Abstract
Background
The phase 2 PIONEER‐HCM (Phase 2 Open‐label Pilot Study Evaluating Mavacamten in Subjects With Symptomatic Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction) study showed that mavacamten improved left ventricular outflow tract gradients, exercise capacity, and symptoms in patients with obstructive hypertrophic cardiomyopathy (HCM), but the results of longer‐term treatment are less well described. We report interim results from the PIONEER‐OLE (PIONEER Open‐Label Extension) study, the longest‐term study of mavacamten in patients with symptomatic obstructive HCM.
Methods and Results
Patients who previously completed PIONEER‐HCM (n=20) were eligible to enroll in PIONEER‐OLE. Patients received oral mavacamten, 5 mg once daily (starting dose), with individualized dose titration at week 6. Evaluations included serial monitoring of safety, echocardiography, Kansas City Cardiomyopathy Questionnaire–Overall Summary Score, and serum NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels. Thirteen patients enrolled and received mavacamten (median study duration at data cutoff, 201 weeks). Most patients (92.3%) received β‐blockers concomitantly. Treatment‐emergent adverse events were predominantly mild/moderate. One patient had an isolated reduction in left ventricular ejection fraction to 47%, which recovered and remained normal with continued treatment at a reduced dose. At week 180, mavacamten was associated with New York Heart Association class improvements from baseline (class II to I, n=9; class III to II, n=1; and unchanged, n=2), sustained reductions in left ventricular outflow tract gradients (mean [SD] change from baseline: resting, −50 [55] mm Hg; Valsalva, −70 [41] mm Hg), and serum NT‐proBNP levels (median [interquartile range] change from baseline: −498 [−2184 to −76] ng/L), and improved Kansas City Cardiomyopathy Questionnaire–Overall Summary Score (mean [SD] change from baseline: +17 [16]).
Conclusions
This long‐term analysis supports the continued safety and effectiveness of mavacamten for >3 years in obstructive HCM.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03496168.
Keywords: echocardiography, health status, long‐term follow‐up, safety, symptoms
Subject Categories: Clinical Studies
Nonstandard Abbreviations and Acronyms
- HCM
hypertrophic cardiomyopathy
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- NYHA
New York Heart Association
- QTcF
Fridericia‐corrected QT
- TEAE
treatment‐emergent adverse event
Clinical Perspective.
What Is New?
Mavacamten, a targeted cardiac‐specific myosin inhibitor, was associated with clinically important and sustained improvements in symptoms, left ventricular outflow tract gradients, and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels with no new safety signals after a median time on study of 201 weeks in 13 patients with obstructive hypertrophic cardiomyopathy.
What Are the Clinical Implications?
These data reflect the longest duration of follow‐up on mavacamten treatment reported to date.
Hypertrophic cardiomyopathy (HCM) is a chronic myocardial disease characterized by left ventricular (LV) hypertrophy that cannot be explained by abnormal cardiac loading or systemic infiltrative or metabolic conditions. 1 , 2 , 3 It is often caused by autosomal dominant mutations in genes encoding proteins of the sarcomere, the fundamental contractile unit of cardiomyocytes. 1 Core pathologic features include myocardial hypercontractility, diastolic dysfunction, and dynamic obstruction of the LV outflow tract (LVOT). 1 , 4 LVOT obstruction, which is present in most patients, 5 , 6 can lead to reduced exercise capacity and debilitating symptoms, including exertional dyspnea, chest pain, and syncope; and in some severe cases, it can lead to a need for invasive septal reduction therapy. 2
Obstructive HCM is most commonly treated with β‐blockers or nondihydropyridine calcium channel blockers. 1 , 2 It is now known that excess myosin‐actin cross‐bridging is the primary molecular driver of hypercontractility in HCM, 7 , 8 providing a potential disease‐specific target for new medical therapies.
Mavacamten is a selective, allosteric, and reversible cardiac myosin inhibitor that reduces actin‐myosin cross‐bridge formation and targets the underlying excessive contractility observed in HCM. 9 , 10 , 11 Mavacamten is the first and only cardiac myosin inhibitor approved in 5 continents for the treatment of patients with symptomatic New York Heart Association (NYHA) class II or III obstructive HCM to improve functional capacity and symptoms. 12 , 13 , 14 , 15 , 16 , 17 Approval was based on findings of the randomized, double‐blinded, placebo‐controlled, phase 3 EXPLORER‐HCM (Clinical Study to Evaluate Mavacamten in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy) trial (ClinicalTrials.gov Identifier: NCT03470545). 18 , 19 In the EXPLORER‐HCM trial, mavacamten significantly reduced LVOT gradients and improved disease‐related symptoms, functional capacity, and patient‐reported health status versus placebo over 30 weeks in patients with symptomatic obstructive HCM. 18 , 20 Mavacamten was well tolerated over the 30‐week study period, with 7 patients experiencing an event of LV ejection fraction (LVEF) of <50% (median, 48%), which was reversible after temporary interruption of mavacamten therapy in all patients. 18 In the preceding open‐label, nonrandomized, phase 2 PIONEER‐HCM (Phase 2 Open‐label Pilot Study Evaluating Mavacamten in Subjects With Symptomatic Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction) study (ClinicalTrials.gov Identifier: NCT02842242), 12 weeks of mavacamten in patients with obstructive HCM was associated with reductions from baseline in LVOT gradients and improvements in exercise capacity and symptoms, but the tolerability of longer‐term exposure to treatment was unknown; therefore, the PIONEER‐OLE (PIONEER Open‐Label Extension) study was initiated in 2018 (ClinicalTrials.gov Identifier: NCT03496168). 21 Patients who completed PIONEER‐HCM were allowed to enroll into the PIONEER‐OLE study, which is an ongoing study designed to evaluate the long‐term safety and effectiveness of 5 years of treatment with mavacamten. Here, we report an interim analysis from the PIONEER‐OLE study after a median time on study of 201 weeks. It is the longest duration of follow‐up to date in patients treated with mavacamten.
Methods
Bristol Myers Squibb's policy on data sharing is available online and is located at https://www.bms.com/researchers‐and‐partners/clinical‐trials‐and‐research/disclosure‐commitment.html.
