Abstract
A very frequent missense mutation at codon 97 of human hepatitis B virus (HBV) core antigen (HBcAg) has been found in chronic carriers worldwide. Functional characterization of this mutant revealed one intracellular and two extracellular phenotypes in contrast to wild-type HBV: (i) a 6- to 12-fold decrease in the level of the full-length relaxed circular DNA, a 4- to 5-fold decrease in the plus-strand DNA, and an approximately 1.8-fold decrease in the minus-strand and overall DNA levels in the intracellular viral core particles; (ii) a 5.7-fold increase in the immature secretion of Dane particles, containing minus-strand, single-stranded virion DNA; and (iii) a significant reduction of nonenveloped core particles in the medium. The steady-state levels of mutant and wild-type core proteins expressed from the same vector appeared to be similar. Using a complementation assay and gradient centrifugation analysis, we demonstrated that this mutant core protein alone is necessary and sufficient for immature secretion. The decreased level of intracellular HBV DNA is caused by both the cis defect of the mutant genome and the trans defect of the mutant core protein. We have dissected further the relationship between the intracellular and extracellular phenotypes of mutant F97L. The pleiotropic effects of the HBcAg codon 97 mutation were observed consistently in several different experimental settings. The mechanism and biological significance of these findings are discussed.
Hepatitis B virus (HBV) is a major human infectious pathogen. Worldwide, there are at least 300 million chronic carriers of HBV. Chronic active hepatitis associated with HBV infection often leads to the development of cirrhosis, liver failure, and highly malignant liver cancer (8, 42, 43). Because the reverse transcriptase of HBV is error prone, the fidelity of HBV DNA synthesis is low. Therefore, sequence divergence within the HBV genome during the long-term carriage of HBV in patients is expected. However, unless a selective advantage can be conferred by particular sequence changes, one would not necessarily expect a particular mutant to emerge and predominate during the evolution of disease.
Despite the identification of many naturally occurring HBV variants, most of the variants either have not been characterized functionally or, rather disappointingly, have not been found to exhibit strong aberrant phenotypes in the available assays. A weak phenotype with a borderline difference from the wild type is a very common frustration in the research of variants. Part of the difficulty is due to the lack of a priori knowledge about which functional assay will be more informative for different variants.
A good example to illustrate this problem in the functional characterization of an HBV mutant is the study of naturally occurring mutations in the HBV core antigen (HBcAg). HBcAg is a 22-kDa protein with multiple functions, including interactions with the pregenomic RNA and polymerase during encapsidation (32), with itself to polymerize into a core particle (4), and with viral DNA during reverse transcription and DNA elongation (19); import of relaxed circular (RC) DNA to the nucleus (49); and targeting to the endoplasmic reticulum for envelope formation (6, 34). In addition to serving as a structural component of nucleocapsid particles, HBcAg has been hypothesized to serve as a signal transducer which can sense and transmit the signal of genomic DNA maturation inside the core particle to the machinery of envelope formation for secretion (44). It is thus conceivable that the functional significance associated with a naturally occurring core antigen mutation could be complex and unpredictable. For example, a naturally occurring core internal deletion variant (1, 33, 53) has been shown to contain all of the functional features of defective interfering particles and may play a role in chronicity and pathogenesis in HBV natural infection (58, 59).
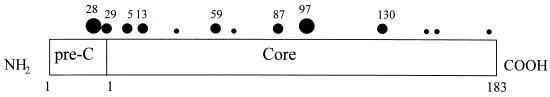
In addition to the deletion mutation of the HBcAg gene, a naturally occurring missense mutation at codon 97 of the HBcAg gene was first reported by Ehata et al. in 1992 (12). In the past 5 years, more than 20 papers have reported mutations at the same position (see, e.g., references 2, 5, 10–13, 16–18, 21, 22, 24, 26, 30, 35–37, 50–52). As shown in Fig. 1, the codon 97 mutation occurs in a hot-spot mutational domain (10, 11, 21), within HBcAg amino acids 80 to 120, with a frequency comparable to (or even higher than) that of the e antigen-negative precore mutation (40). The codon 97 mutation often changes the wild-type amino acid from phenylalanine (or isoleucine) into leucine. Because of the similarity in the hydrophobicities of these amino acids, it is difficult to predict if a major functional difference between the wild type and the codon 97 mutant will occur.
FIG. 1.
Mutational hot spots within HBcAg in chronic carriers. A missense mutation at codon 97 appears to be the most frequent HBcAg mutation in natural infection (10, 11, 21). The sizes of the dots reflect their respective estimated average mutation frequencies found in chronic hepatitis B patients (data are compiled from references 2, 11, 21, 22, and 24): large dots, more than 45%; medium dots, 20 to 35%; and small dots, fewer than 20% of patients are found to have virus with a predominant mutation at this position.
Here, we report a surprising discovery that the seemingly subtle missense mutation at codon 97 of HBcAg results in distinct pleiotropic effects. This mutant not only secretes a significantly increased amount of Dane particles containing the immature replicative intermediate of minus-strand DNA but also has a significantly reduced level of the intracellular viral plus-strand DNA and the RC form. Furthermore, we focused our investigation on the mechanism and relationship between these two distinct phenotypes caused by a common naturally occurring mutation at HBcAg codon 97.
MATERIALS AND METHODS
Preparation of HBV intracellular core particles and viral DNA was as described previously (57). Gradient analysis of secreted viral particles was as detailed elsewhere (58). Procedures for immunoblot analysis of HBcAg were as described by Yuan et al. (58).
Plasmid constructs. (i) pWT, pF97L, p1903, pF97L/1903, pSK/O, pF97L/SK/O, and pECE24.
pWT, described as pSV2NeoHBV2x elsewhere (41), is a replication vector of wild-type HBV with a tandem dimer configuration. Site-directed mutagenesis was performed to introduce specific mutations into the HBV monomer genome, which was subsequently dimerized to mimic the genetic configuration of HBV (38). The oligonucleotide used to create mutation F97L is 5′-GGG CCT AAA GCT CAG GCA ACT-3′. The initiation codon of the core antigen in mutants p1903 and pF97L/1903 was abolished with the oligonucleotide 5′-TTT TGG GGC ATA GAC ATC GAC C-3′. The oligonucleotide used to eliminate the initiation codon of the surface antigen in pSK/O and pF97L/SK/O is 5′-CGC TGA ACA CGG AGA ACA TC-3′. The mutated nucleotides are underlined. The resulting tandem dimers were confirmed by restriction enzyme digestion and DNA sequencing.
(ii) pSVC, pSVC97, and pECE24.
pSVC is a wild-type core antigen expression vector under the control of the simian virus 40 (SV40) enhancer and early promoter. The cloning strategy for pSVC has been described previously (58, 59). The same protocol for constructing pSVC was used for the construction of pSVC97, using mutant pF97L as a PCR DNA template. Plasmid pECE24 is an expression vector for the major surface antigen (34). Except for pECE24 (adw), all HBV constructs were derived from HBV subtype ayw (14).
DNA and RNA probes. (i) Full-length HBV DNA probe.
The full-length 3.1-kb HBV DNA fragment was purified from pWT by EcoRI digestion. Approximately 25 ng of the 3.1-kb DNA fragment was radiolabelled by using a random-primed DNA labelling kit (Boehringer Mannheim Co.). Standard procedures for Southern blot analysis were used in this study.
