Abstract
Introduction
This analysis examined the baseline characteristics and clinical outcomes of patients with chronic kidney disease (CKD) and rapid or non-rapid estimated glomerular filtration rate (eGFR) decline, using retrospective data from DISCOVER CKD (ClinicalTrials.gov, NCT04034992).
Methods
Data (2008–2020) were extracted from UK Clinical Practice Research Datalink, US TriNetX, US Limited Claims and Electronic Health Record Dataset, and Japan Medical Data Vision. Patients with CKD (two consecutive eGFR measures < 75 mL/min/1.73 m2 recorded 90–730 days apart) were included. Rapid eGFR decline was defined as an annual decline of > 4 mL/min/1.73 m2 at 2 years post-index; non-rapid eGFR decline was defined as an annual decline of ≤ 4 mL/min/1.73 m2. Clinical outcomes assessed included all-cause mortality, kidney outcomes (composite risk of kidney failure [progression to CKD stage 5] or > 50% eGFR decline, and kidney failure alone), cardiovascular events—including major adverse cardiovascular events (MACE; non-fatal myocardial infarction/stroke and cardiovascular death)—and all-cause hospitalization.
Results
Across databases, rapid eGFR decline occurred in 13.7% of 804,237 eligible patients. Mean annual eGFR decline ranged between − 6.21 and − 6.86 mL/min/1.73 m2 in patients with rapid eGFR decline versus between − 0.11 and − 0.77 mL/min/1.73 m2 in patients with non-rapid eGFR decline. Rapid eGFR decline was associated with increased comorbidity burden and medication prescriptions. Across databases, the composite risk of kidney failure or > 50% decline in eGFR was significantly greater in patients with rapid versus non-rapid eGFR decline (P < 0.01); all-cause mortality, kidney failure alone, MACE, and all-cause hospitalization each significantly increased in two databases (P < 0.01–0.05).
Conclusion
Understanding patient factors associated with rapid eGFR decline in patients with CKD may help identify individuals who would benefit from proactive management to minimize the risk of adverse outcomes.
Trial Registration
ClinicalTrials.gov identifier, NCT04034992.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02913-x.
Keywords: Chronic kidney disease, DISCOVER CKD, eGFR, Rapid decline, Clinical outcomes
Key Summary Points
| Why carry out this study? |
| Identifying the risk factors for rapid estimated glomerular filtration rate (eGFR) decline in chronic kidney disease (CKD) may help patients at highest risk receive earlier diagnosis and initiation of treatment and more frequent monitoring to slow disease progression and reduce the risk of adverse clinical outcomes. |
| What did the study ask? |
| This analysis investigated the baseline characteristics and clinical outcomes of patients with rapid and non-rapid eGFR decline using retrospective data from the DISCOVER CKD observational cohort study. |
| Data were extracted from four databases: UK Clinical Practice Research Datalink (2008–2019); US TriNetX (2008–2020); US Limited Claims and Electronic Health Record Dataset (2012–2019); and Japan Medical Data Vision (2008–2017). |
| What was learned from the study? |
| Urine albumin-to-creatinine ratios and C-reactive protein levels were both higher, and anemia, atrial fibrillation, and acute kidney injury were all more prevalent, among patients with rapid versus non-rapid eGFR decline, suggesting that preventing or treating these conditions could prevent rapid declines in kidney function. |
| The risk of kidney failure or > 50% decline in eGFR was significantly elevated among patients with rapid versus non-rapid eGFR decline in all four databases; all-cause mortality, kidney failure alone, major adverse cardiovascular events, and all-cause hospitalization were significantly elevated in two databases each. |
Introduction
Rapid estimated glomerular filtration rate (eGFR) decline in patients with chronic kidney disease (CKD) is associated with worse clinical outcomes compared with non-rapid eGFR decline, independent of baseline eGFR [1–4]. Identifying comorbidities and patient characteristics associated with rapid eGFR decline remains challenging in clinical practice. eGFR decline has been associated with increased age, comorbidities including diabetes mellitus and hypertension, genetic mutations, previous eGFR decline, and current eGFR [1, 4–6].
Although risk calculators predicting clinical outcomes such as kidney failure and mortality have been developed from epidemiological data [7, 8], they do not include the rate of eGFR change over time, which may help to contextualize individual risk profiles [9].
Determining the relationships between patient baseline characteristics, rate of eGFR decline, and adverse clinical outcomes is a first step in isolating risk factors for rapid eGFR decline. This could direct prioritization of resources towards those patients at highest risk, ensuring they receive earlier and more intense diagnosis and treatment initiation and more frequent monitoring to reduce the risk of adverse clinical outcomes, and delay or even avoid the need for renal replacement therapy (RRT). Although several publications have reported on this topic, studies comparing patients with rapid or non-rapid eGFR decline using multiple international datasets are lacking. DISCOVER CKD (ClinicalTrials.gov, NCT04034992) is an ongoing international observational cohort study of patients with CKD, aiming to improve our understanding of the epidemiology, and clinical and economic burden of CKD, as well as the determinants of clinical and patient-reported outcomes in real-world CKD settings [10–14]. In this analysis, the baseline characteristics and clinical outcomes of patients with CKD and rapid or non-rapid eGFR decline were evaluated using retrospective data from DISCOVER CKD.
Methods
Patient Population and Study Databases
The data used in this analysis are from a subset of patients from the DISCOVER CKD retrospective cohort [10], extracted from the following databases: UK Clinical Practice Research Datalink (CPRD) [15–17] linked to hospital episode statistics data (2008–2019); US TriNetX, a global federated health research analytics network dataset (2008–2020); US Limited Claims and Electronic Health Record Dataset (LCED; 2012–2019); and Japan Medical Data Vision (MDV; 2008–2017).
