Abstract
Background
Although early‐onset group B β‐hemolytic streptococcus (GBS) infection is rare, it accounts for approximately 30% of neonatal infections, has a high mortality rate, and is acquired through vertical transmission from colonized mothers. Several trials have demonstrated the efficacy of intrapartum antibiotic prophylaxis (IAP) for preventing early‐onset disease (EOD). Vaginal disinfection with chlorhexidine during labour has been proposed as another strategy for preventing GBS EOD in the preterm and term neonate. Chlorhexidine has been found to have no impact on antibiotic resistance, is inexpensive, and applicable to poorly equipped delivery sites.
Objectives
To determine the effectiveness of vaginal disinfection with chlorhexidine during labour in women who are colonized with GBS for preventing early‐onset GBS infection in preterm and term neonates.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 October 2014) and reference lists of retrieved studies.
Selection criteria
Randomized and quasi‐randomized trials comparing vaginal disinfection with chlorhexidine (vaginal wash or gel/cream) versus placebo, or no treatment.
Data collection and analysis
Three review authors independently assessed the trials for inclusion and risk of bias, extracted the data and checked them for accuracy.
Main results
We identified no new trials eligible for inclusion in this update. One study was moved from included to excluded studies from the previous version of the review. Four studies, including 1125 preterm and term infants, met the inclusion criteria and reported on at least one of the outcomes of interest. For the comparison chlorhexidine (vaginal wash or gel) versus placebo or no treatment, two studies (n = 987) were pooled. There was no statistically significant difference in early‐onset GBS disease (sepsis and/or meningitis) comparing chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment; risk ratio (RR), 2.32 (95% confidence interval (CI) 0.34 to 15.63); I‐squared (I²) = 0% or in GBS pneumonia; RR 0.35 (95% CI 0.01 to 8.6); test for heterogeneity not applicable. The outcome of colonization of the neonate with GBS was reported in three studies (n = 328); RR 0.64 (95% CI 0.40 to 1.01; there was substantial between‐study heterogeneity (Chi² = 3.19; P = 0.20; I² = 37%). Maternal mild side effects (stinging or local irritation) (three trials, 1066 women) were more commonly seen in women treated with chlorhexidine (RR 8.50 (95% CI 1.60 to 45.28); there was no heterogeneity (Chi² = 0.01, df = 1 (P = 0.91); I² = 0%). No side effects were reported among the neonates.
For the comparison chlorhexidine vaginal wash verus mechanical washing with placebo or no treatment (one study, n = 79), there was a significant reduction in neonatal colonization with GBS; RR 0.32 (95% CI 0.12 to 0.90). Tests for heterogeneity not applicable. There were no other significant results for this comparison.
For the comparison chlorhexidine gel or cream versus placebo or no treatment, there were no statistically significant results for the outcomes reported on.
The quality of the trials varied and the overall risk of bias was rated as unclear or high. The quality of the evidence using GRADE was very low for the outcomes of the comparison chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment. These outcomes included: early‐onset GBS disease (sepsis and/or meningitis), GBS pneumonia, neonatal colonization with GBS, neonatal mortality due to early‐onset GBS infection and adverse (mild) effects in the mother and the neonate.
Authors' conclusions
The quality of the four included trials varied as did the risk of bias and the quality of the evidence using GRADE was very low. Vaginal chlorhexidine was not associated with reductions in any of the primary outcomes of early‐onset GBS disease (sepsis and/or meningitis) or GBS pneumonia. Vaginal chlorhexidine may reduce GBS colonization of neonates. The intervention was associated with an increased risk of maternal mild adverse effects. The review currently does not support the use of vaginal disinfection with chlorhexidine in labour for preventing early‐onset disease. Results should be interpreted with caution as the methodological quality of the studies was poor. As early‐onset GBS disease is a rare condition trials with very large sample sizes are needed to assess the effectiveness of vaginal chlorhexidine to reduce its occurrence. In the era of intrapartum antibiotic prophylaxis, such trials may be difficult to justify especially in developed countries.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Streptococcus agalactiae; Administration, Intravaginal; Anti‐Infective Agents, Local; Anti‐Infective Agents, Local/administration & dosage; Chlorhexidine; Chlorhexidine/administration & dosage; Labor, Obstetric; Randomized Controlled Trials as Topic; Streptococcal Infections; Streptococcal Infections/prevention & control; Vaginal Creams, Foams, and Jellies; Vaginal Creams, Foams, and Jellies/administration & dosage
Plain language summary
Antibacterial chlorhexidine applied to the vagina during labour to prevent early‐onset group B streptococcal infection in the newborn
There is no evidence to show that washing the vagina with the antibacterial liquid chlorhexidine or using a chlorhexidine gel during labour reduces group B β‐hemolytic streptococcal (GBS) infections in babies.
A woman's vagina normally contains numerous bacteria that generally do not pose any problems to her or her baby. However, occasionally a baby picks up an infection during birth. GBS infection can cause severe illness in babies and rarely a baby may die as a result of the infection. Washing the vagina with chlorhexidine, or applying chlorhexidine gel or cream, during labour was studied in this systematic review as a possible way of reducing infections. The review of four trials included women who were colonized vaginally or rectally with GBS and their 1125 preterm and term infants. It showed that although chlorhexidine may reduce the number of bacteria that pass from the mothers to the babies as the babies pass through the birth canal, or if they suck in (aspirate) contaminated amniotic fluid, the studies were not large enough to say whether chlorhexidine reduced GBS infections or not.
Vaginal disinfection with chlorhexidine did not result in a reduction of early‐onset GBS illness in the newborns such as sepsis, pneumonia, meningitis, or deaths caused by the infection. It may reduce GBS colonization of newborns when compared with mechanical washing with placebo (from one study).
For the comparison chlorhexidine gel or cream versus placebo or no treatment, there were no significant results for the outcomes reported.
Maternal mild side effects such as stinging or local irritation were more common in women treated with chlorhexidine. Different preparations, doses, frequency of dose, and reported outcomes were used. No side effects were reported among the newborns.
The small number of studies that reported on each of the outcomes of interest; and the relatively small sample size (1125 infants), given the low incidence of GBS infection (one to three per 1000 live births) in the general population, meant that the evidence was limited.
There is a need to conduct a large, well‐designed randomized trial that examines the efficacy of vaginal disinfection with chlorhexidine for reducing GBS infection in term and preterm infants and overcomes the methodological limitations of the included studies. Costs associated with treatment with antibiotics and lack of skilled personnel have limited the availability of preventative treatment for women in poorer areas of the world. Chlorhexidine is inexpensive and has no impact on development of antibiotic resistance.
Summary of findings
Summary of findings for the main comparison. Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment.
| Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment | ||||||
| Population: women with vaginal or rectal colonization with GBS during labour and their preterm/term infants Settings: hospitals in The Netherlands, Sweden, Belgium, Norway Intervention: chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment | |||||
| Early onset GBS disease (sepsis and/or meningitis within the first seven days of life)) | Study population | RR 2.32 (0.34 to 15.63) | 987 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 2 per 1000 | 4 per 1000 (1 to 29) | |||||
| Moderate | ||||||
| 1 per 1000 | 2 per 1000 (0 to 16) | |||||
| GBS pneumonia within the first seven days of life | Study population | RR 0.35 (0.01 to 8.6) | 987 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 2 per 1000 | 1 per 1000 (0 to 16) | |||||
| Moderate | ||||||
| 1 per 1000 | 0 per 1000 (0 to 9) | |||||
| Neonatal colonization with GBS within the first seven days of life | Study population | RR 0.64 (0.4 to 1.01) | 328 (3 studies) | ⊕⊝⊝⊝ very low1,3 | ||
| 225 per 1000 | 144 per 1000 (90 to 227) | |||||
| Moderate | ||||||
| 333 per 1000 | 213 per 1000 (133 to 336) | |||||
| Neonatal mortality due to early‐onset GBS infection | See comment | See comment | Not estimable | 190 (1 study) | See comment | The outcome was reported with no events. |
| Adverse (mild) effects in the mother | Study population | RR 8.5 (1.6 to 45.28) | 1066 (3 studies) | ⊕⊝⊝⊝ very low1,3 | ||
| 2 per 1000 | 15 per 1000 (3 to 78) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Adverse (mild) effects in the neonate | See comment | See comment | Not estimable | 1066 (3 studies) | See comment | The outcome was reported with no events. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Most studies contributing data had design limitations. 2 Wide confidence interval crossing the line of no effect and few events. 3 Few events and small sample size.
Background
Description of the condition
During labour an infant may be exposed to a wide spectrum of infectious organisms including group B ß‐hemolytic streptococcus (GBS), or Streptococcus agalactiae. The maternal gastrointestinal tract, vagina and urethra serve as reservoirs for GBS. Since the 1970s, GBS has been a leading bacterial cause of illness and death in newborns (Keenan 1998; Shah 2000).
Although approximately 15% to 35% of all pregnant women are colonized with GBS in the vagina and/or rectum, approximately 65% of the infants born to GBS positive mothers will be colonized with the bacteria, and approximately one to two infants per 1000 births will become infected (Schuchat 1998). An overview in 1992, reported maternal colonization rates from 19 studies in the years from 1980 to 1991 ranging from 1.6% in Israel, to 28% in England (Ohlsson 1992). The transmission rate for GBS colonization from mother to infant varied from 35% in England, to 69% in Brazil. The incidence of early‐onset GBS disease varied from 0.2 per 1000 live births in Israel, to 5.0 per 1000 live births in the US (Ohlsson 1992). In a systematic review on the prevalence of maternal GBS colonization in European countries, Barcaite 2008 identified 21 studies published between 1996 and 2006, that reported on 24,093 women. Group B streptococcus vaginal colonization rates ranged from 6.5% to 36%. The carriage rates were reported to be 19.7% to 29.3% in Eastern Europe, 11% to 21% in Western Europe, 24.3% to 36% in Scandinavia, and 6.5% to 32% in Southern Europe (Barcaite 2008). Similar colonization rates have been found in developing countries (17.8%) (Stoll 1998).
Two forms of GBS disease in infants are well recognized. Early‐onset disease (EOD) is defined as isolation of GBS from a normally sterile site (i.e., blood or cerebrospinal fluid, or both) in an infant less than seven days of age with clinical signs compatible with a systemic infection (Schuchat 1998). The three most common clinical presentations include sepsis, pneumonia, and meningitis with bacteria present in the blood stream, the lungs and/or around the membranes covering the brain. Preverbal infants cannot report symptoms but clinical signs include; hypo‐ or hyperthermia, irritability, crying, breathing difficulties, feeding difficulties, neck stiffness, tense fontanel and dehydration. Group B streptococcus EOD accounts for approximately 30% of neonatal infections; has a high mortality rate and is acquired through vertical transmission from colonized mothers (Freij 1999; Stoll 1996). Exposure of the fetus/neonate to the organism can also occur from aspiration of contaminated amniotic fluid leading to invasive disease in some infants. Perinatal transmission can occur through intact membranes or by acquisition during passage through the birth canal (Schuchat 1998; Schuchat 1999). Infants presenting with meningitis may suffer permanent neurological damage including cognitive delay, cerebral palsy, and sensory damage (Baker 1995).
Late‐onset disease (LOD) usually occurs in infants between one week and up to three months of age, with meningitis being the most common clinical presentation (85% of cases) (Baker 1995). Late‐onset disease is acquired either by vertical transmission (delayed infection after early colonization) (Dillon 1987), or by horizontal transmission due to cross‐infection in the hospital by healthcare workers, or in the community (Noya 1987). The pathogenesis of LOD is less well understood than EOD (Schuchat 1998).
