Abstract
After pseudorabies virus (PRV) infection of murine L929 cells, the cell surface expression of major histocompatibility complex (MHC) class I proteins changes such that the total amount of MHC class I molecules remains relatively constant but the levels of the individual alleles Dk and Kk vary. This is an active process involving at least three PRV gene products that act in an allele-specific manner such that cell surface expression of MHC class I Dk is decreased and that of Kk is increased. Our results indicate that an early gene product mediates the overall reduction in Dk protein and a late gene product which is mutant in the attenuated PRV strain Bartha mediates the increase in Kk protein. We provide additional evidence for a third gene product involved in the regulation of the synthesis of both the Dk and Kk proteins. In addition, we show that the early decrease in the Dk protein is not due to a block in synthesis or processing of the complex through the secretory system.
Pseudorabies virus (PRV) is a pathogen of swine and cattle (25). PRV is a member of the alphaherpesvirus family that includes the human pathogens herpes simplex virus (HSV) and varicella-zoster virus. Like many of the viruses in this family, PRV has the capacity to invade the local peripheral nervous system, where it establishes a latent infection that can be reactivated. Primary infection of older pigs often results in a mild respiratory disease with the virus establishing a latent infection in sensory ganglia innervating nasopharyngeal surfaces. In contrast, primary PRV infection of young pigs often results in lethal encephalitis with the virus invading the spinal cord and brain. PRV also infects a wide variety of mammals and some birds, and invariably, primary infection of these animals results in fatal encephalitis (9).
In order to establish a primary infection and be maintained in the host population, the alphaherpesviruses must be able to evade both innate and acquired immune defenses with some efficiency. The ability of PRV to establish primary, acute, lethal brain infections in diverse hosts suggests that general innate defenses are easily overcome by this virus. Little is known about the molecular mechanisms involved in this process. During a primary PRV infection, infected cells are first recognized by the innate immune system comprising, in part, natural killer (NK) cells, complement, and the interferons. Action by these innate defenses is presumed to limit spread and confine the infection to local mucosal surfaces. It is known that NK cells contribute to the nonspecific clearance of virally infected cells after primary virus infection. Depletion of NK cells from mice before PRV infection results in higher virus titers in the brain and reduced survival. Conversely, when NK cell activity is stimulated by addition of serum thymic factor, virus titers are reduced with a concomitant increase in animal survival (27). Interestingly, in vitro assays have shown that some PRV-infected cell types are sensitive to NK cell-mediated lysis while other cell types are resistant (6, 18). The mechanisms by which NK cells recognize infected cells are under study in many laboratories, but one idea is that cells with reduced levels of major histocompatibility complex (MHC) class I proteins on the cell surface are preferentially attacked by NK cells (16).
If an animal survives PRV infection, the virus is invariably found latent in sensory and autonomic ganglia innervating the tissue where the primary infection occurred (9). Such latently infected animals are immunized and have circulating antibody and cytotoxic T cells (CTLs) that can react with PRV-infected cells (24). When the latent infection reactivates in these immunized animals, infected cells would be recognized by these CTLs through T-cell receptor binding to specific intracellularly derived viral peptides bound to cell surface MHC class I proteins. In this case, action of CTLs would limit local spread of infection and promote clearance of the virus and virus-infected cells.
It is clear that the MHC class I proteins represent a key host defense interface that is critical for both innate and acquired immune defenses. Therefore, as might be expected, MHC class I protein expression is regulated by many viruses. In particular, many herpesviruses encode proteins that modulate MHC class I levels in infected cells (2, 4, 7, 14, 17, 23, 26, 30, 38). Such proteins act at several points in the MHC class I peptide presentation pathway, including transcription, intracellular peptide processing and loading, MHC class I protein stability, and passage through the secretory system to the final destination on the cell surface (12).
In this report, we present evidence for differential regulation of specific classes of MHC class I proteins on the cell surface after primary infection of murine cells by PRV. Despite differential appearance of MHC class I proteins, the total concentration remains relatively unchanged after viral infection. We show that an early PRV gene product initially reduces the cell surface concentration by destabilizing cell surface molecules, not by interfering with the synthesis or processing of the MHC class I complex. Late in infection, a second PRV gene product reduces the synthesis of both Dk and Kk without further reducing cell surface levels of MHC class I. We also show that the PRV gene product that increases Kk levels is a late gene product and is mutant in the attenuated PRV strain Bartha, suggesting that regulation of MHC I is one factor in the virulence of this virus. While the biological significance of these observations is currently under study, we speculate that by affecting the specific composition of MHC class I molecules on the surface of an infected cell, PRV may evade both NK- and T-cell-mediated responses.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
PK15 (swine kidney) cells were maintained in Dulbecco modified Eagle medium plus 10% fetal bovine serum (FBS). L929 (mouse fibroblast) cells (American Type Tissue Collection, Manassas, Va.) were maintained in minimal essential medium plus 10% FBS. All PRV strains were grown in PK15 cells in Dulbecco modified Eagle medium plus 2% FBS. The antibodies used are described in Table 1.
TABLE 1.
Antibodies used in this study
| Antibody | Epitope(s) | Source | Refer-ence |
|---|---|---|---|
| 15-5-5 | H-2Dk | Pharmingen | 28 |
| 16-3-1N | H-2Kk | Gift from A. Bendelac | 28 |
| AF3-12.1.3 | H-2Kk | ATCCa | 19 |
| 11-4.1 | H-2Kk | Pharmingen | 29 |
| 15-3-1S | H-2Dk, H-2Kk | Gift from A. Bendelac | 28 |
| 15-3-1S | β2-microglobulin | Gift from H. Ploegh | |
| 284 | PRV gB | 32 | |
| 282 | PRV gC | 33 | |
| Anti-mouse–PEc | Mouse Fab′ | Jackson Laboratory | |
| Anti-rabbit–FITCb | Rabbit Fab′ | Jackson Laboratory | |
| Anti-goat–PE | Goat Fab′ | Jackson Laboratory | |
| Anti-goat–FITC | Goat Fab′ | Jackson Laboratory |
ATCC, American Type Culture Collection.
