Abstract
Introduction
In EVOLVE-MS-1 (NCT02634307), mean absolute lymphocyte count (ALC) on diroximel fumarate (DRF) declined from baseline by approximately 28% in year 1, then stabilized, similar to ALC decline observed with dimethyl fumarate (DMF). Prior studies reported that clinical efficacy of DMF was not substantially different in patients with and without lymphopenia.
Methods
EVOLVE-MS-1—an open-label, 96-week, phase 3 study—assessed DRF safety and exploratory efficacy in patients with relapsing–remitting multiple sclerosis. This study analyzes efficacy-related outcomes comparing (1) patients with lymphopenia (≥ 1 ALC below lower limit of normal [LLN]) and without (all ALCs ≥ LLN); (2) across quartiles stratified by week 96 ALC decline from baseline: Q1 (≥ 47% decline); Q2 (30% to < 47% decline); Q3 (12% to < 30% decline); Q4 (< 12% decline).
Results
Baseline characteristics were similar between patients without (n = 593) and with lymphopenia (n = 452). At week 96, adjusted annualized relapse rate (ARR; 95% confidence interval) was 0.14 (0.11–0.17) without lymphopenia and 0.12 (0.09–0.15) with lymphopenia. Estimated proportions with 12-week confirmed disability progression (CDP12) at week 96 were 10.2% without and 9.3% with lymphopenia. When stratified by quartiles (Q1–Q4), ARR at week 96 was 0.11 (Q1), 0.09 (Q2), 0.13 (Q3), and 0.17 (Q4). Estimated proportions with CDP12 at week 96 were 9.6% (Q1), 10.2% (Q2), 5.7% (Q3), and 10.9% (Q4). At week 96, no evidence of disease activity was achieved by 47.2% (Q1), 47.8% (Q2), 45.4% (Q3), and 37.3% (Q4) of patients.
Conclusion
In DRF-treated patients in EVOLVE-MS-1, clinical and radiological measurements indicated reduced disease activity regardless of lymphopenia or magnitude of ALC decline from baseline; however, patients who had greater ALC declines appeared to have numerically lower ARR and higher proportions free from relapses and gadolinium-enhancing lesions compared with those with smallest decline. This supports prior evidence that, while lymphopenia may contribute to fumarate efficacy outcomes, it is not the primary mechanism of action.
Trial Registration
ClinicalTrials.gov identifier NCT02634307.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-024-00637-2.
Keywords: Absolute lymphocyte count, Diroximel fumarate, Efficacy, Lymphopenia
Key Summary Points
| Why carry out this study? |
| In the 96-week phase 3 EVOLVE-MS-1 study, mean absolute lymphocyte count (ALC) decreased from baseline in patients on diroximel fumarate (DRF) by approximately 28% in year 1, then stabilized, similar to the ALC decline observed in patients treated with dimethyl fumarate (DMF). |
| Prior studies reported that clinical efficacy of DMF was not substantially different in patients with and without lymphopenia. |
| The correlation of change in ALCs to DRF treatment response has not been previously determined; therefore, the aim of this analysis was to determine if lymphopenia or the magnitude of ALC decline from baseline in patients with multiple sclerosis (MS) treated with DRF correlates with DRF efficacy-related related outcomes. |
| What was learned from this study? |
| In DRF-treated patients in EVOLVE-MS-1, clinical and radiological measurements indicated reduced disease activity regardless of lymphopenia or magnitude of ALC decline from baseline. |
| This supports prior evidence that, while lymphopenia may contribute to fumarate efficacy outcomes, it is not the primary mechanism of action. |
Introduction
Diroximel fumarate (DRF) has been developed for the treatment of relapsing forms of multiple sclerosis (MS). It is a next-generation oral fumarate approved in the USA for adults with relapsing forms of MS and in Europe for adult patients with relapsing–remitting MS (RRMS) [1, 2]. Upon oral administration, DRF undergoes rapid esterase cleavage in the gastrointestinal tract to monomethyl fumarate (MMF), the same pharmacologically active metabolite as for dimethyl fumarate (DMF) [3]. DMF has demonstrated clinical efficacy in both clinical trials and in real-world studies [4–8]. At a dose of 462 mg, oral administration of DRF resulted in MMF systemic exposure bioequivalent to 240 mg DMF; therefore, DRF is expected to exhibit comparable efficacy and safety profiles to DMF [9]. As of December 31, 2023, approximately 44,297 patients have been treated with DRF, representing 61,780 patient-years of exposure. Of these, 1661 patients (1785 patient-years) were from clinical trials [10].
In the 96-week phase 3 EVOLVE-MS-1 study (NCT02634307), comprising 1057 adults with RRMS, DRF demonstrated favorable clinical and radiological outcomes consistent with previous fumarate studies [11, 12]. DRF also showed an improved gastrointestinal tolerability profile compared with DMF in the head-to-head phase 3 EVOLVE-MS-2 study, a randomized, blinded study of DRF and DMF over 5 weeks (NCT03093324) [13]. In the phase 3 EVOLVE-MS-1 study, mean absolute lymphocyte counts (ALCs) in patients receiving DRF declined from baseline by approximately 28% within the first year before stabilizing [12]. This result is similar to the ALC decline observed in the DMF phase 3 clinical trials, where mean ALC declined by approximately 30% from baseline, also typically in the first year of treatment, followed by stabilization [4, 5, 14]. While lymphopenia may contribute to the efficacy of DMF, prior studies reported that clinical efficacy of DMF was not substantially different in patients with and without lymphopenia [14, 15].
