Abstract
Objectives
To describe a case of post-immune checkpoint inhibitor (ICI) opsoclonus-myoclonus-ataxia syndrome (OMAS), with complete clinical remission after treatment.
Methods
A 52-year-old man was admitted because of subacute-onset vertigo, dysarthria, vomiting, and weight loss. He was under atezolizumab (anti–PD-L1) monotherapy (23 cycles) for metastatic small-cell lung cancer, with excellent response.
Results
On examination (1 month after symptom onset), the patient had opsoclonus, dysarthria, severe truncal and gait ataxia, and mild appendicular ataxia without myoclonus (SARA score 26/40). Brain MRI showed mild cerebellar atrophy, and CSF analysis disclosed pleocytosis and oligoclonal bands. Anti-SOX1 antibodies were detected in serum and CSF. Atezolizumab was stopped, and corticosteroids and monthly IV immunoglobulins were administered. Chemotherapy (carboplatin and etoposide) was also started because of cancer progression. Three months later, examination showed regression of the opsoclonus, truncal ataxia, and dysarthria and persistence of very mild gait ataxia (SARA score 3.5/40), which completely regressed at last examination (20 months after onset).
Discussion
The clinical pattern and reversibility bring the present case close to a few patients with paraneoplastic OMAS described before the ICI era. More research is needed to clarify the pathogenesis and outcomes of OMAS in the context of ICI.
Introduction
Neurologic adverse events of immune checkpoint inhibitors (ICIs) include disorders resembling paraneoplastic neurological syndromes (PNSs), which, similarly to their counterparts in ICI-naïve patients, usually have poor neurologic outcomes.1 Outside the ICI context, rapidly progressive cerebellar ataxia may occur in the context of paraneoplastic cerebellar degeneration (PCD, e.g., associated with anti-Yo antibodies), characterized by an irreversible loss of Purkinje cells or in the context of opsoclonus-myoclonus-ataxia syndrome (OMAS), in which neuropathologic findings are variable, with apparent preservation of Purkinje cells in some cases.2-6
Methods
We describe the occurrence of opsoclonus and cerebellar ataxia clinically resembling OMAS in a patient receiving the programmed death-ligand 1 (PD-L1) inhibitor atezolizumab for small-cell lung cancer (SCLC), with clinical remission after ICI discontinuation, immune active treatment, and chemotherapy.
Results
A 52-year-old nonsmoker man was diagnosed with metastatic SCLC (hepatic, adrenal, and osseous). Chemotherapy (carboplatin and etoposide) and atezolizumab were given for 4 cycles, followed by monthly atezolizumab, with excellent response (persistent remission of the thoracic, osseous, and adrenal gland lesions and size reduction of the hepatic lesions at follow-up whole-body CT scans). After 22 courses of atezolizumab, he developed vertigo, dysarthria, nausea, vomiting, and weight loss (8 kilos) over 2–3 weeks and rapidly deteriorated after the 23rd atezolizumab infusion. On examination, 1 month after onset, the patient had opsoclonus, dysarthria, truncal titubation, mild bilateral appendicular ataxia, and severe truncal and gait ataxia without myoclonus (Scale for the Assessment and Rating of Ataxia, SARA score 26/40, Video 1). Blood analysis was unremarkable, and brain MRI showed mild cerebellar atrophy (Figure 1A). Except for eye movement artifacts, EEG was normal. The CSF contained 9 cells/mm3 (predominance of lymphocytes, no malignant cells), 31 mg/dL proteins, 4 mmol/L glucose (range 2.3–4), several oligoclonal bands absent in serum, and normal levels of interleukin-6 (2.6 pg/mL); bacterial, fungal, and neurotropic viruses screening were negative. Anti-SOX1 antibodies were detected in the serum and CSF by immunohistofluorescence on rat brain sections, dot-blot, and cell-based assay. Neurofilament light chain (NfL) levels, measured by an Enzyme-Linked Immunoadsorbent kit (R-PLEX Human Neurofilament L Meso Scale Discovery), were 32 pg/mL in serum and 2,684 pg/mL in CSF. Whole-body fluorodeoxyglucose (FDG)-PET detected a single area of pathologic hypermetabolism in the liver. Atezolizumab was stopped, and IV methylprednisolone (1 g daily for 3 days followed by oral prednisone 1 mg/kg daily), IV immunoglobulins (IVIg, 2 g/kg, 3 monthly cycles), and chemotherapy (carboplatin and etoposide, 6 cycles) were started. Three months after treatment (on daily 7.5 mg of prednisone), examination showed a regression of the opsoclonus, truncal titubation, and dysarthria and the persistence of a very mild gait ataxia (SARA score 3.5/40, Video 2). Brain MRI was stable (Figure 1B), and CSF examination showed normal cell count (3 cells/mm3) and no oligoclonal bands. Anti-SOX1 antibodies were still positive. Four additional IVIg cycles (6–8 weeks apart) were administered, and at last follow-up (20 months after onset), examination was normal except for distal lower limb hypopallesthesia and paresthesia attributed to the chemotherapy. The biopsy of a new hypermetabolic hepatic lesion upon whole-body FDG-PET confirmed SCLC recurrence and prompted subsequent decision of restarting carboplatin and etoposide.
