Abstract
Introduction
The association between sodium-glucose cotransporter-2 (SGLT2) inhibitors and the risk of urogenital infections remains controversial. This study aimed to investigate the association between SGLT2 inhibitors and the incidence of perineal soft tissue infections, including Fournier's gangrene (FG), genital bacterial infections, and urinary tract infections (UTIs), using administrative claims data in Japan.
Methods
In this retrospective cohort study, we utilized the JMDC Claims Database. The study included patients aged 18 years or older diagnosed with type 2 diabetes mellitus, identified by a diagnostic code, who received new prescriptions for SGLT2 inhibitors or dipeptidyl peptidase 4 (DPP-4) inhibitors between April 2014 and August 2020. Using one-to-one propensity score (PS) matching, we compared the incidence of perineal soft tissue infections, including FG, genital bacterial infection, and UTIs between groups treated with SGLT2 and DPP-4 inhibitors. Hazard ratios (HR) and their 95% confidence intervals (CI) were estimated using the Cox proportional hazards model.
Results
We identified 34,897 patients in the SGLT2 inhibitor group and 135,311 patients in the DPP-4 inhibitor group. After one-to-one PS matching, 31,665 pairs were generated. The mean age of the patients was 51 years, with approximately 70% being male. The use of SGLT2 inhibitors was associated with a decreased risk of UTI (HR 0.90, 95% CI 0.83–0.98) and an increased risk of genital bacterial infection (HR 1.23, 95% CI 1.03–1.46) compared to DPP-4 inhibitors. However, no significant association was observed with perineal soft tissue infection (HR 1.05, 95% CI 0.61–1.81).
Conclusions
SGLT2 inhibitors were associated with a reduced risk of UTI and an increased risk of genital bacterial infection. They showed no significant association with perineal soft tissue infection when compared to DPP-4 inhibitors. Future research should explore broader demographics, focusing on the elderly and achieving gender balance, to gain a comprehensive understanding of infection risks.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-024-01613-7.
Keywords: Claims database, Dipeptidyl peptidase 4 inhibitor, Fournier’s gangrene, Sodium-glucose cotransporter-2 inhibitor, Urogenital infection
Key Summary Points
| Why carry out this study? |
| The association between sodium-glucose cotransporter-2 (SGLT2) inhibitors and the risk of urogenital infections remains controversial. |
| This study aimed to investigate the association between SGLT2 inhibitors and the incidence of perineal soft tissue infections, including Fournier's gangrene (FG), genital bacterial infections, and urinary tract infections (UTIs), based on administrative claims data from Japan. |
| What was learned from the study? |
| The use of SGLT2 inhibitors was associated with a lower risk of UTIs and a higher risk of genital bacterial infections compared to DPP-4 inhibitors; however, no significant association was observed with perineal soft tissue infections. |
| This study contributes to the ongoing debate regarding the safety profile of SGLT2 inhibitors and helps people with type 2 diabetes choose the most appropriate antidiabetic medication. |
Introduction
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a relatively new class of oral antihyperglycemic agents that suppress glucose reuptake in the renal tubular system [1]. Various large, randomized, placebo-controlled trials and recent meta-analyses have revealed the beneficial effects of SGLT2 inhibitors on cardiovascular disease, heart failure, and kidney disease outcomes [2–5]. Consequently, the American Diabetes Association recommends SGLT2 inhibitors as first-line medications for patients with diabetes who have these comorbidities [6]. Despite their pivotal role in diabetes management, concerns regarding side effects persist.
While SGLT2 inhibitors are associated with elevated glucose levels in urine, potentially increasing the risk of genitourinary infections [7, 8], the specific associations between SGLT2 inhibitors and certain genital bacterial infections, such as prostatitis or Bartholin's gland abscesses, remain unexplored. Notably, the U.S. Food and Drug Administration issued warnings in 2015 and 2018 due to reported cases of serious urinary tract infections (UTIs) and Fournier’s gangrene (FG), also known as necrotizing fasciitis of the perineum, associated with the use of SGLT2 inhibitors [9, 10]. However, recent meta-analyses contradict these concerns, indicating that SGLT2 inhibitors are not significantly associated with UTI [7, 8]. Additionally, comprehensive observational studies using claims databases have failed to show an elevated risk of FG [11–14]. Thus, the relationship between SGLT2 inhibitors and bacterial infections remains unclear.