The correlation of change in ALCs to DRF treatment response has not been previously determined. Hence, using data from the EVOLVE-MS-1 dataset, the objective of the present study was to determine if lymphopenia or the magnitude of ALC decline from baseline in patients with MS treated with DRF correlates with DRF efficacy-related outcomes.
Methods
Study Design and Treatment
EVOLVE-MS-1 (NCT02634307) was an open-label, 96-week, phase 3 study that assessed the safety, tolerability, and efficacy of DRF in adults with RRMS between December 10, 2015 and November 11, 2021. Participants were either newly initiated on DRF (initial dose titration 231 mg twice-daily [BID] for 7 days; treatment maintenance 462 mg BID from day 8 onward) or had rolled over from completing the EVOLVE-MS-2 study (NCT03093324) of DRF and DMF. The study design for EVOLVE-MS-1 has been described in detail previously [11, 12].
Patients
Patients were eligible for EVOLVE-MS-1 if they were aged 18–65 years, had a confirmed diagnosis of RRMS, and were considered neurologically stable, with no evidence of relapse in the 30 days before screening. Use of prior disease-modifying treatments (DMTs), including DRF and DMF, was permitted. Further details of the inclusion and exclusion criteria for EVOLVE-MS-1 and EVOLVE-MS-2 have been reported previously [9, 13].
The study was conducted in accordance with protocol, the International Council for Harmonization Guideline E6, all applicable local regulatory requirements, and ethical principles based on the Declaration of Helsinki. Before enrollment of patients, the study protocol was reviewed and approved by the Copernicus Group institutional review board and the independent review boards/ethics committees for each study site. A list of these committees has been published previously [11]. All patients provided their written and informed consent ahead of participation in the study.
Assessments and Analysis Populations
Lymphopenia efficacy correlation in the overall DRF patient population in EVOLVE-MS-1 was assessed. Final safety and exploratory efficacy outcomes (clinical and radiological) were evaluated in a subset of patients with ALC values recorded at baseline and week 96. ALCs were collected at each visit (every 4 weeks). Patients were divided into two subgroups. In the first subgroup analysis, efficacy-related outcomes (adjusted annualized relapse rate [ARR], Expanded Disability Status Scale [EDSS], and gadolinium-enhancing [Gd+] lesion reductions) at 2 years in patients treated with DRF were compared between patients with lymphopenia (≥ 1 ALC test below the lower limit of normal [LLN] at any time during the study) or without lymphopenia (all ALCs remained at or greater than the LLN, defined as ALC = 0.91 × 109/L). No patients developed severe, prolonged lymphopenia (< 0.5 × 109/L for ≥ 6 months), as any patients who developed severe lymphopenia (ALC < 0.5 × 109/L) for ≥ 4 weeks had DRF withdrawn, according to the EVOLVE-MS-1 protocol-defined stopping rule.
In the second subgroup analysis, patients were stratified into quartiles based on the decline in ALC from baseline to week 96. The four quartiles were Q1 (≥ 47% ALC decline from baseline); Q2 (30% to < 47% ALC decline from baseline); Q3 (12% to < 30% ALC decline from baseline); and Q4 (< 12% ALC decline from baseline). Change from baseline to week 96 in adjusted ARR, EDSS, Gd+ lesion reductions, zero relapses, and no evidence of disease activity-3 [NEDA-3] was reported for these quartiles.
Statistical Analysis
Demographics and baseline characteristics were summarized using descriptive statistics and compared between the subgroups using standardized mean differences (SMDs) and statistical tests (two-sample t test for continuous variables, proportion and/or chi-square test for categorical variables).
Safety assessments were summarized using descriptive statistics [12]. Adjusted ARR was based on a Poisson regression model adjusted for treatment duration. Adjusted ARR included protocol-defined relapses that occurred during the EVOLVE-MS-1 treatment period (up to 2 years of DRF). Rates were compared with the reported relapse rate for the 12 months before DRF initiation in EVOLVE-MS-1.
Confirmed disability progression (CDP) and magnetic resonance imaging (MRI) outcomes were assessed in patients who received ≥ 1 dose of DRF and completed ≥ 1 post-baseline efficacy assessment [12]. The estimated proportion of patients free from relapse and free from CDP at week 96 was based on the Kaplan–Meier product limit method. The estimated proportion of patients with NEDA-3 (NEDA-3: patients have no relapses, no CDP sustained for 12 weeks as measured on EDSS, no new or enlarging T2 hyperintense lesions, and no new Gd+ lesions) at week 96 was based on the Kaplan–Meier product limit method using relapse, MRI, EDSS data, and the number of evaluable patients for NEDA-3. Gd+ lesion counts in the safety population were summarized by change from baseline at week 96. Number of Gd+ lesions in patients with and without lymphopenia was compared using an ordinal logistic regression model adjusted for baseline covariates. Statistical analyses were carried out using SAS 9.4.