The rapid onset of postnatal disease and its associated high mortality, the morbidity of long‐term neurological sequelae among survivors, and the rising cost of comprehensive healthcare to treat GBS infection has led to numerous strategies to prevent EOD (Adriaanse 1995b; Schuchat 1999). One strategy evaluated extensively over the last two decades is intrapartum antibiotic prophylaxis (IAP) with penicillin or alternative antibiotics such as erythromycin or clindamycin (Ohlsson 1994; Ohlsson 2014; Smaill 1996). The most recent update of the Cochrane review on 'Intrapartum antibiotics for known maternal Group B streptococcal colonization' (Ohlsson 2014) includes three trials (involving 500 women) (Boyer 1986; Matorras 1990; Tuppurainen 1989). The authors conclude that there is lack of evidence from well‐designed and conducted trials to recommend IAP to reduce EOD (Ohlsson 2014). Despite the possible benefits of IAP, increasing antibiotic resistance to erythromycin and clindamycin recommended for women allergic to penicillin is an increasing health concern (Fernandez 1998). In addition, costs associated with treatment have limited the availability of IAP to women in poorer areas of the world. In a review of the relevant cost literature, Benitz and others (Benitz 1999) found that the costs of GBS prevention and treatment ranged from US $9,720 to $22,215 per case prevented. The authors found that the variation in costs was due to the specific screening and IAP protocol used.
The first guidelines for GBS prevention were published in the US in 1992 (AAP 1992; ACOG 1992). Since then numerous different guidelines have been published by various healthcare organizations (AAP 1997; ACOG 1996; CDC 1996; CDC 2002; CDC 2010; Money 2013 (for SOGC); RCOG 2003; Shah 2001; SOGC 1994; SOGC 1997; SOGC 2004). The same literature has been interpreted differently by different professional organizations. Although these current guidelines are based on studies of poor quality (Ohlsson 1994; Ohlsson 2014), there seems to be a temporal association between the introduction of guidelines and a decline in the EOD rate (CDC 2005; CDC 2007; Schrag 2002). The incidence of invasive EOD decreased from 1.8 cases per 1000 live births in the early 1990s to 0.26 cases per 1000 live births in 2010 (Schrag 2013). Mortality has decreased but all cases of EOD cannot be prevented. There has been no reduction in LOD in infants (CDC 2007; Schrag 2013).
Several GBS vaccine candidates have been developed against the nine currently identified GBS serotypes (Johri 2006) and a type III conjugate vaccine has been found to be safe and immunogenic in pregnant women (Johri 2006).
Description of the intervention
Vaginal disinfection with chlorhexidine during labour has been proposed as another strategy for preventing GBS early‐onset infection in the preterm and term neonate (Christensen 1983; Dykes 1987; Schuchat 1999). Chlorhexidine can be applied as a vaginal wash/douching or as a gel/cream application. Chlorhexidine gel is applied around the portio and onto the fornices or by examination gloves lubricated with chlorhexidine cream. Chlorhexidine has been described as a powerful mucous membrane disinfectant, which can result in suppression of GBS organisms.
How the intervention might work
Christensen and others (Christensen 1983) found that the minimum inhibitory concentration of chlorhexidine for GBS is 0.5 to 1 mg/L. Dykes and colleagues (Dykes 1983) demonstrated that chlorhexidine results in a greater than 100‐fold reduction of urethral and vaginal counts of the organism in carriers within one hour of application and for a duration of many hours. Chlorhexidine has been found to have no impact on antibiotic resistance, is inexpensive and simple, and applicable to poorly equipped delivery sites (Schuchat 1999). In general, the disinfectant has been associated with only minor adverse effects such as skin rashes and dermatological hypersensitivity reactions (Garland 1996; Thune 1998). However, adverse reactions such as severe anaphylactic reaction in the adult (Autegarden 1999; Pham 2000) and bradycardia in the neonate have been reported with topical application of chlorhexidine (Quinn 1989).
Why it is important to do this review
Clavisi and others (Clavisi 2000) conducted a systematic review to examine the efficacy of vaginal disinfection with chlorhexidine during labour for reducing infection rates and mortality. However, the review did not focus on the efficacy of the intervention for reducing early‐onset GBS infection in neonates, but examined the effectiveness of chlorhexidine for reducing all types of infection in both the mother and baby. Six randomized controlled trials and two comparative and not randomized or quasi‐randomized controlled trials were included. The time frame of the search strategy was limited, and included trials from January 1990 to October 1998. The authors concluded that "well designed randomised controlled trials do not support the use of chlorhexidine for reducing postnatal infection rates in mothers and babies" (Clavisi 2000).
Lumbiganon and co‐workers (Lumbiganon 2014) in a Cochrane review that was updated in Issue 9, 2014, assessed the effectiveness and side effects of chlorhexidine vaginal douching during labour in reducing maternal and neonatal infections (excluding GBS and human immunodeficiency virus (HIV)). The review is complimentary to the present review. They included three studies (3012 participants) and found no evidence of an effect of vaginal chlorhexidine during labour in preventing maternal and neonatal infections. Although the data suggested a trend in reducing postpartum endometritis, the difference was not statistically significant. The review authors concluded that "there is no evidence to support the use of vaginal chlorhexidine during labour in preventing maternal and neonatal infections". Although all three included trials were of high quality, one trial used only 20 mL of chlorhexidine or sterile water for vaginal irrigation, while the other two trials used 200 mL of chlorhexidine or sterile saline solution. The effectiveness of vaginal chlorhexidine might depend on the volume of the solution used for irrigation. "There is a need for a well‐designed randomized controlled trial using appropriate concentration and volume of vaginal chlorhexidine irrigation solution and with adequate sample size" (Lumbiganon 2014).
Goldenberg and co‐workers conducted a systematic review to determine the potential for chlorhexidine, used as a vaginal and neonatal wash, to reduce adverse outcomes of pregnancy, especially in developing countries (Goldenberg 2006). They summarized the results of every study that they identified (randomized controlled trials and observational studies) in which chlorhexidine was used as a vaginal treatment, with or without wash of the neonate. All pregnancy outcomes except mother‐to‐child transmission of HIV were included. They found that in developed countries in general, although chlorhexidine when used as a vaginal or newborn disinfectant reduced bacterial load including transmission of GBS from mother to the fetus/infant, it has not been shown to reduce life‐threatening maternal or neonatal infections (Goldenberg 2006). They referred to two large non‐randomized studies conducted in Malawi (Taha 1997 ) and Egypt (Bakr 2005) that suggested that important reductions in maternal and neonatal sepsis and neonatal mortality may be achievable with vaginal or neonatal chlorhexidine treatment (Goldenberg 2006). They concluded that further study of this highly promising treatment is indicated (Goldenberg 2006).
The current systematic review attempted to overcome the limitations of the earlier reviews, and conforms to the methods and criteria of The Cochrane Collaboration. The aim of this review was to combine all trials (randomized controlled trials and quasi‐randomized trials) comparing vaginal disinfection with chlorhexidine versus placebo, or to no treatment, to determine the efficacy of the intervention in preventing early‐onset GBS infection in preterm and term neonates.
Objectives
The primary objective of this review was to determine the effectiveness of vaginal disinfection with chlorhexidine (vaginal wash or gel/cream) during labour in women who were colonized with GBS for the prevention of early‐onset group B ß‐hemolytic streptococcus (GBS) infection in preterm and term neonates.
The secondary objectives were to: 1) describe any benefits or adverse effects to the mother when chlorhexidine was used as a vaginal disinfectant (vaginal wash or gel/cream) during labour, and 2) to describe any adverse effects to the neonate when vaginal chlorhexidine was used.
We carried out a primary analysis of all included trials (vaginal wash or gel/cream versus placebo or no treatment) for the primary and secondary objectives. It was hypothesized that mechanical washing of the vagina during labour, irrespective of the solution used, may result in suppression of GBS. Thus, we conducted comparative analyses a priori to compare the effectiveness of mechanical washing with chlorhexidine to mechanical washing with placebo (saline or water) and of chlorhexidine obstetrical gel/cream versus placebo or no treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials that compared the effectiveness of vaginal disinfection with chlorhexidine during labour versus placebo or no treatment for the prevention of early‐onset disease (EOD) in preterm and term neonates.
Types of participants
Women with vaginal or rectal colonization with GBS during labour and their preterm/term infants. Colonization with GBS was demonstrated by culture or rapid screening test.
Types of interventions
Vaginal disinfection with chlorhexidine (vaginal wash or gel/cream application) during, but not before, onset of labour of women colonized with GBS, compared with placebo or no intervention for the prevention of EOD in preterm and term neonates.
Types of outcome measures
Studies that reported on the incidence or occurrence of one or more of the following outcomes amongst all randomized trials.
Primary outcomes
1. Early‐onset GBS disease (sepsis and/or meningitis), defined as a positive blood and/or cerebrospinal fluid (CSF) culture with GBS in an infant less than seven days of age with clinical signs consistent with a systemic infection. 2. GBS pneumonia within the first seven days of life. This was added in this update as a non‐prespecified outcome.
Secondary outcomes
3. Neonatal colonization with GBS within the first seven days of life. 4. Neonatal mortality due to early‐onset GBS infection within the first 28 days of life. 5. Adverse effects in the mother related to vaginal disinfection with chlorhexidine, including dermatological hypersensitivity reactions, anaphylactic shock, and others. 6. Adverse effects in the neonate related to maternal vaginal disinfection including dermatological hypersensitivity reactions, bradycardia, and others. 7. Benefits to the mother related to vaginal disinfection with chlorhexidine, such as decreased incidence of endometritis, urinary infections, and others. 8. Long‐term neurological sequelae, which may include cognitive delay, cerebral palsy, cortical blindness, deafness, and/or hydrocephalus.
Search methods for identification of studies
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 October 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (OVID);
weekly searches of Embase (OVID);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
[For details of additional author searching carried out for the previous version of this review (Stade 2004), please see Appendix 1.]
Searching other resources
We searched cited references from retrieved articles for additional studies. We reviewed abstracts and letters to the editor to identify randomized controlled trials which have not been published. If a randomized controlled trial was identified, we attempted to contact the primary investigator directly to obtain further data. We reviewed editorials, indicating expert opinion, to identify and ensure that no key studies were missed for inclusion in this review.
We did not apply language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review (Stade 2004), please see Appendix 2.
For this update the following methods were used. These methods are based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Three review authors (AO, VS, BS) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We used a form designed by the review group to extract data. For eligible studies, two review authors (AO, VS) extracted the data using the agreed form. We resolved discrepancies through discussion. One review author (AO) entered data into Review Manager software (RevMan 2014) and another review author (VS) checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (AO, VS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor (BS).
(1) Random sequence generation (checking for possible selection bias)
We describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We describe for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We consider that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We describe for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following key outcomes for the main comparison Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment:
Early onset neonatal infection (GBS sepsis and or meningitis) defined as a positive blood and/or CSF culture with GBS within the first seven days.
GBS pneumonia within the first seven days.
Neonatal colonization with GBS within the first seven days.
Neonatal mortality due to early‐onset GBS infection within the first 28 days of life. There was no neonatal mortality reported in any of the studies so this outcome was not included in the GRADE assessment.
Adverse effects (mild, as defined by the authors) in the mother.
Adverse effects (mild, as defined by the authors) in the neonate.
GRADEprofiler (GRADE 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary (typical) risk ratio (RR) with 95% confidence intervals.
