. Freed, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.033628
For Sources of Funding and Disclosures, see page 16.
References
- 1. Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frøkiær J, Eiskjaer H, Jespersen NR, et al. Cardiovascular effects of treatment with the ketone body 3‐hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139:2129–2141. doi: 10.1161/CIRCULATIONAHA.118.036459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gormsen LC, Svart M, Thomsen HH, Søndergaard E, Vendelbo MH, Christensen N, Tolbod LP, Harms HJ, Nielsen R, Wiggers H, et al. Ketone body infusion with 3‐hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: a positron emission tomography study. J Am Heart Assoc. 2017;6:e005066. doi: 10.1161/JAHA.116.005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berg‐Hansen K, Christensen KH, Gopalasingam N, Nielsen R, Eiskjær H, Møller N, Birkelund T, Christensen S, Wiggers H. Beneficial effects of ketone ester in patients with cardiogenic shock: a randomized, controlled, double‐blind trial. JACC Heart Fail. 2023;10:1337–1347. doi: 10.1016/j.jchf.2023.05.029 [DOI] [PubMed] [Google Scholar]
- 4. Nielsen R, Christensen KH, Gopalasingam N, Berg‐Hansen K, Seefeldt J, Homilius C, Boedtkjer E, Andersen MJ, Wiggers H, Møller N, et al. Hemodynamic effects of ketone bodies in patients with pulmonary hypertension. J Am Heart Assoc. 2023;12:e028232. doi: 10.1161/JAHA.122.028232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. White H, Heffernan AJ, Worrall S, Grunsfeld A, Thomas M. A systematic review of intravenous β‐hydroxybutyrate use in humans—a promising future therapy? Front Med (Lausanne). 2021;8:740374. doi: 10.3389/fmed.2021.740374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. 2020;141:1800–1812. doi: 10.1161/CIRCULATIONAHA.119.045033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selvaraj S, Hu R, Vidula MK, Dugyala S, Tierney A, Ky B, Margulies KB, Shah SH, Kelly DP, Bravo PE. Acute echocardiographic effects of exogenous ketone administration in healthy participants. J Am Soc Echocardiogr. 2021;35:305–311. doi: 10.1016/j.echo.2021.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258 [DOI] [PubMed] [Google Scholar]
- 9. Tsai YC, Liao TH, Lee JA. Identification of L‐3‐hydroxybutyrate as an original ketone body in rat serum by column‐switching high‐performance liquid chromatography and fluorescence derivatization. Anal Biochem. 2003;319:34–41. doi: 10.1016/S0003-2697(03)00283-5 [DOI] [PubMed] [Google Scholar]
- 10. Tsai YC, Chou YC, Wu AB, Hu CM, Chen CY, Chen FA, Lee JA. Stereoselective effects of 3‐hydroxybutyrate on glucose utilization of rat cardiomyocytes. Life Sci. 2006;78:1385–1391. doi: 10.1016/j.lfs.2005.07.013 [DOI] [PubMed] [Google Scholar]
- 11. Hsu WY, Kuo CY, Fukushima T, Imai K, Chen CM, Lin PY, Lee JA. Enantioselective determination of 3‐hydroxybutyrate in the tissues of normal and streptozotocin‐induced diabetic rats of different ages. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3331–3336. doi: 10.1016/j.jchromb.2011.07.038 [DOI] [PubMed] [Google Scholar]
- 12. Lehto A, Koch K, Barnstorf‐Brandes J, Viel C, Fuchs M, Klein J. ß‐Hydroxybutyrate improves mitochondrial function after transient ischemia in the mouse. Neurochem Res. 2022;47:3241–3249. doi: 10.1007/s11064-022-03637-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gómora‐García JC, Montiel T, Hüttenrauch M, Salcido‐Gómez A, García‐Velázquez L, Ramiro‐Cortés Y, Gomora JC, Castro‐Obregón S, Massieu L. Effect of the ketone body, D‐β‐hydroxybutyrate, on Sirtuin2‐mediated regulation of mitochondrial quality control and the autophagy–lysosomal pathway. Cells. 2023;12:486. doi: 10.3390/cells12030486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klos M, Morgenstern S, Hicks K, Suresh S, Devaney EJ. The effects of the ketone body β‐hydroxybutyrate on isolated rat ventricular myocyte excitation‐contraction coupling. Arch Biochem Biophys. 2019;662:143–150. doi: 10.1016/j.abb.2018.11.027 [DOI] [PubMed] [Google Scholar]
