Abstract
Circulating extracellular vesicles and particles (EVPs) are being investigated as potential biomarkers for early cancer detection, prognosis, and disease monitoring. However, the suboptimal purity of EVPs isolated from peripheral blood plasma has posed a challenge of in‐depth analysis of the EVP proteome. Here, we compared the effectiveness of different methods for isolating EVPs from healthy donor plasma, including ultracentrifugation (UC)‐based protocols, phosphatidylserine‐Tim4 interaction‐based affinity capture (referred to as “PS”), and several commercial kits. Modified UC methods with an additional UC washing or size exclusion chromatography step substantially improved EVP purity and enabled the detection of additional proteins via proteomic mass spectrometry, including many plasma membrane and cytoplasmic proteins involved in vesicular regulation pathways. This improved performance was reproduced in cancer patient plasma specimens, resulting in the identification of a greater number of differentially expressed EVP proteins, thus expanding the range of potential biomarker candidates. However, PS and other commercial kits did not outperform UC‐based methods in improving plasma EVP purity. PS yielded abundant contaminating proteins and a biased enrichment for specific EVP subsets, thus unsuitable for proteomic profiling of plasma EVPs. Therefore, we have optimized UC‐based protocols for circulating EVP isolation, which enable further in‐depth proteomic analysis for biomarker discovery.
Keywords: biomarkers, early cancer detection, extracellular vesicles and particles (EVPs), proteomics
1. INTRODUCTION
In the past decade, circulating extracellular vesicles (nanoparticles enclosed by a lipid bilayer, such as exosomes) and particles (nanoparticles lacking external membranous structure, such as exomeres) (EVPs) have emerged as promising biomarker reservoirs for early cancer detection, prognosis, and monitoring treatment response in cancer patients (Chen et al., 2017; Hoshino et al., 2015, 2020; Lucotti et al., 2022; Shimada et al., 2021). Tumor cells actively release EVPs to mediate intercellular communication with cells in the local tumor microenvironment as well as with cells in distant organs, including pre‐metastatic niches at future sites of metastasis, through circulation (Lucotti et al., 2022; Pelissier Vatter et al., 2021; Sheehan & D'Souza‐Schorey, 2019; Xu et al., 2018). Tumor‐derived EVPs are released in peripheral blood and hold high potential for identifying biomarkers in minimally invasive liquid biopsies for cancer patients (Colombo et al., 2014; Fraser et al., 2019). In addition to EVPs shed by tumor cells, EVPs are also produced by cells in the tumor microenvironment as well as cells in distant organs that have been systemically influenced by the tumor. These EVPs can also contribute to biomarker reservoirs reflective of disease status. For example, alterations in the protein cargos of EVPs released from a perturbed immune system in cancer patients can be detected in the circulating EVP pool and used to distinguish cancer patients from healthy donor (HD) controls (Hoshino et al., 2020).
An essential step in identifying novel circulating EVP‐based biomarkers is to perform an in‐depth characterization of the molecular cargos carried by EVPs in plasma. Besides cataloging EVP‐associated RNAs (especially microRNAs), proteomic profiling of plasma EVPs via mass spectrometry (MS) has emerged as a predominant strategy for biomarker discovery (Choi et al., 2015; Ko et al., 2018). We recently conducted a comprehensive proteomics study in which we identified EVP‐based biomarkers for cancer detection in multiple cancer types (Hoshino et al., 2020). Despite the success of this study, we observed that the plasma‐derived EVPs isolated using the same differential ultracentrifugation (UC) method, involving two steps of high‐speed UC, yielded a much lower total number of different proteins identified compared to EVPs derived from cell lines and tissue explant cultures. For example, an average of 265 proteins were identified via proteomic MS in plasma EVPs whereas an average of 852 and 1482 proteins were identified in EVPs isolated from human cell lines and tissue explant cultures, respectively. We reasoned that the lower detection rates for plasma‐derived EVP samples were likely due to the high abundance of contaminants uniquely present in these samples but not EVPs isolated from other sources. Therefore, we aimed to optimize our current EVP isolation procedure further to enhance the purity of plasma‐derived EVPs and enable more extensive analysis of their proteomes for the discovery of novel biomarkers.
In this work, we compared the performance of several UC‐based EVP isolation protocols, the phosphatidylserine‐Tim4 interaction‐based affinity capture approach (referred as “PS”) and several commercial kits. Given the additional contribution of non‐tumor tissue‐derived EVPs that also reflect the disease status in cancer patients (Hoshino et al., 2020), we thereby focused on EVP isolation procedures that have been developed based on general biophysical properties of EVPs, such as UC‐based approaches, rather than immunoaffinity capturing (IAC) approaches that reply on the expression of tumor‐specific markers (e.g., EpCAM). Considering EVP heterogeneity and the lack of universal expression of surface markers such as CD9, CD81, and CD63, and especially their absence in exomeres, IAC approaches that rely on the expression of EVP surface markers were not chosen in this study. As phosphatidylserine is a common component of EV membranes and has also been reported in exomeres (although its configuration has not yet been resolved) (Zhang et al., 2018), we sought to compare the PS kit with conventional UC‐based methods for isolating EVPs to perform downstream proteomic profiling via MS. Moreover, we also included several commercial kits that enable isolation of EVPs without any limitation to specific EVP subsets for comparison of their performance. We evaluated EVP yield, purity, and the depth of EVP proteome profiling via MS and showed that by adding an additional UC washing step or a size exclusion chromatography step, the optimized UC‐based procedures yielded better performance than the other methods assessed in this study.
2. MATERIALS AND METHODS
2.1. Plasma samples
Large volume (about 300 mL) of plasma samples from a total of six HDs were purchased from New York Blood Center for method development and comparison (collected using citrate‐phosphate‐dextrose as anti‐coagulant). For the validation study, five patients with newly diagnosed, stage I–III pancreatic cancer and five matching healthy controls were recruited at Memorial Sloan Kettering Cancer Center, and 5–10 mL of blood samples were collected in EDTA tubes (MSKCC, IRB #15‐015, IRB # 17–527). Plasma was then prepared by 10 min centrifugation at 500×g, 20 min centrifugation at 3000×g, and the supernatant was collected and stored at −80°C for EVP isolation. All individuals provided informed consent for sample collection according to protocols approved by the Institutional Review Boards of MSKCC and NIH. The study is compliant with all relevant ethical regulations regarding research involving human participants.
2.2. Purification of EVPs
Plasma samples were thawed at 4°C and centrifuged at 12,000×g for 20 min at 10°C to remove large microvesicles. Supernatant was then transferred to new tubes (Beckman Coulter, 355645) and centrifuged at 100,000×g for 70 min at 10°C using 50.4Ti rotor (Beckman Coulter, 347299) in a Beckman Coulter Optima XE or XPE ultracentrifuge. Pellets were re‐suspended in ice‐cold PBS, and at least 2 mL of PBS was used to wash each sample. Samples were centrifuged again at 100,000×g for 70 min at 10°C. For UC2, the pellets were re‐suspended in 150 µL of PBS. For UC3, the pellets were re‐suspended in 2 mL of ice‐cold PBS and another round of centrifugation was performed at 100,000×g for 70 min at 10°C. The pellets were re‐suspended in 150 µL of PBS. To obtain enough EVP products for a range of downstream assays, we typically initiate with 5 mL of plasma for UC2, 8 mL for UC3, and up to 40 mL for UC2‐qEV.
For UC2‐qEV, EVPs purified by UC2 were re‐suspended in 500 µL of PBS and loaded onto qEV original/70 nm (Izon, ICO‐70) columns which were previously equilibrated with 30 mL of ice‐cold PBS per manufacture's instruction. The void volume (∼3 mL) was discarded, and fractions (0.5 mL per fraction) were collected starting from fraction 7 using the automatic fraction collector (Izon). Fractions 8–10 were EVPs enriched, combined, and concentrated using centrifugal filter units (Millipore, UFC903008) for downstream analysis.
MagCapture Exosome Isolation Kit PS (FUJIFILM Wako Pure Chemical Corporation, 293–77601) was used for PS beads‐based isolation of EVPs. One milliliter of plasma was centrifuged at 12,000×g for 20 min at 10°C, and the supernatant was used for each purification following essentially the manufacturer's protocol.
For qEV‐DGC, 0.5 mL of plasma was applied to the qEV column as described above and EVP‐containing fractions from five runs were pooled and concentrated using centrifugal filter units (Millipore, UFC903008) and diluted with PBS to a final volume of 500 µL. To prepare the discontinuous iodixanol gradient, 40% (w/v), 20% (w/v), 10% (w/v), 5% (w/v) solutions of iodixanol were made by diluting OptiPrep (Cosmo Bio, AXS‐1114542) (60% (w/v)) with sucrose solution (0.25 M, 10 mM Tris, pH 7.5). To set the gradient, 3 mL of 40% iodixanol solution was added to the bottom of a 14 × 95 mm ultra clear tube (Beckman Coulter, 344060). 3 mL of 20% solution was gently layered to the top of 40% solution with a syringe, followed by 3 mL of 10% solution and 2.5 mL of 5% solution. 500 μL of concentrated qEV eluate were overlaid onto the top of the gradient. The gradient was centrifuged at 100,000 x g for 16 h at 10°C using a SW‐40Ti rotor (Beckman Coulter, 331302). 12 fractions of 1 mL were collected with pipettes from top to bottom. Fractions 6‐9, which were enriched for EVPs, were pooled, diluted with 11 mL of PBS, and concentrated using centrifugal filter units (Millipore, UFC903008).
For DGC+Bind‐Elute size exclusion chromatography (BE‐SEC), the discontinuous iodixanol gradient was prepared as described previously. Plasma was centrifuged at 12,000 x g for 20 min, and 500 μL of supernatant was overlaid onto the top of each gradient. The gradient was centrifuged at 100,000×g for 16 h at 10°C using a SW‐40Ti rotor (Beckman Coulter, 331302). Twelve fractions of 1 mL were collected with pipettes from top to bottom. Fractions 6–9 from four gradients (2 mL plasma total) were pooled, diluted with PBS, and concentrated using centrifugal filter units (Millipore, UFC9030). The concentrated EVP sample was further washed with 11 mL of PBS and concentrated using centrifugal filter units twice. A HiScreen Capto Core 700 column (4.7 mL bed volume, Cytiva, 17548115) was connected and run using the AKTAPure system, and a HiTrap Capto Core 700 column (1 mL bed volume, Cytiva, 17548151) was connected to a syringe and operated manually according to the manufacturer's instructions. The columns were washed with distilled water first and then equilibrated with five column volumes of PBS. The concentrated EVP samples were then loaded onto the columns and eluted with PBS. The flow rate was 1 mL/min for the HiScreen column and approximately 1.8 mL/min for the HiTrap column. Fractions were collected and concentrated using centrifugal filter units (Millipore, UFC5030) for BCA quantification, ELISA and EM imaging analysis. Cleaning‐In‐Place (CIP) was performed according to the manufacturer's manual to recover the columns for future use. EVP‐containing fractions, as revealed by CD9 ELISA, were further combined for Western blot analyses.
Four commercial kits were also used for EVP purification: exoEasy Maxi Kit (Qiagen, 76064); Exo‐spin midi (Cell Guidance Systems, EX04‐5); ExoQuick Exosome Isolation and RNA Purification Kit (for Serum & Plasma) (System Biosciences, EQ806A‐1); and miRCURY Exosome Serum/Plasma Kit (Qiagen, 76603). EVP purification was performed following the manufacturer's instructions, and EVPs were re‐suspended in PBS.
2.3. EVP characterization
BCA Protein Assay (Pierce, Thermo Fisher Scientific, 23227) and Nanosight Tracking Analysis (NTA) were conducted upon isolated EVPs following manufacturer's manual for protein quantification and particle size and number measurement, respectively. NS500 nanoparticle characterization system (NanoSight, Malvern Instruments) equipped with a blue laser (405 nm) was utilized for NTA. PS Capture Exosome ELISA Kit (Anti Mouse IgG POD) (FUJIFILM Wako Pure Chemical Corporation, 297–79201) was utilized to evaluate the relative purity of isolated EVPs and recovery rate from plasma. The Nalm6 cell line‐derived EVPs purified by UC2 were used as ELISA standards. Five hundred nanogram of Nalm6 EVPs in 200 µL of PBS was used as the first standard sample, from which the following six standard samples were prepared by serial dilution. The detecting antibody recognizes the CD9 antigen (HI9a). The relative purity of EVPs isolated using each protocol was expressed as the ratio of purified EVP amount (estimated by ELISA) to total protein amount (determined by BCA). Recovery rate was calculated as the ratio of purified EVP amount to the total EVP amount present in the input plasma (both evaluated by ELISA).
2.4. Western blotting
EVPs were first lysed in SDS lysis buffer (Biorad, #1610772EDU). EVPs isolated from an indicated volume of plasma were diluted with reducing sample buffer (Biorad, #1610747), separated on Novex 4%−20% Tris‐glycine gels (Life Technologies, XP04122BOX) and transferred onto polyvinylidene difluoride membranes (Biorad, #1704273). Membranes were sequentially blocked with 1X TBS containing 5% non‐fat milk (w/v) and 0.1% Tween20 (v/v), incubated with primary antibodies overnight at 4°C, rinsed three times and washed four times with 1X PBS containing 0.1% Tween20 (v/v), incubated with secondary antibodies (horseradish peroxidase‐conjugated anti‐mouse (invitrogen, G‐21040) or anti‐rabbit (CST, 7074P2) and washed again to remove unbound antibodies. Bound antibody complexes were detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher, 34078) or SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher, 34095) and visualized using the Bio‐Rad ChemiDoc Touch Imaging System. The primary antibodies used for western blotting include: anti‐Apo AI (Abcam, ab64308); anti‐HSA (Abcam, ab10241); Peroxidase AffiniPure Goat Anti‐Human IgG (Jackson Immunoresearch, 109‐035‐190); anti‐Flotillin‐1(BD, 610820); anti‐Hsp90 (Invitrogen, MA5‐15863); anti‐CD9 (Abcam, ab223052); and anti‐Syntenin‐1 (Santa Cruz, sc‐100336). The signal intensity of bands was quantified using ImageJ. After subtracting the background, intensity value of each band was normalized against the mean intensity of UC2 samples of the same experiment.
2.5. TEM
Negative staining TEM analysis was performed as previously described (Zhang et al., 2018). In brief, 5 µL of EVPs (0.1 µg/µL in PBS) isolated using different methods (EVPs isolated using PS in a concentration less than 0.1 µg/µL in Elution Buffer provided by the kit) were placed on a formvar/carbon‐coated grid and allowed to settle for 1 min. The sample was then blotted and negatively stained with four successive drops of 1.5% aqueous uranyl acetate, blotting between each drop. Grids were air‐dried and imaged with a JEOL JSM 1400 (JEOL, USA, Ltd, Peabody, MA) transmission electron microscope operating at 100 kV. Images were captured on a Veleta 2k × 2k charge‐coupled device camera (Olympus‐SIS, Munich, Germany).
2.6. Proteomic mass spectrometry
Proteomic MS and database search were performed as previously described (Zhang et al., 2018). In brief, 2 µg of isolated EVP samples were vacuum‐dried and re‐dissolved in 30–50 µL of 8 M Urea/50 mM ammonium bicarbonate/10 mm DTT. Proteins were then alkylated using 20 or 30 mM iodoacetamide (Sigma) and digested with Endopeptidase Lys C (Wako) in <4 M urea followed by trypsination (Promega) in <2 M urea. Peptides were desalted and concentrated using Empore C18‐based solid phase extraction. Peptides were separated using a high resolution/high mass accuracy reversed phase C18 column (12 cm/75 mm, 3 mm beads, Nikkyo Technologies) at 200 or 300 nL/min with a gradient increasing from 1% Buffer B/95% buffer A to 40% buffer B/60% Buffer A in typically 90 or 120 min (buffer A: 0.1% formic acid, buffer B: 0.1% formic acid in 80% acetonitrile). Typically, 1.875 µg of samples were injected. Mass spectrometer (Fusion Lumos, Thermo Scientific) was operated in data dependent (DDA) positive ion mode.
Proteome Discoverer 1.4.1.14 (Thermo‐Scientific, 2012)/Mascot 2.5 (Perkins et al., 1999) was used for processing the high resolution/high mass accuracy nano‐LC‐MS/MS data. Human data were queried against the UniProt's Complete HUMAN proteome (February, 2020:74,788 sequences) using the following parameters: Enzyme: Trypsin/P; maximum allowed missed cleavage sites: 2; monoisotopic precursor mass tolerance:10 ppm; monoisotopic fragment mass tolerance: 0.02 Da; dynamic modifications: Oxidation (M), Acetyl (Protein N‐term); static modification: Carbamidomethyl (C). Percolator was used to calculate peptide false discovery rates (FDR), which were calculated per file. An FDR of 1% was applied to each separate LC‐MS/MS file. The sequences of porcine trypsin and Endopeptidase LysC were concatenated to the human databases.
