Abstract
Respiratory viral infections frequently lead to severe respiratory disease, particularly in vulnerable populations such as young children, individuals with chronic lung conditions and older adults, resulting in hospitalisation and, in some cases, fatalities. The innate immune system plays a crucial role in monitoring for, and initiating responses to, viruses, maintaining a state of preparedness through the constant expression of antimicrobial defence molecules. Throughout the course of infection, innate immunity remains actively involved, contributing to viral clearance and damage control, with pivotal contributions from airway epithelial cells and resident and newly recruited immune cells. In instances where viral infections persist or are not effectively eliminated, innate immune components prominently contribute to the resulting pathophysiological consequences. Even though both young children and older adults are susceptible to severe respiratory disease caused by various respiratory viruses, the underlying mechanisms may differ significantly. Children face the challenge of developing and maturing their immunity, while older adults contend with issues such as immune senescence and inflammaging. This review aims to compare the innate immune responses in respiratory viral infections across both age groups, identifying common central hubs that could serve as promising targets for innovative therapeutic and preventive strategies, despite the apparent differences in underlying mechanisms.
Shareable abstract
Comparing innate immune responses to respiratory viral infections in different age groups identifies common central hubs that serve as targets for innovative therapeutic and preventive strategies, despite the apparent differences in underlying mechanisms. https://bit.ly/3xRmRrn
Introduction
Respiratory tract infections (RTIs), particularly in infants and older adults, pose a substantial risk for severe disease, but the underlying mechanisms can vary significantly. In the case of young children, the maturation of innate immunity is a critical factor, whereas older adults experience a decline in immune function along with other physiological impairments, such as reduced mucociliary clearance [1, 2], diminished elastic recoil of the lung, decreased respiratory muscle capacity and heightened aspiration [3].
Microbial exposure drives immune development in newborns
Early life is a period of dynamic change for the immune and respiratory systems, as both are undergoing a period of maturation. Alveolarisation of the lung continues after birth for the first 2 years of life [4]. For the immune system, this involves the development of immunological memory against pathogens, which may take several years [5]. Due to the post-natal development of innate antimicrobial defence pathways, which initially is heavily dependent on the activity of neutrophils, monocytes, dendritic cells (DCs) and epithelial barrier cells, the very young are more susceptible to respiratory pathogens [6], posing an increased risk of hospitalisation due to lower RTIs. Interestingly, abundant microbial exposure in early life seems to reduce this risk. Studies from areas rich in microbial exposure, e.g. living on a farm, suggest that this profoundly affects innate immune responses and poses a protective effect [7].
From birth onwards, the lungs and gut are seeded with microbes. Results from a longitudinal study of 100 children during the first 3 months of life [8], employing mass cytometry and proteomics on peripheral blood, suggested stereotypical immune maturation driven by the development of the gut microbiome. The relevance of early-life microbial exposure is further underlined in children born via caesarean section [9, 10] or those who received antibiotics in early life [11, 12], which were associated with an increased susceptibility to respiratory viruses and a heightened risk of developing childhood asthma. To protect the vulnerable mucosal surfaces in vital organs such as lungs and gut, maintaining immune balance and a state of tonic noninflammatory immune readiness is essential. Host–microbe interactions play a crucial role in establishing this balance [13].
Inflammaging and immunosenescence
As individuals age, the functionality of the immune system declines, a process known as immunosenescence [14–16]. Consequently, older people are at an increased risk for cancer and infectious diseases, alongside a suboptimal development of vaccine responses [17–19]. The molecular mechanisms that drive immunosenescence have been defined well in mice, but their validation in humans is less extensive [20, 21]. Immunosenescence is multi-faceted, affecting the function and phenotype of almost all immune populations. The primary lymphoid organs produce fewer (naïve) immune cells [22]. In addition, proliferating memory cells lead to strong clonal expansions and a reduced pool of cells remaining capable of responding to novel antigens [23, 24]. The accumulation of damage, metabolic changes and chronic exposure to viruses and bacteria translocating from the gut further contributes to immunosenescence. Many of these mechanisms converge through the process of inflammaging, which is the elevated presence of inflammatory cytokines in the body [25]. Many of the inflammatory cytokines found during inflammaging are produced by innate immune populations, such as monocytes, DCs and innate lymphocytes; however, adipocytes can also contribute [26]. There are profound differences in the composition, activation state and functional profile of the innate immune system with advanced age, many of which are expected to impact the response to RTIs [26–31].
Both infants and older adults exhibit a heightened susceptibility to RTIs and experience distinctive variations in innate infection control. This review examines the existing evidence concerning these immune deviations and their significance in viral control and the progression of disease. Ultimately, we explore potential strategies that target innate immune components to enhance the management of RTIs effectively.
Search strategy
This manuscript is structured as a narrative review. The published data in the area is nonhomogeneous and not conducive to meta-analysis. In addition, various high-quality reviews have been published on either respiratory viral infection, on immune development in neonates or immune senescence in elderly. Here, we focus on overlapping aspects in innate immunity against respiratory viral infection between neonates and elderly. To restrict our findings to the state of the art, the search period covers 2000–2023. To find relevant English-language publications in peer-reviewed journals, the following databases were interrogated: PubMed, Google, Scopus and Medline (Ovid). Search terms were variably deployed, depending on the area of focus in the current review. The search algorithm was based on combinations of the following medical subject headings (MeSH): (“Respiratory virus” OR SARS-CoV-2 OR COVID-19 OR “Respiratory Syncytial Virus” OR Rhinovirus OR Influenza) AND (“Innate Immunity’ OR “Trained Immunity” OR “Innate immune cells” OR Neutrophils OR Macrophages OR “Dendritic cells” OR “MAIT cells”) AND (lung OR nose OR Nasal OR “respiratory tract”) AND ((“older adults” OR elderly OR aged) OR (infants OR neonates OR pediatric OR Young)) AND ((resolution OR resolving OR clearance) OR (“lung microbiome”) OR (“Bronchial epithelial cells” OR “nasal epithelial cells”) OR (BCG OR “bacterial lysates” OR “microbial metabolites”) OR (Interferons OR “IFN-stimulated genes”)). This resulted in 864 papers, including 108 reviews, 33 clinical studies and various opinion pieces, of which 207 have been referenced in this review.
Immune surveillance and initial responses to respiratory viruses
Neutrophils in the respiratory tract
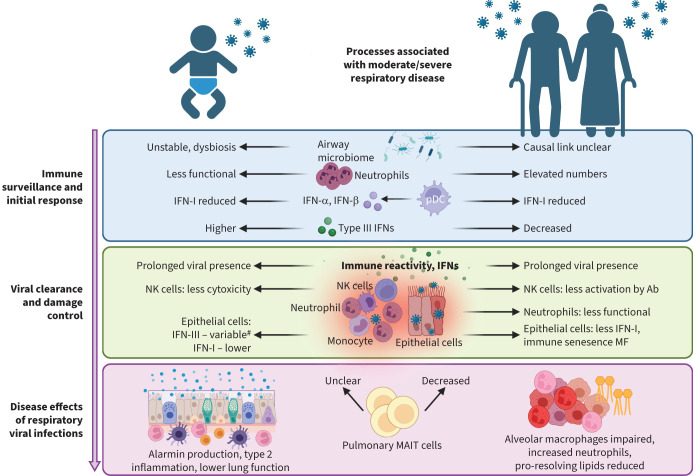
It is challenging to gain an insight into early immune events during the initiation of natural infections in humans, as this should be examined prior to or at least at the first signs of infection. Controlled human infection models are helpful in this regard as the infection initiation is known and planned. Several studies have investigated older adults in these models. For example, controlled infection of older adults with respiratory syncytial virus (RSV) led to a twice higher attack rate compared to young adults, with significantly higher viral titres [32]. The number of baseline neutrophils was related to infection susceptibility and was associated with inhibited tumour necrosis factor-α (TNF-α) and interleukin (IL)-17 responses early post-exposure [33]. Consequently, local inflammatory responses following exposure can be impaired by a pre-existing inflammaging state. In a mouse model, RSV infection in older mice also led to increased viral loads at day 5 [34]. Neutrophils were also found to be less abundant in the nasal mucosa of children compared to adults [35]. However, at an older age, the neutrophil to lymphocyte ratio increases both in blood and the nasal mucosa [35, 36], even in the absence of disease [37]. This might relate to an increased susceptibility to severe viral infections in older individuals. In contrast to older mice, neonatal mice showed a relatively low neutrophilic infiltration in the lungs early after RSV infection, while this was clearly more enhanced in neonatal mice with influenza infection [38, 39]. However, neonatal neutrophils showed poor chemotaxis, endothelial adhesion, phagocytic function and neutrophil extracellular trap (NET) formation in vitro, suggesting that they may not act as effectively as neutrophils from adults [40–42]. However, a recent study [43] showed a greater migration of cord blood neutrophils across RSV-infected epithelial cells compared to adult neutrophils in an in vitro culture model. At baseline, cord blood neutrophils had a higher expression of CD11b, CD64, neutrophil elastase and myeloperoxidase, suggesting that infant neutrophils are not fully impaired in their function, but may respond different to virus-infected epithelium. Some studies have suggested an enhanced viability of migrated neutrophils from infants (e.g. in bronchoalveolar lavage (BAL) [44]), while others report enhanced apoptosis [45, 46]. As these processes will affect protective mechanisms against the virus by clearing obsolete cells and curbing ongoing inflammation, changes in or failure of these mechanisms may affect disease severity and resolution. Therefore, while in neonates the behaviour of neutrophils in the lung may be different from those in adults, in older adults their frequency is much higher, which is strongly associated with an unfavourable outcome (figure 1).
FIGURE 1.
Innate responses during viral infections in young and older individuals. Impairment or deviations in innate immune cell numbers and/or functionalities during different stages of viral infection: 1) during immune surveillance and initiation of infection, 2) viral clearance and damage control, and 3) disease effects with uncontrolled and persistent viral infections in both very young children and older adults. Ab: antibody; IFN: interferon; MAIT: mucosal-associated invariant T; MF: macrophage; NK: natural killer; pDC: plasmacytoid dendritic cell.
Interferons (IFNs) during early infection
An important component of innate immunity in the respiratory tract is the IFN response. This consists of type I, II and III IFNs (IFN-I, IFN-II and IFN-III). IFN-I (IFN-α/IFN-β) is an antiviral cytokine that binds to ubiquitously expressed INF-α/β receptors and induces the expression of hundreds of IFN-stimulated genes (ISGs) that impair viral replication in infected cells and protect the lung from further spreading of viruses [47]. However, IFN-I is also central to inflammatory responses, by inducing recruitment and activation of immune cells to the lungs. This helps to control virus burden but can also cause detrimental immunopathology and contribute to disease severity. IFN-III [48, 49] or IFN-λ acts on similar genes to IFN-I, but it primarily targets lung epithelial cells and protects them against respiratory viruses. Indeed, IFNs could inhibit severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza infection and replication in primary human airway epithelial cells when given before infection [50, 51]. Type II IFN refers to IFN-γ, which is primarily produced by immune cells, such as T- and natural killer (NK) cells. It has antiviral activity and promotes the activation of macrophages and other immune cells [52].
