Abstract
Introduction
Brain tumors remain especially challenging to treat due to the presence of the blood–brain barrier. The unique biophysical properties of nanomaterials enable access to the tumor environment with minimally invasive injection methods such as intranasal and systemic delivery.
Methods
In this review, we will discuss approaches taken in NP delivery to brain tumors in preclinical neuro-oncology studies and ongoing clinical studies.
Results
Despite recent development of many promising nanoparticle systems to modulate immunologic function in the preclinical realm, clinical work with nanoparticles in malignant brain tumors has largely focused on imaging, chemotherapy, thermotherapy and radiation.
Conclusion
Review of early preclinical studies and clinical trials provides foundational safety, feasibility and toxicology data that can usher a new wave of nanotherapeutics in application of immunotherapy and translational oncology for patients with brain tumors.
Keywords: Nanoparticles, Immunotherapy, Biomodulation, Clinical trials
Introduction
Brain tumors remain especially challenging to treat due to both anatomic and intrinsic factors [1, 2]. First, their location behind the blood–brain barrier (BBB) makes pharmacologic treatment challenging. Conventional therapies are primarily confined to local control options such as surgery and radiation [1, 2]. However, surgery alone is often not sufficient to cure these tumors and can cause damage to normal tissue architecture, while radiotherapy carries risks of radiation necrosis, vasculopathy, secondary malignancy, and adverse affects on cognition [1, 2]. Meanwhile chemotherapy has variable effectivity. Temozolomide, while standard therapy for glioblastomas (GBMs) in adults, maintains only ~ 20% of its blood concentration in the CNS [3]. Moreover, temozolomide is associated with systemic side effects, and has not been shown to demonstrably provide benefit against many glioma subtypes (e.g. pediatric high-grade glioma) [4, 5]. Thus, new therapies that overcome delivery limitations are paramount.
Immune therapies have produced impressive clinical benefits in the face of many treatment-resistant tumors [6–8]. However, immunologic treatment of brain tumors is complicated by barriers to delivery and their uniquely immune suppressive nature [9, 10], which have demonstrated an ability to withstand robust combination therapy with traditional chemotherapy, radiation therapy, surgery, and even newer immune therapies [11, 12]. Future treatment of brain tumors will require therapies that can initiate potent antitumor immune responses and eliminate the immunologic resistance of the tumor microenvironment (TME).
Nanotechnology may be a solution to both problems. Nanomaterials provide substantial flexibility of engineering to overcome traditional drug delivery barriers, and enable enhanced detection, delivery and treatment in a myriad of disease applications [13–17]. Their ability to access cancers through the enhanced permeability and retention effect has led to their use as modulators of pharmacokinetics for conventional drugs. Nanomaterials have also been leveraged to maximize particle delivery through the BBB [18–20]. Nanomaterials are particularly attractive in the setting of immune therapies. While increasing sophistication of engineering is enabling nanoparticles to deliver drugs with increasing efficiency, nanomaterials can also be engineered very simply for uptake by immune cells [21, 22]. Despite this promise, a dearth of cross-disciplinary expertise and concerns about their in vivo reactivity and toxicology have stunted the number of new nanoparticle (NP) technologies that have been actively translated into human use [23–26].
In this review, we will discuss approaches taken in NP delivery to treat brain tumors with a special focus on cell targets and injection methods (Fig. 1). We will also review the NPs currently used in human brain tumor trials. These data will provide foundational safety, feasibility and toxicology data that may usher a new wave of nanotherapeutics in application of immunotherapy and translational oncology.
Fig. 1.
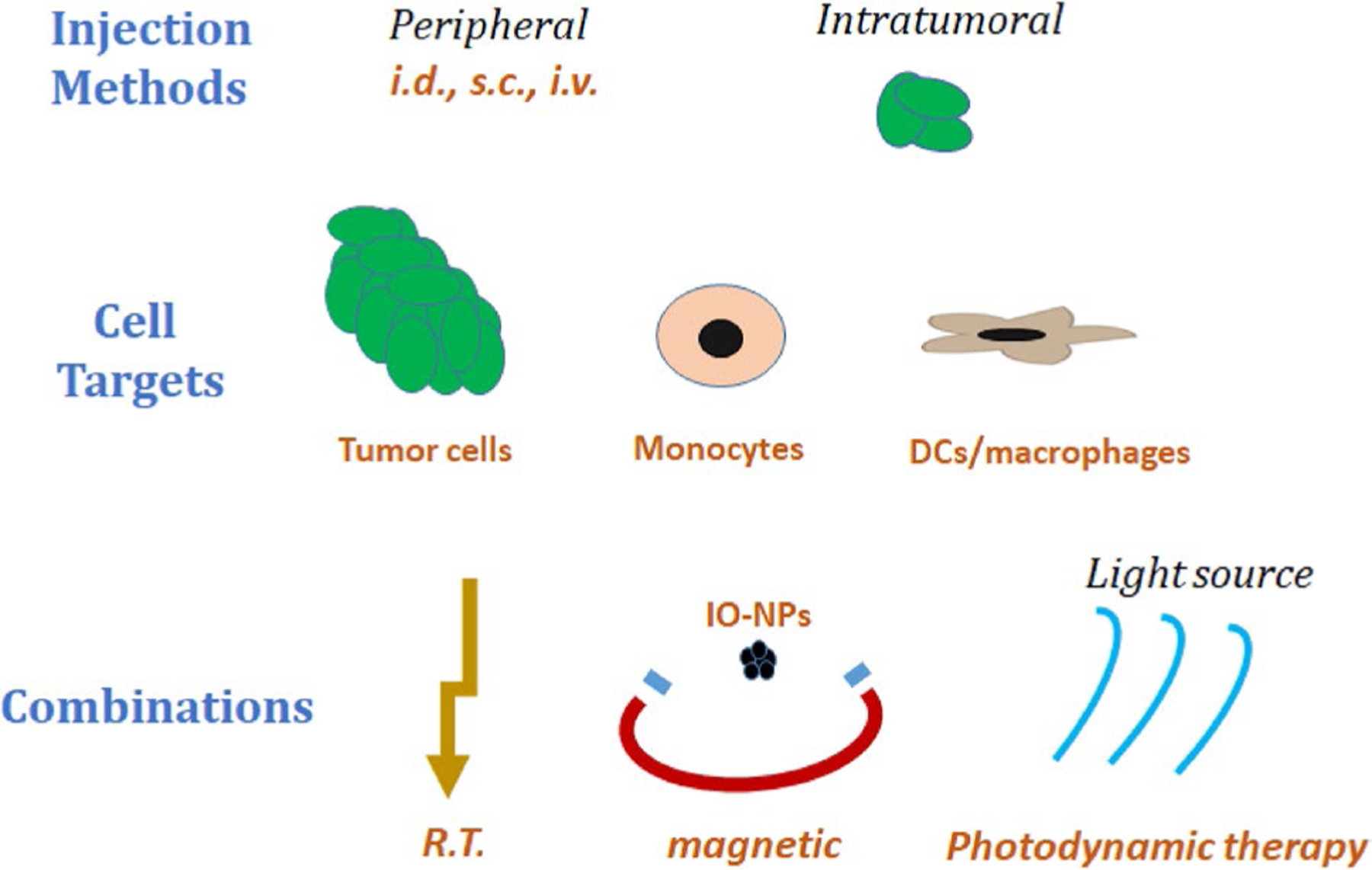
Summary of nanoparticle applications for treatment of brain tumors. Previous clinical work utilized systemic or intratumoral injections to deliver nanoparticles with direct cytotoxic activity on tumor cells either alone or in combination with radiation or magnetic hyperthermia. New preclinical avenues include intranasal nanoparticle delivery, the targeting of immune cells both inside the tumor and in the periphery, and photodynamic therapy to specifically mediate cell death at the tumor site. i.d. intradermal, s.c. subcutaneous, i.v. intravenous, r.t. radiotherapy, IO-NP iron-oxide nanoparticles
Targeting approaches
NP based therapies have been designed for a variety of applications, reflected best in the diversity of their target cells. While the first NP therapies attempted to deliver chemotherapy directly to tumor cells to initiate cytotoxic effects, more recent approaches have targeted nanomaterials to peritumoral immune cells to modify the TME or enable application of heat to the entire tumor with photodynamic therapy, radiation therapy, or magnetic hyperthermia. More recent immunotherapy based approaches do not require NP passage across the BBB, but rather initiate peripheral education and activation of T cells that can then access the TME for induction of anti-tumor immunity. We begin this review with a discussion of each approach.