This analysis included adults aged ≥ 18 years (≥ 20 years for Japan MDV) at the index date, defined as the second of two consecutive eGFR measurements < 75 mL/min/1.73 m2 recorded 90–730 days apart, on or after January 1, 2008. Patients were required to have continuous enrollment in the database of ≥ 12 months before the index date. Patients with < 30 days follow-up from index, a history of kidney transplant or chronic RRT, or a history of type 1 diabetes, polycystic kidney disease, lupus nephritis, or anti-neutrophil cytoplasmic antibody nephritis were excluded from this analysis.
Definition of Rapid and Non-rapid eGFR Decline
The CKD Epidemiology Collaboration equation [18] was used to calculate eGFR from records of serum creatinine measurements. Linear mixed models (separated by database) were used to estimate eGFR trajectories by extracting eGFR measurements recorded in the first 2 years post-index; eGFR values < 5 mL/min/1.73 m2 were excluded from eGFR trajectory calculations as they were assumed to have been misrecorded. On the basis of calculated eGFR trajectories, patients were classified as having rapid or non-rapid eGFR decline. Rapid eGFR decline was defined as annual decline > 4 mL/min/1.73 m2. Non-rapid eGFR decline was defined as annual decline ≤ 4 mL/min/1.73 m2. Groupings were based on published literature [1, 2]. Only patients with ≥ 2 years follow-up were included in the analyses. The baseline (time zero) for these patients was “reset” at 2 years post-index, at which point patient characteristics and outcomes were assessed.
Study Variables and Outcomes
Patient characteristics analyzed included age; sex; laboratory findings, including baseline eGFR and urine albumin-to-creatinine ratio (UACR), although UACR data were not available for the Japan MDV; comorbidities, including type 2 diabetes (T2D), stroke, coronary heart disease (CHD), heart failure (HF), hypertension, hyperkalemia, atrial fibrillation, and anemia; and prescribed medications, including renin-angiotensin-aldosterone system inhibitors (RAASi; comprising angiotensin-converting enzyme inhibitors [ACEi], angiotensin receptor blockers [ARB], and mineralocorticoid receptor antagonists), diuretics, sodium-glucose co-transporter-2 inhibitors (SGLT2i), insulin, potassium binders, and statins. Laboratory data and procedural history were captured at or within 12 months of baseline (index). Prescribed medications were assessed at or within 90 days of baseline. Comorbidity history used data captured at any point in a patient’s medical history.
Risks of clinical outcomes with rapid versus non-rapid eGFR decline were assessed at 2 years post-index, after the initial 2-year window was used to calculate eGFR trajectories. Clinical outcomes included all-cause mortality; kidney outcomes, comprising the composite of kidney failure (progression to CKD stage 5 [sustained eGFR ≤ 15 mL/min/1.73 m2 in patients with eGFR > 15 mL/min/1.73 m2 at baseline or initiation of chronic RRT for > 30 days]) or > 50% decline in eGFR, and kidney failure alone; a composite of CV events (including major adverse CV events [MACE], defined as non-fatal myocardial infarction, non-fatal stroke, and CV death); hospitalization for HF (hHF); and all-cause hospitalization. For analyses of kidney outcomes, patients with baseline eGFR ≤ 15 mL/min/1.73 m2 were excluded. Data on all-cause mortality were not captured in the US LCED, and data on hHF were not captured in the Japan MDV, because these databases are generated from medical records data that did not include this information. The analysis observation period was from index date until death (censored at 3 years of follow-up), loss to follow-up, dialysis initiation, kidney transplant, or database end, whichever occurred first.
Statistical Analyses
Baseline (2 years post-index) characteristics, including prescribed medications, were summarized descriptively.
To compare risks of adverse clinical outcomes, Cox proportional hazards modelling was applied to calculate the adjusted hazard ratios and 95% confidence intervals. Models were adjusted for baseline age, sex, race (UK CPRD and US TriNetX), diastolic and systolic blood pressure (BP), body mass index, cholesterol, eGFR, natural logarithm of number of eGFR tests recorded before 2 years post-index, UACR, hemoglobin, serum potassium, medications, and history of hypertension, T2D, HF, stroke, or myocardial infarction. Risk of all-cause mortality stratified according to eGFR category at baseline was assessed for individual databases, and pooled for the UK CPRD, US TriNetX, and Japan MDV databases (patient confidentiality agreements relating to the US LCED forbade pooling with other databases). To account for missing data, a binary numerical indicator was used (data were coded as missing [0] or not missing [1]) for continuous variables; for categorical variables derived from continuous variables, a new category was added for missing data (if an underlying continuous variable was missing). For prior comorbidities and medications, no prior record (i.e., missing) was defined as lack of evidence of prior comorbidity or medication prescription. Sensitivity analyses included a competing risks analysis of adverse clinical outcomes to determine the potential impact of the competing risk of mortality on results.
Ethical Approval
This study was performed in accordance with ethical principles consistent with the Declaration of Helsinki, International Conference on Harmonisation, Good Clinical Practice, and the applicable legislation on noninterventional studies and observational studies. This study used de-identified data and did not require data collection beyond that of routine clinical care. No identifiable information was collected or examined as part of the study. Ethical and scientific approval for use of CPRD data in the current study was obtained from the Independent Scientific Advisory Committee of CPRD (protocol number 19_172). Informed consent was waived by the East Midlands – Derby Research Ethics Committee because CPRD data are anonymized for research purposes (for further information, see https://cprd.com/safeguarding-patient-data). Ethics approval was not required for use of data from the US TriNetX and Japan Medical Data Vision for this study, in accordance with local or national guidelines.
