Abstract
Introduction
Traditional methods for assessing movement quality rely on subjective standardized scales and clinical expertise. This limitation creates challenges for assessing patients with spinocerebellar ataxia (SCA), in whom changes in mobility can be subtle and varied. We hypothesized that a machine learning analytic system might complement traditional clinician-rated measures of gait. Our objective was to use a video-based assessment of gait dispersion to compare the effects of troriluzole with placebo on gait quality in adults with SCA.
Methods
Participants with SCA underwent gait assessment in a phase 3, double-blind, placebo-controlled trial of troriluzole (NCT03701399). Videos were processed through a deep learning pose extraction algorithm, followed by the estimation of a novel gait stability measure, the Pose Dispersion Index, quantifying the frame-by-frame symmetry, balance, and stability during natural and tandem walk tasks. The effects of troriluzole treatment were assessed in mixed linear models, participant-level grouping, and treatment group-by-visit week interaction adjusted for age, sex, baseline modified Functional Scale for the Assessment and Rating of Ataxia (f-SARA), and time since diagnosis.
Results
From 218 randomized participants, 67 and 56 participants had interpretable videos of a tandem and natural walk attempt, respectively. At Week 48, individuals assigned to troriluzole exhibited significant (p = 0.010) improvement in tandem walk Pose Dispersion Index versus placebo {adjusted interaction coefficient: 0.584 [95% confidence interval (CI) 0.137 to 1.031]}. A similar, nonsignificant trend was observed in the natural walk assessment [coefficient: 1.198 (95% CI − 1.067 to 3.462)]. Further, lower baseline Pose Dispersion Index during the natural walk was significantly (p = 0.041) associated with a higher risk of subsequent falls [adjusted Poisson coefficient: − 0.356 [95% CI − 0.697 to − 0.014)].
Conclusion
Using this novel approach, troriluzole-treated subjects demonstrated improvement in gait as compared to placebo for the tandem walk. Machine learning applied to video-captured gait parameters can complement clinician-reported motor assessment in adults with SCA. The Pose Dispersion Index may enhance assessment in future research.
Trial Registration—ClinicalTrials.gov Identifier
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-024-00625-6.
Keywords: Troriluzole, Spinocerebellar ataxia, Kinematic analysis, Video, Artificial intelligence, Pose dispersion index
Key Summary Points
| This post hoc analysis compared the efficacy of troriluzole versus placebo in adults with SCA who participated in a Phase 3, long-term, randomized, double-blind, placebo-controlled study designed to assess the safety, tolerability, and efficacy of troriluzole 200 mg once per day for 48 weeks in adults with SCA (NCT03701399). |
| Investigators used a novel approach to the assessment of treatment effects—the Pose Dispersion Index—a video-based kinematic analysis of frame-to-frame variation in human motion based on quantitative analysis of upper and lower ambulation that represents intra-individual differences in gait asymmetry and base support. |
| The primary outcome measure was the treatment effect of troriluzole versus placebo on the Pose Dispersion Index at 48 weeks during a tandem walk task. |
| At Week 48, individuals treated with troriluzole exhibited significant improvement versus placebo on the tandem walk Pose Dispersion Index (p = 0.010); a similar but nonsignificant trend was observed in the analysis of paired videos in the natural walk assessment. |
| Pose Dispersion Index scores at baseline for the natural walk were negatively associated (p = 0.041) with frequency of subsequent falls. |
Digital Features
This article is published with digital features, including four videos to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.25663299.
Introduction
Spinocerebellar ataxias (SCAs) are autosomal-dominant, neurodegenerative diseases characterized principally by Purkinje cell death and cerebellar atrophy associated with reduced uptake of extracellular glutamate, mitochondrial dysfunction, transcriptional dysregulation, and channelopathies; at least 48 genetically distinct subtypes are recognized [1–6]. The symptoms of SCA include impaired balance and motor coordination, postural or kinetic tremor, incoordination of limbs, cognitive impairment, dysarthria/dysphagia, and oculomotor dysfunction [7]. The changes in gait may include an increase in step width, greater step dispersion, abnormal trunk oscillations, uncoordinated walking patterns, irregular foot trajectories, and reduced stability, resulting in a lack of smooth cadence [8–12]. Poor gait performance is associated with a greater risk of falling [13–18]. An estimated 84% of patients with SCA fall at least once per year [18], and most (≥ 54%) have experienced fall-related injuries [16, 18]. Factors associated with a higher fall frequency in patients with SCA include disease duration, severity of ataxia, the presence or absence of pyramidal symptoms, the number of non-ataxia symptoms, and the SCA genotype; gait performance can be involved in a number of these factors [16].
Since subtle changes of gait may be among the earliest motor symptoms among individuals with SCA [19], early assessment of changes in gait movement can be an important determinant of disease progression. However, traditional assessments rely solely on clinician observations, which are subjective and susceptible to error [20, 21]; these also capture incremental assessments, which are designed to cover the continuum of disease, not to detect these smallest changes. Human pose estimation—i.e., the calculation of human body keypoint coordinates based on recorded images—can be used to supplement traditional clinician-rated measures [22–24]. This innovation was enabled by the introduction of deep artificial intelligence (AI) machine learning and the evolution of convolutional neural networks and novel model architectures. This technology is readily applicable to videos of human motion and has enabled motion capture based on human pose estimation algorithms absent the need for wearable devices. This technology is deemed to be comparable to marker-based methods in the assessment of symptoms, functional ability, and response to therapeutic interventions [21].
Treatment of patients with SCA is challenging. Although high-intensity motor training (e.g., physiotherapy and kinesitherapy) may improve postural stability and reduce dependence on walking aids [25], clinical management is typically limited to supportive measures. No medications to date have demonstrated effects on disease progression. Previous research suggests that riluzole, a benzothiazole derivative with neuroprotective properties, may be associated with improvements in functional ability in patients with SCA [26, 27], but its benefits are limited by inconsistent exposure, poor solubility, food effects, and serum aminotransferase elevations [28–31].
