Abstract
Introduction
Surgical approaches for tumors of the orbital apex and the spheno-orbital region (SOR) comprehend medial and lateral corridors. The TransOrbital NeuroEndoscopic (TONE) approach has recently been reported as a possible effective alternative to the classic lateral corridors, but literature about is still underestimated.
Research question
The aim of this study was to make a critical appraisal of the results of using the lateral TONE approach in a monocentric consecutive series of SOR tumors.
Material and methods
Data from 38 consecutive patients managed surgically by means of a lateral TONE approach for a tumor involving the orbital apex and the SOR were collected and retrospectively reviewed from 2016, January 1st to 2023, December 31st.
Results
Mean age was 57 ± 14,9 years (23 female). 20 tumors were intraconal, with intradural involvement of SOR in 5 cases. Gross total resection was achieved in 82,9% of the 35 cases treated with a curative intent. Average operative time was 94,8 ± 28,5 and 140,2 ± 43,3 min for extraconal and intraconal tumors, respectively. Meningiomas had an overall prevalence of 31,6%. The complication rate was 21%, of which 87,5% transient. The recurrence rate was 0 for meningiomas and 14,3% for malignant tumors based on a follow-up of 55,3 ± 26,3 and 68,6 ± 17 months, respectively.
Discussion and conclusion
The lateral TONE approach is the approach of choice for tumors involving the lateral compartment of the orbital apex. It is also an effective and minimal invasive option in selected cases of spheno-orbital intradural tumors with no encasement of intracranial vessels.
Keywords: Endoscopic orbitotomy, Intraconal meningiomas, Lateral orbitotomy, Orbital approaches, Spheno-orbital meningiomas, TONE approach, Case series
Highlights
-
•
The lateral TONE approach is an alternative to classic corridors that should be considered for tumors involving the lateral aspects of the orbital apex and SOR.
-
•
Most frequent complications are diplopia, trigeminal paraesthesia, palpebral edema and periorbital ecchymosis.
-
•
More than 87,5% of complications were transient and didn't significantly change the post-operative course.
-
•
The lateral TONE approach is contraindicated in cases of large skull base involvement, encasement of the intracranial vessels, and extension on the medial aspects of the optic nerve.
-
•
A careful anatomical knowledge of the SOR is required for safely perform a lateral TONE approach.
1. Introduction
Surgical approaches for tumors of the orbital apex and the spheno-orbital region (SOR) classically comprehend medial and lateral corridors which are chosen according to the circumferential extension of the lesion surrounding the optic nerve. Transcranial corridors have the great advantage of providing a 270° exposure of both these regions, along with the possibility of being combined with zygomatic osteotomies to access the orbital floor as well. They have been historically utilized to treat tumors involving the lateral orbital wall, orbital roof, optic canal, and posterior aspect of the medial orbital wall. Endoscopic techniques have also been utilized for SOR tumors because they could potentially be less invasive and equally effective as the transcranial approaches (Almeida et al., 2017; Zoia et al., 2018a; Zoia et al., 2018a, 2018a, 2018b; Castelnuovo et al., 2015; Choi et al., 2018; Dallan et al., 2015; Jeon et al., 2018; Kong et al., 2018; Locatelli et al., 2016; Luzzi et al., 2019; Murchison et al., 2011; Peron et al., 2017; Rachinger et al., 2010; Stokken et al., 2016). The refinement of endoscopic endonasal techniques has led to a progressive widening of the spectrum of possible medial corridors to the orbital apex and SOR, much more than the lateral ones. The lateral transorbital neuroendoscopic (TONE) approach has been proposed as a bone-sparing, effective, safe, and thereby minimally invasive, alternative to the transcranial approaches for tumors involving the lateral orbital compartment, orbital apex, and SOR (Almeida et al., 2017; Jeon et al., 2018; Luzzi et al., 2019; Murchison et al., 2011; Park et al., 2020; Balakrishnan and Moe, 2011; Moe et al., 2010; Norris and Cleasby, 1981; Ramakrishna et al., 2016; Zoli et al., 2023). Nevertheless, literature about its technical aspects, effectiveness, and safety profile is still scant.
The purpose of this retrospective case series study was to critically evaluate the feasibility, efficacy, safety, and limitations of the lateral TONE approach for surgical management of neoplastic lesions primarily or secondarily involving the orbital apex and SOR.
A detailed technical description of the approach, along with some illustrative cases has been provided. A non-systematic narrative literature review of similar studies has also been provided to compare literature data with ours.
2. Material and methods
2.1. Inclusion criteria
Patients >18 yo surgically managed at the IRCSS Policlinico San Matteo of Pavia by means of a lateral TONE approach due to a tumor primarily or secondarily involving the orbital apex and the SOR have been included. Observation period went from 2016, January 1st to 2023, December 31st. Based on the institutional database, overall data about demographics, clinical onset, site, operative time, extent of resection (EOR), histology, complications, and recurrence rate were collected and retrospectively reviewed.
2.2. Preoperative study
Preoperative imaging study involved, in all cases, a multiplanar noncontrast-enhanced computed tomography (CT) and a multiplanar gadolinium contrast-enhanced T1-and T2-weighted magnetic resonance imaging (MRI) of the orbits and SORs. In selected cases of intradural involvement, a CT angiography of the intracranial internal carotid arteries was also performed. Clinical assessment included the evaluation of oculomotion, ocular fundus, intraocular pressure, and visual field in every case.
2.3. Postoperative study
A contrast-enhanced MRI was performed on the second to third postoperative day to assess resection rate and rule out complications. After discharge, clinical assessments were done at 1 week, 1-month and 6-months post-op at the outpatient clinic. Further clinical and radiological assessment were defined case by case on the basis of the histological results.
2.4. Selection of the approach
Selection of the lateral TONE approach was mainly based on a “round-the-clock” algorithm, described by Paluzzi et al. (2015), according to the contrast-enhanced orbital coronal MRI. The positions of all treated tumors ranged from 11 to 7 o'clock (for right eye, conversely for left eye).
2.5. Technical Aspects of the Lateral TONE Approach
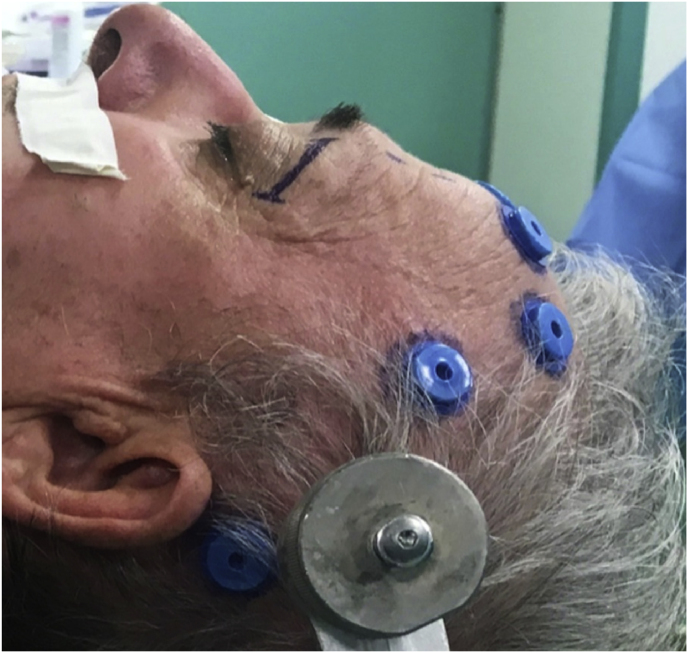
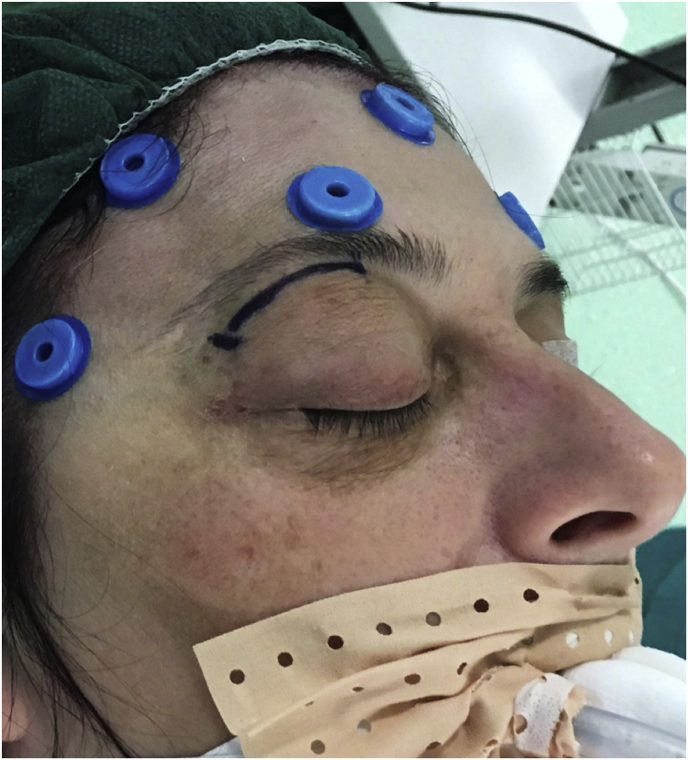
After induction of general anesthesia, the patient is placed in supine position with the head fixed by a three-point head holder. The operating table is placed perpendicular to the longest axis of the anesthesia cart, the latter at the left side of the footboard. The headboard is elevated 20°, and a reverse 10°–20° Trendelenburg position aids in further decreasing central venous pressure. Depending on the affected side, the endoscopic video cart is placed at 1 to 2 o'clock for right lesions or 10 to 11 o'clock for left lesions, to increase visual ergonomics as much as possible. The arm of the neuronavigation platform field generator, constantly used during the procedure, is placed above the endoscopic monitor or in line with the vertex of the patient. Navigation screen can be placed at the nonaffected side.
