Abstract
Vascular calcification (VC) is a complex process of calcium deposition on the arterial wall and atherosclerotic plaques and involves interaction between vascular smooth muscle cells, inflammatory and VC mediators. The latter are independent predictors of cardiovascular morbidity and mortality and potential targets of pharmaceutical therapy. This paper is a narrative review of the complex mechanisms of VC development and in this context the potential anti-atherosclerotic effects of statins. At the initial stages of atherosclerosis VC correlates with atherosclerosis burden and in the long-term with cardiovascular morbidity and mortality. A plethora of animal and clinical studies have proposed statins as the cornerstone of primary and secondary prevention of atherosclerotic cardiovascular disease. Based on coronary computed tomography data, high doses of statins may have negligible or even positive effects on the progression of coronary artery calcification. Growing data support an increase in atherosclerotic plaque calcification in peripheral arteries (e.g., carotids), after long-term, statin-therapy. Despite the paradox of increasing VC, those effects of statins have been associated with higher plaque stability, reducing the risk of consequent adverse events. Statins seem to promote a “favorable” atherosclerotic calcification, suppressing atherosclerotic lesion expansion and their vulnerability. More studies are required to clarify the underlying mechanisms.
Keywords: arterial calcification, statins, atherosclerotic plaque, atherosclerotic plaque calcification, plaque vulnerability
1. Introduction
The term “vascular calcification” (VC) is essentially synonymous with “arterial calcification”, and describes the deposition of calcium phosphate mainly in the form of hydroxyapatite [1], and refers to two different types: atherosclerotic intimal calcification and medial calcification known as Mönckeberg’s sclerosis [2]. These two forms of calcification, which may coexist, present different localization, morphology, predisposing factors and pathophysiological effects, but both lead to increased morbidity and mortality [3].
Atherosclerotic intimal calcification, accompanied by cholesterol deposition, has been associated with atherosclerotic occlusive lesions. This process is the predominant type of calcification observed in the aorta, coronary and peripheral arteries [4, 5] and is correlated with the presence of vascular smooth muscle cells (VSMCs), as well as macrophages in lipid-rich atherosclerotic plaques [6, 7, 8]. Atherosclerotic intimal calcification is associated with classic cardiovascular risk factors like age, male sex, smoking, hypertension, dyslipidemia, diabetes mellitus as well as newer ones, such as inflammation. It is focal and the adjacent vascular wall may be free of lesions. It gradually leads to narrowing of the arterial lumen, while an imbalanced deposition of calcium in certain areas of the atherosclerotic plaques renders them vulnerable, meaning they are susceptible to rupture and consequent thrombosis. Atherosclerotic cardiovascular diseases (ASCVDs) represent the clinical manifestations of atherosclerotic calcification, such as coronary artery disease (CAD) and peripheral arterial disease (PAD), while the most common acute events of plaque destabilization are myocardial infarction and stroke. There is a complex interplay between VC, lipids metabolism and atherosclerosis progression. Statins are widely used for primary and secondary prevention of ASCVDs. Therefore, dyslipidemia alleviation by statins may become an important tool to modify VC development.
Mönckeberg’s arteriosclerosis, or Mönckeberg’s sclerosis (MScl), is a form of arteriosclerosis or vessel hardening, where calcium deposits are found in the muscular middle layer of the arterial walls (the tunica media). This condition occurs as an age-related degenerative process, but it is particularly common in diabetic and uremic patients [9, 10]. The accumulation of calcium phosphate crystals significantly contributes to vascular disease, and worsens further along with the progression of kidney dysfunction, especially in patients undergoing hemodialysis. For this reason, it has long been assumed that the medial VC results from calcium deposits and leads to vascular stiffening [2, 11, 12].
Several non-invasive imaging techniques can be employed for the screening of VC such as x-rays of the abdomen and extremities and two-dimensional ultrasound to identify the presence of calcification in carotid and femoral arteries, and the aorta [13, 14]. However, quantification of the calcification is not feasible through these techniques. Alternatively, electron beam computed tomography (EBCT) and multi-slice computed tomography (MSCT) have emerged as tools for the precise evaluation of VC [15, 16]. More recently, MSCT is capable of the accurate detection and quantification of VC using scores such as the Agatston [17] and volume score [18].
The aim of the present manuscript was a narrative literature review of the currently known pathophysiologic mechanisms of VC and most importantly to evaluate the impact of statins on them. For this purpose, we analyzed the potential mechanistic explanations of statins’ effects on intimal artery calcium deposition within atherosclerotic plaques, on the medial artery wall layer and their relationship with clinical outcomes.
2. Search Strategy
A search was conducted for English language publications in MEDLINE and Embase databases from January 1990 to June 2023. The following broad search terms, including Medical Subject Headings, were used: statins, lipid-lowering, vascular calcification, intimal and medial calcification, atherosclerotic plaques, imaging techniques, calcium index, calcium score, Agatston score, echogenicity, gray-scale median (GSM) score. Except for case studies, all other types of preclinical (in vitro and animal) and clinical studies (randomized, non-randomized, prospective, retrospective) were considered eligible. The articles’ reference list was checked to identify additional relevant papers for inclusion.
3. Pathophysiologic Mechanisms of Atherosclerotic and Arterial Calcification in Relation to Statins
The evidence behind a causal relationship between lipoproteins and bone pathologies is conflicting, but the underlying mechanisms are clearly similar which requires a deeper insight into lipid-lowering drugs, like statins, in VC.
3.1 Vascular Calcification Types
VC resembles bone mineralization and presents into 2 types:
(1) Atherosclerotic-related calcification: this is associated with intimal artery calcification and the early stages of this process are characterized by the development of micro-calcification. Calcium phosphate hydroxyapatite crystals are deposited into the extracellular matrix of atherosclerotic lesions, and their accumulation varies between patients. Spotty distribution of bone mineralization within atherosclerotic plaques has been associated with clinical manifestations, such as acute coronary events and cardiovascular death in the long term [19]. The dynamic process of microcalcification is indicative of the risk of plaque rupture and unfavorable clinical outcomes [20]. On the other hand, macro-calcification of the atherosclerotic plaques, characterized by the deposition of large amounts of calcium, gradually decreases the lumen patency and creates a more stable phenotype [21]. This is a dual-edged sword, because heavily calcified plaques are stiff and less amenable to transcutaneous revascularization, but they appear with a low propensity to rupture. Perhaps, the inhomogeneous texture of the plaques leads to variable resistance to the hemodynamic forces in the bloodstream, which increases their vulnerability. Statins may change the localization of calcium microdeposits enhancing their deposition around the necrotic core of the atherosclerotic plaque which stabilizes the lesions [22].
(2) Medial calcification (MScl): It is usually circumferential, located in the medial layer. Most frequently is observed in diabetes mellitus and chronic kidney disease and its clinical significance is the subject of debate [9, 23]. The clinical repercussions are rare because the reduction of the lumen is minimal, unless it is overlapped by an atherogenic process, where the clinical manifestations become more evident [24, 25]. This is a bone-like morphology of the artery wall. It is believed that the lesion is produced by the fatty degeneration of the VSMCs of the middle layer, forming a mass that undergoes hyaline degeneration which then becomes calcified. MScl is not related to atherosclerosis, and its link with dyslipidemia is not established. Statins cannot alter MScl [26] and there are no current data regarding the impact of statins on arterial calcification. For that reason, our review focused primarily on the intimal artery calcification which relates to dyslipidemia and statins exert significant effects.
3.2 Inflammation
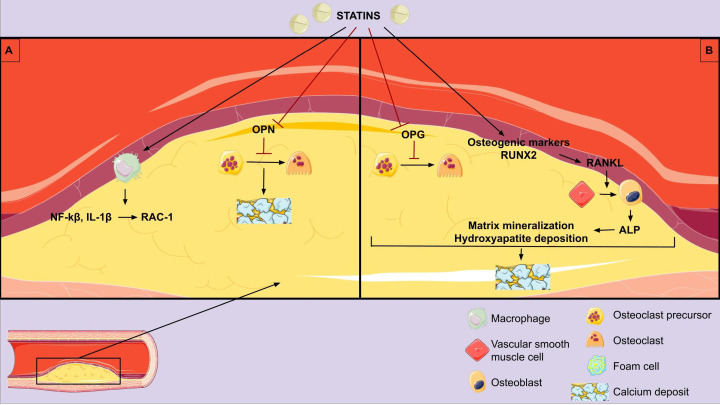
Inflammation plays a critical role in the advancement of atherosclerosis and contributes to approximately 20% to 30% of the remaining risk for adverse cardiovascular events, often linked to the rupture of unstable coronary plaques. This relationship is supported by various studies [27, 28]. Systemic inflammatory disorders are associated with an enhanced risk of adverse events and early ASCVDs [29, 30]. In the early stage of atherosclerosis, inflammation is the predominant pathophysiological mechanism that promotes plaque progression and calcification [31]. The repeated cycles of inflammatory damage and repair ultimately lead to calcification of the initial atherosclerotic plaques, whose progress provides an important estimate of clinical prognosis [12]. Inflammation also plays an important role in the calcification process, while macrophages, neutrophils, and T cells promote extracellular matrix remodeling,osteogenic differentiation and apoptosis of VSMCs [22, 32]. A recent experimental study showed that statin therapy is related to increased coronary artery calcification by stimulating inflammasome and macrophages, leading to nuclear factor-k (NF-k) activation and the secretion of IL-1 mRNA and Rac1-depended IL-1 protein [33, 34]. Racs are small GTPases and signal inflammatory transducers affecting the expression of growth factors and cytokines. The anti-inflammatory actions of statins have long been proven [35]. Statins disrupt Rac1 isoprenylation by inhibiting geranylgeranyl diphosphate synthesis and promote plaque Rac-1 activation and expression of osteogenic markers, alkaline phosphate (ALP) and runt-related transcription factor 2 (RUNX2) [34]. RUNX2 promotes the differentiation of VSMCs to osteoblast-like cells by upregulating receptor activator of NF-k ligand (RANKL) and creating a microenvironment suitable for capture of phosphates for mineralization [36]. Moreover, statin therapy enhances the healing process against atherosclerotic plaque inflammation by activating M2 (anti-inflammatory phenotype) macrophages, resulting in increased plaque calcification across its volume regression [37].