This study aimed to investigate the association between SGLT2 inhibitors and the incidence of perineal soft tissue infections, including FG, genital bacterial infections, and UTIs, using administrative claims data in Japan. Our findings are expected to provide valuable insights into the risks of SGLT2 inhibitors, specifically in relation to perineal soft tissue and urogenital infections. These insights could have a significant impact on clinical decision-making and help improve patient care strategies for individuals with diabetes and comorbidities.
Methods
Ethical statement
The Ethics Committee of Jichi Medical University has approved this study and waived the requirement for informed consent owing to the retrospective design and use of anonymized data (approval number, 21–198). This study was performed in accordance with the relevant guidelines and regulations. The data used in this study were obtained from the JMDC Claims Database, which contains anonymized individual-level, de-identified data from multiple health insurance associations. All personal information was encrypted and anonymized to ensure patient confidentiality. The JMDC Claims Database is a commercially available database. Permission was obtained from the data provider (JMDC Inc.) to access and use the data. The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Study Design and Data Source
We conducted a retrospective cohort study using data from the JMDC Claims Database. This database comprises inpatient, outpatient, and dispensing receipt data obtained from health insurance associations (employee health insurance systems in large companies in Japan) [15]. Therefore, the study predominantly included the working-age population (under 65 years old) and did not encompass self-employed individuals, pensioners, or those aged 75 years or older. As of February 2022, approximately 14 million persons were registered in this database. Each patient was assigned a unique ID, facilitating continuous patient follow-up even if they underwent transfers between hospitals [16]. The database encompasses patient demographics (unique ID, year and month of birth, and sex), diagnostic codes following the 10th edition of the International Classification of Diseases (ICD-10), medical procedures (tests and treatment), medication codes based on the Anatomical Therapeutic Chemical (ATC) classification, prescription durations, healthcare utilization details, and dates of diagnosis, procedures, prescriptions, and hospitalizations. The coding system employed for electronic claims aligns with the system developed by the Japanese Ministry of Health, Labor and Welfare (Japanese electronic claims codes). Most diagnostic and medication codes correspond to the ICD-10 and ATC classification systems, respectively.
Study Cohort
We included all patients aged 18 years or older diagnosed with type 2 diabetes mellitus (identified by diagnostic codes E11 or E14) and prescribed new SGLT2 inhibitors or dipeptidyl peptidase 4 (DPP-4) inhibitors between April 1, 2014, and August 31, 2020. The index date was set as the day of the first prescription of an SGLT2 or DPP-4 inhibitor, following a 180-day look-back period. Exclusions encompassed patients with type 1 diabetes (E10), secondary diabetes (E12 and E13), and individuals undergoing hemodialysis. Additionally, those prescribed SGLT2 or DPP-4 inhibitors only once and individuals diagnosed with necrotizing fasciitis (M72.6) during the look-back period were excluded from the study. Individuals who were prescribed the comparator drugs during the look-back period were also excluded. SGLT2 and DPP-4 inhibitors were identified based on the ATC classifications A10BK and A10BH, respectively.
Outcome Definitions
The outcome measures included perineal soft tissue, genital bacterial, and urinary tract infections. Individuals were categorized as having a perineal soft tissue infection if they were hospitalized and underwent debridement or related surgical procedures within 7 days following a diagnosis of necrotizing fasciitis, cellulitis, or a skin abscess in the perineal area. Genital bacterial infections and UTIs were defined as cases with the respective diagnostic codes and where any antibiotic was prescribed within 7 days of diagnosis. The diagnostic codes for these definitions, using the Japanese electronic claims codes, are provided in Supplementary Table S1.
Analysis
The follow-up time was calculated from the index date until the occurrence of specific events: patients were censored if they discontinued therapy (with a treatment gap of > 30 days), added or switched to the comparator, reached the date of the last claim record, or reached the end of the study period (August 31, 2021). The treatment gap was defined as the duration between the end of one prescription period (prescription date plus the prescribed days) and the start of the subsequent prescription. If a patient was censored due to a treatment gap, the follow-up time was defined as the period from the index date to the last prescription date. Patients with a follow-up duration of 0 days were excluded from the study.
