Abstract
Background:
Exclusion diets for gastrointestinal symptom management have been hypothesized to be a risk factor for avoidant/restrictive food intake disorder (ARFID; a non-body image-based eating disorder). In a retrospective study of pediatric and adult neurogastroenterology patients, we aimed to (1) identify the prevalence and characteristics of an exclusion diet history and (2) evaluate if an exclusion diet history was concurrently associated with presence of ARFID symptoms.
Methods:
We conducted a chart review of 539 consecutive referrals (ages 6-90, 69% female) to adult (n=410; January-December 2016) and pediatric (n=129; January 2016-December 2018) neurogastroenterology clinics. Blinded coders (n=4) retrospectively applied DSM-5 criteria for ARFID and a separate coder assessed documentation of exclusion diet history. We excluded patients with no documentation of diet in the chart (n=35) or who were not orally fed (n=9).
Results:
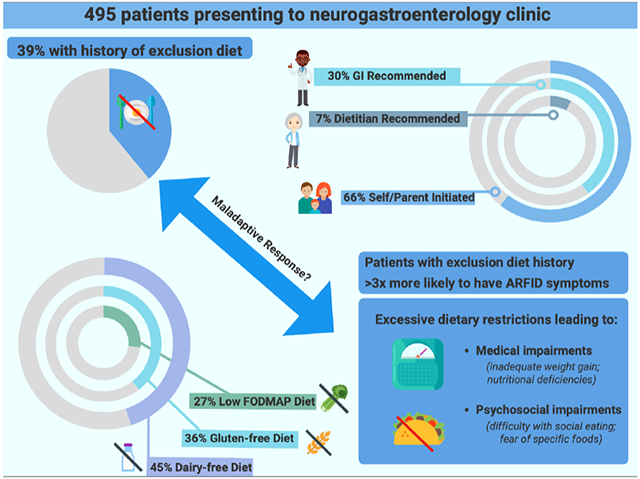
Of 495 patients included, 194 (39%) had an exclusion diet history, and 118 (24%) had symptoms of ARFID. Of reported diets, dairy-free was the most frequent (45%), followed by gluten-free (36%). Where documented, exclusion diets were self-initiated by patients/parents in 66% of cases, and recommended by gastroenterology providers in 30%. Exclusion diet history was significantly associated with the presence of ARFID symptoms (OR=3.12[95% CI 1.92–5.14], p<.001).
Conclusions:
History of following an exclusion diet was common and was most often patient-initiated among pediatric and adult neurogastroenterology patients. As patients with self-reported exclusion diet history were over three times as likely to have ARFID symptoms, providers should be cognizant of this potential association when considering dietary interventions.
Keywords: avoidant/restrictive food intake disorder, disorders of gut-brain interaction, feeding and eating disorders, functional gastrointestinal disorders
Graphical Abstract
INTRODUCTION
Disorders of gut-brain interaction (DGBI; also known as functional gastrointestinal (GI) disorders) encompass an array of sensory and motility disturbances affecting both pediatric and adult patients.(1, 2) Exclusion diets (e.g., dairy-free diet, gluten-free diet) are often used for symptom management in DGBI, but may put some patients at risk for impaired nutrition and/or quality of life.(3, 4)
Dietary management for DGBI is increasingly employed as a first-line intervention or as part of integrated care.(5-7) Exclusion diets may be self-initiated by patients or selected by patients and providers due to their high face validity as a “natural,” non-pharmacologic treatment strategy.(3, 8) In a survey of >1000 gastroenterologists in the United States, over half of providers recommended dietary therapy to adult patients with irritable bowel syndrome (IBS).(9) Avoidance of specific foods is also common in patients with other DBGIs,(10) such as functional dyspepsia,(11, 12) and among pediatric patients with DGBI.(13) However, with the increased use and popularity of exclusion diets, there is growing concern for their potential psychological impact and relationship to disordered eating.(3, 14, 15)
High rates of avoidant/restrictive food intake disorder (ARFID) symptoms in DGBI(16-20) have raised concern that exclusion diets may put some patients at risk for impairments in nutritional status or eating-related quality of life.(3, 8, 21) ARFID is a non-body image-based eating disorder characterized by a reduction in food intake (volume and/or variety) associated with medical (e.g., inability to gain weight, nutrient deficiency) and/or psychosocial (e.g., social eating difficulty) impairments. ARFID symptoms have been reported in 13-40% of patients with DGBI.(8, 17-20) Exclusion diets have been proposed as one potential risk factor for some patients to develop ARFID,(3, 8, 15, 22) but whether this relationship exists remains to be elucidated.
In a retrospective study of pediatric and adult patients presenting for neurogastroenterology evaluation, we aimed to: (1) identify the prevalence and characteristics of current or past exclusion diet use and (2) evaluate if a history of exclusion diet was associated with presence of ARFID symptoms. We hypothesized that patients with a history of exclusion diet would have a significantly increased likelihood of having ARFID symptoms. We also explored the relationships between presence of ARFID symptoms and exclusion diet type (e.g., diary-free diet, gluten-free diet), and recommender (e.g., GI provider, self).
METHODS
Procedures
We conducted a retrospective chart review of patients (N=539, ages 6 – 90 years, 69% female) presenting to our tertiary care center’s pediatric and adult neurogastroenterology clinics. Pediatric patients (n=129, ages 6 – 18, 56% female) comprised consecutive referrals to the pediatric neurogastroenterology clinic from January 2016 to December 2018, and adult patients (n=410, ages 18 – 90, 73% female) comprised consecutive referrals to the adult neurogastroenterology clinic from January through December 2016. Pediatric referrals were collected over a longer timeframe (3 years versus 1 year for adult referrals) to maximize the sample size because of the lower patient volume in the pediatric neurogastroenterology clinic compared to the adult neurogastroenterology clinic.