Continuous data
For continuous data, we would have used the mean difference (MD) if outcomes were measured in the same way between trials. We would have used the standardized mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomized trials
We would have included cluster‐randomized trials in the analyses along with individually‐randomized trials. We would have adjusted their sample sizes using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population (Higgins 2011). If we had used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster‐randomized trials and individually‐randomized trials, we planned to synthesize the relevant information. We would have considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit was considered to be unlikely.
We would have acknowledged heterogeneity in the randomization unit and would have performed a sensitivity analysis to investigate the effects of the randomization unit.
No cluster‐randomized trials were identified for this 2014 update.
Cross‐over trials
It was unlikely that cross‐over designs would have been a valid study design for this review. No cross‐over trials were identified.
Other unit of analysis issues
This review did not focus on multiple pregnancies. If that had been the case, special methods would have been needed to analyze data relating to multiple pregnancies (see the Pregnancy and Childbirth Group Methodological Guidelines and theHandbook sections 9.3.7 and 16.3) (Higgins 2011). In the study by Adriaanse 1995a and co‐workers 1.9% of the total of 1020 participating women (n = 19) carried a twin pregnancy. The authors did not specify how many of these women were GBS carriers and how many were randomized into each of the three treatment groups. No specific information related to the twins was provided. Twin or multiple pregnancies were excluded in the study by Burman 1992. Hennequin 1995 and co‐workers did not provide any information on whether multiple pregnancies were included or not. The study by Stray‐Pedersen 1999 an co‐workers included 13 pairs of twins among 2002 liveborn infants (1.2%). No information related to twin pregnancies was provided regarding the 79 mothers who were GBS carriers. Becasue of the lack of information related to twin pregnancies we did not apply any special statistical methods related to multiple pregnancies.
Dealing with missing data
For included studies, we noted levels of attrition. We would have explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses, and all participants were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We report statistical heterogeneity in each meta‐analysis using the I‐squared (I²) and Chi² statistics using a fixed‐effect model. We report heterogeneity as substantial if the I² was greater than 30% and, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. We did not use the random‐effects model and therefore we do not report on the Tau² statistic (See Handbook [Section 9.5.4]) (Higgins 2011).
Assessment of reporting biases
Had there been 10 or more studies in a meta‐analysis we would have investigated reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we would have performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used the fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we would have used a random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary would have been treated as the average of the range of possible treatment effects and we would have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we would not combine trials.
Had we used random‐effects analyses, the results would have been presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we would have investigated it using subgroup analyses and sensitivity analyses. We would have considered whether an overall summary was meaningful, and if it was, we would have used random‐effects analysis to produce it.
For the two primary outcomes 'early‐onset GBS infection (sepsis and/or meningitis)', and 'pneumonia' within the first seven days of life) there was no substantial (I² > than 30%) heterogeneity and therefore no subgroup analyses were undertaken. The only result with substantial heterogeneity was for the outcome 'Neonatal colonization with GBS within the first seven days of life' in Comparison 01 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment. Using the fixed‐effect model the results were: risk (RR) 0.64 (95% confidence interval (CI) 0.40 to 1.01); the I² value was 37%. Using the random‐effects model the CI widened but the point estimate was essentially the same RR 0.65 (95% CI 0.36 to 1.18); the I² value was 37%. In the discussion section we provide a possible explanation for the heterogeneity in this analysis (See Discussion).
In future updates we will attempt to carry out subgroup analyses for analyses with substantial heterogeneity based on how laboratory samples for GBS were collected, handled, plated and analyzed.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
Because of the paucity of included trials no sensitivity analyses were conducted. In the future if more trials become available we will perform sensitivity analyses based on perceived study quality and study location (industrialized versus non‐industrialized countries). We will carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, with poor‐quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
For this update in 2014 the search of the literature in August 2014 identified three studies for potential inclusion (Cutland 2009; Pereira 2011; Saleem 2010), but all were excluded as they included a co‐intervention of washing the infant with chlorhexidine. One study (Calkin 1996) was moved from included to excluded studies as maternal GBS status during labour was not known. We also retrieved one further report (Rouse 1997) by searching the reference lists of retrieved studies. This was subsequently excluded.
Included studies
We have provided details of the included studies in the table Characteristics of included studies.
Four studies, including 1125 term and preterm infants, met the inclusion criteria and reported on at least one of the outcomes of interest for this systematic review (Adriaanse 1995a; Burman 1992; Hennequin 1995; Stray‐Pedersen 1999). These studies were performed in four countries (the Netherlands, Sweden, Belgium, Norway).
Different methods of administration and preparations of chlorhexidine were used; 0.3 % chlorhexidine digluconate gel (Adriaanse 1995a); lubricated glove with 1 % chlorhexidine digluconate cream (Hennequin 1995); and 0.2 % chlorhexidine acetate/diacetate vaginal wash (Burman 1992; Stray‐Pedersen 1999).
For one study (Adriaanse 1995a), we had made an error in data abstraction in the previous review and when corrected the outcome of 'Neonatal colonization with GBS within the first seven days' no longer showed a statistically significant reduction..
Excluded studies
We have provided details of the excluded studies in the table Characteristics of excluded studies. For this update of the review in 2014, a total of 18 studies were excluded. We excluded studies that lacked randomization (Christensen 1985a; Christensen 1985b; Christensen 1987; Coppens 2000; Dykes 1987; Kaihura 2000; Kollee 1989; Sanderson 1985); included interventions that did not meet inclusion criteria (vaginal chlorhexidine versus ampicillin) (Facchinetti 2002); did not meet the inclusion criteria related to types of intervention (Henrichsen 1994); or GBS status was not ascertained during labour (Rouse 1997). One study (Calkin 1996) was moved from included to excluded studies as maternal GBS status during labour was not known. For the previous update of this review (Stade 2004), we identified four potential randomized controlled trials; one published in abstract form (Mushangwe 2006;) and two ongoing trials (Madhi 2007; Moss 2007) on the use of vaginal chlorhexidine in labour. None of the trials were eligible for inclusion as they did not have as an entry criterion 'maternal colonization with GBS'. According to www.clinicaltrials.gov the recruitment status of the Madhi 2007 is unknown since 2007 and the study by Moss 2007 is no longer listed in the trials registry. As stated above three newly identified trials (Cutland 2009; Pereira 2011; Saleem 2010) were excluded as they included a co‐intervention of washing the infant with chlorhexidine.
Risk of bias in included studies
We have presented the assessment of individual studies in the table of Characteristics of included studies. See Figure 1 and Figure 2 for a summary of the 'Risk of bias' assessments.
1.
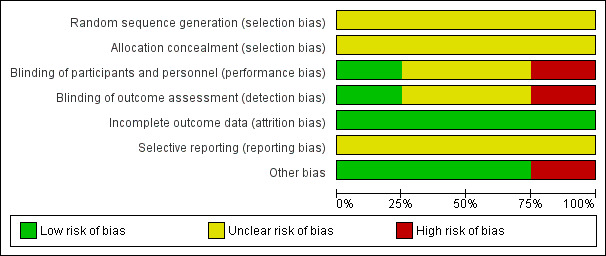
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
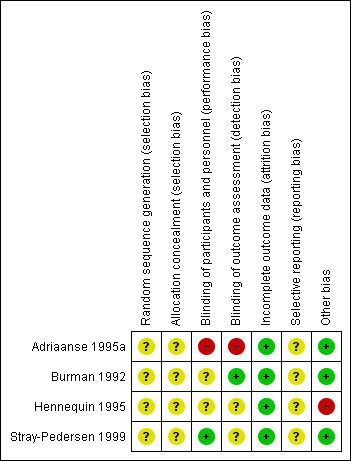
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
In the four included studies the overall risk of bias was unclear (Burman 1992; Stray‐Pedersen 1999) or high (Adriaanse 1995a; Hennequin 1995).
Allocation
We assessed the risk as unclear for random sequence generation and for allocation concealment in all studies (Adriaanse 1995a; Burman 1992; Hennequin 1995; Stray‐Pedersen 1999).
Blinding
We assessed the risk as high for performance and detection bias in the study by Adriaanse 1995a; as unclear for the study by Burman 1992 for performance bias but low for detection bias (one neonatologist made the final decision on infants' diagnoses from their charts before breaking the treatment code); as unclear for both performance and detection bias for the study by Hennequin 1995; and as low risk for performance bias and unclear risk for detection bias for the study by Stray‐Pedersen 1999.
Incomplete outcome data
We assessed the risk of attrition bias as low for all four studies as outcomes (at least one in each study) were reported on all randomized women/infants.
Selective reporting
We assessed the risk as unclear for the four included studies (Adriaanse 1995a; Burman 1992; Hennequin 1995; Stray‐Pedersen 1999) as the protocols for the studies were not available to us and therefore we could not judge if there were any deviations from the protocol in the execution and reporting of the trials.
Other potential sources of bias
We assessed three trials (Adriaanse 1995a; Burman 1992; Stray‐Pedersen 1999) as being free of other bias and assigned a rating of 'Low risk'. We assigned a rating of 'High risk" for bias to the rial by Hennequin 1995 as the study was reported in letter format only and no baseline characteristics were presented so we cannot judge if there were imbalances.
Effects of interventions
See: Table 1
For the update of this review, we identified no additional eligible trials. Four studies met the inclusion criteria. These included a total of 1125 infants, and reported on at least one of the outcomes of interest for this systematic review. We report the findings in three separate comparisons 1) Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment (Comparison 01), 2) Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment (Comparison 02) and 3) Chlorhexidine digluconate gel or cream versus placebo or no treatment (Comparison 03).
Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment (Comparison 01)
Primary outcomes
Early onset GBS disease (sepsis and/or meningitis) within the first seven days of life (Outcome 01.01)Analysis 1.1
1.1. Analysis.
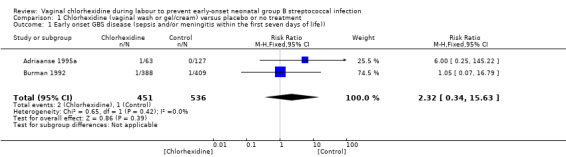
Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 1 Early onset GBS disease (sepsis and/or meningitis within the first seven days of life)).
Two studies (including 987 infants) reported on the outcome of early‐onset GBS infection following chlorhexidine vaginal disinfection. None of the studies reported a statistically significant difference for this outcome. When the studies were combined, there was no statistically significant difference in this outcome; risk ratio (RR) 2.32 (95% confidence interval (CI) 0.34 to 15.63). There was no substantial between‐study heterogeneity for RR (Chi² = 0.65; P = 0.42, I² = 0%).
GBS pneumonia within the first seven days of life (Outcome 01.02)Analysis 1.2
1.2. Analysis.
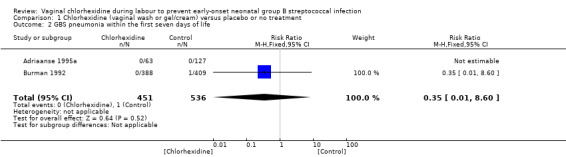
Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 2 GBS pneumonia within the first seven days of life.
Two studies (including 987 infants) reported on the outcome of early‐onset GBS pneumonia following chlorhexidine vaginal disinfection. In one study (Adriaanse 1995a) there was no case of pneumonia in either group. The studies combined showed no statistically significant difference in this outcome; RR 0.35 (95% CI 0.01 to 8.60). Tests for heterogeneity not applicable for RR.