3.3 Vascular Calcification Inhibitors
Until recently, it was commonly believed that VC was a passive and degenerative process. A variety of factors are involved in the active ossification process of the arterial wall, which either act protectively by inhibiting the calcification of the arterial wall: fetuin-A, Matrix GLa Protein (MGP), osteopontin (OPN), osteoprotegerin (OPG), inorganic pyrophosphate (PPi), or by precipitating calcium and phosphorus deposition through the regulation of bone metabolism: vitamin D, parathormone (PTH), PTH related peptide (PTHrP), Receptor Activator of Nuclear factor B (RANK), RANK Ligand (RANKL), bone morphogenetic proteins 2 and 7 (BMP2 and BMP7) [22], involved at different stages of VC process [38, 39]. OPG, a member of the tumor necrosis factor (TNF) family, is known for its ability to inhibit the formation of bone-resorbing cells called osteoclasts [40]. Recent research has revealed that OPG is produced in various tissues, including the heart and arteries [41, 42]. The loss of other local and circulating calcification inhibitors, like fetuin-A, osteopontin, and matrix Gla protein (MGP) may also contribute to VC formation [33]. Those inhibitors behave as decoy ligands for both minerals and calcification proteins. Though osteoclasts stimulation those inhibitors suppress the VC progression rather than actively decalcify the already existing lesions [34]. Both clinical and animal studies have demonstrated a connection between OPG and atherosclerotic cardiovascular diseases (ASCVDs). Moreover, investigations have been conducted to examine the association between OPG, other VC inhibitors (e.g. OPN) and plaque characteristics [43, 44, 45].
Limited data from genetic polymorphisms of some of the aforementioned factors have been found to be involved in 40-50% cases of coronary arterial calcification [46, 47]. Although the data from genetic analysis are limited, they make more robust the evidence for the contribution of bone metabolism to VC. For example, the CD73 gene deficiency generates a loss of its activity and triggers the tissue-nonspecific alkaline phosphatase, a key protein for bone formation and the main conductor of the medial VC [48].
3.4 VMSCs Differentiation and Apoptosis
The medial layer of the vessel wall is composed of smooth muscle cells and elastin-rich extracellular matrix. One of the major mechanisms of arterial calcification includes the differentiation of VSMCs into osteoblast-like cells. This is regulated by the protein Cfba1/Runx2 (core-binding factor subunit 1/runt-related transcription factor 2) [49, 50], high extracellular phosphate levels [51] and BMPs. Additional factors such as oxidative stress, oxidized lipids, and inflammatory cytokines trigger the differentiation of VSMCs and thereby increase the sources of calcium deposition [52]. Under pathological conditions, like calcium overload, high pro-inflammatory milieu, and atherosclerosis, the osteoblast-like VSMCs release matrix vesicles (MVs) in the extracellular matrix leading to mineral deposition forming foci of metallic calcium nuclei [53]. Extracellular MVs are tiny nanoparticles enclosed in phospholipids carrying calcium, phosphate, and matrix proteins. Those MVs either promote or hinder mineralization, depending on their content of inhibitory proteins, such as matrix -carboxyglutamic acid protein (MGP). The latter belongs to a family of vitamin K-dependent proteins and contains -carboxylated glutamate residues [54]. Among others, ALP, produced by cells resembling osteoblasts, plays a role in matrix mineralization and the deposition of hydroxyapatite. It accomplishes this by breaking down inorganic pyrophosphate, a major inhibitor of calcium phosphate nucleation [55]. In addition to differentiation, damaged VSMCs may release apoptotic bodies, which further contribute to VC by accumulating calcium [56]. Experimental studies examining the impact of statins on the VC process have yielded inconsistent findings. For instance, in a model of human VSMCs involving inflammatory VC, statins demonstrated a dose-dependent inhibition of calcification [57]. Conversely, in an experiment where VSMCs from the aortas of Wistar rats were incubated in a specialized calcification medium, atorvastatin showed a dose-dependent stimulation of calcification. Furthermore, atorvastatin also induced significant apoptosis of the VSMCs [58]. In vitro cell culture studies have also indicated that statins stimulate calcium deposition in VSMCs and mesenchymal stem cells [58, 59].
Matrix metalloproteinases (MMPs) have been long validated as important contributors of atherosclerosis development, progression, and destabilization, while preliminary data implicate their role in VC [60]. Statins may act as a stabilizing factor by inhibiting the secretion of MMPs from VSMCs and inflammatory cells, with still unknown impact on VC [61, 62].
3.5 Other Mechanisms
Okuyama et al. [63] declared that despite the current belief that cholesterol reduction with statins decreases atherosclerosis, simultaneously statins lead to coronary artery calcification as mitochondrial toxins impair muscle function in the heart and blood vessels through the depletion of coenzyme Q10 and ‘heme A’, and thereby ATP generation. Statins possess a mechanism that inhibits the synthesis of vitamin , which is the co-factor for matrix Gla-protein activation, which in turn protects arteries from calcification [63]. An experimental study examined the effects of pravastatin on VC in mice and showed that pravastatin-treated groups had a larger number of alkaline phosphate (ALP) positive cells, suggesting a possible increase in osteoblast-like cells in those mice [19]. The pathophysiologic mechanisms of atherosclerotic calcification are summarized in Fig. 1.
Fig. 1.
Pathophysiologic mechanisms of statins on vascular calcification. (A) Anti-inflammatory effect and vascular calcification promotion. (B) Direct vascular calcification promotion. OPN, osteopontin; OPG, osteoprotegerin; NF-B, nuclear factor kappa light chain enhancer of activated B cells; IL-1, interleukin-1; RUNX2, runt-related transcription factor 2; RANKL, receptor activator of nuclear factor kappa- ligand; ALP, alkaline phosphatase.
4. Detection of Arterial and Atherosclerotic Calcification
4.1 X-Ray
Standard single energy and standard dual energy chest x-rays is a low cost, low radiation diagnostic modality that can detect arterial calcifications. The chest x-ray is an already ordered procedure in almost all patients, and advanced processing has been created to enable the detection of coronary arteries calcium [64]. Also, aortic arch and peripheral artery calcification can be detected readily and reproducibly using x-rays and its presence is an independent predictor of arterial and atherosclerotic calcification, indicating CAD severity [65]. Furthermore, breast arterial calcification has been detected during mammogram screening and has been correlated with arterial calcification in the extremities and in other arterial beds, being a predictor of ASCVDs [21]. VC seen in x-rays may implicate an increased atherosclerotic calcification which cannot be distinguished from MScl.
4.2 Computed Tomography (CT)
It continues to be the most reliable and sensitive non-invasive tool for coronary and peripheral artery calcification. Since the 1940s, calcium found in the coronary arteries has served as a surrogate marker of CAD. Its presence and progression have been linked to a higher cardiovascular risk [66]. With the advances in imaging modalities, we can now detect and quantify more accurately coronary artery calcification (CAC) for cardiovascular risk assessment. For CAC quantification there are three semi-quantitative scores available: the mass equivalent score, the volume score, and the commonly utilized Agatston score. Those scoring methods exhibit a strong correlation with each other [67]. Among them, the Agatston scoring method calculates the CAC value by multiplying the area of calcified plaque by the density score. It is a widely known, valid, approach and has been endorsed by all recent international guidelines for major cardiovascular risk assessment [68]. CAC can be identified through electron beam computed tomography (EBCT) [69] and multi-slice CT [70]. These methods offer rapid scanning and processing times, taking approximately a few minutes. Additionally, the radiation dose involved is low, around 1 mSv, and there is no requirement for a contrast agent. Magnetic resonance angiography has also been employed in medical settings. However, assessment of the coronary arteries remains a challenge due to several factors such as the small size of the vessels, extended acquisition time, and the intrinsic motions caused by cardiac contractions and respiration [71]. On the other hand, CT quantification of peripheral arteries has not been clinically applicable and there is less robust evidence with scarce data. In this case, a more qualitative approach is performed in clinical practice.
4.3 Peripheral Arteries Ultrasound
It is considered a radiation free, cost effective and easily repeatable technique [72]. It is well known that there is a strong association of carotid plaque echolucency with the histologic content of vulnerable carotid plaques and the subsequent risk of a cerebrovascular event [73]. Plaque echogenicity is directly associated with the degree of calcification and fibrous tissue, and inversely associated with the lipid content of the plaque. Several scores have been proposed. In the Gray Weale-Nicolaides (GWN) classification lipid-rich plaques appear echolucent, while those with fibrous and calcific content appear echogenic [74, 75]. The Gray Scale Median (GSM) score constitutes the quantified measurement of plaque echogenicity and an important, objective, valid, marker of carotid plaque vulnerability [76] associated with increased cardiovascular mortality [77]. The GSM represents the median of the frequency distribution of tones of pixels included in the plaque areas. In other words, GSM is a median value of pixel brightness of the plaque, ignoring focal variability of the atherosclerotic lesion [76]. There are a number of factors which may influence the GSM score calculation, including the physical distance from the transducer and the consistency of the atherosclerotic plaque [73].
4.4 Intravascular Ultrasound
Also, intravascular ultrasound (IVUS) is an invasive diagnostic modality for detecting coronary calcification and stratifying plaque stability [42]. A high frequency ultrasound transducer–containing catheter is placed within the coronary arterial lumen, which detects calcium as significantly echogenic areas and provides detailed information about the distribution and nature of plaque burden. The sensitivity and specificity of IVUS detecting coronary calcification are 90% and 100%, respectively [32]. However, calcium measurement with IVUS is semi-quantitative technique limited by ultrasound’s inability to penetrate the calcium deposits [19].
4.5 Positron Emission Tomography
Recently, there has been a significant focus on utilizing 18F-NaF positron emission tomography (PET) for the examination of VC in various arteries, including the coronary arteries, the aorta, and the carotid arteries. This technique gained considerable recognition, but its usage remains limited in clinical routine for the understanding of the underlying processes of plaque formation [78, 79].
5. Clinical Significance of Vascular Calcification
VC is considered an actively regulated and complex process. Although highly correlated to increasing age, both types of calcification (intimal and medial) are associated with different pathological conditions such as Type 1 (T1D) and Type 2 diabetes (T2D), [80] metabolic syndrome [81], chronic kidney disease (CKD) [82], and osteoporosis affecting postmenopausal women [83]. In diabetic patients CAC has shown strong predictive value during mid and long-term follow-up compared to established risk scores, like the UK Prospective Diabetes Study (UKPDS) risk score [84]. Nevertheless, the addition of statins is not related to more rapid progression of CAC among diabetic patients [85]. Novel biomarkers have been proposed as indices of coronary calcification in CKD patients, which is of clinical importance [86]. The occurrence of arterial calcification varies significantly based on the age and gender of the individual. Research studies indicated that nearly 90% of men and 67% of women aged over 70 will develop this condition [87, 88, 89]. Factors such as increased body mass index, high blood pressure, imbalanced lipid profile (elevated LDL and TG levels), diabetes mellitus, and metabolic syndrome predispose to arterial calcification. Additionally, genetic predisposition and CKD can also contribute to arterial calcification, as identified in studies conducted by Kronmal et al. and Liu et al. [89, 90].