FITC, fluorescein isothiocyanate.
PE, phycoerythrin.
Inactivation of viral particles.
β-Propiolactone (0.08%; Sigma) was added to PRV strain Becker, and the mixture was incubated for 30 min on ice. The stock was then incubated at 37°C for 4 h and then returned to 4°C for 72 h. The resulting precipitate was then removed by centrifugation. The titer of the resulting stock was determined on PK15 cells.
Fluorescence-activated cell sorter (FACS) analysis.
Cells were infected with a multiplicity of infection (MOI) of 10 in minimal essential medium plus 2% FBS and incubated for 14 to 16 h. Cells (106) were then trypsinized briefly, resuspended in phosphate-buffered saline–3% bovine serum albumin (BSA) with the appropriate primary antibody, and incubated on ice for 30 min. Cells were then resuspended in the appropriate secondary antibody diluted in phosphate-buffered saline plus 3% BSA and incubated for 30 min on ice. The stained cells were then fixed in 1% formaldehyde, and 10,000 cells were analyzed by flow cytometry (FACScan; Becton Dickinson).
Phosphonoacetic acid (PAA) experiments.
Cells were pretreated for 1 h in the presence of PAA (400 μg/ml; Sigma) and then infected with PRV strain Becker in the presence of PAA for 16 h. Cells were then analyzed by flow cytometry as described above.
Citrate wash experiments.
L929 cells were infected and then washed with citrate wash (0.062 M Na2HPO4, 0.132 M citric acid, 0.5% BSA, pH 3) for 2 min at 4 or 8 h postinfection as described by Sugawara et al. (34). The citrate wash was then neutralized by being washed three times with medium. Cells were then incubated in fresh medium at 37°C and analyzed by flow cytometry as described above at 24 h postinfection. For the cycloheximide experiment, cells were treated with citrate wash and then washed as described above. Cycloheximide was then added (500 μg/ml). The cells were harvested at 6 h postwash.
RESULTS
PRV infection has little effect on the overall cell surface expression of MHC class I.
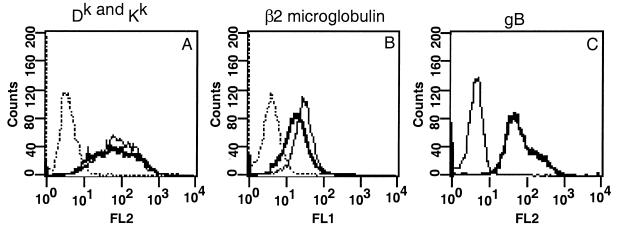
We determined whether the overall cell surface expression of MHC class I was changed in PRV-infected mouse fibroblast (L929) cells. Cells were infected with PRV strain Becker at an MOI of 10 for 16 h and then stained for FACS analysis as described in Materials and Methods. All cells were infected, as shown by expression of the viral glycoprotein gB (Fig. 1C). We used an antibody that recognizes two alleles of MHC class I found on the surface of these cells (Dk and Kk), as well as an antibody against β2-microglobulin which recognizes all MHC class I species. Comparisons of MHC class I concentrations on the surface of these cells showed a slight decrease in both β2-microglobulin and total Dk and Kk expression after PRV infection (Fig. 1A and B). These results show that there is no significant change in overall MHC class I expression on the surface of these cells after PRV infection.
FIG. 1.
PRV infection of L929 cells does not affect overall MHC class I protein levels. L929 cells were infected for 16 h and then stained for FACS analysis, using antibodies against Dk and Kk (monoclonal antibody 16-3-1S) (A) β2-microglobulin (B), or viral glycoprotein gB. Lines: dotted, anti-mouse antibody alone; light, mock-infected cells; dark, infected cells.
The cell surface concentration of two MHC class I alleles changes after PRV infection.
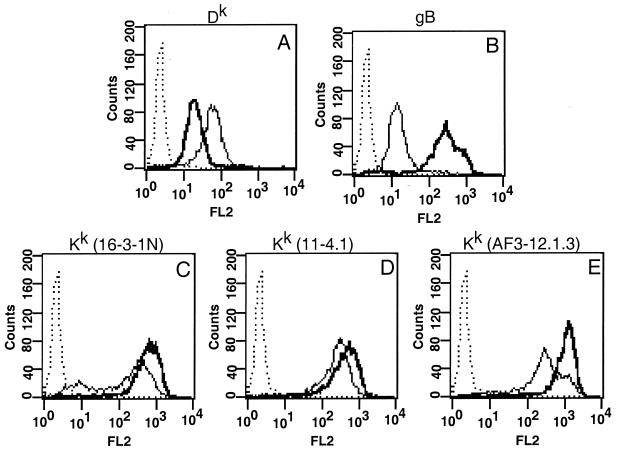
Since previous studies had indicated that MHC class I was reduced in PRV-infected cells (23), we next examined the cell surface expression of specific MHC class I alleles Dk and Kk on infected L929 cells. As shown in Fig. 2B, all cells were infected, as demonstrated by robust expression of the viral glycoprotein gB. After infection, cell surface expression of Dk was reduced by approximately 70% (Fig. 2A). In contrast to the results shown by Mellencamp et al. (23), we found that cell surface expression of Kk was increased by 130% (Fig. 2C, D, and E) by using three separate monoclonal antibodies to Kk (16-3-1N, 11-4.1, and AF3-12.1.S). Similar results were obtained when cells were infected with three different strains of PRV, i.e., Phylaxia, Kaplan, and NIA-3 (data not shown). We conclude that PRV infection modulates the steady-state concentration of MHC class I proteins on the cell surface of murine fibroblast cells such that at late times after infection, the Dk protein is reduced while the Kk protein is increased.