Results
Patients
The full analysis population consisted of 1045 patients with RRMS, of whom 593 did not have lymphopenia (all ALCs ≥ LLN) and 452 patients had lymphopenia (≥ 1 ALC < LLN). Baseline characteristics were similar between patients in both subgroups, except for age, sex, race, and time since diagnosis. Those with lymphopenia were older, mean age (SD) of patients without lymphopenia was 41.0 (10.6) years versus 44.4 (10.7) in patients with lymphopenia. Additionally, a higher percentage of patients with lymphopenia were female: 68.1% of patients without lymphopenia were female compared with 77.2% of patients with lymphopenia. Patients with lymphopenia also had a longer time since diagnosis: mean (SD), 7.1 (7.2) years in patients without lymphopenia versus 8.2 (7.4) in patients with lymphopenia (Table 1). Demographics and baseline characteristics stratified into quartiles by percentage ALC decline at week 96 are shown in Table S1 in the supplementary material.
Table 1.
Demographics and baseline characteristics by ALC count (safety population)
| Characteristics | Without lymphopenia (always ALC ≥ LLN) n = 593 |
With lymphopenia (at least one ALC < LLN) n = 452 |
SMD in absolute value | All patients N = 1045 |
|---|---|---|---|---|
| Age, years, mean (SD) | 41.0 (10.6) | 44.4 (10.6) | 0.32* | 42.5 (10.8) |
| Female, n (%) | 404 (68.1) | 349 (77.2) | 0.20* | 753 (72.1) |
| Race, n (%) | ||||
| White | 530 (89.4) | 432 (95.6) | 0.26* | 962 (92.1) |
| Black or African American | 56 (9.4) | 14 (3.1) | 70 (6.7) | |
| Othera | 7 (1.2) | 6 (1.3) | 13 (1.2) | |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 19 (3.2) | 19 (4.2) | 0.05 | 38 (3.6) |
| Not Hispanic or Latino | 574 (96.8) | 433 (95.8) | 1007 (96.4) | |
| BMI, kg/m2, mean (SD) | 26.7 (6.4) | 26.6 (5.8) | 0.02 | 26.6 (6.1) |
| US region, n (%) | 255 (43.0) | 189 (41.8) | 0.02 | 444 (42.5) |
| Prior DMT, n (%) | 373 (62.9) | 299 (66.2) | 0.07 | 672 (64.3) |
| Time since diagnosis, years, mean (SD) | 7.1 (7.2)b | 8.2 (7.4) | 0.15* | 7.6 (7.3)c |
| No. of relapses in previous year, mean (SD) | 0.7 (0.8) | 0.7 (0.8) | < 0.01 | 0.7 (0.8) |
| EDSS score, mean (SD) | 2.7 (1.5) | 2.7 (1.4) | < 0.01 | 2.7 (1.5) |
| No. of Gd+ lesions, mean (SD) | 1.1 (3.0)b | 1.2 (4.1)d | 0.03 | 1.1 (3.5)e |
| Gd+ lesion free, n (%) | 410 (69.1) | 320 (70.8) | 0.04 | 730 (69.9) |
ALC absolute lymphocyte count, BMI body mass index, DMT disease-modifying therapy, EDSS expanded disability status score, Gd+ gadolinium-enhancing, LLN lower limit of normal, SMD standardized mean difference
*p < 0.05
a“Other” race includes Asian, American Indian, Alaska Native, Native Hawaiian, or other Pacific Islander, and multiple races; subjects who reported multiple races including White were included in White subgroup
bn = 592
cn = 1044
dn = 450
en = 1042
Safety
Adverse events (AEs) were reported in 504 (85.0%) patients without lymphopenia and 424 (93.8%) patients with lymphopenia (Table 2). Most AEs were mild to moderate in severity in patients with and without lymphopenia, in line with the overall population (Table 2). Forty-one (6.9%) patients without lymphopenia and 36 (8.0%) patients with lymphopenia had AEs that led to study treatment discontinuation. The most common AEs in both the without lymphopenia and with lymphopenia groups were flushing (without lymphopenia, n = 152, 25.6%; with lymphopenia, n = 131, 29.0%) and MS relapse (without lymphopenia, n = 115, 19.4%; with lymphopenia, n = 90, 19.9%).
Table 2.
Safety summary in the overall population
| Characteristics | Without lymphopenia (always ALC ≥ LLN) n = 593 |
With lymphopenia (≥ 1 ALC < LLN) n = 452 |
All patients N = 1045 |
|---|---|---|---|
| Any AE, n (%) | 504 (85.0) | 424 (93.8) | 928 (88.8) |
| Mild | 169 (28.5) | 134 (29.6) | 303 (29.0) |
| Moderate | 282 (47.6) | 245 (54.2) | 527 (50.4) |
| Severe | 53 (8.9) | 45 (10.0) | 98 (9.4) |
| AEs leading to treatment discontinuation, n (%) | 41 (6.9) | 36 (8.0) | 77 (7.4) |
| Most common AEs leading to treatment discontinuationa, n (%) | |||
| Lymphopenia | 0 (0.0) | 14 (3.1) | 14 (1.3) |
| MS relapse | 8 (1.3) | 2 (0.4) | 10 (1.0) |
| Lymphocyte count decreased | 0 (0.0) | 7 (1.5) | 7 (0.7) |
| Flushing | 5 (0.8) | 0 (0.0) | 5 (0.5) |
| Diarrhea | 3 (0.5) | 0 (0.0) | 3 (0.3) |
| Any SAE, n (%) | 76 (12.8) | 46 10.2) | 122 (11.7) |
| Deathb, n (%) | 2 (0.3) | 2 (0.4) | 4 (0.4) |
| Most common AEsc, n (%) | |||
| Flushing | 152 (25.6) | 131 (29.0) | 283 (27.1) |
| MS relapse | 115 (19.4) | 90 (19.9) | 205 (19.6) |
| Upper respiratory tract infection | 89 (15.0) | 64 (14.2) | 153 (14.6) |
| Nasopharyngitis | 68 (11.5) | 69 (15.3) | 137 (13.1) |
| Lymphopenia | 3 (0.5) | 121 (26.8) | 124 (11.9) |
| Diarrhea | 52 (8.8) | 57 (12.6) | 109 (10.4) |
| Urinary tract infection | 45 (7.6) | 59 (13.1) | 104 (10.0) |
| Lymphocyte count decreased | 0 | 46 (10.2) | 46 (4.4) |
AE adverse event, ALC absolute lymphocyte count, LLN lower limit of normal, MS multiple sclerosis, SAE serious adverse event
aOccurring in ≥ 0.5% of patients in any subgroup
bAccidental fall, bacterial pneumonia, hypertensive heart disease, and cardiac arrest; none of the deaths were considered related to study drug by the investigator
cOccurring in ≥ 10% of patients
Efficacy-Related Outcomes in Patients with and Without Lymphopenia
In patients without lymphopenia, the ARR (95% CI) was 0.73 (0.66–0.80) at baseline and decreased to 0.14 (0.11–0.17) at week 96 (80.7% reduction; p < 0.0001). The ARR (95% CI) for patients with lymphopenia was 0.67 (0.61–0.74) at baseline and decreased to 0.12 (0.09–0.15) at week 96 (82.5% reduction; p < 0.0001). The number of patients relapse free at week 96 was similar between groups: 83.3% without lymphopenia and 83.0% of patients with lymphopenia (Fig. 1b; Table 3).