(ii) Plus-strand-specific riboprobe.
pGEM-4Z-HBV was constructed to produce plus- or minus-strand-specific RNA probes. pGEM-4Z-HBV contains one copy of the HBV monomer genome in the pGEM-4Z vector (Promega, Madison, Wis.). Use of the T7 or SP6 promoter at either end of the HBV monomer produces either plus-strand- or minus-strand-specific probes, respectively. In vitro transcription of XhoI-linearized pGEM-4Z-HBV DNA with T7 polymerase produces a 3.1-kb RNA fragment which specifically hybridizes with the plus-strand HBV DNA. In vitro transcription to make the 32P-labelled RNA probes was performed by the procedure recommended by the manufacturer (Amersham).
(iii) Minus-strand-specific riboprobe.
The minus-strand-specific RNA probe was synthesized by using HindIII-linearized pGEM-4Z-HBV DNA and SP6 polymerase for in vitro transcription.
Quantitative measurement of different replicative intermediates of HBV DNAs.
Quantitative comparisons of plus-strand-, minus-strand-, and double-strand (ds)-specific signals were made by measuring the images from X-ray film, counting the entire lane from the 4.0-kb position to the bottom of the lane. The banding intensity of the full-length RC form was measured by counting the signal at the 4.0-kb position. The intensity of the total RC-form population was measured by counting the signal from the 4.0-kb position to a position right above the 1.5-kb position of single-stranded (ss) DNA, while the intensity of the ss form was measured by counting the signals at and below the 1.5-kb position. The image was acquired from X-ray film with either a Howtek Scanmaster 3+ machine or a Stratagene Eagle Eye II. The stored image was then analyzed by using the ONE-DScan computer program (Scanalytics Co., Billerica, Mass.).
RESULTS
To investigate the functional significance of the codon 97 mutation in HBcAg, we introduced a mutation at codon 97, changing the amino acid from wild-type (ayw) phenylalanine (F) to mutant leucine (L) (see Materials and Methods). Plasmid DNA of a tandem homodimer of mutant F97L was transfected into the Huh7 human hepatoma cell line via the calcium phosphate technique. At 5 days posttransfection, intracellular core particle-associated DNA was analyzed by Southern blot analysis, while the extracellular viral particles were collected from the medium and analyzed by CsCl gradient centrifugation and Southern blot analysis.
Decreased overall DNA synthesis in mutant F97L core particles.
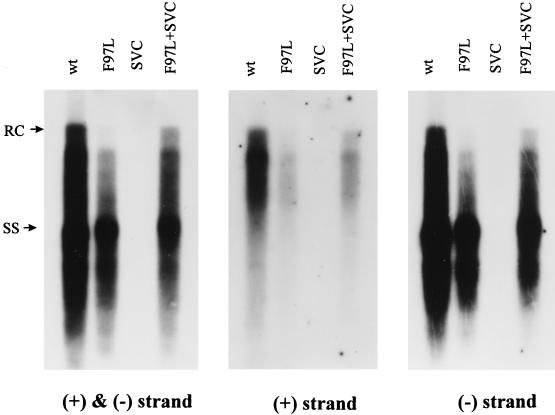
As shown in Fig. 2 (left panel), with the ds probe, the overall HBV DNA content of mutant F97L core particles was decreased by approximately 1.8-fold. However, the most striking difference (6- to 12-fold) came from the absence of apparent full-length RC form at the 4.0 kb-position. When wild-type core protein was provided in trans from the plasmid pSVC, this defect in RC and overall viral DNA synthesis could be partially rescued (Fig. 2, left panel). Cotransfection of pSVC with wild-type virus does not increase HBV DNA synthesis in this system (data not shown).
FIG. 2.
One characteristic intracellular phenotype of mutant F97L is the decrease of overall HBV DNA replication, particularly in plus-strand DNA synthesis. This replication defect of mutant F97L can be partially rescued by providing wild-type (wt) core protein in trans. Ten micrograms of each plasmid DNA was transfected into Huh7 cells. Core particle-associated DNA was harvested 5 days posttransfection and analyzed by Southern blot analysis. Intracellular HBV DNA was then detected with a 3.1-kb HBV ds DNA probe (left panel), a plus-strand-specific riboprobe (middle panel), and a minus-strand-specific riboprobe (right panel) (see Materials and Methods). These three different probes were applied to the same nitrocellulose membrane sequentially. Full-length RC-form DNA at 4.0 kb and ss DNA at 1.5 kb are indicated by arrows.
More severe deficiency in plus-strand DNA synthesis in mutant F97L core particles.
The absence of the full-length RC-form DNA at the 4.0-kb position and the near-normal amount of ss DNA at and below the 1.5-kb position (Fig. 2, left panel), compared to the wild type, imply a specific defect in plus-strand DNA synthesis in mutant F97L. To determine if this is indeed the case, the ds probe in Fig. 2, left panel, was removed and the same filter was rehybridized with radiolabelled, strand-specific riboprobes (see Materials and Methods). The minus-strand-specific riboprobe gave a hybridization pattern (Fig. 2, right panel) similar to that of the ds probe (left panel). However, the difference in plus-strand DNA synthesis between the wild type and mutant F97L was more evident (approximately four- to fivefold according to densitometer quantitation) (see Materials and Methods) (Fig. 2, middle panel). Thus, the plus-strand-specific probe appeared to be the most diagnostic in distinguishing the intracellular phenotype of decreased DNA synthesis in mutant F97L from that of the wild type.
Mutant F97L secretes Dane particles containing predominantly immature HBV DNA (minus-strand, ss DNA).
It is known that the replicative intermediates of minus-strand DNA are precursors to RC DNA. Thus, a loss in the RC form and plus-strand synthesis (Fig. 2) could result in an accumulation of minus-strand DNA prior to plus-strand initiation or elongation. However, we observed no apparent increase or accumulation of minus-strand DNA replicative intermediates in mutant F97L relative to the wild type (Fig. 2, right panel). This puzzle prompted us to examine the extracellular DNA profile of HBV particles in the medium from a mutant F97L-transfected culture.