We employed propensity score (PS) models adjusted for 61 variables, including age, sex, comorbidities, co-medications, procedures, and healthcare utilization during the look-back period (Supplementary Table S2). The look-back period was defined as the 180 days preceding the index date, which was set as the day of the first prescription of an SGLT2 or DPP-4 inhibitor. Both one-to-one and nearest-neighbor matching without replacement were conducted with a caliper width set at 0.20 times the standard deviation of the logit of the PS. The absolute standardized mean difference (ASD) was calculated to assess the balance in variable distribution between the SGLT2 and DPP-4 inhibitor groups, with an ASD greater than 0.1 considered indicative of imbalance.
We conducted separate survival time analyses for three outcomes (UTIs, genital bacterial infections, and perineal soft tissue infections) using the Kaplan–Meier method and log-rank test. Additionally, Cox proportional hazard regression was used to calculate hazard ratios (HRs) for each outcome independently. The threshold for significance was set at P ≤ 0.05. Subgroup analyses for all outcomes were also conducted, considering sex, age group, comedication (biguanides, insulin, and sulfonylureas), and history of major cardiovascular disease. All analyses were performed using R version 3.5.3 (R Foundation, Vienna, Austria).
Results
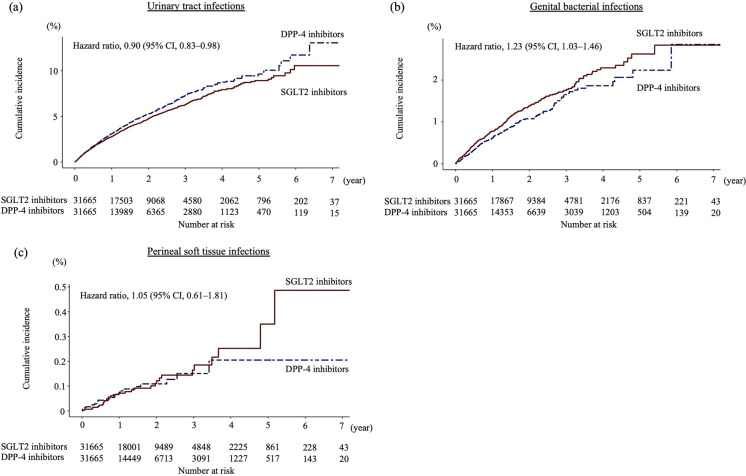
A total of 34,897 and 135,311 patients were included in the SGLT2 inhibitor and DPP-4 inhibitor groups, respectively. Following one-to-one PS matching, 31,665 pairs were generated. Certain covariates, such as age, history of hypertension and liver disease, and antidiabetic medication use, exhibited an imbalance between the two groups before matching. However, all measured characteristics were balanced after matching (Table 1, Supplementary Table S3). Median follow-up lengths for the analyses of UTIs, genital bacterial infections, and perineal soft tissue infections were 1.00 years (interquartile range [IQR] 0.34–2.03), 1.03 years (IQR 0.34–2.02), and 1.03 years (IQR 0.33–1.97), respectively. The median follow-up lengths in the SGLT2 inhibitor vs. DPP-4 inhibitor groups were 1.22 years (IQR 0.42–2.29) vs. 0.86 years (IQR 0.30–1.78), 1.21 years (IQR 0.41–2.28) vs. 0.86 years (IQR 0.29–1.77), and 1.17 years (IQR 0.40–2.22) vs. 0.82 years (IQR 0.28–1.72) for the respective analyses. Detailed outcome analyses are presented in Table 2. After PS matching, SGLT2 inhibitor use was associated with a reduced risk of UTI (HR 0.90, 95% CI 0.83–0.98, P = 0.02) and an increased risk of genital bacterial infection (HR 1.23, 95% CI 1.03–1.46, P = 0.02), but showed no significant association with perineal soft tissue infection (HR 1.05, 95% CI 0.61–1.81, P = 0.9) compared to DPP-4 inhibitor use. PS-matched Kaplan–Meier curves that display the cumulative incidence of each event among users of SGLT2 inhibitors and DPP-4 inhibitors are presented in Fig. 1. In subgroup analyses of UTIs and perineal soft tissue infections, the HRs for sex, age group, comedications, and history of cardiovascular disease were consistent with those observed in the main analyses, although statistical significance was not demonstrated. However, in the subgroup analyses of genital bacterial infections, the use of SGLT2 inhibitors was associated with a significant increase in risk among males, patients aged 40 to 64 years, those with ischemic heart diseases, or those on biguanides (Supplementary Tables S4–S6).
