Abstract
Diabetic foot ulcers (DFU) are a debilitating and life-threatening complication of Diabetes Mellitus. Ulceration develops from a combination of associated diabetic complications, including neuropathy, circulatory dysfunction, and repetitive trauma, and they affect approximately 19–34% of patients as a result. The severity and chronic nature of diabetic foot ulcers stems from the disruption to normal wound healing, as a result of the molecular mechanisms which underly diabetic pathophysiology. The current standard-of-care is clinically insufficient to promote healing for many DFU patients, resulting in a high frequency of recurrence and limb amputations. Biomaterial dressings, and in particular those derived from the extracellular matrix (ECM), have emerged as a promising approach for the treatment of DFU. By providing a template for cell infiltration and skin regeneration, ECM-derived biomaterials offer great hope as a treatment for DFU. A range of approaches exist for the development of ECM-derived biomaterials, including the use of purified ECM components, decellularisation and processing of donor/ animal tissues, or the use of in vitro-deposited ECM. This review discusses the development and assessment of ECM-derived biomaterials for the treatment of chronic wounds, as well as the mechanisms of action through which ECM-derived biomaterials stimulate wound healing.
Keywords: Diabetic foot ulcer, Extracellular matrix, Biomaterials, Wound healing, Decellularisation
Introduction
Diabetes mellitus (DM) is a systemic metabolic disorder which is characterised by hyperglycaemia [1]. Over half a billion people are living with DM worldwide, and the number of patients is projected to increase substantially to over a billion by 2050, thus worsening the social and economic burden of DM and its associated complications [2]. Type 1 diabetes mellitus (T1DM) is an autoimmune condition which is characterised by the destruction of pancreatic insulin-producing β-cells, leading to hyperglycaemia [3]. In contrast, the onset of insulin resistance in T2DM typically occurs as a result of poor lifestyle and hyperglycaemia, leading to the onset of diabetic complications [3, 4]. Diabetic complications can be debilitating and life-threatening, and include retinopathy, nephropathy, neuropathy, cardiovascular dysfunction and chronic wounds [5].
As a result of DM-associated vascular complications, wound healing capacity in DM patients is also severely affected and can lead to the formation of chronic wounds. Diabetic foot ulcers (DFU) are particularly prevalent, affecting approximately 19–34% of DM patients [6]. Ulceration develops from a combination of neuropathy, circulatory dysfunction, and trauma [7]. Anatomical deformities including the claw toe, are particularly susceptible to the development of DFUs and chronic wounds, as a result of DM-induced neuropathy, and areas of high pressure and repetitive trauma in long-term diabetic patients [8, 9]. Due to the non-healing nature of DFUs, and the consistent exposure of the open wound to the environment, microbial infection and severe infection with bone involvement or osteomyelitis, are major risks to patients. Osteomyelitis can ultimately lead to the need for limb amputations, frequently seen in the lower limb [10]. The current standard of care for DFU includes rigorous protocols of debridement, off-loading, and antibiotics to treat infection [11]. However, standard wound-care has proven insufficient for many patients, as 20% of DFU patients eventually require lower limb amputations [6]. Despite the lower severity of DFUs in comparison to other fatal diabetic complications, it remains an urgent global concern due to its associated economic and social burden, significant impact on quality of life, and ever-growing frequency. As such, it is evident that there is an urgent need for the improvement of treatment strategies for DFUs [12].
In recent years, the use of biomaterials in wound healing applications has shown great promise to treat chronic wounds. Biomaterials are classed as either composed of synthetic or naturally-derived materials, either of which may be processed or modified to further enhance their wound healing capacity [13]. These alterations can include chemical modification to control biophysical properties and/or bioactivity [14]. While a wide range of biomaterials have been developed to promote tissue regeneration, biomaterials derived from the extracellular matrix (ECM) have shown particular promise [15]. Owing to their inherent biocompatibility, biodegradation, and minimal immunogenicity, host cells will readily populate and remodel ECM-derived materials [16]. This review focuses on the development of ECM-derived biomaterial systems, and their capacity to enhance repair and regeneration in the context of DFUs. Furthermore, we will discuss the mechanisms of action of ECM-derived biomaterials, and finally point towards promising developments in the field that can lead to the clinical realisation of ECM-derived biomaterials as efficacious therapies for patients.
The molecular basis of diabetic foot ulcers
The onset of DFU is a multifactorial process which is stimulated by repetitive un-noticed trauma, and exacerbated by poor wound healing, peripheral neuropathy, and cardiovascular dysfunction. In DM-induced neuropathy, hyperglycaemia leads to an overproduction of reactive oxygen species (ROS), mitochondrial failure, and subsequent oxidative damage to neurons and Schwann cells [17]. Both motor and sensory neurons are affected in the process, thus leading to muscle atrophy, anatomical deformities, and a loss of sensation. Decreased sensation in the lower limbs can lead to patients ignoring or not noticing repeated trauma and the formation of a wound. This can lead to the development of a chronic wound or DFU over time [7].
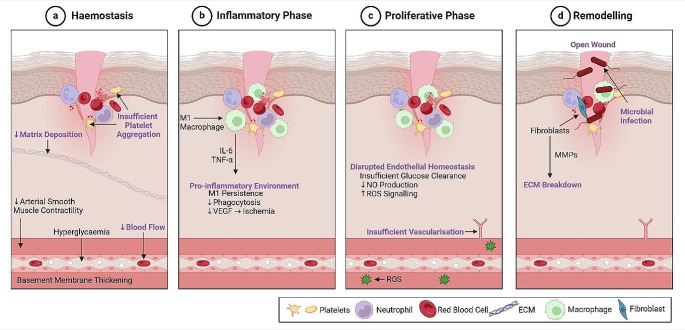
Classical wound healing involves 4 key stages: haemostasis, inflammation, proliferation, and remodelling. These processes involve a highly regulated and balanced interplay between inflammation, cell migration and angiogenesis, and tissue regeneration, leading to ECM deposition, remodelling, and healing. For an in-depth description and discussion of these processes under physiological conditions, the reader is referred to a number of excellent papers [18–21]. While the classical wound healing process ultimately leads to wound closure and functional restoration of the skin in those without underlying conditions, this is not the case for patients with DM. In diabetic patients, many of the key stages of wound healing are dysfunctional due to hyperglycaemia, neuropathy, ischemia and chronic inflammation [22]. Figure 1 depicts a schematic of the phases of wound healing, and how they become disrupted in diabetic patients.
Fig. 1.
Disruption to the phases of wound healing in diabetic patients. The 4 phases of wound healing are negatively affected in diabetic patients. a. During Haemostasis, thickening of the blood vessel walls and reduced contractility of smooth muscle leads to perturbed vasoconstriction and reduced blood flow. Subsequent ischemia leads to insufficient platelet aggregation which prevents progression of blood coagulation after injury and delays healing. Reduced deposition of ECM at this stage also delays healing. b. The inflammatory phase is prolonged, as seen by pro-inflammatory macrophage (M1) persistence. Secretion of pro-inflammatory cytokines e.g., IL-6 and TNF-a leads to reduced phagocytosis and VEGF secretion, thus delaying angiogenesis and exacerbating local ischemia. c. As a result of insulin resistance and disrupted endothelial homeostasis in the previous phases, there is reduced nitric oxide and increased ROS production within the endothelial membrane, further preventing angiogenesis/ vasculogenesis. d. Finally, tissue remodelling is inhibited through insufficient progression of healing. Fibroblasts within a wound release excessive MMPs which are disruptive to ECM components including collagens. Similarly, exposure of the open wound to the environment increases susceptibility to serious infection during healing. Figure was created in Biorender
Further, hyperglycaemia-induced kidney damage contributes to the onset of raised blood pressure and atherosclerosis. This reduces vessel contractility, impeding vasodilation and reducing blood flow. Ultimately, reduced blood flow and thickened capillary membranes inhibit perfusion of peripheral tissue and impede wound repair i.e., a DFU [4].
The first stage of wound healing impacted by DM is haemostasis. Capillary basement membrane thickening, and reduced contractility of arterial smooth muscle, in diabetic patients, diminishes vasoconstriction, obstructing healing from the outset [23]. Platelet plug formation is disrupted as a result of this ischemia, further delaying the coagulation cascade and lowering clot stability. Ultimately, there is less provisional matrix deposition during this phase, and subsequently reduced cellular invasion [24].
In DFUs, the inflammatory phase is prolonged, leading to a pro-inflammatory micro-environment that inhibits regeneration. This includes the secretion of pro-inflammatory cytokines and proteins, including interleukin (IL)-1 and − 6, and tumour necrosis factor alpha (TNF-α) [25, 26]. The persistence of pro-inflammatory (M1) macrophages is coupled with their reduced phagocytosis of microbes and efferocytosis of apoptotic cells and debris, further preventing cessation of the pro-inflammatory phase [27, 28]. In classical wound healing, a transition from M1 to anti-inflammatory (M2) macrophages occurs, promoting resolution of inflammation and tissue vascularisation. However, in DFUs, this transition does not occur, thus hindering vascularisation and angiogenesis, amplifying ischemia, and preventing wound healing [18].
The proliferative phase of wound healing is typically characterised by increased tissue vascularisation, the formation of granulation tissue, and ECM deposition by fibroblasts [22]. Similarly, angiogenesis and the formation of new blood vessels is a critical step of wound healing, which normally facilitates the restoration of endothelial and tissue homeostasis, through antioxidant signalling [18, 29]. However, hyperglycaemia leads to endothelial dysfunction and the inhibition of tissue vascularisation. This includes reduced nitric oxide (vasodilator and vasoprotector) production, and increased reactive oxygen species (ROS) generation [29, 30]. High levels of ROS signalling further contributes to the perturbation of vascularisation and a reduction in the formation of blood vessels [18].
Persistence of a pro-inflammatory and ischemic microenvironment in DM further affects the remodelling phase of wound healing through elevated production of matrix metalloproteinases (MMPs), driving the degradation of ECM components [31]. ECM deposition and collagen fibre reorganisation are essential for tissue repair and the re-establishment of the structural integrity and barrier function of the skin, which includes proper epithelial cell differentiation [32, 33]. As such, excessive MMP activity and ECM degradation inhibits re-epithelisation, and results in the wound remaining open [33]. Furthermore, prolonged exposure of the open wound to the environment increases the risk of infection and biofilm formation, further complicating and hindering repair [34].
Clinically it has been observed that even a DFU that achieves re-epithelisation, is highly likely to recur due to the suboptimal mechanical characteristics of the neotissue [35]. The current rate of recurrence is approximately 42% over 1 year, increasing to 65% after 5 years [36]. Consequently, this leads to the high frequency of amputations as a preventative measure against wound recurrence and systemic infection [37]. However, even after limb amputations, there is a high frequency (~ 43%) of surgical wound complications among patients due to the underlying, untreated pathophysiology [38]. The following section details the current standard of care for the treatment of DFU.
Established therapies for DFUs
The current standard of care for DFUs aims to support wound healing through regular debridement, wound dressings, and pressure off-loading [22]. The removal of necrotic tissue by debridement aids healing and skin regeneration through the elimination of infection, and the removal of the physical barrier for cell migration [11]. Wound dressings and topical solutions including antibiotics or antimicrobial ointments are applied, and regularly changed to maintain a moist environment and prevent infection [22, 39], while pressure off-loading, and the use of a total-contact casts or a boot limits ambulation and prevents DFU aggravation [40]. Other approaches include negative pressure wound therapy (NPWT) and hyperbaric oxygen therapy, which are recommended as adjunctive therapies to other treatments for the removal of infection and promotion of vascularisation [41, 42]. Skin grafts have also shown promise, but their invasive nature coupled with a considerable risk of infection, makes them a less ideal solution [43]. Similarly, the underlying DM pathophysiology increases the likelihood of graft failure [39].
Current therapies predominantly aim to protect wounds from infection and rely on the inherent regeneration capacity of the tissue for wound closure to occur. However, owing to the limited regenerative potential and healing capacity of DFUs, these treatments are insufficient for many diabetic patients [44]. Given that the number of diabetic patients worldwide is rapidly increasing, there is a definitive need for more advanced therapeutic options [45]. The next section will describe how biomaterials have emerged as a promising therapeutic approach, acting as a template to support cell infiltration and neotissue formation, accelerating repair.
Biomaterials as an emerging DFU therapy
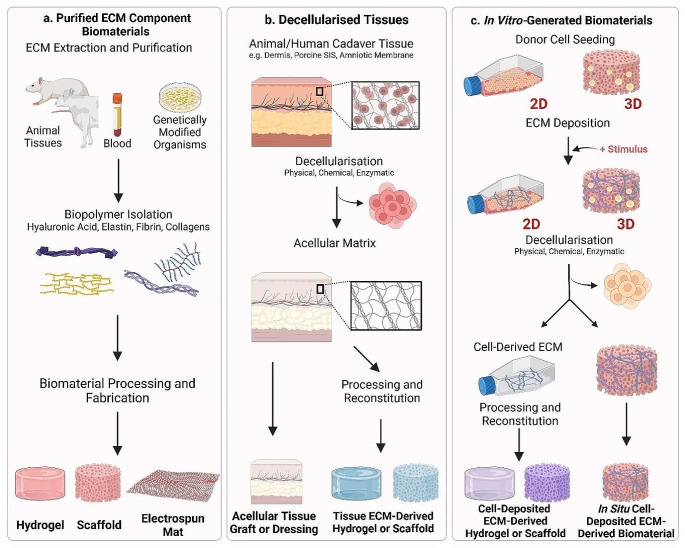
By providing a provisional matrix for cell migration, infiltration, and proliferation, biomaterials can support neotissue formation and healing [13, 46–48]. A number of biomaterials have been assessed in clinical trials as an alternative to standard wound care protocols and have demonstrated great potential. For example, the ‘Integra Dermal Regeneration Template’ (IDRT), a bi-layered collagen/ chondroitin-6-sulphate matrix, has shown significant promise to promote DFU healing [48]. Similarly, Alloderm™, a cadaveric skin-derived dermal substitute is FDA-approved for treatment of DFUs [49]. These ECM-derived biomaterials, in addition to many others at the stage of pre-clinical and in vitro assessment, emphasise the great promise that biomaterials hold to promote wound repair. A schematic summary of the production and application of ECM-derived biomaterials is provided in Fig. 2.