Results
Patient Attrition
The DISCOVER CKD cohort included data from > 1.8 million patients. Overall, 804,237 patients across the four databases were eligible for this analysis (Supplementary Material Fig. S1).
Baseline Characteristics
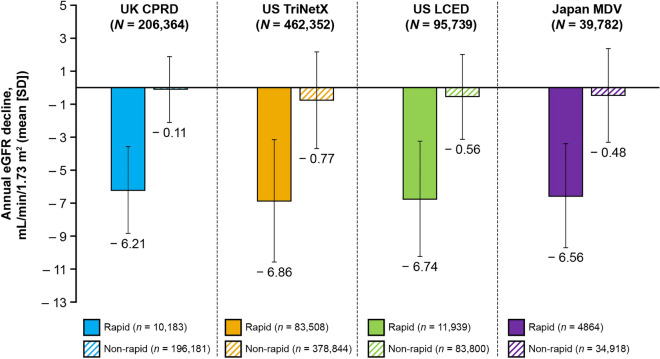
The baseline characteristics of each cohort are summarized in Supplementary Material Table S1. Rapid eGFR decline occurred in 13.7% (n = 110,494) of patients overall, varying from 4.9% in the UK CPRD to 18.1% in the US TriNetX. The mean number of eGFR measurements per patient used to calculate eGFR slopes was 6.9–12.7 and 3.0–7.7 for patients with rapid and non-rapid eGFR decline, respectively. Mean annual eGFR decline ranged between − 6.21 and − 6.86 mL/min/1.73 m2 for patients with rapid eGFR decline, and between − 0.11 and − 0.77 mL/min/1.73 m2 for patients with non-rapid eGFR decline (Fig. 1). The median follow-up time per patient was 3.3–4.5 and 3.6–5.0 years for patients with rapid and non-rapid eGFR decline, respectively. In patients without (i.e., no eGFR measurement in the first 2 years) versus with eGFR slope data, mean age was numerically lower, and a higher proportion were female (Supplementary Material Table S2).
Fig. 1.
Annual eGFR decline in patients with rapid versus non-rapid eGFR decline. CPRD Clinical Practice Research Datalink, eGFR estimated glomerular filtration rate, LCED Limited Claims and Electronic Health Record Dataset, MDV Medical Data Vision, SD standard deviation
In patients with rapid versus non-rapid eGFR decline, mean age and median UACR were numerically higher at baseline. Proportions of male and female patients, mean systolic and diastolic BP, mean high- and low-density lipoprotein (HDL and LDL) values, and median glycated hemoglobin (HbA1c) were comparable between groups (Supplementary Material Tables S1 and S3). Mean total cholesterol levels were numerically higher in patients with non-rapid versus rapid eGFR decline (Supplementary Material Table S3).
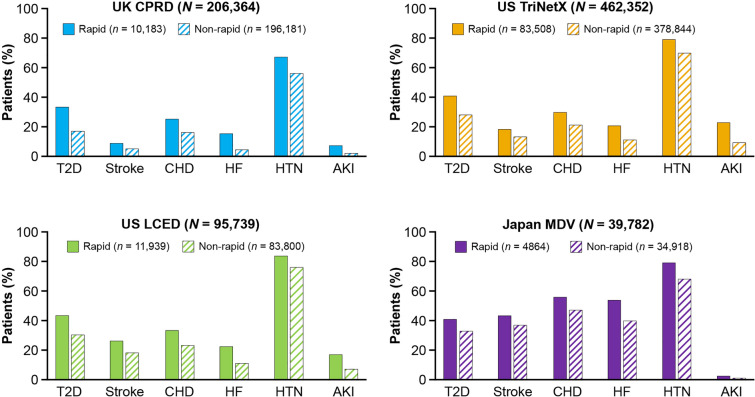
Prevalence of comorbidities was higher in patients with rapid versus non-rapid eGFR decline (Fig. 2; Supplementary Material Table S4). Hypertension was the most prevalent comorbidity in all patients. Patients in Japan had a greater overall comorbidity burden compared with patients in the USA and UK (Fig. 2).
Fig. 2.
Comorbidity burden in patients with rapid versus non-rapid eGFR decline (2 years post-index). CHD was ascertained via diagnostic codes for unstable angina and myocardial infarction. AKI acute kidney injury, CHD coronary heart disease, CKD chronic kidney disease, CPRD Clinical Practice Research Datalink, eGFR estimated glomerular filtration rate, HF heart failure, HTN hypertension, LCED Limited Claims and Electronic Health Record Dataset, MDV Medical Data Vision, T2D type 2 diabetes
The proportion of patients with prescribed medications at baseline was higher in patients with rapid versus non-rapid eGFR decline. The proportion of patients prescribed RAASi was 14.5–58.5% in patients with rapid eGFR decline and 10.8–42.2% in patients with non-rapid eGFR decline (Supplementary Material Table S5).
