Abstract
Background
Periodontitis has not been recognized as a modifiable risk factor for atrial fibrillation (AF). This prospective nonrandomized study investigated whether periodontal treatment improves the AF ablation outcome.
Methods and Results
We prospectively enrolled 288 AF patients scheduled to undergo initial radiofrequency catheter ablation. Each patient underwent periodontal inflamed surface area (PISA; a quantitative index of periodontal inflammation) measurement. All eligible patients were recommended to receive periodontal treatment within the blanking period, and 97 consented. During the mean follow‐up period of 507±256 days, 70 (24%) AF recurrences were documented. Patients who exhibited AF recurrences had a higher PISA than those who did not (456.8±403.5 versus 277.7±259.0 mm2, P=0.001). These patients were categorized into high‐PISA (>615 mm2) and low‐PISA (<615 mm2) groups according to the receiver operating characteristic analysis for AF recurrence (area under the curve, 0.611; sensitivity, 39%; specificity, 89%). A high PISA, as well as female sex, AF duration, and left atrial volume, were the statistically significant predicter for AF recurrence (hazard ratio [HR], 2.308 [95% CI, 1.234–4.315]; P=0.009). In patients with a high PISA, those who underwent periodontal treatment showed significantly fewer AF recurrences (P=0.01, log‐rank test). The adjusted HR of periodontal treatment for AF recurrence was 0.393 (95% CI, 0.215–0.719; P=0.002).
Conclusions
Periodontitis may serve as a modifiable risk factor for AF. PISA is a hallmark of AF recurrence, and periodontal treatment improves the AF ablation outcome, especially for those with poor periodontal condition.
Keywords: modifiable risk factor, periodontal inflamed surface area, periodontitis, radiofrequency catheter ablation, recurrence
Subject Categories: Atrial Fibrillation, Risk Factors, Risk Factors
Nonstandard Abbreviations and Acronyms
- PISA
periodontal inflamed surface area
- PV
pulmonary vein
- RFCA
radiofrequency catheter ablation
Clinical Perspective.
What Is New?
Periodontal inflamed surface area, a quantitative index of periodontal inflammation, predicts atrial fibrillation (AF) recurrence after radiofrequency catheter ablation.
Periodontal treatment during the blanking period can reduce periodontal inflamed surface area and improve the AF ablation outcome.
What Are the Clinical Implications?
Periodontitis should be recognized as a modifiable risk factor for AF, and periodontal examination is beneficial for AF ablation candidates.
Periodontal inflamed surface area can be a hallmark of AF recurrence, and patients with a high periodontal inflamed surface area should be examined more carefully for AF recurrence after ablation.
Periodontal treatment during the blanking period is recommended, especially for those with poor periodontal condition.
Atrial fibrillation (AF) is the most common arrhythmia that increases the risks of stroke, heart failure, and death, thereby shortening healthy life expectancy. 1 Although radiofrequency catheter ablation (RFCA) successfully exhibits a higher AF recurrence–free survival rate compared with antiarrhythmic drugs, 2 , 3 AF recurs after RFCA in 13% to 39% of patients with paroxysmal AF and at an even higher rate in those with nonparoxysmal AF. 4 Therefore, risk factor modification is essential to achieve more reliable sinus rhythm maintenance after RFCA. Currently, obesity, physical inactivity, disordered breathing during sleep, diabetes, hypertension, hyperlipidemia, tobacco intake, alcohol consumption, and caffeine consumption are considered modifiable risk factors for AF, 5 and modifying these factors improves the outcome of AF ablation. 6
Periodontitis, a common infectious and inflammatory disease, reportedly correlates with several systemic diseases, but it has not been recognized as a modifiable risk factor for AF. Several cohort studies have reported that periodontitis is related to AF occurrence. 7 , 8 , 9 , 10 , 11 Two reports, including ours, demonstrated the relationship between periodontitis and AF recurrence after catheter ablation. 12 , 13 However, no reports have investigated whether periodontal treatment can effectively help maintain sinus rhythm after AF ablation. Thus, this prospective study aimed to (1) confirm the negative impact of periodontitis on the AF ablation outcome and (2) investigate whether periodontal treatment improves the AF recurrence rate after ablation.
METHODS
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
This study conformed to the principles of the Declaration of Helsinki and obtained approval from the Hiroshima University Ethics Committee (Approval No. E‐1954). Written informed consent was obtained from all patients before participation.
Study Design
This prospective nonrandomized study was conducted at Hiroshima University Hospital. From April 1, 2020, to July 31, 2022, 330 consecutive patients with AF admitted for an initial RFCA were enrolled in this study.
All patients underwent a periodontal examination 1 day before the RFCA, and the degree of periodontitis was quantified using the periodontal inflamed surface area (PISA). Nonsurgical periodontal treatment during the blanking period, particularly at 1 and 3 months after the RFCA, was recommended. Only those who expressed consent received the periodontal treatment. They were then followed up for AF recurrence at the hospital's outpatient clinic. Figure 1 depicts the study's flowchart of participant selection. One patient refused to undergo periodontal examination. Meanwhile, 10 agreed but could not take the periodontal examination because of time constraints. In addition, 31 were excluded because of left atrial appendage thrombus detected by transesophageal echocardiography before RFCA (n=8), intraoperative complications (n=2), and insufficient follow‐up periods (n=21).
Figure 1. Flowchart of participant selection.