FIG. 2.
MHC class I protein expression is regulated in an allele-specific manner in L929 cells. L929 cells were infected for 16 h and then stained for FACS analysis by using antibodies against Dk (A), gB (B), or Kk (C, D, and E; with monoclonal antibodies 16-3-1N, 11-4.1, and AF3-12.1.3, respectively). Lines: dotted, anti-mouse antibody alone; light, mock-infected cells; dark, infected cells.
Both early and late viral gene products affect cell surface expression of MHC class I proteins.
We next determined if early or late PRV gene products affected cell surface expression of MHC class I proteins. To do so, we relied on the fact that viral late gene expression requires DNA replication, while early gene expression can occur from nonreplicated genomes. By adding PAA, an inhibitor of viral DNA replication, to the cells during infection, late gene expression is prevented, but early genes are still expressed (25).
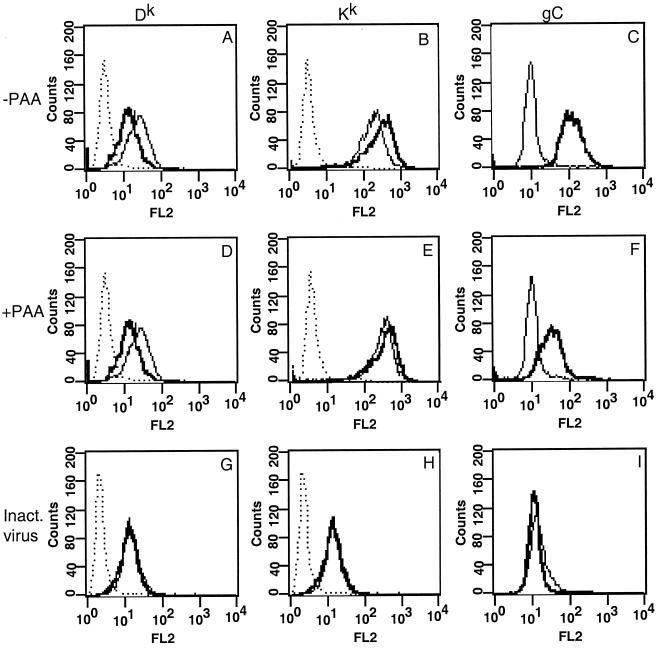
L929 cells were pretreated with PAA at 400 μg/ml for 1 h prior to infection and then infected with PRV strain Becker at an MOI of 10 in the presence of PAA for 16 h. Cells were then stained for MHC class I proteins and analyzed as previously described, except that the late viral glycoprotein gC was used to control for infectivity. As predicted, gC expression was reduced more than 350% after addition of PAA compared to that on untreated cells (compare Fig. 3C with Fig. 3F). In the absence of PAA, Dk was reduced by 44% and Kk was increased by 50% (Fig. 3A and B). In the presence of PAA, Dk surface expression decreased by 60% but Kk expression increased by only 15% compared to that on mock-infected, PAA-treated cells (Fig. 3D and E). Clearly, the addition of PAA had no effect on the reduction of Dk. This observation suggests that an early gene product(s) is involved in this phenomenon. On the other hand, the addition of PAA effectively blocked the increased expression of Kk. This suggests that a late gene product(s) is involved in this process. These experiments provide additional evidence that PRV can regulate MHC class I in an allele-specific manner since the gene product responsible for the phenomenon can be separated into two different kinetic classes.
FIG. 3.
Cell surface expression of Dk is reduced by an early PRV gene product, and Kk is increased by a late PRV gene product. L929 cell were infected with PRV strain Becker (A to F) or β-propiolactone-inactivated PRV strain Becker (G to I) in the presence (D to F) or absence (A to C and G to I) of PAA at 400 μg/ml for 16 h and then stained for FACS analysis by using antibodies against Dk and (A, D, and G), Kk (B, E [with monoclonal antibody 16-3-1N], and H [with monoclonal antibody 11-4.1]), or gC (C, F, and I). Lines: dotted, anti-mouse antibody alone; light, mock-infected cells; dark, infected cells.
While the PAA experiments suggest that an early viral protein is involved in the reduction of MHC class I from the surface of cells, they do not rule out the possibility that a structural component of the virion which is synthesized late in infection mediates this effect upon infection. To examine this possibility, we inactivated viral particles by using β-propiolactone as described in Materials and Methods. This reduced the viral titer to less than 50 PFU/ml (data not shown). We then infected cells at an MOI of 10 as determined before inactivation and compared the amounts of reduction of Dk obtained with inactivated and infectious virus. Cells infected with inactivated virus did not reduce cell surface levels of MHC class I, in contrast to cells infected with infectious virus (Fig. 3G). As expected, cells infected with inactivated virus also did not increase Kk levels (Fig. 3H) or express the viral gB protein (Fig. 3I). Therefore, it is unlikely that a late gene product which is a structural component of the virion is involved in MHC class I regulation.
Cell surface expression of MHC class I alleles after infection by the attenuated Bartha strain of PRV.
