Abstract
Caspases are enzymes with protease activity. Despite being known for more than three decades, caspase investigation still yields surprising and fascinating information. Initially associated with cell death and inflammation, their functions have gradually been revealed to extend beyond, targeting pathways such as cell proliferation, migration, and differentiation. These processes are also associated with disease mechanisms, positioning caspases as potential targets for numerous pathologies including inflammatory, neurological, metabolic, or oncological conditions. While in vitro studies play a crucial role in elucidating molecular pathways, they lack the context of the body’s complexity. Therefore, laboratory animals are an indispensable part of successfully understanding and applying caspase networks. This paper aims to summarize and discuss recent knowledge, understanding, and challenges in caspase knock-out mice.
Keywords: Caspases, Apoptotic, Non-apoptoic, Deficiency, Animal model, Mouse
Introduction
Caspases, also known as cysteine-dependent aspartate-specific proteases (alternatively cysteine-aspartic proteases or cysteine aspartic acid proteases), are enzymes that utilize the sulfur atom in cysteine to catalyze cleavage reaction. Together with the serine protease granzyme B, caspases display specificity for Asp in the P1 position of the caspase recognition motif when processing their substrates [1]. The caspase family is highly evolutionary conserved, underscoring its importance across various organisms [2]. Research on caspases began with the identification of protease activity that generates mature interleukin (IL)-1β from its precursor in extracts of human monocytes, where it plays a crucial role in regulating inflammatory responses [3]. Few years later, unusual cleavage at Asp-X bonds of the interleukin-1β-converting enzyme (ICE), also known as caspase-1, was identified [4] and specified in 1992 [5, 6]. In 1993, the C. elegans Cell death protein-3 (CED-3) and mammalian ICE similarity was revealed and associated with programmed cell death - apoptosis. Along with caspase-1, caspase-2 was one of the first discovered mammalian homologues of CED-3 [7]. By 1998, crucial protein components that participate in apoptosis were defined in humans and laboratory mice [8, 9]. Further research brought discovery of members of caspase family in vertebrates standing behind apoptosis or inflammation, function of which remains mostly unexplained, this applies for caspase-15-18 [10, 11].
The presence of specific caspases varies among species (Table 1). This variability is evident when comparing the mouse model to the humans. For instance, mouse caspase-11 is considered an orthologue of human caspase-4 and caspase-5, sharing 68% and 47% of amino acid sequences, respectively [12]. Mice express full length of caspase-12 [13], while primarily a truncated form is present in humans [14]. Conversely, mice lack caspase-5 [15] and -10 [16] compared to humans. Notably, despite these differences, the cascade of molecular caspase pathways is conserved across eukaryotes (Fig. 1).
Table 1.
Comparison of caspases in different species with focus on classical caspase categorisation. In category „others“ the caspase either does not fit groups above or have not yet been specified. * differentiation of keratinocytes [259], **regulates non-canonical pathway of apoptosis [291], *** blocks CED-3 and apoptosis in germ cells [292], **** blocks CED-3 and apoptosis in somatic cells [292]
| human | mouse M. musculus |
zebrafish D. rerio |
fruitfly D. melanogaster |
worm C. elegans |
|
|---|---|---|---|---|---|
| number of caspases | 13 | 11 | 19 | 7 | 4 |
| inflammatory caspases | -1, -4, -5 -12 | -1, -11, -12 | -1, -19a, -19b, -23 | ||
| initiator caspases | -2, -8, -9, -10 | -2, -8, -9 | -2, -8a, -8b, -9, -10, -20, -22 | Dredd, Dronc, Strica | |
| executor caspases | -3, -6, -7 | -3, -6, -7 | -3a, -3b, -6a, -6b, -6c,-7, -21 | Drice, Dcp-1, Decay, Dam | CED-3 |
| others | -14*, -16 | -14*, − 16 | -17 | CSP-1**, CSP-2***, and CSP-3**** | |
| references | [15] | [15] | [293] | [294] | [19] |
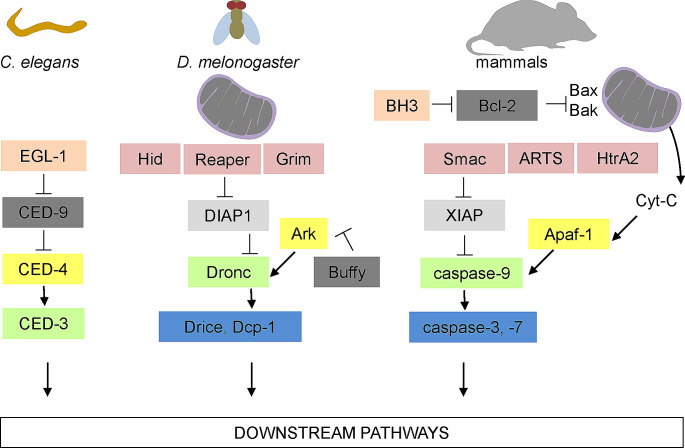
Fig. 1.
Conserved caspase signalling cascades in eukaryotic organisms. In C. elegans, the antagonist EGL-1 inhibits CED-9, leading to the release of CED-4 from the CED-9–CED-4 complex. This liberation promotes the activation of CED-3. In Drosophila, the inhibitors of apoptosis (IAPs) Reaper, Hid, and Grim facilitate the degradation of DIAP1, thereby freeing Drice and Dcp-1. This process also involves the interaction of Dronc with Ark and the formation of the apoptosome, which activates executioner caspases. The activation of the apoptosome might be regulated by proteins such as Buffy. In mammals, Bcl-2 and BH3-only proteins regulate BAX- and BAK-dependent release of cytochrome c from the mitochondria. Cytochrome-c then binds to APAF1 to form the apoptosome. In parallel, IAP antagonists, including DIABLO, HTRA2 and ARTS, translocate from the mitochondria and release caspases from their negative regulation by IAPs. Caspase-9 is subsequently liberated from XIAP and activated by the apoptosome, triggering executioner caspases-3 and − 7. Green: caspase-9 like, yellow: Apaf-1 like, blue: executor caspases, dark grey: Bcl-2 like, light grey: apoptotic inhibitors, pink: BH3-only like, purple: IAP binding. Figure based on Bell and Megeney [284], Fuchs and Steller [285]
Caspase research was developed with help of various laboratory techniques as summarised in Fig. 2. Different organisms (Table 1) were used to investigate caspases and their downstream pathways, particularly, biological activities, potential redundancies, interactions, or impact/s of their deficiency. The mouse is the most relevant in vivo model to search for potential applications in several caspase-related human diseases such as autoimmune, inflammatory, cancer, metabolic, and neurodegenerative pathologies [17].
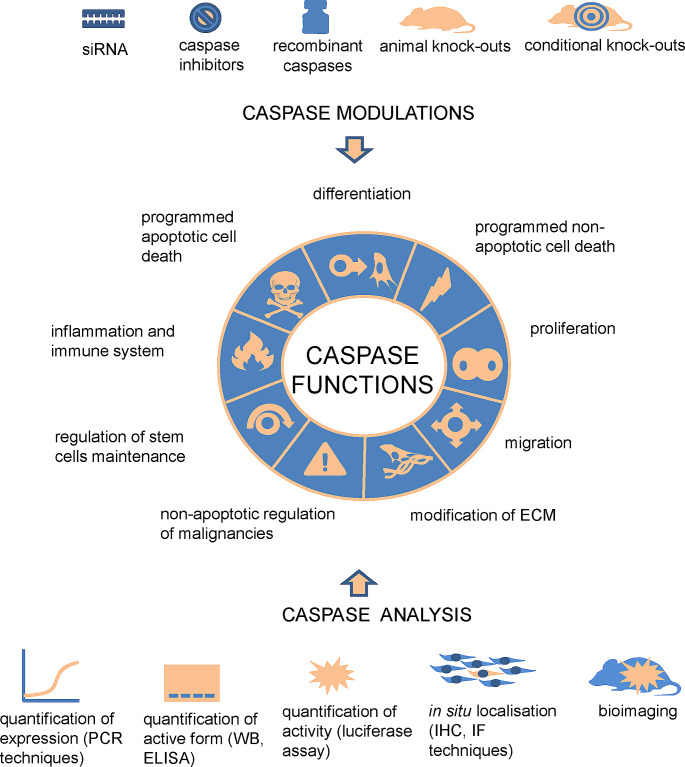
Fig. 2.
Overview of caspase modulation, analysis, and functions. Downstream caspase pathways may be studied with the help of the caspase downregulation at several levels, including inhibition of caspase gene expression by siRNA and inhibition of caspase activity by inhibitors in vitro. Alternatively, recombinant caspases may be used for specification of caspase functions. In vivo investigation relies on deficient mice with null or targeted caspase deletion. Analysis of caspases includes quantification of caspase expression by PCR-based techniques and activity assessment (e.g., western blot, bioluminescence, bioimaging) applied in vitro and in vivo. Detection of caspases by specific antibodies in situ provides information about caspase importance in individual cell types. With the help of these approaches, caspases have been associated with multiple functions such as programmed apoptotic cell death [286], programmed non-apoptotic cell death [287], inflammation and immune system [288], differentiation [16], proliferation [289], regulation of stem cells maintenance [43], non-apoptotic regulation of malignancies [290], modification of ECM [181], migration [28]
Caspase structure and classification
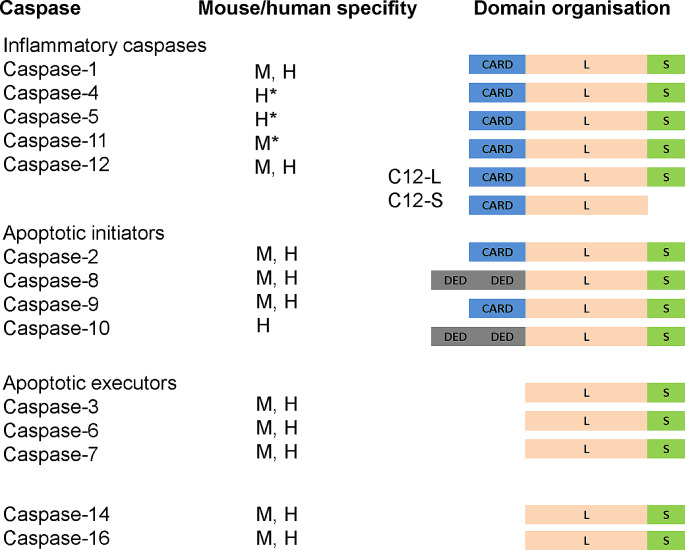
Despite being known for dozens years, there is no clear order in caspase classification. Respectively, the recent classifications are rather artificial, and does not reflect all characteristic of individual caspases. We can also speculate that each caspase has both broad (redundant) and specific (non-redundant) functions. Therefore, any categorisation would inevitably be somewhat inaccurate. When evaluating lethal vs. non-lethal caspases (as shown in Fig. 3), caspase-2, -3, -6, -7, -8, -9, -10 are conventionally associated with apoptosis. Further, there is a group of inflammatory caspases with caspase-1, -4, -5, -11, -12. The mammalian caspases with unknown lethal function are caspase-14 [15, 18] and caspase-16 [10].
Fig. 3.
Classification of caspases. Blue/Grey: long pro-domain containing CARD/DED, pink: long domain L, green: short domain S. The asterisk is used to highlight human/mouse caspase orthologue caspase-4, caspase-5/caspase-11. C12L and C12S stands for full-length and truncated versions, respectively
Pro-apoptotic caspases were further subdivided based on their molecular structure and the relation to the apoptotic machinery. Caspases are mostly expressed as inactive monomers consisting of a pro-domain (long or short), large, and small subunits. The long pro-domain is typical for initiator caspases. It may contain two death effector domains (DED), as seen in caspase-8 and -10. Alternatively, it can have a caspase-activation recruitment domain (CARD), found in caspase-2 and -9. These domains promote dimerization, followed by autoactivation in multiprotein complexes [19]. In contrast, executioner caspases lacking the long pro-domain require cleavage by initiator caspases to reach the activated state [20].
Regarding recent observations on caspase functions, some authors have proposed slight modification(s) to the original classification. In the new system, three caspases stand alone: caspase-2 as a caspase involved in cell cycle, caspase-14 as a caspase involved in cell differentiation, and caspase-12 as caspase with undefined functions [17].
The caspase family can also be subdivided according to amino acids making up the motif (P4, 3, 2, 1) upstream of the cleavage site P1 (Table 2) [21]. Several groups, using different methods, have demonstrated a problem of overlapping substrate specificity among caspases [22]. In the cleavage motif, there are positions where variations are tolerated compared to positions with high selectivity (Table 2). It is important to note that some caspases can cleave certain substrates better than others, sometimes unexpectly based on original analyses [23].
Table 2.
Caspase cleavage motives - based on human caspase research according to Talanian et al. [21]. Caspases exhibit selectivity for Asp (D) in the P1 position, toleration of wide range of amino acids in the P2, a preference for Glu (E) in P3, and a lack of tolerance for charged residues at P1′ (φ symbol stands for preferred Gly, Ala, Thr, Ser and Asn). Most significant differences in caspase specificities are at the P4 positions. ↓ stands for the cleavage site. * Caspase-6 was recently identified to recognise and prefer pentapeptide motif [295]. These groups of caspase roughly reflect groups of initiators, executors, and inflammatory caspases
| caspase | P5 | P4 | P3 | P2 | P1 ↓ | P1´ |
|---|---|---|---|---|---|---|
| -1, -4, -5, -14 | W/Y | E | X | D | Φ | |
| -8, -9, -10 | I/L | E | X | D | Φ | |
| -3, -7 | D | E | X | D | Φ | |
| -6 * | V | E | X | D | Φ | |
| -2 | V/L | D | E | X | D | Φ |
Caspase functions
Caspases have been first recognised as enzymes crucial for apoptotic cell death and inflammation. However, following studies pointed to their functions beyond lethal activities [15, 24–26]. These events include both “non-autonomous” and “autonomous” mechanisms. The former refers to mechanisms that mediate, for example, the compensatory proliferation of cells adjacent to those undergoing apoptosis, while the latter refers to intrinsically mediated activities of caspases that do not result in cell death [27]. Particularly, caspases were associated with proliferation [25], migration [28], differentiation of various cell types [29–32], or even inhibition of cell death [33]. Their substrates include proteins associated to various cellular functions not only the lethal ones but also substrates related to cell adhesion, cytoskeleton, physiology of endoplasmic reticulum (ER) and Golgi apparatus, cell cycle, DNA synthesis and repair, etc. [34]. The cleavage hit mediated by caspases may result in both activation [35] or inactivation [36] of the substrates.
Types and number of substrates is thought to be very different throughout caspase groups. Initiators are thought to cleave few substrates besides their own precursors and other caspases downstream, effectors have a broader spectrum of targets. Among executors, caspase-3 seems to be more promiscuous compared to caspase-7 [37, 38]. The fundamental roles of caspases are summarised in Fig. 2.
Diverse roles of caspases are thought to be associated with various molecular pathways. The apoptotic signalling of caspases is directed by so called extrinsic and intrinsic pathway [39, 40]. The extrinsic pathway regulated by interaction of death receptor (DR) and death ligand results in formation of death-inducing signalling complex (DISC) that activates initiator caspase-8, -10. The intrinsic (mitochondrial) pathway is triggered by internal signals inducing the leakage of cytochrome-c out of mitochondria. Cytochrome-c associates with Apaf-1 and pro-caspase-9, giving rise to a multiprotein complex known as apoptosome, where caspase-9 is activated [41].
Apoptotic pathways are modulated by diverse inhibitory apoptosis proteins (IAPs) and members of the B-cell lymphoma 2 (Bcl-2) protein family, which is divided into three groups: anti-apoptotic proteins (Bcl-2, Bcl-xl, Bcl-w, Mcl-1, Bfl-1/a1), pro-apoptotic BH3-only proteins (Bad, Bid, Bik, Bim, Bmf, Hrk, Noxa, Puma, etc.), and pro-apoptotic pore-formers (Bax, Bak, Bok) [42].
The extrinsic and intrinsic pathways are often interconnected and finally aim to activate central caspase-3 or other executors. In the apoptotic machinery, the executors are not equivalent in their capacity [38]. In short, caspase-3 engagement finally results in caspase-activated DNase (CAD) activation which causes degradation of nuclear DNA. Executors further play role in the cytoskeletal reorganization and formation of cytoplasmic blebs and apoptotic bodies.
In contrast, non-lethal functions of caspases remain mostly unknown, although they may involve processes such as the cleavage of non-caspase substrates by initiators, signalisation of executor pro-caspases, or proteolytic cleavage of transcription factors [30, 43, 44] as illustrated in Fig. 4.
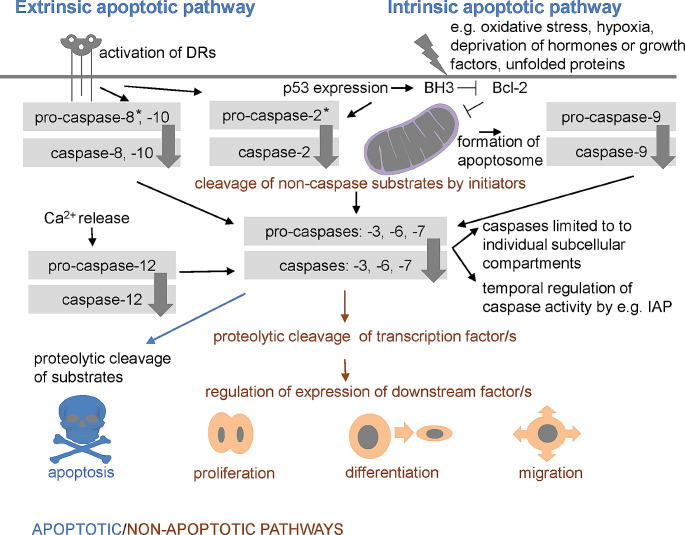
Fig. 4.
Apoptotic and non-apoptotic caspase signalisation. The extrinsic pathway is regulated by death receptors, leading to the activation of caspase-8 and -10. The intrinsic pathway is usually initiated in a cell-autonomous manner, resulting in expression of BH3-only proteins that inhibit anti-apoptotic proteins such as Bcl-2, permeabilization of the mitochondrial outer membrane, formation of apoptosome, and activation of caspase-9. Both pathways aim to activate caspase-3 (or other executors: caspase-6, -7). The extrinsic and intrinsic pathways are often interconnected (e.g. caspase − 8 (and also − 2) cleaves Bid into tBid, which impacts mitochondria). Caspase-12 contributes to Ca2+-dependent apoptosis. Caspase-2 activation occurs in response of both intrinsic and extrinsic stimuli
Initiator caspases
The phenotypes of mice deficient for initiator caspases are listed in Table 3.
Table 3.