2.7. Bioinformatics
For mass spectrometry proteomics data analysis, R (R Core Team, 2021) package DEP (Zhang et al., 2018) was used for the processing of proteomics data. Briefly, data were first filtered, background‐corrected and normalized, and imputed for missing values. Principal component analysis (PCA) was performed, and the first two principal components were used to visualize the proteomics data and evaluate global differences between technical groups. Batch effect was observed between multiple MS runs and was corrected using the removeBatchEffect function from the limma package (Ritchie et al., 2015) before plotting PCA and heatmaps.
Differentially detected proteins were identified based on filtered, background‐corrected and normalized, and imputed data using functions from the limma package. Batch effect was included in the linear model fitted by the lmFit function. Within‐donor correlation was calculated using the duplicateCorrelation function. Log fold‐changes between groups were obtained by makeContrasts function, and contrast was estimated for each gene by contrasts.fit function. Empirical Bayes smoothing of standard errors was performed, and proteins that showed at least a log fold change of 1.5 and an adjusted p value of <0.05 were considered differentially expressed. Heatmaps were created using heatmap.2 function from gplots package.
Gene set enrichment analysis (GSEA) was done using javaGSEA through the Broad Institute. For each comparison, all proteins were ranked by t‐statistic and preranked analysis was used to look at enrichment in top 200 proteins originally detected in human plasma by MS (human protein atlas: https://www.proteinatlas.org/humanproteome/blood+protein/proteins+detected+in+ms), and ECM‐associated proteins (MatrisomeDB) (https://matrisomedb.org). The default parameters were used in all analyses.
Subcellular localization of proteins was determined by QIAGEN Ingenuity Pathway Analysis (QIAGEN IPA). Pathway analysis and tissue‐ and cell type‐specific origin analysis were performed using the web‐based Metascape resource platform.
The MS‐based proteomics raw data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (https://www.ebi.ac.uk/pride) and is available via ProteomeXchange with identifier PXD052726. The proteomics datasets have also been deposited in the Figshare repository. Specifically, the proteomics data pertaining to the comparison of various methods, including UC2, UC3, UC2‐qEV, and PS, can be accessed at the following https://doi.org/10.6084/m9.figshare.22798619. The proteomics data comparing qEV‐DGC and UC are available at DOI: https://doi.org/10.6084/m9.figshare.22798934. Lastly, the proteomics data related to plasma EVP samples from pancreatic cancer patients and healthy controls can be found at https://doi.org/10.6084/m9.figshare.22798940.
2.8. Statistical analysis
To determine statistical significance for assays including BCA, NTA, WB and ELISA, one‐way ANOVA with Geisser‐Greenhouse correction, in accordance with recommended guidelines for within‐subjects analysis, was used, followed with Bonferroni's multiple comparisons test (GraphPad). For identification of differentially detected proteins via mass spectrometry, we incorporated within‐donor correlation by utilizing the duplicateCorrelation function. As a result, these analyses were systematically conducted in a pairwise manner. p value < 0.05 was considered statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3. RESULTS
3.1. Establishing protocols to isolate plasma EVPs with improved purity
To establish and compare different protocols for EVP isolation from plasma, large volumes of plasma from HDs (purchased from New York Blood Center) were used unless specified otherwise. This allowed us to compare the performance of different protocols using the same specimen, thereby avoiding confounding effects from individual variance. The differential UC protocol, one of the most well‐established and commonly used protocols in the field, involves two sequential steps of UC at 100,000 g (referred to as “UC2”) and was included as a reference for performance comparison of EVP isolation procedures (Figure 1a). To improve purity, EVPs isolated via UC2 were either subjected to a third UC step (referred to as “UC3”) or loaded onto a size‐exclusion column (referred to as “UC2‐qEV”). The MagCapture Exosome Isolation Kit PS (FUJIFILM Wako Pure Chemical Corporation, referred to as “PS”) employing the interaction between Tim4 and phosphatidylserine present on the surface of EVPs was compared in parallel. To acquire an ample quantity of EVP products for a range of downstream assays, we typically initiate with 5 mL of plasma for UC2, 8 mL for UC3, up to 40 mL for UC2‐qEV, and 1 mL for PS. BCA assay, Nanoparticle Tracking Analysis (NTA), negative staining transmission electron microscopy (TEM) and Western blotting (WB) analysis were conducted to evaluate the yield and purity of EVP samples isolated through these four protocols.
FIGURE 1.
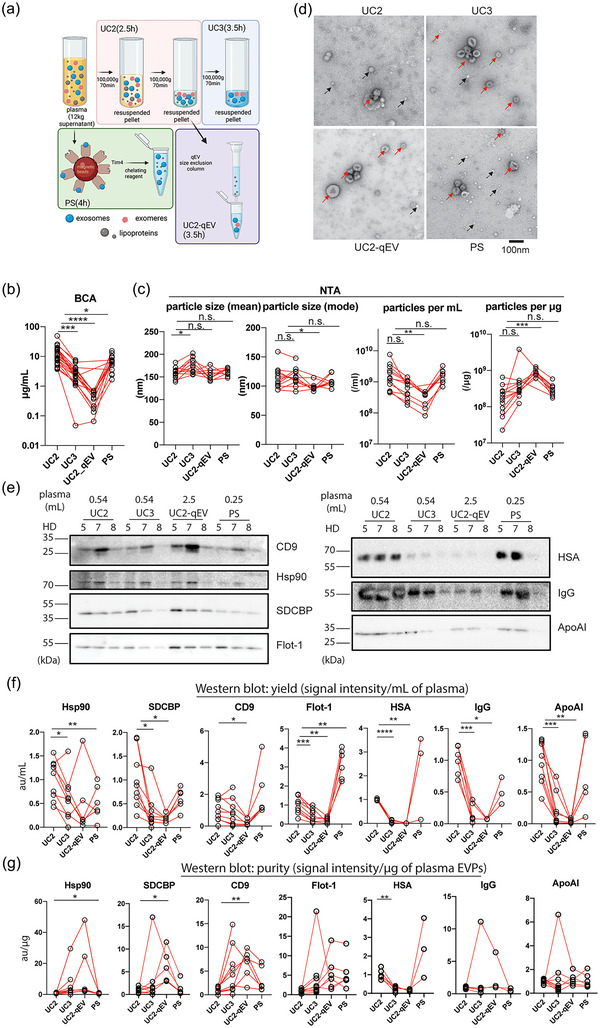
Establishment and performance comparison of protocols for isolating plasma EVPs with improved purity. (a). Diagrams illustrating the four major procedures compared for performance for isolating plasma EVPs. Estimated execution time for each procedure is listed in parenthesis. Other methods examined but not illustrated in this figure include qEV‐DGC (size exclusion chromatography followed by density gradient ultracentrifugation), DGC+BE‐SEC (DGC followed by bind‐elute size exclusion chromatography),exoEasy Maxi Kit (Qiagen), Exo‐spin midi (Cell Guidance Systems), ExoQuick Exosome Isolation and RNA Purification Kit (for Serum & Plasma) (System Biosciences), and miRCURY Exosome Serum/Plasma Kit (Qiagen). (b). Quantification of total protein yield in isolated plasma EVPs using BCA assay. n > = 12. (c) Assessment of particle size (mode and mean) and number (per mL of plasma input and per µg of isolated EVPs) in isolated plasma EVPs using Nanoparticle Tracking Analysis (NTA). n > = 8. (d) Representative TEM images of plasma EVPs isolated using different approaches. Red and black arrows indicate EVPs and particles resembling lipoprotein nanoparticles. Scale bar, 100 nm. (e) Western blotting (WB) analysis of EVP marker proteins and potential contaminants in plasma EVPs isolated from three healthy donors (HD). The volume of input plasma analyzed for each sample is indicated on top of the images. CD9, Tetraspanin CD9; Hsp90, Heat shock protein 90; SDCBP, Syntenin‐1; Flot‐1, Flotillin‐1; HSA, Human serum albumin; IgG, Immunoglobulin G; and ApoAI, Apolipoprotein AI. f and g. Quantification of WB analysis results to evaluate the yield (expressed as EVP marker protein signal/mL of plasma) and purity (expressed as EVP marker protein or contaminant signal/µg of EVPs) of isolated plasma EVPs, respectively. n > = 3. In all graphs, the bar indicates mean. One‐way ANOVA with Geisser‐Greenhouse correction was used, followed with Bonferroni's multiple comparisons test to determine the statistical significance.
The protein concentration of isolated EVPs was determined by BCA assay and then standardized to a unit volume of plasma, serving as a reference. UC2 yielded the highest number of proteins (12 µg/mL) on average, followed by PS (7 µg/mL plasma), UC3 (3 µg/mL), and UC2‐qEV (0.4 µg/mL) (Figure 1b). The particle size and number were determined by NTA (Figure 1c). The size of particles isolated through different protocols was quite similar, with UC3 EVPs being marginally larger and UC2‐qEV EVPs slightly smaller than PS and UC2 EVPs. PS and UC2 yielded similar numbers of particles per unit volume (mL) of plasma, while UC2‐qEV yielded a significantly lower number of particles per mL of plasma (p value = 0.047). UC3 showed a trend toward lower yield though not statistically significant (p value = 0.083). In contrast, when we normalized particle number to total protein amount, we found that UC2‐qEV ranked highest (p value = 0.0001), followed by UC3, PS, and UC2. It should be noted that the detection range of NTA is limited to particles down to 60–70 nm (Zhang et al., 2018), therefore the small extracellular vesicles and exomeres below 60 nm in size cannot be measured accurately by this method. Additionally, NTA is not able to distinguish between EVPs and other particles such as chylomicrons and very low‐density lipoproteins (VLDL) due to their overlap in size.
Next, we examined the morphology and purity of the isolated EVPs by conducting negative staining TEM analysis (Figure 1d). The size range and morphology of EVPs isolated through all four protocols are comparable. Nonetheless, with equivalent or even less protein loaded, we observed more membranous vesicle structures (marked by red arrows) in images of EVPs isolated through UC3, UC2‐qEV and PS compared to UC2. Compared to the dark background of UC2 and PS images, likely due to the presence of highly abundant plasma proteins (the contaminants), the images of EVPs isolated through UC2‐qEV, and to a less extent UC3, showed fewer background contaminants, indicating an improvement in EVP purity. Due to the physical property overlap between lipoprotein particles and EVPs and the higher abundance of lipoproteins in blood (1013–1016 /mL of blood depending on lipoprotein type (Simonsen, 2017) versus 107–109/mL for EVPs (Arraud et al., 2014)), lipoproteins are a major contaminant in plasma‐derived EVPs. Indeed, we observed that the number of bright, bubble‐like structures (examples marked by black arrows), which resemble lipoprotein nanoparticles, are reduced in EVPs isolated through UC3 and UC2‐qEV compared to UC2. In contrast, the PS kit enriched for such structures alongside EVPs, which can be explained by a previous study reporting enrichment of PS in a subset of high‐density lipoproteins (HDL) (Camont et al., 2013).
To evaluate the yield and purity of these EVP samples, we performed WB analysis for four well‐documented EVP markers (i.e., heat shock protein 90/Hsp90, Flotillin‐1/Flot‐1, Syntenin‐1/SDCBP and tetraspanin CD9) as well as three plasma soluble proteins (i.e., human serum albumin/HSA, immunoglobulin G/IgG and apolipoprotein AI/ApoAI) that are potential contaminants. The loading volume for each group (as indicated on top of Figure 1e) was established so most bands were visible and quantifiable. To compare the yield, the abundance of EVP markers was first normalized to the starting volume of plasma (Figure 1f). Our results showed that among the four protocols tested, UC2‐qEV followed by UC3 had the lowest yield of all four tested EVP markers. UC2 protocol recovered higher levels of Hsp90 and SDCBP than other protocols, while the PS kit enriched most for Flot‐1 and CD9. The decrease in the levels of HSA, IgG and ApoAI in EVPs isolated using UC3 and UC2‐qEV, compared to UC2, was more significant than the decrease in the levels of EVP markers, indicating improved EVP purity with UC3 and UC2‐qEV (see below). In contrast, PS retained relatively high levels of HSA, IgG and ApoAI contaminants with large individual variance.
To compare the EVP purity, the abundance of the examined proteins was normalized to the amount of total protein (Figure 1g). The highest enrichment of the four EVP markers was observed in samples prepared by UC2‐qEV followed by UC3. Intriguingly, the PS kit enriched for Flot‐1, and to a less extent CD9, but not SDCBP or Hsp90. Although HSA was significantly depleted by UC3 and UC2‐qEV, the relative abundance of IgG and ApoAI was not significantly changed in EVPs isolated through all four protocols. Notably, the different levels of enrichment of extracellular vesicle markers (Flot‐1, SDCBP and CD9) by the PS kit underscore the heterogeneity of EVPs and implicates enrichment of specific EVP subsets (e.g., Flot‐1‐high subsets) using PS.
In summary, the BCA, NTA, WB, and TEM analyses collectively demonstrate that EVPs isolated using UC2‐qEV and UC3, as compared to UC2, exhibit higher purity but lower yield, whereas the PS kit provides a comparable yield but with a biased enrichment for specific EVP subsets and poor purity.
3.2. Performance evaluation of commercial kits for plasma EVP isolation compared to UC‐based approaches
Several commercial kits for EVP isolation offer convenience by eliminating specific equipment requirements, including an ultracentrifuge. However, their suitability for isolating EVPs from plasma and downstream proteomic profiling has not been evaluated in comparison to the UC‐based approaches. Therefore, in this study, we compared the performance of four commercially available EVP isolation kits, that is, exoEasy Maxi Kit (1.5 mL of plasma was processed), Exo‐spin midi (1 mL of plasma was processed), ExoQuick Exosome Isolation and RNA Purification Kit (for Serum and Plasma, 0.5 mL of plasma was processed), and miRCURY Exosome Serum/Plasma Kit (0.6 mL of plasma was processed), with UC2 and UC3 as described above, in terms of EVP purity. Given that the UC2‐qEV protocol requires a significantly larger quantity of plasma as the starting material, we opted not to include it in this comparison.
We first used the PS ELISA assay to evaluate the EVP isolation efficiency of these commercial kits in comparison to the UC‐based methods. PS+ EVPs were captured using Tim‐4 and detected using antibodies against CD9, one of the most frequently detected markers on plasma EVPs (Hoshino et al., 2020). Although CD9 and PS expressing EVPs may represent only a subset of the plasma EVPs, we used the isolation efficiency of this subset as an indicator of overall EVP isolation efficiency. By calculating the ratio of CD9 abundance in the purified EVPs to the CD9 abundance in the original plasma input, we found that three out of the four kits (excluding exoEasy Maxi Kit) retained the majority of CD9+ EVPs and their isolation efficiency was significantly higher than that of the UC2 and UC3 (Figure S1a). However, when the CD9 abundance was normalized to the amount of the final EVP product, we found that the relative purity of CD9+ EVPs was two (Exo‐spin midi) or three (exoEasy Maxi Kit, ExoQuick Exosome Isolation and RNA Purification Kit for Serum & Plasma, and miRCURY Exosome Serum/Plasma Kit) orders of magnitude lower than UC2 and UC3 (Figure S1a). This was consistent with the TEM analysis of these samples (Figure S1b), which appear dominated by a high proportion of contaminants, such as lipoprotein particles and soluble plasma proteins. Based on these observations, we concluded that none of these kits outperformed UC2 and UC3 with respect to the purity of isolated plasma EVPs. Therefore, we did not further explore their utility for downstream proteomic profiling studies.
3.3. Performance evaluation of density gradient centrifugation in conjugation with size exclusion chromatograph for plasma EVP isolation
Density gradient centrifugation (DGC) is often used for EVP isolation but is labor‐intensive and time‐consuming (Vergauwen et al., 2021). Here, we compared a two‐step protocol combining size exclusion chromatography (SEC) and OptiPrep DGC (referred to as “qEV‐DGC”, 2.5 mL of plasma was processed) with UC2 and UC3. Given the generally low recovery from the DGC step, we concluded that a single DGC step is practical for most clinical specimens. qEV‐DGC is distinct from the UC2‐qEV protocol, which is a three‐step protocol consisting of two UC steps followed by one SEC step. BCA assay showed that qEV‐DGC yielded a larger quantity of proteins than UC2 and UC3 (Figure S1c). The particle size characterized by NTA was comparable among EVPs isolated using UC2, UC3, and qEV‐DGC (Figure S1d). When the particle number was normalized to the volume of plasma, qEV‐DGC isolated a higher number of particles than UC3 or UC2 (Figure S1d). However, when the particle number was normalized to the protein amount, the qEV‐DGC samples showed lower particle numbers per µg of protein than UC2 and UC3 samples, indicating a lower purity of EVPs.
We also conducted CD9 ELISA assay and TEM analysis to evaluate the recovery efficiency and purity of EVPs isolated through qEV‐DGC. As shown in Figure S1(a), qEV‐DGC resulted in a lower yield and lower purity of CD9 expressing EVPs than UC2 and UC3. Similar observations were made using TEM analysis (Figure S1b).