Study Design
The PIONEER‐OLE study (ClinicalTrials.gov Identifier: NCT03496168) is an ongoing, prospective, single‐arm, open‐label, phase 2 study of 5 years of mavacamten treatment conducted at 4 US academic centers. The primary objective of the PIONEER‐OLE study is to assess the long‐term safety and tolerability of mavacamten. Secondary objectives are to assess the long‐term effectiveness of mavacamten on obstructive HCM symptoms, LVOT gradients, cardiovascular biomarkers, and echocardiographic measures of structure and function. The data cutoff date for this interim analysis was May 31, 2022, comprising a median (interquartile range) time on study of 201 (193–209) weeks.
Patients with symptomatic obstructive HCM who had previously completed the 16‐week parent PIONEER‐HCM study (12 weeks of treatment and 4 weeks of washout) 21 were eligible to participate in the PIONEER‐OLE study and were rescreened before PIONEER‐OLE study enrollment to ensure that they continued to meet study eligibility criteria (Figure 1). Patients enrolled in the PIONEER‐OLE study between 7 and 18 months after completing the PIONEER‐HCM study and were not treated with mavacamten during that period. Of the 20 patients who completed the PIONEER‐HCM study, 13 enrolled in the PIONEER‐OLE study, 6 elected to undergo septal reduction therapy in the intervening time between studies, and 1 opted not to enroll in the extension study.
Figure 1. Study design.
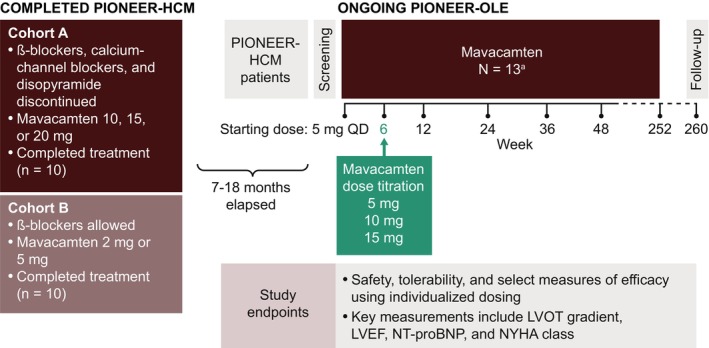
Schematic overview of key design features of the PIONEER‐OLE (Extension Study of Mavacamten in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy Previously Enrolled in PIONEER) study and the parent study, PIONEER‐HCM (Phase 2 Open‐label Pilot Study Evaluating Mavacamten in Subjects With Symptomatic Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction). aOne patient discontinued treatment at week 26. LVEF indicates left ventricular ejection fraction; LVOT, left ventricular outflow tract; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; and QD, once daily.
The study is conducted in accordance with the principles of the Declaration of Helsinki, the International Council for Harmonization Good Clinical Practice guidelines, and applicable local laws and human clinical research regulations. The study protocol and study documents were reviewed and approved by institutional review boards at each center, and all patients provided written, informed consent.
Patients
Eligibility criteria at baseline for the parent PIONEER‐HCM study have been previously published 21 and included age of 18 to 70 years and a diagnosis of HCM, defined as the presence of otherwise unexplained LV hypertrophy (LV wall thickness of ≥15 mm [≥13 mm in those with a family history of HCM]), LVOT obstruction (resting LVOT gradient ≥30 mm Hg and postexercise LVOT gradient ≥50 mm Hg), documented LVEF of at least 55%, and a NYHA class of II to III. Additional inclusion criteria for the PIONEER‐OLE study included a body weight of >45 kg and a body size–adjusted estimated glomerular filtration rate of at least 30 mL/min per 1.73 m2. Background therapy for cardiomyopathy with β‐blockers or calcium channel blockers was allowed if patients had been receiving a stable dose for at least 14 days before screening. Exclusion criteria included a Fridericia‐corrected QT (QTcF) interval of >500 ms or any other ECG abnormality considered by the investigator to pose a risk to patient safety (eg, second‐degree atrioventricular block type II). Patients who had developed any of the following conditions after enrollment into the PIONEER‐HCM study were also excluded: obstructive coronary disease (>70% stenosis in ≥1 arteries) or known moderate or severe aortic valve stenosis, clinically significant malignant disease, or any acute or serious comorbid condition that would pose a risk to patient safety or interfere with study evaluation. Previous or concomitant treatment with cardiotoxic drugs (eg, doxorubicin) or antiarrhythmic drugs with negative inotropic activity (eg, flecainide, propafenone) was prohibited. Use of disopyramide, ranolazine, moderate or potent cytochrome P450 2C19 inhibitors, and potent cytochrome P450 3A4 inhibitors was also prohibited from 14 days before screening onwards.
Treatment
Patients received mavacamten orally once daily and continue to receive mavacamten for a planned treatment duration of up to 5 years (252 weeks). Mavacamten was administered at a starting dose of 5 mg once daily in all patients, with titration at week 6 to individualized target doses (5, 10, or 15 mg) based on modeling of pharmacokinetic data from the PIONEER‐HCM study (steady‐state trough plasma concentration range, 250–500 ng/mL) and week 4 determinations of LVOT gradients (at rest, after Valsalva maneuver, and postexercise), LVEF, and mavacamten plasma concentrations. Mavacamten dose was not increased if ≥1 of the following criteria was met: LVEF of <55%; postexercise LVOT gradient of <30 mm Hg; trough mavacamten plasma concentration of >350 ng/mL; or based on investigator decision. Further dose adjustments based on echocardiographic or pharmacokinetic findings were permitted beyond week 6 after discussion with the medical monitor of the study sponsor.
In case of an exaggerated pharmacologic effect, dose reduction or drug discontinuation was implemented on the basis of the clinical judgment of the investigator at any time during the study. Criteria for temporary discontinuation of mavacamten per protocol were a mavacamten plasma concentration of at least 1000 ng/mL, LVEF <50%, or a prolonged QTcF interval (defined as a 15% increase from baseline or an absolute QTcF interval ≥520 ms [if QRS <120 ms] or ≥550 ms [if QRS ≥120 ms], as determined by core laboratory read ECG). Mavacamten could be resumed at a lower dose if values recovered within normal ranges following temporary interruption. If LVEF, drug plasma concentrations, or QTcF interval persisted out of range at the follow‐up visit, the patient was discontinued from the study.