Fig. 2.
ECM-Derived Biomaterials Production and Application. There are 3 principal production methods for ECM-derived biomaterials, as discussed throughout this review. Purified ECM components, decellularised tissues, and in vitro-generated ECM are then processed to produce a biomaterial for DFU treatment. a. Purified ECM Component Biomaterials are produced from isolated ECM components including collagen, fibrin, hyaluronic acid, and elastin. ECM-derived biomaterials can be produced from single components, or from a combination of components e.g. collagen and hyaluronic acid. b. Decellularised tissue-derived ECM biomaterials are produced from animal or cadaver tissue, and decellularised to produce an acellular matrix. c. In vitro-generated biomaterials can be produced in 2 ways. Firstly, cells are kept in culture as they deposit their ECM over time. Decellularisation then removes the cellular components and preserves their ECM. Secondly, cells are seeded to a biomaterial and allowed to deposit their ECM over time, which is optimised through induction of a stimulus e.g., macromolecular crowding, ascorbic acid, increased cell density. Decellularisation then removes the cellular component, and preserves the deposited ECM, leaving an in situ deposited ECM-derived scaffold which can be implanted to an ulcer. Figure was created in Biorender
Biomaterials are typically classed as either of natural or synthetic origin. Although synthetic biomaterials have proven useful in tissue engineering due to their ease of manufacture and modification, natural biomaterials are more widely used owing to a number of reasons [50]. In particular, mammalian ECM-derived biomaterials are of significant interest due to their inherent biocompatibility, bioactivity, and biodegradation, as well as the presence of cell-recognition motifs [16, 51]. In fact, ECM-derived biomaterials have shown particular promise for tissue regeneration in a wide range of applications, including wound repair [13]. ECM-derived biomaterials may be of allogeneic or xenogeneic origin, and their production includes the extraction and processing of distinct ECM components, decellularisation of whole human or animal tissue, or the processing of ECM deposited in vitro [52]. The following sections will describe these techniques in more detail.
Purified components
Biomaterials composed of isolated ECM components constitute the simplest form of ECM-derived biomaterials for tissue repair. Typically, ECM-derived biopolymers such as collagen, hyaluronic acid (HyA), fibrin, and gelatin, are chemically or physically crosslinked to form a structure to facilitate cell infiltration and tissue repair. These biopolymers are particularly suitable for wound repair owing to their presence in the native ECM, as well as their inherent biocompatibility, biodegradability, and low immunogenicity [52, 53].
Collagen type I is the most abundant component of the ECM and has been widely used as a biomaterial due to its established capacity to facilitate cell migration, infiltration and proliferation [54, 55]. Collagen-based biomaterials have been investigated in a wide variety of forms for the treatment of DFUs, including scaffolds, gels, particles, and films. For example, collagen type I electrospun matrices demonstrated enhanced keratinocyte adhesion, implying its potential to support wound healing [56]. Furthermore, fish skin-derived collagen was electrospun to form a nanofibrous mesh, and facilitated adhesion, proliferation, and differentiation of human keratinocytes in vitro. This further demonstrated the biocompatibility and pro-regenerative capacity of electrospun collagen in the context of wound re-epithelialisation, which was shown in a murine model [57]. Collagen-based biomaterials can also support cell delivery, as demonstrated when mesenchymal stem cells (MSCs) seeded in collagen type I scaffolds enhanced healing in rabbit DFU models, through increased angiogenesis and accelerated wound closure [58]. Further pre-clinical studies which have shown the efficacy of collagen-based biomaterials in wound healing include the Promogran® sponge, made from collagen and oxidised regenerated cellulose. This combination of oxidised regenerated cellulose and collagen has previously shown to be effective for the improvement of haemostasis and the treatment of intraoperative bleeding, further aligning with its efficacy in wound healing [59]. In Promogran®-treated DFUs, the sponge displayed inhibitory effects on protease-driven pathways, thus impeding protease-driven growth factor/ECM degradation, accelerating re-epithelialisation, and shortening healing times, in comparison to commercial hydrocolloid-treated wounds [60]. Similarly, the use of lyophilised collagen particles as a wound dressing has effectively reduced DFU wound size by 45.43%, in comparison to 23.40% of control hydrocolloid dressing-treated wounds [61].
Hyaluronic acid (HyA) is a naturally occurring ECM-derived biopolymer, whose inherent ability to promote wound healing, and regulate inflammation, angiogenesis, and cell migration is well-documented [62–65]. Furthermore, HyA has been widely used to develop biomaterials, in particular hydrogels with great potential for tissue repair [66–70]. The hydrophilic nature of HyA is also of crucial importance in the development of a wound healing therapeutic, due to its ability to maintain a moist wound environment, facilitating epithelial migration [71, 72]. Like collagens, a variety of forms of HyA-derived biomaterials have been assessed for their wound healing capacity, including electrospun scaffolds and hydrogels. Dermal fibroblast infiltration has been seen throughout HyA-based scaffolds in a porosity-dependent manner, implying its suitability as a template for tissue integration and wound healing. The implantation of these electrospun HyA scaffolds in full-thickness mouse wounds also facilitated increased vascularisation and re-epithelialisation in comparison to silicon dressings [73]. Hyaff-11 (a HyA-based wound dressing) has also promoted effective ECM deposition and endothelial cell proliferation in vitro, with up to 50% reduction in DFU wound size, increased endothelial cell proliferation, and ECM deposition (collagens, fibronectin and laminins) in vivo [64, 74, 75].
Fibrin is the primary protein component of blood clots and has been assessed as a biomaterial to drive DFU repair [19]. Its success in wound healing therapies can be partially attributed to the role that it plays in the early stages of wound healing, particularly the formation and stabilisation of the fibrin clot, which acts as a template for cell infiltration [76]. Owing to its pro-regenerative capacity, it has been extensively used in wound healing, with fibrin hydrogels demonstrating immunomodulatory effects on macrophages in vitro, thus driving the resolution of the inflammation [77]. Similarly, fibrin biomaterials have laid the foundation for the development of many hybrid scaffolds. For example, fibrin hydrogels and electrospun membranes (with poly(lactide-co-glycolide)) have been combined to develop a bi-layered scaffold. After implantation in a full-thickness rat wound model, these scaffolds stimulated re-epithelialisation and deposition of collagens, demonstrating wound healing potential [78]. Platelet rich plasma (PRP) which is the fluid component of blood, can be obtained through centrifugation, contains higher amounts of platelets than blood, and is frequently used therapeutically e.g., cartilage repair surgery [79, 80]. Additionally, platelet rich fibrin (PRF), is a PRP-derivative which is rich in platelets, leukocytes, and growth factors within a fibrin matrix. The autologous nature and abundance of growth factors in both blood derivatives make them good candidates for the production of a wound healing biomaterial [81]. In DFU clinical trials, hybrid PRP/PRF scaffolds facilitated significant reduction in wound size in both diabetic and non-diabetic treatment groups [82]. A similar study by Game et al. demonstrated full wound closure in 34.1% of PRF-patch treated DFU patients, in comparison to 22% in the standard care control groups [83].
The inclusion of fibronectin in biomaterials has also been assessed for wound healing applications. Fibronectin meshes were produced by rotary jet spinning and were applied to full-thickness mouse wounds with polymer films as adhesives. The fibronectin meshes increased wound closure and epidermal thickness in comparison to non-treated wounds over 16 days [84]. Gelatin, or hydrolysed collagen, is another product of the ECM which has also been used as a biomaterial for wound healing. Owing to its bioactivity, biodegradability, and easily modifiable nature, it is particularly suitable for wound healing [85]. Gelatin hydrogels have been used as cell-delivery vehicles for wound healing, with laminin and fibronectin deposition shown in vitro, and accelerated wound closure, re-epithelialisation, and vascularisation in preclinical mouse models [86].
Although collagen, HyA, fibrin, fibronectin, and gelatin-based biomaterials have demonstrated promise, more complex biomaterials involving combinations of multiple ECM components may be necessary to drive wound healing. When used in combination, these biomaterials constitute a more complex structure which more faithfully recapitulates the native ECM. This can be seen in collagen-based biomaterials, which have been widely assessed in combination with a range of other ECM components. For example, collagen type I and HyA scaffolds have facilitated increased fibroblast attachment and proliferation in vitro [87]. Similarly, collagen type I-HyA hydrogels have facilitated both endothelial cell and fibroblast attachment, infiltration, and proliferation in vitro, further supporting their potential in wound healing. This was also confirmed in preclinical models, with the highest reduction in wound size after collagen type I-HyA hydrogel treatment [88]. Similarly, the IDRT is a bi-layered matrix which incorporates both collagen type I and chondroitin-6-sulphate. Application of IDRT to DFUs, traumatic wounds, and life-threatening burns has supported a significant decrease in wound size, and accelerated wound closure [48, 89–93].
Elastin has also been used in combination with collagens for wound dressings which have increased flexibility, supporting coverage of curved surfaces such as the toes [57]. In a 2013 clinical study, elastin/ collagen type I scaffolds demonstrated significantly faster wound healing, resulting in shorter hospital stays [94]. Further optimisation of collagen type I/elastin scaffolds, including pore alignment and cross-linking, has also enhanced their wound healing potential through increased fibroblast proliferation [95]. The wound healing potential of a collagen type III/elastin mesh was further substantiated in a case report of full wound closure after traumatic tissue loss in an elderly diabetic patient [96]. Sulphated HyA, chondroitin sulphate, and gelatin have also been combined to form electrospun scaffolds, which mimicked the biological and mechanical cues of native skin. This led to increased proliferation of fibroblasts, keratinocytes, and human MSCs [97]. Finally, clinical evaluation of the pro-healing capacity of PRF/HyA hydrogels demonstrated increased VEGF and decreased IL-6 expression, implying enhanced healing through angiogenesis and reduced inflammation [98].
ECM-derived biomaterials constitute a simple but promising approach to support healing of diabetic wounds, a summary of which is presented in Table 1. However, despite demonstrating clear promise, it is evident that the complexity of the ECM is not fully recapitulated in biomaterials composed of only a small number of ECM components. It is likely that alternate approaches are necessary that can more faithfully recapitulate the composition, complexity and functionality of the native ECM.
Table 1.
Summary table of purified ECM component biomaterials
| Biomaterial Components | Form Factor | In Vitro Characterisation | In Vivo Model | In Vivo Outcome | Reference |
|---|---|---|---|---|---|
| Collagen, ECM protein coating (collagen type 1 or laminin or fibronectin) | Electrospun Scaffold | Increased keratinocyte adhesion and spreading with collagen type 1 and laminin coating | Full-thickness, non-diabetic rat wound model |
Enhanced capillary density, fibroblast proliferation, and dense connective tissue after 1 week in collagen type 1-coated scaffold Epithelialisation complete after 1 week in both |
[56] |
| Collagen | Electrospun Scaffold |
No proliferation of mouse lymphocytes Increased keratinocyte adhesion, proliferation, and differentiation |
Full-thickness, non-diabetic rat wound model |
No significant activation of immune response Enhanced wound healing after 7 days Increased epithelialisation |
[57] |
| Collagen | Scaffold | High cell infiltration to scaffold at high mesenchymal stromal cell (MSC) seeding density | Alloxan-induced hyperglycaemic rabbit wound model |
Enhanced wound closure with higher MSC density Reduced radial diffusion No significant increase in inflammatory cells |
[58] |
| HyA | Scaffold |
Attachment and proliferation of endothelial cells Deposition of endothelial matrix in compacted scaffolds |
N/A | N/A | [74] |
| Gelatin, PEG | Hydrogel |
Enhanced adipose-derived stem cell (ASC) viability, proliferation, migration, and network formation when encapsulated Enhanced stemness and wound healing gene expression at high seeding density |
Stented excisional, splinted wound, non-diabetic mouse model |
Accelerated healing, hydrogel degradation, host cell infiltration remodelling, and vascularisation with ASCs No immune response to degradation products |
[86] |
| HyA, fibrinogen, collagen, gelatin, chondroitin sulphate | Fibrous Hydrogel | Higher porosity facilitated infiltration of fibroblasts | Full-thickness, splinted non-diabetic mouse models |
Accelerated wound closure and re-epithelialisation with higher porosity Increased vascularisation, Increased scaffold degradation and biodegradation |
[73] |
| Gelatin, HyA/ sulphated HyA, Chondroitin-4-sulphate | Scaffold | Increased proliferation and adhesion of fibroblasts, keratinocytes, mesenchymal stem cells | N/A | N/A | [97] |
| Collagen, HyA | Scaffold | No fibroblast toxicity, supported attachment and proliferation | Non-diabetic rabbit intramuscular implantation | Capsule formation, neovascularization, tissue ingrowth and cell infiltration. | [87] |
| Fibronectin | Rotary Jet-Spun Scaffold | N/A | Full-thickness wounds in C57BL/6 mice |
Accelerated wound closure Similar skin morphology Restored epidermal thickness Restored lipid layer Enhanced hair follicle and sebaceous gland recovery |
[84] |
| Fibrin-Alginate | Scaffold | N/A | Full-thickness porcine wound model | Reduced wound area and contraction | [99] |
Decellularised tissue
Decellularised ECM (dECM) consists of whole tissues from animal or human cadavers, which are subsequently treated to remove the cellular components and retain the native ECM structure [100]. A number of physical, chemical and enzymatic protocols have been developed to remove cells and ensure that the remaining ECM retains its complex composition, architecture, and bioactivity [100]. This class of biomaterial aims to provide a template for native cell infiltration and stimulate wound repair, a summary of which can be seen in Table 2. Although the structure of the tissue can be retained in these decellularisation processes, the material may also be reconstituted into other forms including lyophilised scaffolds, injectable hydrogels or electrospun structures [101]. Commonly used tissues for the production of decellularised tissue-derived biomaterials include the dermis, placenta, and porcine small intestinal submucosa (SIS) [9].
