Abstract
Intracellular immunization with RevM10, a transdominant negative form of the Rev protein, efficiently inhibits human immunodeficiency virus (HIV) replication in vitro and gene therapy protocols that use this modality are currently being evaluated in human clinical trials. Development of resistance to this kind of therapy has not been previously reported. Here we show that RevM10-resistant HIV type 1 (HIV-1) variants can be selected by in vitro passage of HIV-1 in a T-lymphoblastoid cell line constitutively expressing RevM10. Unexpectedly, the selected variants showed changes in the Rev response element (RRE) but no changes in Rev. Replacement of the wild-type RRE with a mutated RRE resulted in a virus that showed increased resistance to RevM10. After repeated passages of the resistant variant in cells expressing RevM10, a virus with an additional mutation in the viral vpu gene was selected. Surprisingly, a virus containing only this vpu mutation also showed some resistance to inhibition by RevM10.
The human immunodeficiency virus type 1 (HIV-1) Rev protein is a posttranscriptional regulator that plays an essential role in virus replication. Rev is required for the expression of unspliced and incompletely spliced HIV mRNAs (20, 22, 35, 37). The Rev protein shuttles between the nucleus and the cytoplasm of infected cells (28, 40, 48) and specifically facilitates the nucleocytoplasmic export of intron-containing HIV-1 mRNAs (for reviews, see ;21 and 46). Hence, proteins encoded by these mRNAs (Gag/Pol, Vif, Vpu, Vpr, and Env) require Rev for their expression. In contrast, Rev is dispensable for the expression of viral proteins encoded by fully spliced mRNAs (Tat, Rev, and Nef). Rev action is mediated through a cis-acting element located in the intron-containing RNAs known as the Rev response element (RRE) (13, 20, 22, 37). The RRE is a complex 234-nucleotide (nt) RNA structure that is located within the coding sequence of the HIV env gene. It contains several stem-loops, and Rev binds directly to this element (11, 24).
Extensive studies of the Rev protein have led to the identification of two important functional domains. One of these is an arginine-rich domain that serves in binding to the RRE. This domain also contains a nuclear/nucleolar localization signal (9). The other domain (amino acids 75 to 84) is rich in leucines and was originally termed an “activation” domain (26, 34). This designation was based on experiments showing that certain substitutions in this domain resulted in transdominant negative (TD) proteins that were able to inhibit the function of the wild-type Rev protein (34, 38). One of the most efficient TD Rev proteins with mutations in this domain is known as RevM10. The “activation” domain was later shown to constitute a nuclear export signal (NES) (17, 58). The NES has been shown to interact directly with cellular proteins, including eIF-5A (49) and CRM1 (18). Recent studies suggest that CRM1 is an export receptor for Rev and other cellular proteins containing similar NESs (18, 19, 41, 44, 54).
The absolute requirement of Rev function for HIV replication has made this protein an attractive target for HIV therapy. Over the past few years, several groups have shown that stable expression of the RevM10 protein can efficiently inhibit HIV replication in T-lymphoblastoid cell lines, as well as in primary T cells (5, 6, 15, 33, 36, 45). Gene therapy protocols utilizing RevM10 are currently in Phase I and II clinical trials (47, 61).
Numerous in vitro and in vivo studies have shown that the development of resistance is a recurrent problem in HIV therapy. Resistance is promoted by high levels of HIV replication and the high mutation rate of the HIV genome (10). To determine whether RevM10-resistant variants could be generated in vitro, we continually passaged an HIV-1 isolate in a T-cell line that constitutively expressed RevM10. This led to the selection of viruses that showed increased resistance to inhibition by this protein. The initial resistant variants displayed changes within the viral RRE. Continual passage of an HIV construct that contained these changes generated a virus that showed even greater resistance to RevM10. Sequence analysis of these variants revealed additional mutations in the vpu gene.
MATERIALS AND METHODS
RevM10 and control CEM cell lines.
The cell lines used were a kind gift from Michael Malim, University of Pennsylvania. They were derived from CEM-SS cells by infection with either a control retroviral vector (LXSN) or a vector expressing RevM10 (36).
Viral stocks and infections.
Viral stocks were generated by transfection of 293 cells with plasmids containing proviral DNA [pNL4-3 nef(−)] by the calcium phosphate method. pNL4-3 nef(−) was derived from the previously described pNL4-3 isolate (GenBank accession number M19921) (1). It has a deletion of 97 nt at the start of the nef coding region. Viral supernatants were collected 72 h posttransfection, and titers were determined by limiting dilutions on CEM-SS cells. In the initial selection experiments (Fig. 1A and B), cells were infected with 0.0004 infectious units per cell. For all subsequent infections, the supernatants were tested for reverse transcriptase (RT) activity, and equal RT values were used to infect cells. Cultures were maintained and infected as follows: 5 × 106 cells were infected in a volume of 10 ml of RPMI 1640 (Gibco BRL)–10% fetal bovine serum (Gibco BRL) for 24 h after which they were washed with phosphate-buffered saline (PBS) and resuspended in 5 ml of fresh medium containing 1 mg G418 sulfate (Mediatech, Inc.) per ml. Cultures were then split every 3 to 4 days by removing three-fifths of the medium plus cells and replacing it with fresh medium containing G418. Viral replication was determined by RT assay on aliquots of supernatants collected at various time points as previously described (59), with the following modifications: the RT reaction mixture contained a template primer of poly(rA)-poly(dT)12–18 (5 μg/ml) (Pharmacia), and incorporation of radionucleotide was determined by using a PhosphorImager and ImageQuant software from Molecular Dynamics.