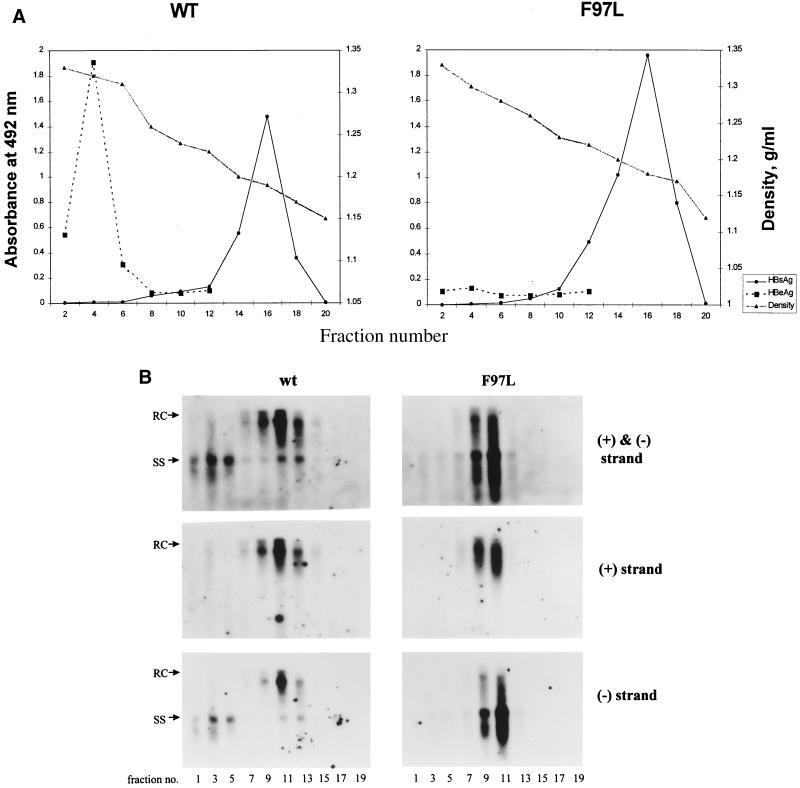
As shown in Fig. 3A, the HBV surface antigen (HBsAg) peaks appear at similar positions for both wild-type and mutant F97L viruses. HBV DNA from odd-numbered fractions was then analyzed by Southern blot analysis with a ds probe (Fig. 3B, upper panels), a plus-strand-specific probe (Fig. 3B, middle panels), and a minus-strand-specific probe (Fig. 3B, lower panels). A striking difference between the wild type and mutant F97L was evident in the ss DNA-specific region below the 1.5-kb position in the Dane particle fractions 9, 11, and 13 (1.24 g/cm3) (Fig. 3B, upper panels). In the case of the wild type, the majority of HBV DNA in these fractions always occurs as the mature RC form above 1.5 kb. In contrast to the case for the wild type, the majority of mutant F97L DNA almost always occurs as the minus-strand, ss DNA at and below 1.5 kb. To be more quantitative, we measured the intensities of the RC and ss DNAs in Fig. 3B (upper panels) by densitometry (see Materials and Methods). The average of the extracellular ratio of the RC to the ss form for the wild type is 3.8, and that for mutant F97L is 0.67 (Table 1). Therefore, the relative amount of immature ss DNA compared to the mature RC DNA has increased by 5.7-fold (3.8/0.67) in mutant F97L compared to the wild type. No apparent difference between the wild type and mutant F97L was observed when the same filters were hybridized with the plus-strand-specific riboprobe (Fig. 3B, middle panels). Therefore, in contrast to the case for the intracellular phenotype, the minus-strand-specific probe appeared to be the most diagnostic in distinguishing between the extracellular phenotypes of the wild type and mutant F97L (Fig. 3B, lower panels).
FIG. 3.
Gradient centrifugation analysis of extracellular viral particles reveals the secretion of immature minus-strand, ss DNA forms in the Dane particle fractions (1.24 g/cm3) for mutant F97L. Conditioned media were collected on days 3 and 5 posttransfection. Viral particles were first purified from the media through a 20% sucrose cushion; this was followed by isopycnic centrifugation in a gradient of 20 to 50% (wt/vol) cesium chloride. (A) Fractions were separated according to their buoyant densities; this was followed by dialysis and enzyme immunoassay for HBsAg and HBcAg (see Materials and Methods). WT, wild type. (B) The extracellular HBV DNA in each fraction was analyzed by Southern blot analysis with a ds-specific 3.1-kb HBV DNA probe (upper panels), a plus-strand-specific riboprobe (middle panels), and a minus-strand-specific riboprobe (lower panels).
TABLE 1.
Summary of the intracellular and extracellular ratios between RC and minus-strand, ss DNA in different experimental settingsa
| Expt setting | RC/ss DNA ratio (mean ± SD)b
|
||
|---|---|---|---|
| Intracellular | Extracellular | Source of data (figure[s]) | |
| Wild-type HBV dimer | 0.71 ± 0.09 | 3.82 ± 0.79 | 2, 3 |
| Mutant F97L dimer | 0.44 ± 0.07 | 0.67 ± 0.20 | 2, 3 |
| p1903 plus SVC | 0.70 | 4.0 | 5, 6 |
| p1903 plus SVC-97 | 0.64 | 1.0 | 5, 6 |
| SK/O plus pECE | 0.35 | 4.50 | 8, 9 |
| F97L/SK/O plus pECE | 0.30 | 0.71 | 8, 9 |
| SK/O | 0.43 | 8 | |
| F97L/SK/O | 0.30 | 8 | |
RC DNA, signals above the 1.5-kb position; ss DNA, signals at and below the 1.5-kb position. Quantitation of the images of RC and ss DNAs was as described in Materials and Methods.
Standard deviations were calculated from three to five independent experimental results with ds-specific probes. Values without standard deviations are averages of at least two independent experimental results.
Mutant F97L appears to release a much-reduced amount of nonenveloped core particles.
In addition to the differences in the enveloped Dane particles, there is a difference between the wild type and mutant F97L in the high-density fractions (fractions 1, 3, and 5 [around 1.35 g/cm3]). Nonenveloped core particles containing predominantly minus-strand DNA have been detected in the medium from wild-type-hepadnavirus-producing cell cultures (7, 39, 45, 48), which is also reproduced in Fig. 3B (upper left panel). However, unlike the case for wild-type HBV, no appreciable amounts of viral DNA (Fig. 3B, upper right panel) or core antigen (Fig. 3A) could be detected in these high-density fractions for mutant F97L.
Comparison of the wild-type and mutant F97L steady-state core protein levels by immunoblot analysis.
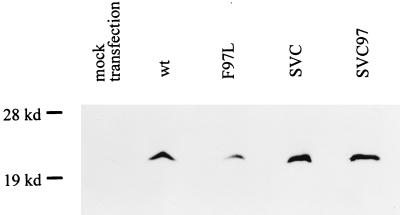
The difference in the intra- and extracellular HBV DNA patterns for the wild type and mutant F97L could be caused by the difference in the stabilities of their respective core proteins. We therefore compared the steady-state levels of each protein produced by wild-type HBV and mutant F97L in the context of an HBV-replicating system (i.e., with the tandem homodimeric form of wild-type and mutant virus DNA in transfection) (Fig. 4). By immunoblot analysis with a rabbit anticore antibody (3), we observed an approximately twofold difference in their steady-state levels of core proteins. This difference appears to be more related to their difference in replication potential, since the level of pre-S1 protein, as an internal control measured by immunoblot analysis (20), was also reduced by about twofold in the mutant F97L-transfected culture (data not shown). Consistent with the reduced amounts of HBV DNA, pre-S1, and core proteins in mutant F97L (Fig. 2 and 4), the 3.5-kb core-specific mRNA of mutant F97L was also reduced by about twofold when measured by both RNase protection assay and Northern blot analysis (data not shown). To determine if the difference in DNA replication results in the apparent difference in core protein levels seen in Fig. 4, both wild-type and mutant F97L core proteins were expressed from the same SV40 enhancer-promoter vectors. As shown in Fig. 4, we found no apparent difference in the steady-state levels of these two different core proteins expressed from the same expression system without concomittant HBV DNA replication. Therefore, the small difference in the core protein level between the wild type and mutant F97L appears to be the result, rather than the cause, of their difference in DNA replication (Fig. 2).
FIG. 4.
Similar steady-state levels of core protein were observed by immunoblot analysis in cells transfected with pSVC and pSVC97 (see Materials and Methods). The small but reproducible difference in the steady-state level of core protein expressed from tandem dimers of wild-type (wt) and mutant F97L parallels production of pre-S1 protein (an internal control) (data not shown) and viral DNA replication (Fig. 2).
The mutant F97L core protein product contributes to the intracellular phenotype of plus-strand deficiency.
