Abstract
Background
Percutaneous heart valve procedures have been increasingly performed over the past decade, yet real‐world mortality data on valvular heart disease (VHD) in the United States remain limited.
Methods and Results
We queried the Centers for Disease Control and Prevention's Wide‐Ranging Online Data for Epidemiologic Research database among patients ≥15 years old from 1999 to 2020. VHD and its subtypes were listed as the underlying cause of death. We calculated age‐adjusted mortality rate (AAMR) per 100 000 individuals and determined overall trends by estimating the average annual percent change using the Joinpoint regression program. Subgroup analyses were performed based on demographic and geographic factors. In the 22‐year study, there were 446 096 VHD deaths, accounting for 0.80% of all‐cause mortality (56 014 102 people) and 2.38% of the total cardiovascular mortality (18 759 451 people). Aortic stenosis recorded the highest mortality of VHD‐related death in both male (109 529, 61.74%) and female (166 930, 62.13%) populations. The AAMR of VHD has declined from 8.4 (95% CI, 8.2–8.5) to 6.6 (95% CI, 6.5–6.7) per 100 000 population. Similar decreasing AAMR trends were also seen for the VHD subtypes. Men recorded higher AAMR for aortic stenosis and aortic regurgitation, whereas women had higher AAMR for mitral stenosis and mitral regurgitation. Mitral regurgitation had the highest change in average annual percent change in AAMR.
Conclusions
The mortality rate of VHD among the US population has declined over the past 2 decades. This highlights the likely efficacy of increasing surveillance and advancement in the management of VHD, resulting in improved outcomes.
Keywords: aortic valvular disease, mitral valvular disease, mortality, trend and disparity, valvular heart disease
Subject Categories: Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- AAMR
age‐adjusted mortality rate
- AAPC
average annual percent change
- APC
average percent change
- AS
aortic stenosis
- AR
aortic regurgitation
- CDC WONDER
Centers for Disease Control and Prevention's Wide‐Ranging Online Data for Epidemiologic Research
- FDA
U.S. Food and Drug Administration
- MR
mitral regurgitation
- MS
mitral stenosis
- SAVR
surgical aortic valve replacement
- TAVR
transcatheter aortic valve replacement
- TEER
transcatheter edge‐to‐edge‐repair
- VHD
valvular heart disease
Clinical Perspective.
What Is New?
There were decreasing trends in the overall age‐adjusted mortality rate due to valvular heart disease and its subtypes from 1999 to 2020.
Aortic stenosis was the most common type of valvular heart disease in patients who died from valvular heart disease.
What Are the Clinical Implications?
Our findings highlight the improving survival of patients with valvular heart disease due to contemporary management algorithms and likely the increased use of transcutaneous aortic valve intervention.
Valvular heart disease (VHD) is one of the most common diseases in the United States, with an estimated prevalence of 2.5% among adults. 1 , 2 , 3 Among VHD, aortic and mitral valvular diseases are the 2 most diagnosed diseases in the recent era. 1 , 4 Management of VHD was limited to the surgical approach in the past. Newer, less invasive percutaneous valvular repair or replacement procedures have been introduced in the past decade. The first transcatheter aortic valve replacement (TAVR) valve was approved by the US Food and Drug Administration (FDA) in 2011, with subsequent iterative expansion of indication for use associated with continued improvement in design and associated procedural outcomes. In 2013, the FDA's approval of the MitraClip for transcatheter edge‐to‐edge repair transformed the management of mitral regurgitation (MR). 5 Recently, in 2022, the FDA further approved the second percutaneous PASCAL transcatheter edge‐to‐edge repair system as a treatment for severe degenerative MR. The use of these transcatheter therapies is rising and has been associated with improved patient outcomes in clinical trials. 6 , 7 , 8
Despite advances in the therapeutic approach to VHD, real‐world data on the impact on population mortality are not well‐established. Thus, we sought to analyze the trend in mortality due to VHD in the United States and to evaluate the changes in mortality following the introduction of these advanced approaches.
Methods
Data Source
We declare that all supporting data are available within the article and the online supplementary files. We conducted a retrospective cross‐sectional analysis using data obtained from the Centers for Disease Control and Prevention's Wide‐Ranging Online Data for Epidemiologic Research (CDC WONDER). This online database contains national mortality and population data in the United States, and our topic of interest is based on the Multiple Cause of Death database. The database is encoded based on death certificates of US residents from January 1, 1999 to December 31, 2020, and contains information on the underlying cause of death along with demographic data. 9 International Classification of Diseases, Tenth Revision (ICD‐10) was used to classify the causes of death for 1999 and beyond. This study approach has been validated in similar research on other topics of interest. 10 , 11 , 12 Our study did not require institutional review board approval because the population data are deidentified and publicly available. This research did not require informed consent because the population data are deidentified and publicly available.
Using the CDC WONDER from 1999 to 2020, we first evaluated demographics for all‐cause mortality in the general population, followed by overall cardiovascular death and, eventually, mortality in VHD with its subtypes. To analyze age‐adjusted mortality rate (AAMR; standardized to 2000 US Census proportions), we selected valvular heart disease (ICD‐10 codes I05–I08 and I34–I37) as the underlying cause of death. The World Health Organization defines the underlying cause of death as the disease or injury that initiates a sequence of events that leads directly to death. 9 Demographic features were used to stratify the study population based on age, sex, race, and geographic region of residence. Subsequently, we explored various subtypes of valvular heart disease, including MR (ICD‐10 I05.1, I34.0, I34.1), mitral stenosis (MS) (ICD‐10 I05.0, I34.2), aortic regurgitation (AR) (ICD‐10 I06.1, I35.1), and aortic stenosis (AS) (ICD‐10 I06.0, I35.0). 1 , 4 Individuals <15 years old or individuals with unknown age at the time of death on the death certificate were excluded from the data query.