Tumor targeting NPs
Direct tumoricidal therapies must overcome the BBB to act specifically on tumor cells without toxicity to normal tissue. This has traditionally been achieved with synthetic NP components. For example, encapsulation of hydrophopic molecules (i.e. camptothecin) inside PLGA NPs increases delivery to brain tumors ten-fold leading to a survival benefit in syngeneic GL261 murine tumors [27]. More recent approaches utilize biomimetic proteolipid NPs, which utilize glioma cell membrane proteins to penetrate the BBB [28]. In one study, this penetration enabled photodynamic therapy leading to a ~ 94% inhibition of tumor growth in an orthotopic model of C6 glioma [28].
Other NPs can be surface modified to enhance targeting to specific cells. For example, coating particles with granzymeb enabled superparamagnetic iron oxide NPs (SPIONs) to infiltrate tumors by binding to Hsp70 [29]. These particles initiated tumor cell death more effectively than granzyme-b alone, leading to prolonged survival in intracranial tumor models for U87 and H1339 [29]. Likewise, coating particles with a peptide recognized by PD-L1 enables NP localization to PD-L1-expressing glioma cells, simultaneously delivering payloads while blocking a critical immunoregulatory axis [30].
External-beam radiotherapy can be applied to further increase particle accumulation at the tumor site. For example, intracranial radiation before intravenous injection of iRGD-conjugated solid lipid-NPs loaded with PD-L1 and EGFR siRNA led to improved NP uptake in tumors and enhanced survival in GL261 preclinical models [31]. Alternatively, photodynamic therapy can also be harnessed to selectively activate NPs in desired locations. For example, administration of non-targeted indocyanine green-loaded phospholipid NPs produces therapeutic effects only in the areas that are exposed to near-IR light [32]. In one study, local application of photodynamic therapy in the TME increased infiltration of T cells and macrophages and increased expression of HSP70, leading to inhibition of tumor growth and extended survival in a rat 9L GBM [32]. This antitumor effect was absent in immunocompromised mice and was achieved without engineering NPs specifically to target tumors [32]. These “up-conversion” NPs nonspecifically accumulate, but are activated in the presence of photons to deliver photothermal therapy only inside tumors [33]. Although promising in preclinical models, photodynamic therapy faces challenges for clinical implementation due to the limited penetration depth of light through a thick tissue like the human skull. Further work may be necessary to specifically activate energy generation among particles through such a light-impenetrable material.
Targeting peri‑tumoral immune cells
NPs can also be delivered to target peri-tumoral immune cells that are known to suppress immune activation, such as M2 macrophages. In one study, particles with a PbAE-mRNA polyplex core coated with PGA-Di-mannose to target M206 on macrophages were engineered to deliver mRNA encoding interferon regulatory factor-5 (IRF-5) and IKKβ (M1-polarizing transcription factors) [34]. Delivery of IVT-derived mRNA encoding these transcription factors was utilized to convert tumor associated macrophages to a pro-inflammatory M1 phenotype [34]. Although this treatment had limited effect as a monotherapy, NP-medi-ated delivery of these transcription factors elicited impressive tumor regression in an established brain tumor model when combined with radiation [34]. Similarly, albumin NPs containing transferrin receptor-binding peptide (T12) with mannose-targeting receptors crossed the BBB and converted M2 macrophages to an M1 phenotype, leading to slightly extended survival in intracranial models for U87 and GL261 [35]. Others have utilized inherent effects of particle components to modify macrophage function. For example, gold-NPs with a polypeptide coating may activate peritumoral microglia and astrocytes to “wall off” tumor growth, effectively exploiting a mechanism utilized by microglia to protect the CNS from infection/inflammation [36]. NP formulations encapsulating chemotherapy (e.g. Nano-Dox) and magnetic NPs in combination with radiotherapy have also been shown to act directly on peritumoral cells such as M2 macrophages/MDSCs in the TME, leading to enhanced efficacy [37, 38].
Combination therapies
These TME modulators can reduce tumor growth as monotherapies but can also be applied to create a TME conducive to systemic immune attack. For example, lipid-NPs coated with the tumor-targeting peptide iRGD encapsulating both a phosphoinositide 3-kinase (PI3K) inhibitor to antagonize regulatory tumor cell populations and an α-GalCer to activate T cells synergistically reprogrammed the TME of intracranial tumors enabling adoptive cellular therapy treatments to prolong survival by ~ 50% in preclinical models [13]. In a similar approach, delivery of antisense oligonucleotides to TGF-beta via systemically administered polybutyl cyanoacrylate NPs coated in polysorbate-80 reduced TGF-beta production in tumors and facilitated improved response to whole tumor cell vaccines [39]. Likewise, gold-NPs enhance the effects of both chemotherapy with doxorubicin and immunotherapy with PD-L1 checkpoint inhibition [40].
More sophisticated multimodal particles have incorporated both targeting and imaging capabilities using a single particle. One group reported significantly improved survival in murine GL261 models using multimodal particles incorporating IONPs for MRI imaging, angiopep for BBB penetration/glioma cell targeting, siTGF-beta for immune modulation and temozolomide for tumor killing [41].
