Abstract
Psoriasis is an inflammatory skin disease characterized by epidermal and immune dysfunctions. Although efficient, current topical treatments display adverse effects, including skin atrophy and burning sensation, leading to poor patient adherence. To overcome these downsides, pickering emulsions were formulated in which the calcitriol-containing dispersed phase was stabilized with either cyclosporin A– or tacrolimus-loaded poly(lactic-co-glycolic) acid nanoparticles. This study aimed to investigate their biological effects on lymphocytes and epidermal cells and their effectiveness in an imiquimod-induced psoriasis-like mouse model. Results showed that both emulsions significantly inhibited nuclear factor of activated T cell translocation in T lymphocytes as well as their IL-2 production, cell activation, and proliferation. In keratinocytes, inhibition of nuclear factor of activated T cell translocation decreased the production of IL-8 and TNF-α. Topical application of emulsions over skin biopsies ex vivo showed accumulation of rhodamin B–coupled poly(lactic-co-glycolic) acid nanoparticles throughout the epidermis by immunofluorescence and significantly decreased the antigen-presenting capacity of Langerhans cells in relation to a reduced expression of activation markers CD40, CD86, and HLA-DR. Using an imiquimod-induced psoriasis model in vivo, pickering emulsions significantly alleviated psoriasiform lesions potentially attributed to the decreased cutaneous expression of T-cell markers, proinflammatory cytokines, chemokines, and specific epidermal cell genes. Altogether, pickering emulsion might be a very efficient formulation for treating inflammatory dermatoses.
Keywords: Coencapsulation, Inflammatory dermatoses, Nanoparticles, Pickering emulsions, Psoriasis
Graphical abstract
Introduction
Inflammatory dermatoses (IDs) refer to skin disorders that arise from the aberrant responses of the immune system toward cutaneous cells. Stress, environment, genetic factors, and dietary habits are involved in ID occurrence. Among dermatoses, psoriasis represents the second most common worldwide chronic ID, with a prevalence ranging from 0.91 to 8.5% in adults (Parisi et al, 2013). More than 85% of patients with psoriasis are affected with the plaque type characterized by marked, thick, red plaques covered with silvery scales that appear mainly on the extensor aspects of elbows and knees, in the lower back, and on the scalp but can also extend to other parts of the body (Di Meglio et al, 2014). Psoriatic lesions result from the activation of T helper (Th) cells, including Th1, Th17, and Th22; their recruitment to the skin; and the subsequent production of proinflammatory cytokines that activate local cutaneous cells, notably keratinocytes and dendritic cells (DCs). The interplay between these cell types, together with other innate immune actors, creates an unlimited loop responsible for angiogenesis and the alteration of keratinocyte differentiation, inducing hyperkeratosis and epidermal hyperplasia, and further recruitment of leukocyte subsets into the skin.
Although psoriasis is not a life-threatening disease, patients with psoriasis suffer from a high level of morbidity, poor QOL, and social exclusion that are highly associated with lesion severity (Nestle et al, 2009). Hence, disease management will greatly vary, with an overall treatment strategy mainly based on psoriasis severity, location, morbidity, and patient tolerability (Griffiths et al, 2021). Current therapeutic approaches recommend topical treatment for mild-to-moderate psoriasis, whereas phototherapy, oral medications, and biologics are used for moderate-to-severe forms. Despite the wide variety of treatments available, prolonged usage induces many adverse effects such as skin atrophy, burning sensations, and organ toxicity, which can ultimately result in patient’s poor adherence to treatment (Lebwohl and Ali, 2001; Zschocke et al, 2014).
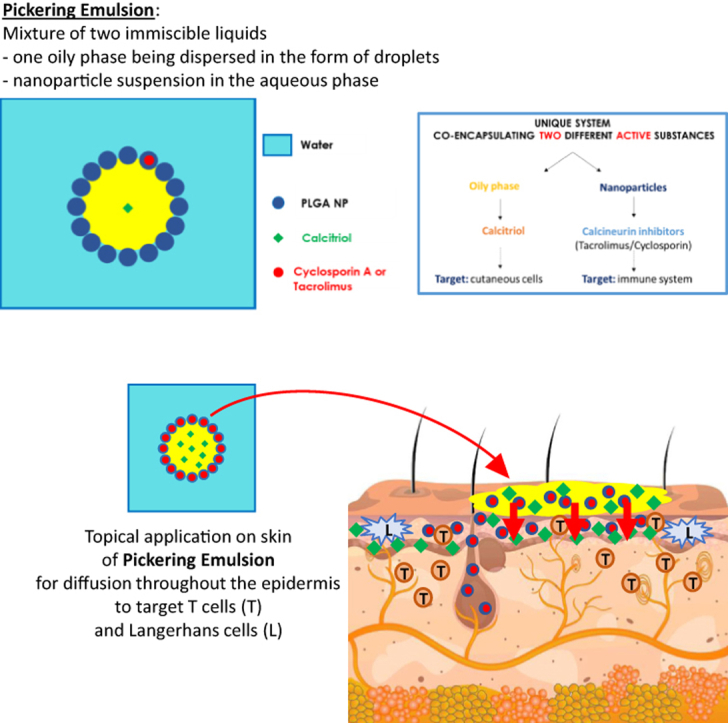
To overcome the actual downsides of topical treatment, recent drug delivery systems have started to emerge in recent years, especially nanoparticle (NP)-based drug delivery system, which has gained a certain interest compared with conventional drug formulations (Mohd Nordin et al, 2021; Pradhan et al, 2018; Saleem et al, 2020; Sindrilaru et al, 2020). Owing to their relatively low size, NP can penetrate through the epithelial barrier and reach the deepest layers to locally release encapsulated active substances (ASs), making them ideal for IDs such as psoriasis. Among NP drug delivery system, pickering emulsions have recently emerged as a promising drug delivery system (Albert et al, 2018; Robin et al, 2022). Compared with classical emulsions, which are stabilized using surfactants that can be toxic for both environment and human health (Cserháti et al, 2002; Branco et al, 2005; Liwarska-Bizukojc et al, 2005; Lémery et al, 2015), pickering emulsions can be stabilized using NP, thus allowing the coencapsulation of 2 potential different ASs, one inside the dispersed phase and one within the NP.
In this study, an oil-in-water emulsion stabilized with poly(lactic-co-glycolic) acid (PLGA) NP was formulated, allowing the coencapsulation of an immunosuppressive drug inside the NP—either cyclosporin A (CsA) or tacrolimus (Tac)—in association with an anti-inflammatory molecule, calcitriol (Cal), in the oily phase. A previous article reported that combining Cal by topical route and CsA by oral route could improve the treatment of psoriasis compared with taking each drug individually (Abe et al, 2006). We hypothesized that coadministration of Cal and CsA by topical route could not only reduce side effects of CsA but might also provide a more efficient response of the calcineurin inhibitor, as previously described by Choi et al (1995) and Duncan et al (1990). Another potent calcineurin inhibitor, Tac, has been developed for treatment of IDs (Luger and Paul, 2007), and this prompted us to test it in comparison with CsA in our study. Pickering emulsions were prepared with both ASs, that is, CsA or Tac and calcipotriol. The aim of the study was to investigate the biological effects of these pickering emulsions in IDs by asserting their immunomodulatory properties on healthy T cells, keratinocytes, and Langerhans cells (LC) in vitro as well as their therapeutic effects in an imiquimod (IMQ)-induced psoriasis-like murine model in vivo.
Results
Pickering emulsions efficiently impaired T-cell activation and proliferation
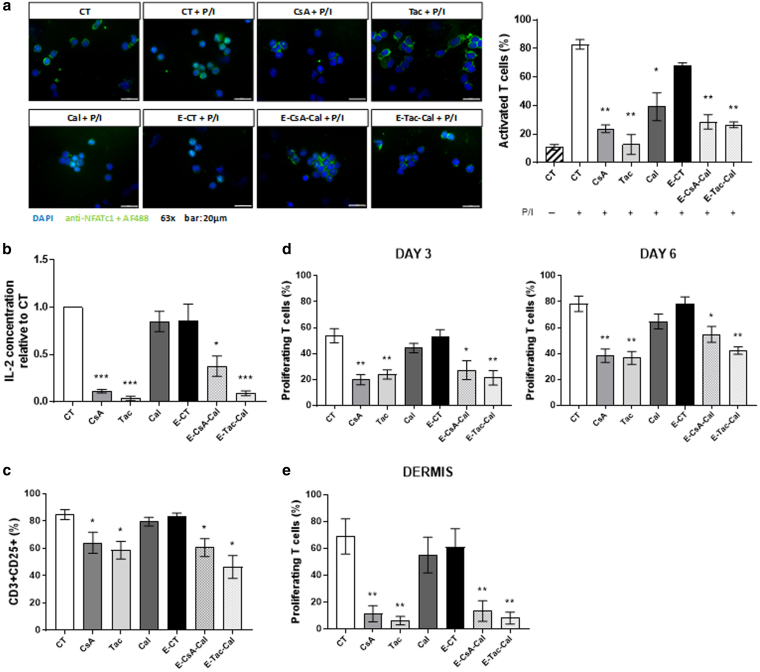
Both CsA and Tac encapsulated in NPs are well-known inhibitors of the calcineurin/nuclear factor of activated T cell (NFAT) signaling pathway. The immunosuppressive effect of the molecules and emulsions were first assessed on NFAT translocation into T-cell nucleus. NFAT translocation was induced by phorbol myristate acetate plus ionomycine (P/I) combination for 30 minutes and quantified as the mean percentage of cells with positive NFAT nuclear localization. In this study, the percentage of NFAT-positive cells was not significantly different between the control activated medium (denoted as CT+P/I) and the activated control emulsion (denoted as E-CT+P/I) (Figure 1a), whereas NFAT translocation was significantly decreased with all the studied preparations. To determine whether the inactivation of NFAT translocation impaired T-cell activation and proliferation, IL-2 production, CD25 expression, and T-cell proliferative ability after 3 and 6 days of TCR activation with CD3xCD28 microbeads were quantified. Compared with control medium, Cal solution and E-CT had no effect on IL-2 production, whereas they were significantly decreased with all the other preparations (Figure 1b). Similarly, all the preparations except Cal solution and E-CT significantly decreased CD25 expression on T cells (Figure 1c) and their proliferation after 3 and 6 days of activation (Figure 1d). Similarly, CD3xCD28-induced proliferation of T cells freshly isolated from normal dermis was also significantly reduced (Figure 1e).