At birth, IFN responses are low and still need to develop. In a human cohort followed prospectively, IFN-α production by DCs increased to adult levels by 1 year of age [53]. In addition, in response to RSV infection, IFN-α production in plasmacytoid DCs (pDCs) from cord blood or peripheral blood mononuclear cells (PBMCs) from children (<1 year) were lower compared to adults [54]. Similar findings were described in RSV-infected neonatal mice, where recruitment of pDCs and production of IFN-I was strongly reduced [55]. Interestingly, deletion of insulin-responsive aminopeptidase (IRAP), a protein involved in regulation of IFN-I production, substantially enhanced IFN-I in neonatal alveolar macrophages, but not in adult ones. Neonatal IRAP-deficient mice infected with RSV cleared the virus more efficiently compared to wild-type mice [56]. This suggests that IRAP is an age-dependent regulator of IFN-I expression and influences protection and immunity to respiratory viruses.
During older age, monocytes/macrophages and conventional DCs (cDCs) showed reduced IFN-I production [57]. In particular, pDCs decline, produce fewer IFN-I and IFN-III responses to influenza virus, and are present at lower frequencies at older age [28, 58]. In contrast to these human data, viral load and IFN-I responses were not different between young and aged mice in the early stages following influenza virus infection [59]. However, this likely depends on the viral agent. A mouse-adapted SARS-CoV-2 strain showed that increased early viral load in aged mice led to death and was linked to impaired IFN responses [60]. Indeed, daily sampling of SARS-CoV-2 infection followed by mathematical modelling indicated that, with increasing age, innate immune responses are less effective in rendering target cells refractory to infection [61]. There was no association between age and viral clearance or growth rate. Early IFN responses in the lung seem key to control viral infection and replication.
Respiratory microbiome
Various bacterial communities are associated with an increased risk of severe RTIs in young children [62, 63]. Nasopharyngeal microbiota in healthy infants showed the biggest dynamics in gene expression profiles during the first days of life, which included Toll-like receptor (TLR) and inflammasome signalling, representing the first colonisation of airway microbiota [64]. This was accompanied by a gradual increase in regulatory responses, such as IL-10. Asymptomatic viral infections were associated with increased IFN-I and IFN-γ expression and preceded the development of risky respiratory microbiota (e.g. Moraxella and Haemophilus species) and increased susceptibility to severe RTI [64]. Indeed, a causal link between respiratory viral infections, IFN-I and changes in microbiota was demonstrated in influenza-infected mice showing that nasal microbiota was modified in an IFN-dependent manner, leading to staphylococcal persistence [65]. In contrast, asymptomatic viral infections were associated with increased IFN signalling in upper respiratory tract and protected against live attenuated influenza vaccine replication in a cohort of children from the Gambia, indicating that asymptomatic viral infections can also protect against secondary incoming viruses [66]. Influenza-like illness was also found to change the nasal microbiota of frail older adults in long-term care homes [67]. However, although gut microbiota was associated with immunosenescence in older adults and the respiratory tract microbiota was shown to change with advancing age, the effect of respiratory tract microbiota on viral infections remains poorly defined in this group [68, 69]. Interestingly, commensal bacteria can also stimulate IFN-I/III expression in respiratory epithelial cells, leading to enhanced antiviral resistance for influenza-A virus [70], as shown by antibiotic treatment and faecal transplantation in mice [71]. Moreover, Streptococcus pneumoniae colonisation was able to dampen nasal inflammatory responses to live attenuated influenza virus in a controlled human co-infection model [72].
These studies suggest that bidirectional interactions between colonised bacteria, asymptomatic viral infections and epithelium influence early IFN-I levels and affect the course of viral RTIs and the composition of microbiota. The timing of the first IFN wave and coincident induction of regulatory responses may be crucial for the clinical outcomes of viral RTIs.
Lessons from the coronavirus disease 2019 (COVID-19) pandemic
Further insights into age-related differences in innate antiviral immunity have emerged from the COVID-19 pandemic. Age is the strongest risk outcome for severe SARS-CoV-2 infections; children have a substantially lower risk of developing severe COVID-19. Of note, approx. 15% of the severe and critically ill COVID-19 patients can be explained by either inborn errors of type 1 IFNs or pre-existing auto-antibodies neutralising IFNs [73–75]. Large-scale epidemiological studies have yielded contradictory results on the effect of old age on SARS-CoV-2 loads [76, 77]. SARS-CoV infection in aged African green monkeys was associated with increased viral load at day 1 post-infection, but not at later time-points [78]. Viral replication of SARS-CoV was similar in aged and young macaques [79].
To understand why SARS-CoV-2 viral load is better controlled in children [77], several single-cell transcriptomics studies were performed comparing airway mucosa and peripheral blood from children and adults. Airway epithelium of children had a higher steady-state expression of IFN-response genes, which may restrict viral replication in children [80]. Indeed, IFN-λ was also upregulated in nasal swabs from infected children, while other IFNs and antimicrobial genes were unaffected, contrasting with findings in infected adults [81]. Children also showed higher basal expression of viral pattern recognition receptors (PRRs) (i.e. melanoma differentiation-associated protein 5 (MDA5)/retinoic acid-inducible gene I (RIG-I)) in epithelial and immune cells, resulting in stronger innate antiviral responses compared adults [82]. Whether this is a consequence of more recent infections in children (and/or changes in microbiota composition which may induce IFNs) remains to be established. Furthermore, nasal populations such as killer cell lectin like receptor C1 (NKG2A)+ cytotoxic T-cells and CD8+ T-cells with a memory phenotype were found in children, suggesting that children mounted stronger early antiviral responses to SARS-CoV-2 infection compared to adults [82]. In contrast, adults displayed a higher cytotoxic immune compartment in peripheral blood, probably because of viral spreading and containment difficulties. This also was accompanied with ISGs in multiple immune cells, adding an inflammatory state to the already cytotoxic immune response and amplifying the pathological effects of systemic immune responses [80]. These studies indicate that a higher level of infection readiness locally in (nasal) mucosal tissues, but without a systemic inflammation-inducing component, is beneficial to curb viral infections early and effectively prevents spreading to lower airways. Notably, higher IFN-III expression in patients with COVID-19 correlated with lower viral load in bronchial aspirates and faster viral clearance. In addition, a higher IFN-III/IFN-I ratio correlated with an improved outcome for critically ill patients [83].
In summary, depending on the virus and host bacterial species, baseline differences in neutrophilic inflammatory status and IFN responses are related to an increased susceptibility to infection (figure 1).
Viral clearance and damage control
Respiratory epithelium: an important gatekeeper
Pathogen clearance and infection resolution in the lung is a delicate and multifaceted process, orchestrated by innate immune cells and respiratory tract cells. Even though an effective immune response is needed to eliminate the pathogen, exaggerated and/or prolonged immune responses can damage the lung tissue and should be avoided. The respiratory epithelium is an important player in the defence against viral infections [84]. Epithelial cells express PRRs and cytokine receptors, produce antimicrobial proteins and mucins, as well as produce IFN responses to viruses [84]. Furthermore, interactions between resident airway macrophages and epithelial cells drive local homeostasis and immune tolerance to harmless stimuli, support antimicrobial responses to dangerous invaders and drive tissue repair following pathogen clearance [85].
IFN-III is considered an important player in immune homeostasis at mucosal sites, where it stimulates pathogen clearance while curbing inflammation to maintain barrier integrity [86]. The activity of IFN-III is restricted by the selective expression of IFN lambda receptor-1 (IFNLR1) on respiratory epithelial cells [87] and certain immune cells [88]. For example, IFN-λ signalling in DCs was crucial for optimal antiviral CD8 T-cell responses as well as an enhanced IL-10 regulatory network in murine influenza virus infections [89]. Importantly, IFN-III was able to restrict the virus within the upper respiratory tract and prevent it from spreading to the lungs [90].
In response to rhinovirus (RV)-C, a subset of ciliated epithelial cells was identified with minimal IFN production despite high levels of virus replication, while other subsets of ciliated cells produced high IFN levels with moderate viral replication. This suggests that ciliated airway epithelial cell composition and IFN responses of infected and uninfected cells influence the risk of more severe viral RTIs, possibly explaining differences found in young versus older individuals [91]. Indeed, bronchial epithelial cultures from paediatric rhesus monkeys showed more viral replication and less IFN-I production in response to influenza (H1N1) virus compared to adult ones. Infected infant rhesus monkeys showed high viral titres on day 1 with prolonged viral presence, as well as prolonged bronchiolitis and alveolitis despite viral clearance [92]. Interestingly, tissues samples collected from children with nonsymptomatic SARS-CoV-2 infections produced IFN-λ faster in vitro than the ones from symptomatic infected children [45], suggesting that an instant readiness of IFN-III may help to protect against viral infections such as SARS-CoV-2. These contrasting data suggest that differential epithelial responses to viruses may explain different disease outcomes in children. In older adults, however, primary nasal epithelial cells cultures infected with influenza virus showed a stronger inflammatory response, but reduced antigen presentation at similar viral loads compared to those from young adults [93]. Single-cell RNA sequencing from older adults infected with SARS-CoV-2 also suggested that interactions between epithelial cells and immune responses were altered, with increased epithelial-derived transforming growth factor-β (TGF-β)/macrophage TGF-β receptor crosstalk and reduced IFN-γ interaction [94]. Epithelium-derived factors, including hyaluron, also drive murine alveolar macrophage dysfunction with age, reducing the ability to respond to granulocyte−macrophage colony-stimulating factor, as shown by adoptive cell transfer and parabiont experiments [95]. In addition, for RSV infection, differences were shown in viral kinetics with a slower viral clearance and enhanced inflammatory responses in epithelial cell cultures from older individuals [96]. Interestingly, similar findings were noted in nasal epithelial cells infected with RV in young children, showing higher viral titres and lower host immune responses [97]. However, no differences were reported for SARS-CoV2 viral titres or immune factors when comparing paediatric and adult bronchial epithelial cells [98]. However, more detailed Single-cell RNA sequencing analysis of the effects of SARS-CoV-2 infection on the differentiation of human nasal epithelial cells in children (<10 year), adults and elderly people (>70 year) revealed age-specific and epithelial cell-specific responses. Notably, there was a strong IFN response in infected paediatric goblet inflammatory cells, reducing viral spread and the appearance of elderly basaloid-like cells (ITGB6hi progenitors) that sustain viral replication and are associated with fibrotic signalling pathways [99]. The studies above in young and older subjects show that epithelial perturbations in protective antiviral inflammatory responses affect their capacity to quickly clear viruses, as well as modulate immune responses affecting clearance and resolution of virus, and pose a threat to the barrier integrity of the lung.
Delayed inflammatory response
Aging is linked to a decreased control and clearance of influenza infections. For example, aged mice showed reduced influenza titres in their lungs compared to younger mice, although the aged mice had prolonged shedding, indicating reduced clearance capacity [100]. Delayed influenza virus clearance was also observed in aged compared to younger macaques in both nasal swabs and BAL fluid [101]. Influenza clearance was also reduced in individuals >65 years in a hospital setting [102]. There are multiple mechanistic studies underway that aim to understand local responses underlying this phenomenon. Older mice showed reduced inflammasome activation in DCs and overcoming this by using nigericin treatment or performing adoptive transfer of DCs from young to older mice improved survival and increased immune infiltration post-infection [103]. Another study found a delayed infiltration of immune cells (cDC2, NK and granulocytes) in the lungs of aged mice following influenza infection, together with delayed cytokine responses [100]. Finally, in aged macaques, higher viral loads in the lungs correlated with increased IFN responses and innate populations [101]. Thus, a weaker initial control could lead to higher and prolonged viral load and then to even higher innate responses.
