Key Points
Question
Is prenatal cannabis use associated with maternal health outcomes during pregnancy?
Findings
In this cohort study of 316 722 pregnancies, prenatal cannabis use was associated with increased risk of gestational hypertension, preeclampsia, weight gain greater and less than guidelines, and placental abruption as well as reduced risk of gestational diabetes. No association was found with eclampsia, placenta previa, placenta accreta, or severe maternal morbidity.
Meaning
The results of this study suggest that the association between prenatal cannabis use and maternal health is complex and there is a need for continued research to understand how prenatal cannabis use affects the health of pregnant individuals.
Abstract
Importance
Many studies have evaluated whether in utero cannabis exposure is associated with fetal and neonatal outcomes, yet little is known about whether prenatal cannabis use is associated with maternal health outcomes during pregnancy.
Objective
To evaluate whether prenatal cannabis use is associated with maternal health outcomes during pregnancy.
Design, Setting, and Participants
This population-based retrospective cohort study included pregnancies in Northern California from January 2011 to December 2019 that lasted 20 weeks or longer and were screened for prenatal cannabis use.
Exposures
Prenatal cannabis use was defined as any self-reported use during early pregnancy or a positive toxicology test result based on universal screening at entrance to prenatal care (approximately 8-10 weeks’ gestation). Self-reported frequency of use (daily, weekly, monthly or less, never, unknown), use defined only by self-report, and use defined only by toxicology test results were examined.
Main Outcomes and Measures
Electronic health record data were used to define the following outcomes: gestational hypertension, preeclampsia, eclampsia, gestational diabetes, gestational weight gain greater and less than guidelines, placenta previa, placental abruption, placenta accreta, and severe maternal morbidity. Adjusted risk ratios (aRRs) were calculated using a modified Poisson regression.
Results
The sample (n = 316 722 pregnancies; 250 221 unique individuals) included 84 039 (26.5%) Asian/Pacific Islander, 20 053 (6.3%) Black, 83 145 (26.3%) Hispanic, and 118 333 (37.4%) White individuals; the mean (SD) age was 30.6 (5.4) years. Overall, 20 053 (6.3%) screened positive for prenatal cannabis use; 2.9% were positive by self-report, 5.3% by toxicology testing, and 1.8% by both. The frequency of cannabis use was 1930 (0.6%) daily, 2345 (0.7%) weekly, 4892 (1.5%) monthly or less, and 10 886 (3.4%) unknown. Prenatal cannabis use was associated with greater risk of gestational hypertension (aRR, 1.17; 95% CI, 1.13-1.21), preeclampsia (aRR, 1.08; 95% CI, 1.01-1.15), weight gain less than (aRR, 1.05; 95% CI, 1.01-1.08) and greater than (aRR, 1.09; 95% CI, 1.08-1.10) guidelines, and placental abruption (aRR, 1.19; 95% CI, 1.05-1.36). The pattern of results was similar when defining prenatal cannabis use only by self-report or only by toxicology testing, and associations between the frequency of prenatal cannabis use and outcomes varied with outcome.
Conclusions and Relevance
The results of this cohort study suggest that prenatal cannabis use was associated with several adverse maternal health outcomes during pregnancy. Continued research is needed to understand whether characteristics of prenatal cannabis use (eg, dose, mode, and timing) moderate these associations.
This cohort study examines the association between prenatal cannabis use and maternal health outcomes during pregnancy.
Introduction
Rates of prenatal cannabis use in the US have increased in recent years,1 corresponding with spreading legalization and rising perceptions of safety.2,3 Pregnant individuals report using cannabis to help with sleep, depression, stress, morning sickness, and pain during pregnancy, and many perceive cannabis to be a safer alternative to prescription medications.2,4,5 However, there is evidence that prenatal cannabis use is associated with moderate increases in the risk of adverse fetal and neonatal health outcomes (eg, lower birthweight, preterm birth, and neonatal intensive care unit admission),6,7,8 and national guidelines recommend that pregnant individuals abstain from using cannabis.9
Whereas many studies have examined how maternal prenatal cannabis use is associated with fetal and neonatal outcomes,6,7,8,10,11,12 less is known about the associations with maternal health during pregnancy. Cannabinoids, including δ9-tetrahydrocannabinol (THC), cross the placenta13,14 and may affect maternal health by binding to the cannabinoid receptors on the placenta, inhibiting migration of the epithelial layer of human placental amnion tissue, disrupting endogenous cannabinoid signaling and estrogen signaling, and affecting placental development and function.15,16,17,18,19,20,21,22,23 Further, maternal cannabis use is associated with increased peripheral vasoconstriction,24 raised maternal heart rate and blood pressure, and increased risk of adverse outcomes (eg, preeclampsia, hypertension).25,26,27
Existing research on the association between prenatal cannabis use or cannabis use disorder and maternal outcomes (eg, gestational diabetes [GD], hypertension, and placental abruption) has limitations that may explain mixed findings.28,29,30,31,32,33 Most studies are limited to self-reported cannabis use, which is known to underestimate use,34,35 and do not adequately account for confounders, such as noncannabis prenatal substance use. Further, previous studies may not be generalizable to current populations due to the changing modes of cannabis administration and increased potency of newer cannabis products.36,37,38,39
In this large, retrospective cohort study, we examined the association between prenatal cannabis use and maternal health outcomes among individuals in a large health care system with universal screening for prenatal cannabis use by self-report and urine toxicology testing, adjusting for a wide range of covariates, including maternal use of other substances.
Methods
Setting
Kaiser Permanente Northern California (KPNC) is an integrated health care delivery system that provides health care to 4.6 million patients, similar to the insured Northern California population.40 Institutional review board approval was obtained from KPNC with waiver of consent and Health Insurance Portability and Accountability Act authorization, and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines were followed.
Cohort and Measures
This population-based retrospective cohort study included singleton pregnancies that began (based on last menstrual period) during 2011 to 2019 and lasted 20 weeks or longer. Eligibility criteria included KPNC membership at any point during pregnancy and 1 or more KPNC prenatal visits. Pregnancies were required to have a response to the self-reported cannabis use question and a THC urine toxicology test (eFigure in Supplement 1). All measures were extracted from KPNC administrative and electronic health record data.
Exposures
The primary exposure was based on universal screening at the entrance to prenatal care (approximately 8-10 weeks’ gestation) via a self-administered questionnaire and urine toxicology test to which patients consented to undergo (eMethods 1 in Supplement 1). Confirmatory testing for the presence of the cannabis metabolite, 11-nor-9-carboxy-δ9-THC, detectable for up to approximately 30 days after last use among those who use regularly, was performed by liquid chromatography-tandem mass spectrometry for all positive immunoassay results. Individuals were classified as having any prenatal use if they self-reported cannabis use since pregnancy or had a positive confirmed toxicology test result. Self-reported frequency of prenatal cannabis use was based on mutually exclusive categories of daily, weekly, monthly or less, and never; we created a category of unknown frequency among those with a positive toxicology test result without self-reported use.
Maternal Outcomes
For all outcomes, International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 diagnosis codes and other diagnostic criteria are provided in eMethods 2 in Supplement 1. Individual outcomes are described in the following sections.
Metabolic Outcomes
Among patients without chronic hypertension, we examined 3 hypertensive disorders of pregnancy: gestational hypertension, preeclampsia, and eclampsia. Gestational hypertension was defined using a combination of diagnoses, anti-hypertensive medication use, and blood pressure measurements. Preeclampsia and eclampsia were ascertained from inpatient diagnoses.