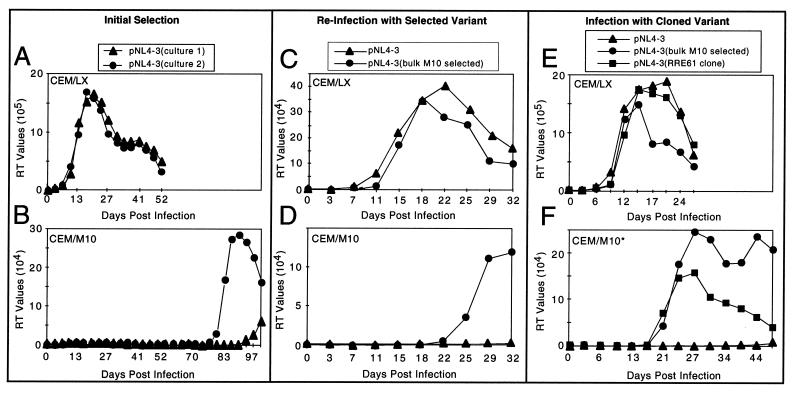
FIG. 1.
Replication of wild-type and mutant viruses in CEM/LX and CEM/M10 cells. Viral replication was determined by measuring supernatant RT activity. (A and B) The initial selection was carried out in duplicate with virus derived from the transfection of 293 cells with a nef(−) derivative of pNL4-3 and infecting cells with 0.0004 infectious units per cell. (C and D) Viral supernatants from day 115 of culture 2 in panel B were used to infect fresh CEM/LX and CEM/M10 cells (●), and pNL4-3 viral supernatant generated from 293 cells was used as a control (▴). Input viral volumes used in this and subsequent infections were adjusted for equal RT activity. (E and F) A pNL4-3 proviral clone containing an RRE fragment with the 61 mutation (■) was transfected into 293 cells to generate a virus stock which was used to infect CEM/LX cells and a subclone of CEM/M10 cells (indicated with an asterisk). Wild-type pNL4-3 viral supernatant (▴) and a RevM10-resistant uncloned viral stock generated from the infection shown in panel D (●) were used as controls.
DNA isolation and PCR.
Genomic DNA from infected cells was isolated, and the regions shown in Fig. 2A were PCR amplified as follows. Cell pellets were resuspended at a concentration of approximately 6 × 106 cells/ml in a solution consisting of 100 mM KCl, 10 mM Tris-HCl (pH 8.3), and 2.5 mM MgCl2. An equal volume of a solution of 10 mM Tris-HCl (pH 8.3), 2.5 mM MgCl2, 1% Tween 20, 1% Nonidet P-40, and proteinase K (120 μg/ml) was added, and the mixture was incubated for 1 h at 60°C. After inactivation of the proteinase K at 95°C for 10 min and cooling on ice, 50 μl of this DNA mixture was added to a tube containing a 50-μl PCR reaction mixture (1 μM concentrations of each primer, 200 μM deoxynucleoside triphosphates, and 2 U of Deep Vent polymerase [New England Biolabs]). PCR conditions of denaturation at 94°C for 1 min, annealing at 58°C for 2 min, and extension at 72°C for 3 min for 35 cycles, with a final extension time of 5 min after the last cycle, were used. The primers used to amplify these regions were 5′-GCGGGATCCGAACAAGCCCAAGAAGAC-3′ (nt 5562 to 5579) and 5′-GCGAATTCACACAGGTACCCCATAATAG-3′ (nt 6334 to 6354) for rev exon 1, 5′-TGACCTGGATGGAG TGGGACAGAGA-3′ (nt 8091 to 8115) and 5′-GCTGCTGTGTT CTACTTGTGATTG-3′ (nt 8814 to 8838) for rev exon 2, and 5′-TAAACATGTGGCAGGA AGTAGG-3′ (nt 7485 to 7506) and 5′-GGCCTGTCGGGTCCCCTCGGG-3′ (nt 8383 to 8403) for RRE. All regions amplified by PCR were sequenced directly or cloned and sequenced. Sequencing was performed with the Sequenase Version 2.0 kit from USB-Amersham Life Science and at the University of Virginia automated sequencing facility on an Applied Biosystems 377 Prism DNA Sequencer by using dye terminator chemistry with Taq polymerase.
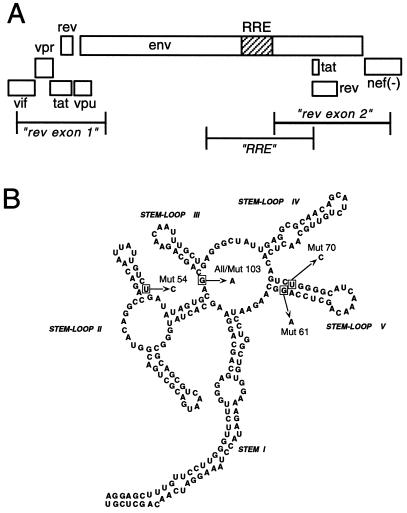
FIG. 2.
Identification of mutations within the HIV RRE that confer resistance to RevM10. (A) Schematic representation of the region of the HIV-1 genome containing the rev and env genes. Open boxes represent individual ORFs. The RRE is shown as a hatched region within the env ORF. Regions that were amplified and sequenced from RevM10-selected provirus DNA are indicated by bracketed lines. (B) Mutations observed in the RRE regions of amplified DNA from RevM10-selected proviruses. The diagram shows the RRE secondary structure with the changes observed indicated as boxed nucleotides. The mutation identified in Mut 103 was also observed in all of the other clones. Mut 54, Mut 61, and Mut 70 contained additional changes as shown. Mut 54 and Mut 103 also contained mutations outside of the RRE region that changed amino acids within Env. The U-to-C change in stem V of Mut 70 also leads to an amino acid change in Env.
Subcloning of CEM/M10 cells.