Table 1.
Selected baseline characteristics of patients before and after propensity score matching
| Before PS matching | After PS matching | |||||
|---|---|---|---|---|---|---|
| SGLT2 inhibitors (n = 34,897) | DPP-4 inhibitors (n = 135,311) | ASD | SGLT2 inhibitors (n = 31,665) | DPP-4 inhibitors (n = 31,665) | ASD | |
| Characteristics | ||||||
| Male sex, n (%) | 24,479 (70.1) | 96,319 (71.2) | 0.023 | 22,400 (70.7) | 22,387 (70.7) | 0.001 |
| Mean (SD) age, years | ||||||
| Age, n (%) | 50.94 (9.66) | 55.76 (9.47) | 0.503 | 51.06 (9.61) | 51.09 (9.84) | 0.003 |
| < 40 years | 4132 (11.8) | 7231 (5.3) | 0.233 | 3683 (11.6) | 3582 (11.3) | 0.010 |
| 40–64 years | 28,357 (81.3) | 104,473 (77.2) | 0.100 | 25,736 (81.3) | 25,935 (81.9) | 0.016 |
| ≥ 65 years | 2408 (6.9) | 23,607 (17.4) | 0.327 | 2246 (7.1) | 2148 (6.8) | 0.012 |
| Diabetic complications | ||||||
| Nephropathy, n (%) | 4660 (13.4) | 13,690 (10.1) | 0.101 | 3751 (11.8) | 3636 (11.5) | 0.011 |
| Retinopathy, n (%) | 4357 (12.5) | 14,858 (11.0) | 0.047 | 3483 (11.0) | 3402 (10.7) | 0.008 |
| Neuropathy, n (%) | 1363 (3.9) | 4470 (3.3) | 0.032 | 982 (3.1) | 979 (3.1) | 0.001 |
| Diabetic medications | ||||||
| α-Glucosidase inhibitors, n (%) | 2586 (7.4) | 15,239 (11.3) | 0.133 | 2130 (6.7) | 2203 (7.0) | 0.009 |
| Meglitinides, n (%) | 847 (2.4) | 3846 (2.8) | 0.026 | 619 (2.0) | 606 (1.9) | 0.003 |
| Sulfonylureas, n (%) | 3668 (10.5) | 26,442 (19.5) | 0.255 | 2957 (9.3) | 2863 (9.0) | 0.010 |
| Biguanides, n (%) | 14,000 (40.1) | 44,788 (33.1) | 0.146 | 11,622 (36.7) | 11,446 (36.1) | 0.012 |
| Thiazolidinediones, n (%) | 2235 (6.4) | 8468 (6.3) | 0.006 | 1779 (5.6) | 1827 (5.8) | 0.007 |
| GLP-1 receptor agonists, n (%) | 3432 (9.8) | 377 (0.3) | 0.447 | 442 (1.4) | 373 (1.2) | 0.019 |
| Insulin, n (%) | 4920 (14.1) | 12,115 (9.0) | 0.162 | 3570 (11.3) | 3504 (11.1) | 0.007 |
| Comorbidities | ||||||
| Hypertension, n (%) | 20,732 (59.4) | 69,149 (51.1) | 0.168 | 18,522 (58.5) | 18,446 (58.3) | 0.005 |
| Ischemic heart disease, n (%) | 3806 (10.9) | 13,046 (9.6) | 0.042 | 3276 (10.3) | 3206 (10.1) | 0.007 |