Following the results of the MESA study, including 6722 patients of four different ethnic groups, Caucasian men were most likely to be identified with higher scores of CAC 0 (70.4%), followed by Chinese men (59.2%), Hispanic men (56.6%) and black men (52%). Caucasian women also presented the higher percentage (44.7%). The calcium index is found to be higher in men compared to women, while it increases steadily with age [91]. After a mean follow-up period of 10 years, a CAC score of zero was shown to be the strongest, and by far, the most negative predictor for the occurrence of a cardiovascular event among other clinical, biochemical, and imaging parameters with known prognostic value [92]. CAC is an independent predictor for all-cause mortality independent of diabetic status. More than 70% of men and 50% of women with T1D are affected by CAD with the predominant risk factor the development of CAC by their mid-forties [93].
Hyperphosphatemia is highly prevalent in patients with CKD—particularly among those with advanced or end-stage renal disease—and it aggravates as the disease progresses and glomerular filtration rates decline. This impairment, especially in CKD patients undergoing hemodialysis (CKD-HD), leads to phosphorus precipitation with serum calcium and consequently to calcium phosphate deposits [94]. Increased rigidity of arterial walls and cardiac calcifications are serious complications and may increase a patient’s cardiovascular risk [95]. Arterial and atherosclerotic calcification are both very common not only in peripheral disease, but as well in coronary arteries among patients with CKD-HD [32, 42], leading to a higher risk of cardiovascular morbidity and mortality in this population [24, 25]. Among patients with advanced as well as end-stage CKD (stage 4 and 5, respectively), 50% of them have cardiovascular diseases and cardiovascular mortality accounts for ~40–50% of all deaths, compared to 26% with normal kidney function [96, 97]. Although, both atherosclerotic and medial calcifications are likely to be related to CKD-HD [98, 99, 100].
Secondary hyperparathyroidism and abnormal phosphate metabolism in CKD patients are important causes of increased VC in these patients [42]. Due to positive phosphate balance in CKD patients, this excess phosphate accumulates in the VSMCs, stimulating proteins involved in bone formation and initiating and promoting calcification [42]. Elevated CAC in these patients is associated with VSMC death in experimental models, which may lead to impaired vascular reactivity and increased plaque rupture [101]. In addition, CKD patients often have co-morbidities related to ASCVD, including albuminuria and chronic inflammation [88]. The high-risk profile of CKD patients is also associated with increased peri-intervention complications.
MScl is a type of arteriosclerosis, but it is controversial whether it extends to the intima layer [102, 103]. Using the measurement of the ankle-branchial index (ABI) 1.3 as a cut-off point for MS diagnosis, its prevalence is estimated at around 0.5% of adult population, while it is 50% more common among women [104] and very rare among individuals younger than 50 years old. However, the presence of CKD and particularly CKD-HD can induce an earlier onset of the disease [2]. In addition to CKD, diabetes mellitus and advanced age are considered the most prominent risk factors for MScl development [105], raising the prevalence of MScl among those subpopulations ranging between 17% and 41.5% [106, 107]. Notably, several studies have documented the association of MScl with increased cardiovascular morbidity and mortality [36, 108]. CAC is mainly localized within both medial and intimal calcification [25].
The interaction between atherosclerotic plaque calcification and its stability remains questionable. Scott et al. [109] have suggested the potential contribution of inflammation in promoting plaque destabilization and microcalcification. The latter represents an early, active stage of VC correlated with an inflammatory state and directly contributed to plaque rupture [110, 111, 112]. On the other hand, histological analysis of atherosclerotic plaque specimens has considered calcified plaques been more stable compared to noncalcified. There is inadequate evidence to support the CAC score as an indicator of atherosclerotic plaque stability [113].
6. Clinical Studies — Effect of Statins on Vascular Calcification
However, statins, which are the mainstay therapy of atherosclerotic cardiovascular disease (ASVD) and reduce the risk of major cardiac events, are associated with increased coronary artery calcium (CAC) scores. Several RCTs demonstrated that statins despite their significant LDL-lowering effect, failed to reduce, but rather increased CAC scores [114, 115, 116, 117]. Statins by decreasing the soft lipid core of a calcified atherosclerotic plaque may increase the density of the plaque and its Agatston calcium score leading to smaller volume [118]. Long-term statin therapy may enhance the downstream step of calcification in the atherosclerotic process [42]. Statins’ effects in the microarchitecture of vascular calcium may be related to increased CAC and stability of the plaque [19, 119]. However, calcium density is inversely associated with event risk, suggesting that statin-induced calcification may contribute to atherosclerotic plaque stability [39, 120].
6.1 Coronary Arteries
Statins, also known as 3-hydroxy-3-methylglutaryl (HMG) CoA reductase inhibitors, have been shown to reduce the risk of ASCVDs in numerous studies [121, 122]. Statins with “pleiotropic” anti-inflammatory actions effectively reduce cardiovascular events, presumably through plaque stabilization [123]. However, these drugs have also been associated with an increase in the progression of coronary and aortic calcification, which is known to be linked to an elevated risk of cardiovascular events [124, 125, 126]. Recent findings by Hanai et al. [127] suggest that lipophilic statins may have a negative impact on kidney function. The use of statins has been linked to a high CAC score, indicating a potential promotion of VC in predisposed individuals. The observed correlation between statin use and increased CAC score in the study by Li et al. [128] may be due to the phenomenon of “confounding by indication”, as statin users were older, had a higher body mass index (BMI), and had a greater burden of diabetes and cardiovascular disease. Although some studies attribute this link to confounding factors, randomized controlled trials have failed to demonstrate a survival advantage of statins in dialysis patients, leaving open the possibility that statins may not prevent or even contribute to arterial calcification. Importantly, even after adjusting for age, diabetes, BMI, and inflammation, the connection between statin use and increased CAC score persisted [129, 130, 131].
Puri et al. [126] found that although statins reduced atheroma volume, they promoted calcification in coronary atheroma. In addition to reducing the lipid-rich core of atherosclerotic plaques, statins may also increase the density of calcification [132] as part of a healing process which could result in plaque stabilization and eventually reduce the incidence of cardiovascular events. In a recent study involving 3483 participants, statin intake was linked to a 31% higher progression of coronary calcification, even after adjustment for cardiovascular risk factors [113]. Based on an old meta-analysis of controlled trials assessing the impact of statins on CAC and coronary stenoses, Henein et al. [133] concluded that the precipitated progression of coronary calcification (CAC growth rate) was due to increased transformation of noncalcified coronary atherosclerotic plaques to calcified plaques. The same authors re-analyzed data from two clinical trials to further investigate the time and dose dependent effects of statins on CAC and whether progression is accompanied by a higher incidence of cardiovascular events [112]. The included trials had the following characteristics: (1) St. Francis Heart Study (SFHS): 419 and 432 patients treated with placebo and 20 mg atorvastatin daily, respectively; CAC assessment at baseline, 2 years and 4–6 years follow-up. (2) EBEAT Study: 164 and 179 patients treated with 10 mg and 80 mg atorvastatin daily, respectively; CAC score assessment at baseline and 12 months. The accumulated data showed a similar CAC increase in the short-term follow-up (12–24 months) between placebo and low-dose atorvastatin, while 80 mg/daily atorvastatin further increased CAC by 12–14% over placebo. In the long term, a high dose of atorvastatin considerably increased the CAC score in both studies, however, that effect was not accompanied by an increase in cardiovascular events. In the SFHS trial patients experiencing cardiovascular events after the second CT scan had less-frequently prescribed statins while they had higher progression of CAC. The authors concluded that statins-induced CAC progression was not an independent predictor of cardiovascular events occurrence indicating probably plaque stabilization rather than plaque expansion [112].
In a recent multinational observational registry titled the Progression of Atherosclerotic Plaque Determined by Computed Tomographic Angiography Imaging (PARADIGM), the researchers collected data from patients who underwent serial coronary computed tomography angiography (CCTA) [134]. Statin administration actually stimulated the calcification of coronary arteries. Surprisingly, this increased calcification was associated with a reduced risk of adverse cardiac events, in agreement with previous reports [114]. In the absence of statin therapy, an increase in the CAC score indicated progression in both previously noncalcified and already calcified plaques. In contrast, the statin-related increase in CAC score indicated “calcification progression” only in previously calcified plaques [122]. In line with the findings from the PARADIGM study, Scott et al. [109] and other researchers found that statin treatment, as a primary prevention measure, correlated to a slower progression of overall coronary atherosclerosis volume, reduction of high-risk plaque features, and increased plaque calcification burden [135]. Participants with higher baseline inflammation experienced a significant increase in coronary calcification over 2 years of statin treatment, illustrating the association between inflammation, microcalcification, and the enhancing effect of statin treatment on coronary calcification. However, CAC scores did not differ between high versus low hs-CRP groups over 2 years. This further confirmed that although the CAC score is a good measure of overall plaque burden and stable end-stage macroscopic calcification, it cannot identify unstable atherosclerotic plaques [109, 112, 136]. In addition, those findings question the link of CAC score with long-term clinical outcomes [137].
Additional clinical studies shed more light on the effects of statins on plaque vulnerability. A previous meta-analysis of nine studies (a total 830 individuals) investigated the possible effect of statin therapy on the composition of coronary atherosclerotic plaques using virtual histology intravascular ultrasound (VH-IVUS) [122]. It was evident that statin administration reduced plaque volume and simultaneously increased dense calcium volume [122]. That increase has been negatively correlated with vessel remodeling in a number of studies, which may be interpreted as a stabilization of atherosclerosis [138, 139]. A recent observational study reported similar findings after the comparison of high-intensity statin treatment with standard medical treatment in patients with CAD regarding changes in plaque morphology [140]. In particular, dense calcium area increased in the high-intensity statin group compared to controls along with smoothing of atherosclerotic plaques and less shear stress and thereby less predisposition to rupture. In parallel to imaging modalities, studies assessing biomarkers have documented a positive effect of statins of VC mediators. In patients with newly diagnosed CAD, 6-months simvastatin therapy significantly reduced VC inhibitors, such as OPG, OPN and fetuin-A [141].
