Abstract
Background
Concomitant atrial fibrillation and end‐stage renal disease is common and associated with an unfavorable prognosis. Although oral anticoagulants have been well established to prevent thromboembolism, the applicability in patients under long‐term dialysis remains debatable. The study aimed to determine the efficacy and safety of anticoagulation in the dialysis‐dependent population.
Methods and Results
An updated network meta‐analysis based on MEDLINE, EMBASE, and the Cochrane Library was performed. Studies published up to December 2022 were included. Direct oral anticoagulants (DOACs, dabigatran, rivaroxaban, apixaban 2.5/5 mg twice daily), vitamin K antagonists (VKAs), and no anticoagulation were compared on safety and efficacy outcomes. The outcomes of interest were major bleeding, thromboembolism, and all‐cause death. A total of 42 studies, including 3 randomized controlled trials, with 185 864 subjects were pooled. VKAs were associated with a significantly higher risk of major bleeding than either no anticoagulation (hazard ratio [HR], 1.47; 95% CI, 1.34–1.61) or DOACs (DOACs versus VKAs; HR, 0.74 [95% CI, 0.64–0.84]). For the prevention of thromboembolism, the efficacies of VKAs, DOACs, and no anticoagulation were equivalent. Nevertheless, dabigatran and rivaroxaban were associated with fewer embolic events. There were no differences in all‐cause death with the administration of VKAs, DOACs, or no anticoagulation.
Conclusions
For dialysis‐dependent populations, dabigatran and rivaroxaban were associated with better efficacy, while dabigatran and apixaban demonstrated better safety. No anticoagulation was a noninferior alterative, and VKAs were associated with the worst outcomes.
Keywords: anticoagulation, atrial fibrillation, chronic kidney disease, dialysis, meta‐analysis
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- ARISTOTLE
Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation
- AXADIA‐AFNET 8
Compare Apixaban and Vitamin K Antagonists in Patients With Atrial Fibrillation and End‐Stage Kidney Disease
- DOAC
direct oral anticoagulant
- RENAL‐AF
Renal Hemodialysis Patients Allocated Apixaban Versus Warfarin in Atrial Fibrillation
- VKA
vitamin K antagonist
Clinical Perspective.
What Is New?
Dabigatran and rivaroxaban exhibited superior efficacy in preventing thromboembolism, while dabigatran and apixaban were associated with lower risk of bleeding.
Opting for no anticoagulation also emerged as a valid noninferior choice.
The use of vitamin K antagonists was consistently linked to the most unfavorable outcomes.
What Are the Clinical Implications?
The strategy of anticoagulation should be individualized for patients undergoing dialysis.
Future randomized trials are warranted to investigate the efficacy and safety of anticoagulation in the setting of advanced renal insufficiency.
Atrial fibrillation (AF) is a common comorbidity in patients with end‐stage renal disease, with a reported prevalence of around 10%. 1 Shared risk factors for AF and chronic kidney disease (CKD) constitute a vicious cycle, and both can lead to a prothrombotic state. 2 Nevertheless, patients with coexisting AF and end‐stage renal disease are associated with a remarkably elevated risk of major bleeding. Although anticoagulation has been well established to improve the prognosis of individuals with AF, randomized controlled trials have predominantly excluded those under maintenance dialysis. Observational studies have also reported contradictory results regarding the administration of vitamin K antagonists (VKAs), direct oral anticoagulants (DOACs), and no anticoagulation. Current evidence is thus inadequate to solidly endorse any anticoagulants or whether to administer anticoagulants in this population.
VKAs have traditionally been used to prevent systemic embolism in patients with AF, but few studies have focused on patients undergoing dialysis. Such patients have an elevated risk of thromboembolism; however, a meta‐analysis found that VKAs were associated with an insignificant protective effect on embolism, potentially due to limited longevity in patients with AF and CKD. 3 In addition, VKAs also contribute to an increased risk of bleeding. Unstable renal function, drug–drug interactions, and dietary factors all affect the prothrombin time when patients are undergoing dialysis. Evidence supporting DOACs in patients on renal replacement therapy is also scarce. Only apixaban has been approved for such patients in limited regions on the basis of a small‐sized pharmacokinetic study involving 8 patients. 4 Furthermore, it is challenging to estimate the bleeding risk in patients undergoing dialysis. An observational study suggested that the HAS‐BLED score was useful only to predict bleeding rate at the catheter puncture site, and clinical parameters of the score, except for renal function impairment, failed to stratify the risk of systemic embolism. 5
The aim of this network meta‐analysis was therefore to elucidate the safety and efficacy of anticoagulants in patients with AF and end‐stage renal disease undergoing dialysis. Different types of DOACs and 2 dosages of apixaban were compared with VKAs as well as no anticoagulation.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The protocol of this study has been registered in the International Prospective Register of Systematic Reviews (No. CRD42022380239). The analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta‐Analyses guidelines. Requirements for institutional review board approval were waived because the data set used in the present study had been previously published.
Data Sources, Search Strategies, and Outcomes
The network meta‐analysis was based on the following inclusion criteria: (1) study group including individuals with both AF and end‐stage renal disease undergoing dialysis; and (2) reported data of all‐cause death or thromboembolism or major bleeding. Ischemic and hemorrhagic stroke were applied as thromboembolic and major bleeding events, respectively, if the information was otherwise unavailable in the study. MEDLINE, EMBASE, and the Cochrane Library were searched from inceptions to December 2022. The search keywords were “oral anticoagulant”, “warfarin”, “vitamin K antagonist”, “factor Xa inhibitor”, “rivaroxaban”, “edoxaban”, “apixaban”, “dabigatran”, “dialysis”, and “atrial fibrillation” as well as related keywords (Table S1). Outcomes of interest in the study were set as all‐cause death, thromboembolism, and major bleeding events. No language restriction was imposed. The target population was set as adult only. Only published manuscripts were considered, whereas gray literature such as conference abstracts were not included. Two investigators (T.W.K. and Z.W.C.) independently reviewed the titles and abstracts to determine eligibility of the manuscripts for inclusion. Confidence in Network Meta‐Analysis was used to assess the quality of the included studies. Disputes on the selected studies at any stage were resolved by consensus from the author panel.
Data Extraction
Demographic and clinical parameters of the included studies were summarized (if available), including age, sex, CHA₂DS₂‐VASc score, HAS‐BLED score, prior stroke, prior bleeding, study type (ie, cohort and randomized trial), dialysis modality, DOAC type and dosing, concurrent use of antiplatelets, follow‐up duration, and study period. In terms of effect sizes, adjusted risk estimates in conjunction with the corresponding 95% CI were extracted, including hazard ratio (HR), subdistribution HR, odds ratio, and the like. When several models with different numbers of covariate adjustments were performed, the most adjusted risk estimates in the multivariable model (eg, logistic regression Fine and Gray model, and Cox proportional hazard model) were chosen. The numbers of patients and outcomes were extracted when the risk estimates were not available in the study. The relative risk was calculated on the basis of data from the tables in the studies with unavailable adjusted risk estimates.
Categorization of Anticoagulants
First, the efficacy and safety of DOACs, VKAs, and no anticoagulation were compared. Second, DOACs were subgrouped into rivaroxaban, dabigatran, and apixaban to investigate their respective effect. Third, apixaban was stratified on the basis of 2 different dosages (2.5 and 5 mg twice daily).
Statistical Analysis
This frequentist‐approach network meta‐analysis was performed with R version 4.3.0 (R Project for Statistical Computing, Vienna, Austria) and the package “netmeta” version 2.8‐2. In studies featuring multiarm groups (ie, 3 groups), each contributed 3 pairwise comparisons. However, only 2 of these pairwise comparisons (eg, rivaroxaban versus VKA and apixaban versus VKA) were presented, leaving the estimate of the third pairwise comparison (eg, rivaroxaban versus apixaban) dependent on the other 2. To address this, and appropriately adjust for correlations in risk estimates arising from studies with multiple arms, the correct standard errors were approximated by assuming they were proportional to the reciprocal of the square root of the patient number (1/sqrt(n)). 6 Network plots were generated to depict network geometry, and the width of connecting bars indicated the abundance of studies compared. Concerning the potential substantial heterogeneity among the included studies, a random‐effects model was applied. The variance of the combined effect sizes was characterized using the τ statistic, which signifies the SD of the true effect sizes. A 2‐sided P value <0.05 was considered to be statistically significant.