Fig. 1.
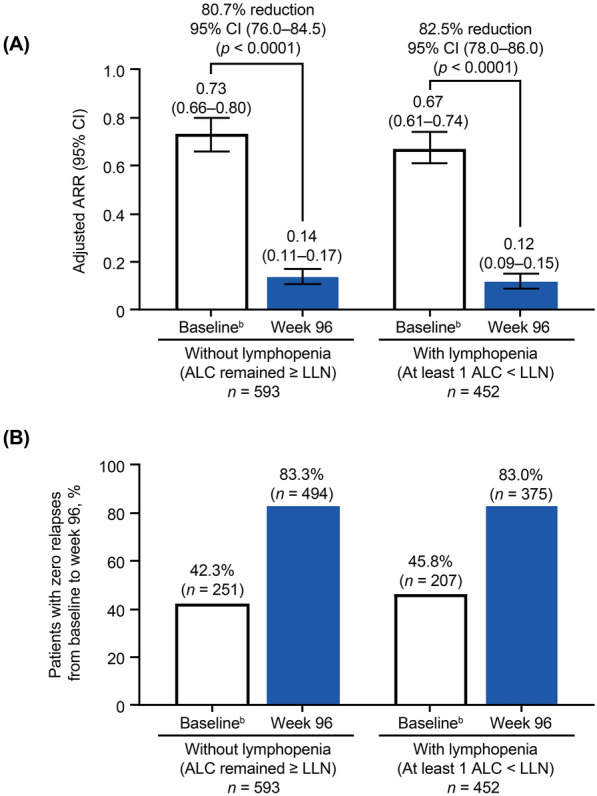
a Adjusted ARRa on treatment compared with baseline and b patients with no relapses from baseline to week 96. “Without lymphopenia” is defined as all ALCs remained ≥ LLN. “With lymphopenia” is defined as ≥ 1 ALC below the LLN at any time during the study.a Calculation of ARR was based on patient-reported relapses in the 12 months before study entry compared with protocol-defined relapses during the study period. Adjusted ARR was based on a Poisson regression model adjusted for treatment duration. bBaseline is the 12 months prior to study entry. ALC absolute lymphocyte count, ARR annualized relapse rate, CI confidence interval, LLN lower limit of normal (ALC = 0.91 × 109/L)
Table 3.
Subgroup analysis 1: number of relapses by ALC count (safety population)
| No. of patients with relapse, n (%) | Without lymphopenia (ALC remained ≥ LLN) | With lymphopenia (at least one ALC < LLN) | ||||
|---|---|---|---|---|---|---|
| Baseline n = 593 |
Week 48 n = 593 |
Week 96 n = 593 |
Baseline n = 452 |
Week 48 n = 452 |
Week 96 n = 452 |
|
| 0 | 251 (42.3) | 524 (88.4) | 494 (83.3) | 207 (45.8) | 398 (88.1) | 375 (83.0) |
| 1 | 259 (43.7) | 60 (10.1) | 76 (12.8) | 190 (42.0) | 49 (10.8) | 56 (12.4) |
| 2 | 73 (12.3) | 8 (1.3) | 16 (2.7) | 49 (10.8) | 5 (1.1) | 18 (4.0) |
| 3 | 6 (1.0) | 0 | 5 (0.8) | 4 (0.9) | 0 | 3 (0.7) |
| ≥ 4 | 4 (0.7) | 1 (0.2) | 2 (0.3) | 2 (0.4) | 0 | 0 |
Baseline: 12 months prior to study entry
ALC absolute lymphocyte count, LLN lower limit of normal
At week 96, the estimated proportions of patients with 12-week CDP (CDP12) per EDSS was similar between groups: 10.2% for patients without lymphopenia and 9.3% for patients with lymphopenia (Fig. 2a). The estimated proportion of patients who achieved NEDA-3 at week 96 was 38.8% for patients without lymphopenia and 43.7% for patients with lymphopenia (Fig. 2b).
