Abstract
Background
Osteochondral autologous transplantation (OAT) has been widely used in the treatment of osteochondral lesion of the talus (OLT). Previous studies have reported successful outcomes following the use of osteochondral autogenous grafts from the intercondylar notch of the knee or a non-weight-bearing region of the femoral condyle. However, donor-site morbidity of the knee joint has been observed in several cases. This study aimed to investigate the outcomes and safety of OAT with autografts from the ipsilateral lateral talar articular facet as an alternative donor site for medial OLT.
Methods
Among 40 patients who underwent OAT, 29 patients were excluded. Eleven patients who underwent OAT with an osteochondral graft harvested from the ipsilateral lateral talar articular facet from 2011 to 2022 were retrospectively analyzed. The size of OLT was measured on ankle magnetic resonance imaging, including coronal length, sagittal length, depth, and area. Clinical outcomes were evaluated using the American Orthopaedic Foot and Ankle Society (AOFAS) ankle-hindfoot scale and a visual analog scale (VAS). Weight-bearing ankle radiographs were obtained postoperatively and at 1 year after surgery.
Results
The average follow-up time after surgery was 64.7 months (range, 14–137 months). The average diameter of lesions was 8.8 mm (range, 8–9.9 mm). The average size of lesions was 51.2 mm2 (range, 33.6–71.3 mm2) , and all lesions included subchondral cysts. The average depth of lesions was 7.3 mm (range, 6.2–9.1 mm). Graft sizes ranged from 8 to 10 mm in diameter (8 mm, n = 1; 10 mm, n = 10) All measured clinical outcomes improved postoperatively, including the AOFAS scores (preoperative, 55.4 ± 9.0; 1-year follow-up, 92.1 ± 7.6; p = 0.001) and VAS scores (preoperative, 5.5 ± 0.7; 1-year follow-up, 1.9 ± 0.8; p = 0.001). All weight-bearing ankle radiographs of the graft and donor sites did not reveal arthritic change in the ankle joint, lateral talar dome collapse, and graft-site delayed union or nonunion at 1 year after surgery.
Conclusions
For a single medial OLT, harvesting autografts from the ipsilateral lateral talar articular facet without knee donor-site morbidities can be a good alternative in OAT for OLT.
Keywords: Ankle, Talus, Osteochondral lesion, Autologous transplantation
An osteochondral lesion of the talus (OLT) causes pain, ankle joint dysfunction, and reduced quality of life. While various treatments for OLT have been introduced, treating OLT still remains challenging. Many studies have shown promising clinical and radiological results with microfracture or multiple drilling.1,2,3,4) However, treatment with reparative fibrocartilage, which has poor biological characteristics, has been demonstrated to be inferior to osteochondral autologous transplantation (OAT), which provides hyaline cartilage for healing OLT.5,6)
The OAT has been recommended as an ideal treatment for large OLTs with full-thickness articular cartilage loss and significant bone loss following failure of bone marrow stimulation. Previous studies have reported successful outcomes following the use of osteochondral autogenous grafts from the intercondylar notch of the knee or a non-weight-bearing region of the femoral condyle.5,6) Nonetheless, donor-site complications of the knee joint, including knee pain, locking, patellar instability, hemarthrosis, deep infection, and functional impairment, have been noted in up to 15%–16% of cases.4,7,8,9,10,11) Additionally, concerns regarding the different mechanical behaviors exhibited by cartilages at the donor and recipient sites have been raised. Several cadaveric studies have shown that donor-site morbidities are attributed to differences in mechanical characteristics, including cartilage thickness, energy dissipation, shear moduli, and friction coefficient, between osteochondral grafts in the distal femur and talus.12,13) On average, the distal femoral cartilage is thicker than the talar cartilage, and energy dissipation tends to be higher in the distal femoral cartilage than in the talar cartilage.13) The importance of considering mismatches between the distal femoral cartilage and talar cartilage has been proposed,12,13) and harvesting from the non-weight-bearing talar articular facet is considered to reduce joint-related donor-site complications.4) There have also been several attempts to use the ipsilateral talar articular facet as a donor site with few donor-site morbidities.14,15,16)