Figure 1.
Effects of Em on healthy T cells.(a) Quantification of NFAT translocation with representative immunofluorescent images of each condition in cytospined human PBMCs activated for 30 minutes with P/I. At least 500 cells were counted for each donor (n = 3 independent human individuals). Bar = 20 μm. Pretreated PBMCs were tested for their ability to (b) produce IL-2, (c) express CD25 at their surface, and (d) proliferate after 3 and 6 days of activation with CD3xCD28 microbeads. (e) Quantification of human dermal T-cell proliferation by flow cytometry analysis of CFSE staining after 7 days of activation with OKT3 mAb directed against CD3. (f) Comparison of control emulsion without active substances (denoted as E-CT), emulsions associating CsA- or Tac-loaded NPs with Cal (E-CsA-Cal or E-Tac-Cal, respectively), or emulsions including only 1 AS (E-CsA, E-Tac, and E-Cal), besides the AS solution described as CsA, Tac, or Cal and control culture medium (denoted as CT) on proliferation of human PBMCs expressed as mean percentage of each successive generation of live cells, from M1 (nonproliferating cells) to M7 (after 6 cell divisions) after 6 days of activation with CD3xCD28 microbeads. Results represent the mean ± SD of 3 independent experiments in a and the mean ± SEM of 6 independent experiments in b–e. Statistical comparison was assessed using the Wilcoxon rank-sum test. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. Cal, calcitriol; CFSE, carboxyfluorescein succinimidyl ester; CsA, cyclosporin A; Em, pickering emulsion; NFAT, nuclear factor of activated T cell; NP, nanoparticle; P/I, phorbol myristate acetate/ionomycin; PMA, phorbol myristate acetate; Tac, tacrolimus.
Pickering emulsions were noncytotoxic for keratinocytes and induced a decrease in production of proinflammatory cytokines
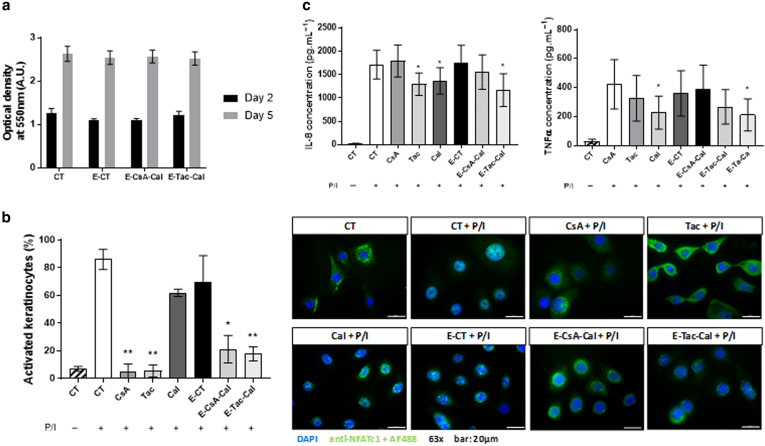
After having shown that the pickering emulsions efficiently impaired T-cell activation and proliferation, the cytotoxicity and effects of these pickering emulsions were assessed on keratinocyte cells. No differences in keratinocyte viability were observed for all the emulsions after 2 and 5 days of exposure (Figure 2a). NFAT translocation in keratinocyte nuclei was also investigated as previously described (Brun et al, 2014). In this study, all preparations except Cal solution and E-CT significantly decreased the translocation of NFAT into keratinocyte nucleus (Figure 2b). The production of 2 proinflammatory cytokines involved in psoriasis pathophysiology, IL-8 and TNF-α (Figure 2c), was also quantified by ELISA in the supernatant after keratinocyte activation. The results revealed that the production of IL-8 was significantly decreased with Cal and Tac solutions and E-Tac-Cal, whereas the production of TNF-α was only decreased with Tac solution and E-Tac-Cal. No differences were observed with either CsA solution or E-CsA-Cal.
Figure 2.
Effects of Em on human keratinocytes.(a) Em cytotoxicity was assessed in vitro by MTT on HaCaT cells after 2 and 5 days of exposure. Their effect on NFAT activation was tested in vitro in human primary keratinocytes by quantifying (b) NFAT translocation after 6 hours of activation or not with P/I (bar = 20 μm) and (c) IL-8 and TNF-α production after 24 h of activation or not with P/I. Results represent the mean ± SD of 3 independent experiments. Statistical comparison was assessed using the student t-test. ∗P < .05and ∗∗P < .01. A.U., arbitrary unit; Em, pickering emulsion; h, hour; NFAT, nuclear factor of activated T cell; P/I, phorbol myristate acetate/ionomycin; PMA, phorbol myristate acetate;
Topical application of pickering emulsion on skin biopsies ex vivo
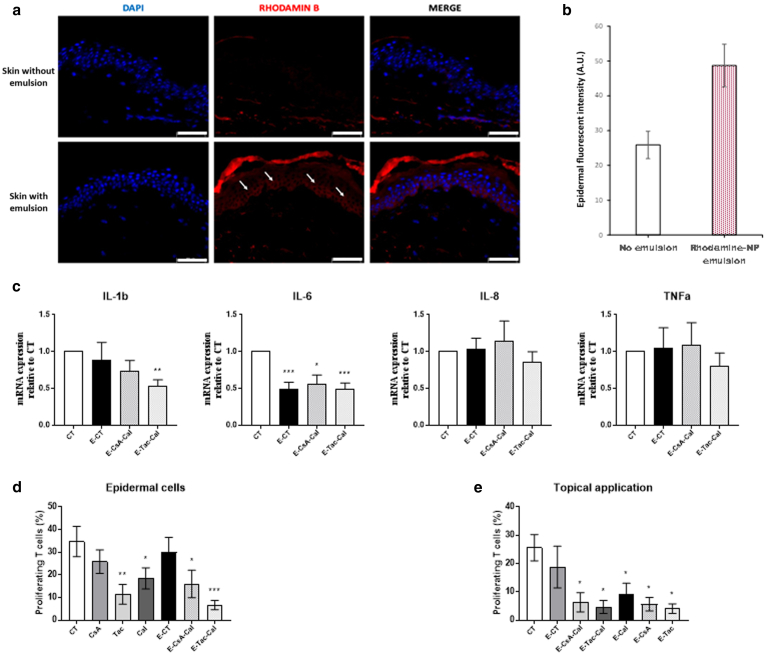
Our data showed that the emulsions were effective on both lymphocytes and keratinocytes. To determine whether the NPs can penetrate the skin and release the AS, emulsions were topically applied for 24 hours on skin explants freshly obtained from healthy skin, and the penetration of rhodamine B–coupled PLGA NP into the epidermis was visualized by immunofluorescence (Figure 3a). Compared with the skin without emulsion, an increased red fluorescence was observed throughout the epidermis until the basal layer of the skin on which emulsions had been applied, suggesting that NPs penetrate through and accumulate within the epidermis. The relative gene expression of IL1β, IL6, IL8, and TNFα by epidermal cells isolated after 24 hours of exposure with the emulsions was then assessed (Figure 3b). A significant downregulation of IL6 expression was observed by the 3 emulsions E-CT, E-CsA-Cal, and E-Tac-Cal. IL-1β expression was reduced by E-Ta-Ca only, and no significant alteration was observed for IL8 and TNFα expressions. Because LCs are located in the epidermis, the antigen-presenting capacity of epidermal cells in the presence of the emulsions was studied using a mixed epidermal cell–lymphocyte reaction. Freshly isolated epidermal cells were treated in vitro with either solutions or emulsions for 24 hours and were washed before they were cocultured with carboxyfluorescein succinimidyl esther–labeled allogenic T cells for 7 days. Compared with the control, a significant downregulation of T-cell proliferation was observed in the presence of epidermal cells pretreated with the emulsions, except the control E-CT (Figure 3c). As shown in Figure 3d, topical application of the emulsions on skin biopsies 24 hours before epidermal cell isolation resulted in a significant decrease of T-cell proliferation in all the emulsion conditions, including the monoemulsions, which contains only one AS, namely E-CsA, E-Tac, and E-Cal (Figure 3d).
Figure 3.
Topical effects of Em on human epidermal cells.(a) Representative immunofluorescent pictures of skin sections after 24 h of topical application of Em stabilized using rhodamin B–coupled PLGA nanoparticles on human skin explants ex vivo. Bar = 50 μm. (b) Mean quantification of the fluorescent intensity of the rhodamine B staining in the layers of the human epidermis except the stratum corneum, as measured by ImageJ software (mean ± SD, n = 3). (c) Ratio of the gene expression of proinflammatory cytokines in isolated epidermal cells after 24 h of topical exposure of human skin with Em on the gene expression of epidermal cells isolated from respective control untreated explants and taken as 1. Quantification of human blood T-cell proliferation after 7 days of coculture with (d) epidermal cells freshly isolated from human skin and pretreated in culture with Em for 24 h before coculture with T cells or (e) with epidermal cells isolated after topical application of Em on human skin for 24 h. Results represent the mean ± SEM of 6 independent experiments (n = 6). Statistical comparison was assessed using the Wilcoxon rank-sum test. ∗P < .05, ∗∗P < 0.01, and ∗∗∗P < .001. Em, pickering emulsion; h, hour; PLGA, poly(lactic-co-glycolic) acid.
