Abstract
Key message
Volatile compounds released from basil prime the tomato wound response by promoting jasmonic acid, mitogen-activated protein kinase, and reactive oxygen species signaling.
Abstract
Within mixed planting systems, companion plants can promote growth or enhance stress responses in target plants. However, the mechanisms underlying these effects remain poorly understood. To gain insight into the molecular nature of the effects of companion plants, we investigated the effects of basil plants (Ocimum basilicum var. minimum) on the wound response in tomato plants (Solanum lycopersicum cv. ‘Micro-Tom’) within a mixed planting system under environmentally controlled chamber. The results showed that the expression of Pin2, which specifically responds to mechanical wounding, was induced more rapidly and more strongly in the leaves of tomato plants cultivated with companion basil plants. This wound response priming effect was replicated through the exposure of tomato plants to an essential oil (EO) prepared from basil leaves. Tomato leaves pre-exposed to basil EO showed enhanced expression of genes related to jasmonic acid, mitogen-activated protein kinase (MAPK), and reactive oxygen species (ROS) signaling after wounding stress. Basil EO also enhanced ROS accumulation in wounded tomato leaves. The wound response priming effect of basil EO was confirmed in wounded Arabidopsis plants. Loss-of-function analysis of target genes revealed that MAPK genes play pivotal roles in controlling the observed priming effects. Spodoptera litura larvae-fed tomato leaves pre-exposed to basil EO showed reduced growth compared with larvae-fed control leaves. Thus, mixed planting with basil may enhance defense priming in both tomato and Arabidopsis plants through the activation of volatile signaling.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00299-024-03285-w.
Keywords: Companion plants, Priming, Tomato, Volatile, Wound signaling
Introduction
Agricultural systems worldwide are dominated by industrial approaches that produce single crops under the control of chemical fertilizers and pesticides (Horrigan et al. 2002). However, these systems carry a substantial risk of degrading topsoil, which is required for crop growth, and produce large amounts of greenhouse gasses, which accelerate global warming (Gao et al. 2022). In addition, excessive nitrogen and phosphorus inputs to agricultural lands pollute rivers and lakes, creating severe environmental problems worldwide (Moss 2007). To meet growing agricultural demands while preserving the global environment, there is a need to rapidly establish agricultural practices that conserve the environment. The transportation of agricultural products also emits large amounts of CO2, such that shortening the distances between agricultural production and consumption areas (i.e., reducing food mileage) is a critical challenge that requires an aggressive shift from conventional large-scale farming to small-scale farming. For vegetable production, it is also necessary to review subsistence-based production systems in private gardens and urban–suburban production systems. Furthermore, the growing health consciousness among consumers is leading to an expectation of safe and secure agricultural products through reduced chemical pesticide use.
Regenerative agriculture, which aims to restore the natural environment while improving the soil for crop growth, has been proposed to ensure global food security while mitigating these problems (Giller et al. 2021). One step toward regenerative agriculture is the implementation of mixed planting, rather than monocultures. Companion planting, in which compatible crops of different species are grown together, originated in the USA, where indigenous Americans planted a mixture of corn, pumpkins, and beans known as the “three sisters” (Pleasant 2016). Companion planting is generally considered beneficial to plants because of its ability to control pests and diseases (Finch et al. 2003; Parker et al. 2013; George et al. 2013; Fu et al. 2015), optimize soil nutrient supply (Mengel 2001), and improve growing space efficiency (Bomford 2004). However, the specific effects of companion plants remain unclear. A typical example of companion planting is a “push–pull” system, in which natural plant–insect communication is harnessed to reduce herbivory by insects (Pickett et al. 2014). In this system, volatiles released from plants repel or disturb feeding insects while attracting their natural enemies. For instance, volatiles released from companion plants have been reported to effectively protect target plants against aphid or whitefly damage under greenhouse conditions (Ben-Issa et al. 2017a, b; Conboy et al. 2019).
Companion plants can help to enhance the defense systems of a target plant species. For example, volatiles released by mint plants were reported to increase pest resistance in soybean or Brassica rapa plants within mixed planting systems (Sukegawa et al. 2018), and volatiles released from injured Solidago canadensis were able to inhibit root nodule symbiosis by nitrogen-fixing bacteria on soybean roots (Takahashi et al. 2021). These findings indicate that volatile signaling is strongly involved in the effects of companion planting on target plants. However, few studies have comprehensively investigated this phenomenon, and molecular research is needed to elucidate its underlying mechanism. An understanding of the mechanisms that drive this effect may help to promote the widespread implementation of companion planting for sustainable agriculture and maximal effectiveness within mixed planting systems.
Therefore, the objective of this study was to clarify the molecular basis for the effects of companion planting on target plants in a tomato–basil mixed planting system, primarily focusing on interplant communication. We investigated the effects of mixed plantings on tomato wound response at the gene expression level, with the aim of elucidating the molecular nature of airborne signaling between tomato and basil. To further elucidate this signaling mechanism, we also used Arabidopsis, analyzed its gene loss-of-function mutants, and compared the results with those obtained in tomatoes.
Our results provide scientific evidence that this beneficial effect of companion planting is mainly driven by interplant communication via plant volatile signaling, primarily mediating MAPK and ROS.
Materials and methods
Plant materials and growth conditions
Tomato (Solanum lycopersicum cv. ‘Micro-Tom’), basil (Ocimum basilicum var. minimum), and Arabidopsis (Columbia ecotype) were used in this study. All plants were grown in soil consisting of a 1:1 ratio of Metro-Mix (Sun Gro Horticulture, Agawam, MA, USA) to vermiculite within a controlled environmental chamber at 23 °C under a 12-h/12-h light/dark photoperiod. A mixed planting system was established by transplanting germinated tomato and basil seeds into 9-cm-diameter pots.
In a tomato jai1-1 experiment, homozygous jai1-1 seedlings were selected from F2 populations following the method previously described by Li et al. (2003). Seeds were germinated on a piece of water-saturated filter paper in a closed petri dishes in the dark at 25 °C. After 4–5 days, when the emerging radical was ∼1 cm in length, the filter paper was resaturated with a solution of 1 mM methyl jasmonate (MeJA). Seedlings were grown in the dark for an additional 24–36 h, and selected the seedlings insensitive to MeJA of those did not show reduced root and hypocotyl growth and anthocyanin accumulation in the hypocotyl.
