Abstract
Breast cancer is the leading cause of cancer mortality in women, and it is on the rise in Iran. Therefore, an early-stage diagnosis of breast cancer is of critical importance. Because ultrasound is one of the available, inexpensive, and minimally invasive techniques for distinguishing malignant from benign masses, a comparison of conventional ultrasound, color Doppler, and spectral Doppler findings can be useful. The purpose of this study was to determine the diagnostic value of sonographic indices, specifically Doppler parameters, in identifying the nature of breast masses. This is a cross-sectional study, with diagnostic value analysis. Before undergoing a biopsy, 80 patients with breast masses underwent B-mode and Doppler breast ultrasound. The ultrasound findings were then compared to pathologic results to determine which groups were malignant or benign. The resulting data were analyzed using statistical tests and diagnostic values with SPSS 22 software. B-mode grey-scale ultrasound indices such as mass shape, mass margin, mass orientation, and posterior features, as well as Doppler indices such as vascularity, RI (Resistive Index), PI (Pulsatility Index), and PSV (Peak Systolic Velocity), were found to be statistically significant with pathological findings. Color Doppler revealed vascularity in 65% of benign and 84% of malignant masses. The diagnostic value results revealed that mass shape, mass margin, mass orientation, and posterior features all play a significant role in predicting lesion malignancy, with a sensitivity of 92%, 58%, 64%, 56%, and specificity of 59%, 66%, 82%, and 84%, respectively. The RI, PI, and PSV indices were significantly higher in malignant masses, and all of them had remarkable diagnostic values in predicting malignancy, with a (Area Under The Curve) AUC of 0.863, 0.882, 0.702, a sensitivity of 84% and 84%, 68%, and a specificity of 83%, 86%, and 62%, respectively, at the optimal cut-off points (0.65, 1.32, 12.40) obtained from the Receiver Operating Characteristics (ROC) curves.
Key Words: breast mass, ultrasound, color Doppler, spectral Doppler, malignancy
Breast cancer is the second most common malignancy in women after lung cancer, and has the highest mortality rate among all women’s cancers.1 The incidence of breast cancer in Iran has become increasingly more common in recent years. Well-established risk factors include family and personal history, obesity, diet, and alcohol, as well as hormonal and sexual factors.2 Studies demonstrated the mean age of presentation to be 55 years in Western countries and 10 years earlier (45 years) in Iran.3
Difficulties in the diagnostic process of cancer and its psychological burden lead to a substantial delay in the first medical visit, resulting in an initial diagnosis at advanced stages.4 Ultrasonography is a non-invasive, less expensive, and widely available modality that could be utilized for a fast, easy, and yet reliable diagnosis. Adding sonography to mammography examination in patients with highly dense breasts increases the sensitivity and specificity of mass detection from 50% to 77.5%.5
Tissue vascularity depends on its metabolic activity.
Most malignant tumors have high metabolic demands in contrast to benign lesions and cause neovascularization.6 The use of color and spectral Doppler ultrasound in the description of breast lesions is becoming more and more popular in recent years. Resistive Index (RI) has been the most popular variant in studies. RI, Pulsatility Index (PI), Peak Systolic Velocity (PSV), and pattern of vascularity distribution (peripheral dominant or central dominant) all could play a role in the differentiation between benign and malignant lesions. In general, more penetrating central vessels and higher quantities of RI, PI, and PSV are considered malignant factors. However, the overlaps with non-malignant lesions should be kept in mind.
The advent of new sonographic techniques like color and spectral Doppler has inspired several studies on improving the sensitivity and specificity of Ultrasound.7,8 Results of studies on color Doppler ultrasound show a diagnostic overlap between neoplasms and highly vascular benign lesions,9 thus the differentiation between benign and malignant masses, only based on Doppler findings, is not recommended. However, the application of both B-mode and Doppler simultaneously increases the sensitivity and specificity of malignant tumor characterization. Currently, color and spectral Doppler parameters are not part of routine ultrasound examinations due to the controversial results in the limited number of studies in the field. Several studies have assessed the advantages of color Doppler, power Doppler, spectral Doppler, and vascular distribution to distinguish malignant tumors from benign lesions, and they have published various results.