Targeting peripheral immune cells outside the TME
Using NPs to target peripheral cells that cross the BBB can be another avenue to indirectly deliver therapy across the BBB. Systemic targeting of peripheral immune cells outside the TME avoids barriers of intratumoral delivery. Our group has leveraged this approach using systemic non-targeted cationic liposomes bearing mRNA encoding tumor antigens [21]. We have shown that these tumor mRNA loaded NPs mediate potent antitumor adaptive immune responses against intracranial tumors [21], and may unlock effects of immune checkpoint inhibitors [22]. Other groups utilized similar approaches to demonstrate that liposomal delivery of microRNAs targeting the immune modulator STAT3 in monocytes/macrophages, achieves antitumor efficacy in mice and activity in canines [42]. Alternatively, subcutaneous administration of NP loaded DCs can also achieve tumor rejection. HSP-70-SPIONs within tumor lysate loaded DCs delayed tumor progression and increased survival in intracranial models for murine C6 glioma [43] while NP loaded DCs bearing grp170/neuritin peptide induced therapeutic levels of cytotoxic T cells [44].
Routes of administration
Although the simplest delivery method of any drug is direct injection into tumors, the unique biophysical properties of nanomaterials have also enabled access to the tumor environment with less invasive injection methods including intranasal and systemic delivery.
Intra‑tumoral injection
Direct intratumoral injection of NPs can act at the disease site and remains superior for therapeutics that require high concentrations of particles in tumors. Most of these therapies seek to induce direct tumor killing. For example, direct injection of iron-oxide (IO) NPs produced by magnetotactic bacteria enables delivery of IONPs needed for tumor heating with alternating magnetic fields leading to inhibition of glioma growth in a U87 model [45]. Direct cytotoxicity can also be combined with immune modulation for prevention of tumor recurrence. This is evidenced by convection-enhanced delivery of lipophilic NPs delivering both Rh-188 with a CXCR4-blocking antibody [46] or direct injection of HDL-mimicking nanodiscs delivering both chemotherapy and immunotherapy with TLR-9 agonists [47]. Direct injection may limit systemic inflammatory response, and secondary side effects, and has been prioritized for virus-like particles derived from cowpea mosaic virus [48]. Although direct injection offers specification of an anatomic target, even greater precision can be achieved by engineering NPs with components to target specific cell types. For glial tumors, this can be achieved by modifying NPs to enhance binding to fibroblast growth factor-inducible 14 (Fn14R) in GBMs [49].
Intranasal
Intranasal injection has great potential for semi-local delivery but has not been utilized as frequently for therapeutic administration. While studies remain few, results appear promising. In one study, intranasal administration of chitosan-siGal-1 NPs produced survival benefits in glioma models by promoting an M1 phenotype in the tumor microenvironment (TME), enabling synergistic antitumor responses when coupled with either temozolomide or immunotherapy with dendritic cell vaccines and PD-1 checkpoint blockade [50, 51]. These early successes warrant further evaluation and utilization in future work.
Systemic delivery
Systemic delivery requires that therapies utilize active or passive uptake in the desired location. For therapies that act on the TME, this requires that particles cross the BBB and, in many cases, specifically infiltrate the tumor. Crossing a BBB composed of endothelial tight junctions is not easily achieved. While specific cell types can pass across the BBB through natural mechanisms, exploiting these can be difficult [52–54]. Since small lipophilic molecules may diffuse across the BBB, nanomaterials can be designed to penetrate or be engineered to cross [20, 55]. Alternatively, since tumors actively disrupt the BBB which is further compromised by standard of care radiotherapy, nanomaterials have preferential advantages in localizing to tumors [56, 57]. NPs can further exploit the leaky capillaries in tumors via the enhanced permeability and retention (EPR) effect, which enables NP retention in the TME [55–57]. Once in the TME, particles can deliver therapeutics to specific cell types based on their surface ligands and physical properties [55–57]. Thus, new nanomaterial designs can leverage these properties to preferentially target tumor cells, or peritumoral immune cells, after systemic administration.
Clinical trials
Clinical evaluation of NP based treatments for GBM has focused predominantly on direct and systemic administrations in adult patients. Despite recent development of many NP systems to modulate immune function in the preclinical realm, clinical work with NPs in GBM has largely focused on imaging, chemotherapy, thermotherapy and radiation. Here, we have grouped these studies based on injection method, treatment modality, and combination approaches, and summarized their results (Table 1).
Table 1.
Summary of clinical trials evaluating nanoparticles for treatment of brain tumors
| Citation | Particle | Delivery | Mechanism | Phase | Result |
|---|---|---|---|---|---|
| Young et al. [60] | Polymeric magnetite NPs encapsulating temozolomide | CED | Chemotherapy | Large Animal Studies | 7/10 canines displayed particle distribution within tumor. Potential clinical improvement in 2 animals |
| Maier-Hauff et al. [62] | Aminosilane coated iron-oxide NPs | Direct Injection | Thermotherapy | Phase I | All patients tolerated the infusion of magnetic nanoparticles and subsequent thermotherapy without complications. |
| Maier-Hauff et al. [63] | Aminosilane coated iron-oxide NPs | Direct Injection | Thermotherapy | Phase II | Median overall survival for recurrent (59/66) GBM patients was 13.4 months following first recurrence |
| Grauer et al. [64] | Aminosilane coated iron-oxide NPs | Coated resection cavity | Thermotherapy | Phase I | Evidence of an inflammatory reaction by histology Prolonged survival in 2/6 patients; 4/6 required surgical removal of particles |
| Schmid et al. [67] | Nab-paclitaxel | i.v. | Chemotherapy | Phase III | No significant survival difference seen between nab-paclitacel alone or nab-paclitacel plus atezolizumab groups in patients with brain metastasis |
| Enochs et al. [69] | Dextran-coated USPIO | i.v. | Imaging enhancement | Part of larger Phase III | USPIO enhancement increased gradually, peaking at 24 h, and remained sharp; distinct from gadolinium-based enhancement |
| Hamilton et al. [75] | Ferumoxytol | i.v. | Imaging enhancement | Pilot Study | Meningiomas with abundant TAMs did not show improved USPIO enhancement |
| Barajas et al. [76] | Ferumoxytol | i.v. | Imaging enhancement | Retrospective analysis | Study of 45 GBM patients demonstrates mismatch between USPIO-and Gd-enhancement to effectively discriminate progression from pseudoprogression |
| \Verry et al. [78] | AGuIX® (Polysiloxane matrix and gadolinium chelates based NP) | i.v. | Radiosensitizer and imaging enhancement | Phase I | Pending |
Direct injection of chemotherapy loaded NPs
NP encapsulation can serve to increase drug penetration and delivery to tumor. Several delivery methods have been explored for increasing local drug concentrations, including convection enhanced delivery (CED) of liposomal chemotherapeutic formulations [58, 59]. CED is a minimally invasive technique for increasing local drug concentration through direct tumor cannulation and the application of continuous positive pressure, which can be achieved via syringe pump [59]. Despite the ability to increase local concentrations of chemotherapeutic agents, more information is needed regarding the distribution patterns and tumor coverage of NPs injected systemically versus via CED. In one small trial, ten canines with spontaneous gliomas were enrolled and treated with polymeric magnetite NPs (PMNPs) encapsulating temozolomide administered through image-guided CED [60]. Nine of the animals received the infusion without incident and 70% showed particle distribution within the tumor after delivery, suggesting CED can accurately target particles to sites of tumor [60]. Two animals showed clinical improvement, with one of them still living ~ 2 years after treatment [60]. In addition, analysis of the tumor microenvironment demonstrated necrosis, hemorrhage, and a substantial infiltration of phagocytic gitter cells [60]. As distribution of particles injected via CED is unpredictable and accurate tumor coverage is requisite for optimal response, similar methods for improving and assessing infusion accuracy should be applied in future clinical trials.