| Heart failure, n (%) | 3078 (8.8) | 8601 (6.4) | 0.093 | 2669 (8.4) | 2616 (8.3) | 0.006 |
| Atrial fibrillation, n (%) | 731 (2.1) | 2973 (2.2) | 0.007 | 644 (2.0) | 606 (1.9) | 0.009 |
| Chronic kidney disease, n (%) | 659 (1.9) | 2842 (2.1) | 0.015 | 574 (1.8) | 544 (1.7) | 0.007 |
| Liver disease, n (%) | 10,967 (31.4) | 33,839 (25.0) | 0.143 | 9921 (31.3) | 9862 (31.1) | 0.004 |
| Cerebrovascular disease, n (%) | 2776 (8.0) | 12,545 (9.3) | 0.047 | 2405 (7.6) | 2302 (7.3) | 0.012 |
| Peripheral artery disease, n (%) | 2543 (7.3) | 9019 (6.7) | 0.024 | 2159 (6.8) | 2188 (6.9) | 0.004 |
| COPD, n (%) | 116 (0.3) | 719 (0.5) | 0.030 | 108 (0.3) | 117 (0.4) | 0.005 |
| Asthma, n (%) | 3834 (11.0) | 11,077 (8.2) | 0.095 | 3454 (10.9) | 3446 (10.9) | 0.001 |
| Autoimmune disease, n (%) | 2352 (6.7) | 9112 (6.7) | < 0.001 | 2190 (6.9) | 2229 (7.0) | 0.005 |
| Cancer, n (%) | 1147 (3.3) | 6855 (5.1) | 0.089 | 1009 (3.2) | 996 (3.1) | 0.002 |
| Psychiatric disease, n (%) | 3970 (11.4) | 12,727 (9.4) | 0.065 | 3573 (11.3) | 3577 (11.3) | < 0.001 |
| Trauma, n (%) | 1889 (5.4) | 6959 (5.1) | 0.012 | 1714 (5.4) | 1704 (5.4) | 0.001 |
| Comedications | ||||||
| Immunosuppressants, n (%) | 221 (0.6) | 1344 (1.0) | 0.040 | 204 (0.6) | 197 (0.6) | 0.003 |
| Oral corticosteroids, n (%) | 2301 (6.6) | 8785 (6.5) | 0.004 | 2137 (6.7) | 2152 (6.8) | 0.002 |
| β-blockers, n (%) | 3551 (10.2) | 11,488 (8.5) | 0.058 | 3162 (10.0) | 3115 (9.8) | 0.005 |
| Calcium channel blockers, n (%) | 8936 (25.6) | 38,000 (28.1) | 0.056 | 8096 (25.6) | 8092 (25.6) | < 0.001 |
| RAAS inhibitors, n (%) | 10,123 (29.0) | 38,921 (28.8) | 0.005 | 8932 (28.2) | 8785 (27.7) | 0.010 |
| Antithrombotics, n (%) | 3170 (9.1) | 15,553 (11.5) | 0.079 | 2765 (8.7) | 2657 (8.4) | 0.012 |
| Antilipidemics, n (%) | 16,727 (47.9) | 61,928 (45.8) | 0.043 | 14,746 (46.6) | 14,807 (46.8) | 0.004 |
| Antibiotics, n (%) | 7373 (21.1) | 23,590 (17.4) | 0.094 | 6773 (21.4) | 6911 (21.8) | 0.011 |
PS propensity score, ASD absolute standardized mean difference, GLP-1 glucagon-like peptide-1, COPD chronic obstructive pulmonary disease, RAAS renin–angiotensin–aldosterone system
Table 2.