The present study aimed to investigate the outcomes and safety of OAT with an ipsilateral lateral talar articular facet as a donor site. Given that the blood supply of the talus is fragile at the base and any damage to the chondral aspect of the talus may lead to avascular necrosis,17) we were concerned that harvesting an osteochondral graft from the same side and adjacent area of the OLT could impair the blood supply and stability of the talus. Therefore, in this study, harvesting from the ipsilateral opposite aspect of the talar articular facet to the OLT, located at the medial talar dome, was first conceived to preserve the blood supply and stability of the talus.17)
METHODS
The Institutional Review Board of Dankook University Hospital approved this study (IRB No. 2023-04-019). Informed consent was waived because of the retrospective nature of the study. Among 40 patients who underwent OAT, 29 patients were excluded. Eleven patients (7 men and 4 women) who underwent OAT with an osteochondral graft harvested from the lateral talar articular facet from 2011 to 2022 were retrospectively analyzed. OLTs were classified into 4 stages by Berndt and Harty18) and 5 stages by Hepple et al.19) using simple radiography and magnetic resonance imaging (MRI), respectively. The inclusion criteria were a single osteochondral lesion, lesions of stage 3 or more in both classifications, lesions located on the medial talar dome, and patients who were refractory to conservative treatment at more than 3 months or bone marrow stimulation surgery such as arthroscopic microfracture. Patients with severe arthritis or deformities of the ankle or foot, infections, or metabolic diseases were excluded.
The size of an OLT was measured on ankle MRI, including coronal length, sagittal length, depth, and area, which was calculated using an ellipse formula (area = abπ = coronal length × sagittal length × 0.79).20) For cases in which follow-up MRI scans were available, the radiological quality of the donor site on follow-up MRI was quantitatively assessed using the MRI scoring system proposed by Henderson et al.21) (Table 1).22)
Table 1. MRI Scoring System of Henderson et al.21).
| Findings | MRI score | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Fill of the repair site | Complete | > 50% Of the defect | < 50% Of the defect | Full-thickness |
| Signal at the repair site | Normal (identical to adjacent articular cartilage) | Nearly normal (slight areas of hyperintensity) | Abnormal (large areas of hyperintensity) | Absent |
| Effusion | Absent | Mild | Moderate | Severe |
| Bone marrow edema | Absent | Mild | Moderate | Severe |
MRI: magnetic resonance imaging.
Clinical outcomes were evaluated using the American Orthopaedic Foot and Ankle Society (AOFAS) ankle-hindfoot scale and a visual analog scale (VAS). Preoperatively, simple weight-bearing ankle radiography and ankle MRI were performed. Weight-bearing ankle radiographs were obtained postoperatively and at 1 year after surgery. The Foot and Ankle Outcome Score (FAOS) was evaluated at 1 year after surgery.
Surgical Protocol
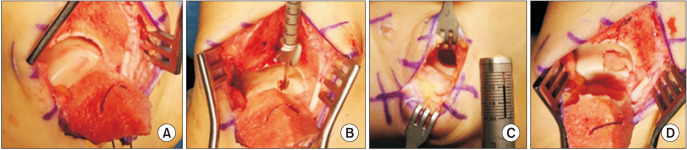
All procedures were performed under general or spinal anesthesia using a thigh pneumatic tourniquet. Unstable lesions on the medial talar domes were detected on arthroscopic images. All lesions were located on the medial talar dome (Fig. 1). A longitudinal incision was made over the medial malleolus. After predrilling of the medial malleolus, a chevron-shaped medial malleolar osteotomy was performed, and the medial malleolus was retracted downward. The OLT was exposed, excised, and prepared.
Fig. 1. Gross intraoperative images of osteochondral autologous transplantation with lateral talar autograft. (A) Exposed osteochondral lesion of the talus after a chevron-shaped medial malleolar osteotomy. (B) Preparation for transplantation. (C) Lateral talar articular facet after harvesting the graft. (D) Posttransplantation appearance.