Clinical Outcomes
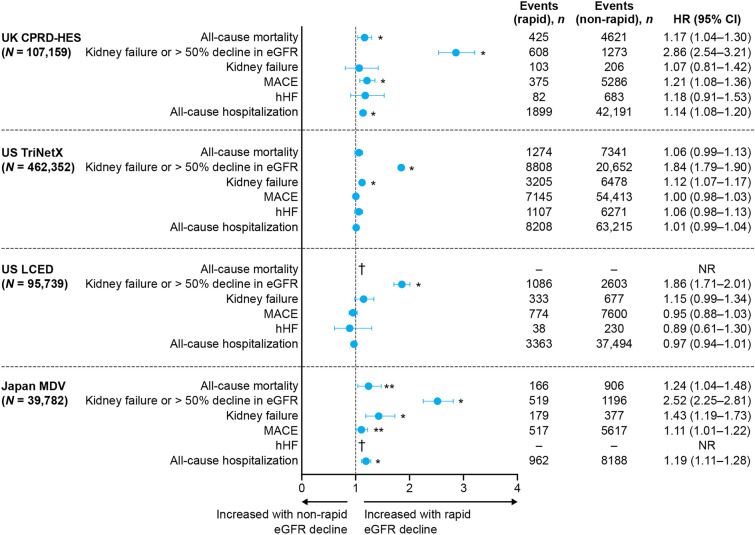
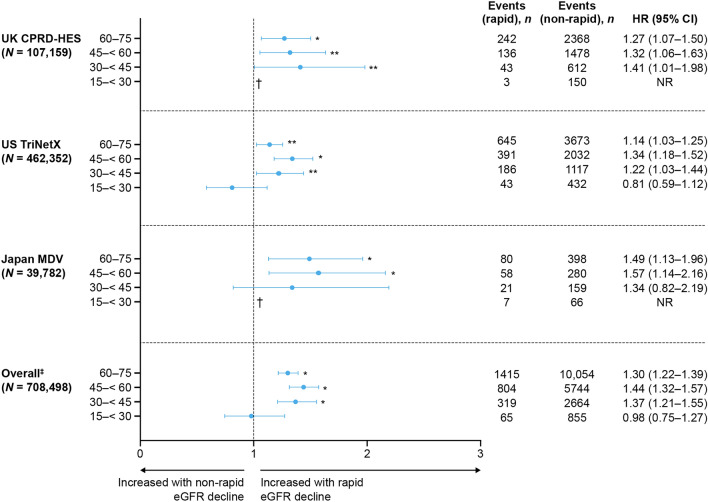
Across databases, the composite risk of kidney failure or > 50% decline in eGFR was significantly greater in patients with rapid versus non-rapid eGFR decline (P < 0.01), while all-cause mortality, kidney failure alone, MACE, and all-cause hospitalizations were each significantly increased in two databases (P < 0.01–0.05; Fig. 3). The risk of all-cause mortality among patients with eGFR ≥ 45 mL/min/1.73 m2 at baseline was significantly higher in those with rapid versus non-rapid eGFR decline (Fig. 4). A competing risks analysis accounting for mortality demonstrated an overall greater risk of adverse clinical outcomes in patients with rapid versus non-rapid eGFR decline (Supplementary Material Fig. S2).
Fig. 3.
Clinical outcomes in patients with rapid versus non-rapid eGFR decline (2 years post-index). *P < 0.01 for rapid versus non-rapid progressors; **P < 0.05 for rapid versus non-rapid progressors. †Data not available. HRs were computed using Cox proportional hazards models adjusted for baseline age, sex, race (where available), diastolic blood pressure, systolic blood pressure, body mass index, high- and low-density lipoprotein, eGFR, urine albumin-to-creatinine ratio, medications, log (natural logarithm) of the number of eGFR tests recorded prior to 2 years post-index, hemoglobin, serum potassium, and history of hypertension, type 2 diabetes, HF, stroke, and MI. MACE includes non-fatal MI, non-fatal stroke, and cardiovascular death, defined by diagnostic codes. CI confidence interval, CPRD Clinical Practice Research Datalink, eGFR estimated glomerular filtration rate, HES hospital episode statistics, hHF hospitalization for heart failure, HR hazard ratio, LCED Limited Claims and Electronic Health Record Dataset, MACE major adverse cardiovascular event, MDV Medical Data Vision, MI myocardial infarction, NR not reported
Fig. 4.
All-cause mortality in patients with rapid versus non-rapid eGFR decline (2 years post-index), stratified by eGFR category. *P < 0.01 for rapid versus non-rapid progressors; **P < 0.05 for rapid versus non-rapid progressors. †Data not available because of low event numbers. ‡Excluding data from LCED, where data on all-cause mortality were not available. Patients with eGFR ≤ 15 mL/min/1.73 m2 were excluded from analyses, owing to confounding by dialysis. HRs were computed using Cox proportional hazards models adjusted for baseline age, sex, race (where available), diastolic blood pressure, systolic blood pressure, body mass index, high- and low-density lipoprotein, eGFR, urine albumin-to-creatinine ratio, medications, log (natural logarithm) of the number of eGFR tests recorded prior to 2 years post-index, hemoglobin, serum potassium, and history of hypertension, type 2 diabetes, heart failure, stroke, and myocardial infarction. CI confidence interval, CPRD Clinical Practice Research Datalink, eGFR estimated glomerular filtration rate, HES hospital episode statistics, HR hazard ratio, LCED Limited Claims and Electronic Health Record Dataset, MDV Medical Data Vision, NR not reported
Discussion
In this analysis, mean annual eGFR decline ranged between − 6.21 and − 6.86 mL/min/1.73 m2 in patients with rapid eGFR decline; in patients with non-rapid eGFR decline, mean annual eGFR decline ranged between − 0.11 and − 0.77 mL/min/1.73 m2, which contrasts slightly with the mean creatinine clearance reported by the Baltimore Longitudinal Study of Aging for individuals without renal disease (− 0.75 mL/min/1.73 m2) [19]. The relationship between age, sex, and eGFR decline is unclear. Previous studies have associated rapid eGFR decline with both older and younger age [1, 2, 20] and both male and female sex [2, 21]. In this analysis, mean age was higher in patients with rapid eGFR decline, but proportions of male and female patients were comparable.