Table 2.
Summary table of decellularised tissue ECM-based biomaterials
| Biomaterial Components | Form Factor | Decellularisation Method | In Vitro Characterisation | In Vivo Model | In Vivo Outcome | Reference |
|---|---|---|---|---|---|---|
| Human dermis | Scaffold | Peracetic acid solution |
Endothelial cell and fibroblast attachment and infiltration ECM deposition Vascular network formation |
N/A | N/A | [105] |
|
Human amniotic membrane, reconstituted fibrin, HyA |
Scaffold |
Triton X-100 SDC Tris buffer Water |
Fibroblast adhesion and proliferation Non-cytotoxic Increased non-fibrotic gene expression Increased ECM deposition |
Full-thickness third-degree burn wounds in New Zealand white rabbits |
Native tissue adhesion Complete epithelialisation Angiogenesis Comparable native skin architecture |
[125] |
| Porcine small intestine submucosa | Scaffold | Mechanical separation from jejunum, tunica serosa and tunica muscularis externa | N/A | Full-thickness rat wound models |
Wound integration and exudate absorbance Horny layer at day 28 Non-significant decrease in wound area Reduced inflammatory cell infiltration Collagen deposition |
[127] |
| Human umbilical cord perivascular cell (HUCPVC)-seeded decellularised human dermis | Scaffold |
Hypo- & hypertonic solution Triton X-100 Potassium chloride Tris buffer Serine protease inhibitor Peracetic acid solution DNase and RNAase |
Enhanced proliferation and migration of HUVPVCs | Streptozotocin-induced diabetic, full-thickness rat wound model |
Accelerated wound closure Collagen fibre deposition and granulation tissue formation after 7 days Complete and thicker re-epithelialisation after 14 days Enhanced vascularisation and VEGFR-2 expression |
[106] |
| Amniotic membrane, chitosan | Scaffold |
PBS Trypsin SDS Water |
Enhanced cell viability | Streptozotocin-induced diabetic, full-thickness mouse wound model |
Accelerated wound healing Thicker granulation tissue Increased early-stage inflammatory cell and fibroblast infiltration Increased neovascularisation Enhanced late-stage healing with hair follicles and sebaceous glands Enhanced collagen deposition and organisation |
[124] |
| Porcine dermis or urinary bladder | Hydrogel |
Mechanical delamination Trypsin Water Ethanol Triton X-100 in EDTA/Tris Peracetic acid |
Fibroblast infiltration and proliferation on both hydrogels High myoblast viability and myotube formation on dermal hydrogel |
Rat partial-thickness abdominal wall defects |
CD68 + cell infiltration Defect remodelling and myogenesis at borders of defect Higher myogenesis in urinary bladder hydrogels |
[108] |
| Human amniotic membrane | Scaffold |
Ethanol Water Triton X-100 Lipase DNase |
N/A | Full-thickness rat wound models |
Reduced wound area Reduced white blood cell count Increased α-smooth muscle actin Reduced TGF-β1 expression |
[118] |
Decellularisation is typically achieved using chemical, physical and enzymatic protocols, usually involving a combination of multiple methods to ensure complete removal of the cellular components [102]. Chemical decellularisation includes a variety of treatments such as detergents which disrupt the cell membrane, for example sodium dodecyl sulphate (SDS), sodium deoxycholate (SDC), and Triton X-100. Enzymatic decellularisation is characterised by the cleavage of DNA, RNA and proteins, using nucleases, proteases, and trypsin [103]. Finally, mechanical decellularisation involves physically disrupting cell-ECM interactions through repetitive cycles of freeze-thawing, increasing hydrostatic pressure, cell scraping, and other disruptive treatments [100]. Although these methods remove much of the cellular components, they are typically insufficient on their own and require an additional decellularisation procedure for complete cell removal. Similarly, decellularised matrices should be evaluated post-treatment to ensure key structural integrity and mechanical properties have not been disrupted [103]. The decellularisation method used must be optimised depending on the tissue used, ensuring efficient retention of ECM components, given the potential for ECM disruption by stronger detergents [104].
Donor dermis sections are decellularised to form acellular dermal matrices (ADM), providing a template for cell infiltration and proliferation. For example, decellularised reticular dermal tissue has allowed for the infiltration of fibroblasts and endothelial cells, in addition to their deposition of key ECM components e.g., collagen IV [105]. Further, decellularised dermal tissue scaffolds have accelerated wound healing in a diabetic rat model, through increased re-epithelialisation and matrix deposition [106]. Upon delivery of human umbilical cord perivascular cells, re-epithelialisation and matrix deposition was further enhanced, in addition to the increased expression of vascular endothelial growth factor (VEGF) receptors and vascularisation [106]. ADMs may also be further processed and reconstituted into lyophilised sponges or injectable hydrogels. For example, pepsin digestion of the decellularised dermis, followed by induction of physiological pH and osmolarity stimulates hydrogel formation [107]. Porcine dermal hydrogels produced using this process have supported cell viability and infiltration, demonstrating their potential in DFU treatment [108]. Finally, cadaveric dermis-derived scaffolds have facilitated host cell invasion in a murine wound model, despite the fact that only 92.1% of DNA was removed during decellularisation [109]. Optimisation of the decellularisation protocol significantly improved DNA removal (99.8%) through the addition of a gamma irradiation step. Although gamma irradiation is widely reported to cause collagen crosslinking, basement membrane damage and radiation-induced fibronectin damage, there was no difference observed in mechanical properties, host cell infiltration, or collagen denaturation [110, 111].
ADMs have demonstrated clinical success in the treatment of diabetic wounds, with a number of studies reporting significant reductions in wound size, and healing times [112, 113]. Similarly, ADMs on the market including Alloderm™, DermACELL™, GraftJacket®, and AlloPatch® offer a promising alternative to full-thickness skin grafts. Alloderm™, derived from cadaveric skin, has been used clinically for the treatment of full-thickness burns, showing decreased fibrosis and scar formation in comparison to other dermal substitutes [49, 114]. In a multicentre assessment of two human-derived ADMs, DermACELL™, and GraftJacket®, it was determined that DermACELL™ enhanced DFU healing in comparison to standard treatment, with 67.9% wound closure and 91.4% reduction in wound size [115]. Notably, this represented a significant improvement in DFU healing in comparison to previous studies [48, 114].
While the skin appears to be the most relevant tissue type from which to extract ECM and develop a biomaterial for wound repair, a number of other tissues have been assessed. This includes the amniotic membrane which has gained significant interest for tissue repair due to the abundance of HyA, collagens and laminins in its ECM [116]. Furthermore, the functions it serves in utero, (tissue development and growth, environmental protection, embryonic development, water retention, angiogenesis, and growth factor/nutrient transfer) closely align with the functions required of a biomaterial for wound repair [117]. The use of decellularised human amniotic membrane in the treatment of full-thickness rat wound models significantly reduced wound size. Similarly, VEGF and α-smooth muscle actin expression was enhanced, indicating the onset of the proliferative and remodelling phase respectively [118]. Biovance® is a clinically approved, decellularised amniotic membrane-based wound dressing which is used for DFU treatment [119]. In the Letendre trial, Biovance®-treated DFU wounds decreased significantly in size over 12 weeks, with at least a 50% reduction in over 80% of participants, including complete closure in 55.5% of participants [120, 121]. Similarly, Omnigen, clinically approved for use in ocular wounds, has significantly reduced wound size when applied to patient DFUs, in comparison to standard care in DFU patients [122]. Epifix is a decellularised tissue matrix which is derived from sections of human chorion and amnion layers, which showed superior DFU healing in comparison to established ADMs [123]. Lastly, the combination of decellularised amnion and chitosan has facilitated proliferation of fibroblasts, in addition to the acceleration of diabetic wound healing, vascularisation, and ECM deposition in mouse models [124].
Despite promising results in decellularised amniotic membrane studies, there are drawbacks such as immunogenicity, difficult handling and manipulation, and the need for adhesives to secure the biomaterial in place. In order to partially overcome these challenges, Ramakrishnan and colleagues reinforced the mechanical properties of decellularised amniotic membrane through the addition of a fibrin-HyA scaffold layer. The scaffold which had increased thickness and tensile strength, was shown to facilitate fibroblast adhesion and proliferation, in addition to endothelial cell recruitment and reduced immunogenic effects [125].
Porcine SIS has also been extensively investigated for the treatment of DFU. Its ECM composition is similar to that of skin, with high collagen, elastin and glycosaminoglycan (GAG) content. Furthermore, it has been shown to facilitate cellular processes important for wound healing, including angiogenesis, cellular proliferation and differentiation [126]. Despite immediate inflammatory signs, the implantation of a SIS-derived sponge to a full-thickness wound in a rat model facilitated increased exudate absorption, re-epithelialisation and inflammatory resolution after 4 weeks, in comparison to established wound dressings [127]. The treatment of DFUs with Oasis®, a SIS-derived 3-layered decellularised matrix, reduced wound size, and aided complete wound healing in 54% of patients, compared to 32% of those treated with standard care [128]. Similarly, the treatment of pressure ulcers, another type of chronic wound, with Oasis® significantly improved healing, with 55% reduction in wound size over 12 weeks. This surpassed the healing of standard care-treated wounds, which failed to exceed 40% incidence [129].
In summary, decellularised tissue-derived biomaterials have demonstrated significant promise in the treatment of DFUs and chronic wounds through the facilitation of increased wound healing, ECM deposition and re-epithelialisation. This is further emphasised by their approval and use in the clinic. However, important considerations to be made include optimisation of the decellularisation protocols to mitigate any potential immunogenic responses. In addition, while a wide range of tissue sources have demonstrated pro-regenerative effects, the optimal source has not been identified, and may differ depending on the type of wound and/or the precise anatomical location.
In vitro-generated ECM biomaterials
Biomaterials generated from in vitro-deposited ECM constitute an innovative approach to wound healing. Two main approaches have been explored, involving the decellularisation and collection of ECM deposited by cells in 2D culture and its incorporation within a biomaterial, or direct deposition of ECM by cells seeded within a pre-existing biomaterial structure, followed by cell removal [16]. There are many benefits to these approaches, particularly their tunability and specificity. For example, the selection of relevant cell types, control over the microenvironment, and stimuli which cells are exposed to, allows for optimisation of the ECM deposition. This level of control is not possible in other methods including tissue decellularisation, as previously outlined [130].
For the production of in vitro-generated ECM biomaterials, cell monolayers are decellularised for the preservation of the deposited ECM, followed by ECM collection and incorporation within a biomaterial [131]. For example, ECM deposited by human induced pluripotent stem cell (hiPSC)-derived fibroblasts was decellularised and subsequently incorporated into a collagen-GAG scaffold by lyophilisation. Higher anti-inflammatory M2 macrophages and VEGF expression was observed in ECM-functionalised scaffolds, indicating their promise to direct the resolution of inflammation and enhance vascularisation [132]. It is also interesting to note the greater capacity of ECM deposited from hiPSC-derived fibroblasts to drive pro-reparative processes in comparison with ECM derived from adult fibroblasts [132]. These effects may be related to the apparent ‘rejuvenation’ of cells, closely resembling their foetal counterparts, thus promoting scarless healing [93]. In another pre-clinical study, scaffolds were produced combining decellularised lung fibroblast-derived ECM and collagen type I. The application of this scaffold in diabetic wound models showed great potential for DFU healing, with higher rates of wound closure, improved vascularisation, and increased collagen deposition in vivo [133]. While these studies emphasised the potential of the technique in DFU therapeutics, they also indicate areas in which the technique can be further enhanced. For example, optimisation of the cellular niche through manipulation of the cellular microenvironment may induce more potent effects on processes related to wound healing such as angiogenesis. For example, hypoxic conditions in human dermal fibroblast cultures significantly increased ECM-deposition in vitro, in addition to increased endothelialisation of cells on a polycaprolactone (PCL) graft, VEGF, and angiotensin-1 expression [134].
Another approach involves the direct seeding of cells on or in an existing biomaterial, in which they deposit ECM over time. Subsequently, decellularisation produces an acellular construct which is laden with cell-deposited ECM. In a comparative study, patient-derived iPSC fibroblasts induced similar ECM deposition to 2D seeding, with enhanced GAG deposition and vascularisation when seeded to a scaffold [135]. Similarly, mesenchymal stromal cells seeded on silk fibroin scaffolds and decellularised, enhanced wound healing in diabetic mouse models. This was demonstrated by reduced wound size over 10 days, increased migration of human umbilical vein endothelial cells (HUVECs) and related expression of angiogenic growth factors, in comparison to silk fibroin controls [136]. However, the decellularisation of pre-optimised scaffolds raises a concern of compromised mechanical properties and structural properties following decellularisation. Thus, care must be taken to optimise the decellularisation process to avoid damaging the structural integrity of the scaffold. To overcome this, ECM-derived scaffolds have been produced using a poly(lactic-co-glycolic) acid template, which was removed after the decellularisation process. This allowed for the formation of pure ECM scaffolds which facilitated high fibroblast viability, attachment, and proliferation indicating their potential in skin regeneration [137].
Although the number of skin-related studies is limited, the success of in vitro-generated ECM biomaterials in the regeneration of other tissues can be extrapolated and applied to the development of DFU therapeutics in the future. For example, Carvahlo et al. seeded co-cultures of MSCs and HUVECs on ECM/PCL electrospun scaffolds for bone tissue engineering. The combination of lyophilised MSC- and HUVEC-ECM with polycaprolactone (PCL) facilitated enhanced osteogenesis and angiogenesis simultaneously [131]. This indicates the potential of this approach, which may be adapted and harnessed for the treatment of DFUs.