Statistical Analysis
We obtained the AAMR for overall VHD and each subtype, stratified by sex, directly from the CDC WONDER database and charted the trends throughout the study period. The AAMR per 100 000 were calculated using the direct method by applying age‐specific rates in a population of interest to the 2000 US Standard Population. 13 This allows for the reduction of confounding effects due to varying age structures and enables meaningful comparisons across different populations. We used the Joinpoint regression program (Joinpoint V4.9.1.0; National Cancer Institute) to evaluate trends of AAMR in each subgroup. This method, as described in previous similar studies, determines the significance of AAMR changes over time using log‐linear regression models where temporal variation occurred. 14 , 15 Annual percent change with 95% CI for the AAMR was calculated using the Monte Carlo permutation test at the identified line segments linking Joinpoint. Afterward, the weighted averages of the annual percent changes, also known as average annual percent change (AAPC), were calculated with corresponding 95% CI, which reflects the summary of the mortality trends in the study period. Statistical significance was set at P≤0.05 using a 2‐tailed t test in all analyses. We also examined the percentage of mortality in each VHD subtype by age and sex groups. The population was further categorized into urban (large central metro, large fringe metro, medium metro, and small metro counties) and rural (micropolitan nonmetro and noncore nonmetro counties) according to the 2013 US Census classifications.
Results
The baseline demographics of patients who met the inclusion criteria are shown in Table 1. In the 22‐year study period from 1999 to 2020, there were 446 096 VHD deaths, accounting for 0.80% of all‐cause mortality (56 014 102 people) and 2.38% of total cardiovascular mortality (18 759 451 people). The different valvular subtypes of VHD deaths are depicted in Figure 1. AS recorded the highest mortality of VHD‐related death in both male (109 529, 61.74%) and female populations (166 930, 62.13%). This was followed by MR (19 001, 10.71%), AR (5995, 3.3%), and MS (2447, 1.38%) in men, and MR (33 907, 12.62%), MS (9655, 3.59%), and AR (5893, 2.19%) in women.
Table 1.
Baseline Characteristics of All‐Cause Mortality, Overall Cardiovascular Death, Valvular Heart Disease and its Subtypes
| Demographic | All‐cause, n (%) | Cardiovascular death, n (%) | VHD, n (%) | AR, n (%) | AS, n (%) | MR, n (%) | MS, n (%) |
|---|---|---|---|---|---|---|---|
| n=56 014 102 | n=18 759 451 | n=446 096 | n=11 888 | n=276 459 | n=52 908 | n=12 102 | |
| Sex | |||||||
| Women | 27 975 534 (49.94) | 9 569 325 (51.01) | 268 684 (60.23) | 5893 (49.57) | 166 930 (60.38) | 33 907 (64.09) | 9655 (79.78) |
| Men | 28 038 568 (50.06) | 9 190 126 (48.99) | 177 412 (39.77) | 5995 (50.43) | 109 529 (39.62) | 19 001 (35.91) | 2447 (20.22) |
| Age of death, y | |||||||
| 15–24 | 696 479 (1.24) | 28 734 (0.15) | 768 (0.17) | 33 (0.28) | 92 (0.03) | 270 (0.51) | 29 (0.24) |
| 25–34 | 1 043 043 (1.86) | 94 568 (0.50) | 2305 (0.52) | 144 (1.21) | 190 (0.07) | 605 (1.14) | 84 (0.69) |
| 35–44 | 1 783 349 (3.18) | 328 697 (1.75) | 4926 (1.10) | 353 (2.97) | 646 (0.23) | 1157 (2.19) | 279 (2.31) |
| 45–54 | 3 869 682 (6.91) | 979 367 (5.22) | 11 315 (2.54) | 771 (6.49) | 2345 (0.85) | 2114 (4.00) | 741 (6.12) |
| 55–64 | 6 885 039 (12.29) | 1 933 512 (10.31) | 23 278 (5.22) | 1209 (10.17) | 7314 (2.65) | 3618 (6.84) | 1383 (11.43) |
| 65–74 | 10 034 288 (17.91) | 3 008 892 (16.04) | 48 599 (10.89) | 1725 (14.51) | 21 048 (7.61) | 6883 (13.01) | 2361 (19.51) |
| 75–84 | 14 723 222 (26.28) | 5 154 065 (27.47) | 118 953 (26.67) | 3020 (25.40) | 69 231 (25.04) | 15 276 (28.87) | 3902 (32.24) |