WB analysis further revealed that qEV‐DGC and UC3 had similar yields for the tested EVP markers, HSA and IgG, which were generally lower than UC2 (Figure S1f). However, qEV‐DGC resulted in a much higher amount of ApoAI in the isolated EVPs than UC3. When the purity was measured as relative abundance of EVP markers per µg of protein, we found that qEV‐DGC and UC2 resulted in comparable enrichment for most EVP markers tested, with a trend lower than UC3 (Figure S1g). Furthermore, qEV‐DGC effectively depleted HSA and IgG but not ApoAI from isolated EVPs, indicating significant contamination by HDL. These results indicated that the combination of qEV and DGC did not outperform UC3.
Recently, a modified version of SEC, known as bind‐elute size exclusion chromatography (BE‐SEC), has been developed and applied to isolate EVPs. BE‐SEC technology employs core beads to prevent larger molecules from entering the core, while smaller molecules (<700 kDa) can enter the core and bind to the hydrophobic and positively charged octylamine ligands (cytiva.com) (Corso et al., 2017). Here, we further assessed the performance of BE‐SEC in conjugation with DGC (2 mL of plasma was processed). As shown in Figure S2, CD9+PS+ ELISA and WB analysis of representative EVP markers such as CD9, Flot‐1, SDCBP, and Hsp90 indicated a lower yield with DGC+BE‐SEC compared to UC2 and UC3. The reduced yield is a limiting factor in this combined method, primarily due to the low recovery from DGC, consistent with our previous testing of the qEV‐DGC method. Evaluation based on the CD9+PS+ ELISA, EM, and WB analysis of both EVP markers and common contaminants revealed that the purity of EVPs isolated using the DGC+BE‐SEC method is not superior to the UC3 method tested in parallel. Therefore, we did not further pursue the methods involving DGC and SEC in this work. An overall evaluation of the performance of all methods investigated in this study is presented in Figure S3.
3.4. UC3 and UC2‐qEV demonstrate superior performance in enriching exosomes and exomeres and depleting plasma soluble proteins and lipoprotein nanoparticles via proteomic analysis
To examine and compare the proteomes of plasma EVPs purified through different protocols, equal amounts of EVPs isolated from six HDs using each protocol were subjected for proteomic mass spectrometry (MS) analysis in a total of three batches (see details in Figure S4a). First, the number of detected proteins significantly increased in samples prepared through UC3 and UC2‐qEV compared to UC2 and PS, with an average of 706 proteins identified by UC2, 1025 proteins by UC3, 1021 proteins by UC2‐qEV, and 649 proteins by PS (Figure 2a). This supports our hypothesis that improved EVP purity can enable a deeper, more comprehensive proteomic analysis of EVPs via MS. PCA revealed clustering of samples primarily according to isolating procedures. EVPs prepared through UC2 and PS formed separate clusters, while UC3 and UC2‐qEV samples co‐mingled (Figure 2b), suggesting a higher degree of similarity.
FIGURE 2.
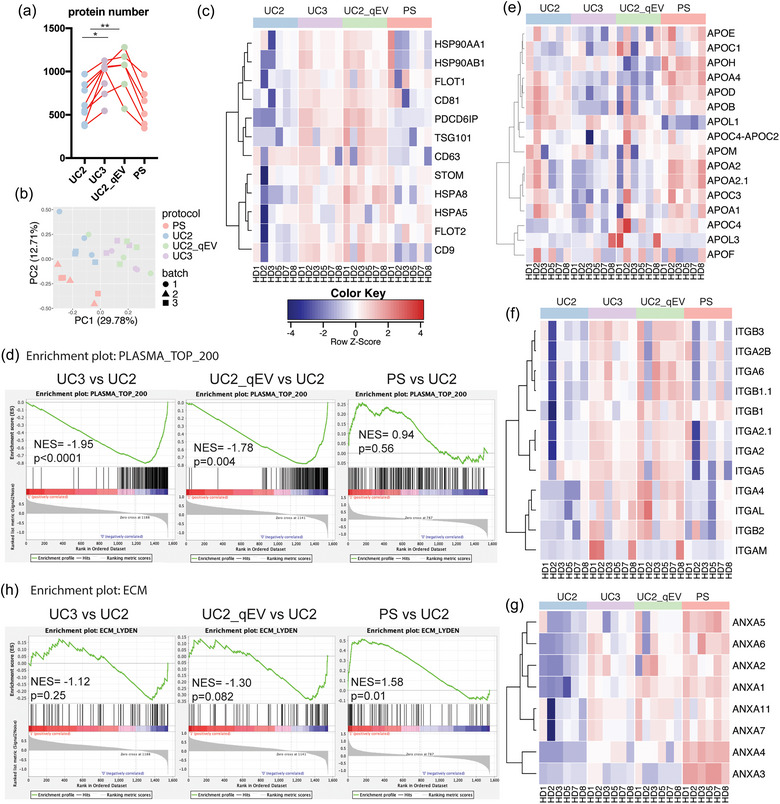
Proteomic profiling reveals the enhanced efficiency of UC3 and UC2‐qEV in enriching EVPs, while demonstrating biased enrichment of PS. (a) Total number of proteins identified in isolated plasma EVPs through proteomic mass spectrometry (MS) analysis. n = 6; the bar indicates mean. (b) Principal component analysis of normalized proteomic MS data sets derived from plasma EVPs isolated using different approaches. (c) Heatmap illustration of the relative abundance of conventional EVP marker proteins in plasma EVPs isolated using different approaches. (d) Gene set enrichment analysis (GSEA) illustrating the enrichment or depletion of the top 200‐ranked plasma proteins in plasma EVPs isolated using different approaches. (e–g) Heatmap illustration of the relative abundance of apolipoproteins, integrins, and annexins in plasma EVPs isolated using different approaches. n = 6 samples per group for (e–g). (h). GSEA of extracellular matrix (ECM)‐associated proteins in plasma EVPs isolated using different methods compared to UC2 approach. n = 6 samples per group.
Next, a closer examination of the proteomes revealed that nearly all the conventional EVP markers (except for CD63) as listed in Figure 2(c) were further enriched using UC3 and UC2‐qEV compared to UC2. Intriguingly, PS specifically enriched Flot‐1, Flot‐2, and HSPA5 but not CD9 and Hsp90 compared to UC2, consistent with the above WB analysis. Other EVP markers, including PDCD6IP/Alix, Tsg101, and CD81, were not enriched by PS compared to UC2. CD63 is unique in that its level was similar across all UC samples but decreased in PS samples. These observations indicate that PS enriches for specific EVP subsets compared to other UC‐based procedures.
Soluble proteins that are highly abundant in plasma are considered a significant source of contamination in plasma EVPs. Therefore, we conducted GSEA on the top 200 most abundant proteins identified in human plasma by MS (human protein atlas: https://www.proteinatlas.org/humanproteome/blood+protein/proteins+detected+in+ms). Strikingly, most of these proteins were significantly depleted by UC3 and UC2‐qEV compared to UC2 (Figure 2d). However, such a depletion was not observed by PS (Figure 2d). Indeed, we noticed that a subset of plasma proteins was further enriched by PS as shown by the heatmap illustration of their relative abundance in all samples (Figure S4b).
Due to their high abundance and similar size and density to EVPs, lipoprotein particles are another major category of contaminants in EVPs isolated from plasma. Upon close examination of the relative abundance of apolipoproteins in the EVP proteomes, we observed a general trend of apolipoprotein depletion by UC3 and UC2‐qEV compared to UC2 (Figure 2e). In contrast, most apolipoproteins were further enriched by PS (Figure 2e), consistent with the TEM and WB analyses described above. These data suggest that PS is less efficient than UC‐based approaches in removing lipoprotein particles from EVPs.
Proteomic MS analysis was also performed on EVPs isolated using qEV‐DGC as described above (Figure S5). However, no significant enrichment of conventional EVP markers or depletion of top‐ranked plasma proteins was observed in EVPs isolated using qEV‐DGC compared to UC2, indicating that UC3 and UC2‐qEV produced higher quality EVPs for downstream proteomic profiling than qEV‐DGC.
3.5. In‐depth characterization of plasma EVP proteomes aided by improved EVP purity
Several classes of proteins of particular interest were further investigated. Integrins (ITGs) are transmembrane proteins present on the surface of exosomes and play important roles in EV biogenesis, targeting, and function in recipient cells (Clayton et al., 2004; Hoshino et al., 2020; Park et al., 2019). Emerging evidence shows that exosomal ITGs represent potential biomarker candidates for disease diagnosis and prognosis (Chen et al., 2021; Hoshino et al., 2020). However, the detection frequency of this family of proteins in plasma EVPs is much lower than in EVPs isolated from cell cultures and tissue explants (Hoshino et al., 2020), thus limiting the exploration of their biomarker potential for liquid biopsies. Our study showed that, compared to UC2, the detection frequency and relative abundance of ITGs were enhanced by UC3 and UC2‐qEV but not PS (Figure 2f). This result further supports the necessity of improved EVP purity to facilitate in‐depth characterization of the EVP proteomes and subsequent biomarker discovery.
Annexins are another protein family identified as exosomal constituents (Hsu et al., 2021; Keklikoglou et al., 2019; Leoni et al., 2015; Maji et al., 2017; Zhang et al., 2018). A closer examination revealed that almost all annexins detected were significantly enriched in EVPs isolated using PS compared to UC2 (Figure 2g). Such enrichment may be attributed to several factors. Firstly, the binding buffer used in the PS protocol contains Ca2+, which may promote the binding of secreted annexins (such as Annexins A1, A2, and A5) to phosphatidylserine on EVPs. Secondly, annexins may be predominantly located in the inner leaflet of the lipid membrane bilayer of phosphatidylserine+ EVs, which could be selectively purified by PS but not by other methods. This finding highlights the potential bias in EVP isolation through PS and its ability to enrich certain EVP subsets carrying specific cargos such as Annexins.
Immunoglobulins constitute a substantial portion of the plasma EVP proteomes, and their differential concentrations in plasma EVP preparations have demonstrated potential for distinguishing cancer from non‐cancer or different types of cancers (Hoshino et al., 2020). Intriguingly, no specific classes of immunoglobulins were greatly enriched by UC3, UC2‐qEV or PS compared to UC2 (Figure S6a). In contrast, some of them were either depleted by UC3 and UC2‐qEV (including IGHG1, IGHG2, IGHG3 and IGHG4) or by PS (including IGHM, IGKC, IGLC3 and IGLC7), although we observed a significant amount of individual variation among the samples.
Another category of proteins which displayed distinct patterns of enrichment is extracellular matrix (ECM) proteins and ECM‐associated proteins. Based on the GSEA and heatmap analysis on the ECM protein set obtained from MatrisomeDB (Figures 2h and S6b), we observed a trend of ECM‐associated proteins being depleted by UC3 and UC2‐qEV (negative NESs) when compared to UC2. In contrast, there was a strong enrichment of these proteins when using PS (positive NES, p = 0.01). For example, we observed a decrease in Fibronectin 1 (FN1), Extracellular Matrix Protein 1 (ECM1), Alpha‐1‐Microglobulin/Bikunin Precursor (AMBP), and Plasminogen (PLG) in EVPs isolated by UC3 and UC2‐qEV compared to UC2, while these proteins were increased in EVPs isolated by PS. In contrast, Transforming Growth Factor‐Beta 1 (TGFB1) showed upregulation with UC3 and UC2‐qEV but not with PS. Collagen Type VI Alpha chain 2 and 3 (COL6A2 and COL6A3) demonstrated upregulation with all three approaches compared to UC2. The levels of S100 family members (including S100A10, S100A11, S100A6 and S100A4) were slightly higher in samples prepared through UC3 and UC2‐qEV than UC2 and PS, while the abundance of S100A9 was comparable across UC3, UC2‐qEV and PS samples and higher than that in UC2 samples. Various proteins including Heparan Sulfate Proteoglycan 2 (HSPG2), Microfibril‐associated glycoprotein 4 (MFAP4), EGF‐containing Fibulin‐like Extracellular Matrix Protein 1 (EFEMP1), and Fibulin 5 (FBLN5) were uniquely enriched by PS.
To further assess the efficacy of different methods in enriching EVPs and depleting contaminants, we investigated the signature proteins associated with exosomes and exomeres that we had previously identified in EVPs derived from several cell lines (Zhang et al., 2018). Using UC2 samples as reference, we observed a significant increase in the abundance of exosome and exomere signature proteins in the products of UC2‐qEV and UC3, but not in PS (Figure S6c), which is consistent with the aforementioned findings.
These observations further highlight the distinct composition of EVP proteomes that result from different isolation procedures. The improved EVP purity enables the detection of low abundance proteins and helps identify potential contaminants present in plasma EVPs.
3.6. Identification of potential contaminants and novel protein cargos in plasma EVPs isolated using UC‐based approaches
Since UC3 and UC2‐qEV effectively enhance the purity of plasma EVPs, we further analyzed the proteins that were specifically enriched or depleted in these samples compared to UC2. This allowed us to define common contaminants in plasma EVPs and potential novel EVP cargos uncovered using these protocols. To accomplish this, we utilized the criteria of log2 fold change (FC) > 1.5 and adjusted p value (padj) < 0.05 to identify the differentially detected proteins (DDPs) between UC2 samples and samples prepared using other protocols. As shown in the Venn diagrams (Figure 3a) and consistent with the aforementioned PCA results, we found that UC3 and UC2‐qEV samples had a higher degree of similarity to each other, with a greater overlap of DDPs in both enriched and depleted proteins, as compared to UC2. On the other hand, the DDPs identified between PS and UC2 had a minimal overlap with those identified by UC3 and UC2‐qEV. Notably, UC2‐qEV exhibited the largest number of DDPs among all comparisons, consistent with the highest purity observed for UC2‐qEV in our other assays.
FIGURE 3.
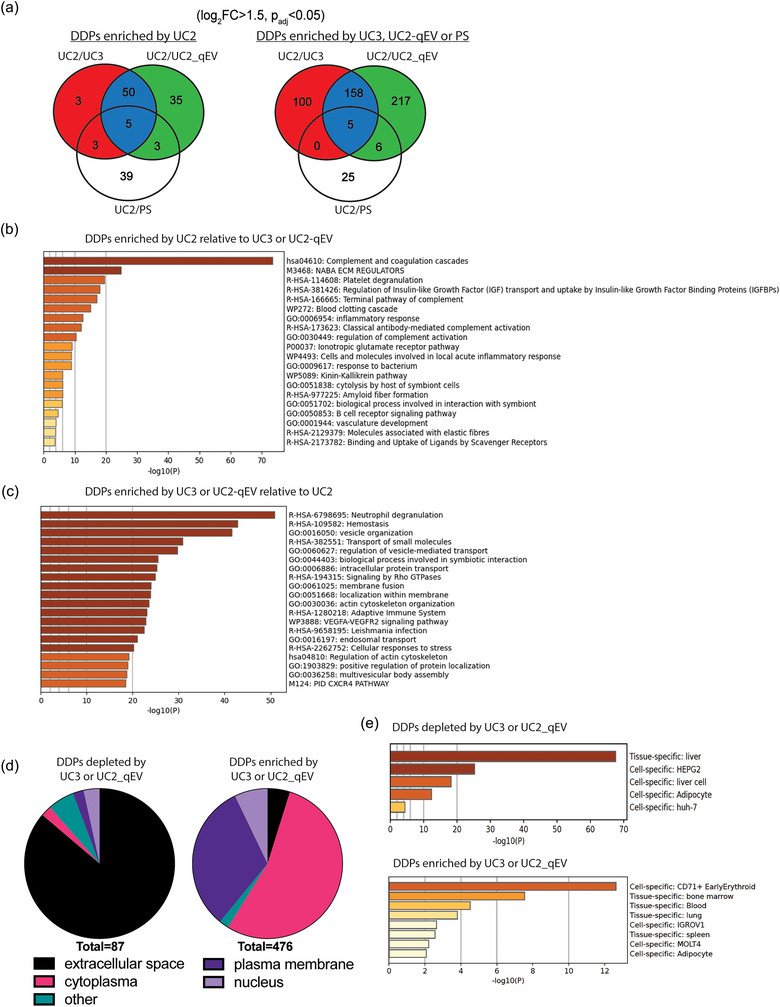
Identification and characterization of differentially detected proteins (DDPs) among plasma EVPs isolated using different UC‐based approaches. (a) Venn diagrams illustrating differentially expressed proteins in plasma EVPs isolated using UC3, UC2‐qEV or PS in comparison with UC2 approach. The criteria for DDP identification include log fold change (FC) > 1.5 and Padj < 0.05. (b, c) Metascape analysis of top pathways associated with DDPs enriched by UC2 (b) or by UC3 or UC2‐qEV (c). Statistically enriched ontology clusters are identified through Metascape Express Analysis of ontologies including GO processes, KEGG pathways, Reactome gene sets, canonical pathways, and CORUM complexes. Top non‐redundant clusters are presented, with the X‐axis illustrating the −log10(p value) to convey their statistical significance. (d). Subcellular localization analysis of DDPs depleted (left chart) or enriched (right chart) by UC3 or UC2‐qEV in comparison with UC2. (e) Enrichment analysis of tissue‐ and cell‐specific pattern genes in DDPs depleted (top panel) or enriched (bottom panel) by UC3 or UC2‐qEV in comparison with UC2. Analysis conducted using PaGenBase through the Metascape online platform.