6.2 Peripheral Arteries
The possible protective effects of statins on patients with PAD has been supported by numerous old studies. The Heart Protection Study included a large number of participants from the UK, a subgroup of whom had PAD and were randomly allocated to simvastatin or placebo [142]. In this sub-group of participants, statin use was associated with a 24% lower probability of major vascular event in comparison with the placebo group (relative risk (RR): 0.76, 95% CI: 0.72, 0.81). Another large observation study of 155647 individuals with incident PAD investigated how statin use may affect amputation and mortality [143]. The results indicated a 33% lower risk of amputation for high-intensity statin users in comparison to antiplatelet-only users (HR: 0.67, 95% CI: 0.61, 0.74). A protective effect was also observed in the low to moderate intensity statin group, but to a lesser extent (HR: 0.81, 95% CI: 0.75, 0.86). After that robust evidence, statins’ administration is highly recommended (level of evidence: IA) in all patients with PAD by both the American Heart Association/American College of Cardiology and the European Society of Cardiology [144, 145]. A population-based cohort study in Spain included 5480 adults with ABI 0.95 and without known ASCVD [146]. They were divided into two groups, based on the use of statin or not and followed up for a median time of 3.6 years. Major cardiovascular events were more common in the non-statin group (24.7 events per 1000 person-years vs 19.7 respectively), since their counterparts receiving statins had a 20% lower probability of major cardiovascular events (HR: 0.80, 95% CI: 0.66, 0.97) and 19% lower probability of overall mortality (HR: 0.81, 95% CI: 0.68, 0.97).
There are limited data regarding the effect of statin use on carotid atherosclerosis and in particular through calcification. A number of studies have demonstrated the stabilizing impact of statins on carotid atherosclerotic plaques [147]. The Rotterdam study, a prospective cohort study of 1740 participants with carotid atherosclerosis undergoing carotid MRI angiography [109]. Statin users had a 73% higher probability of having intra-plaque calcification in comparison to individuals who had never taken statins (OR: 1.73, 95% CI:1.22, 2.44). The duration of statin therapy was an important factor for plaque calcification, since only statin therapy lasting more than 48 months seemed to be associated with calcification with a pronounced protective effect (OR: 1.74, 95% CI: 1.09, 2.77). However, three small (number of participants range: 26–33), observational studies reported no impact of statins on plaque calcification. During 3 years [148], 2 years [149] and 6 months follow-up [150], the administration of either low- or high-dose statins did not significantly alter either the absolute or the percentage of calcification within carotid plaques. However, the inability to reach statistical significance may be due to the study design. Interestingly, the percentage change of calcification between baseline and 6 months correlated with percentage plaque volume change in the last study. Using ultrasound, statin therapy may increase plaque echogenicity, GSM score and hence its stability [75]. In patients with established carotid atherosclerosis (carotid stenosis 40%) but without indication for intervention of 6 months atorvastatin therapy (dose range: 10–80 mg) targeting LDL 100 mg/dL improved lipid profile, reduced inflammatory biomarkers along with VC inhibitors OPN and OPG [151]. Most importantly, intensive lipid-lowering enhanced carotid plaque echogenicity (GSM score elevation) outlining a beneficial impact on plaque stability, in peri-procedural period for carotid revascularization [152]. Among elderly people, the Thoracic Aorta Calcium Score was associated with a two-fold higher risk of stroke [153]. It is not yet clarified how the prescription of statins could contribute to reducing this risk. A summary of the results of statins on atherosclerotic calcification in clinical studies is presented in Table 1, Ref. [73, 109, 114, 115, 116, 117, 121, 122, 124, 125, 126, 131, 132, 133, 134, 135, 151, 152].
Table 1.
A summary of clinical studies investigating the impact of statins on vascular calcification indices.
| Reference | Participants; vascular calcification indices | Study design | Findings |
| Coronary artery disease | |||
| Schmermund A et al. (2006) [115] | 471 pts w/o CAD, w/ 2 CV risk factors, CAC score 30; | • RCT: Group A (N = 235): ATORVA 80 mg; Group B (N = 236): ATORVA 10 mg | Group A vs group B: |
| EBT: CAC | • Duration: 12 mo | ↔ CAC | |
| Kovarnik T et al. (2012) [132] | 89 pts w/ stable angina; | • RCT: Group A (N = 18): Aggressive therapy ATORVA 80 mg/d + EZET 10 mg/d; Group S (N = 71): standard statin therapy (started by GP or ATORVA 10 mg/d statin-naive patients) | Group A vs group S: |
| Coronary VH-IVUS | • Duration: 12 mo | ↑ coronary dense calcification | |
| Henein MY et al. (2011) [133] | 11 studies, 1839 pts; | • Meta-analysis | High dose vs low-dose vs placebo: |
| 6 trials assessing CAC and 5 trials assessing coronary stenoses; | • High dose statins vs low dose statins vs placebo | ↔ coronary calcification | |
| EBT, MDCT: CAC | • Duration: 12–24 mo | ↓ coronary stenosis | |
| Henein M et al. (2015) [124] | 2 clinical trials w/ 1194 pts: St. Francis Heart Study (SFHS) and EBEAT Study; | • Pooled analysis of 2 RCTs | ↑ CAC w/ greater statin doses and prolonged therapy |
| CCTA: CAC score | • SFHS study — group A (N = 432): ATORVA 20 mg/d; group B (N = 419): placebo | ||
| • EBEAT Study — group A (N = 179): ATORVA 80 mg/d; group B (N = 164): ATORVA 10 mg/d | |||
| • Duration: CAC score at baseline, 2 y, 4–6 y in SFHS study and 0 and 12 mo in EBEAT study | |||
| Banach M et al. (2015) [122] | 9 prospective clinical studies, 830 pts, 16 statin treatment arms; | • Systematic review & meta-analysis | All statins: |
| coronary VH-IVUS | • Statin intervention: 737 pts (ATORVA, 10 to 80 mg/day; PRAVA, 10 to 40 mg/day; SIMVA, 20 mg/day; ROSUVA, 10 to 40 mg/day; FLUVA, 60 mg/day; PITAVA, 2 to 4 mg/day) | ↑ coronary dense calcium volume | |
| • Placebo: 93 pts | |||
| Puri R et al. (2015) [126] | 8 RCTs, 3495 participants; | • post-hoc propensity-weighted analysis | HIST vs LIST or no statin group |
| IVUS assessment of coronary calcification and percent atheroma volume (PAV) | • HIST (N = 1545): High intensity statin therapy ATORVA 80 mg/d, ROSUVA 40 mg/d | ↓ PAV | |
| • LIST (N = 1726): Low intensity statin therapy ATORVA 40 mg/d, ROSUVA 20 mg/d, SIMVA 40 mg/d, PRAVA 80 mg/d, LOVA 20 mg/d, FLUVA 40 mg/d | ↑ coronary calcification | ||
| • No-statin therapy (N = 224) | |||
| Dykun I et al. (2016) [125] | 3483 participants; | • Observational study | ↑ CAC |
| EBT: CAC progression | • 230 pts on statins at baseline | ↓ coronary events | |
| • FU median duration: 5 y | |||
| Coronary calcification | |||
| Raggi P et al. (2005) [116] | 475 hyperlipidemic, postmenopausal women; | • RCT: Group A (N = 218): intensive statin ATORVA 80 mg; Group B (N = 257): moderate statin therapy, PRAVA 40 mg | Group A vs group B: |
| EBT: CAC | • Duration: 12 mo | ↔ CAC | |
| ↓ LDL | |||
| Houslay ES et al. (2006) [117] | 102 pts w/ calcific AS; Helical CT: CAC | • RCT: Group A (N = 48): ATORVA 80 mg; Group B (N = 54): matched placebo | Group A vs group B: |
| • Duration: median FU 24 mo | ↔ CAC | ||
| Terry JG et al. (2007) [114] | 80 pts w/ asymptomatic vascular disease, HDL 50 mg/dL, 100 LDL 160 mg/dL, 2 other CV risk factors, CAC score 50; | • RCT: Group A (N = 40): SIMVA 80 mg; Group B (N = 40): placebo | Group A vs group B: |
| MDCT: CAC | • Duration: 12 mo | ↑ CAC, AAC | |
| ↓ TC, TG, LDL | |||
| Andelius L et al. (2018) [131] | 12 studies, 692 participants; | • Systematic review & meta-analysis | Intensive vs moderate statin therapy: |
| CCTA: plaque volume, plaque calcification, calcium intensity signal | • Intensive statin therapy (N = 99) | ↓ non-calcified plaque volume, | |
| • Moderate statin therapy (N = 404) | ↑ calcified plaque volume ↑calcium signal intensity | ||
| • Controls (N = 189) | |||
| • FU: 14.5 9.5 months | |||
| Lee SE et al. (2018) | 1255 pts; | • Prospective multinational registry: Group A (N = 781): statin receivers; Group B (N = 474): statin naïve | Group A vs group B: |
| PARADIGM study [135] | CCTA: CAC progression | • Duration: 2-year interval | ↓ atheroma volume |
| ↓ non-calcified and ↓ high risk plaques | |||
| ↑ plaque calcification | |||
| Lee SE et al. (2019) | 654 pts; | • Prospective multinational registry: Group A (N = 408): statin receivers; Group B (N = 246): non-statin | Group A vs group B: |
| PARADIGM study [134] | CCTA: CAC progression | • Duration: 2-year interval | ↑ calcified plaque volume |
| ↓ non-calcified plaque volume progression | |||
| Scott et al. (2022) | 142 participants; | • Prospective, cohort study, sub-analysis of RIGHT study: Group A (N = 66): high hs-CRP; Group B (N = 76): low hs-CRP | Group A vs B: |
| RIGHT study [109] | CCTA: coronary calcification | • Duration: 2 years | ↑ DCB |
| ↔ NCB | |||
| Carotid artery disease | |||
| Kadoglou NPE et al. (2008) [151] | 97 pts w/ carotid stenosis 40%, w/o indication of revascularization | • Prospective study | ATORVA: |
| 52 age-& sex-matched controls; | • ATORVA (10 mg–80 mg) target LDL-C 100 mg/dL | ↑ GSM score | |
| GSM score | • Controls at baseline | ↓ OPG, OPN | |
| OPG, OPN | • Duration: 6 mo | ||
| Kadoglou NPE et al. (2010) (JVS) [73] | 140 pts w/ moderate carotid stenosis w/o indication of revascularization; | • RCT: Group A (N = 70): Low-dose ATORVA (10 mg–20 mg) target LDL-C 100 mg/dL; Group B (N = 70): High-dose ATORVA (80 mg) target LDL-C 70 mg/dL | High-dose vs Low-dose ATORVA: |
| GSM score | • Duration: 12 mo | ↑ GSM score | |
| OPG, OPN | ↓ OPG, OPN | ||
| Kadoglou NPE et al. (2010) (EJVS) [152] | 113 pts w/ bilateral carotid atherosclerosis; | • Group A (N = 46): Ipsilateral carotid revascularization; Group B (N = 67): Bilateral low-grade stenosis | Group A vs group B: |
| GSM score | • Both groups received ATORVA 10–80mg, LDL target 100 mg/dL | ↑ GSM score contralateral | |
| OPG, OPN | • Duration: 6 mo | ↓ OPG, OPN | |
| Mujaj B et al. (2018). The Rotterdam study [121] | 1740 pts, age 45 years old w/ carotid atherosclerosis; carotid MRI | • prospective population-based cohort study. | Higher dose and longer use of statins: |
| • statin exposure: 30.2% of participants | ↑ carotid plaque calcification | ||
| • Median duration exposure: 48 mo | |||
AAC, abdominal aortic calcium; AS, aortic stenosis; ATORVA, atorvastatin; CAC, coronary artery calcium; CCTA, coronary computed tomography angiography; CV, cardiovascular; CVD, cardiovascular disease; DCB, dense-calcified coronary burden; EBT, electron-beam tomography; EZET, ezetimibe; FLUVA, fluvastatin; FU, follow-up; GP, general practitioner; GSM, grey scale median; HDL, high-density lipoprotein; HIST, high intensity statin therapy; hs-CRP, high-sensitivity C-reactive protein; LIST, low intensity statin therapy; LDL, low density lipoprotein; LOVA, lovastatin; MDCT, multi-detector computed tomography; NCB, noncalcified coronary burden; pts, patients; PAV, percent atheroma volume; PITAVA, pitavastatin; PRAVA, pravastatin; RCT, randomized control trial; ROSUVA, rosuvastatin; SIMVA, simvastatin; TC, total cholesterol; TG, triglycerides; w/, with; w/o, without; VH-IVUS, virtual histology intravascular ultrasound; CT, computed tomography; OPN, osteopontin; OPG, osteoprotegerin.