Fig. 2.
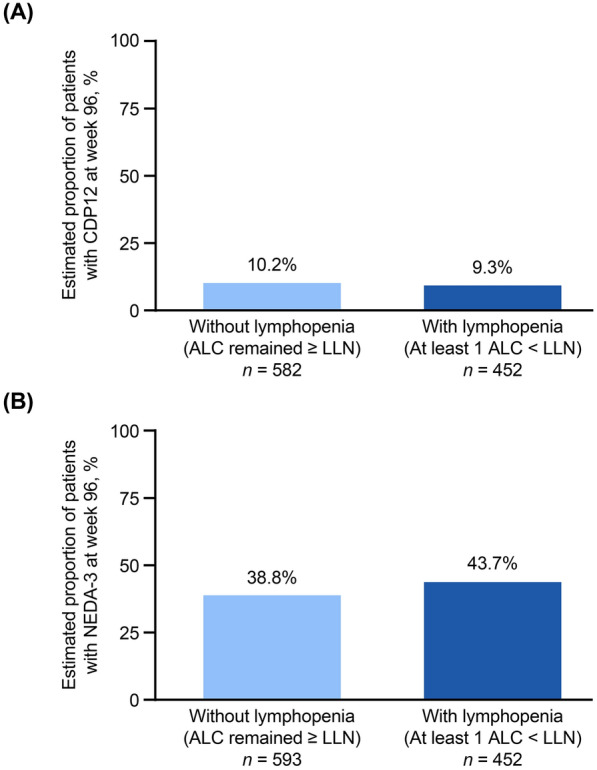
a Estimated proportion of patients with CDP12 and b estimated proportion of patients achieving NEDA-3 at week 96 in patients with and without lymphopenia. “Without lymphopenia” is defined as all ALCs remained ≥ LLN. “With lymphopenia” is defined as ≥ 1 ALC below the LLN at any time during the study. ALC absolute lymphocyte count, CDP12 confirmed disability progression sustained for 12 weeks per EDSS, EDSS expanded disability status score, LLN lower limit of normal (ALC = 0.91 × 109/L), NEDA-3 no evidence of disease activity-3
For patients without and with lymphopenia, mean (SD) number of Gd+ lesions was 0.6 (4.0) and 0.1 (0.8), respectively (Table 4) at week 96 (p < 0.01). In total, 86.8% of patients without lymphopenia and 96.6% of patients with lymphopenia had no lesions at week 96 (Table 4).
Table 4.
Summary of Gd+ lesion count at week 96 and stratified into quartiles by % ALC decline at week 96
| Without lymphopenia (always ALC ≥ LLN) n = 582a |
With lymphopenia (≥ 1 ALC < LLN) n = 452b |
Q1 (≥ 47% decline) n = 198 |
Q2 (30 to < 47% decline) n = 207 |
Q3 (12 to < 30% decline) n = 194 |
Q4 (< 12% decline) n = 202 |
All participants N = 1034c |
|
|---|---|---|---|---|---|---|---|
| No. of Gd+ lesions at week 96, mean (SD) | 0.6 (4.0) | 0.1 (0.8) | 0.1 (0.5) | 0.2 (1.6) | 0.6 (5.5) | 0.5 (2.0) | 0.3 (3.0) |
| Change from baseline at week 96, mean (SD) | − 0.4 (3.1) | − 1.1 (4.4) | − 0.9 (2.3) | − 0.7 (2.3) | − 0.6 (5.8) | − 0.7 (3.9) | − 0.7 (3.8) |
| No. of Gd+ lesions at week 96, n (%)d | |||||||
| 0 | 374/431 (86.8) | 370/383 (96.6) | 189/194 (97.4) | 195/203 (96.1) | 169/191 (88.5) | 171/201 (85.1) | 744/814 (91.4) |
| 1–4 lesions | 47/431 (10.9) | 12/383 (3.1) | 4/194 (2.1) | 7/203 (3.4) | 19/191 (9.9) | 24/201 (11.9) | 59/814 (7.2) |
| 5–8 lesions | 6/431 (1.4) | 0/383 | 1/194 (0.5) | 0/203 | 2/191 (1.0) | 3/201 (1.5) | 6/814 (0.7) |
| ≥ 9 lesions | 4/431 (0.9) | 1/383 (0.3) | 0/194 | 1/203 (0.5) | 1/191 (0.5) | 3/201 (1.5) | 5/814 (0.6) |
ALC absolute lymphocyte count, Gd+ gadolinium-enhancing, MRI magnetic resonance imaging, Q quartile
aOf 582 patients, 431 had baseline and week 96 Gd+ measurements
bOf 452 patients, 383 had baseline and week 96 Gd+ measurements
cOf 1034 patients, 814 had baseline and week 96 Gd+ measurements
dDenominator number of patients with baseline and week 96 Gd+ measurements
Outcomes Across Quartiles Stratified by % ALC Decline from Baseline at Week 96
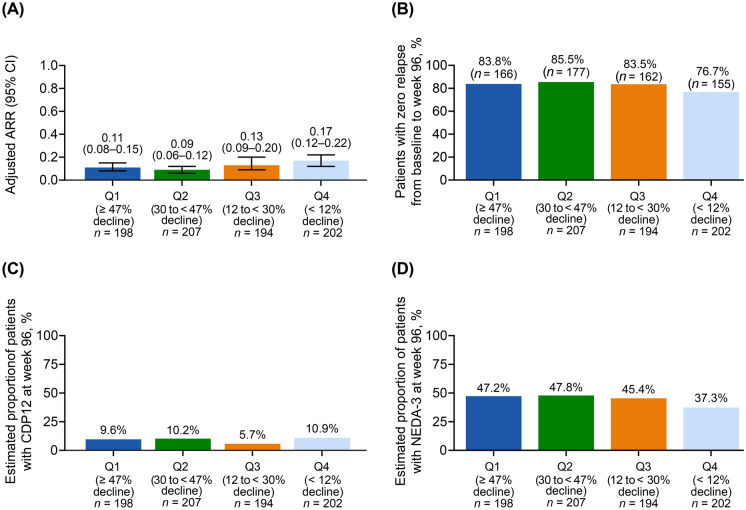
Baseline characteristics and patient demographics were similar across quartiles (see Table S1 in the electronic supplementary material for details). When stratified by quartiles, the mean adjusted ARR at week 96 was 0.11 (Q1), 0.09 (Q2), 0.13 (Q3), and 0.17 (Q4) (Fig. 3a), and the proportions of patients with no relapses from baseline to week 96 were 83.8% (Q1), 85.5% (Q2), 83.5% (Q3), and 76.7% (Q4) (Fig. 3b; Table S2 in the electronic supplementary material). Mean (SD) change in number of Gd+ lesions from baseline to week 96 was consistent across quartiles: − 0.9 (2.3) in Q1, − 0.7 (2.3) in Q2, − 0.6 (5.8) in Q3, and − 0.7 (3.9) in Q4 (Table 4). These findings are consistent with a decrease of 0.7 (3.8) in the overall population.
Fig. 3.
