Abstract
Variations in the polyomavirus major capsid protein VP1 underlie important biological differences between highly pathogenic large-plaque and relatively nonpathogenic small-plaque strains. These polymorphisms constitute major determinants of virus spread in mice and also dictate previously recognized strain differences in sialyloligosaccharide binding. X-ray crystallographic studies have shown that these determinants affect binding to the sialic acids. Here we report results of further experiments designed to test the importance of specific contacts between VP1 and the carbohydrate moieties of the receptor. With minor exceptions, substitutions at positions predicted from crystallography to be important in binding the terminal α-2,3-linked sialic acid or the penultimate sugar (galactose) destroyed the ability of the virus to replicate in cell culture. Substitutions that prevented binding to a branched disialyloligosaccharide were found to result in viruses that were both viable in culture and tumorigenic in the mouse. Conversely, substitutions that allowed recognition and binding of the branched carbohydrate chain inhibited spread in the mouse, though the viruses remained viable in culture. Mice of five different inbred strains, all highly susceptible to large-plaque virus, showed resistance to the spread of polyomavirus strains bearing the VP1 type which binds the branched-chain receptor. We suggest that glycoproteins bearing the appropriate O-linked branched sialyloligosaccharide chains are effective pseudoreceptors in the host and that they block the spread of potentially tumorigenic or virulent virus strains.
Wild-type laboratory strains of polyomavirus are all potent transforming agents in culture, but only some are able to induce the high frequency and broad array of tumors in mice (12) typical of early virus isolates (14, 21). Previous studies with recombinants between a strain producing tumors at a high frequency and a strain producing tumors at a low frequency indicated that a single-site polymorphism in VP1 was a major determinant of tumorigenicity (15), although differences in noncoding sequences between the two strains also had effects (16, 18). Similarly, comparisons between a virulent strain that causes a rapidly lethal infection of newborn mice and its parental strain, which induces tumors at a high frequency, showed a single amino acid difference in VP1 to be the major determinant of virulence (2). Table 1 summarizes the findings with respect to critical amino acid substitutions in the VP1s of three prototype wild-type strains of widely different pathogenicities. It is significant that the important biological determinants at positions 91 and 296 map to regions of VP1 that form parts of the oligosaccharide binding site on the virus surface (32–34).
TABLE 1.
Properties of prototype wild-type strains of polyomavirus
| Virus strain (plaque type) | Pathogenicity | Sialic acid recognitiona
|
VP1 sequence at position:
|
||
|---|---|---|---|---|---|
| Straight chain | Branched chain | 91 | 296 | ||
| RA (small) | Induces few or no tumors | + | + | Gly | Val |
| PTA (large) | Highly tumorigenic | + | − | Glu | Val |
| LID (large) | Virulent | + | − | Glu | Ala |
Straight chain, NeuNAc-(α-2,3)-Gal-(β-1,3)-GalNAc or -GlcNAc-; branched chain, NeuNAc-(α-2,3)-Gal-(β-1,3 or β-1,4)-[(NeuAc-(α-2,6)]-GlcNAc-.
Differences in selectivity and avidity of binding to sialyloligosaccharides dictated by VP1 clearly correlate with pathogenicity. The directions of these correlations are surprising and have interesting implications, in two respects. First, although small-plaque virus strains have broader binding specificities, recognizing branched- as well as straight-chain sialyloligosaccharides (6, 7, 19), they are far less pathogenic than large-plaque strains, which bind only the straight chain (12, 15, 18). The operative change is at position 91 (17). The presence of glutamic acid at this position prevents binding to the branched-chain oligosaccharide (through electrostatic repulsion with the α-2,6-linked sialic acid), while glycine passively accommodates the branched carbohydrate chain (32, 33). These results imply the existence in the mouse of virus inhibitors or pseudoreceptors with branched-chain sialyloligosaccharides. Second, strain PTA disseminates broadly and induces multiple tumors, while its derivative strain LID (26, 31) spreads even more rapidly and kills newborn mice before tumors can develop (2, 4). The important difference between these two large-plaque strains is at position 296, where valine in PTA is replaced by alanine in LID. This seemingly conservative substitution results in the loss of a hydrophobic contact between VP1 and the terminal α-2,3-linked sialic acid. These results imply that weaker binding by the virus to its true receptor translates into a more virulent phenotype (2).
In this study, we first report unsuccessful attempts to identify a unique polyomavirus receptor through the isolation of monoclonal antibodies and then turn to analyzing the recognition of the carbohydrate moiety of the receptor by VP1 as the key to understanding the importance of virus-receptor interactions in pathogenesis. Site-directed mutagenesis is used to introduce substitutions into the VP1s of different wild-type strains, guided by results of X-ray crystallography (32–34). Differences in noncoding sequences among wild-type strains are common, and these can have significant effects on the extent and tissue specificity of virus replication (1, 9, 28–30) as well as on tumor induction (16, 18). By comparing site-directed mutants with parental strains, the effects of VP1 mutations can be assessed in the absence of differential contributions from viral enhancers. Mutations affecting all sites predicted from structural studies to be involved in oligosaccharide binding have been studied. The results show that proper recognition of the terminal sialic acid as well as of the penultimate galactose are critical for viability of both large- and small-plaque strains. Substitutions that allow recognition of the branched disialyloligosaccharide are viable in culture but lead to restricted spread and reduced pathogenicity in the mouse. These results confirm and extend earlier findings on the structural basis for selectivity in sialic acid binding by the virus, the importance of receptor affinity and selectivity, and the likely role of branched-chain sialyloligosaccharides as pseudoreceptors capable of inhibiting virus spread in the mouse.