GD was ascertained from the KPNC Gestational Diabetes Registry,41 which uses diagnoses, medications, and laboratory tests throughout pregnancy to identify GD among those without pregestational diabetes. We examined gestational weight gain (GWG) that was either less or greater than the range recommended by the Institute of Medicine (2009; eMethods 2 in Supplement 1).42
Placental Outcomes and Severe Maternal Morbidity
Placenta previa and accreta were identified by inpatient diagnosis codes; placental abruption was identified by inpatient and outpatient diagnosis codes. Severe maternal morbidity was defined by the US Centers for Disease Control and Prevention criteria using inpatient and outpatient diagnosis codes for 21 pregnancy-related conditions.43,44
Covariates
Data on maternal age at pregnancy onset, self-reported race and ethnicity (Asian/Pacific Islander, Black, Hispanic, White, and other, including American Indian, Alaska Native, and multiracial, which was included as a social construct due to known differences in the prevalence of prenatal cannabis use by race and ethnicity), parity (0, 1, ≥2), maternal insurance (Medicaid vs other), neighborhood deprivation index (quartiles),45 birth year, and prepregnancy body mass index (calculated as weight in kilograms divided by height in meters squared) were extracted from the electronic health record. Month of prenatal care initiation was classified using the Kotelchuck initiation index: inadequate (≥7), intermediate (5-6), adequate (3-4), or adequate plus (1-2).46 Noncannabis prenatal substance use was based on universal screening at entrance to prenatal care and defined as prenatal use of alcohol, nicotine, opioids, stimulants, and anxiety/sleep medications (eMethods 1 in Supplement 1).
For maternal comorbidities, ICD diagnosis codes were used to define pregestational diabetes (type 1 or 2) during the 2 years before pregnancy, mood/anxiety disorder, other psychiatric disorders, and noncannabis substance use disorder diagnoses during the year before pregnancy through the first prenatal visit and nausea and vomiting of pregnancy through the first prenatal visit. Antidepressant medication use was defined as a prescription fill during pregnancy through the first prenatal visit or before pregnancy with the supply lasting past the pregnancy onset date.
Statistical Analysis
We fit extended modified Poisson models with robust standard errors using the extension of the sandwich variance estimator to account for the correlation in the outcomes for multiple pregnancies per person.47 Risk ratios (RRs) and adjusted RRs (aRRs) with 95% CIs were reported. Model 1 was unadjusted. Model 2 was adjusted for maternal sociodemographic characteristics, parity, birth year, prenatal care initiation, prepregnancy body mass index, other noncannabis prenatal substance use (ie, alcohol, nicotine, opioids, stimulants, and anxiety/sleep medications), and comorbidities (ie, pregestational diabetes, nausea/vomiting during pregnancy, mood/anxiety disorders, other psychiatric disorders, substance use disorders, and antidepressant use). We also tested for associations between use frequency and outcomes, followed by a trend test when results suggested an ordering of associations across frequency levels; trend tests excluded the category of frequency unknown, positive toxicology test result.
Sensitivity Analyses
To assess differences in exposure classification, we fit separate models in which the exposure (ie, any prenatal cannabis use) was determined only by self-report status or urine toxicology test results. We also repeated analyses (1) that were limited to the subset of pregnancies without evidence of noncannabis prenatal substance use, (2) among those who entered prenatal care during the first trimester, and (3) after removing calendar year as a covariate, as controlling for calendar time may constitute overadjustment that could bias the estimated associations.
The standardized difference was calculated as the difference in proportions divided by the standard error.48 Analyses were conducted using SAS, version 9.4 (SAS Institute), and R, version 4.0.2 (R Foundation), from October 2023 to May 2024. Two-sided P values of <.05 were considered statistically significant.
Results
The maternal characteristics of the 316 722 pregnancies (from 250 221 unique individuals) were 84 039 (26.5%) Asian/Pacific Islander, 20 053 (6.3%) Black, 83 145 (26.3%) Hispanic, and 118 333 (37.4%) White individuals; 49 015 (15.5%) were younger than 25 years, and 28 803 (9.1%) were insured by Medicaid. Overall, 20 053 (6.3%) screened positive for prenatal cannabis use by either self-report or toxicology testing; 2.9% were positive by self-report, 5.3% were positive by toxicology, and 1.8% were positive by both. Self-report and toxicology testing were completed at a median of 8.3 weeks (IQR,7.0-10.3) and 9.0 weeks (IQR, 7.7-11.4) of gestation, respectively. Pregnancies excluded due to missing data on prenatal cannabis use had sociodemographic characteristics like the final sample but had more missing data (eTable 1 in Supplement 1). The frequency of cannabis use was 1930 (0.6%) daily, 2345 (0.7%) weekly, and 4892 (1.5%) monthly or less, and 10 886 (3.4%) had a positive toxicology test result but did not report cannabis use (Table 1). Pregnancy characteristics by frequency are shown in eTable 2 in Supplement 1. Several maternal outcomes were rare (<5%): preeclampsia (44 363 [4.8%]), eclampsia (384 [0.1%]), placenta previa (3499 [1.1%]), placental abruption (4002 [1.3%]), placenta accreta (499 [0.2%]), and severe maternal morbidity (10 338 [3.3%]). Gestational hypertension (44 363 [14.7%]), GD (36 374 [11.7%]), and GWG less than (48 117 [16.0%]) and greater than (176 898 [58.8%]) guidelines were more common (Table 2).
Table 1. Pregnancy Characteristics Overall and by Prenatal Cannabis Use.
| Pregnancy characteristics | No. (%) | Standardized differencea | ||
|---|---|---|---|---|
| Overall (N = 316 722) | Prenatal cannabis use | |||
| Yes (n = 20 053 [6.3%]) | No (n = 296 669 [93.7%]) | |||
| Age at pregnancy onset, y | ||||
| <18 | 2994 (0.9) | 617 (3.1) | 2377 (0.8) | 0.68 |
| 18-24 | 46 021 (14.5) | 7541 (37.6) | 38 480 (13.0) | |
| 25-30 | 87 882 (27.7) | 5469 (27.3) | 82 413 (27.8) | |
| 31-35 | 113 020 (35.7) | 4250 (21.2) | 108 770 (36.7) | |
| ≥36 | 66 805 (21.1) | 2176 (10.9) | 64 629 (21.8) | |
| Self-reported race and ethnicity | ||||
| Hispanic | 83 145 (26.3) | 5583 (27.8) | 77 562 (26.1) | 0.80 |
| Non-Hispanic | ||||
| American Indian/Alaska Nativeb | 1078 (0.3) | 144 (0.1) | 934 (0.3) | |
| Asian/Pacific Islander | 84 039 (26.5) | 1191 (5.9) | 82 848 (27.9) | |
