Abstract
Purpose
The impact of central nervous system (CNS) prophylaxis in diffuse large B-cell lymphoma (DLBCL) is contentious. The CNS International Prognostic Index (IPI) calculator offers prognostic guidance in identifying those patients who may be at highest risk of disease progression or relapse to the CNS. However, it is unclear whether this tool has guided clinician decision-making in a real-world setting. Studies have suggested that CNS prophylaxis may not offer clinically significant benefit in terms of preventing CNS disease progression. Given this, we investigated the utilization of CNS prophylaxis within our own population and documentation of the CNS-IPI score.
Methods
We retrospectively evaluated patients with newly diagnosed DLBCL between January 1, 2014, and December 31, 2017. Patients were assessed for receipt of CNS prophylaxis in the form of intrathecal (IT) chemotherapy and/or high-dose intravenous (IV) methotrexate. CNS-IPI scores were calculated for all patients who received CNS prophylaxis or those who experienced CNS disease. Long-term outcomes at five years from diagnosis included CNS progression/relapse and survival.
Results
Of 234 patients who met criteria, 20 (8.6%) received either IV methotrexate or IT chemotherapy; most received IT methotrexate. No patients in the IT prophylaxis group developed CNS disease, while two of eight IV methotrexate patients experienced CNS disease involvement. The incidence of CNS progression was 3.7% in the no prophylaxis group and 10% in those who received prophylaxis.
Conclusions
This study revealed low utilization of CNS prophylaxis and CNS-IPI documentation in a community hospital system. Given large differences between groups, claims of CNS prophylaxis efficacy are unable to be made. CNS relapse rates were consistent with existing literature and promote continued evaluation of the utility of current CNS prophylaxis approaches in DLBCL. New unambiguously effective therapeutic approaches are needed and may encourage a higher rate of standardized use.
Keywords: CNS, DLBCL, prophylaxis, intrathecal, chemotherapy, methotrexate
While diffuse large B-cell lymphoma (DLBCL) is estimated to have a five-year relative survival of 64.7%,1 it is also known to be associated with the seldom but often detrimental risk of secondary central nervous system (CNS) relapse. The two-year risk of secondary CNS involvement is reported to be up to 10%, but risk may increase depending upon disease-specific factors such as uterine involvement (four-year 44%).2 The notable potential to experience disease, despite systemic chemotherapy, has warranted investigations into CNS-directed prophylaxis and prediction methods.
In 2016, Schmitz et al attempted to stratify the risk of CNS relapse in DLBCL patients using a tool that can calculate which patients are most appropriate for CNS prophylaxis. The validation of the calculator, known as the CNS-International Prognostic Index (CNS-IPI), focused on 1597 DLBCL patients who received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemoimmunotherapy. The risk calculator considers the following factors: age, lactate dehydrogenase, Eastern Cooperative Oncology Group (ECOG) performance status, disease stage, extranodal involvement, and renal/adrenal involvement. On a scale of 0–6, patients may be classified as low (0–1), intermediate (2–3), or high (4–6) risk of CNS relapse, which is associated with a 0.6%, 3.4%, or 10.2% two-year rate of CNS disease development, respectively.3
Historically, the prevention strategy for CNS relapse has proven difficult to standardize. In 2009, one of the first practice-altering studies, which introduced the addition of rituximab to standard CHOP chemotherapy in patients with CD20 positive DLBCL, was published. Two-year incidence of CNS relapse was reduced to 4.1% from 6.9%. In the same trial, the use of intrathecal methotrexate (IT MTX) did not reduce the risk except for within a small subgroup of patients with testicular involvement.4 Clinical trials are evaluating testicular and other high risk lymphoma patients (NCT00945724, NCT02777736).
Overall, there are no clear data suggesting that IT chemotherapy or HD IV MTX has any proven benefit in reducing secondary CNS disease; further investigation is ongoing regarding lenalidomide and other agents such as ibrutinib. This study expands upon our institution’s prior interim investigation5 by aiming to capture broader CNS prophylaxis utilization rates, CNS-IPI stratification, and patient outcomes within a large community health system.