| 85+ | 16 979 000 (30.31) | 7 231 616 (38.55) | 235 952 (52.89) | 4633 (38.97) | 175 593 (63.52) | 22 985 (43.44) | 3323 (27.46) |
| Race | |||||||
| American Indian or Alaska Native | 345 850 (0.62) | 85 803 (0.46) | 1577 (0.35) | 69 (0.58) | 760 (0.27) | 177 (0.33) | 84 (0.69) |
| Asian or Pacific Islander | 1 178 072 (2.10) | 396 524 (2.11) | 7723 (1.73) | 318 (2.67) | 3700 (1.34) | 1280 (2.42) | 446 (3.69) |
| Black | 6 586 732 (11.76) | 2 256 923 (12.03) | 24 039 (5.39) | 1395 (11.73) | 10 629 (3.84) | 3792 (7.17) | 896 (7.40) |
| White | 47 903 448 (85.52) | 16 020 201 (85.40) | 412 757 (92.53) | 10 106 (85.01) | 261 370 (94.54) | 47 659 (90.08) | 10 676 (88.22) |
| Census region | |||||||
| Region 1 | 10 555 896 (18.85) | 3 697 047 (19.71) | 97 441 (21.84) | 2236 (18.81) | 64 196 (23.22) | 11 030 (20.85) | 2421 (20.00) |
| Region 2 | 13 061 398 (23.32) | 4 388 498 (23.39) | 110 504 (24.77) | 2754 (23.17) | 68 361 (24.73) | 13 254 (25.05) | 2960 (24.46) |
| Region 3 | 21 474 392 (38.34) | 7 087 291 (37.78) | 133 053 (29.83) | 2889 (24.30) | 78 898 (28.54) | 16 308 (30.82) | 3744 (30.94) |
| Region 4 | 10 922 416 (19.50) | 3 586 615 (19.12) | 105 098 (23.56) | 4009 (33.72) | 65 004 (23.51) | 12 316 (23.28) | 2977 (24.60) |
AR indicates aortic regurgitation; AS, aortic stenosis; MR, mitral regurgitation; MS, mitral stenosis; and VHD, valvular heart disease.
Figure 1. State‐level age‐adjusted mortality rate for VHD and trends in AAMR for VHD subtypes, stratified by sex, between 1999 and 2020.
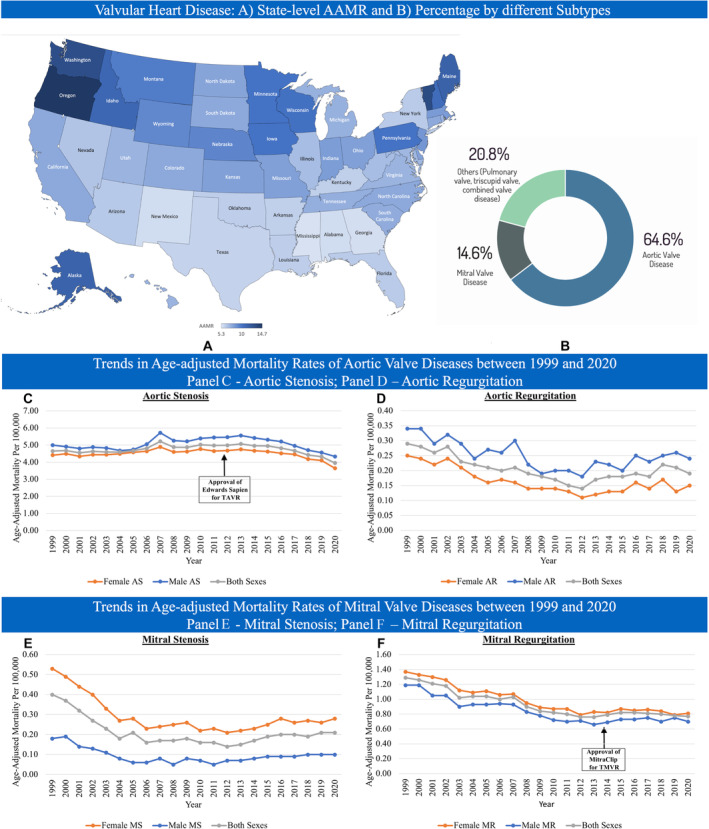
AAMR indicates age‐adjusted mortality rate; AR, aortic regurgitation; AS, aortic stenosis; MR, mitral regurgitation; MS, mitral stenosis; TAVR, transcatheter aortic valve replacement; TMVR, transcatheter mitral valve replacement; and VHD, valvular heart diseases.
Regional Differences
When overall VHD mortality was cross‐examined based on regional differences, the South (census region 3) had the highest percentage of VHD mortality (133 053, 29.83%), followed by the Midwest (census region 2, 24.77%), the West (census region 4, 23.56%), and the Northeast (census region 1, 21.84%) (Table 2). The West had the highest AAMR of VHD (8.57 [95% CI, 8.52–8.63] per 100 000 people), followed by the Midwest (8.27 [95% CI, 8.22–8.32] per 100 000 people), Northeast (8.19 [95% CI, 8.13–8.24] per 100 000 people), and South (6.49 [95% CI, 6.45–6.52] per 100 000 people). When the AAMR of VHD subtypes was analyzed, the rates were the highest in the West (AS, 5.31 [95% CI, 5.27–5.35] per 100 000 people; MR, 1.02 [95% CI, 1.00–1.04] per 100 000 people; MS, 0.24 [95% CI, 0.23–0.24] per 100 000 people), except for AR, which was the highest in the Midwest (0.22 [95% CI, 0.21–0.23] per 100 000 people). The Northeast and South shared the lowest AAMR of AR (0.18 [95% CI, 0.18–0.19] per 100 000 people), and the South had the lowest AAMR for the other VHD subtypes (AS, 3.86 [95% CI, 3.83–3.89] per 100 000 people; MR, 0.79 [95% CI, 0.77–0.80] per 100 000 people; MS, 0.17 [95% CI, 0.16–0.18] per 100 000 people). In terms of urbanization, rural regions had a higher AAMR of overall VHDs compared with urban regions (8.28 [95% CI, 8.22–8.34] versus 7.57 [95% CI 7.55–7.60] per 100 000 people). In rural regions, the AAMR of VHD fluctuated from 8.31 (95% CI, 8.03–8.58) per 100 000 people in 1999 to 7.74 (95% CI, 7.50–7.98) per 100 000 people in 2020, with an AAPC of −0.07 (95% CI, −0.32 to 0.18). On the other hand, the AAMR of VHDs in urban regions had decreased from 8.37 (95% CI, 8.23–8.50) per 100 000 people in 1999 to 6.39 (95% CI, 6.29–6.48) per 100 000 people in 2020, with an AAPC of −0.89 (95% CI, −1.11 to −0.67) (Figures S1–S5).
