Abstract
The CHA2DS2 -VASc score is a vital clinical tool for evaluating thromboembolic risk in patients with atrial fibrillation (AF). This study investigated the efficacy of the CHA2DS2 -VASc score in a cohort of 737 heterogeneous patients (mean age: 63 years) receiving care in cardiac intensive care units (CICUs), with a creatinine-based estimated glomerular filtration rate (eGFR) of ≥60 mL/min/1.73 m2 upon admission and discharge. Incident chronic kidney disease (CKD) was defined as the emergence of a new-onset eGFR<60 mL/min/1.73 m2, accompanied by a decline of >5 mL/min/1.73 m2 compared to that at discharge. The primary endpoint was the incidence of CKD, and the secondary endpoints included all-cause mortality, cardiovascular events, and progression to end-stage kidney disease. In this cohort, 210 (28 %) patients developed CKD. Multivariate analyses revealed that CHA2DS2 -VASc score was a significant independent predictor of incident CKD, regardless of the presence of AF. Integration of CHA2DS2 -VASc scores with eGFR enhanced the predictive accuracy of incident CKD, as evidenced by the improved C-index, net reclassification improvement, and integrated discrimination improvement values (all p < 0.05). Over the 12-month follow-up period, a composite endpoint was observed in 61 patients (8.3 %), with elevated CHA2DS2 -VASc scores being independently associated with this endpoint. In conclusion, CHA2DS2-VASc scores have emerged as robust predictors of both CKD incidence and adverse outcomes. Their inclusion substantially refined the 12-month risk stratification of patients with preserved renal function hospitalized in the CICUs.
Keywords: CHA2DS2-VASc scores, Cardiac intensive care units, Chronic kidney injury, Cardiovascular disease
Highlights
-
•
Twenty-eight percent of patients developed chronic kidney disease (CKD) within 1 year from discharge.
-
•
The CHA2DS2-VASc score was a significant independent predictor of progression to CKD, irrespective of the atrial fibrillation status.
-
•
The addition of CHA2DS2-VASc scores to estimated glomerular filtration rate improved its predictive value for incident CKD.
-
•
Patients with higher CHA2DS2-VASc scores had higher risks of all-cause mortality and cardiovascular events.
1. Introduction
Patients with chronic kidney disease (CKD) face a substantially increased risk of cardiovascular disease (CVD) mortality, which is approximately double that of patients without CKD [1]. Notably, patients with CKD also demonstrate pronounced susceptibility to cardiovascular events, with approximately 50 % of the patients at CKD stages 4/5 experiencing CVD [2,3]. The interplay between CVD and CKD is multifaceted, with shared risk factors suggesting a bidirectional relationship, wherein CVD promotes CKD initiation and exacerbates its progression [4]. The rate of kidney function decline varies widely among patients with CVD, necessitating the early identification of individuals at a heightened risk of incident CKD to enable timely interventions and avert complications.
The CHA2DS2-VASc score, encompassing congestive heart failure, hypertension, age (≥75 years), diabetes, prior stroke, vascular disease, age (65–74 years), and sex, serves as a widely utilized clinical prediction tool for assessing the risk of thromboembolic events in patients with atrial fibrillation (AF) [5]. Previous studies have reported a correlation between increasing CHA2DS2 -VASc scores and an elevated incidence of CKD and end-stage kidney disease (ESKD) in patients with AF [6,7]. Notably, Beyer–Westendorf et al. demonstrated that higher CHA2DS2 -VASc scores are associated with accelerated CKD progression in patients with AF and CKD [8]. Given the close association between CHA2DS2 -VASc score components and renal dysfunction, we hypothesized that this score would accurately predict subsequent CKD in patients with CVD. Furthermore, we hypothesized that the CHA2DS2 -VASc score could significantly predict cardiovascular and all-cause mortality in patients with CKD [9,10].
However, the efficacy of CHA2DS2 -VASc score has not been fully investigated in patients treated in cardiac intensive care units (CICUs). Consequently, this study aimed to evaluate the predictive value of CHA2DS2 -VASc scores for incident CKD and assess their prognostic implications for patients admitted to the CICUs.
2. Materials and methods
This study was conducted at the Department of Cardiology, Fujita Health University School of Medicine (Toyoake, Japan). Approval for the study protocol (protocol number: HM19-264) was obtained from the Ethics Committee of Fujita Health University in accordance with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants.
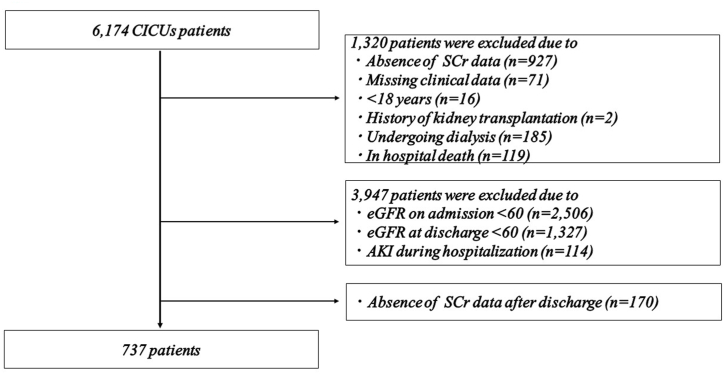
A patient flow diagram detailing the selection process for inclusion in this study is shown in Fig. 1. Among the 6174 patients admitted to the CICUs between December 2009 and December 2018, 1320 were excluded for various reasons: absence of serum creatinine (SCr) data on days 0, 2, and 7 following CICU admission (927 patients), missing clinical data (71 patients), age<18 years (16 patients), history of kidney transplantation (two patients), undergoing dialysis (185 patients), and in-hospital death (119 patients). Additionally, 3974 patients were further excluded for meeting any of the following criteria: SCr-based estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 either upon admission or discharge (3833 patients), development of acute kidney injury (AKI) during hospitalization (114 patients), and absence of creatinine data post-discharge (170 patients). Consequently, a total of 737 patients without AKI who had preserved renal function (eGFR≥60 mL/min/1.73 m2 on both admission and discharge) were included in the final analysis. Physicians independently determined the appropriate therapy and managed patients according to established protocols, utilizing outcome measurements such as symptom improvement, physical examination findings, laboratory data, pulmonary congestion on chest radiography, and echocardiographic findings. Clinical characteristics were extracted from the patients’ medical records upon enrollment.
Fig. 1.
Flow diagram of the study. CICUs, cardiac intensive care unit; SCr, serum creatinine; eGFR, estimated glomerular filtration rate; AKI, acute kidney injury.