A longitudinal incision (approximately 4–5 cm in size) was made on the anteroinferior aspect of the lateral malleolus to harvest the lateral talar articular facet. After dissecting and retracting the inferior extensor retinaculum, the lateral talar articular surface was exposed over the anterior talofibular ligament and anterior border of the lateral malleolus. The anterior talofibular ligament was not divided during dissection. While maintaining ankle plantar flexion, the lateral talar articular facet was harvested using the Osteochondral Autograft Transfer System (The Single-Use Autograft OATS, Arthrex) as far from the superior talar dome as possible. After harvesting, the donor site was filled with demineralized bone matrix (DBM, CGBIO). After over-reaming the OLT, the harvested graft was transplanted into the medial OLT. The rest area that was not covered by the graft was filled with the remnant DBM used in the donor site. The site of medial malleolus osteotomy was reduced and fixed using 2 cannulated screws. After surgery, immobilization with a short leg cast for 4 weeks was followed by rehabilitation, including muscle strengthening and joint range of motion exercise for about 8 weeks. More vigorous activities were recommended only after 3 months postoperatively.
Statistical Analysis
For comparisons between groups, the Student t-test or the Mann-Whitney U-test was used for continuous variables after performing the Shapiro-Wilk normality test. Clinical variables between the preoperative and last follow-up examinations were compared using the paired sample t-test or Wilcoxon signed-rank test. Statistical significance was set at p < 0.05. Data were analyzed using IBM SPSS Statistics version 26.0 statistical software (IBM Corp.), and GraphPad Prism version 8 (GraphPad Software Inc.).
RESULTS
The mean patient age was 47.7 years (range, 23–68 years), and the duration of symptoms was 33.7 months (range, 6–120 months). The average follow-up time after surgery was 64.7 months (range, 14–137 months). The average diameter of lesions was 8.8 mm (range, 8–9.9 mm). The average size of lesions was 51.2 mm2 (range, 33.6–71.3 mm2), and all lesions included subchondral cysts. The average depth of lesions was 7.3 mm (range, 6.2–9.1 mm). Graft sizes ranged from 8 to 10 mm in diameter (8 mm, n = 1; 10 mm, n = 10) (Table 2). Two out of eleven patients had a history of arthroscopic microfracture before OAT.
Table 2. Demographic Data of Patients.
| No. | Sex | Age (yr) | BMI (kg/m2) | Duration of symptom (mo) | Lesion size (coronal × sagittal, mm) | Lesion area (mm2) | Depth (mm) | Graft diameter size (mm) | Previous operation history |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 68 | 19.7 | 48 | 7.0 × 9.5 | 52.5 | 7.3 | 8 | - |
| 2 | F | 60 | 26.6 | 36 | 7.1 × 8.0 | 44.9 | 6.7 | 10 | AS microfracture |
| 3 | F | 63 | 28.4 | 36 | 7.0 × 8.1 | 44.8 | 9.0 | 10 | - |
| 4 | M | 51 | 28.0 | 7 | 6.0 × 9.6 | 45.5 | 6.8 | 10 | - |
| 5 | M | 41 | 26.6 | 36 | 9.6 × 9.5 | 72.0 | 8.5 | 10 | AS microfracture |
| 6 | F | 23 | 19.5 | 24 | 5.0 × 8.5 | 33.6 | 7.1 | 10 | - |
| 7 | F | 63 | 20.0 | 12 | 6.2 × 8.0 | 39.2 | 6.3 | 10 | - |
| 8 | M | 38 | 22.5 | 120 | 8.2 × 7.2 | 46.6 | 6.2 | 10 | - |
| 9 | M | 39 | 27.4 | 6 | 6.9 × 8.0 | 43.6 | 9.1 | 10 | - |
| 10 | M | 40 | 27.5 | 6 | 9.3 × 9.7 | 71.3 | 7.1 | 10 | - |
| 11 | M | 59 | 28.9 | 36 | 8.9 × 9.9 | 69.6 | 6.5 | 10 | - |
BMI: body mass index, AS: arthroscopic.