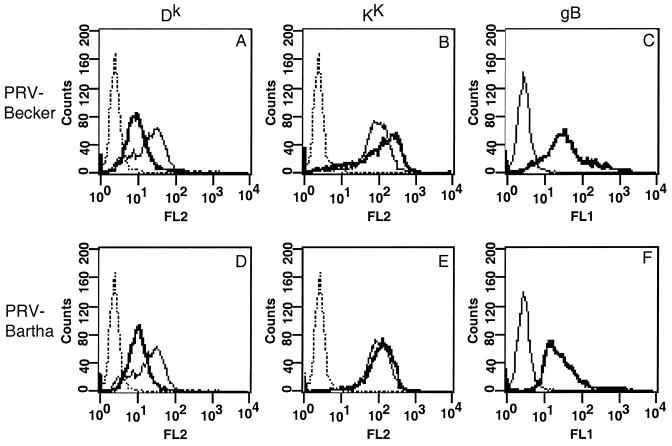
In an initial attempt to map the gene products involved in regulating cell surface expression of MHC class I proteins, we infected L929 cells with the attenuated Bartha strain of PRV. This strain harbors a number of known mutations, including a deletion in the Us region, which encompasses gI, gE, Us9, and Us2, and point mutations in gC, UL21, and gM (8, 20, 21, 32). Comparison of infections of L929 cells with PRV strains Becker and Bartha provided further evidence that different gene products mediate allele-specific MHC class I protein accumulation on the surface of infected cells. Both viruses caused a marked 60% reduction in MHC class I Dk protein on the surface of infected cells (Fig. 4A and D). In contrast, PRV strain Bartha-infected cells showed little or no sign of increased expression of MHC class I Kk (Fig. 4E). The amount of this allele increased by only 15% after infection with PRV strain Bartha, compared to the 70% increase obtained with PRV strain Becker in this experiment (Fig. 4B). All cells were infected, as shown by gB expression (Fig. 4C and F). Thus, PRV strain Bartha is defective in the gene product(s) responsible for increasing Kk on the cell surface.
FIG. 4.
Cell surface expression of Kk is unaffected by infection with PRV vaccine strain Bartha. L929 cells were infected for 16 h with PRV strain Becker (A to C) or Bartha and then stained for FACS analysis by using antibodies against Dk (A and D), Kk (B and E; monoclonal antibody 16-3-1N), or gB (C and F). Lines: dotted, anti-mouse antibody alone; light, mock infected cells; dark, infected cells.
Early decrease in MHC Dk protein on L929 cells is not due to blocked synthesis or processing of the molecule.
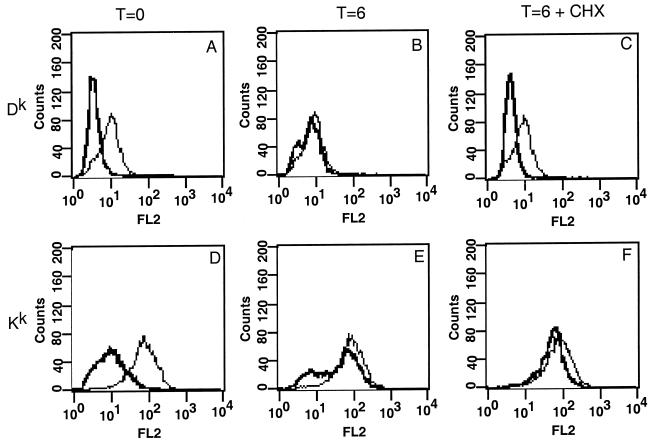
We next determined if PRV infection affects the synthesis or posttranslational processing of the MHC class I proteins. More specifically, we determined if the lack of Dk on the cell surface after infection resulted because MHC heavy chains were no longer synthesized or were blocked in the assembly of the MHC class I–peptide–β2-microglobulin complex or its transport through the secretory system to the cell surface. Previously, Sugawara et al. (34) have shown that treatment of cells by washing with pH 3 citrate buffer removes all detectable MHC class I protein from the cell surface. If a neutral pH is restored, newly synthesized MHC class I molecules quickly repopulate the cell surface. Thus, we can determine if the block in Dk cell surface expression after PRV infection is due to blocked synthesis of the molecule or its transport to the cell surface or if it is due to an alteration in the cell surface stability of the molecule. Eighty percent of the cell surface MHC class I protein is removed by citrate washing of uninfected cells, as shown in Fig. 5A and D. The expression of all cell surface proteins was not affected by citrate washing, as gB expression was not affected by citrate washing (Fig. 6I). In addition, Sugawara et al. (34) have shown that MHC class II protein is not affected by citrate washing. If the cells are then allowed to recover in fresh medium for 6 h, MHC class I protein returns to the cells surface to the same level as before washing (Fig. 5B and E).
FIG. 5.
Effect of low-pH citrate washing on uninfected L929 cells. L929 cells were treated with a citrate wash, new medium was added, and the cells were stained for FACS analysis immediately (A and D) or incubated at 37°C for 6 h in the presence (B and E) or absence (C and F) of cycloheximide (CHX) (500 μg/ml) (C and F) and stained for Dk (A to C) or Kk (D to F; with monoclonal antibody 11-4.1). Lines: light, unwashed cells; dark, washed cells.
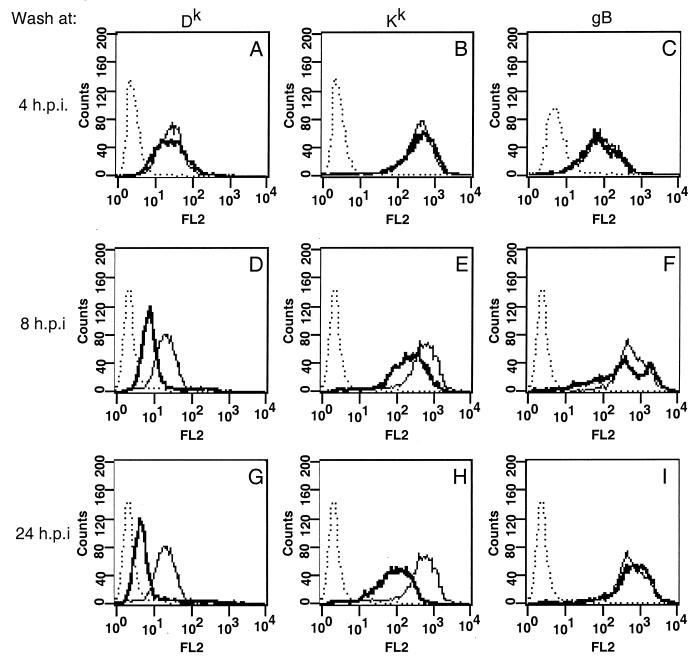
FIG. 6.