In this study, incident CKD was defined as the emergence of a new-onset eGFR<60 mL/min/1.73 m2 with a decline of >5 mL/min/1.73 m2 per year compared to that at discharge, as per established criteria for rapid progression. This criteria was followed to avoid “noise” introduced by natural eGFR fluctuations based on the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [11]. The eGFR was calculated using the Modification of Diet in Renal Disease Study (MDRD) equation recommended by the Japan Chronic Kidney Disease Initiative [12]. AKI was diagnosed according to KDIGO criteria, identifying an increase in SCr by ≥ 0.3 mg/dL within 48 h or an increase in SCr to ≥1.5 times the baseline within 1 week [13].
For each patient, the CHA2DS2 -VASc score was calculated at admission, utilizing the following scoring system: 1 point for congestive heart failure (CHF, recent decompensated HF, objective evidence of moderate to severe left ventricular dysfunction, or hypertrophic cardiomyopathy), hypertension (HT, on antihypertensive therapy), 65–74 year age-group, diabetes mellitus (DM, treatment with oral hypoglycaemic drugs and/or insulin or fasting blood glucose>125 mg/dL), vascular disease (angiographically significant coronary artery disease, previous myocardial infarction [MI], peripheral artery disease, or aortic plaque), and female sex, while 2 points were assigned for age ≥75 years and prior stroke or transient ischemia attack [14,15]. The same criteria for CHA2DS2 -VASc score were used for all patients, irrespective of the presence of AF. Two-dimensional echocardiography was routinely performed to calculate ejection fraction (LVEF) using the modified Simpson's method.
All patients underwent clinical follow-up 12 months after discharge. The primary endpoint was the incidence of CKD. Independently assessed by the researchers, the secondary endpoint was a composite of all-cause mortality and cardiovascular events (admission for acute decompensated HF, acute coronary syndrome [ACS], stroke [ischemic or hemorrhagic], or acute arterial occlusive disease) along with progression to ESKD. Patients who were clinically diagnosed with HF or met the Framingham criteria for HF were considered as having experienced an HF event. Readmissions for ACS presentations included ST-elevation Ml, non ST-elevation MI, and unstable angina (UA). The diagnosis of MI is associated with high-sensitivity cardiac troponin release and is based on the fourth universal definition of MI [16]. UA is defined as myocardial ischemia at rest or with minimal exertion in the absence of acute cardiomyocyte injury or necrosis. Endpoint data were retrieved from the hospital charts.
Statistical analyses were conducted using the JMP version Pro 15 software (SAS Institute Inc., Cary, NC, USA). Categorical variables are presented as numbers and frequencies, while continuous variables are expressed as mean ± standard deviation or median with interquartile ranges, depending on their distribution. Because the serum NT-proBNP and urinary microalbumin data exhibited irregular distributions, log transformation was performed to meet the criteria for normalization following statistical confirmation. Clinical characteristics were compared using chi-square analysis for categorical variables and the Mann–Whitney U test or Student's t-test for continuous variables. Odds ratios and 95 % confidence intervals (CIs) were calculated using logistic analysis. All baseline variables (p < 0.05) identified in univariate analyses were entered into the model to determine the independent predictors of CKD. Similarly, Cox multivariate analysis integrated all baseline variables (p < 0.05) from the univariate analyses to determine the independent predictors of the composite endpoint. Hazard ratios and 95 % CIs were determined using the Cox proportional hazards analysis. Kaplan–Meier curves were constructed and compared using the log-rank test to assess the differences in event-free survival among the groups. To assess the enhanced predictive accuracy of integrating the CHA2DS2 -VASc score with the e-GFR, the C-index, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were calculated. The C-index, defined as the area under the ROC curve (AUC), compares the individual predictive probabilities for endpoints with the incidence of endpoints against the baseline model [17]. The NRI and IDI provide relative indicators of improved predicted probability and average enhancement, respectively, after incorporating the variables into the baseline model [18]. Statistical significance was set at p < 0.05.
3. Results
A total of 737 patients (517 males; mean age, 63 years) were enrolled in this study (Fig. 1). The baseline patient characteristics are summarized in Table 1. In the cohort, 462 patients (63 %) were admitted for ACS, with 158 patients presenting with ST-segment elevation MI, 239 with non-ST-segment elevation MI, and 65 with UA. Additionally, 125 patients (17 %) were admitted for acute decompensated HF, categorized into 72 patients with reduced ejection fraction (LVEF<40 %), 20 with mid-range ejection fraction (LVEF 41–49 %), and 33 with preserved ejection fraction (LVEF≥50 %). Other reasons for admission were arrhythmia (35 patients, 5 %), pulmonary embolism (24 patients, 3 %), acute aortic syndrome (24 patients, 3 %), infective endocarditis (15 patients, 2 %), and other miscellaneous causes (52 patients, 7 %).
Table 1.
Baseline characteristics of study population according to CKD.
| All | CKD (−) | CKD (+) | p-value | |
|---|---|---|---|---|
| Number | 737 | 527 | 210 | |
| Male | 517 (70) | 378 (72) | 139 (66) | 0.14 |
| Age (years) | 63.0 ± 14.4 | 60.5 ± 14.7 | 69.4 ± 11.2 | <0.001 |
| Hypertension | 388 (53) | 268 (51) | 120 (57) | 0.12 |
| Diabetes mellitus | 220 (30) | 144 (27) | 76 (36) | 0.02 |
| Hyperlipidemia | 322 (44) | 231 (44) | 91 (43) | 0.90 |
| Hyperuricemia | 94 (13) | 65 (12) | 29 (14) | 0.59 |
| Previous cerebral infarction or transit ischemic attack | 63 (9) | 33 (6) | 30 (14) | 0.001 |
| Previous myocardial infarction | 90 (12) | 55 (10) | 35 (17) | 0.02 |
| ACS | 462 (63) | 345 (65) | 117 (56) | 0.01 |
| Acute decompensated heart failure | 125 (17) | 74 (14) | 51 (24) | 0.001 |
| AF | 92 (12) | 56 (11) | 36 (17) | 0.02 |
| Systolic blood pressure (mmHg) | 138 ± 27 | 138 ± 26 | 137 ± 28 | 0.56 |
| Heart rate (bpm) | 87 ± 26 | 87 ± 26 | 88 ± 28 | 0.49 |
| Hemoglobin (g/dL) | 13.2 ± 2.0 | 13.4 ± 2.0 | 12.8 ± 2.0 | <0.001 |
| eGFR on admission (mL/min/1.73 m2) | 87.8 ± 20.0 | 91.7 ± 20.5 | 77.8 ± 14.6 | <0.001 |
| eGFR at discharge (mL/min/1.73 m2) | 78.5 ± 17.2 | 81.7 ± 18.5 | 70.4 ± 9.4 | <0.001 |
| NT-proBNP (pg/mL) | 449 (120–1549) | 390 (97–1407) | 640 (184–2206) | 0.008 |
| High-sensitivity Troponin I (ng/mL) | 0.21 (0.02–3.60) | 0.23 (0.02–3.93) | 0.18 (0.03–3.28) | 0.98 |
| Urinary microalbumin (mg/gCr) | 22.6 (9.2–67.7) | 19.7 (8.3–57.8) | 30.8 (13.4–99.0) | 0.80 |
| Ventilation at enrollment | 62 (8) | 40 (8) | 22 (10) | 0.21 |
| IABP or PCPS at enrollment | 44 (6) | 31 (6) | 13 (6) | 0.87 |
| CAG or PCI before admission | 317 (43) | 244 (46) | 73 (35) | <0.01 |
| Left ventricular ejection fraction (%) | 50 ± 12 | 50 ± 11 | 49 ± 13 | 0.25 |
| CHA2DS2-VASc Score | 2 (1–4) | 2 (1–3) | 3 (2–4) | <0.001 |
| Treatment at discharge | ||||
| ARBs or ACE inhibitors | 424 (58) | 311 (59) | 113 (54) | 0.20 |
| Beta-blockers | 401 (54) | 286 (54) | 115 (55) | 0.90 |
| MRA | 117 (16) | 62 (12) | 55 (26) | <0.001 |
| SGLT-2 inhibitors | 117 (16) | 62 (12) | 55 (26) | <0.001 |
| Composite endpoint | 61 (8) | 29 (6) | 32 (15) | <0.001 |
Data are presented as number (%), mean ± standard deviation, or median (interquartile range).