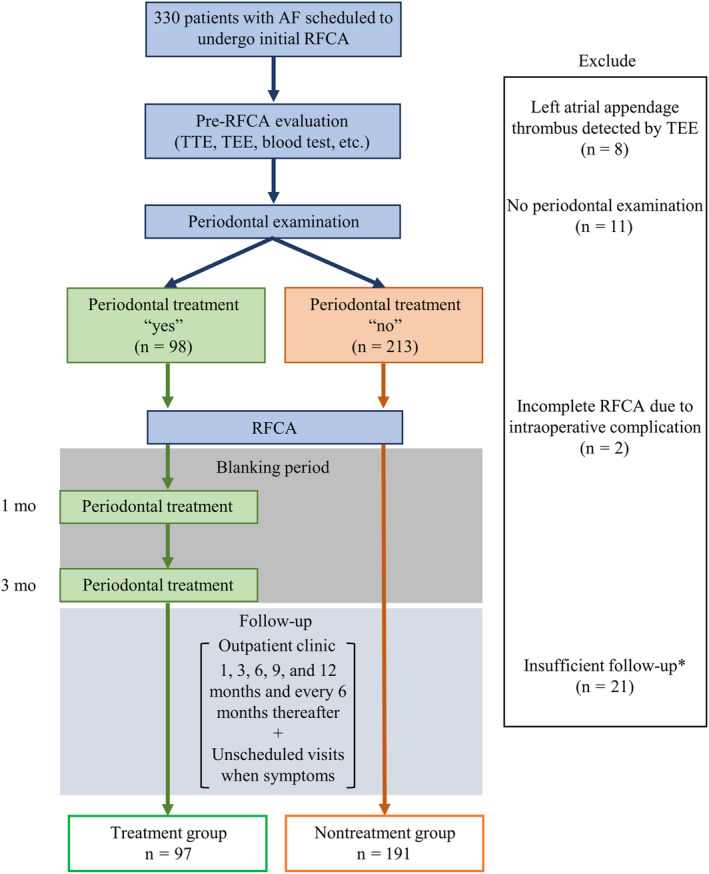
This single‐center prospective, nonrandomized study enrolled 330 patients who were scheduled to undergo initial RFCA. Periodontal examination was performed 1 day before RFCA, and periodontal inflammation was quantified by PISA. Patients were divided into the periodontal treatment group and nontreatment group according to their intentions before RFCA. Nonsurgical periodontal treatment was conducted during the blanking period only in the treatment group. We excluded 42 patients and included 288 patients (treatment group, n=97; nontreatment group, n=191) in the analysis. *Patients who were lost to follow‐up before the 6‐month outpatient visit. AF indicates atrial fibrillation; PISA, periodontal inflamed surface area; RFCA, radiofrequency catheter ablation; TEE, transesophageal echocardiography; and TTE, transthoracic echocardiography.
Baseline Data Acquisition and Pre‐RFCA Evaluation
Baseline clinical characteristics were recorded 1 day before the RFCA. We ascertained patients' smoking and drinking statuses by using a questionnaire, which was answered by the patients before RFCA. Patients who had smoked >100 cigarettes in their lifetime were considered as former smokers. Those who drank ≥3 days a week and consumed ≥20 g of alcohol per day were regarded as habitual drinkers. One day before the RFCA, a peripheral blood test was performed, and results, including the hs‐CRP (high‐sensitivity C‐reactive protein) level, were collected from the patients' medical records. Experienced echocardiographers conducted transthoracic and transesophageal echocardiography in accordance with the American Society of Echocardiography guidelines 14 before the RFCA. Paroxysmal AF was defined as episodes spontaneously terminating within 7 days, whereas nonparoxysmal AF was defined as episodes persisting beyond 7 days.
Periodontal Examination and Treatment
One day before RFCA, a single experienced dental clinician, who was blinded to the patients' clinical information, conducted a complete periodontal examination using a periodontal probe (Hu‐Friedy Mfg, Chicago, IL) in all patients at the hospital's dental outpatient clinic (Department of Periodontal Medicine). Patients' remaining teeth were counted visually. Furthermore, the gingival margin, probing depth, and bleeding on probing in all the periodontal pockets were recorded. The periodontal inflammation degree was quantified by calculating the PISA according to the probing depth and bleeding on probing measurements at each periodontal pocket, as previously described. 15 PISA is the sum of all bleeding areas in the periodontal pocket epithelia, reflecting the quantification of clinical periodontal inflammation degree. 15 In a tooth loss site, where the periodontal pocket has healed and disappeared, the area of the periodontal pocket epithelia is calculated as 0 mm2. Thus, patients with an edentulous jaw were included with PISA values of 0 mm2. The relationship between the number of remaining teeth and PISA is presented in Figure S1. In contrast, although only 3 patients with dental implants were included, none of the patients suffered from peri‐implantitis. After the periodontal examination, all patients underwent supragingival deplaquing using rubber cups and toothpaste.
All eligible patients were informed of their periodontitis status and suggested to undergo periodontal treatment during the blanking period by the dental clinician, regardless of their periodontitis status. Patients who wished to receive periodontal treatment were included in the treatment group, and those who did not were included in the nontreatment group. A patient's decision was not influenced by physicians who were involved in the RFCA and follow‐up. Nonsurgical periodontal treatment was conducted during the blanking period, particularly 1 and 3 months after the RFCA (Figure 1). In brief, each patient received oral hygiene instruction, followed by full‐mouth debridement (supragingival and subgingival), which was performed by an experienced dental clinician, using an ultrasonic device and Gracey curette. After debridement, all teeth surfaces were polished using rubber cups and prophylaxis paste. Patients who received nonsurgical periodontal treatment did not require interruption of anticoagulation, and complications related to the treatment were not observed.
Measurement of Serum Antibody Titers Against Major Periodontal Pathogens
From blood samples obtained from the left atrium before the RFCA, serum immunoglobulin G (IgG) antibody titers against major periodontal pathogens were measured by ELISA. As bacterial antigens, the sonicated preparations of Porphyromonas gingivalis (Su63), Treponema denticola (ATCC35405), Tannerella forsythia (ATCC43037), Aggregatibacter actinomycetemcomitans (Y4), and Fusobacterium nucleatum (ATCC 25586) were applied. The sera from 5 healthy individuals (aged 12–81 years) were pooled and used for calibration. With serial dilutions of this pooled control serum, the standard reaction was expressed as ELISA unit, and 100 ELISA units corresponded to a 1:3200 dilution of the calibrator sample. The following formula was applied to the ELISA unit: diagnostic standardized value=(patient's IgG titer–healthy individuals' mean IgG titer)/2 SD determined using the mean IgG titer of 5 healthy individuals. All samples were conducted twice. According to the SD, the results of serum antibody titers were divided into 2 groups: high‐value (>mean+3 SD) and low‐value (<mean+3 SD) groups.
Measurement of Inflammatory Cytokines and Chemokines
From serum samples obtained from the left atrium before RFCA, inflammatory cytokines and chemokines were quantified using LEGENDplex (BioLegend, San Diego, CA) as per the manufacturer's instructions and then analyzed using FACSVerse flow cytometer (BD Biosciences, Franklin Lakes, NJ) and Qognit software (BioLegend).