| Black | 20 053 (6.3) | 4480 (22.3) | 15 573 (5.2) | |
| White | 118 333 (37.4) | 7750 (38.6) | 110 583 (37.3) | |
| Multiracialb | 4540 (1.3) | 622 (0.2) | 3918 (1.2) | |
| Unknownb | 5534 (1.8) | 283 (0.1) | 5251 (1.7) | |
| Parity | ||||
| 0 | 129 585 (40.9) | 9906 (49.4) | 119 679 (40.3) | 0.25 |
| 1 | 110 434 (34.9) | 5471 (27.3) | 104 963 (35.4) | |
| ≥2 | 65 908 (20.8) | 3408 (17.0) | 62 500 (21.1) | |
| Unknown | 10 795 (3.4) | 1268 (6.3) | 9527 (3.2) | |
| Prenatal care initiation | ||||
| Adequate plus (months 1-2) | 198 083 (62.5) | 11 932 (59.5) | 186 151 (62.7) | 0.13 |
| Adequate (months 3-5) | 99 481 (31.4) | 6619 (33.0) | 92 862 (31.3) | |
| Intermediate (months 5-6) | 11 297 (3.6) | 962 (4.8) | 10 335 (3.5) | |
| Inadequate (months 7+) | 7861 (2.5) | 540 (2.7) | 7321 (2.5) | |
| Insured by Medicaid | 28 803 (9.1) | 5049 (25.2) | 23 754 (8.0) | 0.47 |
| Neighborhood deprivation index, quartile | ||||
| 1 (Least deprivation) | 74 902 (23.6) | 2641 (13.2) | 72 261 (24.4) | 0.41 |
| 2 | 74 934 (23.7) | 3987 (19.9) | 70 947 (23.9) | |
| 3 | 74 907 (23.7) | 5315 (26.5) | 69 592 (23.5) | |
| 4 (Most deprivation) | 74 916 (23.7) | 7510 (37.5) | 67 406 (22.7) | |
| Unknown | 17 063 (5.4) | 600 (3.0) | 16 463 (5.5) | |
| Noncannabis substance use during pregnancy | ||||
| Alcohol | 29 565 (9.3) | 3987 (19.9) | 25 578 (8.6) | 0.33 |
| Nicotine | 14 281 (4.5) | 4499 (22.4) | 9782 (3.3) | 0.60 |
| Opioids | 22 054 (7.0) | 2561 (12.8) | 19 493 (6.6) | 0.21 |
| Stimulants | 1712 (0.5) | 598 (3.0) | 1114 (0.4) | 0.20 |
| Anxiety or sleep medications | 9163 (2.9) | 1370 (6.8) | 7793 (2.6) | 0.20 |
| Prepregnancy BMI categories | ||||
| Underweight (<18.5) | 7164 (2.3) | 537 (2.7) | 6627 (2.2) | 0.24 |
| Normal (18.5-24.9) | 137 375 (43.4) | 6945 (34.6) | 130 430 (44.0) | |
| Overweight (25-29.9) | 83 426 (26.3) | 5275 (26.3) | 78 151 (26.3) | |
| Obesity (≥30) | 73 367 (23.2) | 6433 (32.1) | 66 934 (22.6) | |
| Unknown | 15 390 (4.9) | 863 (4.3) | 14 527 (4.9) | |
| Diagnoses and medications | ||||
| 2 y Before pregnancy | ||||
| Diabetes | 4026 (1.3) | 222 (1.1) | 3804 (1.3) | −0.02 |
| 1 y Before pregnancy through first prenatal visit | ||||
| Mood/anxiety disorder | 34 984 (11.0) | 4514 (22.5) | 30 470 (10.3) | 0.34 |
| Other psychiatric disorder | 7498 (2.4) | 1269 (6.3) | 6229 (2.1) | 0.21 |
| Substance use disorder diagnosis (other than cannabis) | 11 220 (3.5) | 3251 (16.2) | 7969 (2.7) | 0.48 |
| Pregnancy onset through first prenatal visit | ||||
| Nausea and vomiting | 35 639 (11.3) | 4642 (23.1) | 30 997 (10.4) | 0.34 |
| Antidepressant medication use | 6441 (2.0) | 865 (4.3) | 5576 (1.9) | 0.14 |
| Frequency of cannabis use | ||||
| Daily | 1930 (0.6) | 1930 (9.6) | 0 | 1.31 |
| Weekly | 2345 (0.7) | 2345 (11.7) | 0 | |
| Monthly or less | 4892 (1.5) | 4892 (24.4) | 0 | |
| Never | 296 669 (93.7) | 0 | 296 669 (100) | |
| Unknown frequency, positive toxicology results | 10 886 (3.4) | 10 886 (54.3) | 0 | |
| Fetal outcome | ||||
| Live birth | 314 480 (99.3) | 19 880 (99.1) | 294 600 (99.3) | 0.20 |
| Stillbirth | 1510 (0.5) | 114 (0.6) | 1396 (0.5) | |
| Therapeutic abortion | 732 (0.2) | 59 (0.3) | 673 (0.2) | |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Standardized difference is the difference in proportions divided by the standard error; imbalance defined as absolute value greater than 0.20 (small effect size).
These categories were collapsed in the models.
Table 2. Frequency and Risk Ratios of Maternal Outcomes by Any Prenatal Cannabis Use.
| Maternal outcomes | Pregnancies, No. (%) | Risk ratios of maternal outcomes for any prenatal cannabis use vs none (95% CI)a | |||
|---|---|---|---|---|---|
| Overall (N = 316 722) | Prenatal cannabis use | Model 1 | Model 2 | ||
| Yes (n = 20 053) | No (n = 296 669) | ||||
| Metabolic outcomes | |||||
| Hypertensive disordersb | |||||
| Gestational hypertension | 44 363 (14.7) | 3822 (20.3) | 40 541 (14.3) | 1.42 (1.38-1.46) | 1.17 (1.13-1.21) |
| Preeclampsia | 14 378 (4.8) | 1212 (6.4) | 13 166 (4.6) | 1.39 (1.31-1.47) | 1.08 (1.01-1.15) |
| Eclampsia | 384 (0.1) | 34 (0.2) | 350 (0.1) | 1.46 (1.03-2.08) | 1.17 (0.80-1.71) |
| Gestational diabetesc | 36 374 (11.7) | 1521 (7.7) | 34 853 (11.9) | 0.64 (0.61-0.68) | 0.89 (0.85-0.94) |
| Gestational weight gaind | |||||
| Within guidelines | 75 844 (25.2) | 3284 (17.1) | 72 560 (25.8) | NA | NA |
| Less than guidelines | 48 117 (16.0) | 2727 (14.2) | 45 390 (16.1) | 1.18 (1.15-1.21) | 1.05 (1.01-1.08) |
| Greater than guidelines | 176 898 (58.8) | 13 145 (68.6) | 176 898 (58.8) | 1.15 (1.15-1.16) | 1.09 (1.08-1.10) |
| Placental outcomes | |||||
| Placenta previa | 3499 (1.1) | 169 (0.8) | 3330 (1.1) | 0.75 (0.64-0.88) | 1.02 (0.87-1.20) |
| Placental abruption | 4002 (1.3) | 281 (1.4) | 3721 (1.3) | 1.12 (0.99-1.26) | 1.19 (1.05-1.36) |
| Placenta accretae | 499 (0.2) | 37 (0.2) | 462 (0.2) | 1.20 (0.86-1.67) | 1.34 (0.92-1.95) |
| Severe maternal morbidity | 10 338 (3.3) | 718 (3.6) | 9620 (3.2) | 1.10 (1.02-1.19) | 0.97 (0.89-1.05) |
Abbreviation: NA, not applicable.
Model 1: modified Poisson model with robust standard errors (no covariates). Model 2: adjusted for maternal sociodemographic characteristics (age category, race and ethnicity, neighborhood deprivation index), parity, birth year, prenatal care initiation, prepregnancy body mass index category, noncannabis prenatal substance use (alcohol, nicotine, opioids, stimulants, and anxiety/sleep medications), and maternal medical and mental health comorbidities (pregestational diabetes, nausea/vomiting during pregnancy, mood/anxiety disorders, other psychiatric disorders, substance use disorders [other than cannabis], and antidepressant use).
Pregnancies of individuals with chronic hypertension were excluded (n = 14 187).
Pregnancies of individuals with pregestational diabetes were excluded. In addition, gestational diabetes could not be ascertained for pregnancies ending in therapeutic abortion. A total of 4737 pregnancies were excluded. In model 2, pregestational diabetes was not included as a covariate for the gestational diabetes outcome.
Categories determined by the 2009 Institute of Medicine guidelines. Pregnancies with missing weight values were excluded (n = 15 863). Less than guidelines models were fit among pregnancies less than or within guidelines; greater than guidelines models were fit among pregnancies greater than or within guidelines.
Placenta accreta could only be ascertained for pregnancies that were delivered in a Kaiser Permanente Northern California facility and ended in live birth or stillbirth (alive at admission); 10 979 were excluded.