CKD, chronic kidney disease; ACS, acute coronary syndrome; AF, atrial fibrillation; bpm, beats per minute; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; IABP, intra-aortic balloon pumping; PCPS, percutaneous cardio pulmonary support; CAG, coronary angiography; PCI, percutaneous coronary intervention; ARB, angiotensin receptor blocker; ACE, angiotensin converting enzyme; MRA, mineralocorticoid receptor antagonist; SGLT-2, sodium-glucose cotransporter-2.
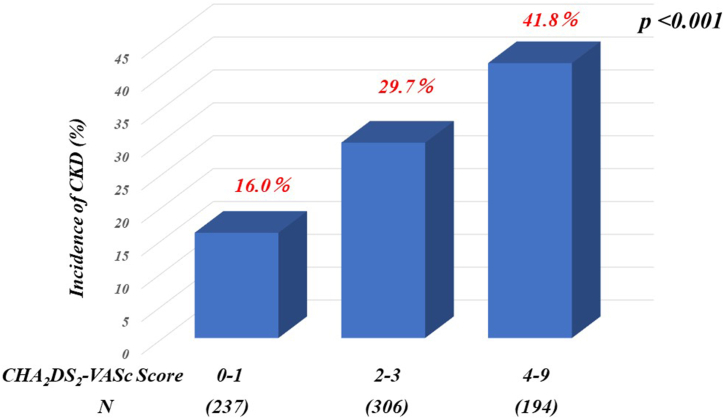
During the 12-month follow-up period after admission, 210 patients (28 %) developed CKD. The characteristics of patients who developed CKD included older age, lower hemoglobin levels, eGFR at admission and discharge, and higher NT-proBNP and CHA2DS2 -VASc scores compared to those who did not develop CKD (Table 1). Notably, many patients who developed CKD had a history of cerebral infarction or transient ischemic attack, MI, DM, acute decompensated HF, AF, or treatment with a mineralocorticoid receptor antagonist (MRA) at discharge. Additionally, patients who developed CKD were less likely to have undergone ACS, coronary angiography, or percutaneous coronary intervention (PCI) before admission than those who did not develop CKD. Patients with incident CKD exhibited a higher risk of composite end points than those without incident CKD (15 % vs. 6 %, p < 0.001). The incidence rate of CKD increased with higher CHA2DS2 -VASc scores (Fig. 2). Multivariate logistic analyses revealed that CHA2DS2 -VASc scores, eGFR on admission, and hemoglobin level were significant independent predictors of incident CKD, regardless of the presence or absence of AF (Table 2). Additionally, we assessed the impact of incorporating the CHA2DS2 -VASc score into the eGFR on admission. As shown in Table 3, integrating CHA2DS2 -VASc score enhanced the prediction of incident CKD beyond that of eGFR alone (p < 0.05), as indicated by a significant increase in the C-index. Furthermore, the inclusion of CHA2DS2 -VASc scores significantly improved patient reclassification and IDI beyond that of eGFR alone (both p < 0.001). In patients without AF, integrating the CHA2DS2 -VASc scores significantly improved the reclassification of patients and the IDI beyond that of eGFR alone (both p < 0.01). Conversely, the C-index did not improve beyond that of eGFR alone.
Fig. 2.
Incidence of CKD according to the CHA2DS2 -VASc score. CKD, chronic kidney disease.
Table 2.
Multivariate logistic analyses of predictors of CKD.
| All |
Without AF |
|||
|---|---|---|---|---|
| Variables | OR (95 % CI) | p-value | OR (95 % CI) | p-value |
| ACS | 0.82 (0.50–1.34) | 0.42 | ||
| Acute decompensated heart failure | 0.71 (0.38–1.35) | 0.30 | ||
| AF | 1.34 (0.80–2.25) | 0.26 | ||
| Hemoglobin (per 1 g/dL increment) | 0.88 (0.80–0.97) | 0.01 | 0.84 (0.76–0.93) | 0.001 |
| eGFR on admission (per 1 mL/min/1.73 m2increment) | 0.95 (0.94–0.96) | <0.001 | 0.95 (0.93–0.96) | <0.001 |
| NT-proBNP (per 10-fold increment) | 1.09 (0.80–1.50) | 0.59 | 1.04 (0.77–1.40) | 0.80 |
| Urinary microalbumin (per 10-fold increment) | 1.31 (0.99–1.72) | 0.05 | 1.39 (1.04–1.86) | 0.02 |
| CAG or PCI before admission | 0.75 (0.50–1.12) | 0.16 | 0.75 (0.50–1.13) | 0.17 |
| CHA2DS2-VASc Score (per 1 point increment) | 1.25 (1.11–1.40) | <0.001 | 1.17 (1.04–1.33) | 0.01 |
The multivariable model was adjusted for all baseline variables with p < 0.05 in univariate analyses.
AF, atrial fibrillation; OR, odds ratio; CI, confidence interval; ACS, acute coronary syndrome; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; CAG, coronary angiography; PCI, percutaneous coronary intervention.
Table 3.