Electrophysiological Study and RFCA Procedures
Antiarrhythmic drug were discontinued for at least 5 half‐lives, except for amiodarone, which was consistently discontinued for at least 2 weeks before the RFCA. The RFCA procedures and electrophysiological study were conducted using 3‐dimensional electroanatomical mapping systems (CARTO 3; Biosense Webster, Diamond Bar, CA; or EnSite Velocity; Abbott, St. Paul, MN). Data S1 further describes these details. Circumferential pulmonary vein (PV) isolation and non‐PV foci ablation (if applicable) were performed, with an end point of disconnecting all PVs and eliminating all non‐PV foci. Cavotricuspid isthmus ablation was also performed in patients with documentation of a typical atrial flutter before or during RFCA. During the electrophysiological study, the atrial signal–to–His bundle interval, His bundle–to–first ventricular activation interval, sinus node recovery time, and atrioventricular nodal conduction at a maximal rate of 1:1 were measured. Four operators with >10 years of experience conducted all these procedures without the knowledge of patient's periodontal status and schedule for periodontal treatment after RFCA.
Follow‐Up and Definition of Recurrence
The blanking period was set at 3 months after RFCA. All patients received antiarrhythmic drugs during the blanking periods at the physicians' discretion, provided there were no contraindications or intolerances observed. After the RFCA, each patient was followed up at the hospital's outpatient clinic at 1, 3, 6, 9, and 12 months and then every 6 months thereafter. At each clinical visit, AF recurrence was assessed by 12‐lead ECG and 24‐hour Holter monitoring. Furthermore, 14‐day event recording or portable ECG monitor recording was employed for those who were suspected with recurrent AF and had no AF evidence according to the 12‐lead ECG and 24‐hour Holter monitoring results. In addition to scheduled outpatient visits, patients with palpitations or pulse irregularity were firmly instructed to visit an outpatient clinic or emergency department to detect AF. The follow‐up period of the patients was defined as the date of the last outpatient visit as of August 1, 2023, starting from the date of RFCA; the last outpatient visit was censored for patients without AF recurrence. We excluded patients who were lost to follow‐up before the 6‐month outpatient visit from the analysis. AF recurrence was defined as >1 AF or atrial tachycardia episode lasting for >30 seconds documented in at least 1 modality. AF episodes detected during the blanking period (<3 months after RFCA) were defined as early recurrence and not included in the recurrence.
Statistical Analysis
Normally distributed continuous data were compared using 1‐way ANOVA, as appropriate, and expressed as means±SD. For nonnormally distributed data, which are expressed as medians with interquartile ranges, nonparametric methods were used. Conversely, categorical variables were compared using the χ 2 test or Fisher's exact test as appropriate, expressing them as percentages of the group total. The optimal cutoff value of PISA for AF recurrence was determined via receiver operating characteristic analysis using the Youden index. We divided the patients into high‐ and low‐PISA‐value groups on the basis of the optimal cutoff value. Moreover, we used the Kaplan–Meier method for assessing the time until AF recurrence, and the log‐rank test for comparing the AF recurrence–free survival rates between patient groups (high PISA versus low PISA and treatment versus nontreatment). The predictors of AF recurrence were determined using the Cox proportional hazards model wherein statistically significant variables from the univariable analysis were included in the multivariable analysis. In the Cox regression analysis, we conducted 2 analyses in which continuous variables were dichotomized or they were treated as continuous variables. Furthermore, for the sensitivity analyses, we applied another PISA cutoff value of 500 mm2 on the basis of the previous reports. 16 , 17 All statistical data were analyzed using JMP software version 15.0 (SAS Institute, Cary, NC), and P values <0.05 were considered statistically significant.
RESULTS
Baseline Characteristics
This study included 288 patients. Among them, 190 (66%) were men, and 163 (57%) had paroxysmal AF. AF recurred in 70 (24%) patients during the mean follow‐up period of 507±256 days. Furthermore, 97 patients received periodontal treatment during the blanking period (treatment group), and 191 did not (nontreatment group). Table 1 shows the baseline patient characteristics between these 2 groups. PISA was significantly higher in the treatment group at baseline than in the nontreatment group. Other baseline characteristics showed no significant differences between these groups. No significant differences were also observed in pre‐ and post‐RFCA antiarrhythmic drug status, RFCA procedures, operators, complications, and electrophysiology findings (Table S1).
Table 1.
Baseline Characteristics Between the Treatment and Nontreatment Groups
| Treatment (n=97) | Nontreatment (n=191) | P value | |
|---|---|---|---|
| Age, y | 67.9±10.2 | 68.3±10.7 | 0.77 |
| Sex, female, n (%) | 38 (39) | 60 (31) | 0.19 |
| Body mass index, kg/m2 | 24.8±3.8 | 24.2±3.8 | 0.24 |
| Nonparoxysmal AF, n (%) | 49 (51) | 76 (40) | 0.08 |
| AF duration, mo (IQR) | 5.0 (3.0–20.5) | 5.0 (3.0–22.5) | 0.37 |
| Habitual drinking, n (%) | 27 (28) | 61 (32) | 0.47 |
| Current or former smoking, n (%) | 52 (54) | 101 (53) | 0.91 |
| Hypertension, n (%) | 66 (68) | 113 (59) | 0.14 |
| Diabetes, n (%) | 12 (12) | 32 (17) | 0.32 |
| Dyslipidemia, n (%) | 51 (53) | 95 (50) | 0.93 |
| Previous stroke, n (%) | 8 (8) | 24 (13) | 0.26 |
| NT‐proBNP, pg/mL (IQR) | 312 (87–948) | 355 (97–949) | 0.79 |
| hs‐CRP, mg/dL (IQR) | 0.08 (0.03–0.14) | 0.06 (0.03–0.12) | 0.39 |
| Creatinine, mg/dL | 0.86±0.29 | 0.91±0.24 | 0.16 |
| LVEF, % | 60.9±5.7 | 60.0±7.8 | 0.35 |
| Left ventricular end‐diastolic dimension, mm | 47.5±4.8 | 47.9±4.9 | 0.50 |
| Left atrial volume, mL | 69.8±22.7 | 69.0±21.9 | 0.77 |
| Number of remaining teeth | 23.1±6.7 | 21.4±8.5 | 0.09 |
| PISA, mm2 | 614.3±457.2 | 311.4±298.8 | <0.0001 |
AF indicates atrial fibrillation; hs‐CRP, high‐sensitivity C‐reactive protein; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and PISA, periodontal inflamed surface area.