Primary Exposure: Any Prenatal Cannabis Use
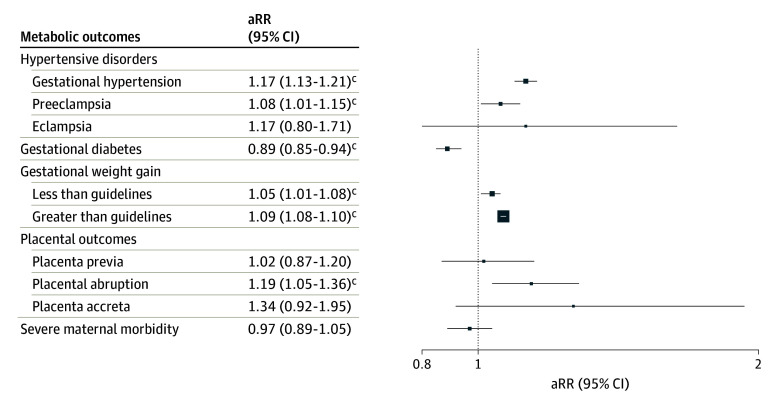
Table 2 shows the unadjusted RRs and fully aRRs for each outcome, and eTable 3 in Supplement 1 shows how results changed by including stepwise covariates. In fully adjusted models, prenatal cannabis use was associated with an increased risk of gestational hypertension (aRR, 1.17; 95% CI, 1.13-1.21) and preeclampsia (aRR, 1.08; 95% CI, 1.01-1.15) but not eclampsia (aRR, 1.17; 95% CI, 0.80-1.71) (Figure).
Figure. Adjusted Risk Ratios (aRRs)a of Maternal Outcomes in Pregnancies With Any Prenatal Cannabis Use vs Noneb.
aRisk ratios were adjusted for maternal sociodemographic characteristics (age category, race and ethnicity, and neighborhood deprivation index), parity, birth year, prenatal care initiation, prepregnancy body mass index category, noncannabis prenatal substance use (alcohol, nicotine, opioids, stimulants, and anxiety/sleep medications), and maternal medical and mental health comorbidities (pregestational diabetes, nausea/vomiting during pregnancy, mood/anxiety disorders, other psychiatric disorders, substance use disorders [other than cannabis], and antidepressant use). Pregestational diabetes was not included as a covariate in the gestational diabetes model as patients with pregestational diabetes were ineligible for this outcome.
bPregnancies of individuals with chronic hypertension were excluded (n = 14 187) from models of hypertensive outcomes. A total of 4737 pregnancies were excluded from the gestational diabetes model (pregnancies of individuals with pregestational diabetes were ineligible, and gestational diabetes could not be ascertained for pregnancies ending in therapeutic abortion). Pregnancies with missing weight values were excluded (n = 15 863) from the gestational weight gain models. Placenta accreta could only be ascertained for pregnancies that were delivered in a Kaiser Permanente Northern California facility and ended in live birth or stillbirth (alive at admission); 10 979 were excluded.
cStatistical significance at the P < .05 level.
In the fully adjusted models, prenatal cannabis use was associated with a significantly decreased risk of GD (aRR, 0.89; 95% CI, 0.85-0.94), an increased risk of GWG less than guidelines (aRR, 1.05; 95% CI, 1.01-1.08) and greater than guidelines (aRR, 1.09; 95% CI, 1.08-1.10), and an increased risk of placental abruption (aRR, 1.19; 95% CI, 1.05-1.36). Prenatal cannabis use was not significantly associated with placenta previa, placenta accreta, or severe maternal morbidity in fully adjusted models.
Secondary Exposure: Frequency of Prenatal Cannabis Use
We found a dose-response association between use frequency and risk of gestational hypertension, with risk decreasing from daily (aRR, 1.24; 95% CI, 1.14-1.36) to weekly (aRR, 1.21; 95% CI, 1.11-1.31) to monthly use (aRR, 1.03; 95% CI, 0.97-1.10) vs never use (trend-test P < .001; Table 3). Unknown frequency (positive toxicology test result but no self-reported use) was also associated with a greater risk of gestational hypertension (aRR, 1.21; 95% CI, 1.16-1.25). No other trends were evident; however, some additional findings for frequency were statistically significant. A greater risk of preeclampsia was found for unknown frequency of use (aRR, 1.14; 95% CI, 1.06-1.24). Increased risk of GWG less than guidelines was found for weekly use (aRR, 1.09; 95% CI, 1.01-1.18), while increased risk of GWG greater than guidelines was found for daily (aRR, 1.06; 95% CI, 1.03-1.09), weekly (aRR, 1.07; 95% CI, 1.04-1.09), monthly or less (aRR, 1.06; 95% CI, 1.04-1.08), and unknown frequency (aRR, 1.11; 95% CI, 1.10-1.12). Greater risk of placental abruption was found for monthly or less use (aRR, 1.31; 95% CI, 1.03-1.67). In contrast, lower risk of GD was found for monthly or less (aRR, 0.88; 95% CI, 0.79-0.98) and unknown frequency of use (aRR, 0.89; 95% CI, 0.83-0.95). There was no association between use frequency and other outcomes.
Table 3. Frequency and Adjusted Risk Ratios (ARRs) of Maternal Outcomes by Frequency of Prenatal Cannabis Use.
| Maternal outcomes by frequency of prenatal cannabis use | Pregnancies, No. (%) | aRR (95% CI)a |
|---|---|---|
| Metabolic outcomes | ||
| Hypertensive disordersb | ||
| Gestational hypertension | ||
| Daily | 402 (22.3) | 1.24 (1.14-1.36) |
| Weekly | 475 (21.3) | 1.21 (1.11-1.31) |
| Monthly or less | 838 (18.0) | 1.03 (0.97-1.10) |
| Never | 40 541 (14.3) | 1 [Reference] |
| Unknown frequency, positive toxicology result | 2107 (20.7) | 1.21 (1.16-1.25) |
| Preeclampsia | ||
| Daily | 129 (7.2) | 1.15 (0.96-1.36) |
| Weekly | 147 (6.6) | 1.11 (0.95-1.30) |
| Monthly or less | 253 (5.4) | 0.89 (0.79-1.01) |
| Never | 13 166 (4.6) | 1 [Reference] |
| Unknown frequency, positive toxicology result | 683 (6.7) | 1.14 (1.06-1.24) |
| Eclampsia | ||
| Daily | 3 (0.2) | 1.11 (0.35-3.49) |
| Weekly | 5 (0.2) | 1.53 (0.61-3.85) |
| Monthly or less | 8 (0.2) | 1.13 (0.54-2.35) |
| Never | 350 (0.1) | 1 [Reference] |
| Unknown frequency, positive toxicology result | 18 (0.2) | 1.13 (0.69-1.83) |
| Gestational diabetesc | ||
| Daily | 149 (7.8) | 0.99 (0.85-1.15) |
| Weekly | 160 (6.9) | 0.87 (0.75-1.00) |
| Monthly or less | 341 (7.1) | 0.88 (0.79-0.98) |
| Never | 34 853 (11.9) | 1 [Reference] |
| Unknown frequency, positive toxicology result | 871 (8.1) | 0.89 (0.83-0.95) |
| Gestational weight gaind | ||
| Less than guidelines | ||
| Daily | 281 (46.0) | 1.05 (0.96-1.14) |
| Weekly | 340 (46.6) | 1.09 (1.01-1.18) |
| Monthly or less | 658 (42.7) | 1.04 (0.98-1.10) |
| Never | 45 390 (38.5) | 1 [Reference] |
| Unknown frequency, positive toxicology results | 1448 (46.3) | 1.04 (1.00-1.08) |
| Greater than guidelines | ||
| Daily | 1230 (78.8) | 1.06 (1.03-1.09) |
| Weekly | 1481 (79.2) | 1.07 (1.04-1.09) |
| Monthly or less | 3070 (77.6) | 1.06 (1.04-1.08) |
| Never | 163 753 (69.3) | 1 [Reference] |
| Unknown frequency, positive toxicology result | 7364 (81.4) | 1.11 (1.10-1.12) |
| Placental outcomes | ||
| Placenta previa | ||
| Daily | 17 (0.9) | 1.10 (0.68-1.77) |
| Weekly | 26 (1.1) | 1.38 (0.93-2.04) |
| Monthly or less | 43 (0.9) | 1.06 (0.78-1.44) |
| Never | 3330 (1.1) | 1 [Reference] |
| Unknown frequency, positive toxicology result | 83 (0.8) | 0.93 (0.74-1.16) |
| Placental abruption | ||
| Daily | 29 (1.5) | 1.26 (0.87-1.84) |
| Weekly | 29 (1.2) | 1.07 (0.74-1.55) |
| Monthly or less | 74 (1.5) | 1.31 (1.03-1.67) |
| Never | 3721 (1.3) | 1 [Reference] |
| Unknown frequency, positive toxicology result | 149 (1.4) | 1.15 (0.98-1.37) |
| Placenta accretae | ||
| Daily | 4 (0.2) | 1.51 (0.54-4.22) |
| Weekly | 5 (0.2) | 1.60 (0.64-4.04) |
| Monthly or less | 7 (0.1) | 1.10 (0.51-2.38) |
| Never | 462 (0.2) | 1 [Reference] |
| Unknown frequency, positive toxicology result | 21 (0.2) | 1.36 (0.85-2.16) |
| Severe maternal morbidity | ||
| Daily | 76 (3.9) | 1.00 (0.80-1.26) |
| Weekly | 77 (3.3) | 0.88 (0.70-1.10) |
| Monthly or less | 180 (3.7) | 1.00 (0.86-1.16) |
| Never | 9620 (3.2) | 1 [Reference] |
| Unknown frequency, positive toxicology result | 385 (3.5) | 0.97 (0.87-1.07) |
Modified Poisson models with robust standard errors were adjusted for maternal sociodemographic characteristics (age category, race and ethnicity, neighborhood deprivation index), parity, birth year, prenatal care initiation, prepregnancy body mass index category, other noncannabis prenatal substance use (alcohol, nicotine, opioids, stimulants, and anxiety/sleep medications), and maternal medical and mental health comorbidities (pregestational diabetes, nausea/vomiting during pregnancy, mood/anxiety disorders, other psychiatric disorders, substance use disorders [other than cannabis], and antidepressant use).