METHODS
Patient Criteria
This retrospective study evaluated all patients in Aurora St. Luke’s Medical Center with a diagnosis of DLBCL between January 1, 2014, and December 31, 2017. To be included in the evaluation, patients were required to be newly diagnosed with DLBCL and to have initiated treatment at an in-system facility. Patients were excluded if their diagnosis was not de-novo DLBCL (eg, transformed follicular lymphoma).
Primary and Secondary Objectives
The primary objective was to report use of CNS prophylaxis among the DLBCL patient population within our health system. Secondary objectives included assessing patient outcomes (CNS progression/relapse and overall survival at five years from diagnosis) and the observations between CNS-IPI and CNS prophylaxis use in those who received CNS prophylaxis.
Data Collection and Analysis
Baseline data collection for all evaluable patients included patient gender, age at diagnosis, disease characteristics, and first-line treatment. Patients were followed until five years from diagnosis to evaluate survival and incidence of documented CNS progression or relapse. Patients who received CNS prophylaxis with first-line therapy—defined as at least one dose of IT chemotherapy via Ommaya or lumbar puncture, HD IV MTX, or oral lenalidomide—were investigated further. If one of these therapies was received beyond the first-line treatment or only after CNS disease was detected, then the patient was still included but not documented as having received prophylaxis.
For the CNS prophylaxis cohort, a CNS-IPI score was calculated based on age, ECOG status, LDH, extranodal sites, disease stage, and kidney/adrenal involvement. CNS-IPI scores correlated with risk as follows: 0–1 low risk (0.6% CNS relapse), 2–3 intermediate risk (3.4% CNS relapse), and 4–6 high risk (10.2% CNS relapse).3 Gene rearrangement or expression (MYC, BCL2, and BCL6) was also recorded.
For the non-prophylaxis cohort, CNS-IPI was not calculated unless the patient had documented relapse involving the CNS. Additionally, in patients who received CNS prophylaxis or those who did not but relapsed in the CNS, chart review was performed for documentation of CNS-IPI by the provider at diagnosis. A year-to-year comparison of CNS prophylaxis trend was performed. This project was reviewed by Aurora Health Care’s Research Subject Protection Program Institutional Review Board and deemed exempt from oversight.
Statistical Analysis