a Adjusted ARRa on treatment, b patients with no relapses from baseline to week 96, c estimated proportion of patients with CDP12 at week 96, and d estimated proportion of patients with NEDA-3 at week 96, stratified by % ALC decline from baseline to week 96b. aCalculation of ARR was based on patient-reported relapses in the 12 months before study entry compared with protocol-defined relapses during the study period. Adjusted ARR was based on a Poisson regression model adjusted for treatment duration. bPatients were stratified into quartiles based on the decline in ALC from baseline to week 96. The four quartiles used were Q1 (≥ 47% ALC decline from baseline); Q2 (30% to < 47% ALC decline from baseline); Q3 (12% to < 30% ALC decline from baseline); Q4 (< 12% ALC decline from baseline). ALC absolute lymphocyte count, ARR annualized relapse rate, CDP12 confirmed disability progression sustained for 12 weeks per EDSS, CI confidence interval, EDSS expanded disability status score, NEDA-3 no evidence of disease activity-3, Q quartile
The estimated proportions of patients with 12-week CDP at week 96 were 9.6% (Q1), 10.2% (Q2), 5.7% (Q3), and 10.9% (Q4) (Fig. 3c). At week 96, the estimated proportions of patients achieving NEDA-3 were 47.2% (Q1), 47.8% (Q2), 45.4% (Q3), and 37.3% (Q4) of patients (Fig. 3d).
Discussion
In this analysis of patients from the EVOLVE-MS-1 study dataset, clinical and radiological measurements indicated reduced disease activity regardless of the presence of lymphopenia. The reduction in ARR at 2 years versus baseline was not substantially different between patients with or without lymphopenia, and the reduction in ARR (80.7% in patients without lymphopenia and 82.5% in patients with lymphopenia) is consistent with what is reported for the overall EVOLVE-MS-1 population (81.6% reduction) [12]. These findings are aligned with the literature for DMF, where clinical efficacy-related outcomes were not reported to be substantially different in patients with and without lymphopenia [14, 15]; ARR at 2 years did not differ substantially between DMF patients with lymphopenia and those without lymphopenia [14]. The mean number of Gd+ lesions was statistically lower in DRF-treated patients with lymphopenia than those without lymphopenia and there were numerical differences in the proportion of patients achieving NEDA-3 between DRF-treated patients with and without lymphopenia, but evidence of clinical benefit was observed in both subgroups.
When stratified by percentage change in ALC from baseline to week 96, clinical benefit was observed across all quartiles, regardless of the magnitude of ALC decline from baseline, consistent with DMF [14]. However, compared with the group with the smallest ALC decline from baseline, the quartiles with greater ALC declines appeared to have a numerically lower ARR and a higher proportion of patients free from relapses and Gd+ lesions.
The lack of difference in DRF efficacy-related outcomes in patients with lymphopenia versus without lymphopenia could be confounded by age. Aging is known to be associated with qualitative and quantitative changes to the innate and adaptive immune systems [16], which in turn impacts disease course, with older patients with MS showing a less inflammatory phenotype than younger patients due to immunosenescence [17]. As a result of these differences in the immune system, response to DMTs may vary in older populations with MS [18]. A real-world study of long-term safety and effectiveness of DMF has demonstrated a favorable risk–benefit profile of DMF in patients ≥ 55 years of age [19]. In this analysis of DRF-treated EVOLVE-MS-1 patients, we observed that patients with lymphopenia were older at baseline (mean age 44 years in patients with lymphopenia versus 41 years in patients without lymphopenia) and had a longer duration of disease (mean time since diagnosis 8 years in patients with lymphopenia versus 7 years in patients without lymphopenia). Despite these modest differences in age at baseline, clinical and radiological indicators of disease activity were similarly reduced in patients on DRF with and without lymphopenia.