Troriluzole is a novel, third-generation tripeptide prodrug of riluzole that has been optimized for improved bioavailability, pharmacokinetics, safety, and dosing. Following oral administration, troriluzole is rapidly absorbed and converted to riluzole, providing high bioavailability, consistent exposure, improved liver safety, once-daily dosing, and no food effect [32], suggesting enhanced clinical benefits compared with riluzole. A previous Phase 3, multicenter, randomized, double-blind, two-arm, placebo-controlled parallel-group study in individuals with SCA (NCT03701399) showed potential for slowing disease progression in adults with SCA using the Scale for the Assessment and Rating of Ataxia (SARA), a clinician-rated assessment of movement [33]. The objective of this study was to compare the effects of troriluzole versus placebo on gait quality by applying a series of deep learning-derived video-based kinematic metrics of frame-to-frame human pose and motion variability, as measured by the Pose Dispersion Index during tandem and natural (normal) walk tasks.
Methods
Ethical Approval
This study was conducted in accordance with Good Clinical Practice and the Helsinki Declaration of 1964, the ethical principles of European Union Directive 2001/20/EC, and the United States Code of Federal Regulations, Title 21, Part 50 (21CFR50). The study was approved by all participating institutions. The main ethics committee for the study was Advarra IRB of 6940 Columbia Gateway Drive, Suite 110, Columbia, MD 21046, USA. Full details of participating institutions and the ethics committees or institutional review boards which approved the study are provided in the supplementary materials. Participants in the original clinical trial signed informed consent, which included information that the trial results might be published; for this post hoc analysis, no separate informed consent was obtained.
Study Conduct
This study was a post hoc analysis of data collected from a Phase 3, multicenter, randomized, double-blind, two-arm, placebo-controlled parallel-group study designed to assess the safety, tolerability, and efficacy of troriluzole in individuals with SCA (NCT03701399). The study included 2 phases: a 48-week randomization phase and a 48-week open-label extension phase. During the randomization phase, participants received troriluzole (140 mg/day) or placebo for 4 weeks and then titrated up to 200 mg/day for the remaining 44 weeks. Participants who agreed to enter the extension phase continued open-label troriluzole 200 mg for up to 96 weeks.
Participants underwent rater assessment on the modified Functional Scale for the Assessment and Rating of Ataxia (f-SARA) at screening, baseline, and through 48 weeks after randomization. Per protocol, participants were evaluated on natural walk and tandem walk tasks based on abilities. For the natural walk task, participants were asked to walk 10 m, followed by a half turn, without pausing, and then return to the starting point. Participants who completed the natural walk task without assistance then underwent the tandem walk task, for which they were asked to complete 10 additional steps with both feet in one line and with no spaces between heel and toe. Although the tasks were performed parallel to the wall and at a distance that enabled participants to use the wall for intermittent support, participants were encouraged to use assistance only when required for safety. The gait tasks were not timed, and participants were instructed to walk at a comfortable pace. Participants were asked to use a similar type of shoe across all exams and to perform the assessment on the same flooring. For the tandem walk assessment, gait scores were based on the initial 10-step gait attempt; more than 1 misstep was scored as a failure.
Videos were filmed of both the natural and tandem walk tasks. The primary planned purpose of recording videos was to enable central adjudication, if required, of scoring the f-SARA gait item. It was not mandated that all subjects consent to being filmed during performance of the gait tasks; 3 of 23 study sites did not collect videos either because local regulations did not permit this (2 sites) or because study participants did not consent to being filmed (1 site).
Participants
Adults aged 18–75 years with a known or suspected diagnosis of SCA 1, 2, 3, 6, 7, 8, or 10 were eligible to participate in Study NCT03701399. Participants had to be able to ambulate 8 m without assistance (canes and other devices were allowed). They also had to have a total score on the f-SARA of at least 3, and a score of at least 1 on the gait subsection of the f-SARA. Individuals with a score of 4 on any individual item of the f-SARA were excluded as were those with Mini-Mental State Examination [34] score less than 24. Full inclusion and exclusion criteria are described elsewhere (https://classic.clinicaltrials.gov/ct2/show/NCT03701399).
Participants in the post hoc analysis were: (1) randomized to troriluzole or placebo; (2) participated in the gait assessment of the ability to complete the natural walk or tandem walk; (3) took at least 1 dose of study medication during the randomization double-blind phase; and (4) provided a non-missing baseline measurement and a non-missing post-baseline efficacy assessment at 48 weeks (or early termination) post-randomization. Eligible participants also had paired videos at screening (Week 0/pre-randomization) and at the last follow-up visit (Week 48 or early termination) post-randomization for the natural or tandem walk that met quality control standards. A convenience sample of patients was used for this analysis based on those sites with readily accessible recordings at the date when the analysis was undertaken, and videos which met quality parameters (patient close enough to the camera enabling reliable extraction of body parts, and stable alignment between camera and gait direction).
Assessments
The f-SARA is a modified version of the standard SARA which was developed to provide semi-quantitative scoring of abnormalities of motor control in cerebellar ataxias [33]. The SARA assesses ataxia severity and disease progression via evaluation of 8 items concerning gait, stance, sitting, speech, and upper and lower limb coordination. The f-SARA was designed to create new response categories that reflect clinically meaningful changes in function and includes the SARA items of gait, stance, sitting, and speech disturbance (axial items 1–4). The gait item of the f-SARA was rated as follows (Figure S1): normal (0: no impairment in walking, turning, and tandem walk); mildly impaired (1: abnormal gait, but no assistance needed with walking and/or fails tandem walk); moderately impaired [2: staggers, but walks independently (intermittent use of wall, examiner’s arm, or cane)]; severely impaired [3: dependent upon assistance (requires constant assistance from an accompanying person or uses walker)]; unable to perform the task (4: unable to walk, even supported). Trained, certified (re-certified at 6 months) observers instructed participants on the specified tasks and rated their abilities according to the specifications of each item.