Considering the similarity between UC3 and UC2‐qEV, we combined the DDPs identified in both protocols versus UC2 for further analysis (see protein lists in Tables 1 and 2). Pathway analysis revealed that DDPs with higher abundance in UC2 products (i.e., potential contaminants in plasma EVPs and depleted by UC3 or UC2‐qEV) were associated with complement system and coagulation cascades (Figure 3b). In contrast, DDPs with lower abundance in UC2 products (i.e., enriched by UC3 or UC2‐qEV and potential EVP cargos undetected previously in plasma EVPs) were associated with many vesicle regulation‐related pathways (Figure 3c). Remarkably, many proteins, including Rab family proteins, transmembrane proteins, and ESCRT members, which are frequently reported as cargo of EVPs isolated from cell culture (e.g., Vesiclepedia, EVpedia, ExoCarta) (Kalra et al., 2012; Kim et al., 2015; Mathivanan & Simpson, 2009), were found among the DDPs enriched by UC3 or UC2‐qEV (see Table 2). We also noted that the DDPs with higher abundance in UC2 samples had an overall higher rank of abundance in plasma (range: 1–1127; median: 71; human protein atlas) than those with higher abundance in UC3 and UC2‐qEV samples (range: 99–3978; median: 1338) (Figure S7).
TABLE 1.
List of proteins enriched by UC2 in comparison to UC3 or UC2‐qEV.
| Symbol | Entrez gene name | Symbol | Entrez gene name |
|---|---|---|---|
| A1BG | alpha‐1‐B glycoprotein | FGG | fibrinogen gamma chain |
| A2M | alpha‐2‐macroglobulin | FN1 | fibronectin 1 |
| AFM | Afamin | GC | GC vitamin D binding protein |
| AHSG | alpha 2‐HS glycoprotein | GPX3 | glutathione peroxidase 3 |
| AMBP | alpha‐1‐microglobulin/bikunin precursor | H2BC12 | H2B clustered histone 12 |
| ANG | angiogenin | HABP2 | hyaluronan binding protein 2 |
| ANGPTL6 | angiopoietin like 6 | HPX | hemopexin |
| APCS | amyloid P component, serum | HRG | histidine rich glycoprotein |
| APOA4 | apolipoprotein A4 | IGHG1 | immunoglobulin heavy constant gamma 1 (G1m marker) |
| APOD | apolipoprotein D | IGHG2 | immunoglobulin heavy constant gamma 2 (G2m marker) |
| ATXN1 | ataxin 1 | IGHG3 | immunoglobulin heavy constant gamma 3 (G3m marker) |
| BANF1 | BAF nuclear assembly factor 1 | IGHG4 | immunoglobulin heavy constant gamma 4 (G4m marker) |
| C1QA | complement C1q A chain | IGHV3OR16‐13 | immunoglobulin heavy variable 3/OR16‐13 (non‐functional) |
| C1QB | complement C1q B chain | IGKV1‐27 | immunoglobulin kappa variable 1‐27 |
| C1QC | complement C1q C chain | IGKV3‐7 | immunoglobulin kappa variable 3‐7 (non‐functional) |
| C1R | complement C1r | IGKV3OR2‐268 | immunoglobulin kappa variable 3/OR2‐268 (non‐functional) |
| C1RL | complement C1r subcomponent like | IGKV6‐21 | immunoglobulin kappa variable 6‐21 (non‐functional) |
| C1S | complement C1s | ITIH1 | inter‐alpha‐trypsin inhibitor heavy chain 1 |
| C2 | complement C2 | ITIH2 | inter‐alpha‐trypsin inhibitor heavy chain 2 |
| C3 | complement C3 | KNG1 | kininogen 1 |
| C4A/C4B | complement C4A (Rodgers blood group) | LPA | lipoprotein(a) |
| C4BPA | complement component 4 binding protein alpha | LRG1 | leucine rich alpha‐2‐glycoprotein 1 |
| C4BPB | complement component 4 binding protein beta | LYZ | lysozyme |
| C5 | complement C5 | MASP1 | MBL associated serine protease 1 |
| C6 | complement C6 | NGEF | neuronal guanine nucleotide exchange factor |
| C7 | complement C7 | PGLYRP2 | peptidoglycan recognition protein 2 |
| C8A | complement C8 alpha chain | PLG | plasminogen |
| C8B | complement C8 beta chain | PON1 | paraoxonase 1 |
| C8G | complement C8 gamma chain | PRG4 | proteoglycan 4 |
| C9 | complement C9 | PROS1 | protein S |
| CFH | complement factor H | PZP | PZP alpha‐2‐macroglobulin like |
| CFHR1 | complement factor H related 1 | RBP4 | retinol binding protein 4 |
| CFHR3 | complement factor H related 3 | RELN | reelin |
| CFI | complement factor I | RNASE4 | ribonuclease A family member 4 |
| CP | ceruloplasmin | SERPINA3 | serpin family A member 3 |
| CPN1 | carboxypeptidase N subunit 1 | SERPINA4 | serpin family A member 4 |
| CPN2 | carboxypeptidase N subunit 2 | SERPINA7 | serpin family A member 7 |
| ECM1 | extracellular matrix protein 1 | SERPINC1 | serpin family C member 1 |
| F12 | coagulation factor XII | SERPIND1 | serpin family D member 1 |
| F13B | coagulation factor XIII B chain | SERPINF2 | serpin family F member 2 |
| F2 | coagulation factor II, thrombin | SVEP1 | sushi, von Willebrand factor type A, EGF and pentraxin domain containing 1 |
| FBLN1 | fibulin 1 | TTR | transthyretin |
| FGA | fibrinogen alpha chain | VTN | vitronectin |
| FGB | fibrinogen beta chain |
TABLE 2.
List of proteins enriched by UC3 or UC2‐qEV in comparison to UC2.
| Symbol | Entrez gene name | Symbol | Entrez gene name |
|---|---|---|---|
| ABCB6 | ATP binding cassette subfamily B member 6 (Langereis blood group) | KIF5B | kinesin family member 5B |
| ABCC1 | ATP binding cassette subfamily C member 1 | KLC1 | kinesin light chain 1 |
| ABCC4 | ATP binding cassette subfamily C member 4 | KPNB1 | karyopherin subunit beta 1 |
| ABCC5 | ATP binding cassette subfamily C member 5 | KRAS | KRAS proto‐oncogene, GTPase |
| ACTB | actin beta | LAMP2 | lysosomal associated membrane protein 2 |
| ACTBL2 | actin beta like 2 | LAMTOR1 | late endosomal/lysosomal adaptor, MAPK and MTOR activator 1 |
| ACTC1 | actin alpha cardiac muscle 1 | LAMTOR5 | late endosomal/lysosomal adaptor, MAPK and MTOR activator 5 |
| ACTR1A | actin related protein 1A | LAP3 | leucine aminopeptidase 3 |
| ACTR2 | actin related protein 2 | LASP1 | LIM and SH3 protein 1 |
| ACVR1 | activin A receptor type 1 | LCN1 | lipocalin 1 |
| ADAM17 | ADAM metallopeptidase domain 17 | LCN2 | lipocalin 2 |
| ADH5 | alcohol dehydrogenase 5 (class III), chi polypeptide | LCP1 | lymphocyte cytosolic protein 1 |
| AGO2 | argonaute RISC catalytic component 2 | LGALS3 | galectin 3 |
| AHCY | adenosylhomocysteinase | LHFPL2 | LHFPL tetraspan subfamily member 2 |
| AHNAK | AHNAK nucleoprotein | LMBRD2 | LMBR1 domain containing 2 |
| AHSP | alpha hemoglobin stabilizing protein | LNPEP | leucyl and cystinyl aminopeptidase |
| AK1 | adenylate kinase 1 | LRRC8D | leucine rich repeat containing 8 VRAC subunit D |
| ALOX12 | arachidonate 12‐lipoxygenase, 12S type | LRSAM1 | leucine rich repeat and sterile alpha motif containing 1 |
| ALPL | alkaline phosphatase, biomineralization associated | LSM4 | LSM4 homolog, U6 small nuclear RNA and mRNA degradation associated |
| AMPD2 | adenosine monophosphate deaminase 2 | MARCHF8 | membrane associated ring‐CH‐type finger 8 |
| ANK1 | ankyrin 1 | MARCKSL1 | MARCKS like 1 |
| ANPEP | alanyl aminopeptidase, membrane | MBP | myelin basic protein |
| ANXA1 | annexin A1 | MDH1 | malate dehydrogenase 1 |
| ANXA11 | annexin A11 | MDH2 | malate dehydrogenase 2 |
| ANXA2 | annexin A2 | MFGE8 | milk fat globule EGF and factor V/VIII domain containing |
| ANXA4 | annexin A4 | MGLL | monoglyceride lipase |
| ANXA6 | annexin A6 | MINK1 | misshapen like kinase 1 |
| AP1B1 | adaptor related protein complex 1 subunit beta 1 | MME | membrane metalloendopeptidase |
| AP1G1 | adaptor related protein complex 1 subunit gamma 1 | MPP1 | MAGUK p55 scaffold protein 1 |
| AP1M1 | adaptor related protein complex 1 subunit mu 1 | MSN | Moesin |
| AP2M1 | adaptor related protein complex 2 subunit mu 1 | MVP | major vault protein |
| APRT | adenine phosphoribosyltransferase | MYO1G | myosin IG |
| AQP1 | aquaporin 1 (Colton blood group) | NAP1L4 | nucleosome assembly protein 1 like 4 |
| ARF6 | ADP ribosylation factor 6 | NAPG | NSF attachment protein gamma |
| ARHGAP1 | Rho GTPase activating protein 1 | NCKAP1 | NCK associated protein 1 |
| ARHGAP17 | Rho GTPase activating protein 17 | NCKAP1L | NCK associated protein 1 like |
| ARHGAP18 | Rho GTPase activating protein 18 | NECTIN2 | nectin cell adhesion molecule 2 |
| ARPC2 | actin related protein 2/3 complex subunit 2 | NME1‐NME2 | NME1‐NME2 readthrough |
| ARPC5 | actin related protein 2/3 complex subunit 5 | NPEPPS | aminopeptidase puromycin sensitive |
| ATIC | 5‐aminoimidazole‐4‐carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase | NPTN | neuroplastin |
| ATP11B | ATPase phospholipid transporting 11B (putative) | NRAS | NRAS proto‐oncogene, GTPase |
| ATP1A1 | ATPase Na+/K+ transporting subunit alpha 1 | NRGN | neurogranin |
| ATP1B3 | ATPase Na+/K+ transporting subunit beta 3 | NSF | N‐ethylmaleimide sensitive factor, vesicle fusing ATPase |
| ATP5F1A | ATP synthase F1 subunit alpha | NT5E | 5′‐nucleotidase ecto |
| ATP5F1B | ATP synthase F1 subunit beta | NUTF2 | nuclear transport factor 2 |
| ATP6AP1 | ATPase H+ transporting accessory protein 1 | OLFM4 | olfactomedin 4 |
| ATP6V0A2 | ATPase H+ transporting V0 subunit a2 | OXSR1 | oxidative stress responsive kinase 1 |
| ATP6V1A | ATPase H+ transporting V1 subunit A | P2RY12 | purinergic receptor P2Y12 |
| ATP6V1B2 | ATPase H+ transporting V1 subunit B2 | PACSIN2 | protein kinase C and casein kinase substrate in neurons 2 |
| ATP6V1E1 | ATPase H+ transporting V1 subunit E1 | PAICS | phosphoribosylaminoimidazole carboxylase and phosphoribosylaminoimidazolesuccinocarboxamide synthase |
| ATP6V1G1 | ATPase H+ transporting V1 subunit G1 | PARK7 | Parkinsonism associated deglycase |
| ATP6V1H | ATPase H+ transporting V1 subunit H | PDCD6 | programmed cell death 6 |
| ATP7A | ATPase copper transporting alpha | PDCD6IP | programmed cell death 6 interacting protein |
| BAG2 | BAG cochaperone 2 | PDE5A | phosphodiesterase 5A |
| BAIAP2 | BAR/IMD domain containing adaptor protein 2 | PDIA6 | protein disulfide isomerase family A member 6 |
| BCAM | basal cell adhesion molecule (Lutheran blood group) | PDLIM1 | PDZ and LIM domain 1 |
| BLMH | bleomycin hydrolase | PEA15 | proliferation and apoptosis adaptor protein 15 |
| BLVRA | biliverdin reductase A | PEBP1 | phosphatidylethanolamine binding protein 1 |
| BLVRB | biliverdin reductase B | PFKP | phosphofructokinase, platelet |
| BROX | BRO1 domain and CAAX motif containing | PGAM1 | phosphoglycerate mutase 1 |
| BTK | Bruton tyrosine kinase | PGD | phosphogluconate dehydrogenase |
| C2orf88 | chromosome 2 open reading frame 88 | PGK1 | phosphoglycerate kinase 1 |
| CA1 | carbonic anhydrase 1 | PGRMC1 | progesterone receptor membrane component 1 |
| CA2 | carbonic anhydrase 2 | PI4K2A | phosphatidylinositol 4‐kinase type 2 alpha |
| CALM1 (includes others) | calmodulin 1 | PIP | prolactin induced protein |
| CALR | calreticulin | PIP4K2A | phosphatidylinositol‐5‐phosphate 4‐kinase type 2 alpha |
| CAND1 | cullin associated and neddylation dissociated 1 | PKM | pyruvate kinase M1/2 |
| CANX | calnexin | PLS3 | plastin 3 |
| CAPN5 | calpain 5 | PLSCR1 | phospholipid scramblase 1 |
| CCL5 | C‐C motif chemokine ligand 5 | PLXNA4 | plexin A4 |
| CCRL2 | C‐C motif chemokine receptor like 2 | PLXNB2 | plexin B2 |
| CCT5 | chaperonin containing TCP1 subunit 5 | PLXNB3 | plexin B3 |
| CCT7 | chaperonin containing TCP1 subunit 7 | PNP | purine nucleoside phosphorylase |
| CD109 | CD109 molecule | PODXL | podocalyxin like |
| CD2 | CD2 molecule | PPBP | pro‐platelet basic protein |
| CD2AP | CD2 associated protein | PPIA | peptidylprolyl isomerase A |
| Symbol | Entrez Gene Name | Symbol | Entrez Gene Name |
| CD3E | CD3 epsilon subunit of T‐cell receptor complex | PPIB | peptidylprolyl isomerase B |
| CD44 | CD44 molecule (Indian blood group) | PPIF | peptidylprolyl isomerase F |
| CD46 | CD46 molecule | PPM1A | protein phosphatase, Mg2+/Mn2+ dependent 1A |
| CD47 | CD47 molecule | PPP1R21 | protein phosphatase 1 regulatory subunit 21 |
| CD48 | CD48 molecule | PRDX5 | peroxiredoxin 5 |
| CD5 | CD5 molecule | PRKACA | protein kinase cAMP‐activated catalytic subunit alpha |
| CD55 | CD55 molecule (Cromer blood group) | PRKACB | protein kinase cAMP‐activated catalytic subunit beta |
| CD58 | CD58 molecule | PRPS1 | phosphoribosyl pyrophosphate synthetase 1 |
| CD6 | CD6 molecule | PSMB1 | proteasome 20S subunit beta 1 |
| CD69 | CD69 molecule | PSMB10 | proteasome 20S subunit beta 10 |
| CD81 | CD81 molecule | PSMB3 | proteasome 20S subunit beta 3 |
| CD82 | CD82 molecule | PSME1 | proteasome activator subunit 1 |
| CD84 | CD84 molecule | PSTPIP2 | proline‐serine‐threonine phosphatase interacting protein 2 |
| CD9 | CD9 molecule | PTGES3 | prostaglandin E synthase 3 |
| CDC37 | cell division cycle 37, HSP90 cochaperone | PTGS1 | prostaglandin‐endoperoxide synthase 1 |
| CDC42 | cell division cycle 42 | PTMA | prothymosin alpha |
| CFL1 | cofilin 1 | PTP4A2 | protein tyrosine phosphatase 4A2 |
| CHMP2A | charged multivesicular body protein 2A | PTPN23 | protein tyrosine phosphatase non‐receptor type 23 |
| CHMP2B | charged multivesicular body protein 2B | PTPRA | protein tyrosine phosphatase receptor type A |
| CHMP4A | charged multivesicular body protein 4A | RAB11B | RAB11B, member RAS oncogene family |
| CHMP4B | charged multivesicular body protein 4B | RAB14 | RAB14, member RAS oncogene family |
| CHMP5 | charged multivesicular body protein 5 | RAB21 | RAB21, member RAS oncogene family |
| CIB1 | calcium and integrin binding 1 | RAB2A | RAB2A, member RAS oncogene family |
| CLC | Charcot‐Leyden crystal galectin | RAB2B | RAB2B, member RAS oncogene family |
| CLEC1B | C‐type lectin domain family 1 member B | RAB35 | RAB35, member RAS oncogene family |