Discrimination and reclassification of CHA2DS2-VASc Score.
| All | ||||||
|---|---|---|---|---|---|---|
|
C-index |
p-value |
NRI |
p-value |
IDI |
p-value |
|
| eGFR on admission | 0.729 | Reference | Reference | Reference | ||
| eGFR on admission + CHA2DS2-VASc Score | 0.752 | 0.04 | 0.506 (0.351–0.661) | <0.001 | 0.037 (0.021–0.054) | <0.001 |
| Without AF | ||||||
|
eGFR on admission |
0.744 |
Reference |
Reference |
Reference |
||
| eGFR on admission + CHA2DS2-VASc Score | 0.760 | 0.12 | 0.487 (0.318–0.656) | <0.001 | 0.028 (0.012–0.043) | 0.001 |
NRI, net reclassification improvement; IDI, integrated discrimination improvement; eGFR, estimated glomerular filtration rate; AF, atrial fibrillation.
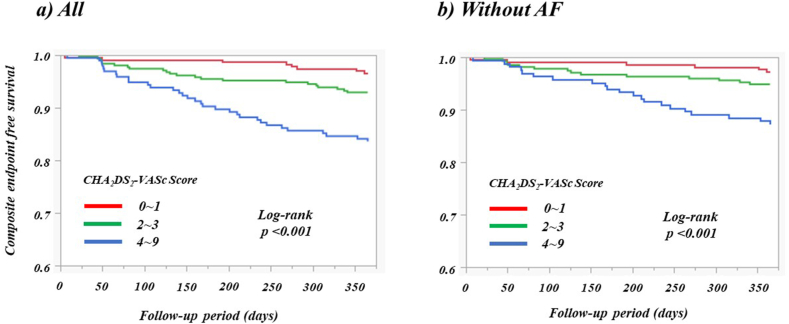
During the 12-month follow-up period, the composite endpoint occurred in 61 patients (8.3 %), with one patient progressing to ESKD (Table 4). Among these cases, all-cause mortality was observed in 11 patients, including five attributed to cardiovascular events. Cardiovascular incidents were primarily driven by acute decompensated HF in 20 patients, ACS in 17, stroke in 11, and acute arterial occlusive disease in one. According to the Cox multivariate analysis, which included all baseline variables (p < 0.05) identified in the univariate analysis, the CHA2DS2 -VASc score emerged as a significant independent predictor of the composite endpoint regardless of the presence or absence of AF (Table 5). Stratification of patients into three groups according to CHA2DS2 -VASc scores revealed a graded escalation in the risk of the composite endpoint, as depicted by the Kaplan–Meier curves, both in the overall patient cohort and in those without AF (Fig. 3; both p < 0.001).
Table 4.
Baseline characteristics of study population according to composite endpoint.
| Composite endpoint (−) | Composite endpoint (+) | p-value | |
|---|---|---|---|
| Number | 676 | 61 | |
| Male | 477 (71) | 40 (66) | 0.42 |
| Age (years) | 62.7 ± 14.3 | 67.4 ± 14.6 | 0.01 |
| Hypertension | 352 (52) | 36 (59) | 0.30 |
| Diabetes mellitus | 197 (29) | 23 (38) | 0.17 |
| Hyperlipidemia | 295 (44) | 27 (44) | 0.93 |
| Hyperuricemia | 64 (12) | 30 (13) | 0.71 |
| Previous cerebral infarction or transit ischemic attack | 48 (7) | 15 (25) | <0.001 |
| Previous myocardial infarction | 73 (11) | 17 (28) | 0.001 |
| ACS | 428 (63) | 34 (56) | 0.25 |
| Acute decompensated heart failure | 103 (15) | 22 (36) | <0.001 |
| AF | 71 (11) | 21 (34) | <0.001 |
| Systolic blood pressure (mmHg) | 138 ± 27 | 133 ± 26 | 0.12 |
| Heart rate (bpm) | 87 ± 26 | 91 ± 29 | 0.24 |
| Hemoglobin (g/dL) | 13.3 ± 2.0 | 12.9 ± 2.1 | 0.21 |
| eGFR on admission (mL/min/1.73 m2) | 87.8 ± 20.2 | 87.4 ± 17.7 | 0.88 |
| eGFR at discharge (mL/min/1.73 m2) | 78.7 ± 17.5 | 76.9 ± 13.1 | 0.43 |
| NT-proBNP (pg/mL) | 414 (118–1408) | 1172 (172–2828) | <0.01 |
| High-sensitivity Troponin I (ng/mL) | 0.20 (0.02–3.68) | 0.37 (0.02–3.12) | 0.98 |
| Urinary microalbumin (mg/gCr) | 21.1 (8.9–64.0) | 41.1 (16.2–112.4) | 0.87 |
| Ventilation at enrollment | 51 (8) | 11 (18) | 0.01 |
| IABP or PCPS at enrollment | 39 (6) | 5 (8) | 0.46 |
| CAG or PCI before admission | 292 (43) | 25 (41) | 0.74 |
| Left ventricular ejection fraction (%) | 50 ± 11 | 46 ± 15 | <0.01 |
| CHA2DS2-VASc Score | 2 (1–3) | 4 (2–5) | <0.001 |
| Treatment at discharge | |||
| ARBs or ACE inhibitors | 390 (58) | 34 (56) | 0.77 |
| Beta-blockers | 361 (53) | 40 (66) | 0.06 |
| MRA | 95 (14) | 22 (36) | <0.001 |
| SGLT-2 inhibitors | 95 (14) | 22 (36) | <0.001 |
| Developed CKD | 178 (26) | 32 (52) | <0.001 |
Data are presented as number (%), mean ± standard deviation, or median (interquartile range).
ACS, acute coronary syndrome; AF, atrial fibrillation; bpm, beats per minute; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; IABP, intra-aortic balloon pumping; PCPS, percutaneous cardio pulmonary support; CAG, coronary angiography; PCI, percutaneous coronary intervention; ARB, angiotensin receptor blocker; ACE, angiotensin converting enzyme; MRA, mineralocorticoid receptor antagonist; SGLT-2, sodium-glucose cotransporter-2; CKD, chronic kidney disease.
Table 5.