Quality of Evidence and Risk of Bias
Confidence in Network Meta‐Analysis was used to assess the quality of evidence for outcomes including the following 6 domains: within‐study bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence. 7 Revised Cochrane Risk of Bias tool and Risk of Bias in Nonrandomized Studies of Interventions were used to evaluate the risk of bias for randomized controlled trials and cohort studies, respectively. Full‐text screening for eligibility assessment and the risk‐of‐bias assessment were conducted independently.
Results
Search Results
The initial search identified a total of 1302 studies, of which 816 were removed due to duplication. Among the preliminarily screened articles, 437 were excluded due to irrelevant titles or abstracts. Full texts of the remaining manuscripts were reviewed, and 7 articles were removed due to contradicting inclusion criteria. Eventually, 42 articles were selected for network meta‐analysis (Figure 1). 5 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 Of them, 8 studies consisted of 3 treatment groups. 17 , 30 , 32 , 33 , 38 , 40 , 43 , 47 Risk of bias was evaluated (Table S2).
Figure 1. Literature search.
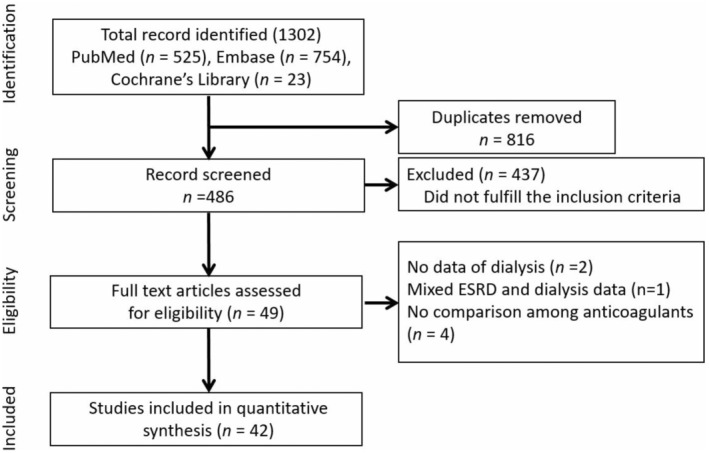
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis flow diagram illustrates the process for study inclusion. ESRD indicates end‐stage renal disease.
Study and Cohort Characteristics
A total of 185 864 subjects were analyzed from the 42 included studies. After excluding 1728 participants from 1 study reported with ratio only, 184 136 individuals from 41 studies were specified with coagulation strategies. Among these patients, 8861 (4.81%) patients received DOACs, 70 047 (38.04%) patients received VKAs, and 105 228 (57.15%) patients were not anticoagulated. Apixaban, rivaroxaban, and dabigatran were used as the DOACs in 8, 2, and 1 study, respectively, whereas 1 study did not specify the type of DOAC. Three studies were randomized controlled trials, 39 , 44 , 45 and the others were observational studies. The modality of renal replacement therapy was predominantly hemodialysis. Concomitant antiplatelet therapy was common. Detailed demographic and clinical parameters are summarized in Table 1.
Table 1.
Demographic and Clinical Characteristics of Included Studies (Original Data Before Matching)
| Comparators | Patient number | Age | Male, % | Dialysis modality (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VKA | DOAC | None | VKA | DOAC | None | VKA | DOAC | None | |||
| Chan, 2009 8 | VKA/none | 508 | 480 | 72.6±0.4 | 71.3±0.6 | 58 | 54 | HD | |||
| Lai, 2009 9 | VKA/none | 51 | 42 | HD | |||||||
| Winkelmayer, 2011 10 | VKA/none | 249 | 2064 | 68.6±12.1 | 70.1±11.9 | 42.60% | 42.50% | HD | |||
| Olesen, 2012 11 | VKA/none | 223 | 678 | HD (77.9), PD (15.4) | |||||||
| Sood, 2013 12 | VKA/none | 2513 | 23 177 | 65.3±13.9 | 59.7±15.5 | 55 | 57 | HD | |||
| Bonde, 2014 13 | VKA/none | HD (72), PD (25.1) | |||||||||
| Chen, 2014 14 | VKA/none | 294 | 2983 | ||||||||
| Shah, 2014 15 | VKA/none | 756 | 870 | 75.3±8.1 | 75.41±8.5 | 61 | 61 | HD + PD | |||
| Wakasugi, 2014 16 | VKA/none | 28 | 32 | 67.8±9.4 | 68.4±8.5 | 57 | 72 | HD | |||
| Chan, 2015 17 |
VKA/D VKA/R |
8064 |
281 244 |
70.6±11 | 68.4±12/66.9±12 | 61.20 | 59.2/60.5 | HD | |||
| Genovesi, 2015 18 | VKA/none | 1838 | 10 446 | x | x | 64.20 | 56.40 | HD | |||
| Shen, 2015 19 | VKA/none | 661 | 2438 | 61.2±12.4 | 62.1±13.6 | 49.70 | 48.70 | HD | |||
| Brancaccio, 2016 20 | VKA/none | 119 | 183 | 71.42±9.67 | 72.58±10.83 | 60 | 57 | HD | |||
| Garg, 2016 21 | VKA/none | 365 | 692 | 75±7 | 78±7 | 55.40 | 51.30 | HD | |||
| Hayashi, 2016 22 | VKA/none | 27 | 55 | 68.8±10.6 | 66.9±11.0 | 69.90 | 62.90 | HD | |||
| Mitsuma, 2016 23 | VKA/none | 69.4±8.3 | 71.9±10.1 | 74 | 71 | HD | |||||
| Toida, 2016 24 | VKA/none | 59 | 82 | HD | |||||||
| Wang, 2016 5 | VKA/none | 30 | 54 | 59.8±10.5 | 62.1±11.8 | 77 | 69 | HD (68.8), PD (31.2) | |||
| Yodogawa, 2016 25 | VKA/none | 134 | 156 | 69.5±10.7 | 70.4±10.2 | 80 | 65 | HD | |||
| Genovesi, 2017 26 | VKA/none | 989 | 3297 | 76 | 76 | 64.20 | 56.40 | HD | |||
| Kai, 2017 27 | VKA/none | 590 | 5150 | 69.2±11.2 | 67.3±13.2 | 63.20 | HD | ||||
| Lee, 2017 28 | VKA/none | 27 | 94 | 68.8±10.6 | 71.2±10.6 | 42.20 | 45.30 | HD | |||
| Lin, 2017 29 | VKA/none | 1838 | 10 446 | NA | |||||||
| Sarratt, 2017 30 |
A 2.5/VKA A 5/VKA |
120 | 40 | 66.5 | 70.9 | 48.30 | 50 | HD | |||
| Yoon, 2017 31 | VKA/none | 2921 | 7053 | 67.8±11 | 66.1±12.6 | 59.90 | 57.50 | HD | |||
| Reed, 2018 32 |
A 2.5/VKA A 5/VKA |
50 | 74 | 62±14.4 | 59.5±14.7 | 62 | 51.40 |
HD (99.2), PD (0.8) |
|||
| Siontis, 2018 33 |
A 2.5/VKA A 5/VKA |
23 172 | 2351 | 68.9±11.5 | 68.2±11.9 | 54.30 | 54.40 | HD (94.6), PD (5.4) | |||
| Voskamp, 2018 34 | VKA/none | 244 | 1474 | 67.6 | 61.3 | 59.80 | 61.60 | HD (64.4), PD (35.6) | |||