Some studies have attempted to explain this paradoxical effect by suggesting that the statins-induced progression of calcification may occur in a way that simultaneously reduces cardiovascular risk, such as by altering the size or density of calcium deposits. For example, a reduction in mineral surface area resulting from the coalescence of small deposits and/or decreased porosity may lower the risk of debonding and subsequent plaque rupture, which could contribute to the reduction in cardiovascular risk observed with statin therapy [117, 154]. To explore this possibility, F-NaF micro-positron emission tomography (µPET) imaging, which can detect fluoride adsorption on the surfaces of actively mineralizing apatite mineral deposits, may serve as a useful tool to quantify the surface area of cardiovascular calcium deposits [155].
7. Conclusions
Several mediators have been involved in arterial and atherosclerotic calcification reflecting a complex process. VC has been associated with high cardiovascular morbidity and mortality, rendering it a potential target of therapy. Statins constitute the cornerstone of primary and secondary prevention of ASCVDs. Most studies using imaging modalities and/or biomarkers have demonstrated that statins promote atherosclerotic plaque calcification in coronary and peripheral arteries in the long term, especially at high doses. Although such an effect seems detrimental at first sight, it has been associated with higher plaque stability and less adverse cardiovascular events. Presumably, statins promote favorable arterial and atherosclerotic calcification. which do not expand atherosclerotic lesions and attenuate their vulnerability. More studies are required to verify those findings and clarify the underlying mechanisms.
Acknowledgment
Not applicable.
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
MS, NV, EK and EC did the literature search, wrote the first draft of the manuscript. NV and EG created images and table. NPEK conceptualized the idea, developed the outline for the review, critically revised the manuscript and figures for submission in its final form. GV made substantial contribution to the conception and design of the work and performed the analysis and interpretation of data. GV critically revised the manuscript and figures for submission in its final form. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Not applicable.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest. Nikolaos P. E. Kadoglou is serving as Guest Editor of this journal. We declare that Nikolaos P. E. Kadoglou had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Zhonghua Sun.
References
- [1].Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney International . 2009;75:890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clinical Journal of the American Society of Nephrology: CJASN . 2008;3:1599–1605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- [3].Shanahan CM. Vascular calcification. Current Opinion in Nephrology and Hypertension . 2005;14:361–367. doi: 10.1097/01.mnh.0000172723.52499.38. [DOI] [PubMed] [Google Scholar]
- [4].McCullough PA, Soman S. Cardiovascular calcification in patients with chronic renal failure: are we on target with this risk factor. Kidney International. Supplement . 2004;66:S18–S24. doi: 10.1111/j.1523-1755.2004.09008.x. [DOI] [PubMed] [Google Scholar]
- [5].Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary Artery Calcification and its Progression: What Does it Really Mean. JACC. Cardiovascular Imaging . 2018;11:127–142. doi: 10.1016/j.jcmg.2017.10.012. [DOI] [PubMed] [Google Scholar]
- [6].Pelisek J, Well G, Reeps C, Rudelius M, Kuehnl A, Culmes M, et al. Neovascularization and angiogenic factors in advanced human carotid artery stenosis. Circulation Journal: Official Journal of the Japanese Circulation Society . 2012;76:1274–1282. doi: 10.1253/circj.cj-11-0768. [DOI] [PubMed] [Google Scholar]
- [7].Trion A, van der Laarse A. Vascular smooth muscle cells and calcification in atherosclerosis. American Heart Journal . 2004;147:808–814. doi: 10.1016/j.ahj.2003.10.047. [DOI] [PubMed] [Google Scholar]
- [8].Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arteriosclerosis, Thrombosis, and Vascular Biology . 2009;29:1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- [9].Couri CEB, da Silva GA, Martinez JAB, Pereira FDA, de Paula FJA. Mönckeberg’s sclerosis - is the artery the only target of calcification. BMC Cardiovascular Disorders . 2005;5:34. doi: 10.1186/1471-2261-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moe SM, O’Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, et al. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney International . 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- [11].Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clinical Journal of the American Society of Nephrology: CJASN . 2009;4:1892–1900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hayden MR, Tyagi SC, Kolb L, Sowers JR, Khanna R. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovascular Diabetology . 2005;4:4. doi: 10.1186/1475-2840-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation . 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- [14].Corciu AI, Siciliano V, Poggianti E, Petersen C, Venneri L, Picano E. Cardiac calcification by transthoracic echocardiography in patients with known or suspected coronary artery disease. International Journal of Cardiology . 2010;142:288–295. doi: 10.1016/j.ijcard.2009.01.021. [DOI] [PubMed] [Google Scholar]
- [15].Stanford W, Thompson BH, Burns TL, Heery SD, Burr MC. Coronary artery calcium quantification at multi-detector row helical CT versus electron-beam CT. Radiology . 2004;230:397–402. doi: 10.1148/radiol.2302020901. [DOI] [PubMed] [Google Scholar]
- [16].Nitta K, Akiba T, Suzuki K, Uchida K, Ogawa T, Majima K, et al. Assessment of coronary artery calcification in hemodialysis patients using multi-detector spiral CT scan. Hypertension Research: Official Journal of the Japanese Society of Hypertension . 2004;27:527–533. doi: 10.1291/hypres.27.527. [DOI] [PubMed] [Google Scholar]
- [17].Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology . 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- [18].Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology . 1998;208:807–814. doi: 10.1148/radiology.208.3.9722864. [DOI] [PubMed] [Google Scholar]
- [19].Doran AC, Terry JG, Carr JJ, Linton MF. Statins and Atherosclerotic Lesion Microcalcification: A New Mechanism for Plaque Stability. Arteriosclerosis, Thrombosis, and Vascular Biology . 2021;41:1306–1308. doi: 10.1161/ATVBAHA.121.315949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proceedings of the National Academy of Sciences of the United States of America . 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Karlöf E, Seime T, Dias N, Lengquist M, Witasp A, Almqvist H, et al. Correlation of computed tomography with carotid plaque transcriptomes associates calcification with lesion-stabilization. Atherosclerosis . 2019;288:175–185. doi: 10.1016/j.atherosclerosis.2019.05.005. [DOI] [PubMed] [Google Scholar]
- [22].Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. The Journal of Biological Chemistry . 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- [23].Sutliff RL, Walp ER, El-Ali AM, Elkhatib S, Lomashvili KA, O’Neill WC. Effect of medial calcification on vascular function in uremia. American Journal of Physiology. Renal Physiology . 2011;301:F78–F83. doi: 10.1152/ajprenal.00533.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McCullough PA, Agrawal V, Danielewicz E, Abela GS. Accelerated atherosclerotic calcification and Monckeberg’s sclerosis: a continuum of advanced vascular pathology in chronic kidney disease. Clinical Journal of the American Society of Nephrology: CJASN . 2008;3:1585–1598. doi: 10.2215/CJN.01930408. [DOI] [PubMed] [Google Scholar]
- [25].Shao JS, Cheng SL, Sadhu J, Towler DA. Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension (Dallas, Tex.: 1979) . 2010;55:579–592. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ellam TJ, Chico TJA. Phosphate: the new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis . 2012;220:310–318. doi: 10.1016/j.atherosclerosis.2011.09.002. [DOI] [PubMed] [Google Scholar]
- [27].Harrington RA. Targeting Inflammation in Coronary Artery Disease. The New England Journal of Medicine . 2017;377:1197–1198. doi: 10.1056/NEJMe1709904. [DOI] [PubMed] [Google Scholar]
- [28].Chen YC, Huang AL, Kyaw TS, Bobik A, Peter K. Atherosclerotic Plaque Rupture: Identifying the Straw That Breaks the Camel’s Back. Arteriosclerosis, Thrombosis, and Vascular Biology . 2016;36:e63–e72. doi: 10.1161/ATVBAHA.116.307993. [DOI] [PubMed] [Google Scholar]
- [29].Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. European Heart Journal . 2015;36:482–489c. doi: 10.1093/eurheartj/ehu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. The New England Journal of Medicine . 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- [31].Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circulation. Cardiovascular Imaging . 2013;6:747–754. doi: 10.1161/CIRCIMAGING.113.000382. [DOI] [PubMed] [Google Scholar]
- [32].Andrews J, Psaltis PJ, Bartolo BAD, Nicholls SJ, Puri R. Coronary arterial calcification: A review of mechanisms, promoters and imaging. Trends in Cardiovascular Medicine . 2018;28:491–501. doi: 10.1016/j.tcm.2018.04.007. [DOI] [PubMed] [Google Scholar]
- [33].Demer LL, Boström KI. Conflicting forces of warfarin and matrix gla protein in the artery wall. Arteriosclerosis, Thrombosis, and Vascular Biology . 2015;35:9–10. doi: 10.1161/ATVBAHA.114.304793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Luegmayr E, Glantschnig H, Wesolowski GA, Gentile MA, Fisher JE, Rodan GA, et al. Osteoclast formation, survival and morphology are highly dependent on exogenous cholesterol/lipoproteins. Cell Death and Differentiation . 2004;11 Suppl 1:S108–S118. doi: 10.1038/sj.cdd.4401399. [DOI] [PubMed] [Google Scholar]
- [35].Kadoglou NPE, Sailer N, Moumtzouoglou A, Kapelouzou A, Gerasimidis T, Kostakis A, et al. Adipokines: a novel link between adiposity and carotid plaque vulnerability. European Journal of Clinical Investigation . 2012;42:1278–1286. doi: 10.1111/j.1365-2362.2012.02728.x. [DOI] [PubMed] [Google Scholar]