The underlying mechanism of DRF-induced lymphopenia is not well understood, and the results of our study demonstrating comparable therapeutic benefit in patients with and without lymphopenia suggest that lymphopenia is not a primary mechanism of action of DRF. Very rare cases of progressive multifocal leukoencephalopathy (PML), an opportunistic viral infection of the brain, have been reported in patients treated with DMF in the setting of lymphopenia, with an incidence of 0.83 per 100,000 person-years of exposure. Currently regular ALC monitoring for all patients, irrespective of age or time on therapy, is recommended [20]. To date, no cases of PML have been seen in patients treated with DRF, though regular ALC monitoring is recommended by the US prescribing information, and DRF interruption should be considered for patients with ALC less than the LLN (< 0.5 × 109/L) persisting for more than 6 months [2, 21].
This study had limitations. EVOLVE-MS-1, an open-label, single-arm study, lacked blinding and a comparator arm, limiting the interpretation of some results. In addition, some patients had prior treatment with fumarates in the EVOLVE-MS-2 study, whereas some patients were naïve to treatment. Relapse data obtained 12 months prior to the study for comparison with on-study relapse outcomes were historical and therefore not protocol-defined. Furthermore, relapse assessment during the study was more stringent than for those reported before the study, which could potentially make the true effect of ARR smaller than estimates of ARR per protocol. In addition, although inclusion criteria did not require a minimum number of reported relapses, regression to the mean could have influenced outcomes.
Conclusions
In patients who received DRF in the EVOLVE-MS-1 phase 3 clinical trial, clinical and radiological measurements indicated reduced disease activity regardless of the presence of lymphopenia or the magnitude of ALC decline from baseline; however, patients who had greater declines in ALC appeared to at least have a numerically lower ARR and higher proportion of patients free from relapses and Gd+ lesions compared with those with the smallest decline in ALC. This finding supports prior evidence that, while lymphopenia may contribute to fumarate efficacy outcomes, it has not been shown to be the primary mechanism by which fumarates exert their therapeutic effects in MS.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of this study.
Medical Writing and Editorial Assistance
Biogen provided funding for medical writing and editorial support in the development of this manuscript; Annabel Campbell, PhD (Excel Medical Affairs), wrote the first draft of the manuscript based on input from authors, and Cara Farrell (Excel Medical Affairs) copyedited and styled the manuscript per journal requirements. Oksana Mokliatchouk (Biogen, Cambridge, MA) and Phoebe Jiang (Biogen, Cambridge, MA) provided additional statistical analyses.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Barry Singer: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Sibyl Wray: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Mark Gudesblatt: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Barbara Bumstead: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Tjalf Ziemssen: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Ashley Bonnell: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Matthew Scaramozza: Concept and design of study, data interpretation, drafting and critically revising the manuscript. Seth Levin: Concept and design of study, data interpretation, drafting and critically revising the manuscript. Mathura Shanmugasundaram: Concept and design of study, data interpretation, drafting and critically revising the manuscript. Hailu Chen: Concept and design of study, data collection, statistical analyses, data interpretation, drafting and critically revising the manuscript. Jason P. Mendoza: Concept and design of study, data interpretation, drafting and critically revising the manuscript. James B. Lewin: Concept and design of study, data interpretation, drafting and critically revising the manuscript. Sai L. Shankar: Concept and design of study, data interpretation, drafting and critically revising the manuscript.
Funding
This study was sponsored by Biogen (Cambridge, MA, USA). Biogen also provided funding for the journal’s Rapid Service Fee.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Barry A Singer: research grant support from AbbVie, Biogen, Bristol Myers Squibb, Greenwich Biosciences, Novartis, and Sanofi; and consulting and/or speaking fees from AbbVie, Alexion, Biogen, Bristol Myers Squibb, Cigna, Cionic, EMD Serono, Genentech, Greenwich Biosciences, Horizon, Janssen, Novartis, Octave Bioscience, Roche, Sanofi, and TG Therapeutics. Sibyl Wray: consulting fees from and advisory boards for Biogen, Celgene, and EMD Serono; speaker bureaus for Biogen, Celgene, EMD Serono, Roche-Genentech, and Sanofi-Genzyme; research support from Biogen, Celgene, EMD Serono, Novartis, Receptos, Roche-Genentech, Sanofi-Genzyme, and TG Therapeutics. Mark Gudesblatt: speaker and consulting for Acorda, Amgen, Biogen, EMD Serono, Medtronic, Novartis, Sanofi-Genzyme, Saol Therapeutics, and Teva Pharmaceuticals. Barbara Bumstead: speaker and consulting for Biogen, BMS, and Genzyme. Tjalf Ziemssen: personal compensation for consulting services and speaker honoraria from Biogen, BMS, Novartis, Roche, Sanofi, and Teva; and financial support for research activities from Biogen, Novartis, Roche, and Sanofi. Ashley Bonnell: speaker for EMD Serono; research support from Biogen, Celgene, EMD Serono, Novartis, Receptos, Roche-Genentech, Sanofi-Genzyme, and TG Therapeutics. Matthew Scaramozza, Seth Levin, Jason P Mendoza, Jim B Lewin, Sai L Shankar: employees of and hold stock/stock options in Biogen. Mathura Shanmugasundaram: was an employee of and may have held stock in Biogen at time of analysis. Currently an employee of Takeda. Hailu Chen: was an employee of and may have held stock in Biogen at time of analysis. Currently an employee of and holds stock/stock options in Alkermes.