MATERIALS AND METHODS
Screening for monoclonal antibodies to polyomavirus receptors.
Hamster anti-mouse cell hybridomas were prepared through Monoclonal Core Facility of Program Project grant PO1-CA50661 (D. Livingston, principal investigator; J. DeCaprio, core director). Golden Armenian hamsters were immunized by three intraperitoneal inoculations of 2 × 106 to 5 × 106 NIH 3T3 or primary baby mouse kidney epithelial cells 1 month apart. Animals were sacrificed, and spleen cells were harvested and fused with NS-1 cells 1 week after the third inoculation. Hybridoma supernatants were screened for their ability to block infection of NIH 3T3 cells by a cytopathic effect (CPE)-protection assay. NIH 3T3 cells were grown in 96-well plates to ∼50% confluence; cells were preincubated at 37°C for 30 min in medium containing 50 μl of supernatant. Wild-type (PTA) virus was then added at a multiplicity of 2 to 5 PFU/cell, and the cultures were monitored for development of CPE over a 5- to 6-day period.
Virus strains.
Genomic clones of viral DNAs of the large-plaque strains PTA (15, 18) and LID (2) and the small-plaque strain RA (15, 18) were used to generate VP1 mutants by oligonucleotide mutagenesis. The EcoRI-BamHI large (3.2-kb) fragments encoding all of VP1 were subcloned into pUC19 (Gibco BRL, Gaithersburg, Md.), and the desired mutations were introduced with a Transformer site-directed mutagenesis kit (Clontech, Palo Alto, Calif.). In most cases, silent mutations were introduced along with the desired mutation to allow discrimination between possible reversion and contamination. Whole virus genomes were reconstituted by ligation of the large fragment confirmed to carry the desired mutations with the PTA wild-type EcoRI-BamHI small (2.1-kb) fragment. The ligation mixtures were transfected into NIH 3T3 cells by electroporation (2 × 105 to 5 × 105 cells in 400 μl; 260 V, 1,050 μF). Transfected cells were plated in Dulbecco’s modified Eagle’s medium with 10% calf serum, and the cultures were monitored as described in Results. Viable mutants were further propagated on primary baby mouse kidney epithelial cells or NIH 3T3 cells. Plaque assays were performed on NIH 3T3 cells with PTA and RA controls to discern the large- versus the small-plaque phenotype as described previously (17).
Mouse strains and animal experiments.
For most experiments, mice of the C3H/BiDa strain were used (obtained from Clarence Reeder, National Cancer Institute, Frederick, Md.). AKR/J, CBA/J, PERA/Ei, and Czech II/Ei mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). Newborn mice (<18 h old) were inoculated intraperitoneally with 50 μl of crude virus of known titer as indicated in Results. Animals were sacrificed at the times indicated below for whole-mouse section hybridization as described previously (13) or maintained for survival studies (2) or tumor development (12). Viral genotypes were confirmed by sequencing viral DNAs amplified from kidneys of infected mice.
RESULTS
The likelihood of occurrence of multiple sialo-glycoprotein receptors.
Cell surface glycoproteins with specific sialic acid linkages are critical determinants for hemagglutination (6, 7) and infection of host cells (19) by polyomavirus. Receptors on NIH 3T3 cells are predominantly N-linked glycoproteins (10), but it is not known whether a single or multiple receptor species exist. We have attempted to answer this question through the isolation and screening of hamster anti-mouse cell monoclonal antibodies. Hybridoma supernatants were screened by preincubation of NIH 3T3 cells followed by viral infection and monitoring for inhibition of development of CPE, a procedure similar to that used successfully in other systems to identify virus receptors (3, 11, 20). Roughly 2,000 hybridomas were screened. Some clones gave preliminary indications of causing a delayed CPE and showed cell surface staining on NIH 3T3 cells by indirect immunofluorescence; however, upon subcloning and further characterization, none proved to be clearly protective. While these negative results do not rule out a single receptor, they suggest the likelihood of multiple glycoprotein receptor species, with each bearing a terminal α-2,3-linked sialic acid(s). Such linkages are commonly found on glycoproteins with N-linked sugars expressed in a variety of cells. Four different β-galactoside α-2,3 sialyltransferases have been cloned from the mouse, with overlapping but distinguishable acceptor specificities, tissue distributions, and developmental patterns of expression (24). These observations are also consistent with the existence of multiple polyomavirus receptor species and with the broad range of cell types (∼30) that polyomavirus is known to infect (12).
Effects of mutations in the receptor binding pockets of VP1.