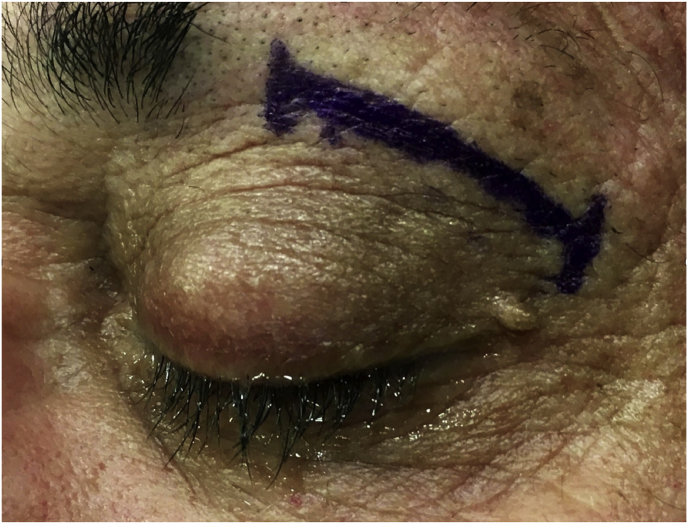
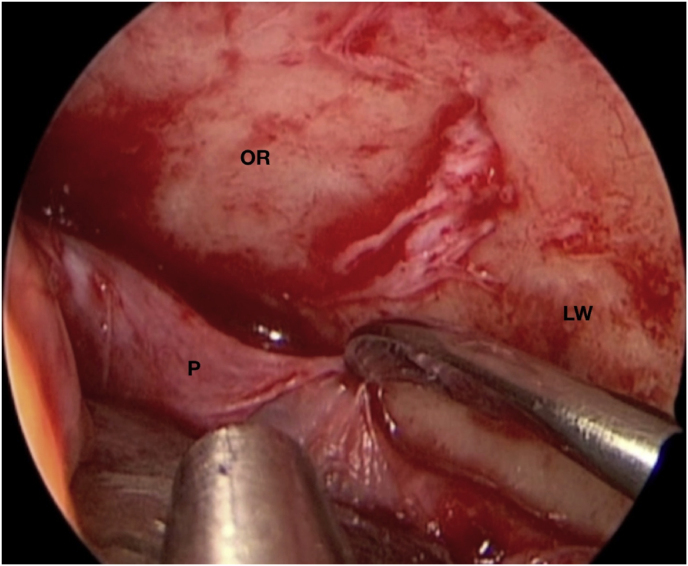
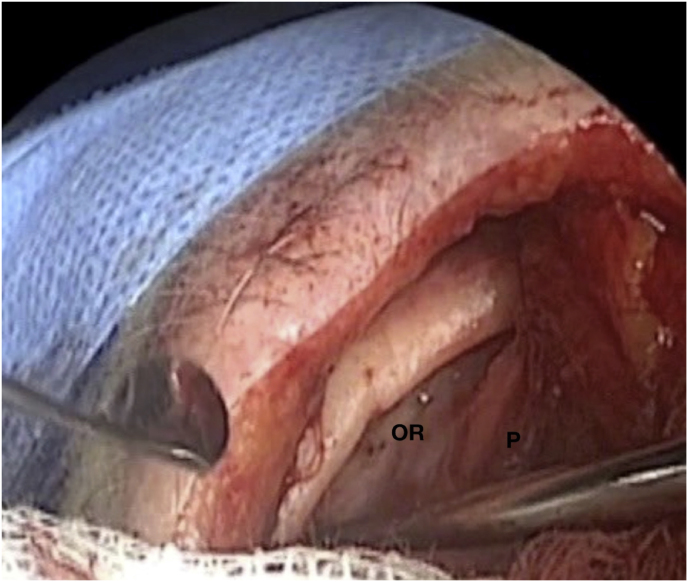
With time, our experience has led us to prefer the “two-surgeons four-hands” technique for the lateral TONE approach. This variation of the “two-nostrils four-hands” technique, reported for transnasal endoscopic procedures for sellar pathologies (Castelnuovo et al., 2006), involves the surgeon performing the main steps of surgery while the assistant holds the retractor for the orbital content, keeping the surgical field clean through constant irrigation and suction. Optionally, the assistant holds the endoscope while the surgeon executes the microsurgical steps of tumor resection using both hands. The surgeon and the assistant are placed on the affected and nonaffected side, respectively. A 3-cm lateral eyebrow skin incision is enough to approach the entire lateral compartment. The subperiosteal plane of the superior orbital rim is reached passing through the point where the orbitalis and frontalis muscle blend, thereby avoiding cutting the muscles. A blunt cranio-caudal and latero-medial subperiosteal dissection of the periorbita allows to gently mobilize the eyeball medially with a hand-held retractor, contextually accessing the orbital apex and the SOR. The endoscope is introduced into the field once the retractor is positioned. The visualization and lighting capabilities of the endoscope minimize the need for retracting the orbital content. At this stage, any bone work, such as drilling the greater sphenoid wing for spheno-orbital meningiomas, with or without hyperostosis, is performed before opening the periorbita or dura. For purely intraorbital tumors, a limited lateral opening of the periorbita proximal to the orbital apex leads to exposure of two well-defined working corridors: a corridor between the superior and lateral rectus muscles, and another one between the lateral rectus and inferior rectus muscles. Spheno-orbital tumors involve exposure of the temporal dura after drilling the greater sphenoid wing or lateral orbital wall, as needed. Opening of the dura corresponding to the superior aspect of the orbital apex allows full exposure of the sphenoidal compartment of the Sylvian fissure, the Sylvian cistern, the M1 segment of the middle cerebral artery, along the origin of the lateral lenticulostriate arteries, and medially to it, the bifurcation point of the internal carotid artery. Debulking and radical resection of the tumor is performed under full endoscopic view using standard microsurgical technique, ensuring meticulous dissection of the arterial and venous vessels eventually affected by those lesions having an intradural extension. The main steps of surgery are possible through the gentle and alternate medialization of the eyeball. For orbital tumors, no material other than fibrin sealant is generally used for reconstruction. Conversely, an inlay dural substitute plus fibrin glue is necessary after removal of the spheno-orbital lesions. Very seldom we used an onlay reconstruction technique. An intradermal running suture of the skin is performed with Monocryl 6-0 for cosmetic purposes.
2.6. Literature review
A non-systematic literature review was conducted for comparative purposes only. MEDLINE database was searched using individual keywords: trans-orbital AND spheno-orbital AND endoscop*. Article of interest were selected on the basis of title and abstract reading, then full texts were obtained and assessed for data extraction. Only article written in English and clinical setting were selected. References were checked screening for other papers of interest.
Data from included study were analyzed in Microsoft Excel (2019) (Microsoft Corp., Redmond, Washington, USA). Collected variables included number of patients, type of tumor, orbital space involvement, intradural involvement, extent of resection, complication rate, type of complication, follow up duration.
This case series has been reported in line with the PROCESS 2023 Guideline (Mathew et al., 2023).
3. Results
3.1. Patients’ data
38 consecutive patients were included (mean age 57 ± 14,9 years). 23 patients were female (mean age 54,3 ± 16,2 years). Proptosis was the clinical onset in 73,7% patients. 20 tumors were intraconal and intradural involvement was documented in 5 cases of spheno-orbital tumors. Table 1 reports the overall clinical data of the present series. To date, no patients were lost at the follow up.
Table 1.
Overall data operated with a lateral transorbital endoscopic approach.