Descriptive statistics were used to report incidence in counts and percentages for CNS prophylaxis use, as well as the comparison of year-to-year utilization observations. Overall survival at five years was calculated using the R Version 2022.07.0 survival package.6 This study primarily focused on patient management, specifically treatment decisions, rather than outcomes. As such, we did not plan a statistical analysis to assess for differences in outcomes.
RESULTS
Baseline
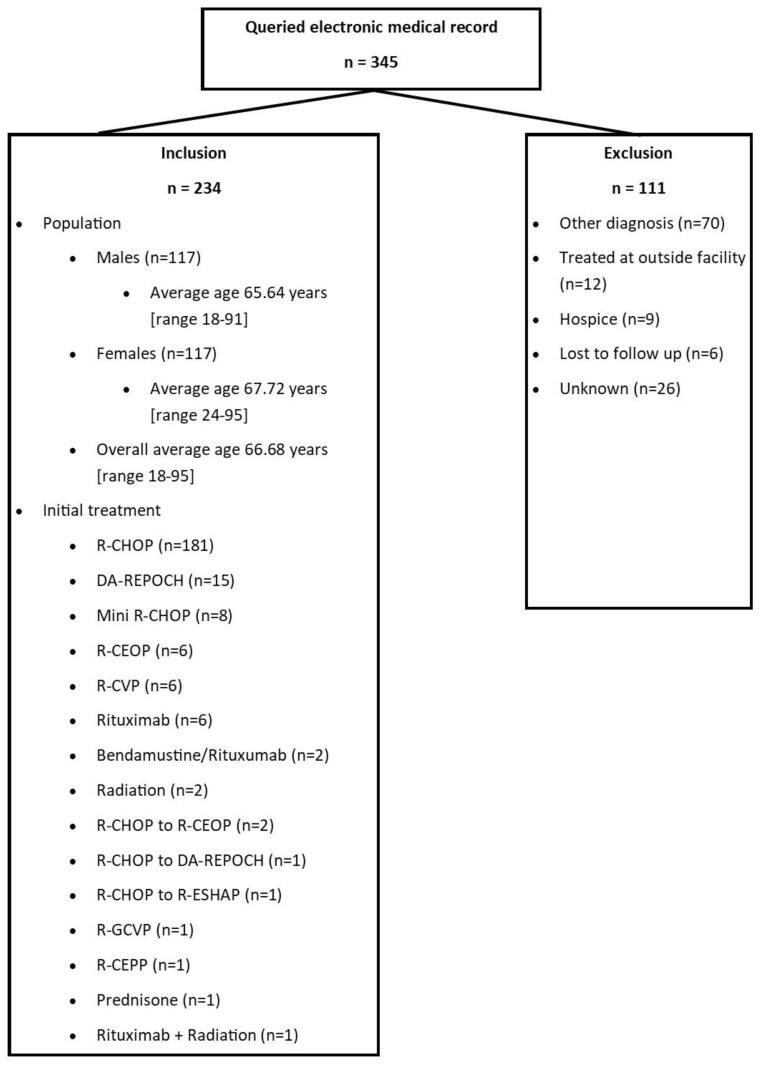
The electronic medical record (EMR) query yielded 345 patients associated with a DLBCL diagnosis between January 1, 2014, and December 31, 2017. Upon chart review, 234 patients were evaluable, as 111 patients met exclusion criteria, a majority of whom did not have a true DLBCL diagnosis. Of the 234 patients observed, the population was a 1:1 male to female ratio with an average age of 65.6 (range: 18–91) years for males and 67.7 (range: 24–95) years for females. First-line systemic therapy consisted of R-CHOP for 181 (77.4%) patients (Figure 1).
Figure 1.
The baseline inclusion/exclusion population and characteristics such as average age and first-line DLBCL treatment.
Primary Objective
Of the entire cohort, 214 patients (91.4%) did not receive any form of CNS prophylaxis. Twenty patients (8.6%) received at least one of the pre-specified forms of CNS prophylaxis. Thirteen of the 20 patients (65%) received IT chemotherapy at a median of 3 [range: 1–5] doses per patient. Thirty-eight of 40 (95%) total IT doses were IT MTX via lumbar puncture, and two doses (5%) were IT cytarabine. One patient received both IT MTX and IT cytarabine for one dose each via Ommaya (Table 1).
Table 1.
Primary and Secondary Objective Results
| Form of Prophylaxis/Patient Number | Doses | Outcome | CNS Progression/Relapse | CNS-IPI | CNS Risk | MYC | BCL2 | BCL6 | Disease Stage |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| IT chemo (n=13) | Median: 3 (range 1–5) | Alive: 9 (69%) Deceased: 4 (31%) | 0 (0%) | Median: 2 (range 1–5) | |||||
| IT MTX (n-13) | |||||||||
|
| |||||||||
| 1* | 1 | Deceased | No | 5 | High | (−) | NA | NA | IVEB |
| 2 | 3 | Alive | No | 1 | Low | NA | NA | NA | IIIAE |
| 3 | 1 | Deceased | No | 2 | Inter | NA | NA | NA | IAE |
| 4 | 4 | Alive | No | 2 | Inter | (+) | (+) | NA | III |
| 5 | 1 | Deceased | No | 5 | High | NA | NA | NA | IV |
| 6 | 4 | Alive | No | 1 | Low | (+) | (+) | (+) | II |
| 7 | 5 | Alive | No | 1 | Low | (+) | (−) | (−) | IAE |
| 8 | 3 | Alive | No | 5 | High | (−) | NA | (−) | IVAE |
| 9 | 4 | Alive | No | 4 | High | (−) | (−) | (−) | IV |
| 10 | 5 | Alive | No | 3 | Inter | (+) | NA | (+) | IV |