Direct injection of NPs for thermotherapy
Intratumorally injected magnetic-NPs can be used as a form of thermotherapy by heating injected particles in an alternating magnetic field. To ensure thermotoxicity is restricted to tumor tissue, PET-CT with an 18F-labeled amino acid tracer has been shown to be useful for defining tumor volume and guiding thermotherapy [61]. In addition, radioactive PET-based imaging may reveal additional tumor tissue beyond that detected by MRI. In another early-phase thermotherapy study investigating the feasibility and safety of aminosilane coated iron-oxide NPs in recurrent GBM, 14 patients were administered the therapy followed by radiotherapy [62]. Overall, the treatment was found to be well-tolerated by all patients and produced some evidence of local tumor control [62]. Median survival was 14.5 months and compared favorably to historical controls, and one patient remained in remission 28 months after treatment [62]. This initial trial was followed by a phase II study evaluating thermotherapy in a larger cohort of 66 patients [63]. The treatment was again fairly well-tolerated and an increase in time-to-recurrence was noted [63]. Among those patients treated for recurrent GBM (59 of 66), median survival following initial tumor recurrence was 13.4 months (compared to 6.2 months for a historic control cohort), and 23.2 months for median overall survival from initial diagnosis [63]. While 41% of patients had received some form of therapy prior to trial enrollment, only tumor volume at the initiation of the study was found to correlate with survival time [63]. It remains unclear whether thermodamage-induced inflammation was the primary mechanism for response in the prolonged survival outcomes observed relative to historic controls.
In a recent human trial, the “NanoPaste” treatment with SPIO nanoparticles was evaluated in 6 patients with a diagnosis of recurrent GBM [64]. In this trial, patients had their resection cavities coated with SPIO-NPs, followed by six 1-hour hyperthermia sessions administered through an alternating magnetic field [64]. Imaging, histopathology, and flow cytometric analysis revealed evidence of an inflammatory reaction seen by tumor flare reaction, prominent macrophage infiltration with NP uptake, increased CD3 + T cells, increased proportion of IFN-γ in T cells, and upregulation of HLA-DR and PD-L1 on myeloid cells and microglia in TME [64]. Although two patients had prolonged responses greater than 23 months, four required surgery to remove deposited NPs [64].
Systemic injection of chemotherapy loaded NPs
A NP based paclitaxel (nab-paclitaxel) treatment, which showed a significant improvement in survival of patients with primary breast cancer when compared with solvent-based paclitaxel (NCT01583426), has also been tested in brain tumors [65–67]. Treatment of advanced solid tumors with cycles of nab-paclitaxel in combination with lapatinib in a phase I clinical trial (NCT00313599) was fairly tolerable [65, 66]. Intravenously administered nab-paclitaxel was then combined with PD-L1 blockade (atezolizumab) for treatment of triple-negative breast cancer, including patients with brain metastasis [67]. An improvement in progression-free survival was noted with the addition of atezolizumab to standard nab-paclitaxel treatment (7.2 months vs. 5.5 months) when considering all treated patients. However, no significant survival difference was achieved in patients with brain metastasis in the intention-to-treat population [67]. Although preclinical evidence suggests that albumin-bound paclitaxel NPs may localize to tumors via enhanced transportation across endothelial cells, next generation therapeutics should include methods to evaluate these effects in human brain tumor patients [68].
Systemic injection of NPs for bio‑imaging
NPs may currently have their most significant role in imaging. There is preclinical evidence that brain tumor margins could be enhanced on MRI from systemically administered ultra-small superparamagnetic iron oxide (USPIO) [69]. USPIO enhancement has been found to increase gradually, peak at 24 h, and remain sharp following administration [69]. This is distinct from gadolinium (Gd)-enhancement, which is immediate and begins decreasing within hours after administration [69]. Ferumoxytol is a clinical USPIO formulation that has been used extensively as an alternative to Gd-based contrast and found to be generally well-tolerated in multi-center review [70]. USPIO-enhanced MRI (FeMRI) has been suggested to function as a non-invasive imaging modality for monitoring macrophage infiltration in the CNS and atherosclerotic plaques [71]. As expected, FeMRI enhancement in CNS lesions was found to differ from that observed with GdMRI [72]. Additionally, FeMRI revealed increased heterogeneity after ischemic brain injury, leading to a suggestion for its use to guide initiation of anti-inflammatory therapy [73].
Whether FeMRI functions to delineate normal tissue from tumor tissue based on leaky vasculature or the presence of macrophages remains unclear. Uptake of USPIO depends highly on context and may be influenced by whether a lesion is metastatic or primary and by the specific tumor type. For example, systemically injected USPIO was recently shown to aid in assessing inflammatory brain lesions and found to be taken up by astrocytes and TAMs (but not tumors) in preclinical tumor xenograft models [74]. Conversely, TAM-rich meningiomas did not show improved USPIO enhancement and tumor vasculature alterations may better associate with FeMRI signal in these tumors [75].
In retrospective analysis of 45 GBM patients, the mismatch between USPIO- and Gd-enhancement was found to effectively discriminate between true disease recurrence and pseudoprogression [76]. In patients with IDH-1 wildtype GBMs, increased mismatch ratios from FeMRI:GdMRI contrast signaled the onset of pseudoprogression and not recurrence; this pattern was reversed for patients with isocitrate dehydrogenase (IDH)-mutant GBMs [76].
Since FeMRI contrast enhancement may be affected by both macrophage infiltration and vascular changes, treatment-specific imaging changes should be systematically investigated. Unfortunately, a clinical trial investigating the effect of anti-VEGF therapy with bevacizumab on MR imaging with gadolinium and ferumoxytol was recently terminated due to insufficient enrollment (NCT00769093). Given the effects of immune infiltrates on contrast enhancement, the effect of checkpoint inhibitors and other immunotherapies on FeMRI and GdMRI enhancement should also be investigated to establish how immune modulation alters enhancement patterns on MRI.