AF Recurrence and Systemic Inflammation
Figure S2 presents the relationship between inflammatory markers and AF recurrences in the nontreatment group. The baseline serum level of hs‐CRP was not significantly different between patients with and without early AF recurrences (0.09 [0.03–0.14] versus 0.06 [0.03–0.11]; P=0.11), but it was significantly higher in patients with AF recurrences within 12 months than in those without (0.10 [0.04–0.24] versus 0.06 [0.03–0.10]; P=0.01). Patients with AF recurrences also showed significantly higher serum levels of interleukin‐1β, interleukin‐4, interleukin‐6, interleukin‐10, and interleukin‐17A, but no inflammatory cytokines/chemokines were related to early AF recurrence.
Periodontitis and AF Recurrence
Figures 2A through 2D depict the relationship between periodontitis and AF recurrence in the nontreatment group. Although PISA was not related to early AF recurrence (Figure 2A), patients with AF recurrence had a significantly higher PISA (Figure 2B). The receiver operating characteristic analysis of PISA for AF recurrence yielded an area under the curve of 0.611 (95% CI, 0.497–0.723). The optimal cutoff value was 615.8 mm2, with 38.9% (95% CI, 22.1–78.6) sensitivity and 89.0% (95% CI, 48.5–98.7) specificity (Figure 2C). The patients were then categorized into 2 groups, high PISA (>615 mm2) and low PISA (<615 mm2), on the basis of the receiver operating characteristic analysis. The high‐PISA group exhibited a significantly lower AF recurrence–free survival rate than the low‐PISA group (Figure 2D). The multivariable Cox regression model adjusted for statistically significant variables in the univariable analysis revealed that high PISA, as well as female sex, AF duration, and left atrial volume, predicted AF recurrence with statistical significance (Table 2 and Figure S3). In another Cox regression model in which the continuous variables including PISA were not dichotomized, we found that PISA predicted recurrence of AF with statistical significance (Table S2). Furthermore, the sensitivity analyses showed consistent results (Figure S4 and Table S3).
Figure 2. Relationship between PISA and AF recurrence.
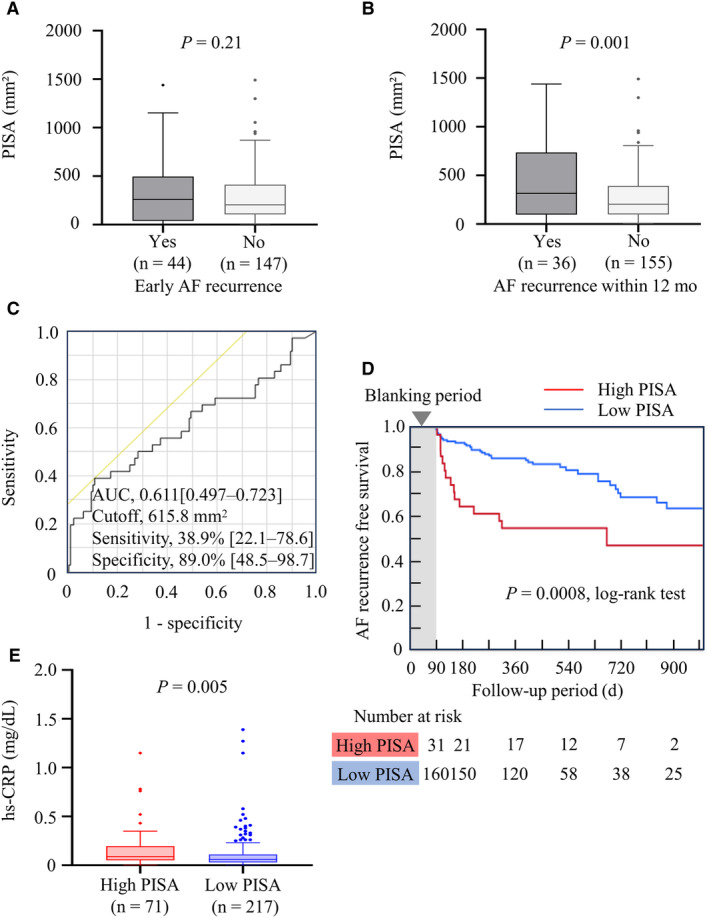
Association of PISA with AF recurrence (A–D) was analyzed in the nontreatment group (n=191). A, PISA and early AF recurrence (360.9±372.1 vs 296.6±272.9 mm2, P=0.21). B, PISA and AF recurrence within 12 months (456.8±403.5 vs 277.7±259.0 mm2; P=0.001). C, The receiver operating characteristic analysis of PISA for AF recurrence within 12 months. Patients were divided into high‐ and low‐PISA groups on the basis of the cutoff value of 615 mm2. D, The Kaplan–Meier analysis of AF recurrence between high‐ and low‐PISA groups. E, Serum hs‐CRP levels between the high‐ and low‐PISA groups (0.09 [IQR, 0.05–0.20] vs 0.06 [IQR, 0.03–0.11] mg/dL; P=0.005, Wilcoxon rank‐sum test, analyzed among all patients [N=288]). AF indicates atrial fibrillation; AUC, area under the curve; hs‐CRP, high‐sensitivity C‐reactive protein; and PISA, periodontal inflamed surface area.
Table 2.
Cox Regression Analyses for AF Recurrence
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | P value | Adjusted HR | 95% CI | P value | |
| Age>75 y (yes or no) | 0.967 | 0.547–1.705 | 0.91 | |||
| BMI>25.0 kg/m2 (yes or no) | 1.073 | 0.621–1.856 | 0.80 | |||
| Sex, female (yes or no) | 1.777 | 1.028–3.070 | 0.04 | 2.262 | 1.252–4.087 | 0.007 |
| Nonparoxysmal AF (yes or no) | 2.138 | 1.247–3.665 | 0.006 | 1.511 | 0.805–2.835 | 0.20 |
| AF duration >2 y* (yes or no) | 2.356 | 1.368–4.058 | 0.002 | 2.263 | 1.294–3.958 | 0.004 |
| Left atrial volume>70 mL* (yes or no) | 2.784 | 1.580–4.905 | 0.0004 | 2.103 | 1.080–4.092 | 0.03 |
| Smoking (yes or no) | 0.724 | 0.424–1.238 | 0.24 | |||
| Habitual drinking (yes or no) | 0.621 | 0.332–1.162 | 0.14 | |||
| Hypertension (yes or no) | 1.703 | 0.957–3.031 | 0.07 | |||
| Diabetes (yes or no) | 1.088 | 0.532–2.227 | 0.82 | |||
| Dyslipidemia (yes or no) | 1.084 | 0.633–1.855 | 0.77 | |||
| Previous cerebral infarction (yes or no) | 1.093 | 0.464–2.572 | 0.84 | |||
| NT‐proBNP >100 pg/mL (yes or no) | 2.725 | 1.164–6.378 | 0.02 | 1.400 | 0.546–3.592 | 0.48 |
| hs‐CRP >0.10 mg/dL (yes or no) | 1.681 | 0.962–2.904 | 0.06 | |||
| Nonpulmonary vein foci (yes or no) | 1.548 | 0.798–3.003 | 0.20 | |||
| Number of remaining teeth <20 (yes or no) | 0.688 | 0.354–1.336 | 0.27 | |||
| High PISA (>615 mm2)* (yes or no) | 2.701 | 1.475–4.947 | 0.001 | 2.308 | 1.234–4.315 | 0.009 |
Univariable and multivariable Cox regression analyses were applied among the nontreatment group (n=191). Variables with P<0.05 in the univariable analysis were included in the multivariable model. AF indicates atrial fibrillation; BMI, body mass index; HR, hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; NT‐proBNP, N‐terminal pro‐B‐type peptide; and PISA, periodontal inflamed surface area.