- [36].Akers EJ, Nicholls SJ, Di Bartolo BA. Plaque Calcification: Do Lipoproteins Have a Role. Arteriosclerosis, Thrombosis, and Vascular Biology . 2019;39:1902–1910. doi: 10.1161/ATVBAHA.119.311574. [DOI] [PubMed] [Google Scholar]
- [37].Shioi A, Ikari Y. Plaque Calcification During Atherosclerosis Progression and Regression. Journal of Atherosclerosis and Thrombosis . 2018;25:294–303. doi: 10.5551/jat.RV17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. American Journal of Physiology. Endocrinology and Metabolism . 2004;286:E686–E696. doi: 10.1152/ajpendo.00552.2003. [DOI] [PubMed] [Google Scholar]
- [39].Derici U, El Nahas AM. Vascular calcifications in uremia: old concepts and new insights. Seminars in Dialysis . 2006;19:60–68. doi: 10.1111/j.1525-139X.2006.00120.x. [DOI] [PubMed] [Google Scholar]
- [40].Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell . 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- [41].Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arteriosclerosis, Thrombosis, and Vascular Biology . 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- [42].D’Amelio P, Isaia G, Isaia GC. The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. Journal of Endocrinological Investigation . 2009;32:6–9. [PubMed] [Google Scholar]
- [43].Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes & Development . 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, et al. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE-/- mice. Arteriosclerosis, Thrombosis, and Vascular Biology . 2006;26:2117–2124. doi: 10.1161/01.ATV.0000236428.91125.e6. [DOI] [PubMed] [Google Scholar]
- [45].Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, et al. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation . 2002;106:1192–1194. doi: 10.1161/01.cir.0000031524.49139.29. [DOI] [PubMed] [Google Scholar]
- [46].Hofmann Bowman MA, McNally EM. Genetic pathways of vascular calcification. Trends in Cardiovascular Medicine . 2012;22:93–98. doi: 10.1016/j.tcm.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].O’Donnell CJ, Kavousi M, Smith AV, Kardia SLR, Feitosa MF, Hwang SJ, et al. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation . 2011;124:2855–2864. doi: 10.1161/CIRCULATIONAHA.110.974899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ichikawa N, Taniguchi A, Kaneko H, Kawamoto M, Sekita C, Nakajima A, et al. Arterial Calcification Due to Deficiency of CD73 (ACDC) As One of Rheumatic Diseases Associated with Periarticular Calcification. Journal of Clinical Rheumatology: Practical Reports on Rheumatic & Musculoskeletal Diseases . 2015;21:216–220. doi: 10.1097/RHU.0000000000000245. [DOI] [PubMed] [Google Scholar]
- [49].Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circulation Research . 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- [50].Burton DGA, Giles PJ, Sheerin ANP, Smith SK, Lawton JJ, Ostler EL, et al. Microarray analysis of senescent vascular smooth muscle cells: A link to atherosclerosis and vascular calcification. Experimental Gerontology . 2009;44:659–665. doi: 10.1016/j.exger.2009.07.004. [DOI] [PubMed] [Google Scholar]
- [51].Willems BA, Furmanik M, Caron MMJ, Chatrou MLL, Kusters DHM, Welting TJM, et al. Ucma/GRP inhibits phosphate-induced vascular smooth muscle cell calcification via SMAD-dependent BMP signalling. Scientific Reports . 2018;8:4961. doi: 10.1038/s41598-018-23353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lee HL, Woo KM, Ryoo HM, Baek JH. Tumor necrosis factor-alpha increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochemical and Biophysical Research Communications . 2010;391:1087–1092. doi: 10.1016/j.bbrc.2009.12.027. [DOI] [PubMed] [Google Scholar]
- [53].Kapustin AN, Shanahan CM. Calcium regulation of vascular smooth muscle cell-derived matrix vesicles. Trends in Cardiovascular Medicine . 2012;22:133–137. doi: 10.1016/j.tcm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- [54].Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. Journal of the American Society of Nephrology: JASN . 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- [55].Fleisch H, Bisaz S. Mechanism of calcification: inhibitory role of pyrophosphate. Nature . 1962;195:911. doi: 10.1038/195911a0. [DOI] [PubMed] [Google Scholar]
- [56].Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circulation Research . 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- [57].Kizu A, Shioi A, Jono S, Koyama H, Okuno Y, Nishizawa Y. Statins inhibit in vitro calcification of human vascular smooth muscle cells induced by inflammatory mediators. Journal of Cellular Biochemistry . 2004;93:1011–1019. doi: 10.1002/jcb.20207. [DOI] [PubMed] [Google Scholar]
- [58].Trion A, Schutte-Bart C, Bax WH, Jukema JW, van der Laarse A. Modulation of calcification of vascular smooth muscle cells in culture by calcium antagonists, statins, and their combination. Molecular and Cellular Biochemistry . 2008;308:25–33. doi: 10.1007/s11010-007-9608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kupcsik L, Meurya T, Flury M, Stoddart M, Alini M. Statin-induced calcification in human mesenchymal stem cells is cell death related. Journal of Cellular and Molecular Medicine . 2009;13:4465–4473. doi: 10.1111/j.1582-4934.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Elahirad S, Elieh Ali Komi D, Kiani A, Mohammadi-Noori E, Vaisi-Raygani A, Mozafari H, et al. Association of Matrix Metalloproteinase-2 (MMP-2) and MMP-9 Promoter Polymorphisms, Their Serum Levels, and Activities with Coronary Artery Calcification (CAC) in an Iranian Population. Cardiovascular Toxicology . 2022;22:118–129. doi: 10.1007/s12012-021-09707-5. [DOI] [PubMed] [Google Scholar]
- [61].Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology . 2003;23:769–775. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- [62].Kadoglou N, Moulakakis KG, Mantas G, Spathis A, Gkougkoudi E, Mylonas SN, et al. Novel Biomarkers and Imaging Indices for the “Vulnerable Patient” with Carotid Stenosis: A Single-Center Study. Biomolecules . 2023;13:1427. doi: 10.3390/biom13091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Okuyama H, Langsjoen PH, Hamazaki T, Ogushi Y, Hama R, Kobayashi T, et al. Statins stimulate atherosclerosis and heart failure: pharmacological mechanisms. Expert Review of Clinical Pharmacology . 2015;8:189–199. doi: 10.1586/17512433.2015.1011125. [DOI] [PubMed] [Google Scholar]
- [64].Song Y, Wu H, Wen D, Zhu B, Graner P, Ciancibello L, et al. Detection of coronary calcifications with dual energy chest X-rays: clinical evaluation. The International Journal of Cardiovascular Imaging . 2021;37:767–774. doi: 10.1007/s10554-020-02072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ma X, Hou F, Tian J, Zhou Z, Ma Y, Cheng Y, et al. Aortic Arch Calcification Is a Strong Predictor of the Severity of Coronary Artery Disease in Patients with Acute Coronary Syndrome. BioMed Research International . 2019;2019:7659239. doi: 10.1155/2019/7659239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC. Cardiovascular Imaging . 2017;10:582–593. doi: 10.1016/j.jcmg.2017.03.005. [DOI] [PubMed] [Google Scholar]
- [67].Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. Journal of the American College of Cardiology . 2018;72:434–447. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mortensen MB, Falk E, Li D, Nasir K, Blaha MJ, Sandfort V, et al. Statin Trials, Cardiovascular Events, and Coronary Artery Calcification: Implications for a Trial-Based Approach to Statin Therapy in MESA. JACC. Cardiovascular Imaging . 2018;11:221–230. doi: 10.1016/j.jcmg.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Becker CR, Knez A, Ohnesorge B, Schoepf UJ, Flohr T, Bruening R, et al. Visualization and quantification of coronary calcifications with electron beam and spiral computed tomography. European Radiology . 2000;10:629–635. doi: 10.1007/s003300050975. [DOI] [PubMed] [Google Scholar]
- [70].Villines TC, Hulten EA, Shaw LJ, Goyal M, Dunning A, Achenbach S, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. Journal of the American College of Cardiology . 2011;58:2533–2540. doi: 10.1016/j.jacc.2011.10.851. [DOI] [PubMed] [Google Scholar]
- [71].Kato S, Kitagawa K, Ishida N, Ishida M, Nagata M, Ichikawa Y, et al. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial. Journal of the American College of Cardiology . 2010;56:983–991. doi: 10.1016/j.jacc.2010.01.071. [DOI] [PubMed] [Google Scholar]
- [72].Jashari F, Ibrahimi P, Bajraktari G, Grönlund C, Wester P, Henein MY. Carotid plaque echogenicity predicts cerebrovascular symptoms: a systematic review and meta-analysis. European Journal of Neurology . 2016;23:1241–1247. doi: 10.1111/ene.13017. [DOI] [PubMed] [Google Scholar]
- [73].Kadoglou NPE, Sailer N, Moumtzouoglou A, Kapelouzou A, Gerasimidis T, Liapis CD. Aggressive lipid-lowering is more effective than moderate lipid-lowering treatment in carotid plaque stabilization. Journal of Vascular Surgery . 2010;51:114–121. doi: 10.1016/j.jvs.2009.07.119. [DOI] [PubMed] [Google Scholar]
- [74].Singh A, Nasir U, Segal J, Waheed TA, Ameen M, Hafeez H. The utility of ultrasound and computed tomography in the assessment of carotid artery plaque vulnerability-A mini review. Frontiers in Cardiovascular Medicine . 2022;9:1023562. doi: 10.3389/fcvm.2022.1023562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kadoglou NP, Khattab E, Velidakis N, Patsourakos N, Lambadiari V. A new approach of statin therapy in carotid atherosclerosis: Targeting indices of plaque vulnerability on the top of lipid-lowering. A narrative review. Kardiologia Polska . 2022;80:880–890. doi: 10.33963/KP.a2022.0155. [DOI] [PubMed] [Google Scholar]
- [76].Mastroiacovo D, Mengozzi A, Dentali F, Pomero F, Virdis A, Camerota A, et al. Enhanced Carotid Plaque Echolucency Is Associated with Reduced Cognitive Performance in Elderly Patients with Atherosclerotic Disease Independently on Metabolic Profile. Metabolites . 2023;13:478. doi: 10.3390/metabo13040478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Jashari F, Ibrahimi P, Johansson E, Grönlund C, Wester P, Henein MY. Carotid IM-GSM is better than IMT for identifying patients with multiple arterial disease. Scandinavian Cardiovascular Journal: SCJ . 2018;52:93–99. doi: 10.1080/14017431.2018.1435903. [DOI] [PubMed] [Google Scholar]