We reviewed electronic medical records beginning at the initial consultation note and obtained demographic and clinical characteristics, including age and BMI at visit, sex, gender identity, race, ethnicity, presenting complaints, and gastrointestinal diagnoses conferred by providers. Masked coders (HBM, CJS, APB, FUR, CB) completed a diagnostic checklist, based on the Diagnostic and Statistical Manual for Mental Disorders, 5th edition (DSM-5)(23) ARFID criteria, to indicate presence or absence of ARFID with moderate to substantial agreement (κ=0.60 for pediatric patients, κ=0.66 for adult patients). Coders conferred “definite ARFID” when cases met all DSM-5 ARFID criteria and “potential ARFID” when criteria were present but not enough information was available to confer or rule out a full diagnosis.(18, 19) These groups were combined and categorized as “ARFID symptoms.”
A separate coder (MA) reviewed each electronic medical record from the initial consultation as well as all preceding hospital and clinic visits for documentation of diet history. Presence of exclusion diet was identified based on patient self-report of historical or ongoing exclusion diet in the medical records. Patients were not considered to have presence of exclusion diet if they had no documentation of history of trying an exclusion diet (i.e., trying a diet prior to initial gastroenterology consultation); this included patients who were advised to initiate an exclusion diet at the initial gastroenterology consultation or subsequent visits. We categorized exclusion diets based on five primary eliminated food groups or ingredients– diary-free, gluten-free, the low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet, low acid diet, and low fat diet. We selected these diet categories as they had been previously described and studied in DGBI(6, 12) and are frequently employed in clinical practice. Exclusion diets which did not fit in the major categories were categorized as “other exclusion diets” (e.g., low histamine, “Candida diet”). When noted by in the medical record, we categorized whether exclusion diet was self-/parent-initiated or provider recommended by patient report. Provider categories were comprised of GI provider, dietitian and “other provider” (e.g., primary care physician, naturopathic provider). We excluded patients from analysis if there was no documentation of diet in the medical record (n=35) or if they received parenteral nutrition or tube feeds (n=9). We did not classify patients as having an exclusion diet if they had: documented Celiac disease and were restricting only gluten, documented food allergies who were restricting only allergic triggers, or documented lactose intolerance and were only restricting dairy.
Institutional Review Board approval (Protocol 2018P002539 and 2018P000504) was obtained prior to study procedure commencement.
Statistical Analysis
We calculated frequency of exclusion diets, types of exclusion diets, and who recommended exclusion diets. We reported descriptive statistics to compare those with and without exclusion diets. For exploratory purposes, we compared frequency of exclusion diet type and who recommended exclusion diet among those with versus without ARFID symptoms. For all descriptive comparisons, we used Kruskal Wallis H-Tests for continuous data (as age and BMI were non-normally distributed) and chi-square tests for categorical data.
To test the hypothesis that history of exclusion diet would be associated with presence of ARFID symptoms, we conducted a multivariate logistic regression with presenting complaints as a priori covariates based on clinical relevance and demographic covariates selected based on univariate screen. We selected presenting complaints as covariates to control for the significant relations previously found between GI presentation and ARFID status.(18, 19)Analyses were performed using R programming (R Core Team 2021). For sensitivity analysis, we also ran the regression models with the pediatric clinic sample and the adult clinic samples separated.
RESULTS
Table 1 includes demographic characteristics of the sample, presenting complaints, and gastrointestinal diagnosis, separated by presence of an exclusion diet history.
Table 1.
Characteristics of neurogastroenterology patients by exclusion diet history (N= 495).
| Exclusion Diet History (n=194) |
No Exclusion Diet History (n=301) |
p-valuea | |
|---|---|---|---|
| Age, mean (SD) | 38.1 (20.5) | 39.1 (22.7) | 0.733 |
| Age group, n (%) | |||
| 6 – 17 years | 37 (19%) | 75 (25%) | -- |
| 18 – 24 years | 25 (13%) | 19 (8%) | -- |
| 25 – 39 years | 44 (23%) | 57 (19%) | -- |
| 40 – 64 years | 63 (32%) | 98 (33%) | -- |
| 65 – 84 years | 24 (12%) | 43 (14%) | -- |
| 85+ years | 1 (1%) | 4 (1%) | -- |
| Pediatric Clinic Patient, n (%) | 40 (21%) | 79 (26%) | -- |
| Sex—female, n (%) | 158 (81%) | 184 (61%) | <0.001 |
| Gender Identity—n (%)b | |||
| Female | 156 (80%) | 179 (59%) | <0.001 |
| Male | 36 (19%) | 119 (40%) | -- |
| Non-binary | 2 (1%) | 1 (0.3%) | |
| Race, n (%)c | |||
| American Indian or Alaskan Native | 1 (0.5%) | 1 (0.3%) | -- |
| Asian | 5 (3%) | 16 (5%) | -- |
| Black or African American | 5 (3%) | 14 (5%) | -- |
| Native Hawaiian or Pacific Islander | 0 (0%) | 0 (0%) | -- |