One germinated seed of tomato and basil or tomato and tomato was transplanted into each pot. The distance between plants was 3 cm. After three weeks of growth, the leaves of each plant were wounded with scissors in an area on each side of the leaf, bordering the main leaf vein.
The loss-of-function of Arabidopsis mutants atmpk3 (SALK_209371) and atmpk6 (SALK_004221) were obtained from the Arabidopsis Biological Resource Center (ABRC).
Insect culture and feeding experiments
Spodoptera litura (S. litura) larvae eclosed from eggs were reared on artificial feed and grown to the second instar stage at 25 °C under a 14-h/10-h light/dark photoperiod. The larvae were carefully put onto the untreated and basil essential oil (EO)-exposed tomato leaves that had excised from seedlings and fed at 23 °C under a 14-h/10-h light/dark photoperiod, then weighed after 3 days to evaluate inhibitory effects on S. litura growth. Thirty larvae were included in each treatment.
Basil EO and volatile compound treatments
Three-week-old tomato and four-week-old Arabidopsis plants grown in 5-cm-diameter pots were placed inside a plant box (7 cm length × 7 cm width × 10 cm height); cotton swabs soaked with basil EO or one of the four tested volatile compounds (linalool, α-terpineol, chavicol, or eugenol) were attached to the bottom of the lid, and the box was closed. After 15 h of exposure, plants were removed from the box and desensitized for 1 h. Then, leaves of each plant were wounded with scissors in one area on each side of the leaf, bordering the main leaf vein. Basil EO was extracted using the hydro distillation method (Tongnuanchan and Benjakul 2014). About 100 g of fresh basil leaves and 200 mL of water are put into a 1000 mL boiling flask and heated using a heating mantle-type heater (200W/100 V) for one hour. After the extraction process, the oil accumulated in the extraction tool was carefully removed and then put into dark-colored vials. Extracted oils were stored at 4 °C until use.
Quantitative polymerase chain reaction (qPCR) analysis
Total RNA was isolated from tomato and Arabidopsis leaves using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized using ReverTra Ace (Toyobo, Osaka, Japan), in accordance with the manufacturer’s instructions. qPCR was performed using the Eco Real-Time PCR System (Illumina, San Diego, CA, USA) using the KAPA SYBR FAST qPCR kit (Sigma-Aldrich, St. Louis, MO, USA). The qPCR cycling protocol consisted of 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Primer sequences used in this analysis are listed in Table S1. Expression levels for each target gene were normalized to the levels of ACTIN (for tomato) and ACTIN2 (for Arabidopsis). The replication size was n = 3 for all experiments.
ROS determination and quantification
Accumulation of ROS in the leaves was visually detected using 3,3-diaminobenzine (DAB). After the wound stress treatment, tomato or Arabidopsis leaves were incubated with DAB solution (1 mg/mL) at pH 3.8 for at least 12 h. After the incubation with DAB solution, the leaves were put into the 100% ethanol solution to decolorize chlorophyll. H2O2 accumulation in leaves was quantified using GIMP2 software (Postor et al. 2013). GIMP2 is a free and open-source image editor that is available for GNU/Linux, macOS, Windows, and other operating systems (https://www.gimp.org). The replication size was n = 3 to 4 for all experiments.
Statistical analysis
One-way ANOVA was performed to compare the effects of mix-planting, EO, and each volatile compound. The significance of differences among the treatments was evaluated using a Tukey’s honest significant difference (HSD post hoc test). Differences referred to in the text were statistically significant at P < 0.05 unless otherwise stated. Statistical analyses were performed using EZR v.1.60 ((Saitama Medical Center, Jichi Medical University, Saitama, Japan). Results are reported as averages ± standard deviations (SD). A two-sided Student’s t test was performed in Fig. 10.
Fig. 10.

Effects of basil EO on the growth of S. litura larvae. a Second instar larvae of S. litura were fed (a) control or (b) basil EO-exposed tomato leaves. b Larval weights were determined at the end of the feeding trial. Bars represent means ± SDs. Significant differences were evaluated using Student’s t test (*P < 0.05; **P < 0.01; ***P < 0.001)
Results
Companion planting with basil induced a priming effect on the tomato wound response
In our experimental system, tomato plants grown with basil were compared to tomato plants grown without basil (Fig. 1a). However, no significant differences, such as plant size, were observed between the two growth conditions (data not shown). To understand the effects of companion basil plants on tomato plants, tomato leaves were subjected to wound stress, followed by analyses of the expression levels of the wound response gene Pin2 (Farmer and Ryan 1992). The results showed that tomato plants grown with basil rapidly exhibited higher Pin2 expression levels than tomato plants grown without basil (Fig. 1b).
Fig. 1.
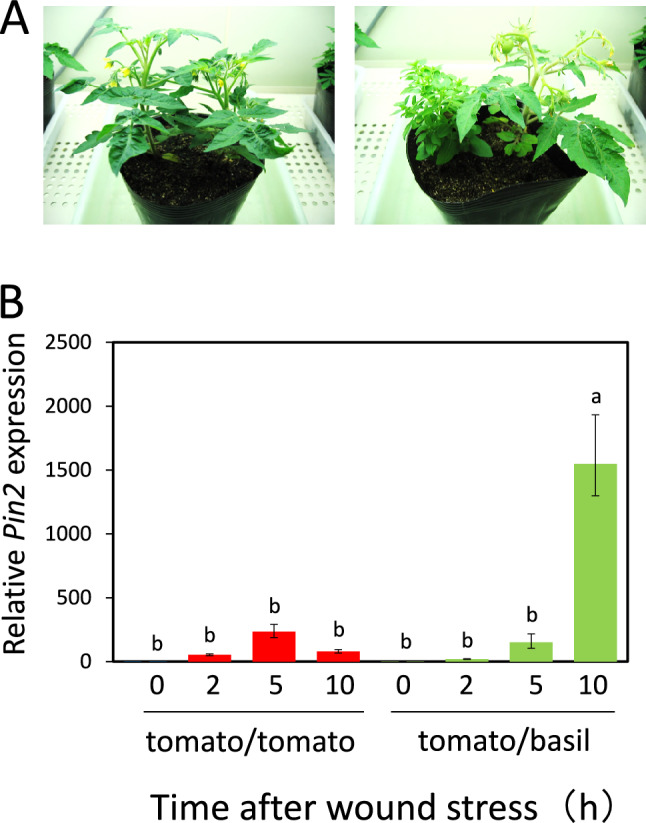
Mixed planting with basil enhanced Pin2 expression in tomato plants under wounding stress. a Tomato plants were grown for 3 weeks with or without basil companion plants. b Effects of basil on expression of the wound response gene Pin2 in tomato leaves. Leaves were wounded on both sides with scissors, sampled at the indicated times, and then subjected to quantitative polymerase chain reaction (qPCR) analysis. Bars represent means ± standard deviations (SDs) from three independent experiments. Different letters indicate significant differences (P < 0.05, one-way analysis of variance [ANOVA] followed by Tukey’s test; n = 3)
We performed two independent experiments to determine whether the root system/rhizosphere contributes to the primed wound response (Figs. S1a, S2a). Both experiments were set up to eliminate direct contact between tomato and basil underground. Since both experiments demonstrated the co-cultivation priming effect in wounded tomato leaves (Figs. S1b, S2b), we considered the basil effect to be mainly due to the airborne signal.