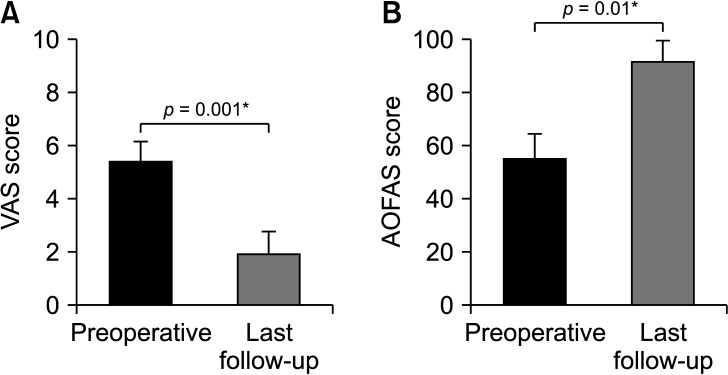
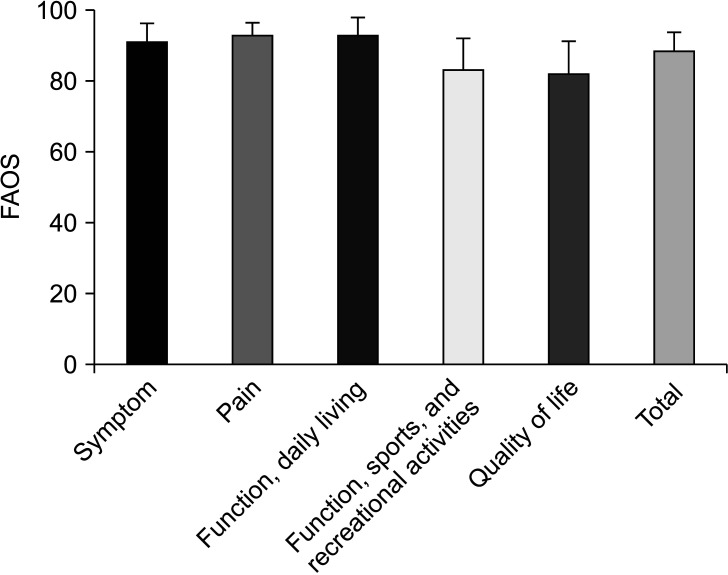
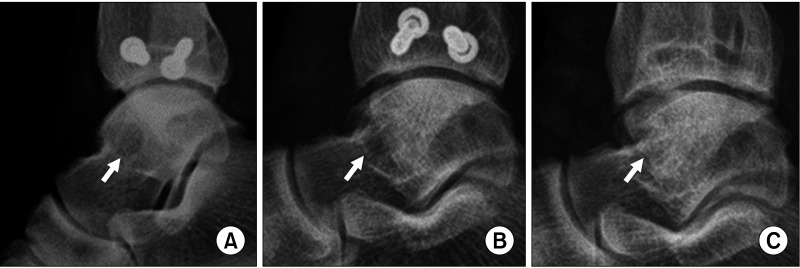
All clinical outcomes showed significant improvements postoperatively, including the AOFAS scores (preoperative, 55.4 ± 9.0; 1-year follow-up, 92.1 ± 7.6; p = 0.001) and VAS scores (preoperative, 5.5 ± 0.7; 1-year follow-up, 1.9 ± 0.8; p = 0.001) (Fig. 2). Although preoperative FAOS was not evaluated, the average FAOS at 1-year follow-up was 89.1 ± 4.9 (Fig. 3). All weight-bearing ankle radiographs of the graft and donor sites did not reveal arthritic change in the ankle joint, lateral talar dome collapse, and graft-site delayed union or nonunion at 1 year after surgery. Donor-site healing was observed on ankle anteroposterior and simple weight-bearing lateral radiographs (Fig. 4).
Fig. 2. Bar graphs show clinical outcomes evaluation by comparing the visual analog scale (VAS; A) and American Orthopaedic Foot and Ankle Society (AOFAS; B) scores. *Statistical significance, p < 0.05.
Fig. 3. Average Foot and Ankle Outcome Score (FAOS), including 5 subscales and total scores at the final follow-up.
Fig. 4. Serial ankle lateral simple radiographs of the lateral talar articular donor site. Radiographs immediately after surgery (A), at 7 months after surgery (B), and at 12 months after surgery (C). Arrows indicate the lateral talar donor site.
Furthermore, no infections, neurovascular injuries, or other complications were noted at the graft and donor sites. In 4 of 11 cases, a follow-up ankle MRI was performed after removing 2 cannulated screws to fix the osteotomy at least 1 year after OAT (Table 3). In the harvested area, the follow-up MRI revealed 50%–100% filling, mild-to-absent bone marrow edema, and mild-to-moderate joint effusion in the harvested area. Furthermore, in 3 out of the 4 patients, the signal of the harvested area on the follow-up MRI indicated normal or nearly normal findings. The average score on the follow-up MRI was 7 points (range, 6–9 points).