Phenotypes of mice lacking initiator caspases. *Mutation in self processing site, d.p.c. day post coitum, HFSC hair follicle stem, NGF nerve growth factor
| mouse strain | development/ phenotype |
apoptotic/ non-apoptotic/unspecified effect | reference | |
|---|---|---|---|---|
| Casp-2 −/− | C57BL/6J | normal development |
decreased number of oocytes, increased number of motoneurons |
[46] |
| Casp-2 −/− | 129/sv×C57BL/6 | normal development | normal apoptosis of thymocytes induced by various stimuli, normal apoptosis of neurons in absence of NGF | [70] |
| Casp-2 −/− | C57BL/6J | reduced sensitivity to heat-shock | reduced cell death | [296] |
| Casp-2 −/− | C57Bl/6 | premature ageing | compromised ability to clear oxidatively damaged cells | [67] |
| Casp-2 −/− /MMTV | 129/B6 | increased tumor acquisition | cell cycle defects, genomic instability | [75] |
| Casp-2 −/− | C57BL/6J | premature ageing | increased oxidative stress | [15] |
| Casp-2 −/− | C57BL/6J | age-related bone loss | increased number of osteoclasts due to reduced apoptosis and increased differentiation | [71, 72] |
| Casp-2 −/− | C57BL/6J | reduced white adipose mass, smaller white adipocytes etc. | altered balance in fuel choice towards increased carbohydrate utilisation due to mild energy stress | [76] |
| Casp-8 −/− | C57BL/6 | abnormalities of heart etc.-prenatally lethal | abnormal receptor-dependent pathway of apoptosis | [68] |
|
Casp-8 −/− (conditional T-cells-specific) |
C57BL/6 | inability of immune response to viral infection | resistance of T-cells to death mediated by anti-CD95 antibody | [90] |
| Casp-8−/−(conditional-several types) | MF-1 | lethal/ non-lethal phenotypes | apoptotic/non-apoptotic engagement in dependence of the targeted cells | [97] |
|
Casp-8 −/− (conditional B cells-specific) |
- | altered responses of B cells to different stimuli |
B cells: abrogation of Fas-mediated apoptosis, failure of induced proliferation, altered ab production |
[91] |
|
Casp-8 −/− (conditional B cells-specific) |
129/J × C57BL/6 | normal cell subpopulations in bone marrow | resistance to DR mediated apoptosis, defective B cells expansion response to TLR4 stimulation, attenuated antibody production upon viral infection | [105] |
|
Casp-8 −/− (conditional keratinocytes-specific) |
C57BL/6 | inflammatory diseases of the skin-lethal by postnatal day 7 |
constitutive phosphorylation of interferon regulatory factor (irf) 3 and tank-binding |
[94] |
|
Casp-8 −/− (conditional epidermis-specific) |
- | epidermal hyperplasia | increased stem cell proliferation and cutaneous inflammation regulated by il-1α | [102] |
|
Casp-8 −/− (conditional hepatocytes-specific) |
- | abnormal hepatocyte reaction to damage | apoptotic and non-apoptotic mechanisms of response to different stimuli | [106, 107] |
|
Casp-8 −/− (conditional macrophage-specific) |
C57BL/6 | mild systemic inflammatory disease | unchecked ripk3 activity | [101] |
|
Casp-8-/- (conditional endothelium -specific) |
- | reduced retina angiogenesis | reduced endothelial proliferation, sprouting, and migration, destabilization cadherin | [32] |
| Casp-8−/−D387A* | C57BL/6 | normal development | reduced Fas-induced apoptosis of T cells, preservation of fas-mediated non-apoptotic signalling | [97] |
| Casp-9 −/− |
129/C57BL/6 129/CD1 |
severe brain defect-prenatally lethal | resistance of different cells to apoptotic stimuli | [124] |
| Casp-9 −/− | C57BL/6 |
severe brain malformation- prenatally lethal |
reduced apoptosis and activation of caspase-3 in brain, resistance of T cells to different apoptotic stimuli | [69] |
| Casp-9 −/− | C57BL/6 | defects and size reduction of the inner ear | decrease of Apaf-1 dependent apoptosis | [116] |
| Casp-9 −/− | C57BL/6 | misrouted axons | abnormal cleavage of sema7a | [122] |
| Casp-9 −/− | C57BL/6 | increased number of oocytes | decreased apoptotic elimination of oocytes during a narrow window between 18.5 and 21.5 d.p.c. | [125] |
|
Casp-9 −/− (conditional HFSC-specific) |
- | accelerated wound repair | induction of apoptotic-engaged state, serving as mitogenic signalling centres by releasing wnt3 | [129] |
Caspase-2
Caspase-2 is thought to be the most evolutionary conserved caspase [45] with a broad expression (brain, heart, kidney, lung and spleen) [46]. Based on the structural properties which include long pro-domain and dimerization during activation, caspase-2 is usually classified as initiator caspase [47]. However, according to research on substrate specificity, caspase-2 rather fits with executor group [21, 48, 49]. Due to the conflicting evidences of its activation (homodimerization, cleavage by other caspases, multiprotein complexes, etc.), function/s or signalling it is called an „orphan“ caspase [50–52]. Regarding apoptosis, caspase-2 can be either pro-apoptotic or anti-apoptotic depending on the cell type, state of growth, and apoptotic stimuli [46, 52]. Interestingly, two splice-variants of caspase-2, pro-apoptotic caspase-2 L, and anti-apoptotic caspase-2 S (included also in DNA repair) were identified to be generated from the same gene in response to pro-apoptotic stimuli [7, 53]. Caspase-2 was also suggested to induce lipoapoptosis, cell death triggered by excessive intracellular accumulation of long-chain fatty acids [54]. Caspase-2-deficient mice did not manifest a phenotype that would support a broad function for caspase-2 in apoptosis [46]. In contrast, this caspase exhibits numerous non-lethal functions, serving as a tumour suppressor [55]/ a cell cycle regulator [52], a regulator of genomic integrity [56, 57], and participating in various cellular processes such as the differentiation [58] and protection of neurons [46], the differentiation of skeletal muscles [59, 60], and osteoblasts in vitro [61, 62]. It might also serve as a therapeutic target for neurological diseases [63], non-alcoholic steatohepatitis [64], metabolic syndrome [54], tautopathies [65], cancer [66], and factor impacting aging [67]. Caspase-2-deficient mice are viable with no gross anatomic abnormalities [46].
Apoptotic effect of caspase-2 deficiency
Caspase-2-deficient mice [46] do not suffer from severe developmental abnormalities, as documented in the case of other initiators [68, 69], implying its crucial function extends beyond apoptosis. Based on caspase-2-deficient mice, it is not clear whether caspase-2 is pro- or anti- apoptotic. Caspase-2 was proposed to stimulate apoptosis of primordial follicles [46]. The number of newly formed primordial follicles containing oocytes was significantly higher in caspase-2-deficient females when compared with wild type (WT) mice, suggesting that apoptotic elimination of foetal germ cell was attenuated in the absence of caspase-2. Furthermore, the oocytes were found to be resistant to cell death induced by chemotherapeutic drugs. This phenomenon, however, was strictly associated with oocytes. Other cell types, such as thymocytes and dorsal root ganglion (DRG) neurons, did not show alterations in apoptotic cell death [70]. In contrast to this, caspase-2 protected motor neurons against naturally occurring cell death during embryonic development, since new-born mice with caspase-2 deficiency had decreased number of motor neurons. The phenomenon might be explained by expression rate of caspase-2 L/caspase-2 S. The short isoform appears to be present in terminally differentiated tissues, such as brain, where it may play a role in survival. Alternatively, caspase-2 loss might be compensated by other caspases up-regulating their expression [46].
Non-apoptotic effect of caspase-2 deficiency
The signs of caspase-2-deficient mice, apart from cell death, indicate a broad functional complexity of this enzyme. While caspase-2-deficient mice had almost the same median lifespan as WT mice, they statistically lived shorter lives [67]. Interestingly, caspase-2 deficiency promoted a number of traits commonly seen in aging animals [15, 67], making these mice potentially interesting models for age-related diseases. Some age-related outcomes may result from significantly increased oxidative damages and reduced activity of antioxidant enzymes in old caspase-2-deficient mice compared to WT animals. The underlying mechanism may include reduced expression of FoxO transcription factors and increased levels of p53 and p21 [16]. The oxidative stress was associated with lower bone mineral density in old (24–26 months) caspase-2-deficient mice compared to WT mice, potentially increasing osteoclast differentiation and reducing apoptosis, leading to enhanced bone resorption [71, 72]. Additionally, lower body fat content and impaired hair growth in caspase-2 deficient mice [67] may be related to oxidative damage.
In the context of neurodegenerative diseases associated with advanced age, mice deficient for caspase-2 showed rescued behavioural and cognitive features of Huntington’s disease (HD) in the YAC128 model. However, they did not exhibit protection from anatomical abnormalities associated with HD, such as specific striatal volume loss [73]. This suggests that different pathways may be involved in the behavioural changes observed in HD. Inhibition of caspase-2 activity could potentially be associated with symptomatic improvement in HD.
Disruption of p53 regulated pathway found in caspase-2-deficient mice may be also related to higher tumour incidence at a sooner age as was seen in caspase-2−/−/MMTV model. Mechanism of caspase-2 action might reside in regulation of cell cycle progression and genomic stability [45, 55, 74, 75]. However, the overall tumour incidence was not observed in caspase-2 deficient mice [67]. Therefore, caspase-2 may be specifically involved in the process of carcinogenesis.
Besides age-related abnormalities, caspase-2 deficiency also altered basal energy metabolism by shifting the balance in fuel choice from fatty acid to carbohydrate usage. Four weeks old caspase-2-deficient mice had increased carbohydrate utilisation and by 17 weeks showed a reduced white adipose mass, smaller white adipocytes, decreased fasting blood glucose and plasma triglycerides but maintained normal insulin levels. In addition, caspase-2-deficient mice placed on a high-fat diet resisted the development of obesity, fatty liver, hyperinsulinemia, and insulin resistance [76].
Caspase-8
Caspase-8 was described as the major initiator of the extrinsic apoptotic pathway [8, 9, 77]. Caspase-8 was identified in cytoplasm as an inactive dimer activated by self-processing [78], which is induced via interaction of the DRs with their ligands. Apart from the apoptosis, caspase-8 is essential for inhibition of necroptosis mediated by Receptor Interacting Serine/Threonine Kinase (RIPK3) and Mixed Lineage Kinase domain-Like (MLKL) [79–82]. Further, it is involved in pyroptosis [83], inflammation [84–86], migration [87, 88], cellular proliferation [89–92], differentiation of osteoblasts [61, 62], myoblasts [29], autophagy [93], and overall cell homeostasis [94]. Caspase-8 is thought to be potential target for treatment of oncologic [95], inflammatory or immune pathologies [96]. Caspase-8-deficient mice performed prenatal lethality around the stage E12.5 resulting from gross abnormalities of vasculature and yolk sac [68].
Apoptotic effect of caspase-8 deficiency
The role of caspase-8 in apoptosis was identified in mesenchymal embryonic fibroblasts (MEF) derived from caspase-8-deficient mice that developed a resistance to extrinsic pathway of programmed cell death [68]. Apoptotic role of this caspase has been further demonstrated in vivo, when caspase-8 specific deletion in hepatocytes using Cre/loxP system protected these cells from Fas-mediated cytotoxicity [97].
Non-apoptotic effect of caspase-8 deficiency
Despite being classified as an apoptotic activator, caspase-8 is vitally important for its anti-lethal effect as a regulator of necroptosis. Interestingly, caspase-8 also appears to regulate inflammatory processes, likely stemming from complex molecular pathways that are not yet fully understood. Deletion of caspase-8 in mice revealed the huge impact of this molecule/protease on the murine embryonic development. The deficiency resulted in degeneration of yolk sac and its vasculature leading to hyperaemia of some blood vessels and organs, congested accumulation of erythrocytes, impaired heart muscle development and neural tube defects [68, 97, 98]. Due to early lethality of caspase-8-deficient mice, following studies focused on targeted deletion of caspase-8 in specific cell populations. Later research explained that lethality of caspase-8-deficient embryos was consequence of an abnormal activity of RIPK3 which is the key component of the necrosome [99]. Caspase-8 inhibits RIPK3 and thus prevents engagement of the final effector MLKL triggering necroptosis [80]. Deletion of RIPK3 or MLKL rescued the embryonic lethality of caspase-8-deficient mice [80, 82]. MLKL deficiency rescued the cardiovascular phenotype but unexpectedly caused perinatal lethality in mice with catalytically inactive caspase-8 (Casp8C362S/C362S,) indicating that CASP8 (C362S) causes necroptosis-independent death at later stages of embryonic development [33].
Despite mice lacking both caspase-8 and RIPK3 not showing any histological abnormalities in utero, embryonic upregulation of the inflammatory genes was detected in several tissues. Interestingly, when focused on the liver, the expression of inflammatory genes starts preferentially in endothelial cells, which were also primarily impacted in caspase-8-deficient mice with fatal consequences [100]. In contrast to increased inflammatory expression in mice lacking both caspase-8 and RIPK3, the loss of caspase-8 in macrophages promotes the onset of a mild systemic inflammatory disease, which could be prevented by the deletion of RIPK3 [101]. Therefore, cell-specific mechanisms probably exist. Regarding the inflammatory processes, mice producing enzymatically inactive caspase-8 developed an inflammatory disease of skin associated with a hyperproliferative state [94], resulting from abnormal signalling regulated by IL1α, which activates both stem cell proliferation and inflammation [102]. Inflammation was also detected in the intestines of mice with conditional deletion of caspase-8 [32].
Caspase-8 deletion further resulted in cellular/humoral alterations of the immune system. Mice with targeted deletion of caspase-8 had significant decrease in the number of peripheral T-cells that were unable to mediate an immune response to viral infection [90]. Notably, in the same mice but older, B and T cell compartments were expanded in the absence of any infection, which resulted in lymphoproliferation and a lethal T cell infiltrating disorder [103]. The impaired function of T-cells was associated with modulation of nuclear factor κB (NF-κB), a key transcription factor for activation of T-cells [104]. NF-κB was also linked with decreased production of antibodies and impaired survival following stimulation of the Toll-like receptors of B-cells in mice with B-cell-specific inactivation of caspase-8 [105].
Generation of mice lacking caspase-8 in hepatocytes (caspase-8Δhepa) demonstrated the role of caspase-8 in liver regeneration after partial hepatectomy. The loss of caspase-8 prevented proteolytic cleavage of the receptor-interacting protein 1 (RIP1) in hepatocytes and subsequently triggered premature activation of NF-κB and c-Jun N-terminal kinase (JNK) related signals which leads to improved liver regeneration [106, 107].
Caspase-9
Caspase-9 is an initiator of the intrinsic apoptotic pathway that becomes activated in apoptosome. Alternatively, activation of caspase-9 without Apaf-1 was induced is some cells by insulin deprivation [108] or caspase-9 can be even cleaved by caspase-3 [109]. In contrast to other caspases, pro-caspase-9 manifests a basal activity that increases with activation level [110]. During apoptosis, caspase-9 cleaves effector caspases [111] or non-caspase substrates (such as vimentin) [111] to dismantle intermediate filaments and amplify the cell death signal [112]. Notably, caspase-9 may also negatively regulate apoptosis with alternatively-spliced truncated caspase-9b form competing with full length caspase-9 [113]. Developmental importance of caspase-9 is supported by its early activation in mouse embryo and early lethality resulting from severe developmental defects [114–116]. Caspase-9 also contributes to necroptosis [117]. Further, caspase-9 was associated with non-lethal functions [118] such as myocyte differentiation and proliferation [119], hematopoietic development [120], immune response to viral infection [121], axon guidance [122] or axon-selective degeneration [44] etc. In a therapeutic invention, caspase-9 may play a central role in pathogenesis of stroke, neurodegenerative diseases, or brain injury caused by hypoxia [123]. Caspase-9 deletion is embryonically or perinatally lethal due to aberrant brain development [69, 124].
Apoptotic effect of caspase-9 deficiency
The most apparent abnormalities of caspase-9 deficiency resided in large brain protrusions and other defects mostly localised in the cortex and forebrain [124]. These alterations were associated with decreased rate of apoptosis and excessive number of neurons. Caspase-9 deficiency further resulted in dramatic decrease in apoptosis in the inner ear epithelium, severe morphogenetic defects, and a significant size reduction of the membranous labyrinth [116].
Furthermore, several cell types performed an abnormal apoptosis when challenged by different apoptotic stimuli. This was, however, not seen in TNF-α induced apoptosis in MEF of caspase-9-deficient mice [124].
Caspase-9 was shown to play a role in oocyte elimination during development. In caspase-9-deficient mice, later phase of oocyte loss was prevented and the total number of oocytes became significantly greater in caspase-9-deficient ovaries at E19.5 when compared to normal mice [125].
In the prenatal formation of tooth, caspase-9-deficient mice displayed inhibition of apoptotic cell death in the primary enamel knot (PEK) [126], the signalling centre of the first molar [127]. Despite PEK regulates the bud-cap transition [128], no impact of the decreased apoptosis was observed during advanced tooth development, indicating that the apoptotic cell death mediated by caspase-9 has been compensated by other molecular mechanisms [126].
Caspase-9 deletion in hair follicle stem cells attenuated the apoptotic process, which surprisingly resulted in increased levels of cleaved caspase-3. These cells were retained in an apoptotic-engaged state, serving as mitogenic signalling centres by releasing Wnt3. Notably, these mice displayed accelerated wound repair and de novo hair follicle regeneration [129].
Non-apoptotic effect of caspase-9 deficiency
Caspase-9 was identified as being important for non-apoptotic aspect(s) of neural development, such as axon-selective degeneration. Interestingly, Apaf-1 was not essential for the process, suggesting either Apaf-1 independent caspase-9 activation [44] or dependence of the phenomenon on pro-caspase form.
Staying with neural system, caspase-9-deficient mice exhibited misrouted axons, impaired synaptic formation, and defects in the maturation of olfactory sensory neurons without affecting the number of these cells. Caspase-9 was shown to be engaged in regulation of active Sema7A levels, which affects axonal path finding, synapse formation and maturation status in the olfactory bulb [122].
Executor caspases
The phenotypes of mice deficient for executor caspases are listed in Table 4.
Table 4.