| Case# | Age, Sex | Dimension (mm) | Clinical Onset | Spheno-Orbital Space Involvement | Intradural Involvement | Extent of Resection | Operative Time (min.) | Histology | Complications | Follow-up (months) | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47, F | 21x20 x39 | Proptosis | Orbital extraconal | No | GTR (Simpson grade 1) | 85 | Fibroblastic meningioma | None | 84 | No |
| 2 | 78, F | 24x20x25 | Proptosis (recurrence) | Spheno-orbital | Yes | GTR (Simpson grade 2) | 70 | Meningothelial meningioma | None | 91 | No |
| 3 | 78, F | 35x55x21 | Proptosis (recurrence) | Orbital intraconal | No | GTR (Simpson grade 1) | 133 | Meningothelial meningioma | None | 84 | No |
| 4 | 49, M | 22x24x23 | Orbital pain | Orbital intraconal | No | GTR | 118 | Fibromyxoid sarcoma | None | 84 | No |
| 5 | 69, M | 26x32x31 | Incidental | Orbital extraconal | No | GTR | 82 | Metastatic poorly differentiated adenocarcinoma | None | 82 | No |
| 6 | 79, M | 17x15x13 | Incidental | Orbital intraconal | No | Biopsy | 121 | Metastatic poorly differentiated adenocarcinoma | None | 86 | – |
| 7 | 56, F | 27x22x28 | Proptosis and orbital mass | Orbital extraconal | No | GTR (Simpson grade 1) | 90 | Meningothelial meningioma | None | 70 | No |
| 8 | 48, F | 22x32x20 | Proptosis (recurrence) | Orbital intraconal | No | GTR (Simpson grade 1) | 156 | Meningothelial meningioma | None | 74 | No |
| 9 | 68, M | 32x28x34 | Proptosis | Orbital extraconal | No | GTR | 94 | Metastatic squamous cell carcinoma | None | 72 | Yes |
| 10 | 69, M | 21x14x18 | Periorbital edema | Orbital extraconal | No | Biopsy | 87 | Metastatic poorly differentiated adenocarcinoma | None | 62 | – |
| 11 | 36, F | 34x45x37 | Proptosis (recurrence) | Orbital intraconal | No | GTR (Simpson grade 1) | 237 | Meningothelial meningioma | orbital abscess | 46 | No |
| 12 | 71, F | 23x25x29 | Proptosis | Orbital intraconal | No | GTR (Simpson grade 1) | 93 | Meningothelial meningioma | None | 54 | No |
| 13 | 42, F | 24x26x19 | Proptosis | Spheno-orbital | Yes | GTR (Simpson grade 1) | 87 | Meningothelial meningioma | None | 48 | No |
| 14 | 67, M | 34x23x28 | Orbital mass | Orbital extraconal | No | STR | 65 | Mucoepidermoid carcinoma | None | 44 | – |
| 15 | 65, F | 15x12x13 | Orbital mass | Orbital intraconal | No | GTR | 108 | Metastatic adenoid cystic carcinoma | None | 50 | No |
| 16 | 63, M | 11x12x10 | Incidental | Orbital intraconal | No | GTR (Simpson grade 1) | 116 | Fibroblastic meningioma | None | 48 | No |
| 17 | 29, F |
18x23x22 | Orbital pain | Orbital intraconal | No | GTR | 98 | Dermoid cyst | Transient Ptosis | 36 | No |
| 18 | 65, M |
30x25x29 | Proptosis | Spheno-orbital | Yes | GTR (Simpson grade 2) | 193 | Meningothelial meningioma | None | 36 | No |
| 19 | 46, F |
22x24x21 | Proptosis | Spheno-orbital | No | GTR | 136 | Mastocytosis | None | 30 | No |
| 20 | 73, F |
28x11x30 | Proptosis | Spheno-orbital | Yes | GTR (Simpson grade 2) | 154 | Fibroblastic meningioma | None | 22 | No |
| 21 | 75, M |
12x13x15 | Incidental | Orbital extraconal | No | GTR | 120 | B cell Lymphoma | None | 24 | No |
| 22 | 25, F |
11x10x29 | Proptosis | Orbital intraconal | No | GTR | 97 | Venous Varix | None | 24 | No |
| 23 | 64, F |
22x21x24 | Proptosis | Orbital intraconal | No | GTR | 124 | Cavernous Haemangioma | Transient Diplopia | 26 | No |
| 24 | 70, F |
12x14x13 | Proptosis | Orbital intraconal | No | GTR | 137 | Cavernous Haemangioma | None | 24 | No |
| 25 | 46, F |
23x25x26 | Proptosis | Orbital intraconal | No | GTR | 156 | Cavernous Haemangioma | Transient Diplopia | 24 | No |
| 26 | 57, M |
18x17x18 | Proptosis | Orbital intraconal | No | GTR | 178 | Cavernous Haemangioma | None | 24 | No |
| 27 | 22, F |
19x23x18 | Incidental | Orbital extraconal | No | GTR | 123 | Dermoid cyst | None | 18 | No |
| 28 | 56, F |
18x16x20 | Proptosis | Orbital intraconal | No | GTR | 144 | Cavernous Haemangioma | None | 6 | No |
| 29 | 72, F |
34x23x33 | Proptosis | Spheno-orbital | Yes | STR (Simpson grade 3) | 192 | Meningothelial meningioma | Transient Ptosis | 6 | No |
| 30 | 64, M | 21x13x15 | Proptosis | Orbital intraconal | No | Biopsy | 90 | IG4-related fibro-inflammatory lesion | None | 24 | – |
| 31 | 45,M | 28x18x24 | Proptosis | Orbital extraconal | Yes | STR | 135 | Mucocele | None | 40 | No |
| 32 | 56,F | 22x14x18 | Proptosis | Orbital extraconal | No | STR | 57 | Epidermoid Cyst | None | 12 | Yes |
| 33 | 57, F | 18x12x15 | Proptosis | Orbital extraconal | No | GTR | 60 | Epidermoid Cyst | Transient facial palsy | 35 | No |