Ethical Approval
The study was conducted in accordance with protocol, the International Council for Harmonization Guideline E6, all applicable local regulatory requirements, and ethical principles based on the Declaration of Helsinki. Before enrollment of patients, the study protocol was reviewed and approved by the Copernicus Group institutional review board and the independent ethics committees for each study site. A list of these committees has been published previously [11]. All patients provided their written and informed consent ahead of participation in the study.
Footnotes
Prior Presentation: The data were presented in abstract and poster form at the joint European Committee for Treatment and Research in Multiple Sclerosis and Americas Committee for Treatment and Research in Multiple Sclerosis 2023 Meeting (11–13 October); Milan, Italy. Mult Scler. 2023;29(3S):340–341.
References
- 1.Vumerity. Summary of product characteristics. Netherlands B.V: Biogen; 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/vumerity-epar-product-information_en.pdf. Accessed 04 Oct 2023.
- 2.Vumerity. Prescribing information. Cambridge, MA: Biogen; 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/211855s009lbl.pdf. Accessed Jan 16, 2024.
- 3.Tecfidera. Prescribing information. Cambridge, MA: Biogen; 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/204063s029lbl.pdf. Accessed May 25, 2023.
- 4.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–107. 10.1056/NEJMoa1114287 [DOI] [PubMed] [Google Scholar]
- 5.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–97. 10.1056/NEJMoa1206328 [DOI] [PubMed] [Google Scholar]
- 6.Cohan SL, Moses H, Calkwood J, et al. Clinical outcomes in patients with relapsing-remitting multiple sclerosis who switch from natalizumab to delayed-release dimethyl fumarate: a multicenter retrospective observational study (STRATEGY). Mult Scler Relat Disord. 2018;22:27–34. 10.1016/j.msard.2018.02.028 [DOI] [PubMed] [Google Scholar]
- 7.Kresa-Reahl K, Repovic P, Robertson D, et al. Effectiveness of delayed-release dimethyl fumarate on clinical and patient-reported outcomes in patients with relapsing multiple sclerosis switching from glatiramer acetate: RESPOND, a prospective observational study. Clin Ther. 2018;40(12):2077–87. 10.1016/j.clinthera.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 8.Gold R, Arnold DL, Bar-Or A, et al. Long-term safety and efficacy of dimethyl fumarate for up to 13 years in patients with relapsing-remitting multiple sclerosis: final ENDORSE study results. Mult Scler. 2022;28(5):801–16. 10.1177/13524585211037909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naismith RT, Wolinsky JS, Wundes A, et al. Diroximel fumarate (DRF) in patients with relapsing-remitting multiple sclerosis: interim safety and efficacy results from the phase 3 EVOLVE-MS-1 study. Mult Scler. 2020;26(13):1729–39. 10.1177/1352458519881761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biogen. Data on file. 2023.
- 11.Wray S, Then Bergh F, Wundes A, et al. Efficacy and safety outcomes with diroximel fumarate after switching from prior therapies or continuing on DRF: results from the phase 3 EVOLVE-MS-1 study. Adv Ther. 2022;39(4):1810–31. 10.1007/s12325-022-02068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer BA, Arnold DL, Drulovic J, et al. Diroximel fumarate in patients with relapsing-remitting multiple sclerosis: final safety and efficacy results from the phase 3 EVOLVE-MS-1 study. Mult Scler. 2023;29(14):1795–807. 10.1177/13524585231205708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naismith RT, Wundes A, Ziemssen T, et al. Diroximel fumarate demonstrates an improved gastrointestinal tolerability profile compared with dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: results from the randomized, double-blind, phase III EVOLVE-MS-2 study. CNS Drugs. 2020;34(2):185–96. 10.1007/s40263-020-00700-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longbrake EE, Mao-Draayer Y, Cascione M, et al. Dimethyl fumarate treatment shifts the immune environment toward an anti-inflammatory cell profile while maintaining protective humoral immunity. Mult Scler. 2021;27(6):883–94. 10.1177/1352458520937282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox RJ, Chan A, Gold R, et al. Characterizing absolute lymphocyte count profiles in dimethyl fumarate-treated patients with MS: patient management considerations. Neurol Clin Pract. 2016;6(3):220–9. 10.1212/CPJ.0000000000000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh SJ, Lee JK, Shin OS. Aging and the immune system: the impact of immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. 2019;19(6):e37. 10.4110/in.2019.19.e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giovannoni G, Popescu V, Wuerfel J, et al. Smouldering multiple sclerosis: the “real MS.” Ther Adv Neurol Disord. 2022;15:17562864211066752. 10.1177/17562864211066751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577. 10.3389/fneur.2017.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao-Draayer Y, Giles K, Balashov K, et al. Safety and effectiveness of delayed-release dimethyl fumarate in patients ≥ 55 years enrolled in the phase IV ESTEEM study. CMSC. Seattle, WA; 2019.
- 20.Lyons J, Hughes R, McCarthy K, et al. Progressive multifocal leukoencephalopathy outcomes in patients with multiple sclerosis treated with dimethyl fumarate. Mult Scler J Exp Transl Clin. 2022;8(4):20552173221132468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer S, Proschmann U, Akgun K, Ziemssen T. Lymphocyte counts and multiple sclerosis therapeutics: between mechanisms of action and treatment-limiting side effects. Cells. 2021;10(11):3177. 10.3390/cells10113177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.