Phenotypes of mice lacking execution caspase
| mouse strain | development/ phenotype |
apoptotic/ non-apoptotic/unspecified effect | reference | |
|---|---|---|---|---|
| Casp-3 −/− | 129 × 1/SvJ | severe brain defects-perinatally lethal | decreased apoptosis of neural precursors | [131] |
| Casp-3 −/− | C57BL/6J | decreased body size, ectopic masses in head | reduced apoptosis in diverse settings | [148] |
| Casp-3 −/− | C57BL/6 | progressing deafness | degeneration of spiral ganglion neurons and a loss of inner and outer hair cells | [153] |
| Casp-3 −/− | C57BL/6 | abnormal development of inner ear | putative defective apoptosis | [144] |
| Casp-3 −/− | C57BL/6J | minimal brain defect | slight resistance to induced cell death | [141] |
| Casp-3 −/− | B6.129S1 | reduction of skeletal muscle mass | defective myoblast differentiation, reduced activation of mst1 | [29] |
| Casp-3 −/− | C57BL/6J | increased number of B cells | increased proliferation of b cells (increased cdk activity and cyclin abundance) | [134] |
| Casp-3 −/− | B6.129S1 | delayed ossification, decreased bone mineral density | over activation of tgf-b/smad2 pathway, upregulation of p53 and p21, downregulation of cdk2 and cdc2 | [30] |
| Casp-3 −/− |
C57BL/6J 129 × 1/SvJ |
slight alterations of molar development | absence of apoptotic bodies in molar tooth germ at E15 | [145] |
| Casp-3 −/− | C57Bl/6 | increased immature hematopoietic cells | impact on hematopoietic stem cell homeostasis | [149] |
| Casp-3 −/− | C57Bl/6 | decreased skin wound healing and liver regeneration | abnormal pge2 production | [189] |
| Casp-3 −/− | C57BL/6J |
small body size at birth, inner ear abnormalities |
putative apoptotic mechanisms | [133] |
| Casp-3 −/− | C57Bl/6 | decreased incidence of induced skin cancer | attenuation of endog | [147] |
| Casp-3 −/− | B6.129S1 | reduced sebaceous glands | downregulation of yap and genes of proliferation | [150] |
| Casp-3 −/− | C57Bl/6 | proliferative glomerular lesions, splenomegaly | expression of inflammation-associated genes | [146] |
| Casp-3 −/− | C57BL/6 | ADHD, signs of autism in males | putative disruption of homeostatic synaptic plasticity | [154, 155] |
| Casp-3 −/− 7 −/− | C57BL/6 | perinatal lethality | exencephaly in 10% of embryos, heart abnormalities | [186] |
|
Casp-3 −/− 7 −/− (conditional myocardium-specific) |
C57BL/6J | hypoplastic heart at birth, myocyte hypertrophy | reduction of myocyte proliferation, increase of glycolytic enzymes | [297] |
| Casp-6 −/− | C57BL/6J | increased susceptibility to influenza infection | altered cell death, zbp1-mediated inflammasome activation, and host defense | [298] |
| Casp-6 −/− | C57BL/6 | abnormal development of B cells |
no difference of apoptosis b cells activation and differentiation of plasma cells |
[167] |
| Casp-6 −/− | C57BL/6 | protection of neurons against stroke | reduced loss of processes and soma of neurons | [168] |
| Casp-6 −/− | FVB/NJ | neuroanatomical and behavioural alterations | protection from excitotoxicity, ngf deprivation and myelin-induced axonal degeneration | [163] |
| Casp-6 −/− | C57BL/6J | alterations in B cells subsets | alteration of il-7 mediated signalling | [172] |
| Casp-6 −/− | C57 | attenuated liver damage in response to I/R | altered regulation of nr4a1/sox9 interaction | [169] |
| Casp-7 −/− | C57BL/6 | normal development | slight survival advantage of MEF after cell death induction | [186] |
| Casp-7 −/− | C57BL/6 | protection from LPS-induced lethality | resistance to LPS-induced lymphocyte apoptosis | [177] |
| Casp-7 −/− | C57BL/6 | abnormal development of hard tissues | alteration of gene expression associated with formation of hard tissues | [31, 179] |
| Casp-7 −/− | C57BL/6 | protection against ON injury-induced RGC loss | increased density of RGCs, reduced thinning of retina resulting from reduced cell death | [187] |
| Casp-7 −/− | C57BL/6 | increased population of mast cells | putative abnormalities of non-apoptotic signalling | [299] |
Caspase-3
Caspase-3 is widely expressed central executor caspase [1, 37]. In vitro investigation of caspase substrates highlighted caspase-3 as promiscuous enzyme with large spectrum of substrates [23]. Due to its central role, variable levels of caspase-3 are ubiquitously expressed in normal tissues [27]. Caspase-3 activation is mediated by both receptor and mitochondrial apoptotic signalling pathways. Additionally, a shorter isoform, caspase-3s, generated by alternative splicing, negatively regulates apoptosis [130]. Beyond crucial function of caspase-3 in apoptotic cell death during development [131–133], caspase-3 was associated with many non-apoptotic events such as regulation of cell cycle [134], cell differentiation [29, 30, 61, 135], stem cell physiology [43], tissue regeneration, and immunomodulation [136, 137]. Caspase-3 is considered as potential target for immunotherapy in distinct tumours [138], neurodegenerative disorders [139], or heart failure [140]. The phenotype of caspase-3-deficient mice was strain-specific. Caspase-3-deficient 129 × 1/SvJ mice died during the perinatal period and exhibited decreased programmed cell death in brain regions resulting in significant neural precursor cell expansion and exencephaly, ectopic, and duplicated neuronal structures. In contrast, caspase-3-deficient C57BL/6J mice reached adulthood, were fertile, and exhibited minimal brain pathology [141].
Apoptotic effect of caspase-3 deficiency
Caspase-3 mediated apoptosis was found to be indispensable for normal development of central nervous system [142] in caspase-3-deficient 129 × 1/SvJ mice. Compensatory activation of other caspase effectors in the caspase-3-deficient C57BL/6J, but not 129 × 1/SvJ, could be explanation for the strain-dependent phenotypes. And indeed, increased activation of caspase-7 was detected in C57BL/6J caspase-3-deficient mice [143]. Alternatively, strain-specific endogenous inhibitors of apoptosis may underlie the variable caspase-3-deficient phenotype [141].
In contrast to the increased mass of neural tissue, caspase-3-deficient eyes were smaller than their WT counterparts. Additionally, caspase-3-deficient mice displayed peripapillary retinal dysplasia, delayed regression of vitreal vasculature, and retarded apoptotic kinetics of the inner nuclear layer. It was assumed that this phenotype is a result of delayed apoptosis in the developing eye [132]. Therefore, in this case, caspase-3-related apoptosis may be more likely to be of regulatory importance (e.g. regulation of number of specific molecular signals-emitting cells) than basically elimination of unwanted cells. Abnormal organ “sculpturing” in caspase-3 deficiency was also case of the inner ear. Caspase-3 knockout mice developed hypomorphism of the vestibular organs resulting in abnormal locomotion and circling behaviour of mice [133]. Other study also pointed to hyperplasia of supporting cells and degeneration of sensory cells resulting in the hearing loss in caspase-3-deficient mice [144]. The role in “sculpting process” would be expected also for apoptosis of developing molar PEK, where caspase-3 was identified. Surprisingly, the absence of caspase-3 on the B57BL/6 background only led to disorganized epithelium of the developing tooth germ [145].
Despite almost normal life span of caspase-3-deficient mice with B57BL/6 background, they also suffered from some defects associated with abnormal apoptosis. These knock-out mice had kidney proliferative glomerular lesions characterized by increased cells and expression of inflammation-associated genes, but renal dysfunction was not observed. Furthermore, these mice had mild splenomegaly compared with WT mice [146].
Mice deficient in caspase-3 performed reduced chemically induced skin carcinogenesis. Thus, caspase-3 seems to facilitate, rather than suppresses, chemical-induced genetic instability and carcinogenesis. This contrasts with typically considered anti-oncogenic role of caspase activation, which ensures the elimination of genetically unstable or damaged cells [147].
Non-apoptotic effect of caspase-3 deficiency
Several studies have described a smaller body size in caspase-3-deficient mice compared to WT mice of the same age [30, 131, 148, 149]. One explanation for this phenomenon could be decreased cell proliferation. Indeed, decreased proliferation potential was identified in bone marrow stromal stem cells [30]. Deletion of caspase-3 further resulted in reduced cell proliferation, decreased cell number, and reduced sebaceous gland size. The underlying mechanism involved caspase-3-mediated cleavage of α-catenin, which facilitated the activation and nuclear translocation of yes-associated protein (YAP). YAP promotes the transcription of genes associated with cell proliferation [150]. Proliferation defects were also identified in hematopoietic cells, as caspase-3 alters signal transduction by limiting activation of the Ras-Raf-MEK-ERK [149], among others, thereby impacting proliferation.
Caspase-3 deletion resulted also in abnormal cell differentiation as was proved in different cell types. Impaired osteoblastic and osteoclastic differentiation was detected in caspase-3-deficient mice. Regarding the molecular signals, over-activated TGF-β/Smad2 pathway, which may lead to the compromised Runx2/Cbfa1 expression, was detected in preosteoblasts. Furthermore, the upregulated expression of p53 and p21, along with downregulated expressions of Cdk2 and Cdc2, and ultimately increased replicative senescence, were identified in caspase-3-deficient mice. These alterations ultimately resulted in delayed ossification and decreased bone mineral density in caspase-3-deficient mice compared to WT mice [30]. The role of caspase-3 in osteoclast differentiation was later confirmed, with primary osteoclasts unable to differentiate in response to RANKL in the absence of pro-caspase-3 [151]. Furthermore, caspase-3 deficiency impacted the differentiation of myoblasts, leading to a total reduction in skeletal muscle mass. This effect was associated with proteolytic function of caspase-3 that activates pro-myogenic Mammalian Sterile Twenty-like kinase (MST1) [29].
Since the process of regeneration includes both proliferation and differentiation, making it unsurprising that mice lacking caspase-3 exhibited deficiencies in skin wound healing and in liver regeneration [152]. Furthermore, the complexity of caspase-3 functions extends to its impact on hematopoietic stem cells homeostasis detected in caspase-3-deficient mice [149].
Caspase-3 seems to be important for cell survival ganglion cells and hair cells. Caspase-3 knockout mice developed deafness with accompanying degeneration of spiral ganglion neurons and hair cells in the inner ear. The ganglion neurons in caspase-3 exhibit morphological features characteristic for necrosis [153]. Neural development of caspase-3-deficient mice was associated with behavioural changes similar to symptoms of attention deficit/hyperactivity disorder (ADHD) or autism-like social interactions [154]. The mechanism of such caspase engagement is poorly understood, however, in vitro results suggest a role of caspase-3 in expression of AMPA receptors mediating synaptic transition [155].
Caspase-6
Caspase-6 structure is similar to other executor caspases [156]. However, its contribution to apoptotic machinery is probably limited or peculiar [157, 158]. Furthermore, the substrate specificity of caspase-6 more closely resembles that of the initiator caspases, caspase-8 and caspase-9 rather than the two executioners, caspase-3 and caspase-7 [156]. In addition to activation by initiators, caspase-6 may be activated by caspase-3 [38]. Additionally, caspase-6 can act downstream of caspase-1 [159]. Caspase-6 participates in inflammasome activation and host defence mechanisms [160, 161]. Recently, it has also been linked with PANoptosis, a process that involves pyroptosis, apoptosis, and necrosis in the context of cancer pathologies [162]. Caspase-6 is extensively expressed in the brain and is associated to neurological disorders such as Alzheimer disease (AD) and HD, where it also seems to have therapeutic potential [163–165]. Gross developmental defects have not been identified in caspase-6-deficient mice [166–168].
Apoptotic effect of caspase-6 deficiency
Only a few apoptotic functions were described in caspase-6-deficient mice. Caspase-6 was associated with participation in ischemia/reperfusion (I/R) injury [169]. The engagement of caspase-6 in programmed cell death was also observed in caspase-6-deficient macrophages infected with influenza A virus (IAV). Impact of caspase-6 deficiency on apoptosis was manifested by attenuated cleavage of initiator caspase-8 and executioner caspase-3 and -7 [160].
Non-apoptotic effect of caspase-6 deficiency
Caspase-6 seems to play a significant role in neurodegeneration and the modulation of immune response. Caspase-6-deficient mice have shown protection from axonal degeneration, leading to improved in functional outcomes during ischemia [168]. However, the impact of caspase-6 on the neural system extends further, as evidenced by age-dependent behavioural changes and region-specific neuroanatomical alterations. These include increases in cortical and striatal volume accompanied by hypoactive phenotype and learning deficits observed in caspase-6-deficient mice [163]. Some of these abnormalities bear resemblance to the morphological or behavioural pathologies of AD and HD, which result from axonal degeneration [163]. The mechanism might be mediated by cleavage of β-amyloid precursor protein (APP) by beta-secretase during trophic factor deprivation. APP binds to DR6 leading to degeneration of axons by caspase-6 [170].
Caspase-6 was revealed in host defence against IAV infection and loss of caspase-6 impaired viral clearance. Reduced Z-DNA-binding protein 1 (ZBP1)-mediated NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome activation was observed in caspase-6-deficient bone marrow-derived macrophages [160]. NLRP3 is known as an intracellular sensor that detects a broad range of microbial motifs and mediates formation of NLRP3 inflammasome leading to activation of caspase-1 and release of cytokines [171].
Caspase-6 was further observed to control the balance between cell proliferation and differentiation by cleaving substrates involved in maintaining B cell quiescence [167]. Increased number of G1 cells in caspase-6-deficient mice did not translate into dysregulation of overall B cell numbers in adult mice, but rather into an elevation of serum immunoglobulin levels [172].
Caspase 7
Caspase-7 was described as an executor of apoptosis [143, 173, 174], functionally distinct from caspase-3 [173], with a recently discovered non-canonical function as death facilitator [175]. Moreover, it was observed to participate in inflammation [176, 177]. Caspase-7 activation during apoptosis is mediated via initiator caspases. Under inflammatory conditions, caspase-7 activation requires caspase-1 inflammasomes [176, 178]. Additionally, various non-lethal functions were further associated with caspase-7, such as bone formation [179], mineralisation of incisor enamel [31], regulation of mast cell population in dermis [180], or modulation of extracellular matrix in vessels [181]. Caspase-7 inhibition has potential application in neurodegenerative disorders such as AD and HD [182] and prevention of lymphocyte cell death in sepsis [183]. Caspase-7 gene has been linked with rheumatoid arthritis [184], and insulin-dependent diabetes mellitus [185]. Caspase-7-deficient mice are born with normal appearance, organ morphology, and lymphoid development [186].
Apoptotic effect of caspase-7 deficiency
Since the caspase-7-deficient mice are mostly normal, it is not easy to judge whether its role in distinct organ systems is very specific, involves fine tuning, or if caspase-7 functions are compensated by other enzymes. In accordance with this, caspase-7-deficient MEFs only exhibited a slight survival advantage as compared with normal MEFs when treated with inducers of apoptosis. The authors of the study speculate about compensation by caspase-3 [186].
In contrast, caspase-7-deficient mice were protected against lipopolysaccharides (LPS)-induced mortality and LPS-induced lymphocyte apoptosis, independently of the excessive production of serum cytokines, showing that caspase-7 is not required for the secretion of pro-inflammatory cytokines and chemokines in this process [177]. Further studies on optic nerve (ON) injury indicated a significant apoptotic role of caspase-7 in the process. Optic nerve crush caused a progressive loss of retinal ganglion cells (RGCs), which was reduced in caspase-7-deficient mice. ON-induced thinning of ganglion cell complex was significantly ameliorated in caspase-7-deficient mice after injury as well [187].
Non-apoptotic effect of caspase-7 deficiency
Caspase-7 deficiency coincided with an altered expression of osteogenic markers, proposing a role of caspase-7 in differentiation of bone cells. Diverse effects were detected in intramembranous vs. endochondral bones. Intramembranous caspase-7-deficient bone showed a statistically significant decrease in volume while mineral density was not altered. Conversely, endochondral bone showed constant volume but a significant decrease in mineral density in the mutant mice [179]. This might point to multiple downstream functions of caspase-7, which are selectively applied in the two models of ossification.
Caspase-7 deficiency further resulted in delayed mineralization and/or hypomineralization of incisor enamel [31]. Notably, caspase-7 has a different localisation in the epithelial cells on the lingual side of rodent incisor where enamel is not secreted (caspase-7 negative) and the labial side of continuously renewing ameloblasts (caspase-7 positive). It is possible that caspase-7 is involved in the modulation of ameloblast functional differentiation by cleaving its direct target Oct4 [188], which was located in the cervical loop, a stem cells niche where progenitors of future ameloblasts reside [189].
Caspase-7 was speculated to regulate the number of mast cells localised in the dermis [180]. Notably, cleaved caspase-7 was observed in mast cells and its deficiency in adult skin resulted in an increased mast cell number.
Inflammatory caspases
The phenotypes of mice deficient for inflammatory caspases are listed in Table 5.
Table 5.
Phenotypes of mice lacking inflammatory caspases. *Mutant mice contain transgenic caspase-11, since caspase-1 and -11 are too close in the genome to be segregated by recombination. Consequently, caspase 1–/– mice lack both caspase-11 and caspase-1. HS haemorrhagic shock
| mouse strain | development/ phenotype |
apoptotic/ non-apoptotic/unspecified effect | reference | |
|---|---|---|---|---|
| Casp-1 −/− | C57BL/6J | normal development | thymocytes resistant to FasL apoptosis, abnormal il-1 distribution | [201] |
| Casp-1 −/− | 129/Sv | normal development | no alterations in apoptosis, defect in il-1 production | [202] |
| Casp-1 −/− | C57BL/6J | decreased brain damage | reduced edema and lesions caused by ischemic injury | [217] |
| Casp-1 −/− | C57BL/6J | protection against ARF | various effects | [198, 214] |
| Casp-1 −/− | C57BL/6J | prolonged response of lungs to LPS | altered regulation of apoptosis, absent il-1β production in neutrophils treated by lps | [205] |
| Casp-1 −/− | C57BL/6J | abnormal reactions to bacterial infection | diverse effects | [210, 211] |
| Casp-1 −/− | C57BL/6J | protection against cisplatin-induced ATN | protection from apoptosis | [216] |
| Casp-1 −/− | C57BL/6J | thicker retina after light exposure and I/R injury | apoptosis of retinal neurons after excessive light exposure and I/R injury | [206] |
| Casp-1 −/− | - | improved myocardial infarction | reduced apoptosis associated with decreased activation of caspase-3 | [204] |
| Casp-1 −/− | C57BL/6J | increased susceptibility to IAV | decreased cytokine production | [209] |
| Casp-1 −/− | C57BL/6J | increased susceptibility to induced tumorigenesis |
reduced apoptosis, increased proliferation |
[207] |
| Casp1 −/− Casp11Tg* | C57BL/6 | altered response to bacterial infection | failure in secretion of il-1b and il-18 in response to various stimuli, lethal response to lps stimulation | [218] |
| Casp-1 −/− | C57BL/6J | liver damage in HS model | altered regulation of cell death | [203] |
| Casp-1 −/− | C57BL/6J | altered metabolism | protection form non-alcoholic steatohepatitis, atherosclerosis, obesity (age and sex-dependent manner) | [198, 219, 220] |
| Casp-1 −/− | C57BL/6J | abnormal reaction to IAV | induction of more severe pneumonia by iav, increased replication rate | [208] |
| Casp-1 −/− 11 −/− 12 −/− | C57BL/6 | no overt abnormalities | no abnormalities in apoptosis, no abnormalities in septic shock (compared to Casp-1−/−11−/−) | [236] |
| Casp-11 −/− | C57BL/6J | normal development | fibroblast resistance to apoptosis, resistance to lethal dose of lps | [221] |
| Casp-11 −/− | C57BL/6J | reduced apoptosis in stroke model | defect in caspase-3 activation | [223] |
| Casp-11 −/− | C57BL/6J | resistance to allergic lung inflammation | decreased levels of leukocytes in bronchoalveolar lavage fluid, fewer infiltrating alveolar eosinophils | [229] |
| Casp-11 −/− | C57BL/6J | increased susceptibility to colitis | impaired il-18 production, epithelial barrier, and proliferation | [232] |
| Casp-11 −/− | C57BL/6 | altered response to bacterial infection | decreased macrophage cell death, protection from lethal dose of lps | [218] |
| Casp-11 −/− | C57BL/6 | abnormal host-defence response | decreased cell migration mediated by actin depolymerization | [234] |
| Casp-11 −/− | C57BL/6 | sensitivity to colitis-associated carcinogenesis | decreased stat1 activity | [233] |
| Casp-12 −/− | 129 X C57BL/6J | normal development | resistance to ER stress-induced apoptosis, defective apoptosis of cortical neurons induced by amyloid-beta protein | [239] |
| Casp-12 −/− | C57BL/6J | resistance to peritonitis and septic shock | dampened production of ifnγ | [243] |
| Casp-12 −/− | C57BL/6J | less severe colonic inflammation | enhanced production of antimicrobial peptides | [254] |
| Casp-12 −/− | C57BL/6J | greater mortality in MNV | higher viral burden and defective type i ifn response | [255] |
| Casp-12 −/− | C57BL/6J | enhanced malaria clearance at blood-stage | enhanced nf-κb activation (via pathway nemo-iκb kinase complex - nf-κb) | [256] |
| Casp-12 −/− | C57BL/6J | severe liver pathology during malaria infection | enhanced pro-inflammatory response (not sufficient to overcome infection) | [257] |
| Casp-12 −/− | C57BL/6J | reduced CCl4-induced hepatic apoptosis | attenuation of activation of caspase-9 and -3 | [251] |
| Mdx-Casp-12 −/− | C57BL/6J |
preserved muscle function in mdx model |
recovery of specific force generation and resistance to muscle fibre degeneration | [250] |
| T17M/Casp-12 −/− | C57BL/6J | preserved vision in retinal pathogenesis | postponed photoreceptor cell death and preservation of retinal structural integrity | [249] |
| Casp-12 −/− | BL6 | obesity and insulin resistance | abnormal nlrp3 inflammasome pathway | [258] |
Caspase-1
Caspase-1 is the best characterized caspase playing an essential role in inflammation [190]. Caspase-1 activation takes place in assembly of multi-protein complex called inflammasome, which is stimulated by several small molecules derived from infection, tissue damage, or metabolic dysfunctions. There are many types of inflammasomes, where NLR families are the most common responsible for host immune responses against infection, trauma or tissue necrosis [191].