| 11 | 4 | Alive | No | 3 | Inter | (+) | (+) | (+) | IIIA |
| 12 | 3 | Deceased | No | 1 | Low | (−) | (−) | NA | III |
| 13 | 1 | Alive | No | 2 | Inter | (−) | (−) | (−) | IIIE |
|
| |||||||||
| IT ARA-C (n=1) | |||||||||
|
| |||||||||
| 1* (via Ommaya) | 1 | Deceased | No | 5 | High | (−) | NA | NA | IVEB |
|
| |||||||||
| HD MTX (n=8) | Median: 2 (range 1–6) | Alive: 7 (87.5%) Deceased: 1 (12.5%) | 2/8 (25%) | Median: 3 (range 2–5) | |||||
|
| |||||||||
| 9** | 1 | Alive | No | 4 | High | (−) | (−) | (−) | IV |
| 14 | 1 | Alive | Yes | 4 | High | (−) | NA | (−) | IV |
| 15 | 3 | Alive | No | 3 | Inter | (−) | NA | NA | IVAE |
| 16 | 3 | Alive | No | 3 | Inter | (−) | NA | NA | IV |
| 17 | 6 | Alive | No | 2 | Inter | NA | NA | NA | III |
| 18 | 1 | Alive | No | 3 | Inter | NA | (−) | (−) | IV |
| 19 | 3 | Deceased | Yes | 2 | Inter | (−) | (−) | (−) | IV |
| 20 | 1 | Alive | No | 5 | High | NA | NA | (−) | IV |
The table illustrates utilization of CNS prophylaxis. Given the low incidence, prophylaxis and disease characteristics are reported per patient. Each patient is reflected by a number. If a patient received more than one form of CNS prophylaxis, the patient is identified by a patient number and an asterisk (i.e. identifier 9* is the same patient in IT MTX and HD MTX groups).
CNS-IPI: CNS International Prognostic Index; MYC: MYC rearrangement; BCL2: BCL2 rearrangement; BCL6: BCL6 rearrangement; Inter: Intermediate risk.
Eight patients received HD IV MTX prophylaxis at a median of 2 [range: 1–6] doses per patient. One patient received both IT MTX and HD IV MTX. No patients received lenalidomide for CNS prophylaxis (Table 1).
Secondary Objectives
Overall survival at five years from diagnosis for the entire cohort was 65% (95% CI: 59.1–71.4). The CNS prophylaxis and no prophylaxis groups had 75% and 64% five-year overall survival, respectively. Survival among the prophylaxis group was 67% among those who received only IT and 85% among those who received only HD MTX. One patient was administered both prophylaxis modalities and was alive at the end of the five-year follow-up period.
The overall relapse incidence among all patients was 4.3% with no documented CNS relapse in the IT prophylaxis group. Two patients within the HD IV MTX group (25%) were documented to have CNS relapse (Table 1). Among all patients who received CNS prophylaxis, four (20%) were documented as having double or triple-hit gene rearrangements. In the no prophylaxis group, eight patients (3.7%) developed CNS involvement, none of whom had double or triple-hit gene rearrangements (Table 1).