CEM/M10 cells were subcloned by limiting dilution, and individual clones were analyzed for RevM10 expression by fluorescence-activated cell sorting (FACS). Internal staining was performed after fixation of cells with 2% formaldehyde in PBS and permeabilization with 0.2% Tween 20 and 10 mM EDTA in PBS as described by Escaich et al. (15). A murine anti-Rev monoclonal antibody, 3H6, which recognizes the basic domain of Rev, was used as the primary antibody at a dilution of 1:2,000 (43). An R-phycoerythrin-conjugated anti-mouse antibody [IgG(H+L) R-PE; catalog number M3004-1; Caltag] was used as the secondary antibody at a dilution of 1:40. FACS analysis was performed on a FACScan using CellQuest software (Becton Dickinson Immunocytometry Systems). A subclone that showed high and uniform expression of RevM10 was selected for further experiments.
Transient transfections.
Transient transfections of CMT3-COS and CMT3-COS cells stably expressing RevΔ78-79 (14) were performed by using a modification of the DEAE-dextran method previously described (23). The gag/pol reporter construct, pSVgag/pol-rre-r (53), was modified by replacing the BH10-RRE with a BglII-BamHI fragment containing either the wild-type pNL4-3 RRE or Mut 61 RRE. Both Rev and secreted alkaline phosphatase (SEAP) (4) were expressed from genes cloned into the previously described vector pCMV (53). The SEAP gene was a kind gift from Michael Malim, University of Pennsylvania. Transfections were performed with 5 μg of the pSVgag/pol reporter constructs and either increasing amounts of pCMV-Rev (Fig. 3A) or 0.25 μg of pCMVRev and 0.5 μg of pCMV-SEAP (Fig. 3B and C). The levels of HIV p24 in the supernatants were determined at 72 h posttransfection by enzyme-linked immunosorbent assay (ELISA) (catalog number NEK-050B; DuPont). SEAP activity in the supernatants was measured by using the Tropix Phospha-Light Chemiluminescent Reporter kit (catalog number BP100).
FIG. 3.
Transient-transfection assays comparing wild-type RRE and RRE-61 by using Rev-dependent reporter constructs. In both panels A and B, p24 in the medium was measured by ELISA at 72 h posttransfection. The percentages shown are the levels of p24 expression from the construct containing RRE-61 relative to the construct containing the wild-type RRE. (A) CMT3-COS cells were cotransfected with the indicated amounts of pCMVRev and 5 μg of a pSVgag/pol reporter construct containing either the wild-type RRE or RRE-61. (B) CMT3-COS cells or CMT3-COS cells stably expressing RevΔ78-79 were cotransfected with 5 μg of the indicated pSVgag/pol reporter construct, 0.25 μg of pCMVRev, and 0.5 μg of pCMV-SEAP. p24 values were adjusted for differences in SEAP expression between the cell lines. (C) The data shown in panel B was replotted as the relative p24 expression for each construct in RevΔ78-79 cells compared to CMT3-COS cells. The values in all panels are the average of duplicate transfections.
RESULTS
Generation of an HIV variant that displays resistance to RevM10.
To determine whether long-term passage of HIV in cells constitutively expressing RevM10 would result in the generation of resistant HIV variants, we infected CEM-RevM10 cells and CEM control cells (36) with HIV-1 at a very low multiplicity of infection (0.0004 infectious units/cell). Two separate cultures were infected in each case, and the cells were maintained in long-term culture by the removal of three-fifths of the cells and medium every 3 days with the addition of the same volume of fresh medium.
Figure 1A demonstrates that both cultures of the infected control cells showed increasing RT values starting at day 15, indicating efficient virus replication. On the other hand, both of the cultures of infected RevM10 cells showed only background RT values for the first 77 days (Fig. 1B). However, starting on this day one of the cultures displayed increasing RT values. Starting on day 97, increasing RT values were also observed in the second culture.
To determine whether the replication in the CEM/M10 cells represented the emergence of a resistant variant, fresh CEM/M10 and control cells were infected with supernatant from the culture showing the highest level of replication. Cells were also infected with a control virus that had not been passaged in CEM/M10 cells. Both viruses replicated with the same kinetics and to the same levels in the control cells (Fig. 1C). In the CEM/M10 cells, however, increasing RT values were observed only in the case of the RevM10-selected variant starting ca. day 22, whereas no replication was observed with the control virus (Fig. 1D). These experiments indicated that the selected virus represented a true variant with increased ability to replicate in the presence of RevM10.
Analysis of proviral DNA from the RevM10-resistant variant.
To determine if RevM10 resistant variants displayed specific mutations in Rev and/or the RRE, DNA was isolated from cells of the culture shown in Fig. 1D at days 32 and 36 after infection. Different regions of proviral HIV DNA were then amplified by PCR, cloned, and sequenced. Analysis of 12 clones containing the first coding exon of Rev and 9 clones containing the second coding exon did not show any changes in the Rev open reading frame (ORF). In contrast, analysis of the RRE region from nine individual clones revealed sequence changes in all of the clones (summarized in Fig. 2B). All of the clones showed a G-to-A change in the central loop of the RRE. This nucleotide substitution does not result in an amino acid change in the overlapping env reading frame. In four of the clones this was the only change observed within the RRE. Five of the clones had additional changes. Three showed a G-to-A change in the stem of stem-loop V, whereas one clone showed a U-to-C change in this stem. Finally, one clone displayed a U-to-C change in the stem of stem-loop IIC.
Transfer of RevM10 resistance through mutations in the RRE.
In order to determine whether the observed changes in the RRE contributed to the RevM10 resistant phenotype, we used fragments containing the RRE and surrounding env sequences (nt 7612 to 8135) from 4 of the mutant clones (clones 54, 61, 70, and 103; Fig. 2) to replace the corresponding region in the wild-type HIV proviral clone. The resulting constructs were transfected into 293 cells. The supernatants from these cells displayed high RT activity in every case (data not shown), indicating that all of the mutated RREs were functional.