Figure. Brain MRI Findings.
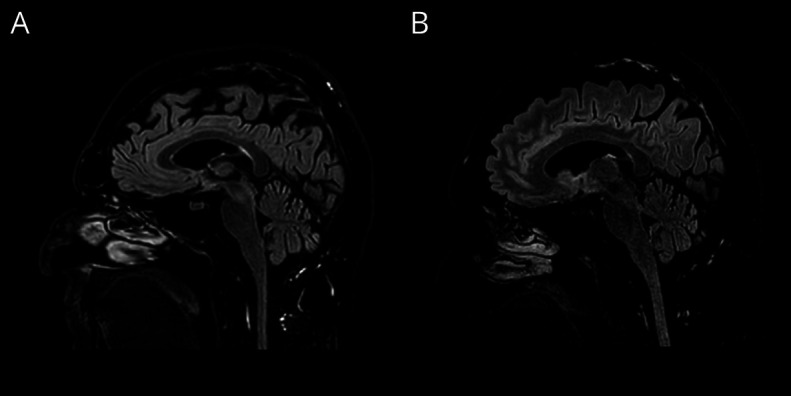
Brain MRI T2/FLAIR scan at (A) 1 month and (B) 4 months after the onset of the neurological syndrome showing stable mild cerebellar atrophy.
Neurological examination 1 month after onset.Download Supplementary Video 1 (28.4MB, mp4) via http://dx.doi.org/10.1212/200287_Video_1
Neurological examination 3 months after treatment.Download Supplementary Video 2 (25.1MB, mp4) via http://dx.doi.org/10.1212/200287_Video_2
Discussion
Rapidly progressive cerebellar ataxia is a rare immune-related adverse event of ICI that can mimic PCD in both its antibody association (e.g., anti-Yo, anti-Hu) and its therapeutic unresponsiveness.1,7 Although opsoclonus may occasionally occur in PCD, the cerebellar ataxia pattern (striking predominance of truncal over limb ataxia) and the clinical reversibility bring the present case closer to OMAS than PCD, adding to a handful of previously published post-ICI OMAS cases.8-10
Outside the ICI context, paraneoplastic OMAS has been mostly associated with SCLC and breast cancers; an autoimmune pathogenesis is suggested by the detection of brain-reactive antibodies in some cases, especially anti-Ri antibodies (the most frequent antibodies in paraneoplastic OMAS) in patients with breast cancers.11 By contrast, no specific antibody association has been described in OMAS occurring in SCLC, as most of these patients are antibody-negative.2,11 The detection of anti-SOX1 antibodies in the present case pairs with a reported post-ICI OMAS case in a SCLC patient with double anti-Hu and anti-SOX1 positivity.10 Of note, anti-Hu (usually at low titers) or anti-SOX1 antibodies are also detectable in a subset of SCLC without PNS; their detection might thus constitute a biomarker of the underlying cancer and not the neurologic syndrome.12,13 However, a previous study14 described the deposition of anti-Hu antibodies in the autoptic specimens of the cortex, brainstem, and cerebellum from a ICI-naïve patient with OMAS, SCLC, and high anti-Hu antibody titers suggesting that, in some cases, the presence of anti-Hu antibodies could be related to the neurologic syndrome. Although the observation that ICI can trigger OMAS also suggests an immune-related origin, it does not clarify the underlying pathogenesis. Similarly to other PNS, cancer-related factors could be necessary to break the immune tolerance toward certain neural antigens in a first step while other factors such as infections or the ICI may contribute to boost the autoimmune response to a clinically apparent level. This “multihit” hypothesis might explain the rarity of OMAS and PNS-like ICI neurotoxicities even in SCLC and other cancers known to constantly express neural antigens.1
Similarly, the effector immune mechanisms in OMAS are uncertain given the heterogeneity of autoptic findings, with variable Purkinje cell loss and inflammatory infiltrates in these patients.2-5 Although it has been suggested that opsoclonus may relate to a dysfunction of neuronal circuits of the brainstem (involving saccadic burst cells and omnipause cells) or the cerebellum (involving the fastigial nucleus and Purkinje cells),15 the neuronal populations involved in these circuits were preserved in many autoptic studies.2,4 This contrasts with PCD6 and suggests that the cytotoxic T-cell–mediated cell toxicity, a central mechanism in PCD, is less prominent in post-ICI OMAS. Although we did not perform a brain biopsy in this patient, the relatively low serum and CSF levels of NfL, an indicator of axonal loss, also suggests only mildly active neuronal damage in this patient, compared with those with other PNS-like encephalitis syndromes (Farina and Joubert, unpublished).