| White | 176 (91%) | 238 (80%) | -- |
| Other/Unknown | 7 (4%) | 27 (9%) | -- |
| Ethnicity—Hispanic/Latinx, n (%)d | 10 (5%) | 26 (9%) | -- |
| BMI, mean (SD)e | 24.2 (6.2) | 24.9 (6.6) | 0.138 |
| BMI, mean (SD) – patients > 18 | 25.4 (6.1) | 26.6 (5.9) | 0.018 |
| BMI percentile, mean (SD) – patients < 18 | 54.5 (31.5) | 57.8 (34.3) | 0.432 |
| ARFID symptoms, n (%) | 73 (38%) | 45 (15%) | <0.001 |
| Presenting Complaints, n (%)f | |||
| Eating-related complaint | 37 (19%) | 41 (14%) | 0.134 |
| Esophageal-related complaint | 75 (39%) | 131 (44%) | 0.328 |
| Stomach-related complaint | 80 (41%) | 124 (41%) | 1 |
| Lower GI-related complaint | 144 (74%) | 182 (60%) | 0.002 |
| Gastrointestinal Diagnosis, n (%)f | |||
| Esophageal Diagnosis | 59 (30%) | 126 (42%) | 0.013 |
| Dyspepsia/Nausea/Vomiting Diagnosis | 67 (35%) | 69 (23%) | 0.006 |
| Lower GI Diagnosis | 131 (68%) | 162 (54%) | 0.003 |
| Chronic Abdominal Pain Diagnosis | 19 (10%) | 29 (10%) | 1 |
Note. ARFID=avoidant/restrictive food intake disorder; BMI=body mass index; GI=gastrointestinal. Eating-related complaints included low weight, weight loss, poor appetite, and food aversion. Esophageal complaints included reflux, regurgitation, belching, globus, choking, cough, dysphagia, heartburn, chest pain/pressure, and other throat-related symptoms. Stomach complaints included nausea, vomiting, post-prandial fullness, early satiety, epigastric pain, and upper abdominal pain. Lower GI complaints included lower abdominal pain, diarrhea, constipation, fecal incontinence, rectal urgency, bloating/distension, rectal pain, anal itching, and flatulence. Esophageal diagnoses include globus, dysphagia, non-cardiac chest pain, gastroesophageal reflux disease, rumination syndrome, and chronic belching. Dyspepsia/nausea/vomiting diagnoses include gastroparesis, functional dyspepsia, cyclic vomiting syndrome, and chronic unspecified nausea and vomiting. Lower GI diagnoses include irritable bowel syndrome, chronic constipation, functional diarrhea, and abdominal bloating/distension.
Comparisons presented for descriptive purposes. Continuous variables analyzed with Kruskal Wallis H-Tests for non-normally distributed data. Categorical variables analyzed with Chi-square Tests.
Gender identity data missing for n=2. Gender identity categories included female, male and non-binary.
Race data missing for n=3.
Ethnicity data missing for n =12.
BMI data missing for n=21.
n=214 had >1 symptom complaint. n=191 had >1 gastrointestinal diagnosis.
Prevalence and Characteristics of Exclusion Diets
Exclusion diet history was present in 194 patients (39%, 194/495), including 40 patients (34% 40/119) in the pediatric neurogastroenterology clinic sample, and 154 cases (41% 154/376) in the adult neurogastroenterology clinic sample. Patients with a history of exclusion diet were more likely to be female (81% vs. 61%, p<0.001), and, in the adult clinic patients only, to have lower mean BMI (p=0.018; but still in “normal range,” on average) (Table 1). Patients with a history of exclusion diet were also more likely to present with a lower gastrointestinal-related complaint (74% vs. 60%, p=0.002) and have a lower gastrointestinal or dyspepsia/nausea/vomiting diagnosis (68% vs. 54%, p=0.003 and 35% vs. 23%, p=0.006, respectively). Esophageal diagnoses were less frequent among patients with history of exclusion diets (30% vs. 42%, p=0.013) (Table 1).
Table 2 displays the frequencies of exclusion diet types. Among patients with exclusion diets in both the pediatric (n=40) and adult clinic (n=154) groups, dairy-free diets were the most frequent exclusion diet (45%, 87/194), although more frequent in the pediatric group (78%, 31/40) than in the adult group (36%, 56/154). In both patient groups, gluten-free diets were the second most frequent exclusion diet (40%, 16/40 in the pediatric group; 35%, 54/154 in the adult group).
Table 2.
Type of Historical Exclusion Diets Among Patients Presenting for Neurogastroenterology Consultation by ARFID Status (n = 194).
| Type of Exclusion Diet, n (%)a | Total | ARFID symptoms (n = 73) |
No ARFID symptoms (n = 121) |
X2 | p-valueg | |
|---|---|---|---|---|---|---|
| Dairy-freea | 87 (45%) | 33 (45%) | 54 (45%) | <0.001h | 1 | |
| Gluten-freeb | 70 (36%) | 25 (34%) | 45 (37%) | 0.067 | 0.795 | |
| Low FODMAP dietc | 53 (27%) | 23 (32%) | 30 (25%) | 0.732 | 0.395 | |
| Low acid dietd | 20 (10%) | 2 (3%) | 18 (15%) | 6.000 | 0.014 | |
| Low fat diete | 15 (8%) | 9 (12%) | 6 (5%) | 2.511 | 0.113 | |
| Other exclusion dietf | 55 (28%) | 29 (41%) | 26 (21%) | 6.585 | 0.010 | |
| Multiple Exclusion Diets | 80 (41%) | 33 (45%) | 47 (39%) | 0.521 | 0.470 |
Note. ARFID=avoidant/restrictive food intake disorder; FODMAP= fermentable oligosaccharides, disaccharides, monosaccharides and polyols. The total sample (n=194) included adult (n=154) and pediatric (n=40) patients who had a history of an exclusion diet.
n=31 pediatric patients, n=56 adult patients.
n=16 pediatric patients, n=54 adult patients.
n=6 pediatric patients, n=47 adult patients.
n=1 pediatric patients, n=19 adult patients.
n=1 pediatric patients, n=14 adult patients.
n=14 pediatric patients, n=41 adult patients.
Analyzed with Chi-square Tests for descriptive purposes.