Multivariate predictors of composite endpoint.
| All |
Without AF |
|||
|---|---|---|---|---|
| Variables | RR (95 % CI) | p-value | RR (95 % CI) | p-value |
| Hyperuricemia | 2.33 (1.24–4.37) | 0.01 | 3.16 (1.52–6.59) | <0.01 |
| Acute decompensated heart failure | 0.83 (0.39–1.77) | 0.63 | 0.54 (0.20–1.45) | 0.22 |
| AF | 3.55 (2.01–6.26) | <0.001 | ||
| NT-proBNP (per 10-fold increment) | 0.95 (0.59–1.52) | 0.82 | 0.89 (0.48–1.61) | 0.69 |
| Urinary microalbumin (per 10-fold increment) | 1.08 (0.72–1.62) | 0.71 | 1.09 (0.67–1.73) | 0.73 |
| Ventilation at enrollment | 1.42 (0.68–2.97) | 0.35 | 2.18 (0.92–5.16) | 0.08 |
| Left ventricular ejection fraction (per 1 % increment) | 0.98 (0.96–1.01) | 0.15 | 0.97 (0.94–1.00) | 0.03 |
| CHA2DS2-VASc Score (per 1 point increment) | 1.44 (1.25–1.67) | <0.001 | 1.48 (1.23–1.79) | <0.001 |
The multivariable model was adjusted for all baseline variables with p < 0.05 in univariate analyses.
AF, atrial fibrillation; RR, relative ratio; CI, confidence interval; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Fig. 3.
Kaplan–Meier curve for composite endpoint. AF, atrial fibrillation.
4. Discussion
In this study, we delved into the relationship between CHA2DS2 -VASc scores and the onset of CKD as well as prognostic implications in patients admitted to CICUs with an eGFR≥60 mL/min/1.73 m2 upon both admission and discharge. Our findings yielded several key insights on this topic. First, 28 % of patients experienced CKD onset within 1 year of discharge. Second, regardless of the presence or absence of AF, the CHA2DS2 -VASc score emerged as a significant independent predictor of CKD progression. Third, integrating the CHA2DS2 -VASc scores with eGFR improved the predictive accuracy for incident CKD, as demonstrated by improvements in the C-index, NRI, and IDI. Forth, patients with elevated CHA2DS2 -VASc scores exhibited heightened risks of all-cause mortality and cardiovascular events, regardless of the AF status. Consequently, our findings suggest that CHA2DS2 -VASc scores have the potential to improve the prediction of CKD progression and may serve as a valuable prognostic tool for individuals hospitalized for CVD with preserved renal function.
The CHA2DS2 -VASc scores have been investigated to predict stroke in patients with AF; however, their relationship with renal impairment in patients with CVD remains unknown. Wang et al. showed that the incidence of CKD increased with rising CHA2DS2 -VASc scores in 8764 patients with AF who did not have CKD [6]. A previous study demonstrated an association between higher CHA2DS2 -VASc scores and faster CKD progression in patients with AF and CKD [8]. CHA2DS2 -VASc scores were independently associated with the risk of contrast-induced AKI in patients with ACS undergoing PCI [[19], [20], [21], [22]]. In our study of patients hospitalized in CICUs with preserved renal function, we observed significant associations between progression to CKD and CHA2DS2 -VASc scores.
Beyond its established role in stroke prediction, the CHA2DS2-VASc score has proven valuable in predicting cardiovascular outcomes across several disease conditions, regardless of AF status [[23], [24], [25]]. Notably, in patients with ACS, the CHA2DS2 -VASc score has emerged as a robust predictor of all-cause mortality and cardiovascular events [[26], [27], [28], [29]]. Furthermore, it has been linked to increased mortality rates and has demonstrated a prognostic accuracy comparable to that of the GRACE scores in patients with coronary artery disease [30] and systolic HF [31]. Consistent with previous research, our current investigation revealed associations between CHA2DS2 -VASc score and adverse clinical outcomes among patients hospitalized in CICUs with preserved renal function [[26], [27], [28], [29], [30], [31]].
The CHA2DS2 -VASc score's ability to predict CKD progression can be attributed to its constituent components, many of which overlap with well-established risk factors for CKD. CHF [32,33], HT [34,35], advanced age [36,37], DM [38], and female sex [39] are widely recognized predisposing factors for CKD. Additionally, prior stroke [40] and vascular diseases [41,42] have been associated with CKD development. The influence of these risk factors on CKD progression is well-documented [[43], [44], [45]], with acceleration observed when multiple factors coexist in an individual [[46], [47], [48]]. Consequently, the association between the individual components of the CHA2DS2 -VASc score and renal function may increase the risk of incident CKD with increasing CHA2DS2 -VASc score. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers induce efferent arteriolar vasodilation, which leads to reduced intraglomerular pressure and renal protection [34]. This renal benefit also applies to patients with HT. Sodium-glucose cotransporter 2 (SGLT2) inhibitors may mediate natriuresis and glucose-induced osmotic diuresis independent of their blood glucose-lowering effects, leading to a reduction in intraglomerular pressure [49,50]. This favorable hemodynamic effect may preserve renal function in patients with or without DM. Therefore, aggressive therapeutic interventions with these drugs may be recommended for patients with high CHA2DS2 -VASc score who have HT and DM.
Our results indicated that CHA2DS2 -VASc score was a significant independent predictor of incident CKD in patients hospitalized in the CICUs. Its straightforward measurement and ready accessibility render the CHA2DS2 -VASc score a practical tool for risk assessment in daily clinical practice. Given that the signs and symptoms of CKD may not be significant until the disease has progressed, utilizing the CHA2DS2 -VASc score can prompt clinicians to be vigilant regarding the potential for declining renal function in patients with high scores. Recent research has highlighted the potential of SGLT2 inhibitors in reducing the risk of kidney disease progression in patients with CKD [51,52] and type 2 diabetes [53]. Moreover, several renal-protective medications such as mineralocorticoid receptor antagonists [54], renin-angiotensin-aldosterone system inhibitors [55], and angiotensin receptor-neprilysin inhibitors [56] have been recommended to mitigate CKD progression in patients with CVD. Considering our results, patients with high CHA2DS2 -VASc scores may benefit from a more aggressive treatment using these drugs, even when renal function appears to be preserved. Furthermore, integrating CHA2DS2 -VASc scores with eGFR improved the predictive accuracy for incident CKD, suggesting that the combined assessment of CHA2DS2 -VASc scores and eGFR may be clinically beneficial.
MDRD equation, utilizing SCr, is commonly employed to estimate eGFR in the Japanese population. However, eGFR calculated using the MDRD equation is affected by muscle mass, protein consumption, physical activity, and ethnicity [57] and has limitations in the assessment of early loss of kidney function [58], which may have influenced our results. Further research is necessary to investigate the utility of the MDRD equation using serum cystatin C.
AKI is a known risk factor for CKD. Of the 114 patients with AKI, 87 (76 %) developed CKD within 1 year. Because we focused on patients with preserved renal function and minimal renal damage, patients with AKI were excluded from this study, which may have introduced a selection bias. Age, a crucial component of the CHA2DS2-VASc score, directly influenced the patient grouping. In the univariate analyses, age was a significant predictor of CKD incidence and composite endpoints. Age was excluded from the multivariate model, as it was found to have a strong correlation with CHA2DS2-VASc scores (r = 0.62, p < 0.001).