Secondary outcomes
Neonatal colonization with GBS within the first seven days of life (Outcome 01.03)Analysis 1.3
1.3. Analysis.
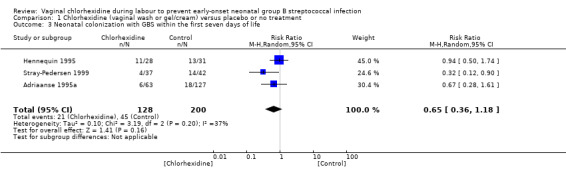
Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 3 Neonatal colonization with GBS within the first seven days of life.
Three studies (including 328 infants) reported on the outcome of infants colonized with GBS. When the studies were combined there was no statistically significant difference in colonization; average RR 0.65 (95% CI 0.36 to 1.18). There was substantial between‐study heterogeneity for RR (Chi² = 3.19; P = 0.20; I² = 37%).
Neonatal mortality due to early‐onset GBS infection (Outcome 01.04)Analysis 1.4
1.4. Analysis.
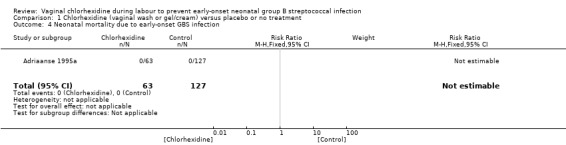
Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 4 Neonatal mortality due to early‐onset GBS infection.
One study (including 190 infants) reported on mortality due to the early‐onset GBS infection. No deaths due to early‐onset GBS infection was reported in this study, and the RR was not estimable. Tests for heterogeneity were not applicable.
Adverse (mild) effects in the mother (Outcome 01.05)Analysis 1.5
1.5. Analysis.
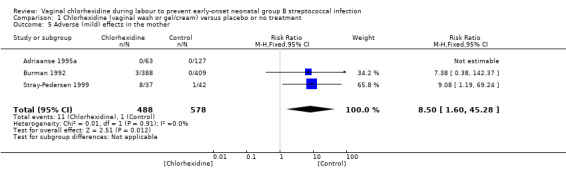
Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 5 Adverse (mild) effects in the mother.
Three studies (including 1066 mothers) reported on this outcome. When the studies were combined, there was a statistically significant increase in the incidence of adverse events following chlorhexidine vaginal disinfection; RR 8.50 (95% CI 1.60 to 45.28). Adverse effects were minor and included stinging or local irritation. There were no major adverse effects reported. There was no substantial between‐study heterogeneity for RR (Chi² = 0.01; P = 0.91; I² = 0%).
Adverse effects in the neonate (Outcome 01.06)Analysis 1.6
1.6. Analysis.
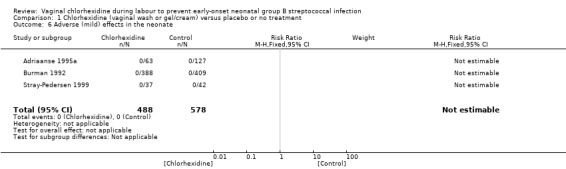
Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 6 Adverse (mild) effects in the neonate.
Three studies (including 1066 infants) reported on adverse effects to the neonate. No adverse effects in the neonates were noted; RR not estimable. Tests for heterogeneity not applicable for RR.
Benefits to the mother
No study reported on any benefits to the mother.
Long‐term neurological sequelae in the infants
Long‐term follow‐up was not reported in any of the studies.
Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment (Comparison 02)
Early onset GBS disease (sepsis and or meningitis within the first seven days of life) (Outcome 02.01)
2.1. Analysis.

Comparison 2 Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment, Outcome 1 Early onset GBS disease (sepsis and/or meningitis) within the first seven days of life.
One study (including 797 infants) reported on GBS sepsis within the first seven days of life. There was no statistically significant effect of the intervention; RR 1.05 (95% CI 0.07 to 16.79). Tests for heterogeneity not applicable.
GBS pneumonia within the first seven days of life (Outcome 02.02)Analysis 2.2
2.2. Analysis.
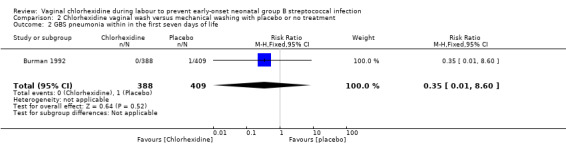
Comparison 2 Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment, Outcome 2 GBS pneumonia within in the first seven days of life.
One study (including 797 infants) reported on GBS pneumonia. There was no statistically significant effect of the intervention; RR 0.35 (95% CI 0.01 to 8.60). Tests for heterogeneity not applicable.
Neonatal colonization with GBS within the first seven days of life (Outcome 02.03)Analysis 2.3
2.3. Analysis.
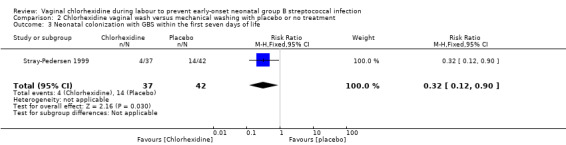
Comparison 2 Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment, Outcome 3 Neonatal colonization with GBS within the first seven days of life.
One study (including 79 infants) reported on infants colonized with GBS. It showed a statistically significant reduction in GBS colonization; RR 0.32 (95% CI 0.12 to 0.90). Test for heterogeneity not applicable.
Neonatal mortality due to early‐onset GBS infection
No study reported on this outcome.
Adverse (mild) effects in the mother (Outcome 02.05)Analysis 2.4
2.4. Analysis.
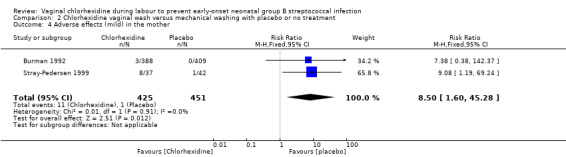
Comparison 2 Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment, Outcome 4 Adverse effects (mild) in the mother.
Two studies (including 876 mothers) reported on adverse (mild) effects in the mother. There was a statistically significantly increased risk for adverse effects in the mother with the use of vaginal wash with chlorhexidine compared to placebo or no treatment; RR 8.50 (95% CI 1.60 to 45.28). There was no substantial heterogeneity for RR (Chi² = 0.01; P = 0.91; I² = 0 %).
Adverse (mild) effects in the neonate (Outcome 02.06)Analysis 2.5
2.5. Analysis.
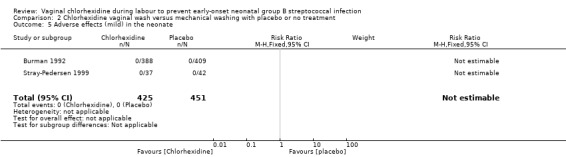
Comparison 2 Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment, Outcome 5 Adverse effects (mild) in the neonate.
Two studies (including 876 neonates) reported on adverse (mild) effects in the neonates. There were no adverse events noted in any of the neonates; RR not estimable. Tests for heterogeneity not applicable for RR.
Chlorhexidine digluconate gel or cream versus placebo or no treatment (Comparison 03)
Early onset GBS disease (sepsis and or meningitis within the first seven days of life) (Outcome 03.01)Analysis 3.1
3.1. Analysis.
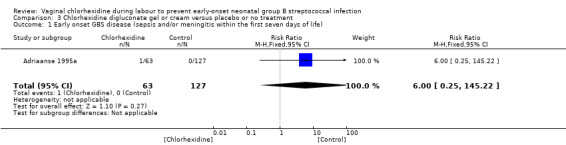
Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 1 Early onset GBS disease (sepsis and/or meningitis within the first seven days of life).
One study (including 190 neonates) reported on early‐onset GBS disease (sepsis and/or meningitis within the first seven days of life). There was no statistically significant difference between the groups; RR 6.0 (95% CI 0.25 to 145.22). Tests for heterogeneity not applicable.
GBS pneumonia within the first seven days of life (Outcome 03.02)Analysis 3.2
3.2. Analysis.
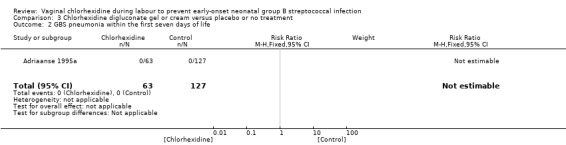
Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 2 GBS pneumonia within the first seven days of life.
One study (including 190 neonates) reported on GBS pneumonia within the first seven days of life. There was no case of pneumonia in either group. There was no statistically significant difference between groups; RR not estimable. Tests for heterogeneity not applicable.
Neonatal colonization with GBS within the first seven days (Outcome 03.03)Analysis 3.3
3.3. Analysis.
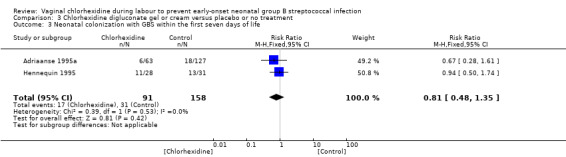
Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 3 Neonatal colonization with GBS within the first seven days of life.
Two studies (including 249 infants) reported on the outcome of infants colonized with GBS within the first seven days. When the studies were combined, there was no statistically significant reduction in colonization for RR 0.81 (95% CI 0.48 to 1.35). There was no statistically significant between‐study heterogeneity for RR (Chi² = 0.39; P = 0.53), I² = 0%).
Neonatal mortality due to early‐onset GBS infection (Outcome 03.04)Analysis 3.4
3.4. Analysis.
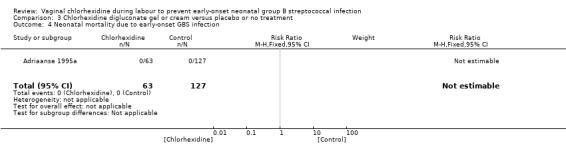
Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 4 Neonatal mortality due to early‐onset GBS infection.
One study (including 190 infants) reported on this outcome. There was no mortality in either group. The RR was not estimable. Tests for heterogeneity not applicable.
Adverse (mild) effects in the mother (Outcome 03.05)Analysis 3.5
3.5. Analysis.
Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 5 Adverse (mild) effects in the mother.
One study (including 190 mothers) reported on this outcome. There were no cases of adverse effects in either group; RR not estimable. Tests for heterogeneity not applicable.
Adverse (mild) effects in the neonate (Outcome 03.06)Analysis 3.6
3.6. Analysis.
Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 6 Adverse (mild) effects in the neonate.
One study (including 190 neonates) reported on this outcome. There were no cases of adverse effects in either group; RR not estimable. Tests for heterogeneity not applicable.
Discussion
Summary of main results
The quality of the evidence was very low (see Quality of the evidence below).
For the main comparison Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment (Comparison 01) there were no statistically significant reductions in early‐onset GBS sepsis, early‐onset GBS pneumonia, early‐onset GBS meningitis, neonatal colonization with GBS within the first seven days of life, or mortality due to early‐onset GBS infection. There was no statistically significant between‐study heterogeneity for these outcomes. Adverse effects to the mother were statistically significantly higher for the intervention group. The adverse effects were minor: stinging or local irritation. No adverse effects to the infant were noted. There was no statistically significant between‐study heterogeneity for any outcomes except for neonatal colonization with GBS within the first seven days of life (three studies, 328 infants) (I² = 37%). For the comparison Chlorhexidine digluconate gel or cream versus placebo or no treatment (Comparison 03) there was no heterogeneity for the outcome neonatal colonization with GBS within the first seven days of life (two studies 249 infants). In Comparison 01 Analysis 1.3 the study by Stray‐Pedersen 1999 appears to be a clear outlier with a significant reduction in neonatal colonization with GBS within the first seven days of life. In Comparison 02 Chlorhexidine wash versus mechanical washing with placebo or no treatment, the study by Stray‐Pedersen 1999 is the only study that reports on the outcome and there is a statistically significant reduction in GBS neonatal colonization; RR 0.32 (95% CI 0.12 to 0.90). The heterogeneity noted when all studies are combined could be due to the different methods of administering chlorhexidine or by different methods of obtaining cultures for GBS, or processing the samples.