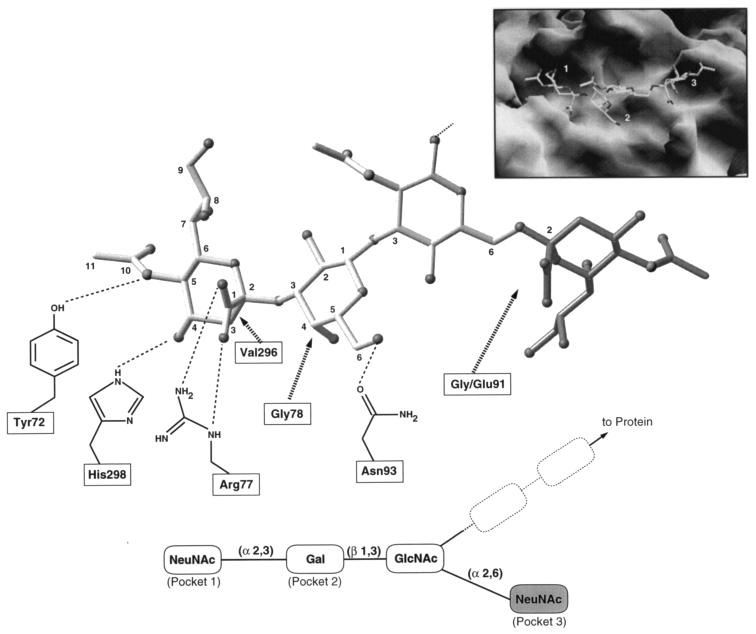
The virus surface shows three distinct pockets, which bind different moieties of sialic acid-containing oligosaccharide receptors (32, 34). Pocket 1 accommodates the terminal sialic acid, and pocket 2 accommodates the penultimate α-2,3-linked galactose. Pocket 3 accommodates the branched sialic acid, which is bound through an α-2,6 linkage to the third sugar, typically N-acetylglucosamine or N-acetylgalactosamine. A fourth pocket can also be discerned but has not been implicated thus far in binding any carbohydrate moiety. Figure 1 shows a view of the pockets on the virus surface along with a schematic representation of interactions between VP1 side chains and the sugars. The VP1 contacts are based on cocrystal structures with purified virus (resolution, 3.65 Å) or recombinant VP1 pentamers (resolution, 1.9 Å) and various sialyloligosaccharides (32–34).
FIG. 1.
Interactions between polyomavirus VP1 and sialyloligosaccharide receptors, as seen in their crystal structures (32–34). The oligosaccharide receptor is shown as a ball-and-stick model, with the straight-chain receptor being represented with light shading and the branching α-2,6-linked sialic acid being represented with darker shading. Hydrogen bonds between VP1 residues and the receptor are shown as broken lines; hydrophobic interactions are represented with arrows. The molecular surface of a polyomavirus VP1 pentamer complexed with the branched oligosaccharide is shown in the inset. The figure was prepared with RIBBONS (M. Carson, University of Alabama at Birmingham) and GRASP (B. Honig and A. Nicholls, Columbia University).
Amino acid substitutions were introduced at each position in VP1 involved in carbohydrate binding. In most instances, conservative substitutions were chosen to alter specific contacts with the sugar and to minimize the possibility of long-range effects that might interfere with VP1 folding or virus assembly. The latter possibilities seem unlikely based on the structure. All of the mutations reside in surface loops of VP1, which are exposed to solvent, and involve sites that are far from either interpentamer contacts or contacts between neighboring VP1s within a pentamer (32, 33). Complete mutant viral genome constructs were made on either an RA or a PTA background and transfected by electroporation into NIH 3T3 cells. Transfection efficiencies were between 1 and 5% based on immunofluorescent staining for T antigen. Transfections with comparable amounts of wild-type viral DNAs were carried out in parallel as positive controls. Transfected cultures were monitored for development of CPE as an indication of virus growth. CPE typically developed in wild-type-transfected cultures within the first week and was complete after 8 to 10 days. In most instances, the VP1 mutants showed little or no CPE even after 3 weeks of incubation. Cultures with or without overt CPE were harvested, and virus yields on NIH 3T3 cells were determined by plaque assay. From any mutant lysate showing plaques, several plaques were picked, the virus was amplified on NIH 3T3 or primary baby mouse kidney cells, and the viral DNA was sequenced to confirm the presence of the mutation and to rule out the possibilities of reversion and wild-type contamination. Experiments on animals were carried out with selected mutants. Table 2 summarizes the results with all the mutants tested.
TABLE 2.