Pregnancies of individuals with chronic hypertension were excluded (n = 14 187).
Pregnancies of individuals with pregestational diabetes were excluded. In addition, gestational diabetes could not be ascertained for pregnancies ending in therapeutic abortion. A total of 4737 pregnancies were excluded. Pregestational diabetes was not included as a covariate.
Categories determined by the 2009 Institute of Medicine guidelines. Pregnancies with missing weight values were excluded (n = 15 863). Less than guidelines models were fit among pregnancies less than or within guidelines; greater than guidelines models were fit among pregnancies greater than or within guidelines.
Placenta accreta could only be ascertained for pregnancies that were delivered in a Kaiser Permanente Northern California facility and ended in live birth or stillbirth (alive at admission); 10 979 were excluded.
Sensitivity Analyses
Results followed the same general pattern when defining prenatal cannabis use by self-report or toxicology testing only (Table 4). Associations for most outcomes were slightly stronger for toxicology tests than for self-report. Results from sensitivity analyses limited to pregnant individuals without evidence of noncannabis prenatal substance use followed the same general pattern but were slightly stronger than the main models (eTable 4 in Supplement 1). Results from sensitivity analyses that were limited to individuals who entered prenatal care during the first trimester and analyses that did not include calendar year as a covariate were similar to the main results (eTable 4 in Supplement 1).
Table 4. Frequency and Adjusted Risk Ratios (aRRs) of Maternal Outcomes by Prenatal Cannabis Use Defined Only by Self-Report or Defined Only by Toxicology Testing.
| Outcome | Overall (N = 316 722 [100%]) | Prenatal cannabis use defined only by self-report | Prenatal cannabis use defined only by toxicology testing | ||||
|---|---|---|---|---|---|---|---|
| Any prenatal cannabis use, No. (%) | aRR (95% CI)a | Any prenatal cannabis use, No. (%) | aRR (95% CI)a | ||||
| Yes (n = 9167 [2.9%]) | No (n = 307 555 [97.1%]) | Yes (n = 16 638 [5.3%]) | No (n = 300 084 [94.7%]) | ||||
| Metabolic outcomes | |||||||
| Hypertensive disordersb | |||||||
| Gestational hypertension | 44 363 (14.7) | 1715 (19.8) | 42 648 (14.5) | 1.10 (1.05-1.15) | 3511 (22.5) | 41 614 (14.5) | 1.19 (1.15-1.23) |
| Preeclampsia | 14 378 (4.8) | 529 (6.1) | 13 849 (4.7) | 0.99 (0.90-1.08) | 1016 (6.5) | 13 362 (4.7) | 1.11 (1.04-1.19) |
| Eclampsia | 384 (0.1) | 16 (0.2) | 368 (0.1) | 1.21 (0.70-2.09) | 27 (0.2) | 357 (0.1) | 1.09 (0.72-1.65) |
| Gestational diabetesc | 36 374 (11.7) | 650 (7.2) | 35 724 (11.8) | 0.91 (0.84-0.98) | 1279 (7.8) | 35 095 (11.9) | 0.89 (0.84-0.94) |
| Gestational weight gaind | |||||||
| Within guidelines | 75 844 (25.2) | 1603 (18.5) | 74 241 (25.4) | NA | 2636 (16.5) | 73 208 (25.7) | NA |
| Less than guidelines | 48 117 (16.0) | 1279 (14.8) | 46 838 (16.0) | 1.05 (1.00-1.09) | 2296 (14.4) | 45 821 (16.1) | 1.05 (1.01-1.08) |
| Greater than guidelines | 176 898 (58.8) | 5781 (66.7) | 171 117 (58.6) | 1.05 (1.04-1.06) | 11 021 (69.1) | 165 877 (58.2) | 1.10 (1.09-1.11) |
| Placental outcomes | |||||||
| Placenta previa | 3499 (1.1) | 86 (0.9) | 3413 (1.1) | 1.15 (0.92-1.44) | 136 (0.8) | 3363 (1.1) | 1.01 (0.84-1.20) |
| Placental abruption | 4002 (1.3) | 132 (1.4) | 3870 (1.3) | 1.22 (1.01-1.47) | 236 (1.4) | 3766 (1.3) | 1.19 (1.03-1.37) |
| Placenta accretae | 499 (0.2) | 16 (0.2) | 483 (0.2) | 1.28 (0.74-2.20) | 34 (0.2) | 465 (0.2) | 1.44 (0.98-2.13) |
| Severe maternal morbidity | 10 338 (3.3) | 333 (3.6) | 10 005 (3.3) | 0.97 (0.87-1.09) | 593 (3.6) | 9745 (3.2) | 0.96 (0.88-1.05) |
Abbreviation: NA, not applicable.
Reference is no prenatal cannabis use. Modified Poisson models with robust standard errors were adjusted for maternal sociodemographic characteristics (age category, race and ethnicity, neighborhood deprivation index), parity, birth year, prenatal care initiation, prepregnancy body mass index category, other noncannabis prenatal substance use (alcohol, nicotine, opioids, stimulants, and anxiety/sleep medications), and maternal medical and mental health comorbidities (pregestational diabetes, nausea/vomiting during pregnancy, mood/anxiety disorders, other psychiatric disorders, substance use disorders [other than cannabis], and antidepressant use).
Pregnancies of individuals with chronic hypertension were excluded (n = 14 187).
Pregnancies of individuals with pregestational diabetes were excluded. In addition, gestational diabetes could not be ascertained for pregnancies ending in therapeutic abortion. A total of 4737 pregnancies were excluded. Pregestational diabetes was not included as a covariate.
Categories determined by the 2009 Institute of Medicine guidelines. Pregnancies with missing weight values were excluded (n = 15 863).
Placenta accreta could only be ascertained for pregnancies that were delivered in a Kaiser Permanente Northern California facility and ended in live birth or stillbirth (alive at admission); 10 979 were excluded.