Video-Based Kinematic Assessment
The original videos were taken in a standardized manner of participants performing the tandem and natural work tasks using pre-programmed camcorders. Video operators stationed cameras directly in front of participants, and the video-based metrics were evaluated until participants performed the half-turn. Videos were processed through a deep neural network architecture that included a multi-stage classifier specifically trained for person pose estimation [35] trained against the normative 40,000 MPII Human Pose dataset [36, 37] that tracked coordinates of key body segments, followed by the computation of interpretable kinematic features reflecting dynamic pose dispersion. For this purpose, an integrated Pose Dispersion Index was estimated, which characterizes gait quality through time and reflects a dynamic global assessment of pose symmetry, balance, and stability during natural and tandem walk tasks based on the continuous data captured in the videos. The Pose Dispersion Index tracks the movement of key body parts (e.g., right and left feet and chest) and the relationship between them over time, and the dynamic symmetry of the individual’s body pose indexed to their base of support (distance between right and left feet). Higher Pose Dispersion Index values indicate better gait stability or a more consistent change in pose and motion across time. For details about the methods used for the video assessments and for calculation of the Pose Dispersion Index, please refer to the Supplementary material.
Endpoints
For the post hoc analysis, the primary endpoint was Pose Dispersion Index values at Week 48 versus screening/Week 0 during a tandem walk task. The secondary endpoint was Pose Dispersion Index values at Week 48 versus screening/Week 0 during a natural walk task. The exploratory endpoint was the relationship between the Pose Dispersion Index at screening and the total number (count) of falls recorded during the randomization period.
Statistical Methods
Categorical variables were tabulated with counts and percentages. Continuous variables were summarized with univariate statistics [e.g., n, mean, standard deviation (SD), standard error (SE), median, 25th–75th percentile] as appropriate. As the sample size for this post hoc analysis was determined by the availability of video assessments at the time the analysis was undertaken, no power calculations were performed. For the primary outcome measure, the effects of troriluzole versus placebo on the Pose Dispersion Index at 48 weeks from screening were assessed using a standard mixed linear model using fixed effect factors, with grouping at the level of each participant, for the following: treatment group, visit week (screening vs. week 48), treatment group-by-visit week interaction, age, sex, baseline f-SARA score, and time since diagnosis as covariates. Coefficients were reported with associated 95% confidence intervals and p values. A similar analysis was performed for the secondary outcome measure. In the exploratory analysis, the association between the Pose Dispersion Index at screening/Week 0 and the total number of subsequent falls was assessed using Poisson regression models with the total number of falls during randomization as the dependent variable, the previously mentioned covariates as the independent variables in the model, and an offset term reflecting each participant’s time of follow-up. Pairwise, unadjusted comparisons across the 2 timepoints (Weeks 0 and 48) were performed using paired t tests. Unadjusted comparisons between 2 unrelated groups were performed using, as appropriate, a Chi-square test (for categorical variables), unpaired t test, or Mann–Whitney U test. A 2-sided alpha level of 0.05 was used, unless specified otherwise. Analyses were performed using Python 3.8 in Visual Studio Code Version: 1.75.1 and based on observed data unless specified otherwise.
Results
Participants
Of the 218 participants in the overall sample, 99 (45.4%) participants provided at least one video of a tandem walk and 67 (30.7%) participants had videos of a tandem walk attempt at Week 0 and Week 48, all of which met quality standards to be included in the analysis. A total of 121 (55.5%) participants provided at least one natural walk video, and 104 (47.7%) participants had natural walk videos at both Week 0 and Week 48, of which videos from 56 (25.7%) participants met quality control. There was some overlap between these samples, with 23 participants contributing video assessments of both natural and tandem walks to the analysis. Demographic and baseline characteristics of video-assessed participants were similar to those in the overall sample enrolled in the overall study (Table 1), except that gait item scores were lower. Furthermore, general equipoise in demographics, f-SARA total, and gait scores, and baseline Pose Dispersion Index was observed between participants in the troriluzole and placebo groups on the tandem and natural walk tasks (Table 2). The median time since diagnosis differed between treatment groups for participants included in the video-based tandem walk analysis.
Table 1.
Characteristics of participants in the overall sample and the post hoc analysis
| Tandem walk | Natural walk | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall n = 218 |
Excludeda n = 151 |
Included (paired videos) n = 67 |
p value | Overall n = 218 |
Excludeda n = 162 |
Included (paired videos) n = 56 |
p value | |
| Treatment group, n (%) | ||||||||
| Placebo | 109 (50.0) | 73 (48.3) | 36 (53.7) | 0.557 | 109 (50.0) | 82 (50.6) | 27 (48.2) | 0.877 |
| Troriluzole | 109 (50.0) | 78 (51.7) | 31 (46.3) | 109 (50.0) | 80 (49.4) | 29 (51.8) | ||
| Age, mean (SD) | 47.6 (12.8) | 48.3 (13.6) | 45.9 (10.7) | 0.157 | 47.6 (12.8) | 47.7 (12.7) | 47.3 (13.3) | 0.864 |
| Sex, n (%) | ||||||||
| Women | 112 (51.4) | 78 (51.7) | 34 (50.7) | 1.000 | 112 (51.4) | 93 (57.4) | 19 (33.9) | 0.004 |
| Men | 106 (48.6) | 73 (48.3) | 33 (49.3) | 106 (48.6) | 69 (42.6) | 37 (66.1) | ||
| Known genotype, n (%) | ||||||||
| SCA1 | 26 (12.0) | 22 (14.7) | 4 (6.0) | 0.512 | 26 (12.0) | 15 (9.3) | 11 (19.6) | 0.061 |
| SCA2 | 67 (30.9) | 44 (29.3) | 23 (34.3) | 67 (30.9) | 53 (32.9) | 14 (25.0) | ||
| SCA3 | 89 (41.0) | 59 (39.3) | 30 (44.8) | 89 (41.0) | 62 (38.5) | 27 (48.2) | ||
| SCA6 | 12 (5.5) | 7 (4.7) | 5 (7.5) | 12 (5.5) | 11 (6.8) | 1 (1.8) | ||
| SCA7 | 10 (4.6) | 8 (5.3) | 2 (3.0) | 10 (4.6) | 9 (5.6) | 1 (1.8) | ||
| SCA8 | 5 (2.3) | 4 (2.7) | 1 (1.5) | 5 (2.3) | 3 (1.9) | 2 (3.6) | ||
| SCA10 | 8 (3.7) | 6 (4.0) | 2 (3.0) | 8 (3.7) | 8 (5.0) | 0 | ||
| Baseline f-SARA | ||||||||
| Score, median (IQR) | 4.0 (4.0, 6.0) | 5.0 (4.0, 7.0) | 4.0 (3.0, 4.5) | < 0.001 | 4.0 (4.0, 6.0) | 4.0 (4.0, 6.0) | 5.0 (4.0, 6.2) | 0.065 |
| Gait item, median (IQR) | 1.0 (1.0, 2.0] | 1.0 (1.0, 2.0) | 1.0 (1.0, 1.0) | < 0.001 | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 0.015 |
| Years since SCA diagnosis, median (IQR) | 3.6 (1.2, 8.1) | 4.0 (1.4, 8.2) | 2.7 (0.8, 5.9) | 0.123 | 3.6 (1.2, 8.1) | 3.4 (1.1, 8.0) | 4.7 (1.3, 8.1) | 0.373 |
SD standard deviation, IQR interquartile range
aVideos not conveniently available, unpaired or not meeting quality control
Table 2.