Effect of low-pH citrate washing on infected L929 cells. L929 cells were infected and treated with a citrate wash at 4 (A to C), 8 (D to F), or 24 (G to I) h postinfection (h.p.i.). Fresh medium was added, and then cells were analyzed by FACS at 24 h postinfection by using antibodies against Dk (A, D, and G), Kk (B, E, and H; with monoclonal antibody 16-3-1N), or gB (C, F, and I). Lines: dotted, anti-mouse antibody alone; light, mock-infected cells; dark, infected cells.
PRV-infected L929 cells were washed with citrate at 4 and 8 h postinfection and then allowed to recover in fresh medium until 24 h postinfection. Cells were then analyzed by flow cytometry as described previously. If cells were exposed to low-pH citrate washing at 4 h postinfection, when Dk levels are first seen to decrease (data not shown), both Dk and Kk returned to approximately the same level as in unwashed infected cells (Fig. 6A and B). However, if cells were treated with citrate at 8 h postinfection, neither Dk nor Kk returned to pretreatment levels (Fig. 6D and E). Treatment of the cells with citrate did not affect the infectivity of the virus, as shown by expression of gB (Fig. 6C, F, and I). These results suggest that the early decrease in Dk expression is not due to blocking the synthesis of the complex or of its transport to the cell surface. Moreover, the data suggest that there is a second gene product responsible for blocking the cell surface expression of both Dk and Kk later in infection.
In order to demonstrate that MHC class I proteins returning to the cell surface were due to new synthesis of the complex, we treated uninfected L929 cells with citrate, added fresh medium, and then added cycloheximide to prevent any new protein synthesis. If only new MHC class I molecules were returning to the cell surface after citrate washing, then addition of cycloheximide to the medium would prevent MHC class I expression. We treated the cells, added cycloheximide, and then analyzed MHC class I protein levels 6 h after exposure to citrate. If cells are treated and then directly analyzed, approximately 80% of both Dk and Kk is removed (Fig. 5B and E). When the cells are allowed to recover in fresh medium in the absence of cycloheximide, MHC class I levels return to normal (Fig. 5C and F). However, if cycloheximide is added to the cells, Dk does not return to normal levels (Fig. 5B), suggesting that new synthesis of Dk is required to replenish Dk after citrate treatment. In contrast, Kk levels returns to normal in the presence or absence of cycloheximide (Fig. 5E), suggesting that new synthesis of Kk is not required to replenish Kk on the cell surface. These experiments suggest that cell surface expression of Dk is not reduced by inhibition of the synthesis of the complex or its transport through the secretory system early in infection. Late in infection, a second gene product shuts off the synthesis of both MHC class I alleles.
DISCUSSION
After PRV infection of murine L929 cells, the cell surface expression of MHC class I proteins changes such that the total amount remains relatively constant but the amounts of the Dk and Kk alleles vary. This is an active process involving at least three PRV gene products which act in an allele-specific manner such that MHC class I Dk is decreased and Kk is increased. Our results indicate that an early gene product which is functional in the attenuated PRV strain Bartha mediates the overall reduction of Dk and a late gene product which is defective in PRV strain Bartha mediates the increase in Kk. We provide additional evidence for a third gene product which reduces the synthesis of both the Dk and Kk proteins. In addition, we show that the early decrease in Dk is not due to blocking the synthesis of MHC class I or of transport of the complex through the secretory system.
Our results directly contradict those of Mellencamp et al. (23), who showed that the intracellular and cell surface concentrations of both the Kk and Dk proteins were reduced after PRV infection. We cannot explain this discrepancy. We have confirmed our results by using three different antibodies against Kk, including AF3-12.1.S, the antibody used by Mellencamp et al. While some experiment-to-experiment variability exists, we have never observed the cell surface concentration of the Kk protein to be less than that found on mock-infected cells, even at early points during infection. We have not used the same viral strain used by Mellencamp et al. (the Indiana Funkhauser strain), but we have confirmed our results with four different PRV strains (Becker, NIA-3, Kaplan, and Phylaxia). It appears unlikely that strain differences are responsible for the difference between our results. One possible difference in our experiments could be the medium and serum in which the cells were grown, as these variables are known to affect MHC class I protein expression (data not shown). Unfortunately, Mellencamp et al. did not state the growth conditions used in their experiments, and we cannot reproduce their experimental protocol precisely.
The viral genes involved in MHC class I regulation remain to be identified. PRV does not contain easily identifiable homologs to the gene products of other herpesviruses known to affect cell MHC class I protein expression. However, after using the viral DNA synthesis inhibitor PAA, we suggest that an early PRV gene product is involved in reduction of the cell surface concentration of the MHC class I Dk protein. It is also possible that the immediate-early protein IE180 or a structural component of the virion which is synthesized late in infection is responsible. To test the first idea, we transfected a plasmid expressing the IE180 protein into PK15 cells and observed a marked increase and not a decrease in the amount of MHC class I protein on the cell surface (data not shown). This most likely reflects the promiscuous transcriptional activator activity of this protein. The simple idea that the IE180 protein reduces MHC class I expression cannot be correct. We tested the second idea by using inactivated virus preparations and found that these preparations did not reduce the cell surface levels of Dk. Thus, viral structural proteins which are synthesized late in infection are unlikely to be involved in this phenomenon.