Table 3. Follow-up Magnetic Resonance Imaging Scores.
| No. | Score | ||||
|---|---|---|---|---|---|
| Fill of the repair site | Signal at the repair site | Bone marrow edema | Effusion | Total score | |
| 4 | 2 | 2 | 1 | 1 | 6 |
| 7 | 2 | 3 | 2 | 2 | 9 |
| 9 | 1 | 2 | 1 | 2 | 6 |
| 11 | 2 | 2 | 1 | 2 | 7 |
| Average | 1.75 | 2.25 | 1.25 | 1.75 | 7 |
The number of each patient corresponds to the patient number in Table 1.
DISCUSSION
Various treatment strategies and techniques have been developed for OLTs. Common nonsurgical treatments for OLTs include immobilization, rest, and using medications such as nonsteroidal anti-inflammatory drugs.23) Surgical treatments include excision of the fragment,3) curettage with or without drilling,1,24) microfracture,2,25) filling with a cancellous bone graft,5,26) autologous chondrocyte implantation (ACI),27) and OAT.6,15) For smaller lesions, bone marrow stimulation procedures such as arthroscopic drilling and microfracture are generally accepted as the first-line treatment.1) As this technique results in fibrocartilaginous tissue formation for repairing the osteochondral defect, it has not shown satisfactory outcomes for larger lesions, those with subchondral cysts, or cases with failed bone marrow stimulation.23,27) These outcomes resulted in the development of surgical techniques to reconstruct the hyaline cartilage, such as osteochondral allograft implantation,28) mosaicplasty,29) ACI,27) and OAT.5,6,30) Among these techniques, successful outcomes have been obtained following the use of osteochondral autogenous grafts from the intercondylar notch of the knee or a non-weight-bearing region of the femoral condyle.5,6,30) Nonetheless, donor-site complications of the knee joint have been noted in up to 15%–16% of cases.4,7,8,9,10,11) Therefore, harvesting from the non-weight-bearing talar articular facet is considered to reduce joint-related donor-site complications.4)
In 2002, Sammarco and Makwana15) first reported harvesting osteochondral autologous transplants from the local talar articular facets. The technique involved creating a wedge-shaped bone block on the anterior plafond of the distal tibia. After removing the bone block, the anterior talar articular facet was harvested. This study demonstrated good short-term results without donor-site complications. Among the 12 patients, 1 patient underwent secondary surgery for arthroscopic debridement of the anterior aspect of the tibial bone block. However, the distal tibial articular surface healed without any defect overall.
In 2016, Georgiannos et al.31) introduced this technique after primary bone marrow stimulation failed. In most cases, the osteochondral graft was harvested from the anterior articular facet on the same side of the OLT by creating a bone block in the anterior tibia. Two of the 46 patients underwent secondary surgery for arthroscopic anterior tibial bone block osteophyte excision. Despite some osteophyte-related complications, there were no complications at the graft or donor site at the talus.
In 2022, Wan et al.14) reported a technique similar to OAT, using the ipsilateral talar articular facet as the donor site. In this study, the medial talar articular facet was the harvest site for the OLT of the medial talar dome through medial malleolar osteotomy. In contrast, in lateral talar dome lesion cases, the graft was harvested from the lateral talar articular facet through fibular osteotomy. Two of the 24 patients underwent secondary surgery with arthroscopic debridement of soft-tissue impingement from the medial osteotomy site. However, there were significant improvements in their clinical scores, including VAS and AOFAS scores.
In the 3 studies mentioned above, an osteochondral autologous graft harvested from the ipsilateral talar articular facet for OLT treatment was suggested, and most cases showed significant improvements in clinical scores. However, complications of impingement around the osteotomy site or lateral malleolus were noted. A wedge-shaped bone block of the anterior surface of the distal tibia was used to expose the osteochondral lesions. In the present study, osteochondral lesions were exposed through a chevron-shaped medial malleolar osteotomy, and osteochondral grafts were harvested from the lateral talar articular facet. Subsequent simple ankle radiographs showed no impinging osteophytes at the medial osteotomy site. Unlike previous studies in which the graft was harvested from the ipsilateral talar articular facet on the same side of the OLT’s location, this study used the ipsilateral lateral talar articular facet as the donor site for the medial OLT. Although an additional incision was made to harvest the osteochondral graft from the lateral talar articular facet, the approach to the lateral talar articular facet did not take a substantially long time because the additional incision was similar to the incision used in the modified Brostrom operation. Furthermore, in all 11 cases, no complications such as infection, pain, and other wound problems related to the additional incision on the lateral ankle were observed.