Exact value = 7.5651x10−31
Of 194 patients with exclusion diet history, 169 (n=38 pediatric clinic patients, n=131 adult clinic patients) had documented information about who recommended the exclusion diet (n=25 had no documentation of recommender). Most exclusion diets were self- or parent-initiated (66%, 112/169), but more frequently so in adult clinic patients (70%, 92/131) than in the pediatric clinic patients (53%, 20/38) (Table 3). Among the pediatric clinic group in particular, exclusion diets were also frequently recommended by providers, with 45% of diets (17/38) recommended by a GI provider and 13% of diets (5/38) recommended by a dietitian. Of note, there was overlap within exclusion diet history, with some patients having more than one type of exclusion diet (n=81), and/or diets recommended by multiple providers (e.g., recommended by GI provider and dietitian). Some diets were also self/parent-initiated and provider recommended (n=23).
Table 3.
Who Recommended Exclusion Diet Among Patients Presenting for Neurogastroenterology Consultation by ARFID Status (n = 169).a
| Who Recommended Exclusion Diet, n (%)b | Total | ARFID symptoms (n = 70) |
No ARFID symptoms (n = 99) |
X2 | p-valuec | |
|---|---|---|---|---|---|---|
| Self-Initiatedd | 112 (66%) | 54 (77%) | 58 (59%) | 5.514 | 0.019 | |
| GI Provider Recommendede | 51 (30%) | 19 (27%) | 32 (32%) | 0.305 | 0.581 | |
| Other Recommendedf | 20 (12%) | 5 (7%) | 15 (15%) | 1.812 | 0.178 | |
| Dietitian Recommendedg | 11 (7%) | 6 (9%) | 5 (5%) | 0.357 | 0.550 |
Note. ARFID=avoidant/restrictive food intake disorder; GI=gastrointestinal.
n=25 did not have documentation of who recommended dietary restriction.
n=23 had exclusion diet recommended by > 1 provider.
Analyzed with Chi-square Tests for descriptive purposes.
n=20 pediatric patients, n=92 adult patients
n=17 pediatric patients, n=34 adult patients
n=3 pediatric patients, n=17 adult patients
n=5 pediatric patents, n=6 adult patients
Association Between Exclusion Diets and ARFID Symptoms
Univariate comparisons showed ARFID symptoms were significantly more frequent in patients with exclusion diet history compared to patients without exclusion diet history (38% vs 15%; Table 2). In the pediatric clinic group, 30 (25%, 30/119) had ARFID symptoms, with the following frequencies for motivations behind avoidant/restrictive eating: 17 (47%) fear of aversive consequences, 10 (33%) lack of interest/low appetite, and 3 (10%) sensory sensitivity [n=7 had multiple presentations]. In the adult clinic group, 88 (23%, 88/376) had ARFID symptoms, with the following frequencies for motivations behind avoidant/restrictive eating: 81 (92%) fear of aversive consequences, 20 (23%) lack of interest/low appetite, and 0 (0%) sensory sensitivity [n=13 had multiple presentations].
Logistic regression (Table 4) confirmed that exclusion diet history was significantly associated with the presence of ARFID symptoms (OR=3.12, 95% CI 1.92–5.14, p<0.001). This association remained similar when the model was run with the pediatric clinic group alone (OR=5.74, 95% CI 1.88—19.33, p=0.003) and the adult clinic group alone (OR=2.86, 95% CI 1.59—5.26, p<0.001). Of the covariates included, female sex, BMI, presenting with eating-related complaints, or presenting with stomach-related complaints were also significant predictors of ARFID symptom presence.
Table 4.
Multivariate Analysis of the Association of Exclusion Diet History with ARFID symptom presence.a
| OR | 95% CI | p-value | |
|---|---|---|---|
| Exclusion diet history, yes/no | 3.12 | 1.92 – 5.14 | <0.001 |
| Age | 1 | 0.99 – 1.01 | 0.732 |
| Female sex, yes/no | 1.90 | 1.06 – 3.51 | 0.035 |
| BMI | 0.94 | 0.90 – 0.98 | 0.007 |
| Eating-related complaints, yes/no | 4.39 | 2.47 – 7.89 | <0.001 |
| Esophageal-related complaints, yes/no | 0.70 | 0.41 – 1.18 | 0.182 |
| Stomach-related complaints, yes/no | 1.78 | 1.08 – 2.92 | 0.023 |
| Lower GI-related complaints, yes/no | 0.99 | 0.58 – 1.70 | 0.975 |
Note. Presenting complaints were selected as a priori covariates. Other variables (age, sex, BMI) were selected based on univariate screen (i.e., they were significantly different between those with and without ARFID symptoms—see Table 1).
Model provided a good fit [X2(8)=91.98, p<0.0001].
In exploratory comparisons, patients with ARFID symptoms were more likely to report history of self-initiated diet (77% vs. 59%, p=0.023), while rates of other provider-recommended diets did not significantly differ between those with and without ARFID symptoms (Table 3). Having a history of an “other exclusion diet” was significantly associated with having ARFID symptoms, while history of low acid diet was significantly associated with not having ARFID symptoms.
DISCUSSION
While food avoidance and exclusion diet use can be adaptive and helpful for symptom management for some patients, there is growing concern that exclusion diets may put a subset of patients at risk for ARFID.(3, 4, 8, 22) In this retrospective study of pediatric and adult patients with DGBI, we found that more than one third (39%) of patients had a history of following an exclusion diet, most of which were self-initiated (66%), with dairy-free diets being most common (45%). Importantly, we found that self-reported exclusion diet history was significantly associated with ARFID symptom presence, which has implications for screening and management in DGBI.