The citrate wash experiments provided evidence that a late viral gene product is required to block the synthesis or transport of both the Dk and Kk proteins. One candidate protein is the virion host shutoff (vhs) homolog encoded by the UL41 gene that is known to reduce the synthesis of many host proteins after HSV type 1 infection, including MHC class I proteins (31, 36). Host protein synthesis is reduced in PRV-infected cells, but it is not clear that the PRV UL41 homolog is responsible for this effect. In L929 cells, host protein synthesis is virtually unchanged at 4 h postinfection but is markedly reduced by 8 h postinfection (data not shown). This observation is consistent with the kinetics of MHC class I shutoff, as demonstrated by the citrate wash experiments. Alternatively, PRV may encode a second gene that specifically reduces the synthesis or transport of both MHC class I alleles through the secretory system.
The attenuated PRV strain Bartha is defective in the expression or action of the late gene product that increases cell surface expression of the Kk protein. We have attempted to identify this Bartha mutation by infecting L929 cells with recombinant PRV strain Becker containing some of the individual known mutations in strain Bartha. Such viruses included a strain Becker recombinant expressing the strain Bartha gC-encoding gene and Becker viruses with the genes for gE, gI, and Us9 deleted. We have not been able to identify the Bartha mutation by this approach. Since PRV strain Bartha has not been completely sequenced, we cannot conclude that the mutation maps to gM, Ul21, or Us2, as other, unidentified, mutations may be involved.
We have ruled out several mechanisms that might be responsible for the early decrease in Dk on the cell surface by using low-pH citrate washes to remove MHC class I proteins from the surface of cells at different points during infection. First, we have demonstrated that MHC class I proteins can be synthesized and transported through the secretory system to the cell surface at early times postinfection when the initial MHC class I decrease is seen. Thus, PRV does not reduce cell surface expression of MHC class I proteins by interfering with transcription of the MHC class I heavy chain or by retaining the complex in the endoplasmic reticulum (ER), as has been found for other viruses (12). Second, the citrate wash results might reflect reduced stability of the MHC class I complex on the surface of infected cells. In this case, PRV infection might interfere with peptide binding to MHC class I heavy-chain complexes, as has been shown to occur for both HSV and human cytomegalovirus (CMV) (12). It is known that MHC class I heavy chains exhibit reduced stability when they are on the cell surface without peptide. As a result, differential appearance of MHC class I proteins may reflect the stabilities of specific MHC class I proteins when no longer complexed with peptide (1). In this regard, previous studies have shown that Kk molecules are particularly stable in the absence of peptide (35). Thus, despite interference with peptide transport into the ER, Kk molecules without peptides would still be present on the cell surface in relatively normal amounts. The increased stability of Kk molecules with no peptide over Dk proteins with no peptide would result in apparent allele-specific regulation of MHC class I appearance on the cell surface.
Cell surface expression of MHC class I Kk protein increased late in infection, despite an apparent decrease in intracellular synthesis (Fig. 2B, C, and D and 6F). This finding may reflect two independent mechanisms affecting the localization of the Kk protein. First, we believe that the Kk protein can be transported to the cell surface in the absence of protein synthesis because the Kk protein returns to the surface after citrate washing in the presence of cycloheximide (Fig. 5E). Second, from experiments done by Tirabassi et al. (37), we know that PRV infection blocks internalization of viral proteins from the plasma membrane after 6 h of infection. If PRV infection promotes a general inhibition of endocytosis late after infection, then proteins on the cell surface would be stabilized and a synthesis block would not be detectable by examination of cell surface molecules. This idea is consistent with our observations of Dk expression throughout infection. Cell surface expression of Dk falls to approximately 70% of the levels in mock-infected cells by 4 h postinfection yet stays at this level even though synthesis of Dk is decreased. The concentration of Dk protein may be stabilized at the cell surface late in infection because it cannot be internalized and turned over. Since Kk molecules would continue to be transported to the cell surface in the absence of any new synthesis, more Kk than Dk protein would be seen after endocytosis was inhibited.
Differential regulation of MHC class I proteins is not unique to PRV infection and has been demonstrated after infection with both RNA and DNA viruses. For example, mouse hepatitis virus (a coronavirus) infection results in increased expression of the Dd allele and a decrease in the Kd allele in murine cerebral endothelial cells (15). The adenovirus E3 19K protein binds more tightly to some human MHC class I alleles than to others, which results in a small fraction of the Aw68, B27, and Bw58 alleles escaping intracellular retention (3). The Us11 and Us2 proteins of human CMV bind to specific mouse MHC class I proteins and cause their degradation by dislocating the newly synthesized heavy chains from the ER into the cytoplasm. Us11 binds to Kb, Dd, Db, and Ld, whereas Us2 only binds to Dd and Db (22). In addition, HSV has been shown to differentially affect the association of MHC heavy chains with β2-microglobulin such that the B51 allele does not associate with β2-microglobulin in HSV-infected human fibroblast cells while the A29 allele assembles normally with both β2-microglobulin and peptide. Neither allele becomes normally sialylated; this is a characteristic of MHC class I heavy chains reaching the cell surface (13). We have also found that in PRV-infected swine kidney (PK15) cells, MHC class I proteins recognized by different monoclonal antibodies are differentially expressed, such that one MHC class I protein is decreased early in infection and one MHC class I protein is decreased late in infection (unpublished results).