Characteristics of participants in the troriluzole and placebo treatment groups
| Tandem walk | Natural walk | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall n = 67 |
Placebo n = 36 |
Troriluzole n = 31 |
p value | Overall n = 56 |
Placebo n = 27 |
Troriluzole n = 29 | p value | |
| Age, mean (SD) | 45.9 (10.7) | 44.5 (11.6) | 47.5 (9.4) | 0.258 | 47.3 (13.3) | 47.6 (14.0) | 47.0 (12.8) | 0.877 |
| Sex, n (%) | ||||||||
| Women | 34 (50.7) | 18 (50.0) | 16 (51.6) | 1.000 | 37 (66.1) | 18 (66.7) | 19 (65.5) | 1.000 |
| Men | 33 (49.3) | 18 (50.0) | 15 (48.4) | 19 (33.9) | 9 (33.3) | 10 (34.5) | ||
| Known genotype | ||||||||
| SCA1 | 4 (6.0) | 1 (2.8) | 3 (9.7) | 0.551 | 11 (19.6) | 4 (14.8) | 7 (24.1) | 0.559 |
| SCA2 | 23 (34.3) | 14 (38.9) | 9 (29.0) | 14 (25.0) | 9 (33.3) | 5 (17.2) | ||
| SCA3 | 30 (44.8) | 15 (41.7) | 15 (48.4) | 27 (48.2) | 13 (48.1) | 14 (48.3) | ||
| SCA6 | 5 (7.5) | 3 (8.3) | 2 (6.5) | 1 (1.8) | 0 | 1 (3.4) | ||
| SCA7 | 2 (3.0) | 2 (5.6) | 0 | 1 (1.8) | 0 | 1 (3.4) | ||
| SCA8 | 1 (1.5) | 0 | 1 (3.2) | 2 (3.6) | 1 (3.7) | 1 (3.4) | ||
| SCA10 | 2 (3.0) | 1 (2.8) | 1 (3.2) | 0 | 0 | 0 | ||
| Baseline f-SARA | ||||||||
| Score, median (IQR) | 4.0 (3.0, 4.5) | 4.0 (3.0, 4.2) | 4.0 (3.0, 4.5) | 0.473 | 5.0 (4.0, 6.2) | 5.0 (4.0, 6.0) | 5.0 (4.0, 7.0) | 0.739 |
| Gait item, median (IQR) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 0.127 | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 3.0) | 0.724 |
| Years since SCA diagnosis, median (IQR) | 2.7 (0.8, 5.9) | 1.7 (0.6, 4.3) | 3.6 (1.4, 9.0) | 0.040 | 4.7 (1.3, 8.1) | 4.0 (0.6, 7.7) | 5.0 (2.7, 9.2) | 0.213 |
| Pose Dispersion Index | 1.0 (0.6, 1.5) | 1.3 (0.6, 1.5) | 0.8 (0.6, 1.4) | 0.352 | 3.0 (1.6, 4.2) | 2.7 (1.6, 4.1) | 3.3 (2.0, 4.5) | 0.408 |
Efficacy
Change in Pose Dispersion Index
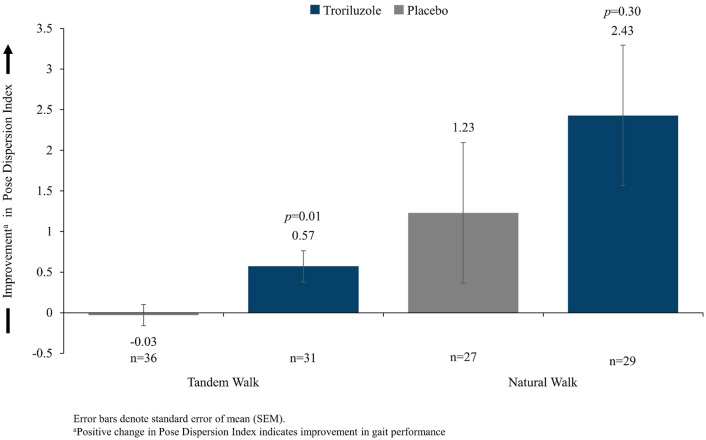
Participants who received troriluzole experienced a significant increase (p = 0.010) in Pose Dispersion Index values on the tandem walk that was independent of age, sex, baseline f-SARA score, and/or years since diagnosis (Table 3; Fig. 1), indicating an improvement in gait performance. For the natural walk task, participants who were treated with troriluzole experienced numerically greater improvement (p = 0.300) in Pose Dispersion Index values than those who were treated with placebo (Table 3; Fig. 1).
Table 3.