LCs have decreased proinflammatory markers after pickering emulsion exposure
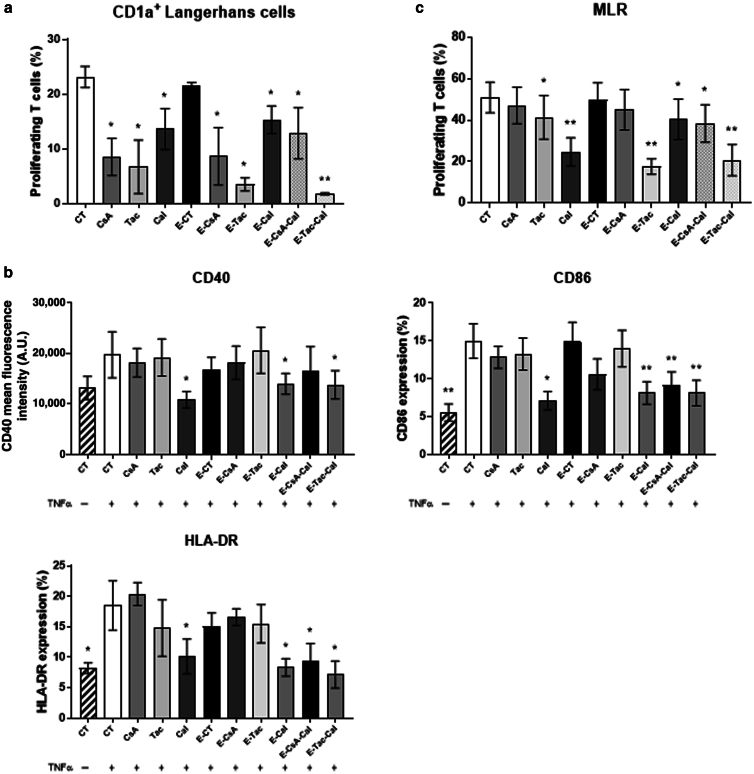
The decrease of T-cell proliferation after 7 days of coculture with pre-exposed epidermal cells led us to test the effect of emulsions on LCs. To determine whether the antigen-presenting capacity of LCs was impeded, freshly isolated CD1a+ LCs were pretreated in vitro for 24 hours before carrying out a mixed epidermal cell–lymphocyte reaction with allogenic T cells. As shown in Figure 1a, T-cell proliferation was decreased in the presence of LCs pretreated with both solutions and emulsions (Figure 1d). We then hypothesized that the expression of activation markers on LC’s surface may be decreased. To address this hypothesis, we used a differentiation model where circulating monocytes are differentiated into monocyte-derived LCs (moLCs). Flow cytometry analysis revealed that the expressions of 3 markers of activation, namely CD40, CD80, and CD86, were significantly downregulated only in the presence of Cal solution and Cal-based emulsions, namely E-CsA-Cal, E-Tac-Cal, and E-Cal (Figure 4b). Finally, T-cell proliferation after 7 days of coculture with pretreated moLCs was also significantly decreased, suggesting that in agreement with our previous results, topical application of our emulsions impeded the antigen-presenting capacity of LCs through reduced expression of activation markers.
Figure 4.
Effect of Em on human Langerhans cells. (a) Quantification of T-cell proliferation after 7 days of coculture with CD1a+ LCs isolated from epidermal cells freshly obtained from human skin and pretreated with either CsA, Tac, or Cal solutions or Em. (b) Quantification of activation markers expressed on moLCs differentiated from human blood and exposed during 48 hours to Em and TNF-α activation in culture. (c) Quantification of human blood T-cell proliferation by CFSE staining after 7 days of coculture with pretreated moLCs. Results represent the mean ± SEM of 6 independent experiments. Statistical comparison was assessed using the Wilcoxon rank-sum test. ∗P < .05 and ∗∗P < 0.01. A.U., arbitrary unit; Cal, calcitriol; CFSE, carboxyfluorescein succinimidyl ester; CsA, cyclosporin A; Em, pickering emulsion; MLR, mixed lymphocyte reaction; moLC, monocyte-derived Langerhans cell; Tac, tacrolimus.
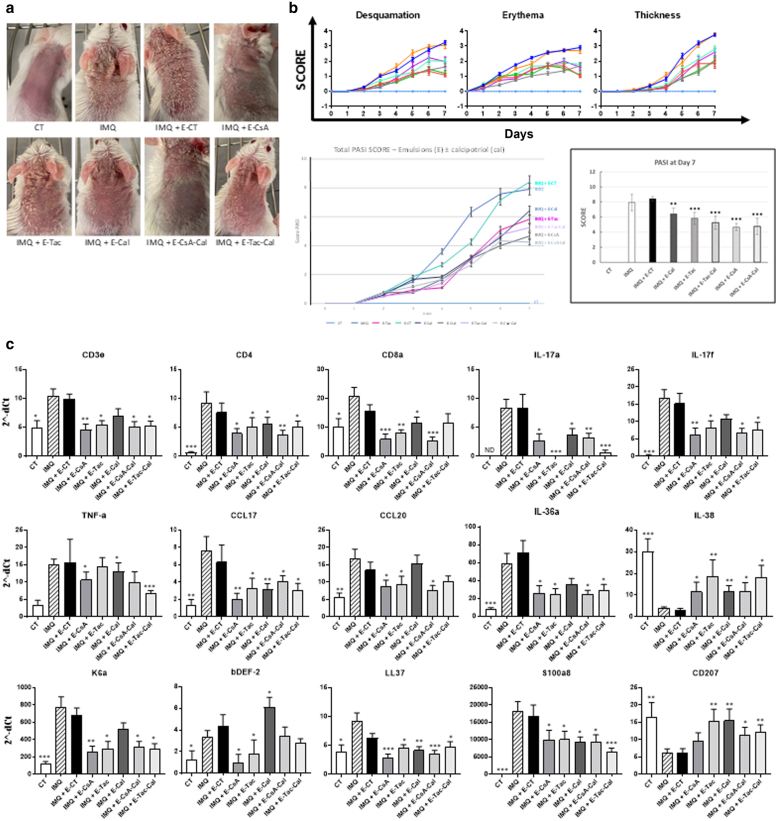
Pickering emulsions exhibit therapeutic effects in IMQ-induced psoriasis-like murine model in vivo
To our knowledge, the combination of CsA- or Tac-loaded NPs with Cal has never been reported in an IMQ-induced psoriasis-like mouse model. To investigate whether the present pickering emulsions elaborated were effective in treating inflammatory skin features, the original model from van der Fits et al (2009) was used by daily applying pickering emulsions on the right ear and on the shaved back 2 hours before treatment with 62.5 mg IMQ-containing cream (Aldara 5%) on the same locations for 7 consecutive days. Clinical observation showed that the lesions were less intense in all treated groups, except that of E-CT, compared with IMQ reference (Figure 5a). Indeed, no significant differences were observed after topical application of E-CT (9.85 ± 0.33) compared with the PASI score of the IMQ-treated group (9.90 ± 0.25). In contrast, the PASI score was significantly decreased with E-CsA (6.6 ± 0.25) and E-Tac (6.17 ± 0.73) (Figure 5b), and a more potent effect was observed with E-Cal (4.23 ± 0.35), E-CsA-Cal (3.94 ± 0.47), and E-Tac-Cal (5.30 ± 0.71). To confirm the alleviation of psoriatic lesions, the expression of psoriasis-associated genes were quantified from skin biopsies using microfluidic qPCR (Figure 5c). Compared with IMQ group, topical application of pickering emulsions, except E-CT, significantly decreased the expression of T-cell markers (CD3, CD4, CD8) and T-cell chemokines CCL17 and CCL20 (Lee et al, 2017), suggesting reduced cutaneous T-cell infiltration. Concomitantly, the decreased expressions of genes associated with keratinocytes, including K6a, S100a8, and LL37, were associated with the decreased expressions of psoriasis-associated proinflammatory cytokines (IL-17A, IL-17F, IL-36α, and TNF-α) (Blauvelt and Chiricozzi, 2018; Christmann et al, 2020; Gao et al, 2020). Conversely, they also significantly increased the expressions of the anti-inflammatory cytokine IL-38 and cell marker CD207. Noteworthy, gene expression of β-defensin 2 was significantly decreased with E-CsA and E-Tac and increased with E-Cal, whereas it remained unaltered with E-CsA-Cal and E-Tac-Cal.
Figure 5.
Effects of Em on IMQ-induced psoriasis-like mouse model.(a) Representative images of psoriatic lesions on dorsal mouse skin in each treatment group after 7 days of treatment. (b) Evaluation of mouse clinical scores (desquamation, erythema, and thickness of the back skin) was assessed daily using a scale from 0 to 4 for 7 consecutive days for each group of 6 mice studied together. In additon, the cumulative total score PASI (scaling plus erythema plus thickness) is depicted as total PASI score over 7 days (low left panel) and as PASI score at day 7 for each treatment (low right panel), for a final total group of 18 mice per treatment, showing an increase of Em clinical efficiency by the presence of calcitriol in oil phase compared with emulsions without it. (c) Gene expression of cytokines was quantified from murine skin biopsies of the first 6 mice investigated for each group of treatment on day 7 using microfluidic qPCR. The results are shown as the gene expression of T-cell markers (CD3, CD4, CD8) and T-cell chemokines CCL17 and CCL20; the gene expression of psoriasis-associated proinflammatory cytokines (IL17A, IL17F, IL36α, and TNFα); as well as the one associated with epidermal cells, including K6a, β-DEF-2, LL37, S100a8, the anti-inflammatory IL38, or the dendritic cell–associated CD207 (Langerin). Symbols in b and in c indicate the mean value ± SEM of 18 mice and 6 mice per group, respectively, with ∗P < .05, ∗∗P < 0.01, and ∗∗∗P < 0.001 compared with the IMQ-applied-only mice. Statistical analysis was assessed using for experiment involving multiple groups, 1-way ANOVA followed by Turkey multiple comparison test, except for analysis of clinical scores, which used 2-way ANOVA followed by Dunnett’s multiple comparison test. B-DEF-2, β-defensin 2; Em, pickering emulsion; IMQ, imiquimod; K6a, keratin 6a.