- 15. Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JRB, Ussher JR, Muoio DM, et al. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res. 2019;115:1606–1616. doi: 10.1093/cvr/cvz045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zou Z, Sasaguri S, Gopalrao Rajesh K, Suzuki R, Saguri S. Dl‐3‐hydroxybutyrate administration prevents myocardial damage after coronary occlusion in rat hearts. Am J Physiol Heart Circ Physiol. 2002;283:1968–1974. doi: 10.1152/ajpheart.00250.2002 [DOI] [PubMed] [Google Scholar]
- 17. Lyhne MD, Schultz JG, Dragsbaek SJ, Hansen JV, Mortensen CS, Kramer A, Nielsen‐Kudsk JE, Andersen A. Closed chest biventricular pressure‐volume loop recordings with admittance catheters in a porcine model. J Vis Exp. 2021:486. doi: 10.3791/62661 [DOI] [PubMed] [Google Scholar]
- 18. Bastos MB, Burkhoff D, Maly J, Daemen J, Den Uil CA, Ameloot K, Lenzen M, Mahfoud F, Zijlstra F, Schreuder JJ, et al. Invasive left ventricle pressure‐volume analysis: overview and practical clinical implications. Eur Heart J. 2020;41:1286–1297. doi: 10.1093/eurheartj/ehz552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sørensen LK, Rittig NF, Holmquist EF, Jørgensen KA, Jørgensen JOL, Møller N, Johannsen M. Simultaneous determination of β‐hydroxybutyrate and β‐hydroxy‐β‐methylbutyrate in human whole blood using hydrophilic interaction liquid chromatography electrospray tandem mass spectrometry. Clin Biochem. 2013;46:1877–1883. doi: 10.1016/j.clinbiochem.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 20. Støttrup NB, Løfgren B, Birkler RD, Nielsen JM, Wang L, Caldarone CA, Kristiansen SB, Contractor H, Johannsen M, Bøtker HE, et al. Inhibition of the malate‐aspartate shuttle by pre‐ischaemic aminooxyacetate loading of the heart induces cardioprotection. Cardiovasc Res. 2010;88:257–266. doi: 10.1093/cvr/cvq205 [DOI] [PubMed] [Google Scholar]
- 21. Jespersen NR, Hjortbak MV, Lassen TR, Støttrup NB, Johnsen J, Tonnesen PT, Larsen S, Kimose HH, Bøtker HE. Cardioprotective effect of succinate dehydrogenase inhibition in rat hearts and human myocardium with and without diabetes mellitus. Sci Rep. 2020;10:10344. doi: 10.1038/s41598-020-67247-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jespersen NR, Yokota T, Støttrup NB, Bergdahl A, Pælestik KB, Povlsen JA, Dela F, Bøtker HE. Pre‐ischaemic mitochondrial substrate constraint by inhibition of malate‐aspartate shuttle preserves mitochondrial function after ischaemia–reperfusion. J Physiol. 2017;595:3765–3780. doi: 10.1113/JP273408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seefeldt JM, Lassen TR, Hjortbak MV, Jespersen NR, Kvist F, Hansen J, Bøtker HE. Cardioprotective effects of empagliflozin after ischemia and reperfusion in rats. Sci Rep. 2021;11:9544. doi: 10.1038/s41598-021-89149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thorell J, Stone‐Elander S, Halldin C, Widén L. Synthesis of [1‐11C]‐beta‐hydroxybutyric acid. Acta Radiol Suppl. 1991;376:94. [PubMed] [Google Scholar]
- 25. Boedtkjer E, Aalkjaer C. The solution to bicarbonate. Am J Physiol Heart Circ Physiol. 2022;322:H685–H686. doi: 10.1152/ajpheart.00057.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kroon M, Groeneveld ABJ, Smulders YM. Cardiac output measurement by pulse dye densitometry: comparison with pulmonary artery thermodilution in post‐cardiac surgery patients. J Clin Monit Comput. 2005;19:395–399. doi: 10.1007/s10877-005-6865-y [DOI] [PubMed] [Google Scholar]
- 27. Gopalasingam N, Christensen KH, Berg Hansen K, Nielsen R, Johannsen M, Gormsen LC, Boedtkjer E, Nørregaard R, Møller N, Wiggers H. Stimulation of the hydroxycarboxylic acid receptor 2 with the ketone body 3‐hydroxybutyrate and niacin in patients with chronic heart failure: hemodynamic and metabolic effects. J Am Heart Assoc. 2023;12:e029849. doi: 10.1161/JAHA.123.029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uriel N, Sayer G, Annamalai S, Kapur NK, Burkhoff D. Mechanical unloading in heart failure. J Am Coll Cardiol. 2018;72:569–580. doi: 10.1016/j.jacc.2018.05.038 [DOI] [PubMed] [Google Scholar]
