Abstract
Introduction
Early, simple predictors for long-term survival in Parkinson’s disease (PD) may help identify patients at elevated risk and are crucial for more personalized treatment.
Methods
This large, retrospective study examined whether higher levodopa equivalent daily dose (LEDD) a year after diagnosis predicts long-term survival.
Results
Mortality risk was increased among 292 patients receiving ≥ 600 mg LEDD versus 2233 patients receiving < 600 mg LEDD (hazard ratio 1.5; 95% confidence interval 1.3–1.7), particularly among patients aged < 75 years (1.8; 1.4–2.4).
Conclusion
In PD, higher LEDD can be an early risk marker of increased mortality, probably because it reflects more severe disease.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02924-8.
Keywords: Antiparkinson’s agents, Drug dosage calculations, Mortality, Parkinson’s disease
Key Summary Points
| Why carry out this study? |
| There is a need for a quantifiable, early, and simple predictor for long-term survival in patients with Parkinson’s disease (PD). |
| Our analysis assessed the relationship between treatment intensity (measured by the dose of oral levodopa equivalent in the first year following PD diagnosis) and long-term survival. |
| What was learned from the study? |
| Patients receiving ≥ 600 mg levodopa equivalent daily dose (LEDD) in the first year following diagnosis had higher mortality risk compared with patients receiving < 600 mg LEDD. |
| Treatment intensity in the first year after a PD diagnosis may serve as an early indicator of increased risk of mortality. |
Introduction
Identifying early and simple predictors for survival in patients with Parkinson's disease (PD) is crucial to understand and prevent PD-related deaths. Survival prediction, however, is limited by the complexity of PD, even in earlier phases, and the lack of an optimal progression biomarker [1, 2]. Identifying a vast and generalizable predictor may enable a more individually targeted treatment.
Known clinical predictors of mortality in PD include, among others, dementia at baseline, dysphagia, and postural instability [3]. Due to the variability of the disease, and difficulties quantifying some of these predictors, the total dose of oral anti-PD medication (referred to here as “treatment intensity”) at an early stage of disease may serve as a good indicator for disease severity. Treatment intensity is usually measured by the levodopa equivalent daily dose (LEDD), an artificial summary of the total daily anti-PD medications a patient receives [4]. This surrogate marker was previously suggested for the identification of patients with advanced PD [5, 6].
This study assesses the relationship between treatment intensity, reflected by LEDD, in the first year following PD diagnosis and long-term patient survival.
Methods
Study Design, Settings, and Population
This population-based, retrospective, cohort study utilized data from Maccabi Healthcare Services (MHS). MHS is the second largest health insurance provider in Israel, representing 26% of the Israeli population with < 1% annual moving-out rate. This study was conducted in accordance with the principles of the International Council for Harmonisation guidelines for Good Clinical Practice, which have their origin in the Declaration of Helsinki. The study was approved by the MHS-IRB committee (0047–20-MHS). Data were de-identified and are, therefore, exempt from obtaining members’ informed consent.
With emphasis on specificity, members were included in the study only if they had ≥ 1 valid PD diagnosis. “Valid diagnosis” was one of the following: (1) inpatient diagnosis; (2) diagnosis given by a neurologist; (3) ≥ 2 diagnoses given by a family physician; or (4) a chronic diagnosis in the electronic medical record, actively defined as such by the family physician. The index date was defined as the first PD diagnosis (regardless of treatment initiation) or anti-PD therapy initiation (to avoid bias from late documentation of the diagnosis), whichever occurred earlier. Patients were included if their index date occurred between 2005–2010 and if they also had ≥ 5-year membership in the health plan prior to the index date and up to 10 years of follow-up (unless prior death). We excluded patients with diagnosis of other parkinsonian syndromes (Supplementary Material Table S1) if given during the first 3 years post-index date.