| CLEC4M | C‐type lectin domain family 4 member M | RAB7A | RAB7A, member RAS oncogene family |
| CLIC1 | chloride intracellular channel 1 | RAB8A | RAB8A, member RAS oncogene family |
| CLIC2 | chloride intracellular channel 2 | RAB8B | RAB8B, member RAS oncogene family |
| CLIC4 | chloride intracellular channel 4 | RABEP1 | rabaptin, RAB GTPase binding effector protein 1 |
| CLINT1 | clathrin interactor 1 | RAC1 | Rac family small GTPase 1 |
| CMIP | c‐Maf inducing protein | RAC2 | Rac family small GTPase 2 |
| CNDP2 | carnosine dipeptidase 2 | RALB | RAS like proto‐oncogene B |
| CNNM2 | cyclin and CBS domain divalent metal cation transport mediator 2 | RAN | RAN, member RAS oncogene family |
| CNNM4 | cyclin and CBS domain divalent metal cation transport mediator 4 | RAP1A | RAP1A, member of RAS oncogene family |
| CNP | 2′,3′‐cyclic nucleotide 3′ phosphodiesterase | RAP1B | RAP1B, member of RAS oncogene family |
| COL6A3 | collagen type VI alpha 3 chain | RAP2B | RAP2B, member of RAS oncogene family |
| CORO1B | coronin 1B | RAP2C | RAP2C, member of RAS oncogene family |
| COTL1 | coactosin like F‐actin binding protein 1 | RASGRP2 | RAS guanyl releasing protein 2 |
| CPNE1 | copine 1 | RDX | radixin |
| CRIP1 | cysteine rich protein 1 | REEP5 | receptor accessory protein 5 |
| CRIP2 | cysteine rich protein 2 | RFFL | ring finger and FYVE like domain containing E3 ubiquitin protein ligase |
| CRKL | CRK like proto‐oncogene, adaptor protein | RFTN1 | raftlin, lipid raft linker 1 |
| CRYZL1 | crystallin zeta like 1 | RGS18 | regulator of G protein signaling 18 |
| CS | citrate synthase | RHAG | Rh associated glycoprotein |
| CSK | C‐terminal Src kinase | RHCE/RHD | Rh blood group D antigen |
| CTSA | cathepsin A | RHOA | ras homolog family member A |
| CYBRD1 | cytochrome b reductase 1 | RHOF | ras homolog family member F, filopodia associated |
| CYC1 | cytochrome c1 | ROCK2 | Rho associated coiled‐coil containing protein kinase 2 |
| CYCS | cytochrome c, somatic | S100A10 | S100 calcium binding protein A10 |
| CYRIB | CYFIP related Rac1 interactor B | S100A11 | S100 calcium binding protein A11 |
| DAAM1 | dishevelled associated activator of morphogenesis 1 | S100A7 | S100 calcium binding protein A7 |
| DBI | diazepam binding inhibitor, acyl‐CoA binding protein | S100A9 | S100 calcium binding protein A9 |
| DBNL | drebrin like | SCAMP2 | secretory carrier membrane protein 2 |
| DCD | dermcidin | SDCBP | syndecan binding protein |
| DDAH2 | dimethylarginine dimethylaminohydrolase 2 | SEC24C | SEC24 homolog C, COPII coat complex component |
| DDT | D‐dopachrome tautomerase | SELL | selectin L |
| DERA | deoxyribose‐phosphate aldolase | SELP | selectin P |
| DNAJA2 | DnaJ heat shock protein family (Hsp40) member A2 | SEMA7A | semaphorin 7A (John Milton Hagen blood group) |
| DNAJA4 | DnaJ heat shock protein family (Hsp40) member A4 | SEPTIN11 | septin 11 |
| DNAJB1 | DnaJ heat shock protein family (Hsp40) member B1 | SEPTIN6 | septin 6 |
| DNAJB4 | DnaJ heat shock protein family (Hsp40) member B4 | SERINC3 | serine incorporator 3 |
| DNAJC5 | DnaJ heat shock protein family (Hsp40) member C5 | SERPINB9 | serpin family B member 9 |
| DNAJC7 | DnaJ heat shock protein family (Hsp40) member C7 | SKP1 | S‐phase kinase associated protein 1 |
| DNM1 | dynamin 1 | SLC11A2 | solute carrier family 11 member 2 |
| DOCK10 | dedicator of cytokinesis 10 | SLC12A4 | solute carrier family 12 member 4 |
| DPP4 | dipeptidyl peptidase 4 | SLC12A6 | solute carrier family 12 member 6 |
| DPYSL2 | dihydropyrimidinase like 2 | SLC12A7 | solute carrier family 12 member 7 |
| DYNLL2 | dynein light chain LC8‐type 2 | SLC16A1 | solute carrier family 16 member 1 |
| ECE1 | endothelin converting enzyme 1 | SLC16A7 | solute carrier family 16 member 7 |
| EFR3A | EFR3 homolog A | SLC1A4 | solute carrier family 1 member 4 |
| EHD1 | EH domain containing 1 | SLC1A5 | solute carrier family 1 member 5 |
| EIF5A | eukaryotic translation initiation factor 5A | SLC22A16 | solute carrier family 22 member 16 |
| ELOC | elongin C | SLC25A3 | solute carrier family 25 member 3 |
| EMCN | endomucin | SLC25A5 | solute carrier family 25 member 5 |
| EMILIN1 | elastin microfibril interfacer 1 | SLC29A1 | solute carrier family 29 member 1 (Augustine blood group) |
| ENDOD1 | endonuclease domain containing 1 | SLC2A1 | solute carrier family 2 member 1 |
| ENO1 | enolase 1 | SLC3A2 | solute carrier family 3 member 2 |
| ENO2 | enolase 2 | SLC40A1 | solute carrier family 40 member 1 |
| ENPEP | glutamyl aminopeptidase | SLC43A1 | solute carrier family 43 member 1 |
| Symbol | Entrez Gene Name | Symbol | Entrez Gene Name |
| EPB41 | erythrocyte membrane protein band 4.1 | SLC43A3 | solute carrier family 43 member 3 |
| ERBIN | erbb2 interacting protein | SLC44A2 | solute carrier family 44 member 2 |
| ESD | esterase D | SLC4A1 | solute carrier family 4 member 1 (Diego blood group) |
| EZR | ezrin | SLC7A1 | solute carrier family 7 member 1 |
| F2R | coagulation factor II thrombin receptor | SLC7A5 | solute carrier family 7 member 5 |
| FABP4 | fatty acid binding protein 4 | SLC9A3R1 | SLC9A3 regulator 1 |
| FABP5 | fatty acid binding protein 5 | SLC9A3R2 | SLC9A3 regulator 2 |
| FAM151A | family with sequence similarity 151 member A | SNTB1 | syntrophin beta 1 |
| FAM234B | family with sequence similarity 234 member B | SNX12 | sorting nexin 12 |
| FAS | Fas cell surface death receptor | SNX3 | sorting nexin 3 |
| FBN1 | fibrillin 1 | SOD1 | superoxide dismutase 1 |
| FCGR2A | Fc gamma receptor IIa | SOD2 | superoxide dismutase 2 |
| FCGRT | Fc gamma receptor and transporter | SRGN | serglycin |
| FDPS | farnesyl diphosphate synthase | ST13 | ST13 Hsp70 interacting protein |
| FHL1 | four and a half LIM domains 1 | STAM | signal transducing adaptor molecule |
| FIS1 | fission, mitochondrial 1 | STAM2 | signal transducing adaptor molecule 2 |
| FKBP1A | FKBP prolyl isomerase 1A | STAT5A | signal transducer and activator of transcription 5A |
| FLII | FLII actin remodeling protein | STEAP3 | STEAP3 metalloreductase |
| FLOT2 | flotillin 2 | STIM1 | stromal interaction molecule 1 |
| FYB1 | FYN binding protein 1 | STIP1 | stress induced phosphoprotein 1 |
| GABARAPL2 | GABA type A receptor associated protein like 2 | STK10 | serine/threonine kinase 10 |
| GAPDH | glyceraldehyde‐3‐phosphate dehydrogenase | STK24 | serine/threonine kinase 24 |
| GCA | grancalcin | STK26 | serine/threonine kinase 26 |
| GDI1 | GDP dissociation inhibitor 1 | STX12 | syntaxin 12 |
| GDI2 | GDP dissociation inhibitor 2 | STX16 | syntaxin 16 |
| GGT1 | gamma‐glutamyltransferase 1 | STX4 | syntaxin 4 |
| GLIPR2 | GLI pathogenesis related 2 | STX6 | syntaxin 6 |
| GMPR | guanosine monophosphate reductase | STX8 | syntaxin 8 |
| GNA13 | G protein subunit alpha 13 | SUSD3 | sushi domain containing 3 |
| GNAI1 | G protein subunit alpha i1 | SYNGR2 | synaptogyrin 2 |
| GNAI2 | G protein subunit alpha i2 | SYTL4 | synaptotagmin like 4 |
| GNAI3 | G protein subunit alpha i3 | TALDO1 | transaldolase 1 |
| GNB1 | G protein subunit beta 1 | TBXAS1 | thromboxane A synthase 1 |
| GNG10 | G protein subunit gamma 10 | TCP1 | t‐complex 1 |
| GNG11 | G protein subunit gamma 11 | TESC | tescalcin |
| GNG2 | G protein subunit gamma 2 | TFRC | transferrin receptor |
| GNG5 | G protein subunit gamma 5 | TGFBRAP1 | transforming growth factor beta receptor associated protein 1 |
| GNG7 | G protein subunit gamma 7 | TKT | transketolase |
| GOLGA7 | golgin A7 | TM4SF4 | transmembrane 4 L six family member 4 |
| GPI | glucose‐6‐phosphate isomerase | TM9SF2 | transmembrane 9 superfamily member 2 |
| GPRC5C | G protein‐coupled receptor class C group 5 member C | TMEM9B | TMEM9 domain family member B |
| GPX1 | glutathione peroxidase 1 | TNIK | TRAF2 and NCK interacting kinase |
| GPX4 | glutathione peroxidase 4 | TPM3 | tropomyosin 3 |
| GRK6 | G protein‐coupled receptor kinase 6 | TPM4 | tropomyosin 4 |
| GSR | glutathione‐disulfide reductase | TRBC1 | T cell receptor beta constant 1 |
| GSTM5 | glutathione S‐transferase mu 5 | TRPC6 | transient receptor potential cation channel subfamily C member 6 |
| GSTO1 | glutathione S‐transferase omega 1 | TSG101 | tumor susceptibility 101 |
| GSTP1 | glutathione S‐transferase pi 1 | TSPAN14 | tetraspanin 14 |
| GTPBP2 | GTP binding protein 2 | TSPAN32 | tetraspanin 32 |
| GYPC | glycophorin C (Gerbich blood group) | TSPAN4 | tetraspanin 4 |
| HBA1/HBA2 | hemoglobin subunit alpha 2 | TWF2 | twinfilin actin binding protein 2 |
| HBB | hemoglobin subunit beta | TXN | thioredoxin |
| HBD | hemoglobin subunit delta | TXNL1 | thioredoxin like 1 |
| HEBP2 | heme binding protein 2 | UBA7 | ubiquitin like modifier activating enzyme 7 |
| HPRT1 | hypoxanthine phosphoribosyltransferase 1 | UBASH3B | ubiquitin associated and SH3 domain containing B |
| HPSE | heparanase | UBC | ubiquitin C |
| HSD17B4 | hydroxysteroid 17‐beta dehydrogenase 4 | UBE2L3 | ubiquitin conjugating enzyme E2 L3 |
| HSP90AB1 | heat shock protein 90 alpha family class B member 1 | UBE2V1 | ubiquitin conjugating enzyme E2 V1 |
| HSP90B1 | heat shock protein 90 beta family member 1 | UMAD1 | UBAP1‐MVB12‐associated (UMA) domain containing 1 |
| HSPA2 | heat shock protein family A (Hsp70) member 2 | USP8 | ubiquitin specific peptidase 8 |
| HSPA4 | heat shock protein family A (Hsp70) member 4 | VAMP3 | vesicle associated membrane protein 3 |
| HSPA6 | heat shock protein family A (Hsp70) member 6 | VAMP5 | vesicle associated membrane protein 5 |
| HSPA8 | heat shock protein family A (Hsp70) member 8 | VAMP7 | vesicle associated membrane protein 7 |
| HSPD1 | heat shock protein family D (Hsp60) member 1 | VAMP8 | vesicle associated membrane protein 8 |
| HSPE1 | heat shock protein family E (Hsp10) member 1 | VNN1 | vanin 1 |
| ICAM1 | intercellular adhesion molecule 1 | VPS11 | VPS11 core subunit of CORVET and HOPS complexes |
| ICAM3 | intercellular adhesion molecule 3 | VPS28 | VPS28 subunit of ESCRT‐I |
| IDH1 | isocitrate dehydrogenase (NADP(+)) 1 | VPS35 | VPS35 retromer complex component |
| IGSF8 | immunoglobulin superfamily member 8 | VPS37B | VPS37B subunit of ESCRT‐I |
| IPO5 | importin 5 | VPS4A | vacuolar protein sorting 4 homolog A |
| IQGAP1 | IQ motif containing GTPase activating protein 1 | VPS4B | vacuolar protein sorting 4 homolog B |
| IQGAP2 | IQ motif containing GTPase activating protein 2 | VPS8 | VPS8 subunit of CORVET complex |
| IST1 | IST1 factor associated with ESCRT‐III | VTA1 | vesicle trafficking 1 |
| ITGA4 | integrin subunit alpha 4 | VTI1B | vesicle transport through interaction with t‐SNAREs 1B |
| ITGA6 | integrin subunit alpha 6 | WARS1 | tryptophanyl‐tRNA synthetase 1 |
| ITGAL | integrin subunit alpha L | WASF2 | WASP family member 2 |
| ITGAM | integrin subunit alpha M | WASF3 | WASP family member 3 |
| ITGB1 | integrin subunit beta 1 | WBP2 | WW domain binding protein 2 |
| ITGB2 | integrin subunit beta 2 | WDSUB1 | WD repeat, sterile alpha motif and U‐box domain containing 1 |
| KALRN | kalirin RhoGEF kinase | XPO1 | exportin 1 |
| KATNAL2 | katanin catalytic subunit A1 like 2 | YKT6 | YKT6 v‐SNARE homolog |
| KEL | Kell metallo‐endopeptidase (Kell blood group) | YWHAE | tyrosine 3‐monooxygenase/tryptophan 5‐monooxygenase activation protein epsilon |
| KIF2A | kinesin family member 2A | ZDHHC2 | zinc finger DHHC‐type palmitoyltransferase 2 |
The subcellular localization analysis of DDPs revealed that the majority of DDPs depleted by UC3 or UC2‐qEV were secreted proteins (Figure 3d). In contrast, most DDPs enriched by UC3 or UC2‐qEV were localized either at the plasma membrane or within the cytoplasm, consistent with the idea that they could be packaged within EVPs.
Moreover, searching the PaGenBase database for tissue‐ and time‐specific pattern genes (Pan et al., 2013) (http://bioinf.xmu.edu.cn/PaGenBase/) showed that a large portion of the DDPs enriched using UC2 were proteins produced by the liver, which is the main source of secreted plasma proteins (Trefts et al., 2017) (Figure 3e). In contrast, DDPs enriched using UC3 or UC2‐qEV contained an increased proportion of house‐keeping and immune‐related proteins from tissues such as blood, bone marrow, and spleen (Figure 3e). Overall, the use of UC3 and UC2‐qEV effectively eliminates many highly abundant secreted proteins in plasma samples and enhances the detection of specific low‐abundance EVP‐associated proteins.
3.7. Distinct plasma EVP protein profiles obtained by PS compared to UC‐based approaches
As shown in Figure 3(a), the EVP proteome yielded by PS is distinct from UC‐based approaches. A comparison was conducted between the proteomes acquired using PS and all three UC‐based methods, and DDPs were identified using the same criteria as described above. DDPs enriched by UC‐based methods and PS are listed in Tables 3 and 4, respectively (as well as Figure S8). Pathway analysis revealed that UC‐based methods preferentially enriched proteins involved in pathways such as neutrophil degranulation, regulation of opsonization, and 20S proteasome (Figure 4a). In addition, several EVP signature proteins previously identified, including Integrin Alpha 5 (ITGA5), Joining Chain of Multimeric IgA and IgM (JCHAIN), and Galectin 3 Binding Protein (LGALS3BP) (Hoshino et al., 2020; Zhang et al., 2018), were consistently enriched using UC‐based protocols (Figure S8a).
TABLE 3.