As treatment of advanced breast cancers is currently a radical mastectomy and the patient is normally exposed to unavoidable physical and mental complications and surgical risks, studies to confirm the high sensitivity and specificity of Doppler ultrasound in a timely and more reliable diagnosis of breast cancer seem to be of crucial importance.
Material and Methods
Approval
The study was approved by the "institutional review board" and "research ethics committee". Informed consent was obtained from all patients verbally.
Study design and population
In this diagnostic value and cross-sectional study, the target population consisted of 80 women with solid breast masses who were candidates for Core-needle biopsy in Shahid Akbarabadi and Firoozgar Hospital in Tehran, from January to December 2020.
Patients with solid breast mass that is indicated for biopsy were included in this study.
Patients with a cystic lesion, those who had received prior neoadjuvant chemotherapy or surgery, or the ones with a previous pathologic study as well as those without consent for biopsy or joining the study, were excluded from our target group.
Data recording
Ultrasound examination was performed by expert radiologists using a Philips ultrasound device with a linear high-frequency probe. A targeted biopsy was done by the interventionist. Pathologic study as the gold standard diagnostic tool was performed by an expert pathologist. Morphology of each breast mass in B-mode gray-scale sonography, considering ACR-BIRADS was evaluated. Doppler sonography features of the lesion, such as vascularity, vascular pattern, RI, PI, PSV, and AT criteria measurements were also included. Sonographic results were compared to the final pathologic results (gold standard).
Statistical analysis
For statistical analysis, the software SPSS version 22.0 was used. Categorical variables were compared, using the Chi-square test. P-values of 0.05 or less were considered statistically significant. ROC (Receiver Operating Characteristic) curves were used to determine the optimal cut-off points to predict malignant breast masse with the highest specificity and sensitivity.
Results
80 women, aged 13 to 77, were included in this study. Among these patients, 44 patients (55%) had benign pathology and 36 patients (45%) showed malignant histology. The frequency of B-mode and Doppler parameters based on pathologic reports were gathered in Table 1. The statistical correlation between B-mode, color, and spectral Doppler parameters with pathologic results was also delineated in Table 1.
There was a significant relationship between grey scale B-mode parameters including mass shape, mass margin, mass orientation, and posterior features with pathologic results (p value<0.05).
The relationship between the lesion vascularity and the pathologic nature of masses was statistically significant in the 95% confidence interval (p value<0.05). Twenty masses were avascular and 60 masses were vascular in color Doppler evaluation, among 20 completely avascular lesions, 75% were pathologically benign and only 5 masses were malignant.
The relationship between vascularity type and pathology was not significant. However, it should be noted that only three patients had central vascularity, and all of them were malignant. However, this quantity could not be evaluated statistically.
Pulsatility Index, Resistive Index, and Peak systolic velocity were significantly higher in malignant tumors compared to benign lesions. However, the relationship between Acceleration Time and pathology was not significant.
The mean value and the Standard Deviation (SD) of the spectral Doppler parameters are shown in Table 2, based on the pathologic nature of the mass. Resistive Index (RI) averages were 0.60 for benign lesions and 0.74 for malignant lesions. The mean value of PI was 1.05 and 1.59 in benign and malignant lesions, respectively. Mean PSV values for benign and malignant lesions were 12.79 and 20.7, respectively. The average value of Acceleration Time (AT) was measured to be 75.45 for malignant lesions and 57.90 for benign ones.
To find out the diagnostic value of spectral Doppler ultrasound in distinguishing between malignant and benign lesions, we found that spectral indices, including PSV (AUC=0.702), RI (AUC=0.863), and PI (AUC=0.882) were capable of differentiating malignant from benign lesions. The optimal cut-off points for RI, PI, and PSV were 0.65 (sensitivity=84%, and specificity= 83%), 1.32 (sensitivity=84%, and specificity=86%), 12.4 (sensitivity=68%, and specificity=62%), respectively (Figure 1, Table 3).