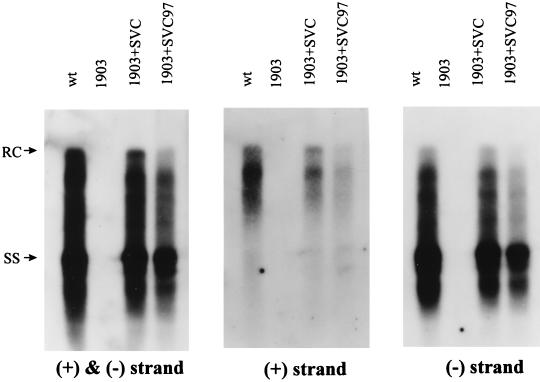
As seen earlier (Fig. 2), the deficiency in synthesis of the plus-strand and RC forms of mutant F97L can be partially rescued by cotransfection with the wild-type core protein expression plasmid pSVC. This suggests that at least part of the intracellular plus-strand deficiency of mutant F97L is caused by the mutant core protein itself. Indeed, as shown in Fig. 5 (middle panel), a reproducible difference in plus-strand synthesis, albeit only twofold or so, was observed when the same HBV dimer construct with the core AUG abolished (p1903) (with the wild-type sequence for codon 97) was rescued either by wild-type (pSVC) or by mutant F97L (pSVC97) core protein. Consistent with this difference in plus-strand synthesis (Fig. 5, middle panel), a small and reproducible difference in overall DNA synthesis (left panel) and minus-strand DNA synthesis (right panel) was also observed.
FIG. 5.
Complementation assay for the trans defect of mutant F97L core protein in the intracellular phenotype of plus-strand deficiency. The mutant core protein results in a less-than-twofold decrease in overall DNA synthesis (left panel) as well as a reproducible twofold decrease in plus-strand synthesis (middle panel). The experimental procedures were as described in the legend to Fig. 2. Specific probes used in each experiment are indicated below each panel. wt, wild type.
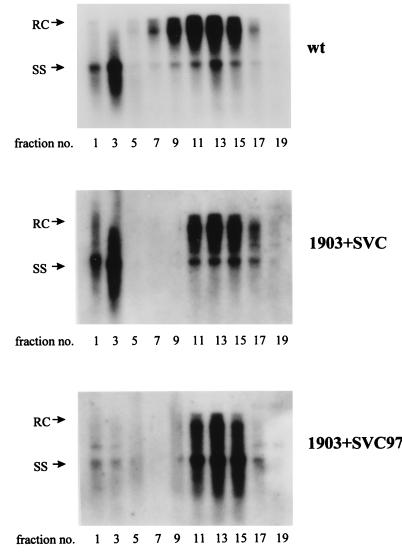
The core protein of mutant F97L is necessary and sufficient for the extracellular phenotype of immature secretion.
To determine whether the mutant core protein contributes to the extracellular phenotype of immature secretion, we compared the gradient profiles of viruses produced by cotransfection of p1903 plus pSVC or p1903 plus pSVC97. As shown in Fig. 6, mutant core from pSVC97 (lower panel), but not wild-type core from pSVC (middle panel), can reproduce the immature secretion phenotype of mutant F97L as seen in Fig. 3B (upper panel). Therefore, mutant F97L core protein alone is necessary and sufficient for the immature secretion. We also noted that the naked core particles in the higher-density fractions 1 and 3 were greatly diminished when mutant 1903 with the core AUG abolished was rescued with the mutant F97L core protein (SVC97) (lower panel), compared to the rescue with wild-type core protein (middle panel).
FIG. 6.
Complementation assay for the trans defect of mutant F97L core protein in the extracellular phenotype of immature secretion. Cotransfection of the F97L mutant core protein alone is necessary and sufficient to result in the secretion of Dane particles containing predominantly the immature form of minus-strand ss DNA. The experimental procedures for the replication assay and gradient centrifugation analysis were as described in the legend to Fig. 3. wt, wild type.
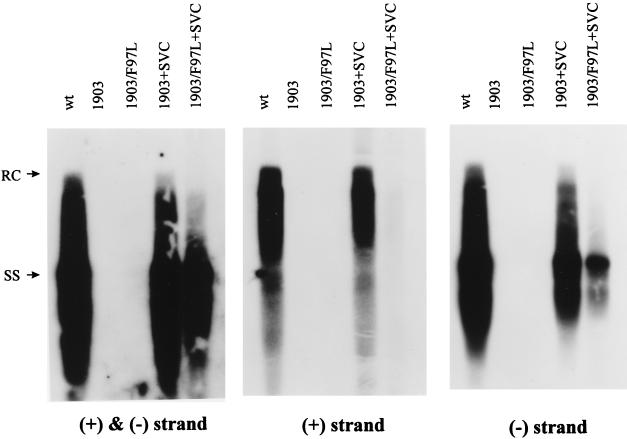
The cis effect of mutation F97L also contributes to the intracellular phenotype of plus-strand deficiency.
In addition to the trans defect of a mutant core protein (Fig. 5 and 6), another potential consequence of mutation F97L is to create a cis defect in the mutant genome. To examine this possibility, we compared the replication activities of the single mutant 1903 and the double mutant 1903/F97L in the presence of the same wild-type core protein expressed from pSVC. Any difference in their replication activities will be due to the cis effect of the F97L nucleotide change, since the core protein used in both cotransfections is the same (wild type). As shown in Fig. 7, although the cis effect of mutation F97L is only a 1.8-fold reduction in overall DNA synthesis (left panel), its effect on intracellular plus-strand synthesis in this complementation assay is a decrease of close to 10-fold (middle panel) and its effect on minus-strand synthesis is a decrease of about 2.4-fold (right panel), compared to its wild type counterpart. Taken together, the results in Fig. 2, 5, and 7 suggest that the intracellular plus-strand deficiency is in part due to the cis defect of the mutant genome. In addition to its effect on plus-strand synthesis, mutation F97L seems to affect minus-strand synthesis in this experimental setting. Dane particles produced from cotransfecting mutant 1903/F97L and pSVC exhibited a gradient profile similar to that for wild-type HBV, albeit at a significantly reduced level, and did not have the immature secretion phenotype (56). Therefore, while there is some cis effect on the intracellular plus-strand DNA level, there is no apparent cis effect on the immature secretion phenotype.
FIG. 7.
Complementation assay for the cis defect of the mutant F97L genome in the intracellular phenotype of plus-strand deficiency. Wild-type (wt) core protein was used to trans complement both the single mutant 1903 and the double mutant 1903/F97L. The probes used for each blot are shown below each panel.
Dissection of the relationship between the intracellular plus-strand deficiency and extracellular immature secretion phenotype by blockage of secretion.