A general reduction in the amount of MHC class I protein on the surface of PRV-infected cells may help the virus to evade detection by circulating T cells and thus allow the virus to cause disease in the central nervous system. There is some data to support this notion. An HSV type 1 mutant defective in the ICP47-encoding gene, the gene responsible for reduced cell surface expression of MHC class I, is less virulent presumably because CD8+ T cells are able to limit the infection (11). However, by reducing overall cell surface levels of MHC class I protein, virally infected cells would become more sensitive to NK cell-mediated lysis (16). By lowering the concentration of some, but not all, MHC class I proteins on the cell surface, PRV-infected cells may escape detection by NK cells. In fact, it is known that NK cell-mediated cell lysis is not increased during PRV infection in some cell types (5; data not shown). The ability to evade NK cell recognition is critical in murine CMV infection, in which a mutant with a change in the MHC class I homolog-encoding gene (m144) exhibits restricted replication in mice containing NK cells but replicates normally in mice depleted of NK cells (10). Thus, although murine CMV decreases cell surface levels of MHC class I protein by a variety of mechanisms, a second gene product that mimics MHC class I protein is required to evade NK cells for a productive infection to occur.
In summary, we have presented evidence that PRV infection affects the steady-state concentration of MHC class I proteins on the cell surface in a differential fashion. The concentration of the MHC class I Kk protein increases after infection, while the MHC class I Dk protein decreases after infection. In addition, at least three PRV gene products may be involved in this phenomenon. The biological implications of these findings in animal infections are not known. However, we speculate that the ability to alter the composition of specific MHC class I molecules on the surface of an infected cell might enable the virus to evade both NK- and T-cell-mediated responses after primary infection and reactivation from latency.
ACKNOWLEDGMENTS
We thank Albert Bendelac, Hidde Ploegh, H.-J. Rziha, and Armin Saalmüller for helpful discussions; Albert Bendelac and Hidde Ploegh for antibodies; Andrew Beavis for help with the FACScan; and all members of the Enquist lab for their help and support.
R.L.S.-T. is supported by NIH training grant 5T32GM07388. This work was supported by grant 1R0133506NDS and NATO Collaborative Research Grant CRG 951341 to L.W.E.
REFERENCES
- 1.Baas E J, van Santen H-M, Kleijmeer M J, Geuze H J, Peters P J, Ploegh H L. Peptide-induced stabilization and intracellular localization of empty HLA class I complexes. J Exp Med. 1992;176:147–156. doi: 10.1084/jem.176.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beersma M F C, Bijlmakers M O, Ploegh H L. Human cytomegalovirus down-regulates HLA class I expression by reducing the stability of class I H chains. J Immunol. 1993;151:4455–4464. [PubMed] [Google Scholar]
- 3.Beier D C, Cox J H, Vining D R, Cresswell P, Engelhard V H. Association of human class I MHC alleles with the adenovirus E3/19K protein. J Immunol. 1994;152:3862–3872. [PubMed] [Google Scholar]
- 4.Campbell A E, Slater J S. Down-regulation of major histocompatibility complex class I synthesis by murine cytomegalovirus early gene expression. J Virol. 1994;68:1805–1811. doi: 10.1128/jvi.68.3.1805-1811.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinsakchai S, Molitor T W. Immunobiology of pseudorabies virus infection in swine. Vet Immunol Immunopathol. 1994;43:107–116. doi: 10.1016/0165-2427(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 6.Chinsakchai S, Molitor T W. Replication and immunosuppressive effects of pseudorabies virus on swine peripheral blood mononuclear cells. Vet Immunol Immunopathol. 1992;30:247–260. doi: 10.1016/0165-2427(92)90142-d. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J I. Infection of cells with varicella-zoster virus down-regulates surface expression of class I major histocompatibility complex antigens. J Infect Dis. 1998;155:1390–1393. doi: 10.1086/517821. [DOI] [PubMed] [Google Scholar]
- 8.Dijkstra J M, Mettenleiter T C, Klupp B G. Intracellular processing of pseudorabies virus glycoprotein M (gM): gM of strain Bartha lacks N-glycosylation. Virology. 1997;237:113–122. doi: 10.1006/viro.1997.8766. [DOI] [PubMed] [Google Scholar]
- 9.Enquist L W, Husak P J, Banfield B W, Smith G A. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 10.Farell H E, Vally H, Lynch D M, Fleming P, Shellam G R, Scalzo A A, Davis-Poynter N J. Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature. 1997;386:510–514. doi: 10.1038/386510a0. [DOI] [PubMed] [Google Scholar]
- 11.Goldsmith K, Chen W, Johnson D C, Hendricks R L. Infected cell protein (ICP) 47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J Exp Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hengel H, Koszinowski U H. Interference with antigen processing by viruses. Curr Opin Immunol. 1997;9:470–476. doi: 10.1016/s0952-7915(97)80097-0. [DOI] [PubMed] [Google Scholar]
- 13.Hill A B, Barnett B C, McMichael A J, McGeogh D J. HLA class I molecules are not transported to the cell surface in cells infected with herpes simplex virus types 1 and 2. J Immunol. 1994;152:2736–2741. [PubMed] [Google Scholar]
- 14.Jennings S R, Rice P L, Kloszewski E D, Anderson R W, Thompson D L, Tevethia S S. Effect of herpes simplex virus types 1 and 2 on surface expression of class I major histocompatibility complex antigens on infected cells. J Virol. 1985;56:757–766. doi: 10.1128/jvi.56.3.757-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph J, Knobler R L, Lublin F D, Hart M N. Differential modulation of MHC class I antigen expression on mouse brain endothelial cells by MHV-4 infection. J Neuroimmunol. 1989;22:241–253. doi: 10.1016/0165-5728(89)90022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kärre K. Natural killer cells and the MHC class I pathway of peptide presentation. Semin Immunol. 1993;5:127–145. doi: 10.1006/smim.1993.1016. [DOI] [PubMed] [Google Scholar]