Essential oil (EO) prepared from basil leaves primed the wound response in wounded tomato leaves
To determine whether the observed wound response priming effect was caused by volatiles released from the aboveground parts of basil plants, we exposed tomato plants to purified EO extracted from basil leaves. Tomato plants were placed in plant boxes (Fig. 2a) and exposed to basil EO for 15 h; their leaves were then subjected to wound stress. Next, we examined Pin2 gene expression in each leaf. Tomato plants that were exposed to basil EO exhibited similar wound response priming to the findings in tomato plants grown with basil plants (Fig. 2b).
Fig. 2.

Effects of basil essential oil (EO) on the wounding response in tomato plants. a Schematic representation of the experimental setup. Plants were placed inside a box; cotton swabs soaked with 5 mL of basil EO (5 mL of water for controls) were attached to the bottom of the lid, and the box was closed. After 15 h of exposure, the box was opened, and the plant leaves were wounded with scissors after a desensitization period. b Effects of basil EO on Pin2 gene expression in tomato leaves. Tomato leaves were sampled at the indicated times and subjected to qPCR analyses. Bars represent means ± SDs from three independent experiments. Different letters indicate significant differences (P < 0.05, one-way ANOVA followed by Tukey’s test; n = 3)
Effects of individual volatile compounds in basil EO on tomato wound response priming
Next, we investigated which volatile components of the basil EO are involved in the induction of wound response priming. Tomato plants were exposed to four major volatile compounds (Politeo et al. 2007) — linalool, α-terpineol, chavicol, and eugenol—at various concentrations (Fig. 2a). Because a previous study showed that (Z)-3-hexenol induced a priming effect in corn seedlings attacked by insects (Engelberth et al. 2004), we included this compound in our experiments. The results showed that linalool, α-terpineol, and chavicol exhibited a priming effect on wound-induced Pin2 expression compared with the control (Fig. 3), whereas no significant priming effect was observed for eugenol or (Z)-3-hexenol.
Fig. 3.
Effects of five volatile compounds on the wound response in tomato plants. Tomato plants were pre-exposed to each compound at the indicated concentrations for 15 h, then wounded with scissors. At 10 h after wounding, tomato leaves were sampled and subjected to qPCR analysis. Bars represent means ± SDs from three independent experiments. Different letters indicate significant differences (P < 0.05, one-way ANOVA followed by Tukey’s test; n = 3). Leaves were sampled and their Pin2-transcript levels were analyzed by qPCR
Basil EO strengthened jasmonic acid (JA) signaling in tomato plants
Because tomato Pin2 gene expression is controlled by JA (Farmer and Ryan 1992), we investigated whether basil EO affects the expression of JA-related genes during wound stress. In tomato plants with a loss-of-function mutation in JAI1, the tomato homolog of Arabidopsis COI1, we found that jai1-1 mutants showed strong inhibition of Pin2 expression after experiencing wound stress (Fig. 4). Next, we examined the effects of basil EO on the induction of JA synthesis genes during the short-term response to leaf wounding. We found that the expression of three essential genes, LYPOXYGENASE D (LOXD), ALLENE OXIDE SYNTHASE (AOS), and ALLENE OXIDE CYCLASE (AOC), were directly and substantially induced by basil EO (Fig. 5). Furthermore, a similar strong priming response was observed for the expression of MYC2, a key factor in JA signaling (Boter et al. 2004; Du et al. 2014), as well as PSY, a precursor to the plant peptide hormone systemin (Ryan and Pearce 1998) (Fig. 6).
Fig. 4.
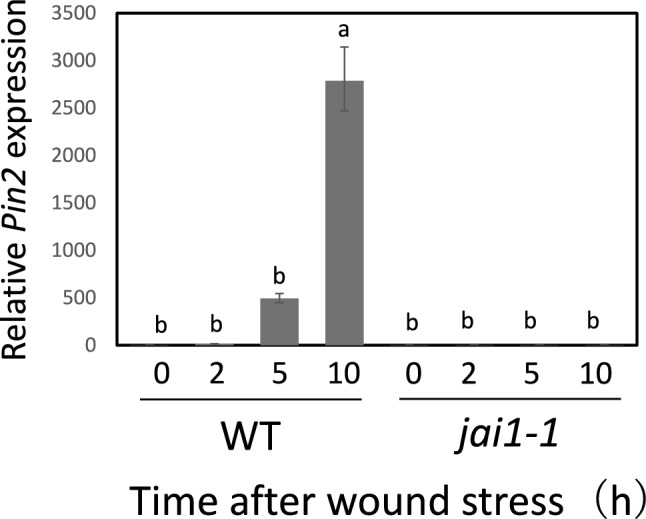
Basil EO promotes jasmonic acid (JA) signaling in tomato plants. Effect of JA-insensitive jai1-1 mutation on wound-induced Pin2 expression. Wild-type (WT) and jai1-1 mutant plants were pre-exposed to basil EO for 15 h, then wounded with scissors. Tomato leaves were sampled at the indicated times, then subjected to qPCR analysis. Bars represent means ± SDs from three independent experiments. Different letters indicate significant differences (P < 0.05, one-way ANOVA followed by Tukey’s test; n = 3)
Fig. 5.