| Phan, 2019 35 | VKA/none | 115 | 361 | 67.3±10.8 | 62.9±13.3 | 58.30 | 57.60 | PD | |||
| Tan, 2019 36 | VKA/none | 1651 | 4114 | 73.9 | 75.1 | 43.60 | 43 | HD (96.7), PD (3.3) | |||
| Agarwal, 2020 37 | VKA/none | 6682 | 16 089 | 71.4±8.7 | 74.3±8.7 | 96.10 | 95.20 | NA | |||
| Mavrakanas, 2020 38 |
A 5/none A 2.5/none |
521 | 10 976 | 68±11 | 67±13 | 54 | 54 | HD (89.4), PD (10.6) | |||
| De Vriese, 2021 39 | R/VKA | 44 | 46 | 80.3 | 79.9 | 56.80 | 76.10 | HD | |||
| Ionescu, 2021 40 |
A 2.5/VKA A 5/VKA |
563 | 144 | 67.2±13.5 | 68.8±16 | 59.10 | 56.30 | HD | |||
| See, 2021 41 | DOACs/VKA | 448 | 448 | 75.2±10.9 | 74.3±10.9 | 47.50% | 49.60 | ||||
| Kang, 2021 42 | VKA/none | 177 | 547 | ||||||||
| Kim, 2021 43 |
VKA/none A 2.5/none |
27 | 62 | ||||||||
| Pokorney, 2022 44 | A/VKA | 72 | 82 | 68 | 69 | 69.40 | 58.50 | HD | |||
| Reinecke, 2022 45 | A 2.5/VKA | 48 | 49 | 74.8±7.9 | 74.7±8.1 | 75.50 | 64.60 | HD | |||
| Sy, 2022 46 | VKA/none | 3458 | 20 670 | 75±9 | 78±9 | 96 | 95 | HD | |||
| Wetmore, 2022 47 |
A 2.5/VKA A 5/VKA |
12 517 | 4639 | NA | 62.5 | 61.40 | HD | ||||
| Akbar, 2023 48 | VKA/none | 44 | 44 | 51.2±14.08 | 53.2±11.83 | 25 | 11.83 | 27.27 | HD (92.3), PD (7.7) | ||
| Prior bleeding, % | Prior stroke, % | CHA₂DS₂‐VASc, % | Concomitant antiplatelet therapy, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VKA | DOAC | None | VKA | DOAC | None | VKA | DOAC | None | VKA | DOAC | None | |
| Chan, 2009 8 | 1 | |||||||||||
| Lai, 2009 9 | ||||||||||||
| Winkelmayer, 2011 10 | 6.8 | 16.9 | 23.6 | |||||||||
| Olesen, 2012 11 | ||||||||||||
| Sood, 2013 12 | 6.7 | 6.5 | 11.8 | 23 | 0 | |||||||
| Bonde, 2014 13 | ||||||||||||
| Chen, 2014 14 | ||||||||||||
| Shah, 2014 15 | 9 | 16 | 5 | 26 | 35 | |||||||
| Wakasugi, 2014 16 | 26 | 61 | 47 | |||||||||
| Chan, 2015 17 | 5.3 |
6.9 8.5 |
11.2 14.6 |
5.6 3.4 |
3.1 | |||||||
| Genovesi, 2015 18 | 11.9 | 26.3 | 14.1 | 20.2 | 71.8 | |||||||
| Shen, 2015 19 | 2.2 | 6 | 26.8 | 21.5 | 23 | |||||||
| Brancaccio, 2016 20 | 16 | 27 | 32 | |||||||||
| Garg, 2016 21 | 23 | 91.5 | 86.4 | |||||||||
| Hayashi, 2016 22 | 49 | 41.3 | ||||||||||
| Mitsuma, 2016 23 | 33 | 29 | 22 | 48 | 31 | |||||||
| Toida, 2016 24 | ||||||||||||
| Wang, 2016 5 | 10 | 17 | 15.9 | 3.9±1.7 | 3.7±1.6 | 3.40 | 2.4 | |||||
| Yodogawa, 2016 25 | 3 | 7 | 2 | 40 | 54 | |||||||
| Genovesi, 2017 26 | 11.9 | 26.3 | 14.1 | 23.90 | 68.6 | |||||||
| Kai, 2017 27 | 30.1 | 32.8 | 25.2 | 5.2±1.7 | 5±1.8 | 39.60 | 45.2 | |||||
| Lee, 2017 28 | 5.4 | 7.6 | 5.8 | 89.70 | 78 | |||||||
| Lin, 2017 29 | ||||||||||||
| Sarratt, 2017 30 | 13.3 | 15 | 15 | 5 | 5 | 44.20 | 37.5 | |||||
| Yoon, 2017 31 | 70.20 | 86.6 | ||||||||||
| Reed, 2018 32 | 5.40 | 4±1.4 | 4.1±1.2 | 36 | 28.4 | |||||||
| Siontis, 2018 33 | 9.2 | 9.9 | 33.20 | 5.27±1.77 | 5.24±1.79 | 6.60 | 7.3 | |||||
| Voskamp, 2018 34 | 9.80 | 25.7 | ||||||||||
| Phan, 2019 35 | 23.50 | 24.4 | 21.1 | 4.6±1.6 | 4.2±1.8 | 40 | 50.7 | |||||
| Tan, 2019 36 | 22.5 | 32.5 | 22.6 | 25.90 | 25.1 | |||||||
| Agarwal, 2020 37 | 23.9 | 38.4 | 43.7 | 5.1±2.1 | 6±2 | 11.40 | 5.6 | |||||
| Mavrakanas, 2020 38 | 49 | 58 | 34 | 4 | 23 | 25 | ||||||
| De Vriese, 2021 39 | 27.3 | 19.6 | 32.60 | 4.8±1.5 | 4.7±1.4 | 31.8 | 32.6 | |||||
| Ionescu, 2021 40 | 23.8 | 20.8 | 27.10 | 50.8 | 5 | |||||||
| See, 2021 41 | 3.4 | 3.6 | 19.60 | 4.7±1.9 | 4.5±1.9 | |||||||
| Kang, 2021 42 | ||||||||||||
| Kim, 2021 43 | ||||||||||||
| Pokorney, 2022 44 | 2.8 | 9.8 | 20.70 | 4 | 4 | NA | 47.1 | 39.3 | ||||
| Reinecke, 2022 45 | 4.54±1.49 | 4.5±1.62 | 34.7 | 33.3 | ||||||||
| Sy, 2022 46 | 37 | 38 | 66 | 6 | 7 | |||||||
| Wetmore, 2022 47 | 19.40 | 4.5±1.7 | 4.3±1.7 | 16.8 | 17.1 | |||||||
| Akbar, 2023 48 | 1.14 | 23.86 | 6.81 | |||||||||
A indicates apixaban; D, dabigatran; DOAC, direct oral anticoagulant; HD, hemodialysis; NA, not applicable; PD, peritoneal dialysis; R, rivaroxaban; and VKA, vitamin K antagonist.
VKAs, DOACs, and No Anticoagulation
The outcomes were assessed among patients who received VKAs, DOACs, or no anticoagulation (Table 2; Figure 2). Compared with no anticoagulation, the use of VKAs (pooled relative risk, 1.47 [95% CI, 1.34–1.61]) but not DOACs (HR, 1.08 [95% CI, 0.92–1.27]) was associated with a remarkably elevated rate of major bleeding. In addition, the use of DOACs was associated with a significantly lower risk of bleeding compared with VKAs (HR, 0.74 [95% CI, 0.64–0.84]). There were no pronounced differences in embolism between VKAs (HR, 1.03 [95% CI, 0.89–1.19]) or DOACs (HR, 0.92 [95% CI, 0.67–1.28]) and no anticoagulation. VKAs (HR, 1.03 [95% CI, 0.91–1.17]), DOACs (HR, 0.96 [95% CI, 0.71–1.29]), and no anticoagulation demonstrated equivalent rates of all‐cause death. The values of τ statistics were 0.112, 0.275, and 0.266 for bleeding, embolism, and all‐cause death, respectively (data not shown in Figure 2).
Table 2.
