Abstract
Aggressive angiomyxoma (AAM) is a rare benign tumor that arises from connective tissue, prominently located in the vulva, vagina, perineum, and pelvis and is mainly found in women aged about 20-40 years old. Giant intraabdominal tumors have rarely been described. These tumors develop slowly over time and are often difficult to diagnose due to various clinical findings, especially in the early stages. Even though surgery is the primary treatment method, the possibility of complete resection is sometimes limited because the tumor tends to infiltrate nearby structures, leading to local recurrence. Only about 10% of AAM cases can be accurately diagnosed before treatment, which causes ineffective outcomes. This article demonstrates a case of giant intra-abdominal AAM precisely diagnosed by suspicious signs on CT and MRI scans before starting treatment.
Keywords: Angiomyxoma, Computed tomography, Magnetic resonance imaging
Introduction
Aggressive angiomyxoma (AAM) is a low-grade mesenchymal tumor, first described in 1983 by Steeper et Rosai [1]. In our literature search, around 300 cases have been reported, with prevalent sizes ranging from 8 cm to 20 cm [2]. There is a high occurrence among females compared to males, with a ratio of 6:1, and females of reproductive age are more likely affected [3]. The vast majority of AAMs are found in the pelvic floor, vulva, and vagina. Hence, they can be clinically misdiagnosed with other benign lesions: Bartholin cyst, vulvar abscess, vaginal cyst, or femoral hernia, possibly leading to treatment failure [4]. Furthermore, a considerable tumor can compress multiple adjacent organs in the pelvis, such as the rectum, urethra, and bladder, resulting in various clinical manifestations, thereby complicating diagnosis. Although the histopathological test has been the gold standard test up to date, the biopsy is sometimes challenging. It poses potential risks composed of bleeding, infection, and damage to pelvis organs due to the deep location of the tumor. Ultrasound is often used for initial examination but is not an effective assessment. Both computed tomography (CT) and magnetic resonance imaging (MRI) are essential for determining tumor characteristics consisting of location, size, and composition and the tumor association with pelvis organs, which optimize diagnosis and treatment [5]. Imaging features of alternating hypointense bands forming layered patterns on T2 weight (T2W) were observed in 83% of cases [6]. A literature review by Wang and colleagues found that only 7 out of 65 AAM patients were accurately diagnosed initially, accounting for 10.77% of cases [7]. However, by combining factors of epidemiology, clinical progression, and tumor characteristics on image findings, diagnosing AAM pretreatment is not unachievable. This article presents features of radiological examination, particularly on MRI, that support the diagnosis of AAM precisely before treatment.
Case reports
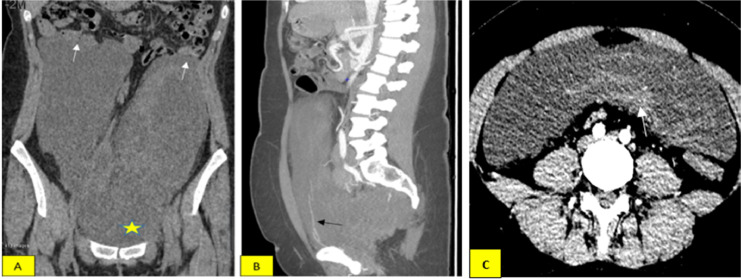
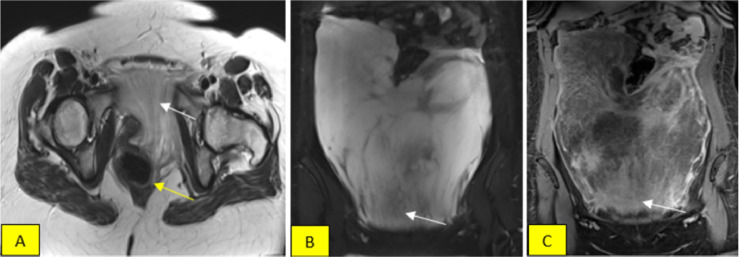
A 29-year-old female patient with no past medical history complained of abdominal distension over a month, accompanied by dull abdominal pain in the right iliac region. Clinical examination revealed a soft tissue mass in the abdomen, mainly in the hypochondriac and left iliac region. A CT scan showed a large mass measuring 30 × 23 × 6.5 cm in the abdomen, hypodense on the unenhanced images, intense, and heterogeneous enhancement on the postcontrast (Fig. 1A), with small vessels within the lesion (Fig. 1B). The vast mass displaced intra-abdominal and pelvis organs, pushing the intestinal to deviate upward. Still, no signs of invasion (Fig. 1A) or enlarged lymph nodes surrounded the mass. Tumor markers tests indicated no significant findings; mainly, the antigen carcinogen (ACE) was 0.9 ng/L, CA19-9 was 6 U/mL, CA 125 was 35 U/mL, and CA 15.3 was 6 U/mL. The mass has characteristics of low signal relative to muscle on the T1-weighted (T1W) MRI and exhibits strong and irregular enhancement after contrast administration with layers appearance, primarily concentrated in the lower portion (Fig. 2C). The tumor was heterogeneous hyperintense on T2W and T2 fat-suppressed (T2FS) sequences. Beneath the tumor, alternating low-signal-intensity bands suggested collagen fibrils on the myxoid lesion background (Figs. 2A and B) corresponding to the intense enhancing part after contrast agent injection (Fig. 2C). The mass compressed and pushed the rectum, urethra, and uterus to the right, while a small part spread downward to the left vulva. The constellation of clinicopathological and imaging characteristics can prompt the diagnosis of AAM.