Table 4 summarizes the area under the curve (AUC), sensitivity, and specificity for B-mode and color-Doppler indices. The B-mode variables have an acceptable ability to differentiate between benign and malignant breast masses. Based on the results, the diagnostic value of them was statically significant (p value <0.05).
Discussion
In our study, the mean age of patients was 43.03±12.10. The youngest was 13 years old and the oldest was 77 years old.
Among the indicators related to B-mode ultrasound, the variables of mass shape, mass margin, mass orientation, and posterior features were significantly related to the pathology results in the Chi-square test. According to the results of Table 1, Oval and round mass shapes were seen more frequently in benign masses and irregular shapes were more common in malignant masses. The mass margin including circumscribed and micro lobulated were found mostly in benign masses and speculated in malignant masses. The parallel orientation was in favor of benign lesions. Posterior shadowing was seen with higher frequency in malignant masses.
Table 1.
Evaluation of relationship between B-mode, color and spectral Doppler parameters with pathology results.
| Variable | Type | Pathology | P | |
|---|---|---|---|---|
| Benign | Malignant | value | ||
| (%) | (%) | |||
| Mass shape | Oval | 28.8 | 3.8 | 0.00 |
| Round | 3.7 | 0.0 | ||
| Irregular | 22.5 | 41.2 | ||
| Mass margin | Circumscribed | 16.2 | 1.2 | 0.00 |
| Indistinct | 10.0 | 7.5 | ||
| Angular | 10.0 | 10.0 | ||
| Micro lobulated | 16.2 | 7.5 | ||
| Speculated | 2.5 | 18.8 | ||
| Mass orientation | Parallel | 45.0 | 16.2 | 0.00 |
| Not parallel | 10.0 | 28.8 | ||
| Posterior features | No features | 46.3 | 20.0 | 0.00 |
| Shadowing | 3.7 | 20.0 | ||
| Enhancement | 5.0 | 5.0 | ||
| Vascularity | Present | 36.2 | 38.8 | 0.03 |
| Absent | 18.7 | 6.3 | ||
| Vascularity pattern | Peripheral | 21.6 | 20 | 0.22 |
| Central | 0.0 | 5.0 | ||
| Combined | 26.7 | 26.7 | ||
| PI | Lower than 1.3 | 36.7 | 8.3 | 0.00 |
| 1.3 and higher | 11.7 | 43.3 | ||
| RI | Lower than 0.65 | 36.7 | 8.3 | 0.00 |
| 0.65 and higher | 11.7 | 43.3 | ||
| PSV (cm/s) | Lower than 12.4 | 30.0 | 16.7 | 0.02 |
| 12.4 and higher | 18.3 | 35 | ||
| AT (ms) | Lower than 70 | 30.0 | 23.3 | 0.18 |
| 70 and higher | 18.3 | 28.4 | ||
Mass shape variable had a high sensitivity (92%) in predicting malignant lesions. As a result, oval and round masses are more likely to rule out malignancy. Also, the variables of mass orientation and posterior features had high specificity of 82% and 84%, respectively, which indicates that the masses are not parallel and have posterior shadowing increasing the probability of malignancy. In Keshavarz’s study, cystic solid masses, not circumscribed margin and mixed echogenicity were significantly higher in malignant masses.10 Also in the study of Shobeiri et al., 6.5% of benign masses and 93.5% of malignant masses had posterior shadowing.11 In our study, 15.8% of benign masses and 84.2% of malignant masses had this feature, respectively. In their study, like us, posterior shadowing had a high specificity (93%). In their study, unlike our study, mass margin had high sensitivity and specificity (89% and 93%, respectively). Ibrahim also stated in his study that mass margin and posterior features are important parameters in predicting malignancy.12 In this study, we utilized Doppler ultrasound abilities to distinguish malignant tumors from benign lesions. 80 patients with 80 breast masses were examined and 60 masses had detectable vascularity. We found that malignant masses (86%) show vascularity more than benign lesions (64%), however, the low specificity (34%) and the high sensitivity (86%) of this variant in our study, are compatible with a low probability of malignancy in avascular lesions. Our results showed a statistically significant difference between malignant and benign lesions vascularity with pathologic nature of masses which is consistent with previous studies, i.e., Badau et al. (malignant=89%, benign=56%),13 Sevensson et al. (malignant=95%, benign=46%),9 and McNicholas et al. (malignant=87%, benign=68%).14 However, specificity and sensitivity for vascularity detection in malignant masses in studies conducted by Shobeiri et al. (spe:87%, sen:63%),11 Del Cura et al. (spe:64%, sen:68%)15 and Ahmadinejad et al. (spe:90%, sen:68%)16 are not consistent with our results.