In a cohort of naturally influenza-infected individuals, similar viral loads in adults and children were observed; however, children showed higher innate responses in nasal fluid. When adjusting for age and viral load, increased nasal monocyte chemoattractant protein-3 (MCP-3), IFN-α and plasma IL-10 at enrolment predicted progression towards severe disease, while increased plasma IL-10, MCP-3 and IL-6 predicted hospitalisation; this correlated with monocyte recruitment to the site of infection [104]. Thus, local enhanced innate responses, due to uncontrolled viral infection and high-level viral replication, contribute to disease progression irrespective of age.
NK cell functionality
Another key mechanism of early viral control is NK-cell recruitment to the respiratory tract [105]. In severely ill RSV-infected children, a highly active NK-cell subset (CD94-) was detected in peripheral blood, while pro-inflammatory plasma cytokines were lower than in healthy children [106]. Neonatal NK cells show diminished cytotoxicity (i.e. reduced perforin secretion), compared to NK cells from adults in co-culture with RSV-infected cells, despite similar upregulation of expression of CD107 [107]. When explanted lungs were infected with influenza virus, the lung-resident CD56brightCD49a+ NK-cell population showed high CD107 upregulation, indicating responsivity. The NK cells provided significant antiviral and cytotoxic activity following contact with influenza-infected cells (i.e. IFN-γ and granzyme-B release) resulting in cell death [108]. Intranasal delivery of IFN-γ to RSV-infected neonatal mice improved RSV clearance and lung inflammation and was dependent on activation of alveolar macrophages [109]. Following influenza vaccination of older adults, the ability of antibodies to induce NK-cell activation was correlated with protection from later infection. Senescent peripheral blood NK cells, as measured by CD57 expression, were less able to be activated by influenza vaccine-induced antibodies [110]. NK-cell numbers in the lungs of aged mice at steady state and their function (degranulation and IFN-γ production) following influenza infection were decreased [111]. In contrast, lung NK cells were not decreased in a human cohort study; however, relatively few people >80 years were analysed [112]. Most lung NK cells were CD57+, which was correlated with age [112]. Thus, alterations in NK-cell numbers, maturity and/or functionality in very young and in older individuals might contribute to reduced clearance of viral pathogens and resolution of inflammation (figure 1).
Disease effects of respiratory viral infections
Secondary bacterial infections
Uncontrolled viral infections can progress towards pneumonia, with or without secondary bacterial infections. These secondary bacterial infections are often the cause of death; a clear example is provided by the “Spanish flu” [113]. Pneumonia is usually related to increased neutrophils in the lungs, whereby neutrophils are required for initial control of bacterial and fungal pathogens, but sustained recruitment can lead to tissue damage [114]. Following severe COVID-19, granulocytes were also found in the nasal mucosa and were negatively associated with hypoxia. A subset of granulocytes was elevated for at least 6 weeks after hospital discharge [115]. Respiratory viral infections also impair neutrophilic function. For example, even though more neutrophils were recruited to the lungs of older adults during influenza infection, they showed reduced phagocytic capacity [114]. Neutrophilic dysfunction enhanced the susceptibility to secondary infection with S. pneumoniae [116]. The reduced innate control of this bacterium was associated with virus-induced TNF-α, which inhibits neutrophilic killing [117]. Aged alveolar epithelial cells in mice secrete more neutrophil-attracting chemokines during influenza infection and this increased neutrophilic recruitment underlies their susceptibility to influenza mortality [118]. This is likely related to outcome following infection as older adults that had a high level of inflammaging had an increased risk of developing community-acquired pneumonia [119]. In addition, in severely ill RSV-infected children, neutrophils are the predominant cell type in the lung lavage [120]. When neutrophils were co-cultured with aspirate fluid from children with viral/bacterial co-infections, a decreased respiratory burst and killing activity was found against Haemophilus influenzae and Staphylococcus aureus [121]. Furthermore, in the blood and lung lavage of RSV-infected children with a bacterial co-infection, increased suppressive neutrophils (CD16hiCD62Llo) were found [122]. These data suggest that neutrophils are similarly increased in severely ill young and older individuals and linked to mortality, while their functionally seems reduced.
Disease resolution
The removal of apoptotic neutrophils from the lungs is mediated by macrophages through efferocytosis, which was impaired in older adults due to reduced Tim-4 expression and could be reversed by p38 mitogen-activated protein kinase (MAPK) inhibition [123]. Adoptive transfer of alveolar macrophages from young into old mice reduced lung damage following influenza infection [124]. Another mediator of resolution of inflammation are specialised pro-resolving lipid mediators (SPMs), which are produced by macrophages, and which reduced inflammation and damage during viral and bacterial lung infections [125]. These SPMs were reduced in homeostasis and following zymosan challenge in aged mice, which led to increased neutrophil influx and delayed resolution of inflammation [126]. The resolution of inflammation measured in circulation during pneumonia was also reduced in older adults [127].
An impaired IFN response with aging or in the very young might also be related to increased inflammation and damage, irrespective of its effects on viral control. IFN-I can reduce the excessive recruitment of neutrophils to the lung, potentially via modifying monocyte-derived cytokines [57]. IFN-α production by interstitial macrophages was able to suppress in situ inflammatory monocyte proliferation during influenza virus infection and confer disease tolerance [128]. IFN-I also induced neutrophil apoptosis, efferocytosis and induction of anti-inflammatory cell types, such as “M2 macrophages” and myeloid-derived suppressor cells in later stages of infection [129–132]. Indeed, individuals with severe COVID-19 showed reduced IFN-α and ISG expression in blood and reduced ISG responses in nasal mucosa [133, 134]. In contrast, children showed faster resolving responses, possibly due to robust and early IFN responses to SARS-CoV-2 [135]. Individuals with severe influenza infection showed a similar reduced IFN-I response accompanied with a progression to neutrophilic-associated patterns [136]. In neonatal IFNLR1-deficient mice, infection with pneumonia virus of mice (rodent equivalent of RSV), induced early and pronounced neutrophilia, with enhanced reactive oxygen species production, NETosis and mucus in the airways [137]. This indicates that enhanced IFN-III signalling in neutrophils will diminish their functionality, dampening lung inflammation and damage.
Mucosal associated invariant T (MAIT) cells are an abundant and evolutionarily conserved class of innate-like lymphocytes [138], which comprise up to 10% of human peripheral blood and respiratory mucosal T-cells [139]. Circulating MAIT cells are low in newborns and increase with age into adulthood, but then decrease again in older adults [140]. MAIT cells recognise microbial-derived metabolites derived from the riboflavin biosynthesis pathway, presented by nonclassical MHC-related protein 1 (MR1). Pulmonary MAIT cells have shown some protection from lethal influenza infection by preventing excessive immune infiltration, while not affecting viral load [141]. Of note, this was independent of MR1 sensing and dependent on IFN-γ production, as IFN-γ-deficient MAIT cells showed no protective effect in adoptive transfer. Monocytic IL-18 production following influenza-infected A549 coculture triggered MAIT cell activation [142]. Peripheral MAIT cell numbers were found to be lower in people who succumbed to H7N9 influenza infection and severe COVID-19 [142, 143]. These cells likely homed to the airways as highly activated MAIT cells and invariant NK T-cells were found in the lungs of individuals with COVID-19 where they were associated with an improved clinical outcome [143]. Peripheral MAIT cells are reduced in older adults; however, mucosal numbers still need to be assessed in older adults [144]. MAIT cells are responsive to signals from local microbiota in early life and may have longer lasting consequences for tissue repair and antiviral immunity [145]. Although MAIT cells are abundantly present in the lungs of adults, their presence and role in viral infections in the paediatric lung remains an open question [139, 146].
Lung function decline and chronic inflammatory lung diseases
Viral RTIs can have prolonged effects, persisting long after clearance and resolution of infection. A pooled analysis of five birth cohorts across Europe showed that children with early-life lower RTIs had significantly lower lung function and a higher risk of developing asthma [147]. Furthermore, reduced lung function gain during childhood into adulthood also increased the risk of developing COPD later in life [148]. Two of the most common viral infections in the first year of life include RSV and RV, both associated with the development of recurrent wheeze and childhood asthma [149, 150]. RSV prophylaxis in early life did reduce the risk of developing asthma; however, this was not significant in a meta-analysis of eight combined studies [149]. Possibly a more prolonged delay or avoidance of early life infection is necessary to reach protection against asthma secondary to RSV infection [151]. Prophylaxis for RV is challenging, since >170 different species exist, of which multiple circulate simultaneously [152]. Interestingly, even asymptomatic RV infections in the first year were associated with increased susceptibility of recurrent RTIs [153]. Although viruses, including RSV and RVs, typically induce type 1 responses, various mouse studies have shown that type 2 (inducing) cytokines and allergic sensitisation occur following infection with these viruses in neonatal models. For example, RSV induced TSLP production in epithelial cells and type 2 innate lymphoid cells (ILC2) accumulation in the lung [154]. Recurrent infections in neonatal mice boosted epithelial damage, further potentiating the development of local type 2 immunity [155]. In children hospitalised with RSV, increased levels of thymic stromal lymphopoietin (TSLP), IL-33 and IL-13 were found in nasal aspirates [156]. RV infection with neonatal mice showed increased type 2 inflammatory cytokines such as IL-25, derived from airway epithelial cells, and IL-13, produced by ILC2, leading to mucus hypersecretion and airway hyperresponsiveness [157]. These effects could be blocked by a neutralising antibody against IL-25 [158].
Thus, impaired resolution of inflammation and efferocytosis by alveolar macrophages, altered trafficking of neutrophils and reduced IFNs contribute to prolonged inflammatory signals and pathology in the lung of older adults following infection. In young children, type 2 inflammatory signals and cells are also boosted, increasing the risk of developing chronic inflammatory diseases such as asthma (figure 1).
Innovative solutions to boost effective innate responses
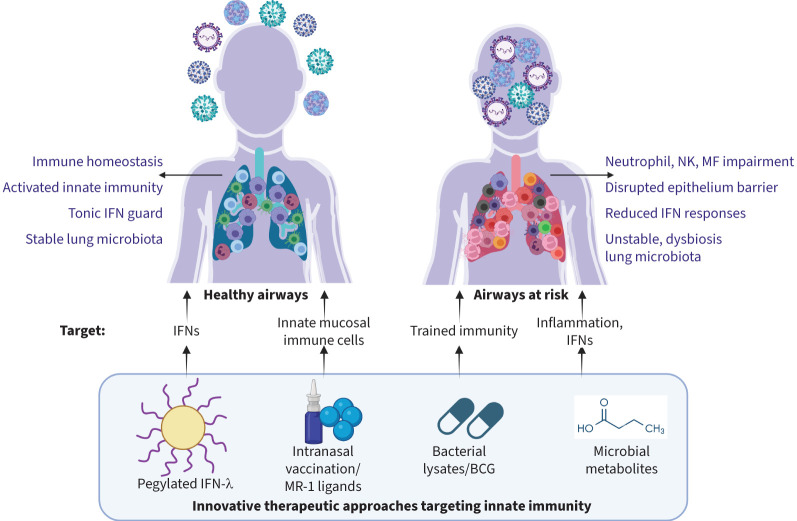
With the clear involvement of innate responses in susceptibility to infections, several innovative solutions have emerged to boost innate immunity in the setting of RTIs, either as a vaccine or immunotherapy (figure 2).