| 34 | 56,F | 21x18x20 | Proptosis | Orbital intraconal | No | GTR | 110 | Cavernous Haemangioma | None | 32 | No |
| 35 | 34, M | 22x16x21 | Proptosis | Orbital extraconal | Yes | GTR | 140 | Cavernous Haemangioma | Transient supraorbital neuralgia | 30 | No |
| 36 | 54, M | 23x13x16 | Proptosis | Orbital intraconal | No | GTR | 160 | Cavernous Haemangioma | None | 20 | No |
| 37 | 56,F | 18x21x21 | Proptosis | Orbital intraconal | No | STR | 240 | Inflammatory pseudotumor | None | 18 | No |
| 38 | 59,M | 25x19x15 | Proptosis | Orbital intraconal | No | STR | 150 | Schwannoma | Transient III nerve palsy | 14 | No |
3.2. Operative time, complications, and recurrence rate
Out of 35 patients treated with a curative intent (3 biopsies), gross total tumor resection was achieved in 82,9% cases. Average operative time was 94,8 ± 28,5 and 140,2 ± 43,3 min for extraconal and intraconal tumors, respectively. Meningiomas had an overall prevalence of 31,6%; 41,6% were intraconal. The remaining lesions consisted of epithelial or mesenchymal tumors. The complication rate was 21% of which 87,5% were transient in nature and didn't require specific treatment (Clavien-Dindo 1 class). The most important was an orbital abscess that completely recovered after 2 months of antibiotic therapy (Clavien-Dindo 2 class). Excluding biopsies, overall average follow-up was 40,9 ± 24,8 months, whereas mean follow-up for meningeal and epithelial-mesenchymal tumors was of 55,3 ± 26,3 and 40 ± 22,8 months, respectively. Recurrence rate was 0% for meningiomas and 14,3% for malignant tumors (Table 1).
3.3. Illustrative case #1: Left intraconal meningioma of the orbital apex
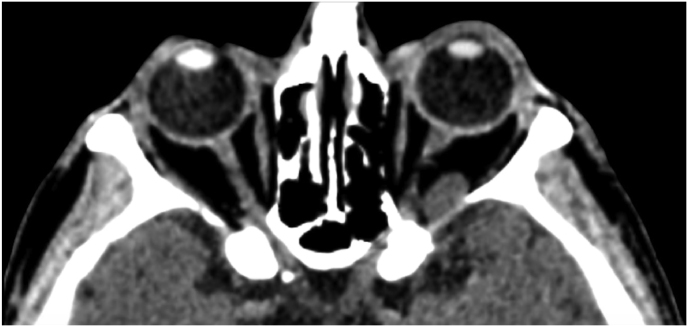
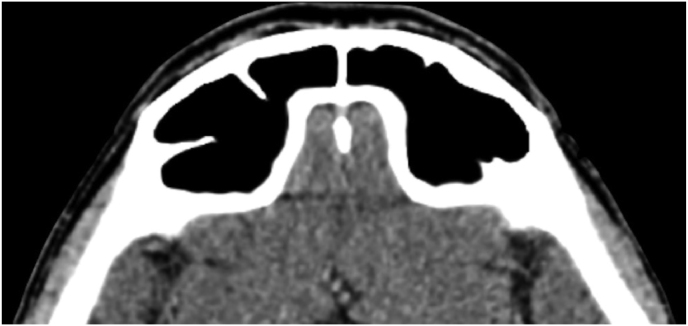
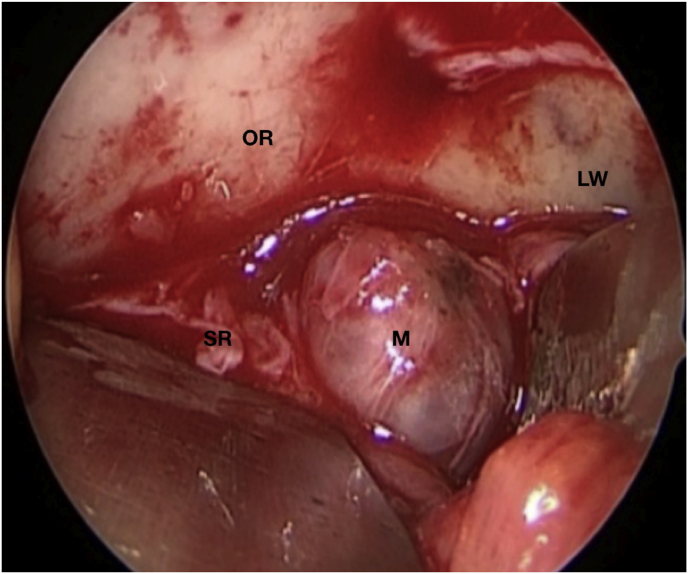
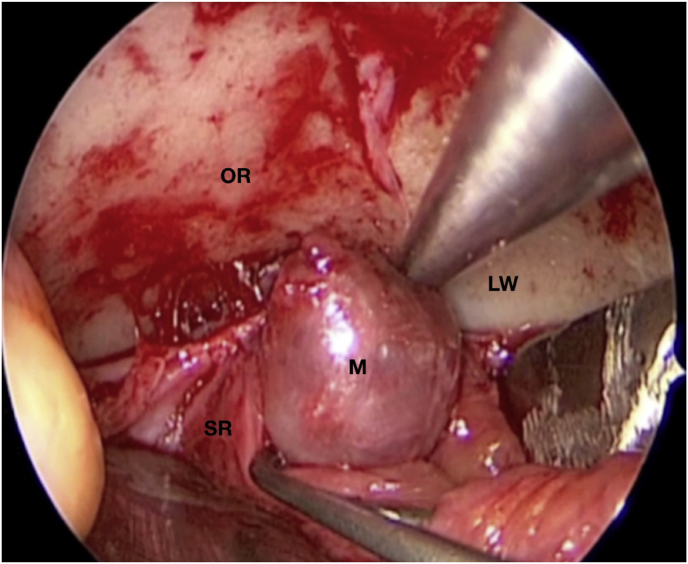
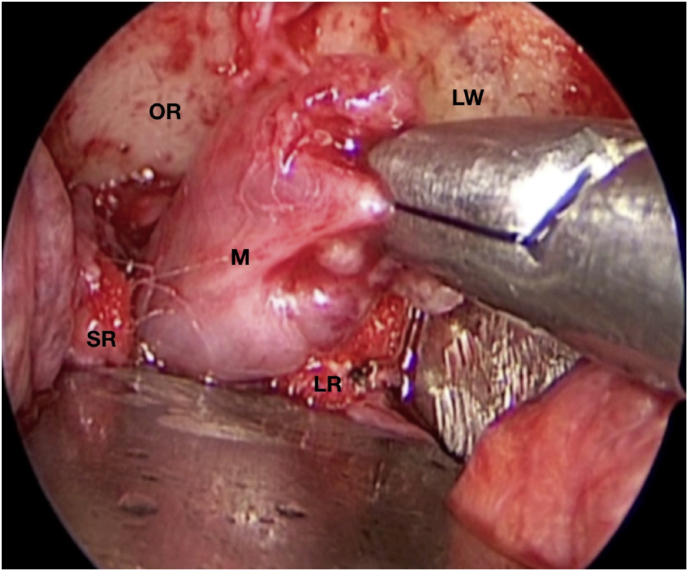
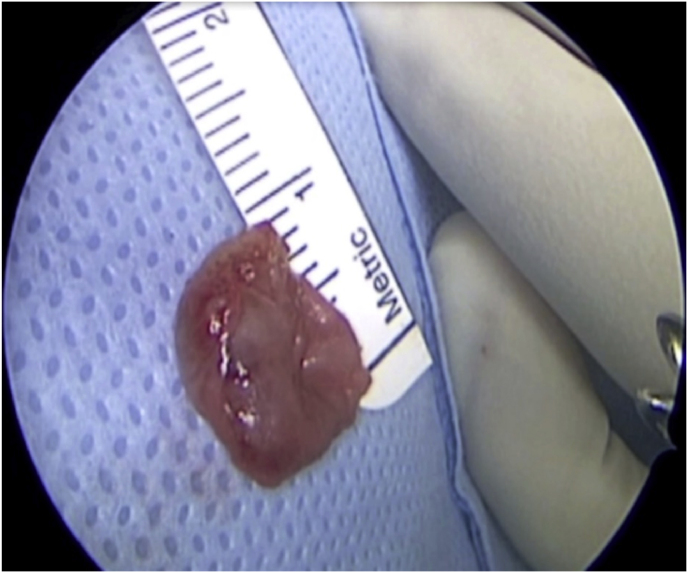
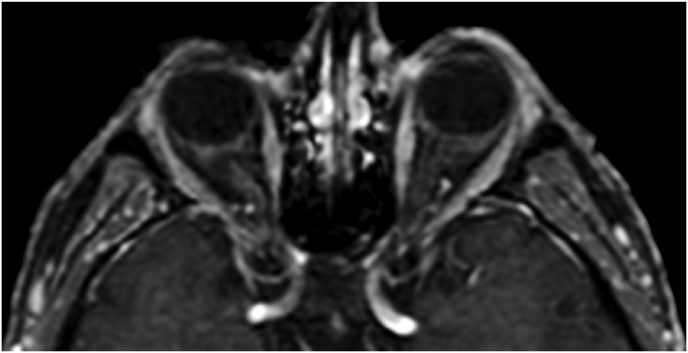
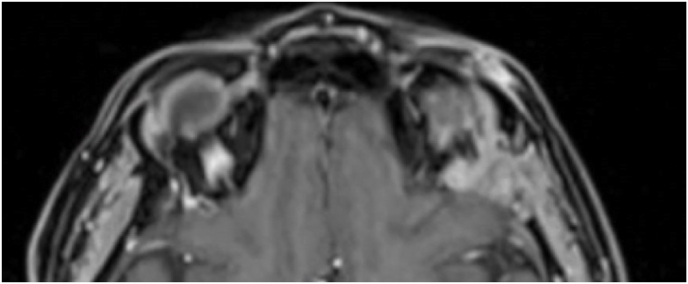
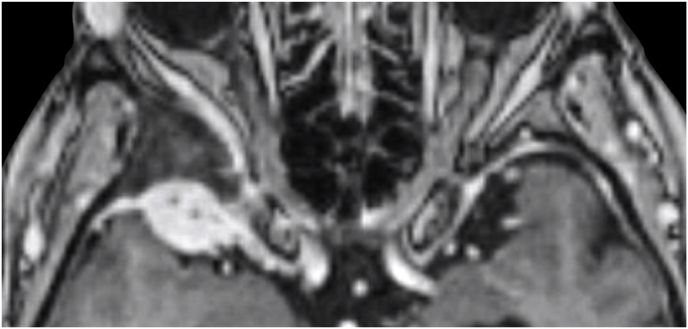
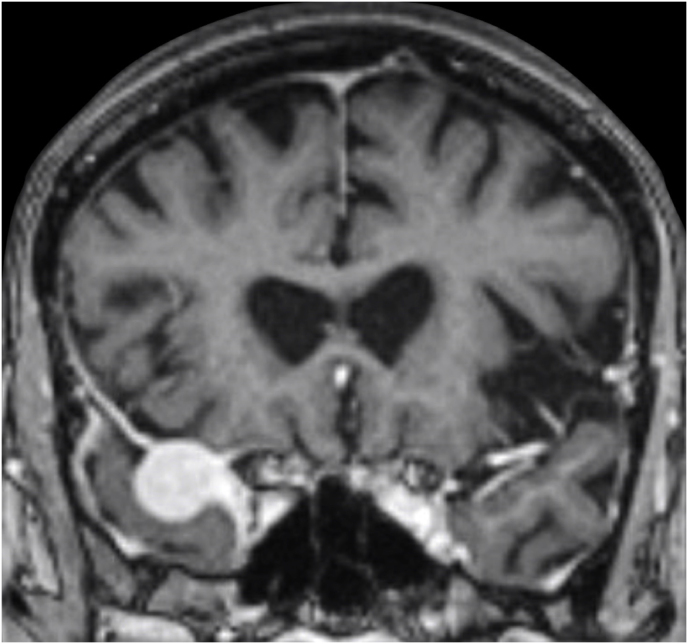
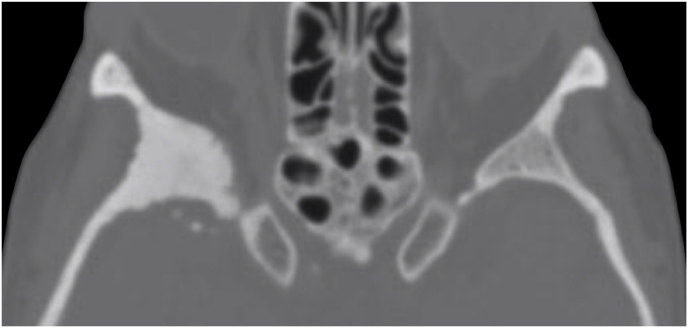
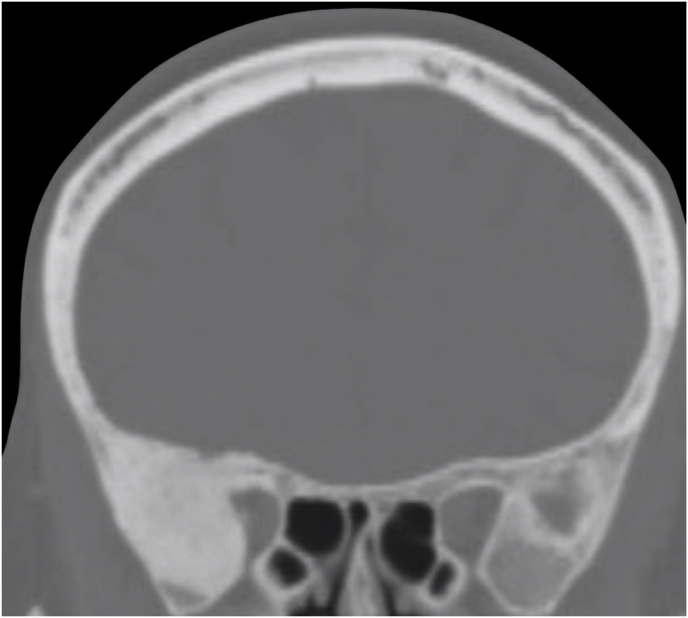
A 63-year-old man underwent a noncontrast-enhanced CT exam because of a mild traumatic brain injury that incidentally revealed an isodense left orbital intraconal lesion. Oculomotion and visual field tests were all unremarkable, while intraocular pressure in the left eye was 21 mm Hg. A coronal T1-weighted gadolinium contrast-enhanced MRI showed an intraconal meningioma of the orbital apex involving the lateral compartment. The patient also showed hyperpneumatization of the frontal sinus, within an anatomical pattern of excessive pneumatization of all paranasal sinuses that would have increased the risk of violating the sinus in the case of an anterolateral transcranial approach. A lateral TONE approach was, therefore, performed; an access route between the superior and the lateral rectus muscles made it possible to achieve gross total tumor resection (Simpson grade 1). The patient was discharged neurologically intact on the second postoperative day. Pathology was conclusive for fibroblastic meningioma. Cosmetic outcome was good, and no recurrences had occurred at third-year follow-up (Fig. 1).
Fig. 1.
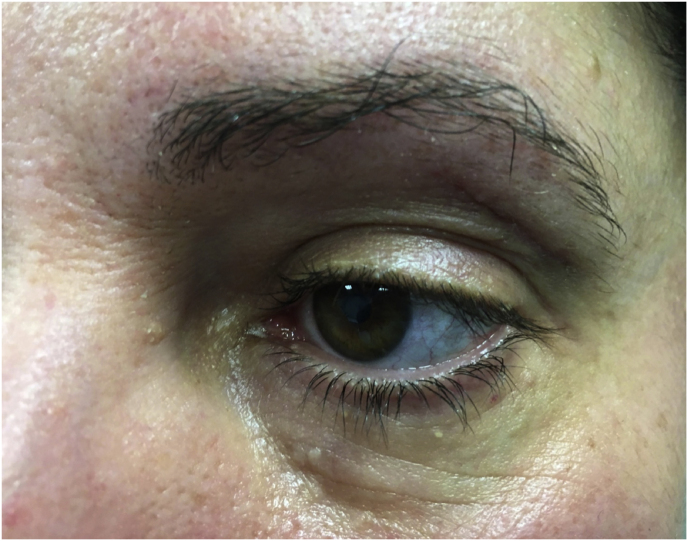
(a) Axial CT of the orbital region showing an isodense left intraconal lesion; Axial (b) and sagittal (c) T1-weighted gadolinium contrast-enhanced MRI showing a hyperintense left intraconal meningioma at the orbital apex involving the lateral compartment; (d) Axial head CT revealing hyperpneumatization of the frontal sinus; (e) Intraoperative image showing patient placed in supine position. The head was fixed with a three-point skull clamp; (f) Intraoperative photograph showing the lateral eyebrow skin incision; (g–j) The “two-surgeons and four-hands” technique allowed excision and complete removal of the tumor (Simpson grade I). OR: orbital roof; LW: lateral orbital wall; M: meningioma; P: periorbita; SR: superior rectus muscle; LR: lateral rectus muscle; (k) photograph of the operative specimen; (l) Axial T1-weighted gadolinium contrast-enhanced MRI of the orbits at 6-month follow-up revealing complete tumor removal, preserved integrity of all the intraconal structures, and no evidence of recurrence; (m) Patient photograph obtained 6 months after surgery and showing good cosmetic results in the affected eye.