While many researchers have tried to differentiate malignant lesions from benign ones by using blood flow in the breast mass, the results of these studies seem to be controversial. Several studies have shown that the color Doppler signal is not always a malignant feature. In general, vascularization increases in high-grade malignant tumors and high-cellular benign lesions, because low-grade malignant tumors may not have detectable vascularity.9,17
Table 2.
Comparison of mean and standard deviation of spectral Doppler indices according to pathology
| Pathology | RI | PI | PSV | AT |
|---|---|---|---|---|
| Benign | 0.60± | 1.05± | 12.79± | 57.90± |
| 0.08 | 0.29 | 10.43 | 33.22 | |
| Malignant | 0.74± | 1.59± | 20.7± | 75.45± |
| 0.09 | 0.41 | 14.10 | 45.41 | |
| Overall | 0.67± | 1.33± | 16.88± | 66.97± |
| 0.11 | 0.45 | 13.00 | 40.63 |
Table 3.
Optimal cut-off points of spectral Doppler indices and the results of spectral Doppler ultrasound diagnostic value.
| Variable | AUC | Specificity (%) | Sensitivity (%) | Cut-off point | P value |
|---|---|---|---|---|---|
| RI | 0.863 | 83 | 84 | 0.65 | 0.00 |
| PI | 0.882 | 86 | 84 | 1.32 | 0.00 |
| PSV | 0.702 | 62 | 68 | 12.4 | 0.00 |
| AT | 0.602 | 65 | 55 | 70 | 0.07 |
Ahmadinejad et al. also states that with the increasing sensitivity of new ultrasound devices, the presence or absence of blood flow is not enough to differentiate breast lesions, and we must use other indicators, such as quantitative Doppler spectral variables and B mode findings.16 In our experience, excessive pressure of the probe during ultrasound examinations on the breast tissue, especially in superficial masses, could affect the detection of vascularity of the mass.
The patterns of vascularity (including peripheral, central, and combined) were not significantly correlated with pathologic results in our statistical analysis. Although the central pattern was seen in only three masses, all of which were malignant, this number is not statistically valuable. Peripheral and combined patterns were also seen almost equally in malignant and benign masses. These findings were inconsistent with previous studies including Keshavarz et al., Kwak et al., Sevensson et al., Ibrahim et al., and Reza et al.,9,10,18-20 in which peripheral patterns of vasculature favored benign masses and central prominent vasculature and penetrating vessels were a predictor of malignancy.
Figure 1.
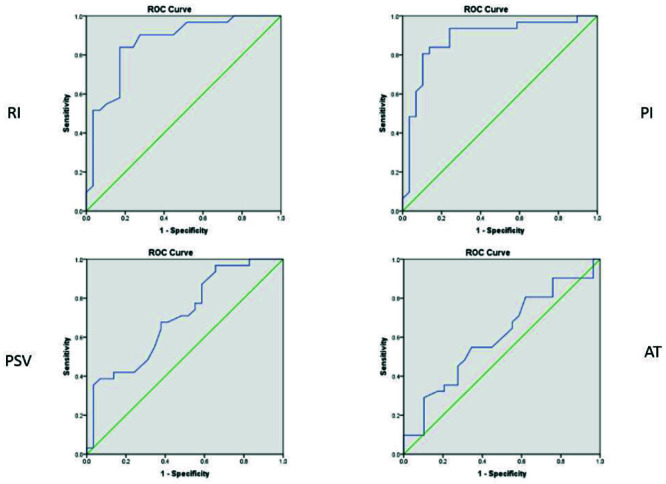
ROC curves of RI, PI, PSV, AT variables.