FIGURE 2.
Innovative therapeutic approaches targeting innate immunity during respiratory tract infections (RTIs). Several strategies have evolved targeting reduced interferon (IFN) production, innate mucosal immune populations, trained innate immunity and excessive inflammation during RTIs. BCG: bacille Calmette–Guérin; MF: macrophage; NK: natural killer.
Immunological agents
While reduced efferocytosis is associated with pneumonia, one study found that patients who were taking statins, a commonly prescribed medication for atherosclerosis and coronary heart diseases, showed increased efferocytosis following pneumonia [159]. This suggests that alveolar macrophage-mediated clearance of apoptotic neutrophils can be improved with a relatively simple solution.
IFN-III drives strong antiviral activity against various respiratory viruses in mice but also helps to curb unwanted and prolonged local inflammation [160]. Pegylated-IFN-λ has been given therapeutically to mice both before and after influenza infection. It was able to prevent infection and reduce inflammation [88], i.e. by enhancing disease resolution [161] through alveolar macrophages [162], and by enhancing humoral and adaptive immune responses when given as adjuvant during vaccination [163]. Similar observations were made in mice with SARS-CoV-2, when IFN-λ was given prophylactically or therapeutically via the nose [164]. During the COVID-19 pandemic, clinical trials with pegylated-IFN-λ showed a more rapid decline of SARS-CoV-2 transcripts over time [165], as well as a significantly reduced risk of hospitalisation [166]. Analysis of immune cells expressing IFNLR1 showed enhanced ISGs, while the development of specific antibodies and virus-specific T-cells was not hampered. The authors observed a delayed T-cell immunity development in older adults compared to young adults, while the decline of viral transcripts was equal, suggesting that pegylated-IFN-λ can overcome delays in adaptive immunity to accelerate viral clearance in older adults [167].
Vaccines and microbial products
Intranasal and, especially, live-attenuated vaccines might also induce innate immunity. For example, live-attenuated influenza vaccination of young adults induced not only influenza-specific CD4 and CD8 T-cells in the lung, but also increased IFN-γ-producing T-cell receptor-γδ T-cells upon in vitro restimulation with influenza vaccine [72].
One of the best-known examples of a vaccine that can induce innate responses is the bacille Calmette–Guérin (BCG) vaccine. Understanding its effects on innate immunity led to a new field, called trained innate immunity [168]. In mouse models, BCG vaccination led to ISG expression in lung myeloid and epithelial cells, secondary to CD4 T-cell-derived IFN-γ. This conferred protection against SARS-CoV-2 infection [169]. A double-blind randomised clinical trial vaccinating older adults with BCG or placebo upon hospital discharge following COVID-19 infection found a protective effect of BCG vaccine against re-infection [170]. However, a placebo-controlled clinical trial that tested BCG administration in the absence of infection did not find any protective effect on the cumulative incidence of RTIs during a year follow-up. Although it improved cytokine responses and antibody induction following infection, this suggests that timing of administration might be key in conferring protection [171]. BCG was also tested against RSV infection in neonatal calves and in mice. Administration of recombinant BCG-expressing RSV nucleoprotein reduced clinical disease upon RSV challenge, although viral burden was unaffected in calves but reduced in mice. The vaccination strategy induced IgA and virus-neutralisation activity as well as T-cell immunity in both animals [172, 173].
Orally administered bacterial lysates of mixed respiratory pathogenic bacteria have been used for decades to prevent RTIs [174] in children [175, 176] and in vulnerable adults [177–179]. Infection incidence, duration and severity were studied in randomised clinical trials. Wheezing episodes and/or asthma exacerbations in children were reduced [180]. In adults with chronic bronchitis and COPD, bacterial lysates reduced exacerbations triggered by RTIs [178, 179]. The underlying mechanism is only partly understood. Initially it was sought to act as an oral “vaccine”, mounting humoral and adaptive immunity against specific respiratory pathogens. However, recently, it was suggested that reduced frequency and duration in RTIs in children [181] was linked to innate immune training. Lipopolysaccharide (LPS) stimulation of PBMCs showed reduced production of pro-inflammatory cytokines (TNF-α, IL-6 and CCL3) following treatment. Pathway analysis and differentially gene correlation analysis on RNA-transcriptomics showed 1) rewiring of immune responses following LPS stimulation resulting in increased coordination of TLR4 with IFN signalling pathways and 2) segregation of TNF-α and IFN-γ in separate expression modules in the treatment group – two cytokines that were collectively shown to exaggerate inflammatory sequelae in the respiratory tract [182, 183]. Uncoupling these processes would facilitate swift innate responses to pathogens, but without the risk of uncontrolled inflammatory pathways. A similar innate immune training was suggested in studies with mice [184, 185].
In one murine study, the MAIT ligand 5-OP-RU was administered together with intraperitoneal influenza, which led to MAIT cell proliferation and accumulation in multiple tissues, including the lungs of both young and old mice [186]. This was associated with heterosubtypic protection from subsequent influenza A virus challenge and similar MAIT cell induction was seen when 5-OP-RU was administered together with an intranasal influenza vaccine or an intramuscular recombinant vesicular stomatitis virus-based SARS-CoV-2 vaccine.
Gut microbial metabolites
The gut microbiota seem crucial for defence against respiratory viruses. Antibiotic-treated mice displayed impaired defence against influenza infection [187], which was dependent on microbiota-driven tonic IFN signals in lung epithelium [188]. Microbiota can act at distal sites, since metabolites, such as short-chain fatty acids (SCFAs) produced in the gut, can enter the circulation and influence processes the lung; this is called the gut–lung axis [189]. Indeed, SCFAs can affect viral infections in the airways, e.g. a fibre-rich diet increased specific monocyte precursors, leading to more anti-inflammatory and tissue-protective macrophages in the lungs through propionate [190], as well as enhanced, lung cytotoxic T-cell metabolism, instrumental against experimental influenza infections [191]. Both oral and nasal administration of acetate reduced viral load and pulmonary inflammation in RSV-infected mice and induced antiviral responses in cells from children with RSV bronchiolitis [192, 193]. In addition, the flavonoid-derived metabolite desaminotyrosine reduced lung pathology in antibiotic-treated mice prior to influenza infection [194]. Several studies point towards roles for IFNs, intracellular PRRs (e.g. RIG-I) and reduced host defence causing pathology and tissue damage [191, 193]. SCFAs have been tested in patients, for example to improve metabolic (obesity and diabetes) or colonic health. However, systemic immune effects showed mixed results. A lower inflammatory status of circulating monocytes following oral ethyl-butyrate supplementation in obese individuals was noted [195], while in patients with long-standing type 1 diabetes, innate peripheral blood immune responses were unchanged [196]. Several studies used high-fibre diets in healthy subjects, older people or pre-diabetics to increase butyrate production. Decreased circulating inflammatory cytokines (IL-1β, IL-6, TNFα), as well as increased IL-10 (in healthy subjects) were found [197–199]. However, there were also studies in which fibre-rich diets did not affect circulating inflammatory markers [200]. Here, elevated gut SCFA levels did not always translate into elevated systemic SCFA levels, in particularly in patients. This may be related to their inflammatory status, as inflammatory cytokines, such as TNF-α, reduce butyrate uptake from the gut [201].
Thus, several immune or microbial agents can be recognised to act on various aspects of innate immunity, i.e. boosting IFN responses and/or innate mucosal immune populations, training innate immunity and/or dampening excessive inflammation, and harbour potential as host-directed therapies.
Summarising discussion
In this review, our focus is on innate responses throughout various stages of viral infections. We acknowledge that these responses are complemented by adaptive cellular and humoral immune components, each with specific shortcomings in young infants and older adults, as well as by adaptive immune cell subsets that employ innate functions, such as neonatal regulatory B-cells [202]. However, detailed discussions on these aspects are available elsewhere [203, 204]. Impaired innate responses are closely associated with the loss of viral control, significantly contributing to exaggerated and harmful immune responses during severe RTIs and may offer interesting possibilities for new treatment options. Additionally, optimising innate defences at mucosal surfaces as a host-directed prevention strategy holds great promise. A comparison between the young and the old reveals several central innate hubs.
During immune surveillance at mucosal surfaces and at the initiation of viral responses, neutrophils take centre stage. In the young, neutrophils in the lung exhibit a different behaviour to viruses, while in older adults, baseline levels are elevated. Although neutrophils are not the primary defenders against viral infections, their presence and functionality are an important factor in early infections and linked to severe disease. Tonic IFN production serves as a guarding system at mucosal surfaces. In both young children and older adults, IFN-I (predominantly pDC-derived) is reduced, whereas IFN-III is more and mostly reduced during aging. Importantly, IFN-III is instrumental in fighting the virus, but without exaggerated and damaging inflammatory responses. This divergence, with children expressing more IFN-III, could partially explain why they experience less severe SARS-CoV-2 infections than adults. In children, the lung microbiota may contribute to better or less mucosal immune fitness, while in older adults their role in worse viral infections is less clear.
The persistence of viral presence at mucosal surfaces affects both children and older adults. The epithelium, either or not infected with the virus, may play an important role in clearing the infection and curbing the downstream inflammation. In children, continued viral presence may be associated with reduced IFN-I production, while in older adults, the picture is more complex. Next to reduced IFN-I, factors include diminished antigen presentation capacity by epithelial cells, distorted interaction with macrophages, reduced immune cell recruitment, reduced inflammasome activity and reduced IFN-γ levels. An additional contributing factor is attributed to NK cells. In young children, NK cells exhibit lower cytotoxicity, whereas in older adults, their antibody-dependent functionality is diminished. Overall, a picture is emerging of local enhanced innate responses, due to uncontrolled viral infection and viral replication, in which disease progression occurs irrespective of age.
Uncontrolled infections progress to severe disease with potential long-term consequences. While the risk of secondary bacterial infections is not age-dependent, it is linked to severe and uncontrolled viral infections. In children, prolonged viral presence is associated with type 2 alarmin production, inducing ILC2 and other type 2 immune cells, leading to an increased risk of wheeze, childhood asthma and decreased lung function. In older adults, impaired immune components important for resolving inflammation, such as IFNs, macrophages, neutrophil clearance and pro-resolving lipids, may contribute to virus-induced pneumonia and prolonged disease.
Various strategies have been proposed to address these impairments, which were tested in preclinical models and in humans. These approaches include pegylated-IFN-λ, bacterial lysates, BCG vaccination, intranasal vaccination, MR-1 ligands and microbial metabolites. These aim to enhance IFN responses, induce innate trained immunity, boost mucosal immune populations and reduce excessive inflammation. Additionally, biologicals such as anti-IL-6 or anti-TNF may help mitigate cytokine storm development [205, 206]. The effectiveness and necessity of these approaches in different age groups, particularly in young children versus older adults, requires further investigation. Nonetheless, these strategies offer unique opportunities to bolster pandemic preparedness and protect vulnerable populations.
Footnotes
Provenance: Commissioned article, peer reviewed.