Substitutions in receptor binding pockets of polyomavirus VP1
| Residue | Interaction with oligosaccharidea | Strain | Mutation | Viabilityb | |
|---|---|---|---|---|---|
| 1 | R77 | Salt bridge between R77 and carboxyl of | PTA or RA | R77E | Nonviable |
| NeuNAc-1; key interaction in attracting and orienting NeuNAc-1 | PTA or RA | R77Q | Nonviable | ||
| Y72 | Hydrogen bond linking the phenolic | PTA or RA | Y72F | Viable (+) | |
| oxygen of Y72 with amide nitrogen of NeuNAc-1 | PTA or RA | Y72A | Nonviable | ||
| H298 | Hydrogen bond to 0-4 of NeuNAc-1 | PTA or RA | H298Q | Nonviable | |
| V296 | van der Waals contact with C-3 and 0-4 | PTA | V296G | Viable (+), virulent | |
| of NeuNAc-1 | PTA | V296I | Viable (++), tumorigenic | ||
| 2 | G78 | Absence of a side chain important in accommodating the galactose | PTA or RA | G78V | Nonviable |
| N93 | Hydrogen bond with 0-4 of galactose | PTA or RA | N93A | Nonviable | |
| 3 | E91 | Electrostatic repulsion of NeuNAc-2 | PTA | E91L | Viable, tumorigenic |
Viability is scored based on kinetics and the extent of development of CPE and on the titers of virus recovered from transfected cultures. Nonviable indicates no observable CPE and no virus recovered (<20 PFU/ml). (++) indicates viability equivalent to that of wild-type virus; (+) indicates a delayed and reduced CPE and virus titers at least 1 order of magnitude less than that of the wild type. See Fig. 1 and the text.
In pocket 1, substitutions for R77 that removed the salt bridge to the negatively charged sialic acid were lethal, as expected. These results stress the critical role of this salt bridge in sugar binding. Removal of the hydrogen bond between Y72 and the N-acetyl, however, was not necessarily lethal. The Y72F mutant was viable, though it grew less well than wild-type virus. Evaluation of the structure indicates that a water molecule can substitute for the tyrosine hydroxyl group in the binding pocket. The Y72A mutant, on the other hand, was not viable. Since the Y72 side chain stacks against the R77 guanidinium group in the wild-type structure and is important for proper orientation of the R77 side chain, removal of the phenyl group is likely to affect the conformation of the crucial R77 side chain and therefore also carbohydrate binding. Although extension of the N-acetyl side chain through metabolic incorporation of synthetic analogues partially blocks infection by polyomavirus (22), the shorter and naturally occurring N-glycolyl neuraminic acid (23) was expected to interact normally with the virus. The H298Q mutant was nonviable, indicating that the hydrogen bond to the O-4 of the terminal sugar is an important interaction for binding. Although Q298 can in principle hydrogen bond to O-4, such an interaction is ruled out because to do so Q298 would have to assume a conformation that would result in unfavorable contacts with other residues.
Substitutions for V296 are viable but with different biological consequences. The V296I PTA mutant (PTA-V296I) is expected to preserve the important van der Waals contact with the sugar ring (2). This mutant induced multiple tumors in each of six mice inoculated and is thus essentially similar to PTA encoding V296. Computer modeling indicated that the I296 side chain can assume a conformation similar to that of the V296 side chain and in which the additional methyl group does not contact the sugar. PTA-V296G remained viable but grew more slowly and to lower titer than PTA in culture. However, in animals, this mutant resembles PTA-V296A and LID (encoding A296) in acquiring a virulent phenotype. The V296G substitution, introducing what is likely to be an even looser fit of the sugar with complete loss of the van der Waals contact, appears to be even more virulent than LID. None of 32 mice receiving 104 PFU of PTA-V296G survived 32 days, while in a previous study four of five mice receiving a similar dose of LID survived (2).
Two substitutions in pocket 2 (G78V and N93A) appeared to be nonviable. These results are consistent with the cocrystal structures which show a hydrogen bond between N93 and O-6 of the galactose and also indicate that any side chain at position 78 would collide with this sugar (32, 34). In pocket 3, the substitution E91L on a PTA background preserved normal viability in culture and also the essential tumorigenic behavior of PTA, with four of four mice developing multiple tumors with an average latency period of less than 70 days. Electrostatic repulsion between E91 and the carboxyl of the branched sialic acid is the likely basis for the failure of large-plaque strains to bind the branched chain (32, 34). Although the L91 substitution does not result in unfavorable contacts with the branched sugar, the presence of the hydrophobic and completely solvent-exposed leucine might trigger a more extended structural rearrangement that partially buries this side chain, thereby altering the pocket shape so that it can no longer accommodate the branched sugar.
Substitution of glycine for glutamic acid-91 in large-plaque strains reduces tumorigenicity and virulence in C3H/BiDa mice.
The expected consequence of the E→G substitution at position 91 in pathogenic large-plaque strains was to allow binding of oligosaccharide chains bearing branched-chain sialic acids of the type NeuNAc-(α-2,3)-Gal-(β-1,3)-[NeuNAc-(α-2,6)]-GlcNAc. Introduction of this mutation into the highly tumorigenic strain PTA resulted in decreased tumorigenicity (Table 3). At the highest dose tested, PTA-E91G was able to induce tumors in 100% of the animals but with a latency period twice as long as that of PTA at an equivalent dose. Moreover, when the doses of virus were reduced, the frequency of tumor induction by PTA-E91G fell to 0 while that of PTA was still 100%. Similarly, when the E91G substitution was introduced into the virulent LID strain, the virus became avirulent. Twelve of 12 newborn mice inoculated with ∼106 PFU of LID-E91G survived, in contrast to mice inoculated with LID itself, which kills 100% of recipients at the same dose or even 10-fold lower doses of virus (2, 8).
TABLE 3.