Adjusted mixed linear model of Pose Dispersion Index during a tandem walk task and natural walk task
| Coefficient | SE | z | P >|z| | 95% CI | |
|---|---|---|---|---|---|
| Tandem walk | |||||
| Intercept | 0.752 | 0.395 | 1.902 | 0.057 | − 0.023, 1.526 |
| Arm (troriluzole or placebo) | − 0.092 | 0.172 | − 0.539 | 0.590 | − 0.429, 0.244 |
| Visit (Week 0 or Week 48) | − 0.022 | 0.155 | − 0.140 | 0.889 | − 0.326, 0.282 |
| Arm × visit interaction | 0.584 | 0.228 | 2.561 | 0.010 | 0.137, 1.031 |
| Sex | 0.208 | 0.135 | 1.539 | 0.124 | − 0.057, 0.473 |
| Baseline f-SARA | 0.039 | 0.057 | 0.691 | 0.490 | − 0.072, 0.151 |
| Age (years) | 0.004 | 0.006 | 0.633 | 0.527 | − 0.008, 0.016 |
| Years since diagnosis | − 0.016 | 0.012 | − 1.309 | 0.190 | − 0.040, 0.008 |
| Individual participant | 0.041 | 0.099 | – | – | – |
| Natural walk | |||||
| Intercept | 0.197 | 1.533 | 0.129 | 0.898 | − 2.807, 3.202 |
| Arm (troriluzole or placebo) | 0.345 | 0.824 | 0.419 | 0.676 | − 1.270, 1.960 |
| Visit (Week 0 or Week 48) | 2.425 | 0.802 | 3.023 | 0.002 | 0.853, 3.997 |
| Arm × visit interaction | 1.198 | 1.155 | 1.037 | 0.300 | − 1.067, 3.462 |
| Sex | 0.045 | 0.615 | 0.073 | 0.942 | − 1.160, 1.249 |
| Baseline f-SARA | 0.307 | 0.162 | 1.896 | 0.058 | − 0.010, 0.625 |
| Age (years) | 0.045 | 0.022 | 2.008 | 0.045 | 0.001, 0.089 |
| Years since diagnosis | − 0.104 | 0.064 | − 1.613 | 0.107 | − 0.230, 0.022 |
| Individual participant | 0.000 | 0.501 | – | – | – |
SE standard error, CI confidence interval
Fig. 1.
Change in the Pose Dispersion Index during a tandem walk task and a natural walk task at week 48
Results for individual participants on the tandem walk task and the natural walk task are presented in the supplementary material (Figure S2).
One sample pair of individual participant videos, processed for human pose estimation, is included in the supplementary material for each of the natural and tandem walk tasks.
Video 1: Participant A Natural Walk at Screening (MP4 7821 KB)
Video 2: Participant A Natural Walk at Week 48 (MP4 7693 KB)
Video 3: Participant B Tandem Walk at Screening (MP4 8335 KB)
Video 4: Participant B Tandem Walk at Week 48 (MP4 7523 KB)
Pose Dispersion Index and Number of Falls
In adjusted Poisson regression models (Table 4), lower Pose Dispersion Index values (greater variability in gait) at baseline were associated with a higher risk of subsequent falls during randomization. This was statistically significant for the natural walk-derived Pose Dispersion Index (p = 0.041) but not for the tandem walk-derived Pose Dispersion Index (p = 0.231). These associations were independent of age, sex, baseline f-SARA scores, time since diagnosis, and treatment group.
Table 4.
Adjusted Poisson Regression Modelsa of Fall Counts During Weeks 0 through 48
| Coefficient | SE | z | P >|z| | 95% CI | |
|---|---|---|---|---|---|
|
Natural walk Pose Dispersion Index (per unit increase) |
− 0.356 | 0.174 | − 2.039 | 0.041 | − 0.697, − 0.014 |
|
Tandem walk Pose Dispersion Index (per unit increase) |
− 0.744 | 0.622 | − 1.197 | 0.231 | − 1.963, 0.475 |
SE standard error, CI confidence interval
aAll models further adjusted for age, sex, baseline f-SARA score, time since diagnosis, and treatment arm with offset term for each participant’s follow-up time
Discussion
This was a post hoc analysis of data from a Phase 3, multicenter, randomized, double-blind, two-arm, placebo-controlled parallel-group study designed to assess the safety, tolerability, and efficacy of troriluzole in adults with SCA. The analysis compared the effects of troriluzole with placebo on gait quality using a series of deep learning-derived video-based kinematic metrics and a novel endpoint, the Pose Dispersion Index, and explored the association between Pose Dispersion Index scores at screening and the total number of falls during the 48-week follow-up period. The Pose Dispersion Index represents a dynamic global assessment of gait quality over time, and in the current application is relevant to those ataxias particularly impacting performance of gait, resulting in unwieldy and uncoordinated movements of the trunk, shoulders, and limbs.
To our knowledge, this represents the first assessment of the treatment effects of a medication on gait using a video-based AI measurement. On the primary endpoint using this measure, the effects of troriluzole versus placebo on the Pose Dispersion Index at 48 weeks, participants treated with troriluzole exhibited significant improvement in tandem walk relative to placebo.
The analysis of paired videos in the natural walk assessment yielded a trend of improvement among participants in the troriluzole treatment group. Although the Pose Dispersion Index at screening was negatively associated with the frequency of falls [i.e., a more stable gait at baseline (higher Pose Dispersion Index value) was associated with fewer subsequent falls], troriluzole had no effect on the experience of falls.
Acknowledging the post hoc nature of the analysis in a reduced sample, these findings suggest that troriluzole treatment confers improvement in cerebellar and motor function, as defined by the machine learning video-assessed metrics captured in the Pose Dispersion Index.
A higher Pose Dispersion Index at baseline was associated with a lower frequency of subsequent falls for both the natural and tandem walk assessments, an effect which reached significance for the natural walk. The magnitude of reduction in the number of falls associated with each unit increase in the Pose Dispersion Index was greater for the tandem walk task; however, the greater variance in the data for the tandem walk impacted the test for statistical significance which is sensitive to variance. Furthermore, to attempt the tandem walk, study participants had to have first successfully completed the natural walk. Accordingly, those study participants who performed the tandem walk may have been at lower risk of falls compared to the overall study population.