Comparative analysis of the association between the use of SGLT2 inhibitors and DPP-4 inhibitors in terms of the risk of urogenital bacterial infections
| Outcome | Before PS matching | After PS matching | ||
|---|---|---|---|---|
| SGLT2 inhibitors (n = 34,897) | DPP-4 inhibitors (n = 135,311) | SGLT2 inhibitors (n = 31,665) | DPP-4 inhibitors (n = 31,665) | |
| Urinary tract infections | ||||
| No of events | 1293 | 5377 | 1130 | 1038 |
| Incidence rate† | 24.35 | 27.84 | 23.73 | 27.44 |
| Hazard ratio (95% CI) | 0.88 (0.83–0.93) | 0.90 (0.83–0.98) | ||
| Genital bacterial infections | ||||
| No of events | 374 | 889 | 321 | 216 |
| Incidence rate† | 6.88 | 4.46 | 6.59 | 5.56 |
| Hazard ratio (95% CI) | 1.55 (1.37–1.74) | 1.23 (1.03–1.46) | ||
| Perineal soft tissue infections | ||||
| No of events | 31 | 101 | 30 | 23 |
| Incidence ratea | 0.57 | 0.50 | 0.61 | 0.59 |
| Hazard ratio (95% CI) | 1.15 (0.77–1.72) | 1.05 (0.61–1.81) | ||
PS propensity score, SGLT2 sodium glucose cotransporter 2, DPP-4 dipeptidyl peptidase-4, CI confidence interval
aPer 1000 person-years of follow-up
Fig. 1.
Propensity score-matched Kaplan–Meier curves depicting the cumulative incidences of main outcomes among users of SGLT2 inhibitors and DPP-4 inhibitors. Urinary tract infection events were identified using Japanese electronic claims codes for urinary tract infections and antibiotic prescriptions. Genital bacterial infections were similarly identified. Perineal soft tissue infections include necrotizing fasciitis, cellulitis, or skin abscesses in the perineal area. Necrotizing fasciitis was defined according to the corresponding Japanese claims codes, hospitalization, and surgical treatments, such as debridement or related procedures. Cellulitis and skin abscesses were identified using the Japanese claims codes and antibiotic prescriptions. SGLT2 sodium glucose cotransporter 2, DPP-4 dipeptidyl peptidase-4, CI confidence interval
Discussion
Compared to DPP-4 inhibitors, the use of SGLT2 inhibitors was associated with a reduced risk of UTIs and an elevated risk of genital bacterial infections; however, no significant relationship was observed with perineal soft tissue infections.
Initial concerns regarding an elevated risk of UTIs with SGLT2 inhibitors have been addressed by recent studies, demonstrating no significant association between SGLT2 inhibitor use and UTIs [7, 8]. Only a few studies have shown a relationship between SGLT2 inhibitor use and a lower incidence of UTIs, aligning with our findings. Notably, the EMPA-REG OUTCOME trial revealed a significant reduction in UTI incidence among women using SGLT2 inhibitors compared to the placebo group [2]. Similarly, a retrospective cohort study using a claims database in the United States found that SGLT2 inhibitors were associated with a lower risk of UTI compared to the group using glucagon-like peptide-1 receptor agonists [17]. Another retrospective cohort study utilizing an electronic database in Hong Kong highlighted that individuals treated with SGLT2 inhibitors exhibited a lower rate of UTIs caused by Gram-negative organisms compared to the control group [18].
There are several potential reasons for the observed decrease in UTI incidence among patients with diabetes treated with SGLT2 inhibitors in this study. Clinicians may have expanded their advice to patients using SGLT2 inhibitors, advocating increased hydration to mitigate UTI risks. Given the widespread concern among clinicians regarding the increased UTI risk among users of SGLT2 inhibitors, there may have been a tendency to refrain from prescribing SGLT2 inhibitors to patients at an elevated risk of UTIs. Although we made substantial efforts to address this issue by extensively adjusting for UTI risk factors, the possibility of unmeasured confounding factors cannot be entirely ruled out. Moreover, while SGLT2 inhibitors may possess a greater glucose-lowering effect compared to DPP-4 inhibitors, the disease severity remains unknown due to the absence of hemoglobin A1c (HbA1c) values in this database. Additionally, the database included a limited number of older individuals, who are typically at a higher risk for UTIs. Subgroup analyses of UTI focusing on older individuals showed similar results across different sex and age groups, indicating the potential relevance of the findings for high-risk populations. To the best of our knowledge, this study represents the first to identify an association between SGLT2 inhibitors and genital bacterial infections, although it is well-documented that genital mycotic infection is a common adverse effect of SGLT2 inhibitor use. High glucose concentrations in urine are known to accelerate the growth rate of Escherichia coli [19]. SGLT2 inhibitors facilitate both sodium and glucose excretion in the proximal tubules, potentially reducing skin hypertonicity by lowering tissue sodium content [20]. These mechanisms could potentially compromise the skin barrier, heightening susceptibility to soft tissue infection. However, a pooled post hoc analysis from two randomized trials showed that canagliflozin did not increase the risk of non-genital soft tissue infections compared to placebo (HR 0.97, 95% CI 0.85–1.11) [21]. Additionally, several retrospective cohort studies using a claims database showed no significant association between SGLT2 inhibitor use and FG [11–14]. Consistent with these findings, our results did not indicate an association between SGLT2 inhibitor use and the risk of perineal soft tissue infections.