Outcomes of the Included Studies
| Comparators | Major bleeding | Thromboembolism | All‐cause death | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VKA | DOAC | None | RR (95% CI) | VKA | DOAC | None | RR (95% CI) | VKA | DOAC | None | RR (95% CI) | ||
| Chan, 2009 8 | VKA/none | NA | 1.74 (1.11–2.72) | 333 | 425 | 1.1 (0.93–1.30) | |||||||
| Lai, 2009 9 | VKA/none | NA | 5 | 16 | NA | ||||||||
| Winkelmayer, 2011 10 | VKA/none | 11 | 21 | 2.38 (1.15–4.96) | 135 | 29 | 0.92 (0.61–1.37) | 181 | 750 | 1.06 (0.90–1.24) | |||
| Olesen, 2012 11 | VKA/none | 1.27 (0.91–1.77) | 0.44 (0.26–0.74) | NA | |||||||||
| Sood, 2013 12 | VKA/none | 1.30 (1.11–1.52) | 1.1 (0.9–1.4) | 1.16 (1.07–1.26) | |||||||||
| Bonde, 2014 13 | VKA/none | NA | 2.01 (1.74–2.33) | 0.85 (0.72–0.99) | |||||||||
| Chen, 2014 14 | VKA/none | NA | 1.017 (0.673–1.537) | NA | |||||||||
| Shah, 2014 15 | VKA/none | 149 | 126 | 1.41 (1.09–1.81) | 52 | 55 | 1.17 (0.79–1.75) | NA | |||||
| Wakasugi, 2014 16 | VKA/none | 3 | 4 | 0.85 (0.19–3.64) | 8 | 5 | 1.94 (0.63–5.93) | 9 | 9 | 1.00 (0.40–2.52) | |||
| Chan, 2015 17 |
VKA/D VKA/R |
1.48 (1.21–1.81) 1.38 (1.03–1.83) |
1.71 (0.97–2.99) 1.80 (0.89–3.64) |
NA NA |
|||||||||
| Genovesi, 2015 18 | VKA/none | 3.96 (1.15–13.68) | 0.12 (0.00–3.59) | NA | |||||||||
| Shen, 2015 19 | VKA/none | 29 | 192 | 0.82 (0.37–1.81) | 63 | 503 | 0.68 (0.47–0.99) | 832 | 4595 | 1.01 (0.92–1.11) | |||
| Brancaccio, 2016 20 | VKA/none | NA | NA | 661 | 2438 | 0.76 | |||||||
| Garg, 2016 21 | VKA/none | 26 | 26 | 1.53 (0.94–2.51) | 13 | 21 | 0.93 (0.48–1.82) | 97 | 145 | 1.03 (0.91–1.15) | |||
| Hayashi, 2016 22 | VKA/none | 60 | 54 | 2.53 (0.66–3.90) | 23 | 43 | 0.64 (0.32–1.28) | 86 | 120 | 1.08 (0.75–1.57) | |||
| Mitsuma, 2016 23 | VKA/none | 7 | 9 | 3 | 5 | 8 | 27 | ||||||
| Toida, 2016 24 | VKA/none | 3 | 6 | 5 | 13 | NA | |||||||
| Wang, 2016 5 | VKA/none | 22 | 24 | 8 | 11 | 44 | 64 | ||||||
| Yodogawa, 2016 25 | VKA/none | 3 | 1 | 2 | 5 | 1.07 (0.20–5.74) | 6 | 15 | |||||
| Genovesi, 2017 26 | VKA/none | 1.16 (0.48–2.82) | 0.44 (0.16–1.20) | 0.91 (0.56–1.48) | |||||||||
| Kai, 2017 27 | VKA/none | 20 | 12 | 0.8 (0.2–2.9) | 58 | 88 | 0.67 (0.45–0.99) | 433 | 496 | 0.78 (0.67–0.89) | |||
| Lee, 2017 28 | VKA/none | 8 | 37 | 0.84 (0.32–2.19) | 48 | 151 | 0.92 (0.57–1.48) | 350 | 1051 | 1.04 (0.88–1.23) | |||
| Lin, 2017 29 | VKA/none | 3.69 (0.93–14.67) | NA | 6.62 (2.56–17.16) | |||||||||
| Sarratt, 2017 30 |
A 2.5/VKA A 5/VKA |
7 7 |
0 0 |
NA NA |
NA NA |
||||||||
| Yoon, 2017 31 | VKA/none | 84 | 50 | 1.56 (1.10–2.22) | 204 | 201 | 0.95 (0.78–1.15) | NA | |||||
| Reed, 2018 32 |
A 2.5/VKA A 5/VKA |
11 11 |
0 4 |
0.15 (0.05–0.46) |
NA NA |
NA NA |
|||||||
| Siontis, 2018 33 |
A 2.5/VKA A 5/VKA |
1034 1317 |
54 75 |
0.71 (0.53–0.95) 0.71 (0.56–0.91) |
160 213 |
26 55 |
0.64 (0.42–0.97) 1.11 (0.82–1.50) |
310 421 |
48 111 |
0.63 (0.46–0.85) 1.07 (0.87–1.33) |
|||
| Voskamp, 2018 34 | VKA/none | NA | NA | 141 | 540 | 1.2 (1.0–1.5) | |||||||
| Phan, 2019 35 | VKA/none | 2 | 3 | 2.00 (0.32–12.8) | 10 | 11 | 2.3 (0.9–5.4) | 32 | 98 | 0.80 (0.53–1.20) | |||
| Tan, 2019 36 | VKA/none | 407 | 1559 | 1.63 (1.45–1.83) | 93 | 644 | 0.93 (0.74–1.16) | 476 | 3349 | 0.72 (0.65–0.80) | |||
| Agarwal, 2020 37 | VKA/none | 1.34 (1.25–1.43) | 1.23 (1.15–1.32) | 0.93 (0.89–0.98) | |||||||||
| Mavrakanas, 2020 38 |
A 5/none A 2.5/none |
4.61 (1.91–11.15) 2.02 (0.58–7.04) |
2.24 (1.03–4.86) 1.11 (0.43–2.85) |
NA NA |
|||||||||
| De Vriese, 2021 39 | R/VKA | 24 | 43 | 0 | 0 | 32 | 57 | ||||||
| Ionescu, 2021 40 |
A 2.5/VKA A 5/VKA |
9 9 |
0 0 |
37 37 |
7 0 |
NA NA |
|||||||
| See, 2021 41 | DOACs/VKA | 0.98 (0.64–1.51) | 1.21 (0.76–1.92) | NA | |||||||||
| Kang, 2021 42 | VKA/none | 1.59 (1.08–2.36) | 0.986 (0.716–1.356) | 1.151 (0.908–1.459) | |||||||||
| Kim, 2021 43 |
VKA/none A 2.5/none |
4.85 (1.12–21.1) 5.35 (0.73–39.35) |
NA 1.76 (0.17–18.03) |
0.26 (0.09–0.81) 0.71 (0.20–2.57) |
|||||||||
| Pokorney, 2022 44 | A/VKA | 7 | 9 | 2 | 1 | 13 | 21 | ||||||
| Reinecke, 2022 45 | A 2.5/VKA | 6 | 5 | 1 | 0 | 12 | 9 | ||||||
| Sy, 2022 46 | VKA/none | 1.38 (1.25–1.52) | 1.44 (1.23–1.69) | NA | |||||||||
| Wetmore, 2022 47 |
A 2.5/VKA A 5/VKA |
1226 1226 |
117 127 |
0.68 (0.55–0.84) 0.67 (0.55–0.81) |
421 421 |
54 52 |
0.85 (0.62–1.17) 0.89 (0.65–1.21) |
741 716 |
6096 6096 |
0.97 (0.98–1.05) 0.85 (0.78–0.92) |
|||
| Akbar, 2023 48 | VKA/none | 3 | 3 | 0.564 (0.034–9.386) | 30 | 16 | 0.435 (0.103–1.846) | 36 | 40 | 0.782 (0.494–1.237) | |||
A indicates apixaban; D, dabigatran; DOAC, direct oral anticoagulant; NA, not applicable; R, rivaroxaban; RR, relative risk; and VKA, vitamin K antagonist.
Figure 2. Summaries of the network meta‐analysis for the efficacy and safety assessment among patients receiving no anticoagulants, VKAs, and DOACs on the risk of (A) major bleeding, (B) thromboembolism, and (C) all‐cause death.