Discussion
The relative lack of research on how prenatal cannabis use is associated with maternal health vs offspring health is notable. This study leveraged data from a large health care system to examine the associations between cannabis use during early pregnancy and maternal health, adjusting for clinical and sociodemographic factors. Prenatal cannabis use was associated with greater risk of gestational hypertension, preeclampsia, GWG outside of guidelines, and placental abruption and lower risk of GD. Prenatal cannabis use was associated with a greater risk of eclampsia, but the result did not reach statistical significance, possibly due to the rarity of this outcome. Associations were slightly stronger for most outcomes when examining cannabis use measured by toxicology testing vs self-report, but the pattern was similar across exposure definitions. Further, results followed a similar pattern when the sample was limited to individuals without any noncannabis substance use during pregnancy, suggesting that results were not due to co-occurring substance use. The disparities in prenatal cannabis use by race and ethnicity, age, and neighborhood deprivation have the potential to exacerbate existing inequities in maternal health outcomes during pregnancy.
Our finding of greater risk of gestational hypertension and preeclampsia associated with prenatal cannabis use differs from prior studies that found no association or an inverse association between prenatal cannabis use based on self-report or urine toxicology testing and these outcomes.30,31,33,49,50 However, the results were consistent with several studies that defined prenatal cannabis exposure based on cannabis-related diagnoses,28,51 reflecting heavier or problematic use. For example, a large, retrospective cohort study of California hospital discharge data found that pregnant individuals with vs without a cannabis use disorder diagnosis had higher risk of gestational hypertension (aRR, 1.19; 95% CI, 1.06-1.34) and preeclampsia (aRR, 1.16; 95% CI, 1.04-1.28).51 In our study, there was evidence of a dose-response association between the frequency of self-reported cannabis use and gestational hypertension, with the greatest risk associated with daily cannabis use. Together, the findings suggest that more frequent prenatal cannabis use may motivate these associations, and differences in exposure measurement may partially explain the inconsistencies in the limited existing literature. Studies of US adults have found that cannabis use, especially more frequent use, was associated with increased odds of myocardial infarction and stroke.52,53,54,55 Future studies are needed to determine if associations with gestational hypertension and preeclampsia are replicable. If so, research could evaluate whether individuals with prenatal cannabis use might benefit from interventions for preventing preeclampsia (eg, low-dose aspirin).
Prenatal cannabis use was also associated with greater risk of GWG that is greater than guidelines, which is associated with hypertensive disorders of pregnancy,56 and could partially mediate the association between prenatal cannabis use and gestational hypertension and preeclampsia. However, prenatal cannabis use was also associated with GWG that was less than guidelines, even after adjusting for potentially confounding factors (eg, nausea and vomiting). GWG outside of recommendations is associated with health issues for pregnant individuals and their children,42 and future studies are needed to explore its potential mediating effects.
Prior studies have found inconsistent associations between prenatal cannabis use and GD. We found a lower risk of GD associated with prenatal cannabis use that was consistent with 3 retrospective cohort studies that found an inverse association between prenatal cannabis use or cannabis-related diagnoses and GD.30,32,33 Other studies have found no association or a positive association between prenatal cannabis use and GD.31,57 Results were consistent with a recent meta-analysis in adults that found a lower risk of developing type 2 diabetes among individuals with vs without cannabis use (aRR, 0.48; 95% CI, 0.39-0.59).58 Hypothesized mechanisms included cannabis-related attenuated inflammatory response, stress signaling, and reactive oxygen species formation, which may be stronger for nonsmoked modes of administration given the potential of smoking to increase oxidative stress.58 In frequency analyses, a statistically significant lower risk was only found among those who self-reported using monthly or less, which does not provide evidence for a dose-response association. Future research is also needed to understand the potential mechanisms underlying lower risk of GD among those who use cannabis infrequently during early pregnancy, who may be different in key unmeasured ways that we were unable to adjust for, and test whether findings differ based on modes of use.
Whereas several prior studies have not found an association between prenatal cannabis use and placental abruption,31,33,49 our finding of a greater risk of placental abruption being associated with prenatal cannabis use was consistent with results from a prior large, population-based pregnancy cohort study of self-reported cannabis use with a matched design that found higher risk of placental abruption among those with vs without prenatal cannabis use (aRR, 1.72; 95% CI, 1.54-1.92).30 Cannabis-related diagnoses during pregnancy have been positively associated with placenta previa32 and severe maternal morbidity51; however, we did not find an association between use and placenta previa, accreta, or severe maternal morbidity.
In this and our prior studies, we have documented low sensitivity of self-reported prenatal cannabis use.35 There are real and perceived risks of disclosing prenatal substance use. Prenatal cannabis use can have greater consequences for individuals from disadvantaged backgrounds due to inequities in who gets tested and reported to child protective services and law enforcement,59,60,61 and some individuals may avoid prenatal care due to these concerns.62,63 In California, prenatal cannabis use is not sufficient to make a child abuse or neglect report.64 This allows KPNC to offer universal prenatal substance use screening and linkage to further assessment, education, and patient-centered, supportive, nonstigmatizing prenatal substance use intervention linked with prenatal care.65,66,67 Routine testing for prenatal cannabis use is not recommended in states with punitive policies that criminalize or penalize prenatal substance use.
Limitations
This study had limitations. Our sample was limited to insured pregnant patients in a large health care organization in Northern California. The findings may not generalize to uninsured patients or those outside of California, a state that legalized medicinal cannabis in 1996 and adult use in November 2016 (with legal adult use sales beginning in January 2018). The prenatal cannabis use screening occurred at entrance to prenatal care, and we were unable to determine whether prenatal use occurred only before pregnancy recognition or continued later into pregnancy. It is possible that urine toxicology tests detected prepregnancy cannabis use. However, this is unlikely, given that toxicology tests were given at a median of 9.0 weeks’ gestation. Further, important aspects of cannabis use were not assessed, including the mode of administration, potency, products used, and reasons for use. Urine toxicology tests are more likely to detect heavy vs infrequent cannabis use, and the duration that cannabis is detectable could vary with mode of use. Although we adjusted for many covariates, unmeasured confounders may have affected results. Additional research is needed to determine whether the associations found in this study are causal and whether they vary depending on the trimester of use, modes of administration, and product strength.