Discussion
As one of the most prevalent worldwide ID, psoriasis is a chronic autoimmune disease characterized by the presence of thickened, red, and silvery plaques whose appearance results from the pathological interplay between keratinocytes and both adaptive and innate immune cells. This dysregulated crosstalk involves the expression of proinflammatory cytokines such as IL-6, IL-8, IL-17, IL-23, and TNF-α, which initiates, amplifies, and maintains a never-ending inflammatory state. Over the last decades, many key pathomechanisms have been undiscovered, which led to the development of a wide variety of treatment options, from topical ointments to phototherapies and systemic treatments, including biotherapies. However, although the effectiveness of several treatments has been repeatedly proven, long-term adherence to treatment remains a major problem. Patients are prone to stop their treatment mostly because of adverse effects such as skin atrophy, burning sensations, or infections (Abraham and Roga, 2014). Other factors are also involved, such as vehicle texture, perceived lack of efficacy, time consuming, and difficulty of use or excessive cost (Bewley and Page, 2011; Teixeira et al, 2021). Therefore, more convenient drug formulations are needed to ensure better patient adherence and clinical outcome.
To date, almost no data have been published about the use of an emulsion as a therapeutic agent with encapsulation of an AS in NP (Marto et al, 2016). However, biocompatible and biodegradable NPs must be used for AS encapsulation. Accordingly, polymers such as PLGA were shown to be of interest because this latter will be degraded in lactic and glycolic acids, which are not cytotoxic and metabolized by the Krebs cycle (Danhier et al, 2012; Petros and DeSimone, 2010). The progressive degradation of NPs could also allow for a controlled AS release within the tissue and ensure a long-term effect (Alvarez-Román et al, 2004; Lademann et al, 2007). Several studies have already reported the topical application of PLGA NPs and showed that they were accumulating throughout the epidermis and might also penetrate through the appendages of the skin, including hair follicles or sweat gland channels (Das and Khuda-Bukhsh, 2016; Sun et al, 2017; Zhang et al, 2010). Substituting the emulsifiers currently used in classical emulsions with the presently used PLGA NPs enables coencapsulation of 2 distinct ASs into a single entity. Although many studies have reported the use of PLGA NP as a therapeutic approach for IDs, including psoriasis, no reports have investigated the effects of this pickering emulsion.
In this work, the biological effects of pickering emulsions coencapsulating a calcineurin inhibitor, either CsA or Tac, in combination with an anti-inflammatory molecule, Cal, were studied in vitro and in vivo. We first demonstrated that both CsA and Tac were released from the NPs by assessing their immunomodulatory properties on T cells and keratinocytes in vitro. This was confirmed by physicochemical analyses in vitro (data not shown). The expression of NFAT/calcineurin signaling pathway has been demonstrated in both lymphoid- and epidermal-targeted cells (Al-Daraji et al, 2002; Macian, 2005). In T cells, activation of NFAT/calcineurin pathway through TCR activation triggers T-cell activation, differentiation, and proliferation and secretion of proinflammatory cytokines. In accordance, both E-CsA-Cal and E-Tac-Cal significantly decreased NFAT translocation in T cells as demonstrated by the downregulation of NFAT-dependent IL-2 production, CD25 expression, and cell proliferation. In accordance with previous studies, the downregulation of IL-8 and TNF-α production did also prove the downregulation of NFAT in keratinocytes (Kaunisto et al, 2015; Maldonado-Pérez et al, 2009). Thus, the downregulation of NFAT translocation and subsequent NFAT-dependent events suggest that both CsA and Tac are released from the NPs. Moreover, the decrease of IL-8 production could be linked to Cal release as previously reported in keratinocytes (Koizumi et al, 1997; Miodovnik et al, 2012).
Immunofluorescent observation showed an increased red fluorescence in the epidermis, suggesting the penetration and accumulation of NPs in the epidermis. Other studies also reported the penetration of PLGA NPs with diameter ranging from 145 to 155 nm (da Silva et al, 2013; Srivastava et al, 2013), close to the ones used in study. We also confirmed the penetration of PLGA NPs by showing that topical application of pickering emulsions on skin explants ex vivo induced a decrease in both IL-1β and IL-6 expressions, although the IL-6 expression was also decreased with E-CT, suggesting a potential effect of the formulation itself. Similarly, topical application of glycolic acid was shown to suppress IL-6 expression after NF-κB stimulation with UVB (Tang et al, 2017) as well as with lactic acid after lipopolysaccharide induction (Xu et al, 2013). However, because the expression was quantified in this study in unstimulated conditions, the mechanism of E-CT activity still remains unclear. Of interest, epidermal cells exposed to AS either by topical application or incubated after their isolation from the epidermis exhibited a reduced ability to induce T-cell proliferation. This could be attributed to a decreased surface expression of activation markers on LCs due to the AS presence, especially under Cal-based preparations. Accordingly, Cal has been known to inhibit DC maturation by decreasing the expression of activation markers, such as CD86 and major histocompatibility complex class II, also altering their ability to migrate (Bscheider and Butcher, 2016; Eftekharian et al, 2010; Griffin et al, 2001). Furthermore, previous studies have documented the inhibitory properties of calcineurin inhibitors on the antigen-presenting capacity of DCs (Naranjo-Gómez et al, 2006; Tiefenthaler et al, 2004). Lin et al (2021) also reported that DCs were able to capture NPs and transport them into skin-draining lymph nodes, which may impede T-cell activation, differentiation, and proliferation in skin-draining lymph nodes and limit their trafficking toward affected cutaneous areas.
Beside penetration/permeation experiments, the development of pickering formulation is reinforced by stability evaluation, in accordance with data reporting that this type of emulsions proved to be stable over 55 days at least (constant droplet size and same macroscopic aspect) (Beladjine et al, 2023). To pursue the study, the integrity of the drugs could be also verified by high-performance liquid chromatography, after the emulsion being first broken by centrifugation and the NP being dissolved in an organic solvent.
If the use of pickering emulsion is compared with the one of single-agent formulations, which enable the delivery of only 1 substance, the combination of active ingredients in the NPs and in the emulsion droplets will enhance the delivery of both ASs topically within the epidermis. Indeed, these pickering emulsions provide a therapeutic oil-in-water formulation that offers several advantages for the topical administration of drugs. Indeed, they allow the encapsulation of at least 2 therapeutic agents (in the oil phase and/or the water phase) and offer potential advantages such as the protection of the drugs from degradation, the preservation of their activity, an improved epidermal penetration of the drugs, or the controlled and sustained release of the drugs. Delivery might occur by accumulation in the stratum corneum or in the hair follicles, which could act as short-term or long-term reservoir, respectively (Jain et al, 2011), and PLGA-CsA NPs can be effective as a topical delivery vehicle because NPs were mainly concentrated in hair follicles and thus penetrated the dermis and epidermis.
Investigation of the effect of the pickering emulsions on psoriasis-like lesions was assessed using the IMQ-induced psoriasis-like mouse model, which is a conventionally accepted model to study psoriasis in vivo. This model consists of the topical application of IMQ (Aldara 5%), which triggers psoriasiform lesions through the activation of innate and adaptive immune cells, as well as of keratinocytes (van der Fits et al, 2009). In IMQ-induced psoriasiform skin, CD4+ and CD8+ T cells were shown to migrate into the skin after their activation in lymph nodes by IMQ-activated DCs and CD207+ LCs and in response to the chemokines expressed by keratinocytes, such as CCL17 and CCL20. They produce high amounts of proinflammatory cytokines, including IL-17, IL-36α, and TNF-α, which activate cutaneous cells, including keratinocytes. In response to these cytokines, keratinocytes overexpress antimicrobial peptides (β-defensin 2, LL37) and alarmins (S100a8) and hyperproliferate, with a subsequent switch of keratin expression, in particular with the keratin 6a overexpression. Hence, the alleviation of psoriatic lesions by topical application of emulsions, as demonstrated by reduction in PASI score, was associated with decreased expressions of all the genes mentioned earlier. Concurrently, the expression of the anti-inflammatory cytokine IL-38 increased, which further reinforces the alleviation of lesion severity. Surprisingly, the PASI score with E-Cal was similar to that with E-CsA-Cal and E-Tac-Cal. However, this reflects a limitation of the model. Indeed, the initiation of psoriatic lesions in this murine model was driven by the activation of DCs, including LCs and plasmacytoid DCs, by IMQ. DCs migrate out of the skin to induce T-cell proliferation and secretion of proinflammatory Th1/Th17 cytokines (Gibson et al, 2002; Xiao et al, 2017; Yan et al, 2022). Our results on LC activation suggested a potent early Cal response, which is consistent with the significant increased expression of CD207 in mice treated with pickering emulsions, indicating LC retention in the skin. Altogether, the combination of calcineurin inhibitors and Cal significantly decreased psoriasiform lesions.