Median UACR was higher in patients with rapid eGFR decline, consistent with previous findings [2, 22, 23]. Acute kidney injury, a risk factor for accelerated CKD progression [24], anemia, atrial fibrillation, and elevated mean C-reactive protein were also more common in patients with rapid eGFR decline, which may therefore be avoided by preventing or treating these conditions.
Elevated HbA1c is associated with rapid eGFR decline in patients with T2D [25]. In this analysis, T2D was more common in patients with rapid eGFR decline, but mean HbA1c was similar between patients with rapid and non-rapid eGFR decline. HbA1c recordings in patients without T2D may have impacted the calculated means.
Elevated BP, total cholesterol, HDL, LDL and proteinuria are risk factors for rapid eGFR decline [1, 2, 26–31]. BP is especially sensitive to eGFR, although strategies for managing BP in CKD vary widely in the real world [32]. In this analysis, mean systolic and diastolic BP, and HDL and LDL were comparable between patients with rapid and non-rapid eGFR decline despite hypertension and other comorbidities being more common in patients with rapid decline. Mean total cholesterol was higher in patients with non-rapid eGFR decline. Our contradictory findings may be because more patients with rapid eGFR decline were receiving BP-lowering medications and/or statins. Alternatively, alongside the greater burden of CV comorbidities in patients with rapid eGFR decline, it is possible that higher statin use in these patients is accelerating eGFR decline.
Higher medication prescriptions in patients with rapid eGFR decline likely reflect their greater comorbidity burden, with diuretics used to manage many comorbidities including hypertension, CHD, and HF. Despite guidelines recommending that RAASi are prescribed at optimal doses [33], RAASi use was low overall (10.8–58.5%) and less than that reported elsewhere for patients with rapid or non-rapid eGFR decline (66–71%) [2]. Although low use of RAASi in our study could be due to suboptimal medication capture in the databases, it is more likely due to hyperkalemia-related RAASi discontinuation [34–37], even though this is associated with increased healthcare resource utilization and a higher risk of cardiorenal events [38–41]. Guidelines now recommend taking measures to reduce serum potassium, including the use of novel potassium binders, to restore and maintain normokalemia when necessary and enable the prescription of RAASi at optimal doses [33]. Elsewhere, it has been suggested that discontinuing RAASi in patients with advanced CKD may slow eGFR decline [42, 43]. This matter remains under debate: findings from the STOP-ACEi trial suggest that RAASi discontinuation has no clinically relevant impact on eGFR decline [44], while current KDIGO guidelines recommend continuing RAASi in patients with CKD even when eGFR falls [45].
In patients with rapid eGFR decline, the risk of kidney failure or > 50% eGFR decline was significantly increased across all databases (as anticipated); the risks of all-cause mortality, kidney failure alone, MACE, and all-cause hospitalization were each significantly increased in two databases, consistent with previous findings [2, 46, 47]. Although several factors may elevate the risk of adverse outcomes in patients with rapid eGFR decline, Cox proportional hazards models adjusted for key comorbidities in our analysis suggest that eGFR decline rate is a key determinant.
The clinical outcomes assessed showed between-country differences. In patients with rapid eGFR decline, the risk of kidney failure alone was significantly increased in the US TriNetX and Japan MDV databases but not in the UK CPRD or US LCED databases. Similarly, the risk of MACE and all-cause hospitalization was increased in the UK CPRD and the Japan MDV databases but not in the US TriNetX or US LCED databases. This could be due to low baseline risk (most patients had stage 2–3a CKD and median UACR was relatively low), relatively short follow-up, differences in patient characteristics (primary care data in the UK versus secondary data in the USA and Japan), differences in local treatment guidelines, or variations in clinical practice and adherence to treatment guidelines.
Notably, the risk of all-cause mortality was lowest in patients with stage 4 CKD. This may be related to a ceiling effect of rapidly worsening renal function in patients who already had poor kidney function, or potentially due to a greater proportion of patients with stage 4 CKD being under specialist care and improved management. In two databases, risk of all-cause mortality stratified by baseline eGFR category (not reported in US LCED) was significantly increased in patients with rapid eGFR decline and eGFR ≥ 45 mL/min/1.73 m2. Patients with mild-to-moderate CKD may therefore benefit from additional monitoring and proactive management to minimize the risk of adverse clinical outcomes. Previous studies have demonstrated that augmenting RAASi with SGLT2i can improve clinical outcomes and reduce the risk of mortality in patients with CKD [48–51]. SGLT2i use was very low overall, although prescriptions were more common in patients with rapid eGFR decline. This is likely due to the higher prevalence of T2D in these patients, with SGLT2i having shown that they can improve clinical outcomes in patients with CKD and T2D [48–51].
Strengths of this analysis include the very large cohort size (> 800,000 patients), regional scale (patients from the UK, USA, and Japan), and inclusion of patients with a wide range of baseline eGFR values. Additional strengths include the granularity of data, the large number of covariates and outcomes captured, and the use of data from primary and secondary healthcare databases.