Caspase-1 acts on the cleavage of downstream substrates, including the maturation of the inflammatory cytokines, IL-1β and IL-18, which are among its most important functions [192]. In addition, caspase-1 activation occurs in pyroptosis, a rapid caspase-1-dependent form of cell death frequently induced by infected macrophages. During this process, cleavage of gasdermin D occurs, serving as a pore-forming protein in the formation of channels for secretion of IL-1β and IL-18 [192, 193]. Some authors also suggest a role for caspase-1 in apoptosis [192]. Further, caspase-1 is present in a variety of cell types and is involved in numerous cellular processes such as myoblast differentiation and fusion to multinucleated myotubes [194], neural cell differentiation, or chondrogenesis [195, 196]. Caspase-1 was also associated with the regulation of glucose and lipid metabolism [197], making it a potential target molecule in the treatment of metabolism-related disorders, such as obesity [198], diabetes or osteoarthritis [199], cancer, and non-alcoholic fatty liver disease [200]. Caspase-1-deficient mice are born live with no apparent spontaneous developmental defects [201].
Apoptotic effect of caspase-1 deficiency
Caspase-1-deficient mice did not show major defects in apoptosis [201, 202] but manifested higher levels of liver damage, cell death, and neutrophil influx in haemorrhagic shock. This phenotype indicated hepatoprotective role of caspase-1, due to its ability to regulate cell death pathways by binding anti-apoptotic proteins Bcl-2 and Bcl-xL [203].
In contrast, caspase-1-deficient mice displayed a significant reduction in mortality after myocardial infarction suggesting a pro-apoptotic role of caspase-1 in the heart. When considering the underlying mechanism, caspase-1 was suspected to cleave caspase-9 and -3, but not caspase-8, indicating activation of the intrinsic apoptotic pathway [204]. This correlates with study where caspase-1-deficient neutrophils were susceptible to Fas-mediated apoptosis. Further, delayed LPS-mediated apoptosis was observed in WT neutrophils but not in those deficient in caspase-1 [205]. A pro-apoptotic effect was observed in studies involving retinal neurons injured by excessive light exposure and I/R, where reduced apoptosis was observed [206], as well as in a model of colitis-associated colorectal cancer [207].
Non-apoptotic effect of caspase-1 deficiency
Given the major role assigned to inflammation, caspase-1-deficient mice display distinct reactions when exposed to viral and bacterial stimuli in comparison with WT mice. For instance, upon challenge with IAV, caspase-1-deficient mice exhibited a 40% mortality rate, contrasting with the 10% observed in WT mice, leading to severe diffuse alveolar damage in the lungs of caspase-1-deficient mice [208]. This increased susceptibility to IAV infection was associated with decreased cytokine production [209]. Similarly, the absence of caspase-1 led to increased susceptibility to Salmonella typhimurium infection [210]. Conversely, treatment of caspase-1-deficient mice with LPS injection resulted in survival advantage compared to WT mice [202], and an improved clinical status was was observed in caspase-1-deficient mice with Pneumococcal meningitis and Pseudomonas aeruginosa corneal infection [211, 212]. These findings suggest that caspase-1 operates specifically in response to various stimuli and individual cell characteristics should also be taken into account.
Mice deficient for caspase-1 were defective in the secretion of IL-1β, IL-18, or pro-IL1α [201, 202]. Due to the inability to process pro-IL-18, caspase-1-deficient mice injected with LPS exhibit defective interferon (IFNγ) production. Since IFNγ is an important regulator of cell proliferation, caspase-1-deficient mice show a higher proliferation rate in splenocytes after LPS stimulation [213]. Altered levels of pro-inflammatory cytokines were also observed in other organs and tissues affected by various insults. For instance, in acute renal failure (ARF)/acute tubular necrosis (ATN), caspase-1-deficient mice display an improved phenotype compared to WT mice [214–216]. These mice do not show the increase in IL-18 observed in WT mice during ARF; instead, they exhibit decreased neutrophil infiltration [214]. Furthermore, lower brain IL-1β levels protect caspase-1-deficient mice from ischemia [217, 218].
Besides changing of inflammatory status, caspase-1 deficiency also resulted in increased proliferation of colonic epithelial cells in a model of colitis-associated colorectal cancer [207].
Another category of caspase-regulated processes is the metabolism. Caspase-1-deficient mice develop obesity depending on age and sex when kept on high-fat diet. This phenotype was attributed to lower levels of IL-18, as IL-18-deficient mice show a similar tendency [198]. The absence of caspase-1 further decreased the harmful effect of high fat diet on the liver [219]. Moreover, caspase-1 deficiency improved the phenotype in atherosclerosis-prone apolipoprotein E-deficient (Apoe−/−) mice displaying poor lipoprotein clearance, resulting in atherosclerotic plaques. In this case, caspase-1 promoted atherosclerosis by enhancing the inflammatory status of the lesion [220].
Caspase-11
The functions of caspase-11 remain unclear. While its expression in healthy mice was low, it is highly inducible upon different stimuli [12], including injection of LPS [221]. Unlike other caspases, caspase-11 requires a transcription-dependent signal to up-regulate its cellular expression prior to its activation [12]. In contrast to caspase-1, caspase-11 activation does not require an upstream sensory complex and can be directly activated by LPS [222]. Despite being classified as an inflammatory caspase, it also shares some characteristic with initiator group [223]. The main function of caspase-11 is the induction of non-canonical pathway of pyroptosis [224]. Once this process is activated, caspase-11 cleaves the major substrate protein gasdermin D [225]. Unlike caspase-1, caspase-11 cleaves gasdermin D independently of inflammasome mediators [12, 223]. Caspase-11 also participates in apoptosis [226] where it cleaves caspase-3 [223]. Additionally, it regulates autophagy in response to bacterial insults [227] and modulates intracellular trafficking by influencing of actin polymerization and cell migration [228]. Furthermore, caspase-11 was revealed to play a role in the pathophysiology of asthma and allergy [229]. It also can be involved in brain injury-induced neuronal pyroptosis [230]. Caspase-11-deficient mice are born live without significant developmental defects [221].
Apoptotic effect of caspase-11 deficiency
Caspase-11 exhibited reduced population of apoptotic cells after being subjected to middle cerebral artery occlusion, a mouse model of stroke [223]. The decreased apoptosis was assigned to decreased activation of caspase-3. Caspase-11 further contributed to macrophage death during Salmonella typhimurium infection [231]. Importantly, the process was not dependent on IL-1β/IL-18 maturation and caspase-11 was shown activated in non-canonical inflammasome during this process.
Non-apoptotic effect of caspase-11 deficiency
Caspase-11-deficient mice were found to be protected from sepsis induced by LPS, and they manifested defective secretion of interleukins [221]. Based on this observation, caspase-11 was indicated to interact with caspase-1 and promote its activation [221]. Since caspase-1 and caspase-11 are located close to each other on the chromosome, caspase-1-deficient mice also lacked caspase-11, making it difficult to separate their functions. Further studies using genetically targeted mice provided insight into the specific roles of caspase-11. Thus caspase-11, rather than caspase-1, may be the critical effector of deleterious inflammatory responses [218].
Caspase-11-deficient mice manifested increased susceptibility to inflammatory disease such as colitis due to impaired IL-18 production, resulting in reduced intestinal epithelial barrier integrity and decreased cell proliferation [232]. Additionally, they were more sensitive to colitis-associated carcinogenesis, showing increased expression of proteins associated with early-stage of angiogenesis. The heightened susceptibility of caspase-11-deficient mice was associated with decreased Signal Transducer and Activator of Transcription 1 (STAT1) activity [233]. On the other hand, caspase-11-deficiency conferred protection from allergic lung inflammation. These mice showed decreased levels of leukocyte numbers in bronchoalveolar lavage fluid and had fewer infiltrating alveolar eosinophils [229].
Caspase-11 was suggested to play role in regulation of lymphocyte migration during inflammation. Caspase-11 interacts with actin interacting protein 1 (Aip1), an activator of cofilin-mediated actin depolymerisation [234].
Caspase-12
Despite being initially classified as an inflammatory caspase, caspase-12 function has not yet been sufficiently explained [235, 236]. Caspase-12 differs from inflammatory caspase-1 and -11 in several aspects. It does not participate in the maturation of IL-1β and is not present in macrophages, which are typical models of inflammatory cells [237]. Some authors speculate about its function in cell death induced by ER stress, which frequently occurs due to the accumulation of misfolded proteins and changes in calcium homeostasis [238, 239]. Activation of caspase-12 was detected in some models of apoptotic induction [240, 241], while in others, it was not [239, 242]. Some studies documented a suppressive effect of caspase-12 on caspase-1, which would then enhance vulnerability to sepsis [243]. However, this function of caspase-12 has also been questioned [244]. These contradictory scientific outcomes make caspase-12 difficult to classify and characterize its physiological function. Furthermore, the activation of caspase-12 is not fully understood. In some circumstances, it has been observed to be activated by calpain, TRAF2, and caspase-7 [245–247].
Caspase-12 was detected in various tissues during development, but its constitutive expression was associated with only some cell types, such as epithelia or primary fibroblasts [180, 240, 245]. Interestingly, caspase-12 was found in developing bone and may regulate the expression of osteogenic markers such as Alpl, Bglap, and Phex [248]. Mice, unlike humans, express full length caspase-12 which can undergo proteolytic cleavage [240]. Multiple roles of caspase-12 thus were hypothesized in mice. In humans, most people express truncated form of caspase-12 lacking catalytic domain and only about 20% African descent people express full length protease which is a risk factor for developing sepsis [14]. From the clinical aspect, caspase-12 has shown potential in the treatment of inherited retinopathy [249] and Duchenne muscular dystrophy (DMD) [250]. Additionally, it is speculated to play a role in neurological diseases due to its putative engagement as ER stress sensor [239]. Remarkably, caspase-12-deficient mice are born live without significant developmental defects [239].
Apoptotic effect of caspase-12 deficiency
Engagement of caspase-12 in apoptosis was dependent on different stimuli [239]. The reduction of apoptosis as observed in different disease model in caspase-12-deficient mice could be either beneficial or harmful for treatment of pathologies [239, 249, 251]. Caspase-12-deficient cortical neurons were defective in ER apoptosis induced by amyloid-β protein and thus caspase-12 may contribute to amyloid-β neurotoxicity [239]. Mechanism of ER induced apoptotic pathway was not understood. One of the hypotheses speculates that an imbalance in Ca2+ homeostasis can cause calpain translocation to the ER leading to activation of caspase-12 [252]. Caspase-12 then induces the caspase-3-dependent apoptotic pathway through the activation of caspase-9 [253].
Caspase-12 ablation in T17M retinas (model of retinal pathology) resulted in postponed photoreceptor cell death and preservation of retinal structural integrity with the scenarios where ER stress-IRE1-TRAF2-Csp12-Csp3/7 and the calcium-induced active calpain-caspase-12-Csp-3/7 pathways contribute to retinal pathogenesis in T17M mice through activation of caspase-12 [249].
In carbon tetrachloride-induced hepatocytes, reduced apoptosis was observed in caspase-12-deficient mice compared to WTs, resulting in decreased liver damage. This phenotype was accompanied by attenuated activation of caspase-9 and -3, supporting caspase-12 action on caspase-3 directly and/or indirectly via caspase-9 activation [251].
Non-apoptotic effect of caspase-12 deficiency
Similar to its apoptotic functions, the non-apoptotic roles of caspase-12 were dependent on specific cell types and stimuli. Caspase-12-deficient mice have been observed to have increased resistance to polymicrobial sepsis and peritonitis [243], as well as to some bacterial infections [254], when compared to WT mice. The survival advantage of the caspase-12-deficient mice resulted from more efficient clearance of bacterial infection than in WT littermates. This was accompanied by increased levels of pro-inflammatory cytokines, including IFNγ, which was critical for the process [243]. Consistently, improved pathogen clearance was associated to NF-κB activation [254].
In contrast to bacterial infection, caspase-12-deficient mice exhibit greater mortality during West Nile virus (WNV) infection compared to WT mice. This was accompanied by exacerbated neurological symptoms, higher viral burden and defective IFNβ response [255]. Despite increased level of pro-inflammatory cytokines [256], caspase-12-deficient mice were not universally protected from malaria infection [257].
Higher level of obesity was observed on a high-fat diet in caspase-12-deficient mice compared to their WT counterparts. They increased liver weight, serum cholesterol, liver triglycerides and elevated liver damage. They also developed glucose intolerance and insulin resistance. This phenotype might be dependent on the NLRP3 inflammasome, since Casp12−/−Nlrp3−/− mice did not develop obesity and were similar with WT mice [258].
Deletion of caspase-12 improved phenotype of mdx mice, model for DMD. ER stress is heightened in dystrophic muscles and contributes to the pathology of DMD. Mdx−/−Casp-12−/− mice had a 75% recovery of both specific force generation and resistance to eccentric contractions. The compensatory hypertrophy normally found in Mdx−/− muscles was normalized when caspase-12 was deleted. The mechanism by which caspase-12 deletion preserves Mdx−/− muscle function is not known. Possible mechanisms may include an improvement in regeneration, protection of contractile proteins from degradation but also apoptotic aspect cannot be excluded [250].
Caspase with differentiation function
The phenotypes of mice deficient for caspase-14 are listed in Table 6.
Table 6.
Phenotypes of mice lacking caspase-14
| mouse strain | development/ phenotype |
apoptotic/ non-apoptotic/unspecified effect | reference | |
|---|---|---|---|---|
| Casp-14 −/− | Swiss Webster X 129 | shinier and more lichenified skin | abnormal cleavage of profilaggrin | [266] |
| Casp-14 −/− | Swiss Webster X 129 | reduced epidermal barrier | defect in the terminal filaggrin degradation | [260] |
| Casp-14 −/− | Swiss Webster X 129 | predisposed parakeratosis | abnormal differentiation and the maintenance of stratum corneum | [268] |
| Casp-14 −/− | Swiss Webster X 129 | enhanced antibacterial response | imbalance of the skin-resident bacterial communities | [269] |
Caspase-14
Caspase-14 stands out as a unique member of the caspase family, distinct from both apoptotic and inflammatory groups of caspases. Activation of caspase-14 primarily occurs in epithelial cells of the skin and hair follicles undergoing a special type of cell death called cornification [259]. Its crucial role lies in the processing of profilaggrin to filaggrin and later into hygroscopic amino acids, which act as one of the elements of natural moisturizing factors, thereby contributing to the maintenance of the skin barrier against water loss [260]. The clinical relevance of caspase-14 is particularly evident during the terminal differentiation of skin keratinocytes and the maintenance of normal stratum corneum [261]. In contrast to other caspases expressed ubiquitously in various cells, caspase-14 was located specifically in cornifying epithelia and hair follicles, Hassall’s bodies of the thymus gland, and in the forestomach of rodents [259, 262]. Although the regulation of the caspase-14 gene has not been fully elucidated, it is speculated to be tightly connected with processes of epidermal differentiation [263].
Furthermore, caspase-14 expression has been described in various types of cancer and diabetic retinopathy [263] In the context of the skin diseases, increased expression of caspase-14 has been found in cancerous lesions, while decreased expression was associated with psoriasis or atopic dermatitis [264, 265]. Notably, caspase-14-deficient mice born live, are fertile, and live as long as WT mice [260, 266].
Apoptotic effect of caspase-14 deficiency
Caspase-14 was not associated with activation in response to apoptotic stimuli [267].
Non-apoptotic effect of caspase-14 deficiency
Caspase-14 deficiency particularly impacted skin cornification [263]. The skin of new-born caspase-14-deficient mice was shinier and more lichenified than in WT mice [266]. Furthermore, caspase-14-deficient mice have decreased epidermal hydration, higher transepidermal water loss, and three times lower levels of natural moisturizing factors [260, 266]. This is because, in caspase-14-deficient mice, processing of profilaggrin, the only known substrate of caspase-14, into fillagrin was initiated but not completed, resulting in the accumulation of filaggrin fragments, which leads to various aberrant phenotypes [260].
The skin of caspase-14-deficient mice was also more sensitive to different stimuli compared to WT mice. Following repetitive treatment by acetone, a higher incidence of large parakeratotic plaques was observed in caspase-14-deficient mice compared to WTs [268]. Additionally, the skin of caspase-14-deficient mice exhibited heightened sensitivity to the formation of cyclobutene pyrimidine dimers after UVB irradiation, resulting in increased levels of UVB-induced apoptosis [266]. Furthermore, caspase-14 ablation resulted in an increase in bacterial richness and diversity during steady-state conditions and caspase-14-deficient mice showed enhanced antibacterial response compared to WT mice when challenged with bacteria [269].
Comparison of knockout animals
Mice deficient in caspases displayed some interesting phenotype similarities. The most apparent resemblance was seen in decreased elimination of neurons, resulting in excessive neural tissue incompatible with life in caspase-3 or -9-deficient mice [69, 131, 270]. A similar observation was made in Apaf-1 deficient mice [271], highlighting the crucial role of the intrinsic apoptotic pathway for neural development and viability. Caspase-8-deficient mice also exhibited abnormalities of neural system; however, the lethality observed in caspase-8 null mice resulted from uncontrolled necroptosis [101]. Therefore, the extrinsic pathway likely plays a minor or specific role in this process. Mice lacking the caspase-3 or -9 (or also Apaf-1) suffered from anatomic and functional abnormalities of the inner ear, which was again attributed to the decreased levels of the intrinsic pathway of apoptosis [116, 133, 144].
Insufficient apoptosis further impacted caspase-2 or -9-deficient ovaries [46, 125], albeit with different timing. Conversely, deficiency of the anti-apoptotic Bcl-2 resulted in decreased oocyte population [272], further supporting the importance of mitochondria in this process. Surprisingly, all caspase-deficient mice, except caspase-14, showed some alterations in apoptosis depending on the stimuli or cell types (see Tables 3, 4 and 5). This suggests existence of various pathways that may be employed in specific cells and situations.
Deficiency of inflammatory caspases resulted in altered response to various types of infection, with either beneficial or deteriorative effects for the mutant mice (see Table 5). As observed in caspase-1 or -11-deficient mice, the phenotype may result from impaired cytokine production [202, 232]. On the contrary, caspase-12-deficient mice showed an increased pro-inflammatory response [257], highlighting the specific character of caspase-12.
In terms of non-lethal functions, caspase-3 or -7 deficiency has been linked to abnormalities in differentiation and the formation of hard tissues [30, 31, 179]. Although both deficient models have shown alterations in osteogenic gene expression [30, 179], the function of these caspases may also involve the degradation of stem-cell-specific factors [43, 188]. Caspase-3 or -12 deficiency have been implicated in skeletal muscle function [29, 250], but unrelated mechanisms were suggested to be involved. Caspase-1, -3 or -8 have been observed to regulate the proliferation [102, 134, 150, 207] of various cell types, making it difficult to determine a general mechanism for this process. Additionally, caspase-1, -2 or caspase-12 deficient mice have shown alterations in metabolism and the development of obesity, with specificities for each caspase [76, 198, 258]. Impaired synaptic formation and plasticity have been observed in caspase-9-deficient [122] or caspase-3-deficient mice [154, 155]. Furthermore, behavioural alterations have been detected in caspase-3 or caspase-6-deficient mice [154, 155, 163]. While caspase-1 or -2-deficiency has been associated with increased susceptibility to tumor induction [75, 207], deficiency of caspase-3 has been linked inversely to a decreased incidence of cancer [147]. These data highlight the diverse roles of caspases, not only as tumour inhibitors but also as tumour inducers, across various pathways. In general, the apoptotic pathway seems to be more conserved compared to non-apoptotic mechanisms, and disruption of the apoptotic function of caspases results in more devastating effect compared to abnormalities resulting from their non-apoptotic roles.