List of proteins enriched by UC‐based methods in comparison to PS.
| Symbol | Entrez Gene Name | Symbol | Entrez Gene Name |
|---|---|---|---|
| A2M | alpha‐2‐macroglobulin | JCHAIN | joining chain of multimeric IgA and IgM |
| ACTR1A | actin related protein 1A | KALRN | kalirin RhoGEF kinase |
| ADIPOQ | adiponectin, C1Q and collagen domain containing | KCTD12 | potassium channel tetramerization domain containing 12 |
| AHCY | adenosylhomocysteinase | LAMP2 | lysosomal associated membrane protein 2 |
| AHNAK | AHNAK nucleoprotein | LAMTOR5 | late endosomal/lysosomal adaptor, MAPK and MTOR activator 5 |
| ALDH16A1 | aldehyde dehydrogenase 16 family member A1 | LAP3 | leucine aminopeptidase 3 |
| ANGPTL3 | angiopoietin like 3 | LCN2 | lipocalin 2 |
| ANPEP | alanyl aminopeptidase, membrane | LECT2 | leukocyte cell derived chemotaxin 2 |
| AP2M1 | adaptor related protein complex 2 subunit mu 1 | LGALS3BP | galectin 3 binding protein |
| APOL1 | apolipoprotein L1 | MASP2 | MBL associated serine protease 2 |
| ARHGDIA | Rho GDP dissociation inhibitor alpha | MGLL | monoglyceride lipase |
| ATP1B3 | ATPase Na+/K+ transporting subunit beta 3 | MME | membrane metalloendopeptidase |
| ATP6V1B2 | ATPase H+ transporting V1 subunit B2 | MVP | major vault protein |
| ATP6V1G1 | ATPase H+ transporting V1 subunit G1 | MYH1 | myosin heavy chain 1 |
| BAIAP2 | BAR/IMD domain containing adaptor protein 2 | MYH2 | myosin heavy chain 2 |
| BANF1 | BAF nuclear assembly factor 1 | MYH4 | myosin heavy chain 4 |
| C1QTNF3 | C1q and TNF related 3 | MYH7 | myosin heavy chain 7 |
| C4BPA | complement component 4 binding protein alpha | MYH7B | myosin heavy chain 7B |
| C4BPB | complement component 4 binding protein beta | MYO1C | myosin IC |
| CALHM5 | calcium homeostasis modulator family member 5 | MYO1G | myosin IG |
| CCT2 | chaperonin containing TCP1 subunit 2 | NPTN | neuroplastin |
| CCT5 | chaperonin containing TCP1 subunit 5 | NRAS | NRAS proto‐oncogene, GTPase |
| CD46 | CD46 molecule | NT5E | 5′‐nucleotidase ecto |
| CD48 | CD48 molecule | OLFM4 | olfactomedin 4 |
| CD5L | CD5 molecule like | OSTF1 | osteoclast stimulating factor 1 |
| CD69 | CD69 molecule | PAICS | phosphoribosylaminoimidazole carboxylase and phosphoribosylaminoimidazolesuccinocarboxamide synthase |
| CHMP4B | charged multivesicular body protein 4B | PANX1 | pannexin 1 |
| CHMP5 | charged multivesicular body protein 5 | PEBP1 | phosphatidylethanolamine binding protein 1 |
| CLIC2 | chloride intracellular channel 2 | PIGR | polymeric immunoglobulin receptor |
| CLIC4 | chloride intracellular channel 4 | PLXNB2 | plexin B2 |
| CLINT1 | clathrin interactor 1 | PODXL | podocalyxin like |
| COLEC10 | collectin subfamily member 10 | PRKCD | protein kinase C delta |
| COLEC11 | collectin subfamily member 11 | PRKCQ | protein kinase C theta |
| CRIP2 | cysteine rich protein 2 | PSMA2 | proteasome 20S subunit alpha 2 |
| CRKL | CRK like proto‐oncogene, adaptor protein | PSMA3 | proteasome 20S subunit alpha 3 |
| CRYZL1 | crystallin zeta like 1 | PSMA4 | proteasome 20S subunit alpha 4 |
| CTTN | cortactin | PSMA6 | proteasome 20S subunit alpha 6 |
| DBI | diazepam binding inhibitor, acyl‐CoA binding protein | PSMA7 | proteasome 20S subunit alpha 7 |
| DERA | deoxyribose‐phosphate aldolase | PSMB1 | proteasome 20S subunit beta 1 |
| DNM2 | dynamin 2 | PSMB3 | proteasome 20S subunit beta 3 |
| DPP4 | dipeptidyl peptidase 4 | PSMB8 | proteasome 20S subunit beta 8 |
| ELOC | elongin C | PTP4A2 | protein tyrosine phosphatase 4A2 |
| EMCN | endomucin | PZP | PZP alpha‐2‐macroglobulin like |
| ENPP4 | ectonucleotide pyrophosphatase/phosphodiesterase 4 | RAB2A | RAB2A, member RAS oncogene family |
| EPS8 | epidermal growth factor receptor pathway substrate 8 | RAP2C | RAP2C, member of RAS oncogene family |
| ERN1 | endoplasmic reticulum to nucleus signaling 1 | RELN | reelin |
| EXTL2 | exostosin like glycosyltransferase 2 | RHOF | ras homolog family member F, filopodia associated |
| FCGBP | Fc gamma binding protein | RP2 | RP2 activator of ARL3 GTPase |
| FCN1 | ficolin 1 | RRAS | RAS related |
| FCN2 | ficolin 2 | S100A10 | S100 calcium binding protein A10 |
| FCN3 | ficolin 3 | S100A12 | S100 calcium binding protein A12 |
| FKBP1A | FKBP prolyl isomerase 1A | SERINC3 | serine incorporator 3 |
| FMNL1 | formin like 1 | SERPINB1 | serpin family B member 1 |
| FTH1 | ferritin heavy chain 1 | SLC22A16 | solute carrier family 22 member 16 |
| GNG7 | G protein subunit gamma 7 | SLC7A5 | solute carrier family 7 member 5 |
| GPRC5C | G protein‐coupled receptor class C group 5 member C | SLC9A3R2 | SLC9A3 regulator 2 |
| GPX4 | glutathione peroxidase 4 | SOD2 | superoxide dismutase 2 |
| H4C1 | H4 clustered histone 1 | SPPL2A | signal peptide peptidase like 2A |
| HSP90B1 | heat shock protein 90 beta family member 1 | ST8SIA4 | ST8 alpha‐N‐acetyl‐neuraminide alpha‐2,8‐sialyltransferase 4 |
| IDH1 | isocitrate dehydrogenase (NADP(+)) 1 | STAM2 | signal transducing adaptor molecule 2 |
| IGHM | immunoglobulin heavy constant mu | STAT3 | signal transducer and activator of transcription 3 |
| IGHV2‐26 | immunoglobulin heavy variable 2‐26 | STAT5A | signal transducer and activator of transcription 5A |
| IGHV2‐5 | immunoglobulin heavy variable 2‐5 | STX6 | syntaxin 6 |
| IGHV2‐70 | immunoglobulin heavy variable 2‐70 | SVEP1 | sushi, von Willebrand factor type A, EGF and pentraxin domain containing 1 |
| IGHV3OR15‐7 | immunoglobulin heavy variable 3/OR15‐7 (pseudogene) | TCP1 | t‐complex 1 |
| IGHV4‐4 | immunoglobulin heavy variable 4‐4 | TGFB1I1 | transforming growth factor beta 1 induced transcript 1 |
| IGKV1‐16 | immunoglobulin kappa variable 1‐16 | TMOD3 | tropomodulin 3 |
| IGLV1‐51 | immunoglobulin lambda variable 1‐51 | TPD52L2 | TPD52 like 2 |
| IGLV2‐11 | immunoglobulin lambda variable 2‐11 | TPP2 | tripeptidyl peptidase 2 |
| IGLV2‐14 | immunoglobulin lambda variable 2‐14 | TSPAN2 | tetraspanin 2 |
| IGLV2‐23 | immunoglobulin lambda variable 2‐23 | TTN | titin |
| IGLV2‐8 | immunoglobulin lambda variable 2‐8 | UBA7 | ubiquitin like modifier activating enzyme 7 |
| IGLV3‐16 | immunoglobulin lambda variable 3‐16 | UMAD1 | UBAP1‐MVB12‐associated (UMA) domain containing 1 |
| IGLV3‐27 | immunoglobulin lambda variable 3‐27 | USP8 | ubiquitin specific peptidase 8 |
| IGLV4‐60 | immunoglobulin lambda variable 4‐60 | VAMP7 | vesicle associated membrane protein 7 |
| INPP5A | inositol polyphosphate‐5‐phosphatase A | VPS37B | VPS37B subunit of ESCRT‐I |
| IQGAP1 | IQ motif containing GTPase activating protein 1 | WASF3 | WASP family member 3 |
| ITGA5 | integrin subunit alpha 5 |
TABLE 4.
List of proteins enriched by PS in comparison to UC‐based methods.
| Symbol | Entrez Gene Name | Symbol | Entrez Gene Name |
|---|---|---|---|
| A1BG | alpha‐1‐B glycoprotein | GC | GC vitamin D binding protein |
| ABCB9 | ATP binding cassette subfamily B member 9 | GPLD1 | glycosylphosphatidylinositol specific phospholipase D1 |
| AFM | afamin | GPX3 | glutathione peroxidase 3 |
| AMBP | alpha‐1‐microglobulin/bikunin precursor | HPX | hemopexin |
| ANXA3 | annexin A3 | HRNR | hornerin |
| ANXA4 | annexin A4 | HSPG2 | heparan sulfate proteoglycan 2 |
| ANXA5 | annexin A5 | IGKV3‐7 | immunoglobulin kappa variable 3‐7 (non‐functional) |
| APCS | amyloid P component, serum | ITLN1 | intelectin 1 |
| APOA4 | apolipoprotein A4 | ITLN2 | intelectin 2 |
| BTD | biotinidase | KNG1 | kininogen 1 |
| C2 | complement C2 | KRT13 | keratin 13 |
| C8G | complement C8 gamma chain | LAMTOR4 | late endosomal/lysosomal adaptor, MAPK and MTOR activator 4 |
| CETP | cholesteryl ester transfer protein | LPA | lipoprotein(a) |
| CFD | complement factor D | LRG1 | leucine rich alpha‐2‐glycoprotein 1 |
| CFH | complement factor H | LRRC4 | leucine rich repeat containing 4 |
| CFHR1 | complement factor H related 1 | MFAP4 | microfibril associated protein 4 |
| CLEC3B | C‐type lectin domain family 3 member B | MGP | matrix Gla protein |
| COL6A1 | collagen type VI alpha 1 chain | MST1 | macrophage stimulating 1 |
| CP | ceruloplasmin | ORM1 | orosomucoid 1 |
| CPN1 | carboxypeptidase N subunit 1 | PCOLCE | procollagen C‐endopeptidase enhancer |
| CRNN | cornulin | PGLYRP2 | peptidoglycan recognition protein 2 |
| CRP | C‐reactive protein | PIP | prolactin induced protein |
| DMTN | dematin actin binding protein | PLG | plasminogen |
| ECM1 | extracellular matrix protein 1 | RARRES2 | retinoic acid receptor responder 2 |
| EFEMP1 | EGF containing fibulin extracellular matrix protein 1 | SAA1 | serum amyloid A1 |
| EPB41L3 | erythrocyte membrane protein band 4.1 like 3 | SAA2 | serum amyloid A2 |
| F10 | coagulation factor X | SERPINA1 | serpin family A member 1 |
| F13B | coagulation factor XIII B chain | SERPINA4 | serpin family A member 4 |
| F9 | coagulation factor IX | SERPINA6 | serpin family A member 6 |
| FBLN1 | fibulin 1 | SERPINA7 | serpin family A member 7 |
| FBLN2 | fibulin 2 | SERPINC1 | serpin family C member 1 |
| FBLN5 | fibulin 5 | SERPINF1 | serpin family F member 1 |
| FGA | fibrinogen alpha chain | SERPINF2 | serpin family F member 2 |
| FGB | fibrinogen beta chain | SLC43A1 | solute carrier family 43 member 1 |
| FGG | fibrinogen gamma chain | TIMD4 | T cell immunoglobulin and mucin domain containing 4 |
| FN1 | fibronectin 1 | TTR | transthyretin |
FIGURE 4.
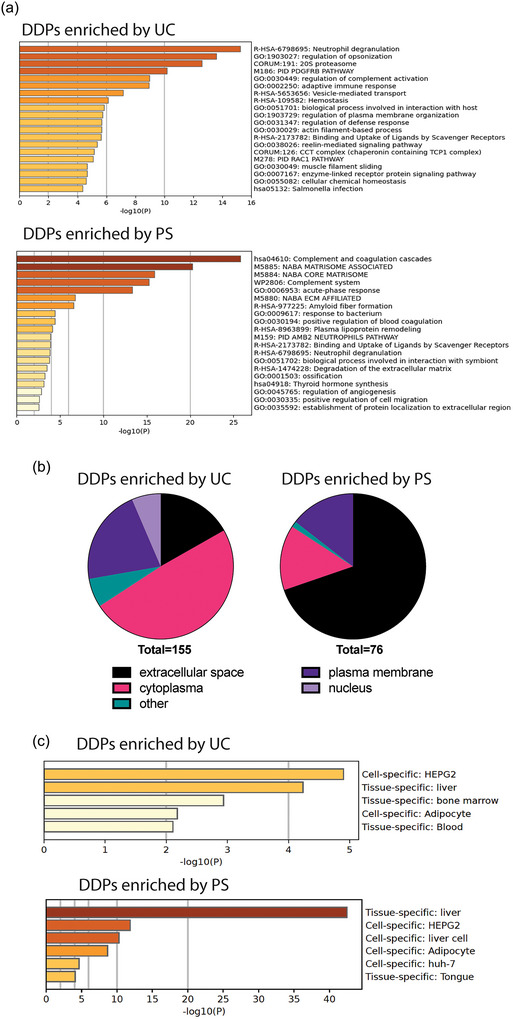
Distinct proteomic compositions of plasma EVPs isolated through PS and UC‐based approaches. (a) Metascape analysis of top pathways associated with DDPs enriched by UC‐based approaches (top panel) and PS (bottom panel), respectively. (b) Subcellular localization analysis of DDPs enriched by UC‐based approaches (left chart) and PS (right chart), respectively. (c) Enrichment analysis of tissue‐ and cell‐specific pattern genes in DDPs enriched by UC‐based approaches (top panel) and PS (bottom panel), respectively.
The pathways associated with DDPs enriched by PS compared to UC‐based methods were significantly different, with the top pathways including complement and coagulation cascades and NABA_core_matrisome, which is an ensemble of core ECM proteins such as ECM glycoproteins, collagens, and proteoglycans (Figure 4a), consistent with the results shown earlier (Figure S6b).
Subcellular localization analysis revealed that a much larger proportion of DDPs enriched using PS were secreted proteins, while DDPs enriched using the UC‐based methods consisted of larger proportions of cytoplasmic, nuclear, and plasma membrane‐associated proteins (Figure 4b). Furthermore, tissue‐ and cell type‐origin analysis indicated that PS further enriched liver‐derived, extracellular proteins (Figure 4c).
Therefore, the heterogeneity of EVPs and the different principles for EVP isolation result in the production of distinct EVP subsets and contaminants when using PS versus UC‐based methods. These observations highlight the need for future studies to perform single EVP analysis for further classification of the heterogeneous EVPs and identification of contaminants.
3.8. Confirming the superior performance of UC3 in isolating EVPs from the plasma of patients with pancreatic cancer
After establishing that UC3 and UC2‐qEV approaches yield EVPs with higher purity than UC2 or PS, we proceeded to validate the performance of these optimized methods in human cancer patient samples. Although UC3 and UC2‐qEV showed comparable purity and proteome profiles, UC2‐qEV requires a larger volume of plasma than UC3, which can be a limitation when using patient samples collected retrospectively. Therefore, we limited our comparison to UC3 and UC2 for cancer patient specimens.
Plasma samples were collected from five early‐stage pancreatic cancer (PC) patients and five HDs (see Table S1). These blood samples were collected at the time of surgery from patients who were recently diagnosed with pancreatic cancers. These patients had not received any prior treatment and had no previous cancer diagnoses. Additionally, no metastasis was detected in these patients. The samples were collected in EDTA‐tubes and processed using UC2 and UC3 in parallel. In both HD and PC samples, we observed EVPs with higher purity, despite lower protein yield, using UC3 compared to UC2 (Figure S9a, b). Importantly, proteomic MS revealed a 25%–30% increase in the total number of proteins identified in EVPs isolated using UC3 compared to UC2 in both PC and HD samples (Figure S9c).
Proteomics analysis further confirmed the enrichment of conventional EVP markers (Figure S9d) and signature proteins associated with exosomes (Figure S9e) in EVPs isolated by UC3 from both HD and PC groups. Furthermore, GSEA confirmed the depletion of contaminating plasma proteins when using UC3 compared to UC2 (Figure S9f). As expected, we also observed enrichment of ITG family members and depletion of apolipoproteins using UC3 in both PC and HD specimens (Figure S9g, h). Due to the small number of exomere‐associated signature proteins detected in these samples, we could not perform GSEA to evaluate whether they were further enriched by UC3.