Limitations of this analysis include those that are inherent to retrospective studies, in that conclusions about causal relationships between baseline characteristics and rapid eGFR decline cannot be made. Additionally, approximately one-third of patients from the overall DISCOVER CKD cohort were excluded from this analysis on the basis of the eligibility criteria. A high proportion of laboratory data were missing (including UACR and HbA1c measurements), and the proportion of missing data tended to be higher in patients with non-rapid eGFR decline. Furthermore, our findings are only applicable to patients meeting the analysis eligibility criteria within specified countries and healthcare systems. There is also the potential for coding errors, as data were not collected for research purposes. For example, data on race and ethnicity were only available in the UK CPRD and US TriNetX databases, medication prescription data were not fully captured, and there was no means of verifying adherence to medications. Differences between the data sources (primary versus secondary healthcare data) could also impact interpretation of our findings. Finally, although our data suggest associations between some patient baseline characteristics and rapid eGFR decline, there may be other unmeasured factors that are contributing, including genetic predisposition, environmental toxins, smoking status, race, occupational history, and BMI.
Conclusion
To the best of our knowledge, this is the most robust analysis of eGFR slopes performed to date in a global CKD population. Rapid eGFR decline was associated with increased comorbidity burden and medication prescriptions, and increased risk of adverse clinical outcomes. These findings emphasize the importance of routine monitoring of eGFR, as well as the often-overlooked UACR parameter, to identify patients at high risk of rapid eGFR decline who may benefit from proactive and more intense management, and early diagnosis and treatment, to minimize risks of adverse clinical outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Katie Groschwitz, PhD, Saeed Banaama, MD, and Matthew Young, DPhil, and editorial support was provided by Sharmin Saleque, MSc, all of Core (a division of Prime, London, UK), supported by AstraZeneca according to Good Publication Practice guidelines (10.7326/M22-1460). The Sponsor was involved in the study design, collection, analysis, and interpretation of data. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Author Contributions
Matthew Arnold and Claudia Cabrera were responsible for the study design. Matthew Arnold was responsible for data acquisition and analysis. Hiddo Heerspink, Stephen Nolan, Juan-Jesus Carrero, Matthew Arnold, Roberto Pecoits-Filho, Juan José García Sánchez, Eric Wittbrodt, Claudia Cabrera, Carolyn S.P. Lam, Hungta Chen, Eiichiro Kanda, Mitja Lainscak, Carol Pollock, and David C. Wheeler contributed to the development of the manuscript, including reviewing, data interpretation, and approving the final version of the submitted manuscript.
Funding
This analysis and all publication costs for this work, including the journal's Open Access and Rapid Service Fees, were funded by AstraZeneca.
Data Availability
The datasets generated during and/or analyzed during the current study are available upon reasonable request in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.
Declarations
Conflict of Interest
Hiddo Heerspink reports grants and other fees from AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Dimerix, Gilead, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novo Nordisk, and Retrophin. Stephen Nolan, Matthew Arnold, Juan José García Sánchez, Eric Wittbrodt, Claudia Cabrera, and Hungta Chen are employees of, and may hold stock and/or stock options in, AstraZeneca. Juan-Jesus Carrero reports institutional grants from Astellas, AstraZeneca, and Vifor Pharma; speaker fees from AstraZeneca, Abbott, and Nutricia; and consultancy for AstraZeneca and Bayer. Roberto Pecoits-Filho is an employee of Arbor Research Collaborative for Health, which receives global support for the ongoing Dialysis Outcomes and Practice Patterns Study Programs (provided without restriction on publications by a variety of funders; for details see https://www.dopps.org/AboutUs/Support.aspx). Roberto Pecoits-Filho also reports research grants from Fresenius Medical Care, non-financial support from Akebia, AstraZeneca, Bayer, Boehringer, Novo Nordisk, and FibroGen; as well as personal fees from Travere Therapeutics and consulting fees from George Clinical outside the submitted work. Carolyn S.P. Lam is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from AstraZeneca, Bayer, Boston Scientific, Medtronic, Roche Diagnostics, and Vifor Pharma; has served as a consultant or on the advisory board/steering committee/executive committee for Abbott Diagnostics, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Biofourmis, Boehringer Ingelheim, Boston Scientific, Corvia Medical, Cytokinetics, Darma Inc., Eko.ai Pte Ltd, Jana Care, Janssen Research & Development LLC, Medtronic, Menarini Group, Merck, MyoKardia, Novartis, Novo Nordisk, Radcliffe Group Ltd, Roche Diagnostics, Sanofi, Stealth BioTherapeutics, The Corpus, Vifor Pharma, and WebMD Global LLC; and serves as co-founder and non-executive director of Us2.ai Pte Ltd. Eiichiro Kanda is a consultant for AstraZeneca. Mitja Lainscak is supported by the Slovenian Research Agency; has received research support from Roche Diagnostics; has served as a consultant or on the advisory board/steering committee for AstraZeneca, Boehringer Ingelheim, Novartis, and Vifor Pharma; has received personal fees from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Novartis, Sanofi, Servier, and Vifor Pharma. Carol Pollock reports advisory board membership for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Vifor Pharma; as well as speaker fees for AstraZeneca, Janssen-Cilag, Novartis, Otsuka, and Vifor Pharma. David C. Wheeler reports personal fees and non-financial support from AstraZeneca, as well as personal fees from Astellas, Bayer, Boehringer Ingelheim, GSK, Janssen, Mundipharma, Napp, Reata Pharmaceuticals, Tricida, and Vifor Fresenius.