Table 2.
Census Population and Cardiovascular and Valvular Heart Disease Deaths by Region
| Population | Northeast (region 1), n (%) | Midwest (region 2), n (%) | South (region 3), n (%) | West (region 4), n (%) | Total, n (%) |
|---|---|---|---|---|---|
| Total population | 989 744 746 | 1 174 560 460 | 1 998 181 533 | 1 247 162 472 | 5 409 649 211 |
| All‐cause mortality | 10 555 896 (18.85) | 13 061 398 (23.32) | 21 474 392 (38.34) | 10 922 416 (19.50) | 56 014 102 (100) |
| Cardiovascular disease deaths | 3 697 047 (19.71) | 4 388 498 (23.39) | 7 087 291 (37.78) | 3 586 615 (19.12) | 18 759 451 (100) |
| Valvular heart disease deaths | 97 441 (21.84) | 110 504 (24.77) | 133 053 (29.83) | 105 098 (23.56) | 446 096 (100) |
| Aortic regurgitation deaths | 2236 (18.81) | 2754 (23.17) | 2889 (24.30) | 4009 (33.72) | 11 888 (100) |
| Aortic stenosis deaths | 64 196 (23.22) | 68 361 (24.73) | 78 898 (28.54) | 65 004 (23.51) | 276 459 (100) |
| Mitral regurgitation deaths | 11 030 (20.85) | 13 254 (25.05) | 16 308 (30.82) | 12 316 (23.28) | 52 908 (100) |
| Mitral stenosis deaths | 2421 (20.00) | 2960 (24.46) | 3744 (30.94) | 2977 (24.60) | 12 102 (100) |
Trends and AAPCs in AAMR Between 1999 and 2020
Figure 2 shows the trend in AAMR of overall VHD from 1999 to 2020. The AAMR of VHD decreased from 8.36 (95% CI, 8.24–8.48) per 100 000 people in 1999 to 6.61 (95% CI, 6.52–6.70) per 100 000 people in 2020, with an AAPC of −0.75 (95% CI, −0.97 to −0.52). When stratified by VHD subtypes, similar decreasing trends were seen in AS (from 4.65 [95% CI, 4.56–4.75] to 3.95 [95% CI, 3.88–4.02] per 100 000 people), AR (from 0.29 [95% CI, 0.26–0.31] to 0.19 [95% CI, 0.17–0.20] per 100 000 people), MR (from 1.29 [95% CI, 1.24–1.34] to 0.77 [95% CI, 0.74–0.80] per 100 000 people), and MS (from 0.40 [95% CI, 0.37–0.42] to 0.21 [95% CI, 0.19–0.23] per 100 000 people). During the study period, the AAMR of AS and AR were higher in men than in women. Female patients had higher AAMR of MR and MS than male patients. The AAPC in AAMR was higher in mitral diseases (MR, –2.47 [95% CI, −2.99 to −1.96] and MS, –2.37 [95% CI, −4.00 to −0.72]) than in aortic diseases (AR, –1.89 [95% CI, −2.94 to −0.83] and AS, −0.17 [95% CI, −0.61 to 0.26]) (Table 3). Most of the deaths seen in the VHD and its subtypes happened in the age group of ≥85 years old, except for MS, which occurred in the age group of 75 to 84 years old (Figure 3).
Figure 2. Trends in age‐adjusted mortality rate for VHDs, stratified by sex, between 1999 and 2020.
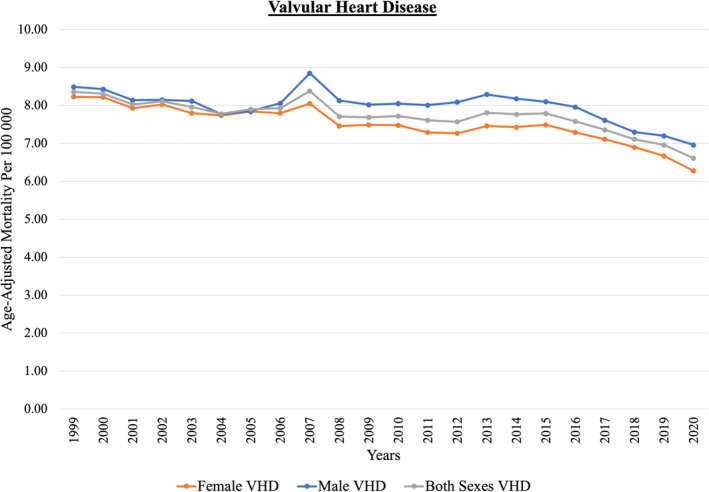
VHD indicates valvular heart disease.