Systemic injection of radiotherapy sensitizing NPs
In addition to NPs as contrast MRI agents and delivery vehicles for chemotherapy, radiosensitization is another potential NP application in brain tumors. While preclinical data suggests potential [77], clinical trials for NP-based radiosensitizers in brain tumors are ongoing. To this end, the NANO-RAD trial (NCT02820454) utilizes a gadolinium-based theranostic agent (AGuIX) in combination with whole brain radiation therapy [78]. This intravenously administered particle has a short half life of around 2.5 h in non-human primates [79] but preferentially accumulates in tumors via the EPR effect [78]. A phase-II trial is also planned (NCT03818386), as well as a trial coupling AGuIX with stereotactic radiotherapy (NCT04094077).
Future trials
Clinical trials evaluating NP based applications for brain tumors have so far focused on enhanced imaging contrast, chemotherapy delivery, thermotherapy and radiosensitization. These studies have largely considered tolerability and overall antitumor effects of these therapies, with less focus on particle pharmacokinetics and effects on intratumoral and peripheral immunity. Future studies should include therapies that combine multiple modalities and modulate intratumoral immunity or produce systemic immune responses. These may include particles specially formulated for active targeting to tumor, for example with IL-13 receptor or anti-EGFRvIII moieties on the NP surface [80–82], or those specifically targeted to immune cells outside the tumor. In each of these studies, it will be important to assess particle localization in human patients and evaluate the effects of each therapy on the intratumoral and systemic immune response.
Conclusions
The treatment of intracranial tumors is complicated by poor BBB penetration of many therapeutic agents and by an inability to identify rogue tumor cells at infiltrating margins. NPs are particularly suited to address these limitations as they display enhanced tumor permeation and retention, enable increased drug delivery, act as contrast agents on MRI, and can sensitize tumors to radiotherapy. While clinical trials exploring these agents have been used in several applications, few have been successfully translated as treatment mainstays. However, given the boon of preclinical data and foundational feasibility and safety/toxicology features, newer studies can be rationally designed to unlock therapeutic activity in brain tumors. The difficulty in accessing the CNS from conventional methodologies may be addressed with new engineering designs allowing innovative NP formulations to tackle grand challenges in neuro-oncology.
Funding
A.J.G is supported by the National Institutes of Health (NCI F30-CA228280). K.A.D. is supported by the National Institutes of Health (NCI F30-CA221345). D.A.M. is supported by the National Institutes of Health (NCI R01CA195563, 1R01-CA175517). E.J.S. is supported by the Rally Foundation, CureSearch for Children’s Cancer, Florida Department of Health (Bankhead-Coley and Live Like Bella Cancer Research Programs), US Department of Defense (W81XWH-17-1-0510) National Institutes of Health (NCI K08CA199224) and St. Baldrick’s Foundation (Hannah’s Heroes Scholar Award).
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain Radiation Oncology T G, National Cancer Institute of Canada Clinical Trials G (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. 10.1016/S1470-2045(09)70025-7 [DOI] [PubMed] [Google Scholar]
- 3.Schreck KC, Grossman SA (2018) Role of temozolomide in the treatment of cancers involving the central nervous system. Oncology (Williston Park) 32:555–560 [PubMed] [Google Scholar]
- 4.Wolff JE, Finlay JL (2004) High-dose chemotherapy in childhood brain tumors. Onkologie 27:239–245. 10.1159/000077973 [DOI] [PubMed] [Google Scholar]
- 5.Cohen KJ, Pollack IF, Zhou T, Buxton A, Holmes EJ, Burger PC, Brat DJ, Rosenblum MK, Hamilton RL, Lavey RS, Heideman RL (2011) Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol 13:317–323. 10.1093/neuonc/noq191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC, Investigators K- (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME (2011) Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17:4550–4557. 10.1158/1078-0432.CCR-11-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter DL, Levine BL, Kalos M, Bagg A, June CH (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365:725–733. 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H (2011) The brain tumor microenvironment. Glia 59:1169–1180. 10.1002/glia.21136 [DOI] [PubMed] [Google Scholar]
- 10.Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66:605–612. 10.1158/0008-5472.CAN-05-4005 [DOI] [PubMed] [Google Scholar]
- 11.Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, Ashby L, Mechtler L, Goldlust SA, Iwamoto F, Drappatz J, O’Rourke DM, Wong M, Hamilton MG, Finocchiaro G, Perry J, Wick W, Green J, He Y, Turner CD, Yellin MJ, Keler T, Davis TA, Stupp R, Sampson JH, investigators AIt (2017) Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol 18:1373–1385. 10.1016/S1470-2045(17)30517-X [DOI] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, Stephan SB, Ene CI, Smith TT, Holland EC, Stephan MT (2018) Nanoparticles that reshape the tumor milieu create a therapeutic window for effective T-cell therapy in solid malignancies. Cancer Res 78:3718–3730. 10.1158/0008-5472.CAN-18-0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83:761–769. 10.1038/sj.clpt.6100400 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Li N, Suh H, Irvine DJ (2018) Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun 9:6. 10.1038/s41467-017-02251-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Jiang W, Luo K, Song H, Lan F, Wu Y, Gu Z (2013) Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 3:595–615. 10.7150/thno.5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toraya-Brown S, Sheen MR, Zhang P, Chen L, Baird JR, Demidenko E, Turk MJ, Hoopes PJ, Conejo-Garcia JR, Fiering S (2014) Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors. Nanomedicine 10:1273–1285. 10.1016/j.nano.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva GA (2008) Nanotechnology approaches to crossing the blood–brain barrier and drug delivery to the CNS. BMC Neurosci 9(Suppl 3):S4. 10.1186/1471-2202-9-S3-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saraiva C, Praca C, Ferreira R, Santos T, Ferreira L, Bernardino L (2016) Nanoparticle-mediated brain drug delivery: overcoming blood-brain barrier to treat neurodegenerative diseases. J Cont Rel 235:34–47. 10.1016/j.jconrel.2016.05.044 [DOI] [PubMed] [Google Scholar]