Conclusions
In this cohort study, prenatal cannabis use was associated with a greater risk of gestational hypertension, preeclampsia, GWG outside of Institute of Medicine guidelines, and placental abruption, but it was also associated with a lower risk of GD. The findings suggest a complex association between prenatal cannabis use and maternal health and highlight the need for continued research to understand the mechanisms through which prenatal cannabis use is associated with the health of pregnant individuals. Prenatal cannabis use is a risk factor for adverse neonatal outcomes.6,7,8 As we continue to learn about the potential harms and benefits of prenatal cannabis use, clinicians must provide coordinated, nonstigmatizing care and education to support pregnant individuals in making informed decisions about cannabis use.68
eFigure. STROBE Diagram of Patient Selection
eMethods 1. Screening Methods for Prenatal Substance Use
eMethods 2. Lists of ICD-9/10 Diagnosis Codes and Other Diagnostic Criteria Used to Identify Maternal Health Outcomes During Pregnancy
eTable 1. Socio-Demographic Characteristics of Individuals Included Versus Excluded for Missing Prenatal Cannabis Use Data
eTable 2. Pregnancy Characteristics, Overall and by Frequency of Prenatal Cannabis Use
eTable 3. Risk Ratios of Maternal Outcomes by Any Prenatal Cannabis Use with Adjustment in Steps
eTable 4. Sensitivity Analyses
Data sharing statement
References
- 1.Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 2019;322(2):167-169. doi: 10.1001/jama.2019.7982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foti TR, Green A, Altschuler A, et al. Patient perceptions of prenatal cannabis use and implications for clinicians. Obstet Gynecol. 2023;142(5):1153-1161. doi: 10.1097/AOG.0000000000005295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz TD, Allshouse AA, Hogue CJ, et al. Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. Am J Obstet Gynecol. 2017;217(4):478.e1-478.e8. doi: 10.1016/j.ajog.2017.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayrampour H, Zahradnik M, Lisonkova S, Janssen P. Women’s perspectives about cannabis use during pregnancy and the postpartum period: an integrative review. Prev Med. 2019;119:17-23. doi: 10.1016/j.ypmed.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 5.Chang JC, Tarr JA, Holland CL, et al. Beliefs and attitudes regarding prenatal marijuana use: perspectives of pregnant women who report use. Drug Alcohol Depend. 2019;196:14-20. doi: 10.1016/j.drugalcdep.2018.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baia I, Domingues RMSM. The effects of cannabis use during pregnancy on low birth weight and preterm birth: a systematic review and meta-analysis. Am J Perinatol. 2024;41(1):17-30. doi: 10.1055/a-1911-3326 [DOI] [PubMed] [Google Scholar]
- 7.Lo JO, Shaw B, Robalino S, et al. Cannabis use in pregnancy and neonatal outcomes: a systematic review and meta-analysis. Cannabis Cannabinoid Res. 2024;9(2):470-485. doi: 10.1089/can.2022.0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avalos LA, Adams SR, Alexeeff SE, et al. Neonatal outcomes associated with in utero cannabis exposure: a population-based retrospective cohort study. Am J Obstet Gynecol. 2023:S0002-9378(23)02034-3. doi: 10.1016/j.ajog.2023.11.1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Committee on Obstetric Practice . Committee opinion No. 722: marijuana use during pregnancy and lactation. Obstet Gynecol. 2017;130(4):e205-e209. doi: 10.1097/AOG.0000000000002354 [DOI] [PubMed] [Google Scholar]
- 10.Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal marijuana use and adverse neonatal outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2016;128(4):713-723. doi: 10.1097/AOG.0000000000001649 [DOI] [PubMed] [Google Scholar]
- 11.Gunn JK, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e009986. doi: 10.1136/bmjopen-2015-009986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchand G, Masoud AT, Govindan M, et al. Birth outcomes of neonates exposed to marijuana in utero: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(1):e2145653. doi: 10.1001/jamanetworkopen.2021.45653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackard C, Tennes K. Human placental transfer of cannabinoids. N Engl J Med. 1984;311(12):797. doi: 10.1056/NEJM198409203111213 [DOI] [PubMed] [Google Scholar]
- 14.Lee CC, Chiang CN. Maternal-fetal transfer of abused substances: pharmacokinetic and pharmacodynamic data. NIDA Res Monogr. 1985;60:110-147. [PubMed] [Google Scholar]
- 15.Paul SE, Rogers CE, Bogdan R. Pre-natal cannabis exposure: associations with development and behaviour. In: D’Souza DC, Castle DJ, Murray RM, eds. Marijuana and Madness. 3rd ed. Cambridge University Press; 2023:267-278. doi: 10.1017/9781108943246.026 [DOI] [Google Scholar]
- 16.Banerjee S, Deacon A, Suter MA, Aagaard KM. Understanding the placental biology of tobacco smoke, nicotine, and marijuana (THC) exposures during pregnancy. Clin Obstet Gynecol. 2022;65(2):347-359. doi: 10.1097/GRF.0000000000000691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correa F, Wolfson ML, Valchi P, Aisemberg J, Franchi AM. Endocannabinoid system and pregnancy. Reproduction. 2016;152(6):R191-R200. doi: 10.1530/REP-16-0167 [DOI] [PubMed] [Google Scholar]
- 18.Lo JO, Hedges JC, Girardi G. Impact of cannabinoids on pregnancy, reproductive health, and offspring outcomes. Am J Obstet Gynecol. 2022;227(4):571-581. doi: 10.1016/j.ajog.2022.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinky PD, Bloemer J, Smith WD, et al. Prenatal cannabinoid exposure and altered neurotransmission. Neuropharmacology. 2019;149:181-194. doi: 10.1016/j.neuropharm.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 20.Roberts VHJ, Schabel MC, Boniface ER, et al. Chronic prenatal delta-9-tetrahydrocannabinol exposure adversely impacts placental function and development in a rhesus macaque model. Sci Rep. 2022;12(1):20260. doi: 10.1038/s41598-022-24401-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rokeby ACE, Natale BV, Natale DRC. Cannabinoids and the placenta: receptors, signaling and outcomes. Placenta. 2023;135:51-61. doi: 10.1016/j.placenta.2023.03.002 [DOI] [PubMed] [Google Scholar]
- 22.Scheyer AF, Melis M, Trezza V, Manzoni OJJ. Consequences of perinatal cannabis exposure. Trends Neurosci. 2019;42(12):871-884. doi: 10.1016/j.tins.2019.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson R, DeJong K, Lo J. Marijuana use in pregnancy: a review. Obstet Gynecol Surv. 2019;74(7):415-428. doi: 10.1097/OGX.0000000000000685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grotenhermen F. Cannabis-associated arteritis. Vasa. 2010;39(1):43-53. doi: 10.1024/0301-1526/a000004 [DOI] [PubMed] [Google Scholar]
- 25.Battaglia FC, Regnault TR. Placental transport and metabolism of amino acids. Placenta. 2001;22(2-3):145-161. doi: 10.1053/plac.2000.0612 [DOI] [PubMed] [Google Scholar]
- 26.Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth—a review. Placenta. 2002;23(suppl A):S28-S38. doi: 10.1053/plac.2002.0791 [DOI] [PubMed] [Google Scholar]
- 27.Illsley NP. Glucose transporters in the human placenta. Placenta. 2000;21(1):14-22. doi: 10.1053/plac.1999.0448 [DOI] [PubMed] [Google Scholar]
- 28.Bandoli G, Jelliffe-Pawlowski L, Schumacher B, et al. Cannabis-related diagnosis in pregnancy and adverse maternal and infant outcomes. Drug Alcohol Depend. 2021;225:108757. doi: 10.1016/j.drugalcdep.2021.108757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. 2016;215(4):506.e1-7. doi: 10.1016/j.ajog.2016.05.044 [DOI] [PubMed] [Google Scholar]
- 30.Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322(2):145-152. doi: 10.1001/jama.2019.8734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koto P, Allen VM, Fahey J, Kuhle S. Maternal cannabis use during pregnancy and maternal and neonatal outcomes: A retrospective cohort study. BJOG. 2022;129(10):1687-1694. doi: 10.1111/1471-0528.17114 [DOI] [PubMed] [Google Scholar]
- 32.Petrangelo A, Czuzoj-Shulman N, Balayla J, Abenhaim HA. Cannabis abuse or dependence during pregnancy: a population-based cohort study on 12 million births. J Obstet Gynaecol Can. 2019;41(5):623-630. doi: 10.1016/j.jogc.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 33.Warshak CR, Regan J, Moore B, Magner K, Kritzer S, Van Hook J. Association between marijuana use and adverse obstetrical and neonatal outcomes. J Perinatol. 2015;35(12):991-995. doi: 10.1038/jp.2015.120 [DOI] [PubMed] [Google Scholar]