- 29. Fujimoto N, Onishi K, Tanabe M, Dohi K, Funabiki K, Kurita T, Yamanaka T, Nakajima K, Ito M, Nobori T, et al. Nitroglycerin improves left ventricular relaxation by changing systolic loading sequence in patients with excessive arterial load. J Cardiovasc Pharmacol. 2005;45:211–216. doi: 10.1097/01.fjc.0000152034.84491.fc [DOI] [PubMed] [Google Scholar]
- 30. Haber HL, Simek CL, Bergin JD, Sadun A, Gimple LW, Powers ER, Feldman MD. Bolus intravenous nitroglycerin predominantly reduces afterload in patients with excessive arterial elastance. J Am Coll Cardiol. 1993;22:251–257. doi: 10.1016/0735-1097(93)90841-N [DOI] [PubMed] [Google Scholar]
- 31. Mierziak J, Burgberger M, Wojtasik W. 3‐Hydroxybutyrate as a metabolite and a signal molecule regulating processes of living organisms. Biomol Ther. 2021;11:1–21. doi: 10.3390/biom11030402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Rijt WJ, Van Hove JLK, Vaz FM, Havinga R, Allersma DP, Zijp TR, Bedoyan JK, Heiner‐Fokkema MR, Reijngoud DJ, Geraghty MT, et al. Enantiomer‐specific pharmacokinetics of D,L‐3‐hydroxybutyrate: implications for the treatment of multiple acyl‐CoA dehydrogenase deficiency. J Inherit Metab Dis. 2021;44:926–938. doi: 10.1002/jimd.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reed WD, Ozand PT, Carter WP. Enzymes of L‐(+)‐3‐hydroxybutyrate metabolism in the rat. Arch Biochem Biophys. 1980;205:94–103. [DOI] [PubMed] [Google Scholar]
- 34. Lincoln BC, Des RC, Brunengraber H. Metabolism of S‐3‐hydroxybutyrate in the perfused rat liver. Arch Biochem Biophys. 1987;259:149–156. doi: 10.1016/0003-9861(87)90480-2 [DOI] [PubMed] [Google Scholar]
- 35. Scofield RF, Brady PS, Schumann WC, Kumaran K, Ohgaku S, Margolis JM, Landau BR. On the lack of formation of L‐(+)‐3‐hydroxybutyrate by liver. Arch Biochem Biophys. 1982;214:268–272. doi: 10.1016/0003-9861(82)90030-3 [DOI] [PubMed] [Google Scholar]
- 36. Stern JR, Del Campillo A. Enzymatic racemization of β‐hydroxybutyryl‐S‐CoA and the stereospecificity of enzymes of the fatty acid cycle1. J Am Chem Soc. 1955;77:1073–1074. doi: 10.1021/ja01609a105 [DOI] [Google Scholar]
- 37. Wang X, Levi AJ, Halestrap AP, Substrate APH. Substrate and inhibitor specificities of the monocarboxylate transporters of single rat heart cells. Am J Physiol. 1996;270:476–484. doi: 10.1152/ajpheart.1996.270.2.H476 [DOI] [PubMed] [Google Scholar]
- 38. King LM, Sidell RJ, Wilding JR, Radda GK, Clarke K. Free fatty acids, but not ketone bodies, protect diabetic rat hearts during low‐flow ischemia. Am J Physiol Heart Circ Physiol. 2001;280:1173–1181. doi: 10.1152/ajpheart.2001.280.3.H1173 [DOI] [PubMed] [Google Scholar]
- 39. Klos ML, Hou W, Nsengimana B, Weng S, Yan C, Xu S, Devaney E, Han S. Differential effects of beta‐hydroxybutyrate enantiomers on induced pluripotent stem derived cardiac myocyte electrophysiology. Biomol Ther. 2022;12:1500. doi: 10.3390/biom12101500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomsen HH, Rittig N, Johannsen M, Møller AB, Jørgensen JO, Jessen N, Møller N. Effects of 3‐hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS‐induced inflammation in humans: anticatabolic impact of ketone bodies. Am J Clin Nutr. 2018;108:857–867. doi: 10.1093/ajcn/nqy170 [DOI] [PubMed] [Google Scholar]