Table 3.
Age‐Adjusted Mortality Rate for Valvular Heart Disease With Its Subtypes in 1999 and 2020, and Average Annual Percent Change by Age Group
| Diseases | Overall | 15–64 years old | ≥65 years old | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1999 AAMR | 2020 AAMR | Average annual percent change, (95% CI) | 1999 AAMR | 2020 AAMR | Average annual percent change, (95% CI) % | 1999 AAMR | 2020 AAMR | Average annual percent change, (95% CI) % | |
| Valvular heart diseases | 8.36 (8.24 to 8.48) | 6.61 (6.52 to 6.70) | −0.75 (−0.97 to −0.52) | 1.07 (1.02 to 1.11) | 0.88 (0.84 to 0.92) | −0.96 (−1.61 to −0.31) | 46.38 (45.66 to 47.10) | 36.45 (35.93 to 36.97) | −0.73 (−1.00 to −0.45) |
| Aortic regurgitation | 0.29 (0.26 to 0.31) | 0.19 (0.17 to 0.20) | −1.89 (−2.94 to −0.83) | 0.06 (0.05 to 0.07) | 0.05 (0.04 to 0.06) | 0.14 (−2.24 to 2.59) | 1.47 (1.35 to 1.60) | 0.91 (0.83 to 0.99) | −2.41 (−3.30 to −1.50) |
| Aortic stenosis | 4.65 (4.56 to 4.75) | 3.95 (3.88 to 4.02) | −0.17 (−0.61 to 0.26) | 0.22 (0.20 to 0.24) | 0.19 (0.18 to 0.21) | −0.06 (−0.78 to 0.66) | 27.77 (27.22 to 28.33) | 23.52 (23.10 to 23.93) | −0.18 (−0.64 to 0.28) |
| Mitral regurgitation | 1.29 (1.24 to 1.34) | 0.77 (0.74 to 0.80) | −2.47 (−2.99 to −1.96) | 0.23 (0.21 to 0.25) | 0.13 (0.12 to 0.15) | −2.97 (−3.56 to −2.37) | 6.79 (6.51 to 7.06) | 4.08 (3.91 to 4.25) | −2.39 (−2.91 to −1.86) |
| Mitral stenosis | 0.40 (0.37 to 0.42) | 0.21 (0.19 to 0.23) | −2.37 (−4.00 to −0.72) | 0.12 (0.10 to 0.14) | 0.05 (0.04 to 0.06) | −4.61 (−8.46 to −0.59) | 1.84 (1.70 to 1.98) | 1.06 (0.97 to 1.14) | −1.80 (−3.1 to −0.5) |
AAMR indicates age‐adjusted mortality rate.
Figure 3. Percent of mortality due to cardiovascular disease and valvular heart disease with its subtypes in different age groups.
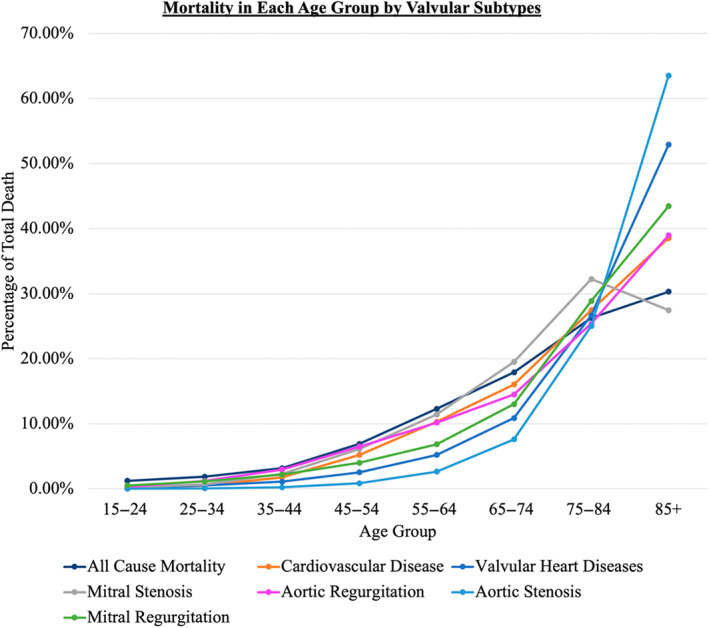
Proportionate Mortality
Overall, the proportionate mortality rate of cardiovascular death (total cardiovascular mortality divided by total all‐cause mortality) has decreased from 40.55% in 1999 to 27.66% in 2020. The proportionate mortality of VHD (total VHD mortality divided by total cardiovascular mortality) showed an increment from 1.87% in 1999 to 2.32% in 2020. The proportionate mortality of VHD increased from 2.19% to 2.76% and 1.50% to 1.91% among men and women, respectively. This increment in proportionate mortality rate was seen in AS and AR, but MR and MS showed a decrease in proportionate mortality rate.
Trends in AAMR of Infective Endocarditis
Additional analysis was performed to assess trends in the AAMR of infective endocarditis during the study period (Figure S2). Our analysis revealed a mild decreasing trend in the AAMR of infective endocarditis from 5.41 (95% CI, 5.31–5.51) per 100 000 people in 1999 to 5.32 (95% CI, 5.23–5.40) per 100 000 people in 2020.