To analyze the replication of virus containing the different RRE mutations, the 293 cell supernatants were used to infect CEM/M10 and CEM/LX control cells. For these experiments, we used a subclone of the original CEM/M10 cells derived by limiting dilutions of these cells (see Materials and Methods). The cells in this subclone (CEM/M10*) express higher levels of RevM10 than most of the nonclonal cells used in the original selection experiments, as analyzed by FACS after internal staining with a Rev-specific antibody (data not shown). Figures 1E and F show the replication of the virus containing the clone 61 RRE mutation in CEM control cells and CEM/M10* cells. These panels also show the replication of a control wild-type virus and an uncloned virus obtained from day 27 of the second passage of the RevM10 selected stock (Fig. 1D). In all three cases, similar replication curves were seen in the control cells (Fig. 1E). In CEM/M10* cells, however, the wild-type virus failed to show any replication, while both the virus from the RevM10 selected stock and the virus containing the 61 mutation replicated efficiently. These results clearly indicate that the two RRE changes present in clone 61 were sufficient for a RevM10-resistant phenotype. Neither of the nucleotide substitutions in RRE clone 61 resulted in a change in the amino acid sequence of Env.
Surprisingly, the other three RRE mutant clones (clones 54, 70, and 103) failed to show any replication in either control cells or RevM10 cells (data not shown). However, further sequence analysis showed that clones 54 and 103 contained additional mutations outside of the RRE region which altered conserved amino acids in the gp120 and gp41 proteins. Mutant 70 did not contain any additional changes, but the U-to-C change in the stem of stem-loop V that was present in this clone also changed a conserved amino acid in the gp41 protein. These changes might explain why these mutants displayed virus production in 293 cells but failed to replicate as viruses in CEM cells. It also possible that some of these changes were the result of PCR errors.
A reporter construct containing RRE-61 displays less inhibition by TD Rev proteins than a construct containing a wild-type RRE.
One possible explanation for the ability of a virus containing RRE-61 to replicate more efficiently in cells containing RevM10 could be that this RRE is more responsive to limiting amounts of the Rev protein. To test this, we performed transient-transfection assays with Rev-dependent reporter constructs that contain the HIV gag and pol genes. These constructs produce HIV-1 pseudovirions in the presence of a functional RRE in cis and the Rev protein in trans (53). gag/pol constructs containing either the RRE-61 or a wild-type RRE were cotransfected into CMT3-COS cells with increasing amounts of a plasmid encoding the Rev protein. The amount of virus particles produced was then measured by a p24 ELISA. The results of this experiment (Fig. 3A) gave no indication that RRE-61 responds better than the wild-type RRE to low amounts of Rev. In fact, the results showed that RRE-61 was less responsive to Rev at all of the concentrations tested.
To directly test whether an RRE carrying the 61 mutation was less inhibited by a TD Rev protein compared to the wild-type RRE, we next performed transfections in CMT3-COS cells, as well as in CMT3-COS cells constitutively expressing a TD Rev protein (RevΔ78-79). RevΔ78-79 contains a 2-amino-acid deletion within the NES and was previously shown to inhibit Rev function as efficiently as did RevM10 (14). In each transfection, the reporter plasmids were cotransfected with plasmids expressing the wild-type Rev protein and SEAP (4). The latter plasmid served as a control for transfection efficiency and nonspecific effects of the TD Rev protein. Figure 3B shows the results of this experiment presented as levels of particle p24 produced after adjustment for differences in SEAP values between the two cell lines.
Again, the RRE-61 showed lower activity than the wild-type RRE in the CMT3-COS cells (67%). However, in the TD Rev-expressing cells, the activity of RRE-61 was almost double that of the wild-type RRE (172%). Figure 3C presents these data as a ratio of activity of each construct in the TD Rev cells to the activity in CMT3-COS cells. As can be seen in this figure, the expression of p24 in the RevΔ78-79 cells from the construct containing the wild-type RRE was only ca. 13% compared to what was observed in the control cells (87% inhibition). In contrast, RRE-61 showed 33% expression in the RevΔ78-79 cells compared to the control cells (67% inhibition). While these differences are small, they were reproduced in several independent experiments performed in duplicate (data not shown). These results show that RRE-61 is less sensitive to inhibition by TD Rev proteins than the wild-type RRE in the context of a subgenomic reporter, in spite of the fact that this mutant does not respond better to low amounts of Rev protein.
We also performed experiments in which the gag/pol reporter plasmid containing RRE-61 was transfected into control cells in the absence of the Rev protein. No p24 activity was ever observed in these experiments, showing that RRE-61 does not allow Rev-independent expression (data not shown).
Mutations in vpu contribute to RevM10 resistance.
Upon continued passage of the CEM/M10* cells infected with virus containing the RRE-61 mutation, we noticed increased levels of virus in the supernatant after an initial drop in replication (Fig. 4A). This suggested that variants with increased RevM10 resistance might have been selected. To further analyze this, the supernatant from day 68 of this culture was used to infect fresh CEM/M10* cells. Replication in these cells peaked at 16 days after infection, suggesting that an efficiently replicating virus had emerged (data not shown). On day 24 after infection, the cells were cocultivated with fresh CEM/M10* cells. After 10 days of culture, DNA was isolated from the infected cells, and selected regions of the HIV genome were PCR amplified and sequenced. Bulk sequencing of the uncloned PCR products, as well as sequencing of individual clones, did not reveal additional changes in the RRE beyond the original 61 mutation, nor were any mutations observed in the rev gene. However, a single nucleotide insertion in the vpu ORF was detected in the sequencing of a bulk, uncloned PCR product covering this region (Fig. 4B). No heterogeneity was detected at this position, indicating that the insertion was present in a majority of the integrated HIV proviral DNA. In addition, sequencing of several individual clones containing this product showed the same change (not shown). The nucleotide insertion that was observed should result in the expression of a truncated Vpu protein (see Fig. 4B).