Finally, a major limitation of cell-derived ECM is the duration of culture required to produce a sufficient yield. As such, a range of approaches are being explored to enhance and accelerate ECM deposition. For example, macromolecular crowding is a particularly promising technique based on the excluded-volume effect, such that the addition of an inert macromolecule enhances ECM deposition [138]. Ficoll and carrageenan have been identified as promising macromolecules to enhance ECM deposition of iPSC-derived fibroblasts and human adipose-derived stem cells in vitro, and have subsequently been used to produce ECM-derived biomaterials that support healing in murine wound models [139, 140]. Similarly, addition of ascorbic acid to culture media facilitates ECM deposition through the upregulation of collagen gene transcription and synthesis, and inhibition of their degradation [141]. A summary of in vitro generated ECM-derived biomaterials is depicted in Table 3.
Table 3.
Summary table of in vitro generated ECM-derived biomaterials
| Biomaterial Components | Form Factor | Decellularisation Method | In Vitro Characterisation | In Vivo Model | In Vivo Outcome | Reference |
|---|---|---|---|---|---|---|
|
Fibroblast-deposited ECM, collagen |
Scaffold |
0.25% Triton X-100 Ammonium hydroxide DNase & RNase |
Increased fibroblast migration α-SMA negative quiescent fibroblasts ECM deposition Upregulation of tissue remodelling markers |
Full-thickness wounds, leptin receptor db/db mice |
Increased wound healing, blood vessel area, remodelling and hair follicles Increased wound closure High mature blood vessel area Increased collagen deposition Reduced dermal/epidermal thickness |
[133] |
|
Fibroblast-deposited ECM, collagen, chondroitin-6-sulphate |
Scaffold |
Water Snap-freeze Lyophilisation |
Extended healthy and DFU fibroblast proliferation and GAG deposition Increased ECM deposition and component variety of all ECM-derived scaffolds Enhanced inflammatory response |
N/A | N/A | [132] |
|
Patient-derived fibroblast- deposited ECM, collagen, chondroitin-6-sulphate |
Scaffold |
Water Freeze-thaw DNase |
Enhanced healthy and patient-matched DFU fibroblast proliferation Increased vessel area and GAG deposition in decellularised scaffold |
N/A | N/A | [135] |
| Mesenchymal stromal cell-deposited ECM, silk fibroin | Scaffold | Water | Enhanced HUVEC migration and angiogenic factor secretion | Punch biopsy wounds on leptin receptor db/db mice | Improved wound healing of 49 ± 0.8% compared to 50 ± 2.4% in not decellularised control in 3 days Increased expression of angiogenic and remodelling genes | [136] |
| Fibroblast-deposited ECM within Poly (lactic-co-glycolic acid) scaffold | Scaffold |
Freeze-thaw Ammonium hydroxide |
High fibroblast viability Even fibroblast distribution Dermal regeneration |
N/A | N/A | [137] |
Mechanisms of action
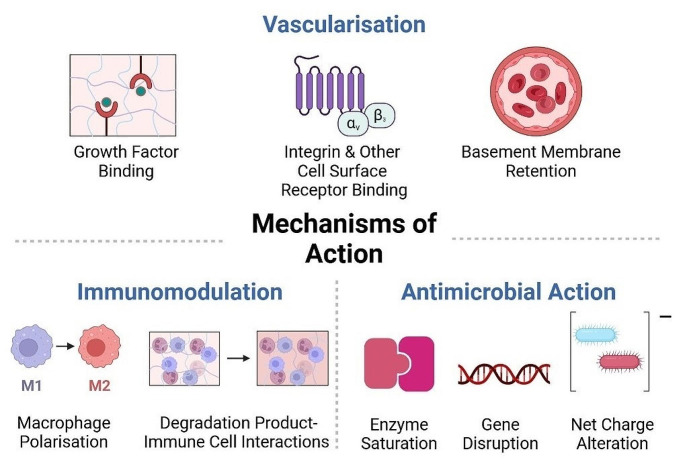
ECM-derived biomaterials have demonstrated great promise for tissue repair in a wide range of applications including the treatment of chronic wounds. A summary of the clinical studies associated with many of these ECM-derived biomaterials can be seen in Table 4. In order to fully harness the promise of ECM-derived biomaterials, it is essential that the mechanisms of action of these materials are fully understood. Generally, biomaterials aim to support tissue repair by providing a structure or template that supports host cell infiltration and proliferation. In addition to this, ECM-derived biomaterials support tissue repair through a number of mechanisms associated with their inherent bioactivity, including immunomodulation, promotion of vascularisation and antimicrobial activity. Each of these mechanisms, and how they relate to wound repair, are discussed below, with a visual summary in Fig. 3.
Table 4.
Summary table of ECM-derived biomaterials clinical studies
| Biomaterial Components | Form Factor | Patient Cohort | Clinical Outcome | Reference |
|---|---|---|---|---|
| Purified ECM Component Biomaterials | ||||
| Collagen, Chondroitin-6-Sulphate, Silicone | Scaffold | DM patients with ≥ 1 DFU with grade 1/2 Wagner classification | Accelerated wound closure | [48] |
| Collagen, Chondroitin-6-Sulphate, Silicone | Scaffold | Full-thickness traumatic wounds | 78% wound closure in treatment group | [91] |
| Collagen, Chondroitin-6-Sulphate, Silicone | Scaffold | Patients with surgically excised burn wounds | Comparable vascularisation and scaffold anchorage to wound for epidermal growth | [92] |
| Collagen, Chondroitin-6-Sulphate, Silicone | Scaffold | Acute and chronic burn patients | 88% full restoration of articular motion and flexible skin coverage | [93] |
| Collagen, Elastin | Scaffold | Type-2 diabetic patients with chronic DFUs | Shorter hospitalisation period and accelerated healing | [94] |
| Collagen, Elastin | Scaffold | Traumatic skin loss recovery in a diabetic, hypertensive, and ischemic heart disease patient | Reduced loss of substance, complete closure, decreased limb oedema, functional rehabilitation | [96] |
| Collagen, Oxidised Regenerated Cellulose | Scaffold | DM patients with DFU for ≥ 6 weeks | Reduced level of MMP-2, gelatinase, elastase in wound exudate and reduced wound size | [60] |
| Collagen Type I | Particles | Patients with non-healing ulcers without bone exposure | 37.29% wound reduction compared to 14.29% in the control group | [61] |
| Platelet Rich Fibrin | Combined platelet-derived scaffold and injectable PRP gel |
Diabetic and non-diabetic advanced wound patients |
Diabetic patient wound size reduced to less than 25% after 4 weeks Enhanced wound healing in both diabetic and non-diabetic patients |
[82] |
| Platelet Rich Fibrin |
Patch (Autologous fibrin) |
DM patients with ≥ 1 DFU and a baseline HbA1c of ≤ 108 mmol/mol | Accelerated and enhanced wound healing | [83] |
| Platelet Rich Fibrin, HyA | Hydrogel | DM patients with 3-month duration DFU with grade 2 Wagner classification | Increased VEGF and decreased IL-6 after 7 days | [98] |
| Collagen, Chondroitin-6-Sulphate |
Hydrogel (Granular collagen mixed with saline) |
Diabetic patients with DFUs with grade 3 Wagner classification |
Accelerated and 86.95% complete healing in treatment group | [90] |
| Collagen, Chondroitin-6-Sulphate |
Hydrogel (Granular collagen mixed with saline) |
Diabetic patients with DFUs with grade 3 Wagner classification |
Decreased infection, accelerated healing compared to IDRT, reduced expression of inflammatory biomarkers, increased expression of ECM remodelling and angiogenesis | [89] |
| HyA | Scaffold | Diabetic patients with DFUs with grade 1/2 Wagner classification | 65.3% complete healing compared to 49.6% in control group | [64] |
| HyA | Scaffold | Diabetic patients with DFUs for ≥ 1 month | Accelerated wound closure in treatment group | [75] |
| Decellularised Tissue Biomaterials | ||||
| Decellularised human dermis |
Scaffold (Chemical and enzymatic decellularisation, and gamma irradiation) |
Volunteer full-thickness punch biopsy wounds | Increased dermal thickness, reduced dermal fibrosis | [114] |
| Decellularised human dermis |
Dressing (Chemical decellularisation and lyophilisation) |
Diabetic patients with a 1 full-thickness DFU with Wagner classification of 1 or 2 | Higher complete healing rate than control, reduced wound area compared to all groups | [115] |
| Decellularised human dermis |
Hydrogel (Dermis in aqueous gelatin solution) |
Patients with chronic full-thickness wounds which failed to heal over 3 weeks | 76.3% complete healing in 12 weeks, accelerated granulation and re-epithelialisation | [112] |
| Decellularised human dermis |
Hydrogel (Dermis in aqueous gelatin solution) |
Diabetic patients with DFUs with Wagner classification grade 2 or 3 for at least 4 weeks | 56.52% complete healing over 60 days compared to 23.08% in control group, accelerated wound healing compared to control | [113] |
| Decellularised human amniotic membrane |
Scaffold (Ocular ulcer treatment) (Chemical decellularisation) |
Diabetic patients with ≥ 1 DFU | 27% healing compared to 6.3% in control group, higher wound area reduction | [122] |
| Porcine small intestine submucosa |
3-Layered Scaffold (Chemical decellularisation) |
Diabetic patients with ≥ 1 DFU for > 6 weeks to 1year | 54% healing compared to 32% in control group, higher wound area reduction at all visits | [128] |
| Decellularised human dermis |
Scaffold (Chemical decellularisation) |
Full-thickness burn patient | Keratinocyte and fibroblast infiltration, neovascularisation, wound re-epithelialisation, increased dermal elasticity | [49] |
| Decellularised human amniotic membrane |
Scaffold (Chemical decellularisation) |
DM patients with chronic DFUs with Wagner classification of 1 or 2 | 55% re-epithelialisation, 50% reduction in wound size in 33.3% of patients | [121] |
Fig. 3.
The mechanisms of action through which biomaterials stimulate wound healing consist of angiogenesis, immunomodulation, and antimicrobial action. Through growth factor binding, integrin and other cell-surface receptor binding, and the retention of basement membrane proteins and architecture, biomaterials stimulate angiogenesis. Through macrophage polarisation, and cellular interactions with biomaterial degradation products, biomaterials elicit immunomodulatory effects. Finally, through the inhibition of hyaluronidase, gene disruption and alteration of the bacterial cell wall net charge, biomaterials can induce wound healing through antimicrobial action. Figure was created in Biorender
Immunomodulation
Inflammation and its timely resolution are crucial to DFU healing, and tissue repair in general. As an initial response to injury, inflammation plays a key role in initiating the repair response, ensuring the removal of necrotic debris and invading microbes [142]. However, in compromised wound healing and chronic wounds such as DFUs, the inflammatory response fails to resolve in a timely manner, manifested by the persistence of M1-type macrophages in the wound. In a healing wound, alteration of the macrophage phenotype from M1 to M2 shifts the balance of the wound microenvironment from pro-inflammatory to anti-inflammatory, supporting inflammatory resolution and wound healing [25]. ECM-derived biomaterials have been shown to play a key role in this process, displaying an innate capacity to drive macrophage polarisation towards a pro-healing phenotype [18]. In the context of ECM-derived biomaterials, an inflammatory cascade is initiated after scaffold implantation to a wound site. This includes infiltration of a variety of immune cells including macrophages and T-cells, which stimulate paracrine signalling and macrophage polarisation [143]. However, there are many contributary factors which may affect the immune response to ECM biomaterials. This can include the degradation products of the material, its tissue origin, and the efficiency of decellularisation.
Degradation products play a key role in ECM biomaterial-driven immunomodulation as the interactions between degradation products and immune cells (including T-regulatory, T-helper cells, and macrophages) drives the downstream immune reaction [144]. Studies have investigated the potential of ECM-derived biomaterials and their degradation products to promote skeletal muscle regeneration and induce macrophage polarisation. After treatment with SIS-ECM degradation products, a high frequency of Fizz1, a marker for M2 activation, was detected in bone marrow derived macrophages, indicating the anti-inflammatory immunomodulatory capacity of SIS-ECM [145]. Similarly, the ECM tissue-of-origin contributes to macrophage polarisation, as seen by the differential capacity of decellularised tissues to induce M2 polarisation in vitro, with the M2 phenotype potently driven by SIS, bladder, and colonic tissue ECM-derived scaffolds. Interestingly, a pro-inflammatory effect was seen after treatment with dermal-ECM, represented by iNOS+, Fizz1-, and cluster determinant molecule (CD)-206 expression [146].
Furthermore, the efficacy of the decellularisation protocol is a key determinant of the immunomodulatory capacity of a decellularised tissue-derived biomaterial. Remnants of foreign body antigens can stimulate the persistence of a pro-inflammatory, non-healing, M1 phenotype [147]. In contrast, more intensive or thorough methods of decellularisation, elicit a predominant M2 phenotype in decellularised SIS-derived biomaterials [148]. Moreover, it has been recently determined that the implantation of decellularised tissue ECM-derived biomaterials can drive the foreign body response towards a modulatory phenotype. Decellularised cardiac muscle and bone ECM-based biomaterials stimulated T-helper cell and IL-4 driven macrophage M2 transition, initiating paracrine signalling, remodelling and healing in a volumetric muscle loss model [149]. Similarly, a porcine SIS-derived material induced a substantial reduction of classical Inflammatory Bowel Disease markers in a murine model. This occurred by modulation of the M1 macrophage polarisation and reduction in TNF-α expression [150].
Specific purified ECM components including HyA have also been shown to induce M2 macrophage polarisation [18]. High molecular weight HyA in particular, has been shown to drive M2 macrophage polarisation, which is associated with the expression of anti-inflammatory cytokines such as IL-10 [151]. When high molecular weight HyA binds to CD44, phagocytosis is initiated, promoting the timely resolution of inflammation, and healing [152]. This effect can be further enhanced through functionalisation of HyA through the addition of sulphate groups. This was seen through the regulation of oxidative stress and upregulation of associated antioxidants such as superoxide dismutase (SOD)-2 and − 3 [153]. The anti-inflammatory effects of HyA were further demonstrated by a sulphated, high molecular weight HyA/Collagen hydrogel. In a diabetic mouse model, it was observed that wounds which were treated with the sulphated HyA/Collagen hydrogel had lower expression of pro-inflammatory genes including IL-1β and NLR family pyrin domain containing 3 (NLRP3), higher expression of IL-10, and higher rates of wound healing, in comparison to non-sulphated controls [154].