- 41. Koutnik AP, Poff AM, Ward NP, DeBlasi JM, Soliven MA, Romero MA, Roberson PA, Fox CD, Roberts MD, D'Agostino DP. Ketone bodies attenuate wasting in models of atrophy. J Cachexia Sarcopenia Muscle. 2020;11:973–996. doi: 10.1002/jcsm.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weckx R, Goossens C, Derde S, Pauwels L, Vander Perre S, Van den Berghe G, Langouche L. Efficacy and safety of ketone ester infusion to prevent muscle weakness in a mouse model of sepsis‐induced critical illness. Sci Rep. 2022;12:10591. doi: 10.1038/s41598-022-14961-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khalafbeigui F, Suga H, Sagawa K. Left ventricular systolic pressure‐volume area correlates with oxygen consumption. Am J Phys Heart Circ Phys. 1979;237:H566–H569. doi: 10.1152/ajpheart.1979.237.5.H566 [DOI] [PubMed] [Google Scholar]
- 44. Monzo L, Sedlacek K, Hromanikova K, Tomanova L, Borlaug BA, Jabor A, Kautzner J, Melenovsky V. Ketone body metabolism in failing heart. Metabolism. 2020;115:154452. doi: 10.1016/j.metabol.2020.154452 [DOI] [PubMed] [Google Scholar]
- 45. Ho KL, Karwi QG, Wagg C, Zhang L, Vo K, Altamimi T, Uddin GM, Ussher JR, Lopaschuk GD. Ketones can become the major fuel source for the heart but do not increase cardiac efficiency. Cardiovasc Res. 2021;117:1178–1187. doi: 10.1093/cvr/cvaa143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luong TV, Nielsen EN, Falborg L, Kjærulff MLG, Tolbod LP, Søndergaard E, Møller N, Munk OL, Gormsen LC. Intravenous and oral whole body ketone dosimetry, biodistribution, metabolite correction and kinetics studied by (R)‐[1‐11C]β‐hydroxybutyrate ([11C]OHB) PET in healthy humans. EJNMMI Radiopharm Chem. 2023;8:8. doi: 10.1186/s41181-023-00198-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, Magor‐Elliott S, Hiyama S, Stirling M, Clarke K. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:1–13. doi: 10.3389/fphys.2017.00848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Offermanns S. Hydroxy‐carboxylic acid receptor actions in metabolism. Trends Endocrinol Metab. 2017;28:227–236. doi: 10.1016/j.tem.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 49. Newman JC, Verdin E. β‐Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr. 2017;37:51–76. doi: 10.1146/annurev-nutr-071816-064916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Møller N, Jørgensen JOL, Møller J, Bak JF, Pørksen N, Alberti KGMM, Schmitz O. Substrate metabolism during modest hyperinsulinemia in response to isolated hyperketonemia in insulin‐dependent diabetic subjects. Metabolism. 1990;39:1309–1313. doi: 10.1016/0026-0495(90)90189-J [DOI] [PubMed] [Google Scholar]
- 51. Soto‐Mota A, Norwitz NG, Evans R, Clarke K, Barber TM. Exogenous ketosis in patients with type 2 diabetes: safety, tolerability and effect on glycaemic control. Endocrinol Diab Metab. 2021;4:e00264. doi: 10.1002/edm2.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Svart M, Rittig N, Pedersen SB, Jessen N, Møller N. Oral 3‐hydroxybutyrate ingestion decreases endogenous glucose production, lipolysis, and hormone‐sensitive lipase phosphorylation in adipose tissue in men: a human randomized, controlled, crossover trial. Diabet Med. 2021;38:e14385. doi: 10.1111/dme.14385 [DOI] [PubMed] [Google Scholar]
- 53. Thorell J‐O, Stone‐Elander S, König WA, Halldin C, Widén L. Synthesis of racemic, R‐ and S‐[1‐11C]‐β‐hydroxybutyric acid. J Labelled Comp Radiopharm. 1991;29:709–718. doi: 10.1002/jlcr.2580290612 [DOI] [Google Scholar]
- 54. Drandarov K, Schubiger PA, Westera G. Automated no‐carrier‐added synthesis of [1‐11C]‐labeled d‐ and l‐enantiomers of lactic acid. Appl Radiat Isot. 2006;64:1613–1622. doi: 10.1016/j.apradiso.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 55. Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.RES.41.1.19 [DOI] [PubMed] [Google Scholar]
- 56. Christensen NL, Jakobsen S, Schacht AC, Munk OL, Alstrup AKO, Tolbod LP, Harms HJ, Nielsen S, Gormsen LC. Whole‐body biodistribution, dosimetry, and metabolite correction of [11C]palmitate: a PET tracer for imaging of fatty acid metabolism. Mol Imaging. 2017;16:153601211773448. doi: 10.1177/1536012117734485 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2
Figures S1–S3
References 53–56