Patients were categorized into two cohorts based on their LEDD [4] during the first year post-index date: (1) the low-dose cohort (< 600 mg LEDD), and (2) the high-dose cohort (≥ 600 mg LEDD). The 600 mg cutoff was chosen based on findings from another study that demonstrated increased risk for motor complications in patients with early PD who were receiving ≥ 600 mg of levodopa daily [7].
Variables and Measurements
Patients’ demographics included sex and age at index date. Data on baseline comorbidities, including vascular (cerebral and cardiac) disease [8], diabetes [9], hypertension [10], chronic kidney disease [11], and mild cognitive impairment/dementia [12], were extracted using the MHS-validated registries. Pre-index–reported signs of interest included tremor, constipation, dysphagia, urinary symptoms, sexual problems, psychosis, orthostatic hypotension, anxiety, depression, and gait impairment. The end of follow-up was defined as the earlier of death date, leaving MHS date, or end of study (December 31, 2019).
Statistical Analyses
Descriptive statistics are presented using frequencies and proportions for categorical variables and mean values with standard deviations (SDs) or medians with interquartile ranges (IQRs). To compare baseline characteristics between cohorts, independent t tests for continuous variables and Fisher’s exact test for categorical variables were applied.
Cumulative case-fatality rates were assessed using Cox proportional hazards regression. Hazard ratios (HRs) and their 95% confidence intervals (CIs) are presented. From a visual inspection of the Kaplan–Meier curve, the proportionality assumption of the Cox proportional model was violated at approximately 2 years of follow-up. After excluding deaths occurring within the first 2 years of follow-up, proportionality was verified using the Schoenfeld residuals test (p = 0.16).
Analyses were adjusted for all baseline characteristics, regardless of the difference found in the univariate analyses. Subgroup analysis was stratified according to age at index date (< or ≥ 75 years, the median age of disease onset in this study). A two-tailed p value < 0.05 was considered significant. All analyses were performed using IBM-SPSS statistical software version 27 (Armonk, NY, USA). Figures were created using ggplot2, survival, and survminer packages in R statistical software version 4.1.1 (R Project for Statistical Computing).
Results
Of 17,085 MHS members with at least one idiopathic PD diagnosis, 2525 were eligible for the study based on predefined criteria (Supplementary Material Fig. S1). Most patients [2233/2525 (88.4%); mean (SD) age 74.4 (10.9) years; 54.7% males] were in the low-dose cohort and 292/2525 patients [11.6%; age 75.4 (8.8) years; 65.1% males] were in the high-dose cohort. At baseline, compared with the low-dose cohort, patients treated with higher LEDD were more likely to have hypertension and gait impairment, and were less likely to report dysphagia and tremor (Table 1).
Table 1.
Baseline characteristics comparison between patients with PD who are treated with low dose or high dose of anti-PD oral medications
| Characteristic | Low dose (n = 2233) |
High dose (n = 292) |
p value |
|---|---|---|---|
| Age at first PD indication, years | |||
| Mean (SD) | 74.4 (10.9) | 75.4 (8.8) | 0.074 |
| Median (IQR) | 75.9 (68.5–82.1) | 75.8 (72–81.3) | |
| Range | 23.1–101.8 | 31.3–94.3 | |
| < 75 | 1040 (46.6) | 130 (44.5) | 0.274 |