There are strengths and limitations to the analysis. Strengths include a study sample for the video-based gait assessments that was demographically and clinically representative of the total study population at screening, and which demonstrated general equipoise between the troriluzole and placebo treatment groups for the tandem walk and natural walk assessments. The use of marker-less motion capture methods and the Pose Dispersion Index introduces a novel method of patient assessment which complements traditional approaches to movement analysis [21], which involve patient self-reports, clinician observations, and visually assessed rating scales. This new method can provide insights into smaller granular changes in gait which potentially translate into clinically meaningful differences for patients. The tandem walk is given less emphasis in conventional ataxia assessments, as both natural and tandem gait are considered together in clinical scales. Accordingly, in our study, the specific application of the Pose Dispersion Index to the tandem walk enables a more intensive investigation of tandem walk performance.
An intrinsic limitation to this study is that it was a post hoc analysis on a relatively small ‘convenience’ sample of available videos which also met quality standards, representing 25.7% and 30.7% of study participants for the natural walk and tandem walk, respectively. This reflects that the videos were initially planned to enable central adjudication of the clinical assessment of the f-SARA gait item, and, accordingly, many videos did not meet the quality metrics needed to support the current analysis. Future studies for which the estimation of the Pose Dispersion Index is a pre-specified analysis will enable the availability of a higher proportion of videos which meet quality control. Video gait assessments were only available for screening and Week 48 study visits; accordingly, patterns of change in Pose Dispersion Index throughout the time course of the double-blind study period could not be analyzed. For technical reasons, only the first part of the natural walk task until the half-turn was included in the video-based gait analysis. Being located further away from the camera, as the study participant begins to walk back, the spatial resolution does not permit sensitive detection of body parts to ensure the reliable detection of the pose, body part location, and their inter-relationship. The turning motion also affects the metrics of the analysis: for example, the arm which was previously on the left side crosses to the right side of the screen, rendering the measurements uninterpretable when the direction of movement changes. Inclusion of just the first part of the natural walk for the video-based analysis of gait introduces a potential bias to the analysis as less data are used, compared to the full observation of the participant’s gait by the clinical assessor.
Only those study participants who completed the natural walk task were asked to perform the tandem walk. Accordingly, those participants doing the tandem walk may have been less severely affected by SCA, with a greater propensity to benefit from active treatment, thus impacting the likelihood of a significant treatment effect for the tandem walk. Inclusion criteria for the Phase 3 trial included that participants have an f-SARA score of at least 3 points, a score of at least 1 point on the gait item, and that none of the individual f-SARA items have a score of 4 points. While such inclusion criteria are suitable in the setting of a pivotal efficacy trial to ensure enrolment of a trial population which has the potential to benefit from active treatment, the selected population did not represent the full spectrum of ataxia severity. It is currently unknown how the Pose Dispersion Index would perform in a broader ataxia patient population.
Another limitation is the use of an open-access dataset for pose estimation that was pre-trained in a normative healthy population [38, 39]. This was done to avoid overfitting that would have been introduced by fine-tuning the model using the videos used for treatment estimation.
Further research is needed to understand the feasibility of using the Pose Dispersion Index for patient assessment in routine clinical practice. Areas for future research also include understanding the clinical meaningfulness of the Pose Dispersion Index from the patient and caregiver perspective, how numeric changes in the Pose Dispersion Index link to specific patient impacts, and the clinical meaningfulness of an improvement in the measure when there has not been a statistically significant change in conventional clinical measures such as the SARA.
Conclusions
Video-based kinematic analysis of gait provides a complementary method to clinician-reported assessment in movement disorders. Using this novel approach, troriluzole treated subjects demonstrated improvement in gait as compared to placebo for the tandem walk. A higher Pose Dispersion Index, indicating more stable gait, was associated with lower frequency of subsequent falls in exploratory analyses.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the study participants for their involvement.
Medical Writing and Editorial Assistance
Medical writing services were provided by Christopher Caiazza and funded by Biohaven Pharmaceuticals, Inc.
Author Contributions
Study conception and design and data collection: Gilbert L’Italien, Evangelos Oikonomou, Rohan Khera, Michele Potashman, Melissa Beiner. and Vladimir Coric. Data analysis: Evangelos Oikonomou, and Rohan Khera. Interpretation and critical analysis: All. Manuscript: All authors commented upon previous versions of the manuscript and read and approved the final version.
Funding
The study was funded by Biohaven Pharmaceuticals, Inc. The journal Rapid Service fee is funded by Biohaven Bioscience Ireland Ltd.
Data Availability
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Evangelos Oikonomou receives support from the National Heart, Lung, and Blood Institute of the National Institutes of Health (under award 1F32HL170592) outside the submitted work. He is an academic co-founder of Evidence2Health LLC, a co-inventor in patent applications (US17/720,068, 63/177,117, 63/580,137, 63/606,203, WO2018078395A1, WO2020058713A1) and has been an ad hoc consultant for Caristo Diagnostics Ltd. Rohan Khera is an Associate Editor of JAMA. He receives support from the National Heart, Lung, and Blood Institute of the National Institutes of Health (under award K23HL153775) and the Doris Duke Charitable Foundation (under award, 2022060) outside the submitted work. He also receives research support, through Yale, from Bristol-Myers Squibb and Novo Nordisk. He is a coinventor of U.S. Provisional Patent Applications 63/177,117, 63/428,569, 63/346,610, 63/484,426, 63/508,315, 63/580,137 and 63/606,203, and an academic co-founder of Evidence2Health, LLC and ENSIGHT-AI, Inc. Jeremy Schmahmann has served on the editorial board for The Cerebellum, Editorial Board,1999. Consultancy: Biohaven Pharmaceuticals. Site PI: Biohaven clinical Trials in ataxia and multiple system atrophy. Research Support, Commercial Entities: Biohaven Pharma support of clinical trials. Research Support, Academic Entities: National Ataxia Foundation. Research Support, Foundations and Societies: National Ataxia Foundation, 2019, PI License fee payments, Technology or Inventions: Brief Ataxia Rating Scale (BARS), and Brief Ataxia Rating Scale revised (BARS2). Copyright held by The General Hospital Corporation. Cerebellar Cognitive Affective/Schmahmann syndrome Scale. Copyright held by The General Hospital Corporation. Patient-Reported Outcome Measure of Ataxia. Copyright held by The General Hospital Corporation. Cerebellar Neuropsychiatric Rating Scale. Copyright held by The General Hospital Corporation. Susan Perlman has nothing to disclose. Gilbert L’Italien, Michele Potashman, Melissa Beiner, Grant Maclaine and Vladimir Coric are employed by and own stock and stock options in Biohaven, Ltd.