Patients with psoriasis have a low incidence of cutaneous infection, which may be accountable to the enhanced expression of antimicrobial peptides, in particular β-defensin 2, which is highly expressed in response to IL-17A (Hu et al, 2016; Kolbinger et al, 2017). According to literature (Bartley, 2010; Youssef et al, 2011), we observed that β-defensin 2 expression was significantly increased with Cal (E-Cal) compared with IMQ. Inversely, both immunosuppressive agents (E-CsA and E-Tac) decreased its expression, which may be related to the downregulation of IL-17A. However, the combination of both molecules in a unique emulsion (E-CsA-Cal and E-Tac-Cal) did not alter its expression. Thus, compared with topical corticosteroids that can worsen skin infections, our pickering emulsions could alleviate psoriatic lesions and avoid skin infection by maintaining high levels of β-defensin 2.
To further investigate penetration/permeation of our pickering emulsions through the epidermis, several studies might be assessed, including analysis of penetration of NPs in the epidermis under the stratum corneum by microscopy techniques, for example, confocal laser scanning microscopy with NPs marked with a fluorescent probe (Alvarez-Román et al, 2004), closed to what we performed in our work, or electron microscopy (Cross et al, 2007). Moroever, the ASs such as CsA or Tac might be detected by immunostaining in the epidermis using specific antibodies against these molecules, although no antibody useful for immunohistochemistry analysis has been developed yet, to our knowledge. Furthermore, isolation of the stratum corneum and the lower viable epidermal layers by successive tape striping and further extraction could be used for high-performance liquid chromatography analysis to detect active molecules throughout the epidermis after topical application of the emulsions. For perspective, penetration/permeation experiments would also include Franz cells and tape stripping experiments, with in situ labeling for the latter technique.
Altogether, our data suggest that coencapsulation of a calcineurin inhibitor with Cal in pickering emulsions could be an effective therapeutic approach for IDs such as psoriasis. Our drug association efficiently decreased the proinflammatory responses of key cells in psoriasis pathophysiology, including T cells, keratinocytes, and LCs, which contributed to the overall improvement of psoriasiform lesions in vivo. Owing to the use of nanotechnology in the treatment of psoriasis, we believe that it will be possible to increase the efficiency of topical drug delivery and limit the systemic use of immunosuppressive drugs in these long-term–treated patients. Further pickering emulsions combining corticoids with calcipotriol might be of interest because it would be interesting to formulate emulsions with other ASs such as some innovative pathway inhibitors that have been recently assessed in IDs. Moreover, the combination of other innovative drugs in pickering emulsions could be of interest for IDs, including psoriasis and atopic dermatitis, and could be considered for the purpose of better treatment of dermatoses in the future.
Materials and Methods
Pickering emulsions and reagents
Pickering emulsions were prepared at Institut Galien Paris-Saclay (Orsay, France) and comprised a control emulsion without ASs (ie, E-CT) and emulsions associating CsA- or Tac-loaded NPs with Cal (E-CsA-Cal or E-Tac-Cal, respectively) or including only one AS (ie, E-CsA, E-Tac, and E-Cal), besides the AS solution described as CsA, Tac, or Cal. The preparation protocol and physicochemical characterizations of these emulsions stabilized with PLGA NPs were prepared according to Albert et al (2018). Briefly, NPs were prepared by nanoprecipitation, with PLGA and CsA (or Tac) dissolved in an organic solvent (acetonitrile). The solution was added dropwise with a syringe pump into milli-Q water, and the organic solvent was then evaporated with a rotary evaporator, up to a final volume corresponding to an NP concentration of 25 mg/ml. The oil-in-water emulsions were then formed by mixing the NP suspension with oil (water–oil ratio of 80/20) with a rotor-stator homogenizer. ASs were added either during the preparation of the NP (CsA or Tac in the organic solvent containing the PLGA) or during the preparation of the emulsions (Cal in the oil phase).
Other reagents included RPMI medium supplemented with 10% fetal bovine serum (Gibco, Life Technologies), 1% L-glutamine (Gibco, Life Technologies), 1% penicillin/streptomycin (Gibco, Life Technologies), 1% amphotericin B (Gibco, Life Technologies), 1% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Gibco, Life Technologies), and 0.2% plasmocin (InvivoGen). This complete medium was used to study blood-isolated PBMCs over a 7-day culture. Complete DMEM was prepared for HaCaT cells with the same supplements for complete RMPI medium but with 5% sodium pyruvate (Gibco, Life Technologies) instead of amphotericin B. Epidermal cells were cultured in Keratinocyte Growth Medium (Lonza). PUCK’s buffer was prepared with sterile water supplemented with 0.8% sodium chloride, 0.04% potassium chloride, 0.035% sodium carbonate, 0.1% glucose monohydrate, and 0.02% EDTA.
Animal experiments
BALB/cJRj female mice aged 6 weeks were purchased from Janvier Labs and were allowed for 7 days to acclimate before starting experiments. The care and treatment of the mice were in accordance with the guidelines established by the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. All animal protocols were approved by the Comité d’Ethique Experimentation Animal Paris-Nord (number 121) (project number APAFIS #23806-2020012712108370). Mice were housed at the animal facility (UMS Saint-Louis US53/UAR2030, Institut de Recherche Saint-Louis, Paris, France) in accordance with animal welfare and ethical guidelines (accreditation number B75-10-08). In this project lasting a total of 3 years, the number of mice (432) used was calculated to allow reduction as much as possible while ensuring the relevance of the results. To obtain statistically relevant results, each group must consist of 5–6 mice, and the experiment must be reproduced 3 times to overcome the variability of therapeutic preparations. Eight treatment groups were defined: a group without induction of the pathology, 1 untreated group, and 6 groups receiving different therapeutic combinations. The use of groups of 18 mice per condition in this study enables sufficiently high statistical power to statistically validate the results obtained, and repeating the experiments in 3 independent procedures for the 8 groups raises the total number to 432 BALB/cJRj mice. Briefly, mice aged 7 weeks were randomly divided into 8 groups (n = 5–6 mice per experiment per group to reach a total of 16–18 per group): control (denoted as CT) without IMQ, IMQ used as psoriasis control (IMQ), IMQ + E-CT, IMQ + E-CsA, IMQ + E-Tac, IMQ + E-Cal, IMQ + E-CsA-Cal, and IMQ + E-Tac-Cal. For all the mice, the back was shaved before 62.5 mg of IMQ-containing cream (Aldara 5%, MEDA Pharma GmbH) were topically applied every day on the shaved dorsal back skin and right ear skin (6.25 mg) for 7 consecutive days. Emulsions were applied 2 hours before each IMQ application. Note, the control group (denoted as CT) received topical Vaseline instead of IMQ. The severity of psoriasis-like lesions was daily evaluated by a double-blind independent lecture using an objective scoring system on the basis of the clinical PASI. Erythema, scaling, and increase in ear thickness measured with a caliper micrometer and related to the first measure were scored independently from 0 to 4 as follows: 0, none; 1, slight; 2, moderate; 3, marked; and 4, very marked. The cumulative score (erythema plus scaling plus thickening) served to indicate the severity of inflammation (scale 0–12). After killing, skin biopsies of the back were collected for further experiments, including RNA extraction for cytokine RNA PCR.
Human blood and tissue samples
Human healthy skin from breast reduction mammoplasty was obtained from the Department of Plastic Surgery, Saint-Louis Hospital (Paris, France) and was collected in compliance with ethics recommendations and after a written informed consent was obtained from each donor according to the approval by the Institutional Review Board CPP-Ile de France (number 911556). Freshly obtained skin samples were used for isolation of epidermal cells, LCs, or dermal T cells as well as for topical application of emulsions for 24 hours. For studies of normal lymphocytes, blood samples from healthy volunteers were obtained from the Etablissement Français du Sang (Paris, France).
PBMC and monocyte isolation
Human PBMCs were isolated from buffy coats of healthy volunteers by gradient centrifugation using Lymphocyte Separation Medium (Eurobio Scientific, Les Ulis, France). Isolated PBMCs were either rested overnight in complete RPMI medium for further experiments or directly used to purify monocytes by positive selection using CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer’s instructions. Purified population with at least 90% of monocytes was considered pure after assessment by flow cytometry using anti-CD14 antibody.
Dermal T-cell isolation
Dermal T cells were isolated from healthy skin by digesting skin biopsies with collagenase P. Briefly, skin biopsies were immerged in complete RMPI medium supplemented with 1 mg.ml–1 collagenase P and incubated for 18 hours at 37 °C. The cell suspension was then filtered through a 30-μm cell strainer, counted, and then rested to allow the separation between adherent and nonadherent cells. After 7 days of culture, the nonadherent cells in the supernatant, which comprise dermal T cells, were harvested and used for proliferation experiments.