Differentially expressed proteins (DEPs) in EVPs isolated from PC and HD plasma have the potential to serve as biomarker candidates to distinguish cancer patients from healthy controls and enabling early detection. To identify such candidates, we applied stringent criteria of >5‐fold difference in abundance and at least 40% detection frequency (i.e., detected in at least two out of five samples per group). Importantly, 14% of total proteins (58 DEPs out of an average of 422 total proteins, Table S2) were found to be differentially expressed between PC and HD EVPs isolated through UC3, which was much higher than the 9% (29 DEPs out of an average of 327 total proteins, Table S3) identified between PC and HD EVPs isolated through UC2 (Figure S9i). These findings strongly support that by improving EVP quality with UC3, a more in‐depth analysis of plasma‐derived EVP proteomes can expand the pool of candidates for biomarker discovery. While the inclusion of this small set of patient samples further confirms the improvements in EVP purity and protein detection achieved through our optimized methods, we must acknowledge that this sample size is inadequate for the identification and validation of cancer‐specific biomarkers due to the inherent variability among individual patient samples and the presence of numerous confounding factors within these samples. Although beyond the scope of this study, larger patient cohorts should be examined in the future to rigorously test this hypothesis.
4. DISCUSSION
In this study, we compared different methods for isolating circulating EVPs from plasma, including modified UC‐based approaches, the phosphatidylserine‐Tim 4 interaction‐based affinity capturing kit, and several other commercial kits. Our results showed that the modified UC‐based approaches, namely, UC3 and UC2‐qEV, yielded EVPs with significantly improved purity compared to the commonly used UC2 method. This was demonstrated by increased levels of conventional EVP markers, systemic enrichment of exosome‐ and exomere‐associated signatures, and identification of a large number of low‐abundance proteins. Importantly, many of the newly identified or further enriched proteins were plasma membrane‐bound or cytoplasmic proteins and functionally associated with vesicle‐related pathways, supporting their identity as bona fide plasma EVP cargo. Future studies using single EVP analysis could further validate and confirm these newly identified proteins as plasma EVP cargo. Significantly, using UC3, we identified a larger set of DEPs between cancer patients and healthy controls, which provides opportunities for potential biomarker discovery. Future studies with larger cohorts of cancer patients and controls will be needed to further validate the utility of UC3 for identifying potential diagnostic and prognostic biomarkers.
Specifically, by comparing the optimized UC3 and UC2‐qEV with the traditional UC2 approach, we observed significant reduction in contaminants, notably apolipoproteins and top‐ranked secreted plasma proteins in the samples isolated through UC3 and UC2‐qEV. However, we observed no consistent trend of depletion or enrichment for most immunoglobulins in the plasma EVPs. The configuration of immunoglobulins (i.e., soluble vs. EVP‐associated, externally bound to vs. internally packaged into EVPs) for each class and isotype of immunoglobulins is a complex area of research that requires further assessment. Further research is necessary to elucidate the functional regulation of immunoglobulins associated with EVPs and their translational potential as biomarkers under different pathological conditions.
Our analysis revealed a distinct proteome composition of plasma EVPs isolated using the PS approach. Specifically, we observed a biased enrichment for EVP subsets carrying FLOT1 and FLOT2, but not those carrying tetraspanins (such as CD81, CD9 and CD63), Tsg101, or PDCD6IP/Alix. Furthermore, compared to UC2, PS was found to enrich for certain exosome signatures, but not those of exomeres, likely due to the lower level and configuration of phosphatidylserine present in exomeres (Zhang et al., 2018). Notably, the PS approach led to higher levels of contaminants, including apolipoproteins and highly abundant secreted plasma proteins compared to UC2. Furthermore, annexin family members and many ECM‐associated proteins were found specifically enriched in plasma EVPs isolated using PS, likely a consequence of the high Ca2+ concentration in the experimental condition, which modulates the molecular interactions of annexins and several ECM and ECM‐associated proteins.
Our findings underscore the heterogeneity of plasma EVPs and the potential for isolation bias toward certain EVP subsets through different isolation procedures. Therefore, it is crucial to utilize consistent sample handling procedures and conditions for studies cross‐comparing and validating plasma EVP biomarkers. Various factors, such as the type of anticoagulants used for plasma preparation and the concentrations of calcium and other ions in the buffer, can influence molecular interactions and thus affect the proteomic constitution of the isolated EVPs and study outcomes. Hence, careful consideration of these variables should be taken into account when designing and interpreting experiments involving plasma EVPs.
It is important to note that the choice of EVP isolation strategy should be based on the research question, experimental goals, and the specific characteristics and heterogeneity of the EVPs being studied. In the present work, we focused on evaluating the performance of different procedures for improving the purity of plasma EVP isolation for downstream proteomic profiling via MS, which is critical for biomarker discovery. However, the choice of EVP isolation strategy should be tailored to the nature of the samples (e.g., plasma, cell culture, or tissue) and the endpoint assays, which may present specific requirements for the purity, quantity, and functionality preservation of isolated EVPs. Therefore, it is important to carefully consider the trade‐offs between the isolation efficiency, purity, and the preservation of the biological properties of the isolated EVPs, and choose a suitable isolation method accordingly.
Our study shows that UC3 and UC2‐qEV improve EVP purity but at the expense of yield. However, whereas UC2‐qEV requires a larger input plasma volume, UC3 can typically provide sufficient material for downstream proteomic analysis using 2–5 mL of plasma, making it suitable for most clinical scenarios. We also found that frozen plasma UC2 samples can be further purified by adding one UC washing step to achieve better EVP purity and enable further in‐depth proteomic profiling, although the total yield is compromised compared to samples freshly processed via UC3 (data not shown). This allows us to further purify previously banked patient plasma EVP samples. Furthermore, both UC‐based modified procedures only require an ultracentrifuge and no specific isolating platforms or instruments, making them readily adapted by most research and clinical laboratories conducting biomarker discovery studies involving large cohorts of patient samples. Once biomarkers are identified, more user‐friendly and scalable methods should be developed to allow for automation, facilitating high‐throughput processing, and routine clinical implementation.
Lastly, while this study reports on a modified procedure to improve plasma EVP purity and the depth of proteomic profiling, it is important to highlight the potential loss of certain subsets that can occur when the recovery rate is compromised in pursuit of higher purity. To mitigate this issue, employing multiple strategies for circulating EVP isolation and characterization in large cohort studies can be beneficial. These complementary approaches can enhance the potential for biomarker discovery.
AUTHOR CONTRIBUTIONS
Zurong Wan: Conceptualization; data curation; formal analysis; investigation; methodology; writing—original draft; writing—review and editing. Jinghua Gu: Formal analysis; methodology; writing—review and editing. Uthra Balaji: Formal analysis. Linda Bojmar: Data curation; investigation; writing—review and editing. Henrik Molina: Resources; software. Søren Heissel: Resources; software. Alexandra E. Pagano: Resources; software. Christopher Peralta: Resources; software. Lee Shaashua: Formal analysis. Dorina Ismailgeci: Data curation. Hope K. Narozniak: Data curation. Yi Song: Data curation. William R. Jarnagin: Data curation; writing—review and editing. David P. Kelsen: Data curation; writing—review and editing. Jaqueline Bromberg: Investigation; writing—review and editing. Virginia Pascual: Conceptualization; formal analysis; funding acquisition; investigation; supervision; writing—review and editing. Haiying Zhang: Conceptualization; formal analysis; funding acquisition; investigation; methodology; supervision; writing—original draft; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare conflicts of interest.
Supporting information
Supporting Information
Supplementary Figure 1. Performance evaluation of commercial kits and a protocol combining size exclusion and density gradient centrifugation (qEV‐DGC) for plasma EVP isolation in comparison to UC‐based approaches. (a) Quantification of CD9 ELISA analysis results to evaluate the yield (isolation efficiency, expressed as CD9 signal in EVPs/CD9 signal in plasma input) and purity (expressed as CD9 signal in EVPs/µg of EVPs) of isolated plasma EVPs. n > = 3. (b) Representative TEM images of plasma EVPs isolated using different approaches. Red and black arrows indicate EVPs and particles resembling lipoprotein nanoparticles. Scale bar, 200 nm. (c) Quantification of total protein yield in isolated plasma EVPs using BCA assay. n > = 3. (d) Measurement of particle size and number in isolated plasma EVPs using NTA. n > = 3. (e) WB analysis of EVP marker proteins and potential contaminants in plasma EVPs isolated from three healthy donors. The volume of input plasma analyzed for each sample is indicated on top of the images. f and g. Quantification of WB analysis results to evaluate the yield and purity of isolated plasma EVPs, respectively. n > = 3. In all graphs, the bar indicates mean. One‐way ANOVA with Geisser‐Greenhouse correction was used, followed with Bonferroni's multiple comparisons test to determine the statistical significance.
Supplementary Figure 2. Performance evaluation of a protocol combining density gradient centrifugation and bind‐elute size exclusion chromatography (DGC+BE‐SEC) for plasma EVP isolation in comparison to UC‐based approaches. (a) Quantification of fractions from DGC‐HiTrap and DGC‐HiScreen size exclusion chromatography for total proteins by BCA and abundance of CD9+PS+ EVPs by ELISA. (b) Quantification of CD9 ELISA analysis results to evaluate the yield (isolation efficiency, expressed as CD9 signal in EVPs/CD9 signal in plasma input) and purity (expressed as CD9 signal in EVPs/µg of EVPs) of isolated plasma EVPs. UC2, UC3 and DGC, n = 3; DGC‐Hi‐Trap, n = 1; DGC‐Hi‐Screen, n = 2. (c) Representative TEM images of plasma EVPs isolated using different approaches. Scale bar, 100 nm. (d) WB analysis of EVP marker proteins and potential contaminants in plasma EVPs isolated from healthy donors. The volume of input plasma analyzed for each sample is indicated on top of the images. Note: EVPs isolated from a smaller volume of plasma were loaded for the DGC‐Hi‐Trap method due to sample dilution and the gel's load capacity.
Supplementary Figure 3. Performance metrics for the methods utilized in this study. Note: “na” indicates data not available due to the lack of technical repeats of the same biological samples.
Supplementary Figure 4. UC3 and UC2‐qEV, but not PS, exhibits substantial depletion of the top 200‐ranked plasma proteins compared to UC2. (a) Healthy donor plasma samples analyzed in different batches. (b) Heatmap illustration of the relative abundance of the top 200‐ranked plasma proteins in plasma EVPs isolated using different approaches (n = 6 per group). A hierarchical cluster of proteins enriched in PS compared to UC‐based methods are highlighted in red.
Supplementary Figure 5. Proteomic profiling of plasma EVPs isolated using qEV‐DGC in comparison to UC2 and UC3 approaches. (a) Total number of proteins identified in plasma EVPs through proteomic MS analysis. n = 3; the bar indicates mean. (b) PCA of normalized proteomic MS data sets derived from plasma EVPs isolated using different approaches. (c) Heatmap illustration of the relative abundance of conventional EVP marker proteins in plasma EVPs isolated using different approaches. (d) GSEA of the top 200‐ranked plasma proteins in plasma EVPs isolated using qEV‐DGC versus UC2 approaches. n = 3 samples per group for b‐d.
Supplementary Figure 6. Examination of distinct categories of proteins in plasma EVPs isolated using different approaches. (a–c). Heatmap illustration of the relative abundance of immunoglobulins (a), ECM‐associated proteins (b), and signature proteins associated with exosomes and exomeres (c) in plasma EVPs isolated using different approaches compared to UC2. n = 6 samples per group.
Supplementary Figure 7. Differentially detected proteins in plasma EVPs isolated using UC3 and UC2‐qEV compared to UC2. Heatmap illustration of the relative abundance of DDPs enriched by UC2 (a) and UC3 and UC2‐qEV (b).
Supplementary Figure 8. Distinct proteomic compositions of plasma EVPs isolated through UC‐based approaches and PS. (a, b) Heatmap illustration of the relative abundance of proteins differentially enriched in plasma EVPs isolated through UC‐based approaches (including UC2, UC3 and UC2‐qEV) (a) and PS (b).
Supplementary Figure 9. Performance comparison between UC2 and UC3 in isolating EVPs from pancreatic cancer patient plasma. (a) BCA quantification of total protein yield in plasma EVPs isolated from pancreatic cancer (PC) patients and healthy donors (HD) using UC2 and UC3 approaches. The bar indicates mean. (b) Representative TEM images of plasma EVPs isolated from PC patients and HDs using UC2 and UC3 approaches. Red and black arrows indicate EVPs and particles resembling lipoprotein nanoparticles. Scale bar, 200 nm. (c) Total number of proteins identified in isolated plasma EVPs through proteomic MS analysis. The bar indicates mean. (d) Heatmap illustration of the relative abundance of conventional EVP marker proteins in plasma EVPs isolated from PC patients and HDs using UC2 and UC3 approaches. (e, f) GSEA illustrating a trend of enrichment of exosome signatures (e) and significant depletion of top 200‐ranked plasma proteins (f) in plasma EVPs isolated from both PC patients and HDs using the UC3 method compared to UC2. (g, h). Heatmap illustration of the relative abundance of apolipoproteins (g) and ITGs (h) in plasma EVPs isolated from PC patients and HDs using UC2 and UC3 approaches. n = 5 samples per group for (a‐h). (i). The percentage of differentially expressed proteins (DEPs) identified between PC and HD plasma EVPs isolated using UC2 and UC3 approaches, respectively.
Supplementary Table 1. Clinicopathological characteristics and demographic information on pancreatic cancer patients and healthy donors from whom de‐identified plasma specimens were collected for EVP isolation and characterization.
Supplementary Table 2. List of DEPs identified in plasma EVPs isolated using UC3 from both pancreatic cancer patients and healthy donors.
Supplementary Table 3. List of DEPs identified in plasma EVPs isolated using UC2 from both pancreatic cancer patients and healthy donors.
ACKNOWLEDGEMENTS
The authors thank colleagues David Lyden, Irina Matei, Candia Kenific, Nancy Boudreau, Larry Remmel, and Katia Manova for their feedback on the project and reading the manuscript. The authors gratefully acknowledge support from the National Cancer Institute (CA232093, CA163117, CA224175, CA163120, CA169538, CA210240, CA207983 and AI144301 (NIAID) to David Lyden, and CA218513 to David Lyden and Haiying Zhang), the Thompson Family Foundation (to David Lyden), the Tortolani Foundation (to David Lyden), the Pediatric Oncology Experimental Therapeutics Investigator's Consortium, the Malcolm Hewitt Weiner Foundation, the Manning Foundation, the Sohn Foundation, the Theodore A. Rapp Foundation, the Hartwell Foundation, the AHEPA Vth District Cancer Research Foundation, Alex's Lemonade Stand Foundation, the Breast Cancer Research Foundation, the Feldstein Medical Foundation, the Tortolani Foundation, the Daedalus Fund Selma and Lawrence Ruben Science to Industry Bridge Award, and the Children's Cancer and Blood Foundation (all to David Lyden), the Swedish Cancer Society project grant (21 1824 Pj 01 H to Linda Bojmar), the Swedish Research Society Starting Grant (2021‐02356 to Linda Bojmar), the Swedish Society for Medical Research (S21‐0079, to Linda Bojmar), the National Institute of Allergy and Infectious Diseases (AI144301, to Virginia Pascual), the Drukier Institute for Children's Health at Weill Cornell Medicine (to Virginia Pascual), Sohn Conferences Foundation and the Leona M. and Harry B. Helmsley Charitable Trust (to Proteomics Resource Center at The Rockefeller University).
Wan, Z. , Gu, J. , Balaji, U. , Bojmar, L. , Molina, H. , Heissel, S. , Pagano, A. E. , Peralta, C. , Shaashua, L. , Ismailgeci, D. , Narozniak, H. K. , Song, Y. , Jarnagin, W. R. , Kelsen, D. P. , Bromberg, J. , Pascual, V. , & Zhang, H. (2024). Optimization of ultracentrifugation‐based method to enhance the purity and proteomic profiling depth of plasma‐derived extracellular vesicles and particles. Journal of Extracellular Biology, 3, e167. 10.1002/jex2.167
Contributor Information
Virginia Pascual, Email: vip2021@med.cornell.edu.
Haiying Zhang, Email: haz2005@med.cornell.edu.