Ethical Approval
This study was conducted in accordance with Good Clinical Practice, as defined by the International Conference on Harmonization; the Helsinki Declaration of 1964; and in accordance with the ethical principles underlying European Union Directive 2001/20/EC and the US Code of Federal Regulations, Title 21, Part 50 (21CFR50). The study was approved by all participating institutions. The main ethics committee for the study was Advarra IRB of 6940 Columbia Gateway Drive, Suite 110, Columbia, MD 21046, USA. Full details of participating institutions and the ethics committees or institutional review boards which approved the study are provided in the supplementary materials. Participants in the original clinical trial signed informed consent which included information that the trial results might be published; for this post hoc analysis, no separate informed consent was obtained.
Footnotes
Prior Presentation: This work has been previously presented by poster at (1) 2024 Muscular Dystrophy Association Clinical & Scientific Conference, March 3–6, Orlando, FL, USA; and (2) American Academy of Neurology 2024 Annual Meeting, April 13–18, Denver, CO, USA.
Change history
6/8/2024
The original online version of this article was revised to correct the digital features link.
References
- 1.Hekman KE, Gomez CM. The autosomal dominant spinocerebellar ataxias: emerging mechanistic themes suggest pervasive Purkinje cell vulnerability. J Neurol Neurosurg Psychiatry. 2015;86(5):554–61. 10.1136/jnnp-2014-308421. 10.1136/jnnp-2014-308421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson KJ, Watchon M, Laird AS. Aberrant cerebellar circuitry in the spinocerebellar ataxias. Front Neurosci. 2020;14:707. 10.3389/fnins.2020.00707. 10.3389/fnins.2020.00707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prestori F, Moccia F, D’Angelo E. Disrupted calcium signaling in animal models of human spinocerebellar ataxia (SCA). Int J Mol Sci. 2019. 10.3390/ijms21010216. 10.3390/ijms21010216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan R, Yau WY, O’Connor E, Houlden H. Spinocerebellar ataxia: an update. J Neurol. 2019;266(2):533–44. 10.1007/s00415-018-9076-4. 10.1007/s00415-018-9076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang CY, Yang CC, Soong BW, Yu CY, Chen SH, Huang HP, et al. Modeling spinocerebellar ataxias 2 and 3 with iPSCs reveals a role for glutamate in disease pathology. Sci Rep. 2019;9(1):1166. 10.1038/s41598-018-37774-2. 10.1038/s41598-018-37774-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt J, Schmidt T, Golla M, Lehmann L, Weber JJ, Hübener-Schmid J, et al. In vivo assessment of riluzole as a potential therapeutic drug for spinocerebellar ataxia type 3. J Neurochem. 2016;138(1):150–62. 10.1111/jnc.13606. 10.1111/jnc.13606 [DOI] [PubMed] [Google Scholar]
- 7.Klockgether T, Mariotti C, Paulson HL. Spinocerebellar ataxia. Nat Rev Dis Primers. 2019;5(1):24. 10.1038/s41572-019-0074-3. 10.1038/s41572-019-0074-3 [DOI] [PubMed] [Google Scholar]
- 8.Earhart GM, Bastian AJ. Selection and coordination of human locomotor forms following cerebellar damage. J Neurophysiol. 2001;85(2):759–69. 10.1152/jn.2001.85.2.759. 10.1152/jn.2001.85.2.759 [DOI] [PubMed] [Google Scholar]
- 9.Ilg W, Golla H, Thier P, Giese MA. Specific influences of cerebellar dysfunctions on gait. Brain. 2007;130(Pt 3):786–98. 10.1093/brain/awl376. 10.1093/brain/awl376 [DOI] [PubMed] [Google Scholar]
- 10.Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist. 2004;10(3):247–59. 10.1177/1073858404263517. 10.1177/1073858404263517 [DOI] [PubMed] [Google Scholar]
- 11.Hoogkamer W, Bruijn SM, Sunaert S, Swinnen SP, Van Calenbergh F, Duysens J. Toward new sensitive measures to evaluate gait stability in focal cerebellar lesion patients. Gait Posture. 2015;41(2):592–6. 10.1016/j.gaitpost.2015.01.004. 10.1016/j.gaitpost.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 12.Aprigliano F, Martelli D, Kang J, Kuo SH, Kang UJ, Monaco V, et al. Effects of repeated waist-pull perturbations on gait stability in subjects with cerebellar ataxia. J Neuroeng Rehabil. 2019;16(1):50. 10.1186/s12984-019-0522-z. 10.1186/s12984-019-0522-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329–42. 10.1002/mds.21720. (quiz 472). 10.1002/mds.21720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schniepp R, Wuehr M, Schlick C, Huth S, Pradhan C, Dieterich M, et al. Increased gait variability is associated with the history of falls in patients with cerebellar ataxia. J Neurol. 2014;261(1):213–23. 10.1007/s00415-013-7189-3. 10.1007/s00415-013-7189-3 [DOI] [PubMed] [Google Scholar]
- 15.Stolze H, Klebe S, Zechlin C, Baecker C, Friege L, Deuschl G. Falls in frequent neurological diseases–prevalence, risk factors and aetiology. J Neurol. 2004;251(1):79–84. 10.1007/s00415-004-0276-8. 10.1007/s00415-004-0276-8 [DOI] [PubMed] [Google Scholar]
- 16.Fonteyn EM, Schmitz-Hübsch T, Verstappen CC, Baliko L, Bloem BR, Boesch S, et al. Falls in spinocerebellar ataxias: results of the EuroSCA Fall Study. Cerebellum. 2010;9(2):232–9. 10.1007/s12311-010-0155-z. 10.1007/s12311-010-0155-z [DOI] [PubMed] [Google Scholar]
- 17.van de Warrenburg BPC, Steijns JAG, Munneke M, Kremer BPH, Bloem BR. Falls in degenerative cerebellar ataxias. Mov Disord. 2005;20(4):497–500. 10.1002/mds.20375. 10.1002/mds.20375 [DOI] [PubMed] [Google Scholar]