Outcomes
Treatment‐emergent adverse events (TEAEs) were described using the Medical Dictionary for Regulatory Activities (MedDRA; version 21.0) and characterized by severity (mild, moderate, severe, life‐threatening, or fatal), causality (related or unrelated to study drug as judged by the investigator), and whether or not the event was serious. Predefined safety end points included cardiovascular death, sudden death, cardiovascular hospitalization, heart failure attributable to systolic dysfunction (defined as a symptomatic LVEF <50%), myocardial infarction, ventricular arrhythmias (ventricular tachycardia, ventricular fibrillation, ventricular flutter, or torsade de pointes), syncope, seizures, stroke, LVEF <50% as measured by echocardiography, and QT and QTcF intervals over time. Adverse events of special interest were defined as LVEF of ≤30%, symptomatic overdose, and pregnancy.
Efficacy end points included an evaluation of symptoms related to obstructive HCM using the 4‐point NYHA functional classification and the patient‐reported Kansas City Cardiomyopathy Questionnaire (KCCQ). 22 The KCCQ is a 23‐item, validated, disease‐specific instrument that assesses physical limitations, symptoms, quality of life, social interference, and self‐efficacy over a 2‐week recall period. 22 , 23 The KCCQ–Overall Summary Score combines the symptom, physical limitation, social limitation, and quality‐of‐life scales to provide a summary of patient health status. 24 The KCCQ has been validated as an instrument that is sensitive to clinical change in patients with HCM. 23 Scores range from 0 to 100, with higher scores representing better health status. Other efficacy end points were serum NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels (a marker of ventricular wall stress), LVOT gradients (resting, Valsalva, and postexercise), and frequency of septal reduction therapy.
Procedures
On‐site clinic visits were scheduled at baseline, weeks 4, 6, 8, and 12, and every 12 weeks until week 156, and then every 24 weeks thereafter. Between clinic visits, patients were contacted by telephone at week 18 and then every 12 weeks thereafter to assess status. Clinic visit assessments included standard safety monitoring (adverse events, physical examinations, vital signs, hematology and chemistry panels, and 12‐lead ECGs), evaluation of NYHA functional class, resting echocardiogram, completion of the KCCQ, and blood sample collection to determine NT‐proBNP levels.
Resting echocardiographic assessments were performed at weeks 4, 8, and 12, then every 12 weeks to week 48, and then every 24 weeks thereafter (with the exception of an additional assessment at week 156); data for visits up to week 180 are included in the present analysis. Postexercise stress echocardiography was performed at select clinical visits (weeks 4, 48, 72, and 156) after resting echocardiographic assessments had been completed. Before protocol amendment 3 (January 22, 2021), echocardiograms were read and interpreted by a central laboratory (Cardiovascular Imaging Core Laboratory, Brigham and Women's Hospital, Boston, MA). From protocol amendment 3 onwards, echocardiograms were site‐read for LVOT gradients (rest, Valsalva, and postexercise) and LVEF starting at week 144, which dictated dosing decisions and temporary discontinuations. Echocardiograms were then sent to the central laboratory for secondary analysis.
Statistical Analysis
There was no sample size calculation or testing for statistical hypotheses. All reported analyses were performed in the safety population, defined as all patients who received at least 1 dose of the study drug. For this interim analysis, safety data are reported until data cutoff (May 31, 2022), and efficacy data are reported to week 180. Efficacy and safety end points were summarized by protocol‐defined visit using descriptive summary statistics. For exposure‐adjusted incidence rates, the exposure time to first event was calculated as total duration of exposure up to the first occurrence of the event. For participants with no event, the time was censored at the last follow‐up time within the treatment‐emergent period. For participants with multiple events, the time to first event was considered. Efficacy data were presented as actual values, changes from baseline, and, for some parameters, as shift tables. Basic statistical analysis was performed with SAS, version 9.4 or higher, although no formal statistical hypothesis testing was performed per protocol.
Results
Study Population
From May 9, 2018 to November 15, 2018, 13 patients were enrolled in the PIONEER‐OLE study and constitute the safety population. At data cutoff (May 31, 2022), 12 patients (92.3%) remained on study treatment. One patient discontinued the treatment at week 26 because of a serious adverse event unrelated to the study drug (cholangiocarcinoma) described below.
Patient demographics and key characteristics at baseline are presented in Table 1. Patients ranged in age from 27 to 72 years (median, 62 years) and were predominantly men (69.2%). The median (range) duration of diagnosis of obstructive HCM was 7.4 (4.6–22.4) years. At baseline, patients were classified as NYHA class II (92.3%) or NYHA class III (7.7%), and 12 of 13 (92.3%) were receiving concomitant β‐blocker therapy. No patients were receiving calcium channel blockers at baseline.
Table 1.
Demographics and Baseline Characteristics (Safety Population)
| Characteristic | Mavacamten (n=13) |
|---|---|
| Age, median (range), y | 62 (27–72) |
| Sex, n (%) | |
| Male | 9 (69.2) |
| Female | 4 (30.8) |
| Race, n (%) | |
| White | 12 (92.3) |
| Black or African American | 1 (7.7) |
| Body mass index, kg/m2 | 30.9 (5.4) |
| Heart rate, beats/min | 61.8 (10.6) |
| Blood pressure, mm Hg | |
| Systolic | 123.2 (10.9) |
| Diastolic | 72.6 (7.1) |
| NYHA functional class, n (%) | |
| Class II | 12 (92.3) |
| Class III | 1 (7.7) |
| KCCQ‐OSS | 72 (19) |
| Background HCM therapy while on study drug, n (%) | |
| β‐Blockers | 12 (92.3) |
| Calcium channel blockers | 0 (0.0) |
| LVEF (resting), % | 72.0 (4.9) |
| LVOT gradient, mm Hg | |
| Resting | 67.3 (42.8) |
| Valsalva | 89.9 (30.7) (n=12) |
| Post‐exercise | 127.5 (33.4) |
| NT‐proBNP, median (IQR), ng/L | 594 (351–1168) |
| Mitral regurgitation present, n (%) | 13 (100) |
| Systolic anterior motion present, n (%) | 12 (92.3) |
| CYP2C19 phenotype, n (%) | |
| Poor metabolizer | 1 (7.7) |
| Intermediate metabolizer | 1 (7.7) |
| Normal metabolizer | 7 (53.8) |
| Rapid metabolizer | 2 (15.4) |
| Ultrarapid metabolizer | 1 (7.7) |
| Not determined | 1 (7.7) |
| Time on study, median (IQR), wk | 200.9 (193.3–208.9) |
Data presented are mean (SD) unless otherwise stated. CYP indicates cytochrome P450; HCM, hypertrophic cardiomyopathy; IQR, interquartile range; KCCQ‐OSS, Kansas City Cardiomyopathy Questionnaire–Overall Summary Score; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and NYHA, New York Heart Association.