In 2006, Kreuz et al.16) harvested the osteochondral graft from the talar articular surface on the same side of the OLT, and the graft’s diameter could not exceed 8 mm. However, in this study, because the lateral talar articular facet was used as the donor site for medial OLT, there was sufficient space to harvest a graft measuring up to 10 mm in diameter, with no additional osteotomy required. Although there were no complications, such as necrosis or collapse of the grafted site, harvesting from the other side of the OLT in the same talus improved safety and stability.
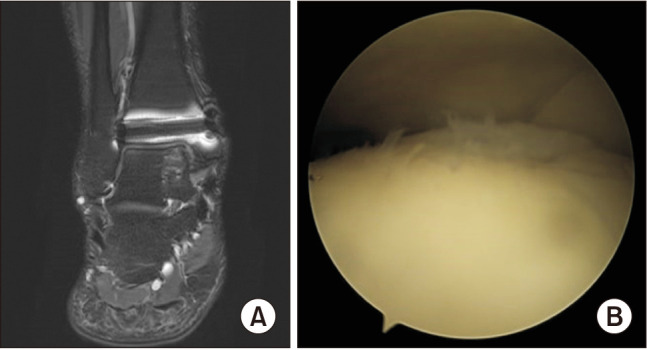
Radiographs of all patients revealed no complications at the transplantation, donor, or osteotomy sites. In this study, follow-up ankle MRI was not routinely performed. However, in 4 of the 11 cases, a follow-up ankle MRI was performed after removing 2 cannulated screws to fix the osteotomy at least 1 year after OAT. A follow-up ankle MRI revealed medial talar graft-site healing and lateral talar donor-site healing. In evaluating lateral talar donor site, the average score on follow-up MRI was 7 points (range, 6–9 points) according to the scoring system by Henderson et al.21) Similarly, Iwasaki et al.22) analyzed follow-up MRI scans of the donor site in the knee after autologous osteochondral mosaicplasty for capitellar osteochondritis dissecans according to the scoring system by Henderson et al.21) and reported an overall score of 6.7 points on followup MRI in the harvested area. There were no other lateral talar dome complications around the donor site (Fig. 5). As 9 of the 11 patients complained of some discomfort on the affected side of the ankle, a second-look ankle arthroscopic examination was performed at the time of screw removal. Mild synovial hypertrophy was commonly observed, and no other specific findings were observed after synovectomy in any of these cases. After screw removal, those patients did not complain of any discomfort. The graft appeared well-attached to the original bed and maintained continuity with the surrounding cartilage in follow-up MRI and arthroscopy (Fig. 6). Furthermore, there were significant improvements in the clinical scores, including the VAS and AOFAS scores, between the preoperative and final follow-up.
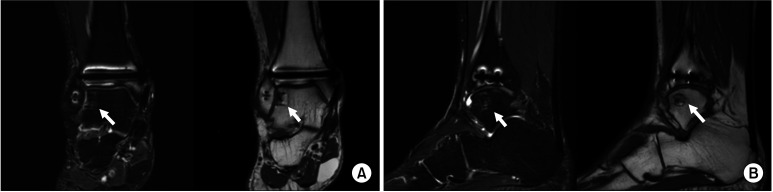
Fig. 5. Follow-up ankle magnetic resonance imaging at 12 months after osteochondral autologous transplantation, focusing on the lateral talar donor site. (A) T2 fat suppression and T1-weighted coronal view. (B) T2 fat suppression and T1-weighted sagittal view. Although a slight signal change was observed, there was no evidence of donor-site complications such as lateral talar dome collapse, osteonecrosis, or infection. Arrows indicate the lateral talar articular facet donor site.
Fig. 6. Follow-up ankle magnetic resonance imaging (MRI) and arthroscopy focusing on the medial talar graft site. (A) Ankle MRI, T2 fat suppression coronal view. (B) Ankle arthroscopy, medial talar graft site.