Tumor development in mice inoculated with PTA or PTA-E91Ga
| Virus dose (PFU/animal) | PTA
|
PTA-E91G
|
||
|---|---|---|---|---|
| No. of mice with tumor(s)/total no. of mice | Avg latency (days) | No. of mice with tumor(s)/total no. of mice | Avg latency (days) | |
| 2 × 105 to 5 × 105 | 9/9 | 71 | 16/16 | 153 |
| 1 × 102 to 3 × 102 | 22/22 | 92 | 2/29 | 135 |
| 1 × 101 to 3 × 101 | 20/20 | 143 | 0/39 | >180 |
Newborn C3H/BiDi mice were inoculated within 24 h of birth intraperitoneally with the virus doses indicated. The fractions of mice developing at least one tumor are given, along with the average times at which mice came to necropsy as an indication of latency. The experiment with PTA-91EG was terminated at 6 months.
The E91G substitution reduced the degrees of virus spread of large-plaque strains in newborn C3H/BiDa mice. Figure 2 shows results of whole-mouse-section hybridization in which the standard wild-type strains were compared with their substitution mutants. By 7 days, the spread of PTA was extensive and became even more extensive with the addition of the virulence determinant (PTA-V296A), similar to results with LID. The E91G substitution in each of the large-plaque viruses led to attenuation of spread to a degree similar to that of the small-plaque strain RA-encoding 91G. Virus titers in kidney homogenates were approximately 100-fold higher in 7-day-old PTA-infected mice than in PTA-E91G-infected or RA-infected mice. The E91G substitution in all three strains altered the plaque size phenotype from large to small, as expected.
FIG. 2.
Whole-mouse-section hybridization. Newborn C3H/BiDa mice were inoculated with 2 × 105 to 5 × 105 PFU of viruses of the indicated strains and sacrificed at 7 days. See Materials and Methods.
Newborn mice of different strains specifically block the spread of PTA-E91G.
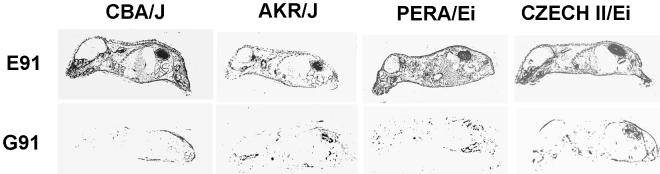
Our experiments on mice were carried out primarily with the susceptible C3H/BiDa strain. Susceptibility to tumor induction in this strain is based on its major histocompatability type and the expression of an endogenous mouse mammary tumor virus superantigen, which together prevent the development of effective T-cell responses to polyomavirus tumors (25). Three other unrelated but highly susceptible standard inbred strains (AKR/J, CBA/J, and RF/J) also carry these host determinants. Two recently wild-derived inbred strains (PERA/Ei and Czech II/Ei) are also highly susceptible to tumor induction by PTA but have genetic bases for susceptibility distinctly different from that of the standard inbred strains (unpublished data). To investigate how general the resistance to the spread of small-plaque viruses is among different mouse strains, newborn mice of four of these strains were inoculated with the large-plaque virus PTA or A2 (both 91E) or PTA-E91G and examined by whole-mouse-section hybridization (Fig. 3). The results for each strain were similar to those with C3H/BiDa mice (Fig. 2). Particularly striking is the effect of the E91G substitution in preventing high-level virus replication in the kidneys of all mouse strains tested. These observations indicate that similar or identical mechanisms operate in different host strains to discern the difference in VP1 types and to selectively block the spread of the 91G type of virus.
FIG. 3.
Whole-mouse-section hybridization with different mouse strains. Newborn mice of the indicated strains were inoculated with 2 × 105 to 5 × 105 PFU of wild-type PTA or A2, each encoding E91 (top in each pair), or PTA-E91G (bottom in each pair) and sacrificed at 10 to 12 days. See Materials and Methods.
Substitution of glutamic acid for glycine-91 in the small-plaque RA strain increases virus spread and pathogenicity.
The predicted effect of replacing glycine-91 with glutamic acid in the relatively nonpathogenic small-plaque strain RA is to block the ability of the virus to bind the branched disialyloligosaccharide. If glycoproteins bearing the branched oligosaccharide act in the intact host as pseudoreceptors, the effect of this substitution would be to increase virus spread and pathogenicity. This prediction was tested by whole-mouse-section hybridization of neonatally infected C3H/BiDa mice (Fig. 4, top row). The G91E substitution clearly led to greater virus spread, particularly in the kidney, which is the major site of amplification and also the critical target in causing death (2, 4). Titers of virus recovered from kidneys were roughly 50-fold higher in mice infected with RA-G91E than in those infected with RA, and the plaque type went from small to large in the substitution mutant.
FIG. 4.
Whole-mouse-section hybridization. Newborn C3H/BiDa mice were inoculated with the indicated virus strains. Shown in the top row (left to right) are results with RA (5 × 106 to 10 × 106 PFU after 7 days), RA-G91E (2 × 105 to 5 × 105 PFU after 7 days), and RA-G91E V296A (2 × 105 to 5 × 105 PFU after 7 days). Shown in the bottom row (left to right) are results with RA (2 × 106 to 5 × 106 PFU after 12 days) and RA-V296A (2 × 106 to 5 × 106 PFU after 12 days). See Results.