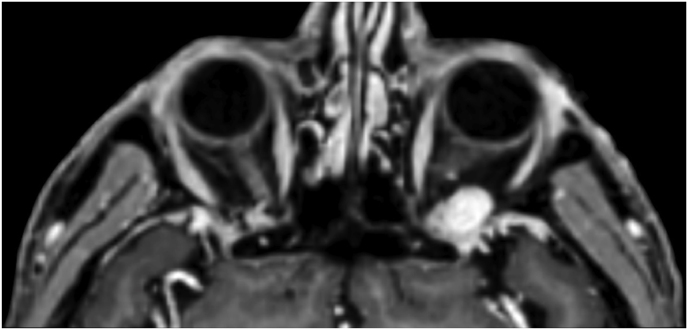
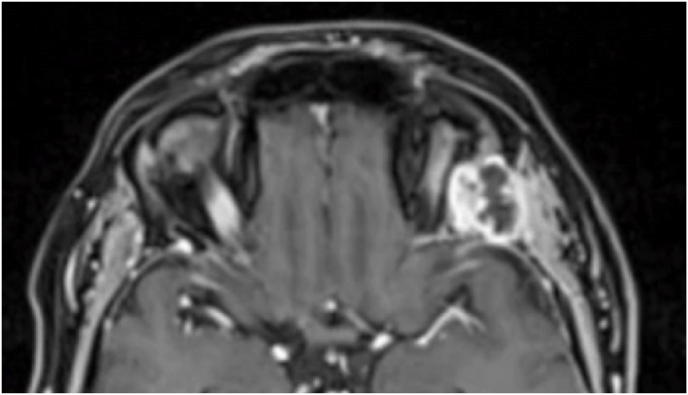
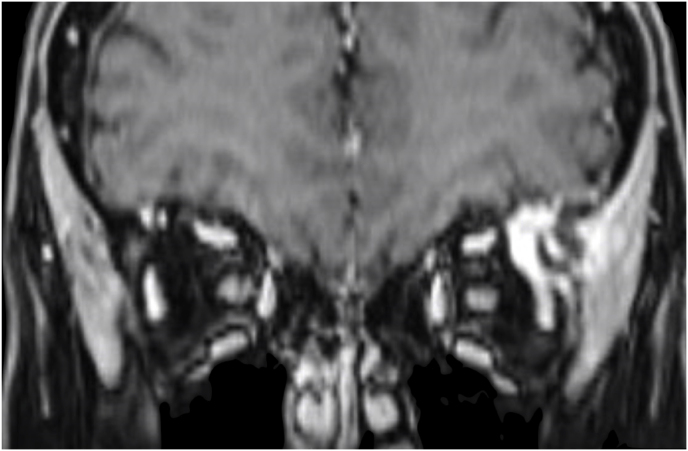
3.4. Illustrative case #2: Left extraconal meningioma of the Lateral wall involving the orbital apex
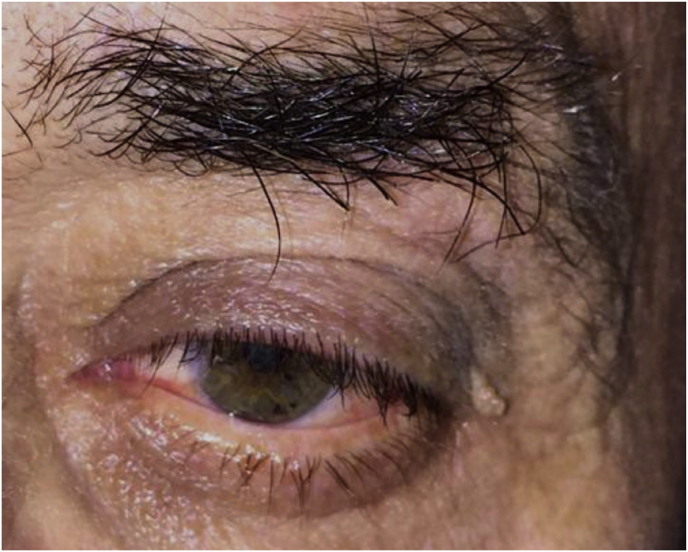
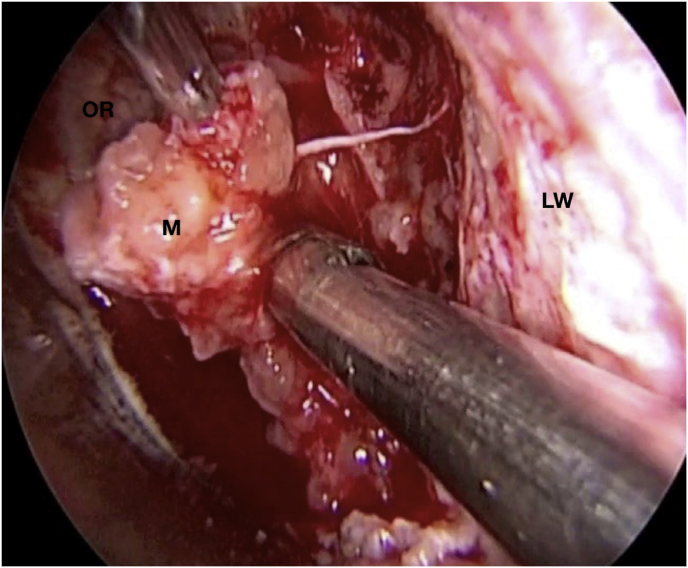
A 56-year-old female was diagnosed with a left extraconal meningioma of the lateral wall involving the orbital apex causing progressive proptosis. The extraconal location as well as the presumptive ease of dissection of the lesion made the patient an ideal candidate for the lateral TONE approach, performed without complications (Simpson grade 1). Pathology revealed a meningothelial meningioma that did not recur until the last MRI follow-up performed after 2 years and 10 months (Fig. 2).
Fig. 2.
Axial (a) and coronal (b) T1-weighted gadolinium contrast-enhanced MRI of the orbit showing a hyperintense left extraconal meningioma of the lateral wall involving the orbital apex; (c) Endoscopic intraoperative photograph obtained during tumor excision. OR: orbital roof; M: meningioma; LW: lateral orbital wall; (d) Patient photograph obtained 6 months after surgery and showing a good cosmetic result in the affected eye; (e) Post-op T1-weighted gadolinium contrast.enhanced MRI of the orbit.