Vaginal disinfection with chlorhexidine did not result in a reduction of early‐onset GBS morbidity. This finding may be due to the methodological quality of the studies, particularly the lack of clarity around incidence of morbidity outcomes; the limited number of studies that report on each of the outcomes of interest; and the relatively small sample size (n = 1125 infants) given the low incidence of GBS infection (one to three per 1000 live births) in the general population (Schuchat 1999). There is a need to conduct a large, multi‐centre, double‐blinded randomized trial that examines the efficacy of vaginal disinfection with chlorhexidine for reducing GBS infection in term and preterm infants and overcomes the methodological limitations of the studies reviewed. Such a study may particularly benefit settings where costs associated with treatment and lack of skilled personnel have limited the availability of intrapartum chemoprophylaxis to women in poorer areas of the world.
We examined the effectiveness of vaginal disinfection for the prevention of group B ß‐hemolytic streptococcus (GBS) infection in term and preterm infants in this systematic review. The review addressed the limitations of two earlier systematic reviews (Clavisi 2000; Goldenberg 2006). Specifically, the current review focused on the efficacy of the intervention for reducing early‐onset GBS infection in neonates and did not include other types of neonatal infection; included only randomized or quasi‐randomized controlled trials; and ensured that the time frame of the search strategy was not limited, but rather extended over 30 years.
Overall completeness and applicability of evidence
This systematic review includes 1125 infants born to mothers, who were enrolled in trials in four different countries of the world. As early‐onset GBS disease is a rare condition in newborn infants very large cohorts of GBS carrier mothers need to be enrolled in trials to be able to ascertain if vaginal chlorhexidine applications do have any impact on the condition or not.
Quality of the evidence
The methodological quality of the included trials varied. No study was of high quality and elements of bias could not be excluded in any of the studies, mainly due to the fact that information was lacking regarding 'random sequence generation' and 'allocation concealment'. In addition the intervention was performed unblinded to group assignment and there was lack of clarity around blinding of outcomes. The quality of the evidence using GRADE was very low for early‐onset neonatal infection, GBS pneumonia within the first seven days, neonatal colonization with GBS within the first seven days and adverse effects in the mother of comparison chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment. These outcomes were downgraded due to the design limitations and wide confidence interval crossing the line of no effect with few events, or small sample size.
Potential biases in the review process
We are not aware of any bias in our review process.
Agreements and disagreements with other studies or reviews
In a recent randomized controlled study (n = 108), Facchinetti 2002 found similar rates of neonatal GBS colonization when intrapartum ampicillin versus chlorhexidine vaginal flushing was used. This finding suggests the need for further research comparing the effectiveness of chlorhexidine, ampicillin, and placebo or no treatment for reduction of neonatal GBS colonization.
Clavisi and others (Clavisi 2000) conducted a systematic review to examine the efficacy of vaginal disinfection with chlorhexidine during labour for reducing infection rates and mortality. However, the review did not focus on the efficacy of the intervention for reducing early‐onset GBS infection in neonates, but examined the effectiveness of chlorhexidine for reducing all types of infection in both the mother and baby. Six randomized controlled trials and two comparative and not randomized or quasi‐randomized controlled trials were included. The time frame of the search strategy was limited, and included trials from January 1990 to October 1998. The authors concluded that "well designed randomised controlled trials do not support the use of chlorhexidine for reducing postnatal infection rates in mothers and babies" (Clavisi 2000).
In a Cochrane review that excluded GBS and HIV infections Lubiganon and co‐workers (Lumbiganon 2014) found no evidence to support the use of vaginal chlorhexidine during labour in preventing maternal and neonatal infections. They concluded like we did that there is a need for a well‐designed randomized controlled trial using appropriate concentration and volume of vaginal chlorhexidine irrigation solution and with an adequate sample size.
Goldenberg and co‐workers conducted a systematic review to determine the potential for chlorhexidine, used as a vaginal and neonatal wash, to reduce adverse outcomes of pregnancy, especially in developing countries (Goldenberg 2006). They summarized the results of every study that they identified (randomized controlled trials and observational studies) in which chlorhexidine was used as a vaginal treatment, with or without wash of the neonate. All pregnancy outcomes except mother‐to‐child transmission of HIV were included. They found that in developed countries in general, although chlorhexidine when used as a vaginal or newborn disinfectant reduced bacterial load including transmission of GBS from mother to the fetus/infant, it has not been shown to reduce life‐threatening maternal or neonatal infections (Goldenberg 2006). They referred to two large non‐randomized studies conducted in Malawi (Taha 1997) and Egypt (Bakr 2005) that suggested that important reductions in maternal and neonatal sepsis and neonatal mortality may be achievable with vaginal or neonatal chlorhexidine treatment (Goldenberg 2006). They concluded that further study of this highly promising treatment is indicated (Goldenberg 2006).
Authors' conclusions
Implications for practice.
This update of the review did not identify any additional eligible trials. The results of the review are based on few trials of variable quality and small sample sizes given the low incidence of invasive early‐onset GBS disease. Vaginal disinfection with chlorhexidine resulted in a possible reduction in group B ß‐hemolytic streptococcus (GBS) colonization of neonates, but was not associated with reductions in other important outcomes: early‐onset GBS disease (sepsis and/or meningitis), early‐onset GBS pneumonia, or mortality. The intervention was associated with an increased risk of maternal (mild) adverse effects. Thus, the review currently does not support the use of vaginal disinfection with chlorhexidine in labour for the prevention of early‐onset GBS morbidity in preterm and term infants.
Implications for research.
The results of this updated systematic review suggest the need for further research. As early‐onset GBS disease is a rare condition trials with very large sample sizes are needed to assess the effectiveness of vaginal chlorhexidine to reduce its occurrence. In the era of intrapartum antibiotic prophylaxis, such a trial may be difficult to justify especially in developed countries. Trials of vaginal chlorhexidine in low‐resource countries where intrapartum antibiotics are not administered may be reasonable to undertake. Acknowledging the increasing concern regarding drug‐resistant bacteria such trials may be justified in developed countries too.
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2014 | New search has been performed | Search updated. Three new reports identified (Cutland 2009; Pereira 2011; Saleem 2010) but all excluded as they included a co‐intervention of washing the infant with chlorhexidine after birth. For one study (Adriaanse 1995a), we had made an error in data abstraction in the previous review and when corrected the outcome of 'Neonatal colonization with GBS within the first seven days' no longer showed a statistically significant reduction. One study previously included now excluded because maternal GBS status during labour was not known (Calkin 1996). A 'Summary of findings' table was incorporated. |
| 31 October 2014 | New citation required and conclusions have changed | The exclusion of Calkin 1996 did not change the overall conclusions but because of our different interpretation of the outcome data from the previous update for one study (Adriaanse 1995a), the outcome of 'Neonatal colonization with GBS within the first seven days' no longer showed a statistically significant reduction. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 11 February 2008 | Amended | Converted to new review format. |
| 1 September 2007 | New search has been performed | The update of the literature search in September 2007 identified two studies published in abstract form and two ongoing studies. All four were randomized controlled trials of the use of vaginal chlorhexidine in labour to prevent neonatal infection. Neither of the two studies published in abstract form, nor the two ongoing studies have identified maternal colonization with group B streptococcus as an entry criterion. Therefore, no new information was found and our conclusions remain the same as in the original review. |
| 1 April 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Marianne Bracht, RN: translation of articles from Spanish into English.
We would like to thank Erika Ota for her support in the creation of the 'Summary of findings' table for this update. Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
Dr. Anna‐Karin Dykes provided additional information regarding the Christensen 1987 study.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Additional searching carried out in previous review versions
For the Stade 2004 version of this review, authors also searched CENTRAL (The Cochrane Library, 2007, Issue 3), MEDLINE (1966 to September 2007), EMBASE (1980 to September 2007), CINAHL (1982 to September 2007) and Latin American and Caribbean Health Science (LILACS) (1982 to September 2007) using the following keywords and free text terms "chlorhexidine" or "vaginal‐creams‐foams‐and‐jellies" or "vaginal gel" or "vaginal wash" or "vaginal disinfection" and "streptococcus" or "streptococcus‐agalactiae" or "streptococcal‐infections" or "group B strep*" and labor or labour or infant‐newborn or birth or childbirth.
Appendix 2. Methods used in previous update
The original review and this update were undertaken by three review authors, all of whom are content experts. One review author conducted the literature search. The studies that were found as a result of the search strategy described earlier were screened by two of the review authors, discarding the studies that were clearly ineligible but aiming to be overly inclusive rather than risk losing relevant studies. We evaluated trials under consideration for methodological quality using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). Blinding of randomization, intervention and outcome measurement, and completeness of follow‐up were ranked as Grade A: adequate; Grade B: uncertain; Grade C: inadequate. Two review authors conducted independent assessments to determine whether the studies met the inclusion criteria, with disagreement resolved by discussion. When discussion was not enough to resolve the disagreement, we referred the matter to the third review author.
The two review authors then independently abstracted information from the results sections of the included studies. We resolved discrepancies by discussion or referral to the third review author. One review author checked and entered the data. Where possible, we sought missing data from the authors.
We performed statistical analyses for dichotomous data using relative risk, and 95% confidence intervals. We calculated the risk difference and reported the numbers needed to treat to benefit and number needed to harm. We used a chi‐square test and the I‐squared analysis to test for heterogeneity, and applied a fixed‐ or random‐effects model accordingly. We conducted subgroup analysis to compare the effectiveness of vaginal washing with chlorhexidine versus vaginal washing with placebo (saline or water). We conducted a subgroup analysis based on methods of chlorhexidine administration and preparation (vaginal washing with chlorhexidine acetate/diacetate versus application of chlorhexidine digluconate gel or cream) to check for heterogeneity across studies or to examine sensitivity of combined results, or both.
Data and analyses
Comparison 1. Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early onset GBS disease (sepsis and/or meningitis within the first seven days of life)) | 2 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.34, 15.63] |
| 2 GBS pneumonia within the first seven days of life | 2 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.60] |
| 3 Neonatal colonization with GBS within the first seven days of life | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.36, 1.18] |
| 4 Neonatal mortality due to early‐onset GBS infection | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adverse (mild) effects in the mother | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.50 [1.60, 45.28] |
| 6 Adverse (mild) effects in the neonate | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early onset GBS disease (sepsis and/or meningitis) within the first seven days of life | 1 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.07, 16.79] |
| 2 GBS pneumonia within in the first seven days of life | 1 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.60] |
| 3 Neonatal colonization with GBS within the first seven days of life | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.90] |
| 4 Adverse effects (mild) in the mother | 2 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.50 [1.60, 45.28] |
| 5 Adverse effects (mild) in the neonate | 2 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Chlorhexidine digluconate gel or cream versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early onset GBS disease (sepsis and/or meningitis within the first seven days of life) | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.25, 145.22] |
| 2 GBS pneumonia within the first seven days of life | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Neonatal colonization with GBS within the first seven days of life | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.35] |
| 4 Neonatal mortality due to early‐onset GBS infection | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adverse (mild) effects in the mother | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse (mild) effects in the neonate | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adriaanse 1995a.
| Methods | Multi‐centre randomized, double‐blind study that used both a placebo and control group. I. Blinding of randomization ‐ yes. II. Blinding of intervention ‐ yes. III. Complete follow‐up ‐ yes. IV. Blinding of outcome measurement(s) ‐ cannot tell. | |
| Participants | 190 women colonized with GBS in labour and their term neonates. Exclusion criteria: known GBS carrier‐ship, use of antibiotics during the 4 weeks before admission, planned cesarean section, antepartum fetal death, suspected congenital abnormalities, and preterm labour. Study period February 1991 through November 1992 in two hospitals in the city of Nijmegen, The Netherlands. |
|
| Interventions | 190 women in labour, who later were proven to be GBS carriers, were randomized to 1 of 3 groups. 63 women treated with 10 mL of 0.3% chlorhexidine digluconate gel. 70 were treated with 10 mL of placebo gel. The chlorhexidine or the placebo gel was applied around the portio and onto the fornices. If labour still continued, a second application was given after 10 h. 57 women served as controls without any treatment. |
|
| Outcomes | 6 infants were colonized in the treated group.