FIG. 4.
Generation of a variant containing a mutation in vpu after long-term passage of a virus containing the RRE-61 mutation in CEM/M10 cells. (A) The culture infected with virus containing the RRE-61 mutation shown in Fig. 1F was continued for 88 days. Viral supernatant from day 68 (indicated by arrow) was used to infect fresh CEM/M10* cells and, after 24 days, supernatant was passaged into fresh CEM/M10* cells. DNA was isolated from these cells at 10 days postinfection. HIV-specific sequences were amplified from the cellular DNA by PCR, and sequencing was performed directly off the PCR products by using an automated sequencer. (B) The nucleotide and amino acid sequences of the wild-type and mutant vpu ORFs are shown. Dots indicate nucleotides common to both the wild type and the mutant sequence. The A insertion of the mutant sequence is shown as by a dash (–) in the wild-type sequence. The ATG of the overlapping Env reading frame is underlined. Termination codons are indicated by an asterisk.
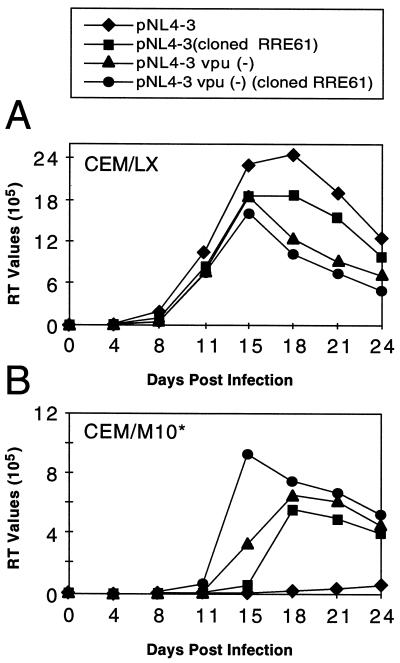
To directly determine whether the observed vpu mutation increased resistance to RevM10, we introduced the mutation into proviral clones containing either the wild-type RRE or the RRE-61 mutation. Replication of virus produced from these clones was compared in both CEM/M10* and control cells to that of the wild-type control virus and virus containing only the RRE-61 mutation. All of the viruses showed similar replication in the control cells (Fig. 5A), and all but the wild-type virus replicated well in the CEM/M10* cells (Fig. 5B). However, the irus containing both the vpu insertion and the RRE-61 mutation peaked somewhat earlier and at higher levels in the CEM/M10* cells, a finding consistent with a more resistant phenotype.
FIG. 5.
Replication of viral variants containing vpu and RRE mutations in CEM/LX and CEM/M10* cells. The viral stocks used for these infections were obtained by transfection of 293 cells with proviral clones. Viral replication was determined by measuring the supernatant RT activity. In all cases, the multiplicity of infection was standardized based on the RT activity.
DISCUSSION
Our results show that RevM10-resistant variants can be selected by passaging HIV in RevM10 cells. This is the first time that resistance to this kind of intracellular immunization has been reported. The resistant variants showed no mutations in the rev gene but displayed changes in the RRE. In addition, continued passage of these variants in RevM10 cells led to mutations in the viral vpu gene.
The original virus that was selected in the RevM10 cells contained changes in the RRE but, interestingly, no changes were observed in stem-loop II, the region that has been shown to be the primary binding site for Rev (3, 27, 30, 56). Rather, many of the selected mutations occurred in stem-loop V. This region is highly conserved among HIV-1 isolates, and the stem is also completely conserved between HIV-1 and HIV-2 (32). While studies with subgenomic reporter constructs have concluded that stem-loop II is the only RRE region required for Rev function (27), other studies have indicated that sequences throughout the RRE are also important for Rev binding and function in an as-yet-undefined manner (12, 13, 25, 39, 42). For example, mutations predicted to disrupt stem-loop V result in a nonfunctional RRE (13), and oligonucleotides complementary to the stem of stem-loop V have been shown to be ninefold more active in blocking Rev-RRE function in vivo compared to oligonucleotides directed to stem-loop II (16). In addition, oligonucleotides directed to the stem of stem-loop V are also capable of completely disrupting preformed Rev-RRE complexes in vitro (8).
Our results are thus another indication that stem-loop V plays an important role in Rev function. One possibility is that stem-loop V may act as a binding site for a cellular cofactor involved in Rev-mediated RNA transport which becomes available only after binding of Rev to the RRE. In support of this notion, secondary structural analysis of the RRE in the presence or absence of Rev indicated that the loop of stem-loop V becomes more sensitive to modification with DMS and kethoxal when Rev is bound (30). Further experiments are needed to more fully elucidate the role played by stem-loop V within the context of the whole viral genome.
It was surprising that a virus containing only the insertion mutation in vpu replicated as well in CEM/M10* cells as a virus containing the RRE-61 mutation (Fig. 5B). Vpu is a viral accessory protein that is not essential for replication in most cell culture systems. The protein has been shown to downregulate surface expression of CD4 (57, 60) and major histocompatibility complex class I molecules (29) and to promote the release of virus from infected cells (31, 55).