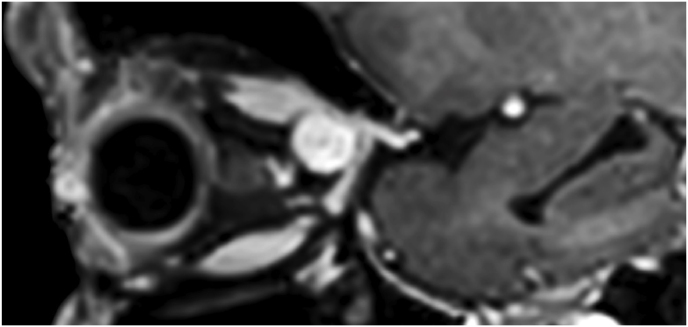
3.5. Illustrative case #3: right spheno-orbital meningioma with hyperostosis of the greater sphenoid wing
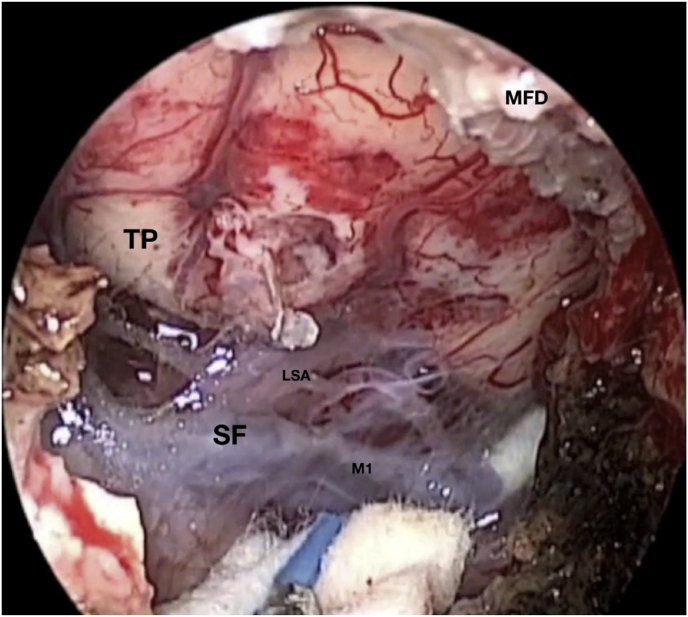
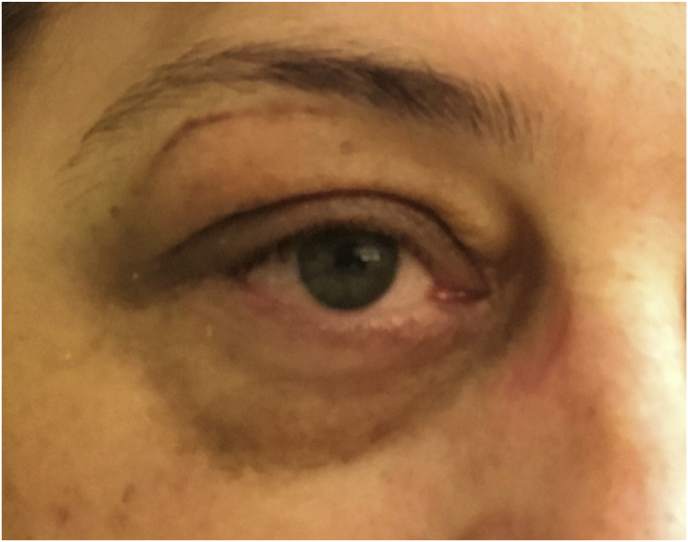
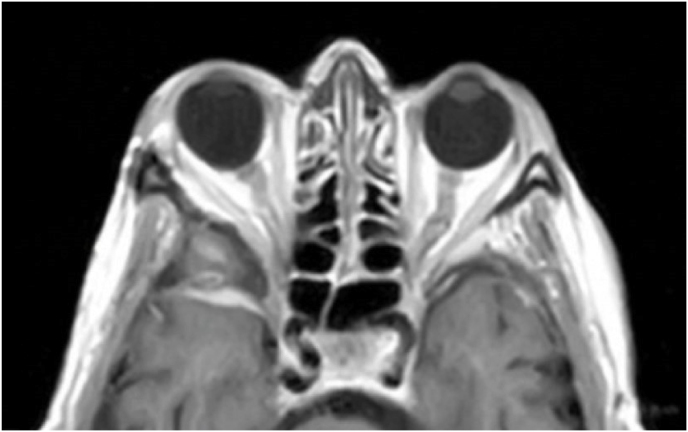
A 42-year-old female had a progressive exophthalmos associated with a slight decrease in visual acuity in the right eye. Contrast-enhanced MRI displayed a right spheno-orbital meningioma with a clear dural tail involving the orbital apex and an initial extension within the Sylvian fissure. MRI angiography showed an intimate relationship between the mass and the M1 segment of the middle cerebral artery, that appeared, however, not to be encased. A CT showed massive hyperostosis of the lateral wall of the orbit and greater sphenoid wing. The patient underwent a lateral TONE approach that involved, as a first step, wide transorbital drilling of the greater sphenoid wing. The subsequent opening of the dura just above the orbital apex allowed for total exposure of the meningioma that was easily debulked and dissected, along with the nearby dura, thereby performing a Simpson grade 1 tumor removal. At the end of surgery, the endoscopic view also allowed for a careful review of the integrity of the perforating arteries from the M1 segment of the middle cerebral artery. A collagen dura substitute and fibrin glue were used to reconstruct the defect in the middle fossa dura. Postoperatively, oculomotion remained unaffected, visual impairment in the right eye completely disappeared, and the cosmetic results were optimal.
Pathology documented a meningothelial meningioma and no relapses had occurred after 3 years (Fig. 3).
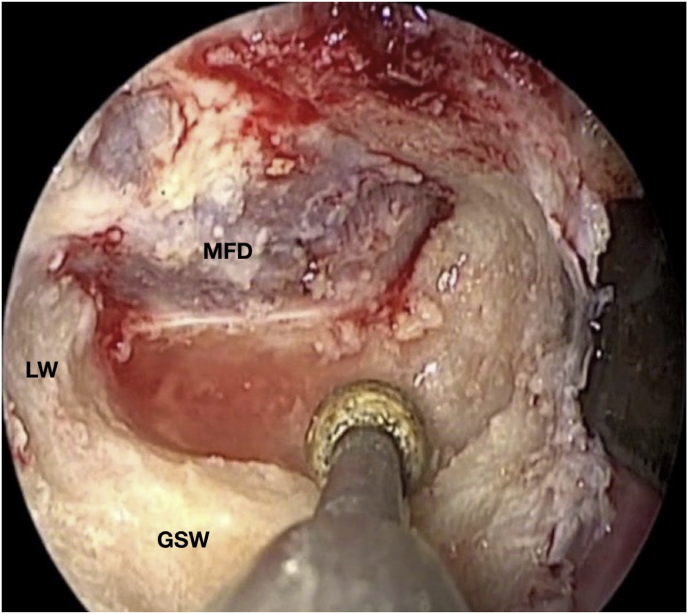
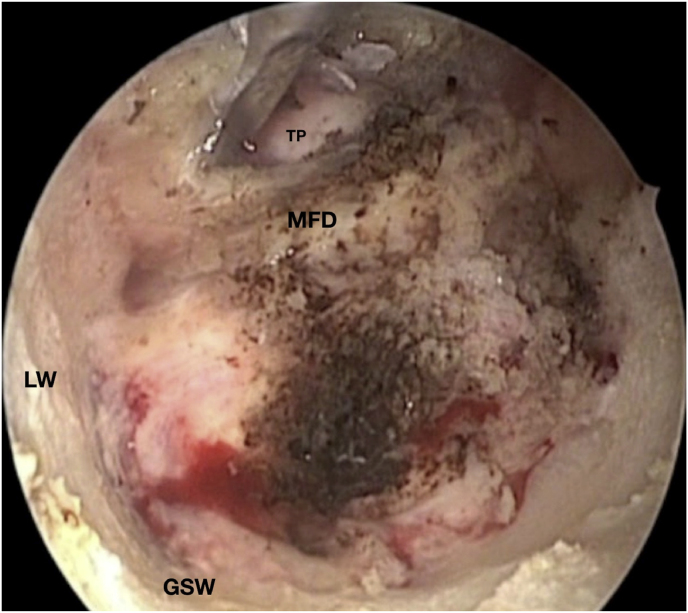
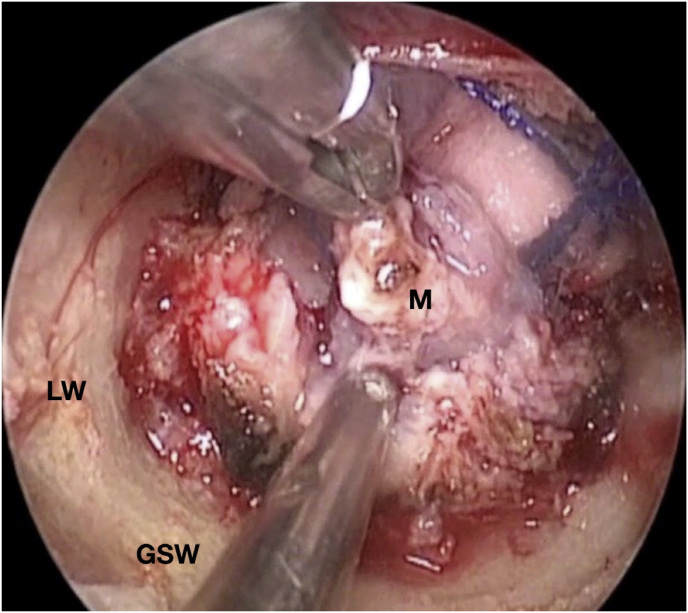
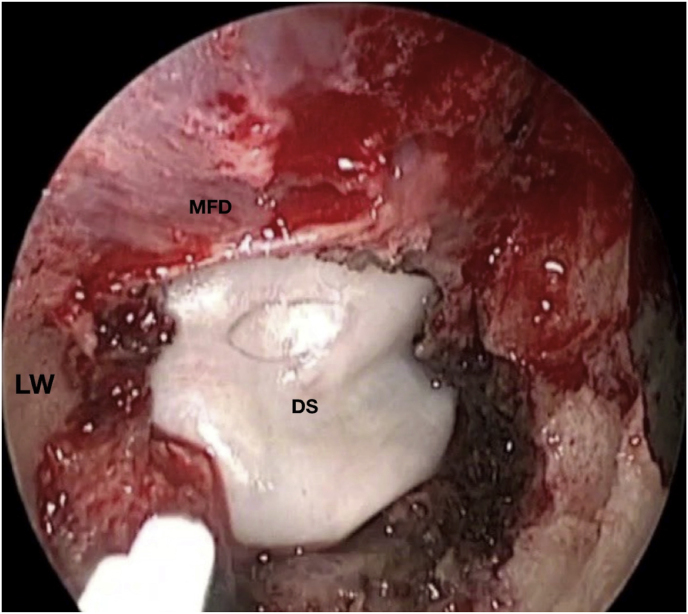
Fig. 3.
Axial (a) and coronal (b) T1-weighted gadolinium contrast-enhanced MRI of the right spheno-orbital region showing a spheno-orbital meningioma with a clear dural tail involving the orbital apex; Axial (c) and coronal (d) non-enhanced bone-window CT showing massive hyperostosis of the lateral wall of the right orbit and greater sphenoid wing; (e) Intraoperative image showing the patient placed in a supine position with the head fixed by a three-point head clamp; (f) Intraoperative image showing the subperiosteal dissection of the periorbita after skin incision; (g) Extensive drilling of the sphenoid ridge allowed exposure of the middle fossa dura; (h) the opening of the spheno-orbital dura was made just above the orbital apex, and the meningioma was fully exposed, debulked and dissected (i); (j) Intraoperative photograph at the end of surgery showing the sphenoidal compartment of the Sylvian fissure, the Sylvian cistern, the M1 segment of middle cerebral artery, along the origin of the lateral lenticulostriate arteries; (k) onlay reconstruction of the spheno-orbital dural defect with collagen dura substitute and fibrin glue; (l) image of the patient obtained 6 months after surgery revealing a good cosmetic outcome; (m) Post-op T1-weighted gadolinium contrast.enhanced MRI of the spheno-orbital region
OR: orbital roof; P: periorbita; MFD: middle cranial fossa dura; LW: lateral orbital wall; GSW: greater sphenoid wing; TP: Temporal pole; M: meningioma; SF: Sylvian fissure; M1: M1 segment of the middle cerebral artery; LSA: lateral lenticulostriate arteries; DS: dural substitute.