Discussion
This is a 22‐year analysis of mortality data from the Centers for Disease Control and Prevention on the trend in the mortality rate of VHD and its subtypes. Our analysis showed that (1) VHD accounted for 0.8% of the all‐cause mortality and 2.4% of the cardiovascular mortality in the United States during the study period. (2) There was a decreasing trend in the overall AAMR of VHD and its subtypes, with MR recording the highest annual percentage decrease in AAMR. (3) AS was the most common type of VHD in both female (62.1%) and male (61.7%) patients who died from VHD. (4) AAMR of aortic valve diseases was higher in men, and women had higher AAMR of mitral valve diseases throughout the study period.
Mortality is an objective indicator of a population's health. It is thus crucial to analyze the underlying cause of mortality and determine its trend so that better patient care can be delivered. One‐third of the total deaths reported to the Centers for Disease Control and Prevention occurred because of cardiovascular events. VHD only accounted for 2.4% of total cardiovascular deaths. This is in line with a 30‐year multinational study. 16
The overall AAMR of VHD decreased from 1999 to 2020, possibly related primarily to advances in aortic valve therapy. AS is the most common type of VHD in the developed world, and the incidence is rising in accordance with the aging population. 17 , 18 , 19 Among reported deaths related to VHD, AS accounted for nearly two‐thirds of the cases in both women and men. The AAMR of AS did not change much until 2012, when it started to decrease. This is likely due to the approval and increasing use of TAVR starting from 2012. 20 , 21 , 22 TAVR has been widely performed in the United States and has gradually surpassed surgical aortic valve replacement (Figure S3), with a higher proportion of TAVR versus surgical aortic valve replacement use in higher‐risk patients. When stratified by age, our analysis further revealed a higher AAPC in patients >65 years old than those younger. Clinical trials of TAVR have not included patients with a bicuspid aortic valve, which affects younger patients, although observational data suggest equivalent early outcomes. 23 The 2019 US FDA approval for TAVR in low‐risk patients expanded the approval to include patients regardless of aortic valve anatomy, paving the way for TAVR in bicuspid valves. Further randomized controlled trials are needed to better assess the role of TAVR and the long‐term outcomes in this population, especially given the potential long‐term consequences of TAVR being used in young patients as the initial procedure. 23 , 24 , 25
The AAPC of decreased AAMR was the highest in mitral valve diseases, particularly MR. There may be multiple explanations for this. Mitral valve disease can be primary or secondary and is sensitive to associated cardiac function. Unlike AS, the prevalence of significant mitral valve disease, although greater than AS, is poorly defined and complicated by the presence of multiple subtypes. Improvements in outcomes of mitral regurgitation are therefore sensitive to multiple factors. Firstly, overall improvements in the algorithm for the management of MR in contemporary guidelines, including optimization of medical therapy for heart failure, and timely surgical and percutaneous interventions have been widely adopted. 26 , 27 , 28 , 29 Secondly, guidelines have recommended closer follow‐up surveillance of patients with MR since 1998, facilitating timely intervention. Thirdly, decreases in the proportion of MR and most of MS mortality can be explained by the decreasing prevalence of rheumatic heart disease in the United States, due to improved prevention. 30 , 31 , 32 The trend toward earlier surgical intervention has been supported by long‐term benefits in existing studies. 33 , 34 Additionally, the introduction of percutaneous repair of the mitral valve as an alternative treatment option for high‐risk surgical patients has afforded these patients a safer and equally effective strategy compared with the surgical approach. 35 Despite the potential benefits of percutaneous mitral valve procedures, the impact of these procedures on mortality attributed to MR is likely small given the small proportion of patients with MR who have undergone percutaneous repair since FDA approval. 36 , 37 Further studies on the impact of percutaneous mitral intervention on overall mitral regurgitation outcomes are needed to explore contemporary trends as the use of percutaneous mitral valve procedures slowly increases (Figures S4 and S5). 35 , 38 , 39
A steady decrease in the AAMR of MS was observed in our analysis from 1999 to early 2000s, with a plateau in mortality from the early 2000s. This initial change is likely associated with the significant reduction in rheumatic heart disease over the latter half of the 20th century in the United States in addition to the introduction of the Inoue balloon catheter in the mid‐1990s providing a percutaneous option for therapy. The subsequent plateau is likely related to the lack of further evolution in management options coupled with the aging of the population and a proportional rise in patients with MS secondary to mitral annular calcification, for which no good percutaneous options are available.
Interestingly, our analysis showed that the mortality of men is higher than women across all types of aortic valve diseases. This is in contrast with mitral valve disease, where women have higher mortality.
Limitations
Our study has several limitations. The main limitation stems from the nature of the CDC WONDER database, which uses death certificates. These vital statistics data are subject to human error, which includes inaccurate assessment of the cause of death, misclassification of demographics, data loss, or errors during compilation. These reporting biases can lead to underreporting of VHD‐related mortality, especially when mortality was not directly attributed to VHD itself. Secondly, the use of ICD‐10 codes as filter criteria without access to associated clinical parameters limits our ability to further verify or understand clinical associations. Hence, the temporal relationship between the staging and cause of valvular heart disease and its mortality rate cannot be ascertained. Thirdly, this population study by its nature excluded individual‐level data, such as comorbidity burden, duration of disease, medical treatment, or prior interventions, which are important confounders for mortality. Next, our study has a narrow set of definitions to classify VHD subtypes. We chose a diagnosis solely involving single‐valve disease to isolate the impact of demographic data on particular valves, and this may lead to an underestimation of the actual nationwide burden of VHD mortality. Lastly, mortality data for tricuspid valve disease were unable to be explored due to data suppression with the low number of cases reported. Despite these limitations, our study sufficiently demonstrates the demographic and temporal relationship between VHD in the United States over the past 22 years. It provides valuable insights into the effectiveness of contemporary VHD management strategies. This study approach has been validated in similar research on another topic of interest. 10 , 11 , 12
Conclusions
Our study emphasized the contemporary trend of death due to VHD and its subtypes in the recent era.