In view of these well-documented functions for vpu, it is not easy to explain how the loss of Vpu expression could contribute to a RevM10-resistant phenotype. However, a recent report showed that expression of Vpu appears to make infected cells more sensitive to apoptosis through the Fas/FasL pathway (7). Preliminary experiments in our laboratory suggest that Fas expression is upregulated in T-cell lines expressing RevM10 compared to control cells and that RevM10 expression may lead to increased cell death and slower growth (data not shown). A slow-growth phenotype for RevM10 has also been reported in yeast cells (50). It is thus possible that RevM10 expression affects cellular gene expression in a way that may increase the susceptibility of T cells to apoptosis via the Fas/FasL pathway. Expression of Vpu could enhance this effect and thus a Vpu-negative virus might have a specific selective advantage in cells containing RevM10.
The vpu gene lies in a region of the HIV genome where changes might be expected to have cis-acting effects on the expression of other viral genes. The gene is positioned just downstream of several 3′ splice sites as well as the tat/rev 5′ splice site, near elements that have already been shown to regulate alternative splicing (2). vpu is also the first ORF of a bicistronic mRNA that encodes both Vpu and Env (52). It is thus possible that the observed mutation gives the virus a selective advantage in the presence of RevM10 either by increasing the levels of Rev or Env mRNA or by allowing increased envelope protein expression. In fact, a recent publication demonstrated that a mutation in the Vpu coding region led to increased levels of Env protein, as well as some increase in Env RNA expression (51).
This is the first report to show that specific virus variants with increased resistance to RevM10 can be selected. In view of these findings it will be of clear interest to investigate whether similar mutations can be detected in patients undergoing clinical trials that use this modality. The RRE mutants that were selected might also be useful tools in the quest for a better understanding of Rev and RRE function.
ACKNOWLEDGMENTS
We thank Joy Niesen and Susan Prasad for expert technical assistance, William Ross of the University of Virginia FACS Core Facility for help with FACS analysis, and Michael Malim for the CEM/LX and CEM/M10 cell lines and the SEAP plasmid.
This work was funded by NIAID grants AI34721 and AI38186 and was also supported by funds from the Charles H. Ross, Jr., and Myles H. Thaler endowments to the University of Virginia.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M. Production of acquired immunodeficiency syndrome-associated retrovirus in human and non-human cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amendt B A, Si Z H, Stoltzfus C M. Presence of exon splicing silencers within human immunodeficiency virus type 1 tat exon 2 and tat-rev exon 3: evidence for inhibition mediated by cellular factors. Mol Cell Biol. 1995;15:4606–4615. doi: 10.1128/mcb.15.8.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel D P, Zapp M L, Green M R, Szostak J W. HIV-1 Rev regulation involves recognition of non-Watson-Crick base pairs in viral RNA. Cell. 1991;67:529–536. doi: 10.1016/0092-8674(91)90527-6. [DOI] [PubMed] [Google Scholar]
- 4.Berger J, Hauber J, Hauber R, Geiger R, Cullen B R. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988;66:1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- 5.Bevec D, Dobrovnik M, Hauber J, Böhnlein E. Inhibition of human immunodeficiency virus type 1 replication in human T cells by retroviral-mediated gene transfer of a dominant-negative Rev trans-activator. Proc Natl Acad Sci USA. 1992;89:9870–9874. doi: 10.1073/pnas.89.20.9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonyhadi M L, Moss K, Voytovich A, Auten J, Kalfoglou C, Plavec I, Forestell S, Su L, Böhnlein E. RevM10-expressing T cells derived in vivo from transduced human hematopoietic stem-progenitor cells inhibit human immunodeficiency virus replication. J Virol. 1997;71:4707–4716. doi: 10.1128/jvi.71.6.4707-4716.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casella C R, Rapaport E L, Finkel T H. Vpu increases susceptibility of human immunodeficiency virus type 1-infected cells to Fas killing. J Virol. 1999;73:92–100. doi: 10.1128/jvi.73.1.92-100.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin D J. Inhibition of human immunodeficiency virus type 1 Rev-Rev-Response element complex formation by complementary oligonucleotides. J Virol. 1992;66:600–607. doi: 10.1128/jvi.66.1.600-607.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochrane A W, Chen C H, Rosen C A. Specific interaction of the human immunodeficiency virus Rev protein with a structured region in the env mRNA. Proc Natl Acad Sci USA. 1990;87:1198–202. doi: 10.1073/pnas.87.3.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 11.Daly T J, Cook K S, Gray G S, Maione T E, Rusche J R. Specific binding of HIV-1 recombinant Rev protein to the Rev-responsive element in vitro. Nature. 1989;342:816–819. doi: 10.1038/342816a0. [DOI] [PubMed] [Google Scholar]
- 12.Dayton E T, Konings D A, Powell D M, Shapiro B A, Butini L, Maizel J V, Dayton A I. Extensive sequence-specific information throughout the CAR/RRE, the target sequence of the human immunodeficiency virus type 1 Rev protein. J Virol. 1992;66:1139–1151. doi: 10.1128/jvi.66.2.1139-1151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dayton E T, Powell D M, Dayton A I. Functional analysis of CAR, the target sequence for the Rev protein of HIV-1. Science. 1989;246:1625–1629. doi: 10.1126/science.2688093. [DOI] [PubMed] [Google Scholar]