The unique capability of ECM-derived biomaterials to tune the immune response during wound healing is key factor which makes them particularly useful in DFU therapeutics. The interactions which occur between immune cells, biomaterials and their degradation products, can drive immunomodulation through mitigation of the foreign body response and macrophage polarisation.
Vascularisation
Vascularisation of the wound bed, and the restoration of oxygen/nutrient delivery, is critical to wound healing [155]. In general, the ECM contains an abundance of pro-angiogenic components, growth factors, and signalling molecules which support and/or promote vascularisation through integrin-mediated endothelial cell-matrix interactions [156]. In particular, αvβ3 and αvβ5 integrins expressed by endothelial cells, play key roles in vascularisation through the promotion of endothelial cell proliferation [157]. Similarly, ECM components play a key role in growth factor retention and signalling to promote vascularisation. Fibroblast growth factor (FGF) for example, is a known pro-angiogenic growth factor that binds to heparan sulphate present in the ECM and propagates downstream signalling required for endothelial cell proliferation, migration and blood vessel formation [158]. For example, perlecan, a heparan sulphate proteoglycan typically present in basement membranes, facilitates angiogenesis through FGF and VEGF binding [159]. Conjugation of heparan sulphate to collagen-based biomaterials enhances growth factor retention within the hydrogel, supporting blood vessel formation as observed in the chorioallantoic membrane assay [160].
ECM-derived biomaterials in general, have shown significant angiogenic potential, for example, purified ECM component biomaterials containing fibrin, HyA, collagens, and gelatin. The presence of αvβ3 and heparin binding domains on fibrin allows for cell attachment, biomaterial infiltration, and subsequent angiogenesis [161]. Similarly, in fibrin-based biomaterials, plasmin-mediated fibrinolysis and the breakdown of the fibrin complex forms multiple fragments, with fragment E playing a pro-angiogenic role in wound healing [162]. A number of in vivo studies have demonstrated the ability of fibrin and collagen hydrogels to facilitate angiogenesis in ischemic tissue, for example in a rat model of myocardial infarction [163].
Further, low molecular weight HyA is known to initiate neo-vascularisation through cell surface receptor binding, initiation of the inflammatory cascade, and eventual endothelial cell proliferation [164]. Cell surface receptors such as CD44 and receptor for HA-mediated motility (RHAMM) bind to HA oligomers, thus inducing mitogenesis and endothelial cell proliferation through mitogen-activated protein (MAP) kinase signal transduction [165]. The modification of HyA-based biomaterials is a common approach to the stimulation of angiogenesis/vascularisation. For example, the addition of RGD binding sites to HyA has facilitated increased vessel lumen formation, neovascularisation, and vascular patterning in vitro [166]. Similarly, the delivery of pro-angiogenic growth factors including VEGF and PDGF (platelet-derived growth factor) has been possible through their adhesion to binding sites on laminin (multiple isoforms)-fibrin based biomaterials. The delivery of these growth factors through heparin binding domains on α-chain laminin-type G and their inclusion in fibrin matrices, successfully promoted wound healing in DFU mouse models [167].
Decellularised tissue matrices similarly provide a platform to promote angiogenesis. Through the retention of ECM components and growth factors, decellularised tissues can stimulate pro-angiogenic processes [168]. Interestingly, the decellularisation protocol used can also have a potent effect on the formation of new vessels. This includes maintaining the architectural integrity of the ECM, allowing for effective recellularisation and vascularisation [169]. Similarly, retention of the vascular basement membrane proteins can be a critical predictor of the angiogenic capacity of decellularised tissue-derived ECM biomaterials, due to their abundance of collagens, fibronectin, proteoglycans etc. [170]. This was further seen in vitro, with immobilised and aligned ECM components mimicking the basement membrane. The aligned ECM protein components facilitated the formation of new blood vessels through HUVEC adhesion and proliferation [171]. Finally, the incorporation of cell-derived ECM has displayed pro-angiogenic potential when incorporated in collagen scaffolds. HUVEC seeding on iPSC-derived fibroblast ECM-collagen-GAG scaffolds resulted in increased tube formation and vascularisation, indicating their pro-angiogenic potential in wound healing [135].
Antimicrobial action
The non-healing nature of DFUs is exacerbated by the presence of bacterial or microbial colonies which elevate and extend the inflammatory response [120]. Although limited, there is some evidence supporting the antimicrobial action of ECM components, most notably HyA [172]. In a dose- and molecular weight-dependant manner, reduced growth of S. aureus and various other bacterial pathogens, has been demonstrated on HyA-based biomaterials, in comparison to biomaterials which are commonly used in orthopaedic implants [173]. Although yet to be fully elucidated, one proposed antimicrobial mechanism of HyA is related to bacterial synthesis of hyaluronidase. Normally, bacterial synthesis of hyaluronidase facilitates local degradation of HyA, allowing bacterial colonisation and infection within a wound. However, it has been speculated that the presence of a higher concentration of HyA saturates the hyaluronidase enzyme, thus reducing bacterial hyaluronidase activity, bacterial proliferation, and preventing attachment within the wound site [174]. Similarly, the amide and carboxyl groups present on the HyA macromer elicit a combined negative charge that repels the negatively charged bacterial cell wall, and prevents the attachment of microbes [175]. Liu et al., fabricated a HyA-based hydrogel which was enzymatically crosslinked with Ɛ-polylysine. This combination of HyA and Ɛ-polylysine effectively reduced the amount of E. coli and S. aureus in infected rat wounds in this instance [176].
Similarly, other ECM-components including laminin and fibronectin have also been reported to elicit antimicrobial activity through bacterial DNA disruption. Through heparin-binding, several α4 and α5 laminin peptide chains initiate membrane lysis and DNA destruction of E. coli and S. aureus [177]. Finally, the collagen IV α3 subunit causes intracellular damage to bacteria including S. aureus by inducing damage to the extracellular membrane and releasing the cellular contents [178]. The innate antimicrobial capacity of biomaterials can be further enhanced through several methods including surface modifications, pH manipulation, and the addition of specific antimicrobial agents. For example, the addition on chitosan, a natural polysaccharide derived from crustaceans with known antimicrobial activity, or conjugation of antimicrobial peptides (AMPs) [179–181].
Discussion
Although the current standard of care for diabetic wounds contains rigorous protocols for wound dressing, antibiotic treatment, and pressure off-loading, these approaches are insufficient to resolve the chronic wounds of diabetic patients. This is evident from the frequency of limb amputations worldwide as a direct result of DFUs. In recent years, the growing potential of biomaterials for the treatment of DFU has come to the fore, with a particular focus on the development of ECM-derived biomaterials [13]. The ECM-derived biomaterials discussed within this review include biomaterials composed of isolated ECM components, and the combination of multiple components. Similarly, the decellularisation of tissues, and the incorporation of in vitro-deposited ECM in biomaterials, allows for the inclusion of a full biological repertoire of ECM components within a biomaterial, similar to that of the native tissue. These ECM-derived biomaterials support wound healing through vascularisation, immunomodulation, and protection against microbes, in comparison to the current standard of care.
While this review discusses a number of highly promising studies, further optimisation of decellularisation techniques is necessary to fully understand and harness the potential of ECM-derived biomaterials in wound healing. For example, in vitro cell-deposited ECM is a relatively recent and novel technique and is less studied as a result. Similarly, future studies should focus on the functionalisation of ECM-derived biomaterials to further support their therapeutic effect, including the addition of therapeutic agents to further their inherent capacity to drive immunomodulation, angiogenesis, and antimicrobial action [155, 182, 183]. Recent examples of this include the loading of decellularised ovine pericardial-derived scaffolds with resveratrol, which enhanced their antibacterial, swelling, and water retention capacities [184]. Similarly, the addition of zinc oxide and vitamin c to decellularised caprine small intestine submucosa hydrogels enhanced burn wound healing through increased wound contraction, reduced inflammatory cell infiltration, and increased collagen deposition in rabbit models [185].
Other potential avenues for the optimisation and understanding of ECM-derived biomaterials for wound healing include their assessment in microphysiological systems, or so-called organs-on-a-chip [186]. The development of microfluidic systems to interrogate the bioactivity and regenerative capacity of ECM-derived biomaterials using human cells would allow for more high throughput analysis of ECM-derived biomaterials in more physiologically relevant systems with human cells and incorporation of biophysical stimuli such as fluid flow [187]. Similarly, it would facilitate more accurate assessment of pro-angiogenic biomaterials and the vasculature in vitro, through the induction of biophysical signals such as shear stress, fluid flow, offering efficient, reproducible, and complementary systems to preclinical animal testing [188, 189]. For example, the pro-angiogenic capacity of human fibroblast-deposited ECM/ collagen type I composite hydrogels has been assessed in microfluidic devices that incorporate fluid flow [190]. This type of high throughput system can act as a bridge to refine and optimise ECM-derived biomaterials prior to preclinical studies, reducing the use of animal models while also providing a 3D test-bed that recapitulates critical aspects of human physiology.
Moreover, through analysis of reported healing rates, it has become evident that DFU severity is heterogenous due to a variety of contributing factors including patient age, overall health, and lifestyle, thus preventing a ‘one size fits all’ solution [6]. Incorporating organ-on-a-chip and advanced stem cell technologies in DFU research has the potential to facilitate the development of disease or even patient-specific or personalised medicine approaches, and the stratification of patients by wound severity using ‘on-chip’ physiology. The incorporation of patient-specific cells would facilitate a deeper understanding of the cellular processes within the DFU of a specific patient, and how ECM components and complementary therapeutics could contribute to its healing through inflammatory resolution, angiogenesis, and antimicrobial action.
Concluding remarks
ECM-derived biomaterials show great promise to improve on the current standard of care for the treatment of DFU. However, a number of hurdles must be overcome before this becomes a reality. This includes the optimisation of tissue sources, and approaches to their subsequent processing and biomaterial development, which are yet to be defined. The biomaterials discussed within this review incorporate the ECM in a variety of ways, ultimately aiming to develop regenerative biomaterials to support healing. In this way, ECM-derived biomaterials stimulate processes such as immunomodulation, angiogenesis, and antimicrobial action to drive wound healing. The translation of these biomaterials to pre-clinical and clinical DFU studies will reduce the burden of DFUs, ultimately restoring some quality of life to DM patients.
Acknowledgements
We acknowledged funding from the Anatomical Society and this is expected by the Anatomical Society.
Author contributions
FQ and SB designed the work and supervised it, contributed to the writing of the contents, and to revise the manuscript. LH was the main writer of the contents and the main author of the revision. TH contributed to the writing of the contents.
Funding
Open Access funding provided by the IReL Consortium
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
Ethics approval and informed consent are not applicable to this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fabio Quondamatteo and Shane Browne share Joint Senior Authorship.
Contributor Information
Fabio Quondamatteo, Email: fabioquondamatteo@rcsi.ie.
Shane Browne, Email: shanebrowne@rcsi.ie.