There were decreasing trends in the overall mortality rate due to VHD and its subtypes over the past 2 decades. This highlights the improving survival of patients with VHD due to contemporary management algorithms and likely the increased use of transcutaneous aortic valve intervention.
Sources of Funding
None.
Disclosures
None.
Supporting information
Figures S1–S5
This work was presented at the American College of Cardiology Scientific Session, April 6–8, 2024, in Atlanta, Georgia.
This article was sent to Saket Girotra, MD, SM, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030895
For Sources of Funding and Disclosures, see page 9.
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8 [DOI] [PubMed] [Google Scholar]
- 2. Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol. 2014;30:962–970. doi: 10.1016/j.cjca.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 3. Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8:162–172. doi: 10.1038/nrcardio.2010.202 [DOI] [PubMed] [Google Scholar]
- 4. Tung M, Nah G, Tang J, Marcus G, Delling FN. Valvular disease burden in the modern era of percutaneous and surgical interventions: the UK Biobank. Open Heart. 2022;9:e002039. doi: 10.1136/openhrt-2022-002039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panaich SS, Eleid MF. Current status of MitraClip for patients with mitral and tricuspid regurgitation. Trends Cardiovasc Med. 2018;28:200–209. doi: 10.1016/j.tcm.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 6. Bartus K, Sadowski J, Litwinowicz R, Filip G, Jasinski M, Deja M, Kusmierczyk M, Pawlak S, Jemielity M, Jagielak D, et al. Changing trends in aortic valve procedures over the past ten years‐from mechanical prosthesis via stented bioprosthesis to TAVI procedures‐analysis of 50,846 aortic valve cases based on a Polish National Cardiac Surgery Database. J Thorac Dis. 2019;11:2340–2349. doi: 10.21037/jtd.2019.06.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kolte D, Vlahakes GJ, Palacios IF, Sakhuja R, Passeri JJ, Inglessis I, Elmariah S. Transcatheter versus surgical aortic valve replacement in low‐risk patients. J Am Coll Cardiol. 2019;74:1532–1540. doi: 10.1016/j.jacc.2019.06.076 [DOI] [PubMed] [Google Scholar]
- 8. Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, Alu MC, Madhavan MV, Chau KH, Russo M, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77:1149–1161. doi: 10.1016/j.jacc.2020.12.052 [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . Multiple Cause of Death 1999–2020 . 2023.
- 10. Bevan GH, Zidar DA, Josephson RA, Al‐Kindi SG. Mortality due to aortic stenosis in the United States, 2008‐2017. JAMA. 2019;321:2236–2238. doi: 10.1001/jama.2019.6292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janus SE, Chami T, Mously H, Hajjari J, Hammad T, Castro‐Dominguez Y, Fakorede F, White Solaru K, Shishehbor MH, Al‐Kindi SG, et al. Proportionate and absolute vascular disease mortality by race and sex in the United States from 1999 to 2019. J Am Heart Assoc. 2022;11:e025276. doi: 10.1161/JAHA.121.025276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parcha V, Patel N, Kalra R, Suri SS, Arora G, Arora P. Mortality due to mitral regurgitation among adults in the United States: 1999‐2018. Mayo Clin Proc. 2020;95:2633–2643. doi: 10.1016/j.mayocp.2020.08.039 [DOI] [PubMed] [Google Scholar]
- 13. Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;47:1–16. [PubMed] [Google Scholar]
- 14. Ariss RW, Minhas AMK, Issa R, Ahuja KR, Patel MM, Eltahawy EA, Michos ED, Fudim M, Nazir S. Demographic and regional trends of mortality in patients with acute myocardial infarction in the United States, 1999 to 2019. Am J Cardiol. 2022;164:7–13. doi: 10.1016/j.amjcard.2021.10.023 [DOI] [PubMed] [Google Scholar]
- 15. Ariss RW, Minhas AMK, Lang J, Ramanathan PK, Khan SU, Kassi M, Warraich HJ, Kolte D, Alkhouli M, Nazir S. Demographic and regional trends in stroke‐related mortality in young adults in the United States, 1999 to 2019. J Am Heart Assoc. 2022;11:e025903. doi: 10.1161/JAHA.122.025903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990‐2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonow RO, Greenland P. Population‐wide trends in aortic stenosis incidence and outcomes. Circulation. 2015;131:969–971. doi: 10.1161/CIRCULATIONAHA.115.014846 [DOI] [PubMed] [Google Scholar]
- 18. Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85:483–500. doi: 10.4065/mcp.2009.0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N, Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta‐analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 20. Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G, Deeb GM, Thourani VH, Cohen DJ, Desai N, et al. STS‐ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76:2492–2516. doi: 10.1016/j.jacc.2020.09.595 [DOI] [PubMed] [Google Scholar]