- 14.Dundr M, Leno G H, Lewis N, Rekosh D, Hammarskjöld M-L, Olson M O. Location of the HIV-1 Rev protein during mitosis: inactivation of the nuclear export signal alters the pathway for postmitotic reentry into nucleoli. J Cell Sci. 1996;109:2239–2251. doi: 10.1242/jcs.109.9.2239. [DOI] [PubMed] [Google Scholar]
- 15.Escaich S, Kalfoglou C, Plavec I, Kaushal S, Mosca J D, Böhnlein E. RevM10-mediated inhibition of HIV-1 replication in chronically infected T cells. Hum Gene Ther. 1995;6:625–634. doi: 10.1089/hum.1995.6.5-625. [DOI] [PubMed] [Google Scholar]
- 16.Fenster S D, Wagner R W, Froehler B C, Chin D J. Inhibition of human immunodeficiency virus type-1 env expression by C-5 propyne oligonucleotides specific for Rev-Response element stem-loop V. Biochemistry. 1994;33:8391–8398. doi: 10.1021/bi00194a002. [DOI] [PubMed] [Google Scholar]
- 17.Fischer U, Huber J, Boelens W C, Mattaj I W, Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 18.Fornerod M, Ohno M, Yoshida M, Mattaj M. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–11. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 20.Hadzopoulou-Cladaras M, Felber B K, Cladaras C, Athanassopoulos A, Tse A, Pavlakis G N. The Rev (trs/art) protein of human immunodeficiency virus type 1 affects viral mRNA and protein expression via a cis-acting sequence in the env region. J Virol. 1989;63:1265–1274. doi: 10.1128/jvi.63.3.1265-1274.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammarskjöld M-L. Regulation of retroviral RNA export. Semin Cell Dev Biol. 1997;8:83–90. doi: 10.1006/scdb.1996.0127. [DOI] [PubMed] [Google Scholar]
- 22.Hammarskjöld M-L, Heimer J, Hammarskjöld B, Sangwan I, Albert L, Rekosh D. Regulation of human immunodeficiency virus env expression by the rev gene product. J Virol. 1989;63:1959–1966. doi: 10.1128/jvi.63.5.1959-1966.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammarskjöld M-L, Wang S-C, Klein G. High-level expression of the Epstein-Barr virus EBNA1 protein in CV1 cells and human lymphoid cells using a SV40 late replacement vector. Gene. 1986;43:41–50. doi: 10.1016/0378-1119(86)90006-5. [DOI] [PubMed] [Google Scholar]
- 24.Heaphy S, Dingwall C, Ernberg I, Gait M J, Green S M, Karn J, Lowe A D, Singh M, Skinner M A. HIV-1 regulator of virion expression (Rev) protein binds to an RNA stem-loop structure located within the Rev response element region. Cell. 1990;60:685–693. doi: 10.1016/0092-8674(90)90671-z. [DOI] [PubMed] [Google Scholar]
- 25.Holland S M, Ahmad N, Maitra R K, Wingfield P, Venkatesan S. Human immunodeficiency virus Rev protein recognizes a target sequence in Rev-responsive element RNA within the context of RNA secondary structure. J Virol. 1990;64:5966–5975. doi: 10.1128/jvi.64.12.5966-5975.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hope T J, Bond B L, McDonald D, Klein N P, Parslow T G. Effector domains of human immunodeficiency virus type 1 Rev and human T-cell leukemia virus type I Rex are functionally interchangeable and share an essential peptide motif. J Virol. 1991;65:6001–6007. doi: 10.1128/jvi.65.11.6001-6007.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X J, Hope T J, Bond B L, McDonald D, Grahl K, Parslow T G. Minimal Rev-response element for type 1 human immunodeficiency virus. J Virol. 1991;65:2131–2134. doi: 10.1128/jvi.65.4.2131-2134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalland K H, Szilvay A M, Brokstad K A, Saetrevik W, Haukenes G. The human immunodeficiency virus type 1 Rev protein shuttles between the cytoplasm and nuclear compartments. Mol Cell Biol. 1994;14:7436–7444. doi: 10.1128/mcb.14.11.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerkau T, Bacik I, Bennink J R, Yewdell J W, Hünig R, Schimpl A, Schubert U. The human immunodeficiency virus type 1 (HIV-1) vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J Exp Med. 1997;185:1295–1305. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kjems J, Brown M, Chang D D, Sharp P A. Structural analysis of the interaction between the human immunodeficiency virus Rev protein and the Rev response element. Proc Natl Acad Sci USA. 1991;88:683–687. doi: 10.1073/pnas.88.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klimkait T, Strebel K, Hoggan M D, Martin M A, Orenstein J M. The human immunodeficiency virus type 1-specific protein Vpu is required for efficient virus maturation and release. J Virol. 1990;64:621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis N, Williams J, Rekosh D, Hammarskjold M L. Identification of a cis-acting element in human immunodeficiency virus type 2 (HIV-2) that is responsive to the HIV-1 Rev and human T-cell leukemia virus types I and II Rex proteins. J Virol. 1990;64:1690–1697. doi: 10.1128/jvi.64.4.1690-1697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Woffendin C, Yang Z, Nabel G J. Regulated expression of a dominant negative form of Rev improves resistance to HIV replication in T cells. Gene Ther. 1994;1:32–37. [PubMed] [Google Scholar]
- 34.Malim M H, Bohnlein S, Hauber J, Cullen B R. Functional dissection of the HIV-1 Rev trans-activator—derivation of a trans-dominant repressor of Rev function. Cell. 1989;58:205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- 35.Malim M H, Cullen B R. Rev and the fate of pre-mRNA in the nucleus: implications for the regulation of RNA processing in eukaryotes. Mol Cell Biol. 1993;13:6180–6189. doi: 10.1128/mcb.13.10.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malim M H, Freimuth W W, Liu J, Boyle T J, Lyerly H K, Cullen B R, Nabel G J. Stable expression of a transdominant Rev protein in human T cells inhibits human immunodeficiency virus replication. J Exp Med. 1992;176:1197–1201. doi: 10.1084/jem.176.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malim M H, Hauber J, Le S V, Maizel J V, Cullen B R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338:254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- 38.Malim M H, McCarn D F, Tiley L S, Cullen B R. Mutational definition of the human immunodeficiency virus type 1 Rev activation domain. J Virol. 1991;65:4248–4254. doi: 10.1128/jvi.65.8.4248-4254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malim M H, Tiley L S, McCarn D F, Rusche J R, Hauber J, Cullen B R. HIV-1 structural gene expression requires binding of the Rev trans-activator to its RNA target sequence. Cell. 1990;60:675–683. doi: 10.1016/0092-8674(90)90670-a. [DOI] [PubMed] [Google Scholar]