- 20.Song Q, Song H, Xu J, Huang J, Hu M, Gu X, Chen J, Zheng G, Chen H, Gao X (2016) Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood–brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm 13:3976–3987. 10.1021/acs.molpharmaceut.6b00781 [DOI] [PubMed] [Google Scholar]
- 21.Sayour EJ, De Leon G, Pham C, Grippin A, Kemeny H, Chua J, Huang J, Sampson JH, Sanchez-Perez L, Flores C, Mitchell DA (2017) Systemic activation of antigen-presenting cells via RNA-loaded nanoparticles. Oncoimmunology 6:e1256527. 10.1080/2162402X.2016.1256527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sayour EJ, Grippin A, De Leon G, Stover B, Rahman M, Karachi A, Wummer B, Moore G, Castillo-Caro P, Fredenburg K, Sarkisian MR, Huang J, Deleyrolle LP, Sahay B, Carrera-Justiz S, Mendez-Gomez HR, Mitchell DA (2018) Personalized tumor RNA loaded lipid-nanoparticles prime the systemic and intratumoral milieu for response to cancer immunotherapy. Nano Lett 18:6195–6206. 10.1021/acs.nanolett.8b02179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adiseshaiah PP, Hall JB, McNeil SE (2010) Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2:99–112. 10.1002/wnan.66 [DOI] [PubMed] [Google Scholar]
- 24.Xue HY, Liu S, Wong HL (2014) Nanotoxicity: a key obstacle to clinical translation of siRNA-based nanomedicine. Nanomedicine (Lond) 9:295–312. 10.2217/nnm.13.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kedmi R, Ben-Arie N, Peer D (2010) The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials 31:6867–6875. 10.1016/j.biomaterials.2010.05.027 [DOI] [PubMed] [Google Scholar]
- 26.Grippin AJ, Sayour EJ, Mitchell DA (2017) Translational nanoparticle engineering for cancer vaccines. Oncoimmunology 6:e1290036. 10.1080/2162402X.2017.1290036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Householder KT, DiPerna DM, Chung EP, Wohlleb GM, Dhruv HD, Berens ME, Sirianni RW (2015) Intravenous delivery of camptothecin-loaded PLGA nanoparticles for the treatment of intracranial glioma. Int J Pharm 479:374–380. 10.1016/j.ijpharm.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 28.Jia Y, Wang X, Hu D, Wang P, Liu Q, Zhang X, Jiang J, Liu X, Sheng Z, Liu B, Zheng H (2019) Phototheranostics: active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano 13:386–398. 10.1021/acsnano.8b06556 [DOI] [PubMed] [Google Scholar]
- 29.Shevtsov M, Stangl S, Nikolaev B, Yakovleva L, Marchenko Y, Tagaeva R, Sievert W, Pitkin E, Mazur A, Tolstoy P, Galibin O, Ryzhov V, Steiger K, Smirnov O, Khachatryan W, Chester K, Multhoff G (2019) Granzyme B functionalized nanoparticles targeting membrane Hsp70-positive tumors for multimodal cancer theranostics. Small 15:e1900205. 10.1002/smll.201900205 [DOI] [PubMed] [Google Scholar]
- 30.Sun Z, Zhang Y, Cao D, Wang X, Yan X, Li H, Huang L, Qu X, Kong C, Qin H, Wang M, Xu W, Liang L (2018) PD-1/PD-L1 pathway and angiogenesis dual recognizable nanoparticles for enhancing chemotherapy of malignant cancer. Drug Deliv 25:1746–1755. 10.1080/10717544.2018.1509907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erel-Akbaba G, Carvalho LA, Tian T, Zinter M, Akbaba H, Obeid PJ, Chiocca EA, Weissleder R, Kantarci AG, Tannous BA (2019) Radiation-induced targeted nanoparticle-based gene delivery for brain tumor therapy. ACS Nano 13:4028–4040. 10.1021/acsnano.8b08177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata S, Shinozaki N, Suganami A, Ikegami S, Kinoshita Y, Hasegawa R, Kentaro H, Okamoto Y, Aoki I, Tamura Y, Iwadate Y (2019) Photo-immune therapy with liposomally formulated phospholipid-conjugated indocyanine green induces specific antitumor responses with heat shock protein-70 expression in a glioblastoma model. Oncotarget 10:175–183. 10.18632/oncotarget.26544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai YC, Vijayaraghavan P, Chiang WH, Chen HH, Liu TI, Shen MY, Omoto A, Kamimura M, Soga K, Chiu HC (2018) Targeted delivery of functionalized upconversion nanoparticles for externally triggered photothermal/photodynamic therapies of brain glioblastoma. Theranostics 8:1435–1448. 10.7150/thno.22482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, Holland EC, Stephan MT (2019) Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun 10:3974. 10.1038/s41467-019-11911-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao P, Wang Y, Kang X, Wu A, Yin W, Tang Y, Wang J, Zhang M, Duan Y, Huang Y (2018) Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophage-mediated immunotherapy. Chem Sci 9:2674–2689. 10.1039/c7sc04853j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxena T, Lyon JG, Pai SB, Pare D, Amero J, Karumbaiah L, Carroll SL, Gaupp E, Bellamkonda RV (2019) Engineering controlled peritumoral inflammation to constrain brain tumor growth. Adv Healthc Mater 8:e1801076. 10.1002/adhm.201801076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li TF, Li K, Wang C, Liu X, Wen Y, Xu YH, Zhang Q, Zhao QY, Shao M, Li YZ, Han M, Komatsu N, Zhao L, Chen X (2017) Harnessing the cross-talk between tumor cells and tumor-associated macrophages with a nano-drug for modulation of glioblastoma immune microenvironment. J Control Release 268:128–146. 10.1016/j.jconrel.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 38.Wu C, Muroski ME, Miska J, Lee-Chang C, Shen Y, Rashidi A, Zhang P, Xiao T, Han Y, Lopez-Rosas A, Cheng Y, Lesniak MS (2019) Repolarization of myeloid derived suppressor cells via magnetic nanoparticles to promote radiotherapy for glioma treatment. Nanomedicine 16:126–137. 10.1016/j.nano.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 39.Schneider T, Becker A, Ringe K, Reinhold A, Firsching R, Sabel BA (2008) Brain tumor therapy by combined vaccination and antisense oligonucleotide delivery with nanoparticles. J Neuroimmunol 195:21–27. 10.1016/j.jneuroim.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 40.Ruan S, Xie R, Qin L, Yu M, Xiao W, Hu C, Yu W, Qian Z, Ouyang L, He Q, Gao H (2019) Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with anti-PD-L1 antibody for improved glioma treatment. Nano Lett. 10.1021/acs.nanolett.9b03968 [DOI] [PubMed] [Google Scholar]
- 41.Qiao C, Yang J, Shen Q, Liu R, Li Y, Shi Y, Chen J, Shen Y, Xiao Z, Weng J, Zhang X (2018) Traceable nanoparticles with dual targeting and ROS response for RNAi-based immunochemotherapy of intracranial glioblastoma treatment. Adv Mater 30:e1705054. 10.1002/adma.201705054 [DOI] [PubMed] [Google Scholar]