Although the preservation of neurons might partially explain why clinical remission in OMAS is more frequent than in other PNS,2 outcomes in OMAS are diverse, and patients may experience a relentlessly progressive course, sometimes resulting in fatal outcomes within a few weeks.2-4 While treatment of the cancer seems to be fundamental for the prognosis of paraneoplastic OMAS,2 it is not known whether immunosuppression plays a role in improving the neurologic outcome. In the setting of ICI treatment, we recommend following the oncological guidelines, which establish to promptly interrupt ICI and administer high-dose corticosteroids, in addition to which up-front administration of IVIg or plasma exchange may be also useful to accelerate clinical improvement,1 as in the present and another post-ICI case.9 Interestingly, both patients had a mild form of OMAS (without myoclonus or encephalopathy), which may have facilitated their recovery, although clinical remission has been described also in full-blown paraneoplastic OMAS.2,5 As exemplified by the present case, PNS antibodies usually associated with T-cell–mediated neuronal loss and poor outcomes1,6 do not discriminate well treatment-responsive patients in the context of OMAS; thus, the value of alternative biomarkers, such as NfL levels, should be investigated to better stratify these patients. In addition, investigating whether the positivity of PNS antibodies before ICI treatment increases the risk of developing PNS-like ICI neurotoxicities remains a research priority.1
In conclusion, clinicians should be aware that OMAS may occur in patients with ICI-treated SCLC, being potentially curable; more research is needed to clarify its underlying pathogenesis and effector mechanisms.
Acknowledgment
The authors thank Véréna Landel (DRS, Hospices Civils de Lyon) for help in English editing.
Appendix. Authors
| Name | Location | Contribution |
| Antonio Farina, MD | French Reference Centre on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron; MeLiS-UCBL-CNRS UMR 5284. INSERM U1314, Université Claude Bernard Lyon 1, France | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Macarena Villagrán-García, MD | French Reference Centre on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron; MeLiS-UCBL-CNRS UMR 5284. INSERM U1314, Université Claude Bernard Lyon 1, France | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Marie Benaiteau, MD | French Reference Centre on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron; MeLiS-UCBL-CNRS UMR 5284. INSERM U1314, Université Claude Bernard Lyon 1, France | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Florian Lamblin, MD | Department of Neurology, University Hospital of La Réunion, Saint-Pierre, France | Drafting/revision of the manuscript for content, including medical writing for content |
| Anthony Fourier, PhD | Lyon Neuroscience Research Center (CRNL), Université de Lyon, CNRS, INSERM, France | Drafting/revision of the manuscript for content, including medical writing for content |
| Jérôme Honnorat, MD, PhD | French Reference Centre on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron; MeLiS-UCBL-CNRS UMR 5284. INSERM U1314, Université Claude Bernard Lyon 1, France | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Bastien Joubert, MD, PhD | French Reference Centre on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron; MeLiS-UCBL-CNRS UMR 5284. INSERM U1314, Université Claude Bernard Lyon 1, France | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
Investissements d'Avenir programme (ANR-18-RHUS-0012) supported by a public grant overseen by the Agence Nationale de la Recherche (ANR).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Farina A, Villagrán-García M, Vogrig A, et al. Neurological adverse events of immune checkpoint inhibitors and the development of paraneoplastic neurological syndromes. Lancet Neurol. 2024;23(1):81-94. doi: 10.1016/S1474-4422(23)00369-1 [DOI] [PubMed] [Google Scholar]