Exposure
At data cutoff, the median (interquartile range) duration of mavacamten treatment was 200.9 (193.3–208.9) weeks. Following planned dose titration per protocol at week 6, mavacamten doses were 5 mg/d (n=4), 10 mg/d (n=6), and 15 mg/d (n=3) and remained stable for the duration of the observation period in most patients (Table S1). After week 6, 3 patients (23.1%) had a mavacamten dose decrease because of reductions in LVEF: 1 patient had a dose reduction from 10 to 5 mg (at week 156 for LVEF 55%), and 2 patients had dose reductions from 15 to 10 mg (1 patient at week 156 and the other after the week 180 visit for LVEFs of 51% and 47%, respectively). For the 2 patients who experienced reductions in LVEF that remained >50%, these were attributable to investigator‐driven dose reductions. The patient with LVEF of <50% was asymptomatic. During the observation period, 1 patient discontinued β‐blocker therapy at week 91.
Safety
Of the predefined safety end points, there were no cardiovascular hospitalizations, cardiovascular deaths, or sudden deaths during the observation period. In addition, there were no reports of myocardial infarction, syncope, seizures, stroke, or adverse events of special interest. LVEF remained >50% in all patients at all time points, except for 1 patient who had a drug‐related asymptomatic LVEF reduction to 47% at week 180 (based on site‐read results), which resulted in temporary treatment interruption for 55 days and dose reduction from 15 to 10 mg/d, per protocol. Previous core‐read LVEF readings in this patient ranged from 79% at baseline to 52% at week 156. After the event of LVEF of <50% at week 180, site‐read LVEF recovered to 64% within 35 days following temporary treatment interruption. The patient subsequently resumed mavacamten at a reduced dose of 10 mg and maintained an LVEF of >50% thereafter. The same patient subsequently had another temporary treatment interruption because of mavacamten plasma concentrations of >1000 ng/mL (1230 ng/mL) at week 204, while the patient was strictly compliant with study protocol and with no change in the patient's condition, and no new intercurrent illness or new medication reported. The patient's LVEF was 62% at week 204 based on site‐read results. Mavacamten plasma concentrations decreased to 45.4 ng/mL 23 days after temporary dose interruption. Approximately 9 weeks after interruption, the patient resumed treatment at 5 mg/d, and the mavacamten concentration was 149 ng/mL at week 228. There was 1 other temporary interruption, resulting in a total of 3 temporary treatment interruptions in 2 patients. The other patient requiring interruption was receiving mavacamten, 5 mg/d, and experienced an increase in QTcF interval of >15% (19.3%) from 410 ms at baseline to 489 ms at week 156; treatment was resumed 34 days later at the same 5‐mg/d dose and QTcF intervals ranged from 433 to 452 ms at subsequent visits. One case of ventricular tachycardia (nonsustained ventricular tachycardia: 9‐beat run at 188 beats/minute) not requiring any intervention was reported, which was considered unrelated to mavacamten.
Exposure‐adjusted incidence of TEAEs of clinical interest and special situations are presented in Table S2. The exposure‐adjusted incidence of major adverse cardiovascular events was 4.58 per 100 patient‐years (1 subdural hematoma and 1 troponin increase), cardiac failure was 2.14 per 100 patient‐years (1 transient case of LVEF=47%), and atrial fibrillation was 2.17 per 100 patient‐years (1 case of atrial fibrillation).
Overall, 144 TEAEs in 13 patients were reported, which were predominantly mild (65.3%) or moderate (27.8%) in severity (Table 2). The most frequently reported TEAEs, regardless of causality, were fatigue (5 patients) and arthralgia, nasopharyngitis, and upper respiratory tract infection (4 patients each). Eleven TEAEs (7.6%) in 6 patients (46.2%) were considered related to mavacamten (Table 2). Six serious TEAEs leading to hospitalizations were reported in 5 patients (38.5%), none of which were considered related to mavacamten and none resulted in mavacamten dose modification (lumbar vertebral fracture and spinal compression fracture in 1 patient, and subdural hematoma, lumbar radiculopathy, acute cholecystitis, and cholangiocarcinoma in 1 patient each). In the overall safety population, no clinically meaningful changes from baseline were noted in safety clinical laboratory assessments, physical examinations, or vital signs.
Table 2.
Summary of TEAEs (Safety Population)
| Mavacamten (n=13) | ||
|---|---|---|
| Patients, n (%) | Events, n (%) | |
| TEAE | 13 (100.0) | 144 (100.0) |
| Mild | 2 (15.4) | 94 (65.3) |
| Moderate | 5 (38.5) | 40 (27.8) |
| Severe | 5 (38.5) | 9 (6.3)* , † |
| Life‐threatening | 1 (7.7)* | 1 (0.7) |
| Serious TEAE | 5 (38.5) | 6 (4.2)‡ |
| Study drug–related TEAE | 6 (46.2) | 11 (7.6)§ |
| Temporary treatment interruption attributable to a TEAE | 2 (15.4)∥ | 3 (2.1) |
| Treatment discontinuation attributable to a TEAE | 1 (7.7)* | 1 (0.7) |
| Study discontinuation attributable to a TEAE | 1 (7.7)* | 1 (0.7) |
TEAE indicates treatment‐emergent adverse event.
One male patient with a history of ulcerative colitis experienced 3 severe TEAEs: epigastric pain, biliary obstruction, and elevated aspartate aminotransferase; the patient was later diagnosed with a serious TEAE of cholangiocarcinoma. The patient discontinued treatment and the study.
Another patient experienced 2 severe TEAEs, a first lumbar vertebra extension fracture, and sixth and seventh thoracic vertebra burst fractures.