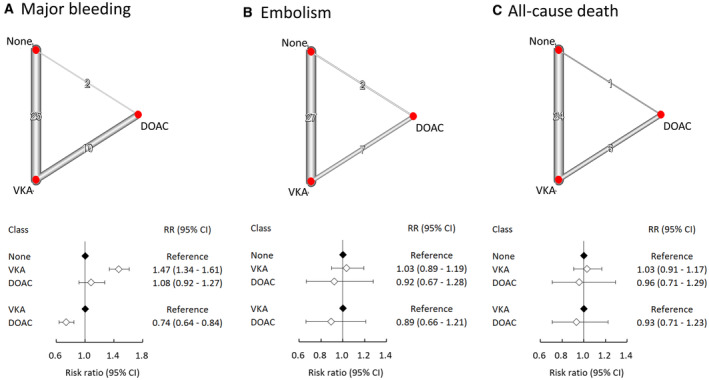
Left: network diagram; middle: forest plot. DOAC indicates direct oral anticoagulant; RR, relative risk; and VKA, vitamin K antagonist.
Different DOACs
DOACs were stratified according to the different types for further comparisons (Figure 3). Compared with VKAs, the use of dabigatran (HR, 0.70 [95% CI, 0.53–0.93]). apixaban (HR, 0.72 [95% CI, 0.61–0.86]) and rivaroxaban (HR, 0.80 [95% CI, 0.61–1.04]) were associated with a lower risk of bleeding, though statistical significance was achieved only with the former 2. In addition, dabigatran and rivaroxaban were associated with a lower risk of embolism, albeit not statistically significant, compared with either no anticoagulation or VKAs. Nevertheless, the risk of all‐cause death remained comparable between any 2 groups. The values of τ statistics were 0.109, 0.275, and 0.270 for bleeding, embolism, and all‐cause death, respectively (data not shown in Figure 3).
Figure 3. Summaries of the network meta‐analysis for the efficacy and safety assessment among patients receiving different DOACs on the risk of (A) major bleeding, (B) thromboembolism, and (C) all‐cause death.
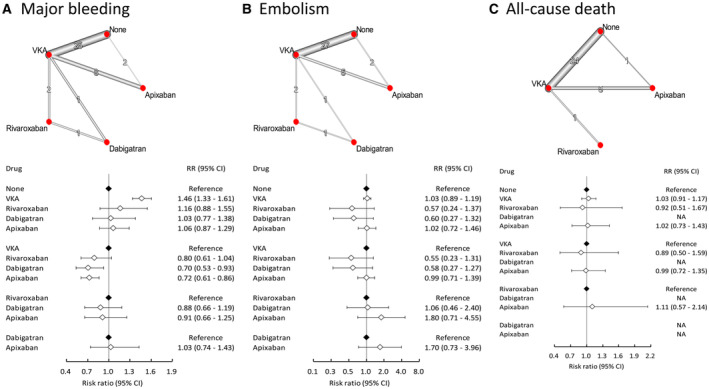
Left: network diagram; middle: forest plot. CI indicates confidence interval; RR, relative risk; and VKA, vitamin K antagonist.
Different Dosages of Apixaban
Apixaban was further subgrouped into 2.5 and 5 mg twice daily according to respective study protocols (Figure 4). A minimal difference was found between either dosage of apixaban regarding bleeding rate. However, apixaban 5 mg twice daily was correlated with numerically better preventive effect against thromboembolism (HR, 0.87 [95% CI, 0.55–1.38]) and all‐cause death (HR, 0.77 [95% CI, 0.51–1.15]) compared with apixaban 2.5 mg twice daily, although the difference was not statistically significant. The values of τ statistics were 0.099, 0.282, and 0.272 for bleeding, embolism, and all‐cause death, respectively (data not shown in Figure 4).
Figure 4. Summaries of the network meta‐analysis for the efficacy and safety assessment among patients receiving a different dose of apixaban on the risk of (A) major bleeding, (B) thromboembolism, and (C) all‐cause death.
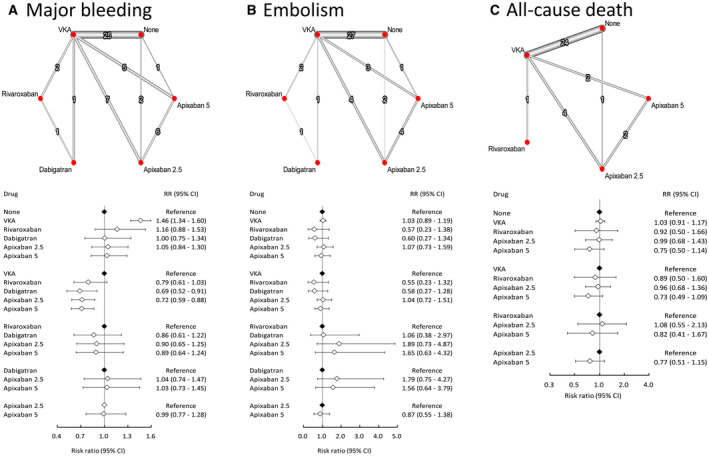
Left: network diagram; middle: forest plot. RR indicates relative risk; and VKA, vitamin K antagonist.
Net Clinical Benefit
To further clarify the safety and efficacy profiles of different anticoagulants, all comparators were classified into quadrants to illustrate the risk estimates of bleeding and embolism (Figure 5). VKAs were associated with elevated rates of bleeding and embolism. Dabigatran and rivaroxaban were associated with more bleeding but fewer embolism events. Apixaban of either dosage was associated with suboptimal outcomes in bleeding and embolism, whereas apixaban 5 mg daily was associated with a better effect in preventing embolism.
Figure 5. Net clinical benefit of different anticoagulants that combined (A) and separated (B) different doses of apixaban.
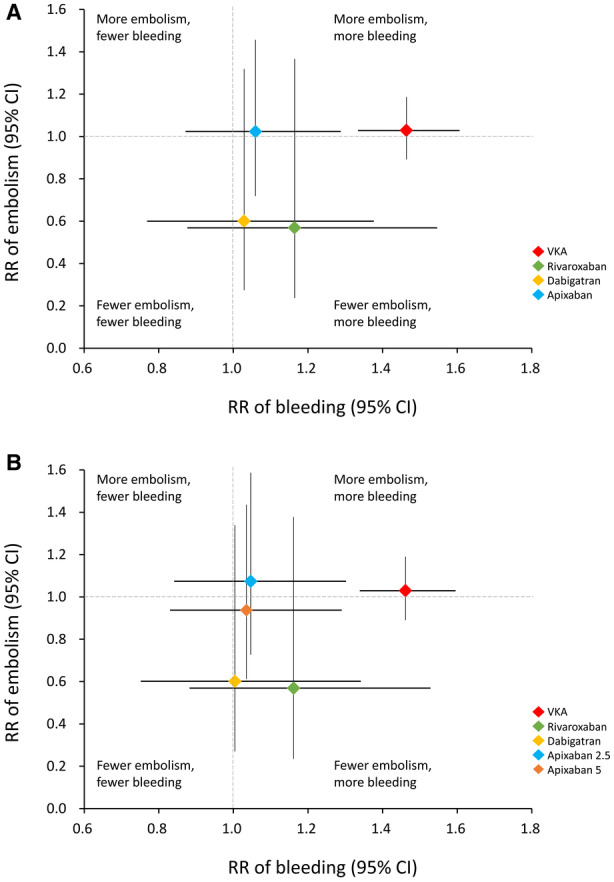
RR indicates relative risk; and VKA, vitamin K antagonist.
With only 1 study included concerning dabigatran, 17 the efficacies and safety profiles were reevaluated following its exclusion. Despite this removal, comparable outcomes were maintained (Figures S1 through S3).
Discussion
To our knowledge, this is the most updated network meta‐analysis to evaluate the efficacy and safety of anticoagulation in patients with AF receiving renal replacement therapy. The major findings of this study are (1) VKA was associated with the least net clinical benefit compared with DOACs or no anticoagulation, (2) apixaban 5 mg twice daily was associated with the lowest risk of all‐cause death, (3) dabigatran and rivaroxaban demonstrated the greatest efficacy in preventing thromboembolism, and (4) dabigatran and apixaban were associated with better safety.