11 were colonized in the placebo group.
7 were colonized in the control group.
1 infant with GBS septicaemia in the treatment group, and none in the control group.
No incidence of pneumonia in either group. No incidence of mortality in either group. No incidence of adverse effects to mother or infant in either group. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Women were randomly assigned, when labour had clearly started, to one of three groups according to a predetermined block (10:10:10) allocation scheme." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Two groups were treated in a double‐blind manner, with either a chlorhexidine or a placebo gel". A third group that received no treatment must have been known to staff. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Two groups were treated in a double‐blind manner, with either a chlorhexidine or a placebo gel". A third group that received no treatment must have been known to staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data provided for all GBS carrier mothers and their infants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for this study was not available to us so we cannot judge if there were any deviations from the protocol in the execution and reporting of the trial. |
| Other bias | Low risk | Appears free of other bias. |
Burman 1992.
| Methods | Multi‐centre randomized, double‐blind, placebo‐controlled study. I. Blinding of randomization ‐ yes. II. Blinding of intervention ‐ yes. III. Complete follow‐up ‐ yes. IV. Blinding of outcome measurement(s) ‐ yes. | |
| Participants | 797 women of at least 37 weeks' gestation colonized with GBS and their term neonates. Exclusion criteria: Preterm deliveries, planned C/S, pregnancy complications after the 30th week of gestation requiring hospital admission, twin or multiple pregnancies, suspected fetal congenital malformations, known or suspected allergy to chlorhexidine, previous birth of an infant with GBS infection, antibiotics during the two weeks before admission, and antepartum fetal death. The trial took place at 10 Swedish hospitals. Study period not stated. |
|
| Interventions | 388 women were treated with 0.2% diacetate chlorhexidine solution vaginal wash.
409 women were treated with placebo (sterile saline) vaginal wash. Each flushing of the vagina was with 60 mL of solution contained in 2 x 30 mL plastic ampoules that were attached to a 20 cm plastic catheter. Applicatiopn was started on arrival in the delivery room and repeated every 6 h until delivery. Women were considered treated if at least 1 hour passed between the first flushing and delivery and if no more than 6 h had passed between applications or between the last application and delivery. |
|
| Outcomes | 1 infant with early‐onset GBS infection in the treatment group and 1 in the placebo group. No infants with GBS pneumonia in the treatment group and 1 in the placebo group. No infant with GBS meningitis in the treatment group and 1 in the placebo group. The one infant with meningitis in the placebo group had sepsis too. 3 women in the treatment group reported adverse effects (2 women reported slight vaginal stinging after 2 flushings, and 1 reported local irritation for 2 hours after 5 chlorhexidine flushings). No adverse effects were observed among infants. | |
| Notes | Colonization rates were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Women were assigned randomly to receive chlorhexidine or placebo according to a predefined block (25:25) allocation scheme." |
| Allocation concealment (selection bias) | Unclear risk | "Women were assigned randomly to receive chlorhexidine or placebo according to a predefined block (25:25) allocation scheme." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear if mothers and healthcare providers were blinded to group assignment. However, the study is announced as a prospective, randomized, double‐blind, placebo‐controlled study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 1 neonatologist (N.W.S.) made the final decision on infants' diagnoses from their charts before breaking the treatment code. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized mothers and their infants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for this study was not available to us so we cannot judge if there were any deviations from the protocol in the execution and reporting of the trial. |
| Other bias | Low risk | Appears free of other bias. |
Hennequin 1995.
| Methods | Single‐centre, randomized controlled trial without the use of a placebo.
I. Blinding of randomization ‐ yes (method not described).
II. Blinding of intervention ‐ cannot tell.
III. Complete follow‐up ‐ yes.
IV. Blinding of outcome measurement(s) ‐ cannot tell. Study period not reported. Single centre: Free University of Brussels Brugmann and Queen Fabiola Children’s Hospital, Brussels, Belgium. |
|
| Participants | 59 women colonized with GBS during labour and their infants. | |
| Interventions | 28 women had vaginal exams performed with gloves lubricated with 5 mL of chlorhexidine 1% digluconate cream.
31 women served as controls and were examined with uncoated gloves.
No placebo was used. The number of vaginal exams in each group was not reported. |
|
| Outcomes | 11 out of 28 newborns (39%) were colonized with GBS in the treated group. 13 out of 31 newborns (42%) were colonized with GBS in the control group. | |
| Notes | Adverse effects were not reported.
Unclear whether infants were preterm or term. The study was published as a letter to the editors and very limited information about the trial was provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random." |
| Allocation concealment (selection bias) | Unclear risk | "No statement." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The outcome of colonization reported for all infants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for this study was not available to us so we cannot judge if there were any deviations from the protocol in the execution and reporting of the trial. |
| Other bias | High risk | This study was reported in letter format only and no baseline characteristics were presented so we can not judge if there were imbalances. |
Stray‐Pedersen 1999.
| Methods | Single‐centre, randomized placebo‐controlled study at Aker University Hospital, Oslo, Norway. I. Blinding of randomization ‐ yes. II. Blinding of intervention ‐ yes. III. Complete follow‐up ‐ yes. IV. Blinding of outcome measurement(s) ‐ cannot tell. | |
| Participants | 79 women colonized with GBS during labour and their preterm/term infants. Study period: dates not stated but the randomized controlled trial lasted 5 months. Exclusion criteria: fetal death, antibiotic treatment during the last week before delivery, cesarean section, or refusal to participate. |
|
| Interventions | 37 women were treated with vaginal douching with 120 mL of 0.2% diacetate chlorhexidine solution in labour.
42 were treated with vaginal douching with 120 mL of a placebo (sterile saline) in labour. The douching was repeated every 6 h until delivery. |
|
| Outcomes | 4 infants were colonized with GBS in the treatment group. 14 in the placebo group. 8 cases of mild adverse effects to mothers in treatment group and 1 case of mild adverse effects to the mother in the placebo group. The adverse effects were local irritation of short duration in both groups. No cases of adverse effects to the infants in any group. | |
| Notes | Presentation of the data does not allow the review authors to determine incidence of early‐onset GBS infection and GBS pneumonia. Thus we have requested additional data from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided ‐ "a double‐blind randomised trial allocated women to vaginal douching with either aqueous chlorhexidine (2 mg/ml = 0.2%), or a sterile saline solution". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind randomized trial. "Both solutions were colourless, the equipment identical, and neither midwives nor patients knew which solution was employed." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information is provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported for all randomized women. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for this study was not available to us so we cannot judge if there were any deviations from the protocol in the execution and reporting of the trial. |
| Other bias | Low risk | Appears free of other bias. |
GBS: group B ß‐hemolytic streptococcus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Calkin 1996 | This randomized controlled study ascertained maternal bacterial colonization status on the third day after giving birth. Coloniztion status during labour was not known. Thus the study does not meet our inclusion criteria. |
| Christensen 1985a | This cohort study compared the efficacy of vaginal disinfection with chlorhexidine during labour for reducing colonization of newborns with GBS. In addition to vaginal disinfection with chlorhexidine of the treatment group, all of the women were given a shower using a chlorhexidine‐based soap, and care of all infants included disinfection of the umbilicus with a solution containing chlorhexidine. Thus the study does not meet criteria for inclusion. |
| Christensen 1985b | This was a cohort study that compared the effectiveness of single vaginal washing with chlorhexidine during labour to no treatment for the prevention of GBS colonization in term infants. The study thus does not meet criteria for inclusion. |
| Christensen 1987 | This study has been published in abstract form only. One of the co‐authors Dr. Anna‐Karin Dykes informed us that this study was a cohort study not a randomized controlled trial. |
| Coppens 2000 | This study was a non‐randomized controlled study that compared the effectiveness of 0.2 % diacetate chlorhexidine solution with placebo (tap water) for reducing GBS colonization in infants. |
| Cutland 2009 | This randomized controlled study investigated the effect of chlorhexidine vaginal wipes or external genitalia water wipes during active labour. The newborn infants received full‐body (intervention group) or foot (control group) washes with chlorhexidine at birth, respectively. In a subset of mothers (n = 5144) maternal lower vaginal swabs and neonatal skin swabs after delivery were collected to assess colonization with potentially pathogenic bacteria. The mothers did not meet our inclusion criterion of GBS colonization at onset of labour. In addition, there was a co‐intervention of washes of the infant with chlorhexidine. |
| Dykes 1987 | This was a cohort study that investigated the effect of vaginal washing with chlorhexidine at delivery on the colonization of the mother's urogenital tract with GBS 4 days later. This study did not meet inclusion criteria related to design. |
| Facchinetti 2002 | This randomized controlled study investigated the efficacy of intrapartum vaginal flushings with chlorhexidine compared with ampicillin in preventing GBS transmission to neonates. This study thus did not meet inclusion criteria related to design. |
| Henrichsen 1994 | This randomized study compared 2 different methods of vaginal disinfection with chlorhexidine for preventing neonatal infection. This study did not meet the inclusion criteria related to 'Types of intervention'. |
| Kaihura 2000 | This study was a non‐randomized controlled study that compared the effectiveness of cleansing the birth canal with chlorhexidine 0.25% to cleansing the external genitals with cetrimide 1% and chlorhexidine 0.1% for reducing GBS sepsis in infants. |
| Kollee 1989 | This study was a non‐randomized controlled trial that compared the effectiveness of vaginal disinfection with chlorhexidine gel to no treatment for reducing GBS colonization in infants. |
| Madhi 2007 | This ongoing study of the use of chlorhexidine wipes of the birth canal to prevent neonatal sepsis does not a have maternal colonization with GBS as an entry criterion. The study therefore does not meet the inclusion criteria for this review. The recruitment status of this study is unknown. NCT 00136370 (www.clinicaltrials.gov accessed September 15, 2014). |
| Moss 2007 | This ongoing study of the use of chlorhexidine wash of the vagina and the newborn to prevent neonatal sepsis does not a have maternal colonization with GBS as an entry criterion. The study therefore does not meet the inclusion criteria for this review. The recruitment status of this study is unknown. www.clinicaltrials.gov accessed September 15, 2014 and no reference to this study was found. No publication of this study was found in PubMed accessed September 15, 2014. |
| Mushangwe 2006 | This study, published in abstract form, reports on the use of chlorhexidine washing of the vagina in labour. Maternal colonization with GBS was not an entry criterion. The study therefore does not meet the inclusion criteria for this review. |
| Pereira 2011 | This trial compared 1 % chlorhexidine vaginal and neonatal washing with no washing. The mothers' GBS colonization status was not known at study entry and there was a co‐intervention of washing the infants. Therefore this study did not meet our inclusion criteria. |
| Rouse 1997 | In this randomized controlled trial prophylaxis for early‐onset neonatal GBS sepsis was administered to mothers with certain risk factors for GBS. Vaginal cultures for GBS were not obtained. Therefore this study did not meet our inclusion criteria. |
| Saleem 2010 | This trial compared chlorhexidine vaginal and neonatal wipes with saline placebo wipes. The mothers' GBS colonization status was not known at study entry and there was a co‐intervention of washing the infants. Therefore, this study did not meet our inclusion criteria. |
| Sanderson 1985 | This was a cohort study that compared the effectiveness of whole body washing by mothers with chlorhexidine during the last 2 weeks of labour to a control group who bathed or showered in their normal manner without chlorhexidine. This study thus did not meet inclusion criteria related to design or intervention. |
GBS: group B ß‐hemolytic streptococcus
Differences between protocol and review
For this update GBS pneumonia within the first seven days of life was added as a non‐prespecified outcome.