- [78].Joshi NV, Vesey AT, Williams MC, Shah ASV, Calvert PA, Craighead FHM, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet (London, England) . 2014;383:705–713. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- [79].Derlin T, Tóth Z, Papp L, Wisotzki C, Apostolova I, Habermann CR, et al. Correlation of inflammation assessed by 18F-FDG PET, active mineral deposition assessed by 18F-fluoride PET, and vascular calcification in atherosclerotic plaque: a dual-tracer PET/CT study. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine . 2011;52:1020–1027. doi: 10.2967/jnumed.111.087452. [DOI] [PubMed] [Google Scholar]
- [80].Zwakenberg SR, de Jong PA, Bartstra JW, van Asperen R, Westerink J, de Valk H, et al. The effect of menaquinone-7 supplementation on vascular calcification in patients with diabetes: a randomized, double-blind, placebo-controlled trial. The American Journal of Clinical Nutrition . 2019;110:883–890. doi: 10.1093/ajcn/nqz147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cao HL, Chen XB, Lu JG, Hou ZH, Tang X, Gao Y, et al. Metabolic syndrome and coronary artery calcification: a community-based natural population study. Chinese Medical Journal . 2013;126:4618–4623. [PubMed] [Google Scholar]
- [82].Toussaint ND, Pedagogos E, Lioufas NM, Elder GJ, Pascoe EM, Badve SV, et al. A Randomized Trial on the Effect of Phosphate Reduction on Vascular End Points in CKD (IMPROVE-CKD) Journal of the American Society of Nephrology: JASN . 2020;31:2653–2666. doi: 10.1681/ASN.2020040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhang Y, Feng B. Systematic review and meta-analysis for the association of bone mineral density and osteoporosis/osteopenia with vascular calcification in women. International Journal of Rheumatic Diseases . 2017;20:154–160. doi: 10.1111/1756-185X.12842. [DOI] [PubMed] [Google Scholar]
- [84].Al-Kindi S, Dong T, Chen W, Tashtish N, Neeland IJ, Nasir K, et al. Relation of coronary calcium scoring with cardiovascular events in patients with diabetes: The CLARIFY Registry. Journal of Diabetes and its Complications . 2022;36:108269. doi: 10.1016/j.jdiacomp.2022.108269. [DOI] [PubMed] [Google Scholar]
- [85].Pechlivanis S, Jung D, Moebus S, Lehmann N, Mahabadi AA, Hoffmann P, et al. Pharmacogenetic association of diabetes-associated genetic risk score with rapid progression of coronary artery calcification following treatment with HMG-CoA-reductase inhibitors -results of the Heinz Nixdorf Recall Study. Naunyn-Schmiedeberg’s Archives of Pharmacology . 2021;394:1713–1725. doi: 10.1007/s00210-021-02100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chen W, Fitzpatrick J, Sozio SM, Jaar BG, Estrella MM, Riascos-Bernal DF, et al. Identification of Novel Biomarkers and Pathways for Coronary Artery Calcification in Nondiabetic Patients on Hemodialysis Using Metabolomic Profiling. Kidney360 . 2021;2:279–289. doi: 10.34067/KID.0004422020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wong ND, Vo A, Abrahamson D, Tobis JM, Eisenberg H, Detrano RC. Detection of coronary artery calcium by ultrafast computed tomography and its relation to clinical evidence of coronary artery disease. The American Journal of Cardiology . 1994;73:223–227. doi: 10.1016/0002-9149(94)90223-2. [DOI] [PubMed] [Google Scholar]
- [88].Goel M, Wong ND, Eisenberg H, Hagar J, Kelly K, Tobis JM. Risk factor correlates of coronary calcium as evaluated by ultrafast computed tomography. The American Journal of Cardiology . 1992;70:977–980. doi: 10.1016/0002-9149(92)90346-z. [DOI] [PubMed] [Google Scholar]
- [89].Liu M, Zhang W, Li X, Han J, Chen Y, Duan Y. Impact of age and sex on the development of atherosclerosis and expression of the related genes in apoE deficient mice. Biochemical and Biophysical Research Communications . 2016;469:456–462. doi: 10.1016/j.bbrc.2015.11.064. [DOI] [PubMed] [Google Scholar]
- [90].Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation . 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- [91].Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation . 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- [92].Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA) European Heart Journal . 2018;39:2401–2408. doi: 10.1093/eurheartj/ehy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ. Coronary calcium in adults with type 1 diabetes: a stronger correlate of clinical coronary artery disease in men than in women. Diabetes . 2000;49:1571–1578. doi: 10.2337/diabetes.49.9.1571. [DOI] [PubMed] [Google Scholar]
- [94].Davies MR, Hruska KA. Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney International . 2001;60:472–479. doi: 10.1046/j.1523-1755.2001.060002472.x. [DOI] [PubMed] [Google Scholar]
- [95].Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease. Journal of the American College of Cardiology . 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- [96].Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, et al. Cause of Death in Patients with Reduced Kidney Function. Journal of the American Society of Nephrology: JASN . 2015;26:2504–2511. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet (London, England) . 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- [98].London GM. Cardiovascular calcifications in uremic patients: clinical impact on cardiovascular function. Journal of the American Society of Nephrology: JASN . 2003;14:S305–S309. doi: 10.1097/01.asn.0000081664.65772.eb. [DOI] [PubMed] [Google Scholar]
- [99].Marinelli A, Pistolesi V, Pasquale L, Di Lullo L, Ferrazzano M, Baudena G, et al. Diagnosis of Arterial Media Calcification in Chronic Kidney Disease. Cardiorenal Medicine . 2013;3:89–95. doi: 10.1159/000350764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Coll B, Betriu A, Martínez-Alonso M, Amoedo ML, Arcidiacono MV, Borras M, et al. Large artery calcification on dialysis patients is located in the intima and related to atherosclerosis. Clinical Journal of the American Society of Nephrology: CJASN . 2011;6:303–310. doi: 10.2215/CJN.04290510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mathew RO, Bangalore S, Lavelle MP, Pellikka PA, Sidhu MS, Boden WE, et al. Diagnosis and management of atherosclerotic cardiovascular disease in chronic kidney disease: a review. Kidney International . 2017;91:797–807. doi: 10.1016/j.kint.2016.09.049. [DOI] [PubMed] [Google Scholar]
- [102].Mönckeberg JG. Über die reine Mediaverkalkung der Extremitätenarterien und ihr Verhalten zur Arteriosklerose. Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medizin . 1903;171:141–167. (In German) [Google Scholar]
- [103].Micheletti RG, Fishbein GA, Currier JS, Fishbein MC. Mönckeberg sclerosis revisited: a clarification of the histologic definition of Mönckeberg sclerosis. Archives of Pathology & Laboratory Medicine . 2008;132:43–47. doi: 10.5858/2008-132-43-MSRACO. [DOI] [PubMed] [Google Scholar]
- [104].Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, et al. Medial vascular calcification revisited: review and perspectives. European Heart Journal . 2014;35:1515–1525. doi: 10.1093/eurheartj/ehu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Edmonds ME, Morrison N, Laws JW, Watkins PJ. Medial arterial calcification and diabetic neuropathy. British Medical Journal (Clinical Research Ed.) . 1982;284:928–930. doi: 10.1136/bmj.284.6320.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care . 1994;17:1252–1256. doi: 10.2337/diacare.17.11.1252. [DOI] [PubMed] [Google Scholar]
- [107].Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arteriosclerosis, Thrombosis, and Vascular Biology . 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- [108].Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions . 2014;83:E212–E220. doi: 10.1002/ccd.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Scott C, Lateef SS, Hong CG, Dey AK, Manyak GA, Patel NH, et al. Inflammation, coronary plaque progression, and statin use: A secondary analysis of the Risk Stratification with Image Guidance of HMG CoA Reductase Inhibitor Therapy (RIGHT) study. Clinical Cardiology . 2022;45:622–628. doi: 10.1002/clc.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Vengrenyuk Y, Cardoso L, Weinbaum S. Micro-CT based analysis of a new paradigm for vulnerable plaque rupture: cellular microcalcifications in fibrous caps. Molecular & Cellular Biomechanics: MCB . 2008;5:37–47. [PubMed] [Google Scholar]
- [111].Shi X, Gao J, Lv Q, Cai H, Wang F, Ye R, et al. Calcification in Atherosclerotic Plaque Vulnerability: Friend or Foe. Frontiers in Physiology . 2020;11:56. doi: 10.3389/fphys.2020.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Vancheri F, Longo G, Vancheri S, Danial JSH, Henein MY. Coronary Artery Microcalcification: Imaging and Clinical Implications. Diagnostics (Basel, Switzerland) . 2019;9:125. doi: 10.3390/diagnostics9040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation . 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- [114].Terry JG, Carr JJ, Kouba EO, Davis DH, Menon L, Bender K, et al. Effect of simvastatin (80 mg) on coronary and abdominal aortic arterial calcium (from the coronary artery calcification treatment with zocor [CATZ] study) The American Journal of Cardiology . 2007;99:1714–1717. doi: 10.1016/j.amjcard.2007.01.060. [DOI] [PubMed] [Google Scholar]
- [115].Schmermund A, Achenbach S, Budde T, Buziashvili Y, Förster A, Friedrich G, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation . 2006;113:427–437. doi: 10.1161/CIRCULATIONAHA.105.568147. [DOI] [PubMed] [Google Scholar]
- [116].Raggi P, Davidson M, Callister TQ, Welty FK, Bachmann GA, Hecht H, et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES) Circulation . 2005;112:563–571. doi: 10.1161/CIRCULATIONAHA.104.512681. [DOI] [PubMed] [Google Scholar]