None of the 6 serious TEAEs (subdural hematoma, acute cholecystitis, cholangiocarcinoma, first lumbar vertebra extension fracture, sixth and seventh thoracic vertebra burst fractures, and lumbar radiculopathy) reported in 5 patients (38.5%) were considered to be related to mavacamten; none resulted in mavacamten dose modification, and all led to hospitalization.
Exertional dizziness (2 events), fatigue (2 events), alanine aminotransferase increased (1 event), atrial fibrillation (1 event), drug level increased (1 event), ejection fraction decreased (1 event), dizziness (1 event), lethargy (1 event), and dyspnea (1 event).
One patient experienced treatment interruption because of a subdural hematoma; treatment was resumed. The second patient experienced 1 treatment interruption because of increased drug levels (mavacamten plasma concentration ≥1000 ng/L; there was no change in the patient's condition, no illness, no new medications, and the patient had been strictly compliant in taking mavacamten) and another interruption because of decreased left ventricular ejection fraction (<50%); treatment was resumed after both interruptions.
Efficacy
Measures of mavacamten efficacy at baseline and week 180, and changes from baseline at weeks 48, 96, 144, and 180 are summarized in Table 3, with individual data at baseline, week 12, and week 16 for the PIONEER‐HCM study and at baseline for the PIONEER‐OLE study presented in Table S3. Overall, individual baseline values were similar between the 2 studies for the patients who participated in both studies.
Table 3.
Efficacy Measures at Baseline and Week 180, and Change from Baseline (Safety Population)
| Baseline | Week 180 | Change from baseline | ||||
|---|---|---|---|---|---|---|
| Parameter | (n=13) | (n=12) | At week 48 (n=12)* | At week 96 (n=12)* | At week 144 (n=12)* | At week 180 (n=12)* |
| NYHA functional class, n (%) | ||||||
| Improved by 1 class | … | 10 (76.9) | 8 (61.5) | 9 (69.2) | 11 (84.6) | 10 (76.9) |
| Improved by 2 classes | … | 0 | 1 (7.7) | 1 (7.7) | 0 | 0 |
| Remained the same | … | 2 (15.4) | 3 (23.1) | 2 (15.4) | 1 (7.7) | 2 (15.4) |
| KCCQ‐OSS† | 72 (19) | 88 (14) | 16 (18) | 16 (18) | 18 (16) | 17 (16) |
| NT‐proBNP, median (IQR), ng/L | 594 (351 to 1168) | 155 (76 to 317) | −472 (−2467 to −197) | −463 (−2441 to −195) | −417 (−2444 to −76) | −498 (−2184 to −76) |
| LVOT gradient, mm Hg | ||||||
| Resting | 67 (43) | 17 (19) | −53 (41) | −56 (47) | −55 (49) | −50 (55) |
| Valsalva | 90 (31) (n=12) | 19 (20) | −66 (30) (n=11) | −63 (41) (n=10) | −70 (43) (n=11) | −70 (41) (n=11) |
| Post‐exercise | 128 (33) | 24 (24) (week 156)‡ | −85 (43) (n=11) | −87 (53) (n=11) (week 72)‡ | −102 (39) (week 156)‡ | … |
| LVEF, resting, % | 72 (5) | 66 (9) (n=10) | −2 (6) | −6 (8) (n=11) | −8 (7) | −6 (9) (n=10) |
| LAVI, resting, mL/m2 | 41.8 (16.5) | 30.7 (6.4) | −10.1 (14.0) | −14.3 (16.4) | −9.8 (16.3) | −11.4 (15.9) |
| Maximum LV wall thickness, mm | 20.9 (2.1) | 18.0 (2.1) | −1.4 (2.2) | −0.7 (2.9) | −2.0 (2.1) | −2.8 (2.5) |
| LV posterior wall thickness, resting, mm | 11.7 (2.1) | 11.4 (1.1) | −0.5 (1.9) | −0.4 (1.8) | −0.5 (1.7) | −0.2 (2.2) |
| Interventricular septum thickness, resting, mm | 16.6 (2.9) | 15.2 (2.6) | −1.2 (2.3) | 0.5 (2.4) | 0.8 (2.1) | −1.4 (3.3) |
| Lateral E/e’, resting | 12.8 (2.9) | 9.4 (3.1) (n=11) | −3.4 (3.4) (n=11) | −2.5 (2.7) (n=10) | −3.9 (3.1) | −3.3 (2.9) (n=11) |
Data presented are mean (SD) unless otherwise stated. Echocardiographic data shown were read by the central cardiac laboratory. E/e’ indicates ratio between early mitral inflow velocity/mitral annular early diastolic velocity; IQR, interquartile range; KCCQ‐OSS, Kansas City Cardiomyopathy Questionnaire–Overall Summary Score; LAVI, left atrial volume index; LV, left ventricular; LVEF, LV ejection fraction; LVOT, LV outflow tract; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and NYHA, New York Heart Association.
One patient discontinued the study because of a diagnosis of cholangiocarcinoma (unrelated to study drug).
Scores range from 0 to 100, with higher scores reflecting better health status.
Stress echocardiography was performed per protocol only at weeks 48, 72, and 156 but not at weeks 96, 144, or 180.
A marked and sustained improvement in NYHA functional class was noted from week 8 onwards compared with baseline. At week 180, 10 of 12 patients (83.3%) had improved by 1 NYHA functional class (9 patients had improved from NYHA class II to NYHA class I, and 1 patient had improved from NYHA class III to NYHA class II; Figure 2). There was no change in NYHA class compared with baseline at week 180 for 2 of 12 patients (16.7%; NYHA class II at baseline for both patients); however, both patients fluctuated between NYHA class I and class II throughout the study. One of these patients received a stable dose of mavacamten from week 6 to week 180, whereas the other patient had a temporary dose interruption at week 156 followed by dose reduction from 15 to 10 mg 55 days later. From week 8 onwards, only 1 patient was classified as NYHA class III. This occurred during an unscheduled visit ≈3 weeks after temporary interruption of study drug related to the COVID‐19 pandemic. This patient subsequently returned to NYHA class I at the following visit, 18 weeks after resuming treatment. The KCCQ–Overall Summary Score increased from a mean (SD) baseline value of 72 (19) to 88 (14) at week 180 (Figure S1). Pronounced reductions in serum NT‐proBNP levels were also observed at all time points, with a median (interquartile range) value of 594 (351–1168) ng/L at baseline, decreasing to 155 (76–317) ng/L at week 180 (Figure 3; Figure S2).