- 42.Yaghi NK, Wei J, Hashimoto Y, Kong LY, Gabrusiewicz K, Nduom EK, Ling X, Huang N, Zhou S, Kerrigan BC, Levine JM, Fajt VR, Levine G, Porter BF, Marcusson EG, Tachikawa K, Chivukula P, Webb DC, Payne JE, Heimberger AB (2017) Immune modulatory nanoparticle therapeutics for intracerebral glioma. Neuro Oncol 19:372–382. 10.1093/neuonc/now198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shevtsov MA, Nikolaev BP, Yakovleva LY, Parr MA, Marchenko YY, Eliseev I, Yudenko A, Dobrodumov AV, Zlobina O, Zhakhov A, Ischenko AM, Pitkin E, Multhoff G (2015) 70-kDa heat shock protein coated magnetic nanocarriers as a nanovaccine for induction of anti-tumor immune response in experimental glioma. J Control Release 220:329–340. 10.1016/j.jconrel.2015.10.051 [DOI] [PubMed] [Google Scholar]
- 44.Yuan B, Shen H, Su T, Lin L, Chen T, Yang Z (2015) A novel nanoparticle containing neuritin peptide with grp170 induces a CTL response to inhibit tumor growth. J Neurooncol 125:23–32. 10.1007/s11060-015-1884-0 [DOI] [PubMed] [Google Scholar]
- 45.Alphandery E, Idbaih A, Adam C, Delattre JY, Schmitt C, Guyot F, Chebbi I (2017) Chains of magnetosomes with controlled endotoxin release and partial tumor occupation induce full destruction of intracranial U87-Luc glioma in mice under the application of an alternating magnetic field. J Control Release 262:259–272. 10.1016/j.jconrel.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 46.Sehedic D, Chourpa I, Tetaud C, Griveau A, Loussouarn C, Avril S, Legendre C, Lepareur N, Wion D, Hindre F, Davodeau F, Garcion E (2017) Locoregional confinement and major clinical benefit of (188)re-loaded CXCR4-targeted nanocarriers in an orthotopic human to mouse model of glioblastoma. Theranostics 7:4517–4536. 10.7150/thno.19403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadiyala P, Li D, Nunez FM, Altshuler D, Doherty R, Kuai R, Yu M, Kamran N, Edwards M, Moon JJ, Lowenstein PR, Castro MG, Schwendeman A (2019) High-density lipoprotein-mimicking nanodiscs for chemo-immunotherapy against glioblastoma multiforme. ACS Nano 13:1365–1384. 10.1021/acsnano.8b06842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerstetter-Fogle A, Shukla S, Wang C, Beiss V, Harris PLR, Sloan AE, Steinmetz NF (2019) Plant virus-like particle in situ vaccine for intracranial glioma immunotherapy. Cancers (Basel). 10.3390/cancers11040515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider CS, Perez JG, Cheng E, Zhang C, Mastorakos P,-Hanes J, Winkles JA, Woodworth GF, Kim AJ (2015) Minimizing the non-specific binding of nanoparticles to the brain enables active targeting of Fn14-positive glioblastoma cells. Biomaterials 42:42–51. 10.1016/j.biomaterials.2014.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Woensel M, Mathivet T, Wauthoz N, Rosiere R, Garg AD, Agostinis P, Mathieu V, Kiss R, Lefranc F, Boon L, Belmans J, Van Gool SW, Gerhardt H, Amighi K, De Vleeschouwer S (2017) Sensitization of glioblastoma tumor micro-environment to chemo- and immunotherapy by Galectin-1 intranasal knockdown strategy. Sci Rep 7:1217. 10.1038/s41598-017-01279-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Woensel M, Wauthoz N, Rosiere R, Mathieu V, Kiss R, Lefranc F, Steelant B, Dilissen E, Van Gool SW, Mathivet T, Gerhardt H, Amighi K, De Vleeschouwer S (2016) Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J Control Release 227:71–81. 10.1016/j.jconrel.2016.02.032 [DOI] [PubMed] [Google Scholar]
- 52.Mahad D, Callahan MK, Williams KA, Ubogu EE, Kivisakk P, Tucky B, Kidd G, Kingsbury GA, Chang A, Fox RJ, Mack M, Sniderman MB, Ravid R, Staugaitis SM, Stins MF, Ransohoff RM (2006) Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain 129:212–223. 10.1093/brain/awh655 [DOI] [PubMed] [Google Scholar]
- 53.Takeshita Y, Ransohoff RM (2012) Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol Rev 248:228–239. 10.1111/j.1600-065X.2012.01127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ransohoff RM, Kivisakk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3:569–581. 10.1038/nri1130 [DOI] [PubMed] [Google Scholar]
- 55.Sattiraju A, Xiong X, Pandya DN, Wadas TJ, Xuan A, Sun Y, Jung Y, Solingapuram Sai KK, Dorsey JF, Li KC, Mintz A (2017) Alpha particle enhanced blood brain/tumor barrier permeabilization in glioblastomas using integrin alpha-v beta-3 targeted liposomes. Mol Cancer Ther. 10.1158/1535-7163.MCT-16-0907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baumann BC, Kao GD, Mahmud A, Harada T, Swift J, Chapman C, Xu X, Discher DE, Dorsey JF (2013) Enhancing the efficacy of drug-loaded nanocarriers against brain tumors by targeted radiation therapy. Oncotarget 4:64–79. 10.18632/oncotarget.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou K, Bostrom M, Ek CJ, Li T, Xie C, Xu Y, Sun Y, Blomgren K, Zhu C (2017) Radiation induces progenitor cell death, microglia activation, and blood-brain barrier damage in the juvenile rat cerebellum. Sci Rep 7:46181. 10.1038/srep46181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis ME, Chen ZG, Shin DM (2008) Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 7:771–782. 10.1038/nrd2614 [DOI] [PubMed] [Google Scholar]
- 59.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH (1994) Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 91:2076–2080. 10.1073/pnas.91.6.2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young JS, Bernal G, Polster SP, Nunez L, Larsen GF, Mansour N, Podell M, Yamini B (2018) Convection-enhanced delivery of polymeric nanoparticles encapsulating chemotherapy in canines with spontaneous supratentorial tumors. World Neurosurg 117:e698–e704. 10.1016/j.wneu.2018.06.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plotkin M, Gneveckow U, Meier-Hauff K, Amthauer H, Feussner A, Denecke T, Gutberlet M, Jordan A, Felix R, Wust P (2006) 18F-FET PET for planning of thermotherapy using magnetic nanoparticles in recurrent glioblastoma. Int J Hyperthermia 22:319–325. 10.1080/02656730600734128 [DOI] [PubMed] [Google Scholar]
- 62.Maier-Hauff K, Rothe R, Scholz R, Gneveckow U, Wust P, Thiesen B, Feussner A, von Deimling A, Waldoefner N, Felix R, Jordan A (2007) Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol 81:53–60. 10.1007/s11060-006-9195-0 [DOI] [PubMed] [Google Scholar]