Our study had certain limitations that warrant consideration. First, the study design was retrospective, which may have introduced bias. Retrospective analyses were conducted at a single center, potentially limiting the generalizability of our findings. Second, the definition of CKD relies solely on a decrease in eGFR, which may not fully encompass all aspects of renal insult, particularly changes in urine protein levels induced by medical therapy. This approach may overlook certain renal pathologies that are influenced by variations in urine protein levels. Third, the retrospective design and nonrandomized nature of the treatments in our study complicate the evaluation of their effects on the primary endpoint. Thus, we did not evaluate the drug treatment using multivariate analysis. Patients who developed CKD underwent MRA more frequently than those who did not. Therefore, differences in medications may have potentially confounded our results. However, upon including medications into our multivariate analyses, CHA2DS2 -VASc scores remained independent and significant predictors of incident CKD. Consequently, we believe that medication did not significantly influence our results. Finally, we used a GFR estimation formula for Japanese patients to calculate renal function. Given that the CKD-EPI formula, considered the international standard, tends to overestimate renal function in Japanese patients, the Japanese Society of Nephrology recommends a GFR estimation formula using SCr levels in Japanese patients in daily clinical practice [12]. Therefore, the generalizability of our findings to non-Japanese populations remains unclear.
In conclusion, CHA2DS2 -VASc score is a potent predictor of incident CKD and adverse outcomes. Its incorporation into clinical practice could substantially improve the 12-month risk stratification of patients with preserved renal function hospitalized in the CICUs.
Ethical approval statement
This study was approved by the Institutional Ethics Review Committee of the Fujita Health University, Japan (approval number: HM19-264).
Data availability
The data pertaining to this study have not been deposited in a publicly accessible repository and will be available upon reasonable request from the corresponding author.
Funding
This research did not receive any specific funding.
CRediT authorship contribution statement
Eirin Sakaguchi: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis, Data curation, Conceptualization. Hiroyuki Naruse: Writing – review & editing, Resources, Methodology, Data curation, Conceptualization. Yuya Ishihara: Writing – review & editing, Investigation. Hidekazu Hattori: Writing – review & editing, Investigation. Akira Yamada: Writing – review & editing, Investigation. Hideki Kawai: Writing – review & editing, Investigation. Takashi Muramatsu: Writing – review & editing, Investigation. Fumihiko Kitagawa: Writing – review & editing, Investigation. Hiroshi Takahashi: Formal analysis. Junnichi Ishii: Writing – review & editing, Investigation. Masayoshi Sarai: Writing – review & editing, Investigation. Masanobu Yanase: Writing – review & editing, Supervision. Yukio Ozaki: Writing – review & editing, Supervision. Kuniaki Saito: Writing – review & editing, Supervision. Hideo Izawa: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful for the support of the staff of the Department of Cardiology at Fujita Health University, Japan.
References
- 1.Sarnak M.J., Amann K., Bangalore S., Cavalcante J.L., Charytan D.M., Craig J.C., Gill J.S., Hlatky M.A., Jardine A.G., Landmesser U., Newby L.K., Herzog C.A., Cheung M., Wheeler D.C., Winkelmayer W.C., Marwick T.H. Conference participants. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019;74:1823–1838. doi: 10.1016/j.jacc.2019.08.1017. [DOI] [PubMed] [Google Scholar]
- 2.Stevens P.E., O'Donoghue D.J., de Lusignan S., Van Vlymen J., Klebe B., Middleton R., Hague N., New J., Farmer C.K. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007;72:92–99. doi: 10.1038/sj.ki.5002273. [DOI] [PubMed] [Google Scholar]
- 3.Morales J., Handelsman Y. Cardiovascular outcomes in patients with diabetes and kidney disease: JACC review topic of the week. J. Am. Coll. Cardiol. 2023;82:161–170. doi: 10.1016/j.jacc.2023.04.052. [DOI] [PubMed] [Google Scholar]
- 4.Taal M.W., Brenner B.M. Predicting initiation and progression of chronic kidney disease: developing renal risk scores. Kidney Int. 2006;70:1694–1705. doi: 10.1038/sj.ki.5001794. [DOI] [PubMed] [Google Scholar]
- 5.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 6.Wang C.J., Hsieh Y.P., Kor C.T., Chiu P.F. The CHA2DS2-VASc score predicts chronic kidney disease among patients with atrial fibrillation. Int. Urol. Nephrol. 2020;52:1523–1531. doi: 10.1007/s11255-020-02514-x. [DOI] [PubMed] [Google Scholar]
- 7.Huang P.S., Cheng J.F., Chen J.J., Wu C.K., Wang Y.C., Hwang J.J., Tsai C.T. CHA2DS2VASc score predicts risk of end stage renal disease in patients with atrial fibrillation: long-term follow-up study. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyer-Westendorf J., Kreutz R., Posch F., Ay C. The CHA2DS2-VASc score strongly correlates with glomerular filtration rate and predicts renal function decline over time in elderly patients with atrial fibrillation and chronic kidney disease. Int. J. Cardiol. 2018;253:71–77. doi: 10.1016/j.ijcard.2017.10.110. [DOI] [PubMed] [Google Scholar]
- 9.Hsu P.C., Lee W.H., Chen S.C., Tsai Y.C., Chen Y.C., Chu C.Y., Lin T.H., Voon W.C., Lai W.T., Sheu S.H., Su H.M. Using CHADS2 and CHA2DS2-VASc scores for mortality prediction in patients with chronic kidney disease. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-76098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goudis C., Daios S., Korantzopoulos P., Liu T. Does CHA2DS2-VASc score predict mortality in chronic kidney disease? Intern Emerg Med. 2021;16:1737–1742. doi: 10.1007/s11739-021-02799-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens P.E., Levin A. Kidney disease: improving global outcomes chronic kidney disease guideline development work group members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K., Yamagata K., Tomino Y., Yokoyama H., Hishida A. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Section 2: AKI definition. Kidney Int. Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Camm A.J., Kirchhof P., Lip G.Y., Schotten U., Savelieva I., Ernst S., Van Gelder I.C., Al-Attar N., Hindricks G., Prendergast B., Heidbuchel H., Alfieri O., Angelini A., Atar D., Colonna P., De Caterina R., De Sutter J., Goette A., Gorenek B., Heldal M., Hohloser S.H., Kolh P., Le Heuzey J.Y., Ponikowski P., Rutten F.H. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European society of Cardiology (ESC) Eur. Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 15.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.A., Dilaveris P.E., Fauchier L., Filippatos G., Kalman J.M., La Meir M., Lane D.A., Lebeau J.P., Lettino M., Lip G.Y.H., Pinto F.J., Thomas G.N., Valgimigli M., Van Gelder I.C., Van Putte B.P., Watkins C.L., ESC Scientific Document Group 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D., ESC Scientific Document Group Fourth universal definition of myocardial infarction (2018) Eur. Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 17.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 18.Pencina M.J., D'Agostino RB Sr, D'Agostino R.B., Jr., Vasan R.S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 19.Kurtul A., Yarlioglues M., Duran M. Predictive value of CHA2DS2-VASC score for contrast-induced nephropathy after percutaneous coronary intervention for acute coronary syndrome. Am. J. Cardiol. 2017;119:819–825. doi: 10.1016/j.amjcard.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Li J., Wang Z., Zhang B., Zheng D., Lu Y., Li W. Predictive value of combining the level of fibrinogen and CHA2DS2-VASC Score for contrast-induced acute kidney injury in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Int. Urol. Nephrol. 2022;54:2385–2392. doi: 10.1007/s11255-022-03149-w. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y., Qiu H., Wang Z., Shen G., Li W. Predictive value of systemic immune-inflammatory index combined with CHA2DS2-VASC score for contrast-induced acute kidney injury in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Int. Urol. Nephrol. 2023;55:2897–2903. doi: 10.1007/s11255-023-03571-8. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhary A.K., Pathak V., Kunal S., Shukla S., Pathak P. CHA2DS2-VASc score as a novel predictor for contrast-induced nephropathy after percutaneous coronary intervention in acute coronary syndrome. Indian Heart J. 2019;71:303–308. doi: 10.1016/j.ihj.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melgaard L., Gorst-Rasmussen A., Lane D.A., Rasmussen L.H., Larsen T.B., Lip G.Y. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA. 2015;314:1030–1038. doi: 10.1001/jama.2015.10725. [DOI] [PubMed] [Google Scholar]
- 24.Cetin M., Cakici M., Zencir C., Tasolar H., Baysal E., Balli M., Akturk E. Prediction of coronary artery disease severity using CHADS2 and CHA2DS2-VASc scores and a newly defined CHA2DS2-VASc-HS score. Am. J. Cardiol. 2014;113:950–956. doi: 10.1016/j.amjcard.2013.11.056. [DOI] [PubMed] [Google Scholar]
- 25.Paoletti Perini A., Bartolini S., Pieragnoli P., Ricciardi G., Perrotta L., Valleggi A., Vergaro G., Michelotti F., Boggian G., Sassone B., Mascioli G., Emdin M., Padeletti L. CHADS2 and CHA2DS2-VASc scores to predict morbidity and mortality in heart failure patients candidates to cardiac resynchronization therapy. Europace. 2014;16:71–80. doi: 10.1093/europace/eut190. [DOI] [PubMed] [Google Scholar]
- 26.Kim K.H., Kim W., Hwang S.H., Kang W.Y., Cho S.C., Kim W., Jeong M.H., Other Korean Working Group in Myocardial Infarction Registry Investigators The CHA2DS2VASc score can be used to stratify the prognosis of acute myocardial infarction patients irrespective of presence of atrial fibrillation. J. Cardiol. 2015;65:121–127. doi: 10.1016/j.jjcc.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Castini D.C., Persampieri S., Sabatelli L., Valli F., Ferrante G., Zambelli D., Toriello F., Provenzale G., Gentile D., Bursi F., Centola M., Carugo S. Incremental value of renal dysfunction addition to the CHA2DS2-Vasc score for mortality prediction in patients with acute coronary syndrome. Cardiology. 2021;146:538–546. doi: 10.1159/000515986. [DOI] [PubMed] [Google Scholar]
- 28.Scudiero F., Zocchi C., De Vito E., Tarantini G., Marcucci R., Valenti R., Migliorini A., Antoniucci D., Marchionni N., Parodi G. Relationship between CHA2DS2-VASc score, coronary artery disease severity, residual platelet reactivity and long-term clinical outcomes in patients with acute coronary syndrome. Int. J. Cardiol. 2018;262:9–13. doi: 10.1016/j.ijcard.2018.03.086. [DOI] [PubMed] [Google Scholar]
- 29.Borovac J.A., Kwok C.S., Mohamed M.O., Fischman D.L., Savage M., Alraies C., Kalra A., Nolan J., Zaman A., Ahmed J., Bagur R., Mamas M.A. The predictive value of CHA2DS2-VASc score on in-hospital death and adverse periprocedural events among patients with the acute coronary syndrome and atrial fibrillation who undergo percutaneous coronary intervention: a 10-year national inpatient sample (NIS) analysis. Cardiovasc Revasc Med. 2021;29:61–68. doi: 10.1016/j.carrev.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Huang F.Y., Huang B.T., Pu X.B., Yang Y., Chen S.J., Xia T.L., Gui Y.Y., Peng Y., Liu R.S., Ou Y., Chen F., Zhu Y., Chen M. CHADS2, CHA2DS2-VASc and R2CHADS2 scores predict mortality in patients with coronary artery disease. Intern Emerg Med. 2017;12:479–486. doi: 10.1007/s11739-017-1608-x. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y.L., Cheng C.L., Huang J.L., Yang N.I., Chang H.C., Chang K.C., Sung S.H., Shyu K.G., Wang C.C., Yin W.H., Lin J.L., Chen S.M., TSOC-HFrEF Registry investigators and committee Mortality prediction using CHADS2/CHA2DS2-VASc/R2CHADS2 scores in systolic heart failure patients with or without atrial fibrillation. Medicine (Baltim.) 2017;96 doi: 10.1097/MD.0000000000008338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kottgen A., Russell S.D., Loehr L.R., Crainiceanu C.M., Rosamond W.D., Chang P.P., Chambless L.E., Coresh J. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J. Am. Soc. Nephrol. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 33.Bagshaw S.M., Cruz D.N., Aspromonte N., Daliento L., Ronco F., Sheinfeld G., Anker S.D., Anand I., Bellomo R., Berl T., Bobek I., Davenport A., Haapio M., Hillege H., House A., Katz N., Maisel A., Mankad S., McCullough P., Mebazaa A., Palazzuoli A., Ponikowski P., Shaw A., Soni S., Vescovo G., Zamperetti N., Zanco P., Ronco C., Acute Dialysis Quality Initiative Consensus Group Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol. Dial. Transplant. 2010;25:1406–1416. doi: 10.1093/ndt/gfq066. [DOI] [PubMed] [Google Scholar]