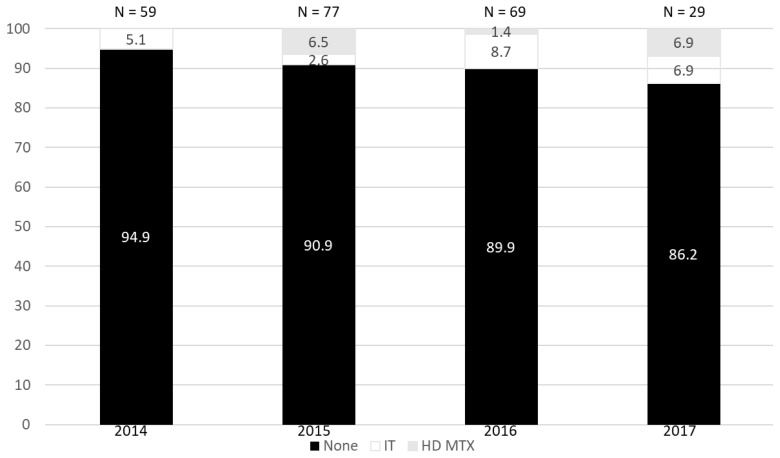
Among the patients who received CNS prophylaxis, the median CNS-IPI was 3 [range: 1–5]. Within this group, the CNS-IPI risk of the two patients who relapsed was 2 (intermediate risk) and 4 (high risk). Of the patients who did not receive CNS prophylaxis but developed CNS disease, the median CNS-IPI was 3 [range: 2–4]. A year-to-year usage evaluation demonstrated varied choice of prophylaxis, but a subtle comparative increase in prophylaxis over time—5.1% use in 2014 compared to 13.8% in 2017 (Figure 2). Chart review of patients who received CNS prophylaxis revealed that 12 of 20 (60%) had CNS-IPI documentation within the EMR.
Figure 2.
Year-to-year comparison of IT and HD MTX usage.
DISCUSSION
The patient population in this retrospective observational study was well balanced and reflective of real-world diagnoses with respect to gender and age distributions. Most patients received guideline-directed frontline DLBCL therapy with R-CHOP, while others received various other systemic regimens based on patient specific characteristics or physician preference. Our CNS prophylaxis utilization was low at 8.6% compared to reported use between 14–35% in similar studies.7,8 Intrathecal methotrexate was the predominant agent. Despite HD MTX being used less often, a majority of recent studies have focused evaluations on its role in CNS prophylaxis. Nearly all of these claim no difference in CNS relapse with the use of HD MTX versus none.9,10 At present, proposed management strategies suggest HD MTX in high-risk patients but sideline the use of IT chemotherapy unless there is sanctuary site involvement or the inability to receive HD MTX.9
Overall survival was consistent with known data in the DLBCL population. Patients who received CNS prophylaxis experienced a higher incidence of five-year survival than those who did not. However, this finding may be confounded by the drastic difference in sample sizes. Survival following CNS relapse is reported to be a median of 1.6 years.11 Half of our study population who had CNS relapse died within five years, all of which occurred within 1.6 years from initial DLBCL diagnosis.
The incidence of secondary CNS disease among the entire population was low (4.3%) and comparable to prior reports. Eyre and colleagues7 assessed 690 DLBCL patients with only 14.3% receiving IT MTX prophylaxis, 2% receiving HD IV MTX, and 2.5% receiving a combination of the two modalities. Among these, 4.6% of patients who received prophylaxis experienced CNS relapse, compared to 2.3% of patients without prophylaxis. Furthermore, Puckrin et al8 shared data of 326 patients in which HD MTX was given to patients with high-risk CNS-IPI, testicular involvement, or double hit lymphoma. Prophylactic HD MTX was administered to 35.3% of selected patients. The incidence of CNS relapse was similar between HD MTX prophylaxis versus none—11.2% versus 12.2%, respectively (P=0.82). Hall et al12 evaluated 114 DLBCL patients among whom 25% received IT prophylaxis (high-dose intravenous methotrexate [HD IV MTX] was not assessed). Overall, there was no statistically significant difference between CNS relapse in the patients who received IT chemotherapy versus those who did not (1 vs 3, respectively; P=1). Recently, a large multicenter analysis among academic institutions evaluated 1162 patients for incidence and outcomes of CNS prophylaxis. Their findings, like other studies, showed prevalent use of CNS prophylaxis (IT chemo in 77% of patients and HD MTX in 20%) with no significant difference in CNS relapse rates between IT or systemic modalities—5.4 vs 6.8%, respectively, P=0.4.13 This study did not assess CNS relapse rates to a non-prophylaxis group. Nonetheless, relapse rates were consistent with comparable evaluations.