- 40.Meyer B E, Malim M H. The HIV-1 Rev trans-activator shuttles between the nucleus and the cytoplasm. Genes Dev. 1994;8:1538–1547. doi: 10.1101/gad.8.13.1538. [DOI] [PubMed] [Google Scholar]
- 41.Neville M, Stutz F, Lee L, Davis L I, Rosbash M. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr Biol. 1997;7:767–775. doi: 10.1016/s0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- 42.Olsen H S, Nelbock P, Cochrane A W, Rosen C A. Secondary structure is the major determinant for interaction of HIV Rev protein with RNA. Science. 1990;247:845–848. doi: 10.1126/science.2406903. [DOI] [PubMed] [Google Scholar]
- 43.Orsini M J, Thakur A N, Andrews W W, Hammarskjold M L, Rekosh D. Expression and purification of the HIV type 1 Rev protein produced in Escherichia coli and its use in the generation of monoclonal antibodies. AIDS Res Hum Retroviruses. 1995;11:945–953. doi: 10.1089/aid.1995.11.945. [DOI] [PubMed] [Google Scholar]
- 44.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 45.Plavec I, Agarwal M, Ho K E, Pineda M, Auten J, Baker J, Matsuzaki H, Escaich S, Bonyhadi M, Böhnlein E. High transdominant RevM10 protein levels are required to inhibit HIV-1 replication in cell lines and primary T cells: implication for gene therapy of AIDS. Gene Ther. 1997;4:128–139. doi: 10.1038/sj.gt.3300369. [DOI] [PubMed] [Google Scholar]
- 46.Pollard V W, Malim M H. The HIV-1 Rev protein. Annu Rev Microbiol. 1998;52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 47.Ranga U, Woffendin C, Verma S, Xu L, June C H, Bishop D K, Nabel G J. Enhanced T cell engraftment after retroviral delivery of an antiviral gene in HIV-infected individuals. Proc Natl Acad Sci USA. 1998;95:1201–1206. doi: 10.1073/pnas.95.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richard N, Iacampo S, Cochrane A. HIV-1 Rev is capable of shuttling between the nucleus and cytoplasm. Virology. 1994;204:123–131. doi: 10.1006/viro.1994.1516. [DOI] [PubMed] [Google Scholar]
- 49.Ruhl M, Himmelspach M, Bahr G M, Hammerschmid F, Jaksche H, Wolff B, Aschauer H, Farrington G K, Probst H, Bevec D, et al. Eukaryotic initiation factor 5A is a cellular target of the human immunodeficiency virus type 1 Rev activation domain mediating trans-activation. J Cell Biol. 1993;123:1309–1320. doi: 10.1083/jcb.123.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saavedra C A, Hammell C M, Heath C V, Cole C N. Yeast heat shock mRNAs are exported through a distinct pathway defined by Rip1p. Genes Dev. 1997;11:2845–2856. doi: 10.1101/gad.11.21.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schubert U, Bour S, Willey R L, Strebel K. Regulation of virus release by the macrophage-tropic human immunodeficiency virus type 1 AD8 isolate is redundant and can be controlled by either vpu or env. J Virol. 1999;73:887–896. doi: 10.1128/jvi.73.2.887-896.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz S, Felber B K, Fenyo E M, Pavlakis G N. Env and Vpu proteins of human immunodeficiency virus type 1 are produced from multiple bicistronic mRNAs. J Virol. 1990;64:5448–5456. doi: 10.1128/jvi.64.11.5448-5456.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith A J, Cho M I, Hammarskjöld M L, Rekosh D. Human immunodeficiency virus type 1 Pr55gag and Pr160gag-pol expressed from a simian virus 40 late replacement vector are efficiently processed and assembled into viruslike particles. J Virol. 1990;64:2743–2750. doi: 10.1128/jvi.64.6.2743-2750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stade K, Ford C S, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 55.Terwilliger E F, Cohen E A, Lu Y C, Sodroski J G, Haseltine W A. Functional role of human immunodeficiency virus type 1 vpu. Proc Natl Acad Sci USA. 1989;86:5163–5167. doi: 10.1073/pnas.86.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiley L S, Malim M H, Tewary H K, Stockley P G, Cullen B R. Identification of a high-affinity RNA-binding site for the human immunodeficiency virus type 1 Rev protein. Proc Natl Acad Sci USA. 1992;89:758–762. doi: 10.1073/pnas.89.2.758. . (Erratum, 89:1997.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vincent M J, Raja N U, Jabbar M A. Human immunodeficiency virus type 1 vpu protein induces degradation of chimeric envelope glycoproteins bearing the cytoplasmic and anchor domains of CD4: role of the cytoplasmic domain in vpu-induced degradation in the endoplasmic reticulum. J Virol. 1993;67:5538–5549. doi: 10.1128/jvi.67.9.5538-5549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen W, Meinkoth J L, Tsien R Y, Taylor S S. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 59.Willey R L, Bonifacino J S, Potts B J, Martin M A, Klausner R D. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc Natl Acad Sci USA. 1988;85:9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willey R L, Maldarelli F, Martin M A, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woffendin C, Ranga U, Yang Z, Xu L, Nabel G J. Expression of a protective gene-prolongs survival of T cells in human immunodeficiency virus-infected patients. Proc Natl Acad Sci USA. 1996;93:2889–2894. doi: 10.1073/pnas.93.7.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]