Future perspective of caspase research
Caspases have been recognized as great promising targets for treating various human diseases [273–275]. However, the efficacy, specificity, and side effects of pharmacological caspase inhibitors, the primary tools in clinical studies and future applications, remain significant challenges [275]. Despite recent advancement such as nanoparticle delivery systems and CRISPR/Cas9 gene editing showing promising potential in caspase treatment [276, 277], many questions about caspases themselves remain unanswered. The main challenge lies in describing and understanding the multiple and sometimes contradictory functions of caspases. This is closely related to further identification of their substrates and downstream pathways, including caspase regulators. Notably, the switch between lethal and non-lethal functions is considered a fundamental question, with several mechanisms proposed to regulate the process, such as subcellular localisation [278], availability of specific substrates [279], compensation by anti-apoptotic proteins [280, 281], various levels of activation [282], the biological status of cells (cell type, state of growth/differentiation), cellular context (environment), caspase activation vs. non-activated state, and mechanisms of activation. Many of these aspects may be further explored using mouse models, with temporal (Tamoxifen-inducible gene deletion) and/or spatial regulation (Cre/loxP recombination system) of caspase deficiency helping to specify caspase engagement in the development of different organs. However, it is important to note that while mouse models are valuable, there are limitations to directly extrapolating their findings to human research. For instance, caspase-8 deficiency is incompatible with life in mice but only results in immunological disorders in human [283]. Finally, the focus of the field should be on translational work and complementing mechanistic models drawn from experimentation in mice.
Conclusion
Mouse caspase models has greatly contributed to the progress in explaining the traditional but also emerging roles of these cysteine-aspartate proteases in the last decades. We hope that this comprehensive overview of available mouse models will not only acknowledge their hitherto utility but also emphasize their importance in enhancement of the development within this research field associated with basic as well as applied research.
Abbreviations
- AD
Alzheimer disease
- ADHD
Attention deficit/hyperactivity disorder
- Aip1
Actin interacting protein 1
- Apoe
Apolipoprotein E-deficient
- APP
β-amyloid precursor protein
- ARF
Acute renal failure
- ATN
Acute tubular necrosis
- Bcl-2
B-cell lymphoma 2
- CAD
Caspase-activated DNase
- CARD
Caspase-activation recruitment domain
- Casp
Caspase
- CED
Cell death protein
- d.p.c.
Day post coitum
- DED
Death effector domain
- DISC
Death-inducing signalling complex
- DMD
Duchenne muscular dystrophy
- DR
Death receptor
- DRG
Dorsal root ganglion
- ER
Endoplasmic reticulum
- HD
Huntington disease
- HFSC
Hair follicle stem cells
- HS
Haemorrhagic shock
- I/R
Ischemia/reperfusion
- IAP
Inhibitor of apoptosis protein
- IAV
Influenza A virus
- ICE
Interleukin-1β-converting enzyme
- IFN
Interferon
- IL
Interleukin
- JNK
C-Jun N-terminal kinase
- LPS
Lipopolysaccharides
- MEF
Mesenchymal embryonic fibroblasts
- MLKL
Mixed Lineage Kinase domain-Like
- MST1
Mammalian Sterile Twenty-like kinase
- NF-κB
Nuclear factor κB
- NGF
Nerve growth factor
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- ON
Optic nerve
- PEK
Primary enamel knot
- RGCs
Retinal ganglion cells
- RIP1
Receptor-interacting protein 1
- RIPK3
Receptor Interacting Serine/Threonine Kinase 3
- STAT1
Signal Transducer and Activator of Transcription 1
- WNV
West Nile virus
- WT
Wild type
- YAP
Yes-associated protein
- ZBP1
Z-DNA-binding protein 1
Author contributions
ES and BV wrote the manuscript. EM, YC and EJ revised it critically. ES prepared illustrations. EM received the funding. All authors approved the content of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The CR-US scientific cooperation was supported by the Inter-Excellence project Inter-Action LTAUSA19033 (www.msmt.cz).
Open access publishing supported by the National Technical Library in Prague.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Julien O, Wells JA (2017) Caspases and their substrates. Cell Death Differ 24:1380–1389. 10.1038/cdd.2017.44 10.1038/cdd.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahoo G, Samal D, Khandayataray P, Murthy MK (2023) A review on caspases: key regulators of biological activities and apoptosis. Mol Neurobiol 60:5805–5837. 10.1007/S12035-023-03433-5 10.1007/S12035-023-03433-5 [DOI] [PubMed] [Google Scholar]
- 3.Kostura MJ, Tocci MJ, Limjuco G, et al (1989) Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A 86:5227–5231. 10.1073/pnas.86.14.5227 10.1073/pnas.86.14.5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard AD, Kostura MJ, Thornberry N, et al (1991) IL-1-converting enzyme requires aspartic acid residues for processing of the IL-1 beta precursor at two distinct sites and does not cleave 31-kDa IL-1 alpha. J Immunol 147:2964–2969. 10.4049/jimmunol.147.9.2964 10.4049/jimmunol.147.9.2964 [DOI] [PubMed] [Google Scholar]
- 5.Thornberry NA, Bull HG, Calaycay JR, et al (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768–774. 10.1038/356768A0 10.1038/356768A0 [DOI] [PubMed] [Google Scholar]
- 6.Cerretti DP, Kozlosky CJ, Mosley B, et al (1992) Molecular cloning of the interleukin-1 beta converting enzyme. Science 256:97–100. 10.1126/SCIENCE.1373520 10.1126/SCIENCE.1373520 [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Kinoshita M, Noda M, et al (1994) Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1 beta-converting enzyme. Genes Dev 8:1613–1626. 10.1101/GAD.8.14.1613 10.1101/GAD.8.14.1613 [DOI] [PubMed] [Google Scholar]
- 8.Alnemri ES, Livingston DJ, Nicholson DW, et al (1996) Human ICE/CED-3 protease nomenclature. Cell 87:171. 10.1016/S0092-8674(00)81334-3 10.1016/S0092-8674(00)81334-3 [DOI] [PubMed] [Google Scholar]
- 9.Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911. 10.1101/GAD.13.15.1899 10.1101/GAD.13.15.1899 [DOI] [PubMed] [Google Scholar]
- 10.Eckhart L, Ballaun C, Hermann M, et al (2008) Identification of novel mammalian caspases reveals an important role of gene loss in shaping the human caspase repertoire. Mol Biol Evol 25:831–841. 10.1093/MOLBEV/MSN012 10.1093/MOLBEV/MSN012 [DOI] [PubMed] [Google Scholar]
- 11.Eckhart L, Ballaun C, Uthman A, et al (2005) Identification and characterization of a novel mammalian caspase with proapoptotic activity. J Biol Chem 280:35077–35080. 10.1074/JBC.C500282200 10.1074/JBC.C500282200 [DOI] [PubMed] [Google Scholar]
- 12.Agnew A, Nulty C, Creagh EM (2021) Regulation, activation and function of Caspase-11 during health and disease. Int J Mol Sci 22:1–20. 10.3390/IJMS22041506 10.3390/IJMS22041506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer H, Koenig U, Eckhart L, Tschachler E (2002) Human caspase 12 has acquired deleterious mutations. Biochem Biophys Res Commun 293:722–726. 10.1016/S0006-291X(02)00289-9 10.1016/S0006-291X(02)00289-9 [DOI] [PubMed] [Google Scholar]
- 14.Saleh M, Vaillancourt JP, Graham RK, et al (2004) Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429:75–79. 10.1038/NATURE02451 10.1038/NATURE02451 [DOI] [PubMed] [Google Scholar]
- 15.Shalini S, Dorstyn L, Dawar S, Kumar S (2015) Old, new and emerging functions of caspases. Cell Death Differ 22:526–539. 10.1038/cdd.2014.216 10.1038/cdd.2014.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalini S, Dorstyn L, Wilson C, et al (2012) Impaired antioxidant defence and accumulation of oxidative stress in caspase-2-deficient mice. Cell Death Differ 19:1370–1380. 10.1038/cdd.2012.13 10.1038/cdd.2012.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Opdenbosch N, Lamkanfi M (2019) Caspases in cell death, inflammation, and disease. Immunity 50:1352–1364. 10.1016/J.IMMUNI.2019.05.020 10.1016/J.IMMUNI.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakamaki K, Satou Y (2009) Caspases: evolutionary aspects of their functions in vertebrates. J Fish Biol 74:727–753. 10.1111/J.1095-8649.2009.02184.X 10.1111/J.1095-8649.2009.02184.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamkanfi M, Declercq W, Kalai M, et al (2002) Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ 9:358–361. 10.1038/SJ.CDD.4400989 10.1038/SJ.CDD.4400989 [DOI] [PubMed] [Google Scholar]
- 20.Ramirez MLG, Salvesen GS (2018) A primer on caspase mechanisms. Semin Cell Dev Biol 82:79–85. 10.1016/j.semcdb.2018.01.002 10.1016/j.semcdb.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talanian R V., Quinlan C, Trautz S, et al (1997) Substrate specificities of caspase family proteases. J Biol Chem 272:9677–9682. 10.1074/JBC.272.15.9677 10.1074/JBC.272.15.9677 [DOI] [PubMed] [Google Scholar]
- 22.Poreba M, Strozyk A, Salvesen GS, Drag M (2013) Caspase substrates and inhibitors. Cold Spring Harb Perspect Biol 5:a008680–a008680. 10.1101/cshperspect.a008680 10.1101/cshperspect.a008680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McStay GP, Salvesen GS, Green DR (2007) Overlapping cleavage motif selectivity of caspases: implications for analysis of apoptotic pathways. Cell Death Differ 2008 152 15:322–331. 10.1038/sj.cdd.4402260 10.1038/sj.cdd.4402260 [DOI] [PubMed] [Google Scholar]
- 24.Lamkanfi M, Festjens N, Declercq W, et al (2007) Caspases in cell survival, proliferation and differentiation. Cell Death Differ 14:44–55. 10.1038/sj.cdd.4402047 10.1038/sj.cdd.4402047 [DOI] [PubMed] [Google Scholar]
- 25.Connolly PF, Jäger R, Fearnhead HO (2014) New roles for old enzymes: killer caspases as the engine of cell behavior changes. Front Physiol 5:149. 10.3389/fphys.2014.00149 10.3389/fphys.2014.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoshan-Barmatz V, Arif T, Shteinfer-Kuzmine A (2023) Apoptotic proteins with non-apoptotic activity: expression and function in cancer. Apoptosis 28:730–753. 10.1007/S10495-023-01835-3 10.1007/S10495-023-01835-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eskandari E, Eaves CJ (2022) Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J Cell Biol 221. 10.1083/JCB.202201159 [DOI] [PMC free article] [PubMed]
- 28.Mashima T, Naito M, Noguchi K, et al (1997) Actin cleavage by CPP-32/apopain during the development of apoptosis. Oncogene 14:1007–1012. 10.1038/SJ.ONC.1200919 10.1038/SJ.ONC.1200919 [DOI] [PubMed] [Google Scholar]
- 29.Fernando P, Kelly JF, Balazsi K, et al (2002) Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci U S A 99:11025–11030. 10.1073/PNAS.162172899 10.1073/PNAS.162172899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura M, Chen X-D, Allen MR, et al (2004) A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest 114:1704–13. 10.1172/JCI20427 10.1172/JCI20427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matalova E, Lesot H, Svandova E, et al (2013) Caspase-7 participates in differentiation of cells forming dental hard tissues. Dev Growth Differ 55:615–21. 10.1111/dgd.12066 10.1111/dgd.12066 [DOI] [PubMed] [Google Scholar]
- 32.Tisch N, Freire-Valls A, Yerbes R, et al (2019) Caspase-8 modulates physiological and pathological angiogenesis during retina development. J Clin Invest 129:5092–5107. 10.1172/JCI122767 10.1172/JCI122767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fritsch M, Günther SD, Schwarzer R, et al (2019) Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575:683–687. 10.1038/S41586-019-1770-6 10.1038/S41586-019-1770-6 [DOI] [PubMed] [Google Scholar]
- 34.Fischer U, Jänicke RU, Schulze-Osthoff K (2003) Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ 10:76–100. 10.1038/sj.cdd.4401160 10.1038/sj.cdd.4401160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enari M, Sakahira H, Yokoyama H, et al (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43–50. 10.1038/34112 10.1038/34112 [DOI] [PubMed] [Google Scholar]
- 36.Van Raam BJ, Ehrnhoefer DE, Hayden MR, Salvesen GS (2013) Intrinsic cleavage of receptor-interacting protein kinase-1 by caspase-6. Cell Death Differ 20:86–96. 10.1038/CDD.2012.98 10.1038/CDD.2012.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter AG, Jänicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104. 10.1038/sj.cdd.4400476 10.1038/sj.cdd.4400476 [DOI] [PubMed] [Google Scholar]
- 38.Slee EA, Adrain C, Martin SJ (2001) Executioner caspase-3, -6, and – 7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276:7320–6. 10.1074/jbc.M008363200 10.1074/jbc.M008363200 [DOI] [PubMed] [Google Scholar]
- 39.Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. 10.1080/01926230701320337 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43:582–592. 10.1002/cbin.11137 10.1002/cbin.11137 [DOI] [PubMed] [Google Scholar]
- 41.Jan R, Chaudhry G e. S (2019) Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharm Bull 9:205–218. 10.15171/APB.2019.024 [DOI] [PMC free article] [PubMed]
- 42.Kale J, Osterlund EJ, Andrews DW (2018) BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ 25:65–80. 10.1038/cdd.2017.186 10.1038/cdd.2017.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujita J, Crane AM, Souza MK, et al (2008) Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell 2:595–601. 10.1016/J.STEM.2008.04.001 10.1016/J.STEM.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cusack CL, Swahari V, Hampton Henley W, et al (2013) Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat Commun 4. 10.1038/NCOMMS2910 [DOI] [PMC free article] [PubMed]
- 45.Kumar S (2009) Caspase 2 in apoptosis, the DNA damage response and tumour suppression: enigma no more? Nat Rev Cancer 9:897–903. 10.1038/NRC2745 10.1038/NRC2745 [DOI] [PubMed] [Google Scholar]
- 46.Bergeron L, Perez GI, Macdonald G, et al (1998) Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev 12:1304–1314. 10.1101/GAD.12.9.1304 10.1101/GAD.12.9.1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baliga BC, Read SH, Kumar S (2004) The biochemical mechanism of caspase-2 activation. Cell Death Differ 11:1234–1241. 10.1038/SJ.CDD.4401492 10.1038/SJ.CDD.4401492 [DOI] [PubMed] [Google Scholar]
- 48.Guo Y, Srinivasula SM, Druilhe A, et al (2002) Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J Biol Chem 277:13430–13437. 10.1074/JBC.M108029200 10.1074/JBC.M108029200 [DOI] [PubMed] [Google Scholar]
- 49.Van De Craen M, Declercq W, Van Den Brande I, et al (1999) The proteolytic procaspase activation network: an in vitro analysis. Cell Death Differ 6:1117–1124. 10.1038/SJ.CDD.4400589 10.1038/SJ.CDD.4400589 [DOI] [PubMed] [Google Scholar]
- 50.Bouchier-Hayes L, Green DR (2012) Caspase-2: the orphan caspase. Cell Death Differ 19:51–57. 10.1038/CDD.2011.157 10.1038/CDD.2011.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forsberg J, Zhivotovsky B, Olsson M (2017) Caspase-2: an orphan enzyme out of the shadows. Oncogene 36:5441–5444. 10.1038/ONC.2017.169 10.1038/ONC.2017.169 [DOI] [PubMed] [Google Scholar]
- 52.Brown-Suedel AN, Bouchier-Hayes L (2020) Caspase-2 substrates: to apoptosis, cell cycle control, and beyond. Front cell Dev Biol 8. 10.3389/FCELL.2020.610022 [DOI] [PMC free article] [PubMed]
- 53.Wang L, Miura M, Bergeron L, et al (1994) Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell 78:739–750. 10.1016/S0092-8674(94)90422-7 10.1016/S0092-8674(94)90422-7 [DOI] [PubMed] [Google Scholar]
- 54.Machado M V., Michelotti GA, Jewell ML, et al (2016) Caspase-2 promotes obesity, the metabolic syndrome and nonalcoholic fatty liver disease. Cell Death Dis 7. 10.1038/CDDIS.2016.19 [DOI] [PMC free article] [PubMed]
- 55.Ho LH, Taylor R, Dorstyn L, et al (2009) A tumor suppressor function for caspase-2. Proc Natl Acad Sci U S A 106:5336–5341. 10.1073/PNAS.0811928106 10.1073/PNAS.0811928106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim IR, Murakami K, Chen NJ, et al (2009) DNA damage- and stress-induced apoptosis occurs independently of PIDD. Apoptosis 14:1039–1049. 10.1007/S10495-009-0375-1 10.1007/S10495-009-0375-1 [DOI] [PubMed] [Google Scholar]
- 57.Manzl C, Krumschnabel G, Bock F, et al (2009) Caspase-2 activation in the absence of PIDDosome formation. J Cell Biol 185:291–303. 10.1083/JCB.200811105 10.1083/JCB.200811105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pistritto G, Papaleo V, Sanchez P, et al (2012) Divergent modulation of neuronal differentiation by caspase-2 and – 9. PLoS One 7:e36002. 10.1371/journal.pone.0036002 10.1371/journal.pone.0036002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boonstra K, Bloemberg D, Quadrilatero J (2018) Caspase-2 is required for skeletal muscle differentiation and myogenesis. Biochim Biophys Acta Mol cell Res 1865:95–104. 10.1016/J.BBAMCR.2017.07.016 10.1016/J.BBAMCR.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 60.Dehkordi, Mahshid H, Tashakor A, O’Connell E, Fearnhead HO (2020) Apoptosome-dependent myotube formation involves activation of caspase-3 in differentiating myoblasts. Cell Death Dis 11:308. 10.1038/s41419-020-2502-4 10.1038/s41419-020-2502-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mogi M, Togari A (2003) Activation of caspases is required for osteoblastic differentiation. J Biol Chem 278:47477–82. 10.1074/jbc.M307055200 10.1074/jbc.M307055200 [DOI] [PubMed] [Google Scholar]
- 62.Kratochvílová A, Veselá B, Ledvina V, et al (2020) Osteogenic impact of pro-apoptotic caspase inhibitors in MC3T3-E1 cells. Sci Rep 10. 10.1038/S41598-020-64294-9 [DOI] [PMC free article] [PubMed]
- 63.Xu ZX, Tan JW, Xu H, et al (2019) Caspase-2 promotes AMPA receptor internalization and cognitive flexibility via mTORC2-AKT-GSK3β signaling. Nat Commun 10. 10.1038/S41467-019-11575-1 [DOI] [PMC free article] [PubMed]
- 64.Machado M V., Michelotti GA, De Almeida Pereira T, et al (2015) Reduced lipoapoptosis, hedgehog pathway activation and fibrosis in caspase-2 deficient mice with non-alcoholic steatohepatitis. Gut 64:1148–1157. 10.1136/GUTJNL-2014-307362 10.1136/GUTJNL-2014-307362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao X, Kotilinek LA, Smith B, et al (2016) Caspase-2 cleavage of tau reversibly impairs memory. Nat Med 22:1268–1276. 10.1038/NM.4199 10.1038/NM.4199 [DOI] [PubMed] [Google Scholar]