- 34.Ku E., Lee B.J., Wei J., Weir M.R. Hypertension in CKD: core curriculum 2019. Am. J. Kidney Dis. 2019;74:120–131. doi: 10.1053/j.ajkd.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 35.Hanratty R., Chonchol M., Havranek E.P., Powers J.D., Dickinson L.M., Ho P.M., Magid D.J., Steiner J.F. Relationship between blood pressure and incident chronic kidney disease in hypertensive patients. Clin. J. Am. Soc. Nephrol. 2011;6:2605–2611. doi: 10.2215/CJN.02240311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey A.S., Inker L.A., Coresh J. Chronic kidney disease in older people. JAMA. 2015;314:557–558. doi: 10.1001/jama.2015.6753. [DOI] [PubMed] [Google Scholar]
- 37.Taal M.W. Chronic kidney disease in older people - diagnosis, aetiology and consequences. Curr. Opin. Nephrol. Hypertens. 2015;24:475–479. doi: 10.1097/MNH.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 38.Drawz P., Rahman M. Chronic kidney disease. Ann. Intern. Med. 2015;162:ITC1–16. doi: 10.7326/AITC201506020. [DOI] [PubMed] [Google Scholar]
- 39.Yu M.K., Katon W., Young B.A. Associations between sex and incident chronic kidney disease in a prospective diabetic cohort. Nephrology. 2015;20:451–458. doi: 10.1111/nep.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee M., Saver J.L., Chang K.H., Liao H.W., Chang S.C., Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ. 2010;341 doi: 10.1136/bmj.c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wattanakit K., Folsom A.R., Selvin E., Coresh J., Hirsch A.T., Weatherley B.D. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J. Am. Soc. Nephrol. 2007;18:629–636. doi: 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- 42.O'Hare A.M., Glidden D.V., Fox C.S., Hsu C.Y. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999-2000. Circulation. 2004;109:320–323. doi: 10.1161/01.CIR.0000114519.75433.DD. [DOI] [PubMed] [Google Scholar]
- 43.Sepanlou S.G., Barahimi H., Najafi I., Kamangar F., Poustchi H., Shakeri R., Hakemi M.S., Pourshams A., Khoshnia M., Gharravi A., Broumand B., Nobakht-Haghighi A., Kalantar-Zadeh K., Malekzadeh R. Prevalence and determinants of chronic kidney disease in northeast of Iran: results of the Golestan cohort study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazancioğlu R. Risk factors for chronic kidney disease: an update. Kidney Int. Suppl. 2013;3:368–371. doi: 10.1038/kisup.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levin A. Identification of patients and risk factors in chronic kidney disease--evaluating risk factors and therapeutic strategies. Nephrol. Dial. Transplant. 2001;16(Suppl 7):57–60. doi: 10.1093/ndt/16.suppl_7.57. [DOI] [PubMed] [Google Scholar]
- 46.Nugent R.A., Fathima S.F., Feigl A.B., Chyung D. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin. Pract. 2011;118:c269–c277. doi: 10.1159/000321382. [DOI] [PubMed] [Google Scholar]
- 47.Fraser S.D., Roderick P.J., May C.R., McIntyre N., McIntyre C., Fluck R.J., Shardlow A., Taal M.W. The burden of comorbidity in people with chronic kidney disease stage 3: a cohort study. BMC Nephrol. 2015;16:193. doi: 10.1186/s12882-015-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai W.C., Wu H.Y., Peng Y.S., Ko M.J., Wu M.S., Hung K.Y., Wu K.D., Chu T.S., Chien K.L. Risk factors for development and progression of chronic kidney disease: a systematic review and exploratory meta-analysis. Medicine (Baltim.) 2016;95 doi: 10.1097/MD.0000000000003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cherney D.Z.I., Dekkers C.C.J., Barbour S.J., Cattran D., Abdul Gafor A.H., Greasley P.J., Laverman G.D., Lim S.K., Di Tanna G.L., Reich H.N., Vervloet M.G., Wong M.G., Gansevoort R.T., Heerspink H.J.L., Diamond investigators. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020;8:582–593. doi: 10.1016/S2213-8587(20)30162-5. [DOI] [PubMed] [Google Scholar]
- 50.Heerspink H.J.L., Kosiborod M., Inzucchi S.E., Cherney D.Z.I. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94:26–39. doi: 10.1016/j.kint.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 51.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., Chertow G.M., Greene T., Hou F.F., Mann J.F.E., McMurray J.J.V., Lindberg M., Rossing P., Sjöström C.D., Toto R.D., Langkilde A.M., Wheeler D.C., DAPA-CKD Trial Committees and Investigators Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 52.The EMPA-KIDNEY Collaborative Group. Herrington W.G., Staplin N., Wanner C., Green J.B., Hauske S.J., Emberson J.R., Preiss D., Judge P., Mayne K.J., Ng S.Y.A., Sammons E., Zhu D., Hill M., Stevens W., Wallendszus K., Brenner S., Cheung A.K., Liu Z.H., Li J., Hooi L.S., Liu W., Kadowaki T., Nangaku M., Levin A., Cherney D., Maggioni A.P., Pontremoli R., Deo R., Goto S., Rossello X., Tuttle K.R., Steubl D., Petrini M., Massey D., Eilbracht J., Brueckmann M., Landray M.J., Baigent C., Haynes R. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2023;388:117–127. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perkovic V., Jardine M.J., Neal B., Bompoint S., Heerspink H.J.L., Charytan D.M., Edwards R., Agarwal R., Bakris G., Bull S., Cannon C.P., Capuano G., Chu P.L., de Zeeuw D., Greene T., Levin A., Pollock C., Wheeler D.C., Yavin Y., Zhang H., Zinman B., Meininger G., Brenner B.M., Mahaffey K.W., Investigators CREDENCE Trial. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 54.Jering K.S., Zannad F., Claggett B., Mc Causland F.R., Ferreira J.P., Desai A., Barkoudah E., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Cardiovascular and renal outcomes of mineralocorticoid receptor antagonist use in PARAGON-HF. JACC Heart Fail. 2021;9:13–24. doi: 10.1016/j.jchf.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Capes S.E., Gerstein H.C., Negassa A., Yusuf S. Enalapril prevents clinical proteinuria in diabetic patients with low ejection fraction. Diabetes Care. 2000;23:377–380. doi: 10.2337/diacare.23.3.377. [DOI] [PubMed] [Google Scholar]
- 56.Pontremoli R., Borghi C., Perrone Filardi P. Renal protection in chronic heart failure: focus on sacubitril/valsartan. Eur Heart J Cardiovasc Pharmacother. 2021;7:445–452. doi: 10.1093/ehjcvp/pvab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevens L.A., Coresh J., Greene T., Levey A.S. Assessing kidney function--measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 58.Levey A.S., de Jong P.E., Coresh J., El Nahas M., Astor B.C., Matsushita K., Gansevoort R.T., Kasiske B.L., Eckardt K.U. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data pertaining to this study have not been deposited in a publicly accessible repository and will be available upon reasonable request from the corresponding author.