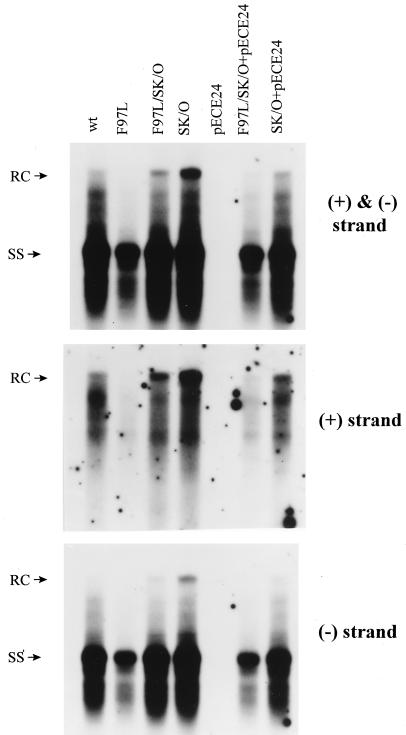
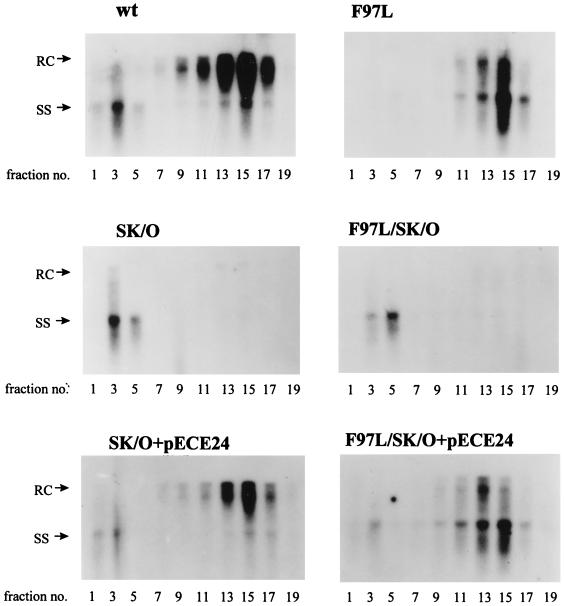
HBsAg is known to be required for secretion of Dane particles. To dissect the potential cause-effect relationship between the intra- and extracellular phenotypes of mutant F97L, we blocked the secretion of Dane particles by abolishing (knockout) the AUG initiation codon of the mutant F97L HBsAg gene (pF97L/SK/O). As shown in Fig. 8 (upper panel), we observed a significant accumulation of both RC and plus-strand DNAs in the single mutant SK/O and the double mutant F97L/SK/O relative to their parental counterparts (wild type and mutant F97L, respectively). Furthermore, the effect of mutation SK/O can be reversed by cotransfection with an HBsAg expression vector, pECE24 (34). The result that the amount of accumulated RC form of the double mutant F97L/SK/O is about twofold less than that for the single mutant SK/O suggests that the intracellular plus-strand deficiency of mutant F97L cannot be caused entirely, if at all, by immature secretion (Fig. 8, upper panel). In other words, the results support the possibility that there is an intrinsic deficiency in plus-strand synthesis of mutant F97L, irrespective of secretion status (Fig. 8, middle panel).
FIG. 8.
The intracellular plus-strand deficiency phenotype of mutant F97L persists when secretion is blocked by abrogating the initiation codon of HBsAg (upper panel; compare lanes F97L/SK/O and SK/O). The partially restored wild-type (wt)-like phenotype of double mutant F97L/SK/O can be reversed to a mutant F97L-like phenotype by supplying HBsAg in trans from the expression vector pECE24. The Southern blot analysis was performed as described in the legend to Fig. 2. In contrast to the plus-strand deficiency phenotype (middle panel), the intracellular minus-strand DNA levels are similar in mutant F97L/SK/O and mutant SK/O (lower panel). The intracellular minus-strand deficiency is seen only when the virus is secretion competent (lower panel; compare lanes F97L/SK/O+pECE24 and SK/O+pECE24).
Dissection of the relationship between the intracellular minus-strand deficiency and the extracellular immature secretion phenotype by reversing the blockage of secretion.
As shown in the lower panel of Fig. 8, mutant SK/O and mutant F97L/SK/O accumulated similar levels of minus-strand DNA in the absence of secretion. However, when HBsAg was provided from pECE24 to allow secretion, mutant F97L/SK/O accumulated an approximately twofold-reduced level of minus-strand DNA relative to that in mutant SK/O. Thus, a substantial part of the minus-strand DNA of mutant F97L/SK/O was lost when secretion was made possible (see Discussion).
Reappearance of extracellular nonenveloped core particles in the absence of HBsAg synthesis in double mutant F97L/SK/O.
The extracellular HBV particles collected from six different transfections from Fig. 8 were further compared by gradient centrifugation analysis (Fig. 9). As expected, the secretion of both wild-type and mutant F97L Dane particles is dependent on HBsAg. However, to our surprise, the high-density nonenveloped core particles, which were much reduced in mutant F97L (Fig. 3B and Fig. 9, upper panels), reappeared in the absence of HBsAg synthesis in double mutant F97L/SK/O (Fig. 9, middle panels). When HBsAg was provided to this double mutant F97L/SK/O, the signal intensity of the naked core particle fractions was severely diminished, while the Dane particle fractions reappeared, containing a majority of the immature minus-strand HBV DNA (Fig. 9, lower panels). We also noted that cotransfection of pECE with SK/O appeared to result in a decrease in the naked core particles (Fig. 9, bottom left panel).
FIG. 9.
Reappearance of extracellular nonenveloped core particles in the absence of HBsAg synthesis in mutant F97L/SK/O in gradient analysis. Secretion of wild-type (wt) and mutant F97L Dane particles is dependent on HBsAg. The ds-specific HBV probe was used for Southern hybridization.
DISCUSSION
We have observed characteristic intracellular and extracellular phenotypes associated with the frequent naturally occurring mutation at HBcAg codon 97 (Fig. 1, 2, and 3). The intracellular phenotype has been observed consistently in four different experimental settings (Fig. 2, 5, 7, and 8), and the extracellular phenotypes have been observed consistently in three different experimental settings (Fig. 3, 6, and 9). The very abrupt disappearance of the full-length RC signals in mutant F97L (Fig. 2) suggests that it would be interesting to examine the DNA priming specificity of mutant F97L in the future (32). It should be noted that the term immature secretion, instead of premature secretion, was used for the description of the extracellular phenotype of mutant F97L Dane particles; whether such Dane particles with mutant core protein actually are secreted earlier (kinetically faster) than those of wild-type virus remains to be investigated in the future.
Genome maturation signal and envelope formation.
Summers and Mason (44) proposed that duck hepatitis B virus (DHBV) DNA replication inside the core particles can transmit a genome maturation signal to the surface of core particles, which in turn recruit the envelopment machinery. Recent studies using a DHBV polymerase mutant supported this hypothesis (54). On the other hand, studies using artificially created human HBV core or polymerase mutants demonstrated that a genome maturation signal may not always be required for envelopment and secretion (15, 31). The immature secretion of the naturally occurring mutant F97L (Fig. 3) and an artificially created core deletion mutant (31) suggests that such a genome maturation signal, while necessary in the wild-type duck system, either is not required or can be bypassed for human HBV mutants. Alternatively, while the preferential export of the RC form in wild-type HBV could reflect a mechanism of positive selection for more-mature capsids, our preliminary studies here are also consistent with a mechanism of negative selection against the export of the immature capsids in wild-type HBV.
Nonselective or less selective export of minus-strand DNA and core-envelope interaction.
When HBsAg is not provided, mutant SK/O and mutant F97L/SK/O accumulate similar levels of intracellular minus-strand DNA (Fig. 8, lower panel). However, when secretion was made possible by providing HBsAg from pECE24 to mutant SK/O and mutant F97L/SK/O, about 40% of the minus-strand DNA in mutant F97L/SK/O was lost (Fig. 8, lower panel). It is therefore tempting to hypothesize that the mutant core particles probably can interact with the envelope machinery in a way that leads to the export of mutant core particles. The less selective or nonselective export of the intracellular minus-strand DNA can explain the distorted distribution between the mature RC form and the less mature minus-strand DNA in the Dane particle population of mutant F97L.