- 66.Fava LL, Schuler F, Sladky V, et al (2017) The PIDDosome activates p53 in response to supernumerary centrosomes. Genes Dev 31:34–45. 10.1101/gad.289728.116 10.1101/gad.289728.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Padalecki SS, Chaudhuri AR, et al (2007) Caspase-2 deficiency enhances aging-related traits in mice. Mech Ageing Dev 128:213–221. 10.1016/J.MAD.2006.11.030 10.1016/J.MAD.2006.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varfolomeev EE, Schuchmann M, Luria V, et al (1998) Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9:267–276. 10.1016/S1074-7613(00)80609-3 10.1016/S1074-7613(00)80609-3 [DOI] [PubMed] [Google Scholar]
- 69.Kuida K, Haydar TF, Kuan CY, et al (1998) Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94:325–37. 10.1016/s0092-8674(00)81476-2 10.1016/s0092-8674(00)81476-2 [DOI] [PubMed] [Google Scholar]
- 70.O’Reilly RC, Noelle DC, Braver TS, Cohen JD (2002) Prefrontal cortex and dynamic categorization tasks: representational organization and neuromodulatory control. Cereb Cortex 12:246–257. 10.1093/cercor/12.3.246 10.1093/cercor/12.3.246 [DOI] [PubMed] [Google Scholar]
- 71.Sharma R, Callaway D, Vanegas D, et al (2014) Caspase-2 maintains bone homeostasis by inducing apoptosis of oxidatively-damaged osteoclasts. PLoS One 9. 10.1371/JOURNAL.PONE.0093696 [DOI] [PMC free article] [PubMed]
- 72.Callaway DA, Riquelme MA, Sharma R, et al (2015) Caspase-2 modulates osteoclastogenesis through down-regulating oxidative stress. Bone 76:40–48. 10.1016/J.BONE.2015.03.006 10.1016/J.BONE.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carroll JB, Lerch JP, Franciosi S, et al (2011) Natural history of disease in the YAC128 mouse reveals a discrete signature of pathology in Huntington disease. Neurobiol Dis 43:257–265. 10.1016/j.nbd.2011.03.018 10.1016/j.nbd.2011.03.018 [DOI] [PubMed] [Google Scholar]
- 74.Puccini J, Dorstyn L, Kumar S (2013) Caspase-2 as a tumour suppressor. Cell Death Differ 20:1133–1139. 10.1038/CDD.2013.87 10.1038/CDD.2013.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parsons MJ, McCormick L, Janke L, et al (2013) Genetic deletion of caspase-2 accelerates MMTV/c-neu-driven mammary carcinogenesis in mice. Cell Death Differ 20:1174–1182. 10.1038/CDD.2013.38 10.1038/CDD.2013.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson CH, Nikolic A, Kentish SJ, et al (2017) Caspase-2 deficiency enhances whole-body carbohydrate utilisation and prevents high-fat diet-induced obesity. Cell Death Dis 8:e3136. 10.1038/cddis.2017.518 10.1038/cddis.2017.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muzio M, Chinnaiyan AM, Kischkel FC, et al (1996) FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death–inducing signaling complex. Cell 85:817–827. 10.1016/S0092-8674(00)81266-0 10.1016/S0092-8674(00)81266-0 [DOI] [PubMed] [Google Scholar]
- 78.Hughes MA, Harper N, Butterworth M, et al (2009) Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol Cell 35:265–279. 10.1016/J.MOLCEL.2009.06.012 10.1016/J.MOLCEL.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 79.Oberst A, Dillon CP, Weinlich R, et al (2011) Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471:363–368. 10.1038/NATURE09852 10.1038/NATURE09852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaiser WJ, Upton JW, Long AB, et al (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471:368–373. 10.1038/NATURE09857 10.1038/NATURE09857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tenev T, Bianchi K, Darding M, et al (2011) the ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell 43:432–448. 10.1016/J.MOLCEL.2011.06.006 10.1016/J.MOLCEL.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 82.Alvarez-Diaz S, Dillon CP, Lalaoui N, et al (2016) The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity 45:513–526. 10.1016/J.IMMUNI.2016.07.016 10.1016/J.IMMUNI.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pang J, Vince JE (2023) The role of caspase-8 in inflammatory signalling and pyroptotic cell death. Semin Immunol 70. 10.1016/J.SMIM.2023.101832 [DOI] [PubMed]
- 84.Gringhuis SI, Kaptein TM, Wevers BA, et al (2012) Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol 13:246–254. 10.1038/NI.2222 10.1038/NI.2222 [DOI] [PubMed] [Google Scholar]
- 85.Gurung P, Anand PK, Malireddi RKS, et al (2014) FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol 192:1835–1846. 10.4049/JIMMUNOL.1302839 10.4049/JIMMUNOL.1302839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Phillips FC, Gurung P, Kanneganti TD (2016) Microbiota and caspase-1/caspase-8 regulate IL-1β-mediated bone disease. Gut Microbes 7:334–341. 10.1080/19490976.2016.1182289 10.1080/19490976.2016.1182289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Helfer B, Boswell BC, Finlay D, et al (2006) Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res 66:4273–4278. 10.1158/0008-5472.CAN-05-4183 10.1158/0008-5472.CAN-05-4183 [DOI] [PubMed] [Google Scholar]
- 88.Torres VA, Mielgo A, Barbero S, et al (2010) Rab5 mediates caspase-8-promoted cell motility and metastasis. Mol Biol Cell 21:369–376. 10.1091/MBC.E09-09-0769 10.1091/MBC.E09-09-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alam A, Cohen LY, Aouad S, Sékaly RP (1999) Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J Exp Med 190:1879–1890. 10.1084/JEM.190.12.1879 10.1084/JEM.190.12.1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salmena L, Lemmers B, Hakem A, et al (2003) Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev 17:883–895. 10.1101/GAD.1063703 10.1101/GAD.1063703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beisner DR, Ch’en IL, Kolla R V., et al (2005) Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol 175:3469–3473. 10.4049/JIMMUNOL.175.6.3469 10.4049/JIMMUNOL.175.6.3469 [DOI] [PubMed] [Google Scholar]
- 92.Pellegrini M, Bath S, Marsden VS, et al (2005) FADD and caspase-8 are required for cytokine-induced proliferation of hemopoietic progenitor cells. Blood 106:1581–1589. 10.1182/BLOOD-2005-01-0284 10.1182/BLOOD-2005-01-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu L, Alva A, Su H, et al (2004) Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 304:1500–1502. 10.1126/SCIENCE.1096645 10.1126/SCIENCE.1096645 [DOI] [PubMed] [Google Scholar]
- 94.Kovalenko A, Kim JC, Kang TB, et al (2009) Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med 206:2161–2177. 10.1084/JEM.20090616 10.1084/JEM.20090616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stupack DG (2013) Caspase-8 as a therapeutic target in cancer. Cancer Lett 332:133–140. 10.1016/J.CANLET.2010.07.022 10.1016/J.CANLET.2010.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Orning P, Lien E (2021) Multiple roles of caspase-8 in cell death, inflammation, and innate immunity. J Leukoc Biol 109:121–141. 10.1002/JLB.3MR0420-305R 10.1002/JLB.3MR0420-305R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang T-B, Ben-Moshe T, Varfolomeev EE, et al (2004) Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol 173:2976–2984. 10.4049/JIMMUNOL.173.5.2976 10.4049/JIMMUNOL.173.5.2976 [DOI] [PubMed] [Google Scholar]
- 98.Sakamaki K, Inoue T, Asano M, et al (2002) Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ 9:1196–1206. 10.1038/SJ.CDD.4401090 10.1038/SJ.CDD.4401090 [DOI] [PubMed] [Google Scholar]
- 99.Morgan MJ, Kim YS (2022) Roles of RIPK3 in necroptosis, cell signaling, and disease. Exp Mol Med 54:1695–1704. 10.1038/S12276-022-00868-Z 10.1038/S12276-022-00868-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang TB, Jeong JS, Yang SH, et al (2018) Caspase-8 deficiency in mouse embryos triggers chronic RIPK1-dependent activation of inflammatory genes, independently of RIPK3. Cell Death Differ 25:1107–1117. 10.1038/S41418-018-0104-9 10.1038/S41418-018-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cuda CM, Misharin A V., Khare S, et al (2015) Conditional deletion of caspase-8 in macrophages alters macrophage activation in a RIPK-dependent manner. Arthritis Res Ther 17. 10.1186/S13075-015-0794-Z [DOI] [PMC free article] [PubMed]
- 102.Lee P, Lee DJ, Chan C, et al (2009) Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature 458:519–523. 10.1038/NATURE07687 10.1038/NATURE07687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salmena L, Hakem R (2005) Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder. J Exp Med 202:727–732. 10.1084/JEM.20050683 10.1084/JEM.20050683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Su H, Bidère N, Zheng L, et al (2005) Requirement for caspase-8 in NF-κB activation by antigen receptor. Science (80-) 307:1465–1468. 10.1126/SCIENCE.1104765/SUPPL_FILE/SU.SOM.PDF 10.1126/SCIENCE.1104765/SUPPL_FILE [DOI] [PubMed] [Google Scholar]
- 105.Lemmers B, Salmena L, Bidere N, et al (2007) Essential role for Caspase-8 in toll-like receptors and NF-κB signalling. J Biol Chem 282:7416–7423. 10.1074/jbc.M606721200 10.1074/jbc.M606721200 [DOI] [PubMed] [Google Scholar]
- 106.Liedtke C, Bangen JM, Freimuth J, et al (2011) Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology 141:2176–2187. 10.1053/J.GASTRO.2011.08.037 10.1053/J.GASTRO.2011.08.037 [DOI] [PubMed] [Google Scholar]
- 107.Freimuth J, Bangen JM, Lambertz D, et al (2013) Loss of caspase-8 in hepatocytes accelerates the onset of liver regeneration in mice through premature nuclear factor kappa B activation. Hepatology 58:1779–1789. 10.1002/HEP.26538 10.1002/HEP.26538 [DOI] [PubMed] [Google Scholar]
- 108.An HK, Chung KM, Park H, et al (2020) CASP9 (caspase 9) is essential for autophagosome maturation through regulation of mitochondrial homeostasis. Autophagy 16:1598–1617. 10.1080/15548627.2019.1695398 10.1080/15548627.2019.1695398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Denault J-B, Drag M, Salvesen GS, et al (2007) Small molecules not direct activators of caspases. Nat. Chem. Biol. 3:519; author reply 520 10.1038/nchembio0907-519 [DOI] [PubMed] [Google Scholar]
- 110.Stennicke HR, Salvesen GS (1999) Catalytic properties of the caspases. Cell Death Differ 6:1054–9. 10.1038/sj.cdd.4400599 10.1038/sj.cdd.4400599 [DOI] [PubMed] [Google Scholar]
- 111.Nakanishi K, Maruyama M, Shibata T, Morishima N (2001) Identification of a caspase-9 substrate and detection of its cleavage in programmed cell death during mouse development. J Biol Chem 276:41237–41244. 10.1074/JBC.M105648200 10.1074/JBC.M105648200 [DOI] [PubMed] [Google Scholar]
- 112.Byun Y, Chen F, Chang R, et al (2001) Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ 8:443–450. 10.1038/SJ.CDD.4400840 10.1038/SJ.CDD.4400840 [DOI] [PubMed] [Google Scholar]
- 113.Srinivasula SM, Ahmad M, Guo Y, et al (1999) Identification of an endogenous dominant-negative short isoform of caspase-9 that can regulate apoptosis. Cancer Res 59:999–1002 [PubMed] [Google Scholar]
- 114.Laguna A, Aranda S, Barallobre MJ, et al (2008) The protein kinase DYRK1A regulates caspase-9-mediated apoptosis during retina development. Dev Cell 15:841–853. 10.1016/J.DEVCEL.2008.10.014 10.1016/J.DEVCEL.2008.10.014 [DOI] [PubMed] [Google Scholar]
- 115.Sanders EJ, Parker E (2002) The role of mitochondria, cytochrome c and caspase-9 in embryonic lens fibre cell denucleation. J Anat 201:121–35. 10.1046/j.1469-7580.2002.00081.x 10.1046/j.1469-7580.2002.00081.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cecconi F, Roth KA, Dolgov O, et al (2004) Apaf1-dependent programmed cell death is required for inner ear morphogenesis and growth. Development 131:2125–35. 10.1242/dev.01082 10.1242/dev.01082 [DOI] [PubMed] [Google Scholar]
- 117.Molnár T, Pallagi P, Tél B, et al (2021) Caspase-9 acts as a regulator of necroptotic cell death. FEBS J 288:6476–6491. 10.1111/FEBS.15898 10.1111/FEBS.15898 [DOI] [PubMed] [Google Scholar]
- 118.Avrutsky MI, Troy CM (2021) Caspase-9: a multimodal therapeutic target with diverse cellular expression in human disease. Front Pharmacol 12. 10.3389/FPHAR.2021.701301 [DOI] [PMC free article] [PubMed]
- 119.Murray TVA, McMahon JM, Howley BA, et al (2008) A non-apoptotic role for caspase-9 in muscle differentiation. J Cell Sci 121:3786–3793. 10.1242/JCS.024547 10.1242/JCS.024547 [DOI] [PubMed] [Google Scholar]
- 120.Lu EP, McLellan M, Ding L, et al (2014) Caspase-9 is required for normal hematopoietic development and protection from alkylator-induced DNA damage in mice. Blood 124:3887–3895. 10.1182/BLOOD-2014-06-582551 10.1182/BLOOD-2014-06-582551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rongvaux A, Jackson R, Harman CCD, et al (2014) Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159:1563. 10.1016/J.CELL.2014.11.037 10.1016/J.CELL.2014.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ohsawa S, Hamada S, Kuida K, et al (2010) Maturation of the olfactory sensory neurons by Apaf-1/caspase-9-mediated caspase activity. Proc Natl Acad Sci U S A 107:13366–71. 10.1073/pnas.0910488107 10.1073/pnas.0910488107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kuida K (2000) Caspase-9. Int J Biochem Cell Biol 32:121–124. 10.1016/S1357-2725(99)00024-2 10.1016/S1357-2725(99)00024-2 [DOI] [PubMed] [Google Scholar]
- 124.Hakem R, Hakem A, Duncan GS, et al (1998) Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94:339–52. 10.1016/s0092-8674(00)81477-4 10.1016/s0092-8674(00)81477-4 [DOI] [PubMed] [Google Scholar]
- 125.Ene AC, Park S, Edelmann W, Taketo T (2013) Caspase 9 is constitutively activated in mouse oocytes and plays a key role in oocyte elimination during meiotic prophase progression. Dev Biol 377:213–223. 10.1016/J.YDBIO.2013.01.027 10.1016/J.YDBIO.2013.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Setkova J, Matalova E, Sharpe PT, et al (2007) Primary enamel knot cell death in Apaf-1 and caspase-9 deficient mice. Arch Oral Biol 52:15–9. 10.1016/j.archoralbio.2006.07.006 10.1016/j.archoralbio.2006.07.006 [DOI] [PubMed] [Google Scholar]
- 127.Thesleff I, Keränen S, Jernvall J (2001) Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv Dent Res 15:14–18. 10.1177/08959374010150010401 10.1177/08959374010150010401 [DOI] [PubMed] [Google Scholar]
- 128.Jernvall J, Åberg T, Kettunen P, et al (1998) The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development 125:161–169. 10.1242/DEV.125.2.161 10.1242/DEV.125.2.161 [DOI] [PubMed] [Google Scholar]
- 129.Ankawa R, Goldberger N, Yosefzon Y, et al (2021) Apoptotic cells represent a dynamic stem cell niche governing proliferation and tissue regeneration. Dev Cell 56:1900–1916.e5. 10.1016/J.DEVCEL.2021.06.008 10.1016/J.DEVCEL.2021.06.008 [DOI] [PubMed] [Google Scholar]
- 130.Huang Y, Shin NH, Sun Y, Wang KKW (2001) Molecular cloning and characterization of a novel caspase-3 variant that attenuates apoptosis induced by proteasome inhibition. Biochem Biophys Res Commun 283:762–769. 10.1006/BBRC.2001.4871 10.1006/BBRC.2001.4871 [DOI] [PubMed] [Google Scholar]
- 131.Kuida K, Zheng TS, Na S, et al (1996) Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384:368–372. 10.1038/384368a0 10.1038/384368a0 [DOI] [PubMed] [Google Scholar]
- 132.Zeiss CJ, Neal J, Johnson EA (2004) Caspase-3 in postnatal retinal development and degeneration. Invest Ophthalmol Vis Sci 45:964–70. 10.1167/iovs.03-0439 10.1167/iovs.03-0439 [DOI] [PubMed] [Google Scholar]
- 133.Makishima T, Hochman L, Armstrong P, et al (2011) Inner ear dysfunction in caspase-3 deficient mice. BMC Neurosci 12:102. 10.1186/1471-2202-12-102 10.1186/1471-2202-12-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Woo M, Hakem R, Furlonger C, et al (2003) Caspase-3 regulates cell cycle in B cells: a consequence of substrate specificity. Nat Immunol 4:1016–1022. 10.1038/NI976 10.1038/NI976 [DOI] [PubMed] [Google Scholar]
- 135.Fernando P, Brunette S, Megeney LA (2005) Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. FASEB J 19:1671–1673. 10.1096/FJ.04-2981FJE 10.1096/FJ.04-2981FJE [DOI] [PubMed] [Google Scholar]
- 136.Boland K, Flanagan L, Prehn JHM (2013) Paracrine control of tissue regeneration and cell proliferation by Caspase-3. Cell Death Dis 4. 10.1038/CDDIS.2013.250 [DOI] [PMC free article] [PubMed]
- 137.Fan W, Dai Y, Xu H, et al (2014) Caspase-3 modulates regenerative response after stroke. Stem Cells 32:473–486. 10.1002/STEM.1503 10.1002/STEM.1503 [DOI] [PubMed] [Google Scholar]
- 138.Zhou Z, Xu S, Jiang L, et al (2022) A systematic Pan-cancer analysis of CASP3 as a potential target for Immunotherapy. Front Mol Biosci 9. 10.3389/FMOLB.2022.776808 [DOI] [PMC free article] [PubMed]
- 139.Khan S, Ahmad K, Alshammari EMA, et al (2015) Implication of Caspase-3 as a common therapeutic target for multineurodegenerative disorders and its inhibition using nonpeptidyl natural compounds. Biomed Res Int 2015. 10.1155/2015/379817 [DOI] [PMC free article] [PubMed]
- 140.Yang B, Ye D, Wang Y (2013) Caspase-3 as a therapeutic target for heart failure. Expert Opin Ther Targets 17:255–263. 10.1517/14728222.2013.745513 10.1517/14728222.2013.745513 [DOI] [PubMed] [Google Scholar]
- 141.Leonard JR, Klocke BJ, D’sa C, et al (2002) Strain-dependent neurodevelopmental abnormalities in caspase-3-deficient mice. J Neuropathol Exp Neurol 61:673–677. 10.1093/JNEN/61.8.673 10.1093/JNEN/61.8.673 [DOI] [PubMed] [Google Scholar]
- 142.Nicholson DW (1999) Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ 6:1028–1042. 10.1038/SJ.CDD.4400598 10.1038/SJ.CDD.4400598 [DOI] [PubMed] [Google Scholar]
- 143.Houde C, Banks KG, Coulombe N, et al (2004) Caspase-7 expanded function and intrinsic expression level underlies strain-specific brain phenotype of caspase-3-null mice. J Neurosci 24:9977–84. 10.1523/JNEUROSCI.3356-04.2004 10.1523/JNEUROSCI.3356-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takahashi K, Kamiya K, Urase K, et al (2001) Caspase-3-deficiency induces hyperplasia of supporting cells and degeneration of sensory cells resulting in the hearing loss. Brain Res 894:359–67. 10.1016/s0006-8993(01)02123-0 10.1016/s0006-8993(01)02123-0 [DOI] [PubMed] [Google Scholar]