Fig. 1.
(A) Unenhanced coronal CT images indicate a huge mass displaces intra-abdominal, partly compressing nearby loop of intestinal (white arrow), relatively homogeneous hypointense mass, and no sign of bleeding or calcification inside (star shape). (B) The sagittal arterial phase with MIP reformatted after administration shows heterogeneous enhancement; there is a clear observation of vessels within the mass (black arrow). (C) Axial arterial phase, the mass shows a lesion with heterogeneous hypodensity, with inside areas of contrast enhancement (arrow).
Fig. 2.
(A and B) Coronal and sagittal with T2W and T2FS sequences demonstrate alternating layers of high-low intensity concentrated in the lower portion (white arrows in Figs. A and B), pointing to collagen lesions on the myxoid background. (A) depicts a small portion of the tumor extending downward the pelvic floor, pushing the rectum slightly toward the right (yellow arrow). (C) Coronal T1 fat-suppressed reveals intense enhancement predominantly in the lower part (arrow), thus potentially determining the orientation for biopsy.
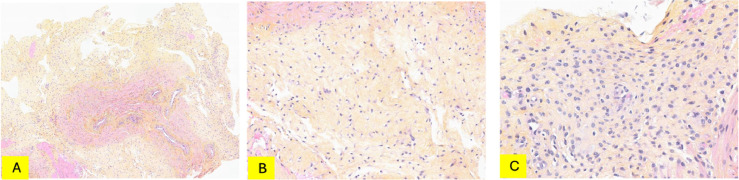
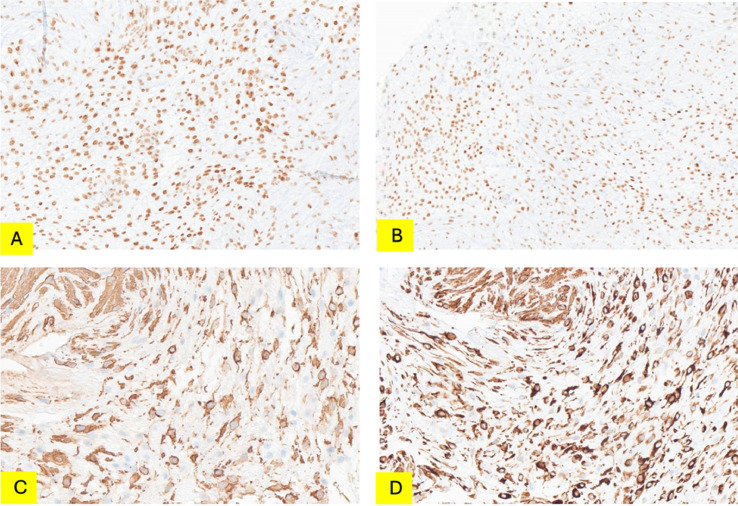
Subsequently, the patient underwent ultrasound-guided biopsy. Histologic examination revealed smooth muscular tissue containing numerous vessels invaded by tumor cells in a hypocellular fibrous stroma background (Fig. 3). The tumor cells were medium in size and had cytoplasmic processes. The nuclei were oval-shaped, distinct nucleoli. Immunohistochemical staining of the tumor showed positivity for CD34, beta catenin, HMGA2, estrogen, and progesterone receptors; negative for S100, SOX10, MUC4, STAT6, EMA, MyoD1, MDM2 (Fig. 4). These results aid in confirming AAM.
Fig. 3.
HPS staining. (A) Smooth muscular tissue containing numerous vessels. Vessels are infiltrated by hypocellular growth in a fibrous background (magnification x5). (B) The tumor cells are medium in size with cytoplasmic processes (magnification x15.6). (C) Round or oval nuclei, distinct nucleoli (magnification x28.4). HPS, Hémalun, Phloxine, Safran.
Fig. 4.
IHC staining: (A) HMGA2—intense nuclear staining of tumor cells. (B) ER—intense nuclear staining of tumor cells. (C) SMA—positive with a part of spindle cells. (D) Desmin—intense cytoplasmic staining of tumor cells. IHC, immunohistochemical.