This study has several limitations. First, the database used did not include individuals aged 75 years or older and comprised only a limited number of individuals aged between 65 and 74 years. Moreover, the dataset predominantly consisted of male participants. To minimize the influence of these biases, we made adjustments for age and sex, and performed additional subgroup analyses specifically targeting older individuals. While the outcomes observed were consistent for both sex and age, further research is certainly necessary. Second, despite our efforts to enhance diagnostic specificity by defining outcomes alongside therapies or procedures, there remains a potential for outcome misclassification. This limitation is partly due to the absence of validation studies for the outcomes of this study. Third, the low incidence of perineal soft-tissue infections may have led to insufficient statistical power to draw definitive conclusions. Finally, our analysis may have been affected by several unmeasured variables that could have influenced our results, such as the duration and severity of diabetes mellitus, as well as activities of daily living including tobacco or alcohol use. However, a previous study demonstrated that adjustments using claims-based proxy information can account for the most unmeasured characteristics, including laboratory test results like HbA1c levels, duration of diabetes, and smoking status among patients with diabetes [22]. We included a comprehensive set of relevant variables to minimize the potential impact of confounding factors.
Conclusions
In comparison to DPP-4 inhibitors, SGLT2 inhibitors exhibited a reduced risk of UTIs and an elevated risk of genital bacterial infections, while no significant association was observed with perineal soft tissue infections. Future investigations should encompass a broader range of population demographics, with a particular focus on the elderly and achieving a more balanced gender representation. This will enable a comprehensive understanding of the risks of infection.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Authorship
All named authors meet the International Committee of Medical Journal Editors’ (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Takanori Imai, Naoki Kanda, Hideki Hashimoto, and Shuji Hatakeyama designed the study. Takanori Imai, Naoto Kato, Naoki Kanda, and Hayato Yamana analyzed the data. Takanori Imai, Naoto Kato, and Naoki Kanda performed statistical analyses. Takanori Imai, Naoki Kanda, Hideki Hashimoto, and Shuji Hatakeyama interpreted the data. Takanori Imai and Shuji Hatakeyama drafted the manuscript. All the authors critically revised the manuscript for intellectual content. All the authors have read and approved the final manuscript.
Funding
This work, including the journal's Rapid Service Fee, was supported in part by the Foundation for the Development of the Community (research grant), JSPS KAKENHI (grant number: 24K10538), and programs for Progress of the next Cross-ministerial Strategic Innovation Promotion Program (SIP) on “Integrated Health System” (grant number: JPJ012425). The funders had no role in the study design, data collection, data analysis, data interpretation, or manuscript writing.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Takanori Imai, Naoto Kato, Naoki Kanda, Hideki Hashimoto, Hayato Yamana, and Shuji Hatakeyama declare no competing interests.
Ethical Approval
The Ethics Committee of Jichi Medical University has approved this study and waived the requirement for informed consent owing to the retrospective design and use of anonymized data (approval number, 21–198). This study was performed in accordance with the relevant guidelines and regulations.