Conflict of interest: H.H. Smits reports support for the present manuscript from Netherlands Lung Foundation and Netherlands National Science Council (NWO). In addition, H.H. Smits reports the following leadership roles, outside the submitted work: board member of Netherlands Respiratory Society (NRS) and treasurer of Netherlands Society for Immunology (NVVI). S.P. Jochems reports grants from Horizon Europe and ZonMW, outside the submitted work.
Support statement: Supported by the Netherlands Science Council ZonMW (Grant: Vici-09150182210043). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Bailey KL. Aging diminishes mucociliary clearance of the lung. Adv Geriatr Med Res 2022; 4: e220005. doi: 10.20900/agmr20220005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho JC, Chan KN, Hu WH, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med 2001; 163: 983–988. doi: 10.1164/ajrccm.163.4.9909121 [DOI] [PubMed] [Google Scholar]
- 3.Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis 2004; 4: 112–124. doi: 10.1016/S1473-3099(04)00931-4 [DOI] [PubMed] [Google Scholar]
- 4.Narayanan M, Owers-Bradley J, Beardsmore CS, et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med 2012; 185: 186–191. doi: 10.1164/rccm.201107-1348OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B Biol Sci 2015; 282: 20143085. doi: 10.1098/rspb.2014.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007; 7: 379–390. doi: 10.1038/nri2075 [DOI] [PubMed] [Google Scholar]
- 7.Stein MM, Hrusch CL, Gozdz J, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med 2016; 375: 411–421. doi: 10.1056/NEJMoa1508749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olin A, Henckel E, Chen Y, et al. Stereotypic immune system development in newborn children. Cell 2018; 174: 1277–1292.e14. doi: 10.1016/j.cell.2018.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong Z, Chen M, Dai S, et al. Association of cesarean section with asthma in children/adolescents: a systematic review and meta-analysis based on cohort studies. BMC Pediatr 2023; 23: 571. doi: 10.1186/s12887-023-04396-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung JY, Li AM, Leung GM, et al. Mode of delivery and childhood hospitalizations for asthma and other wheezing disorders. Clin Exp Allergy 2015; 45: 1109–1117. doi: 10.1111/cea.12548 [DOI] [PubMed] [Google Scholar]
- 11.Murk W, Risnes KR, Bracken MB. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics 2011; 127: 1125–1138. doi: 10.1542/peds.2010-2092 [DOI] [PubMed] [Google Scholar]
- 12.Cait A, Wedel A, Arntz JL, et al. Prenatal antibiotic exposure, asthma, and the atopic march: a systematic review and meta-analysis. Allergy 2022; 77: 3233–3248. doi: 10.1111/all.15404 [DOI] [PubMed] [Google Scholar]
- 13.Hornef MW, Torow N. “Layered immunity”and the “neonatal window of opportunity” – timed succession of non-redundant phases to establish mucosal host–microbial homeostasis after birth. Immunology 2020; 159: 15–25. doi: 10.1111/imm.13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol 2018; 19: 10–19. doi: 10.1038/s41590-017-0006-x [DOI] [PubMed] [Google Scholar]
- 15.Ciabattini A, Nardini C, Santoro F, et al. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol 2018; 40: 83–94. doi: 10.1016/j.smim.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 16.Esposito S, Franco E, Gavazzi G, et al. The public health value of vaccination for seniors in Europe. Vaccine 2018; 36: 2523–2528. doi: 10.1016/j.vaccine.2018.03.053 [DOI] [PubMed] [Google Scholar]
- 17.Daniel P, Woodhead M, Welham S, et al. Mortality reduction in adult community-acquired pneumonia in the UK (2009–2014): results from the British Thoracic Society audit programme. Thorax 2016; 71: 1061–1063. doi: 10.1136/thoraxjnl-2016-208937 [DOI] [PubMed] [Google Scholar]
- 18.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373: 415–427. doi: 10.1056/NEJMoa1500245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lian J, Yue Y, Yu W, et al. Immunosenescence: a key player in cancer development. J Hematol Oncol 2020; 13: 151. doi: 10.1186/s13045-020-00986-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franceschi C, Garagnani P, Vitale G, et al. Inflammaging and “Garb-aging”. Trends Endocrin Met 2017; 28: 199–212. doi: 10.1016/j.tem.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 21.Thevaranjan N, Puchta A, Schulz C, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017; 21: 455–466.e4. doi: 10.1016/j.chom.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanes RE, Gustafson CE, Weyand CM, et al. Lymphocyte generation and population homeostasis throughout life. Semin Hematol 2017; 54: 33–38. doi: 10.1053/j.seminhematol.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol 2004; 5: 133–139. doi: 10.1038/ni1033 [DOI] [PubMed] [Google Scholar]
- 24.Britanova OV, Putintseva EV, Shugay M, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol 2014; 192: 2689–2698. doi: 10.4049/jimmunol.1302064 [DOI] [PubMed] [Google Scholar]
- 25.Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci 2000; 908: 244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 26.Pereira B, Xu XN, Akbar AN. Targeting inflammation and immunosenescence to improve vaccine responses in the elderly. Front Immunol 2020; 11: 583019. doi: 10.3389/fimmu.2020.583019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunha LL, Perazzio SF, Azzi J, et al. Remodeling of the immune response with aging: immunosenescence and its potential impact on COVID-19 immune response. Front Immunol 2020; 11: 1748. doi: 10.3389/fimmu.2020.01748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jing Y, Shaheen E, Drake RR, et al. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum Immunol 2009; 70: 777–784. doi: 10.1016/j.humimm.2009.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak J, Dobrovolny J, Novakova L, et al. The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in men and women of reproductive age. Scand J Immunol 2014; 80: 271–275. doi: 10.1111/sji.12193 [DOI] [PubMed] [Google Scholar]
- 30.Rauh MJ, Cook EK, Bowdish DME. Myeloid-derived suppressor cells in aged humans. In: Fulop T, Franceschi C, Hirokawa K, et al. eds. Handbook of Immunosenescence: Basic Understanding and Clinical Implications. New York, Springer International Publishing, 2017; pp. 733–744. [Google Scholar]
- 31.Metcalf TU, Cubas RA, Ghneim K, et al. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 2015; 14: 421–432. doi: 10.1111/acel.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ascough S, Dayananda P, Kalyan M, et al. Divergent age-related humoral correlates of protection against respiratory syncytial virus infection in older and young adults: a pilot, controlled, human infection challenge model. Lancet Healthy Longev 2022; 3: E405–E416. doi: 10.1016/S2666-7568(22)00103-9 [DOI] [PubMed] [Google Scholar]
- 33.Habibi MS, Thwaites RS, Chang M, et al. Neutrophilic inflammation in the respiratory mucosa predisposes to RSV infection. Science 2020; 370: eaba9301. doi: 10.1126/science.aba9301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pennings JLA, Mariman R, Hodemaekers HM, et al. Transcriptomics in lung tissue upon respiratory syncytial virus infection reveals aging as important modulator of immune activation and matrix maintenance. Sci Rep 2018; 8: 16653. doi: 10.1038/s41598-018-35180-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiné J, Carniel BF, Solórzano C, et al. Dynamic changes in innate immune and T cell function and composition at the nasal mucosa across the human lifespan. bioRxiv 2019; preprint [ 10.1101/576744] [DOI] [Google Scholar]
- 36.Li J, Chen Q, Luo X, et al. Neutrophil-to-lymphocyte ratio positively correlates to age in healthy population. J Clin Lab Anal 2015; 29: 437–443. doi: 10.1002/jcla.21791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer KC, Ershler W, Rosenthal NS, et al. Immune dysregulation in the aging human lung. Am J Respir Crit Care Med 1996; 153: 1072–1079. doi: 10.1164/ajrccm.153.3.8630547 [DOI] [PubMed] [Google Scholar]
- 38.Makris S, Johansson C. R848 or influenza virus can induce potent innate immune responses in the lungs of neonatal mice. Mucosal Immunol 2021; 14: 267–276. doi: 10.1038/s41385-020-0314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lines JL, Hoskins S, Hollifield M, et al. The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol 2010; 185: 2980–2988. doi: 10.4049/jimmunol.0903075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filias A, Theodorou GL, Mouzopoulou S, et al. Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr 2011; 11: 29. doi: 10.1186/1471-2431-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinberger B, Laskin DL, Mariano TM, et al. Mechanisms underlying reduced responsiveness of neonatal neutrophils to distinct chemoattractants. J Leukoc Biol 2001; 70: 969–976. [PMC free article] [PubMed] [Google Scholar]
- 42.Allgaier B, Shi M, Luo D, et al. Spontaneous and Fas-mediated apoptosis are diminished in umbilical cord blood neutrophils compared with adult neutrophils. J Leukoc Biol 1998; 64: 331–336. doi: 10.1002/jlb.64.3.331 [DOI] [PubMed] [Google Scholar]
- 43.Robinson E, Sawhney S, Cortina-Borja M, et al. Neutrophil responses to RSV infection show differences between infant and adult neutrophils. Thorax 2024; 79: 545–552. doi: 10.1136/thorax-2023-220081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones A, Qui JM, Bataki E, et al. Neutrophil survival is prolonged in the airways of healthy infants and infants with RSV bronchiolitis. Eur Respir J 2002; 20: 651–657. doi: 10.1183/09031936.02.00278902 [DOI] [PubMed] [Google Scholar]
- 45.Beucher G, Blondot M-L, Celle A, et al. Bronchial epithelia from adults and children: SARS-CoV-2 spread via syncytia formation and type III interferon infectivity restriction. Proc Natl Acad Sci USA 2022; 119: e2202370119. doi: 10.1073/pnas.2202370119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang SZ, Smith PK, Lovejoy M, et al. The apoptosis of neutrophils is accelerated in respiratory syncytial virus (RSV)-induced bronchiolitis. Clin Exp Immunol 1998; 114: 49–54. doi: 10.1046/j.1365-2249.1998.00681.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makris S, Paulsen M, Johansson C. Type I interferons as regulators of lung inflammation. Front Immunol 2017; 8: 259. doi: 10.3389/fimmu.2017.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wack A, Terczyńska-Dyla E, Hartmann R. Guarding the frontiers: the biology of type III interferons. Nat Immunol 2015; 16: 802–809. doi: 10.1038/ni.3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witte K, Witte E, Sabat R, et al. IL-28A, IL-28B, and IL-29: promising cytokines with type I interferon-like properties. Cytokine Growth Factor Rev 2010; 21: 237–251. doi: 10.1016/j.cytogfr.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 50.Lokugamage KG, Hage A, de Vries M, et al. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J Virol 2020; 94: e01410-20. doi: 10.1128/jvi.01410-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plotnikova M, Lozhkov A, Romanovskaya-Romanko E, et al. IFN-λ1 displays various levels of antiviral activity in vitro in a select panel of RNA viruses. Viruses 2021; 13: 1602. doi: 10.3390/v13081602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang S, Brown HM, Hwang S. Direct antiviral mechanisms of interferon-gamma. Immune Netw 2018; 18: e33. doi: 10.4110/in.2018.18.e33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corbett NP, Blimkie D, Ho KC, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One 2010; 5: e15041. doi: 10.1371/journal.pone.0015041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marr N, Wang TI, Kam SH, et al. Attenuation of respiratory syncytial virus-induced and RIG-I-dependent type I IFN responses in human neonates and very young children. J Immunol 2014; 192: 948–957. doi: 10.4049/jimmunol.1302007 [DOI] [PubMed] [Google Scholar]