Basil EO enhances wound-induced expression of JA biosynthesis-related genes in tomato plants. Effects of basil EO on the wound-induced expression of JA biosynthesis-related genes LYPOXYGENASE D (LOXD), ALLENE OXIDE SYNTHASE (AOS), and ALLENE OXIDE CYCLASE (AOC). WT plants were pre-exposed to basil EO for 15 h, then wounded with scissors. Tomato leaves were sampled at the indicated times, then subjected to qPCR analysis. Bars represent means ± SDs from three independent experiments. Different letters indicate significant differences (P < 0.05, one-way ANOVA followed by Tukey’s test; n = 3)
Fig. 6.
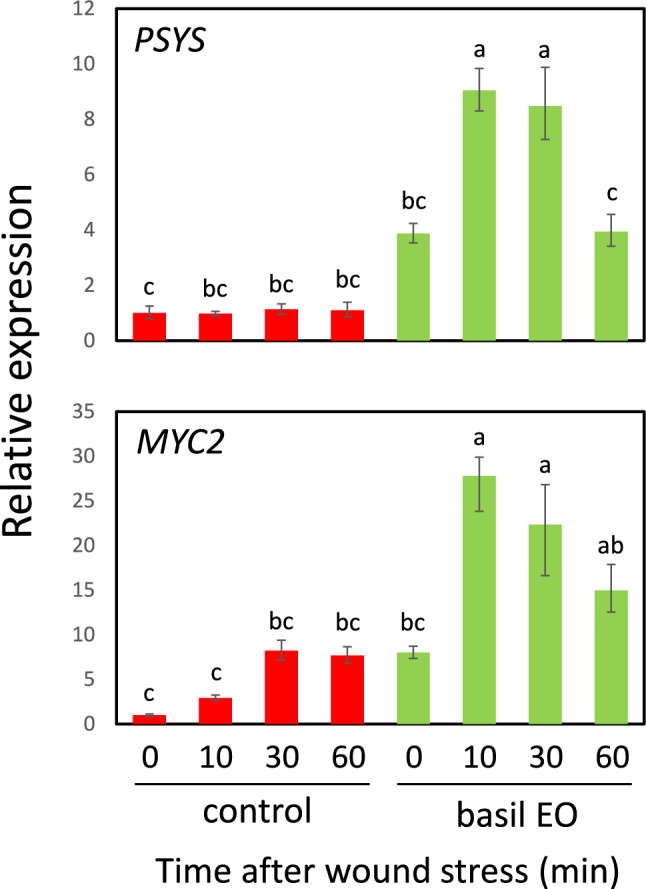
Basil EO enhances wound-induced expression of JA signaling-related genes in tomato plants. Effects of basil EO on the wound-induced expression of JA signaling-related genes PROSYSTEMIN (PSYS) and MYC2 in tomato plants. WT plants were pre-exposed to basil EO for 15 h, then wounded with scissors. Tomato leaves were sampled at the indicated times, then subjected to qPCR analysis. Bars represent means ± SDs from three independent experiments. Different letters indicate significant differences (P < 0.05, one-way ANOVA followed by Tukey’s test; n = 3)
Basil EO induced the expression of MAPK- and ROS-related genes
MAPK is involved in plant-wound signaling and controls endogenous JA levels (Seo et al. 2007). The accumulation of MAPK proteins in cells has been associated with priming induction in plant-stress signaling (Conrath 2011). Therefore, we examined whether basil EO promotes expression of the tomato MAPK genes SIMPK1, SIMPK2, and SIMPK3 after wound stress. SlMPK1 and SlMPK2 are the orthologs of Arabidopsis AtMPK6, and are 95% identical at the amino acid level (Stulemeijer et al. 2009). SlMPK3 is the orthologs of Arabidopsis AtMPK3 and is transcriptionally upregulated in response to wounding (Higgins et al. 2007). These MAPKs were shown to function in the systemin-mediated defense response against herbivorous insects (Kandoth et al. 2009). The results of this experiment did not confirm wound-related induction of SIMPK1 and SIMPK2 expression in control plants; however, the expression of these genes was significantly induced by pre-exposure to basil EO (Fig. 7). In contrast, SIMPK3 was transiently expressed after wounding, with a peak at 30 min, and a priming effect was observed in plants pre-exposed to basil EO.
Fig. 7.

Basil EO induces and promotes the expression of mitogen-activated protein kinase (MAPK) genes in tomato plants. Effects of basil EO on the wound-induced expression of three MAPK genes (SIMPK1, SIMPK2, and SIMPK3) in tomato plants. WT plants were pre-exposed to basil EO for 15 h, then wounded with scissors. Tomato leaves were sampled at the indicated times, then subjected to qPCR analysis. Bars represent means ± SDs from three independent experiments. Different letters indicate significant differences (P < 0.05, one-way ANOVA followed by Tukey’s test; n = 3)
ROS have also been proposed as strong candidates for controlling priming responses in plants (Pastora et al. 2013). Therefore, we examined the effect of basil EO on ROS accumulation in tomato leaves under wound stress via 3,3′-diaminobenzidine (DAB) staining. The results showed that ROS accumulation was upto threefold higher in basil EO-exposed leaves than in control leaves (Fig. 8a, b). Higher expression levels of Wfi1, a key gene for ROS production in tomatoes (Song et al. 2018), were also observed in basil EO-exposed leaves after wounding (Fig. 8c).
Fig. 8.