Ethical Approval
This study was performed in accordance with ethical principles consistent with the Declaration of Helsinki, International Conference on Harmonisation, Good Clinical Practice, and the applicable legislation on noninterventional studies and observational studies. This study used de-identified data and did not require data collection beyond that of routine clinical care. No identifiable information was collected or examined as part of the study. Ethical and scientific approval for use of CPRD data in the current study was obtained from the Independent Scientific Advisory Committee of CPRD (protocol number 19_172). Informed consent was waived by the East Midlands – Derby Research Ethics Committee because CPRD data are anonymized for research purposes (for further information, see https://cprd.com/safeguarding-patient-data). Ethics approval was not required for use of data from the US TriNetX and Japan Medical Data Vision for this study, in accordance with local or national guidelines.
References
- 1.Go AS, Yang J, Tan TC, et al. Contemporary rates and predictors of fast progression of chronic kidney disease in adults with and without diabetes mellitus. BMC Nephrol. 2018;19(1):146. 10.1186/s12882-018-0942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali I, Chinnadurai R, Ibrahim ST, Green D, Kalra PA. Predictive factors of rapid linear renal progression and mortality in patients with chronic kidney disease. BMC Nephrol. 2020;21(1):345. 10.1186/s12882-020-01982-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naimark DM, Grams ME, Matsushita K, et al. Past decline versus current eGFR and subsequent mortality risk. J Am Soc Nephrol. 2016;27(8):2456–66. 10.1681/ASN.2015060688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovesdy CP, Coresh J, Ballew SH, et al. Past decline versus current eGFR and subsequent ESRD risk. J Am Soc Nephrol. 2016;27(8):2447–55. 10.1681/ASN.2015060687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukmark T, Sukmark S. Predictors of faster progression in chronic kidney disease. J Med Assoc Thai. 2014;97(8):812–9. [PubMed] [Google Scholar]
- 6.Vigil A, Condés E, Camacho R, et al. Predictors of a rapid decline of renal function in patients with chronic kidney disease referred to a nephrology outpatient clinic: a longitudinal study. Adv Nephrol. 2015;2015:1–8. 10.1155/2015/657624 [DOI] [Google Scholar]
- 7.Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164–74. 10.1001/jama.2015.18202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grams ME, Sang Y, Ballew SH, et al. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate. Kidney Int. 2018;93(6):1442–51. 10.1016/j.kint.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosansky SJ. Renal function trajectory is more important than chronic kidney disease stage for managing patients with chronic kidney disease. Am J Nephrol. 2012;36(1):1–10. 10.1159/000339327 [DOI] [PubMed] [Google Scholar]
- 10.Pecoits-Filho R, James G, Carrero JJ, et al. Methods and rationale of the DISCOVER CKD Global Observational Study. Clin Kidney J. 2021;14:1570–8. 10.1093/ckj/sfab046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollock C, James G, Garcia Sanchez JJ, et al. Healthcare resource utilisation and related costs of patients with CKD from the UK: a report from the DISCOVER CKD retrospective cohort. Clin Kidney J. 2022;15(11):2124–34. 10.1093/ckj/sfac168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Arnold M, James G, Padman R. Developing a common data model approach for DISCOVER CKD: a retrospective, global cohort of real-world patients with chronic kidney disease. PLoS One. 2022;17(9):e0274131. 10.1371/journal.pone.0274131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James G, Garcia Sanchez JJ, Carrero JJ, et al. Low adherence to Kidney Disease: Improving Global Outcomes 2012 CKD clinical practice guidelines despite clear evidence of utility. Kidney Int Rep. 2022;7(9):2059–70. 10.1016/j.ekir.2022.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollock C, James G, Garcia Sanchez JJ, et al. Cost of end-of-life inpatient encounters in patients with chronic kidney disease in the United States: a report from the DISCOVER CKD retrospective cohort. Adv Ther. 2022;39(3):1432–45. 10.1007/s12325-021-02010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical Practice Research Datalink. Medicines and healthcare products regulatory agency. https://cprd.com. Accessed 19 Apr 2024
- 17.Ghosh RE, Crellin E, Beatty S, Donegan K, Myles P, Williams R. How Clinical Practice Research Datalink data are used to support pharmacovigilance. Ther Adv Drug Saf. 2019;10:2042098619854010. 10.1177/2042098619854010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–7. 10.1053/j.ajkd.2010.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muntner P. Longitudinal measurements of renal function. Semin Nephrol. 2009;29(6):650–7. 10.1016/j.semnephrol.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 20.Toyama T, Kitagawa K, Oshima M, et al. Age differences in the relationships between risk factors and loss of kidney function: a general population cohort study. BMC Nephrol. 2020;21(1):477. 10.1186/s12882-020-02121-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151–64. 10.1038/nrneph.2017.181 [DOI] [PubMed] [Google Scholar]
- 22.Nichols GA, Deruaz-Luyet A, Brodovicz KG, Kimes TM, Rosales AG, Hauske SJ. Kidney disease progression and all-cause mortality across estimated glomerular filtration rate and albuminuria categories among patients with vs. without type 2 diabetes. BMC Nephrol. 2020;21(1):167. [DOI] [PMC free article] [PubMed]
- 23.Hoefield RA, Kalra PA, Baker PG, et al. The use of eGFR and ACR to predict decline in renal function in people with diabetes. Nephrol Dial Transplant. 2011;26(3):887–92. 10.1093/ndt/gfq526 [DOI] [PubMed] [Google Scholar]