- 145.Matalova E, Sharpe PT, Lakhani SA, et al (2006) Molar tooth development in caspase-3 deficient mice. Int J Dev Biol 50:491–497. 10.1387/IJDB.052117EM 10.1387/IJDB.052117EM [DOI] [PubMed] [Google Scholar]
- 146.Suzuki T, Ichii O, Nakamura T, et al (2020) Immune-associated renal disease found in caspase 3-deficient mice. Cell Tissue Res 379:323–335. 10.1007/S00441-019-03084-W/FIGURES/8 10.1007/S00441-019-03084-W/FIGURES/8 [DOI] [PubMed] [Google Scholar]
- 147.Liu X, He Y, Li F, et al (2015) Caspase-3 promotes genetic instability and carcinogenesis. Mol Cell 58:284–296. 10.1016/J.MOLCEL.2015.03.003 10.1016/J.MOLCEL.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Woo M, Hakem R, Soengas MS, et al (1998) Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev 12:806–19. 10.1101/gad.12.6.806 10.1101/gad.12.6.806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Janzen V, Fleming HE, Riedt T, et al (2008) Hematopoietic stem cell responsiveness to exogenous signals is limited by caspase-3. Cell Stem Cell 2:584–594. 10.1016/J.STEM.2008.03.012 10.1016/J.STEM.2008.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yosefzon Y, Soteriou D, Feldman A, et al (2018) Caspase-3 regulates YAP-dependent cell proliferation and organ size. Mol Cell 70:573–587.e4. 10.1016/J.MOLCEL.2018.04.019 10.1016/J.MOLCEL.2018.04.019 [DOI] [PubMed] [Google Scholar]
- 151.Szymczyk K, Freeman T, Adams C, et al (2006) Active caspase-3 is required for osteoclast differentiation. J Cell Physiol 209:836–844. 10.1002/JCP.20770 10.1002/JCP.20770 [DOI] [PubMed] [Google Scholar]
- 152.Li F, Huang Q, Chen J, et al (2010) Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal 3. 10.1126/SCISIGNAL.2000634 [DOI] [PMC free article] [PubMed]
- 153.Morishita H, Makishima T, Kaneko C, et al (2001) Deafness due to degeneration of cochlear neurons in caspase-3-deficient mice. Biochem Biophys Res Commun 284:142–149. 10.1006/bbrc.2001.4939 10.1006/bbrc.2001.4939 [DOI] [PubMed] [Google Scholar]
- 154.Lo SC, Scearce-Levie K, Sheng M (2016) Characterization of social behaviors in caspase-3 deficient mice. Sci Rep 6. 10.1038/SREP18335 [DOI] [PMC free article] [PubMed]
- 155.Lo SC, Wang Y, Weber M, et al (2015) Caspase-3 deficiency results in disrupted synaptic homeostasis and impaired attention control. J Neurosci 35:2118–2132. 10.1523/JNEUROSCI.3280-14.2015 10.1523/JNEUROSCI.3280-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thornberry NA, Rano TA, Peterson EP, et al (1997) A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 272:17907–17911. 10.1074/JBC.272.29.17907 10.1074/JBC.272.29.17907 [DOI] [PubMed] [Google Scholar]
- 157.Zheng TS, Hunot S, Kuida K, Flavell RA (1999) Caspase knockouts: matters of life and death. Cell Death Differ 6:1043–1053. 10.1038/SJ.CDD.4400593 10.1038/SJ.CDD.4400593 [DOI] [PubMed] [Google Scholar]
- 158.Ruchaud S, Korfali N, Villa P, et al (2002) Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. EMBO J 21:1967–1977. 10.1093/EMBOJ/21.8.1967 10.1093/EMBOJ/21.8.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guo H, Pétrin D, Zhang Y, et al (2006) Caspase-1 activation of caspase-6 in human apoptotic neurons. Cell Death Differ 13:285–292. 10.1038/SJ.CDD.4401753 10.1038/SJ.CDD.4401753 [DOI] [PubMed] [Google Scholar]
- 160.Zheng M, Karki R, Vogel P, Kanneganti TD (2020) Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell 181:674–687.e13. 10.1016/J.CELL.2020.03.040 10.1016/J.CELL.2020.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ladha S, Qiu X, Casal L, et al (2018) Constitutive ablation of caspase-6 reduces the inflammatory response and behavioural changes caused by peripheral pro-inflammatory stimuli. Cell Death Discov 4. 10.1038/S41420-018-0043-8 [DOI] [PMC free article] [PubMed]
- 162.Qi L, Wang L, Jin M, et al (2023) Caspase-6 is a key regulator of cross-talk signal way in PANoptosis in cancer. Immunology 169:245–259. 10.1111/IMM.13633 10.1111/IMM.13633 [DOI] [PubMed] [Google Scholar]
- 163.Uribe V, Wong BKY, Graham RK, et al (2012) Rescue from excitotoxicity and axonal degeneration accompanied by age-dependent behavioral and neuroanatomical alterations in caspase-6-deficient mice. Hum Mol Genet 21:1954–1967. 10.1093/HMG/DDS005 10.1093/HMG/DDS005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Angel A, Volkman R, Royal TG, Offen D (2020) Caspase-6 knockout in the 5xFAD model of Alzheimer’s disease reveals favorable outcome on memory and neurological hallmarks. Int J Mol Sci 21. 10.3390/IJMS21031144 [DOI] [PMC free article] [PubMed]
- 165.Wang XJ, Cao Q, Zhang Y, Su XD (2015) Activation and regulation of caspase-6 and its role in neurodegenerative diseases. Annu Rev Pharmacol Toxicol 55:553–572. 10.1146/ANNUREV-PHARMTOX-010814-124414 10.1146/ANNUREV-PHARMTOX-010814-124414 [DOI] [PubMed] [Google Scholar]
- 166.Zheng TS, Hunot S, Kuida K, et al (2000) Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat Med 6:1241–7. 10.1038/81343 10.1038/81343 [DOI] [PubMed] [Google Scholar]
- 167.Watanabe C, Shu GL, Zheng TS, et al (2008) Caspase 6 regulates b cell activation and differentiation into plasma cells. J Immunol 181:6810–6819. 10.4049/JIMMUNOL.181.10.6810 10.4049/JIMMUNOL.181.10.6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Akpan N, Serrano-Saiz E, Zacharia BE, et al (2011) Intranasal delivery of caspase-9 inhibitor reduces caspase-6-dependent axon/neuron loss and improves neurological function after stroke. J Neurosci 31:8894–8904. 10.1523/JNEUROSCI.0698-11.2011 10.1523/JNEUROSCI.0698-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sheng M, Weng Y, Cao Y, et al (2023) Caspase 6/NR4A1/SOX9 signaling axis regulates hepatic inflammation and pyroptosis in ischemia-stressed fatty liver. Cell Death Discov 9. 10.1038/S41420-023-01396-Z [DOI] [PMC free article] [PubMed]
- 170.Nikolaev A, McLaughlin T, O’Leary DDM, Tessier-Lavigne M (2009) APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 457:981–989. 10.1038/NATURE07767 10.1038/NATURE07767 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 171.Swanson K V., Deng M, Ting JPY (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19:477–489. 10.1038/S41577-019-0165-0 10.1038/S41577-019-0165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Watanabe C, Shu GL, Giltiay N V., Clark EA (2018) Regulation of B-lineage cells by caspase 6. Immunol Cell Biol 96:1072–1082. 10.1111/IMCB.12172 10.1111/IMCB.12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Walsh JG, Cullen SP, Sheridan C, et al (2008) Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci U S A 105:12815–9. 10.1073/pnas.0707715105 10.1073/pnas.0707715105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Choudhury S, Bhootada Y, Gorbatyuk O, Gorbatyuk M (2013) Caspase-7 ablation modulates UPR, reprograms TRAF2-JNK apoptosis and protects T17M rhodopsin mice from severe retinal degeneration. Cell Death Dis 4. 10.1038/CDDIS.2013.34 [DOI] [PMC free article] [PubMed]
- 175.Nozaki K, Maltez VI, Rayamajhi M, et al (2022) Caspase-7 activates ASM to repair gasdermin and perforin pores. Nature 606:960–967. 10.1038/S41586-022-04825-8 10.1038/S41586-022-04825-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Akhter A, Gavrilin MA, Frantz L, et al (2009) Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog 5. 10.1371/JOURNAL.PPAT.1000361 [DOI] [PMC free article] [PubMed]
- 177.Lamkanfi M, Moreira LO, Makena P, et al (2009) Caspase-7 deficiency protects from endotoxin-induced lymphocyte apoptosis and improves survival. Blood 113:2742–2745. 10.1182/BLOOD-2008-09-178038 10.1182/BLOOD-2008-09-178038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lamkanfi M, Kanneganti TD, van Damme P, et al (2008) Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics 7:2350–2363. 10.1074/MCP.M800132-MCP200 10.1074/MCP.M800132-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Svandova E, Lesot H, Vanden Berghe T, et al (2014) Non-apoptotic functions of caspase-7 during osteogenesis. Cell Death Dis 5:e1366. 10.1038/cddis.2014.330 10.1038/cddis.2014.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Veselá B, Matalová E (2015) Expression of apoptosis-related genes in the mouse skin during the first postnatal catagen stage, focused on localization of Bnip3L and caspase-12. Connect Tissue Res 56:326–335. 10.3109/03008207.2015.1040546 10.3109/03008207.2015.1040546 [DOI] [PubMed] [Google Scholar]
- 181.Cowan KN, Leung WCY, Mar C, et al (2005) Caspases from apoptotic myocytes degrade extracellular matrix: a novel remodeling paradigm. FASEB J 19:1848–1850. 10.1096/FJ.05-3706FJE 10.1096/FJ.05-3706FJE [DOI] [PubMed] [Google Scholar]
- 182.Hermel E, Gafni J, Propp S, et al (2004) Specific caspase interactions and amplification are involved in selective neuronal vulnerability in Huntington’s disease. Cell Death Differ 11:424–438. 10.1038/sj.cdd.4401358 10.1038/sj.cdd.4401358 [DOI] [PubMed] [Google Scholar]
- 183.Hotchkiss RS, Nicholson DW (2006) Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 6:813–822. 10.1038/NRI1943 10.1038/NRI1943 [DOI] [PubMed] [Google Scholar]
- 184.Teixeira VH, Jacq L, Lasbleiz S, et al (2008) Genetic and expression analysis of CASP7 gene in a European Caucasian population with rheumatoid arthritis. J Rheumatol 35:1912–1918 [PubMed] [Google Scholar]
- 185.Babu SR, Bao F, Roberts CM, et al (2003) Caspase 7 is a positional candidate gene for IDDM 17 in a Bedouin Arab family. Ann N Y Acad Sci 1005:340–343. 10.1196/ANNALS.1288.054 10.1196/ANNALS.1288.054 [DOI] [PubMed] [Google Scholar]
- 186.Lakhani SA, Masud A, Kuida K, et al (2006) Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311:847–851. 10.1126/SCIENCE.1115035 10.1126/SCIENCE.1115035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Choudhury S, Liu Y, Clark AF, Pang IH (2015) Caspase-7: a critical mediator of optic nerve injury-induced retinal ganglion cell death. Mol Neurodegener 10. 10.1186/S13024-015-0039-2 [DOI] [PMC free article] [PubMed]
- 188.Musch T, Öz Y, Lyko F, Breiling A (2010) Nucleoside drugs induce cellular differentiation by caspase-dependent degradation of stem cell factors. PLoS One 5. 10.1371/JOURNAL.PONE.0010726 [DOI] [PMC free article] [PubMed]
- 189.Li L, Kwon HJ, Harada H, et al (2011) Expression patterns of ABCG2, Bmi-1, Oct-3/4, and Yap in the developing mouse incisor. Gene Expr Patterns 11:163–170. 10.1016/J.GEP.2010.11.001 10.1016/J.GEP.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 190.Yuan J, Shaham S, Ledoux S, et al (1993) The C. Elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell 75:641–652. 10.1016/0092-8674(93)90485-9 10.1016/0092-8674(93)90485-9 [DOI] [PubMed] [Google Scholar]
- 191.Molla MD, Akalu Y, Geto Z, et al (2020) Role of Caspase-1 in the pathogenesis of inflammatory-associated chronic noncommunicable diseases. J Inflamm Res 13:749–764. 10.2147/JIR.S277457 10.2147/JIR.S277457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Denes A, Lopez-Castejon G, Brough D (2012) Caspase-1: is IL-1 just the tip of the ICEberg? Cell Death Dis 3. 10.1038/CDDIS.2012.86 [DOI] [PMC free article] [PubMed]
- 193.Fernandes-Alnemri T, Wu J, Yu JW, et al (2007) The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 14:1590–1604. 10.1038/SJ.CDD.4402194 10.1038/SJ.CDD.4402194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Barnoy S, Kosower NS (2003) Caspase-1-induced calpastatin degradation in myoblast differentiation and fusion: cross-talk between the caspase and calpain systems. FEBS Lett 546:213–217. 10.1016/S0014-5793(03)00573-8 10.1016/S0014-5793(03)00573-8 [DOI] [PubMed] [Google Scholar]
- 195.Vaisid T, Kosower NS, Barnoy S (2005) Caspase-1 activity is required for neuronal differentiation of PC12 cells: cross-talk between the caspase and calpain systems. Biochim Biophys Acta 1743:223–230. 10.1016/J.BBAMCR.2005.01.001 10.1016/J.BBAMCR.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 196.Ramesova A, Vesela B, Svandova E, et al (2021) Caspase-1 inhibition impacts the formation of chondrogenic nodules, and the expression of markers related to osteogenic differentiation and lipid metabolism. Int J Mol Sci 22. 10.3390/IJMS22179576 [DOI] [PMC free article] [PubMed]
- 197.Kotas ME, Jurczak MJ, Annicelli C, et al (2013) Role of caspase-1 in regulation of triglyceride metabolism. Proc Natl Acad Sci U S A 110:4810–4815. 10.1073/PNAS.1301996110 10.1073/PNAS.1301996110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang H, Capell W, Yoon JH, et al (2014) Obesity development in caspase-1-deficient mice. Int J Obes (Lond) 38:152–155. 10.1038/IJO.2013.59 10.1038/IJO.2013.59 [DOI] [PubMed] [Google Scholar]
- 199.An S, Hu H, Li Y, Hu Y (2020) Pyroptosis plays a role in osteoarthritis. Aging Dis 11:1146–1157. 10.14336/AD.2019.1127 10.14336/AD.2019.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fève B, Bastard JP (2009) The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 5:305–311. 10.1038/NRENDO.2009.62 10.1038/NRENDO.2009.62 [DOI] [PubMed] [Google Scholar]
- 201.Kuida K, Lippke JA, Ku G, et al (1995) Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267:2000–3. 10.1126/science.7535475 10.1126/science.7535475 [DOI] [PubMed] [Google Scholar]
- 202.Li P, Allen H, Banerjee S, et al (1995) Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80:401–11. 10.1016/0092-8674(95)90490-5 10.1016/0092-8674(95)90490-5 [DOI] [PubMed] [Google Scholar]
- 203.Menzel CL, Sun Q, Loughran PA, et al (2011) Caspase-1 is hepatoprotective during trauma and hemorrhagic shock by reducing liver injury and inflammation. Mol Med 17:1031. 10.2119/MOLMED.2011.00015 10.2119/MOLMED.2011.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Merkle S, Frantz S, Schön MP, et al (2007) A role for caspase-1 in heart failure. Circ Res 100:645–653. 10.1161/01.RES.0000260203.55077.61 10.1161/01.RES.0000260203.55077.61 [DOI] [PubMed] [Google Scholar]
- 205.Rowe SJ, Allen L, Ridger VC, et al (2002) Caspase-1-deficient mice have delayed neutrophil apoptosis and a prolonged inflammatory response to lipopolysaccharide-induced acute lung injury. J Immunol 169:6401–6407. 10.4049/JIMMUNOL.169.11.6401 10.4049/JIMMUNOL.169.11.6401 [DOI] [PubMed] [Google Scholar]
- 206.Arai J, Katai N, Kuida K, et al (2006) Decreased retinal neuronal cell death in caspase-1 knockout mice. Jpn J Ophthalmol 50:417–425. 10.1007/s10384-006-0352-y 10.1007/s10384-006-0352-y [DOI] [PubMed] [Google Scholar]
- 207.Hu B, Elinav E, Huber S, et al (2010) Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A 107:21635–21640. 10.1073/PNAS.1016814108 10.1073/PNAS.1016814108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Huang CH, Chen CJ, Yen CT, et al (2013) Caspase-1 deficient mice are more susceptible to influenza a virus infection with PA variation. J Infect Dis 208:1898–1905. 10.1093/INFDIS/JIT381 10.1093/INFDIS/JIT381 [DOI] [PubMed] [Google Scholar]
- 209.Thomas PG, Dash P, Aldridge JRJ, et al (2009) The intracellular sensor NLRP3 mediates key innate and healing responses to influenza a virus via the regulation of caspase-1. Immunity 30:566–575. 10.1016/j.immuni.2009.02.006 10.1016/j.immuni.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lara-Tejero M, Sutterwala FS, Ogura Y, et al (2006) Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med 203:1407. 10.1084/JEM.20060206 10.1084/JEM.20060206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Koedel U, Winkler F, Angele B, et al (2002) Role of Caspase-1 in experimental pneumococcal meningitis: evidence from pharmacologic caspase inhibition and caspase-1-deficient mice. Ann Neurol 51:319–329. 10.1002/ANA.10103 10.1002/ANA.10103 [DOI] [PubMed] [Google Scholar]
- 212.Thakur A, Barrett RP, Hobden JA, Hazlett LD (2004) Caspase-1 inhibitor reduces severity of pseudomonas aeruginosa keratitis in mice. Invest Ophthalmol Vis Sci 45:3177–3184. 10.1167/IOVS.04-0041 10.1167/IOVS.04-0041 [DOI] [PubMed] [Google Scholar]
- 213.Fantuzzi G, Puren AJ, Harding MW, et al (1998) Interleukin-18 regulation of interferon γ production and cell proliferation as shown in interleukin-1β–converting enzyme (Caspase-1)-deficient mice. Blood 91:2118–2125. 10.1182/BLOOD.V91.6.2118 10.1182/BLOOD.V91.6.2118 [DOI] [PubMed] [Google Scholar]
- 214.Melnikov VY, Ecder T, Fantuzzi G, et al (2001) Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest 107:1145–1152. 10.1172/JCI12089 10.1172/JCI12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang W, Faubel S, Ljubanovic D, et al (2005) Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am J Physiol Renal Physiol 288. 10.1152/AJPRENAL.00130.2004 [DOI] [PubMed]
- 216.Faubel S, Ljubanovic D, Reznikov L, et al (2004) Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int 66:2202–2213. 10.1111/J.1523-1755.2004.66010.X 10.1111/J.1523-1755.2004.66010.X [DOI] [PubMed] [Google Scholar]
- 217.Schielke GP, Yang GY, Shivers BD, Betz AL (1998) Reduced ischemic brain injury in interleukin-1β converting enzyme- deficient mice. J Cereb Blood Flow Metab 18:180–185. 10.1097/00004647-199802000-00009 10.1097/00004647-199802000-00009 [DOI] [PubMed] [Google Scholar]
- 218.Kayagaki N, Warming S, Lamkanfi M, et al (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121. 10.1038/NATURE10558 10.1038/NATURE10558 [DOI] [PubMed] [Google Scholar]
- 219.Dixon LJ, Flask CA, Papouchado BG, et al (2013) Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS One 8. 10.1371/JOURNAL.PONE.0056100 [DOI] [PMC free article] [PubMed]
- 220.Gage J, Hasu M, Thabet M, Whitman SC (2012) Caspase-1 deficiency decreases atherosclerosis in apolipoprotein E-null mice. Can J Cardiol 28:222–229. 10.1016/J.CJCA.2011.10.013 10.1016/J.CJCA.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 221.Wang S, Miura M, Jung Y, et al (1998) Murine Caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92:501–509. 10.1016/S0092-8674(00)80943-5 10.1016/S0092-8674(00)80943-5 [DOI] [PubMed] [Google Scholar]
- 222.Shi J, Zhao Y, Wang Y, et al (2014) Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514:187–192. 10.1038/NATURE13683 10.1038/NATURE13683 [DOI] [PubMed] [Google Scholar]