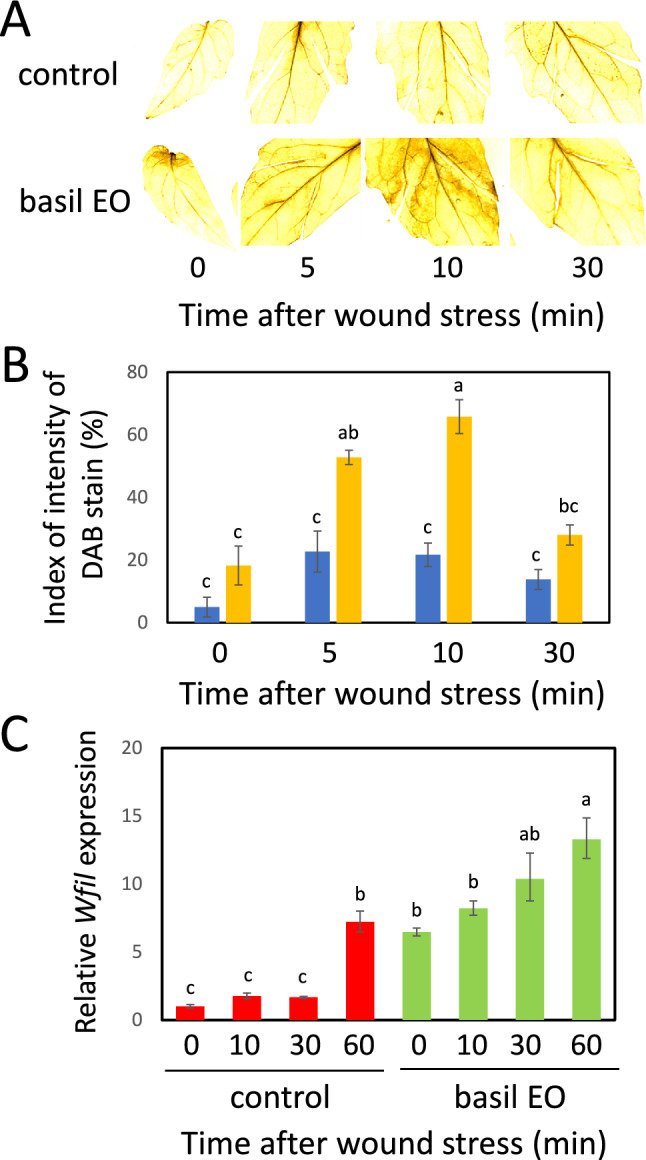
Effects of basil EO on wound-induced reactive oxygen species (ROS) accumulation in tomato leaves. a DAB (3,3′-diaminobenzidine) staining of wounded tomato leaves. Tomato plants were pre-exposed to basil EO for 15 h, then wounded with scissors. b DAB staining intensity in wounded leaves was quantified using GIMP software. Blue and orange bars represent control and basil EO treatments, respectively. c qPCR analysis of transcript levels of the tomato NADPH-oxidase gene Wfi1 in wounded tomato leaves. Tomato plants were pre-exposed to basil EO for 15 h, then wounded with scissors. Tomato leaves were sampled at the indicated times, then subjected to qPCR analysis. Bars represent means ± SDs from three independent experiments. Different letters indicate significant differences (P < 0.05, one-way ANOVA followed by Tukey’s test; n = 3)
Basil EO promoted the wound response in Arabidopsis leaves
Because basil EO primed the tomato wound response, we investigated whether a similar effect could occur in Arabidopsis. We exposed Arabidopsis plants to basil EO and subjected them to wound stress. In Arabidopsis leaves exposed to basil EO, we observed enhanced expression of the wound response gene VSP2 (Fig. 9). We also found that loss-of-function mutations affecting the Arabidopsis MAPK genes AtMPK3 and AtMPK6 eliminated the priming effect of basil EO on the wound response (Fig. 9). Furthermore, basil EO did not appear to enhance ROS accumulation in wounded leaves of atmpk3 or atmpk6 plants (Fig. S3).
Fig. 9.
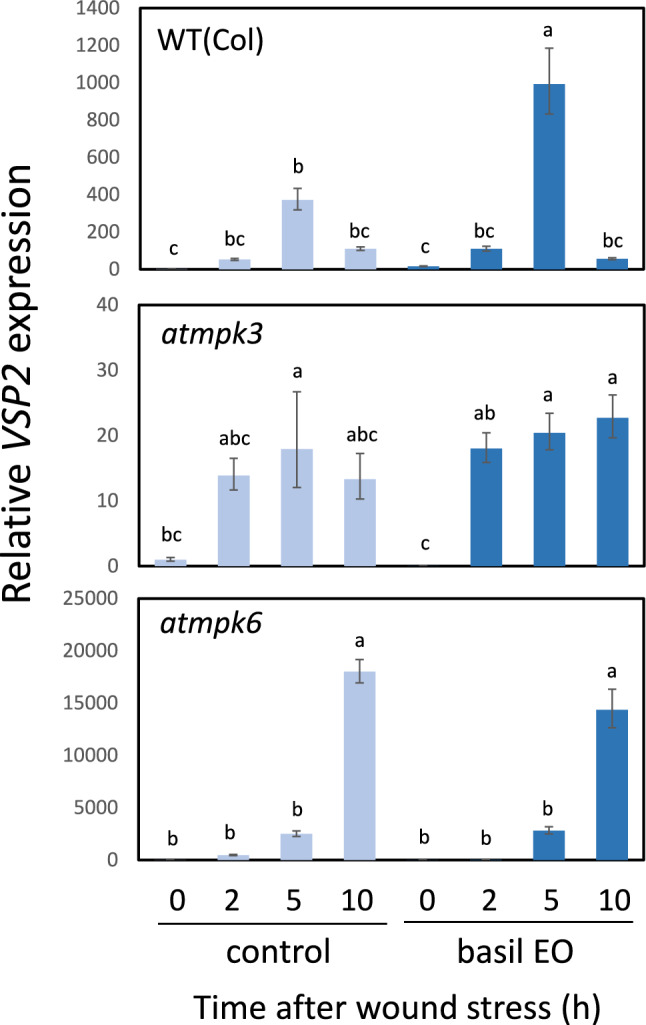
Basil EO promotes the wound response in Arabidopsis through a mechanism mediated by MAPK genes. Effects of basil EO on expression of the wound response gene VSP2 in Arabidopsis. WT, atmpk3, and atmpk6 plants were pre-exposed to basil EO for 15 h, then wounded with scissors. Arabidopsis leaves were sampled at the indicated times, then subjected to qPCR analysis. Bars represent means ± SDs from three independent experiments. Different letters indicate significant differences (P < 0.05, one-way ANOVA followed by Tukey’s test; n = 3)
S. litura larvae-fed basil EO-exposed tomato leaves exhibited growth inhibition
Next, we evaluated whether the higher expression of the wound response gene Pin2 induced by basil EO in tomatoes could promote plant resistance to insect feeding. In this experiment, young S. litura larvae were fed tomato leaves, and changes in their growth were measured after the feeding period. The results showed that larvae-fed basil EO-exposed tomato leaves were smaller than larvae-fed control leaves (Fig. 10a). The weight of larvae-fed leaves pre-exposed to basil EO was approximately half of the control larvae weight (Fig. 10b).