| ≥ 75 | 1193 (53.4) | 162 (55.5) | |
| Sex | |||
| Male | 1222 (54.7) | 190 (65.1) | < 0.001 |
| Female | 1011 (45.3) | 102 (34.9) | |
| Cardiovascular disease | 624 (27.9) | 89 (30.5) | 0.201 |
| Hypertension | 1634 (73.2) | 229 (78.4) | 0.031 |
| Cerebrovascular disease | 184 (8.2) | 23 (7.9) | 0.47 |
| Diabetes | 644 (28.8) | 95 (32.5) | 0.109 |
| Chronic kidney disease | 1349 (60.4) | 176 (60.3) | 0.506 |
| Anxiety/depression/mood disorders | 935 (41.9) | 114 (39.0) | 0.195 |
| Mild cognitive impairment/dementia | 295 (13.2) | 34 (11.6) | 0.259 |
| Constipation | 555 (24.9) | 63 (21.6) | 0.124 |
| Psychosis | 121 (5.4) | 18 (6.2) | 0.339 |
| Urinary symptoms | 402 (18.0) | 43 (14.7) | 0.095 |
| Sexual problems | 87 (3.9) | 15 (5.1) | 0.193 |
| Dysphagia | 67 (3.0) | 3 (1.0) | 0.03 |
| Orthostatic hypotension | 21 (0.9) | 3 (1.0) | 0.537 |
| Tremor | 471 (21.1) | 45 (15.4) | 0.013 |
| Gait abnormalities | 184 (8.2) | 46 (15.8) | < 0.001 |
| First fall | 481 (21.5) | 64 (21.9) | 0.467 |
Data are presented as n (%) unless otherwise noted
IQR interquartile range, PD Parkinson’s disease
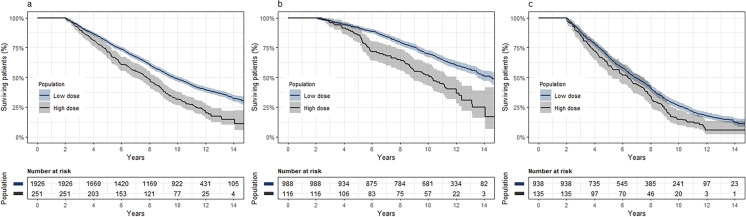
The survival analysis included 2177/2525 patients [86.2%; low dose, 1926/2233 (86.3%); high dose, 251/292 (86.0%)] who had ≥ 2 years of follow-up post-index date. Throughout the follow-up period (median, 9.3 years; IQR, 5.5–11.7; range, 2–15), 1381/2177 (63.4%) patients died [low dose, 1183/1926 (61.4%); high dose, 198/251 (78.9%)]. Compared with the low-dose cohort, patients treated with high LEDD had a significantly higher mortality risk (adjusted HR, 1.5; 95% CI, 1.3–1.7; p < 0.001; Fig. 1a). After stratification by age at index date, compared with patients in the low-dose cohort, those treated with higher LEDD had 1.8-fold and 1.3-fold increased risks for mortality among those aged < 75 years (95% CI, 1.4–2.4; p < 0.001; Fig. 1b) and ≥ 75 years (95% CI, 1.1–1.6; p = 0.007; Fig. 1c) at baseline, respectively.
Fig. 1.
Kaplan–Meier survival curves for all-cause mortality, by low (< 600 mg) and high (≥ 600 mg) levodopa equivalent daily dose in the member population with at least 2 years of follow-up: a entire cohort; b age at baseline < 75 years; c age at baseline ≥ 75 years; shading 95% confidence interval
Discussion
In this nationwide, population-based study, we observed an increased mortality risk in patients with PD receiving higher LEDD treatment during the first year after diagnosis. This observation aligns with findings from a previous, much smaller, study of 133 patients with early PD [13]. A greater risk was observed in patients who were aged < 75 years at baseline. Results were robust after adjustment for sex, age, and baseline comorbidities and symptoms.
While the reduced life expectancy of patients with PD compared with the general population is well established [2, 13, 14], identifying predictors of increased mortality within the population of patients with PD is more complex due to the large disease heterogeneity. Previously documented factors include clinical, genetic, dopaminergic, neuroimaging, and cerebrospinal fluid biomarkers [3]. Treatment intensity of oral medication is a potential marker that may incorporate many of these features, and can serve as a marker for disease severity. Indeed, increased LEDD (> 1000 mg) was previously suggested as a marker to identify patients with advanced PD manifested by increased risk for mortality, hospitalizations, and disability [5].