There was discordance between CNS prophylaxis utilization rates and CNS-IPI scores. Among the 20 patients who received prophylaxis, the median CNS-IPI of 3 is below the recommended CNS-IPI score for prophylaxis by multiple international guidelines.9 This may suggest a more aggressive prophylactic approach on a patient or provider level. However, when considering those who developed CNS disease, one patient with a CNS-IPI of 4 did not receive prophylaxis. It is unclear from documentation if the decision for CNS prophylaxis was based on risk stratification alone versus provider discretion, as 40% of patients who received prophylaxis did not have documented CNS-IPI scores. Additionally, variability in prophylaxis prescribing does not allow a proper assessment of the prognostic CNS-IPI risk calculator. In terms of the utility of the CNS-IPI calculator, Eyre et al noted that there was no discernible difference between CNS relapse with or without prophylaxis when their findings were adjusted based on CNS-IPI scores.7
These data are limited by the retrospective nature of the study, especially when assessing decision-making patterns. While the goal of our study was not to compare the efficacy between clinical approaches, we do note minimal clinical variation in the incidence of relapse between prophylaxis and non-prophylaxis groups. These data are limited by the fact that location of disease (with exception of renal/adrenal involvement) is not taken into consideration. While these factors likely played a significant role in the systemic chemoimmunotherapy choices, the CNS-IPI score and retrospective chart review are unable to capture a complete picture of the considerations surrounding the decision to use CNS prophylaxis.
Aside from IT and HD IV chemotherapy approaches, the oral agent lenalidomide is also being investigated given its known penetration to the CNS. One study added lenalidomide to standard R-CHOP (R2CHOP) first-line therapy in 136 patients and observed only one case of two-year relapse (incidence 0.7%), much less than that of the historical, more typical ~5% relapse across all DLBCL patients.14 Further combined R2CHOP analyses regarding efficacy in CNS disease prevention are pending. An alternative approach of stem cell transplant may also be viable. Previously, a group of high-risk patients were treated with higher intensity chemoimmunotherapy followed by consolidative autologous transplantation as opposed to CNS prophylaxis; these patients experienced a trend towards less CNS relapse (6.0% vs 13.7%, P=0.15). However, one criticism of the findings was the limited use of prophylaxis (35%) from which to base the results.8 Ultimately, CNS relapse remains a concern among clinicians. Effective approaches are needed to prevent CNS disease and aid in standardizing treatment approaches.
CONCLUSIONS
These data correspond with the growing level of evidence published in the literature. Uniquely, this study reports practices within a large community health system, unlike a large portion of published findings. Our data have led us to internally reconsider CNS prophylaxis strategies, in addition to re-evaluating the body of literature surrounding this topic. Given our findings, we plan to further review our internal data to include years after those reported in this study with similar long-term follow up to detect potential statistical differences regarding risks versus benefits of CNS prophylaxis strategies. We would encourage other cancer centers and health systems to evaluate their own CNS prophylaxis and CNS-IPI data within the DLBCL population.
Patient-Friendly Recap.
The effectiveness of preventive measures like chemotherapy or high-dose methotrexate against diffuse large B-cell lymphoma (DLBCL) progression to the brain and central nervous system (CNS) remains contentious.
Use of such interventions in our analysis was low but may be chosen per the discretion of the treating physician based on a variety of disease risk factors.
Further research into more effective means by which to prevent CNS disease is needed, despite relatively low rates of CNS progression in our DLBCL population.
Footnotes
Author Contributions: Study design: Atienza, Thompson. Data acquisition or analysis: Williams, Atienza, Aranda, Flint, Thompson. Manuscript drafting: Williams, Thompson. Critical revision: Thompson, Sana, Medlin, Gul, Sanchez.