Table 4.
Results of diagnostic value of B-mode ultrasound and color Doppler to determine lesion malignancy.
| Variable | AUC | Specificity (%) | Sensitivity (%) | P value |
|---|---|---|---|---|
| Vascularity | 0.602 | 34 | 86 | 0.12 |
| Vascularity pattern | 0.504 | 55 | 61 | 0.95 |
| Mass shape | 0.754 | 59 | 92 | 0.00 |
| Mass margin | 0.736 | 66 | 58 | 0.00 |
| Mass orientation | 0.721 | 82 | 64 | 0.00 |
| Posterior features | 0.715 | 84 | 56 | 0.00 |
In the present study, the mean values of RI, PI, PSV, and AT in benign masses were 0.60, 1.05, 12.79, and 57.90, respectively, while in malignant masses, they were 0.74, 1.59, 20.70, and 75.45, respectively. The mean values of all the above-mentioned indices were much higher in malignant masses. This difference was significant for RI, PI, and PSV indices. Spectral values are comparable to several previous studies. In Shobeiri et al, the mean RI and PSV in benign masses were 0.63 and 4.66, respectively, while in malignant masses, they were 0.99 and 11.22, respectively.11 The mean RI in the Davoudi and Sarkar study was 0.65 and 0.68 in benign and 0.71 and 0.82 in malignant masses, respectively.21,22 Keshavarz et al. in their study expressed the mean of RI, PI, and PSV in benign masses to be 0.63, 0.90, and 11.69, while in malignant masses, they were 0.73, 1.09, and 18.9, respectively.10 In all studies similar to ours, the above variables were remarkably associated with pathology. Although the relationship of the AT index is not significant, as mentioned, its mean value was higher in malignant masses. Mesaki and colleagues investigated the role of AT in this subject and found out that it was significantly lower in benign lesions with a threshold of 14 ms.23 However, in our study, no noteworthy relationship was found.
In evaluating the diagnostic value of spectral Doppler ultrasound, the sensitivity and the specificity of RI, PI, PSV and AT indices in optimal cut-off points are (spe=83% - sen=84%), (spe=86% - sen=84%) =), (spe=62%- sen=68%) and (spe =65% -sen =55%). The Area Under the Curve (AUC) was 0.863, 0.882, 0.702, and 0.602, respectively, indicating the high and significant diagnostic values of RI, PI, and PSV in predicting breast mass malignancy. In the Keshvarz study, the optimal cutoff points for RI, PI, and PSV were 0.68, 0.93, and 12.5, respectively. Sensitivity, specificity, and AUC of the above three indicators were also remarkable in his study.10 In the Shobeiri study, the cut-off points for RI, PSV, and EDV were 0.7, 20, and 3, respectively. The sensitivity and the specificity of RI in their study were both 100%. PSV sensitivity was 21% and specificity was 100%.11 In the Del Cura study, RI greater than 1 and PI greater than 4 were both 99% sensitive with less than 15% specificity in favor of malignancy, regardless of other ultrasound grayscale findings.15 In other studies, Stanzani (RI greater than 0.73), Lee (RI greater than 0.78), and Fux (RI greater than 0.70)24-26 were predictors of malignancy. In Choi’s study, RIs above 0.70 with sensitivity and specificity of 80.9% and 89.1% were in favor of malignancy, respectively.27 In the Parveen study, RI criteria above 0.70 had sensitivity and specificity of 92.4% and 88.7%, respectively.28 In Schmillevitch’s study, RI above 0.69 had a sensitivity of 84% and a specificity of 88% in predicting malignancy.29 The difference between cut-off points and sensitivity and specificity in different studies can be due to the type of tumor behavior, grade, and subtype of malignancy based on pathology, which can affect the pattern of vascularity.21 Further studies can be performed by pathologic subtypes and grade. Also, studies with a higher statistical population can help improve the results.