Surgical resection and possible risks, including the removal of adjacent organs, were evaluated during medical consultation. However, the patient refused surgery due to her desire to give birth.
Discussion
AAM is an uncommon benign soft-tissue neoplasm listed as a “Tumor of Uncertain Differentiation” in the 2013 edition of the World Health Organization classification of soft tissue and bone tumors. Usually, it gradually arises in the pelvis and perineum area of women of childbearing age [2,8]. Although there have been 3 cases of AAM diagnosed with pulmonary metastasis, the term “aggressive” does not refer to the malignancy level of the tumor, but rather represents its local invasion, leading to a recurrence rate after surgery within 5 years of up to 85% [2].
The clinical diagnosis of AAM is challenging due to 65% of AAM cases being asymptomatic, while the remaining 35% present with varied and non-specific symptoms among patients. Some clinical manifestations that may occur include abdominal pain or vague discomfort in the pelvic and perineal area. When the tumor compresses local organs such as the rectum, bladder, and urethra, it can lead to urinary dysfunction, such as polyuria [9].
Abdominal ultrasound is AAM's initial imaging diagnostic examination, showing a hypoechoic lesion with heterogeneous internal echogenicity, fibrillary internal structure, and the possibility of detecting internal blood flow signals. However, ultrasound alone is not sufficient for a comprehensive evaluation of the tumor. CT Scan reveals a low but heterogeneous density mass compared to surrounding muscle tissues, slightly irregular and enhanced after contrast administration, and the possibility of observing dilated, tortuous vessels within the tumor, indicating a highly vascularized lesion (seen in 44% of cases). From this, preoperative embolization treatment may enhance treatment effectiveness [[10], [11], [12]]. In our clinical case, we observe the intra-tumoral blood vessels in the CT's maximum intense projection (MIP) mode (Fig. 1B). MRI is considered an optimal diagnostic method for AAM. On T2W imaging, the high signal intensity of the tumor is explained by its highwater content and loose myxoid matrix [13]. In addition, hyperintense and hypointense bands are observed within the T2-weighted high-signal tumor background, concentrated in the lower area of the lesion. Their layered and swirling appearance after administering gadolinium indicates collagen fiber injury on the myxoid background (Figs. 2A and B), a specific diagnostic feature for AAM (present in 83% of cases) [6]. Calcification and cystic degeneration are rare findings, with 6% and 19%, respectively. These 2 signs are not observed in the present study. Furthermore, on MRI, the detailed evaluation of the tumor's involvement with local organs such as the rectum, bladder, uterus, and urethra optimize the treatment strategy for the patient.
In the pathological examination, tumor cells are scattered throughout a myxoid-rich matrix. The blood vessels within the tumor are often arranged in a disorganized manner. Although immunohistochemical staining is not specific for AAM, intense positive staining for CD34, estrogen, and progesterone receptors and moderate positive staining for desmin help distinguish AAM from other tumors [5]. Moreover, the tumor cells are commonly positive for estrogen and progesterone receptors, suggesting the role of hormones in the development of the tumor and providing an explanation for its higher incidence in women of reproductive age. Therefore, hormone therapy is a factor that can be considered for women who desire conservative treatment [11].
The differential diagnosis of AAM mainly includes other mesenchymal neoplasms, particularly angiomyofibroblastoma and superficial angiomyxoma. Angiomyofibroblastoma is a benign lesion that predominantly arises in the superficial soft tissue with a distinct border and small size (<5 cm) [14]. In contrast, AAM is located in the pelvis and perineum, with a large size (>10 cm), and tends to invade local organs. Superficial angiomyxoma is another harmless lesion that develops in the subcutaneous layer and is commonly found in the head and neck region. In terms of histology, superficial angiomyxoma are often lack large blood vessels in comparison with AAM [11].
Surgery is considered an optimal treatment, although tumors are often significant in size when detected and tend to invade adjacent structures, making their complete removal a challenge. This feature can lead to residual damage and local recurrence after treatment, which can occur up to 72% within 2-4 years following surgery [6]. Furthermore, due to its location in the pelvis and perineum, there is a risk of partial or complete removal of organs associated with the tumor, especially reproductive organs, which increases the probability of surgical complications and poses long-term psychological effects on patients of reproductive age [5]. Chemotherapy and radiation therapy are not recommended for the treatment of AAM. In some cases, preoperative embolization can reduce the size and decrease the risk of post-operative bleeding. However, this remains a controversial issue as insufficient data is available to confirm its effectiveness. Recently, hormone therapy has been highly considered based on the histological characteristics of tumor cells that are positive for estrogen and progesterone receptors. This method can help patients avoid surgery and the potential risk of having their reproductive organs removed in some cases, which can impact the patient's desire for future fertility [5].