Dissecting the complicated relationships between the intracellular and extracellular phenotypes.
Due to the undefined nature of the released naked or nonenveloped core particles, we will focus our discussion only on the relationship between the intracellular phenotype and the immature secretion of Dane particles. As shown in Fig. 8 (upper panel), abrogating HBsAg synthesis in mutant F97L (F97L/SK/O) can significantly restore the intracellular pattern of HBV DNA synthesis. This result indicates that mutant F97L retains the potential to make the full-length RC DNA. The difference in the RC form and replication activity between mutant SK/O and mutant F97L/SK/O suggests that mutation F97L creates an intrinsic defect in the intracellular plus-strand DNA synthesis regardless of secretion status (Fig. 8, middle panel). This result is entirely consistent with the cis defect demonstrated in Fig. 7. In theory, the twofold trans defect of the mutant core protein in plus-strand synthesis (Fig. 5) could be caused indirectly by immature secretion leading to a diminishing intracellular HBV DNA pool. Alternatively, the trans defect of F97L core protein could be manifested through its direct effect on the polymerase activity in HBV DNA synthesis. The preliminary results in Fig. 9 appear to support the former possibility (see below).
Quantitative aspects of plus-strand deficiency.
As shown in Fig. 2 (middle panel), plus-strand DNA synthesis of the mutant F97L tandem dimer is approximately four- to fivefold lower than that of wild-type virus. Based on the complementation assay (Fig. 5, middle panel), the mutant core protein alone contributes to approximately a twofold decrease in the plus-strand DNA level. Indeed, when wild-type core protein was provided to mutant F97L, the deficiency in plus-strand synthesis could be partially restored (Fig. 2). Taken together, by subtracting these differences, one would expect that the cis defect of the F97L mutant genome by itself would contribute to only a two- to threefold decrease in plus-strand synthesis. It is therefore puzzling that there is a close-to-10-fold difference in plus-strand synthesis between p1903 and p1903/F97L after complementation with pSVC (Fig. 7, middle panel). It is possible, though, that the cis defect in both plus- and minus-strand DNA synthesis could be amplified out of proportion in this particular experimental setting of trans complementation. The cis effect of the F97L mutation does not necessarily prove the existence of a cis element for DNA synthesis in this portion of the HBV core gene, however. Such speculation about a cis element requires more careful examination.
Intracellular and extracellular ratios between RC and single-strand DNAs.
As discussed above, it is difficult to have perfectly consistent quantitative data from different experimental settings, particularly when different figures with different probes and exposure times of X-ray film are compared with each other. However, quantitative comparisons can be better attempted with reference to an internal control. As shown in Table 1, we compared the ratio of the RC form to the ss form intra- and extracellularly. The intracellular RC/ss DNA ratio in mutant F97L is about 1.6-fold lower than that in wild-type HBV. However, the extracellular RC/ss DNA ratio in mutant F97L is about 5.7-fold (3.8/0.67) lower than that of wild-type HBV. By comparing the intracellular and extracellular RC/ss DNA ratios in wild-type HBV, it appears that the RC form is generally enriched by 5.3-fold (3.8/0.71) in the secreted Dane particles. However, only minor enrichment for the RC form in Dane particles with mutation F97L was seen in all of the different experimental settings (0.67/0.44, 1.0/0.64, and 0.71/0.30) (Table 1). Similar ratios were obtained in different experimental settings (Table 1). These findings are consistent with the nonselective or less selective export of minus-strand DNA for mutant F97L virus.
DNase sensitivity and the structural integrity of intracellular core particles and extracellular Dane particles.
We consider it highly unlikely that the decreased level of the intracellular HBV DNA of mutant F97L is caused by the instability of core particles and the exogenous nuclease digestion during the viral DNA preparation (25, 31, 55), for the following reasons. (i) In complementation experiments with core AUG knockout mutants 1903 and 1903/F97L (Fig. 7), both mutants were rescued with the same wild-type core protein from the same SV40 expression vector. Thus, the integrity of the core particles in both cotransfections should be the same. Contrary to this prediction, we observed significant differences in both plus-strand and minus-strand DNA synthesis between mutant 1903 and mutant 1903/F97L. (ii) If the plus-strand and RC-form deficiency is caused by nuclease degradation, which should not discriminate between plus- and minus-strand DNAs, one would expect to have detected a significant amount of degraded plus-strand signal lower than the 1.5-kb region. Contrary to prediction, despite the abundant presence of the minus-strand DNA lower than the 1.5-kb region in mutant F97L, there is no significant plus-strand signal in this region (Fig. 2 and 5). (iii) A similar intracellular phenotype has been observed in an HBV RNase H mutant (15), where the core particle structure was unlikely to be affected.
One major difference between the hepadnavirus core gene insertion mutants described by Kock et al. (25); the deletion mutants described by Yu and Summers (55), Beames and Lanford (3), and Nassal (31); and our naturally occurring HBcAg missense mutant F97L is the degree of perturbation of the native structure of the core protein molecules and their assembled particles. The insertion and deletion mutations are generally considered to have more drastic effects, while the missense mutation in the case of mutant F97L is likely to be more subtle. In this regard, our results and the results of Kock et al. (25) are not necessarily contradictory.
When DNase treatment was not included in the preparation of extracellular Dane particles of mutant F97L, we observed reproducibly in several independent experiments the same phenotype of immature secretion in gradient centrifugation analysis (data not shown). Therefore, unlike the case for the class II core deletion-insertion mutants, immature secretion of the naturally occurring mutant F97L is not a phenotype dependent on DNase treatment (25).
Biological significance of viral attenuation with HBcAg codon 97 mutation.
Secretion of immature forms of woodchuck hepatitis virus DNA has been observed in one of three naturally infected woodchucks treated experimentally with acyclovir (46). It remains to be seen if the immature secretion phenotype of the HBV codon 97 mutant can be found in the sera of patients with chronic HBV infection. The fact that the codon 97 mutation is very often found in natural infection (Fig. 1) suggests that it is most likely to be infectious in vivo. It is also worth noting that codon 97 happens to fall within a potent T-cell epitope (23, 27, 29, 47), suggesting an immune escape nature of the codon 97 mutation. One major outcome of plus-strand deficiency and immature secretion is the attenuation of the total yield of mutant virus production. While the putative immune escape mutation could change the quality of core antigen, the pleiotropic effects of mutation F97L could provide the mutant virus a mechanism to control the quantity of all of the HBV-specific antigens. In summary, we hypothesize that codon 97 mutation, by lowering the quantity of antigen presentation, could dampen the immune response (9, 28), leading to a more successful maintenance of chronic infection in human HBV carriers.
ACKNOWLEDGMENTS
We thank colleagues in C. Shih’s laboratory for careful reading of the manuscript and J. Tsai for densitometer analysis. We also thank W. Gerlich for pre-S1 antibody, R. Lanford for anticore antibody, and J. Ou for pECE24.
W.E.W. was supported in part by the J. McLaughlin Predoctoral Fellowship and an NIH T32 Predoctoral Training Grant in Tropical and Emerging Infectious Diseases. C.S. was a recipient of an NIH Research Career Development Award when this work was initiated. The main part of this work was completed with support from NIH grant RO1-CA-70336 to C.S.