Our study cannot distinguish between LEDD dosage as a marker for disease severity and the less likely possibility that higher dosage contributes directly to mortality risk. Another, more likely, possibility is that the higher medication dosage in the first year post-diagnosis may result from a delayed diagnosis, which occurs mostly in males and patients presenting with gait disturbances [15], similar to our findings in the high-dose cohort. In addition, the higher prevalence of gait impairments and lower prevalence of tremor in the high-dose population suggests a higher prevalence of the postural instability and gait disorders PD subtype. The postural instability and gait disorders subtype is usually characterized by a faster and more aggressive disease progression and, therefore, requires a higher dosage of medication early in the course of disease [16].
One of the strengths of this predictor, compared with previously suggested published algorithms to predict mortality risk in PD, is its availability and generalizability. A predictive algorithm for disease progression (manifested in motor progression) based on longitudinal clinical, molecular, and genetic data has been developed [17]. However, this model was constructed using information not necessarily available in most datasets. Additional strengths of the current study are (1) sizable cohort, (2) long follow-up period (up to 15 years), and (3) the use of incident cases to avoid selection bias.
Aside from its retrospective nature and reliance on database analysis, our study has several limitations. First, we lack the cause of death of patients in the MHS database; thus, we were not able to differentiate between PD-related and non-PD-related deaths. This might explain the weaker association measured in patients aged ≥ 75 years due to increased risk of competing causes of death. Second, we were unable to assess the risk for mortality throughout the follow-up period due to the violation of the proportionality assumption in the first 2 years; however, the current analysis included 13 years (vs. 2 years), 86% of the study population, and 80% of overall deceased patients. Third, the inclusion of patients with PD diagnoses from only family physicians could result in the inclusion of patients misdiagnosed with PD. However, only a small minority of patients in this analysis were diagnosed solely by family physicians, minimizing the risk of bias from misdiagnosis. Additionally, our analysis was not able to distinguish between different PD subtypes or levels of motor impairment, both of which may contribute to the difference in mortality rates between the groups.
Conclusion
Treatment intensity of oral anti-PD medications during the first year, measured by LEDD ≥ 600 mg, may be an objective, robust, and accessible indicator for higher risk of mortality. This indicator could be used in addition to physicians' clinical judgment to identify patients with PD who require more intensive follow-up and optimization of PD treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing and Editorial Assistance
Editorial support was provided by Alicia Salinero, PhD, ISMPP CMPPTM, of JB Ashtin, and funded by AbbVie.
Authorship
All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Author Contributions
Yael Barer, Olga Sánchez-Soliño, Meital Grabarnik-John, Lars Bergmann, and David Arkadir contributed to the study concept/design. Yael Barer, Gabriel Chodick, and Sivan Gazit contributed to data acquisition. Yael Barer, Olga Sánchez-Soliño, Gabriel Chodick, and Sivan Gazit contributed to the statistical analysis of the data. All authors contributed to interpretation of the data, and participated in writing, review, and critique of the manuscript. All authors had access to the data and participated in the development, review, critique, and approval of the manuscript throughout the editorial process, and approved the final manuscript draft submitted for publication.
Funding
AbbVie funded this study and participated in the study design, research, data collection, analysis, interpretation of data, reviewing, and approval of the publication. Funding for the Advances in Therapy Rapid Service Fee and Open Access Fee is provided by AbbVie.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/, then select “Home”.
Declarations
Conflict of Interest
Yael Barer, Gabriel Chodick, Sivan Gazit are employees of Maccabi Healthcare Services and report no other conflict of interest. Olga Sánchez-Soliño, Lars Bergmann, Connie H. Yan, Neta li Feurestein-Ganor, and Shiran Naftelberg Blonder are employees of AbbVie and may or may not own AbbVie stock. Meital Grabarnik-John was an employee of AbbVie at the time of the project and during most of the manuscript preparation and may or may not own AbbVie stock. David Arkadir reports no conflict of interest.