Epidermal cell isolation
Epidermal cells were isolated from human skin biopsies using trypsin digestion. Briefly, skin biopsies were immerged in PUCK’s bufferm supplemented with 0.25% trypsin and incubated for 18 hours at 4 °C. Floating epidermis was then harvested with forceps, immerged in PUCK’s 10% fetal bovine serum, and constantly agitated for 30 minutes at 37 °C to dissociate epidermal cells. The epidermal cell suspension was then filtered through a 30-μm cell strainer, counted, and either directly seeded in 100 mm2 petri dish to culture keratinocyte in Keratinocyte Growth Medium or used to purify LCs by positive selection using the CD1a microbeads (Miltenyi Biotec), according to the manufacturer’s instructions. For keratinocyte experiments, the cells were seeded in 6-well plates at 2.5 × 105 cells per well in Keratinocyte Growth Medium until 80% confluence ; afterwhich, cells were pretreated for 1 hour with the various preparations and then activated for 24 hours with 50 nM phorbol myristate acetate and 1 μM ionomycin (P/I) before collecting the supernatants for cytokine dosage. For topical experiments, the emulsions were topically applied on 1-cm2 skin biopsies and incubated at 37 °C for 24 hours before either epidermal cell isolation, as described earlier, or cryoconservation in optimal cutting temperature compound. Sections of 5-μm thickness were then analyzed by immunochemistry.
moLCs
Differentiation of circulating monocytes into moLCs was adapted from Otsuka et al (2018). Briefly, isolated monocytes were seeded in a 24-well plate at 1 × 106 cells per well and cultured in complete RMPI medium in the presence of 100 ng.ml−1 human recombinant GM-CSF (Peprotech), 10 ng.ml−1 human recombinant IL-4 (Peprotech), and 10 ng.ml−1 human recombinant TGF-β (Peprotech). Half of the medium was changed every 2 days with fresh cytokines without IL-4. After 5 days of culture, the various preparations were added, and the cells were activated with 20 ng.ml−1 human recombinant TNF-α. After 2 days of activation, the cells were harvested by vigorous flushing.
Cell proliferation, mixed epidermal cell–lymphocyte reaction, and mixed lymphocyte reaction
PBMCs and dermal T cells were stained with carboxyfluorescein succinimidyl esther (InvitroGen) according to the manufacturer’s instructions, seeded in 96-well round bottom plate at 105 cells per well in complete RPMI medium, and pretreated for 1 hour with the various preparations. PBMCs were then activated with CD3- and CD28-coated microbeads for 6 days, whereas OKT3 mAb directed against CD3+ was used to activate dermal T cells for 7 days. Supernatants of CD3- and CD28-coated microbeads-activated PBMCs were collected on day 3 for IL-2 dosage. Carboxyfluorescein succinimidyl esther–stained PBMCs were also cocultured with epidermal cells or isolated LCs, previously pretreated for 24 hours with the various preparations, in a mixed epidermal cell–lymphocyte reaction or with pretreated moLCs in a mixed lymphocyte reaction for 7 days. All cells were then harvested, and their proliferation was analyzed by flow cytometry.
Flow cytometry
Carboxyfluorescein succinimidyl esther–stained cells were labeled with allophycocyanin-conjugated CD3 mouse anti-human mAb (BD Bioscience) and resuspended in propidium iodide just before analysis on a Canto II flow cytometer (BD Bioscience). Furthermore, moLC phenotype was analyzed on an LSR Fortessa (BD Bioscience) after staining with the following mouse anti-human mAbs: BV510 fixable viability dye (BD Bioscience), FITC-conjugated CD1a, PerCP-eFluor 710–conjugated CD80 (B7-1), allophycocyanin-conjugated CD207 (Langerin), SB600-conjugated CD86 (B7-2), SB780-cojugated CD14, phycoerythrin-conjugated CD197 (CCR7), phycoerythrin-Cy7–conjugated CD40, and eFluor 450–conjugated HLA-DR (all from Thermo Fisher Scientific).
Pickering emulsion cytotoxicity study
The keratinocyte cell line HaCaT was used to assess PEm cytotoxicity. Cells were seeded in a 96-well plate at 2 × 103 cells in complete DMEM per well and then exposed to the preparations after 48 hours of culturing. The cytotoxicity was evaluated on days 2 and 5 by MTT assay. The optical density was measured using a model 680 microplate reader (Bio-Rad Laboratory).
Cytokine quantification by ELISA
IL-2 concentration in T-cell supernatants and IL-6, IL-8, and TNF-α concentrations in keratinocyte supernatants were determined by ELISA sandwich technique (Kit ELISA Sandwich Standard Format, PeproTech), according to the manufacturer's instructions. The optical density was quantified with a model 680 microplate reader (Bio-Rad Laboratory).
Real-time quantitative PCR and microfluidic PCR analysis
Total RNA was extracted either from human epidermal cells using the RNeasy Mini Kit (Qiagen) or from mouse back skin biopsies using the RNeasy Plus Universal Mini Kit (Qiagen) after grinding with Tissue Lyzer (Qiagen), according to the manufacturer’s instructions. RNA concentrations were measured using the NanoDrop spectrophotometer 2000c (Thermo Fisher Scientific). cDNA was then synthesized using the Thermoscript RT-PCR system kit (Invitrogen). Quantification of human and mouse gene expressions was performed respectively by real-time quantitative PCR on a Roche Lightcycler 480 II and by microfluidic qPCR at the qPCR-HD-Genomic Paris Centre platform. Gene probes used for PCR amplification were specifically designed for studying the gene expression of T-cell markers (CD3, CD4, CD8) and T-cell chemokines CCL17 and CCL20; the gene expression of psoriasis-associated proinflammatory cytokines (IL17A, IL17F, IL36α, and TNFα); as well as the one associated with epidermal cells, including keratin 6a, β-defensin 2, LL37, S100a8, the anti-inflammatory IL38, or the DC-associated CD207 (Langerin). Gene probes are listed in Table 1. The fold change in the expression of the target gene relative to the control was normalized to GAPDH or β-actin using the ΔΔCt method.
Table 1.
Human and Murine Gene Sequences
| Host | Genes | Probes | Sequences |
|---|---|---|---|
| h | GAPDH | F | GCTCCTCCTGTTCGACAGTCA |
| R | ACCTTCCCCATGGTGTCTGA | ||
| h | IL1β | F | TACCTGTCCTGCGTGTTGAA |
| R | TCTTTGGGTAATTTTTGGGATCT | ||
| h | IL6 | F | TGGCTGAAAAAGATGGATGCT |
| R | GATGATTTTCACCAGGCAAGTCT | ||
| h | IL8 | F | AGACAGCAGAGCACACAAGC |
| R | ATGGTTCCTTCCGGTGGT | ||
| h | TNFα | F | GAGCCTCTTCTCCTTCCTGAT |
| R | GCCAGAGGGCTGATTAGAGA | ||
| m | β-act | F | GTGACGTTGACATCCGTAAAGA |
| R | GCCGGACTCATCGTACTCC | ||
| m | CD3ε | F | ATGCGGTGGAACACTTTCTGG |
| R | GCACGTCAACTCTACACTGGT | ||
| m | CD4 | F | AGGTGATGGGACCTACCTCTC |
| R | GGGGCCACCACTTGAACTAC | ||
| m | CD8a | F | CCGTTGACCCGCTTTCTGT |
| R | CGGCGTCCATTTTCTTTGGAA | ||
| m | IL17A | F | TTTAACTCCCTTGGCGCAAAA |
| R | CTTTCCCTCCGCATTGACAC | ||
| m | IL17F | F | TGCTACTGTTGATGTTGGGAC |
| R | AATGCCCTGGTTTTGGTTGAA | ||
| m | IL36α | F | TAGTGGGTGTAGTTCTGTAGTGTGC |
| R | GTTCGTTCAAGAGTGTCCAGATAT | ||
| m | IL38 | F | CCTGGCGTGTGTAAAGACAA |
| R | CAGATCCCAAGCTTCTCTGG | ||
| m | CCL17 | F | CCGAGAGTGCTGCCTGGATTA |
| R | CACAGATGAGCTTGCCCTGGA | ||
| m | CCL20 | F | CAGGCAGAAGCAAGCAACTAC |
| R | AGCTTCATCGGCCATCTGTC | ||
| m | CD207 | F | CCGAAGCGCACTTCACAGT |
| R | GCAGATACAGAGAGGTTTCCTCA | ||
| m | K6a | F | AGAGAGGGGTCGCATGAACT |
| R | TCATCTGTTAGACTGTCTGCCTT | ||
| m | S100a8 | F | AAATCACCATGCCCTCTACAAG |
| R | CCCACTTTTATCACCATCGCAA | ||
| m | β-DEF2 | F | TCTCTGCTCTCTGCTGCTGATATGC |
| R | AGGACAAATGGCTCTGACACAGTACC | ||
| m | LL37 | F | GCTGTGGCGGTCACTATCAC |
| R | TGTCTAGGGACTGCTGGTTGA | ||
| m | TNFα | F | CCCTCACACTCAGATCATCTTCT |
| R | GCTACGACGTGGGCTACAG |
Abbreviations: F, forward; h, human; m, murine; R, reverse.
Immunofluorescence analysis
NFATc1 staining was performed on 5 × 104 T cells cytospined on Superfrost slides and on 5 × 103 keratinocytes seeded on Lab-Tek chamber slides. NFAT translocation was induced with P/I in T cells for 30 minutes and in keratinocytes for 6 hours. Cells were then fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, stained with mouse anti-human NFATc1 primary antibody (MA3-024, Thermo Fisher Scientific) and goat anti-mouse Alexa Fluor 488–conjugated secondary antibody, and then counterstained with antifade mounting medium with DAPI (Vectashield, Vector Laboratories). Slides of topically treated skin biopsies and conserved in optimal cutting temperature compound were cryosectionned using a cryostat (Leica). Skin sections were then mounted on Superfrost slides and counterstained with DAPI.
Statistical analysis
All statistical analyses were performed using GraphPad Prism statistical software, version 7.04. For in vitro experiments, statistical comparison was assessed using the Wilcoxon rank-sum test. ∗P < .05 and ∗∗P < .01. For in vivo experiment involving multiple groups, 1-way ANOVA followed by Turkey multiple comparison test was used, except for analysis of clinical scores, which used 2-way ANOVA followed by Dunnett’s multiple comparison test. P < .05 was considered significant.