Dominance of pseudoreceptor recognition over virulence.
When the virulence determinant at position 296 was introduced into RA, there was no discernable increase in virus spread (Fig. 4, bottom row); animals inoculated with RA or RA-V296A were sacrificed at 12 rather than 7 days, and blots were exposed for longer than usual to look for signs of increased spread. The same substitution on the RA-G91E background produced a rapidly spreading virus. This RA-G91E V296A double mutant (Fig. 4, top right) killed 100% of newborn C3H/BiDa mice (n = 11) within 21 days at a dose of ∼106 PFU/animal and thus acquired a degree of virulence similar to that of LID (2). These results are in line with those for LID-E91G and PTA-E91G V296A, which are nonvirulent despite the fact that they encode alanine at position 296 (see Fig. 2 and the text above). Together, they demonstrate the effective dominance of pseudoreceptor recognition (91G) over virulence (296A) when both specificities are present in the same VP1. The former determinant allows the virus to bind to branched-chain receptors and leads to attenuated spread, while the latter promotes more rapid and extensive virus spread but only on a 91E virus background.
Relative affinities of different VP1s for straight- and branched-chain sialyloligosaccharides.
A relative order of affinities of different VP1s for straight- and branched-chain sugars can be derived from crystal-soaking experiments (32). Apparent dissociation constants are in the millimolar range (Table 4). Affinities for the straight chain are essentially the same for large (91E)- and small (91G)-plaque strains, indicating that highly pathogenic and relatively nonpathogenic strains bind equally well to the true receptor. The small-plaque virus binds with roughly equal affinities to straight and branched sugars, while the large-plaque virus binds with reduced affinity to the branched sugar. Between large-plaque strains, the virulent LID (296A) binds more weakly than the tumorigenic PTA (296V) to the straight-chain sugar. As discussed below, these results can readily be interpreted in a manner consistent with the biological results.
TABLE 4.
Estimates of carbohydrate dissociation constants for different polyomavirus strainsa
| Plaque size | Relevant characteristics of virus strain | Dissociation constant (mM) for:
|
|
|---|---|---|---|
| Straight-chain receptor | Branched-chain receptor | ||
| Small | Gly 91, Val 296 | 5–10 | 5–10 |
| Large | Glu 91, Val 296 | 5–10 | ≪5 |
| Glu 91, Ala 296 | <5 | NAb | |
Dissociation constants are estimates based on crystal-soaking experiments (32). Straight-chain receptor, 3′-sialyllactose; branched-chain receptor, NeuNAc-(α-2,3)-Gal-(β-1,3)-GlcNAc-[(α-2,6)-NeuNAc]-(β-1,3)-Gal-(β-1,4)-Glc.
NA, not applicable.
DISCUSSION
The ability of polyomavirus VP1 to discriminate between straight- and branched-chain sialyloligosaccharides on the cell surface is a powerful determinant of biological behavior in an infected mouse. The structural basis for this discrimination is clear based on the VP1 E-G polymorphism at position 91 (references 32 and 34 and the present results). The importance of receptor discrimination is seen in the facts that newborn mice inoculated with as much as 107 PFU of wild-type RA (91G) frequently develop no tumors but that those receiving as little as 1 to 10 PFU of wild-type PTA (91E) usually develop multiple tumors following extensive virus amplification in the host (reference 12 and unpublished results). PTA must be able to discriminate against binding the branched sugar in order to spread and induce tumors efficiently and, likewise, for LID to cause early death. Substitution of G91 in the VP1s of both viruses greatly reduces virus spread and pathogenicity by allowing binding to the branched oligosaccharide. RA has undiminished affinity for the straight-chain receptor and must therefore owe its relative inability to spread and cause disease to a failure to discriminate and consequently not bind to the branched chain. Substitution of E91 in RA, effectively blocking binding to the branched chain, clearly enhances its ability to spread in newborn mice. While most of these results were carried out with the C3H/BiDa mouse strain (12, 13, 15–18), mice of four other strains known to be susceptible to tumor induction by large-plaque strains also showed strong resistance to the spread of virus carrying the VP1 type that allows recognition of branched-chain receptors.
The most straightforward interpretation of these findings is that glycoproteins bearing branched-chain sialyloligosaccharides, while they may serve as functional receptors for small-plaque strains in cultured mouse cells (10), act predominantly as pseudoreceptors or inhibitors of virus spread in the intact host. These molecules may be present at high densities on cell surfaces in vivo and effectively trap or route the virus to some intracellular site(s) that is nonproductive in terms of infection. Pseudoreceptor molecules may also act intracellularly by blocking virus release, or they may be present extracellularly (e.g., in basement membrane or extracellular matrix), where they may simply bind and sequester the virus of the appropriate VP1 specificity. Branched oligosaccharides with the structure NeuNAc-(α-2,3)-Gal-(β-1,3 or β-1,4)-[NeuNAc-(α-2,6)]-GlcNAc- are found in O-linked carbohydrates (5, 27) but not, as far as we are aware, in N-linked glycoproteins. These observations suggest that while the true receptors are primarily or exclusively N linked (10), the putative pseudoreceptors are O-linked glycoproteins or possibly glycolipids.