It has been commonly accepted that the OAT is a suitable treatment option for patients whose size of the OLT is over 150 mm2 in area or 15 mm in diameter or who have subchondral cystic lesions.32) Hannon et al.33) reported that bone marrow stimulation is not a proper treatment option for patients whose size of the OLT is over 10 mm in diameter and 5 mm in depth. Scranton34) identified that OLT sized 8 to 12 mm in diameter is ideal for OAT. In the present study, all lesions included subchondral cysts and were over 5 mm in depth and 8 mm in diameter.
There were several limitations in this study. First, this study was retrospective and used data from a single medical center. Second, the total number of patients was small, and the duration of the research was relatively short. Finally, not all patients took follow-up ankle computed tomography (CT) or MRI. Although ankle CT is a valuable tool for confirming the bony union of the grafted and donor sites, other complications, including arthritic change, were not detected in all patients on simple radiography, and there were no nonunion-related symptoms.
In conclusion, in this study, harvesting autografts from the ipsilateral lateral talar articular facet for medial OLTs was an effective technique with good clinical results. For a single medial OLT, harvesting autografts from the ipsilateral lateral talar articular facet without knee donor-site morbidities can be a good alternative in OAT for OLTs.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Barnes CJ, Ferkel RD. Arthroscopic debridement and drilling of osteochondral lesions of the talus. Foot Ankle Clin. 2003;8(2):243–257. doi: 10.1016/s1083-7515(03)00016-0. [DOI] [PubMed] [Google Scholar]
- 2.Becher C, Thermann H. Results of microfracture in the treatment of articular cartilage defects of the talus. Foot Ankle Int. 2005;26(8):583–589. doi: 10.1177/107110070502600801. [DOI] [PubMed] [Google Scholar]
- 3.Verhagen RA, Struijs PA, Bossuyt PM, van Dijk CN. Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin. 2003;8(2):233–242. doi: 10.1016/s1083-7515(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 4.Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238–246. doi: 10.1007/s00167-009-0942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolker D, Murray M, Wilson M. Osteochondral defects of the talus treated with autologous bone grafting. J Bone Joint Surg Br. 2004;86(4):521–526. [PubMed] [Google Scholar]
- 6.Scranton PE, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br. 2006;88(5):614–619. doi: 10.1302/0301-620X.88B5.17306. [DOI] [PubMed] [Google Scholar]
- 7.Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med. 2009;37 Suppl 1:105S–1011S. doi: 10.1177/0363546509351481. [DOI] [PubMed] [Google Scholar]
- 8.Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A Suppl 2:25–32. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- 9.Reddy S, Pedowitz DI, Parekh SG, Sennett BJ, Okereke E. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med. 2007;35(1):80–85. doi: 10.1177/0363546506290986. [DOI] [PubMed] [Google Scholar]
- 10.Gautier E, Kolker D, Jakob RP. Treatment of cartilage defects of the talus by autologous osteochondral grafts. J Bone Joint Surg Br. 2002;84(2):237–244. doi: 10.1302/0301-620x.84b2.11735. [DOI] [PubMed] [Google Scholar]
- 11.LaPrade RF, Botker JC. Donor-site morbidity after osteochondral autograft transfer procedures. Arthroscopy. 2004;20(7):e69–e73. doi: 10.1016/j.arthro.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Buckley MR, Bonassar LJ, Cohen I. Localization of viscous behavior and shear energy dissipation in articular cartilage under dynamic shear loading. J Biomech Eng. 2013;135(3):31002. doi: 10.1115/1.4007454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henak CR, Ross KA, Bonnevie ED, et al. Human talar and femoral cartilage have distinct mechanical properties near the articular surface. J Biomech. 2016;49(14):3320–3327. doi: 10.1016/j.jbiomech.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Wan DD, Huang H, Hu MZ, Dong QY. Results of the osteochondral autologous transplantation for treatment of osteochondral lesions of the talus with harvesting from the ipsilateral talar articular facets. Int Orthop. 2022;46(7):1547–1555. doi: 10.1007/s00264-022-05380-7. [DOI] [PubMed] [Google Scholar]