Conclusion
AAM is an uncommon but benign mesenchymal neoplasm that undergoes a slow development process but exhibits a high recurrence rate after treatment. It commonly occurs in women of reproductive age with nonspecific clinical signs and symptoms. On MRI, specific diagnostic features to consider for AAM include a large mass located in the pelvis that compresses adjacent organs rather than invading them; a lesion with high but heterogeneous signal intensity, characterized by hyperintense and hypointense bands concentrated in the lower area of the lesion, creating layers on a T2W background; and strong, heterogeneous enhancement after gadolinium administration. Furthermore, MRI is strongly advised for therapeutic strategy as well as long-term follow-up after treatment due to the high recurrence rate after treatment of this soft tissue mass.
Author's contributions
Trinh AD, Nguyen DH, and Nguyen MD: Case file retrieval and case summary preparation. Trinh AD, Nguyen DH, and Nguyen MD: preparation of manuscript and editing. All authors read and approved the final manuscript.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
Our institution does not require ethical approval for reporting individual cases or case series.
Patient consent
Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Steeper TA, Rosai J. Aggressive angiomyxoma of the female pelvis and perineum: report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm*. Am J Surg Pathol. 1983;7(5):463–476. doi: 10.1097/00000478-198307000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Zhao H, Xie Y, Jin M. Clinicopathological features and differential diagnosis of aggressive angiomyxoma of the female pelvis: 5 case reports and literature review. Medicine (Baltimore) 2017;96(20):e6820. doi: 10.1097/MD.0000000000006820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fetsch JF, Laskin WB, Lefkowitz M, Kindblom LG, Meis-Kindblom JM. Aggressive angiomyxoma: a clinicopathologic study of 29 female patients. Cancer. 1996;78(1):79–90. doi: 10.1002/(SICI)1097-0142(19960701)78. 1<79::AID-CNCR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Sozutek A, Irkorucu O, Reyhan E, et al. A giant aggressive angiomyxoma of the pelvis misdiagnosed as incarcerated femoral hernia: a case report and review of the literature. Case Rep Surg. 2016;2016:1–6. doi: 10.1155/2016/9256749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H, Sun P, Xu R, Wang L, Shi Y. Aggressive angiomyxoma in pregnancy: a case report and literature review. J Int Med Res. 2020;48(7) doi: 10.1177/0300060520936414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Umairi RS, Kamona A, Al-Busaidi FM. Aggressive angiomyxoma of the pelvis and perineum: a case report and literature review. Oman Med J. 2016;31(6):456–458. doi: 10.5001/omj.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Bu X, Liu Y, Xing Y, Tong Q. Characteristics and treatment strategies of aggressive angiomyxoma in women: a retrospective review of 87 cases. Front Surg. 2023;10 doi: 10.3389/fsurg.2023.966971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abduljabbar A, Wazzan M. Recurrent aggressive angiomyxoma presented with perianal mass and typical imaging swirl sign. Int J Surg Case Rep. 2020;72:486–489. doi: 10.1016/j.ijscr.2020.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Zhu L, Chang X, Chen J, Lang J. Clinicopathological features and treatment analysis of rare aggressive angiomyxoma of the female pelvis and perineum – a retrospective study. Pathol Oncol Res. 2017;23(1):131–137. doi: 10.1007/s12253-016-0109-y. [DOI] [PubMed] [Google Scholar]
- 10.Outwater EK, Marchetto BE, Wagner BJ, Siegelman ES. Aggressive angiomyxoma: findings on CT and MR imaging. Am J Roentgenol. 1999;172(2):435–438. doi: 10.2214/ajr.172.2.9930798. [DOI] [PubMed] [Google Scholar]
- 11.Sutton BJ, Laudadio J. Aggressive angiomyxoma. Arch Pathol Lab Med. 2012;136(2):217–221. doi: 10.5858/arpa.2011-0056-RS. [DOI] [PubMed] [Google Scholar]
- 12.Surabhi VR, Garg N, Frumovitz M, Bhosale P, Prasad SR, Meis JM. Aggressive angiomyxomas: a comprehensive imaging review with clinical and histopathologic correlation. Am J Roentgenol. 2014;202(6):1171–1178. doi: 10.2214/AJR.13.11668. [DOI] [PubMed] [Google Scholar]
- 13.Heffernan EJ, Hayes MM, Alkubaidan FO, Clarkson PW, Munk PL. Aggressive angiomyxoma of the thigh. Skeletal Radiol. 2008;37(7):673–678. doi: 10.1007/s00256-008-0465-0. [DOI] [PubMed] [Google Scholar]
- 14.Jeyadevan NN, Sohaib SAA, Thomas JM, Jeyarajah A, Shepherd JH, Fisher C. Imaging features of aggressive angiomyxoma. Clin Radiol. 2003;58(2):157–162. doi: 10.1053/crad.2002.1127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.