Ethics Statement
Human healthy skin from breast reduction mammoplasty was obtained from the Department of Plastic Surgery, Saint-Louis Hospital (Paris, France) and was collected in compliance with ethics recommendations and after a written informed consent was obtained from each donor according to the approval by the Institutional Review Board CPP-Ile de France (number 911556). A global convention has been signed between Inserm laboratories from U976 and EFS Paris: thus human volunteers that give their blood sign a consent enabling anonymous use of their blood for transfusion or research experiments. There is no access to these personnal data but just the guarantee of a viral free normal blood.
The care and treatment of the mice were in accordance with the guidelines established by the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. All animal protocols were approved by the Comité d’Ethique Experimentation Animal Paris-Nord (number 121) (project number APAFIS #23806-2020012712108370). Mice were housed at the animal facility (UMS Saint-Louis US53/UAR2030, Institut de Recherche Saint-Louis, Paris, France) in accordance with animal welfare and ethical guidelines (accreditation number B75-10-08).
Data Availability Statement
Detailed data are available upon request from LM at laurence.michel@inserm.fr.
ORCIDs
Maxime Sintès: http://orcid.org/0000-0003-3763-9986
Petra Kovjenic: http://orcid.org/0009-0007-0452-8098
Liasmine Haine (Hablal): http://orcid.org/0009-0006-7884-4950
Kevin Serror: http://orcid.org/0000-0002-2517-1039
Mohamed Beladjine: http://orcid.org/0000-0001-5695-0038
Véronique Parietti (Montcuquet): http://orcid.org/0000-0001-8314-7427
Marine Delagrange: http://orcid.org/0009-0002-6596-4268
Bertrand Ducos: http://orcid.org/0000-0002-5322-1339
Jean-David Bouaziz1: http://orcid.org/0000-0002-4993-2461
David Boccara: http://orcid.org/0000-0002-7365-5943
Maurice Mimoun: http://orcid.org/0009-0009-5609-1478
Armand Bensussan: http://orcid.org/0000-0002-0409-2497
Martine Bagot: http://orcid.org/0000-0002-0400-1954
Nicolas Huang: http://orcid.org/0000-0003-2750-3778
Laurence Michel: http://orcid.org/0000-0002-5952-3282
Conflict of Interest
The authors state no conflict of interest.
Acknowledgments
We thank the team of the institute's microscopy and cytometry platform of Saint-Louis Institute conducted by SETTERBLAD N for their availability in our work. This work was supported by the Agence Nationale de la Recherche (ANR-16-CE09-0003, ANR-20-CE18-0033-02), the Ministère de l’Enseignement Supérieur, de la Recherche et de l'Innovation, and LEO Foundation (Research prize).
Author Contributions
Conceptualization: MS, NH, LM; Data Curation: MS, PK, LH(H), BD, NH, LM; Formal Analysis: MS, PK, LH(H), MD, BD, NH, LM; Funding Acquisition: NH, LM; Investigation: MS, PK, LH(H), MD, BD, NH, LM; Methodology: MS, PK, LH(H), MB, MD, BD, NH, LM; Project Administration: NH, LM; Resources: KS, VP(M), BD, MM, NH, LM; Software: MD, BD, LM; Supervision: NH, LM; Validation: PK, LH(H), NH, LM; Visualization: MS, MB, BD, MB, NH, LM; Writing – Original Draft Preparation: MS, NH, LM; Writing – Review and Editing: MS, PK, LH(H), KS, AB, J-DB, MB, NH, LM
Declaration of Generative Artificial Intelligence (AI) or Large Language Models (LLMs)
No use of generative artificial intelligence or large language models in writing process.
accepted manuscript published online XXX; corrected published online XXX
References
- Abe M., Syuto T., Hasegawa M., Sogabe Y., Yokoyama Y., Ishikawa O. Daily versus intermittent application of high-concentration tacalcitol ointment in combination with low-dose cyclosporin for psoriasis vulgaris. J Dermatol. 2006;33:108–111. doi: 10.1111/j.1346-8138.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- Abraham A., Roga G. Topical steroid-damaged skin. Indian J Dermatol. 2014;59:456–459. doi: 10.4103/0019-5154.139872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert C., Huang N., Tsapis N., Geiger S., Rosilio V., Mekhloufi G., et al. Bare and sterically stabilized PLGA nanoparticles for the stabilization of pickering emulsions. Langmuir. 2018;34:13935–13945. doi: 10.1021/acs.langmuir.8b02558. [DOI] [PubMed] [Google Scholar]
- Al-Daraji W.I., Grant K.R., Ryan K., Saxton A., Reynolds N.J. Localization of calcineurin/NFAT in human skin and psoriasis and inhibition of calcineurin/NFAT activation in human keratinocytes by cyclosporin A. J Invest Dermatol. 2002;118:779–788. doi: 10.1046/j.1523-1747.2002.01709.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Román R., Naik A., Kalia Y.N., Guy R.H., Fessi H. Skin penetration and distribution of polymeric nanoparticles. J Control Release. 2004;99:53–62. doi: 10.1016/j.jconrel.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Bartley J. Vitamin D: emerging roles in infection and immunity. Expert Rev Anti Infect Ther. 2010;8:1359–1369. doi: 10.1586/eri.10.102. [DOI] [PubMed] [Google Scholar]
- Beladjine M., Albert C., Sintès M., Mekhloufi G., Gueutin C., Nicolas V., et al. Pickering emulsions stabilized with biodegradable nanoparticles for the co-encapsulation of two active pharmaceutical ingredients. Int J Pharm. 2023;637 doi: 10.1016/j.ijpharm.2023.122870. [DOI] [PubMed] [Google Scholar]
- Bewley A., Page B. Maximizing patient adherence for optimal outcomes in psoriasis. J Eur Acad Dermatol Venereol. 2011;25(Suppl. 4):9–14. doi: 10.1111/j.1468-3083.2011.04060.x. [DOI] [PubMed] [Google Scholar]
- Blauvelt A., Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55:379–390. doi: 10.1007/s12016-018-8702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco N., Lee I., Zhai H., Maibach H.I. Long-term repetitive sodium lauryl sulfate-induced irritation of the skin: an in vivo study. Contact Dermatitis. 2005;53:278–284. doi: 10.1111/j.0105-1873.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- Brun C., Demeaux A., Guaddachi F., Jean-Louis F., Oddos T., Bagot M., et al. T-plastin expression downstream to the calcineurin/NFAT pathway is involved in keratinocyte migration. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bscheider M., Butcher E.C. Vitamin D immunoregulation through dendritic cells. Immunology. 2016;148:227–236. doi: 10.1111/imm.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.K., Flynn G.L., Amidon G.L. Percutaneous absorption and dermal delivery of cyclosporin A. J Pharm Sci. 1995;84:581–583. doi: 10.1002/jps.2600840512. [DOI] [PubMed] [Google Scholar]
- Christmann C., Zenker S., Martens L., Hübner J., Loser K., Vogl T., et al. Interleukin 17 promotes expression of alarmins S100A8 and S100A9 during the inflammatory response of keratinocytes. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.599947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S.E., Innes B., Roberts M.S., Tsuzuki T., Robertson T.A., McCormick P. Human skin penetration of sunscreen nanoparticles: in-vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacol Physiol. 2007;20:148–154. doi: 10.1159/000098701. [DOI] [PubMed] [Google Scholar]
- Cserháti T., Forgács E., Oros G. Biological activity and environmental impact of anionic surfactants. Environ Int. 2002;28:337–348. doi: 10.1016/s0160-4120(02)00032-6. [DOI] [PubMed] [Google Scholar]
- da Silva C.L., Del Ciampo J.O., Rossetti F.C., Bentley M.V., Pierre M.B. PLGA nanoparticles as delivery systems for protoporphyrin IX in topical PDT: cutaneous penetration of photosensitizer observed by fluorescence microscopy. J Nanosci Nanotechnol. 2013;13:6533–6540. doi: 10.1166/jnn.2013.7789. [DOI] [PubMed] [Google Scholar]
- Danhier F., Ansorena E., Silva J.M., Coco R., Le Breton A., Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- Das S., Khuda-Bukhsh A.R. PLGA-loaded nanomedicines in melanoma treatment: future prospect for efficient drug delivery. Indian J Med Res. 2016;144:181–193. doi: 10.4103/0971-5916.195024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio P., Villanova F., Nestle F.O. Psoriasis. Cold Spring Harb Perspect Med. 2014;4:a015354. doi: 10.1101/cshperspect.a015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J.I., Payne S.N., Winfield A.J., Ormerod A.D., Thomson A.W. Enhanced percutaneous absorption of a novel topical cyclosporin A formulation and assessment of its immunosuppressive activity. Br J Dermatol. 1990;123:631–640. doi: 10.1111/j.1365-2133.1990.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Eftekharian M.M., Zarnani A.H., Moazzeni S.M. In vivo effects of calcitriol on phenotypic and functional properties of dendritic cells. Iran J Immunol. 2010;7:74–82. [PubMed] [Google Scholar]
- Gao J., Chen F., Fang H., Mi J., Qi Q., Yang M. Daphnetin inhibits proliferation and inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced psoriasis-like skin lesion in mice. Biol Res. 2020;53:48. doi: 10.1186/s40659-020-00316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S.J., Lindh J.M., Riter T.R., Gleason R.M., Rogers L.M., Fuller A.E., et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- Griffin M.D., Lutz W., Phan V.A., Bachman L.A., McKean D.J., Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths C.E.M., Armstrong A.W., Gudjonsson J.E., Barker J.N.W.N. Psoriasis. Lancet. 2021;397:1301–1315. doi: 10.1016/S0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
