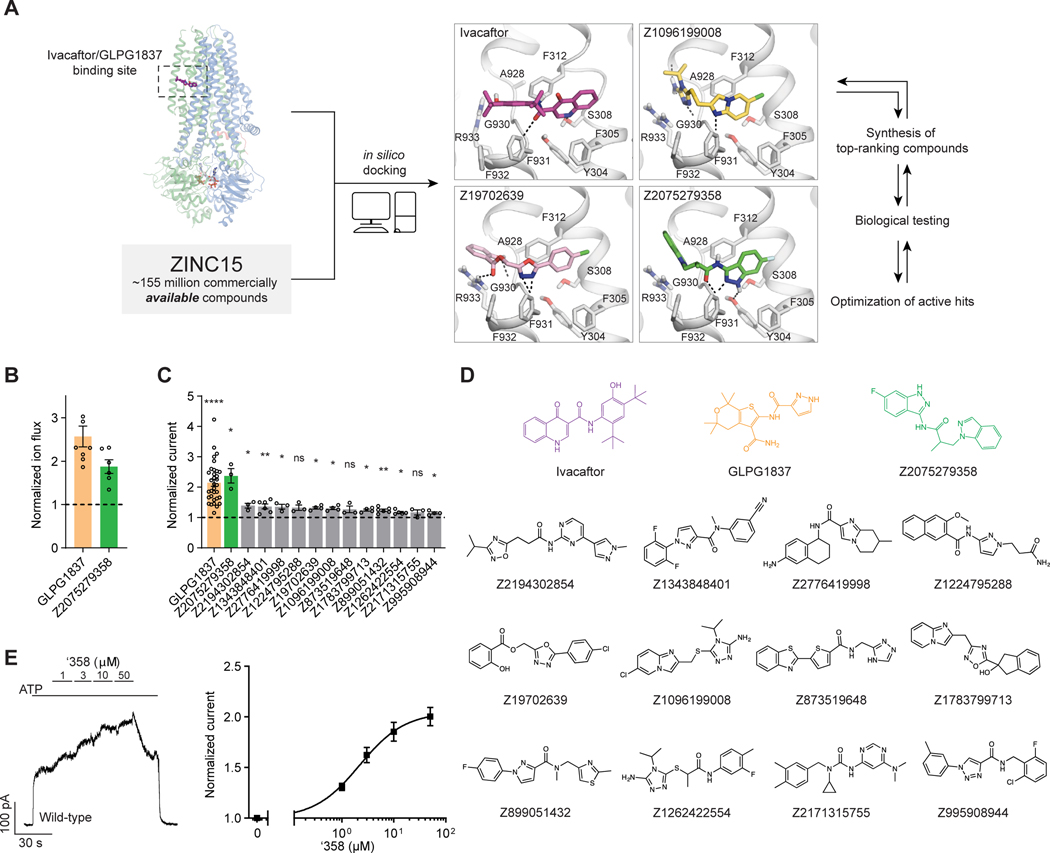
Figure 1: Ultra-large docking screen identifies CFTR potentiators.
(A) The workflow of this study. (B) Compound Z2075279358 (‘358) potentiates ΔF508 CFTR. CFBE41o− cells homozygous for ΔF508 CFTR were cultured with 1 μM lumacaftor to facilitate surface-expression and pre-treated with 20 μM forskolin to activate ΔF508 CFTR by PKA-phosphorylation. The relative potentiation was calculated as the ratio of flux rates with and without 1 μM potentiator. Data points represent the means and standard errors (SEs) of 6 to 8 measurements (each shown as a dot). (C) Potentiation activity of 10 μM GLPG1837 or 5 μM compound against WT CFTR fused to a carboxy-terminal GFP tag. Inside-out membrane patches containing WT CFTR were excised from CHO cells and then fully phosphorylated by protein kinase A (PKA) in the presence of 3 mM ATP. The fold stimulation is defined as the ratio of the current in the presence and absence of added compound. Data represent means and SEs of 3–33 patches with individual measurements shown as dots. Statistical significance relative to absence of compound was tested by two-tailed Student’s t-test with Benjamini-Hochberg correction (ns: not significant; *P < 0.05; **P < 0.01; ****P = 4.2×10−14). (D) The 2D structures of the potentiators GLPG1837, ivacaftor, and the positive hits from the initial screen. (E) Representative macroscopic current trace and dose-response curve of WT CFTR in response to perfusion with ‘358. CFTR-containing membrane patches were fully phosphorylated by PKA. The current in the presence of 3 mM ATP before titration was used to normalize the current potentiated by different concentrations of ‘358. The EC50 is estimated to be 2.2 ± 0.6 μM by fitting the dose-responses with the Hill equation. Data represent means and SEs from 3 patches.