- 17.Kimman T G, Bianchi A T J, de Bruin T G M, Mulder W A M, Priem J, Voermans J M. Interaction of pseudorabies virus with immortalized porcine B cells: influence on surface class I and II major histocompatibility and immunoglobulin M expression. Vet Immunol Immunopathol. 1995;45:253–263. doi: 10.1016/0165-2427(94)05344-r. [DOI] [PubMed] [Google Scholar]
- 18.Kimman T G, Bruin T G M D, Voermans J J M, Bianchi A T J. Cell-mediated immunity to pseudorabies virus: cytolytic effector cells with characteristics of lymphokine-activated killer cells lyse virus-infected glycoprotein gB- and gC-transfected L14 cells. J Gen Virol. 1996;77:978–990. doi: 10.1099/0022-1317-77-5-987. [DOI] [PubMed] [Google Scholar]
- 19.Loken M R, Stall A M. Flow cytometry as an analytical and preparative tool in immunology. J Immunol Methods. 1982;50:R85–R112. doi: 10.1016/0022-1759(82)90161-2. [DOI] [PubMed] [Google Scholar]
- 20.Lomniczi B, Watanabe S, Ben-Porat T, Kaplan A S. Genetic basis of the neurovirulence of pseudorabies virus. J Virol. 1984;52:198–205. doi: 10.1128/jvi.52.1.198-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lomniczi B, Watanabe S, Ben-Porat T, Kaplan A S. Genome location and identification of functions defective in the Bartha vaccine strain of pseudorabies virus. J Virol. 1987;61:796–801. doi: 10.1128/jvi.61.3.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machold R P, Wiertz E J H J, Jones T R, Ploegh H L. The HCMV gene products US11 and US2 differ in their ability to attack allelic forms of murine major histocompatibility complex (MHC) class I heavy chains. J Exp Med. 1997;185:363–366. doi: 10.1084/jem.185.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellencamp M W, O’Brien P C M, Stevenson J R. Pseudorabies virus-induced suppression of major histocompatibility complex class I antigen expression. J Virol. 1991;65:3365–3368. doi: 10.1128/jvi.65.6.3365-3368.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mettenleiter T C. Immunobiology of pseudorabies virus (Aujesky’s disease) Vet Immunol Immunopathol. 1996;54:221–229. doi: 10.1016/s0165-2427(96)05695-4. [DOI] [PubMed] [Google Scholar]
- 25.Mettenleiter T C. Pseudorabies virus (Aujesky’s disease): state of the art. Acta Vet Hung. 1994;42:153–177. [PubMed] [Google Scholar]
- 26.Nataraj C, Eidmann S, Hariharan M J, Sur J H, Perry G A, Srikumaran S. Bovine herpesvirus 1 downregulates the expression of bovine MHC class I molecules. Viral Immunol. 1997;10:21–34. doi: 10.1089/vim.1997.10.21. [DOI] [PubMed] [Google Scholar]
- 27.Onodera T, Yoshihara K, Suzuki T, Tsuda T, Ikeda T, Aawaya A, Kobayashi H, Yukawa M. Resistance to pseudorabies virus with enhanced interferon production and natural killer cell activity in mice treated with serum thymic factor. Microbiol Immunol. 1994;38:47–53. doi: 10.1111/j.1348-0421.1994.tb01743.x. [DOI] [PubMed] [Google Scholar]
- 28.Ozato K, Henkart P, Cornelius J, Sachs D. Spatially distinct allodeterminants of the H-2Kk molecule as detected by monoclonal anti-H-2 antibodies. J Immunol. 1981;126:1780–1785. [PubMed] [Google Scholar]
- 29.Ozato K, Mayer N, Sachs D H. Hybridoma cell lines secreting monoclonal antigens to mouse H-2 and Ia antigens. J Immunol. 1980;124:533–540. [PubMed] [Google Scholar]
- 30.Pereira R, Tscharke D C, Simmons A. Upregulation of class I major histocompatibility complex gene expression in primary sensory neurons, satellite cells and Schwann cells of mice in response to acute but not latent herpes simplex virus infection in vivo. J Exp Med. 1994;180:841–850. doi: 10.1084/jem.180.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Read G S. Control of mRNA stability during herpes simplex virus infections. In: Hartford J B, Morris D R, editors. mRNA metabolism and post-transcriptional gene regulation. New York, N.Y: Wiley-Liss; 1997. pp. 311–321. [Google Scholar]
- 32.Robbins A K, Ryan J P, Whealy M E, Enquist L W. The gene encoding the gIII envelope protein of pseudorabies virus vaccine strain Bartha contains a mutation affecting protein localization. J Virol. 1989;63:250–258. doi: 10.1128/jvi.63.1.250-258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan J P, Whealy M E, Robbins A K, Enquist L W. Analysis of pseudorabies virus glycoprotein gIII localization and modification by using novel infectious viral mutants carrying unique EcoRI sites. J Virol. 1987;61:2962–2972. doi: 10.1128/jvi.61.10.2962-2972.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugawara S, Abo T, Kumagai K. A simple method to eliminate the antigenicity of surface class I MHC molecules from the membrane of viable cells by acid treatment at pH 3. J Immunol Methods. 1987;100:83–90. doi: 10.1016/0022-1759(87)90175-x. [DOI] [PubMed] [Google Scholar]
- 35.Tan L, Andersen M H, Elliot T, Haurum J S. An improved assembly assay for peptide binding to HLA-B*2705 and H-2Kk class I MHC molecules. J Immunol Methods. 1997;209:25–36. doi: 10.1016/s0022-1759(97)00142-7. [DOI] [PubMed] [Google Scholar]
- 36.Tigges M A, Leng S, Johnson D C, Burke R L. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with INF-γ or when virion host shutoff functions are disabled. J Immunol. 1996;156:3901–3910. [PubMed] [Google Scholar]
- 37.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeildler R, Eissner G, Meissner P, Uebel S, Tampe R, Lazis S, Hammerschmidt W. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus-encoded interleukin-10. Blood. 1997;90:2390–2397. [PubMed] [Google Scholar]