Figure 2. New York Heart Association (NYHA) functional classification by visit.
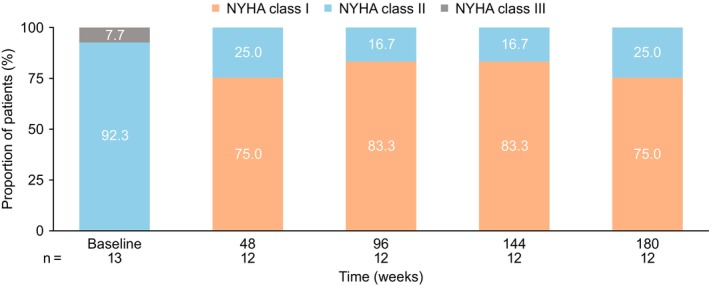
Proportion of patients at each visit categorized according to the NYHA functional classification system. Class I is defined as no limitation of physical activity: ordinary physical activity does not cause undue fatigue, palpitation, or dyspnea (shortness of breath). Class II is defined as slight limitation of physical activity, comfortable at rest: ordinary physical activity results in fatigue, palpitation, or dyspnea. Class III is defined as marked limitation of physical activity, comfortable at rest: less than ordinary activity causes fatigue, palpitation, or dyspnea. Class IV is defined as unable to perform any physical activity without discomfort, symptoms of heart failure at rest: if any physical activity is undertaken, discomfort increases. Note: baseline is defined as last nonmissing measurement before dosing.
Figure 3. Serum N‐terminal NT‐proBNP (pro‐B‐type natriuretic peptide) levels by visit.
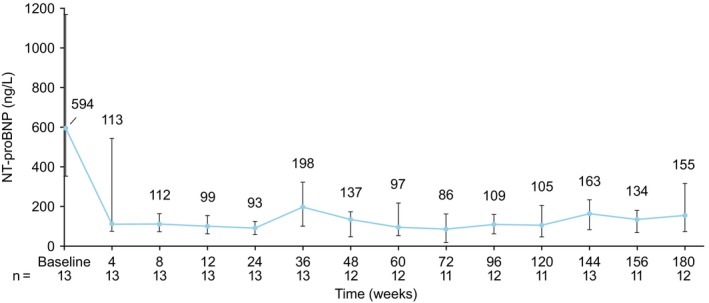
Median (interquartile range) serum NT‐proBNP levels by visit. Note: variability at week 36 was attributable to an elevated value in 1 patient who discontinued study treatment at week 26 because of a serious adverse event unrelated to the study drug (cholangiocarcinoma) and who contributed their end‐of‐study measure at this visit.
Echocardiographic measures are presented in Table 3, Figure 4, and Figure S3. At week 180, treatment with mavacamten resulted in sustained long‐term improvement in LVOT gradients (resting, Valsalva, and postexercise [week 156]) from baseline (Figure 4; Figure S3). Mean [SD] LVOT gradients were <30 mm Hg at last assessment (resting, 17 [19] mm Hg and Valsalva, 19 [20] mm Hg at week 180; postexercise, 24 [24] mm Hg at week 156). The proportion of patients with resting LVOT gradients of <30 mm Hg versus at least 30 mm Hg at week 180 was 75% (9/12 patients) and 25% (3/12 patients), respectively. The proportion of patients with Valsalva LVOT gradients of <50 mm Hg versus at least 50 mm Hg at week 180 was 91.7% (11/12 patients) and 8.3% (1/12 patients), respectively. The proportion of patients with post‐exercise LVOT gradients of <50 mm Hg versus at least 50 mm Hg at week 156 was 83.3% (10/12 patients) and 16.7% (2/12 patients), respectively. LVOT gradients above threshold values at week 180 (resting and Valsalva) and week 156 (postexercise) are provided in Table S4. Minimal reductions in LVEF occurred; however, values remained at least at 50% at week 180 (Figure 4; Figure S3). Improved metrics of LV filling were also observed, with reductions in left atrial volume index (mean [SD], 41.8 [16.5] mL/m2 at baseline; 30.7 [6.4] mL/m2 at week 180) (Figure 4; Figure S3) and resting lateral ratio between early mitral inflow velocity/mitral annular early diastolic velocity (mean [SD], 12.8 [2.9] at baseline; 9.4 [3.1] at week 180) (Figure S4). Reductions in maximum LV wall thickness were observed (mean [SD], 20.9 [2.1] mm at baseline; 18.0 [2.1] mm at week 180) (Figure 4; Figure S3) with reductions in interventricular septal wall thickness and stable posterior wall thickness (Table 3). Mitral regurgitation was present in 9 patients (75%) at week 180 compared with 13 patients (100%) at baseline, and no patients had systolic anterior motion at week 180 compared with 12 patients (92.3%) at baseline. No patient underwent septal reduction therapy during the study observation period.
Figure 4. Echocardiographic outcomes by visit.