Ethical Approval
This study was conducted in accordance with the principles of the International Council for Harmonisation guidelines for Good Clinical Practice, which have their origin in the Declaration of Helsinki. The study was approved by the MHS-IRB committee (0047-20-MHS). Data were de-identified and are, therefore, exempt from obtaining informed consent.
References
- 1.Greenland JC, Williams-Gray CH, Barker RA. The clinical heterogeneity of Parkinson’s disease and its therapeutic implications. Eur J Neurosci. 2019;49(3):328–38. 10.1111/ejn.14094. 10.1111/ejn.14094 [DOI] [PubMed] [Google Scholar]
- 2.Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1615–22. 10.1002/mds.25898 [DOI] [PubMed] [Google Scholar]
- 3.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–303. 10.1016/S0140-6736(21)00218-X [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–53. 10.1002/mds.23429 [DOI] [PubMed] [Google Scholar]
- 5.Barer Y, Gurevich T, Chodick G, et al. Advanced-stage Parkinson’s disease: from identification to characterization using a nationwide database. Mov Disord Clin Pract. 2022;9(4):458–67. 10.1002/mdc3.13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonini A, Stoessl AJ, Kleinman LS, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018;34(12):2063–73. 10.1080/03007995.2018.1502165 [DOI] [PubMed] [Google Scholar]
- 7.Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. NEJM. 2004;351(24):2498–508. 10.1056/NEJMoa033447 [DOI] [PubMed] [Google Scholar]
- 8.Shalev V, Chodick G, Goren I, Silber H, Kokia E, Heymann AD. The use of an automated patient registry to manage and monitor cardiovascular conditions and related outcomes in a large health organization. Int J Cardiol. 2011;152(3):345–9. 10.1016/j.ijcard.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 9.Chodick G, Heymann AD, Shalev V, Kookia E. The epidemiology of diabetes in a large Israeli HMO. Eur J Epidemiol. 2003;18(12):1143–6. 10.1023/B:EJEP.0000006635.36802.c8 [DOI] [PubMed] [Google Scholar]
- 10.Weitzman D, Chodick G, Shalev V, Grossman C, Grossman E. Prevalence and factors associated with resistant hypertension in a large health maintenance organization in Israel. Hypertension. 2014;64(3):501–7. 10.1161/HYPERTENSIONAHA.114.03718 [DOI] [PubMed] [Google Scholar]
- 11.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–31. 10.1001/jama.2014.6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben Zaken S, Radomysky Z, Koren G. Association between serum magnesium levels and Alzheimer’s disease or mixed dementia patients: a population-based retrospective controlled study. J Alzheimers Dis Rep. 2020;4(1):399–404. 10.3233/ADR-200220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoogland J, Post B, de Bie R. Overall and disease related mortality in Parkinson’s disease–a longitudinal cohort study. J Parkinson’s Dis. 2019;9(4):767–74. 10.3233/JPD-191652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bugalho P, Ladeira F, Barbosa R, et al. Motor and non-motor function predictors of mortality in Parkinson’s disease. J Neural Transm (Vienna). 2019;126(11):1409–15. 10.1007/s00702-019-02055-3 [DOI] [PubMed] [Google Scholar]
- 15.Breen DP, Evans JR, Farrell K, Brayne C, Barker RA. Determinants of delayed diagnosis in Parkinson’s disease. J Neurol. 2013;260:1978–81. 10.1007/s00415-013-6905-3 [DOI] [PubMed] [Google Scholar]
- 16.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord. 2006;21(8):1123–30. 10.1002/mds.20897 [DOI] [PubMed] [Google Scholar]
- 17.Latourelle JC, Beste MT, Hadzi TC, et al. Large-scale identification of clinical and genetic predictors of motor progression in patients with newly diagnosed Parkinson’s disease: a longitudinal cohort study and validation. Lancet Neurol. 2017;16(11):908–16. 10.1016/S1474-4422(17)30328-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/, then select “Home”.