References
- 1.Quondamatteo F (2014) Skin and diabetes mellitus: what do we know? Cell Tissue Res 355:1–21. 10.1007/s00441-013-1751-2 [DOI] [PubMed] [Google Scholar]
- 2.Ong KL, Stafford LK, McLaughlin SA et al (2023) Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of Disease Study 2021. Lancet 402:203–234. 10.1016/S0140-6736(23)01301-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skyler JS (2004) Diabetes Mellitus: Pathogenesis and treatment strategies. J Med Chem 47:4113–4117. 10.1021/jm0306273 [DOI] [PubMed] [Google Scholar]
- 4.Packer CF, Ali SA, Manna B (2022) Diabetic Ulcer. In: StatPearls. StatPearls Publishing, Treasure Island (FL) [PubMed] [Google Scholar]
- 5.Forbes JM, Cooper ME (2013) Mechanisms of Diabetic complications. Physiol Rev 93:137–188. 10.1152/physrev.00045.2011 [DOI] [PubMed] [Google Scholar]
- 6.McDermott K, Fang M, Boulton AJM et al (2022) Etiology, epidemiology, and disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 46:209–221. 10.2337/dci22-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandyk DF (2018) The diabetic foot: pathophysiology, evaluation, and treatment. Semin Vasc Surg 31:43–48. 10.1053/J.SEMVASCSURG.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 8.Kimura T, Thorhauer ED, Kindig MW et al (2020) Neuropathy, claw toes, intrinsic muscle volume, and plantar aponeurosis thickness in diabetic feet. BMC Musculoskelet Disord 21:485. 10.1186/s12891-020-03503-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yammine K, Mouawad J, Honeine MO, Assi C (2024) Interphalangeal Resection Arthroplasty for the Prevention and Treatment of Diabetic Deformities and ulcers of the toes: a systematic review and Meta-analysis. Foot Ankle Orthop 9:24730114241256373. 10.1177/24730114241256373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giurato L, Meloni M, Izzo V, Uccioli L (2017) Osteomyelitis in diabetic foot: a comprehensive overview. World J Diabetes 8:135–142. 10.4239/wjd.v8.i4.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayya D, O’Neill OJ, Huedo-Medina TB et al (2022) Debridement of Diabetic Foot Ulcers. Adv Wound Care 11:666. 10.1089/WOUND.2021.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magliano DJ, Boyko EJ IDF Diabetes Atlas 10th edition scientific committee (2021) IDF DIABETES ATLAS, 10th ed. International Diabetes Federation, Brussels
- 13.Liu J, Zheng H, Dai X et al (2017) Biomaterials for promoting Wound Healing in Diabetes. J Tissue Sci Eng 08. 10.4172/2157-7552.1000193
- 14.Kasiewicz LN, Whitehead KA (2017) Recent advances in biomaterials for the treatment of diabetic foot ulcers. Biomater Sci 5:1962–1975. 10.1039/C7BM00264E [DOI] [PubMed] [Google Scholar]
- 15.Phang SJ, Basak S, Teh HX et al (2022) Advancements in Extracellular Matrix-based biomaterials and biofabrication of 3D organotypic skin models. ACS Biomater Sci Eng 8:3220–3241. 10.1021/acsbiomaterials.2c00342 [DOI] [PubMed] [Google Scholar]
- 16.Xing H, Lee H, Luo L, Kyriakides TR (2020) Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol Adv 42:107421. 10.1016/j.biotechadv.2019.107421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman EL, Callaghan BC, Pop-Busui R et al (2019) Diabetic neuropathy. Nat Rev Dis Primer 5:1–18. 10.1038/s41572-019-0092-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigues M, Kosaric N, Bonham CA, Gurtner GC (2019) Wound Healing: a Cellular Perspective. Physiol Rev 99:665–706. 10.1152/physrev.00067.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridiandries A, Tan JTM, Bursill CA (2018) The role of chemokines in Wound Healing. Int J Mol Sci 19:3217. 10.3390/ijms19103217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Y, Richmond A (2015) Chemokine regulation of Neutrophil Infiltration of skin wounds. Adv Wound Care 4:631–640. 10.1089/wound.2014.0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alhajj M, Goyal A (2022) Physiology, Granulation tissue. In: StatPearls. StatPearls Publishing, Treasure Island (FL) [PubMed] [Google Scholar]
- 22.Baltzis D, Eleftheriadou I, Veves A (2014) Pathogenesis and treatment of impaired Wound Healing in Diabetes Mellitus: New insights. Adv Ther 31:817–836. 10.1007/s12325-014-0140-x [DOI] [PubMed] [Google Scholar]
- 23.La Sala L, Prattichizzo F, Ceriello A (2019) The link between diabetes and atherosclerosis. Eur J Prev Cardiol 26:15–24. 10.1177/2047487319878373 [DOI] [PubMed] [Google Scholar]
- 24.Li XH, Guan LY, Lin HY et al (2016) Fibrinogen: a marker in Predicting Diabetic Foot Ulcer Severity. J Diabetes Res 2016:2358321. 10.1155/2016/2358321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess JL, Wyant WA, Abdo Abujamra B et al (2021) Diabetic Wound-Healing Science. Med (Mex) 57:1072. 10.3390/medicina57101072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beidler SK, Douillet CD, Berndt DF et al (2009) Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg 49:1013–1020. 10.1016/j.jvs.2008.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavlou S, Lindsay J, Ingram R et al (2018) Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol 19:24. 10.1186/s12865-018-0261-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanna S, Biswas S, Shang Y et al (2010) Macrophage dysfunction impairs resolution of inflammation in the wounds of Diabetic mice. PLoS ONE 5:e9539. 10.1371/journal.pone.0009539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts AC, Porter KE (2013) Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diab Vasc Dis Res 10:472–482. 10.1177/1479164113500680 [DOI] [PubMed] [Google Scholar]
- 30.Clyne AM (2021) Endothelial response to glucose: dysfunction, metabolism, and transport. Biochem Soc Trans 49:313–325. 10.1042/BST20200611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazaro Jl, Izzo V, Meaume S et al (2016) Elevated levels of matrix metalloproteinases and chronic wound healing: an updated review of clinical evidence. J Wound Care 25:277–287. 10.12968/jowc.2016.25.5.277 [DOI] [PubMed] [Google Scholar]
- 32.Mahmoud NN, Hamad K, Al Shibitini A et al (2024) Investigating inflammatory markers in Wound Healing: understanding implications and identifying artifacts. ACS Pharmacol Transl Sci 7:18–27. 10.1021/acsptsci.3c00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diller RB, Tabor AJ (2022) The role of the Extracellular Matrix (ECM) in Wound Healing: a review. Biomimetics 7:87. 10.3390/biomimetics7030087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pouget C, Dunyach-Remy C, Pantel A et al (2020) Biofilms in Diabetic Foot Ulcers: significance and clinical relevance. Microorganisms 8:1–15. 10.3390/MICROORGANISMS8101580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong DG, Boulton AJM, Bus SA (2017) Diabetic Foot Ulcers and their recurrence. N Engl J Med 376:2367–2375. 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Wang C, Zheng L (2024) Bibliometric analysis of systematic review and meta-analysis on diabetic foot ulcer. Heliyon 10. 10.1016/j.heliyon.2024.e27534 [DOI] [PMC free article] [PubMed]
- 37.Bakker K, van Houtum WH, Riley PC (2005) 2005: the International Diabetes Federation focuses on the diabetic foot. Curr Diab Rep 5:436–440. 10.1007/s11892-005-0051-y [DOI] [PubMed] [Google Scholar]
- 38.Park YU, Eim SH, Seo YW (2024) Prevalence and risk factors of wound complications after transtibial amputation in patients with diabetic foot. World J Diabetes 15:629–637. 10.4239/wjd.v15.i4.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCartan B, Dinh T (2012) The Use of Split-Thickness skin grafts on Diabetic Foot ulcerations: a Literature Review. Plast Surg Int 2012:1–6. 10.1155/2012/715273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lazzarini PA, Jarl G (2021) Knee-high devices are gold in closing the Foot Ulcer gap: a review of Offloading treatments to Heal Diabetic Foot Ulcers. Med (Mex) 57:941. 10.3390/medicina57090941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji S, Liu X, Huang J et al (2021) Consensus on the application of negative pressure wound therapy of diabetic foot wounds. Burns Trauma 9. 10.1093/BURNST/TKAB018 [DOI] [PMC free article] [PubMed]
- 42.Chen P, Vilorio NC, Dhatariya K et al (2024) Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev 40:e3644. 10.1002/dmrr.3644 [DOI] [PubMed] [Google Scholar]
- 43.Prohaska J, Cook C (2023) Skin grafting. In: StatPearls. StatPearls Publishing, Treasure Island (FL) [PubMed] [Google Scholar]
- 44.Swoboda L, Held J (2022) Impaired wound healing in diabetes. J Wound Care 31:882–885. 10.12968/jowc.2022.31.10.882 [DOI] [PubMed] [Google Scholar]
- 45.Santema TB, Poyck PPC, Ubbink DT (2016) Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev 2016. 10.1002/14651858.CD011255.PUB2 [DOI] [PMC free article] [PubMed]
- 46.Dai C, Shih S, Khachemoune A (2020) Skin substitutes for acute and chronic wound healing: an updated review. J Dermatol Treat 31:639–648. 10.1080/09546634.2018.1530443 [DOI] [PubMed] [Google Scholar]
- 47.Murray RZ, West ZE, Cowin AJ, Farrugia BL (2019) Development and use of biomaterials as wound healing therapies. Burns Trauma 7. 10.1186/s41038-018-0139-7. s41038-018-0139–7 [DOI] [PMC free article] [PubMed]
- 48.Driver VR, Lavery LA, Reyzelman AM et al (2015) A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen 23:891–900. 10.1111/WRR.12357 [DOI] [PubMed] [Google Scholar]
- 49.Wainwright DJ (1995) Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 21:243–248. 10.1016/0305-4179(95)93866-I [DOI] [PubMed] [Google Scholar]
- 50.Keane TJ, Badylak SF (2014) Biomaterials for tissue engineering applications. Semin Pediatr Surg 23:112–118. 10.1053/j.sempedsurg.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 51.Pierschbacher MD, Ruoslahti E (1984) Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309:30–33. 10.1038/309030a0 [DOI] [PubMed] [Google Scholar]
- 52.Cramer MC, Badylak SF (2020) Extracellular matrix-based biomaterials and their influence upon cell behavior. Ann Biomed Eng 48:2132–2153. 10.1007/s10439-019-02408-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naomi R, Fauzi MB (2020) Cellulose/Collagen Dressings for Diabetic Foot Ulcer: a review. Pharmaceutics 12:881. 10.3390/pharmaceutics12090881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeschke MG, Sandmann G, Schubert T, Klein D (2005) Effect of oxidized regenerated cellulose/collagen matrix on dermal and epidermal healing and growth factors in an acute wound. Wound Repair Regen 13:324–331. 10.1111/j.1067-1927.2005.130316.x [DOI] [PubMed] [Google Scholar]
- 55.Browne S, Zeugolis DI, Pandit A (2013) Collagen: finding a solution for the source. Tissue Eng Part A 19:1491–1494. 10.1089/ten.tea.2012.0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rho KS, Jeong L, Lee G et al (2006) Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 27:1452–1461. 10.1016/j.biomaterials.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 57.Zhou T, Wang N, Xue Y et al (2016) Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids Surf B Biointerfaces 143:415–422. 10.1016/j.colsurfb.2016.03.052 [DOI] [PubMed] [Google Scholar]
- 58.O’Loughlin A, Kulkarni M, Creane M et al (2013) Topical Administration of Allogeneic Mesenchymal Stromal Cells Seeded in a collagen Scaffold augments Wound Healing and increases angiogenesis in the Diabetic rabbit Ulcer. Diabetes 62:2588–2594. 10.2337/db12-1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kleine J, Leisz S, Ghadban C et al (2021) Variants of oxidized regenerated cellulose and their distinct effects on neuronal tissue. Int J Mol Sci 22:11467. 10.3390/ijms222111467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ulrich D, Smeets R, Unglaub F et al (2011) Effect of oxidized regenerated Cellulose/Collagen matrix on proteases in Wound Exudate of patients with Diabetic Foot Ulcers. J Wound Ostomy Cont Nurs 38:522. 10.1097/WON.0b013e31822ad290 [DOI] [PubMed] [Google Scholar]
- 61.Chalimidi KR, Kumar Y, Kini UA (2015) Efficacy of Collagen Particles in Chronic non Healing Ulcers. J Clin Diagn Res JCDR 9:PC01–PC03. 10.7860/JCDR/2015/11782.6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taddeucci P, Pianigiani E, Colletta V et al (2004) An evaluation of Hyalofill-F plus compression bandaging in the treatment of chronic venous ulcers. J Wound Care 13:202–204. 10.12968/jowc.2004.13.5.26613 [DOI] [PubMed] [Google Scholar]
- 63.Mahedia M, Shah N, Amirlak B (2016) Clinical evaluation of Hyaluronic Acid Sponge with Zinc versus Placebo for Scar reduction after breast surgery. Plast Reconstr Surg – Glob Open 4:e791. 10.1097/GOX.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caravaggi C, De Giglio R, Pritelli C et al (2003) HYAFF 11-based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: a prospective, multicenter, controlled, randomized clinical trial. Diabetes Care 26:2853–2859. 10.2337/diacare.26.10.2853 [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Han G, Guo B, Huang J (2016) Hyaluronan oligosaccharides promote diabetic wound healing by increasing angiogenesis. Pharmacol Rep PR 68:1126–1132. 10.1016/j.pharep.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 66.Rohrer L, Kato S, Browne SA et al (2024) Acrylated Hyaluronic-Acid Based Hydrogel for the Treatment of Craniofacial Volumetric Muscle Loss. 10.1089/ten.tea.2023.0241. Tissue Eng Part A [DOI] [PubMed]
- 67.Dienes J, Browne S, Farjun B et al (2021) Semisynthetic hyaluronic acid-based hydrogel promotes recovery of the injured tibialis anterior skeletal muscle form and function. ACS Biomater Sci Eng 7:1587–1599. 10.1021/acsbiomaterials.0c01751 [DOI] [PubMed] [Google Scholar]
- 68.Burdick JA, Prestwich GD (2011) Hyaluronic acid hydrogels for Biomedical Applications. Adv Mater 23:H41–H56. 10.1002/adma.201003963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Browne S, Hossainy S, Healy K (2020) Hyaluronic Acid Macromer Molecular Weight dictates the Biophysical Properties and in Vitro Cellular response to Semisynthetic Hydrogels. ACS Biomater Sci Eng 6:1135–1143. 10.1021/acsbiomaterials.9b01419 [DOI] [PubMed] [Google Scholar]
- 70.Zheng Shu X, Liu Y, Palumbo FS et al (2004) In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 25:1339–1348. 10.1016/j.biomaterials.2003.08.014 [DOI] [PubMed] [Google Scholar]