Our findings on the frequency and characteristics of exclusion diet use add to the literature in DGBI, and to our knowledge is the first to report use of specific exclusion diets in a pediatric DGBI population. Patients with DGBI commonly try to identify dietary causes for symptoms. In fact, up to 90% of children/adolescents(24, 25) and adults(26, 27) with IBS attribute their symptoms to food. In patients of all ages, foods rich in carbohydrates and fat are most frequently indicated as symptom-producing.(24, 25, 28) Thus, dairy-free, gluten-free and the low FODMAP diets have been embraced as treatment strategies in DGBI,(29, 30) and were the most commonly reported exclusion diets in our study.
The relatively high rate of history of exclusion diet use is notable, especially given the limited evidence for many of the diets reported in our sample. The most rigorously studied exclusion diet in DGBI is the low FODMAP diet,(31-34) but its efficacy is limited to adult patients with IBS.(31, 35) There is minimal evidence for gluten-free(31, 36, 37) or dairy-free diets in IBS,(38) and evidence supporting exclusion diets in other DGBIs, such as a low fat diet for functional dyspepsia, is scarce.(39, 40) In pediatrics, very few studies have addressed exclusion diets in DGBI,(41-44) and the rare, small randomized controlled trials show mixed results.(45, 46) Given the minimal support for exclusion diets beyond the low FODMAP diet, the high frequency of exclusion diet history use is surprising, but may be explained by the fact that most diets were patient-, rather than provider-initiated.
Most exclusion diets were self-initiated by patients and/or their parents (66%). This is consistent with gastroenterologists reporting that over half of their patients with irritable bowel syndrome attempt to self-manage their symptoms with dietary interventions, most often using a “trial and error” approach.(9) The frequency of self-initiated diets may also be due growing availability of online resources about dietary interventions and the increased production and marketing of specialty food products (e.g., gluten-free, vegan), but concerns remain about both the quality of this content(47) and lack of dietetic supervision.(48, 49) We also found that almost half of exclusion diets had been documented as recommended by a provider, with 30% of all reported diets recommended by a GI provider. While gastroenterologist-prescribed exclusion diets may be a preferred, safer alternative to patient-initiated diets, patients may have difficultly applying these recommendations without closer dietitian guidance and supervision.(50, 51) Gastroenterologists may also not be able to fully evaluate exclusion diet appropriateness—for example, a retrospective study in adults showed that over 70% of patients who screened positive for ARFID were recommended a low FODMAP diet by their GI provider.(52) In fact, exclusion diets may put a subset of patients at risk for developing de novo problems with eating, such as ARFID.
While prior studies have shown that disordered eating can be found in diet-controlled chronic disease, including DGBIs,(20, 53-55) our study is the first to our knowledge to examine exclusion diets as a retrospective correlate for ARFID. Our finding that patients with exclusion diet history were over three times as likely to have ARFID symptoms raises concerns about additional potential adverse outcomes of dietary management strategies. Exclusion diets, including a gluten-free diet and the low FODMAP diet, have been linked to poor diet quality and inadequate nutrient intake.(4, 49, 56, 57) Although studies have been conflicting,(58) in some, low FODMAP diet resulted in decreased caloric intake(32, 59) and weight loss.(60) Exclusion diets can also negatively affect quality of life (e.g., difficulty eating outside the home).(58, 61-63) It is possible that the association we found between ARFID and exclusion diets exists because both are independent markers of DGBI disease severity. However, when exclusion diets are associated with medical/nutritional and/or quality of life impairments, we argue this indicates that the patient’s current dietary restrictions are a problem, and likely meet criteria for ARFID.
The reason for the strong association between exclusion diet history and ARFID symptoms is not known. Some patients with DGBI may perceive some symptom improvement with an exclusion diet, but anxiety around their symptoms returning can lead to extreme or prolonged dietary restrictions and dysfunctional illness beliefs, such as overestimation of negative consequences of expanding their diet.(15, 64) This maladaptive response to exclusion diets can spiral into a negative feedback loop, as the nutritional and psychological consequences of ARFID may promote sensory and motility disturbances, thus worsening DGBI symptoms.(15, 65)
It is also possible that exclusion diet history and ARIFD symptoms had a strong association because of inadequate supervision. Our exploratory findings showed that self-initiated, but not provider-recommended, exclusion diets were significantly more frequent in those with ARFID symptoms. Unsupervised patients may lack clear expectations for exclusion diets and timelines for re-introduction of food. For the low FODMAP diet in particular, dietitian guidance has been identified as an important factor to reduce risk of negative outcomes,(49) with one study showing that patients who were not seen by dietitian were less able to follow the three phases of the diet, and over one-fourth of patients followed the restrictive phase of the diet for longer than recommended.(51)
While further prospective research is needed to understand how some patients develop ARFID in the context of DGBI, our study provides preliminary evidence that use of exclusion diets may be related to ARFID, and that ARFID may develop in patients attempting to follow diets without provider guidance. However, as we cannot demonstrate causality, it is possible that we found a relationship between exclusion diet history and ARFID because some patients may have had ARFID prior to their DGBI. DGBIs are common among patients with eating disorders,(66, 67) including those with ARFID.(68) Patients who have established disordered eating practices when they are diagnosed with DGBI may be more likely to try exclusion diets. However, it is highly plausible that ARFID developed in the context of DGBI in our sample. Other research has shown that patients with diet-controlled health conditions are more likely to develop disordered eating after the onset of dietary therapy, (54, 64) perhaps due to the inherent focus on food intake with dietary regimens. (53, 55, 64) In patients with ARFID and DGBI, most patients cite a fear around gastrointestinal symptom consequences as a driver for their disordered eating(18-20) and this, rather than other ARFID presentations (i.e., sensory sensitivity to food characteristics, low appetite/lack of interest in eating), was most common among our patients.