Conclusions
This study was performed to confirm the high sensitivity and specificity of Doppler ultrasound indices, To prevent the inevitable complications of breast cancer with a quick and timely diagnosis. Doppler ultrasound will also increase the reliability of patients’ follow-ups and reduce the number of preoperative biopsies. Although the standard method for differentiating benign and malignant breast lesions is an excisional biopsy, less invasive diagnostic methods are necessary to reduce the number of biopsies reduce patient anxiety, and lower costs.
List of abbreviations
- RI
Resistive Index
- PI
Pulsatility Index
- PSV
Peak Systolic Velocity
- EDV
End Diastolic Velocity
- AT
Acceleration Time
- sen
sensitivity
- spe
specificity
- US
Ultrasound
- AUC
Area Under The Curve
- ROC
Receiver Operating Characteristics
Footnotes
Conflict of interest
The authors declare no potential conflict of interest, and all authors confirm accuracy.
Contributor Information
Bahareh Mehdikhani, Email: bahare.mehdikhani@gmail.com.
Milad Benam, Email: miladbenam.md@gmail.com.
Afrooz Moradkhani, Email: afroozmoradkhani@gmail.com.
Ayda Roostaee, Email: ayda152001@yahoo.com.
Seyedeh Sabahat Bahman, Email: sabahat88.b@gmail.com.
Pooyan Barmayoon, Email: pooyan.b808@gmail.com.
Ghazaleh Dezyani, Email: dezyanighazal@gmail.com.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
- 1.Wolman I. Berek and Novak’s Gynecology 15th Edition: Lippincott Williams and Wilkins, 2012. [Google Scholar]
- 2.Wagner HN, Szabo Z, Buchanan JW. Principles of nuclear medicine. Pennsylvania: W.B. Saunders,. 1995, p 235. [Google Scholar]
- 3.Yavari P, Mosavizadeh M, Sadrol-Hefazi B, Mehrabi Y. Reproductive characteristics and the risk of breast cancer--a case-control study in Iran. Asian Pac J Cancer Prev 2005;6:370-5. [PubMed] [Google Scholar]
- 4.Sandler MP, Patton JA, Gottschalk A. Diagnostic nuclear medicine. Pennsylvania: Williams & Wilkins, 1996. [Google Scholar]
- 5.Ozdemir A, Ozdemir H, Maral I, et al. Differential diagnosis of solid breast lesions: contribution of Doppler studies to mammography and gray scale imaging. J. Ultrasound Med 2001;20:1091–101. [DOI] [PubMed] [Google Scholar]
- 6.Hashmi A, Ackerman S, Irshad A. Color Doppler sonography: characterizing breast lesions. Imaging Med 2010;2:151–63. [Google Scholar]
- 7.William EB. Fundamental of Diagnostic Radiology. 4th ed. Philadelphia: Williams & Wilkins,. 2012; pp 535-7. [Google Scholar]
- 8.Rumack CM, Wilson SR, Charboneau JW. Diagnostic ultrasound. 3th ed Philadelphia,. 2011; pp 773-86. [Google Scholar]
- 9.Svensson WE, Pandian AJ, Hashimoto H. The use of breast ultrasound color Doppler vascular pattern morphology improves diagnostic sensitivity with minimal change in specificity. Ultraschall Med 2010;31:466–74. [DOI] [PubMed] [Google Scholar]
- 10.Keshavarz E, Zare Mehrjardi M, Karimi M A, et al. Diagnostic value of spectral doppler ultrasound in detecting breast malignancies: an original article. Int J Cancer Manag 2018;11:e8200. [Google Scholar]
- 11.Shobeiri E, Panahi F. Comparison of the diagnostic value of the gray scale and color Doppler ultrasonography in the diagnosis of solid breast masses. SJKU 2014;19:77-83. [Google Scholar]
- 12.Bassett LW, Ysrael M, Gold RH, Ysrael C. Usefulness of mammography and sonography in women less than 35 years of age. Radiology 1991;180:831-5. [DOI] [PubMed] [Google Scholar]