Contributions of authors
Arne Ohlsson: design of review; editing text of protocol; evaluation of methodologic quality of included trials; and editing text of review.
Vibhuti Shah: design of review; editing text of protocol; identification of trials for inclusion; evaluation of methodologic quality of included trials; abstraction of data; and editing text of review.
Brenda Stade: design of review; writing text of protocol; literature search and identification of trials for inclusion; evaluation of methodologic quality of included trials;abstraction of data; verifying and entering data into Review Manager; and writing text of review.
All three review authors contributed to the 2004 update of this review (Stade 2004).
For the 2014 update, all three review authors assessed the identified trials for inclusion or exclusion. Arne Ohlsson became the contact author; revised the background and methods sections. Arne Ohlsson and Vibhuti Shah extracted data from all included trials and completed the 'Risk of bias' tables. Vibhuti Shah developed the 'Summary of findings' table. Vibhuti Shah and Brenda Stade edited the text and checked the 'Risk of bias' table and the 'Summary of findings' table for accuracy.
Sources of support
Internal sources
St. Michael's Hospital, Toronto, Canada.
Mount Sinai Hospital, Toronto, Canada.
External sources
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Arne Ohlsson, Vibhuti Shah and Brenda Stade have no conflicts of interest to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Adriaanse 1995a {published data only}
- Adriaanse AH, Kollee LA, Muytjens HL, Nijhuis JG, Haan A, Eskes TK. Randomized study of vaginal chlorhexidine disinfection during labour to prevent vertical transmission of group B streptococci. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;61:135‐41. [DOI] [PubMed] [Google Scholar]
- Adriaanse AH, Kollée LAA, Muytjens HL, Nijhuis JG, Eskes TKAB. Randomized study of vaginal chlorhexidine disinfection during labor to prevent vertical transmission of group B streptococci. International Journal of Gynecology and Obstetrics 1994;46(Suppl 1):83. [DOI] [PubMed] [Google Scholar]
Burman 1992 {published data only}
- Burman L, Christensen P, Christensen K, Fryklund B, Helgesson AM, Svenningsen N, et al. Prevention of excess neonatal morbidity associated with group B streptococci by vaginal chlorhexidine disinfection during labour. Lancet 1992;340:65‐9. [DOI] [PubMed] [Google Scholar]
Hennequin 1995 {published and unpublished data}
- Hennequin Y, Tecco L, Vokaer A. Use of chlorhexidine during labor: how effective against neonatal group B streptococci colonization?. Acta Obstetricia et Gynecologica Scandinavica 1995;74:168. [DOI] [PubMed] [Google Scholar]
Stray‐Pedersen 1999 {published data only}
- Stray‐Pedersen B, Bergan T, Hafstad A, Normann E, Grogaard J, Vangdal M. Vaginal disinfection with chlorhexidine during childbirth. International Journal of Antimicrobial Agents 1999;12:245‐51. [DOI] [PubMed] [Google Scholar]
- Stray‐Pedersen B, Hafstad A, Grogaard J, Normann E, Vangdal M, Storvoll G. The effect of intrapartal vaginal douching with chlorhexidine on maternal and neonatal infectious morbidity. Proceedings of the 12th European Congress of Perinatal Medicine; 1990 September 11‐14; Lyon, France. 1990:126.
References to studies excluded from this review
Calkin 1996 {published data only}
- Calkin S. Chlorhexidine swabbing in labour. Modern Midwife 1996;6:28‐33. [PubMed] [Google Scholar]
Christensen 1985a {published data only}
- Christensen KK, Christensen P, Dykes AK, Kahlmeter G. Chlorhexidine for prevention of neonatal colonization with group B streptococci. III. Effect of vaginal washing with chlorhexidine before rupture of the membranes. European Journal of Obstetrics & Gynecology and Reproductive Biology 1985;19:231‐6. [DOI] [PubMed] [Google Scholar]
Christensen 1985b {published data only}
- Christensen KK, Dykes AK, Christensen P. Reduced colonization of newborns with group B streptococci following washing of the birth canal with chlorhexidine. Journal of Perinatal Medicine 1985;13:239‐43. [DOI] [PubMed] [Google Scholar]
Christensen 1987 {published data only}
- Christensen KK, Dykes AK, Christensen P. Prevention of infant colonization with group B streptococci and neonatal disease. Proceedings of the Xth International Lancefield Symposium on Streptococci and Streptococcal Disease; 1987 Sept; Koln, Germany. 1987:256.
Coppens 2000 {published data only}
- Coppens H, Verguts L, Vervliet J, Kersemans L. Intrapartal chlorhexidine lavage as prevention of neonatal colonization with group B streptococci [Intrapartale spoeling met chloorhexidine als preventie van neonatale kolonisatie met group B streptokokken (GBS)]. Nederlands Tijdschrift voor Geneeskunde 2000;56:1558‐62. [Google Scholar]
Cutland 2009 {published data only}
- Cutland CL, Madhi SA, Zell ER, Kuwanda L, Laque M, Groome M, et al. Chlorhexidine maternal‐vaginal and neonate body wipes in sepsis and vertical transmission of pathogenic bacteria in South Africa: a randomised, controlled trial. Lancet 2009;374(9705):1909‐16. [DOI] [PubMed] [Google Scholar]
Dykes 1987 {published data only}
- Dykes AK, Christensen KK, Christensen P. Chlorhexidine for prevention of neonatal colonization with group B streptococci. IV. Depressed puerperal carriage following vaginal washing with chlorhexidine during labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 1987;24:293‐7. [DOI] [PubMed] [Google Scholar]
Facchinetti 2002 {published data only}
- Facchinetti F, Piccinini F, Mordini B. Prophylaxis of group B streptococcus vertical transmission by using vaginal chlorhexidine. American Journal of Obstetrics and Gynecology 1999; Vol. 180, issue 1 Pt 2:S84.
- Facchinetti F, Piccinini F, Mordini B, Volpe A. Chlorhexidine vaginal flushings versus systemic ampicillin in the prevention of vertical transmission of neonatal group B streptococcus, at term. Journal of Maternal‐Fetal & Neonatal Medicine 2002;11:84‐8. [DOI] [PubMed] [Google Scholar]
Henrichsen 1994 {published data only}
- Henrichsen T, Lindemann R, Svenningsen L, Hjelle K. Prevention of neonatal infections by vaginal chlorhexidine disinfection during labour. Acta Paediatrica 1994;83:923‐6. [DOI] [PubMed] [Google Scholar]
Kaihura 2000 {unpublished data only}
- Kaihura C, Barbara, G, Benassi G, Ricci L, Bedocchi L, Rossi T, et al. Lavage of the birth canal with chlorhexidine: a new valid method for the prevention of perinatal infections [Il lavaggio del canale del parto con clorexidina: una nuova valida metodica di prevenzione delle infezioni perinatali]. Acta Bio‐Medica de L'Ateneo Parmense 2000;71(Suppl 1):567‐71. [PubMed] [Google Scholar]
Kollee 1989 {published data only}
- Kollee LAA, Speyer I, Kuijck MAP, Koopman R, Dony JM, Bakker JH, et al. Prevention of group B streptococci transmission during delivery by vaginal application of chlorhexidine gel. European Journal of Obstetrics & Gynecology and Reproductive Biology 1989;31:47‐51. [DOI] [PubMed] [Google Scholar]
Madhi 2007 {published data only}
- Madhi S. Prevention of perinatal sepsis (PoPS): evaluation of chlorhexidine wipes of birth canal and newborn (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 8 September 2007] 2007.
Moss 2007 {published data only}
- Moss N. Chlorhexidine vaginal and infant wash in Pakistan (ongoing trial). www.clinicaltrials.gov (accessed September 8 2007).