- 63.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A (2011) Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 103:317–324. 10.1007/s11060-010-0389-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grauer O, Jaber M, Hess K, Weckesser M, Schwindt W, Maring S, Wolfer J, Stummer W (2019) Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J Neurooncol 141:83–94. 10.1007/s11060-018-03005-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chien AJ, Illi JA, Ko AH, Korn WM, Fong L, Chen LM, Kashani-Sabet M, Ryan CJ, Rosenberg JE, Dubey S, Small EJ, Jahan TM, Hylton NM, Yeh BM, Huang Y, Koch KM, Moasser MM (2009) A phase I study of a 2-day lapatinib chemosensitization pulse preceding nanoparticle albumin-bound Paclitaxel for advanced solid malignancies. Clin Cancer Res 15:5569–5575. 10.1158/1078-0432.CCR-09-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Untch M, Jackisch C, Schneeweiss A, Conrad B, Aktas B, Denkert C, Eidtmann H, Wiebringhaus H, Kummel S, Hilfrich J, Warm M, Paepke S, Just M, Hanusch C, Hackmann J, Blohmer JU, Clemens M, Darb-Esfahani S, Schmitt WD, Dan Costa S, Gerber B, Engels K, Nekljudova V, Loibl S, von Minckwitz G, German Breast G (2016) Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol 17:345–356. 10.1016/S1470-2045(15)00542-2 [DOI] [PubMed] [Google Scholar]
- 67.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA, Investigators IMT (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121. 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 68.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, Noker P, Yao R, Labao E, Hawkins M, Soon-Shiong P (2006) Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 12:1317–1324. 10.1158/1078-0432.CCR-05-1634 [DOI] [PubMed] [Google Scholar]
- 69.Enochs WS, Harsh G, Hochberg F, Weissleder R (1999) Improved delineation of human brain tumors on MR images using a long-circulating, superparamagnetic iron oxide agent. J Magn Reson Imaging 9:228–232 [DOI] [PubMed] [Google Scholar]
- 70.Nguyen KL, Yoshida T, Kathuria-Prakash N, Zaki IH, Varallyay CG, Semple SI, Saouaf R, Rigsby CK, Stoumpos S, Whitehead KK, Griffin LM, Saloner D, Hope MD, Prince MR, Fogel MA, Schiebler ML, Roditi GH, Radjenovic A, Newby DE, Neuwelt EA, Bashir MR, Hu P, Finn JP (2019) Multicenter safety and practice for off-label diagnostic use of ferumoxytol in MRI. Radiology. 10.1148/radiol.2019190477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corot C, Petry KG, Trivedi R, Saleh A, Jonkmanns C, Le Bas JF, Blezer E, Rausch M, Brochet B, Foster-Gareau P, Baleriaux D, Gaillard S, Dousset V (2004) Macrophage imaging in central nervous system and in carotid atherosclerotic plaque using ultrasmall superparamagnetic iron oxide in magnetic resonance imaging. Invest Radiol 39:619–625. 10.1097/01.rli.0000135980.08491.33 [DOI] [PubMed] [Google Scholar]
- 72.Manninger SP, Muldoon LL, Nesbit G, Murillo T, Jacobs PM, Neuwelt EA (2005) An exploratory study of ferumoxtran-10 nanoparticles as a blood–brain barrier imaging agent targeting phagocytic cells in CNS inflammatory lesions. AJNR Am J Neuroradiol 26:2290–2300 [PMC free article] [PubMed] [Google Scholar]
- 73.Saleh A, Schroeter M, Ringelstein A, Hartung HP, Siebler M, Modder U, Jander S (2007) Iron oxide particle-enhanced MRI suggests variability of brain inflammation at early stages after ischemic stroke. Stroke 38:2733–2737. 10.1161/STROKEAHA.107.481788 [DOI] [PubMed] [Google Scholar]
- 74.McConnell HL, Schwartz DL, Richardson BE, Woltjer RL, Muldoon LL, Neuwelt EA (2016) Ferumoxytol nanoparticle uptake in brain during acute neuroinflammation is cell-specific. Nanomedicine 12:1535–1542. 10.1016/j.nano.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamilton BE, Woltjer RL, Prola-Netto J, Nesbit GM, Gahramanov S, Pham T, Wagner J, Neuwelt EA (2016) Ferumoxytol-enhanced MRI differentiation of meningioma from dural metastases: a pilot study with immunohistochemical observations. J Neurooncol 129:301–309. 10.1007/s11060-016-2175-0 [DOI] [PubMed] [Google Scholar]
- 76.Barajas RF, Hamilton BE, Schwartz D, McConnell HL, Pettersson DR, Horvath A, Szidonya L, Varallyay CG, Firkins J, Jaboin JJ, Kubicky CD, Raslan AM, Dogan A, Cetas JS, Ciporen J, Han SJ, Ambady P, Muldoon LL, Woltjer R, Rooney WD, Neuwelt EA (2019) Combined iron oxide nanoparticle ferumoxytol and gadolinium contrast enhanced MRI define glioblastoma pseudoprogression. Neuro Oncol 21:517–526. 10.1093/neuonc/noy160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lux F, Tran VL, Thomas E, Dufort S, Rossetti F, Martini M, Truillet C, Doussineau T, Bort G, Denat F, Boschetti F, Angelovski G, Detappe A, Cremillieux Y, Mignet N, Doan BT, Larrat B, Meriaux S, Barbier E, Roux S, Fries P, Muller A, Abadjian MC, Anderson C, Canet-Soulas E, Bouziotis P, Barberi-Heyob M, Frochot C, Verry C, Balosso J, Evans M, Sidi-Boumedine J, Janier M, Butterworth K, McMahon S, Prise K, Aloy MT, Ardail D, Rodriguez-Lafrasse C, Porcel E, Lacombe S, Berbeco R, Allouch A, Perfettini JL, Chargari C, Deutsch E, Le Duc G, Tillement O (2018) AGuIX((R)) from bench to bedside-transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br J Radiol. 10.1259/bjr.20180365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verry C, Sancey L, Dufort S, Le Duc G, Mendoza C, Lux F, Grand S, Arnaud J, Quesada JL, Villa J, Tillement O, Balosso J (2019) Treatment of multiple brain metastases using gadolinium nanoparticles and radiotherapy: NANO-RAD, a phase I study protocol. BMJ Open 9:e023591. 10.1136/bmjopen-2018-023591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kotb S, Piraquive J, Lamberton F, Lux F, Verset M, Di Cataldo V, Contamin H, Tillement O, Canet-Soulas E, Sancey L (2016) Safety evaluation and imaging properties of gadolinium-based nanoparticles in nonhuman primates. Sci Rep 6:35053. 10.1038/srep35053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madhankumar AB, Slagle-Webb B, Mintz A, Sheehan JM, Connor JR (2006) Interleukin-13 receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol Cancer Ther 5:3162–3169. 10.1158/1535-7163.MCT-06-0480 [DOI] [PubMed] [Google Scholar]
- 81.Madhankumar AB, Slagle-Webb B, Wang X, Yang QX, Antonetti DA, Miller PA, Sheehan JM, Connor JR (2009) Efficacy of interleukin-13 receptor-targeted liposomal doxorubicin in the intracranial brain tumor model. Mol Cancer Ther 8:648–654. 10.1158/1535-7163.MCT-08-0853 [DOI] [PubMed] [Google Scholar]
- 82.Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, Wu X, Mao H (2010) EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res 70:6303–6312. 10.1158/0008-5472.CAN-10-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]