REFERENCES
- 1.Ackrill A M, Naoumov N V, Eddleston A L W F, Williams R. Specific deletions in the hepatitis B virus core open reading frame in patients with chronic active hepatitis B. J Med Virol. 1993;41:165–169. doi: 10.1002/jmv.1890410213. [DOI] [PubMed] [Google Scholar]
- 2.Akarca U S, Lok A S F. Naturally occurring hepatitis B virus core gene mutations. Hepatology. 1995;22:50–60. [PubMed] [Google Scholar]
- 3.Beames B, Lanford R E. Carboxy-terminal truncations of the HBV core protein affect capsid formation and the apparent size of the encapsidated HBV RNA. Virology. 1993;194:597–607. doi: 10.1006/viro.1993.1299. [DOI] [PubMed] [Google Scholar]
- 4.Birnbaum F, Nassal M. Hepatitis B virus nucleocapsid assembly: primary structure requirements in the core protein. J Virol. 1990;64:3319–3330. doi: 10.1128/jvi.64.7.3319-3330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozkaya H, Ayola B, Lok A S F. High rate of mutations in the hepatitis B core gene during the immune clearance phase of chronic hepatitis B virus infection. Hepatology. 1996;24:32–37. doi: 10.1002/hep.510240107. [DOI] [PubMed] [Google Scholar]
- 6.Bruss V, Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci USA. 1991;88:1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C, Jeng K S, Hu C P, Lo S J, Su T S, Ting L P, Chou C K, Han S H, Pfatt E, Salfeld J, Schaller H. Production of hepatitis B virus in vitro by transient expression of cloned HBV DNA in a hepatoma cell line. EMBO J. 1987;6:675–680. doi: 10.1002/j.1460-2075.1987.tb04807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D S. From hepatitis to hepatoma: lessons from type B viral hepatitis. Science. 1993;262:369–370. doi: 10.1126/science.8211155. [DOI] [PubMed] [Google Scholar]
- 9.Chisari F V, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 10.Chuang W L, Omata M, Ehata T, Yokosuka O, Ito Y, Ohto M. Concentrating missense mutations in core gene of hepatitis B virus. Dig Dis Sci. 1993;38:594–600. doi: 10.1007/BF01316786. [DOI] [PubMed] [Google Scholar]
- 11.Chuang W L, Omata M, Ehata T, Yokosuka O, Ito Y, Imazeki F, Lu S N, Chang W Y, Ohto M. Precore mutations and core clustering mutations in chronic hepatitis B virus infection. Gastroenterology. 1993;104:263–271. doi: 10.1016/0016-5085(93)90861-6. [DOI] [PubMed] [Google Scholar]
- 12.Ehata T, Omata M, Yokosuka O, Hosoda K, Ohto M. Variations in codons 84-101 in the core nucleotide sequence correlate with hepatocellular injury in chronic hepatitis B virus infection. J Clin Invest. 1992;89:332–338. doi: 10.1172/JCI115581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehata T, Omata M, Chuang W L, Yokosuka O, Ito Y, Hosoda K, Ohto M. Mutations in core nucleotide sequence of hepatitis B virus correlate with fulminant and severe hepatitis. J Clin Invest. 1993;91:1206–1213. doi: 10.1172/JCI116281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galibert F, Mandart E, Fitoussi F, Tiollais P, Charnay P. Nucleotide sequence of the hepatitis B virus genome cloned in E. coli. Nature. 1979;281:646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- 15.Gerelsaikhan T, Tavis J E, Bruss V. Hepatitis B virus nucleocapsid envelopment does not occur without genomic DNA synthesis. J Virol. 1996;70:4269–4274. doi: 10.1128/jvi.70.7.4269-4274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gotoh K, Mima S, Uchida T, Shikata T, Yoshizawa K, Irie M, Mizui M. Nucleotide sequence of hepatitis B virus isolated from subjects without serum anti-hepatitis B core antibody. J Med Virol. 1995;46:201–206. doi: 10.1002/jmv.1890460306. [DOI] [PubMed] [Google Scholar]
- 17.Gray A H, Fang J W S, Davis G L, Mizokami M, Wu P C, Williams R, Schuster S M, Lau J Y N. Variations of hepatitis B virus core gene sequence in Western patients with chronic hepatitis B virus infection. J Viral Hepatitis. 1997;4:371–378. doi: 10.1046/j.1365-2893.1997.00075.x. [DOI] [PubMed] [Google Scholar]
- 18.Gunther S, Baginski S, Kissel H, Reinke P, Kruger D H, Will H, Meisel H. Accumulation and persistence of hepatitis B virus core gene deletion mutants in renal transplant patients are associated with end-stage liver disease. Hepatology. 1996;24:751–758. doi: 10.1002/hep.510240401. [DOI] [PubMed] [Google Scholar]
- 19.Haton T, Zhou S, Standring D N. RNA- and DNA-binding activities in hepatitis B virus capsid protein: a model for their roles in viral replication. J Virol. 1992;66:5232–5241. doi: 10.1128/jvi.66.9.5232-5241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heermann K H, Goldmann U, Schwartz W, Seiffarth T, Baumgarten H, Gerlich W H. Large surface proteins of hepatitis B virus containing the pre-S sequence. J Virol. 1984;52:396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosono S, Tai P C, Wang W, Ambrose M, Hwang D, Yuan T T, Peng B H, Yang C S, Lee C S, Shih C. Core antigen mutations of human hepatitis B virus in hepatomas accumulate in MHC class II-restricted T cell epitopes. Virology. 1995;212:151–162. doi: 10.1006/viro.1995.1463. [DOI] [PubMed] [Google Scholar]
- 22.Hur G M, Lee Y I, Sun D J, Lee J H, Lee Y I. Gradual accumulation of mutations in precore/core region of HBV in patients with chronic active hepatitis: implication of clustering changes in a small region of the HBV core region. J Med Virol. 1996;48:38–46. doi: 10.1002/(SICI)1096-9071(199601)48:1<38::AID-JMV6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 23.Jung M C, Diepolder H M, Spengler U, Wierenga E A, Zachoval R, Zachoval R M, Hoffmann R M, Eichenlaub D, Frosner G, Will H, Pape G R. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD4+ T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J Virol. 1995;69:3358–3368. doi: 10.1128/jvi.69.6.3358-3368.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karasawa T, Shirasawa T, Okawa Y, Kuramoto A, Shimada N, Aizawa Y, Zeniya M, Toda G. Association between frequency of amino acid changes in core region of hepatitis B virus (HBV) and the presence of precore mutation in Japanese HBV carriers. J Gastroenterol. 1997;32:611–622. doi: 10.1007/BF02934110. [DOI] [PubMed] [Google Scholar]
- 25.Kock J, Wieland S, Blum H E, von Weizsacker F. Duck hepatitis B virus nucleocapsids formed by N-terminally extended or C-terminally truncated core proteins disintegrate during viral DNA maturation. J Virol. 1998;72:9116–9120. doi: 10.1128/jvi.72.11.9116-9120.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMillan J S, Bowden D S, Angus P W, McCaughan G W, Locarnini S A. Mutations in the hepatitis B virus precore/core and core promoter in patients with severe recurrent disease following liver transplantation. Hepatology. 1996;24:1371–1378. doi: 10.1002/hep.510240610. [DOI] [PubMed] [Google Scholar]
- 27.Menne S, Maschke J, Tolle T K, Lu M, Roggendorf M. Characterization of T cell response to woodchuck hepatitis core protein and protection of woodchucks from infection by immunization with peptides containing a T cell epitope. J Virol. 1997;71:65–74. doi: 10.1128/jvi.71.1.65-74.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milich D. Immune response to the hepatitis B virus: infection, animal models, vaccination. Viral Hepatitis. 1997;3:63–103. [Google Scholar]