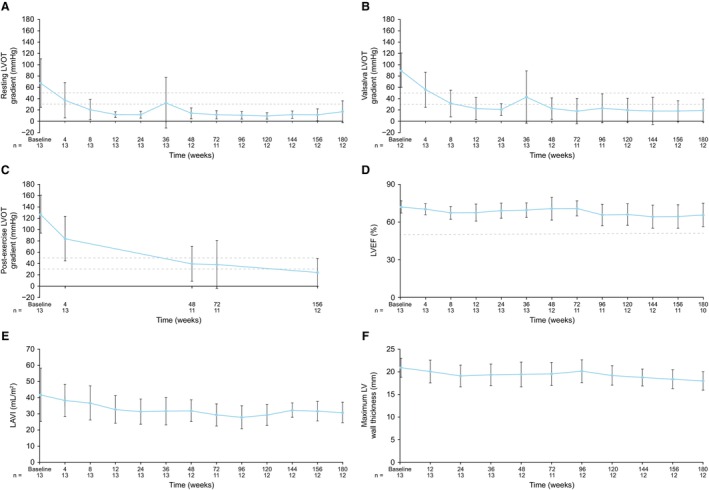
A, Resting left ventricular outflow tract (LVOT) gradient. B, Valsalva LVOT gradient. C, Postexercise LVOT gradient. D, Left ventricular ejection fraction (LVEF). E, Left atrial volume index (LAVI). F, Maximal left ventricular (LV) wall thickness. Graphs show mean (SD) values by visit. Dashed lines in A through C indicate LVOT gradients of 30 mm Hg (considered to be the threshold for obstruction) and 50 mm Hg (considered to be the threshold for septal reduction therapy).1 Dashed line in D indicates an LVEF of 50% (considered to be the threshold for LV systolic dysfunction). 1Note: baseline is defined as last nonmissing measurement before dosing. Variability in resting and Valsalva LVOT gradients at week 36 was attributable to elevated values in 2 patients: 1 patient who discontinued study treatment at week 26 because of a serious adverse event unrelated to the study drug (cholangiocarcinoma) and who contributed their end‐of‐study measure at this visit, and another patient who had an ongoing adverse event of common cold. For week 180, the latter patient also contributed to the upward trend in resting LVOT; they had a temporary treatment interruption at the time of week 180 visit, attributable to a serious adverse event of subdural hematoma.
Discussion
After completion of the phase 2 PIONEER‐HCM study, 21 13 patients were eligible and agreeable to receive long‐term treatment with mavacamten under the PIONEER‐OLE study protocol, the first study ever to investigate the long‐term safety and efficacy of a cardiac myosin inhibitor in symptomatic obstructive HCM. At the time of data cutoff for this interim analysis, 12 patients had received mavacamten for almost 4 years (median, 201 weeks), the longest duration of follow‐up on mavacamten reported to date. These results suggest that after the initiation and titration phases of mavacamten treatment, longer‐term efficacy, safety, and tolerability are achievable with currently available doses.
Mavacamten was generally well tolerated, and the safety profile was consistent with other studies in patients with obstructive HCM. 18 , 25 Most adverse events were mild in nature and considered unrelated to mavacamten. There were no serious adverse events related to mavacamten. Small reductions from baseline in LV systolic function were documented in the overall study population, consistent with the mechanism of action of mavacamten. An isolated asymptomatic LVEF reduction of <50% was documented in 1 patient, resulting in temporary discontinuation from mavacamten per protocol. LVEF recovered, and the patient was able to successfully resume mavacamten at a reduced dose.
Frequent echocardiographic assessments supported both a stable response and the safety of mavacamten. Rapid and sustained improvements were seen from baseline in LVOT gradients (rest, Valsalva, and postexercise) and metrics of diastolic function (reductions in left atrial volume index and lateral ratio between early mitral inflow velocity/mitral annular early diastolic velocity). These changes were accompanied by pronounced reductions in serum NT‐proBNP levels from week 4 onwards, indicating decreased wall stress. These observations are consistent with the findings of the EXPLORER‐HCM study at 30 weeks 18 , 26 and the EXPLORER long‐term extension cohort of the MAVA‐LTE (Long‐term Safety Extension Study of Mavacamten in Adults with Hypertrophic Cardiomyopathy Who Have Completed the MAVERICK‐HCM or EXPLORER‐HCM Trials) study at 84 weeks. 25 The current study shows the durability of mavacamten effects on the myocardium over a median of 201 weeks of treatment without any new safety signals. Echocardiographic frequency every 6 months beyond week 48 following initial dose titration demonstrated stable outcomes, supporting a less frequent monitoring schedule once a stable dose of mavacamten has been achieved. Future analysis in the MAVA‐LTE study is needed to confirm this observation.
Marked improvements in symptoms of obstructive HCM were also documented over the study observation period, according to both investigator‐assessed (NYHA functional class) and patient‐reported (KCCQ) measures. Most patients showed stable and sustained improvements of 1 NYHA class at week 180 compared with baseline. Over the same period, mean KCCQ–Overall Summary Score increased by 17 points from baseline, a change that sits between threshold scores denoting a moderate‐to‐large (10 points) and large‐to‐very large (20 points) clinical improvement. 23 , 24 This magnitude of change is similar to what has been observed in the EXPLORER‐HCM trial, but, given the open‐label nature of the PIONEER‐OLE study, we are unable to provide placebo‐corrected change in KCCQ scores.
These results should be interpreted in the context of some limitations, which include small sample size and lack of a placebo or control group. However, these data provide assurance that, over an almost 4‐year period, mavacamten continued to be safe and effective.
Conclusions
These longest‐term results after a median (interquartile range) time receiving mavacamten of 201 (193–209) weeks show continued safety, tolerability, and efficacy of mavacamten in patients with symptomatic obstructive HCM. After initial titration, dosing remained stable over time. Mavacamten was associated with sustained improvements from baseline in symptoms, quality of life, LVOT obstruction, cardiac biomarkers, and multiple measures of cardiac structure and function. Favorable changes in echocardiographic parameters were suggestive of favorable cardiac remodeling during treatment with mavacamten. No patients required septal reduction therapy during the study observation period. Patient follow‐up is continuing for years 4 and 5.
Sources of Funding
This study was funded by Bristol Myers Squibb.
Disclosures
Dr Masri has received research grants from Ionis/Akcea, Pfizer, Ultromics, and the Wheeler Foundation; and fees from Alnylam, Attralus, Bristol Myers Squibb, Cytokinetics, Eidos, Haya, Intellia, Ionis, Pfizer, and Tenaya. Dr Lester is on the Philips Advisory Board. Dr Hegde's institution has received fees for core laboratory services (or consulting services) from Bristol Myers Squibb. Drs Sehnert, Balaratnam, and Shah are employees of Bristol Myers Squibb and own stocks of Bristol Myers Squibb. Dr Wang has received consulting fees from Bristol Myers Squibb, Cytokinetics, and BioMarin; has been involved in speakers' bureaus for Bristol Myers Squibb; and declares institutional research grants from Bristol Myers Squibb and Cytokinetics. Shawna Fox is an employee of IQVIA, a partner providing statistics services to Bristol Myers Squibb. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
Figures S1–S4
Acknowledgments
The investigators and study team thank the patients who participated in the PIONEER‐HCM and PIONEER‐OLE studies and their families, as well as the individual site teams. All authors contributed to and approved the manuscript. Writing and editorial assistance was provided by Katherine Ward (MBiochem) of Oxford PharmaGenesis, Oxford, UK, funded by Bristol Myers Squibb.