Discussion
Companion planting, which exploits compatibility between plant species to increase productivity per unit area and adaptability to environmental stresses, is expected to offer sustainable agriculture with reduced environmental impact. However, many studies have failed to produce results demonstrating these benefits, possibly due to the lack of scientific data supporting the effectiveness of companion plants, or the lack of well-established conditions and methods to detect their effectiveness. Clarification of the scientific basis for the benefits of companion plants is needed to establish effective strategies for their use in agricultural production.
In the present study, we established a mixed planting system consisting of tomato and basil plants to elucidate the molecular basis underlying the beneficial effects of companion plants on target plants. This experimental system showed that basil companion plants significantly enhanced the wounding response in tomato plants, which has previously been described as a priming effect (Mauch-Mani et al. 2017). We focused on plant–plant interactions through volatiles released from aboveground parts. Subsequently, we demonstrated that an EO prepared from basil leaves could prime the wounding response in tomato plants.
In plants, energy allocation to growth and stress responses typically follows a trade-off relationship, such that the induction of stress adaptation actively suppresses plant growth (Karasov et al. 2017). However, stress response priming induction has minimal effects on plant growth; it allows rapid and decisive responses to irregularly encountered stresses (Frost et al. 2008). Several molecular mechanisms are involved in the induction of plant-stress response priming (Pastora et al. 2013). Our experiments showed that basil volatiles induce MAPK expression and ROS production, both of which constitute essential mediators of plant-stress signaling (Meng and Zhang 2013). In Arabidopsis, benzothiadiazole activates plant-stress responses by inducing the expression of AtMPK3 and AtMPK6, leading to enhanced expression of downstream disease resistance genes (Beckers et al. 2009). In addition, thiamine (i.e., vitamin B1) enhances the accumulation of ROS and callose during pathogen infection, resulting in H2O2-dependent induction of defense gene expression (Ahn et al. 2007). These chemicals may promote the accumulation of intracellular signaling factors and enhance downstream signaling (Pastora et al. 2013). The observed priming effect of basil volatiles, which enhanced the tomato wound response, is presumably driven by a similar mechanism. Basil volatiles promoted the expression of JA-related genes after wounding. Because MAPKs reportedly function as essential signal mediators in wound and JA-related responses (Seo et al. 2007; Takahashi et al. 2007), it is reasonable to speculate that basil volatiles activate or enhance MAPK-mediated JA signaling. Our findings suggest that ROS also function as critical mediators of volatile signaling. Several studies have demonstrated that ROS function both upstream and downstream of MAPKs (e.g., Jalmi and Sinha 2015).
We observed a similar priming effect in Arabidopsis exposed to basil EO. Loss-of-function analysis of Arabidopsis MAPKs strongly suggested that AtMPK3 and AtMPK6 are involved in basil EO-dependent defense priming. Although this effect was less pronounced than the effect observed in tomato plants, we detected a slight increase in ROS among wounded Arabidopsis leaves exposed to basil EO. This increase was not observed in atmpk3 and atmpk6 mutants, suggesting that MAPKs function upstream of ROS. We attempted to analyze the effects of basil EO on ROS accumulation in atrborD:atrborF, a double loss-of-function mutant of NADPH oxidoreductase; however, unfavorable growth conditions prevented us from completing the experiment. Further analyses of MAPK- and ROS-mediated pathways, including MAPK activation, are required. Although the involvement of other mechanisms for wound response priming has not been investigated, basil is expected to play a role in inducing this priming effect by amplifying intracellular signaling factors (e.g., MAPKs or ROS) in tomato plants through the release of volatiles.
The mechanism by which plants recognize volatiles as signals (i.e., their specific receptors) remains poorly understood. Thus far, ethylene is the only volatile compound that has been confirmed to act as a plant signal (Lacey and Binder 2014). However, beginning with studies of the poplar eavesdropping effect (Baldwin and Schultz 1983), various studies have revealed the potential for plant-derived volatile compounds to function as specific chemical signals. Recent studies have demonstrated that β-caryophyllene, released from insect-damaged plants, specifically binds to the transcriptional regulatory protein TOPLESS in tobacco cells and induces the expression of stress response-related genes (Nagashima et al. 2019). Stirling et al. (2024) have found that a petunia karrikin-insensitive receptor, PhKAI2ia, stereospecifically perceives ( −)-germacrene D to control its pistil development and seed yield. Airborne methyl salicylate is perceived and converted into salicylic acid by SABP2 in neighboring plants. Gong et al (2023) have revealed that methyl salicylate (MeSA), salicylic acid-binding protein-2 (SABP2), the transcription factor NAC2 and salicylic acid-carboxylmethyltransferase-1 (SAMT1) form a signaling circuit to mediate airborn defense against aphids and viruses. (Z)-3-Hexenol is another strong candidate for airborn defense to convert (Z)-3-hexenyl β-vicianoside in received tomato plants, and Sugimoto et al (2023) have identified that a uridine diphosphate-glycosyltransferase UGT91R1 catalyzes its production. SABP2 and UGT91R1 do not function as specific receptors but catalyze to generate other signaling molecules or valid substances in plant cells.
Intriguingly, plants may recognize the volatile signal as a blend of multiple compounds, rather than as a single compound (Kikuta et al. 2011). In the present study, we confirmed that four volatile compounds contained in basil EO play roles in the induction of wound response priming in tomato plants. In a future study, we will examine how different combinations of these four compounds influence wound responses in tomato and Arabidopsis plants. Our preliminary results indicate that mixed planting with basil substantially increases the symbiosis of mycorrhizal fungi in tomato plant roots (data not shown). Several studies have revealed that mycorrhizal fungi can prime disease resistance in plants (Pozo and Azcón-Aguilar 2007; Sabine et al. 2012). Interplant networks composed of mycorrhizal fungi mycelia are also suspected to function as communication tools in salicylic acid and JA signaling (Song et al. 2010, 2014). The ability of companion planting to enhance plant-stress adaptation through mycorrhizal fungi requires further study. Elucidation of the molecular origins of both above- and belowground interplant communication would substantially contribute to the global implementation of companion planting and future development of sustainable agriculture.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported in part by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (JSPS-KAKENHI; grant number 15K07294). We are grateful to Dr. Kenji Matsui (Yamaguchi university) for sharing tomato jai1-1 mutant.
Author contributions
ST and RY conceived and designed the project. ST, CW, SS, HM and MI performed experiments. ST, CW, SS, HM, MI and RY analyzed the data. RY wrote the manuscript, with contributions from all authors.