- 29.Milich D R, McLachlan A, Moriarty A, Thornton G B. Immune response to hepatitis B virus core antigen (HBcAg): localization of T cell recognition sites within HBcAg/HBeAg. J Immunol. 1987;139:1223–1231. [PubMed] [Google Scholar]
- 30.Miska S, Gunther S, Vassilev M, Meisel H, Pape G, Will H. Heterogeneity of hepatitis B virus C-gene sequences: implications for amplification and sequencing. J Heptol. 1993;18:53–61. doi: 10.1016/s0168-8278(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 31.Nassal M. The arginine-rich domain of the hepatitis B virus core protein is required for pregenome encapsidation and productive viral positive-strand DNA synthesis but not for virus assembly. J Virol. 1992;66:4107–4116. doi: 10.1128/jvi.66.7.4107-4116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nassal M, Schaller H. Hepatitis B virus replication—an update. J Viral Hepatitis. 1996;3:217–226. doi: 10.1111/j.1365-2893.1996.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto H, Tsuda F, Mayumi M. Defective mutants of hepatitis B virus in the circulation of symptom-free carriers. Jpn J Exp Med. 1987;57:217–221. [PubMed] [Google Scholar]
- 34.Ou J-H, Rutter W J. Regulation of secretion of the hepatitis B virus major surface antigen by the pre-S1 protein. J Virol. 1987;61:782–786. doi: 10.1128/jvi.61.3.782-786.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollicino T, Campo S, Raimondo G. PreS and core gene heterogeneity in hepatitis B virus (HBV) genomes isolated from patients with long-lasting HBV chronic infection. Virology. 1995;208:672–677. doi: 10.1006/viro.1995.1198. [DOI] [PubMed] [Google Scholar]
- 36.Pollicino T S, Zanetti A R, Cacciola I, et al. Pre-S2 defective hepatitis B virus infection in patients with fulminant hepatitis. Hepatology. 1997;26:495–499. doi: 10.1002/hep.510260235. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Frias F, Buti M, Jardi R, Cotrina M, Viladomiu L, Esteban R, Guardia J. Hepatitis B virus infection: precore mutants and its relation to viral genotypes and core mutations. Hepatology. 1995;22:1641–1647. doi: 10.1002/hep.1840220605. [DOI] [PubMed] [Google Scholar]
- 38.Roychoudhury S, Faruqi A, Shih C. Pregenomic RNA encapsidation analysis of eleven missense and nonsense polymerase mutants of human hepatitis B virus. J Virol. 1991;65:3617–3624. doi: 10.1128/jvi.65.7.3617-3624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sells M A, Chen M L, Acs G. Production of hepatitis B virus particles in HepG2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci USA. 1987;84:1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shafritz D A. Variants of hepatitis B virus associated with fulminant liver disease. N Engl J Med. 1991;324:1737–1739. doi: 10.1056/NEJM199106133242411. [DOI] [PubMed] [Google Scholar]
- 41.Shih C, Li L S, Roychoudhury S, Ho M H. In vitro propagation of human hepatitis B virus in a rat hepatoma cell line. Proc Natl Acad Sci USA. 1989;86:6323–6327. doi: 10.1073/pnas.86.16.6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shih C, Tai P-C, Whitehead W, Hosono S, Lee C-S, Yang C-S. Hepatitis B and C viruses and liver cancer. In: Bertino J R, editor. Encyclopedia of cancer. II. New York, N.Y: Academic Press, Inc.; 1996. pp. 824–834. [Google Scholar]
- 43.Slagle B L, Lee T H, Butel J S. Hepatitis B virus and hepatocellular carcinoma. Prog Med Virol. 1992;39:167. [PubMed] [Google Scholar]
- 44.Summers J, Mason W S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 45.Sureau C, Romet-Lemonne J-L, Mullins J I, Essex M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell. 1986;47:37–47. doi: 10.1016/0092-8674(86)90364-8. [DOI] [PubMed] [Google Scholar]
- 46.Tencza M G, Newbold J E. Heterogeneous response for a mammalian hepadnavirus infection to acyclovir: drug-arrested intermediates of minus-strand viral DNA synthesis are enveloped and secreted from infected cells as virion-like particles. J Med Virol. 1997;51:6–16. [PubMed] [Google Scholar]
- 47.Tsai S L, Chen M H, Yeh C T, Chu C M, Lin A N, Chiou F H, Chang T H, Liaw Y F. Purification and characterization of a naturally processed hepatitis B virus peptide recognized by CD8 cytotoxic T lymphocytes. J Clin Invest. 1996;97:577–584. doi: 10.1172/JCI118450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuttleman J, Pugh J C, Summers J. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986;58:17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuttleman J, Pourcel C, Summers J. Formation of the pool of covalently closed viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 50.Uchida T, Aye T T, Becher S O, Hirashima M, Shikata T, Komine F, Moriyama M, Arakawa Y, Takase S, Mima S. Detection of precore/core-mutant hepatitis B virus genome in patients with acute or fulminant hepatitis without serological markers for recent HBV infection. J Hepatol. 1993;18:369–372. doi: 10.1016/s0168-8278(05)80283-1. [DOI] [PubMed] [Google Scholar]
- 51.Uchida T, Aye T T, Shihata T, Yano M, Yatsuhashi H, Koga M, Mima S. Evolution of the hepatitis B virus gene during chronic infection in seven patients. J Med Virol. 1994;43:148–154. doi: 10.1002/jmv.1890430209. [DOI] [PubMed] [Google Scholar]
- 52.Valliammai T, Thyagarajan S P, Zuckerman A J, Harrison T J. Precore and core mutations in HBV from individuals in India with chronic infection. J Med Virol. 1995;45:321–325. doi: 10.1002/jmv.1890450315. [DOI] [PubMed] [Google Scholar]
- 53.Wakita T, Kakumu S, Shibata M, Yoshioka K, Ito Y, Shinagawa T, Ishikawa T, Takayanagl M, Morishima T. Detection of pre-C and core region mutants of hepatitis B virus in chronic hepatitis B virus carriers. J Clin Invest. 1991;88:1793–1801. doi: 10.1172/JCI115500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei Y, Tavis E, Ganem D. Relationship between viral DNA synthesis and virion envelopment in hepatitis B viruses. J Virol. 1996;70:6455–6458. doi: 10.1128/jvi.70.9.6455-6458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu M, Summers J. A domain of the hepadnavirus capsid protein is specifically required for DNA maturation and virus assembly. J Virol. 1991;65:2511–2517. doi: 10.1128/jvi.65.5.2511-2517.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan, T.-T., and C. Shih. Unpublished results.
- 57.Yuan T T, Faruqi A, Shih J W K, Shih C. The mechanism of natural occurrence of two closely-linked HBV precore predominant mutations. Virology. 1995;211:144–156. doi: 10.1006/viro.1995.1387. [DOI] [PubMed] [Google Scholar]
- 58.Yuan T-T, Lin M H, Qiu S M, Shih C. Functional characterization of naturally occurring variants of human hepatitis B virus containing the core internal deletion mutation. J Virol. 1998;72:2168–2176. doi: 10.1128/jvi.72.3.2168-2176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan T-T, Lin M H, Chen D S, Shih C. A defective interference-like phenomenon of human hepatitis B virus in chronic carriers. J Virol. 1998;72:578–584. doi: 10.1128/jvi.72.1.578-584.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]