3.6. Literature review
10 studies were included in this non-systematic literature review based on the pre-specified criteria. The considered parameters were similar to those in the present study (see Discussion).
4. Discussion
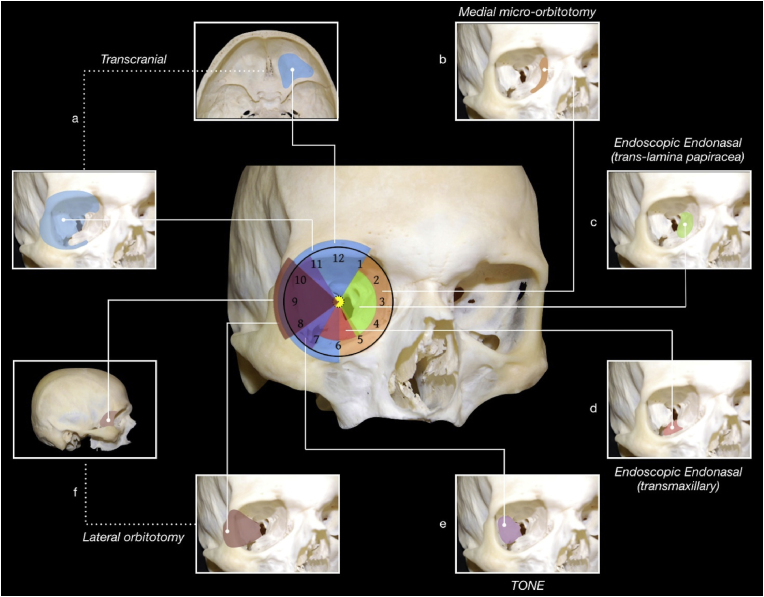
Approaches for tumors of the orbital apex and SOR are chosen according to a simple and effective “round-the-clock” scheme (Fig. 4), within which the lateral TONE approach provides an easy access the lateral compartment of the orbit and orbital apex, but also anterior and middle skull base, and lateral wall of the cavernous sinus.
Fig. 4.
Overview of the main approaches to the orbital and spheno-orbital region based on the “round-the-clock” scheme
TONE: transorbital neuroendoscopic (approach); ON: optic nerve.
The avoidance of the brain retraction and manipulation of the temporalis muscle, with all the related risks of complications, could makes this approach advantageous when compared to the conventional transcranial corridors. A further strength lies in its potentially better cosmetic outcome, which basically derive from its minimal invasiveness.
Unlike the trans-lamina papyracea and transmaxillary endoscopic endonasal approaches, the lateral TONE approach is characterized by a greater freedom, a working corridor which is not strictly limited by the optic nerve and internal carotid artery, and an advantage in dealing with those tumors more laterally extending.
Furthermore, for most of the lesions affecting this area, the transorbital route allows for a more direct access, along with a better visualization of the esocranial surface of the greater and lesser sphenoid wing.
The evaluation of the results of this retrospective case series has led to confirm the feasibility of the TONE approach in surgical management of a spectrum of tumors affecting these regions. The reported data of 82,9% GTR confirms, notably, its effectiveness in terms of the EOR of these lesions. For meningiomas, a Simpson grade 1 removal was achieved in 75% of cases, this data indicating that the extent of resection is comparable to that of conventional transcranial approaches. Although no severe optic neuropathies were present preoperatively in our series, literature reports a good clinical and ophthalmological outcome related to the endoscopic transorbital surgery for cranioorbital tumors (Kong et al., 2018). The same data were found, in terms of recovery from proptosis, by the analysis of our cohort.
The complication rate of 21% of which 87,5% Clavien-Dindo 1 also defines a safe profile of the TONE approach.
Particularly for the risk of cerebrospinal fluid leaks regarding the spheno-orbital meningiomas, it is rationale to suppose that the ocular globe, especially in those cases were the periorbita is preserved, may act as a barrier able to counteract the pressure gradient from the violated intracranial compartment toward the orbit.
From a technical point of view, preference for a lateral eyebrow skin incision comes from the finding of a better cosmetic outcome compared with eyelid incision, which has been used conversely in the first 4 treated cases. It must be highlighted that, in all the treated cases, increased illumination and magnification of the surgical field provided by the endoscopic view allowed for unequalled lateral visualization of the orbital apex and sphenoidal ridge as well as of the sphenoidal compartment of the Sylvian fissures in case of intradural tumors.
In our series, we have been usual to perform by default an inlay dural reconstruction with dural substitute and fibrin glue, considering it adequate for a large part of intradural lesions limited in size. However, larger dural defects could benefit from more complex duraplasty techniques as dual inlay-onlay reconstruction, with or without interposition grafts.
Table 2 shows the results of the literature review of the previously reported case series, which involved removal of neoplastic lesions through a lateral TONE approach.
Table 2.
Literature review of the main previously reported case series (>3 cases) on lateral transorbital endoscopic approach.
| Author, Year | PMID | # of Patients | Type of Tumors | Orbital Space Involvement |
# of lesions w/Intradural Involvement | Extent of Resection (#/%) |
Complication Rate (#/%) | Type of Complications | Average Follow-up (months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Extraconal | Intraconal | GTR/NTR | STR | ||||||||
| Kong et al., 2018 (Kong et al., 2018) | 30215555 | 17 | 12 Meningiomas 1 Sebaceous gland carcinoma 1 Cystic teratoma 1 Intraconal Schwannoma 1 Osteosarcoma 1 Plasmacytoma |
16 | 1 | 13 | 13 (76.4 %) | 4 (25.5 %) | 2 (11.1 %) | Periorbital swelling | 5.3 3.7 |
| Dallan et al., 2018 (Dallan et al., 2018) | 29462449 | 14 | Spheno-orbital Meningioma | 8 | 3 | 3 | 5 (35.7 %) | 9 (64.3 %) | 10 (71.43%) | Necrosis of the upper eyelid, diplopia, V1 hypoesthesia, V2 hypoesthesia, left periorbital emphysema, palpebral edema | 25 |
| Jeon et al., 2018 (Jeon et al., 2018) | 30544350 | 9 | 4 Trigeminal Schwannomas 2 Meningiomas 1 Chondrosarcoma 1 Metastatic lesion 1 Dermoid cyst |
NA | NA | 6 | 7 (77.8 %) | 2 (22.2 %) | 1 (11%) | Ptosis | NA |
| Park et al., 2020 (Park et al., 2020) | 31226689 | 25 | Trigeminal Schwannomas | – | – | 17 | 19 (76 %) | 6 (24 %) | 3 (12 %) | Middle cerebral artery vasospasm, infection, medial gaze palsy | NA |
| Locatelli et al., 2020 (Locatelli et al., 2020) | 35433179 | 18 | 18 meningioma | 18 | 8 | 14 | 7 (38,9%) | 11 (61,1%) | 3 (16,7%) | 1 visual deficit, 2 trigeminal hypoesthesia | 31.5 (6–84) |
| Kong et al., 2020 (Sakashita et al., 2009) | 32502989 | 41 | 39 grade I and 2 grade 2 meningiomas | NA | NA | 41 | 21 (51,2%) | 20 (48,8%) | 4 (9,8%) | 2 CSF leak, 2 wound complications | 16 |
| In Woo et al. (2021) (Dallan et al., 2018) | 33226438 | 18 | 17 meningioma grade I, 1 meningioma grade II | 18 | 8 | 10 | 3 (16,7%) | 15 (83,3%) | 8 (44%) | 3 transient EOM deficit, 3 trigeminal hypoesthesia, 1 CSF leak, 1 wound infection | 20 10 |
| Di Somma et al., 2023 (Locatelli et al., 2020) | 37410915 | 20 | 5 spheno-orbital meningiomas, 1 intradiploic Meningioma, 2 intraconal lesions, 1 temporal pole lesion, 2 trigeminal schwannoma, 3 cavernous sinus lesions, and 6 petroclival lesions | 5 | 2 | 12 | NA | NA | 4 (20%) | 1 limitation of eye abduction, 1 ocular neuropathic pain, 2 slight enophthalmus | NA |
| Han X et al., 2023 (Di Somma et al., 2023) | 36947242 | 16 | 8 meningiomas, 2 hemangiomas, 1 low-grade glioma, 1 instance of inflammatory hyperplasia tissue, 1 Langerhans cell histiocytosis, 1 epidermoid cyst, 1 trigeminal schwannoma, and 1 bone fibrosis hyperplasia | 6 | 7 | 3 | 13 (81,3%) | – | 0 (0%) | – | NA |
| Zoli et al., 2023 (Zoli et al., 2023) | 37331478 | 22 | 16 meningioma grade I, 3 meningioma grade II, 1 fibrousdysplasia, 1 Plasmocytoma, 1 solitary fibrous tumor | 22 | 12 | 18 | 8 (36.4%) | 14 (63.4%) | 5 (22,7%) | 1 CSDH,1 permanent EOM deficit,3 trigeminal hypoesthesia | 46 (6–90) |
| Present study | 38 | 12 meningiomas, 8 cavernous haemangioma, 5 metastasis, 4 dermoid cysts, 1 lymphoma, 1 fibromyxoid sarcoma, 2 inflammatory lesion, 1 mastocytosis, 1 mucocele, 1 mucoepidermoid carcinoma, 1 schwannoma, 1 venous varix | 12 | 20 | 7 | 29/35 (82,9%) | 6/35 (17,1%) | 8/38 (21%) | 1 orbital abscess, 2 transient ptosis, 2 transient diplopia, 1 transient facial palsy, 1 transient III nerve palsy, 1 transient supraorbital neuralgia | 40,9 ± 24,8 | |
NA: data not available; GTR: gross total removal; NTR: near total removal; STR: subtotal removal.
Based on the main series, the estimated rate of gross total/near total tumor resection ranged from 16,7% to 81,3%, this data being in line with our results.