Although CKD is associated with an elevated risk of thromboembolism, 49 the use of anticoagulation in individuals with AF and end‐stage renal disease undergoing dialysis has long been under debate. Studies comparing warfarin, DOACs, and no anticoagulation have reported discordant results in such patients. VKAs have been traditionally administered to individuals with long‐term dialysis to prevent thromboembolism secondary to AF. A retrospective study suggested that VKAs could mitigate all‐cause death and ischemic stroke 27 ; however, characterization of the potential confounding factors was not feasible due to the nonrandomized nature of the study. In addition, dose adjustment, albeit tailored by prothrombin time, remains clinically challenging due to the concomitant use of heparin during dialysis and altered metabolism caused by frequent uremia. 50 In a pooled analysis of 14 observational studies, VKAs were shown to be associated with an increased rate of bleeding and no significant improvement in the risk of thromboembolism in patients undergoing long‐term dialysis. 51 Suboptimal compliance with VKA therapy in such patients is another major concern. 19 In the present study, we comprehensively included all studies comparing VKAs with either no anticoagulation or DOACs and confirmed a higher bleeding rate as well as no significant mitigation in thromboembolism with VKA therapy.
DOACs have emerged as an alternative in patients with CKD, but the extended application under renal replacement therapy remains questionable. To date, 3 randomized trials have assessed the use of VKAs and DOACs in subjects undergoing long‐term dialysis. The RENAL‐AF (Renal Hemodialysis Patients Allocated Apixaban Versus Warfarin in Atrial Fibrillation) study did not identify any superiority between apixaban and warfarin regarding bleeding events in patients undergoing dialysis. 44 However, the sample size in the study was relatively small. The AXADIA‐AFNET 8 (Compare Apixaban and Vitamin K Antagonists in Patients With Atrial Fibrillation and End‐Stage Kidney Disease) study reported that the efficacy of DOACs was equivalent to that of warfarin, yet both groups presented with high cardiovascular risk. 45 The Valkyrie study demonstrated that VKAs were associated with higher risks of major bleeding and cardiovascular events compared with low‐dose rivaroxaban. 38 However, the time in therapeutic range of the individuals was suboptimal in these studies and may have compromised interpretation of the results.
To date, only the cautious use of apixaban 5 mg twice daily has been approved by the Food and Drug Administration for patients undergoing dialysis. However, the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial and most cohort studies have excluded patients undergoing dialysis, and thus the evidence remains weak. In a previous meta‐analysis by Kuno et al, 52 apixaban 5 mg twice daily was surprisingly shown to reduce death compared with warfarin or no anticoagulation. In addition, apixaban 2.5 or 5 mg twice daily was further proposed to be associated with less bleeding but equivalent efficacy in preventing thromboembolism than warfarin. However, only 1 study on the use of apixaban was included in that study, whereas our study included 6 studies with apixaban. In our analysis, apixaban 5 mg twice daily was associated with a more preferred net clinical benefit. We also studied the combined effect of apixaban with either a usual dose or a low dose. Consistent with the previous study, apixaban was demonstrated to be better than warfarin regarding safety among patients with advanced CKD. 53 We further found that apixaban was comparable with no anticoagulation regarding safety and efficacy. Our pooled analysis added a randomized controlled trial involving rivaroxaban and 7 studies with apixaban. However, the resultant major bleeding rate was greater only in the patients who received rivaroxaban, whereas the risk in the patients who received dabigatran was comparable to those who received apixaban of either dose.
We recognized a previous network meta‐analysis, which preliminarily assessed the applicability of DOACs in patients undergoing dialysis. 52 In that study, DOACs failed to demonstrate efficacy in preventing thromboembolism, while dabigatran and rivaroxaban, in conjunction with VKAs, potentiated the risk of bleeding. The AXADIA‐AFNET 8 trial 45 and 9 other cohort studies were published subsequently and were incorporated into our study. Additionally, we included all literature reporting outcomes of anticoagulation in patients undergoing dialysis, including subgroup analyses, to enhance the statistical power. This updated meta‐analysis offers valuable evidence to assist in selecting anticoagulants, particularly in the context of long‐term dialysis.
In our analysis, we also classified the respective anticoagulants into quadrants to illustrate their net clinical benefits. Neither DOACs nor VKAs were associated with fewer bleeding or embolism events, and consequently the need to administer anticoagulants in patients undergoing long‐term dialysis remains equivocal. Moreover, AF has not been well established as an independent risk factor for stroke in this population. 54 We thus propose that not using anticoagulants is also a reasonable strategy in patients with end‐stage renal disease undergoing dialysis.
Future studies should aim to identify which clinical parameters can be used to tailor anticoagulant use in such patients individually. Among the enrolled studies in our analysis, 5 applied the CHA2DS2‐VASc score and 2 the HAS‐BLED score to stratify the patients undergoing anticoagulant therapy. In the few studies evaluating warfarin use, prothrombin time was used to assess the safety and efficacy of VKAs. However, the other enrolled studies reported discordant characteristics to evaluate the risk of bleeding and thromboembolism. In addition, none of the included randomized trials further subgrouped patients with either parameter. Therefore, we could not identify clinical characteristics to guide anticoagulant use in such patients. Future prospective studies are warranted to determine the specific conditions for administering anticoagulants.
Our study has several limitations. First, anticoagulation in dialysis has been studied mostly in observational studies that retrospectively assessed different drugs without appropriate dose adjustment and using administrative codes or billing data, resulting in noise. The enrolled studies are hence predominantly observational in nature, which could potentially confound the interpretation of the associations. In addition, the sample size in each study was relatively small, which may have limited the significance of the effect of anticoagulation therapy. The findings were based on low‐quality evidence. Second, studies regarding DOACs other than apixaban were very limited. No study specifically regarding edoxaban was included in the present network meta‐analysis, and we therefore could not investigate its effect. The only study including dabigatran was administered using an ultra‐low dose. 17 Even though a pharmacodynamic study validated the bioavailability of DOACs under dialysis, 55 discrepancy in the protocol regarding dose adjustment for patients undergoing renal replacement therapy may have limited generalization of the results. Third, the patient‐level data of each included study were unavailable. Analysis with prestratification according to different clinical parameters was therefore not possible. Fourth, concomitant antiplatelet therapy, as often required by the common presence of comorbidities in such patients, may have masked the effect of anticoagulants. Interpretation of the results should hence be prudent. Finally, our analysis yielded only 1 study that reported outcomes related to dabigatran, showing unexpectedly promising efficacy and safety despite its predominantly renal excretion. Given the significance of this finding, we opted to retain it. Moreover, similar pooled outcomes were observed even when the sole study on dabigatran was excluded. Further research is essential to validate the efficacy and optimal dosage of dabigatran in patients undergoing long‐term dialysis.
In conclusion, the findings of this study updated the latest evidence for the use of anticoagulants in patients with end‐stage renal disease undergoing dialysis. Among DOACs, dabigatran and rivaroxaban were associated with a lower risk of thromboembolism, whereas fewer bleeding events were demonstrated in the patients receiving apixaban. VKAs were associated with worse safety and efficacy. There was no significant difference in the prognosis in the patients who did not receive anticoagulants compared with those who received either DOACs or VKAs. The anticoagulation strategy should therefore be tailored individually.
Sources of Funding
None.
Disclosures
Dr Lin, one of the corresponding authors of this article, is an associate editor of the Journal of the American Heart Association. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figures S1–S3
Acknowledgments
The authors thank Alfred Hsing‐Fen Lin, MS; Ben Yu‐Lin Chou, MPH; and Zoe Ya‐Zhu Syu, MPH, Raising Statistics Consultant Inc, for the assistance in statistical analysis of this study.
This manuscript was sent to Jong‐Ho Park, MD, PhD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.034176
For Sources of Funding and Disclosures, see page 14.
Contributor Information
Zheng‐Wei Chen, Email: librajohn7@hotmail.com.
Yen‐Hung Lin, Email: austinr34@gmail.com.