Periodontitis and Systemic Inflammation
The serum hs‐CRP level was significantly higher in the high‐PISA group than in the low‐PISA group (Figure 2E). The high‐PISA group also showed significantly higher serum levels of interleukin‐1β, interleukin‐4, interleukin‐6, interleukin‐10, interleukin‐17A, and tumor necrosis factor‐α (Figure 3).
Figure 3. PISA and inflammatory cytokines/chemokines.
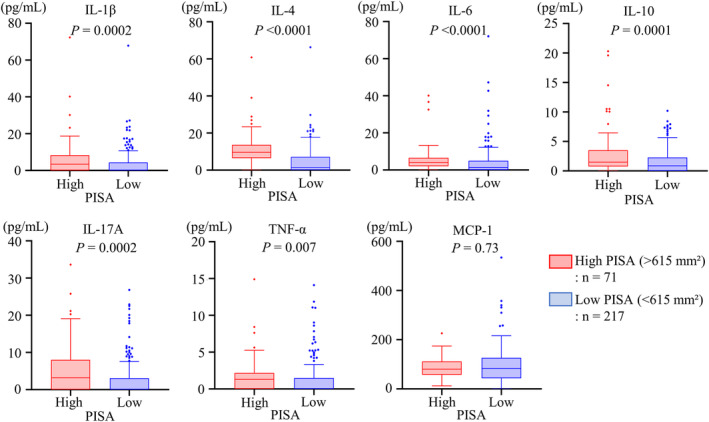
Serum levels of inflammatory cytokines and chemokines were compared between the high‐ and low‐PISA groups and analyzed using the Wilcoxon rank‐sum test. IL indicates interleukin; MCP‐1, monocyte chemoattractant protein‐1; PISA, periodontal inflamed surface area; and TNF‐α, tumor necrosis factor‐ α.
Periodontal Treatment and AF Recurrence
Periodontal treatment during the blanking period improved PISA, especially for patients with high PISA at baseline (614.3±457.2–322.0±295.2 mm2; Figure 4A). Figures 4B through 4D show the Kaplan–Meier analyses of AF recurrence between the treatment and nontreatment groups (Figure 4B, all patients; 4C, high‐PISA group; 4D, low‐PISA group). The treatment group showed significantly fewer AF recurrences than the nontreatment group within 12 months, but the differences throughout the long follow‐up period were not significant. However, the difference was more pronounced and significant throughout the entire follow‐up period in patients with high PISA. Moreover, Table 3 shows the univariable and multivariable hazard ratios (HRs) of periodontal treatment and other significant confounders for AF recurrence. Significant confounders in Table 2 were adjusted in the multivariable model, and the periodontal treatment was significantly associated with fewer cases of AF recurrence after RFCA. The sensitivity analyses showed consistent results (Figure S5 and Table S4).
Figure 4. Periodontal treatment and AF recurrence.
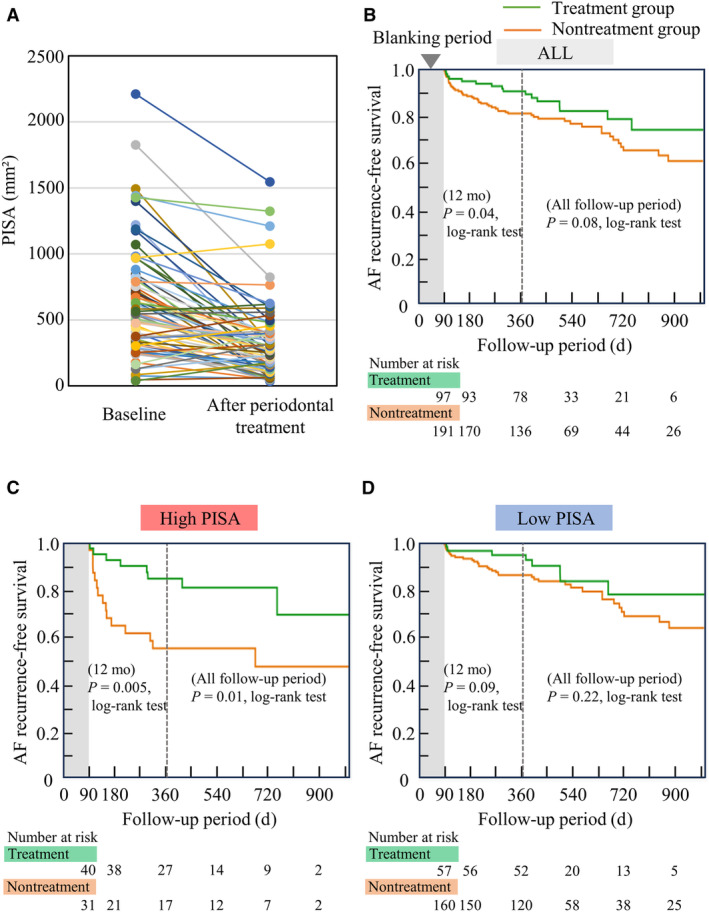
A, PISA was improved by the periodontal treatment during the blanking period (614.3±457.2–322.0±295.2 mm2). B through D, Kaplan–Meier analysis of AF recurrence between the treatment and nontreatment groups. B, All patients (N=288); C, high PISA (>615 mm2) group (n=71); D, low PISA (<615 mm2) group (n=217). Results of the log rank tests were presented for AF recurrences within 12 months and the follow‐up period. AF indicates atrial fibrillation; and PISA, periodontal inflamed surface area.