- [117].Houslay ES, Cowell S, Prescott R, Reid J, Burton J, Northridge D, et al. Progressive coronary calcification despite intensive lipid-lowering treatment: a randomised controlled trial. Heart . 2006;92:1207–1212. doi: 10.1136/hrt.2005.080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Shekar C, Budoff M. Calcification of the heart: mechanisms and therapeutic avenues. Expert Review of Cardiovascular Therapy . 2018;16:527–536. doi: 10.1080/14779072.2018.1484282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Pan W, Jie W, Huang H. Vascular calcification: Molecular mechanisms and therapeutic interventions. MedComm . 2023;4:e200. doi: 10.1002/mco2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Healy A, Berus JM, Christensen JL, Lee C, Mantsounga C, Dong W, et al. Statins Disrupt Macrophage Rac1 Regulation Leading to Increased Atherosclerotic Plaque Calcification. Arteriosclerosis, Thrombosis, and Vascular Biology . 2020;40:714–732. doi: 10.1161/ATVBAHA.119.313832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Mujaj B, Bos D, Selwaness M, Leening MJG, Kavousi M, Wentzel JJ, et al. Statin use is associated with carotid plaque composition: The Rotterdam Study. International Journal of Cardiology . 2018;260:213–218. doi: 10.1016/j.ijcard.2018.02.111. [DOI] [PubMed] [Google Scholar]
- [122].Banach M, Serban C, Sahebkar A, Mikhailidis DP, Ursoniu S, Ray KK, et al. Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Medicine . 2015;13:229. doi: 10.1186/s12916-015-0459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Jr, Kastelein JJP, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. The New England Journal of Medicine . 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- [124].Henein M, Granåsen G, Wiklund U, Schmermund A, Guerci A, Erbel R, et al. High dose and long-term statin therapy accelerate coronary artery calcification. International Journal of Cardiology . 2015;184:581–586. doi: 10.1016/j.ijcard.2015.02.072. [DOI] [PubMed] [Google Scholar]
- [125].Dykun I, Lehmann N, Kälsch H, Möhlenkamp S, Moebus S, Budde T, et al. Statin Medication Enhances Progression of Coronary Artery Calcification: The Heinz Nixdorf Recall Study. Journal of the American College of Cardiology . 2016;68:2123–2125. doi: 10.1016/j.jacc.2016.08.040. [DOI] [PubMed] [Google Scholar]
- [126].Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. Journal of the American College of Cardiology . 2015;65:1273–1282. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
- [127].Hanai K, Babazono T, Uchigata Y. Impact of statins on serial coronary calcification during atheroma progression and regression. Clinical and Experimental Nephrology . 2016;21:633–642. [Google Scholar]
- [128].Li Y, Lee Y, Wolfe RA, Morgenstern H, Zhang J, Port FK, et al. On a preference-based instrumental variable approach in reducing unmeasured confounding-by-indication. Statistics in Medicine . 2015;34:1150–1168. doi: 10.1002/sim.6404. [DOI] [PubMed] [Google Scholar]
- [129].Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. The New England Journal of Medicine . 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- [130].Wanner C, Krane V, März W, Olschewski M, Mann JFE, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. The New England Journal of Medicine . 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- [131].Andelius L, Mortensen MB, Nørgaard BL, Abdulla J. Impact of statin therapy on coronary plaque burden and composition assessed by coronary computed tomographic angiography: a systematic review and meta-analysis. European Heart Journal. Cardiovascular Imaging . 2018;19:850–858. doi: 10.1093/ehjci/jey012. [DOI] [PubMed] [Google Scholar]
- [132].Kovarnik T, Mintz GS, Skalicka H, Kral A, Horak J, Skulec R, et al. Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration: HEAVEN study. Circulation Journal: Official Journal of the Japanese Circulation Society . 2012;76:176–183. doi: 10.1253/circj.cj-11-0730. [DOI] [PubMed] [Google Scholar]
- [133].Henein MY, Owen A. Statins moderate coronary stenoses but not coronary calcification: results from meta-analyses. International Journal of Cardiology . 2011;153:31–35. doi: 10.1016/j.ijcard.2010.08.031. [DOI] [PubMed] [Google Scholar]
- [134].Lee SE, Sung JM, Andreini D, Budoff MJ, Cademartiri F, Chinnaiyan K, et al. Differential association between the progression of coronary artery calcium score and coronary plaque volume progression according to statins: the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography Imaging (PARADIGM) study. European Heart Journal. Cardiovascular Imaging . 2019;20:1307–1314. doi: 10.1093/ehjci/jez022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A, et al. Effects of Statins on Coronary Atherosclerotic Plaques: The PARADIGM Study. JACC. Cardiovascular Imaging . 2018;11:1475–1484. doi: 10.1016/j.jcmg.2018.04.015. [DOI] [PubMed] [Google Scholar]
- [136].Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation . 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- [137].Wong ND. Evolution of Coronary Calcium Screening for Assessment of Atherosclerotic Cardiovascular Disease Risk and Role in Preventive Cardiology. Current Atherosclerosis Reports . 2022;24:949–957. doi: 10.1007/s11883-022-01073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Nozue T, Fukui K, Yamamoto S, Kunishima T, Umezawa S, Onishi Y, et al. Time course of statin-induced changes in coronary atherosclerosis using intravascular ultrasound with virtual histology. Coronary Artery Disease . 2013;24:481–486. doi: 10.1097/MCA.0b013e32836325ac. [DOI] [PubMed] [Google Scholar]
- [139].García-García HM, Mintz GS, Lerman A, Vince DG, Margolis MP, van Es GA, et al. Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention: Journal of EuroPCR in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology . 2009;5:177–189. doi: 10.4244/eijv5i2a29. [DOI] [PubMed] [Google Scholar]
- [140].Gu SZ, Costopoulos C, Huang Y, Bourantas C, Woolf A, Sun C, et al. High-intensity statin treatment is associated with reduced plaque structural stress and remodelling of artery geometry and plaque architecture. European Heart Journal Open . 2021;1:oeab039. doi: 10.1093/ehjopen/oeab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Kadoglou NPE, Kottas G, Lampropoulos S, Vitta I, Liapis CD. Serum levels of fetuin-A, osteoprotegerin and osteopontin in patients with coronary artery disease: effects of statin (HMGCoA-reductase inhibitor) therapy. Clinical Drug Investigation . 2014;34:165–171. doi: 10.1007/s40261-013-0157-y. [DOI] [PubMed] [Google Scholar]
- [142].Heart Protection Study Collaborative Group Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. Journal of Vascular Surgery . 2007;45:645–645. doi: 10.1016/j.jvs.2006.12.054. [DOI] [PubMed] [Google Scholar]
- [143].Arya S, Khakharia A, Binney ZO, DeMartino RR, Brewster LP, Goodney PP, et al. Association of Statin Dose with Amputation and Survival in Patients with Peripheral Artery Disease. Circulation . 2018;137:1435–1446. doi: 10.1161/CIRCULATIONAHA.117.032361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation . 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Aboyans V, Ricco JB, Bartelink MLEL, Bjorck M, Brodmann M, Cohnert T, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS) Revista Espanola De Cardiologia (English Ed.) . 2018;71:111. doi: 10.1016/j.rec.2017.12.014. [DOI] [PubMed] [Google Scholar]
- [146].Ramos R, García-Gil M, Comas-Cufí M, Quesada M, Marrugat J, Elosua R, et al. Statins for Prevention of Cardiovascular Events in a Low-Risk Population with Low Ankle Brachial Index. Journal of the American College of Cardiology . 2016;67:630–640. doi: 10.1016/j.jacc.2015.11.052. [DOI] [PubMed] [Google Scholar]
- [147].Kadoglou NPE, Sfyroeras GS, Spathis A, Gkekas C, Gastounioti A, Mantas G, et al. Galectin-3, Carotid Plaque Vulnerability, and Potential Effects of Statin Therapy. European Journal of Vascular and Endovascular Surgery: the Official Journal of the European Society for Vascular Surgery . 2015;49:4–9. doi: 10.1016/j.ejvs.2014.10.009. [DOI] [PubMed] [Google Scholar]
- [148].Zhao XQ, Dong L, Hatsukami T, Phan BA, Chu B, Moore A, et al. MR imaging of carotid plaque composition during lipid-lowering therapy a prospective assessment of effect and time course. JACC. Cardiovascular Imaging . 2011;4:977–986. doi: 10.1016/j.jcmg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Underhill HR, Yuan C, Zhao X-Q, Kraiss LW, Parker DL, Saam T, et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: A high-resolution magnetic resonance imaging trial. American Heart Journal . 2008;155:584. doi: 10.1016/j.ahj.2007.11.018. e1–584.e8. [DOI] [PubMed] [Google Scholar]
- [150].Migrino RQ, Bowers M, Harmann L, Prost R, LaDisa JF., Jr Carotid plaque regression following 6-month statin therapy assessed by 3T cardiovascular magnetic resonance: comparison with ultrasound intima media thickness. Journal of Cardiovascular Magnetic Resonance: Official Journal of the Society for Cardiovascular Magnetic Resonance . 2011;13:37. doi: 10.1186/1532-429X-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Kadoglou NPE, Gerasimidis T, Golemati S, Kapelouzou A, Karayannacos PE, Liapis CD. The relationship between serum levels of vascular calcification inhibitors and carotid plaque vulnerability. Journal of Vascular Surgery . 2008;47:55–62. doi: 10.1016/j.jvs.2007.09.058. [DOI] [PubMed] [Google Scholar]
- [152].Kadoglou NPE, Gerasimidis T, Kapelouzou A, Moumtzouoglou A, Avgerinos ED, Kakisis JD, et al. Beneficial changes of serum calcification markers and contralateral carotid plaques echogenicity after combined carotid artery stenting plus intensive lipid-lowering therapy in patients with bilateral carotid stenosis. European Journal of Vascular and Endovascular Surgery: the Official Journal of the European Society for Vascular Surgery . 2010;39:258–265. doi: 10.1016/j.ejvs.2009.11.013. [DOI] [PubMed] [Google Scholar]
- [153].Cho IJ, Chang HJ, Cho I, Heo R, Lee SE, Shim CY, et al. Association of Thoracic Aorta Calcium Score with Exercise Blood Pressure Response and Clinical Outcomes in Elderly Individuals: Differential Impact of Aorta Calcification Compared with Coronary Artery Calcification. Journal of the American Heart Association . 2016;5:e003131. doi: 10.1161/JAHA.115.003131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Hoshino T, Chow LA, Hsu JJ, Perlowski AA, Abedin M, Tobis J, et al. Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. American Journal of Physiology. Heart and Circulatory Physiology . 2009;297:H802–H810. doi: 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine . 2010;51:1826–1829. doi: 10.2967/jnumed.110.077933. [DOI] [PMC free article] [PubMed] [Google Scholar]