References
- 1. Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, Acquistapace I, Stella A, Bonforte G, DeVecchi A, et al. Prevalence of atrial fibrillation and associated factors in a population of long‐term hemodialysis patients. Am J Kidney Dis. 2005;46:897–902. doi: 10.1053/j.ajkd.2005.07.044 [DOI] [PubMed] [Google Scholar]
- 2. Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, Ojo A, Teal VL, Jensvold NG, Robinson NL, et al. Chronic renal insufficiency cohort (CRIC) study group. Chronic kidney disease and prevalent atrial fibrillation: the chronic renal insufficiency cohort (CRIC). Am Heart J. 2010;159:1102–1107. doi: 10.1016/j.ahj.2010.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Randhawa MS, Vishwanath R, Rai MP, Wang L, Randhawa AK, Abela G, Dhar G. Association between use of warfarin for atrial fibrillation and outcomes among patients with end stage renal disease: a systematic review and meta‐analysis. JAMA Netw Open. 2020;3:e202175. doi: 10.1001/jamanetworkopen.2020.2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang X, Tirucherai G, Marbury TC, Wang J, Chang M, Zhang D, Song Y, Pursley J, Boyd RA, Frost C. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56:628–636. doi: 10.1002/jcph.628 [DOI] [PubMed] [Google Scholar]
- 5. Wang TK, Sathananthan J, Marshall M, Kerr A, Hood C. Relationships between anticoagulation, risk scores and adverse outcomes in dialysis patients with atrial fibrillation. Heart Lung Circ. 2016;25:243–249. doi: 10.1016/j.hlc.2015.08.012 [DOI] [PubMed] [Google Scholar]
- 6. Woods BS, Hawkins N, Scott DA. Network meta‐analysis on the log‐hazard scale, combining count and hazard ratio statistics accounting for multi‐arm trials: a tutorial. BMC Med Res Methodol. 2010;10:54. doi: 10.1186/1471-2288-10-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, Salanti G. An approach for assessing confidence in the results of a network meta‐analysis. PLoS Med. 2020;17:e1003082. doi: 10.1371/journal.pmed.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–2233. doi: 10.1681/ASN.2009030319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lai HM, Aronow WS, Kalen P, Adapa S, Patel K, Goel A, Vinnakota R, Chugh S, Garrick R. Incidence of thromboembolic stroke and of major bleeding in patients with atrial fibrillation and chronic kidney disease treated with and without warfarin. Int J Nephrol Renov Dis. 2009;2:33–37. doi: 10.2147/ijnrd.s7781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662–2668. doi: 10.2215/CJN.04550511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olesen JB, Lip GY, Kamper AL, Hommel K, Køber L, Lane DA, Lindhardsen J, Gislason GH, Torp‐Pedersen C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367:625–635. doi: 10.1056/NEJMoa1105594 [DOI] [PubMed] [Google Scholar]
- 12. Sood MM, Larkina M, Thumma JR, Tentori F, Gillespie BW, Fukuhara S, Mendelssohn DC, Chan K, de Sequera P, Komenda P, et al. Major bleeding events and risk stratification of antithrombotic agents in hemodialysis: results from the DOPPS. Kidney Int. 2013;84:600–608. doi: 10.1038/ki.2013.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonde AN, Lip GY, Kamper AL, Hansen PR, Lamberts M, Hommel K, Hansen ML, Gislason GH, Torp‐Pedersen C, Olesen JB. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol. 2014;64:2471–2482. doi: 10.1016/j.jacc.2014.09.051 [DOI] [PubMed] [Google Scholar]
- 14. Chen JJ, Lin LY, Yang YH, Hwang JJ, Chen PC, Lin JL. Anti‐platelet or anti‐coagulant agent for the prevention of ischemic stroke in patients with end stage renal disease and atrial fibrillation—a nation‐wide database analyses. Int J Cardiol. 2014;177:1008–1011. doi: 10.1016/j.ijcard.2014.09.140 [DOI] [PubMed] [Google Scholar]
- 15. Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, Humphries KH, Tu JV, Behlouli H, Guo H, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129:1196–1203. doi: 10.1161/CIRCULATIONAHA.113.004777 [DOI] [PubMed] [Google Scholar]
- 16. Wakasugi M, Kazama JJ, Tokumoto A, Suzuki K, Kageyama S, Ohya K, Miura Y, Kawachi M, Takata T, Nagai M, et al. Association between warfarin use and incidence of ischemic stroke in Japanese hemodialysis patients with chronic sustained atrial fibrillation: a prospective cohort study. Clin Exp Nephrol. 2014;18:662–669. doi: 10.1007/s10157-013-0885-6 [DOI] [PubMed] [Google Scholar]
- 17. Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131:972–979. doi: 10.1161/CIRCULATIONAHA.114.014113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Genovesi S, Rossi E, Gallieni M, Stella A, Badiali F, Conte F, Pasquali S, Bertoli S, Ondei P, Bonforte G, et al. Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant. 2015;30:491–498. doi: 10.1093/ndt/gfu334 [DOI] [PubMed] [Google Scholar]
- 19. Shen JI, Montez‐Rath ME, Lenihan CR, Turakhia MP, Chang TI, Winkelmayer WC. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis. 2015;66:677–688. doi: 10.1053/j.ajkd.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brancaccio D, Neri L, Bellocchio F, Barbieri C, Amato C, Mari F, Canaud B, Stuard S. Patients' characteristics affect the survival benefit of warfarin treatment for hemodialysis patients with atrial fibrillation. A historical cohort study. Am J Nephrol. 2016;44:258–267. doi: 10.1159/000448898 [DOI] [PubMed] [Google Scholar]
- 21. Garg L, Chen C, Haines DE. Atrial fibrillation and chronic kidney disease requiring hemodialysis—does warfarin therapy improve the risks of this lethal combination? Int J Cardiol. 2016;222:47–50. doi: 10.1016/j.ijcard.2016.07.118 [DOI] [PubMed] [Google Scholar]
- 22. Hayashi M, Abe T, Iwai M, Matsui A, Yoshida T, Sato Y, Kanno Y; Warfarin Study Group . Safety of warfarin therapy in chronic hemodialysis patients: a prospective cohort study. Clin Exp Nephrol. 2016;20:787–494. doi: 10.1007/s10157-015-1205-0 [DOI] [PubMed] [Google Scholar]
- 23. Mitsuma W, Matsubara T, Hatada K, Imai S, Saito N, Shimada H, Miyazaki S. Clinical characteristics of hemodialysis patients with atrial fibrillation: the RAKUEN (registry of atrial fibrillation in chronic kidney disease under hemodialysis from Niigata) study. J Cardiol. 2016;68:148–155. doi: 10.1016/j.jjcc.2015.08.023 [DOI] [PubMed] [Google Scholar]
- 24. Toida T, Sato Y, Nakagawa H, Komatsu H, Uezono S, Yamada K, Ishihara T, Hisanaga S, Kitamura K, Fujimoto S. Risk of cerebral infarction in Japanese hemodialysis patients: Miyazaki dialysis cohort study (MID study). Kidney Blood Press Res. 2016;41:471–478. doi: 10.1159/000443448 [DOI] [PubMed] [Google Scholar]
- 25. Yodogawa K, Mii A, Fukui M, Iwasaki YK, Hayashi M, Kaneko T, Miyauchi Y, Tsuruoka S, Shimizu W. Warfarin use and incidence of stroke in Japanese hemodialysis patients with atrial fibrillation. Heart Vessel. 2016;31:1676–1680. doi: 10.1007/s00380-015-0777-7 [DOI] [PubMed] [Google Scholar]
- 26. Genovesi S, Rebora P, Gallieni M, Stella A, Badiali F, Conte F, Pasquali S, Bertoli S, Ondei P, Bonforte G, et al. Effect of oral anticoagulant therapy on mortality in end stage renal disease patients with atrial fibrillation: a prospective study. J Nephrol. 2017;30:573–581. doi: 10.1007/s40620-016-0364-8 [DOI] [PubMed] [Google Scholar]
- 27. Kai B, Bogorad Y, Nguyen LN, Yang SJ, Chen W, Spencer HT, Shen AY, Lee MS. Warfarin use and the risk of mortality, stroke, and bleeding in hemodialysis patients with atrial fibrillation. Heart Rhythm. 2017;14:645–651. doi: 10.1016/j.hrthm.2017.01.047 [DOI] [PubMed] [Google Scholar]