Both large- and small-plaque strains have been studied in the laboratory and referred to as wild types based on their growth and transforming properties in culture. It is not clear, however, which type of strain predominates in the wild. Is virus of the small-plaque type able to establish persistent infections and to be selected for based on its low pathogenicity, or is it the potentially pathogenic large-plaque strains with a clear replication advantage that exist preferentially in nature? From the host standpoint, how important is the presence of the putative pseudoreceptor(s) in downregulating pathogenicity? Published accounts of polyomavirus isolation do not resolve these questions, in part because the earliest isolations occurred prior to the development of the plaque assay and also because of the apparently high rate of mutation of the plaque size phenotype when the virus is propagated in culture. These questions are of interest given the importance of the VP1 polymorphism at position 91 in determining not only plaque type in culture but also virus spread in the natural host. New attempts at virus isolation together with PCR and sequence analysis of viral DNAs in tissues of naturally infected wild mice should help to resolve these questions.
ACKNOWLEDGMENTS
This work has been supported by Public Health Service research grants PO1-CA50661 and R35-CA44343 to T.L.B. and CA13202 to S.C.H.
We acknowledge contributions from the following: Rod Bronson for pathology reports; Jean Dahl, Jianmin Gan, Cathy Riney, and Rebbeca Dowgiert for help in generating and screening of hybridomas; Robert Liu for construction of several mutants; and John Carroll, Sherrie Witt, and John Fung for various aspects of the mouse work. Informative discussions on carbohydrate structures with James Paulson, Verne Reinhold, and M. Howard Chen are gratefully acknowledged. We also acknowledge CHESS for access to the F1 beamline.
REFERENCES
- 1.Amalfitano A, Martin L G, Fluck M M. Different roles for two enhancer domains in the organ- and age-specific pattern of polyomavirus replication in the mouse. Mol Cell Biol. 1992;12:3628–3635. doi: 10.1128/mcb.12.8.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer P H, Bronson R T, Fung S C, Freund R, Stehle T, Harrison S C, Benjamin T L. Genetic and structural analysis of a virulence determinant in polyoma VP1. J Virol. 1995;69:7925–7931. doi: 10.1128/jvi.69.12.7925-7931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergelson J M, Shepley M P, Chan B M, Hemler M E, Finberg R W. Identification of the integrin VLA-2 as a receptor for echovirus 1. Science. 1992;255:1718–1720. doi: 10.1126/science.1553561. [DOI] [PubMed] [Google Scholar]
- 4.Bolen J B, Fisher S E, Chowdhury K, Shan C, Williams J E, Dawe C J, Israel M A. A determinant of polyomavirus virulence enhances virus growth in cells of renal origin. J Virol. 1985;53:335–339. doi: 10.1128/jvi.53.1.335-339.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockhausen I, Schutzbach J, Kuhns W. Glycoproteins and their relationship to human disease. Acta Anat. 1998;161:36–78. doi: 10.1159/000046450. [DOI] [PubMed] [Google Scholar]
- 6.Cahan L D, Paulson J C. Polyoma virus recognizes specific sialyloligosaccharide receptors on eythrocytes. Virology. 1980;103:505–509. doi: 10.1016/0042-6822(80)90208-1. [DOI] [PubMed] [Google Scholar]
- 7.Cahan L D, Singh R, Paulson J C. Sialyloligosaccharide receptors of binding variants of polyoma virus. Virology. 1983;130:281–289. doi: 10.1016/0042-6822(83)90083-1. [DOI] [PubMed] [Google Scholar]
- 8.Carroll J P, Fung J S, Bronson R T, Razvi E, Benjamin T L. Radiation-resistant and radiation-sensitive forms of host resistance to polyomavirus. J Virol. 1999;73:1213–1218. doi: 10.1128/jvi.73.2.1213-1218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M C, Redenius D, Osati-Ashtiani F, Fluck M M. Enhancer-mediated role for polyomavirus middle T/small T in DNA replication. J Virol. 1995;69:326–333. doi: 10.1128/jvi.69.1.326-333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M H, Benjamin T L. Roles of N-glycans with α2,6 as well as α2,3 linked sialic acid in infection by polyoma virus. Virology. 1997;233:440–442. doi: 10.1006/viro.1997.8596. [DOI] [PubMed] [Google Scholar]
- 11.Colonno R J, Callahan P L, Long W J. Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses. J Virol. 1986;57:7–12. doi: 10.1128/jvi.57.1.7-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawe C J, Freund R, Mandel G, Balmer-Hofer K, Talmage D A, Benjamin T L. Variations in polyoma virus genotype in relation to tumor induction in mice: characterization of wild type strains with widely differing tumor profiles. Am J Pathol. 1987;127:243–261. [PMC free article] [PubMed] [Google Scholar]
- 13.Dubensky T W, Freund R, Dawe C J, Benjamin T L. Polyomavirus replication in mice: influences of VP1 type and route of inoculation. J Virol. 1991;65:342–349. doi: 10.1128/jvi.65.1.342-349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eddy B E. Polyoma virus. Virol Monogr. 1969;7:1–114. [Google Scholar]