Funding
Open access funding provided by Kagoshima University. The Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (JSPS-KAKENHI; grant number 15K07294).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahn I, Kim S, Lee Y, Suh S (2007) Vitamin B1-induced priming is dependent on hydrogen peroxide and the NPR1 gene in Arabidopsis. Plant Physiol 143:838–848. 10.1104/pp.106.092627 10.1104/pp.106.092627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Schultz JC (1983) Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science 221:277–279. 10.1126/science.221.4607.277 10.1126/science.221.4607.277 [DOI] [PubMed] [Google Scholar]
- Beckers GJM, Jaskiewicz M, Liu Y et al (2009) Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. Plant Cell 21:944–953. 10.1105/tpc.108.062158 10.1105/tpc.108.062158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Issa R, Gomez L, Gautier H (2017a) Companion plants for aphid pest management. InSects 8:112. 10.3390/insects8040112 10.3390/insects8040112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Issa R, Gautier H, Gomez L (2017b) Influence of neighbouring companion plants on the performance of aphid populations on sweet pepper plants under greenhouse conditions. Agric for Entomol 19:181–191. 10.3390/insects8040112 10.3390/insects8040112 [DOI] [Google Scholar]
- Bomford MK (2004) Yield, pest density, and tomato flavor effects of companion planting in garden-scale studies incorporating tomato, basil, and brussels sprout (Morgantown, WV: West Virginia University). 10.33915/etd.2105
- Boter M, Ruíz-Rivero O, Abdeen A et al (2004) Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev 18(13):1577–1591. 10.1101/gad.297704 10.1101/gad.297704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy NJA, McDaniel T, Ormerod A et al (2019) Companion planting with French marigolds protects tomato plants from glasshouse whiteflies through the emission of airborne limonene. PLoS ONE 14:e0213071. 10.1371/journal.pone.0213071 10.1371/journal.pone.0213071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U (2011) Molecular aspects of defense priming. Trends Plant Sci 16(10):524–531. 10.1016/j.tplants.2011.06.004 10.1016/j.tplants.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Du M, Zhao J, Tzeng DTW, Liu Y et al (2014) MYC2 orchestrates a hierarchical transcriptional cascade that regulates jasmonate-mediated plant immunity in tomato. Plant Cell 29(8):1883–1906. 10.1105/tpc.16.00953 10.1105/tpc.16.00953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Alborn HT, Schmelz EA et al (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci U S A 101(6):1781–1785. 10.1073/pnas.0308037100 10.1073/pnas.0308037100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4(2):129–134. 10.1105/tpc.4.2.129 10.1105/tpc.4.2.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch S, Billiald H, Collier R (2003) Companion planting–do aromatic plants disrupt host-plant finding by the cabbage root fly and the onion fly more effectively than non-aromatic plants? Entomol Experimentalis Applicata 109(3):183–195. 10.1007/978-981-10-4325-3_10 10.1007/978-981-10-4325-3_10 [DOI] [Google Scholar]
- Frost CJ, Mescher MC, Carlson JE et al (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146:818–824. 10.1104/pp.107.113027 10.1104/pp.107.113027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Wu X, Zhou X et al (2015) Companion cropping with potato onion enhances the disease resistance of tomato against Verticillium dahliae. Front Plant Sci 11(6):726. 10.3389/fpls.2015.00726 10.3389/fpls.2015.00726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Tian H, Zhang Z et al (2022) Warming-induced greenhouse gas fluxes from global croplands modified by agricultural practices: a meta-analysis. Sci Total Environ 820:153288. 10.1016/j.scitotenv.2022.153288 10.1016/j.scitotenv.2022.153288 [DOI] [PubMed] [Google Scholar]
- George DR, Collier RH, Whitehouse DM (2013) Can imitation companion planting interfere with host selection by Brassica pest insects? Agric for Entomol 15(1):106–109. 10.1111/j.1461-9563.2012.00598.x 10.1111/j.1461-9563.2012.00598.x [DOI] [Google Scholar]
- Giller KE, Hijbeek R, Andersson JA et al (2021) Regenerative agriculture: an agronomic perspective. Outlook Agri 50(1):13–25. 10.1177/0030727021998063 10.1177/0030727021998063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Wang Y, He L et al (2023) Molecular basis of methyl-salicylate-mediated plant airborne defence. Nature 622(7981):139–148. 10.1038/s41586-023-06533-3 10.1038/s41586-023-06533-3 [DOI] [PubMed] [Google Scholar]
- Higgins R, Lockwood T, Holley S et al (2007) Changes in extracellular pH are neither required nor sufficient for activation of mitogen-activated protein kinases (MAPKs) in response to systemin and fusicoccin in tomato. Planta 225(6):1535–1546. 10.1007/s00425-006-0440-8 10.1007/s00425-006-0440-8 [DOI] [PubMed] [Google Scholar]
- Horrigan L, Lawrence RS, Walker P (2002) How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ Health Perspect 110(5):445–456 10.1289/ehp.02110445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalmi SK, Sinha AK (2015) ROS mediated MAPK signaling in abiotic and biotic stress-striking similarities and differences. Front Plant Sci 6:769. 10.3389/fpls.2015.00769 10.3389/fpls.2015.00769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoth PK, Ranf S, Pancholi SS et al (2009) Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc Natl Acad Sci USA 104(29):12205–12210. 10.1073/pnas.0700344104 10.1073/pnas.0700344104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov TL, Chae E, Herman JJ et al (2017) Mechanisms to mitigate the trade-off between growth and defense. Plant Cell 29(4):666–680. 10.1105/tpc.16.00931 10.1105/tpc.16.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta Y, Ueda H, Nakayama K et al (2011) Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants. Plant Cell Physiol 52(3):588–596. 10.1093/pcp/pcr017 10.1093/pcp/pcr017 [DOI] [PubMed] [Google Scholar]