This study had several limitations related to its retrospective chart design, and specifically reliance on free-text documentation of diet in notes. Patient self-report of diet can be inaccurate,(69) and we were not able to evaluate important factors around exclusion diet use (e.g., the duration of diet, adherence to diet, and motivation for trialing dietary therapy). Exclusion diets in pediatric and adult populations differ because of parental involvement and potential growth and development implications, but we were not able to explore this due to our smaller sample of pediatric patients. Our categorization of ARFID symptom presence was also limited by the retrospective design, and there was not information to definitively confer an ARFID diagnosis for 83 cases of the combined ARFID symptom group. Finally, while we controlled for age and gastrointestinal presenting complaints in our multivariate analysis, our inability to examine differential risk by gastrointestinal diagnosis is a limitation.
Our findings are novel and enhance our understanding of exclusion diets among patients with DGBI, and should provoke more critical investigation of their use and relationship with ARFID. Exclusion diets may be helpful and/or non-problematic for many patients, but their use may be overly restrictive when not indicated or backed by evidence, particularly in pediatrics where the data supporting exclusion diets for DGBI is especially limited. Providers should consider screening patients’ dietary histories closely, being mindful of self-initiated diets and preexisting fear or anxiety around food intake (see Chey et al.(21) and Burton Murray et al. for guidance).(70) Before recommending exclusion diets, providers should consider the psychological and nutritional effects, and consider the risk level of certain diets.(70) Ideally, exclusion diets should be implemented with dietitian guidance, and patients should be followed regularly to assess their response, and to determine when foods can be re-introduced. Future longitudinal research is needed, especially to identify what factors put some DGBI patients, but not others, at risk for ARFID development.
ACKNOWLEDGMENTS
The authors thank Casey J. Silvernale, Abbey P. Bailey, Fatima U. Rao and Corey Baker for their assistance with coding the original data set.
FUNDING
This manuscript was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, K23 DK131334 (HBM) and K23 DK120945 (KDS).
Footnotes
DISCLOSURES
MA and ENM have no personal or financial conflicts to declare. HBM and JJT receive royalties from Oxford University Press for their forthcoming book on rumination syndrome. KS has received research support from Ironwood and Urovant, has served as a speaker for Shire, and has served as a consultant to Arena, Gelesis, GI Supply, and Shire. BK has received research support from AstraZeneca, Takeda, Gelesis, Medtronic, Genzyme and has served as a consultant to Shire, Takeda, and Ironwood. JJT and KTE receive royalties from Cambridge University Press for the sale of their books, Cognitive-Behavioral Therapy for Avoidant/Restrictive Food Intake Disorder: Children, Adolescents, and Adults and The Picky Eater’s Recovery Book: Overcoming Avoidant/Restrictive Food Intake Disorder.
REFERENCES
- 1.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021;160:99–114 e3. [DOI] [PubMed] [Google Scholar]
- 2.Robin SG, Keller C, Zwiener R, et al. Prevalence of Pediatric Functional Gastrointestinal Disorders Utilizing the Rome IV Criteria. J Pediatr 2018;195:134–139. [DOI] [PubMed] [Google Scholar]
- 3.Chey WD. Elimination Diets for Irritable Bowel Syndrome: Approaching the End of the Beginning. Am J Gastroenterol 2019;114:201–203. [DOI] [PubMed] [Google Scholar]
- 4.Scarlata K, Catsos P, Smith J. From a Dietitian's Perspective, Diets for Irritable Bowel Syndrome Are Not One Size Fits All. Clin Gastroenterol Hepatol 2020;18:543–545. [DOI] [PubMed] [Google Scholar]
- 5.Fikree A, Byrne P. Management of functional gastrointestinal disorders. Clin Med (Lond) 2021;21:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moayyedi P, Quigley EM, Lacy BE, et al. The Effect of Dietary Intervention on Irritable Bowel Syndrome: A Systematic Review. Clin Transl Gastroenterol 2015;6:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim BJ, Kuo B. Gastroparesis and Functional Dyspepsia: A Blurring Distinction of Pathophysiology and Treatment. J Neurogastroenterol Motil 2019;25:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton Murray H, Staller K. When Food Moves From Friend to Foe: Why Avoidant/Restrictive Food Intake Matters in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- 9.Lenhart A, Ferch C, Shaw M, et al. Use of Dietary Management in Irritable Bowel Syndrome: Results of a Survey of Over 1500 United States Gastroenterologists. J Neurogastroenterol Motil 2018;24:437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manning LP, Biesiekierski JR. Use of dietary interventions for functional gastrointestinal disorders. Curr Opin Pharmacol 2018;43:132–138. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013;108:18–37; quiz 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncanson KR, Talley NJ, Walker MM, et al. Food and functional dyspepsia: a systematic review. J Hum Nutr Diet 2018;31:390–407. [DOI] [PubMed] [Google Scholar]
- 13.van Tilburg MA, Felix CT. Diet and functional abdominal pain in children and adolescents. J Pediatr Gastroenterol Nutr 2013;57:141–8. [DOI] [PubMed] [Google Scholar]
- 14.McGowan A, Harer KN. Irritable Bowel Syndrome and Eating Disorders: A Burgeoning Concern in Gastrointestinal Clinics. Gastroenterol Clin North Am 2021;50:595–610. [DOI] [PubMed] [Google Scholar]