- 21. Itchhaporia D. TAVR 20 years later: a story of disruptive transformation. J Am Coll Cardiol. 2022;79:1314–1316. doi: 10.1016/j.jacc.2022.02.017 [DOI] [PubMed] [Google Scholar]
- 22. Young MN, Kearing S, Malenka D, Goodney PP, Skinner J, Iribarne A. Geographic and demographic variability in transcatheter aortic valve replacement dispersion in the United States. J Am Heart Assoc. 2021;10:e019588. doi: 10.1161/JAHA.120.019588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vincent F, Ternacle J, Denimal T, Shen M, Redfors B, Delhaye C, Simonato M, Debry N, Verdier B, Shahim B, et al. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Circulation. 2021;143:1043–1061. doi: 10.1161/CIRCULATIONAHA.120.048048 [DOI] [PubMed] [Google Scholar]
- 24. Kang JJ, Fialka NM, El‐Andari R, Watkins A, Hong Y, Mathew A, Bozso SJ, Nagendran J. Surgical vs transcatheter aortic valve replacement in bicuspid aortic valve stenosis: a systematic review and meta‐analysis [published online April 28, 2023]. Trends Cardiovasc Med. doi: 10.1016/j.tcm.2023.04.004 [DOI] [PubMed] [Google Scholar]
- 25. Sa MP, Jacquemyn X, Sultan I. Transcatheter aortic valve implantation in bicuspid aortic valve stenosis [published online May 6, 2023]. Trends Cardiovasc Med. doi: 10.1016/j.tcm.2023.05.001 [DOI] [PubMed] [Google Scholar]
- 26. Bonow RO, Carabello B, De Leon AC Jr, Edmunds LH Jr, Fedderly BJ, Freed MD, Gaasch WH, McKay CR, Nishimura RA, O'Gara PT, et al. Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation. 1998;98:1949–1984. doi: 10.1161/01.CIR.98.18.1949 [DOI] [PubMed] [Google Scholar]
- 27. Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients with Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748 [DOI] [PubMed] [Google Scholar]
- 28. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. doi: 10.1161/CIR.0000000000000503 [DOI] [PubMed] [Google Scholar]
- 29. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. doi: 10.1161/CIR.0000000000000031 [DOI] [PubMed] [Google Scholar]
- 30. Kumar RK, Antunes MJ, Beaton A, Mirabel M, Nkomo VT, Okello E, Regmi PR, Remenyi B, Sliwa‐Hahnle K, Zuhlke LJ, et al. Contemporary diagnosis and management of rheumatic heart disease: implications for closing the gap: a scientific statement from the American Heart Association. Circulation. 2020;142:e337–e357. doi: 10.1161/CIR.0000000000000921 [DOI] [PubMed] [Google Scholar]
- 31. Nascimento BR, Beaton AZ. Rheumatic heart disease and socioeconomic development. Lancet Glob Health. 2019;7:e1297–e1299. doi: 10.1016/S2214-109X(19)30369-9 [DOI] [PubMed] [Google Scholar]
- 32. Steer AC, Carapetis JR. Prevention and treatment of rheumatic heart disease in the developing world. Nat Rev Cardiol. 2009;6:689–698. doi: 10.1038/nrcardio.2009.162 [DOI] [PubMed] [Google Scholar]
- 33. Suri RM, Vanoverschelde JL, Grigioni F, Schaff HV, Tribouilloy C, Avierinos JF, Barbieri A, Pasquet A, Huebner M, Rusinaru D, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013;310:609–616. doi: 10.1001/jama.2013.8643 [DOI] [PubMed] [Google Scholar]
- 34. Zhou T, Li J, Lai H, Zhu K, Sun Y, Ding W, Hong T, Wang C. Benefits of early surgery on clinical outcomes after degenerative mitral valve repair. Ann Thorac Surg. 2018;106:1063–1070. doi: 10.1016/j.athoracsur.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 35. Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355 [DOI] [PubMed] [Google Scholar]
- 36. De Backer O, Piazza N, Banai S, Lutter G, Maisano F, Herrmann HC, Franzen OW, Sondergaard L. Percutaneous transcatheter mitral valve replacement: an overview of devices in preclinical and early clinical evaluation. Circ Cardiovasc Interv. 2014;7:400–409. doi: 10.1161/CIRCINTERVENTIONS.114.001607 [DOI] [PubMed] [Google Scholar]
- 37. Yasmin F, Najeeb H, Fareed Siddiqui H, Hamayl Zeeshan M, Mehdi A, Sohaib Asghar M, Shaikh A, Aamir M. Current practices and considerations for transcatheter mitral valve implantation based on risk stratification among patients with mitral valve regurgitation. Curr Probl Cardiol. 2023;48:101413. doi: 10.1016/j.cpcardiol.2022.101413 [DOI] [PubMed] [Google Scholar]
- 38. Mack M, Carroll JD, Thourani V, Vemulapalli S, Squiers J, Manandhar P, Deeb GM, Batchelor W, Herrmann HC, Cohen DJ, et al. Transcatheter mitral valve therapy in the United States: a report from the STS/ACC TVT registry. Ann Thorac Surg. 2022;113:337–365. doi: 10.1016/j.athoracsur.2021.07.030 [DOI] [PubMed] [Google Scholar]
- 39. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, et al. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S5