- 28. Lee KH, Li SY, Liu JS, Huang CT, Chen YY, Lin YP, Hsu CC, Tarng DC. Association of warfarin with congestive heart failure and peripheral artery occlusive disease in hemodialysis patients with atrial fibrillation. J Chin Med Assoc. 2017;80:277–282. doi: 10.1016/j.jcma.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 29. Lin MC, Streja E, Soohoo M, Hanna M, Savoj J, Kalantar‐Zadeh K, Lau WL. Warfarin use and increased mortality in end stage renal disease. Am J Nephrol. 2017;46:249–256. doi: 10.1159/000481207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sarratt SC, Nesbit R, Moye R. Safety outcomes of apixaban compared with warfarin in patients with end stage renal disease. Ann Pharmacother. 2017;51:445–450. doi: 10.1177/1060028017694654 [DOI] [PubMed] [Google Scholar]
- 31. Yoon CY, Noh J, Jhee JH, Chang TI, Kang EW, Kee YK, Kim H, Park S, Yun HR, Jung SY, et al. Warfarin use in patients with atrial fibrillation undergoing hemodialysis: a nationwide population‐based study. Stroke. 2017;48:2472–2479. doi: 10.1161/STROKEAHA.117.017114 [DOI] [PubMed] [Google Scholar]
- 32. Reed D, Palkimas S, Hockman R, Abraham S, Le T, Maitland H. Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res Pract Thromb Haemost. 2018;2:291–298. doi: 10.1002/rth2.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, Tilea A, Stack AG, Balkrishnan R, Yao X, et al. Outcomes associated with apixaban use in patients with end stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138:1519–1529. doi: 10.1161/CIRCULATIONAHA.118.035418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Voskamp PWM, Rookmaaker MB, Verhaar MC, Dekker FW, Ocak G. Vitamin K antagonist use and mortality in dialysis patients. Nephrol Dial Transplant. 2018;33:170–176. doi: 10.1093/ndt/gfx199 [DOI] [PubMed] [Google Scholar]
- 35. Phan D, Yang SJ, Shen AY, Lee MS. Effect of warfarin on ischemic stroke, bleeding, and mortality in patients with atrial fibrillation receiving peritoneal dialysis. Am J Cardiovasc Drugs. 2019;19:509–515. doi: 10.1007/s40256-019-00347-3 [DOI] [PubMed] [Google Scholar]
- 36. Tan J, Bae S, Segal JB, Zhu J, Alexander GC, Segev DL, McAdams‐DeMarco M. Warfarin use and the risk of stroke, bleeding, and mortality in older adults on dialysis with incident atrial fibrillation. Nephrology. 2019;24:234–244. doi: 10.1111/nep.13207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agarwal MA, Potukuchi PK, Sumida K, Naseer A, Molnar MZ, George LK, Koshy SK, Streja E, Thomas F, Kalantar‐Zadeh K, et al. Clinical outcomes of warfarin initiation in advanced chronic kidney disease patients with incident atrial fibrillation. JACC Clin Electrophysiol. 2020;6:1658–1668. doi: 10.1016/j.jacep.2020.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus no anticoagulation in patients undergoing long‐term dialysis with incident atrial fibrillation. Clin J Am Soc Nephrol. 2020;15:1146–1154. doi: 10.2215/CJN.11650919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De Vriese AS, Caluwé R, Van Der Meersch H, De Boeck K, De Bacquer D. Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol. 2021;32:1474–1483. doi: 10.1681/ASN.2020111566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ionescu F, Cooper C, Petrescu I, George J, Mansuri S. Safety of apixaban compared to warfarin in hemodialysis patients: do antiplatelets make a difference? Eur J Haematol. 2021;106:689–696. doi: 10.1111/ejh.13599 [DOI] [PubMed] [Google Scholar]
- 41. See LC, Lee HF, Chao TF, Li PR, Liu JR, Wu LS, Chang SH, Yeh YH, Kuo CT, Chan YH, et al. Effectiveness and safety of direct oral anticoagulants in an Asian population with atrial fibrillation undergoing dialysis: a population‐based cohort study and meta‐analysis. Cardiovasc Drugs Ther. 2021;35:975–986. doi: 10.1007/s10557-020-07108-4 [DOI] [PubMed] [Google Scholar]
- 42. Kang Y, Choi HY, Kwon YE, Shin JH, Won EM, Yang KH, Oh HJ, Ryu DR. Clinical outcomes among hemodialysis patients with atrial fibrillation: a Korean nationwide population‐based study. Kidney Res Clin Pract. 2021;40:99–108. doi: 10.23876/j.krcp.20.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim MR, Kim DG, Shin HW, Kim SH, Kim JS, Yang JW, Han BG, Choi SO, Lee JY. Survival benefit of anticoagulation therapy in end stage kidney disease patients with atrial fibrillation: a single center retrospective study. Medicina. 2021;58:58. doi: 10.3390/medicina58010058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pokorney SD, Chertow GM, Al‐Khalidi HR, Gallup D, Dignacco P, Mussina K, Bansal N, Gadegbeku CA, Garcia DA, Garonzik S, et al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation. 2022;146:1735–1745. doi: 10.1161/CIRCULATIONAHA.121.054990 [DOI] [PubMed] [Google Scholar]
- 45. Reinecke H, Engelbertz C, Bauersachs R, Breithardt G, Echterhoff HH, Gerß J, Haeusler KG, Hewing B, Hoyer J, Juergensmeyer S, et al. A randomized controlled trial comparing apixaban with the vitamin K antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA‐AFNET 8 study. Circulation. 2023;147:296–309. doi: 10.1161/CIRCULATIONAHA.122.062779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sy J, Wenziger C, Marroquin M, Kalantar‐Zadeh K, Kovesdy C, Streja E. Warfarin use, stroke, and bleeding risk among pre‐existing atrial fibrillation US veterans transitioning to dialysis. Nephron. 2022;146:360–368. doi: 10.1159/000521494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wetmore JB, Weinhandl ED, Yan H, Reyes JL, Herzog CA, Roetker NS. Apixaban dosing patterns versus warfarin in patients with nonvalvular atrial fibrillation receiving dialysis: a retrospective cohort study. Am J Kidney Dis. 2022;80:569–579.e1. doi: 10.1053/j.ajkd.2022.03.007 [DOI] [PubMed] [Google Scholar]
- 48. Akbar MR, Febrianora M, Iqbal M. Warfarin usage in patients with atrial fibrillation undergoing hemodialysis in Indonesian population. Curr Probl Cardiol. 2023;48:101104. doi: 10.1016/j.cpcardiol.2022.101104 [DOI] [PubMed] [Google Scholar]
- 49. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 50. Yang F, Hellyer JA, Than C, Ullal AJ, Kaiser DW, Heidenreich PA, Hoang DD, Winkelmayer WC, Schmitt S, Frayne SM, et al. Warfarin utilisation and anticoagulation control in patients with atrial fibrillation and chronic kidney disease. Heart. 2017;103:818–826. doi: 10.1136/heartjnl-2016-309266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harel Z, Chertow GM, Shah PS, Harel S, Dorian P, Yan AT, Saposnik G, Sood MM, Molnar AO, Perl J, et al. Warfarin and the risk of stroke and bleeding in patients with atrial fibrillation receiving dialysis: a systematic review and meta‐analysis. Can J Cardiol. 2017;33:737–746. doi: 10.1016/j.cjca.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 52. Kuno T, Takagi H, Ando T, Sugiyama T, Miyashita S, Valentin N, Shimada YJ, Kodaira M, Numasawa Y, Briasoulis A, et al. Oral anticoagulation for patients with atrial fibrillation on long‐term hemodialysis. J Am Coll Cardiol. 2020;75:273–285. doi: 10.1016/j.jacc.2019.10.059 [DOI] [PubMed] [Google Scholar]
- 53. Stanifer JW, Pokorney SD, Chertow GM, Hohnloser SH, Wojdyla DM, Garonzik S, Byon W, Hijazi Z, Lopes RD, Alexander JH, et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020;141:1384–1392. doi: 10.1161/CIRCULATIONAHA.119.044059 [DOI] [PubMed] [Google Scholar]
- 54. Murray AM, Seliger S, Lakshminarayan K, Herzog CA, Solid CA. Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol. 2013;24:1166–1173. doi: 10.1681/ASN.2012080841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28:2241–2248. doi: 10.1681/ASN.2016090980 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3