- Lacey RF, Binder BM (2014) How plants sense ethylene gas–the ethylene receptors. J Inorg Biochem 133:58–62. 10.1016/j.jinorgbio.2014.01.006 10.1016/j.jinorgbio.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC et al (2003) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16:126–143 10.1105/tpc.017954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch-Mani B, Baccelli I, Luna E et al (2017) Defense priming: an adaptive part of induced resistance. Annu Rev Plant Biol 68:485–512. 10.1146/annurev-arplant-042916-041132 10.1146/annurev-arplant-042916-041132 [DOI] [PubMed] [Google Scholar]
- Meng X, Zhang S (2013) MAPK cascades in plant disease resistance signaling. Ann Rev Phytopathol 51(1):245-266. 10.1146/annurev-phyto-082712-102314 10.1146/annurev-phyto-082712-102314 [DOI] [PubMed] [Google Scholar]
- Mengel K (2001) Alternative or complementary role of foliar supply in mineral nutrition. International Symposium on Foliar Nutrition of Perennial Fruit Plants. Acta Sci Pol-Hortoru 594:33–47 [Google Scholar]
- Moss B (2007) Water pollution by agriculture. Philos Trans R Soc Lond B Biol Sci 363(1491):659–666 10.1098/rstb.2007.2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima A, Higaki T, Koeduka T et al (2019) Transcriptional regulators involved in responses to volatile organic compounds in plants. J Biol Chem 294(7):2256–2266. 10.1074/jbc.ra118.005843 10.1074/jbc.ra118.005843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JE, Snyder WE, Hamilton GC et al (2013) Companion planting and insect pest control Weed and Pest Control-Conventional and New Challenges (IntechOpen)10.5772/55044
- Pastora V, Lunab E, Mauch-Manic B et al (2013) Primed plants do not forget. Environ Exp Bot 94:46–56. 10.1016/j.envexpbot.2012.02.013 10.1016/j.envexpbot.2012.02.013 [DOI] [Google Scholar]
- Pickett JA, Woodcock CM, Midega CAO et al (2014) Push–pull farming systems. Curr Opin Biotechnol 26:125–132. 10.1016/j.copbio.2013.12.006 10.1016/j.copbio.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Pleasant JM (2016) Food yields and nutrient analyses of the three sisters: a haudenosaunee cropping system. Ethnobiol Lett 7:87–98 [Google Scholar]
- Politeo O, Jukic M, Milos M (2007) Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem 101:379–385. 10.1016/j.foodchem.2006.01.045 10.1016/j.foodchem.2006.01.045 [DOI] [Google Scholar]
- Postor V, Luna E et al (2013) Fine tuning of reactive oxygen species homeostasis regulates primed immune responses in Arabidopsis. MPMI 26:1334–1344. 10.1094/MPMI-04-13-0117-R 10.1094/MPMI-04-13-0117-R [DOI] [PubMed] [Google Scholar]
- Pozo MJ, Azcón-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10(4):393–398. 10.1016/j.pbi.2007.05.004 10.1016/j.pbi.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Ryan CA, Pearce G (1998) Systemin: a polypeptide signal for plant defensive genes. Annu Rev Cell Dev Biol 14:1–17. 10.1146/annurev.cellbio.14.1.1 10.1146/annurev.cellbio.14.1.1 [DOI] [PubMed] [Google Scholar]
- Sabine C, Martinez-Medina JA, Lopez-Raez JA et al (2012) Mycorrhiza-induced resistance and priming of plant defenses. J Chem Ecol 38:651–664. 10.1007/s10886-012-0134-6 10.1007/s10886-012-0134-6 [DOI] [PubMed] [Google Scholar]
- Seo S, Katou S, Seto H et al (2007) The mitogen-activated protein kinases WIPK and SIPK regulate the levels of jasmonic and salicylic acids in wounded tobacco plants. Plant J 49(5):899–909. 10.1111/j.1365-313x.2006.03003.x 10.1111/j.1365-313x.2006.03003.x [DOI] [PubMed] [Google Scholar]
- Song YY, Zeng RS, Xu JF et al (2010) Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS ONE 5(10):e13324. 10.1371/journal.pone.0013324 10.1371/journal.pone.0013324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YY, Ye M, Li C et al (2014) Hijacking common mycorrhizal networks for herbivore-induced defence signal transfer between tomato plants. Sci Rep 4:3915. 10.1038/srep03915 10.1038/srep03915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LX, Xu XC, Wang FN et al (2018) Brassinosteroids act as a positive regulator for resistance against root-knot nematode involving RESPIRATORY BURST OXIDASE HOMOLOG-dependent activation of MAPKs in tomato. Plant Cell Environ 41(5):1113–1125. 10.1111/pce.12952 10.1111/pce.12952 [DOI] [PubMed] [Google Scholar]
- Stirling SA, Guercio AM, Patrick RM et al (2024) Volatile communication in plants relies on a KAI2-mediated signaling pathway. Science 383(6689):1318–1325. 10.1126/science.adl4685 10.1126/science.adl4685 [DOI] [PubMed] [Google Scholar]
- Stirling SA, Guercio AM, Patrick RM et al (2024) Volatile communication in plants relies on a KAI2-mediated signaling pathway. Science 383(6689):1318–1325 10.1126/science.adl4685 [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Ono E, Inaba T et al (2023) Identification of a tomato UDP-arabinosyltransferase for airborne volatile reception. Nat Commun 14(1):677. 10.1038/s41467-023-36381-8 10.1038/s41467-023-36381-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulemeijer IJE, Stratmann JW, Joosten MHAJ (2009) Tomato mitogen-activated protein kinases LeMPK1, LeMPK2, and LeMPK3 are activated during the Cf-4/Avr4-induced hypersensitive response and have distinct phosphorylation specificities. Plant Physiol 144(3):1481–1494. 10.1104/pp.107.101063 10.1104/pp.107.101063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukegawa S, Shiojiri K, Higami T et al (2018) Pest management using mint volatiles to elicit resistance in soy: mechanism and application potential. Plant J 96:910–920. 10.1111/tpj.14077 10.1111/tpj.14077 [DOI] [PubMed] [Google Scholar]
- Takahashi F, Yoshida R, Ichimura K et al (2007) The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 19(3):805–818. 10.1105/tpc.106.046581 10.1105/tpc.106.046581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Shiojiri K, Yamawo A (2021) Aboveground plant-to-plant communication reduces root nodule symbiosis and soil nutrient concentrations. Sci Rep 11:12675. 10.1038/s41598-021-92123-0 10.1038/s41598-021-92123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongnuanchan P, Benjakul S (2014) Essential oils: extraction, bioactivities, and their uses for food preservation. J Food Sci 79:1231–1249. 10.1111/1750-3841.12492 10.1111/1750-3841.12492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.