- 15.Werlang ME, Sim LA, Lebow JR, et al. Assessing for Eating Disorders: A Primer for Gastroenterologists. Am J Gastroenterol 2021;116:68–76. [DOI] [PubMed] [Google Scholar]
- 16.Peters JE, Basnayake C, Hebbard GS, et al. Prevalence of disordered eating in adults with gastrointestinal disorders: A systematic review. Neurogastroenterol Motil 2021:e14278. [DOI] [PubMed] [Google Scholar]
- 17.Burton Murray H, Jehangir A, Silvernale CJ, et al. Avoidant/restrictive food intake disorder symptoms are frequent in patients presenting for symptoms of gastroparesis. Neurogastroenterol Motil 2020;32:e13931. [DOI] [PubMed] [Google Scholar]
- 18.Murray HB, Bailey AP, Keshishian AC, et al. Prevalence and Characteristics of Avoidant/Restrictive Food Intake Disorder in Adult Neurogastroenterology Patients. Clin Gastroenterol Hepatol 2020;18:1995–2002 e1. [DOI] [PubMed] [Google Scholar]
- 19.Murray HB, Rao FU, Baker C, et al. Prevalence and Characteristics of Avoidant/Restrictive Food Intake Disorder in Pediatric Neurogastroenterology Patients. J Pediatr Gastroenterol Nutr 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton Murray H, Riddle M, Rao F, et al. Eating disorder symptoms, including avoidant/restrictive food intake disorder, in patients with disorders of gut-brain interaction. Neurogastroenterol Motil 2021:e14258. [DOI] [PubMed] [Google Scholar]
- 21.Chey WD, Hashash JG, Manning L, et al. AGA Clinical Practice Update on the Role of Diet in Irritable Bowel Syndrome: Expert Review. Gastroenterology 2022. [DOI] [PubMed] [Google Scholar]
- 22.Harer KN, Eswaran SL. Irritable Bowel Syndrome: Food as a Friend or Foe? Gastroenterol Clin North Am 2021;50:183–199. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 24.Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil 2016;28:1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chumpitazi BP, Weidler EM, Lu DY, et al. Self-Perceived Food Intolerances Are Common and Associated with Clinical Severity in Childhood Irritable Bowel Syndrome. J Acad Nutr Diet 2016;116:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes P, Corish C, O'Mahony E, et al. A dietary survey of patients with irritable bowel syndrome. J Hum Nutr Diet 2014;27 Suppl 2:36–47. [DOI] [PubMed] [Google Scholar]
- 27.Bohn L, Storsrud S, Tornblom H, et al. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol 2013;108:634–41. [DOI] [PubMed] [Google Scholar]
- 28.Simrén M, Månsson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001;63:108–15. [DOI] [PubMed] [Google Scholar]
- 29.El-Salhy M, Gundersen D. Diet in irritable bowel syndrome. Nutr J 2015;14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chey WD, Keefer L, Whelan K, et al. Behavioral and Diet Therapies in Integrated Care for Patients With Irritable Bowel Syndrome. Gastroenterology 2021;160:47–62. [DOI] [PubMed] [Google Scholar]
- 31.Kamal A, Pimentel M. Influence of Dietary Restriction on Irritable Bowel Syndrome. Am J Gastroenterol 2019;114:212–220. [DOI] [PubMed] [Google Scholar]
- 32.Bohn L, Storsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology 2015;149:1399–1407 e2. [DOI] [PubMed] [Google Scholar]
- 33.Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbone F, Van den Houte K, Besard L, et al. Diet or medication in primary care patients with IBS: the DOMINO study - a randomised trial supported by the Belgian Health Care Knowledge Centre (KCE Trials Programme) and the Rome Foundation Research Institute. Gut 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dionne J, Ford AC, Yuan Y, et al. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPs Diet in Treating Symptoms of Irritable Bowel Syndrome. Am J Gastroenterol 2018;113:1290–1300. [DOI] [PubMed] [Google Scholar]
- 36.Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013;145:320–8 e1-3. [DOI] [PubMed] [Google Scholar]
- 37.Rej A, Sanders DS, Shaw CC, et al. Efficacy and Acceptability of Dietary Therapies in Non-Constipated Irritable Bowel Syndrome: A Randomized Trial of Traditional Dietary Advice, the Low FODMAP Diet and the Gluten-Free Diet. Clin Gastroenterol Hepatol 2022. [DOI] [PubMed] [Google Scholar]
- 38.Parker TJ, Woolner JT, Prevost AT, et al. Irritable bowel syndrome: is the search for lactose intolerance justified? Eur J Gastroenterol Hepatol 2001;13:219–25. [DOI] [PubMed] [Google Scholar]
- 39.Duboc H, Latrache S, Nebunu N, et al. The Role of Diet in Functional Dyspepsia Management. Front Psychiatry 2020;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duncanson K, Burns G, Pryor J, et al. Mechanisms of Food-Induced Symptom Induction and Dietary Management in Functional Dyspepsia. Nutrients 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newlove-Delgado TV, Martin AE, Abbott RA, et al. Dietary interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev 2017;3:CD010972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boradyn KM, Przybylowicz KE, Jarocka-Cyrta E. Low FODMAP Diet Is Not Effective in Children with Functional Abdominal Pain: A Randomized Controlled Trial. Ann Nutr Metab 2020;76:334–344. [DOI] [PubMed] [Google Scholar]
- 43.Llanos-Chea A, Fasano A. Gluten and Functional Abdominal Pain Disorders in Children. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ockeloen LE, Deckers-Kocken JM. Short- and long-term effects of a lactose-restricted diet and probiotics in children with chronic abdominal pain: a retrospective study. Complement Ther Clin Pract 2012;18:81–4. [DOI] [PubMed] [Google Scholar]