- 2.Anderson NE, Budde-Steffen C, Rosenblum MK, et al. Opsoclonus, myoclonus, ataxia, and encephalopathy in adults with cancer: a distinct paraneoplastic syndrome. Medicine (Baltimore). 1988;67(2):100-109. doi: 10.1097/00005792-198803000-00003 [DOI] [PubMed] [Google Scholar]
- 3.Hormigo A, Dalmau J, Rosenblum MK, River ME, Posner JB. Immunological and pathological study of anti-Ri-associated encephalopathy. Ann Neurol. 1994;36(6):896-902. doi: 10.1002/ana.410360615 [DOI] [PubMed] [Google Scholar]
- 4.Ridley A, Kennard C, Scholtz CL, Büttner-Ennever JA, Summers B, Turnbull A. Omnipause neurons in two cases of opsoclonus associated with oat cell carcinoma of the lung. Brain. 1987;110(Pt 6):1699-1709. doi: 10.1093/brain/110.6.1699 [DOI] [PubMed] [Google Scholar]
- 5.Ohara S, Iijima N, Hayashida K, Oide T, Katai S. Autopsy case of opsoclonus–myoclonus–ataxia and cerebellar cognitive affective syndrome associated with small cell carcinoma of the lung. Movement Disord. 2007;22(9):1320-1324. doi: 10.1002/mds.21326 [DOI] [PubMed] [Google Scholar]
- 6.Winklehner M, Bauer J, Endmayr V, et al. Paraneoplastic cerebellar degeneration with P/Q-VGCC vs Yo autoantibodies. Neurol Neuroimmunol Neuroinflamm. 2022;9(4):e200006. doi: 10.1212/NXI.0000000000200006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farina A, Villagrán-García M, Ciano-Petersen NL, et al. Anti-Hu antibodies in patients with neurologic side effects of immune checkpoint inhibitors. Neurol Neuroimmunol Neuroinflamm. 2023;10(1):e200058. doi: 10.1212/NXI.0000000000200058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogrig A, Muñiz-Castrillo S, Joubert B, et al. Central nervous system complications associated with immune checkpoint inhibitors. J Neurol Neurosurg Psychiatry. 2020;91(7):772-778. doi: 10.1136/jnnp-2020-323055 [DOI] [PubMed] [Google Scholar]
- 9.Maller B, Peguero E, Tanvetyanon T. Ipilimumab/nivolumab-related opsoclonus-myoclonus-ataxia syndrome variant in a patient with malignant pleural mesothelioma. J Immunother. 2018;41(9):411-412. doi: 10.1097/CJI.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 10.Arai H, Utsu Y, Horio J, Furukawa S, Kikkawa Y. Paraneoplastic opsoclonus-myoclonus syndrome with anti-hu and anti-SOX-1 antibodies after immune-checkpoint inhibitor treatment combined with chemotherapy in a patient with small-cell lung cancer. Intern Med. 2022;61(1):71-74. doi: 10.2169/internalmedicine.7167-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armangué T, Sabater L, Torres-Vega E, et al. Clinical and immunological features of opsoclonus-myoclonus syndrome in the era of neuronal cell surface antibodies. JAMA Neurol. 2016;73(4):417-424. doi: 10.1001/jamaneurol.2015.4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalmau J, Furneaux HM, Gralla RJ, Kris MG, Posner JB. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer—a quantitative western blot analysis. Ann Neurol. 1990;27(5):544-552. doi: 10.1002/ana.410270515 [DOI] [PubMed] [Google Scholar]
- 13.Arnaldos-Pérez C, Vilaseca A, Naranjo L, et al. Algorithm to improve the diagnosis of paraneoplastic neurological syndromes associated with SOX1 antibodies. Front Immunol. 2023;14:1173484. doi: 10.3389/fimmu.2023.1173484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hersh B, Dalmau J, Dangond F, Gultekin S, Geller E, Wen PY. Paraneoplastic opsoclonus-myoclonus associated with anti-Hu antibody. Neurology. 1994;44(9):1754-1755. doi: 10.1212/wnl.44.9.1754 [DOI] [PubMed] [Google Scholar]
- 15.Wong A. An update on opsoclonus. Curr Opin Neurol. 2007;20(1):25-31. doi: 10.1097/WCO.0b013e3280126b51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neurological examination 1 month after onset.Download Supplementary Video 1 (28.4MB, mp4) via http://dx.doi.org/10.1212/200287_Video_1
Neurological examination 3 months after treatment.Download Supplementary Video 2 (25.1MB, mp4) via http://dx.doi.org/10.1212/200287_Video_2