- 13.Buadu L, Murakami J, Murayama S, et al. Colour Doppler sonography of breast masses: a multiparameter analysis. Clin. Radiol 1997;52:917–23. [DOI] [PubMed] [Google Scholar]
- 14.McNicholas MM, Mercer PM, Miller JC, et al. Color Doppler sonography in the evaluation of palpable breast masses. AJR Am J Roentgenol 1993;161:765-71. [DOI] [PubMed] [Google Scholar]
- 15.Del Cura JL, Elizagaray E, Zabala R, et al. The use of unenhanced Doppler sonography in the evaluation of solid breast lesions. Am J Roentgenol 2005;184: 1788–94. [DOI] [PubMed] [Google Scholar]
- 16.AhmadiNejad P, Shahriaran S, GhasemiPhiroozabadi A, Giti M. Evaluation of solid breast lesions with color doppler sonography and power doppler imaging. Tehran University Medical Journal 2002;60:277-82. [Google Scholar]
- 17.Busilacchi P, Draghi F, Preda L, Ferranti C. Has color Doppler a role in the evaluation of mammary lesions? J Ultrasound 2012;15:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwak JY, Kim EK, Kim MJ, et al. Power Doppler sonography: evaluation of solid breast lesions and correlation with lymph node metastasis. Clin Imaging 2008;32:167–71. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim R, Rahmat K, Fadzli F, et al. Evaluation of solid breast lesions with power Doppler: value of penetrating vessels as a predictor of malignancy. Singapore Med J 2016;57:634-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raza S, Baum J. Solid breast lesions: evaluation with power Doppler US. Radiology 1997;203:164. [DOI] [PubMed] [Google Scholar]
- 21.Davoudi Y, Borhani B, Rad M, Matin M. The role of Doppler sonography in distinguishing malignant from benign breast lesions. J Med Ultrasound 2014;22:92-5. [Google Scholar]
- 22.Sarkar SS. To determine role of colour doppler ultrasound in breast lesions. J Adv Med Dent Scie Res 2020;8:203-6. [Google Scholar]
- 23.Mesaki K, Hisa N, Kubota K, et al. Differentiation of benign and malignant breast tumors using Doppler spectral parameters including acceleration time index. Oncol Rep 2003;10:945-50. [PubMed] [Google Scholar]
- 24.Lee WJ, Chu JS, Houng SJ, et al. Breast cancer angiogenesis: a quantitative morphologic and Doppler imaging study. Ann Surg Oncol 1995;2:246–51. [DOI] [PubMed] [Google Scholar]
- 25.Stanzani D, Chala LF, Barros N, et al. Can Doppler or contrast-enhanced ultrasound analysis add diagnostically important information about the nature of breast lesions? Clinics (Sao Paulo) 2014; 69:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu X, Lu K, Zhang J, et al. Significance of resistance index and pulsatility index in differential diagnosis of breast neoplasm. Zhongguo Yi XueKeXue Yuan XueBao 1998;20:454-8. [PubMed] [Google Scholar]
- 27.Choi H Y, Kim HY, Baek SY, et al. Significance of resistive index in color Doppler ultrasonogram: differentiation between benign and malignant breast masses. Clin Imaging 1999;23:284-8. [DOI] [PubMed] [Google Scholar]
- 28.Parveen I, Javed K, Elahi B, et al. Evaluation of breast lesions with Doppler ultrasound: Diagnostic accuracy of resistive index as a predictor of malignancy. Professional Med J 2020;27:825-30. [Google Scholar]
- 29.Schmillevitch J, Guimara˜es Filho He´lio A, De Nicola H, et al. Utilization of vascular resistance index in the differentiation between benign and malignant breast nodules. Radiol Bras 2009;42: 241e4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