- 55.Cormier SA, Shrestha B, Saravia J, et al. Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection. J Virol 2014; 88: 9350–9360. doi: 10.1128/jvi.00818-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drajac C, Laubreton D, Marquant Q, et al. Control of IFN-I responses by the aminopeptidase IRAP in neonatal C57BL/6 alveolar macrophages during RSV infection. Mucosal Immunol 2021; 14: 949–962. doi: 10.1038/s41385-021-00402-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng E, Balint E, Poznanski SM, et al. Aging and interferons: impacts on inflammation and viral disease outcomes. Cells 2021; 10: 708. doi: 10.3390/cells10030708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prakash S, Agrawal S, Cao JN, et al. Impaired secretion of interferons by dendritic cells from aged subjects to influenza: role of histone modifications. Age 2013; 35: 1785–1797. doi: 10.1007/s11357-012-9477-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith CA, Kulkarni U, Chen J, et al. Influenza virus inoculum volume is critical to elucidate age-dependent mortality in mice. Aging Cell 2019; 18: e12893. doi: 10.1111/acel.12893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beer J, Crotta S, Breithaupt A, et al. Impaired immune response drives age-dependent severity of COVID-19. J Exp Med 2022; 219: e20220621. doi: 10.1084/jem.20220621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ke R, Martinez PP, Smith RL, et al. Daily longitudinal sampling of SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness. Nat Microbiol 2022; 7: 640. doi: 10.1038/s41564-022-01105-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bosch A, de Steenhuijsen Piters WAA, van Houten MA, et al. Maturation of the infant respiratory microbiota, environmental drivers, and health consequences. A prospective cohort study. Am J Respir Crit Care Med 2017; 196: 1582–1590. doi: 10.1164/rccm.201703-0554OC [DOI] [PubMed] [Google Scholar]
- 63.Man WH, Clerc M, de Steenhuijsen Piters WAA, et al. Loss of microbial topography between oral and nasopharyngeal microbiota and development of respiratory infections early in life. Am J Respir Crit Care Med 2019; 200: 760–770. doi: 10.1164/rccm.201810-1993OC [DOI] [PubMed] [Google Scholar]
- 64.de Steenhuijsen Piters WAA, Watson RL, de Koff EM, et al. Early-life viral infections are associated with disadvantageous immune and microbiota profiles and recurrent respiratory infections. Nat Microbiol 2022; 7: 224–237. doi: 10.1038/s41564-021-01043-2 [DOI] [PubMed] [Google Scholar]
- 65.Tarabichi Y, Li K, Hu S, et al. The administration of intranasal live attenuated influenza vaccine induces changes in the nasal microbiota and nasal epithelium gene expression profiles. Microbiome 2015; 3: 74. doi: 10.1186/s40168-015-0133-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costa-Martins AG, Mane K, Lindsey BB, et al. Prior upregulation of interferon pathways in the nasopharynx impacts viral shedding following live attenuated influenza vaccine challenge in children. Cell Rep Med 2021; 2: 100465. doi: 10.1016/j.xcrm.2021.100465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bowdish DME, Rossi L, Loeb M, et al. The impact of respiratory infections and probiotic use on the nasal microbiota of frail residents in long-term care homes. ERJ Open Res 2023; 9: 00212-2023. doi: 10.1183/23120541.00212-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeJong EN, Surette MG, Bowdish DME. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe 2020; 28: 180–189. doi: 10.1016/j.chom.2020.07.013 [DOI] [PubMed] [Google Scholar]
- 69.Whelan FJ, Verschoor CP, Stearns JC, et al. The loss of topography in the microbial communities of the upper respiratory tract in the elderly. Ann Am Thorac Soc 2014; 11: 513–521. doi: 10.1513/AnnalsATS.201310-351OC [DOI] [PubMed] [Google Scholar]
- 70.Kim HJ, Jo A, Jeon YJ, et al. Nasal commensal Staphylococcus epidermidis enhances interferon-λ-dependent immunity against influenza virus. Microbiome 2019; 7: 80. doi: 10.1186/s40168-019-0691-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bradley KC, Finsterbusch K, Schnepf D, et al. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep 2019; 28: 245–256.e4. doi: 10.1016/j.celrep.2019.05.105 [DOI] [PubMed] [Google Scholar]
- 72.Carniel BF, Marcon F, Rylance J, et al. Pneumococcal colonization impairs mucosal immune responses to live attenuated influenza vaccine. JCI Insight 2021; 6: e141088. doi: 10.1172/jci.insight.141088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bastard P, Gervais A, Le Voyer T, et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci Immunol 2021; 6: eabl4340. doi: 10.1126/sciimmunol.abl4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Q, Bastard P, Cobat A, et al. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature 2022; 603: 587. doi: 10.1038/s41586-022-04447-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020; 370: eabd4570. doi: 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin Y, Wu P, Tsang TK, et al. Viral kinetics of SARS-CoV-2 following onset of COVID-19 in symptomatic patients infected with the ancestral strain and omicron BA.2 in Hong Kong: a retrospective observational study. Lancet Microbe 2023; 4: e722. doi: 10.1016/S2666-5247(23)00146-5 [DOI] [PubMed] [Google Scholar]
- 77.Jones TC, Biele G, Mühlemann B, et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science 2021; 373: eabi5273. doi: 10.1126/science.abi5273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clay CC, Donart N, Fomukong N, et al. Severe acute respiratory syndrome-coronavirus infection in aged nonhuman primates is associated with modulated pulmonary and systemic immune responses. Immun Ageing 2014; 11: 4. doi: 10.1186/1742-4933-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smits SL, de Lang A, van den Brand JM, et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. Plos Pathog 2010; 6: e1000756. doi: 10.1371/journal.ppat.1000756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshida M, Worlock KB, Huang N, et al. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature 2022; 602: 321–327. doi: 10.1038/s41586-021-04345-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gilbert C, Lefeuvre C, Preisser L, et al. Age-related expression of IFN-λ1 versus IFN-I and beta-defensins in the nasopharynx of SARS-CoV-2-infected individuals. Front Immunol 2021; 12: 750279. doi: 10.3389/fimmu.2021.750279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loske J, Röhmel J, Lukassen S, et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat Biotechnol 2022; 40: 319–324. doi: 10.1038/s41587-021-01037-9 [DOI] [PubMed] [Google Scholar]
- 83.Galani I-E, Rovina N, Lampropoulou V, et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol 2021; 22: 32–40. doi: 10.1038/s41590-020-00840-x [DOI] [PubMed] [Google Scholar]
- 84.Hewitt RJ, Lloyd CM. Regulation of immune responses by the airway epithelial cell landscape. Nat Rev Immunol 2021; 21: 347–362. doi: 10.1038/s41577-020-00477-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Puttur F, Gregory LG, Lloyd CM. Airway macrophages as the guardians of tissue repair in the lung. Immunol Cell Biol 2019; 97: 246–257. doi: 10.1111/imcb.12235 [DOI] [PubMed] [Google Scholar]
- 86.Broggi A, Granucci F, Zanoni I. Type III interferons: balancing tissue tolerance and resistance to pathogen invasion. J Exp Med 2020; 217: e20190295. doi: 10.1084/jem.20190295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mordstein M, Neugebauer E, Ditt V, et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol 2010; 84: 5670–5677. doi: 10.1128/jvi.00272-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galani IE, Triantafyllia V, Eleminiadou E-E, et al. Interferon-λ mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity 2017; 46: 875. doi: 10.1016/j.immuni.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 89.Hemann EA, Green R, Turnbull JB, et al. Interferon-λ modulates dendritic cells to facilitate T cell immunity during infection with influenza A virus. Nat Immunol 2019; 20: 1035–1045. doi: 10.1038/s41590-019-0408-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klinkhammer J, Schnepf D, Ye L, et al. IFN-λ prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. eLife 2018; 7: e33354. doi: 10.7554/eLife.33354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Basnet S, Mohanty C, Bochkov YA, et al. Rhinovirus C causes heterogeneous infection and gene expression in airway epithelial cell subsets. Mucosal Immunol 2023; 16: 386–398. doi: 10.1016/j.mucimm.2023.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clay CC, Reader JR, Gerriets JE, et al. Enhanced viral replication and modulated innate immune responses in infant airway epithelium following H1N1 infection. J Virol 2014; 88: 7412–7425. doi: 10.1128/jvi.00188-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chason KD, Jaspers I, Parker J, et al. Age-associated changes in the respiratory epithelial response to influenza infection. J Gerontol A Biol Sci Med Sci 2018; 73: 1643–1650. doi: 10.1093/gerona/gly126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hou Y, Zhou Y, Jehi L, et al. Aging-related cell type-specific pathophysiologic immune responses that exacerbate disease severity in aged COVID-19 patients. Aging Cell 2022; 21: e13544. doi: 10.1111/acel.13544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McQuattie-Pimentel AC, Ren Z, Joshi N, et al. The lung microenvironment shapes a dysfunctional response of alveolar macrophages in aging. J Clin Invest 2021; 131: e140299. doi: 10.1172/JCI140299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ito K, Daly L, Coates M. An impact of age on respiratory syncytial virus infection in air–liquid-interface culture bronchial epithelium. Front Med 2023; 10: 1144050. doi: 10.3389/fmed.2023.1144050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Usemann J, Alves MP, Ritz N, et al. Age-dependent response of the human nasal epithelium to rhinovirus infection. Eur Respir J 2020; 56: 2000877. doi: 10.1183/13993003.00877-2020 [DOI] [PubMed] [Google Scholar]
- 98.Stölting H, Baillon L, Frise R, et al. Distinct airway epithelial immune responses after infection with SARS-CoV-2 compared to H1N1. Mucosal Immunol 2022; 15: 952–963. doi: 10.1038/s41385-022-00545-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Woodall M, Cujba A-M, Worlock KB, et al. The emergence of goblet inflammatory or ITGB6hi nasal progenitor cells determines age-associated SARS-CoV-2 pathogenesis. bioRxiv 2023; preprint [ 10.1101/2023.01.16.524211] [DOI] [Google Scholar]
- 100.Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res 2009; 10: 112. doi: 10.1186/1465-9921-10-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Josset L, Engelmann F, Haberthur K, et al. Increased viral loads and exacerbated innate host responses in aged macaques infected with the 2009 pandemic H1N1 influenza A virus. J Virol 2012; 86: 11115–11127. doi: 10.1128/JVI.01571-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009; 200: 492–500. doi: 10.1086/600383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stout-Delgado HW, Vaughan SE, Shirali AC, et al. Impaired NLRP3 inflammasome function in elderly mice during influenza infection is rescued by treatment with nigericin. J Immunol 2012; 188: 2815–2824. doi: 10.4049/jimmunol.1103051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oshansky CM, Gartland AJ, Wong SS, et al. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am J Respir Crit Care Med 2014; 189: 449–462. doi: 10.1164/rccm.201309-1616OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verbist KC, Rose DL, Cole CJ, et al. IL-15 participates in the respiratory innate immune response to influenza virus infection. PLoS One 2012; 7: e37539. doi: 10.1371/journal.pone.0037539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Larrañaga CL, Ampuero SL, Luchsinger VF, et al. Impaired immune response in severe human lower tract respiratory infection by respiratory syncytial virus. Pediatr Infect Dis J 2009; 28: 867–873. doi: 10.1097/INF.0b013e3181a3ea71 [DOI] [PubMed] [Google Scholar]