- Hu S.C., Yu H.S., Yen F.L., Lin C.L., Chen G.S., Lan C.C. Neutrophil extracellular trap formation is increased in psoriasis and induces human β-defensin-2 production in epidermal keratinocytes. Sci Rep. 2016;6:31119. doi: 10.1038/srep31119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Mittal A.K., Jain A. Enhanced topical delivery of Cyclosporin-A using PLGA nanoparticles as carrier. Curr Nanosci. 2011;7:524–530. [Google Scholar]
- Kaunisto A., Henry W.S., Montaser-Kouhsari L., Jaminet S.C., Oh E.Y., Zhao L., et al. NFAT1 promotes intratumoral neutrophil infiltration by regulating IL8 expression in breast cancer. Mol Oncol. 2015;9:1140–1154. doi: 10.1016/j.molonc.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H., Kaplan A., Shimizu T., Ohkawara A. 1,25-dihydroxyvitamin D3 and a new analogue, 22-oxacalcitriol, modulate proliferation and interleukin-8 secretion of normal human keratinocytes. J Dermatol Sci. 1997;15:207–213. doi: 10.1016/s0923-1811(97)00609-9. [DOI] [PubMed] [Google Scholar]
- Kolbinger F., Loesche C., Valentin M.A., Jiang X., Cheng Y., Jarvis P., et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923–932.e8. doi: 10.1016/j.jaci.2016.06.038. [DOI] [PubMed] [Google Scholar]
- Lademann J., Richter H., Teichmann A., Otberg N., Blume-Peytavi U., Luengo J., et al. Nanoparticles--an efficient carrier for drug delivery into the hair follicles. Eur J Pharm Biopharm. 2007;66:159–164. doi: 10.1016/j.ejpb.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Lebwohl M., Ali S. Treatment of psoriasis. Part 1. Topical therapy and phototherapy. J Am Acad Dermatol. 2001;45:487–499. doi: 10.1067/mjd.2001.117046. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Jeon Y.J., Choi J.H., Kim H.Y., Kim T.Y. Effects of VitabridC12 on skin inflammation. Ann Dermatol. 2017;29:548–558. doi: 10.5021/ad.2017.29.5.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lémery E., Briançon S., Chevalier Y., Bordes C., Oddos T., Gohier A., et al. Skin toxicity of surfactants: structure/toxicity relationships. Colloids Surf Physicochem Eng ASp. 2015;469:166–179. [Google Scholar]
- Lin Z., Xi L., Chen S., Tao J., Wang Y., Chen X., et al. Uptake and trafficking of different sized PLGA nanoparticles by dendritic cells in imiquimod-induced psoriasis-like mice model. Acta Pharm Sin B. 2021;11:1047–1055. doi: 10.1016/j.apsb.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liwarska-Bizukojc E., Miksch K., Malachowska-Jutsz A., Kalka J. Acute toxicity and genotoxicity of five selected anionic and nonionic surfactants. Chemosphere. 2005;58:1249–1253. doi: 10.1016/j.chemosphere.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Luger T., Paul C. Potential new indications of topical calcineurin inhibitors. Dermatology. 2007;215:45–54. doi: 10.1159/000102119. [DOI] [PubMed] [Google Scholar]
- Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- Maldonado-Pérez D., Brown P., Morgan K., Millar R.P., Thompson E.A., Jabbour H.N. Prokineticin 1 modulates IL-8 expression via the calcineurin/NFAT signaling pathway. Biochim Biophys Acta. 2009;1793:1315–1324. doi: 10.1016/j.bbamcr.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marto J., Ascenso A., Simoes S., Almeida A.J., Ribeiro H.M. Pickering emulsions: challenges and opportunities in topical delivery. Expert Opin Drug Deliv. 2016;13:1093–1107. doi: 10.1080/17425247.2016.1182489. [DOI] [PubMed] [Google Scholar]
- Miodovnik M., Koren R., Ziv E., Ravid A. The inflammatory response of keratinocytes and its modulation by vitamin D: the role of MAPK signaling pathways. J Cell Physiol. 2012;227:2175–2183. doi: 10.1002/jcp.22951. [DOI] [PubMed] [Google Scholar]
- Mohd Nordin U.U., Ahmad N., Salim N., Mohd Yusof N.S. Lipid-based nanoparticles for psoriasis treatment: a review on conventional treatments, recent works, and future prospects [published correction appears in RSC Adv 2022;12:21110] RSC Adv. 2021;11:29080–29101. doi: 10.1039/d1ra06087b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo-Gómez M., Climent N., Cos J., Oliva H., Bofill M., Gatell J.M., et al. Tacrolimus treatment of plasmacytoid dendritic cells inhibits dinucleotide (CpG-)-induced tumour necrosis factor-alpha secretion. Immunology. 2006;119:488–498. doi: 10.1111/j.1365-2567.2006.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle F.O., Kaplan D.H., Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- Otsuka Y., Watanabe E., Shinya E., Okura S., Saeki H., Geijtenbeek T.B.H., et al. Differentiation of Langerhans cells from monocytes and their specific function in inducing IL-22-specific Th Cells. J Immunol. 2018;201:3006–3016. doi: 10.4049/jimmunol.1701402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi R., Symmons D.P., Griffiths C.E., Ashcroft D.M., Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- Petros R.A., DeSimone J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- Pradhan M., Alexander A., Singh M.R., Singh D., Saraf S., Saraf S., et al. Understanding the prospective of nano-formulations towards the treatment of psoriasis. Biomed Pharmacother. 2018;107:447–463. doi: 10.1016/j.biopha.2018.07.156. [DOI] [PubMed] [Google Scholar]
- Robin B., Agnely F., Tsapis N., Huang N. In: Chapter 3. Pickering emulsions: history and fundamentals. Wypych F., de Freitas R.A., editors. Elsevier; Amsterdam: 2022. pp. 61–85.https://www.sciencedirect.com/science/article/pii/B9780323918589000057 Dev. Clay sci. [Google Scholar]
- Saleem S., Iqubal M.K., Garg S., Ali J., Baboota S. Trends in nanotechnology-based delivery systems for dermal targeting of drugs: an enticing approach to offset psoriasis. Expert Opin Drug Deliv. 2020;17:817–838. doi: 10.1080/17425247.2020.1758665. [DOI] [PubMed] [Google Scholar]
- Sindrilaru A., Filip A., Scharffetter-Kochanek K., Crisan D. How can nanoparticle-based technologies revolutionize the topical therapy in psoriasis? Exp Dermatol. 2020;29:1097–1103. doi: 10.1111/exd.14149. [DOI] [PubMed] [Google Scholar]
- Srivastava A.K., Bhatnagar P., Singh M., Mishra S., Kumar P., Shukla Y., et al. Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage [published correction appears in Int J Nanomedicine 2019;14:7001–7002] Int J Nanomedicine. 2013;8:1451–1462. doi: 10.2147/IJN.S26364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Liu Z., Wang L., Cun D., Tong H.H.Y., Yan R., et al. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J Control Release. 2017;254:44–54. doi: 10.1016/j.jconrel.2017.03.385. [DOI] [PubMed] [Google Scholar]
- Tang S.C., Liao P.Y., Hung S.J., Ge J.S., Chen S.M., Lai J.C., et al. Topical application of glycolic acid suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation by modulating NF-κB signaling pathway in keratinocytes and mice skin. J Dermatol Sci. 2017;86:238–248. doi: 10.1016/j.jdermsci.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Teixeira A., Teixeira M., Almeida V., Gaio R., Torres T., Magina S., et al. Does the vehicle matter? Real-world evidence on adherence to topical treatment in psoriasis. Pharmaceutics. 2021;13:1539. doi: 10.3390/pharmaceutics13101539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiefenthaler M., Hofer S., Ebner S., Ivarsson L., Neyer S., Herold M., et al. In vitro treatment of dendritic cells with tacrolimus: impaired T-cell activation and IP-10 expression. Nephrol Dial Transplant. 2004;19:553–560. doi: 10.1093/ndt/gfg594. [DOI] [PubMed] [Google Scholar]
- van der Fits L., Mourits S., Voerman J.S.A., Kant M., Boon L., Laman J.D., et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- Xiao C., Zhu Z., Sun S., Gao J., Fu M., Liu Y., et al. Activation of Langerhans cells promotes the inflammation in imiquimod-induced psoriasis-like dermatitis. J Dermatol Sci. 2017;85:170–177. doi: 10.1016/j.jdermsci.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Xu G., Jiang J., Wang M., Li L., Su J., Ren X. Lactic acid reduces LPS-induced TNF-α and IL-6 mRNA levels through decreasing IKBα phosphorylation. J Integr Agric. 2013;12:1073–1078. [Google Scholar]
- Yan B.X., Chen X.Y., Wang Z.Y., Cui Y.Z., Landeck L., Fu N.C., et al. Mupirocin blocks imiquimod-induced psoriasis-like skin lesion by inhibiting epidermal isoleucyl-tRNA synthetase. Cell Commun Signal. 2022;20:185. doi: 10.1186/s12964-022-00995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef D.A., Miller C.W., El-Abbassi A.M., Cutchins D.C., Cutchins C., Grant W.B., et al. Antimicrobial implications of vitamin D. Dermatoendocrinol. 2011;3:220–229. doi: 10.4161/derm.3.4.15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Gao J., Zhu Q., Zhang M., Ding X., Wang X., et al. Penetration and distribution of PLGA nanoparticles in the human skin treated with microneedles. Int J Pharm. 2010;402:205–212. doi: 10.1016/j.ijpharm.2010.09.037. [DOI] [PubMed] [Google Scholar]
- Zschocke I., Mrowietz U., Lotzin A., Karakasili E., Reich K. Assessing adherence factors in patients under topical treatment: development of the Topical Therapy Adherence Questionnaire (TTAQ) Arch Dermatol Res. 2014;306:287–297. doi: 10.1007/s00403-014-1446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Detailed data are available upon request from LM at laurence.michel@inserm.fr.