- 18.Fonteyn EM, Schmitz-Hübsch T, Verstappen CC, Baliko L, Bloem BR, Boesch S, et al. Prospective analysis of falls in dominant ataxias. Eur Neurol. 2013;69(1):53–7. 10.1159/000342907. 10.1159/000342907 [DOI] [PubMed] [Google Scholar]
- 19.Rochester L, Galna B, Lord S, Mhiripiri D, Eglon G, Chinnery PF. Gait impairment precedes clinical symptoms in spinocerebellar ataxia type 6. Mov Disord. 2014;29(2):252–5. 10.1002/mds.25706. 10.1002/mds.25706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muro-de-la-Herran A, Garcia-Zapirain B, Mendez-Zorrilla A. Gait analysis methods: an overview of wearable and non-wearable systems, highlighting clinical applications. Sensors (Basel). 2014;14(2):3362–94. 10.3390/s140203362. 10.3390/s140203362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wade L, Needham L, McGuigan P, Bilzon J. Applications and limitations of current markerless motion capture methods for clinical gait biomechanics. PeerJ. 2022;10: e12995. 10.7717/peerj.12995. 10.7717/peerj.12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duker AP. Video recording in movement disorders: practical issues. Continuum (Minneap Minn). 2013;19(5):1401-5 Movement Disorders. 10.1212/01.CON.0000436163.63941.d6. 10.1212/01.CON.0000436163.63941.d6 [DOI] [PubMed] [Google Scholar]
- 23.Sambati L, Baldelli L, Calandra Buonaura G, Capellari S, Giannini G, Scaglione CLM, et al. Observing movement disorders: best practice proposal in the use of video recording in clinical practice. Neurol Sci. 2019;40(2):333–8. 10.1007/s10072-018-3639-0. 10.1007/s10072-018-3639-0 [DOI] [PubMed] [Google Scholar]
- 24.Jiang J, Skalli W, Siadat A, Gajny L. Effect of face blurring on human pose estimation: ensuring subject privacy for medical and occupational health applications. Sensors (Basel). 2022. 10.3390/s22239376. 10.3390/s22239376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanza G, Casabona JA, Bellomo M, Cantone M, Fisicaro F, Bella R, et al. Update on intensive motor training in spinocerebellar ataxia: time to move a step forward? J Int Med Res. 2020;48(2):300060519854626. 10.1177/0300060519854626. 10.1177/0300060519854626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ristori G, Romano S, Visconti A, Cannoni S, Spadaro M, Frontali M, et al. Riluzole in cerebellar ataxia: a randomized, double-blind, placebo-controlled pilot trial. Neurology. 2010;74(10):839–45. 10.1212/WNL.0b013e3181d31e23. 10.1212/WNL.0b013e3181d31e23 [DOI] [PubMed] [Google Scholar]
- 27.Romano S, Coarelli G, Marcotulli C, Leonardi L, Piccolo F, Spadaro M, et al. Riluzole in patients with hereditary cerebellar ataxia: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015;14(10):985–91. 10.1016/s1474-4422(15)00201-x. 10.1016/s1474-4422(15)00201-x [DOI] [PubMed] [Google Scholar]
- 28.Groeneveld GJ, van Kan HJ, Toraño JS, Veldink JH, Guchelaar HJ, Wokke JH, et al. Inter- and intraindividual variability of riluzole serum concentrations in patients with ALS. J Neurol Sci. 2001;191(1–2):121–5. 10.1016/s0022-510x(01)00613-x. 10.1016/s0022-510x(01)00613-x [DOI] [PubMed] [Google Scholar]
- 29.Bensimon G, Doble A. The tolerability of riluzole in the treatment of patients with amyotrophic lateral sclerosis. Expert Opin Drug Saf. 2004;3(6):525–34. 10.1517/14740338.3.6.525. 10.1517/14740338.3.6.525 [DOI] [PubMed] [Google Scholar]
- 30.Rilutek (riluzole) tablets. Prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212640s001lbl.pdf. Accessed 21 Nov 2023.
- 31.Riluzole. LiverTox: Clinical and research information on drug-induced liver injury [Internet]. 2018. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. https://www.ncbi.nlm.nih.gov/books/NBK548919/. Accessed 21 Nov 2023. [PubMed]
- 32.Various authors. Medscape Neurology Exchange 2023; complete citation awaiting publication of abstracts in MDedge supplement.
- 33.Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–20. 10.1212/01.wnl.0000219042.60538.92. 10.1212/01.wnl.0000219042.60538.92 [DOI] [PubMed] [Google Scholar]
- 34.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. 10.1016/0022-3956(75)90026-6. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 35.Cao Z, Hidalgo G, Simon T, Wei SE, Sheikh Y. OpenPose: realtime multi-person 2D pose estimation using part affinity fields. IEEE Trans Pattern Anal Mach Intell. 2021;43(1):172–86. 10.1109/tpami.2019.2929257. 10.1109/tpami.2019.2929257 [DOI] [PubMed] [Google Scholar]
- 36.Pishchulin L, Andriluka M, Schiele B. Fine-grained activity recognition with holistic and pose based features. Cham: Springer; 2014. p. 678–89. [Google Scholar]
- 37.Andriluka M, Pishchulin L, Gehler P, Schiele B. 2D Human pose estimation: new benchmark and state of the art analysis. 2014 IEEE conference on computer vision and pattern recognition. 2014. p. 3686–93.
- 38.Vafadar S, Skalli W, Bonnet-Lebrun A, Khalifé M, Renaudin M, Hamza A, et al. A novel dataset and deep learning-based approach for marker-less motion capture during gait. Gait Posture. 2021;86:70–6. 10.1016/j.gaitpost.2021.03.003. 10.1016/j.gaitpost.2021.03.003 [DOI] [PubMed] [Google Scholar]
- 39.Needham L, Evans M, Cosker DP, Wade L, McGuigan PM, Bilzon JL, et al. The accuracy of several pose estimation methods for 3D joint centre localisation. Sci Rep. 2021;11(1):20673. 10.1038/s41598-021-00212-x. 10.1038/s41598-021-00212-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.