- 223.Kang SJ, Wang S, Hara H, et al (2000) Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol 149:613–622. 10.1083/JCB.149.3.613 10.1083/JCB.149.3.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Huang X, Feng Y, Xiong G, et al (2019) Caspase-11, a specific sensor for intracellular lipopolysaccharide recognition, mediates the non-canonical inflammatory pathway of pyroptosis. Cell Biosci 9. 10.1186/S13578-019-0292-0 [DOI] [PMC free article] [PubMed]
- 225.Kayagaki N, Stowe IB, Lee BL, et al (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526:666–671. 10.1038/NATURE15541 10.1038/NATURE15541 [DOI] [PubMed] [Google Scholar]
- 226.Miao N, Wang B, Xu D, et al (2018) Caspase-11 promotes cisplatin-induced renal tubular apoptosis through a caspase-3-dependent pathway. Am J Physiol Renal Physiol 314:F269–F279. 10.1152/AJPRENAL.00091.2017 10.1152/AJPRENAL.00091.2017 [DOI] [PubMed] [Google Scholar]
- 227.Krause K, Caution K, Badr A, et al (2018) CASP4/caspase-11 promotes autophagosome formation in response to bacterial infection. Autophagy 14:1928–1942. 10.1080/15548627.2018.1491494 10.1080/15548627.2018.1491494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Akhter A, Caution K, Abu Khweek A, et al (2012) Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37:35–47. 10.1016/J.IMMUNI.2012.05.001 10.1016/J.IMMUNI.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zasłona Z, Flis E, Wilk MM, et al (2020) Caspase-11 promotes allergic airway inflammation. Nat Commun 11. 10.1038/S41467-020-14945-2 [DOI] [PMC free article] [PubMed]
- 230.Sun Y, Li J, Wu H, et al (2023) GABAB receptor activation attenuates neuronal pyroptosis in post-cardiac arrest brain injury. Neuroscience 526:97–106. 10.1016/J.NEUROSCIENCE.2023.06.001 10.1016/J.NEUROSCIENCE.2023.06.001 [DOI] [PubMed] [Google Scholar]
- 231.Broz P, Ruby T, Belhocine K, et al (2012) Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490:288–291. 10.1038/NATURE11419 10.1038/NATURE11419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Oficjalska K, Raverdeau M, Aviello G, et al (2015) Protective role for caspase-11 during acute experimental murine colitis. J Immunol 194:1252–1260. 10.4049/JIMMUNOL.1400501 10.4049/JIMMUNOL.1400501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Flood B, Manils J, Nulty C, et al (2019) Caspase-11 regulates the tumour suppressor function of STAT1 in a murine model of colitis-associated carcinogenesis. Oncogene 38:2658–2674. 10.1038/S41388-018-0613-5 10.1038/S41388-018-0613-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li J, Brieher WM, Scimone ML, et al (2007) Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat Cell Biol 9:276–286. 10.1038/NCB1541 10.1038/NCB1541 [DOI] [PubMed] [Google Scholar]
- 235.Bolívar BE, Vogel TP, Bouchier-Hayes L (2019) Inflammatory caspase regulation: maintaining balance between inflammation and cell death in health and disease. FEBS J 286:2628–2644. 10.1111/FEBS.14926 10.1111/FEBS.14926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Salvamoser R, Brinkmann K, O’Reilly LA, et al (2019) Characterisation of mice lacking the inflammatory caspases-1/11/12 reveals no contribution of caspase-12 to cell death and sepsis. Cell Death Differ 26:1124–1137. 10.1038/S41418-018-0188-2 10.1038/S41418-018-0188-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lamkanfi M, Kalai M, Vandenabeele P (2004) Caspase-12: an overview. Cell Death Differ 11:365–8. 10.1038/sj.cdd.4401364 10.1038/sj.cdd.4401364 [DOI] [PubMed] [Google Scholar]
- 238.Shiraishi H, Okamoto H, Yoshimura A, Yoshida H (2006) ER stress-induced apoptosis and caspase-12 activation occurs downstream of mitochondrial apoptosis involving Apaf-1. J Cell Sci 119:3958–3966. 10.1242/JCS.03160 10.1242/JCS.03160 [DOI] [PubMed] [Google Scholar]
- 239.Nakagawa T, Zhu H, Morishima N, et al (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103. 10.1038/47513 10.1038/47513 [DOI] [PubMed] [Google Scholar]
- 240.Kalai M, Lamkanfi M, Denecker G, et al (2003) Regulation of the expression and processing of caspase-12. J Cell Biol 162:457–467. 10.1083/JCB.200303157 10.1083/JCB.200303157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kilic M, Schäfer R, Hoppe J, Kagerhuber U (2002) Formation of noncanonical high molecular weight caspase-3 and – 6 complexes and activation of caspase-12 during serum starvation induced apoptosis in AKR-2B mouse fibroblasts. Cell Death Differ 9:125–137. 10.1038/SJ.CDD.4400968 10.1038/SJ.CDD.4400968 [DOI] [PubMed] [Google Scholar]
- 242.Wang X, Shao Z, Zetoune FS, et al (2003) NRADD, a novel membrane protein with a death domain involved in mediating apoptosis in response to ER stress. Cell Death Differ 10:580–591. 10.1038/SJ.CDD.4401208 10.1038/SJ.CDD.4401208 [DOI] [PubMed] [Google Scholar]
- 243.Saleh M, Mathison JC, Wolinski MK, et al (2006) Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 440:1064–1068. 10.1038/NATURE04656 10.1038/NATURE04656 [DOI] [PubMed] [Google Scholar]
- 244.Vande Walle L, Lamkanfi M (2016) Pyroptosis. Curr Biol 26:R568–R572. 10.1016/j.cub.2016.02.019 10.1016/j.cub.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 245.Nakagawa T, Yuan J (2000) Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol 150:887–894. 10.1083/JCB.150.4.887 10.1083/JCB.150.4.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yoneda T, Imaizumi K, Oono K, et al (2001) Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276:13935–13940. 10.1074/JBC.M010677200 10.1074/JBC.M010677200 [DOI] [PubMed] [Google Scholar]
- 247.Rao R V., Hermel E, Castro-Obregon S, et al (2001) Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem 276:33869–33874. 10.1074/JBC.M102225200 10.1074/JBC.M102225200 [DOI] [PubMed] [Google Scholar]
- 248.Vesela B, Kratochvilova A, Svandova E, et al (2020) Caspase-12 is present during craniofacial development and participates in regulation of osteogenic markers. Front cell Dev Biol 8:589136. 10.3389/fcell.2020.589136 10.3389/fcell.2020.589136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bhootada Y, Choudhury S, Gully C, Gorbatyuk M (2015) Targeting Caspase-12 to preserve vision in mice with inherited retinal degeneration. Invest Ophthalmol Vis Sci 56:4725–4733. 10.1167/IOVS.15-16924 10.1167/IOVS.15-16924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Moorwood C, Barton ER (2014) Caspase-12 ablation preserves muscle function in the mdx mouse. Hum Mol Genet 23:5325–5341. 10.1093/HMG/DDU249 10.1093/HMG/DDU249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Liu H, Wang Z, Nowicki MJ (2014) Caspase-12 mediates carbon tetrachloride-induced hepatocyte apoptosis in mice. World J Gastroenterol 20:18189–18198. 10.3748/WJG.V20.I48.18189 10.3748/WJG.V20.I48.18189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Martinez JA, Zhang Z, Svetlov SI, et al (2010) Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells. Apoptosis 15:1480–1493. 10.1007/S10495-010-0526-4 10.1007/S10495-010-0526-4 [DOI] [PubMed] [Google Scholar]
- 253.Morishima N, Nakanishi K, Takenouchi H, et al (2002) An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 277:34287–34294. 10.1074/JBC.M204973200 10.1074/JBC.M204973200 [DOI] [PubMed] [Google Scholar]
- 254.LeBlanc PM, Yeretssian G, Rutherford N, et al (2008) Caspase-12 modulates NOD signaling and regulates antimicrobial peptide production and mucosal immunity. Cell Host Microbe 3:146–157. 10.1016/J.CHOM.2008.02.004 10.1016/J.CHOM.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 255.Wang P, Arjona A, Zhang Y, et al (2010) Caspase-12 controls West Nile virus infection via the viral RNA receptor RIG-I. Nat Immunol 11:912–919. 10.1038/jid.2014.371 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Labbé K, Miu J, Yeretssian G, et al (2010) Caspase-12 dampens the immune response to malaria independently of the inflammasome by targeting NF-kappaB signaling. J Immunol 185:5495–5502. 10.4049/JIMMUNOL.1002517 10.4049/JIMMUNOL.1002517 [DOI] [PubMed] [Google Scholar]
- 257.Miu J, Saleh M, Stevenson MM (2010) Caspase-12 deficiency enhances cytokine responses but does not protect against lethal Plasmodium Yoelii 17XL infection. Parasite Immunol 32:773–778. 10.1111/J.1365-3024.2010.01250.X 10.1111/J.1365-3024.2010.01250.X [DOI] [PubMed] [Google Scholar]
- 258.Skeldon AM, Morizot A, Douglas T, et al (2016) Caspase-12, but not Caspase-11, inhibits obesity and insulin resistance. J Immunol 196:437–447. 10.4049/JIMMUNOL.1501529 10.4049/JIMMUNOL.1501529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lippens S, Kockx M, Knaapen M, et al (2000) Epidermal differentiation does not involve the pro-apoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ 7:1218–24. 10.1038/sj.cdd.4400785 10.1038/sj.cdd.4400785 [DOI] [PubMed] [Google Scholar]
- 260.Hoste E, Kemperman P, Devos M, et al (2011) Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol 131:2233–2241. 10.1038/JID.2011.153 10.1038/JID.2011.153 [DOI] [PubMed] [Google Scholar]
- 261.Raymond AA, Méchin MC, Nachat R, et al (2007) Nine procaspases are expressed in normal human epidermis, but only caspase-14 is fully processed. Br J Dermatol 156:420–427. 10.1111/J.1365-2133.2006.07656.X 10.1111/J.1365-2133.2006.07656.X [DOI] [PubMed] [Google Scholar]
- 262.Lippens S, Kockx M, Denecker G, et al (2004) Vitamin D3 induces caspase-14 expression in psoriatic lesions and enhances caspase-14 processing in organotypic skin cultures. Am J Pathol 165:833–41. 10.1016/S0002-9440(10)63346-9 10.1016/S0002-9440(10)63346-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Markiewicz A, Sigorski D, Markiewicz M, et al (2021) Caspase-14-From biomolecular basics to clinical approach. A review of available data. Int J Mol Sci 22. 10.3390/IJMS22115575 [DOI] [PMC free article] [PubMed]
- 264.Markiewicz A, Sigorski D, Markiewicz M, et al (2023) mRNA expression of caspase 14 in skin epithelial malignancies. Postep Dermatologii i Alergol 40:315–320. 10.5114/ADA.2023.127646 10.5114/ADA.2023.127646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jung M, Choi J, Lee SA, et al (2014) Pyrrolidone carboxylic acid levels or caspase-14 expression in the corneocytes of lesional skin correlates with clinical severity, skin barrier function and lesional inflammation in atopic dermatitis. J Dermatol Sci 76:231–239. 10.1016/J.JDERMSCI.2014.09.004 10.1016/J.JDERMSCI.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 266.Denecker G, Hoste E, Gilbert B, et al (2007) Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol 9:666–74. 10.1038/ncb1597 10.1038/ncb1597 [DOI] [PubMed] [Google Scholar]
- 267.Chien AJ, Presland RB, Kuechle MK (2002) Processing of native caspase-14 occurs at an atypical cleavage site in normal epidermal differentiation. Biochem Biophys Res Commun 296:911–917. 10.1016/S0006-291X(02)02015-6 10.1016/S0006-291X(02)02015-6 [DOI] [PubMed] [Google Scholar]
- 268.Hoste E, Denecker G, Gilbert B, et al (2013) Caspase-14-deficient mice are more prone to the development of parakeratosis. J Invest Dermatol 133:742–750. 10.1038/JID.2012.350 10.1038/JID.2012.350 [DOI] [PubMed] [Google Scholar]
- 269.Kubica M, Hildebrand F, Brinkman BM, et al (2014) The skin microbiome of caspase-14-deficient mice shows mild dysbiosis. Exp Dermatol 23:561–567. 10.1111/EXD.12458 10.1111/EXD.12458 [DOI] [PubMed] [Google Scholar]
- 270.Hakem R, Hakem A, Duncan GS, et al (1998) Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94:339–352. 10.1016/S0092-8674(00)81477-4 10.1016/S0092-8674(00)81477-4 [DOI] [PubMed] [Google Scholar]
- 271.Cecconi F, Alvarez-Bolado G, Meyer BI, et al (1998) Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 94:727–37. 10.1016/s0092-8674(00)81732-8 10.1016/s0092-8674(00)81732-8 [DOI] [PubMed] [Google Scholar]
- 272.Ratts VS, Flaws JA, Kolp R, et al (1995) Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology 136:3665–3668. 10.1210/ENDO.136.8.7628407 10.1210/ENDO.136.8.7628407 [DOI] [PubMed] [Google Scholar]
- 273.McIlwain DR, Berger T, Mak TW (2015) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 7. 10.1101/CSHPERSPECT.A026716 [DOI] [PMC free article] [PubMed]
- 274.Kudelova J, Fleischmannova J, Adamova E, Matalova E (2015) Pharmacological caspase inhibitors: research towards therapeutic perspectives. J Physiol Pharmacol an off J Polish Physiol Soc 66:473–482 [PubMed] [Google Scholar]
- 275.Dhani S, Zhao Y, Zhivotovsky B (2021) A long way to go: caspase inhibitors in clinical use. Cell Death Dis 12:949. 10.1038/s41419-021-04240-3 10.1038/s41419-021-04240-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Duan H, Liu Y, Gao Z, Huang W (2021) Recent advances in drug delivery systems for targeting cancer stem cells. Acta Pharm Sin B 11:55–70. 10.1016/j.apsb.2020.09.016 10.1016/j.apsb.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Karlgren M, Simoff I, Keiser M, et al (2018) CRISPR-Cas9: a new addition to the drug metabolism and disposition tool box. Drug Metab Dispos 46:1776–1786. 10.1124/dmd.118.082842 10.1124/dmd.118.082842 [DOI] [PubMed] [Google Scholar]
- 278.Prokhorova EA, Kopeina GS, Lavrik IN, Zhivotovsky B (2018) Apoptosis regulation by subcellular relocation of caspases. Sci Rep 8. 10.1038/S41598-018-30652-X [DOI] [PMC free article] [PubMed]
- 279.Nakajima Y, Kuranaga E (2017) Caspase-dependent non-apoptotic processes in development. Cell Death Differ 24:1422–1430. 10.1038/cdd.2017.36 10.1038/cdd.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Huesmann GR, Clayton DF (2006) Dynamic role of postsynaptic caspase-3 and BIRC4 in zebra finch song-response habituation. Neuron 52:1061–1072. 10.1016/J.NEURON.2006.10.033 10.1016/J.NEURON.2006.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Grabow S, Kueh AJ, Ke F, et al (2018) Subtle changes in the levels of BCL-2 proteins cause severe craniofacial abnormalities. Cell Rep 24:3285–3295.e4. 10.1016/j.celrep.2018.08.048 10.1016/j.celrep.2018.08.048 [DOI] [PubMed] [Google Scholar]
- 282.Basu S, Rajakaruna S, Menko AS (2012) Insulin-like growth factor receptor-1 and nuclear factor κB are crucial survival signals that regulate caspase-3-mediated lens epithelial cell differentiation initiation. J Biol Chem 287:8384–97. 10.1074/jbc.M112.341586 10.1074/jbc.M112.341586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bazgir N, Tahvildari A, Chavoshzade Z, et al (2023) A rare immunological disease, caspase 8 deficiency: case report and literature review. Allergy Asthma Clin Immunol 19. 10.1186/S13223-023-00778-3 [DOI] [PMC free article] [PubMed]
- 284.Bell RAV, Megeney LA (2017) Evolution of caspase-mediated cell death and differentiation: twins separated at birth. Cell Death Differ 24:1359–1368. 10.1038/CDD.2017.37 10.1038/CDD.2017.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Fuchs Y, Steller H (2015) Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol 16:329–344. 10.1038/nrm3999 10.1038/nrm3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kumar S (2007) Caspase function in programmed cell death. Cell Death Differ 14:32–43. 10.1038/sj.cdd.4402060 10.1038/sj.cdd.4402060 [DOI] [PubMed] [Google Scholar]
- 287.Tian F, Fu X, Gao J, et al (2014) Glutaric acid-mediated apoptosis in primary striatal neurons. Biomed Res Int 2014:484731. 10.1155/2014/484731 10.1155/2014/484731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Jiménez Fernández D, Lamkanfi M (2015) Inflammatory caspases: key regulators of inflammation and cell death. Biol Chem 396:193–203. 10.1515/hsz-2014-0253 10.1515/hsz-2014-0253 [DOI] [PubMed] [Google Scholar]
- 289.Fogarty CE, Bergmann A (2017) Killers creating new life: caspases drive apoptosis-induced proliferation in tissue repair and disease. Cell Death Differ 24:1390–1400. 10.1038/CDD.2017.47 10.1038/CDD.2017.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Olsson M, Zhivotovsky B (2011) Caspases and cancer. Cell Death Differ 18:1441–1449. 10.1038/cdd.2011.30 10.1038/cdd.2011.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Denning DP, Hatch V, Horvitz HR (2013) Both the Caspase CSP-1 and a caspase-independent pathway promote programmed cell death in parallel to the canonical pathway for apoptosis in caenorhabditis elegans. PLoS Genet 9. 10.1371/JOURNAL.PGEN.1003341 [DOI] [PMC free article] [PubMed]
- 292.Geng X, Zhou QH, Kage-Nakadai E, et al (2009) Caenorhabditis elegans caspase homolog CSP-2 inhibits CED-3 autoactivation and apoptosis in germ cells. Cell Death Differ 16:1385–1394. 10.1038/cdd.2009.88 10.1038/cdd.2009.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Spead O, Verreet T, Donelson CJ, Poulain FE (2018) Characterization of the caspase family in zebrafish. PLoS One 13:e0197966. 10.1371/journal.pone.0197966 10.1371/journal.pone.0197966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kietz C, Meinander A (2023) Drosophila caspases as guardians of host-microbe interactions. Cell Death Differ 30:227–236. 10.1038/S41418-022-01038-4 10.1038/S41418-022-01038-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Groborz KM, Kalinka M, Grzymska J, et al (2023) Selective chemical reagents to investigate the role of caspase 6 in apoptosis in acute leukemia T cells. Chem Sci 14:2289–2302. 10.1039/D2SC05827H 10.1039/D2SC05827H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tu S, McStay GP, Boucher LM, et al (2006) In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat Cell Biol 8:72–77. 10.1038/NCB1340 10.1038/NCB1340 [DOI] [PubMed] [Google Scholar]
- 297.Cardona M, López JA, Serafín A, et al (2015) Executioner Caspase-3 and 7 Deficiency reduces myocyte number in the developing mouse heart. PLoS One 10. 10.1371/JOURNAL.PONE.0131411 [DOI] [PMC free article] [PubMed]
- 298.Thant AA, Nawa A, Kikkawa F, et al (2000) Fibronectin activates matrix metalloproteinase-9 secretion via the MEK1-MAPK and the PI3K-Akt pathways in ovarian cancer cells. Clin Exp Metastasis 2000 185 18:423–428. 10.1023/A:1010921730952 10.1023/A:1010921730952 [DOI] [PubMed] [Google Scholar]
- 299.Vesela B, Svandova E, Vanden Berghe T, et al (2015) Non-apoptotic role for caspase-7 in hair follicles and the surrounding tissue. J Mol Histol 46:443–55. 10.1007/s10735-015-9636-1 10.1007/s10735-015-9636-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.