Conflicts of Interest: Dr. Thompson participated on the advisory board for Celgene/Bristol-Meyers Squibb and is an editorial board member for the Journal of Patient-Centered Research and Reviews. Dr. Medlin serves as a consultant for Kite Pharma. The other authors declare no competing financial interests.
References
- 1.National Cancer Institute. Cancer Stat Facts: NHL – Diffuse Large B-Cell Lymphoma (DLBCL) National Cancer Institute, Surveillance, Epidemiology, and End Results Program. 2020. [Accessed November 22, 2023]. https://seer.cancer.gov/statfacts/html/dlbcl.html .
- 2.Savage KJ. Secondary CNS relapse in diffuse large B-cell lymphoma: defining high-risk patients and optimization of prophylaxis strategies. Hematology. 2017;2017:578–86. doi: 10.1182/asheducation-2017.1.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2016;34:3150–6. doi: 10.1200/JCO.2015.65.6520. [DOI] [PubMed] [Google Scholar]
- 4.Boehme V, Schmitz N, Zeynalova S, et al. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) Blood. 2009;113:3896–902. doi: 10.1182/blood-2008-10-182253. [DOI] [PubMed] [Google Scholar]
- 5.Thompson MA, Yoder SA, Robinson LA, et al. Patterns of use of CNS prophylaxis in DLBCL in a large health system. (abstr.) J Clin Oncol. 2017;35(Supplement 15):7562. doi: 10.1200/JCO.2017.35.15_suppl.7562. [DOI] [Google Scholar]
- 6.Therneau TM. A package for survival analysis in R. Published. 2023. https://CRAN.R-project.org/package=survival .
- 7.Eyre TA, Kirkwood AA, Wolf J, et al. Stand-alone intrathecal central nervous system (CNS) prophylaxis provide unclear benefit in reducing CNS relapse risk in elderly DLBCL patients treated with R-CHOP and is associated increased infection-related toxicity. Br J Haematol. 2019;187:185–94. doi: 10.1111/bjh.16070. [DOI] [PubMed] [Google Scholar]
- 8.Puckrin R, El Darsa H, Ghosh S, et al. Lack of effectiveness of intravenous high-dose methotrexate for prevention of CNS relapse in patients with high-risk DLBCL: a retrospective analysis from Alberta, Canada. (abstr.) Blood. 2020;136(Supplement 1):26–7. doi: 10.1182/blood-2020-139025. [DOI] [Google Scholar]
- 9.Wilson MR, Bobillo S, Cwynarski K. CNS prophylaxis in aggressive B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2022;2022:138–45. doi: 10.1182/hematology.2022000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz N, Frontzek F. CNS prophylaxis in DLBCL: time to say goodbye? Blood. 2022;139:315–7. doi: 10.1182/blood.2021014043. [DOI] [PubMed] [Google Scholar]
- 11.Doolittle ND, Abrey LE, Shenkier TN, et al. Brain parenchyma involvement as isolated central nervous system relapse of systemic non-Hodgkin lymphoma: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2008;111:1085–93. doi: 10.1182/blood-2007-07-101402. [DOI] [PubMed] [Google Scholar]
- 12.Hall KH, Valla K, Flowers CR, et al. Intrathecal central nervous system prophylaxis in patients with diffuse large B-cell lymphoma at an academic healthcare system. Clin Lymphoma Myeloma Leuk. 2019;19:89–94. doi: 10.1016/j.clml.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Orellana-Noia VM, Reed DR, Sen JM, et al. CNS prophylaxis during front-line therapy in aggressive non-Hodgkin lymphomas: real-world outcomes and practice patterns from 19 US academic institutions. (abstr.) Blood. 2020;136(Supplement 1):27–8. doi: 10.1182/blood-2020-134798. [DOI] [Google Scholar]
- 14.Ayed AO, Chiappella A, Pederson L, et al. CNS relapse in patients with DLBCL treated with lenalidomide plus R-CHOP (R2CHOP): analysis from two phase 2 studies. Blood Cancer J. 2018;8:63. doi: 10.1038/s41408-018-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]