This article was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030607
For Sources of Funding and Disclosures, see page 10.
References
- 1. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142:e558–e631. doi: 10.1161/CIR.0000000000000937 [DOI] [PubMed] [Google Scholar]
- 2. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:655–668. doi: 10.1056/NEJMra1710575 [DOI] [PubMed] [Google Scholar]
- 4. Spudich JA. Three perspectives on the molecular basis of hypercontractility caused by hypertrophic cardiomyopathy mutations. Pflugers Arch. 2019;471:701–717. doi: 10.1007/s00424-019-02259-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682 [DOI] [PubMed] [Google Scholar]
- 6. Jacoby DL, DePasquale EC, McKenna WJ. Hypertrophic cardiomyopathy: diagnosis, risk stratification and treatment. CMAJ. 2013;185:127–134. doi: 10.1503/cmaj.120138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawana M, Spudich JA, Ruppel KM. Hypertrophic cardiomyopathy: mutations to mechanisms to therapies. Front Physiol. 2022;13:975076. doi: 10.3389/fphys.2022.975076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trivedi DV, Adhikari AS, Sarkar SS, Ruppel KM, Spudich JA. Hypertrophic cardiomyopathy and the myosin mesa: viewing an old disease in a new light. Biophys Rev. 2018;10:27–48. doi: 10.1007/s12551-017-0274-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, Henze M, Kawas R, Oslob JD, Rodriguez HM, et al. A small‐molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. 2016;351:617–621. doi: 10.1126/science.aad3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawas RF, Anderson RL, Ingle SRB, Song Y, Sran AS, Rodriguez HM. A small‐molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J Biol Chem. 2017;292:16571–16577. doi: 10.1074/jbc.M117.776815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, Rogers CS, Gorham JM, Wong FL, Morck MM, et al. Deciphering the super relaxed state of human beta‐cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci USA. 2018;115:E8143–E8152. doi: 10.1073/pnas.1809540115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. CAMZYOS™ (mavacamten) . Canadian product monograph. Bristol‐Myers Squibb Canada; 2022. Accessed February 2, 2024. https://www.bms.com/assets/bms/ca/documents/productmonograph/CAMZYOS_EN_PM.pdf [Google Scholar]
- 13. CAMZYOS® (mavacamten) . Australian product information. Bristol‐Myers Squibb Australia Pty Ltd.; 2022. Accessed February 2, 2024. https://www.tga.gov.au/sites/default/files/2023‐07/auspar‐camzyos‐230703‐pi.pdf [Google Scholar]
- 14. CAMZYOS® (mavacanteno) . Brazilian bula professional. Bristol‐Myers Squibb Farmacêutica LTDA; 2023. Accessed February 2, 2024. https://www.bms.com/assets/bms/brazil/documents/Camzyos_Bula_Profissional.pdf [Google Scholar]
- 15. CAMZYOS® (mavacamtenum) . Professional information, Swissmedic. Bristol‐Myers Squibb SA; 2023. Accessed February 2, 2024. https://www.swissmedicinfo.ch/ShowText.aspx?textType=FI&lang=DE&authNr=68477 [Google Scholar]
- 16. CAMZYOS® (mavacamten) . Korean prescribing information. Bristol Myers Squibb; 2023. Accessed February 2, 2024. 의약품안전나라 〉 통합검색 〉 의약품등 정보검색 (mfds.go.kr) [Google Scholar]
- 17. CAMZYOS® (mavacamten) . In: Macao PI. Bristol Myers Squibb. 2023.
- 18. Olivotto I, Oreziak A, Barriales‐Villa R, Abraham TP, Masri A, Garcia‐Pavia P, Saberi S, Lakdawala NK, Wheeler MT, Owens A, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER‐HCM): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2020;396:759–769. doi: 10.1016/S0140-6736(20)31792-X [DOI] [PubMed] [Google Scholar]
- 19. CAMZYOS (mavacamten) . US prescribing information. Bristol‐Myers Squibb Company; 2022. Accessed February 27, 2024. https://packageinserts.bms.com/pi/pi_camzyos.pdf [Google Scholar]
- 20. Spertus JA, Fine JT, Elliott P, Ho CY, Olivotto I, Saberi S, Li W, Dolan C, Reaney M, Sehnert AJ, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER‐HCM): health status analysis of a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2021;397:2467–2475. doi: 10.1016/S0140-6736(21)00763-7 [DOI] [PubMed] [Google Scholar]
- 21. Heitner SB, Jacoby D, Lester SJ, Owens A, Wang A, Zhang D, Lambing J, Lee J, Semigran M, Sehnert AJ. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann Intern Med. 2019;170:741–748. doi: 10.7326/M18-3016 [DOI] [PubMed] [Google Scholar]
- 22. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 23. Nassif M, Fine JT, Dolan C, Reaney M, Addepalli P, Allen VD, Sehnert AJ, Gosch K, Spertus JA. Validation of the Kansas City Cardiomyopathy Questionnaire in symptomatic obstructive hypertrophic cardiomyopathy. JACC Heart Fail. 2022;10:531–539. doi: 10.1016/j.jchf.2022.03.002 [DOI] [PubMed] [Google Scholar]
- 24. Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;76:2379–2390. doi: 10.1016/j.jacc.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 25. Rader F, Oręziak A, Choudhury L, Saberi S, Fermin D, Wheeler MT, Abraham TP, Garcia‐Pavia P, Zwas DR, Masri A, et al. Mavacamten treatment for symptomatic obstructive hypertrophic cardiomyopathy: interim results from the MAVA‐LTE study. EXPLORER‐LTE cohort. JACC Heart Fail. 2024;12:164–177. doi: 10.1016/j.jchf.2023.09.028 [DOI] [PubMed] [Google Scholar]
- 26. Hegde SM, Lester SJ, Solomon SD, Michels M, Elliott PM, Nagueh SF, Choudhury L, Zemanek D, Zwas DR, Jacoby D, et al. Effect of mavacamten on echocardiographic features in symptomatic patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2021;78:2518–2532. doi: 10.1016/j.jacc.2021.09.1381 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S4