Table 3.
Cox Regression Analysis of Periodontal Treatment and AF Recurrence
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | P value | Adjusted HR | 95% CI | P value | |
| Sex, female (yes or no) | 1.894 | 1.174–3.035 | 0.008 | 2.345 | 1.427–3.853 | 0.0008 |
| Nonparoxysmal AF (yes or no) | 1.974 | 1.231–3.200 | 0.005 | 1.632 | 0.925–2.879 | 0.09 |
| AF duration >2 y (yes or no) | 2.067 | 1.277–3.346 | 0.003 | 2.013 | 1.213–3.292 | 0.005 |
| Left atrial volume>70 mL (yes or no) | 2.157 | 1.332–3.492 | 0.002 | 1.587 | 0.907–2.778 | 0.11 |
| NT‐proBNP >100 pg/mL (yes or no) | 2.461 | 1.220–4.963 | 0.01 | 1.303 | 0.592–2.869 | 0.51 |
| High PISA (>615 mm2) (yes or no) | 1.831 | 1.104–3.037 | 0.02 | 2.022 | 1.175–3.479 | 0.01 |
| Periodontal treatment (yes or no) | 0.614 | 0.350–1.076 | 0.09 | 0.393 | 0.215–0.719 | 0.002 |
Periodontal treatment and other confounders (P<0.05 in Table 2) were included in the multivariable Cox regression model. AF indicates atrial fibrillation; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and PISA, periodontal inflamed surface area.
Periodontal Pathogen and AF Recurrence
Figure S6 illustrates the relationships between periodontal pathogens and AF recurrence according to the high and low values of serum IgG antibody titers. In patients with paroxysmal AF, none of the major periodontal pathogens was related to AF recurrence. In contrast, P gingivalis (HR, 2.06 [95% CI, 1.08–3.94]; P=0.03) and F nucleatum (HR, 2.08 [95% CI, 1.09–3.97]; P=0.03) were related to a higher AF recurrence rate after ablation in those with nonparoxysmal AF.
DISCUSSION
PISA is a quantitative index reflecting the disease activity of periodontitis. This study showed that a high PISA predicted AF recurrence after RFCA. In addition, periodontal treatment during the blanking period reduced PISA, and patients who received periodontal treatment exhibited lower AF recurrence rates after RFCA.
Periodontitis and AF: Previous and Present Insights
The oral–systemic disease connection has gained research interest in recent decades, and the causal relationships between periodontitis and some systemic diseases (eg, atherosclerosis, diabetes, rheumatoid arthritis, nonalcoholic steatohepatitis, and Alzheimer disease) have been clarified. 18 Although periodontitis has not been recognized as a risk factor for AF, the negative impact of periodontitis on AF occurrence and outcome has been extensively reported. Using a large database, Chen et al reported an increased risk of AF occurrence in patients with periodontitis. 7 More recently, similar findings have been presented in some cohort studies. 8 , 9 , 10 , 11 One experimental study involving mongrel canines showed that periodontitis induced by tying ligatures at the premolar increases AF inducibility as well as blood C‐reactive protein levels. 19 Regarding the outcome of AF ablation, we first reported the negative impact of elevated serum IgG antibody titers of P gingivalis, which is a useful surrogate marker of periodontitis, 20 on sinus rhythm maintenance after RFCA. 12 One report from a Japanese institution showed a similar result. 13 Our study found that PISA, a quantitative index of periodontitis, can be a hallmark of AF recurrence after RFCA. To the best of our knowledge, this study is the first to demonstrate the benefit of periodontal treatment for patients who underwent AF ablation. Periodontal treatment during the blanking period prevented AF recurrences in patients with a high PISA. We conducted this study following a nonrandomized design to obtain initial evidence for this issue. Based on the results, a multicenter randomized study is warranted.
Mechanism Linking Periodontitis and AF, and Ablation Outcome
Although the mechanism linking periodontitis and AF pathogenesis has not been elucidated, it may first be explained by systemic inflammation induced by periodontitis. One of the unique points of this study was that periodontal inflammation was quantified by PISA. PISA is the sum of all bleeding areas in periodontal pocket epithelia, and it reflects the quantification of the clinical periodontal inflammation degree. 15 Our results found that the high‐PISA group had significantly higher serum levels of hs‐CRP and inflammatory cytokines than the low‐PISA group, indicating that patients with severe periodontitis have a persistent systemic inflammatory status. PISA increment also reportedly has a positive relationship with inflammatory cytokine elevation both in saliva and blood. 16 , 17 , 21 Furthermore, periodontitis activates the level of nucleotide‐binding oligomerization domain–like receptor family pyrin domain‐containing protein‐3 inflammasome, 22 which has recently been suggested to be pathogenic for AF. 23 Therefore, periodontitis may elicit a long‐standing systemic inflammation that is closely associated with AF development. 24 , 25 , 26 Although the standard cutoff value remains unclear, a PISA of ≈500 mm2 has been suggested to evoke systemic inflammation. 16 Miki et al reported a significant difference in hs‐CRP levels between groups with high PISA (>500 mm2) and low PISA (<500 mm2) in the Japanese population. 17 In this study, we obtained the cutoff value of PISA for AF recurrence as 615.8 mm2 on the basis of the receiver operating characteristic analysis. Furthermore, we applied a PISA cutoff value of 500 mm2 for the sensitivity analyses, and a PISA value of >500 mm2 also predicted AF recurrence (Figure S4 and Table S3). Considering our results and the above‐mentioned studies in the literature, 16 , 17 a PISA value of about 500 to 600 mm2 is likely to be sufficient for eliciting systemic inflammation and for serving as the clinical hallmark. Atrial fibrosis is responsible for conduction disturbances and is a central component of the AF substrate. 27 We have recently reported histological evidence that revealed a positive correlation between PISA and atrial fibrosis using left atrial appendages. 28 It is highly possible for periodontitis to persistently induce systemic inflammation and affect the interaction among systemic inflammation, atrial fibrosis, and AF. Inflammation also plays an important role in AF recurrence after ablation, and some anti‐inflammatory therapies, such as colchicine, can reduce AF recurrences. 29 Although this study did not include the data of changes of inflammatory markers after periodontal treatment, sufficient evidence revealed that periodontal treatment can reduce systemic inflammation. 30 , 31 In contrast, although inflammation might cause early recurrence, 32 PISA and inflammatory markers at baseline were not related to early AF recurrence in this study. Early AF recurrence developed during the blanking period may largely depend on the inflammation elicited by the procedures. 32
Furthermore, the serum IgG antibody titer of specific periodontal pathogens (P gingivalis and F nucleatum) was related to AF recurrence only in nonparoxysmal AF. Structural remodeling and fibrotic changes within the atrium are responsible for AF perpetuation in nonparoxysmal AF, whereas PV triggers are the main initiators of paroxysmal AF. 33 Thus, specific periodontal pathogens might affect AF substrate apart from systemic inflammation. Further pathophysiological study is needed to elucidate the causal relationship between periodontitis and AF through inflammation or another bacteriological aspect.