Mushangwe 2006 {published data only}
- Mushangwe V, Tolosa JE, Pereira L, Mashu A, Bangdiwala S, Rusakaniko S, et al. Chlorhexidine washing of the vagina in labor effectively reduces bacterial colonization: a study by the global network for perinatal & reproductive health. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S66. [Google Scholar]
Pereira 2011 {published data only}
- Pereira L, Chipato T, Mashu A, Mushangwe V, Rusakaniko S, Bangdiwala S, et al. Chlorhexidine washing of the vagina in labor and neonate after birth: an RCT by the global network for perinatal & reproductive health. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S70. [Google Scholar]
- Pereira L, Chipato T, Mashu A, Mushangwe V, Rusakaniko S, Bangdiwala SI, et al. Randomized study of vaginal and neonatal cleansing with 1% chlorhexidine. International Journal of Gynecology & Obstetrics 2011;112(3):234‐8. [DOI] [PubMed] [Google Scholar]
Rouse 1997 {published data only}
- Rouse DJ, Hauth JC, Andrews WW, Mills BB, Maher JE. Chlorhexidine vaginal irrigation for the prevention of peripartal infection: A placebo‐controlled randomized clinical trial. American Journal of Obstetrics and Gynecology 1997;176:617‐22. [DOI] [PubMed] [Google Scholar]
Saleem 2010 {published data only}
- Saleem S, Rouse DJ, McClure EM, Zaidi A, Reza T, Yahya Y, et al. Chlorhexidine vaginal and infant wipes to reduce perinatal mortality and morbidity: a randomized controlled trial. Obstetrics & Gynecology 2010;115(6):1225‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanderson 1985 {published data only}
- Sanderson PJ, Haji TC. Transfer of group B streptococci from mothers to neonates: effect of whole body washing of mothers with chlorhexidine. Journal of Hospital Infection 1984;6:257‐64. [PubMed] [Google Scholar]
Additional references
AAP 1992
- American Academy of Pediatrics, Committee on Infectious Diseases and Committee on Fetus and Newborn. Guidelines for prevention of group B streptococcal infection by chemoprophylaxis. Pediatrics 1992;90:775‐8. [PubMed] [Google Scholar]
AAP 1997
- American Academy of Pediatrics, Committee on Infectious Diseases/Committee on Fetus and Newborn. Revised guidelines for prevention of early‐onset group B streptococcal (GBS) disease. Pediatrics 1997;99:489‐96. [DOI] [PubMed] [Google Scholar]
ACOG 1992
- Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Group B Streptococcal Infections in Pregnancy. ACOG Technical Bulletin no. 170. ACOG, 1992 July. [Google Scholar]
ACOG 1996
- American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Washington DC. Prevention of Early‐Onset Group B Streptococcal Disease in Newborns [Opinion 173]. ACOG, 1996. [PubMed] [Google Scholar]
Adriaanse 1995b
- Adriaanse AH. Prevention of neonatal septicaemia due to group B streptococci. Baillieres Clinical Obstetrics and Gynaecology 1995;9:545‐52. [DOI] [PubMed] [Google Scholar]
Autegarden 1999
- Autegarden JE, Pecquet C, Huet S, Bayrou O, Leynadier F. Anaphylactic shock after application of chlorhexidine to unbroken skin. Contact Dermatitis 1999;40:215. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baker 1995
- Baker CJ, Edwards MS. Group B streptococcal infection. In: Remington J, Klein JO editor(s). Infectious Diseases of the Fetus and Newborn Infant. Philadelphia: Saunders, 1995:980‐1054. [Google Scholar]
Bakr 2005
- Bakr AF, Karkour T. Effect of predelivery vaginal antisepsis on maternal and neonatal morbidity and mortality in Egypt. Journal of Women's Health 2005;14(6):496‐501. [DOI] [PubMed] [Google Scholar]
Barcaite 2008
- Barcaite E, Bartusevicius A, Tameliene R, Kliucinskas M, Maleckiene L, Nadisauskiene R. Prevalence of maternal group B streptococcal colonisation in European countries. Acta Obstetricia et Gynecologica Scandinavica 2008;87(3):260‐71. [DOI] [PubMed] [Google Scholar]
Benitz 1999
- Benitz WE, Gould JB, Druzin ML. Preventing early‐onset group B streptococcal sepsis: strategy development using decision analysis. Pediatrics 1999;103:e76. [DOI] [PubMed] [Google Scholar]
Boyer 1986
- Boyer K, Gotoff S. Prevention of early‐onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. New England Journal of Medicine 1986;314:1665‐9. [DOI] [PubMed] [Google Scholar]
CDC 1996
- Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease: a public health perspective. Morbidity and Mortality Weekly Report 1996;45:(RR‐7):1‐24. [PubMed] [Google Scholar]
CDC 2002
- Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease. Morbidity andMortality Weekly Reports 2002;51(RR11):1‐22. [Google Scholar]
CDC 2005
- Centers for Disease Control and Prevention (CDC). Early‐onset and late‐onset neonatal group B streptococcal disease‐‐United States, 1996‐2004. Morbidity and Mortality Weekly Report 2005;54(47):1205‐8. [PubMed] [Google Scholar]
CDC 2007
- Centers for Disease Control and Prevention (CDC). Perinatal group B streptococcal disease after universal screeningrecommendations‐United States, 2003‐2005. Morbidity and Mortality Weekly Report 2007;56:701‐5. [PubMed] [Google Scholar]
CDC 2010
- Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. Morbidity and Mortality Weekly Report 2010;59(RR‐10):1‐36. [PubMed] [Google Scholar]
Christensen 1983
- Christensen KK, Christensen P, Dykes A, Kahlmeter G, Kurl DN, Linden V. Chlorhexidine for the prevention of neonatal colonization with group B streptococci. I. In vitro effect of chlorhexidine on group B streptococci. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;16:157‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clavisi 2000
- Clavisi O, Shaw S, Anderson, JN. Does cleansing the birth canal at delivery reduce postnatal infection rates?. Medical Journal of Australia 2000;173:550‐1. [PubMed] [Google Scholar]
Dillon 1987
- Dillon HC, Khare S, Gray BM. Group B streptococcal carriage and disease: a 6‐year prospective study. Journal of Pediatrics 1987;110:31‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dykes 1983
- Dykes AK, Christensen KK, Christensen P, Kahlmeter G. Chlorhexidine for prevention of neonatal colonization with group B streptococci. II. Chlorhexidine concentrations and recovery of group B streptococci following vaginal washing in pregnant women. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;16:167‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fernandez 1998
- Fernandez M, Hickman ME, Baker CJ. Antimicrobial susceptibilities of group B streptococci isolated between 1992 and 1996 from patients with bacteremia or meningitis. Antimicrobial Agents and Chemotherapy 1998;42:1517‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freij 1999
- Freij B, McCracken G. Acute infections. In: Avery G, Fletcher M, MacDonald M editor(s). Neonatology: Pathophysiology and Management of the Newborn. 5th Edition. Philadelphia: Lippincott, Williams & Wilkins, 1999:1189‐230. [Google Scholar]
Garland 1996
- Garland JS, Alex CP, Mueller CD, Cisler‐Kahill LA. Local reactions to a chlorhexidine gluconate‐impregnated antimicrobial dressing in very low birth weight infants. Pediatric Infectious Disease Journal 1996;15:912‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goldenberg 2006
- Goldenberg RL, McClure EM, Saleem S, Rouse D, Vermund S. Use of vaginally administered chlorhexidine during labor to improve pregnancy outcomes. Obstetrics & Gynecology 2006;107(5):1139‐46. [DOI] [PubMed] [Google Scholar]
GRADE 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version Version 3.6. The Cochrane Collaboration, 2008.
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd. 2005.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johri 2006
- Johri AK, Paoletti LC, Glaser P, Dua M, Sharma PK, Grandi G, et al. Group B Streptococcus: global incidence and vaccine development. Nature Reviews. Microbiology 2006;4:932‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keenan 1998
- Keenan C. Prevention of neonatal group B streptococcal infection. American Family Physician 1998;57:2713‐20. [PubMed] [Google Scholar]
Lumbiganon 2014
- Lumbiganon P, Thinkhamrop J, Thinkhamrop B, Tolosa JE. Vaginal chlorhexidine during labour for preventing maternal and neonatal infections (excluding Group B Streptococcal and HIV). Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD004070.pub3] [DOI] [PubMed] [Google Scholar]
Matorras 1990
- Matorras R, Garcia‐Perea A, Madero R Usandizaga JA. Maternal colonization by group B streptocci and puerperal infection; analysis of intrapartum chemoprophylaxis. European Journal of Obstetrics & Gynecology and Reproductive Biology 1990;38(3):203‐7. [DOI] [PubMed] [Google Scholar]
Money 2013
- Money D, Allen VM, Society of Obstetricians and Gynaecologists of Canada. The prevention of early‐onset neonatal group B streptococcal disease. Journal of Obstetrics and Gynaecology Canada 2013;35(10):939‐51. [DOI] [PubMed] [Google Scholar]
Noya 1987
- Noya FJ, Rench MA, Metzger TG, Colman G, Naidoo J, Baker C. Unusual occurrence of an epidemic of type Ib/c group B streptococcal sepsis in a neonatal intensive care unit. Journal of Infectious Diseases 1987;155:1135‐44. [DOI] [PubMed] [Google Scholar]
Ohlsson 1992
- Ohlsson A. Perinatal group B streptococcal infections. Canadian Journal of Obstetrics/Gynaecology & Women's Health Care 1992;4:355‐63. [Google Scholar]
Ohlsson 1994
- Ohlsson A, Myhr TL. Intrapartum chemoprophylaxis of perinatal group B streptococcal infections: a critical review of randomized controlled trials. American Journal of Obstetrics and Gynecology 1994;170:1326‐7. [DOI] [PubMed] [Google Scholar]
Ohlsson 2014
- Ohlsson A, Shah VS. Intrapartum antibiotics for known maternal Group B streptococcal colonization. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD007467.pub4] [DOI] [PubMed] [Google Scholar]
Pham 2000
- Pham NH, Weiner JM, Reisner GS, Baldo BA. Anaphylaxis to chlorhexidine. Case report. Implication of immunoglobulin E antibodies and identification of an allergenic determinant. Clinical and Experimental Allergy 2000;30:1001‐7. [DOI] [PubMed] [Google Scholar]
Quinn 1989
- Quinn MW, Bini RM. Bradycardia associated with chlorhexidine spray. Archives of Disease in Childhood 1989;64:892‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
RCOG 2003
- Royal College of Obstetricians and Gynaecologists. Prevention of early onset neonatal group B streptococcal disease. http://www.rcog.org.uk/resources/Public/pdf/GroupB_strep_no36.pdf (accessed 2008) 2003.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schrag 2002
- Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold K, Craig AS, et al. A population‐based comparison of strategies to prevent early‐onset group B streptococcal disease in neonates. New England Journal of Medicine 2002 2002;347:233‐9. [DOI] [PubMed] [Google Scholar]
Schrag 2013
- Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for prevention of perinatal group B streptococcal disease:experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine 2013;31(Suppl 4):D20‐D26. [DOI] [PubMed] [Google Scholar]
Schuchat 1998
- Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clinical Microbiology Reviews 1998;11:497‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuchat 1999
- Schuchat A. Group B streptococcus. Lancet 1999;353:51‐6. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Übertragbarkeit von Studienergebnissen]. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2009;103(6):391‐400. [PubMed: 19839216] [DOI] [PubMed] [Google Scholar]
Shah 2000
- Shah V, Ohlsson A. Perinatal group B streptococcal infections. In: Newell ML, McIntyre J editor(s). Congenital and Perinatal Infections. Prevention, Diagnosis and Treatment. Cambridge: Cambridge University Press, 2000:96‐121. [Google Scholar]
Shah 2001
- Shah V, Ohlsson A, The Canadian Task Force on Preventive Health Care. Prevention of early‐onset Group B Streptococcal(GBS) infection in the newborn: systematic review and recommendations. Technical report. The CanadianTask Force on Preventive Health Care (www.ctfphc.org) (accessed 2008) 2001.
Smaill 1996
- Smaill F. Intrapartum antibiotics for group B streptococcal colonisation. Cochrane Database of Systematic Reviews 1996, Issue 1. [DOI: 10.1002/14651858.CD000115] [DOI] [PubMed] [Google Scholar]
SOGC 1994
- Society of Obstetricians and Gynaecologists of Canada, Canadian Paediatric Society. National consensus statement on the prevention of early onset group B streptococcal infections in the newborn. Journal of the Society of Obstetricians and Gynaecologists of Canada 1994;16:2271‐8. [Google Scholar]
SOGC 1997
- Society of Obstetricians and Gynaecologists. Statement on the prevention of early‐onset group B streptococcal infections in the newborn. Journal of the Society of Obstetricians and Gynaecologists of Canada 1997;19:751‐8. [Google Scholar]
SOGC 2004
- Society of Obstetricians and Gynaecologist of Canada. The prevention of early‐onset group B streptococcal infections in newborns. Journal of Obstetrics and Gynaecology of Canada 2004;26:826‐32. [DOI] [PubMed] [Google Scholar]
Stoll 1996
- Stoll B, Gordon T, Korones S, Shankaran S, Tyson JE, Bauer CR, et al. Early‐onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. Journal of Pediatrics 1996;129:72‐80. [DOI] [PubMed] [Google Scholar]
Stoll 1998
- Stoll BJ, Schuchat A. Maternal carriage of group B streptococci in developing countries. Pediatric Infectious Disease Journal 1998;17(6):499‐503. [DOI] [PubMed] [Google Scholar]
Taha 1997
- Taha TE, Biggar RJ, Broadhead RL, Mtimavalye LAR, Justesen AB, Liomba GN, et al. Effect of cleansing the birth canal with antiseptic solution on maternal and newborn morbidity and mortality in Malawi: clinical trial. BMJ 1997;315(7102):216‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thune 1998
- Thune P. Two patients with chlorhexidine allergy‐anaphylactic reactions and eczema. Tidsskift For Den Norske Laegeforening 1998;118:3295‐6. [PubMed] [Google Scholar]
Tuppurainen 1989
- Tuppurainen N, Hallman M. Prevention of neonatal group B streptococcal disease: intrapartum detection and chemoprophylaxis of heavily colonized parturients. Obstetrics & Gynecology 1989;75:583‐7. [PubMed] [Google Scholar]
References to other published versions of this review
Stade 2004
- Stade BC, Shah VS, Ohlsson A. Vaginal chlorhexidine during labour to prevent early‐onset neonatal group B streptococcal infection.. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003520.pub2] [DOI] [PubMed] [Google Scholar]