- 15.Freund R, Calderone A, Dawe C J, Benjamin T L. Polyomavirus tumor induction in mice: effects of polymorphisms of VP1 and large T antigen. J Virol. 1990;65:335–341. doi: 10.1128/jvi.65.1.335-341.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freund R, Dawe C J, Benjamin T L. Duplication of noncoding sequences in polyoma virus is required for the development of thymic tumors in mice. J Virol. 1988;62:3896–3899. doi: 10.1128/jvi.62.10.3896-3899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freund R, Garcea R L, Sahli R, Benjamin T L. A single-amino-acid substitution in polyomavirus VP1 correlates with plaque size and hemagglutination behavior. J Virol. 1991;65:350–355. doi: 10.1128/jvi.65.1.350-355.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freund R, Mandel G, Carmichael G G, Barncastle J, Dawe C J, Benjamin T L. Polyomavirus tumor induction in mice: influences of viral coding and non-coding sequences on tumor profiles. J Virol. 1987;61:2232–2239. doi: 10.1128/jvi.61.7.2232-2239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried H, Cahan L D, Paulson J C. Polyoma virus recognizes specific sialyloligosaccharide receptors on host cells. Virology. 1981;109:188–192. doi: 10.1016/0042-6822(81)90485-2. [DOI] [PubMed] [Google Scholar]
- 20.Greve J M, Davis G, Meyer A M, Forte C P, Yost S C, Marlor C W, Karmarck M E, McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 21.Gross L G. Oncogenic viruses. 3rd ed. Vol. 2. New York, N.Y: Pergamon Press, Inc.; 1983. The polyoma virus; pp. 737–818. [Google Scholar]
- 22.Herrmann M, von der Lieth C L, Stehling P, Reutter W, Pawlita M. Consequences of a subtle sialic acid modification on the murine polyomavirus receptor. J Virol. 1997;71:5922–5931. doi: 10.1128/jvi.71.8.5922-5931.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawano T, Suzuki A. Molecular cloning of cytidine monophospho-N-acetylneuraminic acid hydroxylase. Regulation of species- and tissue-specific expression of N-glycolyl neuraminic acid. J Biol Chem. 1995;270:16458–16463. doi: 10.1074/jbc.270.27.16458. [DOI] [PubMed] [Google Scholar]
- 24.Kono M, Ohyama Y, Lee Y-C, Hamamoto T, Kojima N, Tsuji S. Mouse β-galactoside α2,3-sialyltransferases: comparison of in vitro substrate specificities and tissue specific expression. Glycobiology. 1997;7:469–479. doi: 10.1093/glycob/7.4.469. [DOI] [PubMed] [Google Scholar]
- 25.Lukacher A E, Ma Y, Carroll J P, Abromson-Leeman S R, Laning J C, Dorf M E, Benjamin T L. Susceptibility to tumors induced by polyoma virus is conferred by an endogenous mouse mammary tumor virus superantigen. J Exp Med. 1995;181:1683–1692. doi: 10.1084/jem.181.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Main J H, Dawe C J. Tumor induction in transplanted tooth buds infected with polyoma virus. J Natl Cancer Inst. 1966;36:1121–1136. [PubMed] [Google Scholar]
- 27.Marth J D. Complexity in O-linked oligosaccharide biosynthesis engendered by multiple polypeptide N-acetylgalactosaminyltransferases. Glycobiology. 1996;6:701–705. doi: 10.1093/glycob/6.7.701. [DOI] [PubMed] [Google Scholar]
- 28.Rochford R, Campbell B A, Villarreal L P. Genetic analysis of the enhancer requirements for polyomavirus DNA replication in mice. J Virol. 1990;64:476–485. doi: 10.1128/jvi.64.2.476-485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rochford R, Moreno J P, Peake M L, Villarreal L P. Enhancer dependence of polyomavirus persistence in mouse kidneys. J Virol. 1992;66:3287–3297. doi: 10.1128/jvi.66.6.3287-3297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochford R, Villarreal L P. Polyomavirus DNA replication in the pancreas and in a transformed pancreas cell line has distinct enhancer requirements. J Virol. 1991;65:2108–2112. doi: 10.1128/jvi.65.4.2108-2112.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe W P, Hartley J W, Estes J D, Huebner R J. Studies of mouse polyoma virus infection. Procedures for quantitation and detection of virus. J Exp Med. 1959;109:379–391. doi: 10.1084/jem.109.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stehle T, Harrison S C. Crystal structures of murine polyomavirus in complex with straight-chain and branched-chain sialyloligosaccharide receptor fragments. Structure. 1996;4:183–194. doi: 10.1016/s0969-2126(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 33.Stehle T, Harrison S C. High-resolution structure of a polyomavirus VP1-oligosaccharide complex: implications for assembly and receptor binding. EMBO J. 1997;16:5139–5148. doi: 10.1093/emboj/16.16.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stehle T, Yan Y, Benjamin T L, Harrison S C. The structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature. 1994;369:160–163. doi: 10.1038/369160a0. [DOI] [PubMed] [Google Scholar]