REFERENCES
- Arraud, N. , Linares, R. , Tan, S. , Gounou, C. , Pasquet, J. M. , Mornet, S. , & Brisson, A. R. (2014). Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. Journal of Thrombosis and Haemostasis, 12(5), 614–627. 10.1111/jth.12554 [DOI] [PubMed] [Google Scholar]
- Camont, L. , Lhomme, M. , Rached, F. , Le Goff, W. , Negre‐Salvayre, A. , Salvayre, R. , Calzada, C. , Lagarde, M. , Chapman, M. J. , & Kontush, A. (2013). Small, dense high‐density lipoprotein‐3 particles are enriched in negatively charged phospholipids: Relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti‐inflammatory, and antiapoptotic functionalities. Arteriosclerosis Thrombosis and Vascular Biology, 33(12), 2715–2723. 10.1161/ATVBAHA.113.301468 [DOI] [PubMed] [Google Scholar]
- Chen, G. Y. , Cheng, J. C. , Chen, Y. F. , Yang, J. C. , & Hsu, F. M. (2021). Circulating exosomal integrin beta3 is associated with intracranial failure and survival in lung cancer patients receiving cranial irradiation for brain metastases: A prospective observational study. Cancers (Basel), 13(3), 380. 10.3390/cancers13030380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, I. H. , Xue, L. , Hsu, C. C. , Paez, J. S. , Pan, L. , Andaluz, H. , Wendt, M. K. , Iliuk, A. B. , Zhu, J. K. , & Tao, W. A. (2017). Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proceedings of National Academy of Science USA, 114(12), 3175–3180. 10.1073/pnas.1618088114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, D. S. , Kim, D. K. , Kim, Y. K. , & Gho, Y. S. (2015). Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrometry Reviews, 34(4), 474–490. 10.1002/mas.21420 [DOI] [PubMed] [Google Scholar]
- Clayton, A. , Turkes, A. , Dewitt, S. , Steadman, R. , Mason, M. D. , & Hallett, M. B. (2004). Adhesion and signaling by B cell‐derived exosomes: the role of integrins. FASEB Journal, 18(9), 977–979. 10.1096/fj.03-1094fje [DOI] [PubMed] [Google Scholar]
- Colombo, M. , Raposo, G. , & Thery, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30, 255–289. 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- Corso, G. , Mager, I. , Lee, Y. , Gorgens, A. , Bultema, J. , Giebel, B. , Wood, M. J. A. , Nordin, J. Z. , & Andaloussi, S. E. (2017). Reproducible and scalable purification of extracellular vesicles using combined bind‐elute and size exclusion chromatography. Science Reports, 7(1), 11561. 10.1038/s41598-017-10646-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, K. , Jo, A. , Giedt, J. , Vinegoni, C. , Yang, K. S. , Peruzzi, P. , Chiocca, E. A. , Breakefield, X. O. , Lee, H. , & Weissleder, R. (2019). Characterization of single microvesicles in plasma from glioblastoma patients. Neuro Oncology, 21(5), 606–615. 10.1093/neuonc/noy187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino, A. , Costa‐Silva, B. , Shen, T. L. , Rodrigues, G. , Hashimoto, A. , Tesic Mark, M. , Molina, H. , Kohsaka, S. , Di Giannatale, A. , Ceder, S. , Singh, S. , Williams, C. , Soplop, N. , Uryu, K. , Pharmer, L. , King, T. , Bojmar, L. , Davies, A. E. , Ararso, Y. , … Lyden, D. (2015). Tumour exosome integrins determine organotropic metastasis. Nature, 527(7578), 329–335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino, A. , Kim, H. S. , Bojmar, L. , Gyan, K. E. , Cioffi, M. , Hernandez, J. , Zambirinis, C. P. , Rodrigues, G. , Molina, H. , Heissel, S. , Mark, M. T. , Steiner, L. , Benito‐Martin, A. , Lucotti, S. , Di Giannatale, A. , Offer, K. , Nakajima, M. , Williams, C. , Nogués, L. , … Lyden, D. (2020). Extracellular vesicle and particle biomarkers define multiple human cancers. Cell, 182(4), 1044–1061e1018. 10.1016/j.cell.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, C. Y. , Hsieh, T. H. , Lin, H. Y. , Lu, C. Y. , Lo, H. W. , Tsai, C. C. , & Tsai, E. M. (2021). Characterization and proteomic analysis of endometrial stromal cell‐derived small extracellular vesicles. Journal of Clinical Endocrinology and Metabolism, 106(5), 1516–1529. 10.1210/clinem/dgab045 [DOI] [PubMed] [Google Scholar]
- Kalra, H. , Simpson, R. J. , Ji, H. , Aikawa, E. , Altevogt, P. , Askenase, P. , Bond, V. C. , Borràs, F. E. , Breakefield, X. , Budnik, V. , Buzas, E. , Camussi, G. , Clayton, A. , Cocucci, E. , Falcon‐Perez, J. M. , Gabrielsson, S. , Gho, Y. S. , Gupta, D. , Harsha, H. C. , … Mathivanan, S. (2012). Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biology, 10(12), e1001450. 10.1371/journal.pbio.1001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keklikoglou, I. , Cianciaruso, C. , Guc, E. , Squadrito, M. L. , Spring, L. M. , Tazzyman, S. , Lambein, L. , Poissonnier, A. , Ferraro, G. B. , Baer, C. , Cassará, A. , Guichard, A. , Iruela‐Arispe, M. L. , Lewis, C. E. , Coussens, L. M. , Bardia, A. , Jain, R. K. , Pollard, J. W. , & De Palma, M. (2019). Chemotherapy elicits pro‐metastatic extracellular vesicles in breast cancer models. Nature Cell Biology, 21(2), 190–202. 10.1038/s41556-018-0256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. K. , Lee, J. , Simpson, R. J. , Lotvall, J. , & Gho, Y. S. (2015). EVpedia: A community web resource for prokaryotic and eukaryotic extracellular vesicles research. Seminars in Cell and Developmental Biology, 40, 4–7. 10.1016/j.semcdb.2015.02.005 [DOI] [PubMed] [Google Scholar]
- Ko, J. , Bhagwat, N. , Black, T. , Yee, S. S. , Na, Y. J. , Fisher, S. , Kim, J. , Carpenter, E. L. , Stanger, B. Z. , & Issadore, D. (2018). miRNA profiling of magnetic nanopore‐isolated extracellular vesicles for the diagnosis of pancreatic cancer. Cancer Research, 78(13), 3688–3697. 10.1158/0008-5472.CAN-17-3703 [DOI] [PubMed] [Google Scholar]
- Leoni, G. , Neumann, P. A. , Kamaly, N. , Quiros, M. , Nishio, H. , Jones, H. R. , Sumagin, R. , Hilgarth, R. S. , Alam, A. , Fredman, G. , Argyris, I. , Rijcken, E. , Kusters, D. , Reutelingsperger, C. , Perretti, M. , Parkos, C. A. , Farokhzad, O. C. , Neish, A. S. , & Nusrat, A. (2015). Annexin A1‐containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. Journal of Clinical Investigation, 125(3), 1215–1227. 10.1172/JCI76693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucotti, S. , Kenific, C. M. , Zhang, H. , & Lyden, D. (2022). Extracellular vesicles and particles impact the systemic landscape of cancer. EMBO Journal, 41(18), e109288. 10.15252/embj.2021109288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji, S. , Chaudhary, P. , Akopova, I. , Nguyen, P. M. , Hare, R. J. , Gryczynski, I. , & Vishwanatha, J. K. (2017). Exosomal Annexin II promotes angiogenesis and breast cancer metastasis. Molecular Cancer Research, 15(1), 93–105. 10.1158/1541-7786.MCR-16-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan, S. , & Simpson, R. J. (2009). ExoCarta: A compendium of exosomal proteins and RNA. Proteomics, 9(21), 4997–5000. 10.1002/pmic.200900351 [DOI] [PubMed] [Google Scholar]
- Pan, J. B. , Hu, S. C. , Shi, D. , Cai, M. C. , Li, Y. B. , Zou, Q. , & Ji, Z. L. (2013). PaGenBase: A pattern gene database for the global and dynamic understanding of gene function. PLoS ONE, 8(12), e80747. 10.1371/journal.pone.0080747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, E. J. , Prajuabjinda, O. , Soe, Z. Y. , Darkwah, S. , Appiah, M. G. , Kawamoto, E. , Momose, F. , Shiku, H. , & Shimaoka, M. (2019). Exosomal regulation of lymphocyte homing to the gut. Blood Advances, 3(1), 1–11. 10.1182/bloodadvances.2018024877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelissier Vatter, F. A. , Cioffi, M. , Hanna, S. J. , Castarede, I. , Caielli, S. , Pascual, V. , Matei, I. , & Lyden, D. (2021). Extracellular vesicle‐ and particle‐mediated communication shapes innate and adaptive immune responses. Journal of Experimental Medicine, 218(8), e20202579. 10.1084/jem.20202579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, D. N. , Pappin, D. J. , Creasy, D. M. , & Cottrell, J. S. (1999). Probability‐based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis, 20(18), 3551–3567. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2021). R: A Language and Environment for Statistical Computing. Retrieved from https://www.R‐project.org/
- Ritchie, M. E. , Phipson, B. , Wu, D. , Hu, Y. , Law, C. W. , Shi, W. , & Smyth, G. K. (2015). limma powers differential expression analyses for RNA‐sequencing and microarray studies. Nucleic Acids Research, 43(7), e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan, C. , & D'Souza‐Schorey, C. (2019). Tumor‐derived extracellular vesicles: Molecular parcels that enable regulation of the immune response in cancer. Journal of Cell Science, 132(20), jcs235085. 10.1242/jcs.235085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, Y. , Matsubayashi, J. , Kudo, Y. , Maehara, S. , Takeuchi, S. , Hagiwara, M. , Kakihana, M. , Ohira, T. , Nagao, T. , & Ikeda, N. (2021). Serum‐derived exosomal PD‐L1 expression to predict anti‐PD‐1 response and in patients with non‐small cell lung cancer. Scientific Reports, 11(1), 7830. 10.1038/s41598-021-87575-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, J. B. (2017). What Are We Looking At? Extracellular Vesicles, Lipoproteins, or Both? Circulation Research, 121(8), 920–922. 10.1161/CIRCRESAHA.117.311767 [DOI] [PubMed] [Google Scholar]
- Trefts, E. , Gannon, M. , & Wasserman, D. H. (2017). The liver. Current Biology, 27(21), R1147–R1151. 10.1016/j.cub.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergauwen, G. , Tulkens, J. , Pinheiro, C. , Avila Cobos, F. , Dedeyne, S. , De Scheerder, M. A. , Vandekerckhove, L. , Impens, F. , Miinalainen, I. , Braems, G. , Gevaert, K. , Mestdagh, P. , Vandesompele, J. , Denys, H. , De Wever, O. , & Hendrix, A. (2021). Robust sequential biophysical fractionation of blood plasma to study variations in the biomolecular landscape of systemically circulating extracellular vesicles across clinical conditions. Journal of Extracellular Vesicles, 10(10), e12122. 10.1002/jev2.12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, R. , Rai, A. , Chen, M. , Suwakulsiri, W. , Greening, D. W. , & Simpson, R. J. (2018). Extracellular vesicles in cancer—implications for future improvements in cancer care. Nature Reviews in Clinical Oncology, 15(10), 617–638. 10.1038/s41571-018-0036-9 [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Freitas, D. , Kim, H. S. , Fabijanic, K. , Li, Z. , Chen, H. , Mark, M. T. , Molina, H. , Martin, A. B. , Bojmar, L. , Fang, J. , Rampersaud, S. , Hoshino, A. , Matei, I. , Kenific, C. M. , Nakajima, M. , Mutvei, A. P. , Sansone, P. , Buehring, W. , … Lyden, D. (2018). Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field‐flow fractionation. Nature Cell Biology, 20(3), 332–343. 10.1038/s41556-018-0040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Smits, A. H. , van Tilburg, G. B. , Ovaa, H. , Huber, W. , & Vermeulen, M. (2018). Proteome‐wide identification of ubiquitin interactions using UbIA‐MS. Nature Protocols, 13(3), 530–550. 10.1038/nprot.2017.147 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supplementary Figure 1. Performance evaluation of commercial kits and a protocol combining size exclusion and density gradient centrifugation (qEV‐DGC) for plasma EVP isolation in comparison to UC‐based approaches. (a) Quantification of CD9 ELISA analysis results to evaluate the yield (isolation efficiency, expressed as CD9 signal in EVPs/CD9 signal in plasma input) and purity (expressed as CD9 signal in EVPs/µg of EVPs) of isolated plasma EVPs. n > = 3. (b) Representative TEM images of plasma EVPs isolated using different approaches. Red and black arrows indicate EVPs and particles resembling lipoprotein nanoparticles. Scale bar, 200 nm. (c) Quantification of total protein yield in isolated plasma EVPs using BCA assay. n > = 3. (d) Measurement of particle size and number in isolated plasma EVPs using NTA. n > = 3. (e) WB analysis of EVP marker proteins and potential contaminants in plasma EVPs isolated from three healthy donors. The volume of input plasma analyzed for each sample is indicated on top of the images. f and g. Quantification of WB analysis results to evaluate the yield and purity of isolated plasma EVPs, respectively. n > = 3. In all graphs, the bar indicates mean. One‐way ANOVA with Geisser‐Greenhouse correction was used, followed with Bonferroni's multiple comparisons test to determine the statistical significance.
Supplementary Figure 2. Performance evaluation of a protocol combining density gradient centrifugation and bind‐elute size exclusion chromatography (DGC+BE‐SEC) for plasma EVP isolation in comparison to UC‐based approaches. (a) Quantification of fractions from DGC‐HiTrap and DGC‐HiScreen size exclusion chromatography for total proteins by BCA and abundance of CD9+PS+ EVPs by ELISA. (b) Quantification of CD9 ELISA analysis results to evaluate the yield (isolation efficiency, expressed as CD9 signal in EVPs/CD9 signal in plasma input) and purity (expressed as CD9 signal in EVPs/µg of EVPs) of isolated plasma EVPs. UC2, UC3 and DGC, n = 3; DGC‐Hi‐Trap, n = 1; DGC‐Hi‐Screen, n = 2. (c) Representative TEM images of plasma EVPs isolated using different approaches. Scale bar, 100 nm. (d) WB analysis of EVP marker proteins and potential contaminants in plasma EVPs isolated from healthy donors. The volume of input plasma analyzed for each sample is indicated on top of the images. Note: EVPs isolated from a smaller volume of plasma were loaded for the DGC‐Hi‐Trap method due to sample dilution and the gel's load capacity.
Supplementary Figure 3. Performance metrics for the methods utilized in this study. Note: “na” indicates data not available due to the lack of technical repeats of the same biological samples.
Supplementary Figure 4. UC3 and UC2‐qEV, but not PS, exhibits substantial depletion of the top 200‐ranked plasma proteins compared to UC2. (a) Healthy donor plasma samples analyzed in different batches. (b) Heatmap illustration of the relative abundance of the top 200‐ranked plasma proteins in plasma EVPs isolated using different approaches (n = 6 per group). A hierarchical cluster of proteins enriched in PS compared to UC‐based methods are highlighted in red.
Supplementary Figure 5. Proteomic profiling of plasma EVPs isolated using qEV‐DGC in comparison to UC2 and UC3 approaches. (a) Total number of proteins identified in plasma EVPs through proteomic MS analysis. n = 3; the bar indicates mean. (b) PCA of normalized proteomic MS data sets derived from plasma EVPs isolated using different approaches. (c) Heatmap illustration of the relative abundance of conventional EVP marker proteins in plasma EVPs isolated using different approaches. (d) GSEA of the top 200‐ranked plasma proteins in plasma EVPs isolated using qEV‐DGC versus UC2 approaches. n = 3 samples per group for b‐d.
Supplementary Figure 6. Examination of distinct categories of proteins in plasma EVPs isolated using different approaches. (a–c). Heatmap illustration of the relative abundance of immunoglobulins (a), ECM‐associated proteins (b), and signature proteins associated with exosomes and exomeres (c) in plasma EVPs isolated using different approaches compared to UC2. n = 6 samples per group.
Supplementary Figure 7. Differentially detected proteins in plasma EVPs isolated using UC3 and UC2‐qEV compared to UC2. Heatmap illustration of the relative abundance of DDPs enriched by UC2 (a) and UC3 and UC2‐qEV (b).
Supplementary Figure 8. Distinct proteomic compositions of plasma EVPs isolated through UC‐based approaches and PS. (a, b) Heatmap illustration of the relative abundance of proteins differentially enriched in plasma EVPs isolated through UC‐based approaches (including UC2, UC3 and UC2‐qEV) (a) and PS (b).
Supplementary Figure 9. Performance comparison between UC2 and UC3 in isolating EVPs from pancreatic cancer patient plasma. (a) BCA quantification of total protein yield in plasma EVPs isolated from pancreatic cancer (PC) patients and healthy donors (HD) using UC2 and UC3 approaches. The bar indicates mean. (b) Representative TEM images of plasma EVPs isolated from PC patients and HDs using UC2 and UC3 approaches. Red and black arrows indicate EVPs and particles resembling lipoprotein nanoparticles. Scale bar, 200 nm. (c) Total number of proteins identified in isolated plasma EVPs through proteomic MS analysis. The bar indicates mean. (d) Heatmap illustration of the relative abundance of conventional EVP marker proteins in plasma EVPs isolated from PC patients and HDs using UC2 and UC3 approaches. (e, f) GSEA illustrating a trend of enrichment of exosome signatures (e) and significant depletion of top 200‐ranked plasma proteins (f) in plasma EVPs isolated from both PC patients and HDs using the UC3 method compared to UC2. (g, h). Heatmap illustration of the relative abundance of apolipoproteins (g) and ITGs (h) in plasma EVPs isolated from PC patients and HDs using UC2 and UC3 approaches. n = 5 samples per group for (a‐h). (i). The percentage of differentially expressed proteins (DEPs) identified between PC and HD plasma EVPs isolated using UC2 and UC3 approaches, respectively.
Supplementary Table 1. Clinicopathological characteristics and demographic information on pancreatic cancer patients and healthy donors from whom de‐identified plasma specimens were collected for EVP isolation and characterization.
Supplementary Table 2. List of DEPs identified in plasma EVPs isolated using UC3 from both pancreatic cancer patients and healthy donors.
Supplementary Table 3. List of DEPs identified in plasma EVPs isolated using UC2 from both pancreatic cancer patients and healthy donors.