- 107.van Erp EA, Feyaerts D, Duijst M, et al. Respiratory syncytial virus infects primary neonatal and adult natural killer cells and affects their antiviral effector function. J Infect Dis 2019; 219: 723–733. doi: 10.1093/infdis/jiy566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cooper GE, Ostridge K, Khakoo SI, et al. Human CD49a+ lung natural killer cell cytotoxicity in response to influenza A virus. Front Immunol 2018; 9: 1671. doi: 10.3389/fimmu.2018.01671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eichinger KM, Egaña L, Orend JG, et al. Alveolar macrophages support interferon gamma-mediated viral clearance in RSV-infected neonatal mice. Respir Res 2015; 16: 122. doi: 10.1186/s12931-015-0282-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boudreau CM, Burke JS, Yousif AS, et al. Antibody-mediated NK cell activation as a correlate of immunity against influenza infection. Nat Commun 2023; 14: 5170. doi:0.1038/s41467-023-40699-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beli E, Clinthorne JF, Duriancik DM, et al. Natural killer cell function is altered during the primary response of aged mice to influenza infection. Mech Ageing Dev 2011; 132: 503–510. doi: 10.1016/j.mad.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dogra P, Rancan C, Ma W, et al. Tissue determinants of human NK cell development, function, and residence. Cell 2020; 180: 749–763.e13. doi: 10.1016/j.cell.2020.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198: 962–970. doi: 10.1086/591708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simmons SR, Bhalla M, Herring SE, et al. Older but not wiser: the age-driven changes in neutrophil responses during pulmonary infections. Infect Immun 2021; 89: e00653-20. doi: 10.1128/IAI.00653-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roukens AHE, Pothast CR, König M, et al. Prolonged activation of nasal immune cell populations and development of tissue-resident SARS-CoV-2-specific CD8+ T cell responses following COVID-19. Nat Immunol 2022; 23: 23–32. doi: 10.1038/s41590-021-01095-w [DOI] [PubMed] [Google Scholar]
- 116.McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun 2006; 74: 6707–6721. 10.1128/IAI.00789-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jochems SP, Marcon F, Carniel BF, et al. Inflammation induced by influenza virus impairs human innate immune control of pneumococcus. Nat Immunol 2018; 19: 1299–1308. doi: 10.1038/s41590-018-0231-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kulkarni U, Zemans RL, Smith CA, et al. Excessive neutrophil levels in the lung underlie the age-associated increase in influenza mortality. Mucosal Immunol 2019; 12: 545–554. doi: 10.1038/s41385-018-0115-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yende S, Tuomanen EI, Wunderink R, et al. Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. Am J Respir Crit Care Med 2005; 172: 1440–1446. doi: 10.1164/rccm.200506-888OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johansson C, Kirsebom FCM. Neutrophils in respiratory viral infections. Mucosal Immunol 2021; 14: 815–827. doi: 10.1038/s41385-021-00397-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grunwell JR, Giacalone VD, Stephenson S, et al. Neutrophil dysfunction in the airways of children with acute respiratory failure due to lower respiratory tract viral and bacterial coinfections. Sci Rep 2019; 9: 2874. doi: 10.1038/s41598-019-39726-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cortjens B, Ingelse SA, Calis JC, et al. Neutrophil subset responses in infants with severe viral respiratory infection. Clin Immunol 2017; 176: 100–106. doi: 10.1016/j.clim.2016 [DOI] [PubMed] [Google Scholar]
- 123.De Maeyer RPH, van de Merwe RC, Louie R, et al. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat Immunol 2020; 21: 615–625. doi: 10.1038/s41590-020-0646-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wong CK, Smith CA, Sakamoto K, et al. Aging impairs alveolar macrophage phagocytosis and increases influenza-induced mortality in mice. J Immunol 2017; 199: 1060–1068. doi: 10.4049/jimmunol.1700397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ferri G, Mucci M, Mattoscio D, et al. Specialized pro-resolving lipid mediators and resolution of viral diseases. Prostaglandins Other Lipid Mediat 2023; 168: 106762. doi: 10.1016/j.prostaglandins.2023.106762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arnardottir HH, Dalli J, Colas RA, et al. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines. J Immunol 2014; 193: 4235–4244. doi: 10.4049/jimmunol.1401313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bruunsgaard H, Skinhoj P, Qvist J, et al. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J Infect Dis 1999; 180: 551–554. doi: 10.1086/314873 [DOI] [PubMed] [Google Scholar]
- 128.Pernet E, Downey J, Vinh DC, et al. Leukotriene B4-type I interferon axis regulates macrophage-mediated disease tolerance to influenza infection. Nat Microbiol 2019; 4: 1389–1400. doi: 10.1038/s41564-019-0444-3 [DOI] [PubMed] [Google Scholar]
- 129.Satyanarayanan SK, El Kebir D, Soboh S, et al. IFN-β is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation. Nat Commun 2019; 10: 3471. doi: 10.1038/s41467-019-10903-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fujimori T, Grabiec AM, Kaur M, et al. The Axl receptor tyrosine kinase is a discriminator of macrophage function in the inflamed lung. Mucosal Immunol 2015; 8: 1021–1030. doi: 10.1038/mi.2014.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taleb K, Auffray C, Villefroy P, et al. Chronic type I IFN is sufficient to promote immunosuppression through accumulation of myeloid-derived suppressor cells. J Immunol 2017; 198: 1156–1163. doi: 10.4049/jimmunol.1502638 [DOI] [PubMed] [Google Scholar]
- 132.Shirey KA, Pletneva LM, Puche AC, et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4Rα-, TLR4–, and IFN-β-dependent. Mucosal Immunol 2010; 3: 291–300. doi: 10.1038/mi.2010.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ziegler CGK, Miao VN, Owings AH, et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell 2021; 184: 4713–4733.e22. doi: 10.1016/j.cell.2021.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020; 369: 718–724. doi: 10.1126/science.abc6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vono M, Huttner A, Lemeille S, et al. Robust innate responses to SARS-CoV-2 in children resolve faster than in adults without compromising adaptive immunity. Cell Rep 2021; 37: 109773. doi: 10.1016/j.celrep.2021.109773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dunning J, Blankley S, Hoang LT, et al. Progression of whole-blood transcriptional signatures from interferon-induced to neutrophil-associated patterns in severe influenza. Nat Immunol 2018; 19: 625–635. doi: 10.1038/s41590-018-0111-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sebina I, Rashid RB, Sikder MAA, et al. IFN-λ diminishes the severity of viral bronchiolitis in neonatal mice by limiting NADPH oxidase-induced PAD4-independent NETosis. J Immunol 2022; 208: 2806–2816. doi: 10.4049/jimmunol.2100876 [DOI] [PubMed] [Google Scholar]
- 138.Le Bourhis L, Guerri L, Dusseaux M, et al. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol 2011; 32: 212–218. doi: 10.1016/j.it.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 139.Long Y, Hinks TSC. MAIT cells in respiratory viral infections in mouse and human. Crit Rev Immunol 2021; 41: 19–35. doi: 10.1615/CritRevImmunol.2021040877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen P, Deng W, Li D, et al. Circulating mucosal-associated invariant T cells in a large cohort of healthy Chinese individuals from newborn to elderly. Front Immunol 2019; 10: 260. doi: 10.3389/fimmu.2019.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Wilgenburg B, Loh L, Chen Z, et al. MAIT cells contribute to protection against lethal influenza infection in vivo. Nat Commun 2018; 9: 4706. doi: 10.1038/s41467-018-07207-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Loh L, Wang Z, Sant S, et al. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc Natl Acad Sci USA 2016; 113: 10133–10138. doi: 10.1073/pnas.1610750113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jouan Y, Guillon A, Gonzalez L, et al. Phenotypical and functional alteration of unconventional T cells in severe COVID-19 patients. J Exp Med 2020; 217: e20200872. doi: 10.1084/jem.20200872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kurioka A, Klenerman P. Aging unconventionally: γδ T cells, iNKT cells, and MAIT cells in aging. Semin Immunol 2023; 69: 101816. doi: 10.1016/j.smim.2023.101816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Constantinides MG, Link VM, Tamoutounour S, et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 2019; 366: eaax6624. doi: 10.1126/science.aax6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lu B, Liu M, Wang J, et al. IL-17 production by tissue-resident MAIT cells is locally induced in children with pneumonia. Mucosal Immunol 2020; 13: 824–835. doi: 10.1038/s41385-020-0273-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oksel C, Granell R, Haider S, et al. Distinguishing wheezing phenotypes from infancy to adolescence. a pooled analysis of five birth cohorts. Ann Am Thorac Soc 2019; 16: 868–876. doi: 10.1513/AnnalsATS.201811-837OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Belgrave DCM, Granell R, Turner SW, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med 2018; 6: 526–534. doi: 10.1016/s2213-2600(18)30099-7 [DOI] [PubMed] [Google Scholar]
- 149.Brunwasser SM, Snyder BM, Driscoll AJ, et al. Assessing the strength of evidence for a causal effect of respiratory syncytial virus lower respiratory tract infections on subsequent wheezing illness: a systematic review and meta-analysis. Lancet Respir Med 2020; 8: 795–806. doi: 10.1016/s2213-2600(20)30109-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lemanske RF, Jr. The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol 2002; 13: 38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x [DOI] [PubMed] [Google Scholar]
- 151.Rosas-Salazar C, Chirkova T, Gebretsadik T, et al. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): a population-based, prospective birth cohort study. Lancet 2023; 401: 1669–1680. doi: 10.1016/S0140-6736(23)00811-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kloepfer KM, Lee WM, Pappas TE, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol 2014; 133: 1301–1307.e1-3. doi: 10.1016/j.jaci.2014.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zuurbier RP, Bogaert D, de Steenhuijsen Piters WAA, et al. asymptomatic viral presence in early life precedes recurrence of respiratory tract infections. Pediatr Infect Dis J 2023; 42: 59–65. doi: 10.1097/inf.0000000000003732 [DOI] [PubMed] [Google Scholar]
- 154.Stier MT, Bloodworth MH, Toki S, et al. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol 2016; 138: 814–824.e11. doi: 10.1016/j.jaci.2016.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tian K, Dangarh P, Zhang H, et al. Role of epithelial barrier function in inducing type 2 immunity following early-life viral infection. Clin Exp Allergy 2023; 54: 109–119. doi: 10.1111/cea.14425 [DOI] [PubMed] [Google Scholar]