- 15.Sammarco GJ, Makwana NK. Treatment of talar osteochondral lesions using local osteochondral graft. Foot Ankle Int. 2002;23(8):693–698. doi: 10.1177/107110070202300803. [DOI] [PubMed] [Google Scholar]
- 16.Kreuz PC, Steinwachs M, Erggelet C, Lahm A, Henle P, Niemeyer P. Mosaicplasty with autogenous talar autograft for osteochondral lesions of the talus after failed primary arthroscopic management: a prospective study with a 4-year follow-up. Am J Sports Med. 2006;34(1):55–63. doi: 10.1177/0363546505278299. [DOI] [PubMed] [Google Scholar]
- 17.Hanselman AE, Cody EA, Easley ME, Adams SB, Parekh SG. Avascular necrosis of the talus after subchondroplasty. Foot Ankle Int. 2021;42(9):1138–1143. doi: 10.1177/10711007211005435. [DOI] [PubMed] [Google Scholar]
- 18.Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41:988–1020. [PubMed] [Google Scholar]
- 19.Hepple S, Winson IG, Glew D. Osteochondral lesions of the talus: a revised classification. Foot Ankle Int. 1999;20(12):789–793. doi: 10.1177/107110079902001206. [DOI] [PubMed] [Google Scholar]
- 20.Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974–1980. doi: 10.1177/0363546509335765. [DOI] [PubMed] [Google Scholar]
- 21.Henderson IJ, Tuy B, Connell D, Oakes B, Hettwer WH. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br. 2003;85(7):1060–1066. doi: 10.1302/0301-620x.85b7.13782. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki N, Kato H, Kamishima T, Suenaga N, Minami A. Donor site evaluation after autologous osteochondral mosaicplasty for cartilaginous lesions of the elbow joint. Am J Sports Med. 2007;35(12):2096–2100. doi: 10.1177/0363546507306465. [DOI] [PubMed] [Google Scholar]
- 23.Canale ST, Belding RH. Osteochondral lesions of the talus. J Bone Joint Surg Am. 1980;62(1):97–102. [PubMed] [Google Scholar]
- 24.Robinson DE, Winson IG, Harries WJ, Kelly AJ. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Br. 2003;85(7):989–993. doi: 10.1302/0301-620x.85b7.13959. [DOI] [PubMed] [Google Scholar]
- 25.Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus): review of the literature and new surgical approach for medial dome lesions. Foot Ankle. 1985;5(4):165–185. doi: 10.1177/107110078500500403. [DOI] [PubMed] [Google Scholar]
- 26.Draper SD, Fallat LM. Autogenous bone grafting for the treatment of talar dome lesions. J Foot Ankle Surg. 2000;39(1):15–23. doi: 10.1016/s1067-2516(00)80059-9. [DOI] [PubMed] [Google Scholar]
- 27.Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 28.El-Rashidy H, Villacis D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: a retrospective review. J Bone Joint Surg Am. 2011;93(17):1634–1640. doi: 10.2106/JBJS.J.00900. [DOI] [PubMed] [Google Scholar]
- 29.Hangody L. The mosaicplasty technique for osteochondral lesions of the talus. Foot Ankle Clin. 2003;8(2):259–273. doi: 10.1016/s1083-7515(03)00017-2. [DOI] [PubMed] [Google Scholar]
- 30.Seow D, Shimozono Y, Gianakos AL, et al. Autologous osteochondral transplantation for osteochondral lesions of the talus: high rate of return to play in the athletic population. Knee Surg Sports Traumatol Arthrosc. 2021;29(5):1554–1561. doi: 10.1007/s00167-020-06216-w. [DOI] [PubMed] [Google Scholar]
- 31.Georgiannos D, Bisbinas I, Badekas A. Osteochondral transplantation of autologous graft for the treatment of osteochondral lesions of talus: 5- to 7-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3722–3729. doi: 10.1007/s00167-014-3389-3. [DOI] [PubMed] [Google Scholar]
- 32.Flynn S, Ross KA, Hannon CP, et al. Autologous osteochondral transplantation for osteochondral lesions of the talus. Foot Ankle Int. 2016;37(4):363–372. doi: 10.1177/1071100715620423. [DOI] [PubMed] [Google Scholar]
- 33.Hannon CP, Bayer S, Murawski CD, et al. Debridement, curettage, and bone marrow stimulation: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1_lsuppl):16S–22S. doi: 10.1177/1071100718779392. [DOI] [PubMed] [Google Scholar]
- 34.Scranton PE. Osteochondral lesions of the talus: autograft and allograft replacement. Tech Foot Ankle Surg. 2004;3(1):25–39. [Google Scholar]