Clinical Implications
Currently, the modifiable risk factors for AF include obesity, physical inactivity, disordered breathing during sleep, diabetes, hypertension, hyperlipidemia, tobacco intake, alcohol consumption, and caffeine consumption. 5 Our study indicates that periodontitis, which can be modified by dental intervention, should also be recognized as a modifiable risk factor for AF. The American Heart Association recommends an integrated work involving primary care physicians, sleep physicians, exercise physiologists, psychologists, endocrine specialists, dietitians, and pharmacists for a comprehensive risk management for AF. 5 Dental specialists should also participate in this team. Providing periodontal examination is important for AF ablation candidates, and PISA can be a hallmark of AF recurrence. Patients with a high PISA should be more carefully examined for AF recurrence after ablation, and providing periodontal treatment during the blanking period is highly beneficial for them. In this study protocol, periodontal treatment was provided only during the blanking period, and its efficacy decreased over 1 year of follow‐up. Thus, regular periodontal care after the blanking period is necessary to maintain the treatment efficacy throughout the remote period. Furthermore, for AF ablation candidates, completing periodontal treatment before AF ablation might have additional merit. Future studies must clarify the optimal protocol of perioperative periodontal treatment.
Study Limitations
This study is limited by its single‐center, nonrandomized study design. In addition, the sample size was small, especially for the treatment group. Receiving periodontal treatment depended on the intention of patients who were informed of their periodontitis status. Although many patients with severe periodontitis were included in the treatment group, this group showed better clinical outcomes overall. In contrast, patients who wished to receive periodontal treatment might be more aware of total risk optimization than those who did not. Moreover, AF recurrences were carefully examined according to clinical symptoms, 12‐lead ECG, 24‐hour Holter recording, and mobile ECG monitoring, but the recurrences might be underestimated, especially for asymptomatic patients. Another limitation is that the periodontitis status was not followed up after the initial examination of the nontreatment group. Inflammatory markers also were not retested after the RFCA.
CONCLUSIONS
Periodontitis serves as a modifiable risk factor for AF. Hence, periodontal examination is beneficial for AF ablation candidates. Periodontal treatment during the blanking period can modify the periodontal inflammation and improve the AF ablation outcome. However, a multicenter randomized study is needed to confirm these results.
Sources of Funding
This study was supported by the Japan Society for the Promotion of Science (Tokyo, Japan) Grant‐in‐Aid for Research Activity Start‐up (21K20924) to Dr S. Miyauchi and Grant‐in‐Aid for Scientific Research (B) (21H03112) to Dr M. Miyauchi. Dr S. Miyauchi also received funding from Johnson and Johnson Medical Research Grant (AS2021A000008479).
Disclosures
None.
Supporting information
Data S1
Tables S1–S4
Figures S1–S6
Acknowledgments
The authors thank Yoko Hayashi (Natural Science Center for Basic Research and Development, Hiroshima University) and Ruri Mikami (research assistant) for supporting the measurement of inflammatory cytokines. We also thank Prof. Yuichi Sakumura (Data Science Center, Nara Institute of Science and Technology) for his advice regarding the statistical analysis. The authors also thank the members of the clerical and medical staff at Hiroshima University Hospital for their assistance and the ENAGO Group (English editing system) for editing a draft of this manuscript.
This manuscript was sent to Luciano A. Sposato, MD, MBA, FRCPC, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.033740
For Sources of Funding and Disclosures, see page 11.
References
- 1. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turagam MK, Musikantow D, Whang W, Koruth JS, Miller MA, Langan MN, Sofi A, Choudry S, Dukkipati SR, Reddy VY. Assessment of catheter ablation or antiarrhythmic drugs for first‐line therapy of atrial fibrillation: a meta‐analysis of randomized clinical trials. JAMA Cardiol. 2021;6:697–705. doi: 10.1001/jamacardio.2021.0852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wynn GJ, Das M, Bonnett LJ, Panikker S, Wong T, Gupta D. Efficacy of catheter ablation for persistent atrial fibrillation: a systematic review and meta‐analysis of evidence from randomized and nonrandomized controlled trials. Circ Arrhythm Electrophysiol. 2014;7:841–852. doi: 10.1161/CIRCEP.114.001759 [DOI] [PubMed] [Google Scholar]
- 4. Balk EM, Garlitski AC, Alsheikh‐Ali AA, Terasawa T, Chung M, Ip S. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol. 2010;21:1208–1216. doi: 10.1111/j.1540-8167.2010.01798.x [DOI] [PubMed] [Google Scholar]
- 5. Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JA, Noseworthy PA, Pack QR, Sanders P, Trulock KM, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750–e772. doi: 10.1161/CIR.0000000000000748 [DOI] [PubMed] [Google Scholar]
- 6. Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136:583–596. doi: 10.1161/CIRCULATIONAHA.116.023163 [DOI] [PubMed] [Google Scholar]
- 7. Chen DY, Lin CH, Chen YM, Chen HH. Risk of atrial fibrillation or flutter associated with periodontitis: a nationwide, population‐based, cohort study. PLoS One. 2016;11:e0165601. doi: 10.1371/journal.pone.0165601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang Y, Woo HG, Park J, Lee JS, Song TJ. Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: a nationwide population‐based cohort study. Eur J Prev Cardiol. 2020;27:1835–1845. doi: 10.1177/2047487319886018 [DOI] [PubMed] [Google Scholar]