- 24.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–8. 10.1038/ki.2011.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheen YJ, Lin JL, Li TC, Bau CT, Sheu WH. Peripheral arterial stiffness is independently associated with a rapid decline in estimated glomerular filtration rate in patients with type 2 diabetes. Biomed Res Int. 2013;2013: 309294. 10.1155/2013/309294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloomfield GS, Yi SS, Astor BC, et al. Blood pressure and chronic kidney disease progression in a multi-racial cohort: the Multi-Ethnic Study of Atherosclerosis. J Hum Hypertens. 2013;27(7):421–6. 10.1038/jhh.2013.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C, Park JT, Chang TI, et al. Low-density lipoprotein cholesterol levels and adverse clinical outcomes in chronic kidney disease: results from the KNOW-CKD. Nutr Metab Cardiovasc Dis. 2022;32(2):410–9. 10.1016/j.numecd.2021.09.037 [DOI] [PubMed] [Google Scholar]
- 28.Melsom T, Norvik JV, Enoksen IT, et al. Association of high-density lipoprotein cholesterol with GFR decline in a general nondiabetic population. Kidney Int Rep. 2021;6(8):2084–94. 10.1016/j.ekir.2021.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen SC, Hung CC, Kuo MC, et al. Association of dyslipidemia with renal outcomes in chronic kidney disease. PLoS One. 2013;8(2):e55643. 10.1371/journal.pone.0055643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turin TC, James M, Ravani P, et al. Proteinuria and rate of change in kidney function in a community-based population. J Am Soc Nephrol. 2013;24(10):1661–7. 10.1681/ASN.2012111118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jafar TH, Stark PC, Schmid CH, et al. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int. 2001;60(3):1131–40. 10.1046/j.1523-1755.2001.0600031131.x [DOI] [PubMed] [Google Scholar]
- 32.Alencar de Pinho N, Levin A, Fukagawa M, et al. Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int. 2019;96(4):983–94. [DOI] [PubMed]
- 33.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4s):S117–S314. [DOI] [PubMed]
- 34.Hundemer GL, Sood MM. Hyperkalemia with RAAS inhibition: mechanism, clinical significance, and management. Pharmacol Res. 2021;172:105835. 10.1016/j.phrs.2021.105835 [DOI] [PubMed] [Google Scholar]
- 35.Kashihara N, Kohsaka S, Kanda E, Okami S, Yajima T. Hyperkalemia in real-world patients under continuous medical care in Japan. Kidney Int Rep. 2019;4(9):1248–60. 10.1016/j.ekir.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wetmore JB, Yan H, Horne L, Peng Y, Gilbertson DT. Risk of hyperkalemia from renin-angiotensin-aldosterone system inhibitors and factors associated with treatment discontinuities in a real-world population. Nephrol Dial Transplant. 2021;36(5):826–39. 10.1093/ndt/gfz263 [DOI] [PubMed] [Google Scholar]
- 37.Pecoits-Filho R, Fliser D, Tu C, et al. Prescription of renin-angiotensin-aldosterone system inhibitors (RAASi) and its determinants in patients with advanced CKD under nephrologist care. J Clin Hypertens (Greenwich). 2019;21(7):991–1001. 10.1111/jch.13563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svensson MK, Murohara T, Lesen E, et al. Hyperkalaemia-related reduction of RAASi treatment associates with more subsequent inpatient care. Nephrol Dial Transplant. 2024. 10.1093/ndt/gfae016. [DOI] [PMC free article] [PubMed]
- 39.Kanda E, Rastogi A, Murohara T, et al. Clinical impact of suboptimal RAASi therapy following an episode of hyperkalemia. BMC Nephrol. 2023;24(1):18. 10.1186/s12882-022-03054-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santoro A, Perrone V, Giacomini E, Sangiorgi D, Alessandrini D, Degli EL. Association between hyperkalemia, RAASi non-adherence and outcomes in chronic kidney disease. J Nephrol. 2021;35(2):463–72. 10.1007/s40620-021-01070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linde C, Bakhai A, Furuland H, et al. Real-world associations of renin-angiotensin-aldosterone system inhibitor dose, hyperkalemia, and adverse clinical outcomes in a cohort of patients with new-onset chronic kidney disease or heart failure in the United Kingdom. J Am Heart Assoc. 2019;8(22):e012655. 10.1161/JAHA.119.012655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2010;25(12):3977–82. 10.1093/ndt/gfp511 [DOI] [PubMed] [Google Scholar]
- 43.Qiao Y, Shin JI, Sang Y, et al. Discontinuation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in chronic kidney disease. Mayo Clin Proc. 2019;94(11):2220–9. 10.1016/j.mayocp.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhandari S, Mehta S, Khwaja A, et al. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387(22):2021–32. 10.1056/NEJMoa2210639 [DOI] [PubMed] [Google Scholar]
- 45.Levin A, Ahmed SB, Carrero JJ, et al. Executive summary of the KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease: known knowns and known unknowns. Kidney Int. 2024;105:684–701. 10.1016/j.kint.2023.10.016 [DOI] [PubMed] [Google Scholar]
- 46.Shlipak MG, Katz R, Kestenbaum B, et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol. 2009;20(12):2625–30. 10.1681/ASN.2009050546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168(20):2212–8. 10.1001/archinte.168.20.2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 49.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 50.Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27–35. 10.1016/S2213-8587(19)30384-5 [DOI] [PubMed] [Google Scholar]
- 51.Garcia Sanchez JJ, Thompson J, Scott DA, et al. Treatments for chronic kidney disease: a systematic literature review of randomized controlled trials. Adv Ther. 2022;39(1):193–220. 10.1007/s12325-021-02006-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available upon reasonable request in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.