- 71.Amorim S, Reis CA, Reis RL, Pires RA (2021) Extracellular Matrix mimics using Hyaluronan-based biomaterials. Trends Biotechnol 39:90–104. 10.1016/j.tibtech.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 72.Graça MFP, Miguel SP, Cabral CSD, Correia IJ (2020) Hyaluronic acid—based wound dressings: a review. Carbohydr Polym 241:116364. 10.1016/j.carbpol.2020.116364 [DOI] [PubMed] [Google Scholar]
- 73.Chantre CO, Gonzalez GM, Ahn S et al (2019) Porous biomimetic hyaluronic acid and extracellular matrix protein nanofiber scaffolds for accelerated cutaneous tissue repair. ACS Appl Mater Interfaces 11:45498–45510. 10.1021/acsami.9b17322 [DOI] [PubMed] [Google Scholar]
- 74.Turner NJ, Kielty CM, Walker MG, Canfield AE (2004) A novel hyaluronan-based biomaterial (Hyaff-11®) as a scaffold for endothelial cells in tissue engineered vascular grafts. Biomaterials 25:5955–5964. 10.1016/j.biomaterials.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 75.Uccioli L, Giurato L, Ruotolo V et al (2011) Two-step autologous grafting using HYAFF scaffolds in treating difficult Diabetic Foot Ulcers: results of a Multicenter, Randomized Controlled Clinical Trial with Long-Term follow-up. Int J Low Extrem Wounds 10:80–85. 10.1177/1534734611409371 [DOI] [PubMed] [Google Scholar]
- 76.Clark RAf (2001) Fibrin and Wound Healing. Ann N Y Acad Sci 936:355–367. 10.1111/j.1749-6632.2001.tb03522.x [DOI] [PubMed] [Google Scholar]
- 77.Tanaka R, Saito Y, Fujiwara Y et al (2019) Preparation of fibrin hydrogels to promote the recruitment of anti-inflammatory macrophages. Acta Biomater 89:152–165. 10.1016/j.actbio.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 78.Bastidas JG, Maurmann N, Oliveira L et al (2023) Bilayer scaffold from PLGA/fibrin electrospun membrane and fibrin hydrogel layer supports wound healingin vivo. Biomed Mater Bristol Engl 18. 10.1088/1748-605X/acb02f [DOI] [PubMed]
- 79.Everts P, Onishi K, Jayaram P et al (2020) Platelet-Rich plasma: New Performance understandings and therapeutic considerations in 2020. Int J Mol Sci 21:7794. 10.3390/ijms21207794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.García JR, Acuña AJ, Villareal JB et al (2024) Next Generation approaches for knee cartilage repair and joint preservation. J Cartil Jt Preserv 100179. 10.1016/j.jcjp.2024.100179
- 81.Pavlovic V, Ciric M, Jovanovic V et al (2021) Platelet-rich fibrin: basics of biological actions and protocol modifications. Open Med 16:446–454. 10.1515/med-2021-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsai H-C, Lehman CW, Chen C-M (2019) Use of platelet-rich plasma and platelet-derived patches to treat chronic wounds. J Wound Care 28:15–21. 10.12968/jowc.2019.28.1.15 [DOI] [PubMed] [Google Scholar]
- 83.Game F, Jeffcoate W, Tarnow L et al (2018) LeucoPatch system for the management of hard-to-heal diabetic foot ulcers in the UK, Denmark, and Sweden: an observer-masked, randomised controlled trial. Lancet Diabetes Endocrinol 6:870–878. 10.1016/S2213-8587(18)30240-7 [DOI] [PubMed] [Google Scholar]
- 84.Chantre CO, Campbell PH, Golecki HM et al (2018) Production-scale fibronectin nanofibers promote wound closure and tissue repair in a dermal mouse model. Biomaterials 166:96–108. 10.1016/j.biomaterials.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nikkhah M, Akbari M, Paul A et al (2016) Gelatin-based Biomaterials for tissue Engineering and Stem Cell Bioengineering. Biomaterials from Nature for Advanced Devices and therapies. Wiley, Ltd, pp 37–62 [Google Scholar]
- 86.Dong Y, A S, Rodrigues M et al (2017) Injectable and tunable gelatin hydrogels enhance Stem Cell Retention and improve cutaneous Wound Healing. Adv Funct Mater 27:1606619. 10.1002/adfm.201606619 [Google Scholar]
- 87.Kirk JF, Ritter G, Finger I et al (2013) Mechanical and biocompatible characterization of a cross-linked collagen-hyaluronic acid wound dressing. Biomatter 3. 10.4161/BIOM.25633 [DOI] [PMC free article] [PubMed]
- 88.Ying H, Zhou J, Wang M et al (2019) In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater Sci Eng C 101:487–498. 10.1016/j.msec.2019.03.093 [DOI] [PubMed] [Google Scholar]
- 89.Campitiello F, Mancone M, Cammarota M et al (2021) Acellular dermal matrix used in Diabetic Foot Ulcers: clinical outcomes supported by biochemical and histological analyses. Int J Mol Sci 22:7085. 10.3390/ijms22137085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Campitiello F, Mancone M, Della Corte A et al (2017) To evaluate the efficacy of an acellular flowable matrix in comparison with a wet dressing for the treatment of patients with diabetic foot ulcers: a randomized clinical trial. Updat Surg 69:523–529. 10.1007/s13304-017-0461-9 [DOI] [PubMed] [Google Scholar]
- 91.Seavey JG, Masters ZA, Balazs GC et al (2016) Use of a bioartificial dermal regeneration template for skin restoration in combat casualty injuries. Regen Med 11:81–90. 10.2217/rme.15.83 [DOI] [PubMed] [Google Scholar]
- 92.Heimbach DM, Warden GD, Luterman A et al (2003) Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil 24:42–48. 10.1097/00004630-200301000-00009 [DOI] [PubMed] [Google Scholar]
- 93.Cuadra A, Correa G, Roa R et al (2012) Functional results of burned hands treated with Integra®. J Plast Reconstr Aesthet Surg 65:228–234. 10.1016/j.bjps.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 94.Jeon H, Kim J, Yeo H et al (2013) Treatment of Diabetic Foot Ulcer using Matriderm in comparison with a skin graft. Arch Plast Surg 40:403–408. 10.5999/aps.2013.40.4.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boekema BKHL, Vlig M, Olde Damink L et al (2014) Effect of pore size and cross-linking of a novel collagen-elastin dermal substitute on wound healing. J Mater Sci Mater Med 25:423–433. 10.1007/s10856-013-5075-2 [DOI] [PubMed] [Google Scholar]
- 96.De Angelis B, Gentile P, Agovino A et al (2013) Chronic ulcers: MATRIDERM® system in smoker, cardiopathic, and diabetic patients. J Tissue Eng 4:2041731413502663. 10.1177/2041731413502663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhowmick S, Rother S, Zimmermann H et al (2017) Biomimetic electrospun scaffolds from main extracellular matrix components for skin tissue engineering application – the role of chondroitin sulfate and sulfated hyaluronan. Mater Sci Eng C 79:15–22. 10.1016/j.msec.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 98.Kartika RW, Alwi I, Suyatna FD et al (2021) Wound Healing in Diabetic Foot Ulcer patients using combined use of platelet Rich Fibrin and Hyaluronic Acid, platelet Rich Fibrin and Placebo: an open label, Randomized Controlled Trial. Acta Med Indones 53:268–275 [PubMed] [Google Scholar]
- 99.Brown SJ, Surti F, Sibbons P, Hook L (2021) Wound healing properties of a fibrin-based dermal replacement scaffold. Biomed Phys Eng Express 8:015025. 10.1088/2057-1976/ac4176 [DOI] [PubMed] [Google Scholar]
- 100.Zhang X, Chen X, Hong H et al (2022) Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Bioact Mater 10:15–31. 10.1016/j.bioactmat.2021.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brown M, Li J, Moraes C et al (2022) Decellularized extracellular matrix: new promising and challenging biomaterials for regenerative medicine. Biomaterials 289:121786. 10.1016/j.biomaterials.2022.121786 [DOI] [PubMed] [Google Scholar]
- 102.Dussoyer M, Michopoulou A, Rousselle P (2020) Decellularized scaffolds for skin repair and regeneration. Appl Sci 10:3435. 10.3390/app10103435 [Google Scholar]
- 103.García-Gareta E, Abduldaiem Y, Sawadkar P et al (2020) Decellularised scaffolds: just a framework? Current knowledge and future directions. J Tissue Eng 11:2041731420942903. 10.1177/2041731420942903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoshiba T, Yunoki S (2023) Comparison of decellularization protocols for cultured cell-derived extracellular matrix-effects on decellularization efficacy, extracellular matrix retention, and cell functions. J Biomed Mater Res B Appl Biomater 111:85–94. 10.1002/jbm.b.35135 [DOI] [PubMed] [Google Scholar]
- 105.Dasgupta A, Orgill D, Galiano RD et al (2016) A novel reticular dermal graft leverages architectural and biological properties to support wound repair. Plast Reconstr Surg Glob Open 4:e1065. 10.1097/GOX.0000000000001065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Milan PB, Lotfibakhshaiesh N, Joghataie MT et al (2016) Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta Biomater 45:234–246. 10.1016/j.actbio.2016.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsieh C-M, Wang W, Chen Y-H et al (2020) A Novel Composite Hydrogel composed of formic acid-decellularized pepsin-soluble Extracellular Matrix Hydrogel and Sacchachitin Hydrogel as Wound Dressing to synergistically accelerate Diabetic Wound Healing. Pharmaceutics 12:538. 10.3390/pharmaceutics12060538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wolf MT, Daly KA, Brennan-Pierce EP et al (2012) A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 33:7028–7038. 10.1016/j.biomaterials.2012.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hogg P, Rooney P, Ingham E, Kearney JN (2013) Development of a decellularised dermis. Cell Tissue Bank 14:465–474. 10.1007/S10561-012-9333-1/FIGURES/7 [DOI] [PubMed] [Google Scholar]
- 110.Hogg P, Rooney P, Leow-Dyke S et al (2015) Development of a terminally sterilised decellularised dermis. Cell Tissue Bank 16:351–359. 10.1007/s10561-014-9479-0 [DOI] [PubMed] [Google Scholar]
- 111.Tuieng RJ, Cartmell SH, Kirwan CC, Sherratt MJ (2021) The effects of Ionising and Non-ionising Electromagnetic Radiation on Extracellular Matrix proteins. Cells 10:3041. 10.3390/cells10113041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim YH, Shim HS, Lee J, Kim SW (2022) A prospective Randomized Controlled Multicenter Clinical Trial comparing paste-type Acellular dermal matrix to Standard Care for the treatment of chronic wounds. J Clin Med 11:2203. 10.3390/jcm11082203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee M, Jun D, Choi H et al (2020) Clinical efficacy of Acellular dermal matrix paste in treating Diabetic Foot Ulcers. Wounds Compend Clin Res Pract 32:50–56 [PubMed] [Google Scholar]
- 114.Greaves NS, Iqbal SA, Hodgkinson T et al (2015) Skin substitute-assisted repair shows reduced dermal fibrosis in acute human wounds validated simultaneously by histology and optical coherence tomography. Wound Repair Regen 23:483–494. 10.1111/wrr.12308 [DOI] [PubMed] [Google Scholar]
- 115.Walters J, Cazzell S, Pham H et al (2016) Healing rates in a Multicenter Assessment of a sterile, Room temperature, Acellular dermal matrix Versus Conventional Care Wound Management and an active Comparator in the treatment of full-thickness Diabetic Foot Ulcers. Eplasty 16:e10 [PMC free article] [PubMed] [Google Scholar]
- 116.Lakmal K, Basnayake O, Hettiarachchi D (2021) Systematic review on the rational use of amniotic membrane allografts in diabetic foot ulcer treatment. BMC Surg 21:87. 10.1186/s12893-021-01084-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Munoz-Torres JR, Martínez-González SB, Lozano-Luján AD et al (2023) Biological properties and surgical applications of the human amniotic membrane. Front Bioeng Biotechnol 10:1067480. 10.3389/fbioe.2022.1067480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Song M, Wang W, Ye Q et al (2017) The repairing of full-thickness skin deficiency and its biological mechanism using decellularized human amniotic membrane as the wound dressing. Mater Sci Eng C Mater Biol Appl 77:739–747. 10.1016/j.msec.2017.03.232 [DOI] [PubMed] [Google Scholar]
- 119.Fetterolf DE, Snyder RJ (2012) Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds 24:299–307 [PubMed] [Google Scholar]
- 120.Holmes C, Wrobel JS, MacEachern MP, Boles BR (2013) Collagen-based wound dressings for the treatment of diabetes-related foot ulcers: a systematic review. Diabetes Metab Syndr Obes Targets Ther 6:17–29. 10.2147/DMSO.S36024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Letendre S, LaPorta G, O’Donnell E et al (2009) Pilot trial of Biovance Collagen-based Wound Covering for Diabetic Ulcers. Adv Skin Wound Care 22:161. 10.1097/01.ASW.0000305463.32800.32 [DOI] [PubMed] [Google Scholar]
- 122.Game F, Gray K, Davis D et al (2021) The effectiveness of a new dried human amnion derived membrane in addition to standard care in treating diabetic foot ulcers: a patient and assessor blind, randomised controlled pilot study. Int Wound J 18:692–700. 10.1111/iwj.13571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zelen CM, Gould L, Serena TE et al (2015) A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J 12:724–732. 10.1111/iwj.12395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang Y, Zhang Y, Yan Y et al (2020) A sponge-like double-layer wound dressing with Chitosan and decellularized bovine amniotic membrane for promoting Diabetic Wound Healing. Polymers 12:535. 10.3390/polym12030535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramakrishnan R, Sreelatha HV, Anil A et al (2020) Human-derived Scaffold Components and Stem cells creating Immunocompatible dermal tissue ensuing regulated Nonfibrotic Cellular Phenotypes. ACS Biomater Sci Eng 6:2740–2756. 10.1021/ACSBIOMATERIALS.9B01961 [DOI] [PubMed] [Google Scholar]
- 126.Shi L, Ronfard V (2013) Biochemical and biomechanical characterization of porcine small intestinal submucosa (SIS): a mini review. Int J Burns Trauma 3:173–179 [PMC free article] [PubMed] [Google Scholar]
- 127.Kim MS, Hong KD, Shin HW et al (2005) Preparation of porcine small intestinal submucosa sponge and their application as a wound dressing in full-thickness skin defect of rat. Int J Biol Macromol 36:54–60. 10.1016/j.ijbiomac.2005.03.013 [DOI] [PubMed] [Google Scholar]