References
- 1.Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018;61:2079–86. 10.1007/s00125-018-4654-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 3.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 4.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57. 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 5.Kanie T, Mizuno A, Takaoka Y, et al. Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis. Cochrane Database Syst Rev. 2021;10:CD013650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S125–43. 10.2337/dc22-S009 [DOI] [PubMed] [Google Scholar]
- 7.Kaze AD, Zhuo M, Kim SC, et al. Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: a meta-analysis. Cardiovasc Diabetol. 2022;21:47. 10.1186/s12933-022-01476-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caparrotta TM, Greenhalgh AM, Osinski K, et al. Sodium-glucose co-transporter 2 inhibitors (SGLT2i) exposure and outcomes in type 2 diabetes: a systematic review of population-based observational studies. Diabetes Ther. 2021;12:991–1028. 10.1007/s13300-021-01004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration (FDA). FDA Drug Safety Communication: FDA Revises Labels of SGLT2 Inhibitors for Diabetes to Include Warnings About too much Acid in the Blood and Serious Urinary Tract Infections. 4 December 2015. http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. (accessed December 27, 2023)
- 10.U.S. Food and Drug Administration (FDA). Safety Announcement: FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes. 29 Aug 2018. https://www.fda.gov/Drugs/DrugSafety/ucm617360.htm. (accessed 16 Jan 2013)
- 11.Wang T, Patel SM, Hickman A, et al. SGLT2 inhibitors and the risk of hospitalization for Fournier's gangrene: a nested case-control study. Diabetes Ther. 2020;11:711–23. 10.1007/s13300-020-00771-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dave CV, Schneeweiss S, Patorno E. Association of sodium-glucose cotransporter 2 inhibitor treatment with risk of hospitalization for Fournier gangrene among men. JAMA Intern Med. 2019;179:1587–90. 10.1001/jamainternmed.2019.2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petruski-Ivleva N, Schneeweiss S, Eapen S, et al. Fournier’s gangrene in patients with type 2 diabetes using second-line antidiabetic medications. Diabetes Obes Metab. 2020;22:267–71. 10.1111/dom.13886 [DOI] [PubMed] [Google Scholar]
- 14.Yang JY, Wang T, Pate V, et al. Real-world evidence on sodium-glucose cotransporter-2 inhibitor use and risk of Fournier’s gangrene. BMJ Open Diabetes Res Care. 2020;8: e000985. 10.1136/bmjdrc-2019-000985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiramatsu K, Barrett A, Miyata Y, PhRMA Japan Medical Affairs Committee Working Group 1. Current status, challenges, and future perspectives of real-world data and real-world evidence in Japan. Drugs Real World Outcomes. 2021;8:459–80. 10.1007/s40801-021-00266-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.JMDC Inc. JMDC Claims Database. https://www.jmdc.co.jp/en/jmdc-claims-database. (accessed 16 Jan 2023)
- 17.Dave CV, Schneeweiss S, Kim D, et al. Sodium-glucose cotransporter–2 inhibitors and the risk for severe urinary tract infections: a population-based cohort study. Ann Intern Med. 2019;171:248–56. 10.7326/M18-3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan GC, Ng JK, Chow KM, et al. SGLT2 inhibitors reduce adverse kidney and cardiovascular events in patients with advanced diabetic kidney disease: a population-based propensity score-matched cohort study. Diabetes Res Clin Pract. 2023;195: 110200. 10.1016/j.diabres.2022.110200 [DOI] [PubMed] [Google Scholar]
- 19.Geerlings SE, Brouwer EC, Gaastra W, et al. Effect of glucose and pH on uropathogenic and non-uropathogenic Escherichiacoli: studies with urine from diabetic and non-diabetic individuals. J Med Microbiol. 1999;48:535–9. 10.1099/00222615-48-6-535 [DOI] [PubMed] [Google Scholar]
- 20.Karg MV, Bosch A, Kannenkeril D, et al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol. 2018;17:5. 10.1186/s12933-017-0654-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang A, Smyth B, Neuen BL, et al. The sodium-glucose cotransporter-2 inhibitor canagliflozin does not increase risk of non-genital skin and soft tissue infections in people with type 2 diabetes mellitus: a pooled post hoc analysis from the CANVAS Program and CREDENCE randomized double-blind trials. Diabetes Obes Metab. 2023;25:2151–62. 10.1111/dom.15091 [DOI] [PubMed] [Google Scholar]
- 22.Patorno E, Gopalakrishnan C, Franklin JM, et al. Claims-based studies of oral glucose-lowering medications can achieve balance in critical clinical variables only observed in electronic health records. Diabetes Obes Metab. 2018;20:974–84. 10.1111/dom.13184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.