- 34.Metz TD, Silver RM, McMillin GA, et al. Prenatal marijuana use by self-report and umbilical cord sampling in a state with marijuana legalization. Obstet Gynecol. 2019;133(1):98-104. doi: 10.1097/AOG.0000000000003028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young-Wolff KC, Sarovar V, Tucker LY, et al. Validity of self-reported cannabis use among pregnant females in Northern California. J Addict Med. 2020;14(4):287-292. doi: 10.1097/ADM.0000000000000581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant KS, Conover E, Chambers CD. Update on the developmental consequences of cannabis use during pregnancy and lactation. Birth Defects Res. 2020;112(15):1126-1138. doi: 10.1002/bdr2.1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bidwell LC, Martin-Willett R, Karoly HC. Advancing the science on cannabis concentrates and behavioural health. Drug Alcohol Rev. 2021;40(6):900-913. doi: 10.1111/dar.13281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pacula RL, Pessar SC, Zhu J, Kritikos A, Smart R. Federal regulation of cannabis for public health in the United States. Accessed January 15, 2024. https://healthpolicy.usc.edu/wp-content/uploads/2022/07/USC-Schaeffer-Center-white-paper_Federal-Regulation-of-Cannabis-for-Public-Health-in-the-United-States.pdf
- 39.Shover CL, Humphreys K. Six policy lessons relevant to cannabis legalization. Am J Drug Alcohol Abuse. 2019;45(6):698-706. doi: 10.1080/00952990.2019.1569669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon NP. Similarity of adult Kaiser Permanente members to the adult population in Kaiser Permanente’s Northern California Service Area: comparisons based on the 2017/2018 cycle of the California Health Interview Survey. Accessed January 15, 2024. https://memberhealthsurvey.kaiser.org/Documents/compare_kp_ncal_chis2017-18.pdf
- 41.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991-2000. Obstet Gynecol. 2004;103(3):526-533. doi: 10.1097/01.AOG.0000113623.18286.20 [DOI] [PubMed] [Google Scholar]
- 42.Institute of Medicine and Natural Research Council . Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; 2009, doi: 10.17226/12584. [DOI] [PubMed] [Google Scholar]
- 43.Kilpatrick SK, Ecker JL; American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine . Severe maternal morbidity: screening and review. Am J Obstet Gynecol. 2016;215(3):B17-B22. doi: 10.1016/j.ajog.2016.07.050 [DOI] [PubMed] [Google Scholar]
- 44.US Centers for Disease Control and Prevention . Identifying severe maternal morbidity (SMM). Accessed March 8, 2024. https://www.cdc.gov/maternal-infant-health/php/severe-maternal-morbidity/icd.html
- 45.Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83(6):1041-1062. doi: 10.1007/s11524-006-9094-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotelchuck M. An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed adequacy of prenatal care utilization index. Am J Public Health. 1994;84(9):1414-1420. doi: 10.2105/AJPH.84.9.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661-670. doi: 10.1177/0962280211427759 [DOI] [PubMed] [Google Scholar]
- 48.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. Accessed May 15, 2024. https://support.sas.com/resources/papers/proceedings12/335-2012.pdf
- 49.Metz TD, Allshouse AA, McMillin GA, et al. Cannabis exposure and adverse pregnancy outcomes related to placental function. JAMA. 2023;330(22):2191-2199. doi: 10.1001/jama.2023.21146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garrison-Desany HM, Ladd-Acosta C, Hong X, et al. Addressing the smoking-hypertension paradox in pregnancy: insight from a multiethnic US birth cohort. Precis Nutr. 2023;2(2):e00035. doi: 10.1097/PN9.0000000000000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prewitt KC, Hayer S, Garg B, et al. Impact of prenatal cannabis use disorder on perinatal outcomes. J Addict Med. 2023;17(3):e192-e198. doi: 10.1097/ADM.0000000000001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeffers AM, Glantz S, Byers AL, Keyhani S. Association of cannabis use with cardiovascular outcomes among US adults. J Am Heart Assoc. 2024;13(5):e030178. doi: 10.1161/JAHA.123.030178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parekh T, Pemmasani S, Desai R. Marijuana use among young adults (18-44 years of age) and risk of stroke: a behavioral risk factor surveillance system survey analysis. Stroke. 2020;51(1):308-310. doi: 10.1161/STROKEAHA.119.027828 [DOI] [PubMed] [Google Scholar]
- 54.Shah S, Patel S, Paulraj S, Chaudhuri D. Association of marijuana use and cardiovascular disease: a behavioral risk factor surveillance system data analysis of 133,706 US adults. Am J Med. 2021;134(5):614-620.e1. doi: 10.1016/j.amjmed.2020.10.019 [DOI] [PubMed] [Google Scholar]
- 55.Ladha KS, Mistry N, Wijeysundera DN, et al. Recent cannabis use and myocardial infarction in young adults: a cross-sectional study. CMAJ. 2021;193(35):E1377-E1384. doi: 10.1503/cmaj.202392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Champion ML, Harper LM. Gestational weight gain: update on outcomes and interventions. Curr Diab Rep. 2020;20(3):11. doi: 10.1007/s11892-020-1296-1 [DOI] [PubMed] [Google Scholar]
- 57.Luke S, Hobbs AJ, Smith M, et al. ; National Maternal Cannabis Working Group . Cannabis use in pregnancy and maternal and infant outcomes: a Canadian cross-jurisdictional population-based cohort study. PLoS One. 2022;17(11):e0276824. doi: 10.1371/journal.pone.0276824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mousavi SE, Tondro Anamag F, Sanaie S. Association between cannabis use and risk of diabetes mellitus type 2: a systematic review and meta-analysis. Phytother Res. 2023;37(11):5092-5108. doi: 10.1002/ptr.7973 [DOI] [PubMed] [Google Scholar]
- 59.Roberts SC, Nuru-Jeter A. Universal screening for alcohol and drug use and racial disparities in child protective services reporting. J Behav Health Serv Res. 2012;39(1):3-16. doi: 10.1007/s11414-011-9247-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bishop D, Borkowski L, Couillard M, Allina A, Baruch S, Wood S. Bridging the divide white paper: pregnant women and substance use: overview of research & policy in the United States. Accessed February 7, 2024. https://hsrc.himmelfarb.gwu.edu/sphhs_centers_jacobs/5
- 61.MacDuffie KE, Kleinhans NM, Stout K, Wilfond BS. Protection versus progress: the challenge of research on cannabis use during pregnancy. Pediatrics. 2020;146(suppl 1):S93-S98. doi: 10.1542/peds.2020-0818R [DOI] [PubMed] [Google Scholar]
- 62.Faherty LJ, Stein BD, Terplan M. Consensus guidelines and state policies: the gap between principle and practice at the intersection of substance use and pregnancy. Am J Obstet Gynecol MFM. 2020;2(3):100137. doi: 10.1016/j.ajogmf.2020.100137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stone R. Pregnant women and substance use: fear, stigma, and barriers to care. Health Justice. 2015;3:2. doi: 10.1186/s40352-015-0015-5 [DOI] [Google Scholar]
- 64.Guttmacher Institute . Substance use during pregnancy. Accessed January 15, 2024. https://www.guttmacher.org/state-policy/explore/substance-use-during-pregnancy
- 65.Armstrong MA, Lieberman L, Carpenter DM, et al. Early Start: an obstetric clinic-based, perinatal substance abuse intervention program. Qual Manag Health Care. 2001;9(2):6-15. doi: 10.1097/00019514-200109020-00004 [DOI] [PubMed] [Google Scholar]
- 66.Goler NC, Armstrong MA, Taillac CJ, Osejo VM. Substance abuse treatment linked with prenatal visits improves perinatal outcomes: a new standard. J Perinatol. 2008;28(9):597-603. doi: 10.1038/jp.2008.70 [DOI] [PubMed] [Google Scholar]
- 67.Goler NC, Armstrong MA, Osejo VM, Hung YY, Haimowitz M, Caughey AB. Early start: a cost-beneficial perinatal substance abuse program. Obstet Gynecol. 2012;119(1):102-110. doi: 10.1097/AOG.0b013e31823d427d [DOI] [PubMed] [Google Scholar]
- 68.Smid MC, Terplan M. What obstetrician-gynecologists should know about substance use disorders in the perinatal period. Obstet Gynecol. 2022;139(2):317-337. doi: 10.1097/AOG.0000000000004657 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. STROBE Diagram of Patient Selection
eMethods 1. Screening Methods for Prenatal Substance Use
eMethods 2. Lists of ICD-9/10 Diagnosis Codes and Other Diagnostic Criteria Used to Identify Maternal Health Outcomes During Pregnancy
eTable 1. Socio-Demographic Characteristics of Individuals Included Versus Excluded for Missing Prenatal Cannabis Use Data
eTable 2. Pregnancy Characteristics, Overall and by Frequency of Prenatal Cannabis Use
eTable 3. Risk Ratios of Maternal Outcomes by Any Prenatal Cannabis Use with Adjustment in Steps
eTable 4. Sensitivity Analyses
Data sharing statement