- 128.Cazzell SM, Lange DL, Dickerson JE, Slade HB (2015) The Management of Diabetic Foot Ulcers with Porcine Small Intestine Submucosa Tri-layer Matrix: a Randomized Controlled Trial. Adv Wound Care 4:711–718. 10.1089/wound.2015.0645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brown-Etris M, Milne CT, Hodde JP (2019) An extracellular matrix graft (Oasis® wound matrix) for treating full-thickness pressure ulcers: a randomized clinical trial. J Tissue Viability 28:21–26. 10.1016/j.jtv.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 130.Satyam A, Tsokos MG, Tresback JS et al (2020) Cell-derived Extracellular Matrix-Rich Biomimetic substrate supports podocyte proliferation, differentiation, and maintenance of native phenotype. Adv Funct Mater 30:1908752. 10.1002/adfm.201908752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carvalho MS, Silva JC, Udangawa RN et al (2019) Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl 99:479–490. 10.1016/j.msec.2019.01.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Santarella F, Sridharan R, Marinkovic M et al (2020) Scaffolds functionalized with Matrix from Induced pluripotent stem cell fibroblasts for Diabetic Wound Healing. Adv Healthc Mater 9:2000307. 10.1002/adhm.202000307 [DOI] [PubMed] [Google Scholar]
- 133.Kim HS, Hwang HJ, Kim HJ et al (2022) Effect of Decellularized Extracellular Matrix Bioscaffolds derived from fibroblasts on skin Wound Healing and Remodeling. Front Bioeng Biotechnol 10. 10.3389/FBIOE.2022.865545/PDF [DOI] [PMC free article] [PubMed]
- 134.Guo J, Huang J, Lei S et al (2023) Construction of Rapid Extracellular Matrix-Deposited Small-Diameter Vascular grafts Induced by Hypoxia in a Bioreactor. ACS Biomater Sci Eng 9:844–855. 10.1021/acsbiomaterials.2c00809 [DOI] [PubMed] [Google Scholar]
- 135.Santarella F, Amaral RJFC, do, Lemoine M et al (2022) Personalized scaffolds for Diabetic Foot Ulcer Healing using Extracellular Matrix from Induced Pluripotent stem-reprogrammed patient cells. Adv NanoBiomed Res 2:2200052. 10.1002/ANBR.202200052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Navone SE, Pascucci L, Dossena M et al (2014) Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Res Ther 5:7. 10.1186/scrt396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lu H, Hoshiba T, Kawazoe N et al (2011) Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials 32:9658–9666. 10.1016/j.biomaterials.2011.08.091 [DOI] [PubMed] [Google Scholar]
- 138.Chen C, Loe F, Blocki A et al (2011) Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv Drug Deliv Rev 63:277–290. 10.1016/j.addr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 139.Marinkovic M, Sridharan R, Santarella F et al (2021) Optimization of extracellular matrix production from human induced pluripotent stem cell-derived fibroblasts for scaffold fabrication for application in wound healing. J Biomed Mater Res A 109:1803–1811. 10.1002/jbm.a.37173 [DOI] [PubMed] [Google Scholar]
- 140.De Pieri A, Korntner SH, Capella-Monsonis H et al (2022) Macromolecular crowding transforms regenerative medicine by enabling the accelerated development of functional and truly three-dimensional cell assembled micro tissues. Biomaterials 287:121674. 10.1016/j.biomaterials.2022.121674 [DOI] [PubMed] [Google Scholar]
- 141.D’Aniello C, Cermola F, Patriarca EJ, Minchiotti G (2017) Vitamin C in Stem Cell Biology: impact on Extracellular Matrix Homeostasis and Epigenetics. Stem Cells Int 2017:8936156. 10.1155/2017/8936156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eming SA, Wynn TA, Martin P (2017) Inflammation and metabolism in tissue repair and regeneration. Science 356:1026–1030. 10.1126/science.aam7928 [DOI] [PubMed] [Google Scholar]
- 143.Sadtler K, Sommerfeld SD, Wolf MT et al (2017) Proteomic composition and immunomodulatory properties of urinary bladder matrix scaffolds in homeostasis and injury. Semin Immunol 29:14–23. 10.1016/j.smim.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dziki JL, Huleihel L, Scarritt ME, Badylak SF (2017) Extracellular Matrix Bioscaffolds as Immunomodulatory Biomaterials. Tissue Eng Part A 23:1152–1159. 10.1089/ten.tea.2016.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sicari BM, Dziki JL, Siu BF et al (2014) The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials 35:8605–8612. 10.1016/j.biomaterials.2014.06.060 [DOI] [PubMed] [Google Scholar]
- 146.Dziki JL, Wang DS, Pineda C et al (2017) Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. J Biomed Mater Res A 105:138–147. 10.1002/jbm.a.35894 [DOI] [PubMed] [Google Scholar]
- 147.Badylak SF, Valentin JE, Ravindra AK et al (2008) Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14:1835–1842. 10.1089/ten.tea.2007.0264 [DOI] [PubMed] [Google Scholar]
- 148.Keane TJ, Londono R, Turner NJ, Badylak SF (2012) Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 33:1771–1781. 10.1016/j.biomaterials.2011.10.054 [DOI] [PubMed] [Google Scholar]
- 149.Sadtler K, Estrellas K, Allen BW et al (2016) Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 352:366–370. 10.1126/science.aad9272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Keane TJ, Dziki J, Sobieski E et al (2017) Restoring mucosal barrier function and modifying macrophage phenotype with an Extracellular Matrix Hydrogel: potential therapy for Ulcerative Colitis. J Crohns Colitis 11:360–368. 10.1093/ecco-jcc/jjw149 [DOI] [PubMed] [Google Scholar]
- 151.Rayahin JE, Buhrman JS, Zhang Y et al (2015) High and low Molecular Weight Hyaluronic Acid differentially influence macrophage activation. ACS Biomater Sci Eng 1:481–493. 10.1021/acsbiomaterials.5b00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hart SP, Rossi AG, Haslett C, Dransfield I (2012) Characterization of the effects of Cross-linking of Macrophage CD44 Associated with increased phagocytosis of apoptotic PMN. PLoS ONE 7:e33142. 10.1371/journal.pone.0033142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jouy F, Lohmann N, Wandel E et al (2017) Sulfated hyaluronan attenuates inflammatory signaling pathways in macrophages involving induction of antioxidants. Proteomics 17:1700082. 10.1002/pmic.201700082 [DOI] [PubMed] [Google Scholar]
- 154.Hauck S, Zager P, Halfter N et al (2021) Collagen/hyaluronan based hydrogels releasing sulfated hyaluronan improve dermal wound healing in diabetic mice via reducing inflammatory macrophage activity. Bioact Mater 6:4342–4359. 10.1016/j.bioactmat.2021.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li R, Liu K, Huang X et al (2022) Bioactive materials promote Wound Healing through Modulation of Cell behaviors. Adv Sci 9:2105152. 10.1002/advs.202105152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Serini G, Valdembri D, Bussolino F (2006) Integrins and angiogenesis: a sticky business. Exp Cell Res 312:651–658. 10.1016/j.yexcr.2005.10.020 [DOI] [PubMed] [Google Scholar]
- 157.Stupack DG, Cheresh DA (2004) Integrins and angiogenesis. Curr Top Dev Biol 64:207–238. 10.1016/S0070-2153(04)64009-9 [DOI] [PubMed] [Google Scholar]
- 158.Veith AP, Henderson K, Spencer A et al (2019) Therapeutic strategies for enhancing angiogenesis in Wound Healing. Adv Drug Deliv Rev 146:97–125. 10.1016/j.addr.2018.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bix G, Iozzo RV (2008) Novel interactions of perlecan: unraveling perlecan’s role in angiogenesis. Microsc Res Tech 71:339–348. 10.1002/jemt.20562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yao C, Markowicz M, Pallua N et al (2008) The effect of cross-linking of collagen matrices on their angiogenic capability. Biomaterials 29:66–74. 10.1016/j.biomaterials.2007.08.049 [DOI] [PubMed] [Google Scholar]
- 161.Brown AC, Barker TH (2014) Fibrin-based biomaterials: modulation of macroscopic properties through rational design at the molecular level. Acta Biomater 10:1502–1514. 10.1016/j.actbio.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thompson WD, Smith EB, Stirk CM et al (1992) Angiogenic activity of fibrin degradation products is located in fibrin fragment E. J Pathol 168:47–53. 10.1002/path.1711680109 [DOI] [PubMed] [Google Scholar]
- 163.Huang NF, Yu J, Sievers R et al (2005) Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng 11:1860–1866. 10.1089/ten.2005.11.1860 [DOI] [PubMed] [Google Scholar]
- 164.Pardue EL, Ibrahim S, Ramamurthi A (2008) Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis 4:203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Slevin M, Kumar S, Gaffney J (2002) Angiogenic oligosaccharides of Hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses *. J Biol Chem 277:41046–41059. 10.1074/jbc.M109443200 [DOI] [PubMed] [Google Scholar]
- 166.Li S, Nih LR, Bachman H et al (2017) Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat Mater 16:953–961. 10.1038/nmat4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ishihara J, Ishihara A, Fukunaga K et al (2018) Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat Commun 9:2163. 10.1038/s41467-018-04525-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hoganson DM, O’Doherty EM, Owens GE et al (2010) The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials 31:6730–6737. 10.1016/j.biomaterials.2010.05.019 [DOI] [PubMed] [Google Scholar]
- 169.Schmitt A, Csiki R, Tron A et al (2017) Optimized protocol for whole organ decellularization. Eur J Med Res 22:31. 10.1186/s40001-017-0272-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mazloomnejad R, Babajani A, Kasravi M et al (2023) Angiogenesis and re-endothelialization in decellularized scaffolds: recent advances and current challenges in tissue engineering. Front Bioeng Biotechnol 11. 10.3389/fbioe.2023.1103727 [DOI] [PMC free article] [PubMed]
- 171.Yu C, Guan G, Glas S et al (2021) A biomimetic basement membrane consisted of hybrid aligned nanofibers and microfibers with immobilized collagen IV and laminin for rapid endothelialization. Bio-Des Manuf 4:171–189. 10.1007/s42242-020-00111-6 [Google Scholar]
- 172.Zamboni F, Wong CK, Collins MN (2023) Hyaluronic acid association with bacterial, fungal and viral infections: can hyaluronic acid be used as an antimicrobial polymer for biomedical and pharmaceutical applications? Bioact Mater 19:458–473. 10.1016/j.bioactmat.2022.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Carlson GA, Dragoo JL, Samimi B et al (2004) Bacteriostatic properties of biomatrices against common orthopaedic pathogens. Biochem Biophys Res Commun 321:472–478. 10.1016/j.bbrc.2004.06.165 [DOI] [PubMed] [Google Scholar]
- 174.Park JH, Park EJ, Yi HS (2017) Wound Healing and anti-inflammatory effects of Topical Hyaluronic Acid Injection in Surgical-Site infection caused by Staphylococcus aureus. Int J Low Extrem Wounds 16:202–207. 10.1177/1534734617714142 [DOI] [PubMed] [Google Scholar]
- 175.Xia Y, Adibnia V, Shan C et al (2019) Synergy between Zwitterionic Polymers and Hyaluronic Acid enhances antifouling performance. Langmuir ACS J Surf Colloids 35:15535–15542. 10.1021/acs.langmuir.9b01876 [DOI] [PubMed] [Google Scholar]
- 176.Liu S, Liu X, Ren Y et al (2020) Mussel-inspired dual-cross-linking hyaluronic Acid/ε-Polylysine hydrogel with Self-Healing and Antibacterial properties for Wound Healing. ACS Appl Mater Interfaces 12:27876–27888. 10.1021/acsami.0c00782 [DOI] [PubMed] [Google Scholar]
- 177.Senyürek I, Klein G, Kalbacher H et al (2010) Peptides derived from the human laminin α4 and α5 chains exhibit antimicrobial activity. Peptides 31:1468–1472. 10.1016/j.peptides.2010.04.016 [DOI] [PubMed] [Google Scholar]
- 178.Abdillahi SM, Maaß T, Kasetty G et al (2018) Collagen VI Contains Multiple Host Defense Peptides with potent in vivo activity. J Immunol 201:1007–1020. 10.4049/jimmunol.1700602 [DOI] [PubMed] [Google Scholar]
- 179.Li G, Lai Z, Shan A (2023) Advances of Antimicrobial peptide-based biomaterials for the treatment of bacterial infections. Adv Sci Weinh Baden-Wurtt Ger 10:e2206602. 10.1002/advs.202206602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McGrath M, Zimkowska K, Genoud KJ et al (2023) A Biomimetic, Bilayered Antimicrobial Collagen-based Scaffold for enhanced Healing of Complex Wound conditions. ACS Appl Mater Interfaces 15:17444–17458. 10.1021/acsami.2c18837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alves PM, Pereira RF, Costa B et al (2022) Thiol–Norbornene Photoclick Chemistry for Grafting antimicrobial peptides onto Chitosan to create Antibacterial Biomaterials. ACS Appl Polym Mater 4:5012–5026. 10.1021/acsapm.2c00563 [Google Scholar]
- 182.Monaghan MG, Borah R, Thomsen C, Browne S (2023) Thou shall not heal: overcoming the non-healing behaviour of diabetic foot ulcers by engineering the inflammatory microenvironment. Adv Drug Deliv Rev 203:115120. 10.1016/j.addr.2023.115120 [DOI] [PubMed] [Google Scholar]
- 183.Browne S, Petit N, Quondamatteo F (2024) Functionalised biomaterials as synthetic extracellular matrices to promote vascularisation and healing of diabetic wounds. Cell Tissue Res 395:133–145. 10.1007/s00441-023-03849-4 [DOI] [PubMed] [Google Scholar]
- 184.Khazaei M, Alizadeh M, Rezakhani L (2024) Resveratrol-loaded decellularized ovine pericardium: ECM introduced for tissue engineering. Biotechnol Appl Biochem 71:387–401. 10.1002/bab.2547 [DOI] [PubMed] [Google Scholar]
- 185.Singh H, Hassan S, Nabi SU et al (2024) Multicomponent decellularized extracellular matrix of caprine small intestine submucosa based bioactive hydrogel promoting full-thickness burn wound healing in rabbits. Int J Biol Macromol 255:127810. 10.1016/j.ijbiomac.2023.127810 [DOI] [PubMed] [Google Scholar]
- 186.Browne S, Gill EL, Schultheiss P et al (2021) Stem cell-based vascularization of microphysiological systems. Stem Cell Rep 16:2058–2075. 10.1016/j.stemcr.2021.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Osaki T, Sivathanu V, Kamm RD (2018) Vascularized microfluidic organ-chips for drug screening, disease models and tissue engineering. Curr Opin Biotechnol 52:116–123. 10.1016/j.copbio.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Esparza A, Jimenez N, Borrego EA et al (2024) Review: human stem cell-based 3D in vitro angiogenesis models for preclinical drug screening applications. Mol Biol Rep 51:260. 10.1007/s11033-023-09048-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weng K-C, Kurokawa YK, Hajek BS et al (2020) Human Induced Pluripotent stem-cardiac-endothelial-tumor-on-a-Chip to assess Anticancer Efficacy and Cardiotoxicity. Tissue Eng Part C Methods 26:44–55. 10.1089/ten.tec.2019.0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Doherty EL, Krohn G, Warren EC et al Human cell-derived Matrix Composite Hydrogels with Diverse Composition for Use in vasculature-on-chip models. Adv Healthc Mater n/a:2400192. 10.1002/adhm.202400192 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.