Average operative time was found to be longer for intraconal tumors than for extraconal ones; however, in both cases these durations were much shorter than those reported for most of the transcranial skull base approaches (Sakashita et al., 2009).
Dallan et al. reported 10 minor complications out of 14 treated patients (71,43%) (Dallan et al., 2018), but this data should be related to the fact that they reported a series of only spheno-orbital meningiomas, where the higher anatomical complexity of the region accounts for this finding. In the remaining studies, the rate of complication ranged from 11% to 44%.
Our experience has progressively led to delineating three main indications for the lateral TONE approach: curative treatment of orbital or spheno-orbital tumors extending from the 7 to the 11 o'clock position of the orbital apex, with or without intracranial involvement; orbital decompression aimed at treating proptosis secondary to large skull base tumors; and biopsy.
An essential aspect for intraoperative orientation concerns the need for a thorough knowledge of the topographic anatomy of the orbital region, along with the anterior and middle cranial fossa.
4.1. Limitations of the Lateral TONE approach
The lateral TONE approach is contraindicated for tumors having prevalent involvement of the medial aspect of the orbit, as well as for cases of wide involvement of the anterior and middle skull base, especially if associated with encasement of intracranial vessels.
4.2. Limitations of the study
This series is limited in numbers and further investigations are needed to validate our results.
5. Conclusions
The lateral TONE approach could be a minimally invasive alternative to transcranial corridors that should be considered for tumors involving the lateral aspects of the orbital apex and SOR.
Its main technical advantages comprehend increased illumination and magnification of the surgical field that offers an unparalleled lateral view of the spheno-orbital neurovascular structures.
The results of the present series suggest the lateral TONE approach as a good option for tumors laterally located and involving the orbital apex. It may be a minimally invasive alternative to transcranial approaches also for selected cases of spheno-orbital meningiomas with no encasement of intracranial vessels.
The lateral TONE approach is contraindicated in cases of large skull base involvement, encasement of the intracranial vessels, and in cases of lesions having a prevalent extension on the medial aspects of the optic nerve. Based on the results of this retrospective case series, further prospective studies of high methodological quality are advocated to better define indications, outcome and potentiality of this emerging alternative approach to the SOR tumors.
Funding
No funding was received for this research.
Authorship contribution statement
Zoia, Bongetta: Investigation,
Luzzi:Writing – original draft.
Mantovani, Mezzini: Writing – review & editing.
Spena, De Bonis: Supervision.
Declaration of competing interest
The authors have no competing interests to declare that are relevant to the content of this article.
Handling Editor: Dr W Peul
References
- Almeida J.P., Omay S.B., Shetty S.R., et al. Transorbital endoscopic eyelid approach for resection of sphenoorbital meningiomas with predominant hyperostosis: report of 2 cases. J. Neurosurg. 2017;128(6):1885–1895. doi: 10.3171/2017.3.JNS163110. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K., Moe K.S. Applications and outcomes of orbital and transorbital endoscopic surgery. Otolaryngology-Head Neck Surg. (Tokyo) 2011;144(5):815–820. doi: 10.1177/0194599810397285. [DOI] [PubMed] [Google Scholar]
- Castelnuovo P., Pistochini A., Locatelli D. Different surgical approaches to the sellar region: focusing on the “two nostrils four hands technique.”. Rhinology. 2006;44(1):2–7. [PubMed] [Google Scholar]
- Castelnuovo P., Turri-Zanoni M., Battaglia P., Locatelli D., Dallan I. Endoscopic endonasal management of orbital pathologies. Neurosurg Clin N Am. 2015;26(3):463–472. doi: 10.1016/j.nec.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Choi K.J., Jang D.W., Abi Hachem R. Endoscopic endonasal approaches to the orbit. Int. Ophthalmol. Clin. 2018;58(2):85. doi: 10.1097/IIO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- Dallan I., Castelnuovo P., Locatelli D., et al. Multiportal combined transorbital transnasal endoscopic approach for the management of selected skull base lesions: preliminary experience. World Neurosurg. 2015;84(1):97–107. doi: 10.1016/j.wneu.2015.02.034. [DOI] [PubMed] [Google Scholar]
- Dallan I., Sellari-Franceschini S., Turri-Zanoni M., et al. Endoscopic transorbital superior eyelid approach for the management of selected spheno-orbital meningiomas: preliminary experience. Oper Neurosurg (Hagerstown). 2018;14(3):243–251. doi: 10.1093/ons/opx100. [DOI] [PubMed] [Google Scholar]
- Di Somma A., De Rosa A., Ferrés A., et al. Endoscopic transorbital approach for the management of spheno-orbital meningiomas: literature review and preliminary experience. World Neurosurg. 2023;176:43–59. doi: 10.1016/j.wneu.2023.03.126. [DOI] [PubMed] [Google Scholar]
- Jeon C., Hong C.K., Woo K.I., et al. Endoscopic transorbital surgery for Meckel's cave and middle cranial fossa tumors: surgical technique and early results. J. Neurosurg. 2018;131(4):1126–1135. doi: 10.3171/2018.6.JNS181099. [DOI] [PubMed] [Google Scholar]
- Kong D.S., Young S.M., Hong C.K., et al. Clinical and ophthalmological outcome of endoscopic transorbital surgery for cranioorbital tumors. J. Neurosurg. 2018;131(3):667–675. doi: 10.3171/2018.3.JNS173233. [DOI] [PubMed] [Google Scholar]
- Locatelli D., Pozzi F., Turri-Zanoni M., et al. Transorbital endoscopic approaches to the skull base: current concepts and future perspectives. J. Neurosurg. Sci. 2016;60(4):514–525. [PubMed] [Google Scholar]
- Locatelli D., Restelli F., Alfiero T., et al. The role of the transorbital superior eyelid approach in the management of selected spheno-orbital meningiomas: in-depth analysis of indications, technique, and outcomes from the study of a cohort of 35 patients. J Neurol Surg B Skull Base. 2020;83(2):145–158. doi: 10.1055/s-0040-1718914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S., Zoia C., Rampini A.D., et al. Lateral transorbital neuroendoscopic approach for intraconal meningioma of the orbital apex: technical nuances and literature review. World Neurosurg. 2019;131:10–17. doi: 10.1016/j.wneu.2019.07.152. [DOI] [PubMed] [Google Scholar]
- Mathew G., Sohrabi C., Franchi T., Nicola M., Kerwan A., Agha R. Preferred reporting of case series in surgery (PROCESS) 2023 guidelines. Int. J. Surg. 2023;109(12):3760–3769. doi: 10.1097/JS9.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe K.S., Bergeron C.M., Ellenbogen R.G. Transorbital neuroendoscopic surgery. Neurosurgery. 2010;67(3 Suppl. Operative):ons16–28. doi: 10.1227/01.NEU.0000373431.08464.43. [DOI] [PubMed] [Google Scholar]
- Murchison A.P., Rosen M.R., Evans J.J., Bilyk J.R. Endoscopic approach to the orbital apex and periorbital skull base. Laryngoscope. 2011;121(3):463–467. doi: 10.1002/lary.21357. [DOI] [PubMed] [Google Scholar]
- Norris J.L., Cleasby G.W. Endoscopic orbital surgery. Am. J. Ophthalmol. 1981;91(2):249–252. doi: 10.1016/0002-9394(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Paluzzi A., Gardner P.A., Fernandez-Miranda J.C., et al. “Round-the-Clock” surgical access to the orbit. J Neurol Surg B Skull Base. 2015;76(1):12–24. doi: 10.1055/s-0033-1360580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.H., Hong S.D., Kim Y.H., et al. Endoscopic transorbital and endonasal approach for trigeminal schwannomas: a retrospective multicenter analysis (KOSEN-005) J. Neurosurg. 2020;133(2):467–476. doi: 10.3171/2019.3.JNS19492. [DOI] [PubMed] [Google Scholar]
- Peron S., Cividini A., Santi L., Galante N., Castelnuovo P., Locatelli D. In: Trends in Reconstructive Neurosurgery. Visocchi M., Mehdorn H.M., Katayama Y., von Wild K.R.H., editors. Springer International Publishing; 2017. Spheno-orbital meningiomas: when the endoscopic approach is better; pp. 123–128. [DOI] [Google Scholar]
- Rachinger W., Grau S., Tonn J.C. Different microsurgical approaches to meningiomas of the anterior cranial base. Acta Neurochir. 2010;152(6):931–939. doi: 10.1007/s00701-010-0646-1. [DOI] [PubMed] [Google Scholar]
- Ramakrishna R., Kim L.J., Bly R.A., Moe K., Ferreira M. Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J. Clin. Neurosci. 2016;24:99–104. doi: 10.1016/j.jocn.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakashita T., Oridate N., Homma A., et al. Complications of skull base surgery: an analysis of 30 cases. Skull Base. 2009;19(2):127–132. doi: 10.1055/s-0028-1096201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokken J., Gumber D., Antisdel J., Sindwani R. Endoscopic surgery of the orbital apex: outcomes and emerging techniques. Laryngoscope. 2016;126(1):20–24. doi: 10.1002/lary.25539. [DOI] [PubMed] [Google Scholar]
- Zoia C., Bongetta D., Pagella F., Antoniazzi E.R., Gaetani P. New surgical option for optic nerve sheath meningiomas: fully endoscopic transnasal approach. Can. J. Ophthalmol. 2018;53(4):e142–e144. doi: 10.1016/j.jcjo.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Zoia C., Bongetta D., Gaetani P. Endoscopic transorbital surgery for spheno-orbital lesions: how I do it. Acta Neurochir. 2018;160(6):1231–1233. doi: 10.1007/s00701-018-3529-5. [DOI] [PubMed] [Google Scholar]
- Zoli M., Sollini G., Rustici A., et al. Endoscopic transorbital approach for spheno-orbital tumors: case series and systematic review of literature. World Neurosurg. 2023 doi: 10.1016/j.wneu.2023.06.026. Published online June 16. S1878-8750(23)00797-0. [DOI] [PubMed] [Google Scholar]