- 45.Chumpitazi BP, Cope JL, Hollister EB, et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther 2015;42:418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nurko S, Benninga MA, Solari T, et al. Pediatric Aspects of Nutrition Interventions for Disorders of Gut-Brain Interaction. Am J Gastroenterol 2022;117:995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alfaro-Cruz L, Kaul I, Zhang Y, et al. Assessment of Quality and Readability of Internet Dietary Information on Irritable Bowel Syndrome. Clin Gastroenterol Hepatol 2019;17:566–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Keeffe M, Lomer MC. Who should deliver the low FODMAP diet and what educational methods are optimal: a review. J Gastroenterol Hepatol 2017;32 Suppl 1:23–26. [DOI] [PubMed] [Google Scholar]
- 49.Halmos EP, Gibson PR. Controversies and reality of the FODMAP diet for patients with irritable bowel syndrome. J Gastroenterol Hepatol 2019;34:1134–1142. [DOI] [PubMed] [Google Scholar]
- 50.Trott N, Aziz I, Rej A, et al. How Patients with IBS Use Low FODMAP Dietary Information Provided by General Practitioners and Gastroenterologists: A Qualitative Study. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuck CJ, Reed DE, Muir JG, et al. Implementation of the low FODMAP diet in functional gastrointestinal symptoms: A real-world experience. Neurogastroenterol Motil 2020;32:e13730. [DOI] [PubMed] [Google Scholar]
- 52.Harer KN, Jagielski CH, Riehl ME, et al. 272 – Avoidant/Restrictive Food Intake Disorder Among Adult Gastroenterology Behavioral Health Patients: Demographic and Clinical Characteristics. Gastroenterology 2019;156. [Google Scholar]
- 53.Quick VM, Byrd-Bredbenner C, Neumark-Sztainer D. Chronic illness and disordered eating: a discussion of the literature. Adv Nutr 2013;4:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conviser JH, Fisher SD, McColley SA. Are children with chronic illnesses requiring dietary therapy at risk for disordered eating or eating disorders? A systematic review. Int J Eat Disord 2018;51:187–213. [DOI] [PubMed] [Google Scholar]
- 55.Quick VM, McWilliams R, Byrd-Bredbenner C. Case-control study of disturbed eating behaviors and related psychographic characteristics in young adults with and without diet-related chronic health conditions. Eat Behav 2012;13:207–13. [DOI] [PubMed] [Google Scholar]
- 56.Staudacher HM, Ralph FSE, Irving PM, et al. Nutrient Intake, Diet Quality, and Diet Diversity in Irritable Bowel Syndrome and the Impact of the Low FODMAP Diet. J Acad Nutr Diet 2020;120:535–547. [DOI] [PubMed] [Google Scholar]
- 57.Staudacher HM, Kurien M, Whelan K. Nutritional implications of dietary interventions for managing gastrointestinal disorders. Curr Opin Gastroenterol 2018;34:105–111. [DOI] [PubMed] [Google Scholar]
- 58.O'Keeffe M, Jansen C, Martin L, et al. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol Motil 2018;30. [DOI] [PubMed] [Google Scholar]
- 59.Melchior C, Algera J, Colomier E, et al. Food Avoidance and Restriction in Irritable Bowel Syndrome: Relevance for Symptoms, Quality of Life and Nutrient Intake. Clin Gastroenterol Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- 60.Frieling T, Heise J, Krummen B, et al. Tolerability of FODMAP - reduced diet in irritable bowel syndrome - efficacy, adherence, and body weight course. Z Gastroenterol 2019;57:740–744. [DOI] [PubMed] [Google Scholar]
- 61.Casellas F, Aparici A, Perez MJ, et al. Perception of lactose intolerance impairs health-related quality of life. Eur J Clin Nutr 2016;70:1068–72. [DOI] [PubMed] [Google Scholar]
- 62.Silvester JA, Weiten D, Graff LA, et al. Living gluten-free: adherence, knowledge, lifestyle adaptations and feelings towards a gluten-free diet. J Hum Nutr Diet 2016;29:374–82. [DOI] [PubMed] [Google Scholar]
- 63.Guadagnoli L, Mutlu EA, Doerfler B, et al. Food-related quality of life in patients with inflammatory bowel disease and irritable bowel syndrome. Qual Life Res 2019;28:2195–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Satherley R, Howard R, Higgs S. Disordered eating practices in gastrointestinal disorders. Appetite 2015;84:240–50. [DOI] [PubMed] [Google Scholar]
- 65.Burton Murray H, Staller K, Kuo B. Eating disorders: Understanding their symptoms, mechanisms, and relevance to gastrointestinal functional and motility disorders. In: Rao S, Parkman HP, McCallum R, editors. Handbook of Gastrointestinal Motility and Functional Disorders. Thorophare, NJ: SLACK Incorporated; In press. [Google Scholar]
- 66.Santonicola A, Siniscalchi M, Capone P, et al. Prevalence of functional dyspepsia and its subgroups in patients with eating disorders. World J Gastroenterol 2012;18:4379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boyd C, Abraham S, Kellow J. Appearance and disappearance of functional gastrointestinal disorders in patients with eating disorders. Neurogastroenterol Motil 2010;22:1279–83. [DOI] [PubMed] [Google Scholar]
- 68.Murray HB, Kuo B, Eddy KT, et al. Disorders of gut-brain interaction common among outpatients with eating disorders including avoidant/restrictive food intake disorder. Int J Eat Disord 2021;54:952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Archer E, Marlow ML, Lavie CJ. Controversy and debate: Memory-Based Methods Paper 1: the fatal flaws of food frequency questionnaires and other memory-based dietary assessment methods. J Clin Epidemiol 2018;104:113–124. [DOI] [PubMed] [Google Scholar]
- 70.Murray HB, Doerfler B, Harer KN, et al. Psychological considerations in the dietary management of patients with DGBI. Am J Gastroenterol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]