- 9. Sen S, Redd K, Trivedi T, Moss K, Alonso A, Soliman EZ, Magnani JW, Chen LY, Gottesman RF, Rosamond W, et al. Periodontal disease, atrial fibrillation and stroke. Am Heart J. 2021;235:36–43. doi: 10.1016/j.ahj.2021.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Im SI, Heo J, Kim BJ, Cho KI, Kim HS, Heo JH, Hwang JY. Impact of periodontitis as representative of chronic inflammation on long‐term clinical outcomes in patients with atrial fibrillation. Open Heart. 2018;5:e000708. doi: 10.1136/openhrt-2017-000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Struppek J, Schnabel RB, Walther C, Heydecke G, Seedorf U, Lamprecht R, Smeets R, Borof K, Zeller T, Beikler T, et al. Periodontitis, dental plaque, and atrial fibrillation in the Hamburg City health study. PLoS One. 2021;16:e0259652. doi: 10.1371/journal.pone.0259652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miyauchi S, Tokuyama T, Shintani T, Nishi H, Hamamoto Y, Ouhara K, Furusho H, Miyauchi M, Komatsuzawa H, Nakano Y. Periodontitis and the outcome of atrial fibrillation ablation: Porphyromonas gingivalis is related to atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2021;32:1240–1250. doi: 10.1111/jce.14952 [DOI] [PubMed] [Google Scholar]
- 13. Tashiro A, Yonetsu T, Aoyama N, Shiheido‐Watanabe Y, Niida T, Miyazaki S, Maejima Y, Goya M, Isobe M, Iwata T, et al. Periodontitis was associated with worse clinical outcomes after catheter ablation for paroxysmal atrial fibrillation. Front Cardiovasc Med. 2023;9:1061243. doi: 10.3389/fcvm.2022.1061243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 15. Nesse W, Abbas F, van der Ploeg I, Spijkervet FK, Dijkstra PU, Vissink A. Periodontal inflamed surface area: quantifying inflammatory burden. J Clin Periodontol. 2008;35:668–673. doi: 10.1111/j.1600-051X.2008.01249.x [DOI] [PubMed] [Google Scholar]
- 16. Schöffer C, Oliveira LM, Santi SS, Antoniazzi RP, Zanatta FB. C‐reactive protein levels are associated with periodontitis and periodontal inflamed surface area in adults with end‐stage renal disease. J Periodontol. 2021;92:793–802. doi: 10.1002/JPER.20-0200 [DOI] [PubMed] [Google Scholar]
- 17. Miki K, Kitamura M, Hatta K, Kamide K, Gondo Y, Yamashita M, Takedachi M, Nozaki T, Fujihara C, Kashiwagi Y, et al. Periodontal inflamed surface area is associated with hs‐CRP in septuagenarian Japanese adults in cross‐sectional findings from the SONIC study. Sci Rep. 2021;11:14436. doi: 10.1038/s41598-021-93872-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76:2089–2100. doi: 10.1902/jop.2005.76.11-S.2089 [DOI] [PubMed] [Google Scholar]
- 19. Yu G, Yu Y, Li YN, Shu R. Effect of periodontitis on susceptibility to atrial fibrillation in an animal model. J Electrocardiol. 2010;43:359–366. doi: 10.1016/j.jelectrocard.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 20. Papapanou PN, Neiderud AM, Sandros J, Dahlén G. Checkerboard assessments of serum antibodies to oral microbiota as surrogate markers of clinical periodontal status. J Clin Periodontol. 2001;28:103–106. doi: 10.1034/j.1600-051x.2001.280116.x [DOI] [PubMed] [Google Scholar]
- 21. Tang V, Hamidi B, Janal MN, Barber CA, Godder B, Palomo L, Kamer AR. Periodontal inflamed surface area (PISA) associates with composites of salivary cytokines. PLoS One. 2023;18:e0280333. doi: 10.1371/journal.pone.0280333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Isola G, Polizzi A, Santonocito S, Alibrandi A, Williams RC. Periodontitis activates the NLRP3 inflammasome in serum and saliva. J Periodontol. 2022;93:135–145. doi: 10.1002/JPER.21-0049 [DOI] [PubMed] [Google Scholar]
- 23. Yao C, Veleva T, Scott L Jr, Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu‐Taha I, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. doi: 10.1161/CIRCULATIONAHA.118.035202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen MJ, Arora R, Jalife J. Atrial myopathy. JACC Basic Transl Sci. 2019;4:640–654. doi: 10.1016/j.jacbts.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. doi: 10.1016/j.jacc.2012.04.063 [DOI] [PubMed] [Google Scholar]
- 26. Goette A. Is periodontitis a modifiable risk factor for atrial fibrillation substrate? JACC Clin Electrophysiol. 2023;9:54–56. doi: 10.1016/j.jacep.2022.09.013 [DOI] [PubMed] [Google Scholar]
- 27. Nattel S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin Electrophysiol. 2017;3:425–435. doi: 10.1016/j.jacep.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 28. Miyauchi S, Nishi H, Ouhara K, Tokuyama T, Okubo Y, Okamura S, Miyamoto S, Oguri N, Uotani Y, Takasaki T, et al. Relationship between periodontitis and atrial fibrosis in atrial fibrillation: histological evaluation of left atrial appendages. JACC Clin Electrophysiol. 2023;9:43–53. doi: 10.1016/j.jacep.2022.08.018 [DOI] [PubMed] [Google Scholar]
- 29. Varghese B, Feldman DI, Chew C, Valilis E, Blumenthal RS, Sharma G, Calkins H. Inflammation, atrial fibrillation, and the potential role for colchicine therapy. Heart Rhythm. 2021;O2(2):298–303. doi: 10.1016/j.hroo.2021.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luthra S, Orlandi M, Hussain SB, Leira Y, Botelho J, Machado V, Mendes JJ, Marletta D, Harden S, D'Aiuto F. Treatment of periodontitis and C‐reactive protein: a systematic review and meta‐analysis of randomized clinical trials. J Clin Periodontol. 2023;50:45–60. doi: 10.1111/jcpe.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, Jia R, Zhang Y, Sun X, Mei Y, Zou R, Niu L, Dong S. Effect of non‐surgical periodontal treatment on cytokines/adipocytokines levels among periodontitis patients with or without obesity: a systematic review and meta‐analysis. BMC Oral Health. 2023;23:717. doi: 10.1186/s12903-023-03383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gottlieb LA, Dekker LRC, Coronel R. The blinding period following ablation therapy for atrial fibrillation: proarrhythmic and antiarrhythmic pathophysiological mechanisms. JACC Clin Electrophysiol. 2021;7:416–430. doi: 10.1016/j.jacep.2021.01.011 [DOI] [PubMed] [Google Scholar]
- 33. Kottkamp H. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J. 2013;34:2731–2738. doi: 10.1093/eurheartj/eht194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S4
Figures S1–S6
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