- 156.Saravia J, You D, Shrestha B, et al. Respiratory syncytial virus disease is mediated by age-variable IL-33. PLoS Pathog 2015; 11: e1005217. doi: 10.1371/journal.ppat.1005217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hong JY, Bentley JK, Chung Y, et al. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol 2014; 134: 429–439. doi: 10.1016/j.jaci.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Han M, Rajput C, Hong JY, et al. The innate cytokines IL-25, IL-33, and TSLP cooperate in the induction of type 2 innate lymphoid cell expansion and mucous metaplasia in rhinovirus-infected immature mice. J Immunol 2017; 199: 1308–1318. doi: 10.4049/jimmunol.1700216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wootton DG, Diggle PJ, Court J, et al. Recovery from pneumonia requires efferocytosis which is impaired in smokers and those with low body mass index and enhanced by statins. Thorax 2016; 71: 1052–1054. doi: 10.1136/thoraxjnl-2016-208505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou JH, Wang YN, Chang QY, et al. Type III interferons in viral infection and antiviral immunity. Cell Physiol Biochem 2018; 51: 173–185. doi: 10.1159/000495172 [DOI] [PubMed] [Google Scholar]
- 161.Davidson S, McCabe TM, Crotta S, et al. IFNλ is a potent anti-influenza therapeutic without the inflammatory side effects of IFNα treatment. EMBO Mol Med 2016; 8: 1099–1112. doi: 10.15252/emmm.201606413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mallampalli RK, Adair J, Elhance A, et al. Interferon lambda signaling in macrophages is necessary for the antiviral response to influenza. Front Immunol 2021; 12: 735576. doi: 10.3389/fimmu.2021.735576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sabbaghi A, Zargar M, Zolfaghari MR, et al. Protective cellular and mucosal immune responses following nasal administration of a whole gamma-irradiated influenza A (subtype H1N1) vaccine adjuvanted with interleukin-28B in a mouse model. Arch Virol 2021; 166: 545–557. doi: 10.1007/s00705-020-04900-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chong Z, Karl CE, Halfmann PJ, et al. Nasally delivered interferon-λ protects mice against infection by SARS-CoV-2 variants including omicron. Cell Rep 2022; 39: 110799. doi: 10.1016/j.celrep.2022.110799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Feld JJ, Kandel C, Biondi MJ, et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med 2021; 9: 498–510. doi: 10.1016/s2213-2600(20)30566-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reis G, Moreira Silva EAS, Medeiros Silva DC, et al. Early treatment with pegylated interferon lambda for COVID-19. N Engl J Med 2023; 388: 518–528. doi: 10.1056/NEJMoa2209760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Santer DM, Li D, Ghosheh Y, et al. Interferon-λ treatment accelerates SARS-CoV-2 clearance despite age-related delays in the induction of T cell immunity. Nat Commun 2022; 13: 6992. doi: 10.1038/s41467-022-34709-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Netea MG, Domínguez-Andrés J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol 2020; 20: 375–388. doi: 10.1038/s41577-020-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee A, Floyd K, Wu S, et al. BCG vaccination stimulates integrated organ immunity by feedback of the adaptive immune response to imprint prolonged innate antiviral resistance. Nat Immunol 2023; 25: 41–53. doi: 10.1038/s41590-023-01700-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell 2020; 183: 315–323 e319. doi: 10.1016/j.cell.2020.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Moorlag S, Taks E, Ten Doesschate T, et al. Efficacy of BCG vaccination against respiratory tract infections in older adults during the coronavirus disease 2019 pandemic. Clin Infect Dis 2022; 75: e938–e946. doi: 10.1093/cid/ciac182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Díaz FE, Guerra-Maupome M, McDonald PO, et al. A recombinant BCG vaccine is safe and immunogenic in neonatal calves and reduces the clinical disease caused by the respiratory syncytial virus. Front Immunol 2021; 12: 664212. doi: 10.3389/fimmu.2021.664212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Céspedes PF, Rey-Jurado E, Espinoza JA, et al. A single, low dose of a cGMP recombinant BCG vaccine elicits protective T cell immunity against the human respiratory syncytial virus infection and prevents lung pathology in mice. Vaccine 2017; 35: 757–766. doi: 10.1016/j.vaccine.2016.12.048 [DOI] [PubMed] [Google Scholar]
- 174.Ballarini S, Ardusso L, Ortega Martell JA, et al. Can bacterial lysates be useful in prevention of viral respiratory infections in childhood? The results of experimental OM-85 studies. Front Pediatr 2022; 10: 1051079. doi: 10.3389/fped.2022.1051079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schaad UB. OM-85 BV, an immunostimulant in pediatric recurrent respiratory tract infections: a systematic review. World J Pediatr 2010; 6: 5–12. doi: 10.1007/s12519-010-0001-x [DOI] [PubMed] [Google Scholar]
- 176.Steurer-Stey C, Lagler L, Straub DA, et al. Oral purified bacterial extracts in acute respiratory tract infections in childhood: a systematic quantitative review. Eur J Pediatr 2007; 166: 365–376. doi: 10.1007/s00431-006-0248-3 [DOI] [PubMed] [Google Scholar]
- 177.Orcel B, Delclaux B, Baud M, et al. Oral immunization with bacterial extracts for protection against acute bronchitis in elderly institutionalized patients with chronic bronchitis. Eur Respir J 1994; 7: 446–452. doi: 10.1183/09031936.94.07030446 [DOI] [PubMed] [Google Scholar]
- 178.Pan L, Jiang XG, Guo J, et al. Effects of OM-85 BV in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Clin Pharmacol 2015; 55: 1086–1092. doi: 10.1002/jcph.518 [DOI] [PubMed] [Google Scholar]
- 179.Huang Y, Pei Y, Qian Y, et al. A meta-analysis on the efficacy and safety of bacterial lysates in chronic obstructive pulmonary disease. Front Med 2022; 9: 877124. doi: 10.3389/fmed.2022.877124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.de Boer GM, Żółkiewicz J, Strzelec KP, et al. Bacterial lysate therapy for the prevention of wheezing episodes and asthma exacerbations: a systematic review and meta-analysis. Eur Respir Rev 2020; 29: 190175. doi: 10.1183/16000617.0175-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Troy NM, Strickland D, Serralha M, et al. Protection against severe infant lower respiratory tract infections by immune training: mechanistic studies. J Allergy Clin Immunol 2022; 150: 93–103. doi: 10.1016/j.jaci.2022.01.001 [DOI] [PubMed] [Google Scholar]
- 182.Coyne CB, Vanhook MK, Gambling TM, et al. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell 2002; 13: 3218–3234. doi: 10.1091/mbc.e02-03-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8: 958–969. doi: 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Antunes KH, Cassão G, Santos LD, et al. Airway administration of bacterial lysate OM-85 protects mice against respiratory syncytial virus infection. Front Immunol 2022; 13: 867022. doi: 10.3389/fimmu.2022.867022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Salzmann M, Haider P, Kaun C, et al. Innate immune training with bacterial extracts enhances lung macrophage recruitment to protect from betacoronavirus infection. J Innate Immun 2022; 14: 293–305. doi: 10.1159/000519699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rashu R, Ninkov M, Wardell CM, et al. Targeting the MR1–MAIT cell axis improves vaccine efficacy and affords protection against viral pathogens. Plos Pathog 2023; 19: e1011485. doi: 10.1371/journal.ppat.1011485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ichinohe T, Pang IK, Kumamoto Y, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA 2011; 108: 5354–5359. doi: 10.1073/pnas.1019378108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bradley KC, Finsterbusch K, Schnepf D, et al. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Reports 2019; 28: 245–256.e4. doi: 10.1016/j.celrep.2019.05.105 [DOI] [PubMed] [Google Scholar]
- 189.Wypych TP, Wickramasinghe LC, Marsland BJ. The influence of the microbiome on respiratory health. Nat Immunol 2019; 20: 1279–1290. doi: 10.1038/s41590-019-0451-9 [DOI] [PubMed] [Google Scholar]
- 190.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014; 20: 159–166. doi: 10.1038/nm.3444 [DOI] [PubMed] [Google Scholar]
- 191.Trompette A, Gollwitzer, ESPattaroni, C, et al. Dietary fiber confers protection against flu by shaping Ly6c− patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 2018; 48: 992–1005.e8. doi: 10.1016/j.immuni.2018.04.022 [DOI] [PubMed] [Google Scholar]
- 192.Antunes KH, Fachi JL, de Paula R, et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat Commun 2019; 10: 3273. doi: 10.1038/s41467-019-11152-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Antunes KH, Stein RT, Franceschina C, et al. Short-chain fatty acid acetate triggers antiviral response mediated by RIG-I in cells from infants with respiratory syncytial virus bronchiolitis. EBioMedicine 2022; 77: 103891. doi: 10.1016/j.ebiom.2022.103891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Steed AL, Christophi GP, Kaiko GE, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017; 357: 498–502. doi: 10.1126/science.aam5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cleophas MCP, Ratter JM, Bekkering S, et al. Effects of oral butyrate supplementation on inflammatory potential of circulating peripheral blood mononuclear cells in healthy and obese males. Sci Rep 2019; 9: 775. doi: 10.1038/s41598-018-37246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.de Groot PF, Nikolic T, Imangaliyev S, et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: a randomised controlled trial. Diabetologia 2020; 63: 597–610. doi: 10.1007/s00125-019-05073-8 [DOI] [PubMed] [Google Scholar]
- 197.Vulevic J, Drakoularakou A, Yaqoob P, et al. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr 2008; 88: 1438–1446. doi: 10.3945/ajcn.2008.26242 [DOI] [PubMed] [Google Scholar]
- 198.Guigoz Y, Rochat F, Perruisseau-Carrier G, et al. Effects of oligosaccharide on the faecal flora and non-specific immune system in elderly people. Nutrition Research 2002; 22: 13–25. doi: 10.1016/S0271-5317(01)00354-2 [DOI] [Google Scholar]
- 199.Peterson CM, Beyl RA, Marlatt KL, et al. Effect of 12 wk of resistant starch supplementation on cardiometabolic risk factors in adults with prediabetes: a randomized controlled trial. Am J Clin Nutr 2018; 108: 492–501. doi: 10.1093/ajcn/nqy121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bach Knudsen KE, Lærke HN, Hedemann MS, et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018; 10: 1499. doi: 10.3390/nu10101499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ferrer-Picon E, Dotti I, Corraliza AM, et al. Intestinal inflammation modulates the epithelial response to butyrate in patients with inflammatory bowel disease. Inflamm Bowel Dis 2020; 26: 43–55. doi: 10.1093/ibd/izz119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhivaki D, Lemoine S, Lim A, et al. Respiratory syncytial virus infects regulatory B cells in human neonates via chemokine receptor CX3CR1 and promotes lung disease severity. Immunity 2017; 46: 301–314. doi: 10.1016/j.immuni.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang H, Weyand CM, Goronzy JJ. Hallmarks of the aging T-cell system. FEBS J 2021; 288: 7123–7142. doi: 10.1111/febs.15770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arruvito L, Raiden S, Geffner J. Host response to respiratory syncytial virus infection. Curr Opin Infect Dis 2015; 28: 259–266. doi: 10.1097/qco.0000000000000159 [DOI] [PubMed] [Google Scholar]
- 205.Aliyu M, Zohora FT, Anka AU, et al. Interleukin-6 cytokine: an overview of the immune regulation, immune dysregulation, and therapeutic approach. Int Immunopharmacol 2022; 111: 109130. doi: 10.1016/j.intimp.2022.109130 [DOI] [PubMed] [Google Scholar]
- 206.Kirillova A, Lado A, Blatt N. Application of monoclonal antibody drugs in treatment of COVID-19: a review. Bionanoscience 2022; 12: 1436–1454. doi: 10.1007/s12668-022-00997-9 [DOI] [PMC free article] [PubMed] [Google Scholar]